Abstract
The yeast Yarrowia lipolytica is distantly related to Saccharomyces cerevisiae, can be genetically modified, and can grow in both haploid and diploid states in either yeast, pseudomycelial, or mycelial forms, depending on environmental conditions. Previous results have indicated that the STE and RIM pathways, which mediate cellular switching in other dimorphic yeasts, are not required for Y. lipolytica morphogenesis. To identify the pathways involved in morphogenesis, we mutagenized a wild-type strain of Y. lipolytica with a Tn3 derivative. We isolated eight tagged mutants, entirely defective in hyphal formation, from a total of 40,000 mutants and identified seven genes homologous to S. cerevisiae CDC25, RAS2, BUD6, KEX2, GPI7, SNF5, and PPH21. We analyzed their abilities to invade agar and to form pseudomycelium or hyphae under inducing conditions and their sensitivity to temperature and to Calcofluor white. Chitin staining was used to detect defects in their cell walls. Our results indicate that a functional Ras-cyclic AMP pathway is required for the formation of hyphae in Y. lipolytica and that perturbations in the processing of extracellular, possibly parietal, proteins result in morphogenetic defects.
The yeast-to-hypha morphological transition is typical of many fungi and seems to be important for the pathogenesis of fungi such as Ustilago maydis (44) and Candida albicans (23). Several groups have reported that strains of C. albicans that cannot form hyphae are avirulent in mice (16, 34, 41, 69). Thus, the characterization of the genes involved in dimorphism may lead to the discovery of new treatments for pathogenic fungi.
C. albicans lacks a sexual cycle and is a diploid organism (51); therefore, other yeasts are usually used as models because they are easier to manipulate. Saccharomyces cerevisiae was the first model used to unravel the mechanisms underlying the dimorphic transition in yeasts and remains the best model (25). Numerous genes involved in the regulation of pseudofilamentous growth in S. cerevisiae have been identified (3, 15, 45, 52). These studies identified three pathways that couple afferent signals to cellular switches. The major pathway is the STE or mitogen-activated protein (MAP) kinase pathway, which mediates the mating pheromone response. In this pathway, at least four components participate in induction of filamentous growth of diploid cells and invasiveness of haploid cells (40). The second pathway is the cyclic AMP (cAMP) pathway (42), in which Ras2p and protein kinase A (Tpk2p) have prominent roles. The third pathway is less well understood and may involve the Rim101p zinc finger transcription factor (38). However, because S. cerevisiae does not display true hyphal growth, several issues could not be properly addressed in this yeast. Moreover, several studies have suggested there are major differences between C. albicans and S. cerevisiae, especially concerning the respective contributions of the three pathways that trigger the dimorphic switch: in S. cerevisiae the MAP kinase pathway seems to be the most important, whereas the cAMP-protein kinase A pathway is predominant in C. albicans (20). Indeed, disruption of the MAP kinase pathway dramatically impairs the dimorphic switch in S. cerevisiae, whereas disruption of the cAMP pathway has only slight effects (40, 43). In C. albicans, the opposite is true (11, 22).
Therefore, investigation of the genetic determinants of morphogenesis in different yeasts should identify which elements are conserved and how the pathway evolved in fungi. Thus, a genetic study of dimorphism was undertaken with another yeast that can be genetically modified, Yarrowia lipolytica (53). Like C. albicans, Y. lipolytica displays a complete yeast-to-hypha transition. Conversely, it has a well-explored sexual cycle that facilitates genetic analysis, and efficient transformation systems have been developed (4). Its budding patterns and germ tube formation are well understood (29). Unexpectedly, mutations in the STE and RIM pathways do not affect morphogenesis in this yeast (17).
In this study, we used a recently developed gene-tagging approach to identify genes required for filamentous growth in Y. lipolytica (49, 57). We report the preliminary analysis of six genes involved in morphogenesis in Y. lipolytica and a seventh gene that has already been identified (XPR6) (18).
MATERIALS AND METHODS
Yeast strains and microbial techniques.
The Y. lipolytica isogenic strains used in this study were W29 [MatA] and PO1a [MatA, ura3-302, leu2-270] (4). They were grown on YPD (4) or on YNB medium composed of 1.7 g of yeast nitrogen base (Difco) per liter without amino acids or ammonium sulfate. When required, 1 g of glutamate per liter, 10 g of glucose per liter, 50 μg of uracil per ml, and 50 μg of leucine per ml were added. Hyphal induction was tested with a mixture of 1% serum (horse serum; Sigma) and 2% agarose. Agar invasion tests were carried out as previously described (67). All cultures were grown at 28°C, except as otherwise stated. Escherichia coli DH5α was grown at 37°C in Luria-Bertani medium supplemented with 100 μg of ampicillin per ml when required.
Genetic techniques.
Standard molecular genetic techniques were used (58). Restriction enzymes and polymerases were supplied by Gibco BRL (France) or New England Biolabs. Genomic DNA was prepared from yeast transformants as previously described (4). DNA was digested with SacI, separated on a 0.8% agarose gel, and transferred onto Hybond-N+ nylon membranes for Southern blotting (Amersham Pharmacia Biotech). Probes were labeled with either the ECL (enhanced chemiluminescence) Direct Nucleic Acid Labeling and Detection system (Amersham Pharmacia Biotech) or with [32P]dCTP by use of the MegaPrime kit (Amersham Pharmacia Biotech). A Perkin-Elmer Thermal Cycler 9600 was used for PCRs. Sequencing was carried out on an ABI 373 DNA Sequencer. The GCG package (Genetics Computer Group, University of Wisconsin, Madison) was used for sequence analysis.
Construction of Y. lipolytica mutants.
PO1a, a wild-type strain derivative, was chosen for mutagenesis because its dimorphic phenotype is stronger than inbred lines (unpublished observations). We used a W29 genomic library (ca. 2 kb) randomly mutagenized in E. coli by mTnYl1 (49, 57). mTnYl1 is a Tn3-based transposon that carries the Y. lipolytica gene YlURA3 as a selective marker. Four pools of mutagenized Y. lipolytica DNA were digested separately with NotI to release the transposed inserts. They were used to transform PO1a by the lithium acetate method with minor modifications (49): 2 μl of a dimethyl sulfoxide (DMSO) solution (1:10 in water) was added to 200 μl of competent cells before addition of DNA. The addition of DMSO slightly increased the transformation efficiency of the library to 5 × 103 to 5 × 104 transformants per μg of DNA. Transformed cells were plated on YNB medium supplemented with 0.2% Casamino Acids (Difco), but without uracil.
Isolation and characterization of disrupted loci.
Chromosomal fragments flanking mTnYl1 insertion sites were amplified by reverse PCR (68) on genomic DNA digested with SacI, with either the mtn1/juan1 or mtn6/juan2 primers (Fig. 1) and the Expand Long Template PCR system (Boehringer Mannheim GmbH). The following PCR cycling conditions were used: 2 min at 94°C, followed by 10 cycles of 10 s at 94°C, 30 s at 56°C, and 10 min at 68°C; 20 cycles of 10 s at 94°C, 30 s at 56°C, and 10 min at 68°C with 15 s of ramping; and a final extension step of 15 min at 68°C. Each PCR product was sequenced with the mtn1 and mtn6 primers, and the sequence of the disrupted locus was assembled after trimming one of the 5-bp repeats created by transposition. The sequence on both sides of mTnYl1 was extended by primer walking on both strands. The following web pages were used for blastx analysis and to search for open reading frames (ORFs), respectively: http://www.ncbi.nlm.nih.gov/blast/blast.cgi and http://www3 .ncbi.nlm.nih.gov/gorf/gorf.html.
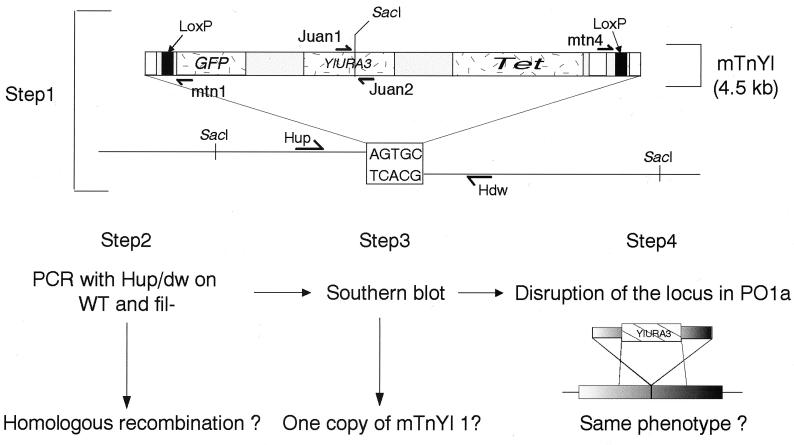
FIG. 1.
General mutation strategy. In step 1, the mTnYl1 flanking regions were sequenced to design the amplification primers Hup and Hdw. In step 2, the homologous integration of mTnYl1 was checked with the two primers. WT, wild type; fil-, Fil−. In step 3, the presence of a single copy of mTnYl1 in the mutants was checked by Southern blot analysis with mTnYl1 as a probe. In step 4, the wild-type locus was disrupted with a cassette derived from the mutated locus and containing the YlURA3 gene as a marker, and the phenotype was verified.
Construction of a plasmid expressing the site-specific recombinase Cre.
The Cre recombinase mediates site-specific excision of DNA flanked by a pair of loxP sites (59). To excise the YlURA3 marker from mTnYl1 integrated in the Y. lipolytica genome (Fig. 1), we designed the replicative plasmid pRRQ2 carrying the LEU2 marker and expressing the CRE gene. A hybrid promoter, hp4d, was excised from pINA1269 by digestion with KpnI and SalI (Table 1) and ligated into pINA1053 that had been cut with KpnI and SalI to yield pRRQ1. Next, the CRE gene from pSH47 (kindly provided by J. H. Hegemann) was excised by digestion with KpnI and SmaI and ligated into pRRQ1 that had been digested with KpnI and PmlI to yield pRRQ2.
TABLE 1.
Characteristics of plasmids used in this study
| Plasmid | Description | Source |
|---|---|---|
| pBluescript | ColE1 lacZ bla | Stratagene |
| pG209 | 1 kb of the YlGPI7 gene in pGEM-T easy | This study |
| pG209URA | YlURA3 gene in pG209 | This study |
| pG354/CRE | 1.3 kb of fil354 locus after mTnYl1 excision in pGEM-T easy | This study |
| pG354/CRE/URA | YlURA3 gene in pG354/CRE | This study |
| pGEM-T easy | oriF1 lacZ bla | Promega Corp., Madison, Wis. |
| pINA156 | YlURA3 in pUC13 | This laboratory collection |
| pINA240 | YlLEU2 ARS68 in pBR322 | This laboratory collection |
| pINA444 | YlLEU2 ARS68 in pBR322 | This laboratory collection |
| pINA1053 | YlLEU2 ARS68 lacZ | This laboratory collection |
| pINA1269 | YlLEU2 ARS68 PH4D promoter XPR2 terminator | This laboratory collection |
| pICT246 | YlLEU2 ARS18; HOY1 with its own promoter | 66 |
| pKSURA | YlURA3 in pBluescript | 70 |
| pMGF1 | YlLEU2, YlPHD1 in pBluescript | A. Dominguez laboratory collection |
| pMR1 | 2.6 kb of the fil316 disruption cassette in pGEM-T easy | This study |
| pMR2 | 0.9-kb fragment of YlRAS2 gene containing I-SceI site in pGEM-T easy | This study |
| pMR3 | 2.1-kb fragment containing YlURA3 in pMR1 at I-SceI site | This study |
| pMR4 | YlPHD1 from p1.69 in pINA240 | This study |
| pMR5 | 2.5 kb of the fil23 disruption cassette in pGEM-T easy | This study |
| pNEB193 | ori F1 lacZ bla | New England Biolabs, Inc., Beverly, Mass. |
| pRRQ1 | 0.8-kb fragment containing promoter from p1269 in pINA1053 | This study |
| pRRQ2 | 1.4-kb fragment containing CRE from pSH47 in pRRQ1 | This study |
| pRRQ10 | HOY1 from pJCT246 in pINA444 | This study |
| pSH47 | YlURA3 ARSH4 CEN6 CRE under GAL1 promoter and CYC1 terminator | 26 |
| pUCURA | 1.2-kb fragment of YlURA3 from pINA156 in pNEB193 | This study |
| p1.69 | HOY1 gene in pBluescript | A. Dominiguez laboratory collection |
Other plasmid construction.
pJCT246, carrying HOY1 and YlLEU2, pMGF1, carrying YlPHD1 and YlLEU2, and p1.69, a pBluescript vector (Stratagene, La Jolla, Calif.) carrying YlPHD1, were kindly provided by A. Dominguez. To construct pMR4 carrying YlPHD1 and YlURA3, the ApaI-XbaI fragment of p1.69, containing YlPHD1, was ligated into pINA240 that had been digested with ApaI and BglII by using an XbaI-BglII adapter. To construct pRRQ10, carrying HOY1 and YlURA3, the HOY1 gene was obtained as two fragments by digesting pJCT246 with BamHI-XhoI and EcoRI-XhoI, and both fragments were ligated into BamHI-EcoRI-digested pINA444.
Construction of disruption cassettes.
Different strategies were used. (i) In the case of Fil316, YlURA3 was inserted between the mTnYl1 flanking regions at the disrupted locus. The fragments were amplified by PCR with PO1a genomic DNA. Two oligonucleotides, 3161up and 3161dw (Table 2), were used to amplify an AvrII-ended 720-bp DNA fragment; two other oligonucleotides, 3162up and 3162dw, were used to amplify a ClaI-ended 760-bp fragment. The YlURA3 gene was extracted from pKSURA (Table 1) by digestion with AvrII and ClaI. These three DNA fragments were ligated and amplified with 3161up and 3162dw to generate a single DNA fragment. The same strategy was used for Fil23 and resulted in a 2.5-kb fragment corresponding to YlURA3 flanked by the 710 bp and 610 bp that flanked mTnYl1 in Fil23. (ii) In the case of Fil345, two primer pairs (3451upL/3451dwSceI and 3452upSceI/3452dwL) were used to amplify mTnYl1-flanking regions (630- and 300-bp DNA fragments, respectively). These were annealed through the I-SceI site and ligated by PCR amplification. The resulting fragment was ligated into pGEM-T easy to yield pMR2. The YlURA3 gene was excised from pKSURA by I-SceI digestion and ligated into pMR2 that had been digested with I-SceI to yield pMR3. pMR3 was digested with EcoRI to release the 2.1-kb disruption cassette. (iii) In the case of fil209, two primers, 209DEL1 and 209DEL2, were used to amplify a 1-kb DNA fragment of the PO1a genome containing the integration locus, which was ligated into pGEM-T easy to yield pG209. YlURA3, rescued from pINA156 by digestion with XbaI and DraI, was inserted into the cloned locus at the naturally occurring PmlI and XbaI restriction sites, yielding pG209Ura. A 2.2-kb disruption cassette was generated from pG209Ura by use of 209DEL1 and 209DEL3. Similarly, in the case of fil246, two oligonucleotides, 246DEL1 and 246DEL2, were used to amplify a 2-kb fragment of PO1a that spanned the insertion site. The YlURA3 gene was extracted from pINA156 by digestion with HincII, and was digested by either PstI or NcoI. These fragments were separately ligated into the 2-kb PCR fragment that had been digested with PmlI. The ligation products were amplified with 246DEL1/URA3-A and 246DEL2/URA3-B, digested with XmaI, which cuts within YlURA3, ligated together, and amplified by PCR with 246DEL1 and 246DEL2 to yield the 3.2-kb disruption cassette. (iv) The fourth strategy involved transforming fil354 with pRRQ2 to excise most of mTnYl1 (Fig. 1), rendering the strain fil354/CRE Ura−. Two oligonucleotides, 354DEL1 and 354DEL2, were used to amplify a 1.3-kb fragment from fil354/CRE genomic DNA with unique BamHI and KpnI sites in the genomic DNA next to an mTnYl1 insertion. This fragment was ligated in pGEM-T easy to yield pG354/CRE. A KpnI-BamHI fragment carrying YlURA3 was ligated into BamHI- and KpnI-digested pG354/CRE to yield pG354/CRE/URA. Amplification with 354DEL1 and 354DEL2 yielded a 2.5-kb disruption cassette.
TABLE 2.
Oligonucleotide sequences of primers used in this study
| Primer | Sequence (5′→3′)a |
|---|---|
| 3161up | AATCTCACGTCCGAATGTCC |
| 3161dw | AATAGCCCTAGGTCTACCACTCGTTTGGTCCC |
| 3162up | AATAGCATCGATAGATCTCATATCGCTTGGCG |
| 3162dw | GATGAACGGCATGAACATGG |
| 3451upL | GTGTTACCGGTATAAGTCGCACG |
| 3451dwSceI | CATTACCCTGTTATCCCTAATAGCATCAAGGGTGGTACCAAGTG |
| 3452upSceI | CTAGGGATAACAGGGTAATTGTTGACCGGTGGTCTGAGTCGACC |
| 3452dwL | TTTGTGTTGTGGAGCTGTGCCATCG |
| 209DEL1 | GACATAGTTACGTGGCTGTCCAAGG |
| 209DEL2 | GATGCTTCTCTGAGGAAAAAGCAAG |
| 209DEL3 | CCCCTCATATTCTCATCTCAATCCC |
| 246DEL1 | CGATAGTGACGCTGACATTCGTCTG |
| 246DEL2 | GGGTCTGAGATGGAACGAAATAAGC |
| URA3-A | CCACCAAAATGCCCTCC |
| URA3-B | TGGTAGTGCAGTGGTGG |
| 354DEL1 | CACCCCACTACACAAACTATGCAAC |
| 354DEL2 | CATCTGATGCTGGCAATCGACGAAC |
| mtn1 | GGAGTTATCCGAAGCGATAGCG |
| mtn6 | GCGGGCTATCCCTATGAC |
| juan1 | GGCGTAGGTGAGTCGTC |
| juan2 | CTCAAGCTCGTGGCAGCC |
| Adapter XbaI | CTAGAGAACCCCTTCG |
| Adapter BglII | TCTTGGGGAAGCCTAG |
Underlined segments represent restriction sites used for cloning.
These cassettes were used to transform PO1a by homologous recombination. All disruptions were confirmed by Southern blot analysis.
Staining procedures.
Chitin was stained with Calcofluor white (1 mg/ml) in the dark at room temperature for 30 min on log-phase cells that had been washed twice in distilled water. After four washings, the cells were suspended in one drop of immunofluorescence mounting solution (100 mg of p-phenylenediamine in 10 ml of phosphate-buffered saline and 90 ml of glycerol) for observation.
Nucleotide sequence accession number.
Sequences were deposited in the GenBank database under accession no. AF321464, AF321465, AF321466, AF321467, AF321468, and AF321469.
RESULTS
Isolation and preliminary characterization of mutants affected in morphology.
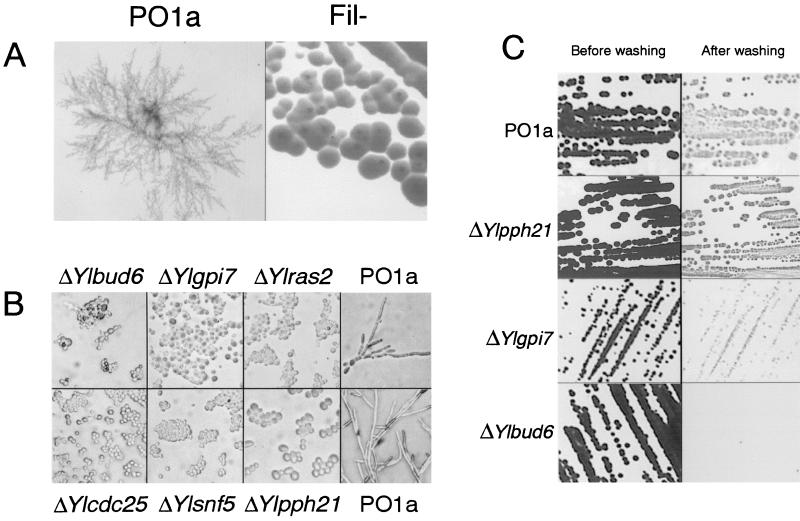
PO1a was mutagenized by use of the mTnYl1 transposed-genomic DNA library of Y. lipolytica (see Materials and Methods). After 7 days, approximately 40,000 transformants were screened visually for smooth colonies; candidate mutants were rechecked after 2 weeks of growth on YPD. A total of 99 mutants with defects in morphogenesis were retained. These mutants were screened a third time after 16 h on 1% serum solid medium; this caused PO1a to switch to the mycelial form immediately (Fig. 2A). Fifty mutants with a clear Fil− phenotype were finally retained.
FIG. 2.
Mutant phenotypes. (A) Colony phenotype of PO1a and Fil− mutants incubated for 2 days on serum medium at 28°C. (B) Cell phenotype of six mutants and PO1a incubated for 2 days on serum medium at 28°C. (C) Invasive phenotype of PO1a and of three mutants incubated for 2 days on YPD medium at 28°C. Results are shown for plates before and after being washed with sterile water.
The mutants were analyzed in three steps (Fig. 1). First, we sequenced the fragments flanking the transposon after reverse PCR (see Materials and Methods). Second, to check whether the disruption cassette integrated by a single homologous recombination event, we designed primer pairs for each locus that annealed approximately 200 bp either side of the transposon integration site (Hup and Hdw [see Fig. 1]) and amplified the target locus from mutant and PO1a genomic DNA. If nonhomologous recombination had occurred, two fragments were amplified: a small fragment (400 to 500 bp) corresponding to the undisrupted wild-type locus and a larger fragment (>5 kb) corresponding to the disrupted locus. If homologous recombination occurred, a single fragment (>5 kb) was amplified (Fig. 1). Third, we checked whether a single copy of the transposon was present in the mutants, by using the transposon as a Southern blot probe.
The first step rejected 11 mutants because no PCR product was obtained for one or both sides of the transposon. The remaining 39 mutants were sequenced, and 7 were found to contain part or all of the plasmid pHSS6 integrated in the genome, suggesting illegitimate integration. Finally, two mutants were dismissed because inverted 5-bp repeats did not flank the transposon, indicating rearrangement during integration.
In the second step, PCR was used to test the 30 remaining mutants for homologous integration. Only 11 mutants yielded the expected pattern; Southern blotting showed that they all resulted from a single integration of mTnYl1 (data not shown).
Preliminary analysis of the sequences flanking mTnYl1 showed that two mutants resulted from independent disruptions of the XPR6 locus, i.e., the Y. lipolytica KEX2 homolog, which is involved in morphogenesis (18), and no ORF could be detected at the loci disrupted in the Fil34 and Fil78 mutants. These four clones were not analyzed further.
Identification of the disrupted genes.
To confirm that the Fil− phenotype resulted from an mTnYl1 insertion and not from a secondary mutation, we tried to redisrupt each of the six loci in PO1a. Different approaches involved either whole genomic DNA from the transposants or amplified mTnYl1-disrupted loci by PCR. These attempts resulted in a high frequency of nonhomologous integration events, so we finally constructed YlURA3 disruption cassettes (see Materials and Methods). At least five independent transformants resulting from homologous integration were analyzed in each case. The phenotypes of the original mutants and of the YlURA3 disruptants were identical for Fil23, Fil209, Fil316, Fil345, and Fil354, but not for Fil243, which was thus discarded. When we tried to construct the YlURA3 disruption cassette in Fil51, the expected PCR products were not obtained, possibly because the corresponding ORF is part of a conserved gene family (see below). Although we could not confirm that its Fil− phenotype was linked to mTnYl1, we tentatively retained Fil51.
Nucleotide sequences flanking mTnYl1 in Fil23, Fil209, Fil316, Fil345, Fil354, and Fil51 were obtained over 400 to 600 bp on both sides and assembled to reconstitute the disrupted ORFs. Preliminary analysis of these partial amino acid sequences suggested that mTnYl1 disrupted the following S. cerevisiae homologs: Bud6p for Fil23, Pph21p for Fil51, Gpi7p for Fil209, Snf5p for Fil316, Ras2p or Ras1p for Fil345, and Cdc25p or Sdc25p for Fil354. None of the disrupting events resulted in an in-frame fusion of green fluorescent protein (GFP), except for YlBUD6, but no GFP expression could be detected in this case.
The results of all of the phenotypic tests undertaken with the six mutant strains (see below) are summarized in Table 3.
TABLE 3.
Phenotypes of the Y. lipolytica Fil− mutants
| Disrupted gene | Calcofluor sensitivity | Abnormal chitin pattern | Agar invasion | Filamentation | Growth at 33°C | Effect of:
|
|
|---|---|---|---|---|---|---|---|
| HOY1 | YIPHD1 | ||||||
| YlCDC25 | No | No | No | Fil− | Yes | Fil− | Fil− |
| YlRAS2 | No | No | No | Fil− | Yes | Fil− | Pseudo+ |
| YlBUD6 | Yes | Yes | No | Fil− | No | Fil− | Fil− |
| YlGPI7 | Yes | No | Low | Fil− | Yes | Fil+ | Fil− |
| YlSNF5 | Slightly | No | No | Fil− | Yes | Fil+ | Fil− |
| YlPPH21 | No | No | Yes | Fil− | Yes | Fil− | Fil− |
The Y. lipolytica BUD6 homolog (YlBUD6)
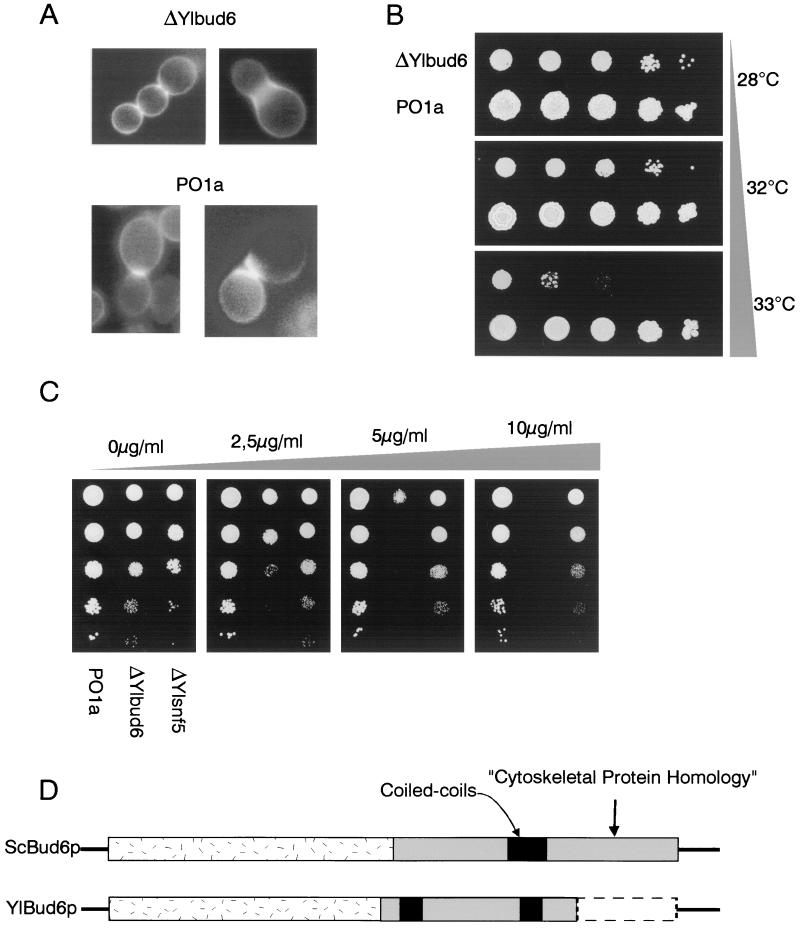
We sequenced 1,980 nucleotides (nt) of the YlBUD6 gene corresponding to 660 amino acids in the N-terminal region of the protein. We found that the amino acid sequence displayed 37% identity to the amino acid sequence of Bud6p, an actin-interacting protein required for bipolar budding, from S. cerevisiae (2, 71). Comparison of the predicted Y. lipolytica and S. cerevisiae amino acid sequences (Fig. 3D) showed that a coiled-coil region (amino acids 415 to 445 and amino acids 582 to 615) within a large domain (amino acids 390 to 630) homologous to cytoskeletal proteins was highly conserved. Studies of S. cerevisiae BUD6 deletion mutants indicated that Bud6p is important for the maintenance of cell shape, bud site location, and polarized growth (2, 61). Deletion of YlBUD6 results in the same morphological defects: cells were rounder than in the wild type, and there were no septa. Moreover, deleted cells form an aberrant pseudomycelium with many buds per cell on YPD medium and no pseudomycelium or hyphae on serum inducing media (Fig. 2B). Cultures lost the typical invasive growth phenotype on YPD medium (Fig. 2C). Diffuse chitin staining was observed in most mutant cells and especially in chain cell structures, but was not localized in the bud neck (Fig. 3A). Staining with rhodamine-phalloidin revealed few or no actin patches (not shown), suggesting that, like in S. cerevisiae, YlBUD6 is involved in actin localization. Also like in S. cerevisiae, mutant cells were thermosensitive (Fig. 3B) and sensitive to Calcofluor white on solid medium (Fig. 3C), indicating cell wall defects.
FIG. 3.
YlBUD6 gene structure and preliminary characterization of ΔYlbud6 mutant. (A) Chitin stain with Calcofluor white of cells disrupted for Ylbud6 and of PO1a cells. (B) Temperature sensitivity test. Serial 1/10 dilutions of cultures grown overnight in liquid YPD medium at 28°C were plated out on YPD medium and incubated at the indicated temperature for 16 h. (C) Calcofluor white sensitivity test. Serial 1/10 dilutions of cultures grown overnight in liquid YPD medium at 28°C were plated out on YPD medium containing the indicated concentrations of Calcofluor white and incubated at 28°C. The results were observed after 16 h. (D) Comparison of ScBud6p and YlBud6p.
The Y. lipolytica RAS2 homolog (YlRAS2).
The complete YlRAS2 sequence encodes a predicted protein of 249 amino acids displaying 43% identity to Ras2p and 40% identity to Ras1p of S. cerevisiae. The YlRAS2 gene contains a 348-bp intron inserted at nt 53 of the ORF, with typical Y. lipolytica splicing sites: 5′-GAGTAG-3′, 5′-TACTAAC-3′, and 5′-CAG-3′ for the donor, internal, and acceptor sites, respectively (54, 64). S. cerevisiae possesses two Ras homologs, RAS1 and RAS2. The complete predicted amino acid sequence for the disrupted ORF in Fil345 is slightly more similar to Ras2p than to Ras1p (see above) and is, therefore, tentatively referred to as Ras2p. The first 170 amino acids of the N-terminal domain of YlRas2p are nearly 90% identical to Ras homologs from other organisms, and the last 4 amino acids, CCVI, conform to the CAAX consensus, which is crucial for the processing of Ras and its anchorage to the plasma membrane (33). The guanine nucleotide-binding site, GXXXXGK (residues 17 to 23 for Ras1p and Ras2p in S. cerevisiae), is also found (GEGGTGK) in YlRas2p between residues 15 and 21. Mutant cells disrupted for YlRAS2 display a complete lack of formation of pseudohyphae or hyphae on YPD or on serum inducing medium (Fig. 2B) and do not invade agar (not shown), but no particular pattern was seen after chitin staining. These cells were neither Calcofluor white nor temperature sensitive.
The Y. lipolytica CDC25 homolog (YlCDC25).
We sequenced 4,518 nt of YlCDC25 corresponding to the 1,506 C-terminal amino acids of the protein, including the conserved GTP exchange factor (GEF) domain that was disrupted by mTnYl1. The predicted amino acid sequence of 1,506 amino acids is 33 and 31.5% identical to Cdc25p and Sdc25p S. cerevisiae proteins, respectively. Although the gene nomenclature is somewhat arbitrary in the absence of the whole gene sequence, we chose YlCDC25 because SDC25 mutations have no obvious phenotype in S. cerevisiae in the presence of a wild-type copy of CDC25 (9). Cells disrupted for YlCDC25 do not undergo pseudohyphal or hyphal transition on YPD or on serum medium (Fig. 2) and are not invasive (data not shown). Chitin staining and Calcofluor white and temperature sensitivity were similar in the mutant and in the wild-type strain.
The Y. lipolytica SNF5 homolog (YlSNF5).
The complete sequence of YlSNF5 encodes a predicted protein of 735 amino acids with 31% identity to Snf5p (905 amino acids) of S. cerevisiae. The N terminus (bp 31 to 324) of Snf5p is rich in glutamine (42%) and proline (11%), whereas the N-terminal sequence of YlSnf5p (bp 1 to 222) is rich in glutamine (28%), glycine (25%), and methionine (21%). Such domains may have a transcriptional activator role, although this region can be greatly reduced in size without loss of Snf5p function. Indeed, in S. cerevisiae, the N-terminal glutamine- and proline-rich region could be trimmed down from 125 Gln and 37 Pro residues to 15 Gln and 9 Pro residues, without any effect on Snf5p function (37). Comparison of Snf5p from S. cerevisiae, humans, and Y. lipolytica revealed that two imperfect repeat motifs spanning amino acids 389 to 436 and 477 to 536 in YlSnf5p were conserved. The roles of these repeat motifs have not yet been elucidated. In Y. lipolytica cells with YlSNF5 deleted, neither pseudofilament or filament formation nor invasive growth could be observed on inducing medium (Fig. 2B). ΔYlsnf5 cells and wild-type strains had similar chitin staining patterns and temperature sensitivities. A slight sensitivity to Calcofluor white was reproducibly observed only at the highest concentration tested (Fig. 3C).
The Y. lipolytica PPH21 homolog (YlPPH21).
The complete sequence of YlPPH21 encodes a predicted protein of 380 amino acids with 74% and 72.5% identity to Pph21p and Pph22p of S. cerevisiae, respectively, and 82% identity over the last 250 amino acids. However, like in mammals or Aspergillus fumigatus, the N-terminal 60-acidic-amino-acid stretch is absent. Cells carrying ΔYlpph21 stay in the yeast form on inducing medium, but retain invasive growth (Fig. 2B and C). This indicates that pseudomyceliation and myceliation are not necessary for agar invasion, as suggested for S. cerevisiae (46). No difference was observed between mutant and wild-type cells for chitin staining nor for Calcofluor white and temperature sensitivity.
The Y. lipolytica GPI7 homolog (YlGPI7).
The complete sequence of YlGPI7 encodes a predicted protein of 860 amino acids with 33% identity with Gpi7p (830 amino acids) of S. cerevisiae. Like GPI7/LAS21, YlGPI7 is predicted to encode an integral membrane protein. Six to eight transmembrane domains (TDs) and three putative glycosylation sites are predicted, which are fewer than the numbers found in Gpi7p, which has 9 to 11 TDs and five putative glycosylation sites. No conserved motifs were identified. YlGPI7 deletion mutants are nonfilamentous on YPD and serum medium and display a reduced invasive growth (Fig. 2B and C). Chitin and actin staining patterns and temperature sensitivities were similar in the mutant and wild-type cells. We noticed that ΔYlgpi7 cells are sensitive to Calcofluor white (data not shown), whereas conflicting data were reported for similar mutants in S. cerevisiae (5, 65).
Suppression of filamentation defects by HOY1 and YlPHD1
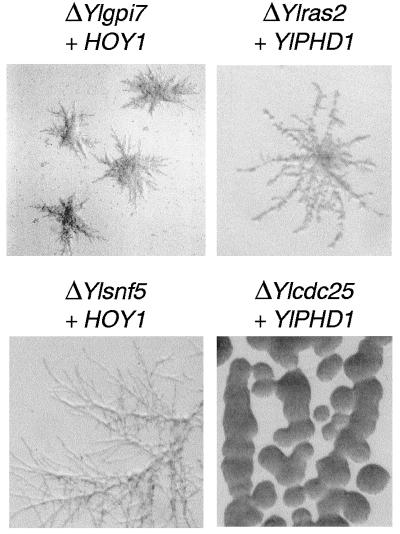
HOY1 and YlPHD1 are known to be involved in morphogenesis in Y. lipolytica. HOY1 is a homeo gene that is required for hyphal formation (66) on solid and liquid media. It appears to be a strong suppressor of morphogenesis defects in several Y. lipolytica mutants. YlPHD1 is homologous to PHD1 of S. cerevisiae (A. Dominguez, personal communication), which encodes a transcription factor that is involved in the regulation of filamentous growth (24) downstream of the cAMP pathway. Both genes were introduced into mutant strains on replicative, centromeric plasmids, which results in multicopy suppression in several instances in Y. lipolytica (7, 28). The introduction of YlPHD1 into ΔYlras2 mutant cells only restored pseudomyceliation on inducing medium (Fig. 4). In agreement with the proposed role of these genes in S. cerevisiae, this may suggest that both genes act in the same pathway, although it does not prove it. Conversely, the introduction of YlPHD1 into cells carrying ΔYlsnf5, ΔYlbud6, ΔYlcdc25, and ΔYlgpi7 did not affect morphogenesis on inducing medium. However, the introduction of HOY1 into Ylsnf5 and Ylgpi7 deletion mutants restored hyphal formation on inducing medium (Fig. 4), whereas it had no effect on other mutants.
FIG. 4.
Suppression of filamentation defects by HOY1 and YlPHD1. Shown are representative clones obtained after transformation of ΔYlgpi7 and ΔYlsnf5 with pRRQ10 and ΔYlras2 and ΔYlcdc25 with pMR4.
DISCUSSION
Fungal dimorphism has received increasing attention because of its potential as a simple experimental model for eukaryotic cell differentiation and its implication in pathogenesis (44). Our current knowledge of the control of this morphological transition is limited to the molecular characterization of the main signaling pathways in yeasts and is mostly from studies of S. cerevisiae (25). Here, we investigated the yeast Y. lipolytica as an alternative model for dimorphism studies.
We used a transposon tagging approach to facilitate the identification of the genes involved in morphogenesis, because earlier studies experienced difficulties in sorting suppressors from cognate genes when morphogenetic mutants were complemented by genomic libraries (66; our unpublished results). We screened 40,000 independent transformants, which is equivalent to two insertions per kilobase of genomic DNA if random mutagenesis occurs and given that the genome of Y. lipolytica is 20 Mb (13). We obtained 50 clones with clearly defective filament formation. This is equivalent to approximately 0.1%, which is similar to what was reported in a study performed with S. cerevisiae (56 mutants out of 100,000 transformants) (46). This indicates that many genes are involved in morphogenesis and that our current screen was not exhaustive. Accordingly, we found one case in which the same gene (XPR6) was independently interrupted twice. Identification of the disrupted loci by reverse PCR was difficult, because only 30 out of 50 mutants could be analyzed. Other approaches, such as rescuing flanking sequence after integration of an E. coli plasmid in mTnYl1 (57), might be more efficient. Further tests showed that only 11 of the 30 mutants resulted from homologous integration. This high level of nonhomologous integration was unexpected in view of our previous results (4) and may reflect strain differences, locus dependence, or changes in the transformation protocol, such as the addition of DMSO to competent cells. Eight Fil− mutants out of the 11 analyzed identified seven different genes, whereas the mutation appeared to be unlinked to mTnYl1 in three cases, which is similar to what was reported in S. cerevisiae (6 out of 45) (46). Seven different ORFs were thus identified, all with clear homologs in S. cerevisiae: RAS2, BUD6, SNF5, PPH21, CDC25, GPI7, and XPR6. Thus, transposon mutagenesis is a valuable tool in Y. lipolytica, but was complicated by the high frequency of illegitimate integration and the inefficient rescue of flanking sequences.
In S. cerevisiae, the dimorphic switch is controlled by at least three signaling pathways: the mating type or STE pathway (30, 43), the cAMP pathway (48), and the pH signaling or RIM101 pathway (38). C. albicans contains homologs of genes from each of these pathways, which all participate in cellular differentiation (11), and other signaling pathways, such as osmosensing (1) or sensing of microaerophilic conditions (62). Interestingly, blocking a single pathway in either S. cerevisiae or C. albicans never suppresses cellular differentiation under all conditions. The situation appears to differ in Y. lipolytica. First, unlike in S. cerevisiae, both haploid and diploid Y. lipolytica forms can undergo the switch from yeast to hyphae and can invade agar, and unlike C. albicans, they are not temperature and pH sensitive (17). Second, previous analyses showed that the RIM and STE genes are not required for agar invasion or myceliation, contrary to what is found in both S. cerevisiae and C. albicans (17, 67). Accordingly, we did not identify any components from these pathways in our screen. Third, the YlTup1p transcriptional factor appears to act as a repressor of the yeast form, like in C. albicans, but unlike in S. cerevisiae, in which Tup1p is required for pseudomyceliation (10, 17). Because two transcriptional factors (Hoy1p and Mhy1p) that do not have any clear homologs in C. albicans or in S. cerevisiae have been found to be essential for the dimorphic transition in Y. lipolytica (32, 66), it was unknown whether a conserved pathway controlling morphogenesis was conserved in the three yeasts.
In this study, we identified seven genes: five that were already known to affect morphogenesis in S. cerevisiae and two new ones, YlSNF5 and YlGPI7.
The role of the cAMP pathway in Y. lipolytica morphogenesis had not been assessed previously. We identified two genes, YlRAS2 and YlCDC25, that are involved in this pathway. It should be stressed that the gene nomenclature is arbitrary at this stage, because we have no evidence for a second RAS gene in Y. lipolytica or for a YlSDC25 paralog. In S. cerevisiae, Cdc25p is an essential GDP/GTP exchange factor (GEF) for Ras2 (8). CDC25 null mutants are lethal in S. cerevisiae, but homozygous CDC25 deletion mutants are viable in C. albicans, although the strains have a partial defect in hyphal formation (19). Similar functions appear to be conserved in Y. lipolytica and C. albicans, because the inactivation of YlCDC25 prevents invasion of the agar and formation of pseudomycelium and hyphae. Activation of Ras2p of S. cerevisiae enhances pseudohyphal growth in the diploid and regulates invasive growth in the haploid through both the STE and cAMP pathways (47, 48). Although there seems to be a single RAS gene in C. albicans, homozygous deletion mutants are viable, unlike RAS1 RAS2 deletion mutants in S. cerevisiae, and are defective in hyphal formation but not in pseudomycelium formation (22). Y. lipolytica phenotypes are much stronger, because YlRAS2 deletion mutants cannot invade agar and do not form pseudomycelium or hyphae. Thus, our results with YlCDC25 and YlRAS2 indicate that the Ras2p-adenylate cyclase pathway is a major pathway in Y. lipolytica morphogenesis.
Expression of the downstream transcriptional factor, YlPhd1p, on a replicative plasmid led to partial suppression of the YlRAS2 defect, restoring formation of pseudomycelium, but not of hyphae. It had no effect in a strain in which the GEF domain of YlCdc25p was interrupted. This may reflect insufficient overexpression of YlPHD1, the existence of other pathways that are required for full activation of hyphal growth, or both. Further work will thus be needed to confirm that YlPhd1p is indeed a target of the cAMP pathway.
The switch from yeast to pseudomycelium is accompanied by a switch in bud site selection in S. cerevisiae haploids and diploids (46) and in C. albicans (29). In clear contrast, both haploid and diploid cells of Y. lipolytica constantly bud in a bipolar manner when in the yeast form or during germ tube emission (29). One of our mutants was affected in a BUD6 homolog and exhibited severe defects in actin patch localization, bud site selection, and cytokinesis and was unable to form hyphae. BUD6 is required in diploid S. cerevisiae cells for bipolar budding and interacts with the bud tip and neck during spindle morphogenesis (60). Our results confirm that bipolar budding is essential for correct cell division of haploids in both the yeast and hyphal forms in Y. lipolytica. They also indicate that bipolar budding and normal spindle organization are required for pseudomyceliation and for agar invasion.
Disruption of the YlXPR6 and YlGPI7 genes resulted in marked deficiency of hyphal formation. Both gene products modify exported proteins and are probably required for the biogenesis of critical cell wall components. Consistent with this hypothesis, KEX2 mutants show abnormal chitin deposition (35). Both Y. lipolytica and C. albicans strains devoid of Xpr6p/Kex2p activity have defects in pseudomycelium formation, but still invade agar, whereas they completely fail to form hyphae (18, 50). Possible targets are precursors of hypha-specific, cell wall-associated proteins, such as Hwp1p (63), or proteins with general cell wall biogenesis activities, such as exo-β-(1-3)-glucanases (14, 21), which all require Xpr6p/Kex2p processing. Conversely, GPI7 is required to produce a functional glycosylphosphatidylinositol (GPI) anchor in S. cerevisiae (6), by adding a side chain to the core structure (5). Over 58 GPI-anchored proteins have been predicted in S. cerevisiae (12), most of which are attached to the cell wall and some of which are required for invasive growth and pseudomyceliation (27). YlGPI7 mutated cells have defective cell wall biogenesis, as suggested by their Calcofluor white sensitivity, and they show a strong defect in formation of pseudohyphae and hyphae and a partial defect in agar invasion. In Y. lipolytica, a ΔYlgpi7 mutation is partially suppressed by overexpression of the transcriptional factor Hoy1p. This suggests that the expression of members of GPI family genes through Hoy1p activation rescues YlGpi7p defects. Our results with YlXPR6 and YlGPI7 confirm that the biogenesis of extracellular proteins, possibly cell wall associated, is critical for morphogenesis in yeasts.
The two remaining genes identified in our screen, YlSNF5 and YlPPH21, probably exert even more pleiotropic effects. S. cerevisiae Snf5p belongs to the SWI-SNF complex, which is a large complex of 2,000 kDa and is highly conserved in all eukaryotes (55). Snf5p is required for the functioning of a variety of sequence-specific transcriptional factors (37), maybe through remodeling of chromatin structure (31). Until now, studies of S. cerevisiae have not shown that SNF5 is involved in morphogenesis. However, Y. lipolytica ΔYlsnf5 mutations are partially suppressed by the overexpression of the transcriptional factor Hoy1p, which may indicate a link between chromatin remodeling and Hoy1p activity. In S. cerevisiae, PPH21 is part of a three-gene family encoding type 2A protein phosphatases (PP2A), in which PPH21 and PPH22 are highly similar and PPH3 is more distantly related (56). In S. cerevisiae, defects in PP2A impair mitosis, actin and chitin organization, and efficiency of “shmoo” formation (39). However, in Y. lipolytica, defects in PPH21 did not result in the delocalization of actin patches, which are normally localized at bud and hyphal tips (36), or in increased sensitivity to Calcofluor white.
Taken together, our results strongly suggest that conserved elements control morphogenesis in distantly related yeasts. They also show that genes hitherto not recognized in S. cerevisiae as involved in morphogenesis are required in Y. lipolytica. Interestingly, both YlGPI7 and YlSNF5 are partially suppressed by overexpression of HOY1, a putative transcriptional regulator without a clear homologue in S. cerevisiae (66). Understanding how these new players fit into the general picture may provide new light on fungal dimorphism.
ACKNOWLEDGMENTS
We thank A. Dominguez for generously providing plasmids bearing HOY1 and YlPHD1 genes and J. H. Hegemann for providing pHS47. We also thank A. Lepingle and A. Auger for sequencing and C. Neuvéglise for help with the use of the mTnYl1 library.
S. Bezzate was supported by the EC grant BIOMED BMH4-CT96-0310, R. Rosas Quijano was supported by EC ALFA grant 5.0118.9, and M. Richard received a CNRS-Aventis grant.
REFERENCES
- 1.Alonso-Monge R, Navarro-Garcia F, Molero G, Diez-Orejas R, Gustin M, Pla J, Sanchez M, Nombela C. Role of the mitogen-activated protein kinase Hog1p in morphogenesis and virulence of Candida albicans. J Bacteriol. 1999;181:3058–3068. doi: 10.1128/jb.181.10.3058-3068.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amberg D C, Zahner J E, Mulholland J W, Pringle J R, Botstein D. Aip3p/Bud6p, a yeast actin-interacting protein that is involved in morphogenesis and the selection of bipolar budding sites. Mol Biol Cell. 1997;8:729–753. doi: 10.1091/mbc.8.4.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andrews P D, Stark M J. Type 1 protein phosphatase is required for maintenance of cell wall integrity, morphogenesis and cell cycle progression in Saccharomyces cerevisiae. J Cell Sci. 2000;113:507–520. doi: 10.1242/jcs.113.3.507. [DOI] [PubMed] [Google Scholar]
- 4.Barth G, Gaillardin C. The dimorphic fungus Yarrowia lipolytica. In: Wolf K, editor. Non-conventional yeasts in biotechnology. Heidelberg, Germany: Springer; 1996. pp. 313–388. [Google Scholar]
- 5.Benachour A, Sipos G, Flury I, Reggiori F, Canivenc-Gansel E, Vionnet C, Conzelmann A, Benghezal M. Deletion of GPI7, a yeast gene required for addition of a side chain to the glycosylphosphatidylinositol (GPI) core structure, affects GPI protein transport, remodeling, and cell wall integrity. J Biol Chem. 1999;274:15251–15261. doi: 10.1074/jbc.274.21.15251. [DOI] [PubMed] [Google Scholar]
- 6.Benghezal M, Lipke P N, Conzelmann A. Identification of six complementation classes involved in the biosynthesis of glycosylphosphatidylinositol anchors in Saccharomyces cerevisiae. J Cell Biol. 1995;130:1333–1344. doi: 10.1083/jcb.130.6.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ben Mamoun C, Beckerich J M, Gaillardin C. The TSR1 gene of Yarrowia lipolytica is involved in the signal recognition particle-dependent translocation pathway of secretory proteins. J Biol Chem. 1996;271:23895–23901. doi: 10.1074/jbc.271.39.23895. [DOI] [PubMed] [Google Scholar]
- 8.Boy-Marcotte E, Buu A, Soustelle C, Poullet P, Parmeggiani A, Jacquet M. The C-terminal part of the CDC25 gene product has Ras-nucleotide exchange activity when present in a chimeric SDC25-CDC25 protein. Curr Genet. 1993;23:397–401. doi: 10.1007/BF00312625. [DOI] [PubMed] [Google Scholar]
- 9.Boy-Marcotte E, Ikonomi P, Jacquet M. SDC25, a dispensable Ras guanine nucleotide exchange factor of Saccharomyces cerevisiae differs from CDC25 by its regulation. Mol Biol Cell. 1996;7:529–539. doi: 10.1091/mbc.7.4.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Braun B R, Johnson A D. Control of filament formation in Candida albicans by the transcriptional repressor TUP1. Science. 1997;277:105–109. doi: 10.1126/science.277.5322.105. [DOI] [PubMed] [Google Scholar]
- 11.Brown A J, Gow N A. Regulatory networks controlling Candida albicans morphogenesis. Trends Microbiol. 1999;7:333–338. doi: 10.1016/s0966-842x(99)01556-5. [DOI] [PubMed] [Google Scholar]
- 12.Caro L H, Tettelin H, Vossen J H, Ram A F, van den Ende H, Klis F M. In silicio identification of glycosyl-phosphatidylinositol-anchored plasma-membrane and cell wall proteins of Saccharomyces cerevisiae. Yeast. 1997;13:1477–1489. doi: 10.1002/(SICI)1097-0061(199712)13:15<1477::AID-YEA184>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 13.Casaregola S, Feynerol C, Diez M, Fournier P, Gaillardin C. Genomic organization of the yeast Yarrowia lipolytica. Chromosoma. 1997;106:380–390. doi: 10.1007/s004120050259. [DOI] [PubMed] [Google Scholar]
- 14.Chambers R S, Broughton M J, Cannon R D, Carne A, Emerson G W, Sullivan P A. An exo-beta-(1,3)-glucanase of Candida albicans: purification of the enzyme and molecular cloning of the gene. J Gen Microbiol. 1993;139:325–334. doi: 10.1099/00221287-139-2-325. [DOI] [PubMed] [Google Scholar]
- 15.De Mattei C R, Davis C P, Konopka J B. Point mutations identify a conserved region of the Saccharomyces cerevisiae AFR1 gene that is essential for both the pheromone signaling and morphogenesis functions. Genetics. 2000;155:43–55. doi: 10.1093/genetics/155.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diez-Orejas R, Molero G, Rios-Serrano I, Vazquez A, Gil C, Nombela C, Sanchez-Perez M. Low virulence of a morphological Candida albicans mutant. FEMS Microbiol Lett. 1999;176:311–319. doi: 10.1111/j.1574-6968.1999.tb13677.x. [DOI] [PubMed] [Google Scholar]
- 17.Dominguez A, Ferminan E, Gaillardin C. Yarrowia lipolytica: an organism amenable to genetic manipulation as a model for analyzing dimorphism in fungi. Contrib Microbiol. 2000;5:151–172. doi: 10.1159/000060349. [DOI] [PubMed] [Google Scholar]
- 18.Enderlin C S, Ogrydziak D M. Cloning, nucleotide sequence and functions of XPR6, which codes for a dibasic processing endoprotease from the yeast Yarrowia lipolytica. Yeast. 1994;10:67–79. doi: 10.1002/yea.320100107. [DOI] [PubMed] [Google Scholar]
- 19.Enloe B, Diamond A, Mitchell A P. A single-transformation gene function test in diploid Candida albicans. J Bacteriol. 2000;182:5730–5736. doi: 10.1128/jb.182.20.5730-5736.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ernst J. Transcription factors in Candida albicans—environmental control and morphogenesis. Microbiology. 2000;146:1763–1774. doi: 10.1099/00221287-146-8-1763. [DOI] [PubMed] [Google Scholar]
- 21.Esteban P F, Casaregola S, Vazquez De Aldana C R, Del Rey F. Cloning and characterization of the EXG1 gene from the yeast Yarrowia lipolytica. Yeast. 1999;15:1631–1644. doi: 10.1002/(SICI)1097-0061(199911)15:15<1631::AID-YEA488>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 22.Feng Q, Summers E, Guo B, Fink G. Ras signaling is required for serum-induced hyphal differentiation in Candida albicans. J Bacteriol. 1999;181:6339–6346. doi: 10.1128/jb.181.20.6339-6346.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fridkin S K, Jarvis W R. Epidemiology of nosocomial fungal infections. Clin Microbiol Rev. 1996;9:499–511. doi: 10.1128/cmr.9.4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gimeno C J, Fink G R. Induction of pseudohypal growth by overexpression of PHD1, a Saccharomyces cerevisiae gene related to transcriptional regulators of fungal development. Mol Cell Biol. 1994;14:2100–2112. doi: 10.1128/mcb.14.3.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gimeno C J, Ljungdahl P O, Styles C A, Fink G R. Unipolar cell divisions in the yeast S. cerevisiae lead to filamentous growth: regulation by starvation and RAS. Cell. 1992;68:1077–1090. doi: 10.1016/0092-8674(92)90079-r. [DOI] [PubMed] [Google Scholar]
- 26.Güldener U, Heck S, Feidler T, Beinhauer J, Hegemann J H. A new efficient gene disruption cassette for repeated use in budding yeast. Nucleic Acids Res. 1996;24:2519–2524. doi: 10.1093/nar/24.13.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo B, Styles C A, Feng Q, Fink G R. A Saccharomyces gene family involved in invasive growth, cell-cell adhesion, and mating. Proc Natl Acad Sci USA. 2000;97:12158–12163. doi: 10.1073/pnas.220420397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He F, Beckerich J M, Gaillardin C. A mutant of 7SL RNA in Yarrowia lipolytica affecting the synthesis of a secreted protein. J Biol Chem. 1992;267:1932–1937. [PubMed] [Google Scholar]
- 29.Herrero A B, Lopez M C, Fernandez-Lago L, Dominguez A. Candida albicans and Yarrowia lipolytica as alternative models for analysing budding patterns and germ tube formation in dimorphic fungi. Microbiology. 1999;145:2727–2737. doi: 10.1099/00221287-145-10-2727. [DOI] [PubMed] [Google Scholar]
- 30.Herskowitch I. MAP kinase pathways in yeast: for mating and more. Cell. 1995;80:187–197. doi: 10.1016/0092-8674(95)90402-6. [DOI] [PubMed] [Google Scholar]
- 31.Hirschhorn J N, Brown S A, Clark C D, Winston F. Evidence that SNF2/SWI2 and SNF5 activate transcription in yeast by altering chromatin structure. Genes Dev. 1992;6:2288–2298. doi: 10.1101/gad.6.12a.2288. [DOI] [PubMed] [Google Scholar]
- 32.Hurtado C A R, Rachubinski R A. MHY1 encodes a C2H2-type zinc finger protein that promotes dimorphic transition in the yeast Yarrowia lipolytica. J Bacteriol. 1999;181:3051–3057. doi: 10.1128/jb.181.10.3051-3057.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kato K, Cox A D, Hisaka M M, Graham S M, Buss J E, Der C J. Isoprenoid addition to Ras protein is the critical modification for its membrane association and transforming activity. Proc Natl Acad Sci USA. 1992;89:6403–6407. doi: 10.1073/pnas.89.14.6403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kobayashi S D, Cutler J E. Candida albicans hyphal formation and virulence: is there a clearly defined role? Trends Microbiol. 1998;6:92–94. doi: 10.1016/s0966-842x(98)01218-9. [DOI] [PubMed] [Google Scholar]
- 35.Komano H, Fuller R S. Shared functions in vivo of a glycosyl-phosphatidylinositol-linked aspartyl protease, Mkc7, and the proprotein processing protease Kex2 in yeast. Proc Natl Acad Sci USA. 1995;92:10752–10756. doi: 10.1073/pnas.92.23.10752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kurischko C, Swoboda R K. Cytoskeletal proteins and morphogenesis in Candida albicans and Yarrowia lipolytica. Contrib Microbiol. 2000;5:173–184. doi: 10.1159/000060353. [DOI] [PubMed] [Google Scholar]
- 37.Laurent B C, Treitel M A, Carlson M. The SNF5 protein of Saccharomyces cerevisiae is a glutamine- and proline-rich transcriptional activator that affects expression of a broad spectrum of genes. Mol Cell Biol. 1990;10:5616–5625. doi: 10.1128/mcb.10.11.5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li W, Mitchell A P. Proteolytic activation of Rim1p, a positive regulator of yeast sporulation and invasive growth. Genetics. 1997;145:63–73. doi: 10.1093/genetics/145.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin F C, Arndt K T. The role of Saccharomyces cerevisiae type 2A phosphatase in the actin cytoskeleton and in entry into mitosis. EMBO J. 1995;14:2745–2759. doi: 10.1002/j.1460-2075.1995.tb07275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu H, Styles C A, Fink G R. Elements of the yeast pheromone response pathway required for filamentous growth of diploids. Science. 1993;262:1741–1744. doi: 10.1126/science.8259520. [DOI] [PubMed] [Google Scholar]
- 41.Lo H J, Kohler J R, DiDomenico B, Loebenberg D, Cacciapuoti A, Fink G R. Nonfilamentous C. albicans mutants are avirulent. Cell. 1997;90:939–949. doi: 10.1016/s0092-8674(00)80358-x. [DOI] [PubMed] [Google Scholar]
- 42.Lorenz C M, Heitman J. Yeast pseudohyphal growth is regulated by GPA2, a G protein alpha homolog. EMBO J. 1997;16:7008–7018. doi: 10.1093/emboj/16.23.7008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Madhani H D, Fink G R. The riddle of MAP kinase signaling specificity. Trends Genet. 1998;14:151–155. doi: 10.1016/s0168-9525(98)01425-5. [DOI] [PubMed] [Google Scholar]
- 44.Mayorga M E, Gold S E. A MAP kinase encoded by the UBC3 gene of Ustilago maydis is required for filamentous growth and full virulence. Mol Microbiol. 1999;34:485–497. doi: 10.1046/j.1365-2958.1999.01610.x. [DOI] [PubMed] [Google Scholar]
- 45.McMillan J N, Longtine M S, Sia R A L, Theesfeld C L, Bardes E S G, Pringle J R, Lew D J. The morphogenesis checkpoint in Saccharomyces cerevisiae: cell cycle control of Swe1p degradation by Hsl1p and Hsl7p. Mol Cell Biol. 1999;19:6929–6939. doi: 10.1128/mcb.19.10.6929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mösch H U, Fink G R. Dissection of filamentous growth by transposon mutagenesis in Saccharomyces cerevisiae. Genetics. 1997;145:671–684. doi: 10.1093/genetics/145.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mösch H U, Kubler E, Krappmann S, Fink G R, Braus G H. Crosstalk between the Ras2p-controlled mitogen-activated protein kinase and cAMP pathways during invasive growth of Saccharomyces cerevisiae. Mol Biol Cell. 1999;10:1325–1335. doi: 10.1091/mbc.10.5.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mösch H U, Roberts R L, Fink G R. RAS2 signals via the CDC42/STE20 mitogen-activated protein kinase module to induce filamentous growth in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1996;93:5352–5356. doi: 10.1073/pnas.93.11.5352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Neuveglise C, Nicauda J M, Ross-Macdonald P, Gaillardin C. A shuttle mutagenesis system for tagging genes in the yeast Yarrowia lipolytica. Gene. 1998;213:37–46. doi: 10.1016/s0378-1119(98)00205-4. [DOI] [PubMed] [Google Scholar]
- 50.Newport G, Agabian N. KEX2 influences Candida albicans proteinase secretion and hyphal formation. J Biol Chem. 1997;272:28954–28961. doi: 10.1074/jbc.272.46.28954. [DOI] [PubMed] [Google Scholar]
- 51.Olaiya A F, Sogin S J. Ploidy determination of Candida albicans. J Bacteriol. 1979;140:1043–1049. doi: 10.1128/jb.140.3.1043-1049.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pan X, Heitman J. Cyclic AMP-dependent protein kinase regulates pseudohyphal differentiation in Saccharomyces cerevisiae. Mol Cell Biol. 1999;19:4874–4887. doi: 10.1128/mcb.19.7.4874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Perez-Campo F M, Nicaud J M, Gaillardin C, Dominguez A. Cloning and sequencing of the LYS1 gene encoding homocitrate synthase in the yeast Yarrowia lipolytica. Yeast. 1996;12:1459–1469. doi: 10.1002/(SICI)1097-0061(199611)12:14%3C1459::AID-YEA26%3E3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 54.Petit T, Gancedo C. Molecular cloning and characterization of the gene HXK1 encoding the hexokinase from Yarrowia lipolytica. Yeast. 1999;15:1573–1584. doi: 10.1002/(SICI)1097-0061(199911)15:15<1573::AID-YEA478>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 55.Quinn J, Fyrberg A M, Ganster R W, Schmidt M C, Peterson C L. DNA-binding properties of the yeast SWI/SNF complex. Nature. 1996;379:844–847. doi: 10.1038/379844a0. [DOI] [PubMed] [Google Scholar]
- 56.Ronne H, Carlberg M, Hu G Z, Nehlin J O. Protein phosphatase 2A in Saccharomyces cerevisiae: effects on cell growth and bud morphogenesis. Mol Cell Biol. 1991;11:4876–4884. doi: 10.1128/mcb.11.10.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ross-Macdonald P, Sheehan A, Roeder S G, Snyder M. A multipurpose transposon system for analysis of protein production, localization, and function in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1997;94:190–195. doi: 10.1073/pnas.94.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 59.Sauer B. Functional expression of the cre-lox site-specific recombination system in the yeast Saccharomyces cerevisiae. Mol Cell Biol. 1987;7:2087–2096. doi: 10.1128/mcb.7.6.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Segal M, Bloom K, Reed S I. Bud6 directs sequential microtubule interactions with the bud tip and bud neck during spindle morphogenesis in Saccharomyces cerevisiae. Mol Biol Cell. 2000;11:3689–3702. doi: 10.1091/mbc.11.11.3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sheu Y J, Barral Y, Snyder M. Polarized growth controls cell shape and bipolar bud site selection in Saccharomyces cerevisiae. Mol Cell Biol. 2000;20:5235–5247. doi: 10.1128/mcb.20.14.5235-5247.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sonneborn A, Bockmuhl D P, Ernst J F. Chlamydospore formation in Candida albicans requires the Efg1p morphogenetic regulator. Infect Immun. 1999;67:5514–5517. doi: 10.1128/iai.67.10.5514-5517.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Staab J F, Bradway S D, Fidel P L, Sundstrom P. Adhesive and mammalian transglutaminase substrate properties of Candida albicans Hwp1. Science. 1999;283:1535–1538. doi: 10.1126/science.283.5407.1535. [DOI] [PubMed] [Google Scholar]
- 64.Strick C A, James L C, O'Donnell M M, Gollaher M G, Franke A E. The isolation and characterization of the pyruvate kinase-encoding gene from the yeast Yarrowia lipolytica. Gene. 1992;118:65–72. doi: 10.1016/0378-1119(92)90249-o. . (Erratum, 140:141, 1994.) [DOI] [PubMed] [Google Scholar]
- 65.Toh-e A, Oguchi T. Las21 participates in extracellular/cell surface phenomena in Saccharomyces cerevisiae. Genes Genet Syst. 1999;74:241–256. doi: 10.1266/ggs.74.241. [DOI] [PubMed] [Google Scholar]
- 66.Torres-Guzman J C, Dominguez A. HOY1, a homeo gene required for hyphal formation in Yarrowia lipolytica. Mol Cell Biol. 1997;17:6283–6293. doi: 10.1128/mcb.17.11.6283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Treton B, Blanchin-Roland S, Lambert M, Lepingle A, Gaillardin C. Ambient pH signalling in ascomycetous yeasts involves homologues of Aspergillus nidulans genes palF and palH. Mol Gen Genet. 2000;263:505–513. doi: 10.1007/s004380051195. [DOI] [PubMed] [Google Scholar]
- 68.Triglia T, Peterson M G, Kemp D J. A procedure for in vitro amplification of DNA segments that lie outside the boundaries of known sequences. Nucleic Acids Res. 1988;16:8186. doi: 10.1093/nar/16.16.8186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tsuchimori N, Sharkey L L, Fonzi W A, French S W, Edwards J E, Jr, Filler S G. Reduced virulence of HWP1-deficient mutants of Candida albicans and their interactions with host cells. Infect Immun. 2000;68:1997–2002. doi: 10.1128/iai.68.4.1997-2002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang H J, Le Dall M-T, Waché Y, Laroche C, Belin J-M, Gaillardin C, Nicaud J-M. Evaluation of acyl coenzyme A oxidase (Aox) isozyme function in the n-alkane-assimilating yeast Yarrowia lipolytica. J Bacteriol. 1999;181:5140–5148. doi: 10.1128/jb.181.17.5140-5148.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zahner J E, Harkins H A, Pringle J R. Genetic analysis of the bipolar pattern of bud site selection in the yeast Saccharomyces cerevisiae. Mol Cell Biol. 1996;16:1857–1870. doi: 10.1128/mcb.16.4.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]