Abstract
Background:
Multiple epidemiological studies have shown that exposure to pesticides is associated with adverse health outcomes. However, the literature on pesticide-related health effects in the Latin American and the Caribbean (LAC) region, an area of intensive agricultural and residential pesticide use, is sparse. We conducted a scoping review to describe the current state of research on the health effects of pesticide exposure in LAC populations with the goal of identifying knowledge gaps and research capacity building needs.
Methods:
We searched PubMed and SciELO for epidemiological studies on pesticide exposure and human health in LAC populations published between January 2007 and December 2021. We identified 233 publications from 16 countries that met our inclusion criteria and grouped them by health outcome (genotoxicity, neurobehavioral outcomes, placental outcomes and teratogenicity, cancer, thyroid function, reproductive outcomes, birth outcomes and child growth, and others).
Results:
Most published studies were conducted in Brazil (37%, ) and Mexico (20%, ), were cross-sectional in design (72%, ), and focused on farmworkers (45%, ) or children (21%, ). The most frequently studied health effects included genotoxicity (24%, ) and neurobehavioral outcomes (21%, ), and organophosphate (OP) pesticides were the most frequently examined (26%, ). Forty-seven percent () of the studies relied only on indirect pesticide exposure assessment methods. Exposure to OP pesticides, carbamates, or to multiple pesticide classes was consistently associated with markers of genotoxicity and adverse neurobehavioral outcomes, particularly among children and farmworkers.
Discussion:
Our scoping review provides some evidence that exposure to pesticides may adversely impact the health of LAC populations, but methodological limitations and inconsistencies undermine the strength of the conclusions. It is critical to increase capacity building, integrate research initiatives, and conduct more rigorous epidemiological studies in the region to address these limitations, better inform public health surveillance systems, and maximize the impact of research on public policies. https://doi.org/10.1289/EHP9934
Introduction
The Latin America and the Caribbean (LAC) region accounts for 14% of global agricultural production and 23% of the world’s exports of agricultural and fisheries commodities.1 The rapid increase of farming in the region in the last decades has been coupled with an extensive use of pesticides (defined as chemical compounds that may either kill, obstruct, or manage the growth of any organism that damages a crop)2,3 and a lack of pesticide use regulations or implementation thereof.4–6 It is estimated that pesticide use in LAC countries accounts for 20% of worldwide consumption3 and that more pesticides are used in Central and South America on a per capita basis (1.84 and of pesticide per person per year, respectively3,7) than in other regions in the world.
Intensive use of pesticides in the LAC region for agricultural and public health vector control purposes8 has resulted in widespread chronic human exposure, particularly among those living in agricultural communities. Pathways of chronic exposure include pesticide drift from treated fields to nearby homes or schools,9–12 take-home exposure,13 and consumption of contaminated food and water.14–17 Elevated occupational exposures in this region are also a concern as workers who apply pesticides or work in treated agricultural fields are exposed to mixtures of pesticides, such as insecticides [e.g., organophosphate (OP) and organochlorine (OC) pesticides],18,19 herbicides (e.g., glyphosate, the most widely used pesticide in the world),20 and fungicides (e.g., chlorothalonil, bisdithiocarbamates, and benzimidazoles).21
Although multiple studies around the world, including those conducted in LAC countries, have shown that pesticides have a negative impact on human health,22,23 public health surveillance and monitoring systems on pesticide use and associated illness are nonexistent or extremely limited in the LAC region.5,24–28 In addition, several pesticides banned in the United States, Europe, and Canada because they were deemed as a potential threat to human health have been or continue to be used in some LAC countries.28–30 Climate change could also exacerbate the health risks of pesticide exposures among LAC populations owing to enhanced chemical toxicity, increased rates of chemical degradation, enhanced volatilization of pesticides to the atmosphere or surface deposition of airborne pesticides, or changes in the frequency and amount of pesticides used.31,32
Promotion of high-quality epidemiological studies with standardized direct exposure assessment methods, the establishment of biomonitoring and environmental surveillance programs, and the development of evidence-based prevention policies and interventions have been suggested as means to protect the health of populations exposed to pesticides.33–35 Still, there is little information on the current state of research on the health effects of pesticides in the LAC region. Previous systematic literature reviews and meta-analyses of studies conducted in LAC populations have focused on one specific class of pesticides or specific active ingredient (e.g., OP pesticides,36,37 pyrethroids,38 glyphosate39), one specific age group (e.g., children37,40–42), or one health outcome (e.g., genotoxicity,43 neurobehavior,36,37,40 or respiratory health41). To address existing gaps of knowledge and identify research capacity building needs in the region, we conducted a scoping review to describe the current state of research on the health effects of pesticide exposure in LAC populations.
Methods
Search Strategy
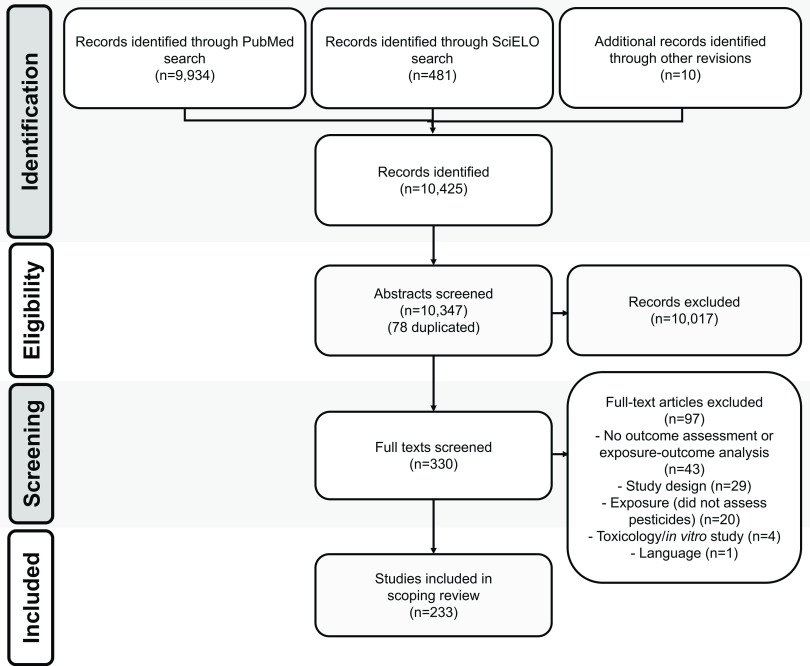
We undertook a scoping review of the literature to identify all primary published data encompassing health effects of occupational or environmental exposure to pesticides in LAC populations. Our methods were guided by the Preferred Reporting Items for Systematic reviews and Meta-Analyses—Extension for Scoping Reviews (PRISMA-ScR) statement.44 We searched PubMed and the Scientific Electronic Library Online (SciELO) for all studies published between January 2007 and December 2021. For PubMed, we used the following search string: (pesticides [All Fields] AND “Latin America” [All Fields]) OR (pesticides [All Fields] AND Aruba [All Fields]) OR (pesticides [All Fields] AND Bahamas [All Fields]) OR (pesticides [All Fields] AND Barbados [All Fields]) OR (pesticides [All Fields] AND “Cayman Islands” [All Fields]) OR (pesticides [All Fields] AND Cuba [All Fields]) OR (pesticides [All Fields] AND Curacao [All Fields]) OR (pesticides [All Fields] AND Dominica [All Fields]) OR (pesticides [All Fields] AND “Dominican Republic” [All Fields]) OR (pesticides [All Fields] AND Grenada [All Fields]) OR (pesticides [All Fields] AND Guadeloupe [All Fields]) OR (pesticides [All Fields] AND Haiti [All Fields]) OR (pesticides [All Fields] AND Jamaica [All Fields]) OR (pesticides [All Fields] AND Martinique [All Fields]) OR (pesticides [All Fields] AND “Puerto Rico” [All Fields]) OR (pesticides [All Fields] AND “Saint Barthélemy” [All Fields]) OR (pesticides [All Fields] AND “Saint Kitts and Nevis” [All Fields]) OR (pesticides [All Fields] AND “Saint Lucia” [All Fields]) OR (pesticides [All Fields] AND “Saint Maarten” [All Fields]) OR (pesticides [All Fields] AND “Saint Vincent and the Grenadines” [All Fields]) OR (pesticides [All Fields] AND “Trinidad and Tobago” [All Fields]) OR (pesticides [All Fields] AND “Turks and Caicos Islands” [All Fields]) OR (pesticides [All Fields] AND “Virgin Islands” [All Fields]) OR (pesticides [All Fields] AND Belize [All Fields]) OR (pesticides [All Fields] AND “Costa Rica” [All Fields]) OR (pesticides [All Fields] AND “El Salvador” [All Fields]) OR (pesticides [All Fields] AND Guatemala [All Fields]) OR (pesticides [All Fields] AND Honduras [All Fields]) OR (pesticides [All Fields] AND Mexico [All Fields]) OR (pesticides [All Fields] AND Nicaragua [All Fields]) OR (pesticides [All Fields] AND Panama [All Fields]) OR (pesticides [All Fields] AND Argentina [All Fields]) OR (pesticides [All Fields] AND Bolivia [All Fields]) OR (pesticides [All Fields] AND Brazil [All Fields]) OR (pesticides [All Fields] AND Chile [All Fields]) OR (pesticides [All Fields] AND Colombia [All Fields]) OR (pesticides [All Fields] AND Ecuador [All Fields]) OR (pesticides [All Fields] AND “French Guiana” [All Fields]) OR (pesticides [All Fields] AND Guyana [All Fields]) OR (pesticides [All Fields] AND Paraguay [All Fields]) OR (pesticides [All Fields] AND Peru [All Fields]) OR (pesticides [All Fields] AND Suriname [All Fields]) OR (pesticides [All Fields] AND Uruguay [All Fields]) OR (pesticides [All Fields] AND Venezuela [All Fields]) AND (“"2007/01/0”"[Date–- Publication]: “"2021/12/3”"[Date–- Publication])) (i.e., names of the 43 LAC countries and territories, as defined by the International Society of Environmental Epidemiology (ISEE) LAC Chapter).45 For SciELO, we used the following search string: ((pesticides AND Latin America)) OR ((pesticides AND Aruba)) OR ((pesticides AND Bahamas)) OR ((pesticides AND Barbados)) OR ((pesticides AND Cayman islands)) OR ((pesticides AND Cuba)) OR ((pesticides AND Curacao)) OR ((pesticides AND Dominica)) OR ((pesticides AND Dominican Republic)) OR ((pesticides AND Grenada)) OR ((pesticides AND Guadeloupe)) OR ((pesticides AND Haiti)) OR ((pesticides AND Jamaica)) OR ((pesticides AND Martinique)) OR ((pesticides AND Puerto Rico)) OR ((pesticides AND Saint Barthelemy)) OR ((pesticides AND saint Kitts and Nevis)) OR ((pesticides AND Saint Lucia)) OR ((pesticides AND Saint Maarten)) OR ((pesticides AND Saint Vincent and the Grenadines)) OR ((pesticides AND Trinidad and Tobago)) OR ((pesticides AND Turks and Caicos islands)) OR ((pesticides AND Virgin Islands)) OR ((pesticides AND Belize)) OR ((pesticides AND Costa Rica)) OR ((pesticides AND El Salvador)) OR ((pesticides AND Guatemala)) OR ((pesticides AND Honduras)) OR ((pesticides AND Mexico)) OR ((pesticides AND Nicaragua)) OR ((pesticides AND Panama)) OR ((pesticides AND Argentina)) OR ((pesticides AND Bolivia)) OR ((pesticides AND Brazil)) OR ((pesticides AND Chile)) OR ((pesticides AND Colombia)) OR ((pesticides AND Ecuador)) OR ((pesticides AND French Guiana)) OR ((pesticides AND Guyana)) OR ((pesticides AND Paraguay)) OR ((pesticides AND Peru)) OR ((pesticides AND Suriname)) OR ((pesticides AND Uruguay)) OR ((pesticides AND Venezuela)) and filtered the results by date of publication. The initial search was conducted on 30 May 2017, with subsequent updates on 1 May 2019, 4 February 2021, and 27 April 2022 (for papers published until 31 December 2021). We also identified potentially relevant citations not retrieved by the initial literature searches by scanning the references of relevant studies throughout the course of title and abstract screening and data abstraction (Figure 1; see Supplemental Material for the list of studies retrieved from PubMed and SciELO).
Figure 1.
PRISMA-ScR flow diagram of study selection. Note: PRISMA-ScR, Preferred Reporting Items for Systematic reviews and Meta-Analyses—Extension for Scoping Reviews; SciELO, Scientific Electronic Library Online.
Study Selection
After removing duplicate records, titles and abstracts of literature search results were scanned for eligibility by two reviewers, with discrepancies resolved by a third reviewer. Studies were selected for full-text review when they met all of our inclusion criteria: a) original full paper that presented unique data from an analytical observational epidemiological study (i.e., cohort, cross-sectional, or case–control study); b) environmental or occupational exposure to pesticides; c) conducted in one of the 43 LAC countries and territories, as defined by the ISEE LAC Chapter45; and d) published in English, Spanish, or Portuguese. We excluded studies if they met one of the following criteria: a) did not report original results (i.e., reviews, meta-analysis, comments, letters, editorials, and case reports); b) were experimental, toxicological, or ecological studies; c) were based on animal or human tissues; or d) reported preliminary results (e.g., conference abstracts or papers that were later updated or revised in a peer-reviewed journal article). Full texts were assessed by two reviewers for final inclusion, with a third reviewer again resolving any discrepancies.
Data Abstraction
We abstracted the following characteristics from the selected publications: bibliographic citation information (i.e., authors, year of publication, and country), characteristics of the study population (i.e., sample size, study area), study design, type of pesticides assessed (e.g., pesticide class or pesticide active ingredient), exposure and health outcome assessment methods, and main study findings. We grouped the studies into eight categories based on the main health outcome assessed: a) genotoxicity, b) neurobehavioral outcomes, c) placental outcomes and teratogenicity, d) cancer, e) thyroid function, f) reproductive outcomes, g) birth outcomes and child growth, and h) other health outcomes.
Because of the expected methodological heterogeneity among the selected studies (e.g., variability in study design; exposure and outcome assessment methods), results were not intended to be combined through meta-analysis. Instead, we conducted a narrative synthesis to highlight the strengths and limitations of the current evidence base and to ultimately draw conclusions about the state of research on the health effects of pesticide exposure in LAC populations, including key challenges moving forward.
Results
The PubMed and SciELO search retrieved 9,934 and 481 citations, respectively, and the review of references from relevant publications yielded 10 additional citations (Figure 1). After removing 78 duplicates, 10,023 publications that did not meet inclusion criteria based on titles/abstracts, and 91 that did not meet inclusion criteria based on full-text reviews, 233 publications were included in this review. Although publications reported on studies from 16 (37%) of the 43 LAC countries and territories, most studies were conducted in Brazil (37%, ) and Mexico (20%, ) (Table 1). Studies were primarily cross-sectional in design (72%, ), and the most frequently studied populations were farmworkers (45%, ) or children (21%, ). Between 2007 and 2021, the average number of publications was , range: 5 in 2008 to 27 in 2020) (Figure S1). Nearly half (47%, ) of the published studies relied solely on indirect pesticide exposure assessment methods (e.g., questionnaire, job status ascertainment via death certificate or surveillance system) (Table 1 and Table S1). Blood was the biological matrix most frequently used to assess pesticide exposure (74%, of the 124 studies that used direct exposure assessment methods). Most published studies focused on OP pesticides (26%, ) or multiple classes of pesticides (32%, ). The most studied health effects included genotoxicity (24%, ) and neurobehavioral outcomes (21%, ) (Table 1 and Table S1).
Table 1.
Characteristics of Latin American and the Caribbean studies on pesticide exposure and health outcomes published between 2007 and 2021 ().
| Characteristic | (%) |
|---|---|
| Study countrya | |
| Argentina | 21 (8.9) |
| Bolivia | 6 (2.6) |
| Brazil | 88 (37.4) |
| Chile | 7 (3.0) |
| Colombia | 9 (3.8) |
| Costa Rica | 14 (6.0) |
| Dominican Republic | 1 (0.4) |
| Ecuador | 17 (7.2) |
| El Salvador | 1 (0.4) |
| Guadeloupe | 14 (6.0) |
| Jamaica | 1 (0.4) |
| Mexico | 46 (19.6) |
| Nicaragua | 4 (1.7) |
| Paraguay | 1 (0.4) |
| Peru | 2 (0.8) |
| Venezuela | 3 (1.3) |
| Study design | |
| Cohort | 41 (17.5) |
| Cross sectional | 167 (71.7) |
| Case–control | 25 (10.7) |
| Study population | |
| Farmworkers | 105 (45.1) |
| Other workers (e.g., vector control program workers) | 9 (3.9) |
| General population | 38 (16.3) |
| Mother–child pairs | 27 (11.6) |
| Pregnant women only | 6 (2.6) |
| Children only | 48 (20.6) |
| Pesticide exposure assessment methodb,c | |
| Indirect | |
| Questionnaire only | 103 (43.1) |
| Other (e.g., job status ascertained via death certificate or surveillance system, residential proximity) | 9 (3.8) |
| Direct | |
| Cholinesterase activityd | 57 (23.8) |
| Pesticides or pesticide metabolites measured in biological matrix | 70 (29.3) |
| Biological matrix used for pesticide exposure assessmente | |
| Blood | 99 (73.9) |
| Breast milk | 2 (1.5) |
| Hair | 4 (3.0) |
| Urine | 28 (20.9) |
| Toenail | 1 (0.7) |
| Pesticides assessedf | |
| Insecticides in general (no class specified) | 5 (1.6) |
| Organophosphates | 81 (26.2) |
| Organophosphates and carbamatesg | 20 (6.5) |
| Organochlorines | 46 (14.9) |
| Pyrethroids | 20 (6.5) |
| Neonicotinoids | 2 (0.6) |
| Herbicides | 21 (6.8) |
| Fungicides | 11 (3.6) |
| Larvicides | 1 (0.3) |
| Rodenticides | 1 (0.3) |
| Natural pesticides | 1 (0.3) |
| Multiple pesticide classes (unspecified) | 100 (32.3) |
| Main health outcomesh | |
| Genotoxicity | 62 (24.0) |
| Neurobehavioral outcomes | 54 (20.9) |
| Placental outcomes and teratogenicity | 13 (5.1) |
| Cancer | 14 (5.4) |
| Thyroid function | 16 (6.2) |
| Reproductive outcomes | 16 (6.2) |
| Birth outcomes and child growth | 13 (5.1) |
| Other effects | 70 (27.1) |
| Kidney functioni | 9 (3.5) |
| Respiratory and allergic outcomesi | 7 (2.7) |
| Liver injuryi | 8 (3.1) |
| Hematological parameters and lipid profilei | 17 (6.6) |
| Acoustic damagei | 8 (3.1) |
| Othersi | 26 (10.1) |
because one published study (Maluf et al.245) was conducted in three countries (Argentina, Brazil, and Mexico).
A total of 125 published studies employed direct exposure assessment methods, with some measuring both cholinesterase activity and pesticide metabolites concentrations. Of these, 81 (65.3%) used data from the direct exposure assessment in exposure–outcome analyses (e.g., some studies measured urinary biomarkers of exposure and ascertained occupational status via questionnaire but only reported exposure–outcome associations using occupational status).
because some published studies employed more than one exposure assessment method (e.g., measurement of cholinesterase activity in blood and urinary pesticide metabolites).
A total of 57 published studies measured cholinesterase activity only; 4 studies measured cholinesterase activity in addition to other pesticide metabolites.
Only for published studies with direct pesticide exposure assessment, but nine studies measured pesticides in more than one biological matrix.
because some published studies assessed multiple pesticide groups.
Exposure assessed via acetylcholinesterase activity monitoring and authors did not differentiate if they were primarily examining organophosphates or carbamates.
because some published studies assessed outcomes from more than one group.
Proportion of published studies that assessed this outcome out of all the studies included in the review (); total studies that assessed other health effects because some assessed multiple outcomes in this category (e.g., several published studies examined liver injury and hematological parameters).
Genotoxicity
Sixty-two publications examined associations of pesticide exposure with cytogenetic or DNA damage (Table 2). Most publications were derived from cross-sectional studies that evaluated DNA damage from accessible tissues, such as blood or buccal cells, via comet assays, telomere attrition, or DNA methylation of candidate genes. Eleven of the 62 publications focused on children. Three of these 11 publications assessed exposure to OC pesticides by measurement of blood or hair OC pesticide concentrations,46–48 whereas the remaining 8 examined exposure to a mixture of pesticides including OP pesticides, pyrethroids, herbicides, or “multiple pesticide classes” via questionnaire.30,49–55 Of the 3 publications that measured blood or hair OC pesticide concentrations,46–48 2 were from cross-sectional studies of school-age Mexican children and reported associations with genotoxic damage—as indicated by DNA damage assessed via comet assay47 or higher frequency of micronuclei and other nuclear abnormalities in buccal cells.48 A third publication from a cross-sectional investigation of mother–child pairs in Mexico reported null associations with DNA and cytogenetic damage measured in maternal blood at delivery and cord blood.46 Five publications examining exposures to more than one pesticide class in children from Mexico,49,51 Argentina,50,54 and Paraguay53 reported associations of higher residential or parental occupational pesticide exposure with cytogenetic damage—assessed via buccal micronuclei and other nuclear abnormalities. Similarly, in a prospective study of school-age children living near a tobacco-producing region in Brazil, researchers found that malondialdehyde, protein carbonyl, and vitamin C levels were higher at the beginning of the pesticide application period than at the leaf harvest period.52 In contrast, 2 publications from small cross-sectional studies of children from Colombia30 and Bolivia55 reported null associations of maternal occupational pesticide exposure—assessed via questionnaire—and urinary atrazine concentrations with cytogenetic damage.
Table 2.
Characteristics of Latin American and the Caribbean studies on pesticide exposure and genotoxicity published between 2007 and 2021 ().
| Study | Year of publication/country | Population and sample size | Study design | Pesticides assessed | Exposure assessment method | Pesticide or metabolite concentrations | Health effect and assessment method/instrument | Results |
|---|---|---|---|---|---|---|---|---|
| Studies on OCs | ||||||||
| Studies in children | ||||||||
| 1. Alvarado-Hernández et al.46 | 2013/Mexico | 50 mother–child (newborns) pairs living in a rural agricultural area | Cross-sectional | OCs | Maternal (collected at delivery) and cord blood HCH, HCB, aldrin, heptachlor epoxide, oxychlordane, chlordane, DDT, DDE, nonachlor, mirex, and endosulfan | Median (P25–P75) ( lipid): Maternal: (243–617); (1,009–2,094); (46–76); (208–528); heptachlor (2,941–5,167); oxychlordane 1,672 (977–2,232); ; ; ; , ; endosulfan (62–118); (153–1,041); (11–341) Cord blood: ; ; ; ; ; ; ; ; ; ; ; ; ; ; |
DNA damage: comet assay Cytogenetic damage: MN, CHBs, NPBs |
Null associations of OC pesticides with markers of cytogenetic or DNA damage. |
| 2. Jasso-Pineda et al.47 | 2015/Mexico | 276 children (6–12 years of age) living in communities with industrial activities (e.g., agriculture) | Cross-sectional | OCs | Questionnaire (drinking water, occupational and parental exposure history) Serum DDT |
( lipid): Range of total blood DDT concentrations in 11 communities: from 12.5 to 21,500 |
DNA damage: comet assay | Children with high total DDT concentrations (defined as higher than the national geometric mean) had a higher DNA damage compared with those with low total DDT concentrations (). Children exposed to PAHs (from biomass combustion) and DDT had the highest DNA damage compared with children in the other three exposure scenarios (high PAHs, high arsenic, and low lead exposure) (). |
| 3. Anguiano-Vega et al.48 | 2020/Mexico | 63 children (6–13 years of age) exposed to pesticides near school/24 controls (6–13 years of age) | Cross-sectional | OCs | Questionnaire (parental occupational exposure history) Hair HCH, aldrin, dieldrin, endrin, chlordane, heptachlor, epoxyheptane, endosulfan, DDD, DDE, DDT |
Total (mean) OCs (): Exposed: 28.2 (0.95) unexposed: 4.4 (0.18) |
Cytogenetic damage: MN, CC Cytotoxicity: KR, PK, BN, KL, LN, AN, TAC |
Higher frequency of PK, BN, KL, LN, and AT abnormalities among exposed children compared with unexposed ( for each). Among all participants, those in the highest tertile of total OC concentrations had higher numbers of TAC compared with those in the lowest tertile (). |
| Studies on OPs or CBs | ||||||||
| Studies in adults | ||||||||
| 4. Franco et al.56 | 2016/Brazil | 161 community health agents/88 controls | Cross-sectional | OPs | Questionnaire (occupational exposure history) | Not applicable | DNA damage: comet assay Transcriptome: LRP1, IGF2R, IGL family, IGJ, CXCL5, CCL3, NSH, LGALS14, NBPF |
Exposed individuals had higher DNA damage than controls (). Higher DNA damage in GSTM1-positive individuals than GSTM1-null individuals (). Sixteen genes with differential gene expression between exposed and controls. Compared with the controls, LRP1 and IGF2R genes were underexpressed and gene IGL family and IGJ were overexpressed in the exposed group. |
| 5. Martinez et al.67 | 2016/Argentina | 27 urban patients with SLE/17 rural patients with SLE/30 urban healthy controls/28 rural healthy controls | Cross-sectional | OPs and CBs | Questionnaire (residential exposure history) Blood AChE, BChEa |
Not applicable | Oxidative stress: CAT, SOD, GSH/GSSG ratio, TBARS | Increase in TBARS (18.3%, ) in rural SLE cases compared with urban SLE cases. |
| 6. Silvério et al.57 | 2017/Brazilb | 94 farmworkers exposed to pesticides including OPs/94 farmworkers exposed to pesticides not including OPs/50 controls | Cross-sectional | OPs | Questionnaire (occupational exposure history) Urinary DAPsa Blood AChE, BChEa |
(): Occupationally exposed to complex mixtures with OPs: ; Occupationally exposed to complex mixtures without OPs: ; Control group: ; |
Cytogenetic damage: MN, BN, NBUDs Cytotoxicity: CC, KR, PN, KL |
Farmworkers exposed to pesticides including OPs had higher NBUDs, CC, and KL than those exposed to pesticides but not OPs (). Both exposed groups had higher MN, BN, CC, KR, PN, and KL than controls (). |
| 7. Simoniello et al.66 | 2017/Argentina | 50 urban patients with SLE/39 rural patients with SLE/54 urban healthy controls/53 rural healthy controls | Cross-sectional | OPs | Questionnaire (residential exposure history) Blood AChE, BChEa |
Not applicable | DNA damage: comet assay Endo sites Oxidative stress: CAT, SOD, TBARS, GSH, GSSG |
Endo sites and SOD (, , , respectively) were higher in rural patients with SLE than urban ones. Rural patients with SLE had increased risk of having oxidative DNA damage than urban patients with SLE (; 95% CI: 1.4, 8.8). |
| 8. Zepeda-Arce et al.58 | 2017/Mexico | 60 sprayers with motor pump (high-exposure group)/126 solid pesticides sprayers (moderate-exposure group)/22 controls | Cross-sectional | OPs, pyrethroids, CBs | Questionnaire (occupational exposure history) Blood AChE, BChEa |
Not applicable | DNA damage: comet assay Oxidative stress: MDA, SOD, CAT, GPx, GR |
No differences in CAT, SOD, GPx, GR activities, DNA damage, and MDA levels between groups. |
| 9. Benitez-Trinidad et al.59 | 2018/Mexico | 127 urban pesticide sprayers/63 controls | Cross-sectional | Multiple pesticide classes | Questionnaire (occupational exposure history) Blood BChEa |
Not applicable | DNA methylation: LINE-1 | Decreased percentage of methylated cytosines in both moderate- and high-exposure groups compared with controls (). Those occupationally exposed had decreased %5mC LINE-1 methylation (; 95% CI: 0.2, 0.8). |
| 10. Xotlanihua-Gervacio et al.60 | 2018/Mexico | 58 spraying brigade workers (high-exposure group)/120 non-sprayer workers (moderate-exposure group)/23 controls | Cross-sectional | OPs, pyrethroids | Questionnaire (occupational exposure history) Urinary DAPsa |
Mean (range) of total DAPs (): ; ; |
Cytogenetic damage: MN, NBUDs, NPBs Oxidative stress: GPx, GR, SOD, CAT |
No differences in MN frequency between exposed workers and controls. A marginal decrease in SOD and CAT activities was observed in the high-exposure group compared with the reference group. |
| 11. Herrera-Moreno et al.61 | 2019/Mexico | 60 spraying brigade workers (high-exposure group)/126 pesticide distributors or occasional farmworkers (moderate-exposure group)/102 controls | Cross-sectional | OPs | Questionnaire (occupational exposure history) Urinary DAPsa |
Mean (range) of total DAPs (): ; ; |
DNA methylation: CDKN2B, CDKN2A | Lower DNA methylation of CDKN2B gene in both pesticide-exposed groups compared with controls (); higher methylation of the CDKN2A promoter in the moderate-exposure group compared with controls (). Association between pesticide exposure and methylation pattern in CDKN2B (; and ; for moderate and high-exposure groups, respectively) and DKN2A (; for moderate-exposure group). |
| 12. Paredes-Céspedes et al.68 | 2019/Mexico | 164 urban mestizo sprayers/189 indigenous persons without occupational pesticide exposure/91 mestizo individuals without occupational pesticide exposure (reference group) | Cross-sectional | OPs | Questionnaire (past and present pesticide exposure) Urinary DAPs |
Mean (range) of total DAPs (): ; ; |
DNA methylation: %5mC of | Increased %5mC in CpG sites 1 and 2 in mestizo sprayers compared with reference and indigenous groups (). Lower %5mC among indigenous group for CpG site 3 compared with reference and mestizo sprayer groups (). No correlations between total urinary DAP concentrations and %5mC in any group. Among the two mestizo groups, self-reported of deltamethrin was associated with decreased odds of having %5mC levels above the GM (; 95% CI: 0.5, 0.9), and self-reported use of temephos was associated with increased odds of having %5mC levels above the GM (; 95% Ci: 1.3, 5.7). |
| 13. Butinof et al.62 | 2019/Argentinab | 47 pesticide applicators/52 unexposed controls | Cross-sectional | OPs, CBs | Questionnaire (occupational exposure history) Blood BChEa |
Not applicable | DNA damage: comet assay Cytogenetic damage: CAs, MN |
Higher CA and MN frequencies and DNA damage in pesticide applicators compared with unexposed ( for each). |
| 14. Bernieri et al.63 | 2020/Brazil | 12 male soybean growers/12 unexposed control males | Cross-sectional | OPs | Questionnaire (occupational history) Blood BChE (measured in samples collected during periods of high and low exposure in the same year)a |
Not applicable | DNA damage (measured in samples collected during periods of high and low exposure in the same year): comet assay | DNA damage index higher in soybean growers during high exposure period compared with the low exposure period and with controls ( for each). No correlation between exposure time and DNA damage. |
| 15. Aiassa et al.65 | 2019/Argentina | 30 pesticide applicators/22 unexposed controls | Cross-sectional | OPs, carbamates | Questionnaire (occupational and environmental exposure history) Blood BChEa |
Not applicable | DNA damage: comet assay Cytogenetic damage: CA, MN |
Higher mean CA, MN, and DNA fragmentation values () in pesticide applicators than in unexposed controls. |
| 16. Valencia-Quintana et al.64 | 2021/Mexico | 54 farmworkers/26 unexposed controls | Cross-sectional | OPs, carbamates | Questionnaire (occupational exposure history) Blood AChE, BChEa |
Not applicable | DNA damage: comet assay Cytogenetic damage: MN Cytotoxicity: KR, KL, CC, PN |
Farmworkers had higher frequency of MN, KR, KL, CC, PN, and all other measured parameters than controls ( for each). |
| Studies on other pesticides or multiple pesticide classes | ||||||||
| Studies in children | ||||||||
| 17. Gómez-Arroyo et al.49 | 2013/Mexico | 125 children (1–13 years of age) living around areas of intensive agriculture/125 controls | Cross-sectional | Multiple pesticide classes | Questionnaire (residential exposure history) | Not applicable | Cytogenetic damage: MN, BN, NBUDs Cytotoxicity: KL, KR |
Exposed children had higher frequency of MN (; 95% CI: 2.7, 3.5), BN (; 95% CI: 3.9, 4.6), KL (; 95% CI: 2.6, 2.7), KR (; 95% CI: 14.8, 20.8), and NBUDs (; 95% CI: 1.5, 1.8) than controls. |
| 18. Bernardi et al.50 | 2015/Argentina | 50 children (4–14 years of age) living near pesticide application areas/ 25 controls | Cross-sectional | OPs, pyrethroids, glyphosate | Questionnaire (residential exposure history) | Not applicable | Cytogenetic damage: MN | Children living from pulverized areas had higher frequency of MN () than children living and controls (). |
| 19. Barrón Cuenca et al.55 | 2015/Bolivia | 41 children with chronic malnourishment/114 cases years of age | Cross-sectional | Multiple pesticide classes | Questionnaire (maternal occupational exposure history) | Not applicable | Cytogenetic damage: MN | Null associations between pesticide exposure and markers of cytogenetic damage. |
| 20. Castañeda-Yslas et al.51 | 2016/Mexico | 34 children of farmworkers (4–11 years of age)/38 child controls (7–14 years of age)/37 female farmworkers/35 controls | Cross-sectional | Multiple pesticide classes | Questionnaire (occupational and parental exposure history) | Not applicable | Cytogenetic damage: MN, BN, NBUDs, NA, LN Cytotoxicity: KR, KL, CC, PN | Frequencies of MN (), LN (), and CC () were higher, and PN () lower in children of farmworkers than in children of controls. Higher MN () and CC (), and lower PN () frequencies in female farmworkers than controls. |
| 21. Nascimento et al.52 | 2017/Brazilb | 40 children (6–12 years of age) living near a tobacco-producing region | Prospective cohort | Multiple pesticide classes | Questionnaire (parental and seasonal exposure history) Blood AChE, BChEa |
Not applicable | Oxidative damage: MDA, PCO, vitamin C | MDA, PCO, and vitamin C () were higher at the beginning of application period than at leaf harvest period. |
| 22. Ruiz-Guzmán et al.30 | 2017/Colombia | 50 children (5–15 years of age) from agricultural villages/13 controls from nearby city | Cross-sectional | OPs, pyrethroids, atrazine, bipyridyl | Questionnaire (parental and residential exposure history) Urinary ATZ and its metabolites ADI and ADDI |
( creatinine): Pelayito: ; ; ; Aguas Negras: |
Cytogenetic damage: MN, NBUDs, apoptotic cells | Null associations of urinary ATZ and its metabolites with MN, NBUDs, or apoptotic cells. |
| 23. Quintana et al.54 | 2017/Argentinab,c,d | 151 mother–newborn pairs living in a rural area/38 mother–newborn pairs from an urban area (controls) | Cross-sectional | OPs | Questionnaire (residential and seasonal exposure history) Cord blood AChE, BChEa |
Not applicable | DNA damage: comet assay Oxidative stress: SOD, CAT |
DNA damage index was higher in RG-SS than controls (), but not significantly different between RG-SS and RG-NSS. SOD activity was lower in RG-SS compared with RG-NSS and controls (). |
| 24. Leite et al.53 | 2019/Paraguay | 43 children (5–10 years of age) living in agricultural community surrounded by transgenic soybean crops/41 children living in agricultural community using biological control of pests | Cross-sectional | Multiple pesticide classes | Questionnaire Blood AChEa |
Not applicable | DNA damage: comet assay Cytogenetic damages: MN, BN, BE Cytotoxicity: KR, KL, CC, PN |
Higher MN, BN, BE, KR, KL, PN, and CC in exposed group compared with control group ( for each). Higher mean values of tail length and tail movement among exposed vs. unexposed group ( for each). |
| Studies in adults | ||||||||
| 25. Jørs et al.69 | 2007/Bolivia | 48 farmworkers/33 controls | Cross-sectional | Multiple pesticide classes | Questionnaire (occupational exposure history) | Not applicable | DNA damage: comet assay Cytogenetic damage: CAs |
Higher DNA damage and frequencies of CAs in farmworkers than in controls (). Number of CAs increased with the intensity of pesticide exposure. |
| 26. Kehdy et al.70 | 2007/Brazil | 29 sanitation workers/30 controls | Cross-sectional | OPs, pyrethroids, rodenticides | Questionnaire (occupational exposure history) | Not applicable | Cytogenetic damages: MN, NPBs, APOP, NECR, NDI | Higher frequencies of MN, NB, and NECR in sanitation workers than in controls (). No difference in APOP frequency between groups. NDI was lower in the sanitation workers than controls (). |
| 27. da Silva et al.86 | 2008/Brazil | 108 vineyard farmworkers/65 controls | Cross-sectional | Multiple pesticide classes | Questionnaire (occupational exposure history) | Not applicable | DNA damage: comet assay Cytogenetic damage: MN, BNMN |
Higher BNMN frequency, DI, and DF in farmworkers compared with controls (. Higher MN frequency in PON1 Gln/Gln individuals in the exposed group, compared with PON1 Arg/– in the exposed group (). |
| 28. Simoniello et al.91 | 2008/Argentina | 27 pesticide applicator farmworkers/27 non-pesticide applicator farmworkers/30 controls | Cross-sectional | Multiple pesticide classes | Occupation (pesticide applicator farmworker, non-pesticide applicator farmworker, non-farmworker) | Not applicable | DNA damage: comet assay, damage index repair assay | Pesticide applicators and non-applicator farmworkers had higher DNA damage than unexposed controls (). |
| 29. Bortoli et al.92 | 2009/Brazil | 29 farmworkers/37 controls | Cross-sectional | Multiple pesticide classes | Questionnaire (occupational exposure history) | Not applicable | Cytogenetic damage: MN | Significantly higher mean MN frequency in farmworkers than in controls (). |
| 30. Martínez-Valenzuela et al.93 | 2009/Mexico | 70 farmworkers/70 controls | Cross-sectional | Multiple pesticide classes | Questionnaire (occupational exposure history) | Not applicable | Cytogenetic damage: SCE, MN, NA, CPK | Significantly higher mean SCE and MN frequencies in farmworkers than in controls ( for each). |
| 31. Remor et al.94 | 2009/Brazilb | 37 farmworkers/20 controls | Cross-sectional | Multiple pesticide classes | Questionnaire (occupational exposure history) Blood BChEa |
Not applicable | DNA damage: comet assay Cytogenetic damage: MN |
Higher DI () and DF () in farmworkers than in controls. MN frequencies were not different between groups. |
| 32. Simoniello et al.95 | 2010/Argentina | 45 farmworkers applicator/50 farmworkers non-applicator/50 controls | Cross-sectional | Multiple pesticide classes | Questionnaire (occupational exposure history) Blood AChE, BChEa |
Not applicable | DNA damage: comet assay Oxidative damage: CAT activity, TBARS |
Increased TBARS levels among farmworkers directly exposed () but not among those indirectly exposed. CAT reduction in both exposed groups respect to controls ( and , respectively). IDEC and IDER increased in both exposed groups (). |
| 33. Paz-y-Miño et al.105 | 2011/Ecuador | 92 exposed from communities with aerial spraying/90 controls | Cross-sectional | GLY | Questionnaire (residence exposure history) | Not applicable | Cytogenetic damage: CAs, karyotype | Levels of cytogenetic damage and DNA alterations were similar between groups. |
| 34. Payán-Rentería et al.96 | 2012/Mexicob | 25 farmworkers and applicators/21 controls | Cross-sectional | OCs, OPs, herbicides | Medical examination Questionnaire (occupational exposure history) Blood AChEa |
Not applicable | DNA damage | Higher circulating DNA fragments () in farmworkers than in controls. |
| 35. Benedetti et al.97 | 2013/Brazil | 81 farmworkers/46 controls | Cross-sectional | Multiple pesticide classes | Questionnaire (occupational exposure history) Blood BChEa |
Not applicable | DNA damage: comet assay Cytogenetic damage: MN, BN, NBUDs Cytotoxicity: CC, KR, KL |
Farmworkers had higher DNA damage (), frequency of MN (), NBUDs (), BN (), and cell death (CC, ; KR, , and KL, ) compared with controls. |
| 36. Khayat et al.71 | 2013/Brazil | 41 farmworkers/32 controls | Cross-sectional | Multiple pesticide classes | Questionnaire (occupational exposure history) | Not applicable | DNA damage: comet assay Cytogenetic damage: MN |
Higher MN () and BN frequencies (), %DNA in the tail (), TM (), OTM () in farmworkers than controls, but not in TL (). |
| 37. Varona-Uribe et al.106 | 2014/Colombia | 223 farmworkers | Cross-sectional | OCs, OPs, CBs, fungicides | Blood OPs: bromophos-ethyl, bromophos-methyl, chlorpyriphos, dimethoate, malathion, methamidophos, methyl parathion, pirimiphos, pirimiphos-methyl, profenofos Blood CBs: aminocarb, bendiocarb, metolcarb, pirimicarb, propoxur Blood OCs: BHC, HCB, heptachlor, heptachloro epoxide, chlordane, endosulfan, DDT DDE, carbofuran, mirex Urinary |
Median (P25–P75) (): , , , , , , , , , , , , , , , , , , , , , , , , , , , , , , |
DNA damage: comet assay | Higher concentrations of , , and HCB (as a mixture) (; 95% CI: 0.33, 2.10) and of pirimiphos-methyl, malathion, bromophos-methyl, and bromophos-ethyl (as a mixture) (; 95% CI: 2.34, 21.60) were associated with higher DNA damage and comet tail length, respectively. |
| 38. Adad et al.98 | 2015/Brazilb | 80 men farmworkers from state association/20 men farmworker from a private company/100 controls | Cross-sectional | Multiple pesticide classes | Questionnaire (occupational exposure history) Blood BChEa |
Not applicable | Cytogenetic damage: MN, BN Cytotoxicity: KR, KL |
Higher frequencies of MN (), KR (state group ; private group ), KL (both exposed groups ), and BN cells (both exposed groups ) in both exposed groups than in controls. |
| 39. Wilhelm et al.72 | 2015/Brazil | 37 floriculturists/37 controls | Cross-sectional | Multiple pesticide classes | Questionnaire (occupational exposure history) | Not applicable | DNA damage: comet assay Cytogenetic damage: MN, NBUDs, BN Cytotoxicity: KR |
MN, NBUDs, BN, and KR frequencies were similar between exposed and controls. Higher DNA damage in the exposed compared with controls ( for DI and DF). |
| 40. Alves et al.73 | 2016/Brazilb | 77 tobacco farmworkers/60 controls | Cross-sectional | Multiple pesticide classes | Questionnaire (occupational exposure history) | Not applicable | DNA damage: comet assay Cytogenetic damage: MN Oxidative stress: SOD |
MN frequency, DF, and DI were higher in farmworkers than controls (). Higher SOD activity in exposed relative to unexposed group (). Higher MN frequency in PON1 Gln/Gln individuals in the exposed group, compared with PON1 Arg/– individuals in the exposed group (). |
| 41. Kahl et al.74 | 2015/Brazil | 62 tobacco farmworkers/62 controls | Cross-sectional | Multiple pesticide classes | Questionnaire (occupational exposure history) | Not applicable | aTL Oxidative stress: TBARS, TEAC |
Farmworkers had higher TEAC () and TBARS (), but lower aTL (, ) than controls. |
| 42. Bianco et al.99 | 2017/Argentina | 76 farmworkers/53 controls | Cross-sectional | Multiple pesticide classes | Questionnaire (occupational exposure history) Blood AChEa |
Not applicable | Cytogenetic damage: CAs | Farmworkers had higher CAs frequency () than controls. |
| 43. Chaves et al.75 | 2017/Brazil | 97 farmworkers/55 controls | Cross-sectional | CBs, OPs, pyrethroids | Questionnaire (occupational and lifestyle exposures history) | Not applicable | Cytogenetic damage: Cas, MN | Increased frequency of CAs () and MN () in farmworkers than in controls. |
| 44. Hilgert Jacobsen-Pereira et al.100 | 2018/Brazil | 50 farmworkers/46 controls from the same agricultural area/29 controls from urban area | Cross-sectional | Multiple pesticide classes | Questionnaire (occupational exposure history) Blood AChE, BChEa |
Not applicable | DNA damage: comet assay. Cytogenetic damage: MN, NBUDs, NPBs Oxidative stress: TBARS, CAT activity |
Higher DI (), MN (), NBUD (), and NPB () frequencies in farmworkers than controls. TBARS level was higher in exposed and in rural controls than urban controls. CAT activity was similar among groups. |
| 45. Tomiazzi et al.76 | 2017/Brazil | 30 nonfarmer smokers/30 nonsmoker farmworkers/30 smokers and farmworkers/30 controls | Cross-sectional | OPs, pyrethroids, glyphosate | Questionnaire (occupational exposure history) | Not applicable | Cytogenetic damage: MN, BNMN Cytotoxicity: KL, KR, CC |
MN frequency and the total cytogenetic abnormalities were higher in all exposed groups than in controls (). |
| 46. Vazquez-Boucard et al.77 | 2017/Mexico | 107 consumers of well or tap water/40 consumers of bottled water (controls) | Case–control | OCs, OPs, neonics | Questionnaire (occupational exposure history) Regional water sampling |
Not applicable | DNA damage: comet assay | Higher DNA damage in those who consumed well or tap water than in control group (). Individuals who consumed well or tap water and worked in agriculture had higher DNA damage than controls (). |
| 47. Marcelino et al.90 | 2017/Brazil | 18 farmworkers/18 unexposed controls | Cross-sectional | Multiple pesticide classes | Questionnaire (occupational exposure history) | Not applicable | DNA damage: comet assay Cytogenetic damage: MN |
Significantly higher DNA and cytogenetic damage in exposed group compared with unexposed group ( for each). |
| 48. Hutter et al.78 | 2018/Dominican Republic | 38 exposed farmworkers/33 control farmworkers | Cross-sectional | Multiple pesticide classes | Questionnaire (occupational exposure history) | Not applicable | Cytogenetic damage: MN, BUD, BN Cytotoxicity: CC, KR, KL, PN |
All cytogenetic damage and cytotoxicity biomarkers were more frequent among farmworkers: MN (; 95% CI: 1.3, 7.4), total MN (; 95% CI: 1.2, 5.2), BUD (; 95% CI: 1.5, 2.5), BN (; 95% CI: 1.2, 1.7), CC (; 95% CI: 1.1, 1.6), KR (; 95% CI: 1.0, 1.4), KL (; 95% CI: 1.1, 1.5), PN (; 95% CI: 2.5, 8.2). |
| 49. Kahl et al.80 | 2018/Brazil | 56 tobacco farmworkers/74 controls | Cross-sectional | Multiple pesticide classes | Questionnaire (occupational exposure history) | Not applicable | DNA damage: comet assay Telomere length Oxidative stress: TEAC, TBARS DNA methylation: global and p16 methylation |
Farmworkers had higher DNA damage (), lower percentage global DNA methylation, shorter telomeres (), and p16 hypermethylation () compared with controls. |
| 50. Cattelan et al.88 | 2018/Brazilb | 84 farmworkers who used pesticides/68 farmworkers who did not use pesticides | Cross-sectional | Multiple pesticide classes | Questionnaire (occupational exposure history) | Not applicable | Cytogenetic damage: MN Oxidative stress: CAT, GPx, GSH, SOD, TBARS, carbonylated protein levels |
Lower mean TBARS (), GPx (), GSH (), and SOD () values in farmworkers who did not use pesticides than in those who did use pesticides. No differences in MN frequency between groups. |
| 51. Kahl et al.89 | 2018/Brazil | 40 tobacco farmworkers/40 unexposed controls | Cross-sectional | Multiple pesticide classes | Questionnaire (occupational exposure history) | Not applicable | Cytogenetic damage: MN, NPB, NBUDs Telomere length DNA methylation: global MTHFR and TERT genotypes |
Higher frequencies of MN, NPB, NBUD, and binucleated cells in farmworkers than controls ( for each). Shorter telomere length () and lower DNA global methylation levels () in exposed group. Allele and genotype frequencies of MTHFR gene were different between exposed and unexposed groups (). No differences between groups for TERT polymorphism frequencies. |
| 52. Claudio et al.79 | 2019/Brazil | 21 male banana farmworkers/20 controls | Cross-sectional | Multiple pesticide classes | Questionnaire (occupational exposure history) | Not applicable | Cytogenetic damage: MN Cytotoxicity: PN, KR, KL |
Higher MN and KR frequencies in farmworkers than controls ( for both), but KL and PN were similar between groups. |
| 53. de Oliveira et al.101 | 2019/Brazil | 76 soybean farmworkers/72 controls | Cross-sectional | Multiple pesticide classes | Questionnaire (occupational exposure history). Blood BChEa |
Not applicable | Cytogenetic damage: MN, BN, NBUDs Cytotoxicity: PN, KR, KL, CC Telomere length XCCR1 Trip/– and PON1 Arg/– genes |
Increased cytogenetic damage (MN and NBUDs ; BN ) and cell death (CC and KR ) in the exposed group compared with controls. Telomere length was similar in both groups. Higher frequencies of BN cells in farmworkers (), and NBUDs in controls () belonging to those carrying XCCR1 Trip/– and PON1 Arg/– genes. |
| 54. Arévalo-Jaramillo et al.81 | 2019/Ecuadorb | 62 women living in 2 agricultural communities/53 unexposed women from control community | Cross-sectional | Multiple pesticide classes | Questionnaire | Not applicable | Cytogenetic damage: MN, NBUDs, notched cells, BN Cytotoxicity: PK, KL, KR, CC |
Lower BN () and higher KL () and KR () among those in the first agricultural community compared with controls. Higher NBUDs () and notched cells () among those in the second agricultural community compared with the controls. Mean MN not statistically different between controls and exposed groups. Increased frequency of KL, KR, and CC cells among individuals with genetic polymorphisms in PON1 and GSTP1 genes. |
| 55. Barrón Cuenca et al.82 | 2019/Bolivia | 297 men and women (17–70 years of age) from three agricultural communities | Cross-sectional | Fungicides, OPs, pyrethroids, herbicides | Questionnaire (occupational history) Urinary metabolites of tebuconazole, chlorpyrifos, permethrin, cypermethrin, cyfluthrin, phenoxy herbicides, bifenthrin, thiabendazole, pyrimethanil |
Mean (IQR) of pesticide metabolites () in total population: ; ; ; ; ; ; ; ; ; |
DNA damage Cytogenetic damage: MN |
Increased MN frequency among those with y active farming compared with those with y active farming. Days of active spraying per month was not associated with genotoxic damage. Increased odds of DNA strand breaks among those with high exposure to 2,4-D (); 95% CI: 1.1, 3.6 for tail movement and ; 95% CI: 1.0, 3.1 for %DNA in tail). Decreased odds of DNA strand breaks among those with high exposure to pyrethroids (; 95% CI: 0.3, 0.9 for %DNA in tail and ; 95% CI: 0.3, 1.0 for tail movement). High exposure to certain mixtures of pesticides (containing mainly 2,4-D or cyfluthrin) was associated with increased DNA strand breaks, but not increased chromosomal aberrations (). Higher levels of DNA strand breaks among participants with certain GSTM1 genotypes. |
| 56. Cepeda et al.83 | 2020/Colombia | 5 farmers/5 unexposed controls | Cross-sectional | Multiple pesticide classes | Questionnaire (pesticide exposure history) | Not applicable | Cytogenetic damage: CA, chromosomal instability | Increased total clonal and non-clonal CAs were observed in pesticide-exposed individuals compared with unexposed individuals (). Higher frequency of fragilities and chromatid/chromosomic breakage in exposed group compared with unexposed group ( for each). |
| 57. Hutter et al.84 | 2021/Ecuador | 34 male farmworkers engaged in conventional farming/37 male unexposed controls engaged in ecological farming | Cross-sectional | Multiple pesticide classes | Questionnaire (occupational exposure history) | Not applicable | Cytogenetic damage: MN, BUD, BN Cytotoxicity: CC, KR, KL, PK, basal cells |
Compared with controls working on ecological farms, those working on conventional farms had higher frequency of MN, BUD, BN, KR, CC, and KL ( for each). |
| 58. Salazar-Flores et al.85 | 2020/Mexico | 113 farmworkers/93 unexposed controls | Cross-sectional | Multiple pesticide classes | Questionnaire (occupational exposure history) | Not applicable | Oxidative stress: GSH, GSSG, GSH/GSSG ratio, carbonyl groups in proteins, nitrates–nitrites, lipoperoxides, membrane fluidity | Lower levels of GSH, GSSG, carbonyl groups in proteins, nitrates–nitrites, lipoperoxides, and membrane fluidity among farmworkers compared with unexposed controls ( for each). No differences in most markers of oxidative stress between farmworkers and controls when farmworkers were grouped in four exposure categories. |
| 59. Lovison Sasso et al.102 | 2021/Brazilb | 50 male farmworkers/50 male controls | Cross-sectional | Multiple pesticide classes | Questionnaire (occupational exposure history) Blood BChEa |
Not applicable | Oxidative stress: GSH, CAT, GR, GPx, SOD, TBARS, carbonylated protein levels | Lower SOD, CAT, GSH, GR, and GPx activity, but higher TBARS and carbonylated protein levels, among exposed group compared with controls ( for each). |
| 60. de Souza Espindola Santos et al.87 | 2021/Brazil | 52 farmworkers/68 non-farmworkers | Cross-sectional | Multiple pesticide classes | Questionnaire (occupational exposure history) | Not applicable | Oxidative stress: CAT, SOD, thiols, GST, GPx, GR, 8-ISO | No differences in biomarkers of oxidative stress between farmworkers and non-farmworkers. |
| 61. Fillippi et al.103 | 2021/Argentinab | 47 pesticide applicators/53 unexposed controls | Cross-sectional | Multiple pesticide classes | Questionnaire (occupational exposure history) Blood HCB, HCH, endosulfan, DDE, DDT, AChE, BChEa |
Not applicable | DNA damage: comet assay Cytogenetic damage: SCE, CA, MN | Pesticide applicators had more DNA damage, as well as higher SCE, CA, MN frequencies, compared with controls ( for each). |
| 62. Mañas et al.104 | 2021/Argentina | 41 adults living in area of intensive agricultural production ( from sprayed fields)/24 unexposed adults ( from sprayed fields) | Cross-sectional | Multiple pesticide classes | Residential proximity to agricultural fields | Not applicable | Cytogenetic damage: CAs, BNMN | Higher frequencies of CAs and BNMN in exposed group compared with unexposed group ( for each). Among exposed group, higher CAs among those living from fields compared with those living (). |
Note: %5mC, percentage 5mC; %DNA, percentage DNA; 2,4-D, 2,4-dichlorophenoxy acetic acid; 8-ISO, 8-isoprostane; 3-BPA, 3-phenoxybenzoic acid; 4F3BPA, 4-fluoro-3-phenoxybenzoic acid; 5-OH-TBZ, 5-hydroxytiabendazole; AChE, acetylcholinesterase; ADI, atrazine desisopropyl; ADDI, atrazine desethyl-desisopropyl; APOP, apoptotic cells; AT, apoptosis; aTL, absolute telomere length; ATZ, atrazine; BChE, butyrylcholinesterase; BE, broken egg; BHC, benzene hexachloride; BN, binucleated cells; BNMN, binucleated cells with micronuclei; BUD, nuclear buds and broken eggs; CAs, chromosomal aberrations; CAT, catalase; CBs, carbamates; CC, condensed chromatin; CCL3, chemokine signaling pathway gene; CDKN2A, cyclin dependent kinase inhibitor 2A; CDKN2B, cyclin dependent kinase inhibitor 2B; CFCA, chloro-3,3,3-trifluoro-1-propen-1-yl]-2,2-dimethylcyclopropanecarboxylic acid; CHBs, chromatin buds; CI, confidence interval; CIN, chromosomal instability; CPK, cell proliferation kinetics; CXCL5, CXC subfamily of chemokine gene; DAP, dialkyl phosphate; DCCA, 3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane carboxylic acid; DDD, dichlorodiphenyldichloroethane; DDE, dichlorodiphenyldichloroethylene; DDT, dichlorodiphenyltrichloroethane; DF, damage frequency; DI, damage index; DNA, deoxyribonucleic acid; ETU, ethylenethiourea; GLY, glyphosate; GM, geometric mean; GPx, glutathione peroxidase; GR, glutathione reductase; GSH, reduced glutathione; GSSG, oxidized glutathione; GST, glutathione -transferase; HCB, hexachlorobenzene; HCH, hexachlorocyclohexane; IDEC, comet assay damage index; IDER, repair test damage index; IGF2R, insulin like growth factor 2 receptor; IGJ, joining chain of multimeric IgA and IgM; IGL, immunoglobulin lambda locus; KL, karyolysis; KR, karyorrhexis; LGALS14, lectin galactoside-binding soluble 14; IQR, interquartile; LINE-1, long interspersed nucleotide element 1; LN, lobulated nucleus; LOD, limit of detection; LRP1, LDL receptor related protein 1; MCPA, 4- chloro-2-methylphenoxy acetic acid; MDA, malondialdehyde; MN, micronuclei; NA, nuclear abnormalities; NBPF, neuroblastoma breakpoint family genes; NBUDs, nuclear buds; NDI, nuclear division index; NECR, necrotic cells; NPBs, nucleoplasmic bridges; NSS, non-spraying season; OC, organochlorine; OH-PYR, 3-hydroxy-pyrimetanil; OP, organophosphate; OR, odds ratio; OTM, olive tail moment; P, percentile; PAHs, polycyclic aromatic hydrocarbons; PCO, protein carbonyls; PK, pyknosis; PN, pyknotic cells; PON1, paraoxonase 1 gene; RBCs, red blood cells; Ref, reference group; RG, rural group; S, Sulfur; SCE, sister chromatids exchanges; SD, standard deviation; SE, standard error; SLE, Systemic Lupus Erythematosus; SOD, superoxide dismutase; SS, spraying season; TAC, total abnormal cells; TBARS, thiobarbituric acid reactive substances; TBE-OH, hydroxy-tebuconazole; TCPy, 3,5,6-trichloro-2-pyridinol; TEAC, total equivalent antioxidant capacity; TL, tail length; TM, tail moment.
Investigators did not use exposure biomarker concentrations in multivariate analyses.
Also included in Table 9 (other health effects).
Also included in Table 4 (placental outcomes and teratogenicity).
Also included in Table 8 (birth outcomes and child growth).
Thirteen publications from cross-sectional studies examined associations of exposure to OP or carbamate pesticides with cytogenetic or DNA damage in adults, primarily among those occupationally exposed (Table 2). One cross-sectional study assessed OP pesticide exposure via questionnaire only and reported higher DNA damage—quantified via comet assay—among workers compared with controls.56 The other 12 studies assessed exposure to OP or carbamate pesticides using urinary dialkyl phosphate (DAP) metabolite concentrations or blood cholinesterase (ChE) measurements, but 11 of them evaluated exposure–outcome associations using predetermined categorical exposure variables based on occupation (e.g., high, moderate, and no exposure)57–65 or residence (e.g., rural or urban).66 Nine of these 11 publications reported associations with genotoxic outcomes, such as changes in DNA methylation patterns of candidate tumor suppressor genes, among moderate- or high-exposure groups.57,59,61–67 Two publications reported no differences in markers of cytogenetic or DNA damage between exposed workers and controls.58,60 The only cross-sectional study that used urinary DAP concentrations in its exposure–outcome analyses reported null associations with DNA methylation but observed group differences when OP pesticide exposure was assessed as a categorical variable.68
Thirty-eight publications examined associations of exposure to pesticides other than OCs, OPs, or carbamates or exposure to multiple pesticide classes with genotoxicity among adults (Table 2). Twenty-five publications estimated occupational pesticide exposure using questionnaire data only and all reported associations of exposure to pesticides with increased cytogenetic damage, including higher frequencies of chromosomal aberrations and micronuclei, DNA damage, oxidative stress, or telomere shortening.69–93 In addition, 10 publications from cross-sectional studies of farmworkers/pesticide applicators and controls assessed pesticide exposure using blood ChE measurements but only evaluated exposure–outcome associations using categorical exposure variables.94–103 All 10 publications reported that occupational pesticide exposure was associated with higher levels of DNA or cytogenetic damage, such as higher frequencies of chromosomal aberrations, nuclear buds, or cell death. Similarly, a publication from a cross-sectional study in Argentina reported increased cytogenetic damage among those living near agricultural fields (),104 whereas a publication from a cross-sectional study in Ecuador reported null associations of residential use of the herbicide glyphosate with chromosomal aberrations frequency and karyogram alterations.105 Last, a publication from a cross-sectional study of rice field workers in Colombia reported associations of two pesticide mixtures (one mixture of OC pesticides and one of carbamates)—assessed via measurement of pesticide metabolites in blood and urine—with DNA damage.106
Overall, studies published to date provide consistent evidence of an association between exposure to different pesticide classes such as OP pesticides and carbamates and genotoxic damage in children and adults living in LAC countries. Notably, most of the studies that have been published were cross-sectional in design, assessed pesticide exposure via questionnaire, and had small sample sizes.
Neurobehavioral Outcomes
Fifty-four publications, primarily derived from cross-sectional studies, examined the potential neurobehavioral effects of pesticide exposure in children, adolescents, and adults (Table 3). Twelve of these 53 publications reported on the association between exposure to OC pesticides and child neurodevelopment107–115; 6 publications focused on the same Mexican cohort,107–109,112–114 5 focused on the same Guadeloupean cohort,110,111,115–117 and 1 was a cross-sectional study from Brazil. Three publications from the prospective cohort study in Mexico reported that higher prenatal dichlorodiphenyltrichloroethane (DDT) exposure—as indicated by measurement of its primary breakdown product dichlorodiphenyldichloroethylene (DDE) in serum—was associated with lower psychomotor development during the first year of life,107 poorer verbal and memory skills and a poorer general cognitive index at 3.5–5 years of age,112 and poorer spatial orientation at 5 years of age.113 A fourth publication from the same cohort study reported that maternal intake of omega-3 and -6 fatty acids during pregnancy modified the association of prenatal DDT exposure with poorer motor and memory skills at 3.5–5 years of age,114 whereas 2 other publications from this cohort reported null associations of prenatal DDT exposure with child neurodevelopment at 1 month109 and at 12–30 months of age.108 Four publications from the prospective Guadeloupean cohort study reported that higher cord blood concentrations of chlordecone—an OC pesticide that was extensively used in banana plantations in the French West Indies—were associated with impaired cognitive and motor function at 7 months of age,110 lower fine motor scores at 18 months of age (among boys only),111,117 and poorer visual contrast sensitivity at 7–8 years of age.115 A fifth publication from the Guadeloupean cohort study reported null associations of prenatal and childhood chlordecone exposure with sex-typed play behavior at 7 years of age.116 The one Brazilian cross-sectional study reported that higher concentrations of several OC pesticide metabolites were associated with poorer performance intelligence quotient, resistance to distraction, or processing speed at 6–16 years of age.118 The only publication that examined the association of OC pesticide exposure—as indicated by measurement of (), DDT, DDE, and dieldrin in serum—with neurodegenerative disorders among adults was from a cross-sectional study conducted in Costa Rica and reported null associations.119
Table 3.
Characteristics of Latin American and the Caribbean studies on pesticide exposure and neurobehavioral outcomes published between 2007 and 2021 ().
| Study | Year of publication/country | Population and sample size | Study design | Pesticides assessed | Exposure assessment method | Pesticide or metabolite concentrations | Health effect and assessment method/instrument | Results |
|---|---|---|---|---|---|---|---|---|
| Studies on OCs | ||||||||
| Studies in children | ||||||||
| 1. Torres-Sánchez et al.107 | 2007/Mexico | 244 mother–child (0–12 months of age) pairs from malaria-endemic zone | Prospective cohort | OCs | Maternal serum DDE before and during each trimester of pregnancy |
(): -DDE: ; ; |
Neurodevelopment: mental and psychomotor development (BSID-II) | Higher DDE during first trimester of pregnancy was associated with lower PDI scores in first year of life ( per 2-fold increase in ; 95% CI: , ). Null associations between DDE and MDI scores. |
| 2. Torres-Sánchez et al.108 | 2009/Mexico | 270 mother–child (12–30 months of age) pairs | Prospective cohort | OCs | Maternal serum DDE during each trimester of pregnancy |
(): -DDE: ; ; ; -DDT: ; ; |
Neurodevelopment: mental and psychomotor development (BSID-II) | Null associations of prenatal DDE with PDI and MDI scores. |
| 3. Bahena-Medina et al.109 | 2011/Mexico | 265 mother–child (1 month of age) pairs | Prospective cohort | OCs | Maternal serum DDE during each trimester of pregnancy |
DDE (): First trimester: ; ; ; ; ; ; Second trimester: ; ; ; ; ; Third trimester: ; ; ; ; ; |
Neurodevelopment: neonatal reflexes (NBAS), neurological soft signs (Graham-Rosenblith scale), mental and psychomotor development (BSID) | Null associations between prenatal DDE exposure and neonatal neurodevelopment. |
| 4. Dallaire et al.110 | 2012/Guadeloupe | 153 mother–child (7 months of age) pairs living near banana plantations | Prospective cohort | OCs (chlordecone) | Questionnaire (infant dietary intake history) Cord blood chlordecone Breast milk chlordecone at 3 months |
chlordecone (): ; |
Neurodevelopment: visual recognition, memory, and processing speed (FTII, TAC, Brunet-Lezine scale) | Those in the highest tertile of cord blood chlordecone concentrations (classified as ) scored lower on the novelty preference test (; 95% CI: , 0) than those in the second tertile of cord chlordecone concentrations (classified as and ) (; 95% CI: , 0.1). Detectable concentrations of chlordecone in cord blood were associated with increased odds of low fine motor scores (; 95% CI: 1.1, 1.5). |
| 5. Boucher et al.111 | 2013/Guadeloupe | 141 mother–child (18 months of age) pairs living near banana plantations | Prospective cohort | OCs (chlordecone) | Questionnaire (infant dietary intake history) Cord blood chlordecone Breast milk chlordecone at 3 months |
chlordecone (): ; |
Neurodevelopment: personal–social, communication, problem-solving, fine motor, and gross motor (ASQ-19) | Higher chlordecone concentrations in cord blood were associated with lower fine motor scores among boys (; ). |
| 6. Torres-Sánchez et al.112 | 2013/Mexico | 203 mother–child (42–60 months of age) pairs | Prospective cohort | OCs | Maternal serum DDE during each trimester of pregnancy | Median (P10–P90) ( lipid): DDE: ; ; ; DDT: ; ; |
Neurodevelopment: verbal, perceptual performance, quantitative, memory, motor skills, and general cognitive index (MSCA) | Higher DDE during third trimester of pregnancy was associated with poorer verbal ( per 2-fold increase in ; 95% CI: , ), quantitative (; 95% CI: , ), and memory (; 95% CI: , ) skills and a poorer general cognitive index (; 95% CI: , ) at 42–60 months of age. |
| 7. Osorio-Valencia et al.113 | 2015/Mexico | 167 mother–child (60 months of age) pairs | Prospective cohort | OCs | Maternal serum DDE during each trimester of pregnancy |
-DDE ( lipid): First trimester: ; ; second trimester: ; ; third trimester: ; |
Neurodevelopment: lateralization and spatial orientation (MSCA) | Higher DDE during second trimester of pregnancy was associated with poorer spatial orientation ( per 2-fold increase in ; 95% CI: , 0.04). |
| 8. Campos et al.118 | 2015/Brazil | 46 children (6–16 years of age) | Cross-sectional | OCs | Child serum HCH, HCB, DDE, DDT, endosulfan, aldrin, endrin, dieldrin, methoxychlor, and mirex | Median (range) (): ; ; ; ; ; ; ; ; ; ; ; ; ; |
Neurodevelopment: cognitive function (WISC-III) | Higher was associated with poorer performance IQ ( per increase ; 95% CI: , 0), resistance to distraction (; 95% CI: , 0), and processing speed (; 95% , ). Higher was associated with poorer resistance to distraction (; 95% , ) and processing speed (; 95% , 0). Higher -DDT was associated with poorer processing speed (; 95% , ). |
| 9. Cordier et al.117 | 2015/Guadeloupea | 75 mother–child (18 months of age) pairs | Prospective cohort | OCs | Cord blood and breast milk chlordecone, cord blood DDE | Median (P25–P75) () in entire study population (111 mother–child pairs): ; cord blood ; |
Neurodevelopment: personal–social, communication, problem-solving, fine motor, and gross motor (ASQ-19) | Association between cord chlordecone and fine motor scores among boys (reported by Boucher et al.111) was not mediated by TSH. |
| 10. Ogaz-Gonzales et al.114 | 2018/Mexico | 142 mother–child (42–60 months of age) pairs | Prospective cohort | OCs | Questionnaire (pesticide use history) Maternal serum DDE during first and third trimester of pregnancy |
Mean (P10–P90) DDE (): |
Neurodevelopment: verbal, perceptual performance, quantitative, memory, motor skills, and general cognitive index (MSCA) | Higher third-trimester maternal DDE was associated with lower motor development in children whose mothers had lower intake of DHA (an omega-3 fatty acid) ( per 2-fold increase in ; 95% CI: , 0.1), but not in children whose mothers had a higher DHA intake. Higher maternal DDE was associated with poorer memory skills in children whose mothers had lower ARA (an omega-6 fatty acid) intake ( per 2-fold increase in ; 95% CI: , ) but not in children whose mothers had a higher ARA intake. |
| 11. Saint-Amour et al.115 | 2020/Guadeloupe | 285 mother–child (7–8 years of age) pairs | Prospective cohort | OCs (chlordecone) | Cord blood and child (7 years of age) blood chlordecone | Median (range) chlordecone (): ; |
Neurodevelopment: visual contrast sensitivity (FrACT) | Higher cord plasma chlordecone (continuous) was associated with lower scores (; 95% CI: , 0). Child chlordecone (continuous) was associated with lower scores among boys (; 95% CI: , 0). |
| 12. Cordier et al.116 | 2020/Guadeloupe | 116 mother–child (7 years of age) pairs | Prospective cohort | OCs (chlordecone) | Cord blood and child (7 years of age) blood chlordecone | Median (range) chlordecone (): ; |
Neurodevelopment: sex-typed play behavior (feminine, masculine, or neutral play reported as a proportion of the complete playing time) | Null associations of cord blood and child chlordecone with sex-typed play behavior. |
| Studies in adults | ||||||||
| 13. Steenland et al.119 | 2014/Costa Rica | 89 adults from historically (and now partially) agricultural area | Cross-sectional | OCs | Questionnaire (occupational exposure history) Serum HCH, DDE, DDT, and dieldrin |
(): Past occupational pesticides exposure: ; ; ; No past occupational pesticides exposure: ; ; ; |
Neurodegeneration: spatial and temporal orientation, short-term memory, attention, calculation, language, praxis (MMSE); tremor-at-rest (UPDRS) | Null associations of serum OC (parent compounds or metabolites) with MMSE and tremor-at-rest. |
| Studies on OPs or CBs | ||||||||
| Studies in children | ||||||||
| 14. Handal et al.130 | 2007/Ecuador | 142 children (24–61 months of age) from 2 communities with industrial flower farms and from a community with local agriculture and crops for food | Cross-sectional | CBs, OPs | Questionnaire (parental occupational and residential exposure history, child outdoor activities) | Not applicable | Neurodevelopment: communication, fine motor, gross motor, problem-solving, personal–social (ASQ), visual–motor skills (Beery-Buktenica VMI developmental test) | Maternal employment in the flower industry at time of child assessment was associated with improved communication (; 95% CI: , 8.5) and problem-solving (; 95% CI: 0.7, 9.4) skills. Pesticide use on domestic crops was also associated with better gross motor (; 95% CI: 0.6, 9.2) and personal–social (; 95% CI: , 9.0) scores, whereas pesticide use within the home was associated with lower communication scores (; 95% CI: , ). Children who played with irrigation water had lower fine motor (; 95% CI: , ) and problem-solving (; 95% CI: , ) scores. |
| 15. Handal et al.121 | 2007/Ecuador | 154 children (3–61 months of age) from 2 communities with industrial flower farms/129 children from a community with local agriculture and crops for food | Cross-sectional | CBs, OPs | Questionnaire (parental occupational and residential exposure history) | Not applicable | Neurodevelopment: communication, fine motor, gross motor, problem solving, personal–social (ASQ) | Children 3–23 months of age from industrial flower farms communities had lower gross motor (, ), fine motor (, ), and socioindividual (, ) scores compared with children from a local agriculture community. Children 24–61 months of age from industrial flower farms communities had lower gross motor scores compared with children of similar ages from a local agriculture community (, ). |
| 16. Handal et al.120 | 2008/Ecuador | 121 children (3–23 months of age) from 2 communities with industrial flower farms and from a community with local agriculture and crops for food | Cross-sectional | CBs, OPs | Questionnaire (maternal occupational exposure history during pregnancy) | Not applicable | Neurodevelopment: communication, fine motor, gross motor, problem solving, personal–social (ASQ), prehension and visual acuity (targeted development tests) | Children whose mothers worked as floriculturists during pregnancy had lower communication (; 95% CI: , 0.3) and fine motor ; 95% CI: , ) scores and had an increased risk of poor visual acuity (; 95% CI: 1.1, 20) than children whose mothers did not. |
| 17. Harari et al.123 | 2010/Ecuador | 84 children (6–8 years of age) living in a floricultural area | Cross-sectional | OPs | Questionnaire (parental occupational and residential exposure history) Urinary DAPsb Blood AChEb |
Not applicable | Neurodevelopment: simple motor speed (finger tapping task), motor coordination (Santa Ana Form Board), attention (CPT), short-term auditory memory (WISC and Stanford-Binet), visual performance (Raven’s test and Stanford-Binet copying test), visual memory (Stanford-Binet copying recall test) | Children whose mothers were exposed to pesticides during pregnancy showed poorer motor speed (; 95% CI: , ), motor coordination (; 95% CI: 1.03, 27.6), visual performance (Raven’s test: ; 95% CI: 0.2, 1.0), and visual memory (; 95% CI: 1.02, 42.9) compared with children of unexposed mothers. Children whose fathers were exposed to pesticides during pregnancy showed poorer visual memory (; 95% CI: 1.8, 101.9) than children of unexposed fathers. Children with current exposure (i.e., at least one detectable urinary DAP metabolite) had longer reaction times compared with children with no exposure (; 95% CI: , 141.7). |
| 18. Muñoz-Quesada et al.127 | 2011/Chile | 25 children (6–11 years of age) from rural communities | Cross-sectional | OPs | Child urinary DAPs | Geometric mean (range) (): ; ; |
Neurodevelopment: cognitive function (WISC-III) | Negative association between urinary DMTP and processing speed (, ). Null associations of other DAP metabolites and WISC-III outcomes. |
| 19. Martos-Mula et al.129 | 2013/Argentina | 42 children (7–10 years of age) living in an agricultural area/29 children living in a nonagricultural area | Cross-sectional | OPs, CBs | Questionnaire Blood AChE, BChE |
Not applicable | Neurodevelopment: associative memory (Digit and Symbol subtest), short-term memory (Digit Memory test), maze test (motor, visuospatial processing), cognitive function (WISC-III), gross motor and balance tests | Children living in an agricultural area had poorer motor function and visuospatial processing than children living in a nonagricultural area (). Null associations between enzyme activities and neurodevelopmental outcomes. |
| 20. Suarez-Lopez et al.122 | 2013/Ecuador | 307 children (4–9 years of age) living in floricultural communities | Cross-sectional | OPs | Questionnaire (parental occupational and residential exposure history) Blood AChE |
Not applicable | Neurodevelopment: attention and inhibitory control, language, memory and learning, sensorimotor, visuospatial processing (NEPSY-II) | Boys, but not girls, in the highest tertile of AChE activity had increased odds of poor neurodevelopment ( 9th percentile) than boys in the lowest tertile (total neurodevelopment ; 95% CI: 0.8, 31.5; attention/executive functioning ; 95% CI: 1.2, 17.4); memory/learning ; 95% CI: 1.2, 31.1). |
| 21. Fortenberry et al.128 | 2014/Mexico | 187 mother–child (6–11 years of age) pairs | Prospective cohort | OPs (chlorpyrifos) | Maternal urinary TCPy during third trimester of pregnancy | Geometric mean (P10–P90) TCPy (): 1.76 (0.45–6.40) | Neurodevelopment: attention and hyperactivity (CRS-R, CPT, BASC-2) | Increased ADHD index for the highest TCPy tertile compared with the lowest tertile for boys (; 95% CI: , 11.3). Increased attention problems for the middle TCPy tertile compared with the lowest tertile for girls (; 95% CI: , 12.4). |
| 22. Suarez-Lopez et al.126 | 2017/Ecuador | 308 children (4–9 years of age) living in floricultural communities | Cross-sectional | OPs | Questionnaire (parental occupational and residential exposure history) Blood AChE |
Not applicable | Neurodevelopment: attention and inhibitory control, language, memory and learning, sensorimotor, visuospatial processing (NEPSY-II) | Children examined sooner after Mother’s Day had lower attention/inhibitory control (score difference per 10.8 d ; 95% CI. 0.10, 0.7), visuospatial processing (0.6; 95% CI: 0.3, 0.9), and sensorimotor (0.4; 95% CI: 0.1, 0.8) scores than children examined later. Further adjustment for AChE activity had overall a small effect on most associations but strengthened associations in the attention and inhibitory control domain by about 16%. |
| 23. Suarez-Lopez et al.124 | 2019/Ecuador | 529 adolescents (11–17 years of age) living in floricultural communities | Cross-sectional | OPs | Blood AChE | Not applicable | Mental health disorders: anxiety (MASC-2) and depression symptoms (CDI-2) | Lower AChE activity was associated with more depression symptoms ( per SD decrease in AChE ; 95% CI: 0, 2.2). Associations were stronger among girls (; 95% CI: 0.1, 3.1) than boys (; 95% CI: , 2.3) and among younger (; 95% CI: , 3.4) than older children (; 95% CI: , 2.0). No associations were observed with anxiety scores. |
| 24. Suarez-Lopez et al.125 | 2021/Ecuador | 300 adolescents (11–17 years of age) living in floricultural communities | Cross-sectional | OPs | Blood AChE | Not applicable | Mental health disorders: anxiety (MASC-2) and depression symptoms (CDI-2) | Lower AChE activity was associated with more depression symptoms ( per 10% decrease in AChE ; 95% CI: 0, 1.9) and increased odds of an elevated depression score (; 95% CI: 1.0, 2.7). These associations were stronger among girls than boys. Adjustment for cortisol, testosterone and dehydroepiandrosterone reduced gender differences by 18%–62%. |
| Studies in adults | ||||||||
| 25. Wesseling et al.136 | 2010/Costa Rica | 78 male banana farmworkers with poisoning/130 non-poisoned workers from company payrolls | Cross-sectional | OPs, CBs | Questionnaire (occupational exposure history, history of OP pesticide poisoning) Blood AChEb |
Not applicable | Mental health disorders: psychological distress and suicidal ideation (BSI) | Farmworkers with history of OP pesticide poisonings had increased odds of somatization (; 95% CI:1.6, 6.0), obsessive-compulsiveness (; 95% CI:1.6, 6.2), interpersonal sensitivity (; 95% CI:1.5, 5.8), depression (; 95% CI: 1.3, 4.7), hostility (; 95% CI: 1.1, 4.6), anxiety (; 95% CI:1 1.4, 4.4), phobia (; 95% CI: 1.0, 3.6), and psychoticism (; 95% CI: 1.1, 4.3). Individuals with history of OP pesticide poisonings had increased odds of having suicidal thoughts in the previous month (; 95% CI: 1.5, 8.8), with increasing risk for those with more poisonings (; 95% CI: 1.7, 14.5). Farmworkers with history of CB pesticide poisonings had increased odds of somatization (; 95% CI: 1.1, 6.2). |
| 26. Muñoz-Quezada et al.132 | 2016/Chile | 93 farmworkers/84 controls | Cross-sectional | OPs | Questionnaire (occupational exposure history) | Not applicable | Neurobehavioral performance: cognitive function (WAIS-IV), visuospatial memory and visual perception (ROCF), visual memory and visuoconstruction skills (BVRT), neurological alterations with frontal involvement (MMSE), and motor performance | Farmworkers had lower WAIS-IV verbal comprehension (; 95% CI: , ), processing speed (; 95% CI: , ), and total IQ (; 95% CI: , ) scores than controls. Farmworkers also had lower MMSE scores (; 95% CI: , ) and poorer discrimination sensitivity (; 95% CI: 0.2, 1.2) and deep reflexes (; 95% CI: 0.0, 2.2) than controls. |
| 27. Corral et al.131 | 2017/Chile | 32 farmworkers/32 individuals living in agricultural communities/38 controls | Cross-sectional | OPs | Questionnaire (occupational exposure history) | Not applicable | Neurobehavioral performance: spatial and temporal orientation, short-term memory, attention, calculation, language, praxis (MMSE), memory and attention (WAIS-R DST), visuoconstruction skills and visual memory (ROCF), divided attention and resistance to interference (Stroop), attention (d2), executive function (FAB), and verbal fluency (Barcelona for Animals and Letter P) | Both farmworkers and people living in agricultural communities had increased odds of poorer (i.e., below cutoff value) executive function (; 95% CI: 5.6, 359.7 and ; 95% CI: 1.7, 32.4, respectively), memory and attention (DST forward: ; 95% CI: 1.6, 14.9, and DST backward: ; 95% CI: 2.4, 22.4; DST forward: ; 95% CI: 1.4, 13.8, and DST backward: ; 95% CI: 1.02, 8.3, respectively), and verbal fluency (animals: ; 95% CI: 1.3, 25.6, and Letter P: ; 95% CI: 4.3, 64.6; animals: ; 95% CI: 1.04, 19.4, and Letter P: ; 95% CI: 2.1, 31.3, respectively) than the unexposed group. |
| 28. Grillo Pizarro et al.135 | 2018/Chile | 55 farmworkers/58 unexposed controls | Cross-sectional | OPs | Questionnaire (occupational and residential exposure history) | Not applicable | Peripheric polyneuropathy | Farmworkers exposed to OP pesticides had increased odds of peripheric polyneuropathy compared with controls (; 95% CI: 1.2, 10.5) |
| 29. Serrano-Medina et al.137 | 2019/Mexico | 140 farmworkers/100 controls | Cross-sectional | OPs | Questionnaire (occupational exposure history) Blood AChE |
Not applicable | Mental health disorders: neuropsychiatric disorders (MINI based on DSM-IV) | Farm work was associated with increased odds of suicide (; 95% CI: 2.4, 11.9), whereas higher AChE activity levels were associated with decreased odds of suicide (, ). |
| 30. Buralli et al.138 | 2020/Brazilc | 42 pesticide applicators/36 farmworkers who did not apply pesticides | Cross-sectional | OPs | Questionnaire (occupational exposure history) Blood AChE, BChEb |
Not applicable | CMD (SRQ-20) | Farmworkers who did not spray pesticides had a higher probability of feeling easily tired (; 95% CI: 1.3, 7.7) and worthless (; 95% CI: 1.7, 31.0) compared with pesticide applicators. |
| 31. Ramírez-Santana et al.134 | 2020/Chile | 87 adults occupationally exposed (OE)/81 environmentally exposed (EE)/100 unexposed controls (RG) | Cross-sectional | OPs, CBs | Questionnaire (occupational and residential exposure history) Blood AChE, BChE, APEH |
Not applicable | Neurobehavioral performance at one (RG) or two (OE and EE) time points: general mental status (MMSE), memory (WMS III, Digit span forward, ROCF memory, 1036 A-B and A-B recall), language (WAIS subtest vocabulary), constructive praxis (ROCF copy, WAIS subtest block design), executive function (Tower of London movements and time resolution tests, WCST perseverative errors, Barcelona test categorical evocation animals and words), attention (WAIS digit span backward, d2 test, Stroop word–color and inhibitory control tests, Trail Making Test A, WAIS symbols), Psychomotricity (Purdue pegboard test, MOART reaction time, MOART finger tapping test), Mood (BDI-II depression inventory, Hamilton anxiety scale) | Both exposure groups (OE and EE) had poorer executive function (Tower of London time, WCST perseverative errors), psychomotricity [MOART reaction time (right and left hand)], and mood (BDI-II depression inventory, Hamilton anxiety scale) than the RG. Seasonal exposure impaired performance in both exposure groups on all tests except those related to attention and mood. During the spray season, BChE activity was associated with decreased scores on tests of logical, auditory, and visual memory; inhibitory control of cognitive interference; constructional and planning abilities; executive function; and motor speed and coordination among those in the EE group. Weaker associations were observed for AChE levels and tests of logical memory, constructional abilities, and fine motor coordination in the EE group. In the OE group, levels of the three biomarkers were associated with worse performance on tests of inhibitory control of cognitive interference (2 tests with AChE, 2 tests with BChE, and 1 test for APEH); results were only significant for AChE. |
| 32. Ramírez-Santana et al.133 | 2020/Chile | 78 adults occupationally exposed (OE)/78 environmentally exposed (EE) | Cross-sectional | OPs, CBs | Questionnaire (occupational and residential exposure history) Blood AChE, BChE |
Not applicable | Changes in neurobehavioral performance from prespray to spraying season in OE and EE: General mental status (MMSE), memory (WMS III, Digit span forward, ROCF memory, 10/36 SRT A-B and A-B recall), language (WAIS subtest vocabulary), constructive praxis (ROCF copy, WAIS subtest block design), executive function (Tower of London movements and time resolution tests, WCST perseverative errors, Barcelona test categorical evocation animals and words), attention (WAIS digit span backward, d2 test, Stroop word–color and inhibitory control tests, Trail Making Test A, WAIS symbols), Psychomotricity (Purdue pegboard test, MOART reaction time, MOART finger tapping test), Mood (BDI-II depression inventory, Hamilton anxiety scale) | AChE inhibition was associated with worse performance on tests of attention (Stroop word–color and inhibitory control test, Trail Making A test) in the EE group and worse performance on tests of memory (WMS) and attention (Trail Making A test) in the OE group. BChE inhibition was associated with worse performance on tests of general mental status (MMSE), memory (WMS III-I, WMS III-II, Digit span forward, 10/36 SRT-A, 10/36 SRT-B, 10/36 SRT-A recall, 10/36 SRT-B recall), language (WAIS), attention (Stroop word–color and inhibitory control tests), executive function (Tower of London movements, WCST perseverative errors, Barcelona tests animals) in the EE group and worse performance on a test of attention (Stroop word–color test) in the OE group. |
| Studies on other pesticides or multiple pesticide classes | ||||||||
| Studies in children | ||||||||
| 33. Eckerman et al.143 | 2007/Brazil | 38 adolescents (10–18 years of age) from rural areas/28 adolescents from urban areas | Cross-sectional | Multiple pesticide classes | Questionnaire (exposure index based on 86 occupational history questions plus number of hours worked per day applying chemical plus number of years worked) | Not applicable | Neurobehavioral: BARS (CPT, MTS, DST, PRT, RTT, SAT, SDL, SDT, TAP) | Compared with adolescents from rural areas, adolescents from urban areas performed better on tests of response speed and coordination (TAP_NP), attention and working memory (DS-F), and complex function (SD_LAT), but worse on a cognition test (SDL) (). Among the youngest age group (10–11 y), rural participants had poorer mean scores in tests of response time and coordination (TAP_P, TAP_NP, TAP_ALT), motivation (PRT), attention and working memory (DS-F; CPT_HLAT; SAT_LAT), reaction time (RT_ALL), complex function (SDT_LAT), and visual memory and delay (MTS_LAT) ( for each) than urban participants. |
| 34. Lu et al.139 | 2009/Costa Rica | 18 children (4–10 years of age) of conventional coffee farmworkers/17 children of organic coffee farmworkers | Cross-sectional | OPs, herbicides, pyrethroids | Child urinary 2,4-D, TCPy, 3-PBA, and IMPY |
(): La Amistad: 2, ; ; ; ; Las Mellizas: ; ; ; ; |
Neurodevelopment: cognition (BARS, figure-drawing task, long-term memory test) | Null associations between urinary pesticide metabolites and neurodevelopmental outcomes. |
| 35. van Wendel et al.140 | 2016/Costa Rica | 140 rural children (6–9 years of age) living near banana and plantain plantations | Cross-sectional | Mn-containing fungicides, OPs, pyrethroids | Questionnaire (parental occupational exposure history) Child urinary TCPy, ETU, and 3-PBA |
Median (P25–P75) (): ; ; |
Neurodevelopment: cognitive function (WISC-IV), behavioral problems (CPRS-R), visual sensory function (LDD-15), visuospatial construction and visual memory (ROCF), verbal memory and learning abilities (CAVLT-2), visual–motor coordination (DTVP-2), fine motor function (WRAVMA), and attention (RTT) | Higher TCPy was associated with poorer working memory in boys ( per 10-fold increase in ; 95% CI: , ) and poorer visual–motor coordination (; 95% CI: , ); oppositional disorders (; 95% CI: 1.8, 28.6) and decreased ability to discriminate colors (; 95% CI: 1.6, 30.3) in boys and girls combined. Higher was associated with poorer verbal learning outcomes (; 95% CI: , ). Higher 3-PBA was associated with poorer processing speed scores, particularly in girls (; 95% CI: , ). |
| 36. Watkins et al.142 | 2016/Mexico | 187 mother–child (2–3 years of age) pairs | Prospective cohort | Pyrethroids | Maternal urinary 3-PBA during third trimester of pregnancy | 3-PBA (): 0.26 | Neurodevelopment: cognitive, language, personal–social, fine and gross motor development (BSID-II) | Children whose mothers had medium and high 3-PBA during pregnancy had lower MDI scores at 24 months than children whose mothers had low 3-PBA (; 95% CI: , 0.8 and ; 95% CI: , 0.8, respectively). Null associations of prenatal 3-PBA with PDI scores at 24 or 36 months. |
| 37. Mora et al.141 | 2018/Costa Rica | 355 mother–child (1 year of age) pairs living near banana plantations aerially sprayed | Prospective cohort | Mn-containing fungicides | Maternal urinary ETU, blood Mn, and hair Mn during pregnancy |
(): ; ; |
Neurodevelopment: cognition, motor function, language, and social–emotional development (BSID-III) | Girls whose mothers had higher urinary ETU during pregnancy had lower social–emotional scores ( per 10-fold points; 95% CI: , 0.4), whereas those whose mothers had higher hair Mn during pregnancy had lower cognitive scores ( per 10-fold points; 95% CI: , 0.1). Among boys, higher hair Mn during pregnancy was associated with lower social–emotional scores ( per 10-fold points; 95% CI: , ). Null associations for blood Mn, language, and motor outcomes. |
| 38. Christian et al.145 | 2018/Jamaica | 298 children (2–8 years of age) with ASD/298 controls without ASD | Case–control | Multiple pesticide classes | Questionnaire (maternal exposure history) | Not applicable | ASD (ADOS, ADI-R) | Maternal exposure to pesticides from 3 months before pregnancy to end of breastfeeding was associated with increased risk of ASD (; 95% CI: 1.1, 2.6), with some evidence of effect modification by exposure to oil-based paints and paint solvents. |
| 39. Friedman et al.144 | 2020/Ecuador | 307 children (4–9 years of age) living in floricultural communities | Cross-sectional | Multiple pesticide classes | Proximity to floricultural crops | Not applicable | Neurodevelopment: attention and inhibitory control, language, memory and learning, sensorimotor, visuospatial processing (NEPSY-II) | For every 100 m closer in proximity to treated floricultural crops, participants had increased odds of low memory/learning (; 95% CI: 1.1, 1.5) and language (; 95% CI: 1.0, 1.2) scores. Compared with those living from crops, those living within of crops had lower language (; 95% CI: , ), attention/inhibitory control (, 95% CI: , ), and memory/learning (; 95% CI: , 0.2) scores. |
| Studies in adults | ||||||||
| 40. Araújo et al.159 | 2007/Brazilc | 102 farmworkers | Cross-sectional | Multiple pesticide classes | Questionnaire (occupational exposure history) Blood AChE |
Not applicable | Neurological symptoms (neurological examination and perception of neurological symptoms) | Null association between inhibition of AChE activity and intoxication symptoms. |
| 41. Steenland et al.146 | 2013/Costa Rica | 400 adults years of age from historically (and now partially) agricultural area | Cross-sectional | Multiple pesticide classes | Questionnaire (occupational exposure history) | Not applicable | Neurodegeneration: spatial and temporal orientation, short-term memory, attention, calculation, language, praxis (MMSE); PD risk (UPDRS) | Exposed subjects performed worse on the MMSE than the nonexposed (), had increased odds of abnormal scores on two UPDRS items (tremor-at-rest ; 95% CI: 1.3–5.2 and finger tapping ; 95% CI: 1.03, 8.4), and had an increased risk of PD (; 95% CI: 0.9, 7.3). |
| 42. Faria et al.152 | 2014/Brazil | 2,400 tobacco farmworkers | Cross-sectional | Fungicides, herbicides, neonicotinoids, OPs, pyrethroids | Questionnaire (occupational exposure history) | Not applicable | Mental health disorders: MPD (SRQ-20) | Increased risk of MPD among those who entered the treated area following application (; 95% CI: 1.3, 2.2) and those who had contact through clothes wet from pesticides (; 95% CI: 1.1, 1.7). Workers from farms in which OPs were used had an increased risk of MPD compared with those who were not exposed (; 95% CI: 1.2, 1.9). Number of poisonings was positively associated with risk of MDP (PR for 1 ; 95% CI: 1.1, 2.2; PR for 2 ; 95% CI: 1.8, 3.4). |
| 43. Portilla-Portilla et al.155 | 2014/Colombia | 49 adults from a rural area | Cross-sectional | Multiple pesticide classes | Questionnaire (occupational and environmental history of exposure to neurotoxic pesticides) | Not applicable | Neurological symptoms (self-reported) | Participants with pesticide exposure had increased odds of irritability (; 95% CI: 1.1, 2.8), dizziness (; 95% CI: 1.1, 4.9), phosphenes (; 95% CI: 1.0, 6.6), epistaxis (; 95% CI: 1.0, 8.3), and fasciculations (; 95% CI: 1.1, 66.9). |
| 44. Campos et al.149 | 2016/Brazil | 869 adults from a rural population | Cross-sectional | Herbicides, natural pesticides, OPs, OCs, pyrethroids | Questionnaire (occupational exposure history) | Not applicable | Mental health disorders: MPD (SRQ-20) and depression (self-reported) | Ever feeling ill after a pesticide application was associated with common mental disorders (; 95% CI: 1.6, 4.3) and self-reported depression (; 95% CI: 1.6, 4.2). Age at onset of pesticide exposure y (; 95% CI: 1.7, 2.8), exposure to pyrethroids (; 95% CI: 1.0, 3.2) and aliphatic alcohol (; 95% CI: 1.04, 3.8), and greater period of exposure to dinitroaniline (; 95% CI: 1.0, 4.7) and sulfonyl urea (; 95% CI: 1.1, 23.0) were associated with self-reported depression. |
| 45. Azevedo and Meyer158 | 2017/Brazil | 51 endemic disease control agents with essential tremor/204 endemic disease control agents with no tremor (controls) | Case–control | Larvicides, OCs, OPs, pyrethroids | Questionnaire (occupational exposure history) | Not applicable | Neurodegeneration: essential tremor | Null association of cumulative pesticide exposure load (calculated by multiplying years of application, frequency of application, and hours worked per day) with essential tremor. Workers who had applied pesticides for 16–16.9 y had increased odds of essential tremor compared with workers who had applied pesticides for y (; 95% CI: 1.3, 18.0). |
| 46. Hansen et al.147 | 2017/Bolivia | 120 male endemic disease control agents | Cross-sectional | Pyrethroids | Questionnaire (occupational exposure history) | Not applicable | Neurobehavioral performance: hand tremor, postural balance, vocabulary (BNT), audiovisual reaction (RTT), cognition (BARS), visual attention (CPT), complex cognitive function (SDT), attention and memory (DST, SDL), and visual memory (MTS) | Higher pesticide spraying intensity was associated with increased odds of poor postural balance among those exposed to pyrethroids (OR per 1-quintile increase in ; 95% CI: 1.1, 13.6). Higher spraying intensity was also associated with worse neurocognitive performance ( per 1-quintile increase for all ; 95% CI: , and for workers exposed to pyrethroids ; 95% CI: , ). Cumulative pesticide exposure was associated with worse neurocognitive performance ( per 1-quintile increase for all ; 95% CI: , and for workers exposed to pyrethroids ; 95% CI: , ). |
| 47. Conti et al.148 | 2018/Brazil | 220 male farmworkers | Cross-sectional | Glyphosate, fungicides, neonicotinoids | Questionnaire (occupational exposure history) | Not applicable | Mental health disorders: depression (BDI-II) | Pesticide exposure was associated with increased odds of more severe depressive symptoms (; 95% CI: 1.2, 25.9). |
| 48. Palzes et al.160 | 2019/Costa Rica | 48 farmworkers | Cross-sectional | Mn-containing fungicides | Hair and toenail Mn |
() Mn: ; |
Cortical brain activity (fNIRS) | Null association of hair and toenail Mn concentrations with brain activity during working memory task. |
| 49. Conti et al.150 | 2020/Brazil | 288 adults from a rural area | Cross-sectional | Multiple pesticide classes | Questionnaire (pesticide exposure in general, did not distinguish between residential and occupational) | Not applicable | Mental health disorders: depression (BDI-II) | Pesticide use was associated with increased odds of depression (, ). |
| 50. Vasconcellos et al.156 | 2020/Brazil | 32 participants with PD | Cross-sectional | Multiple pesticide classes | Questionnaire (occupational exposure history) | Not applicable | PD | 78% of patients with PD had worked in agriculture and 75% had contact with pesticides. |
| 51. Silvestre et al.157 | 2020/Brazil | 88 PD cases/264 controls | Case–control | Multiple pesticide classes | Questionnaire (occupational and environmental exposure history) | Not applicable | PD | Pesticide use at work was associated with increased odds of PD (; 95% CI: 1.6, 7.6). |
| 52. Cruzeiro Szortyka et al.153 | 2021/Brazil | 2,469 tobacco growers | Cross-sectional | Multiple pesticide classes | Questionnaire (occupational exposure history, history of APP, pesticide-related work tasks) | Not applicable | Mental health disorders: suicidal ideation (SRQ), suicide attempts (self-reported) | Performing between 6 and 9 pesticide-related tasks (; 95% CI: 1.0, 3.3) and history of APP (; 95% CI: 1.2, 4.7) were associated with increased prevalence of suicidal ideation. |
| 53. Gonzaga et al.154 | 2021/Brazil | 547 farmworkers (311 occupationally exposed/236 following agroecological practices) | Cross-sectional | Multiple pesticide classes | Questionnaire (occupational exposure history, history of APP) | Not applicable | Mental health disorders: suicidal ideation (SRQ-20) | Occupational pesticide exposure (; 95% CI: 1.2, 4.6) and history of APP (; 95% CI: 3.0, 24.7) were associated with increased odds of suicidal ideation. |
| 54. Farnham et al.151 | 2021/Costa Rica | 300 farmworkers | Cross-sectional | Multiple pesticide classes | Questionnaire (occupational exposure history, history of APP) | Not applicable | Neurological symptoms Mental health disorders: psychological distress and suicidal ideation (BSI) |
Self-reported APP was associated with fainting (; 95% CI: 1.8, 30.7), shaking hands (; 95% CI: 1.6, 7.6), numbness/tingling in hands or feed (; 95% CI: 1.7, 6.3), insomnia (, 95 % CI: 1.3, 4.8), accelerated heartrate (; 95% CI: 1.0, 5.5), dizziness (; 95% CI: 1.2, 4.7), increased irritability/anger (; 95% CI: 1.2, 4.6), low energy (; 95% CI: 1.2, 4.5), and difficulty concentrating (; 95% CI: 1.1, 3.9) during the 12 months prior to the interview. Farmworkers who reported an APP in the 10 y prior to the interview experienced increased odds of hostility (; 95% CI: 1.2, 17.7) and paranoid ideation (; 95% CI: 1.0, 18.2). |
Note: %change, percentage change; 2,4-D, 2,4-dichlorophenoxyacetate; 3-PBA, 3-phenoxybenzoic acid; AChE, acetylcholinesterase; ADHD, attention deficit hyperactivity disorder; ADI-R, Autism Diagnostic Interview–Revised; ADOS, Autism Diagnostic Observation Schedule; APEH, acyl peptide hydrolase; APP, acute pesticide poisoning; ARA, arachidonic acid; ASD, autism spectrum disorder; ASQ, Ages and Stages Questionnaire; BARS, BChE, butyrylcholinesterase; Behavioral Assessment and Research System; BASC-2, Behavior Assessment System for Children-2; BDI-II, Beck’s Depressive Inventory, 2nd edition; BNT, Boston Naming Test; BSI, Brief Symptom Inventory; BSID, Bayley Scales of Infant Development; BSID-II, Bayley Scales of Infant Development, 2nd edition; BVRT, Benton Visual Retention Test; CAVLT-2, Children’s Auditory Verbal Learning Test, 2nd edition; CB, carbamate; CDI-2, Children’s Depression inventory, 2nd edition; CI, confidence interval; CIT, 5-chloro-1,2-dihydro-1-isopropyl-[3H]-1; CMD, common mental disorders; CRS-R, Conners’ Parental Rating Scales–Revised; CPT, Continuous Performance Test; CPRS-R, Conner’s Parent Rating Scale–Revised Short Version; CPT, Continuous Performance Test; CPT_HLAT, Continuous Performance Latency for Hits; DAP, dialkylphosphate; DDE, dichlorodiphenyldichloroethylene; DDT, dichlorodiphenyltrichloroethane; DEP, diethylphosphate; DHA, docosahexaenoic acid; DMP, dimethylphosphate; DMTP, dimethylthiophosphate; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders; DS-F, DST, Digit Span Test; DTVP-2, Frostig Developmental Test of Visual Perception, 2nd edition; EE, environmentally exposed; ETU, ethylenethiourea; FAB, Frontal Assessment Battery; fNIRS, functional near-infrared spectroscopy; FrACT, functional acuity contrast test; FTII, Fagan Tests of Infant Intelligence; GSD, geometric standard deviation; HCH, hexachlorocyclohexane; IMPY, 2-isopropyl-4-methyl-6-hydroxypyrimidinol; IQ, intelligence quotient; LOD, limit of detection; LDD-15, Lanthony Desaturated D-15 Test; MASC-2, Multidimensional Anxiety Scale for Children, 2nd edition; MDI, Mental Development Index; MINI, Mini International Neuropsychiatric Interview Diagnostic Test; MMSE, Mini-Mental State Exam; Mn, manganese; MOART, multi-operational apparatus for reaction time; MPD, minor psychiatric disorders; MSCA, McCarthy Scales of Children’s Abilities; MTS, matching to sample; MTS_LAT, matching to sample latency; NESPY-II, A Developmental Neuropsychological Assessment, 2nd edition; NBAS, Brazelton Neonatal Behavioral Assessment; OC, organochlorine; OE, occupationally exposed; OP, organophosphate; OR, odds ratio; PD, Parkinson’s disease; PDI, Psychomotor Development Index; PR, probability ratio; PRT, Progressive Ratio test; RG, rural group; RGDT, Random Gap Digit test; ROCF, Rey-Osterrieth complex figure test; RT_ALL, reaction time; RTT, reaction time test; SAT, selective attention test; SAT_LAT, selective attention test latency; SD, standard deviation; SDL, serial digit learning; SDT, Symbol Digit test; SDT_LAT, Symbol Digit test latency; SRQ-20, Self-Reporting Questionnaire 20-Item; SRT, spatial recall test; TAC, total abnormal cells; TAP, tapping test; TAP_ALT, tapping with alternate hands; TAP_NP, tapping with non-preferred hand; TAP_P tapping with preferred hand; TCPy, 3,5,6-trichloro-2-pyridinol; TSH, thyroid-stimulating hormone; UPDRS, United Parkinson’s Disease Rating Motor Subscale; VMI, visual motor integration test; WAIS; Weschler Adult Intelligence Scale; WAIS-R; Weschler Adult Intelligence Scale–Revised Version; WCST, Wisconsin Card Sorting test; WISC, Weschler Intelligence Scale for Children; WMS, Wechsler Memory Scale; WRAVMA; Wide Range Assessment of Visual Motor Ability.
Also included in Table 6 (thyroid function).
Investigators did not use exposure biomarker concentrations in multivariate analyses.
Also included in Table 9 (other health outcomes).
Eleven publications examined the association of OP or carbamate pesticides with neurobehavioral outcomes in children or adolescents (Table 3). Six publications from cross-sectional studies in Ecuador reported that children and adolescents who lived in floricultural communities—in which OP pesticides and carbamates are intensively used—or whose mothers worked as floriculturists during pregnancy had adverse neurobehavioral outcomes, including poorer motor or socioindividual skills at 3–61 months of age120,121; attention, executive function, and memory deficits at 4–9 years of age (in boys only)122; impaired motor coordination, visual performance, and visual memory at 6–8 years of age123; and more depression symptoms at 11–17 years of age (particularly among girls).124,125 In line with these findings, a seventh publication reported that Ecuadorian children 4–9 years of age who were examined sooner after the end of an increased pesticide use period had lower attention/inhibitory control, visuospatial processing, and sensorimotor scores than children examined later.126 A publication from a cross-sectional study of Chilean school-age children who lived in agricultural communities reported associations of OP pesticide exposure—as indicated by measurement of urinary DAP metabolites—with poorer processing speed.127 A publication from a prospective cohort study in Mexico reported that prenatal exposure to the OP pesticide chlorpyrifos—assessed by measurement of 3,5,6-trichloro-2-pyridinol (TCPy) in maternal urine samples collected during the third trimester of pregnancy—was associated with increased attention problems in school-age boys and girls.128 Conversely, two cross-sectional studies found null or protective associations of OP pesticide exposure with neurodevelopmental outcomes among children.121,129 In a publication from Ecuador, investigators reported that maternal employment in the flower industry or pesticide use on domestic crops at the time of child assessment was associated with improved communication, gross motor, and problem-solving skills at 24–61 months of age.130 A publication from Argentina reported null associations of OP exposure—assessed via blood ChE levels—with motor function and visuospatial processing at 7–10 years of age, but it also reported worse neurodevelopmental outcomes among children living in an agricultural community compared with those living in a nonagricultural community.129
Eight publications examined the association of OP or carbamate pesticides with neurobehavioral outcomes and neurodegenerative disorders among adults (Table 3). Seven of the studies described in these publications were cross-sectional and found that workers exposed to pesticides (i.e., farmworkers and endemic disease control agents) and adults who lived in agricultural communities had impaired cognitive, executive function, memory and attention, and verbal fluency skills131–134; poorer discrimination sensitivity and deep reflexes132; increased odds of polyneuropathy135; or increased odds of psychological distress and suicidal ideation.136,137 Conversely, a publication from a cross-sectional study in Brazil reported that farmworkers who did not handle/apply pesticides—but who used less personal protective equipment (PPE) and had less training on safe pesticide use practices—had more adverse health outcomes (e.g., feeling easily tired, feeling worthless) than pesticide applicators.138
Seven publications from two prospective cohort studies, four cross-sectional studies, and one case–control study examined the associations of exposure to multiple pesticide classes with child neurodevelopment (Table 3). Of these seven publications, four assessed exposure using direct assessment methods139–142; two examined exposure using predetermined categorical exposure variables based on residence143 or proximity to treated agricultural fields144; and one examined maternal pesticide exposure history via questionnaire.145 A publication from a prospective cohort study in Costa Rica found an association of prenatal exposure to manganese (Mn)-containing fungicides—assessed by measurement of urinary ethylenethiourea (ETU) as well as blood and hair Mn in maternal samples collected during pregnancy—with lower social–emotional and cognitive scores in children at 1 year of age.141 A publication from a prospective cohort study in Mexico reported that prenatal exposure to pyrethroids—as indicated by measurement of 3-phenoxybenzoic acid (3-PBA) in maternal urine samples collected during the third trimester of pregnancy—was associated with lower mental development scores at 24 months of age, but not at 36 months of age.142 Notably, a publication from a cross-sectional study of school-age children in Costa Rica reported that higher urinary 3-PBA concentrations were associated with poorer processing speed scores (particularly in girls), but also that urinary TCPy concentrations were associated with poorer working memory (among boys only), visual–motor coordination, and decreased ability to discriminate colors.140 In contrast, a publication from a small cross-sectional study also conducted in Costa Rica139 reported null associations of exposure to OP pesticides, pyrethroids, and herbicides—assessed via pesticide-specific metabolites (e.g., urinary 3-PBA and TCPy concentrations)—and neurodevelopmental outcomes among children 4–10 years of age. A publication from a study conducted in Jamaica reported that maternal exposure to pesticides from 3 months before pregnancy to the end of breastfeeding was associated with an increased risk of autism spectrum disorder.145 Last, two publications from cross-sectional studies in Ecuador144 and Brazil143 reported that children and adolescents who lived near agricultural fields in which OP pesticides and other pesticide classes were extensively used had poorer neurodevelopmental outcomes compared with those who lived farther from the fields (or in nonagricultural communities), including poorer cognitive skills, motor function, memory/learning, visuospatial processing, or attention/inhibitory control.
Fifteen publications evaluated the neurobehavioral effects of exposure to multiple pesticide classes, predominantly assessed via occupational exposure history, among adults (Table 3). Nine of these publications reported that workers exposed to pesticides (i.e., farmworkers and endemic disease control agents), farmworkers who had experienced an acute pesticide poisoning (APP), and adults who lived in agricultural or rural communities had cognitive impairment146,147; increased odds of minor psychiatric disorders such as depression, anxiety, and somatic disorders148–152; suicidal ideation153,154; or an array of neurological symptoms.151,155 Three publications from cross-sectional and case–control studies conducted in Costa Rica119 and Brazil156,157 reported associations between exposure to multiple classes of pesticides—assessed via questionnaire—and increased odds of Parkinson’s disease. Notably, publications from two studies of Brazilian workers reported null associations of pesticide exposure with essential tremor158 and acute intoxication symptoms.159 A publication from a small cross-sectional study of farmworkers in Costa Rica reported a null association between exposure to Mn-containing fungicides—assessed by measurement of toenail and hair Mn concentrations—and cortical brain activity during a working memory task.160
Overall, studies published to date provide consistent evidence of associations between prenatal and childhood exposure to pesticides such as OP pesticides and carbamates and impaired neurodevelopment in LAC children and adolescents. Some of the adverse neurodevelopmental outcomes that have been reported include poorer cognition, memory, and attention, as well as anxiety and depression. Publications from studies of farmworkers in LAC countries also provide consistent evidence of associations between exposure to multiple classes of pesticides—assessed mainly via questionnaire—and impaired neurobehavioral performance, psychological distress, suicidal ideation, and neurodegenerative disorders.
Placental Outcomes and Teratogenicity
Thirteen publications from seven cross-sectional studies, five case–control studies, and one prospective cohort study reported on the potential placental and teratogenic effects of pesticide exposure (Table 4). Seven of these 13 publications reported on the association of exposure to OC pesticides or multiple pesticide classes with congenital malformations. A case–control study conducted in Mexico reported that children whose mothers had higher serum hexachlorobenzene (HCB), , DDT, or DDE concentrations at delivery had increased odds of cryptorchidism.161 Similarly, publications from studies conducted in Brazil162–164 and Mexico165 reported associations of parental occupational pesticide use or environmental pesticide exposure (e.g., being born in a floricultural community) before or during pregnancy—ascertained via questionnaire—with increased odds of congenital malformations, including male external genital malformations. In contrast, publications from case–control studies in Brazil166 and Guadeloupe167 found null associations between pesticide exposure and malformations in general.
Table 4.
Characteristics of Latin American and the Caribbean studies on pesticide exposure and placental outcomes and teratogenicity published between 2007 and 2021 ().
| Study | Year of publication/country | Population and sample size | Study design | Pesticides assessed | Exposure assessment method | Pesticide or metabolite concentrations | Health effect and assessment method/instrument | Results |
|---|---|---|---|---|---|---|---|---|
| Studies on OCs | ||||||||
| Studies in children | ||||||||
| 1. Bustamante Montes et al.161 | 2010/Mexico | 641 cases with cryptorchidism/41 controls | Case–control | OCs | Maternal serum HCB, HCH, DDT, DDE after delivery | Median (range) (mg/kg): Controls:; ; ; ; ; Cryptorchidism cases:; ; ; ; ; |
Cryptorchidism | Children whose mothers had higher OC pesticide concentrations at delivery had increased odds of cryptorchidism (HCB ; 95% CI: 1.1, 1.3; -DDE ; 95% CI: 1.1, 1.2; -DDT ; 95% CI: 1.1, 1.4; -DDT ; 95% CI: 1.1, 1.2; -DDE/-DDT ; 95% CI: 1.1, 1.8). |
| 2. Rouget et al.167 | 2020/ Guadeloupe | 36 cases with congenital malformations/ 1,052 controls | Case–control | OCs | Maternal plasma (at delivery) and cord plasma chlordecone | Median (range) chlordecone (): Maternal plasma: 0.39 (); cord plasma: 0.20 () |
Congenital malformations | Null associations of maternal and cord plasma chlordecone concentrations with risk of overall malformations or undescended testes. |
| Studies on OPs or carbamates | ||||||||
| Studies in pregnant women | ||||||||
| 3. Acosta-Maldonado et al.171 | 2009/Mexico | 9 pregnant women exposed to pesticides/76 nonexposed pregnant women | Cross-sectional | OPs | Questionnaire (residential and partner exposure history Blood AChEa |
Not applicable | Placental maturity | Pesticide exposure was associated with PMI of central area of placenta (, ). |
| 4. Vera et al.168 | 2012/Argentina | 40 pregnant women living on agricultural farms | Cross-sectional | OPs | Questionnaire (occupational and seasonal exposure histories) Blood AChE and BChE, placental CEa |
Not applicable | Nuclear and mitochondrial lipid composition of placenta | Total cholesterol and SM content of nucleus were higher in PP than RP (). PE content of light mitochondria was lower in PP, whereas CL content was higher (). The CL increased and the PE content decreased in the light mitochondrial fraction, whereas total cholesterol and SM increased in the nuclear fraction () in PP. |
| 5. Bulgaroni et al.170 | 2013/Argentina | 46 pregnant women from a rural area/36 pregnant women from urban area (controls) | Cross-sectional | OPs | Questionnaire (residential and seasonal exposure history) Blood AChE and BChE, placental CEa |
Not applicable | Cytokines, arginase, and ornithine decarboxylase expression in placenta IL-13, , and Placental weight, pw/nw ratio |
IL-13 increased during SS in the rural group (), whereas the expression frequency of () and () increased in the rural group in SS and NSS. The arginase activity (), arginase II protein content (), and ODC expression (), increased in placentas collected during SS compared with those collected during NSS. No differences in placenta weight or pw/nw ratio among groups. |
| 6. Chiapella et al.172 | 2014/Argentinab | 46 mother–newborn pairs from a rural area/24 mother–newborn pairs from an urban area (controls) | Cross-sectional | OPs | Questionnaire (residential and seasonal exposure history) Placental CEa |
Not applicable | Placental weight, pw/nw ratio Placental oxidative status (CAT, GPx, GSH, protein carbonyl, lipid peroxidation, anti-Nfr2 levels) |
No differences in placental antioxidant/oxidant status, placental weight, or placental index between groups. |
| 7. Rivero Osimani et al.169 | 2016/Argentinab | 43 mother–newborn pairs from a rural area/20 mother–newborn pairs from an urban area (controls) | Cross-sectional | OPs | Questionnaire (residential and seasonal exposure history) Placental CEa |
Not applicable | Placental weight Placental CT and SCT mitochondria function (mitochondrial respiratory complex activity, CAT, Mn-SOD, GST, progesterone) and eNOS expression |
No differences in placental parameters between newborns from RG-SS and controls SS (). RG-SS had higher complex IV activity than RG-NSS () and CG (). HNE levels in SCT mitochondria were lower in RG-SS than in CG (). The antioxidant defense enzyme activity in CT and SCT mitochondria was similar among groups and seasons. Progesterone level was lower in RG-SS () than CG, and eNOS expression was lower in RG-NSS () and RG-SS () than CG. |
| 8. Quintana et al.54 | 2017/Argentinab,c,d | 151 mother–newborn pairs living in a rural area/38 mother–newborn pairs from an urban area (controls) | Cross-sectional | OPs | Questionnaire (residential and seasonal exposure history) Cord blood AChE, BChEa |
Not applicable | Placental weight | Higher placental weight and pw/nw for vaginal RG-SS and RG-NSS than in CG (). |
| Studies on other pesticides or multiple pesticide classes | ||||||||
| Studies in children | ||||||||
| 9. Silva et al.166 | 2011/Brazil | 42 cases with congenital malformations/84 controls | Case–control | Multiple pesticide classes | Questionnaire (parental, occupational and residential exposure history) | Not applicable | Congenital malformations | Null association of parental occupational exposure to pesticides and residential proximity to agricultural fields with congenital malformations. |
| 10. Gaspari et al.164 | 2012/Brazil | 2,710 male newborns from an intensive-use pesticide area | Cross-sectional | Herbicides, insecticides | Questionnaire (parental exposure history) | Not applicable | Cryptorchidism, hypospadias, and micropenis | Among the 56 cases of malformations detected (2.1%), 79% of the parents reported living in areas of high pesticide use for vector control; 80% of the mothers and 59% of the fathers were occupationally exposed to pesticides or other EDC before and during pregnancy, and 93% and 89% of the mothers reported residential exposure to pesticides or other EDCs before and during pregnancy, respectively. |
| 11. Oliveira et al.162 | 2014/Brazil | 219 cases with congenital malformations/862 live births controls | Case–control | Multiple pesticide classes | Questionnaire (residential exposure history at the periconceptional period) Exposure index from records (pesticides information systems and pesticide invoices) |
Not applicable | Congenital malformations | Increased odds of congenital malformations in those children in the highest quartile of pesticide exposure in the 6 months prior to conception (; 95% CI: 1.2, 3.6) and in the third and fourth quartiles of pesticide exposure after conception (; 95% CI: 1.0, 2.8; ; 95% CI: 1.1, 3.2, respectively) compared with children in the lowest quartile. |
| 12. Ueker et al.163 | 2016/Brazil | 137 cases with congenital malformations ( years of age at enrollment)/274 controls | Case–control | Multiple pesticide classes | Questionnaire (parental exposure history) | Not applicable | Congenital malformations | Children whose fathers applied pesticides during the 12 months before conception and whose mothers had a low educational level had increased odds of congenital malformations (; 95% CI: 2.2, 32.5). Null association between paternal pesticide use and congenital malformations among children whose mothers had a high educational level. |
| 13. Castillo-Cadena et al.165 | 2017/Mexico | 1,149 newborns from floricultural community/5,069 newborns from urban area (controls) | Prospective cohort | Multiple pesticide classes | Questionnaire (residential exposure history) | Not applicable | Congenital malformations | Congenital malformations were more prevalent among children born in a floricultural community (20%) than among those born in an urban area (6%) (). |
Note: AChE, acetylcholinesterase; BChE, butyrylcholinesterase; CAT, catalase; CE, carboxylesterases; CG, control group; CI, confidence interval; CL, cardiolipin; CT, cytotrophoblast; DDE, dichlorodiphenyldichloroethylene; DDT, dichlorodiphenyltrichloroethane; EDC, endocrine disrupting chemical; eNOS, endothelial nitric oxide synthase; GPx, glutathione peroxidase; GSH, glutathione; GST, glutathione -transferase; HCB, hexa-chlorobenzene; HCH, hexa-chlorocyclohexane; IL-13, interleukin-13; Mn, manganese; HNE, 4-hydroxynonenal; Nfr2, nuclear factor erythroid 2-related; LOD, limit of detection; NSS, non-spraying season; OC, organochlorine; ODC, ornithine decarboxylase; OP, organophosphate; OR, odds ratio; PE, phosphatidylethanolamine; PMI, placental maturity index; PP, pulverization period; pw/nw, placental weight/neonate weight ratio; RG, rural group; RP, recess period; SCT, syncytiotrophoblast; SM sphingomyelin; SOD, superoxide dismutase; SS, spraying season; , transforming growth factor beta; , tumor necrosis factor-alpha.
Investigators did not use exposure biomarker concentrations in multivariate analyses.
Also included in Table 8 (birth outcomes and child growth).
Also included in Table 2 (genotoxicity).
Also included in Table 9 (other health effects).
Six publications, all from cross-sectional studies conducted either in Mexico or Argentina, reported on the associations between exposure to OP or carbamate pesticides and placental outcomes (Table 4). Each of these studies measured blood ChE or placental carboxylesterase activity levels but used predetermined exposure categories (e.g., rural vs. urban) in exposure–outcome analyses. Among the most prevalent outcomes associated with pesticide exposure were alterations in lipid composition and oxidative status of placental mitochondria,168,169 as well as changes in the expression of placental cytokines and levels of placental enzymes (e.g., arginase, ornithine decarboxylase).170 Publications from two cross-sectional studies conducted in Mexico and in Argentina reported that pesticide exposure was associated with a higher placental maturity index171 and higher placental weight.54 Conversely, three publications from Argentina reported largely null associations with placental morphological parameters (e.g., weight, placental weight to neonate weight ratio).169,170,172
To date, a small number of publications have reported on the association of pesticide exposure with placental or teratogenic outcomes in LAC populations and their findings are inconsistent. Some published studies found associations of exposure to OCs, OPs/carbamates, and multiple pesticide classes (retrospectively assessed via questionnaire in case–control studies) with outcomes such as alterations in lipid composition and oxidative stress of placental mitochondria and increased odds of congenital malformations. Other studies observed null associations with outcomes such as placental morphological parameters and risk of malformations.
Cancer
Fourteen publications examined the association of pesticide exposure with cancer or cancer-related mortality in children or adults (Table 5). Thirteen publications reported findings from case–control studies; 12 of these studies used indirect exposure assessment methods (i.e., questionnaires or death certificates indicating occupation at the time of death) and 11 examined multiple pesticide classes. Two studies, 1 case–control and 1 prospective cohort, examined associations of serum OC pesticide concentrations with the risk of prostate cancer or prostate cancer recurrence.173,174
Table 5.
Characteristics of Latin American and the Caribbean studies on pesticide exposure and cancer published between 2007 and 2021 ().
| Study | Year of publication/country | Population and sample size | Study design | Pesticides assessed | Exposure assessment method | Pesticide or metabolite concentrations | Health effect and assessment method/instrument | Results |
|---|---|---|---|---|---|---|---|---|
| Studies in children | ||||||||
| 1. Monge et al.176 | 2007/Costa Rica | 344 childhood leukemia cases ( years of age)/579 controls | Case–control | Herbicides, fungicides, insecticides | Questionnaire (parental occupational exposure during the year before conception, pregnancy, and first year of life of the child) | Not applicable | Childhood leukemia (ALL, AML, other leukemias) | Children whose mothers were occupationally exposed to any pesticide during the year before conception (; 95% CI: 1.0, 5.9), first trimester (; 95% CI: 2.8, 171.5), second trimester (; 95% CI: 1.4, 14.7) of pregnancy, or at any time (; 95% CI: 1.0, 4.8) had increased odds of leukemia. Children whose fathers were occupationally exposed to any pesticide during the second trimester of pregnancy had increased odds of leukemia (; 95% CI: 1.0, 2.3). |
| 2. Hernández-Morales et al.177 | 2009/Mexico | 47 childhood leukemia cases ( years of age)/47 controls | Case–control | Multiple pesticide classes | Questionnaire (parental occupational exposure and residential exposure during the 3 months before pregnancy, pregnancy, at birth, and at the time of diagnosis; residential proximity to agricultural fields) | Not applicable | Childhood leukemia (ALL, AML) | Children whose parents used pesticides inside their homes during the 3 months before pregnancy (; 95% CI: 1.5, 2.9) or during pregnancy (; 95% CI: 1.0, 2.3) had increased odds of leukemia. Children whose parents used pesticides in their gardens during the 3 months before pregnancy (; 95% CI: 1.2, 2.6) or during pregnancy (; 95% CI: 1.0, 2.6) also had increased odds of leukemia. |
| 3. Ferreira et al.179 | 2012/Brazil | 292 childhood leukemia cases ( years of age)/541 controls | Case–control | Herbicides, insecticides | Questionnaire (parental occupational and environmental exposure during the 3 months before pregnancy, pregnancy, and while breastfeeding) | Not applicable | Childhood leukemia (ALL, AML) | Children whose mothers were occupationally or environmentally exposed to chemicals (including pesticides) during pregnancy had increased odds of leukemia (; 95% CI: 1.2, 1.6). |
| 4. Ferreira et al.178 | 2013/Brazil | 252 childhood leukemia cases ( months of age)/423 controls | Case–control | Multiple pesticide classes | Questionnaire (parental occupational and environmental exposure during the 3 months before pregnancy, pregnancy, and while breastfeeding) | Not applicable | Childhood leukemia (ALL, AML) | Children whose mothers were occupationally or environmentally exposed to pesticides during the 3 months before pregnancy had increased odds of ALL (; 95% CI: 1.2, 4.8) and AML (; 95% CI: 1.3, 10.8) at 0–11 months of age and increased odds of AML (; 95% CI: 1.2, 5.1) at 12–23 months of age. Children whose mothers were occupationally or environmentally exposed to pesticides during pregnancy and while breastfeeding had increased odds of AML (e.g., OR for exposures during the ; 95% CI: 1.3, 10.4) at 0–11 months of age. Children whose mothers were occupationally or environmentally exposed to any pyrethroid pesticide during pregnancy had increased odds of ALL (; 95% CI: 1.1, 2.9) and AML (; 95% CI: 1.7, 16.8) at 0–23 months of age. Increased odds of ALL or AML were also observed among children whose mothers were exposed to individual pyrethroids during pregnancy. |
| 5. Hyland et al.175 | 2018/Costa Rica | 251 childhood leukemia cases ( years of age)/577 controls | Case–control | Multiple pesticide classes | Questionnaire (residential use and nearby pesticide applications in the year prior to pregnancy, during pregnancy, while breastfeeding, and during childhood) | Not applicable | Childhood leukemia (ALL) | Boys whose mothers reported using insecticides inside the home in the year before pregnancy (; 95% CI: 1.1, 2.5), during pregnancy (; 95% CI: 1.1, 2.7), and while breastfeeding (; 95% CI: 1.1, 2.7) had increased odds of ALL. Children whose mothers reported a high average frequency of insecticide use inside their homes ( times/y) in the year before pregnancy, during pregnancy, and while breastfeeding had increased odds of ALL compared with children whose mothers reported a low frequency of insecticide use ( times/y) during these exposure periods (e.g., OR for exposure during ; 95% CI: 1.1, 2.3). Maternal report of pesticides sprayed on farms or at companies near the home during pregnancy, while breastfeeding, and during any time period was also associated with childhood ALL (e.g., OR for exposure during ; 95% CI: 1.0, 2.1). |
| Studies in adults | ||||||||
| 6. Ortega Jacome et al.180 | 2010/Brazil | 110 breast cancer cases/101 controls | Case–control | Insecticides | Questionnaire (lifetime residential use of insecticides) | Not applicable | Breast cancer | Women who used insecticides in their homes during adulthood ( years of age) had increased odds of breast cancer (; 95% CI: 1.8, 12.9). |
| 7. Meyer et al.184 | 2011/Brazil | 5,782 deaths by esophagus cancer/5,782 deaths by causes other than neoplasms and diseases of the digestive system | Case–control | Multiple pesticide classes | Death certificates (occupation at the time of death) | Not applicable | Esophageal cancer mortality | Agricultural workers had increased odds of dying from esophageal cancer compared with nonagricultural workers (; 95% CI: 1.2, 1.6). |
| 8. Miranda-Filho et al.185 | 2012/Brazil | 2,040 deaths in males by brain cancer/4,140 deaths in males by causes other than neoplasms and diseases of the central nervous system | Case–control | Multiple pesticide classes | Death certificates (occupation at the time of death) and pesticide sales per region of residence | Not applicable | Brain cancer mortality | Agricultural workers had increased odds of dying from brain cancer compared with nonagricultural workers (; 95% CI: 1.2, 2.7). Slightly increased brain cancer mortality odds were also observed in agricultural workers who resided in municipalities in Rio de Janeiro state in the third (; 95% CI: 1.0, 1.5) and fourth (; 95% CI: 0.9, 1.5) quartiles of per capita use of pesticides. |
| 9. Boccolini et al.186 | 2014/Brazil | 1,1766 stomach cancer deaths cases/11,557 controls who died by causes other than neoplasms and diseases of the digestive system | Case–control | Multiple pesticide classes | Death certificates (occupation at the time of death and pesticide expenditure per agricultural worker) | Not applicable | Stomach cancer mortality | Agricultural workers had increased odds of dying from stomach cancer compared with nonagricultural workers (; 95% CI: 1.3, 1.8). Among agricultural workers, those who resided in the areas with the highest levels of pesticide use had slightly increased odds of stomach cancer (e.g., OR for the highest ; 95% CI: 0.9, 2.1). |
| 10. Segatto et al.182 | 2015/Brazil | 95 cutaneous melanoma cases/96 controls | Case–control | Multiple pesticide classes | Questionnaire (lifetime occupational and residential exposure) | Not applicable | Cutaneous melanoma | Those who were ever exposed to pesticides had increased odds of cutaneous melanoma compared with those who were never exposed (; 95% CI: 1.0, 6.9). Indoor residential pesticide use was associated with increased odds of cutaneous melanoma; exposure for y was associated with increased odds compared with exposure for y (; 95% CI: 1.6, 5.3) and high frequency of indoor pesticide use ( times/y) associated with increased odds compared with low frequency of use ( times/y) ; 95% CI: 1.1, 3.5). Null associations between residential outdoor pesticide exposure and cutaneous melanoma. History of occupational exposure to pesticides was also associated with increased odds of cutaneous melanoma (; 95% CI: 1.9, 6.3). |
| 11. Emeville et al.174 | 2015/Guadeloupe | 576 prostate cancer cases/655 controls | Case–control | OCs | Serum DDT, DDE, chlordecone | Median (P25–P75) (): for controls and for cases; - for controls and for cases; for controls and 2.55 (1.11–5.74 for cases); for controls and 0.43 (0.18–0.94) for cases |
Prostate cancer | DDE concentrations in the highest vs. lowest quintile of exposure were associated with increased odds of prostate cancer [ (95% CI: 1.0, 2.3), ]. Results not shown for other OCs. |
| 12. Boccolini et al.183 | 2016/Brazil | 1,317 non-Hodgkin lymphoma death cases/2,634 controls who died by causes other than neoplasm or hematological diseases | Case–control | Multiple pesticide classes | Death certificates (occupation at the time of death and pesticide expenditure per agricultural worker) | Not applicable | Non-Hodgkin lymphoma | Null association between agricultural work and risk of death by non-Hodgkin lymphoma in the entire study population (; 95% CI: 0.8, 1.3), but increased odds of death by non-Hodgkin lymphoma among agricultural workers 20–39 years of age (; 95% CI: 1.2, 3.1) compared with nonagricultural workers in the same age range. |
| 13. Silva et al.181 | 2019/Brazil | 85 breast cancer cases/266 controls | Case–control | Multiple pesticide classes | Questionnaire (environmental and occupational exposure history) | Not applicable | Breast cancer | Living near cropland with pesticides was associated with increased odds of breast cancer (; 95% CI: 1.8, 3.2). Residential pesticide use and history of working with pesticides were not associated with breast cancer risk. |
| 14. Brureau et al.173 | 2020/Guadeloupe | 340 incident prostate cancer patients who underwent radical prostatectomy | Prospective cohort | OCs | Serum chlordecone, DDE | Median (P25–P75) (): (0.16–0.69); - (0.93–4.68) |
Biochemical recurrence of prostate cancer (defined as two consecutive PSA measurements ) | Highest quartile of chlordecone concentrations was associated with increased risk of biochemical recurrence of prostate cancer compared with those in the lowest quartile (; 95% CI: 1.4, 4.6). DDE concentrations were not associated with risk of biochemical recurrence of prostate cancer. |
Note: ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; CI, confidence interval; DDE, dichlorodiphenyldichloroethylene; DDT, dichlorodiphenyltrichloroethane; HR, hazard ratio; LOD, limit of detection; OC, organochlorine; OR, odds ratio; P, percentile; PSA, prostate-specific antigen.
Five publications reported that children whose mothers were occupationally or environmentally exposed to pesticides before, during, or after pregnancy had increased odds of leukemia.175–179 For instance, in a Brazilian case–control study, children whose mothers were exposed to pyrethroid insecticides during pregnancy had increased odds of acute lymphoblastic leukemia (ALL) and acute myeloid leukemia (AML) at 0–23 months of age.178 Similarly, in a Costa Rican case–control study, boys, but not girls, whose mothers reported using insecticides inside their homes in the year before pregnancy, during pregnancy, and while breastfeeding had increased odds of childhood ALL.175 Maternal report of pesticides sprayed on farms or companies near the home during pregnancy and while breastfeeding was also associated with childhood ALL in the Costa Rican study.175 Another publication from this Costa Rican case–control study reported that children whose fathers were occupationally exposed to any pesticide during pregnancy, but particularly the second trimester, had increased odds of leukemia.176
Five publications examined the association of pesticide exposure with breast cancer,180,181 cutaneous melanoma,182 prostate cancer,174 and prostate cancer recurrence173 in adults. Two publications reported that women who reported using insecticides in their homes during adulthood ( years of age)180 or who lived near agricultural fields181 had increased odds of breast cancer. Another publication found that study participants who were ever exposed to pesticides had increased odds of cutaneous melanoma, with stronger associations among those with indoor residential pesticide exposure, particularly for those with a high frequency of use ( times per year) or long duration of exposure ( y).182 In addition, two studies from Guadeloupe reported associations of serum concentrations of two OC pesticides, DDE and chlordecone, with increased risk of prostate cancer174 or its biochemical recurrence.173
Four publications assessed the association of occupational pesticide exposure with mortality by non-Hodgkin lymphoma183 or esophageal,184 brain,185 or stomach186 cancer in adults using death certificate data to ascertain occupation at the time of death. More specifically, a publication from a study conducted in Brazil reported mostly null associations between agricultural work and the risk of death by non-Hodgkin lymphoma.183 Conversely, three publications reported that farmworkers had increased odds of dying from esophageal, brain, and stomach cancers than non-farmworkers; two of these publications also reported increased odds of dying from brain185 and stomach186 cancer among farmworkers who lived in the areas of greatest pesticide use.
The small number of studies published to date and included in this scoping review provide somewhat consistent evidence of associations between maternal pesticide exposure before or during pregnancy and increased risk of leukemia among LAC children. In addition, eight of nine publications of studies conducted in adults reported evidence of residential or occupational pesticide exposure with an increased risk of various types of cancer or death by cancer. Nevertheless, these findings must be interpreted with caution given that all studies assessed exposure to multiple pesticide classes via questionnaire and examined different types of cancer.
Thyroid Function
Sixteen publications from 10 cross-sectional studies and 6 prospective cohort studies reported on the associations of pesticide exposure with thyroid function (Table 6). Four of these 16 publications examined the potential thyroid effects of OC pesticide exposure—assessed via measurement of OC pesticide metabolites in blood or breast milk—among children.117,187–189 Briefly, a publication from a cross-sectional study of mother–newborn pairs in Bolivia reported null associations of cord blood DDT and DDE concentrations with neonatal thyroid-stimulating hormone (TSH) levels.188 However, a publication from a cross-sectional study of Brazilian children (0–14 years of age) found that higher concentrations of 17 (of 19) OC pesticides, including DDE and DDT but not chlordecone, were associated with increased levels of total triiodothyronine (T3) or free thyroxine (T4), but not with TSH.187 Two publications from a prospective cohort study in Guadeloupe reported associations of early-life chlordecone exposure—as indicated by measurement of chlordecone in cord blood and breast milk samples—with elevated TSH or decreased T3 and T4 at 3 months and at 7 years of age, with some evidence of effect modification by sex.117,189
Table 6.
Characteristics of Latin American and the Caribbean studies on pesticide exposure and thyroid function published between 2007 and 2021 ().
| Study | Year of publication/country | Population and sample size | Study design | Pesticides assessed | Exposure assessment method | Pesticide or metabolite concentrations | Health effect and assessment method/instrument | Results |
|---|---|---|---|---|---|---|---|---|
| Studies on OCs | ||||||||
| Studies in children | ||||||||
| 1. Freire et al.187 | 2012/Brazil | 193 children (0–14 years of age) from an old factory | Cross-sectional | OCs | Questionnaire (occupational and residential history of parents) Serum HCH, HCB, chlordane, trans-nonachlor, heptachlor, DDT, DDE, endosulfan, aldrin, endrin, dieldrin, methoxychlor, mirex |
Median (P20–P80) (): ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; | Serum TSH, total T3, fT4 | Higher concentrations of 17 of 19 OC pesticides were associated with increased total T3 levels [ (95% CI)] for highest quintile of exposure compared with lowest quintile of exposure: ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; . For mirex, exposure was categorized into quartiles and the highest quartile of exposure was associated with increased total T3 levels compared with the lowest quartile (; 95% CI: , 20.7). Highest quartile of DDD (; 95% CI: 0, 0.2), endosulfan 1 (; 95% CI: 0, 0.1), and dieldrin (; 95% CI: , 0.11) were associated with increased fT4 levels. Mostly null associations between OC pesticides and TSH levels. |
| 2. Arrebola et al.188 | 2016/Boliviaa | 200 mother–newborn pairs from agricultural area | Cross-sectional | OCs | Questionnaire (residential exposure history) Cord blood DDT and DDE |
Median (P25–P75) ( lipid): ; |
Serum TSH | Null associations of cord blood DDT and DDE with neonatal TSH levels. |
| 3. Cordier et al.117 | 2015/Guadeloupeb | 111 mother–child (18 months of age) pairs | Prospective cohort | OCs | Cord blood and breast milk chlordecone, cord blood DDE | Median (P25–P75) (): ; ; |
Serum TSH, fT3, fT4 | Cord chlordecone was associated with increased TSH, particularly among boys (). Postnatal chlordecone was associated with decreased fT3 among boys and decreased fT4 among girls (). |
| 4. Ayhan et al.189 | 2021/Guadeloupec,d | 285 mother–child (7 years of age) pairs | Prospective cohort | OCs | Cord and child blood chlordecone, cord blood DDE | Median (P25–P75) (): Cord blood chlordecone: , ; child chlordecone: , ; cord blood DDE: , |
Serum TSH, fT3, fT4 | Third quartile of cord blood chlordecone associated with elevated TSH levels in girls (; 95% CI: 0, 0.4), relative to first quartile. Null associations of cord blood chlordecone with fT3 and fT4. No report on associations of child chlordecone and cord blood DDE with thyroid hormones. |
| Studies in adults | ||||||||
| 5. Freire et al.190 | 2013/Brazil | 608 adolescents and adults ( years of age) living near an abandoned pesticide factory | Cross-sectional | OCs | Questionnaire (residential exposure history) Serum HCH, HCB, chlordane, trans-nonachlor, heptachlor, DDT, DDE, endosulfan, aldrin, endrin, dieldrin, methoxychlor, mirex |
Median (P25–P75) (): Women: ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; Men: ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; |
Serum TSH, total T3, fT4 TPOAb, TGAb |
Among men, higher endosulfan 2 was associated with decreased total T3 levels (; 95% CI: , ); higher was associated with decreased fT4 levels (; 95% CI: , ) and slightly increased TSH levels (; 95% CI: 0.001, 0.007); higher -DDT was associated with decreased fT4 (; 95% CI: , ). Men with detected methoxychlor had an increased risk for presence of TPOAb (; 95% CI: 1.3, 3.8). Among women, higher (; 95% CI: 1.1, 10.2), -DDT (; 95% CI: 0.1, 0.7), endosulfan 2 (; 95% CI: 0.1, 7.0), and methoxychlor (; 95% CI: 1.7, 15.4) was associated with increased total T3 levels; higher HCB (; 95% CI: 0.001, 0.04), heptachlor (; 95% CI: 0.003, 0.03), -DDT (; 95% CI: 0.01, 0.04), and -DDT (; 95% CI: 0.001, 0.01) were associated with increased fT4 among women. Aldrin was associated with the presence of TPOAb (; 95% CI: 1.0, 1.02). |
| 6. Blanco-Muñoz et al.192 | 2016/Mexico | 136 male floriculture workers | Prospective cohort | OCs | Questionnaire (occupational and residential exposure history) Serum -DDE, -DDT |
Median (P25–P75) () Rainy season ; dry season |
Serum TSH, total T3, total T4 | Higher -DDE was associated with increased total T3 (; 95% CI: , 0.03) and total T4 (; 95% CI: 0.0, 0.1) levels. Null association of -DDE with TSH levels. |
| 7. Piccoli et al.191 | 2016/Brazil | 275 men and women from farmworker families | Cross-sectional | OCs, OPs | Questionnaire (residential and occupational exposure history) Serum HCH, HCB, chlordane, heptachlor, heptachlor epoxide B, trans-nonachlor, DDT, DDE, DDD, endosulfan, aldrin, endrin, dieldrin, metoxichlor, mirex, pentachloroanisole Blood AChE, BChE |
Median (P5–P95) (): ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; |
Serum TSH, total T3, fT4 | Farmworkers had higher total T3 (; 95% CI: 1.01, 1.1) than non-farmworkers. Higher was associated with increased TSH levels (; 95% CI: 1.01, 1.5), whereas higher dieldrin was associated with increased fT4 (; 95% CI: 0.9, 1.0). Higher (; 95% CI: 1.0, 1.1), (; 95% CI: 1.0, 1.1), heptachlor epoxide B (; 95% CI: 1.0, 1.2), trans-nonachlor (; 95% CI: 1.0, 1.2), -DDE (; 95% CI: 1.0, 1.1), and endosulfan 2 (; 95% CI: 1.0, 1.3) were associated with increased total T3. AChE and BChE inhibitions were not associated with thyroid hormones. |
| 8. Hernández-Mariano et al.193 | 2017/Mexico | 430 pregnant women living in a floriculture area | Prospective cohort | OCs | Serum DDE during pregnancy | ( lipid) | Serum TSH, total T3, fT3, total T4, fT4 | Women with DDE concentrations had higher total T3 levels (; 95% CI: 0.06, 0.3) than those with . Mostly null associations of serum DDE with total and free T4 and TSH levels. |
| 9. Londoño et al.194 | 2018/Colombia | 819 farmworkers and their partners from plantain and coffee farms | Cross-sectional | OCs, OPs | Serum chlorpyrifos, DDT, DDE, endosulfan, HCB, aldrin, endrin, heptachlor, methoxychlor, chlordane | Median (range) (): ; |
Serum TSH, fT4 TPOAb |
Higher -DDE (; 95% CI: 1.6, 9.2), heptachlor (; 95% CI: 1.0, 3.2), endosulfan 1 (; 95% CI: 1.6, 24.8), and OCs in blood ( ; 95% CI:1.1, 3.3) were associated with increased odds of subclinical hypothyroidism. Null associations of chlorpyrifos concentrations and hypothyroidism. |
| Studies on OPs or CBs | ||||||||
| Studies in children | ||||||||
| 10. Phillips et al.195 | 2021/Ecuador | 80 adolescents (12–17 years of age) living in agricultural areas | Cross-sectional | OPs | Questionnaire Blood AChE |
Not applicable | Serum TSH, fT4 | Lower AChE activity was marginally associated with increased fT4 levels ( per SD decrease in AChE , 90% CI: 0.00, 0.06), but not with TSH (, 90% CI: , 0.36). In girls, lower AChE activity was associated with increased fT4 levels (, 90% CI: 0.01, 0.10) and decreased TSH levels (, 90% CI: , ). Null associations were observed in boys. |
| Studies in adults | ||||||||
| 11. Lacasaña et al.196 | 2010/Mexico | 136 male floriculture workers | Prospective cohort | OPs | Questionnaire (occupational and residential exposure history) Urinary DAPs |
( creatinine): Rainy season: ; ; ; Dry season: ; ; ; |
Serum TSH, total T3, total T4 | Higher (; 95% CI: 0.1, 0.3), (; 95% CI: 0.1, 0.4), and (; 95% CI: 0.1, 0.4) were associated with increased TSH levels. Higher (; 95% CI: 0.1, 0.3) and (; 95% CI: 0.1, 0.3) were associated with increased total T4 levels. Null associations were observed for total T3. |
| 12. Lacasaña et al.197 | 2010/Mexico | 136 male floriculture workers | Prospective cohort | OPs | Questionnaire (occupational and residential exposure history) Urinary DAPs |
( creatinine): Rainy season: ; ; ; Dry season: ; ; ; |
Serum TSH, total T3, total T4 | Interaction between PON1192RR and on TSH (; 95% CI: 0.05, 0.6) and total T3 (; 95% CI: 0.0, 0.2) levels, and between PON1192RR and on TSH (; 95% CI: 0.02, 0.5). No interaction between PON155 polymorphism and DAP metabolite concentrations on hormone levels. |
| 13. Miranda-Contreras et al.200 | 2013/Venezuelac | 64 male farmworkers/35 controls | Cross-sectional | OPs, CBs | Questionnaire (occupational exposure history) Blood AChE, BChE |
Not applicable | Serum TSH, fT4 | Null associations of serum hormones with cholinesterase levels. |
| 14. Torres-Sanchez et al.199 | 2019/Mexico | 381 pregnant women living in a floricultural area | Cross-sectional | OPs | Questionnaire (para-occupational exposure history) Urinary DAPs in a subsample |
Median total DAPs ( creatinine): Para-occupationally ; non–para-occupationally (data not shown in tables) |
Serum TSH, fT4 | Null associations of para-occupational exposure to OP pesticides and urinary DAPs with hypothyroxinemia. No interaction was observed between pesticides para-occupational exposure and PON1 polymorphisms. |
| 15. Bernieri et al.198 | 2019/Brazil | 46 rural farmworkers/27 controls | Cross-sectional | OPs, CBs | Questionnaire (occupational exposure history) Blood BChEe |
Not applicable | Serum fT4, total T3, TSH | Farmworkers had lower TSH () but higher total T3 and fT4 ( for each) than controls. |
| Studies on other pesticides or multiple pesticide classes | ||||||||
| Studies in adults | ||||||||
| 16. Santos et al.201 | 2019/Brazilc | 122 individuals living in small farms | Cross-sectional | Multiple pesticide classes | Questionnaire (occupational, residential, and seasonal exposure history) | Not applicable | Serum TSH, total T3, fT3, total T4, fT4 | Use of dithiocarbamate fungicides in the past week (; 95% CI: , ) and no use of full PPE during the last pesticide application (; 95% CI: , ) was associated with decreased TSH levels. Use of cyhalothrin in the past week was associated with decreased fT4 (; 95% CI: , ) and total T4 (; 95% CI: , ), whereas use of paraquat in the past week was associated with decreased fT3 (; 95% CI: , ). Lifetime use ( y) of OP pesticides was associated with decreased fT4 (; 95% CI: , ) and total T4 ( ; 95% CI: , ). Lifetime use (1–20 y) of mancozeb was associated with decreased total T4 (; 95% CI: , ) and fT3 (; 95% CI: , ). |
Note: AChE, acetylcholinesterase; BChE, butyrylcholinesterase; CBs, carbamate pesticides; CI, confidence interval; CPO, chlorpyrifos; DAP, dialkylphosphate; DDD, dichlorodiphenyldichloroethane; DDE, dichlorodiphenyldichloroethylene; DDT, dichlorodiphenyltrichloroethane; DEP, diethylphosphate; DETP, diethylthiophosphate; DM, dimethyl; DMDTP, dimethyldithiophosphate; DMP, dimethylphosphate; HCB, hexa-chlorobenzene; fT3, free triiodothyronine; fT4; free thyroxine; HCH, hexa-chlorocyclohexane; LOD, limit of detection; OC, organochlorine; OP, organophosphate; OR, odds ratio; P, percentile; PON1, paraoxonase-1; PPE, personal protective equipment; T3, triiodothyronine; T4, thyroxine; TGAb, thyroglobulin antibodies; TPOAb, thyroid peroxidase antibodies; TSH, thyroid-stimulating hormone.
Also included in Table 8 (birth outcomes and child growth).
Also included in Table 3 (neurobehavioral outcomes).
Also included in Table 7 (reproductive outcomes).
Also included in Table 9 (other health effects).
Investigators did not use exposure biomarker concentrations in multivariate analyses.
Five publications reported on the association between exposure to OC pesticides and thyroid function in adults (Table 6). A publication from a cross-sectional study of individuals living near an abandoned pesticide factory in Brazil reported various associations of OC pesticide concentrations with thyroid hormone levels, which differed between men and women.190 For example, among men, higher endosulfan 2 concentrations were associated with decreased T3 levels, whereas higher and DDT concentrations were associated with decreased free T4 levels. Among women, higher , DDT, endosulfan 2, and methoxychlor concentrations were associated with increased T3 levels, whereas higher HCB, heptachlor, and DDT concentrations were associated with increased T4 levels.190 A publication from a cross-sectional study of farmworker families in Brazil also reported associations of several OC pesticide concentrations with increased TSH (i.e., ), total T3 (i.e., , , heptachlor epoxide B, trans-nonachlor, DDE, and endosulfan 2), or free T4 (i.e., dieldrin).191 Two publications from different prospective cohort studies in Mexico reported associations of serum DDE concentrations with increased total T3 or T4 levels among male floriculture workers192 and pregnant women living in a floriculture area.193 In addition, a cross-sectional study of Colombian farmworkers and their partners found associations of serum DDE, heptachlor, endosulfan 1, and three or more OC pesticides with increased odds of subclinical hypothyroidism.194
Six publications examined associations of OP or carbamate pesticide exposure—assessed by measurement of urinary DAP metabolite concentrations or blood ChE activity—with thyroid function (Table 6), but only one focused on children.195 The latter publication from a cross-sectional study of Ecuadorian adolescents living in agricultural areas reported that lower acetylcholinesterase (AChE) activity was associated with increased free T4 and decreased TSH levels among girls, but not boys.195 Two publications from a prospective cohort study of adult floriculture workers in Mexico reported that higher DAP metabolite concentrations were associated with increased TSH and total T4 levels196 and that these associations were modified by paraoxonase 1 (PON1192RR).197 Similarly, a publication from a cross-sectional study in Brazil reported increased TSH, but also decreased T3 and T4 levels, among farmworkers compared with unexposed controls.198 In contrast, two publications from cross-sectional studies in Mexico199 and Venezuela200 reported null associations of occupational or para-occupational exposure to OP pesticides with thyroid hormone levels. Last, a publication from a cross-sectional study in Brazil examined associations between exposure to multiple pesticide classes—ascertained via questionnaire—and thyroid function among adults and reported associations of recent use of dithiocarbamate fungicides with decreased TSH levels, recent use of (pyrethroid insecticide) with decreased free and total T4 levels, and recent use of paraquat (herbicide) with decreased free T3 levels.201 Overall, published studies on the associations of pesticide exposure and thyroid function among LAC populations have reported mixed findings with notorious differences between pesticide active ingredients, age groups, and sexes.
Reproductive Outcomes
Sixteen publications reported on the association of pesticide exposure with reproductive outcomes such as reproductive hormone profiles among adults (Table 7). Four of these 16 publications focused on OC pesticide exposure and used direct pesticide exposure assessment methods.189,202–204 A publication from a prospective cohort study in Guadeloupe reported that higher cord blood chlordecone concentrations were associated with elevated androsterone and testosterone in 7-y-old boys and girls.189 Notably, a publication from a prospective cohort study of male floriculture workers in Mexico reported that higher serum DDE concentrations were associated with decreased prolactin and testosterone, but also with increased inhibin B.202 A publication from a cross-sectional study of individuals living near an abandoned pesticide factory in Brazil (mentioned above) reported that higher serum heptachlor and DDT concentrations were associated with decreased testosterone levels among men and that higher serum aldrin, HCB, DDT, endosulfan 2, and mirex concentrations were associated with increased estradiol levels, decreased luteinizing hormone (LH) levels, or decreased follicle-stimulating hormone (FSH) levels among peri-/postmenopausal women.203 Furthermore, a publication from a case–control study in Brazil reported that infertile women had higher detectable serum DDE concentrations than fertile women.204
Table 7.
Characteristics of Latin American and the Caribbean studies on pesticide exposure and reproductive outcomes published between 2007 and 2021 ().
| Study | Year of publication/country | Population and sample size | Study design | Pesticides assessed | Exposure assessment method | Pesticide or metabolite concentrations | Health effect and assessment method/instrument | Results |
|---|---|---|---|---|---|---|---|---|
| Studies on OCs | ||||||||
| 1. Bastos et al.204 | 2013/Brazil | 15 women seeking help for infertility treatment/21 women spontaneously pregnant | Case–control | OCs | Questionnaire (occupational and reproductive history). Serum HCB, DDT, DDE, DDD |
(): Fertile women: ; ; Infertile women: ; ; |
Fertility | Infertile women had higher detectable serum DDE concentrations than fertile women (). |
| 2. Blanco-Muñoz et al.202 | 2012/Mexico | 84 male floriculture workers | Prospective cohort | OCs | Questionnaire (occupational history) Serum DDE |
Median (range) (): Rainy season: Dry season: |
Serum FSH, LH, prolactin, testosterone, estradiol, inhibin B | -DDE concentrations were negatively associated with prolactin (; 95% CI: , ) and testosterone (; 95% CI: , 0.01), but positively associated with inhibin B (; 95% CI: 0.02, 0.21). Null associations of -DDE with FSH, LH, or estradiol. |
| 3. Freire et al.203 | 2014/Brazil | 604 men and women living near an abandoned pesticide factory | Cross-sectional | OCs | Questionnaire (residential exposure history) Serum HCH, HCB, chlordane, trans-nonachlor, heptachlor, DDT, DDE, DDD, endosulfan, aldrin, endrin, dieldrin, methoxychlor, mirex |
Median (P25–P75) (): Premenopausal women: ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; [] Peri-/postme nopausal women: ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; Men: ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; |
Serum testosterone, estradiol, progesterone, prolactin, LH, FSH | Higher heptachlor and -DDT were associated with decreased testosterone levels among men (; 95% CI: , , and ; 95% CI: , , respectively). Among peri-/postmenopausal women, higher aldrin was associated with increased estradiol levels (; 95% CI: 0.001, 0.01), but decreased LH (; 95% CI: , ) and FSH (; 95% CI: , ) levels. Higher -DDD and endosulfan 1 were associated with decreased LH (; 95% CI: , , and ; 95% CI: , , respectively) and FSH (; 95% CI: , ; ; 95% CI: , , respectively) levels. Higher HCB (; 95% CI: , ), -DDT (; 95% CI: , ), endosulfan 2 (; 95% CI: , ), and mirex (; 95% CI: , ) were also associated with decreased LH levels among this group of women. Among premenopausal women, no associations were found. |
| 4. Ayhan et al.189 | 2021/Guadeloupea,b | 285 mother–child (7 years of age) pairs | Prospective cohort | OCs | Cord and child blood chlordecone, cord blood DDE | Median (P25–P75) (): Cord blood chlordecone: , ; child chlordecone: , ; cord blood DDE: , |
Serum DHEA, TT, DHT, estradiol | Third quartile of cord blood chlordecone was associated with elevated DHEA ( for ; 95% CI: 0.1, 1.0; for ; 95% CI: 0, 0.7), TT (OR for ; 95% CI: 1.1, 9.6; OR for ; 95% CI: 1.3, 8.2), and DHT (OR for ; 95% CI: 1.3, 10.6; OR for ; 95% CI: 1.0, 10.2) levels in boys and girls, relative to first quartile of cord blood chlordecone. |
| Studies on OPs or CBs | ||||||||
| 5. Recio-Vega et al.209 | 2008/Mexico | 19 sprayer farmworkers/16 non-sprayer farmworkers/17 non-farmworkers | Prospective cohort | OPs | Questionnaire (occupational, residential, and seasonal exposure histories). Urinary DAPs |
total DAPs (ppb): Farmworkers but not OP Sprayers exposed to |
Semen quality | Sprayer farmworkers had lower sperm volume (, ) and lower sperm count (, ) than non-farmworkers. During low exposure period, non-sprayer farmworkers had lower rapid progressive motility (, ). During medium exposure period sprayer farmworkers had lower sperm volume (, ). During high exposure period, seminal parameters were similar among all groups. Sperm vitality was lower at higher levels of DMDTP (, ). No other seminal parameters were associated with DAP levels. |
| 6. Yucra et al.208 | 2008/Peru | 31 male farmworkers/31 controls | Cross-sectional | OPs | Questionnaire (occupational exposure history) Urinary DAPs |
(): Nonexposed: ; ; ; ; ; Exposed: ; ; ; ; ; |
Semen quality Serum testosterone, estradiol, FSH, LH |
Higher concentrations of ethylated OP metabolites were associated with lower seminal volume (), whereas higher concentrations of methylated OP metabolites were associated with higher seminal pH (). After controlling for ethylated OP metabolites, exposure to pesticides (yes/no) was associated with increased seminal pH (). After controlling for methylated OP metabolites, exposure to pesticides (yes/no) was associated with increased seminal pH () and decreased seminal fructose levels (). Null associations of pesticide exposure and serum hormone levels. |
| 7. Blanco-Muñoz et al.207 | 2010/Mexico | 104 male floriculture workers | Cross-sectional | OPs | Questionnaire (occupational exposure history) Urinary DAPs |
Median (range) ( creatinine): Low exposure: ; ; ; ; ; ; Medium exposure: ; ; ; ; ; ; total High exposure: ; ; ; ; ; ; |
Serum FSH, LH, prolactin, testosterone, inhibin B, estradiol | Higher DMP (; 95% CI: , ), DEP (; 95% CI: , ), DETP (; 95% CI: , ) and total DAP (; 95%CI: , ) concentrations were associated with decreased inhibin B levels. Higher DEP concentrations were associated with decreased FSH (; 95% CI: , ). Higher DEP (; 95% CI: , 0.004) and total DAP (; 95% CI: 0.000005, 0.0003) concentrations marginally associated with increased testosterone levels. Higher DETP marginally was associated with decreased LH levels (; 95% CI: , 0.0001). |
| 8. Cecchi et al.26 | 2012/Argentinaa | 97 pregnant women living in a rural area with intensive use of pesticides | Prospective cohort | OPs | Questionnaire (residential exposure history) Blood AChE and BChE |
Not applicable | Serum progesterone (measured during spray and prespray season) | Higher AChE activity was associated with increased progesterone levels (; ). |
| 9. Miranda-Contreras et al.200 | 2013/Venezuelab | 64 male farmworkers/35 controls | Cross-sectional | OPs, CBs | Questionnaire (occupational exposure history) Blood AChE and BChE |
Not applicable | Semen quality Sperm chromatin integrity (DFI) Serum testosterone, FSH, LH, PRL |
Farmworkers had higher seminal pH () and lower percentage of live sperm () than controls. Farmworkers with decreased BChE activity had higher DFI (, ). Null associations of serum hormones with cholinesterase levels. |
| 10. Aguilar-Garduño et al.205 | 2013/Mexico | 136 male floricultural workers | Prospective cohort | OPs | Urinary DAPs | Median (GM) total DAPs ( creatinine): ; |
Serum FSH, LH, prolactin, testosterone, estradiol, inhibin B | Higher total DAP concentrations were associated with increased FSH and prolactin levels ( for each) and decreased testosterone () and inhibin B levels (). |
| 11. Silvia et al.210 | 2020/Argentinac | 53 pregnant women living in areas with intensive pesticide application | Cross-sectional | OPs, CBs | Questionnaire (residential exposure history) Blood AChE, BChEd |
Not applicable | Plasma estradiol, progesterone (measured during spray and non-spray season) | Progesterone and estradiol levels did not differ between spray and non-spray seasons. |
| Studies on other pesticides or multiple pesticide classes | ||||||||
| 12. Sanin et al.211 | 2009/Colombia | 2,592 fertile women from regions with different levels of aerial glyphosate spraying | Retrospective cohort | Glyphosate | Questionnaire (residential and occupational exposure history) Ecological exposure index (different levels of exposure according to agricultural practices) |
Not applicable | TTP | Reduced fecundability was not associated with aerial glyphosate spraying. |
| 13. Rojas and Guevara212 | 2014/Venezuela | 180 women of reproductive age | Cross-sectional | Multiple pesticide classes | Questionnaire (occupational exposure history) | Not applicable | Menstrual cycle and bleeding duration | Women who were occupationally exposed to pesticides had longer menstrual cycles than women who did not have contact with pesticides (). Null association between bleeding duration and pesticide exposure. |
| 14. Miranda-Contreras et al.213 | 2015/Venezuela | 64 male farmworkers/64 controls | Cross-sectional | Multiple pesticide classes | Questionnaire (occupational exposure history) | Not applicable | Semen quality Sperm chromatin integrity (DFI) |
Farmworkers had decreased sperm concentration (), vitality (), slow progressive motility (), lower sperm membrane integrity (), and high DFI () compared with controls. |
| 15. Cremonese et al.214 | 2017/Brazil | 99 rural young men/36 urban young men | Cross-sectional | Multiple pesticide classes | Questionnaire (residential, occupational, and reproductive exposure history) Blood AChE and BChE |
Not applicable | Semen quality Genital measurements (AGD, TV) Serum testosterone, LH, FSH, SHBG, prolactin |
Rural men had decreased normal sperm morphology (; 95% CI: 0.6, 0.9), increased sperm count (; 95% CI: 1.01, 2.5), increased TV (; 95% CI: 1.1, 1.5), decreased LH levels (; 95% CI: 0.7, 1.0) and increased T:LH ratio (; 95% CI: 1.1, 1.6) compared with urban men. Farmers who had y working had decreased T:LH ratio (; 95% CI: 0.7, 1.0) and decreased TV (; 95% CI: 0.8, 1.0) (); who had y handling pesticides had decreased LH levels (; 95% CI: 0.7, 1.0), increased T:LH ratio (; 95% CI: 1.1, 1.4), lower normal morphology (; 95% CI: 0.6, 0.9) and increased TV (; 95% CI: 1.1, 1.4) (Ref ), and who had d/y handling pesticides had increased LH levels (; 95% CI: 1.0, 1.4) and lower normal morphology (; 95% CI: 0.8, 1.0) (). Those farmworkers who used pesticides in the high use season had increased prolactin levels (; 95% CI: 1.2, 1.7), and those who did not use PPE had decreased testosterone levels (; 95% CI: 0.8, 1.0) and TV (; 95% CI: 0.8, 1.0). Maternal farming during pregnancy was associated with increased AGD (; 95% CI: 1.01, 1.1) and TV (; 95% CI: 1.02, 1.3). Cholinesterase activities were not associated with reproductive hormones or semen quality. |
| 16. Santos et al.201 | 2019/Brazilb | 122 farmworkers and their families | Cross-sectional | Multiple pesticide classes | Questionnaire (occupational, residential, and seasonal exposure history) | Not applicable | Serum LH, testosterone, estradiol, LH, FSH | Recent use of fungicides in general (; 95% CI: 11, 80), (59%; 95% CI: 13, 123), and phthalimide (95%; 95% CI: 37, 176) was associated with increased LH levels in men. Working in agriculture (1–30 y) was associated with increased testosterone levels in men (20%; 95% CI: 2, 40) (reference group never worked in agriculture). |
Note: %change, percentage change; AChE, acetylcholinesterase; AGD, anogenital distance; BChE, butyrylcholinesterase; CBs, carbamate pesticides; CE, carboxylesterases; CI, confidence interval; DAP, dialkylphosphate; DDD, dichlorodiphenyldichloroethane; DDE, dichlorodiphenyldichloroethylene; DDT, dichlorodiphenyltrichloroethane; DEDTP, Diethyldithiophosphate; DEP, diethylphosphate; DETP, diethylthiophosphate; DFI, fragmentation Index; DHEA, dehydroepiandrosterone; DHT, dihydrotestosterone; DMDTP, dimethyldithiophosphate; DMP, dimethylphosphate; FSH, follicle-stimulating hormone; GSD, geometric standard deviation; HCB, hexa-chlorobenzene; IQR, interquartile range; LH, luteinizing hormone; LOD, limit of detection; OC, organochlorine; OP, organophosphate; P, percentile; PPE, personal protective equipment; PRL, Prolactin; Ref, reference group; SD, standard deviation; SHBG, sex hormone-binding globulin; T:LH, testosterone/luteinizing hormone ratio; TT, total testosterone; TTP, time to pregnancy; TV, testis volume.
Also included in Table 9 (other health outcomes).
Also included in Table 6 (thyroid function).
Also included in Table 8 (birth size and child growth).
Investigators did not use exposure biomarker concentrations in multivariate analyses.
Seven publications examined associations of OP or carbamate pesticide exposure with reproductive outcomes, six ascertained exposure via urinary DAP metabolites or blood ChE levels,200,205–209 and one assigned exposure based on the season of sample collection (spray vs. nonspray)210 (Table 7). A publication from a prospective cohort study in Mexico reported lower sperm volume and count among farmworkers who sprayed OP pesticides compared with non-farmworkers, but mostly null associations between urinary DAP metabolite concentrations and seminal parameters.209 Three publications from cross-sectional studies conducted in Peru,208 Mexico,207 and Venezuela200 reported increased seminal pH, lower percentage of live sperm, and lower seminal fructose levels among farmworkers compared with non-farmworkers. The study conducted in Venezuela also reported that lower butyrylcholinesterase (BChE) activity was associated with an increased damage to sperm chromatin among farmworkers.200 A publication from a cross-sectional study of male floriculture workers in Mexico reported that higher urinary DAP metabolite concentrations were associated with decreased inhibin B, FSH, or LH levels, but also with increased testosterone levels.207 Another publication based on the same study population reported that higher urinary DAP metabolite concentrations were associated with increased FSH and prolactin levels, but decreased testosterone and inhibin B levels.205 Last, although one publication from a prospective cohort study of pregnant women in Argentina reported a weak association between higher AChE activity and increased progesterone levels,206 a cross-sectional study of women in Argentina reported no difference in progesterone and estradiol levels measured in the spray and nonspray seasons.210
Five publications from four cross-sectional studies and one retrospective cohort study reported on the associations of exposure to pesticides other than OCs, OPs, or carbamates or exposure to multiple pesticide classes with reproductive outcomes (Table 7). All studies relied on questionnaires to assess environmental or occupational pesticide exposure,201,211–213 but one of them also measured blood ChE activity.214 Two publications from studies conducted in Brazil214 and Venezuela213 reported associations of pesticide exposure with reduced sperm quality—as indicated by parameters such as decreased sperm concentration and higher sperm DNA fragmentation index—among farmworkers/rural men compared with controls/urban men. The publication from the cross-sectional study conducted in Brazil also reported that men living in rural areas and who mixed or applied pesticides had increased testis volume, decreased LH levels, or increased testosterone:LH ratios compared with men living in rural areas and who did not mix or apply pesticides, but the publication reported null associations of blood ChE activity with reproductive hormones and semen quality.214 A publication from another cross-sectional study in Brazil reported that recent use of fungicides in general, (pyrethroid insecticide), and phthalimide (fungicide) was associated with increased LH levels in men living in agricultural communities.201 A cross-sectional study of reproductive-age women in Venezuela found that women who were occupationally exposed to pesticides had longer menstrual cycles than those who were not exposed.212 Last, a publication from a retrospective cohort of fertile women aerially exposed to glyphosate in Colombia reported null associations with fecundability.211
Overall, publications from studies conducted to date provide some evidence of associations between exposure to pesticides, particularly OC pesticides, OP pesticides, and carbamates, with reproductive outcomes such as infertility, changes in sex hormone levels (e.g., testosterone and estradiol), and alterations in semen quality among adults in LAC countries. Although 10 of 16 studies employed direct exposure assessment methods, most were cross-sectional in design and had small sample sizes, limiting causal inference.
Birth Outcomes and Child Growth
Thirteen publications reported on the association of pesticide exposure with birth outcomes and infant/child growth (Table 8). Of the 13 publications, 7 focused on OC pesticides,188,215–217 4 on OP pesticides or carbamates,54,169,172,210 1 on Mn-containing fungicides,218 and 1 on multiple pesticide classes.219 A publication from a small cross-sectional study in Brazil reported null associations of maternal and newborn contamination indices—estimated using metal and OC pesticide concentrations measured in maternal blood at delivery and cord blood—with birth outcomes.217 In contrast, a publication from a cross-sectional study of mother–newborn pairs from Bolivia reported that higher cord blood DDT concentrations were associated with lower birth weight, whereas higher cord blood DDE concentrations were associated with higher birth weight and shorter gestation length.188 Publications from two prospective cohort studies in Mexico reported null associations of prenatal DDT or DDE exposure with birth outcomes and child growth during the first year of life216 and up to 43 months of age.215 Three publications from a prospective cohort study in Guadeloupe reported that higher cord blood chlordecone concentrations were associated with shorter length of gestation and increased risk of preterm birth,220 lower birth weight in children whose mothers gained a large amount of weight during pregnancy,221 and higher body mass index (BMI) at 3–18 months of age.222
Table 8.
Characteristics of Latin American and the Caribbean studies on pesticide exposure and birth size and child growth published between 2007 and 2021 ().
| Study | Year of publication/country | Population and sample size | Study design | Pesticides assessed | Exposure assessment method | Pesticide or metabolite concentrations | Health effect and assessment method/instrument | Results |
|---|---|---|---|---|---|---|---|---|
| Studies on OCs | ||||||||
| 1. Cupul-Uicab et al.215 | 2010/Mexico | 788 mother–children (13–43 months of age, boys only) pairs from an area where DDT was recently used | Prospective cohort | OCs | Questionnaire (residential and seasonal exposure at prenatal period) Maternal serum DDT and DDE at delivery |
Median (IQR) () |
Weight, height | Null associations of prenatal DDT exposure with height and BMI in boys up to 43 months of age. |
| 2. Garced et al.216 | 2012/Mexico | 253 mother–child (0–12 months of age) pairs | Prospective cohort | OCs | Maternal serum DDE in each trimester of pregnancy |
() -DDE: ; ; -DDT: ; ; |
Weight, length, head circumference during the first year of life | Null associations between prenatal DDE exposure and child growth during the first year of life. |
| 3. Kadhel et al.220 | 2014/Guadeloupe | 818 mother–newborn pairs | Prospective cohort | OCs | Cord blood chlordecone and DDE | Median (P25–P75) () ; . |
Length of gestation, preterm birth | Higher cord blood chlordecone concentrations were associated with shorter length of gestation ( per 10-fold wk; 95% CI: , 0) and increased risk of preterm birth (; 95% CI: 1.0, 2.3). Null associations of cord blood DDE with birth outcomes. |
| 4. Costet et al.222 | 2015/Guadeloupe | 222 mother–child (3–18 months of age) pairs | Prospective cohort | OCs | Questionnaire (dietary intake of food contaminated with chlordecone) Cord plasma and breast milk (3 months of age) chlordecone |
Median (IQR) (): ; |
Body length, weight, BMI at 3, 8, and 18 months of age | Highest tertile of cord blood chlordecone was associated with higher BMI in boys at 3 months of age (; 95% CI: 0, 1.8) and in girls at 8 (; 95% CI: 0, 1.5) and 18 (; 95% CI: , 1.4) months of age. |
| 5. Arrebola et al.188 | 2016/Boliviaa | 200 mother–newborn pairs from an agricultural area | Cross-sectional | OCs | Questionnaire (residential exposure history) Cord blood DDT and DDE |
Median (P25–P75) ( lipid) |
Birth weight, head circumference, birth length, ponderal index, length of gestation | Higher cord blood -DDT was associated with lower birth weight (; 95% CI: , ), whereas higher -DDE was associated with higher birth weight (; 95% CI: 0.003, 0.02). Higher cord blood -DDE was also associated with shorter gestation length ( [95% CI: , ). Higher -DDT was associated with smaller head circumference (; 95% CI: , 0). |
| 6. Motta et al.217 | 2016/Brazil | 40 mother–newborn pairs living in a rural area | Cross-sectional | OCs | Questionnaire (residential, occupational, and domestic exposure history) Maternal blood (at delivery) and cord blood DDT, DDE, HCH, HCB, chlordane |
Data not shown | Birth weight, head circumference, birth length | Null associations of maternal and newborn contamination indices (calculated using both metal and pesticide concentrations) with birth outcomes (). |
| 7. Hervé et al.221 | 2016/Guadeloupe | 593 mother–newborn pairs | Prospective Cohort | OCs | Cord blood chlordecone and DDE | Median (P25–P75) () ; |
Birth weight | Among mothers in the highest quartile of GWG, newborns with low and medium cord blood chlordecone concentrations had greater mean reduction in birth weight, compared with those with low cord blood chlordecone (; 95% CI: , , and ; 95% CI: , 20, respectively). |
| Studies on OPs or CBs | ||||||||
| 8. Chiapella et al.172 | 2014/Argentinab | 46 mother–newborn pairs from a rural area/24 mother–newborn pairs from an urban area (controls) | Cross-sectional | OPs | Questionnaire (residential and seasonal exposure history) Placental CEc |
Not applicable | Birth weight, birth length, head circumference, ponderal index, length of gestation | No differences in fetal growth measurements between exposure groups. |
| 9. Rivero Osimani et al.169 | 2016/Argentinab | 43 mother–newborn pairs from a rural area (RG)/20 mother–newborn pairs from an urban area (controls) | Cross-sectional | OPs | Questionnaire (residential and seasonal exposure history) Placental CEc |
Not applicable | Birth weight, birth length, head circumference, length of gestation | Mean birth weight was lower in RG-SS (difference of 9.6%; ) and RG-NSS (difference of 7.8%; ) compared with the control group. No differences in other birth outcomes between exposure groups. |
| 10. Quintana et al.54 | 2017/Argentinaa,b,e | 151 mother–newborn pairs living in a rural area/38 mother–newborn pairs from an urban area (controls) | Cross-sectional | OPs | Questionnaire (residential and seasonal exposure history) Cord blood AChE, BChE |
Not applicable | Birth weight, birth length, head circumference, length of gestation | Mean birth weight was lower in cesarean RG-SS than cesarean control group (difference of 14%; ). No differences in other birth outcomes between exposure groups. Null associations of cord blood AChE with birth outcomes. |
| 11. Silvia et al.210 | 2020/Argentina | 53 mother–newborn pairs living in areas with intensive pesticide application | Cross-sectional | OPs, CBs | Questionnaire (residential exposure history) Blood AChE, BChEc |
Not applicable | Birth weight, birth length, head circumference, preterm birth, intrauterine growth retardation | No differences in birth weight, length, head circumference, head circumference/weight ratio, and ponderal index between children born during SS and those born during NSS. |
| Studies on other pesticides or multiple pesticide classes | ||||||||
| 12. Mora et al.218 | 2015/Costa Rica | 380 mother–newborn pairs living near banana plantations | Prospective cohort | Mn-containing fungicides | Maternal blood and hair Mn in each trimester of pregnancy |
: ; |
Birth weight, birth length, head circumference, chest circumference, ponderal index, length of gestation | Hair Mn during the second and third trimesters of gestation were associated with chest circumference ( per 10-fold ; (95% CI: 0.2, 1.1), and ; 95% CI: , 1.3, respectively). Null associations between blood Mn and birth outcomes. |
| 13. Cecchi et al.219 | 2021/Argentina | 418 rural mother–newborn pairs living in proximity to intensive pesticide application/358 urban mother–child pairs | Prospective cohort | Multiple pesticides | Questionnaire (previous history of pesticide exposure and residential pesticide exposure) | Not applicable | Birth weight, birth length, head circumference | No differences in birth weight between groups. Birth length () and head circumference () -scores were lower in exposed group than in unexposed group. |
Note: AChE, acetylcholinesterase; BChE, butyrylcholinesterase; BMI, body mass index; CI, confidence interval; DDE, dichlorodiphenyldichloroethylene; DDT, dichlorodiphenyltrichloroethane; GWG, gestational weight gain; HCB, hexa-chlorobenzene; HCH, hexa-chlorocyclohexane; HR, hazard ratio; NSS, non-spraying season; Mn, manganese; OC, organochlorine; OP, organophosphate; OR, odds ratio; RG, rural group; SS, spraying season.
Also included in Table 6 (thyroid function).
Also included in Table 4 (placental outcomes and teratogenicity).
Investigators did not use exposure biomarker concentrations in multivariate analyses.
Also included in Table 2 (genotoxicity).
Also included in Table 9 (other health effects).
Four publications from cross-sectional studies in Argentina examined the association between prenatal OP pesticide exposure and fetal growth. Two of them reported a lower mean birth weight among mother–newborn pairs from a rural area compared with controls,54,169 whereas the other two found no differences in growth parameters between exposure groups.172,223 A publication from a prospective cohort study conducted in Argentina reported lower birth length and smaller head circumference in children living in proximity to pesticide applications compared with those living in an urban area.219 Finally, a publication from a prospective cohort study of mother–newborn pairs living near banana plantations aerially sprayed with Mn-containing fungicides in Costa Rica found that maternal Mn concentrations in hair, but not blood, were positively associated with infant chest circumference.218
Overall, the small number of published studies that have examined the association of pesticide exposure with birth size and child growth in LAC populations have reported mixed findings. More specifically, about half of the studies found some evidence of adverse outcomes and the other half reported null associations.
Other Health Problems
Kidney function.
Nine publications reported on the association between pesticide exposure—ascertained only via questionnaire—and kidney function (Table 9). Notably, six of these nine publications reported null associations with estimated glomerular filtration rate (eGFR) levels or prevalence of chronic kidney disease (CKD).224–229 In contrast, a publication from a cross-sectional study conducted in Nicaragua reported that accidental pesticide inhalation (ever), but not lifetime days of mixing/applying pesticide or lifetime days of working in fields with pesticide use, was associated with reduced eGFR.230 A publication from a cross-sectional study in Mexico reported a reduction in eGFR levels among migrant and seasonal farmworkers (who did not apply or mix pesticides) from preharvest to late harvest, as well as lower GFR levels among farmworkers who worked in conventional fields compared with those who worked in organic fields.231 Last, a publication from a prospective cohort study of school-age children from a tobacco-producing region in Brazil reported increased levels of microalbuminuria at the beginning of the pesticide application period compared with the leaf harvest period, suggesting that children environmentally exposed to xenobiotics in rural areas may suffer from early kidney dysfunction.52
Table 9.
Characteristics of Latin American and the Caribbean studies on pesticide exposure and other health effects published between 2007 and 2021 ().
| Study | Year of publication/country | Population and sample size | Study design | Pesticides assessed | Exposure assessment method | Pesticide or metabolite concentrations | Health effect and assessment method/instrument | Results |
|---|---|---|---|---|---|---|---|---|
| Kidney function | ||||||||
| 1. Sanoff et al.228 | 2010/Nicaragua | 124 renal insufficiency cases/873 controls | Case–control | Multiple pesticide classes | Questionnaire (occupational exposure history) | Not applicable | Renal insufficiency (defined by eGFR) | Null association between pesticide exposure and odds of renal insufficiency. |
| 2. Raines et al.230 | 2014/Nicaragua | 78 cases of reduced eGFR/ 205 controls from area with high prevalence of CKD | Cross-sectional with case–control analysis | Multiple pesticide classes | Questionnaire (occupational and residential exposure history) | Not applicable | eGFR | Null associations of lifetime days mixing/applying pesticides and lifetime days working in fields with pesticide use with reduced eGFR. Accidental pesticide inhalation (ever) was associated with reduced eGFR (; 95% CI: 1.0, 6.9). |
| 3. Vela et al.225 | 2014/El Salvador | 223 subjects ( years of age) from two farming communities | Cross-sectional | Multiple pesticide classes | Questionnaire (occupational exposure history) | Not applicable | CKD (defined by eGFR or markers for renal damage: hematuria, proteinuria with hematuria, microalbuminuria) | Similar prevalence of CKD between farmworkers and non-farmworkers (descriptive analyses only). |
| 4. Wesseling et al.226 | 2016/Nicaragua | 86 male sugarcane cutters/56 male construction workers/52 male small-scale farmers | Cross-sectional | Multiple pesticide classes | Questionnaire (occupational exposure history) | Not applicable | Kidney disfunction (defined by eGFR) | Null association between self-reported (ever) pesticide use and eGFR levels. |
| 5. Nascimento et al.52 | 2017/Brazila | 40 children (6–12 years of age) living in a tobacco-producing region | Prospective cohort | Multiple pesticide classes | Questionnaire (parental exposure history) Blood BChEb |
Not applicable | Kidney function: microalbuminuria, NAG activity Serum vitamin C Hematological parameters: hematocrit, Hb, WBC, RBC, platelets (all parameters measured at two different crop periods: in the beginning of pesticide applications and in the leaf harvest) |
Microalbuminuria levels were higher at the beginning of the application period than during leaf harvest period (). No difference in NAG activity between both periods. Neutrophils, monocytes (), and basophils () were lower, and Hb and lymphocytes were higher during the pesticide application period (). |
| 6. Smpokou et al.227 | 2019/Nicaragua | 57 adults from rural communities | Nested case–control (nested within a prospective cohort) | Herbicides, pyrethroids, OPs, fungicides | Questionnaire (occupational exposure history) Urinary metabolites of fungicides (ETU, OH-PYR, 5-OH-TBZ), OPs (TCPy), pyrethroids (DCCA, 3-PBA); herbicide 2,4-D (measured once or twice) |
Median (P25–P75) ( creatinine): 2,4-D: ; ; 3-PBA: ; DCCA: ; ; ; : ; ; ; TEB-OH: ; ; ; TCP: ; ; ; Glyphosate: ; ; ; 4F3PBA, CFCA, MCPA, OH-PYM, 5-OH-TBZ: ; ; ; |
Decline in kidney function (defined by eGFR; parameters estimated at two time points: baseline (before the harvest or visit 1) and 6 months later (at the end of the harvest or visit 2) | No differences in pesticide metabolite concentrations between those whose kidney function remained stable over the follow-up period and those whose kidney function declined. |
| 7. Ruiz-Alejos et al.224 | 2021/Peru | 1,1514 adults from urban and rural areas | Cross-sectional | Multiple pesticide classes | Questionnaire (environmental and occupational exposure history) | Not applicable | Impaired kidney function (defined by eGFR) | Null association between self-reported pesticide exposure and impaired kidney function. |
| 8. López-Gálvez et al.231 | 2021/Mexico | 101 migrant and seasonal farmworkers (who did not directly apply or mix pesticides)/50 nonagricultural office workers | Cross-sectional | Multiple pesticide classes | Questionnaire (residential and occupational exposure history) during preharvest and late harvest | Not applicable | eGFR | Farmworkers had lower eGFR levels than office workers. eGFR in farmworkers decreased from preharvest to late harvest (). Farmworkers who worked in conventional fields had lower eGFR levels than those who worked in organic fields (). |
| 9. Prudente et al.229 | 2021/Brazil | 208 farmworkers | Cross-sectional | Multiple pesticide classes | Questionnaire (occupational exposure history) Blood BChE |
Not applicable | eGFR | Null association between pesticide exposure and eGFR reduction. |
| Respiratory and allergic outcomes | ||||||||
| 10. Fieten et al.232 | 2009/Costa Rica | 69 indigenous women exposed to pesticides while working in plantain fields/58 indigenous women unexposed | Cross-sectional | OPs and paraquat | Questionnaire (occupational and residential exposure history) | Not applicable | Respiratory symptoms (European Community Respiratory Health Survey), spirometry (FVC, ) | Exposure to chlorpyrifos and terbufos was associated with increased risk of wheeze among nonsmokers (; 95% CI: 1.6, 28.0 and ; 95% CI: 1.4, 25.6, respectively). Exposure to chlorpyrifos was also associated with shortness of breath among nonsmokers (; 95% CI: 1.0, 7.3). Null associations of pesticide exposure with FVC and . |
| 11. Cupul-Uicab et al.237 | 2014/Mexico | 747 mother–children (12–30 months of age, boys only) pairs from an area where DDT was recently used | Prospective cohort | OCs | Questionnaire (residential and seasonal exposure at prenatal period) Maternal serum DDT and DDE at delivery |
Median (IQR) () ; |
LRTIs (physician-diagnosed between birth and 30 months of age and reported by mothers) | Null associations of prenatal DDT exposure with LRTIs in boys up to 30 months of age. |
| 12. Buralli et al.233 | 2018/Brazil | 48 farmworkers and 34 relatives residing in a rural area | Cross-sectional | Multiple pesticide classes | Questionnaire (residential, occupational, and intoxication history) Blood AChE and BChEb |
Not applicable | Respiratory symptoms (European Community Respiratory Health Survey), spirometry FVC, , ratio, ) (all parameters measured at two different crop season and off-season) | Crop season was associated with increased odds of waking up with a cough (; 95% CI: 1.2, 51.1), but not with other respiratory symptoms. Years of working with pesticides or rural work were associated with decreased FVC (; 95% CI: . , (; 95% CI: , ), and (; 95% CI: , ) during the crop season, but also during the off-season ( ; 95% CI: , ; ; 95% CI: , ; ; 95% CI: , ). |
| 13. Díaz-Criollo et al.234 | 2019/Colombia | 217 farmworkers | Cross-sectional | Multiple pesticide classes | Questionnaire (occupational and domestic exposure history) Job-exposure matrix (proxy of chronic exposure) Urinary paraquat |
Mean (range) paraquat (): ; ; | Self-reported respiratory symptoms (e.g., cough, wheezing), spirometry (FVC, , ratio) | Self-reported use of pesticide mixtures containing paraquat, profenofos, and methomyl was associated with increased prevalence of flu (; 95% CI: 1.6, 3.3), whereas use of pesticide mixtures containing paraquat, and profenofos, and methamidophos was associated with thoracic pain (; 95% CI: 1.7, 9.9). Exposure to pesticide mixtures containing paraquat and profenofos (; 95% CI: 1.1, 7.0) or paraquat and glyphosate (; 95% CI: 1.2, 9.8) was associated with increased prevalence of allergic rhinitis. Self-reported use of pesticide mixtures containing paraquat and methamidophos was associated with an obstructive pattern in spirometry (; 95% CI: 1.1, 17.5). Chronic paraquat exposure was associated with self-reported asthma (; 95% CI: 1.0, 1.1). |
| 14. Mora et al.236 | 2020/Costa Rica | 355 mother–child (1 year of age) pairs living near banana plantations aerially sprayed | Prospective cohort | Fungicides, OPs, pyrethroids, herbicides | Questionnaire Urinary metabolites of fungicides (, OH-PYR, 5-OH-TBZ), OPs (TCPy), pyrethroids (DCCA, 3-PBA); herbicide 2,4-D during each trimester of pregnancy |
Median (range) () (pregnancy average): ; ; ; ; ; ; |
LRTIs and wheeze (physician- or nurse-diagnosed in first year of life and reported by mothers at 11–19 months of age) | High () during the first half of pregnancy was associated with increased odds of LRTIs (; 95% CI: 1.0, 6.3), whereas high () during second half of pregnancy was associated with decreased odds of wheezing (; 95% CI: 0.3, 1.0). Null associations of other pesticide metabolites with LRTIs and wheeze. |
| 15. Alhanti et al.235 | 2021/Costa Rica | 266 women living near banana plantations aerially sprayed | Prospective cohort | Fungicides, OPs, pyrethroids, herbicides | Questionnaire (residential pesticide use) Urinary metabolites of fungicides (ETU, OH-PYR, 5-OH-TBZ), OPs (TCPy), pyrethroids (DCCA, 3-PBA); herbicide 2,4-D |
Min–max (): ; ; ; ; ; ; |
Self-reported respiratory outcomes: wheeze, doctor-diagnosed asthma, asthma score Allergic outcomes: rhinitis, eczema, itchy rash |
Current pesticide use in the home was associated with increased odds of diagnosed asthma (; 95% CI: 1.1, 3.9). Higher 5-OH-TBZ was associated with increased odds of a high asthma score (; 95% CI: 1.1, 3.3). Women who worked in agriculture had decreased odds of rhinitis (; 95% CI: 0, 0.9) but increased odds of eczema (; 95% CI: 1.3, 4.9) and itchy rash (; 95% CI: 1.2, 7.7). |
| 16. Rocha et al.317 | 2021/Brazil | 319 cases with uncontrolled asthma/319 controls (ages 6–7 and 13–14 years of age) | Case–control | Multiple pesticide classes | Questionnaire (residential pesticide exposure, exposure to pesticides from nearby agricultural spraying) | Not applicable | Uncontrolled asthma (ISAAC) | Living close to agricultural fields (; 95% CI: 1.5, 11.8), farmworkers in the household (; 95% CI: 2.1, 16.5), and aerial spraying close to the home (; 95% CI: 1.5, 11.9) were associated with increased odds of uncontrolled asthma. |
| Liver injury | ||||||||
| 17. Cecchi et al.206 | 2012/Argentinac | 97 pregnant women living in a rural area with intensive use of pesticides | Prospective cohort | OPs | Questionnaire (residential exposure history) Blood AChE, BChE, b |
Not applicable | Liver enzymes: AST, ALT Other biochemical parameters: albumin, glucose (all parameters were measured during spraying and prespraying season) |
AST levels, AST/ALT ratio, and albumin levels increased by 17% (), 21% (), and 8% (), respectively, during the spraying period compared with the prespraying period. No differences in ALT or glucose levels between study periods. |
| 18. Bahia et al.240 | 2014/Brazil | 354 general population living near OC factory (45 high/103 moderate/206 low exposure) | Cross-sectional | OCs | Questionnaire (residential, occupational, and dietary exposure history) | Not applicable | Liver enzymes: AST, ALT, GGT, alkaline phosphatase Other biochemical parameters: albumin |
Null associations between pesticide exposure and liver function markers. |
| 19. Lermen et al.238 | 2018/Brazil | 73 orange grower farmworkers/30 controls | Cross-sectional | Multiple pesticide classes | Questionnaire (occupational exposure history) Blood BChEb |
Not applicable | Liver enzymes: ALT, AST, GGT Hematological parameters: hematocrit, Hb, WBC, RBC, platelets Other biochemical parameters: creatinine, urea Self-reported symptoms |
ALT (), AST (), and creatinine () were higher in farmworkers than in controls, but only among females. No differences in GGT, urea, and hematological parameters between farmworkers and controls. |
| 20. Ruiz-Arias et al.242 | 2018/Mexico | 55 applicators (high exposure))/119 occasional applicators (moderate exposure)/46 controls | Cross-sectional | OPs, carbamates, pyrethroids | Questionnaire (occupational exposure history) Urinary DAPsb Blood AChE, BChEb Blood |
Geometric mean (95% CI) total DAPs (): ; ; Geometric mean: (): ; ; |
Liver enzymes: AST, ALT, GGT Hematological parameters: hematocrit, Hb Lipid profile: cholesterol, triglycerides, LDL, VLDL, HDL Other biochemical parameters: glucose, albumin |
Higher activity was associated with increased AST, ALT, and GGT levels (). Higher activity was also associated with increased glucose, total lipids, triglycerides, cholesterol, atherogenic index, VLDL, hematocrit, and hemoglobin levels () and with lower HDL levels (). |
| 21. Cattelan et al.88 | 2018/Brazila | 84 farmworkers who used pesticides/68 farmworkers who did not use pesticides | Cross-sectional | Multiple pesticide classes | Questionnaire (occupational exposure history) | Not applicable | Liver enzymes: AST, ALT, GGT, alkaline phosphatase Lipid profile: total cholesterol, HDL, LDL, triglycerides Other biochemical parameters: glucose, albumin, creatinine, total proteins, urea, uric acid |
Lower alkaline phosphatase, total cholesterol, albumin, leucocytes, platelets, and monocytes among farmworkers who had used pesticides compared with those who had not ( for each). |
| 22. Arévalo-Jaramillo et al.81 | 2019/Ecuadora | 62 women living in 2 separate agricultural communities/53 controls (living in a commercial city) | Cross-sectional | Multiple pesticide classes | Questionnaire | Not applicable | Liver enzymes: AST, ALT, GGT Hematological parameters: hematocrit, Hb, WBC, RBC, platelets Lipid profile: cholesterol, triglycerides, HDL, LDL Other biochemical parameters: glucose, urea, creatinine |
Higher percentage of ALT () and AST values () exceeding normal levels in women from the first agricultural community compared with controls. Higher percentage of GGT values exceeding normal levels () in women from the second agricultural community compared with controls. Higher hemoglobin and hematocrit levels, but lower platelet count and cholesterol levels, in exposed women than in controls (). |
| 23. Cestonaro et al.241 | 2020/Brazil | 62 farmworkers/54 unexposed controls | Cross-sectional | OPs, CBs | Questionnaire (occupational exposure history) Blood AChE, BChE |
Not applicable | Liver enzymes: ALT, AST, GGT Hematological parameters: RBC, hematocrit, leukocytes, number and percentage of neutrophils, lymphocytes, eosinophils, monocytes, basophils, platelets Other biochemical parameters: glucose, total bilirubin, direct bilirubin, urea, creatinine, high sensitivity C-reactive protein, total proteins Immunological parameters: complement C3, complement C4, IgA, IgM, IgG Adhesion molecules: percentage of LFA-1, ICAM-1, and L-selectin surface protein expression in lymphocytes and monocytes |
Farmworkers had lower AST and ALT levels, but higher glucose, urea, total protein, IgM, and C3 levels, than controls (). Farmworkers had a higher number of neutrophils and a higher mean platelet volume but lower numbers of lymphocytes, monocytes, and platelets than controls (). Farmworkers showed a decrease in monocyte and an increase in lymphocyte expression for both LFA-1 and ICAM-1 compared with controls. AChE activity was negatively correlated with glucose levels (; ), whereas BChE activity was negatively correlated with IgG levels (; ). Null associations of AChE and BChE activities with liver enzymes. LFA-1 and ICAM-1 surface protein expression in lymphocytes was positively associated with exposure time to pesticides in years ( for both; ), whereas LFA-1 and ICAM-1 surface protein expression in monocytes was inversely associated with exposure time to pesticides ( and , respectively; ). |
| 24. Bernieri et al.239 | 2021/Brazil | 50 soybean farmworkers/63 controls from 2 different areas | Cross-sectional | Multiple pesticide classes | Questionnaire (occupational exposure history) Blood BChEb |
Not applicable | Liver enzymes: AST, ALT, GGT Other biochemical parameters: urea, creatinine Antioxidant capacity (FRAP) (measured during the high and low pesticide exposure periods) |
Farmworkers had higher urea and creatinine levels, but lower ALT levels, than controls (). Farmworkers had higher AST, urea, and creatinine levels, as well as higher blood plasma antioxidant potential values (), during the high pesticide exposure period compared with the low exposure period. |
| Hematological parameters and lipid profiles | ||||||||
| 25. Remor et al.94 | 2009/Brazila | 37 farmworkers/20 controls | Cross-sectional | Multiple pesticide classes | Questionnaire (occupational exposure history) Blood BChE, ALA-Db |
Not applicable | Hematological parameters: hematocrit, Hb, WBC, RBC, platelets Lipid profile: total cholesterol, LDL, triglycerides, HDL |
No differences in hematological parameters or lipid profiles between groups. |
| 26. Maluf et al.245 | 2009/Brazil, Argentina, Mexico | 173 adults with aplastic anemia/692 healthy controls | Case–control | Multiple pesticide classes | Questionnaire (occupational or domestic exposure history to pesticides and other chemical products) | Not applicable | Aplastic anemia (AA) and agranulocytosis by medical diagnosis | Exposure to pesticides was associated with increased odds of AA (; 95% CI: 1.1, 4.7). High frequency of exposure ( times/y) to OP pesticides (; 95% CI: 0.9, 10.1) and pyrethroids (; 95% CI: 1.0, 3.1) was also associated with increased odds of AA. |
| 27. Payán-Rentería et al.96 | 2012/Mexicoa | 25 farmworkers/21 controls | Cross-sectional | OCs, OPs, TRZ | Medical examination Questionnaire (occupational exposure history) Blood AChEb |
Not applicable | Hematological parameters: hematocrit, Hb, WBC, RBC, platelets Lipid profile: LDL, HDL, total cholesterol, triglycerides Other biochemical parameters: albumin and globulin levels, albumin/globulin relation, total bilirubin, direct bilirubin, and indirect bilirubin Self-reported fertility problems, birth defects, and cancer |
Higher hemoglobin () and hematocrit () levels in farmworkers than in controls. No differences in other hematological or biochemistry outcomes between farmworkers and controls. Farmworkers reported more fertility problems, birth defects, and cancer in themselves or their family members than controls. |
| 28. Adad et al.98 | 2015/Brazila | 80 male farmworkers from state association/20 male farmworkers from a private company/100 matched controls | Cross-sectional | Multiple pesticide classes | Questionnaire (occupational exposure history) Blood BChEb |
Not applicable | Hematological parameters: hematocrit, Hb, WBC, RBC, platelets Lipid profile: LDL, HDL, triglyceride |
No differences in hematological parameters or lipid profiles between groups. |
| 29. Alves et al.73 | 2016/Brazila | 77 tobacco farmworkers/60 controls | Cross-sectional | Multiple pesticide classes | Questionnaire (occupational exposure history) | Not applicable | Hematological parameters: hematocrit, Hb, WBC, RBC, platelets | Lower mean levels of band neutrophils () and monocytes () in exposed group compared with control group. All other hematological parameters were similar between groups. |
| 30. Quintana et al.54 | 2017/Argentinaa,d,e | 151 mother–newborn pairs living in a rural area/38 mother–newborn pairs from an urban area | Cross-sectional | OPs | Questionnaire (residential and seasonal exposure history) Cord blood AChE, BChEb |
Not applicable | Hematological parameters: hematocrit, Hb, WBC, RBC, platelets, osmotic fragility | No differences in cord blood hematological parameters between groups. |
| 31. Siller-López et al.251 | 2017/Colombia | 205 coffee harvesters | Cross-sectional | OPs | Questionnaire (occupational exposure history) Blood BChE |
Not applicable | Lipid profile: cholesterol and triglycerides Atherosclerotic cardiovascular disease (ASCVD) Hypertension |
Association between BChE activity and hypercholesterolemia (total cholesterol ) (; ). Null associations of BChE activity with ASCVD and hypertension. Higher prevalence of hypertension was associated with Q allele carriers of PON1192 polymorphism in both dominant model () (; 95% CI: 1.03, 3.4) and recessive model () (; 95% CI: 1.1, 7.4). |
| 32. Cortés-Iza et al.248 | 2017/Colombia | 92 farmworkers | Cross-sectional | OPs, CBs | Questionnaire (occupational exposure history) Blood AChE, BChEb |
Not applicable | Hematological parameters: hematocrit, Hb, WBC, RBC, platelets, MCV, MCH, MCHC, RDW | Leucocytes and monocytes below the reference range in farmworkers exposed to pesticides for 6–9 h and farmworkers who handled pesticides for 1–10 y. MCH above the reference range and RDW below the reference range in farmworkers exposed to pesticides for y. |
| 33. Piccoli et al.249 | 2019/Brazil | 275 farmworkers and their families | Cross-sectional | OCs | Questionnaire (occupational exposure history) Serum HCH, HCB, chlordane, trans-nonachlor, heptachlor, DDT, DDE, endosulfan, aldrin, endrin, dieldrin, methoxychlor, mirex, and pentachloroanisole |
Median (P25–P75) ( lipid): ; ; ; ; ; heptachlor epoxide ; heptachlor epoxide ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; | Hematological parameters: hematocrit, Hb, WBC, RBC, platelets, MCV, MCH, MCHC, RDW | Detectable concentrations of (; 95% CI: 0.8, 0.9), heptachlor (; 95% CI: 0.8, 0.9), -DDT (; 95% CI: 0.8, 1.0), -DDE (; 95% CI: 0.7, 1.0), and -DDE (; 95% CI: 0.8, 1.0) were associated with decreased monocytes. Detectable concentrations of (; 95% CI: 0.7, 1.0), heptachlor (; 95% CI: 0.6, 0.9), trans-nonachlor (; 95% CI: 0.3, 0.9), -DDD (; 95% CI: 0.4, 1.0), -DDD (; 95% CI: 0.6, 0.9), endrin (; 95% CI: 0.6, 0.9), endosulfan 1 (; 95% CI: 0.6, 0.9), and methoxychlor (; 95% CI: 0.5, 1.0) were associated with decreased eosinophils. Detectable concentrations of were associated with decreased hemoglobin levels (; 95% CI: , 0). |
| 34. Dabló et al.247 | 2019/Brazil | 142 farmworkers | Cross-sectional | Multiple pesticides | Questionnaire (occupational exposure history) | Not applicable | Hematological parameters: RBC, hemoglobin, hematocrit, MCV, MCH, MCHC | Lower leukocytes and platelets ( for each), but higher percentage band neutrophils () and percentage neutrophiles () during the pesticide application period compared with the harvest period (period without pesticide exposure). |
| 35. Molina-Pintor et al.266 | 2020/Mexico | 60 sprayers with motor pump (high exposure)/126 solid pesticides sprayers (moderate exposure)/22 controls | Cross-sectional | OPs | Questionnaire (occupational exposure history) Blood BChE Urinary DAPsb |
Mean (range) total DAPs (): Control group: 33.5 (24.6–41.3); moderate-exposure group: 58.5 (24.5–353.3); high-exposure group: 122.5 (25.6–488.4) |
Lipid profile: cholesterol, LDL, VLDL, triglycerides, atherogenic index Other biochemical parameters: glucose, albumin, creatinine |
Lower LDL, cholesterol, and albumin levels among highly exposed group compared with moderately exposed and control groups (). Positive correlations of BChE activity with triglycerides, VLDL, and total lipids among normal-weight individuals. Positive correlations of BChE activity with glucose, cholesterol, atherogenic index, triglycerides, LDL, VLDL, and total lipids among overweight individuals. |
| 36. Jacobsen-Pereira et al.246 | 2020/Brazil | 43 farmworkers/30 unexposed controls | Cross-sectional | Multiple pesticides | Questionnaire (occupational exposure history) | Not applicable | Cellular immune profile: total leukocytes, neutrophils, monocytes, basophils, eosinophils, lymphocytes, cytokines | Higher classical monocytes (), dendritic cells (), total T cells (), central memory CD8 T cells (), effector memory CD8 T cells (), and pro-inflammatory IL-6 () in farmworkers than in controls. Lower total B cells (), regulatory B cells () and plasmablasts () in farmworkers than in controls. |
| Acoustic damage | ||||||||
| 37. Guida et al.252 | 2010/Brazil | 48 male workers exposed to malathion and noise/36 male workers exposed to noise | Cross-sectional | OPs (malathion) | Questionnaire (occupational exposure history) | Not applicable | Hearing loss: DPOAE | Worse performance in workers exposed to noise and malathion at the frequencies of 3 kHz on the left ear (), and 4 kHz on both ears (left ear ; right ear ) than in workers exposed only to noise. |
| 38. Bazilio et al.253 | 2012/Brazil | 33 farmworkers | Cross-sectional | Multiple pesticide classes | Questionnaire (occupational exposure history) | Not applicable | Meatoscopy, audiometry, DPT, GIN (500 Hz and 1 and 2 kHz) | Higher pesticide exposure indices were associated with increased risk of worse performance in DPT for right ear (; 95% CI: 1.1, 3.7) and left ear (; 95% CI: 1.2, 3.1), and in GIN for right ear (; 95% CI: 1.2, 3.4) and left ear (; 95% CI: 0.9, 1.5). |
| 39. Alcarás et al.254 | 2013/Brazil | 25 farmworkers exposed to malathion and noise with normal hearing/30 controls | Cross-sectional | OPs (malathion) | Questionnaire (occupational exposure history) | Not applicable | Hearing loss: TEOAE, DPOAE | For right ear, farmworkers had lower level of response for TEOAE at 1 and 1.5 kHz (), and for DPOAE at 6,000 () and 7,069 Hz () but higher at 750 () and 984 Hz (). For left ear, farmworkers had lower level of response for TEOAE at 1 (), 1 kHz, and total (), and for DPOAE at 3,984, 6,000, and 7,069 Hz () but higher at 750 () and 984 Hz (). |
| 40. de Sena et al.255 | 2013/Brazil | 235 farmworkers/116 controls | Cross-sectional | Multiple pesticide classes | Questionnaire (current or past occupational exposure history) | Not applicable | Hearing loss: airborne tonal thresholds Quality of life: SF-36 Self-reported health status |
Farmworkers had lower scores for physical outcomes and general health status and poorer mental health and emotional regulation than controls ( for each). |
| 41. Garcia et al.256 | 2017/Brazil | 205 students living in an agricultural area | Cross-sectional | Multiple pesticide classes | Questionnaire (residential and occupational exposure history) | Not applicable | Hearing loss: TEOAE and DPOAE | Students in the highest quartile of pesticide exposure had increased odds of failing TEOAE test (; 95% CI: 1.4, 9.9) and showing alterations in cochlear function assessed by DPOAE (; 95% CI: 1.2, 7.2) compared with students in the lowest quartile. |
| 42. Tomiazzi et al.257 | 2019/Brazil | 30 smokers/30 subjects exposed to pesticides for at least 1 y/30 farmers exposed to pesticides who were smokers/37 controls not exposed to pesticides and who never smoked | Cross-sectional | Multiple pesticide classes | Questionnaire (occupational exposure history) | Not applicable | Meatoscopy, pure-tone audiometry (250–8,000 kHz), vocal audiometry (SRT and SRPI), immittance testing | Increased incidence of hearing threshold loss at high frequency and of downward sloping audiometric curve configuration and alteration of stapedial reflex in groups exposed to pesticides compared with controls (). |
| 43. Mattiazzi et al.259 | 2019/Brazil | 71 rural workers exposed to pesticides | Cross-sectional | OPs, CBs | Questionnaire (occupational exposure history) Blood AChE (measured in a subset of 50 workers) |
Not applicable | Hearing loss: air conduction auditory thresholds | Null association between AChE activity and hearing loss. |
| 44. de Souza Alcarás et al.258 | 2021/Brazil | 38 male endemic disease control agents/18 male workers without occupational pesticide exposure | Cross-sectional | Multiple pesticide classes | Questionnaire (occupational exposure history) | Not applicable | Central auditory function: inspection of external acoustic meatus, pure-tone audiometry, acoustic immittance, BAEP, dichotic digits test, TEOAE | Increase in waves III and V absolute latencies, and I–III and I–V interpeak latencies in exposed workers compared with controls with auditory thresholds up to 25 dB HL at the frequencies from 2,000 to 4,000 Hz. Mean dichotic digits test performance was worse in exposed workers than in controls. No differences between groups in the TEOAE test. |
| Other outcomes | ||||||||
| 45. de Souza et al.276 | 2011/Brazil | 298 rural agriculture or livestock workers from three rural counties | Cross-sectional | Multiple pesticide classes | Questionnaire (occupational, para-occupational, and residential exposure history) | Not applicable | Chronic diseases: self-reported symptoms and illness | Pesticide exposure was associated with the report of several diseases, with neurological (; 95% IC: 1.8, 5.4) and oral diseases (; 95% IC: 1.4, 1.6) being the most prevalent. |
| 46. Suarez-Lopez et al.260 | 2013/Ecuador | 138 children (4–9 years of age) living with flower plantation workers/133 living with no agricultural workers | Cross-sectional | OPs, CBs | Questionnaire (residential and parental exposure history) Blood AChE |
Not applicable | Resting heart rate, blood pressure (SBP and DBP) | Decrease in AChE activity was associated with a decrease in SBP (; 95% CI: , ) and in DBP (; 95% CI: , ). Children living with flower workers had lower SBP (; 95% CI: , 0.1). Null associations were found between exposures and heart rate. |
| 47. Saunders et al.318 | 2014/Guadeloupe | 779 pregnant women | Prospective cohort | OCs | Maternal plasma chlordecone at delivery | Data not shown | Gestational diabetes, gestational hypertension, preeclampsia | Higher chlordecone concentrations were associated with decreased risk of gestational hypertension (OR per 10-fold increase in concentrations ; 95% CI: 0.2, 0.6). Null associations of chlordecone exposure with risk of preeclampsia or gestational diabetes. |
| 48. Butinof et al.267 | 2015/Argentina | 880 farmworkers handling pesticides | Cross-sectional | Multiple pesticide classes | Questionnaire (occupational exposure history) | Not applicable | Health status by perception of symptoms as irritation (i.e., skin and eye irritation, nausea, vomiting, respiratory disorders), fatigue/tiredness, headache, nervousness or depression, medical consultation, and hospitalizations | Farmworkers who had worked for y or had mixed/applied pesticides had increased odds of irritation (; 95% CI: 1.1, 2.6; ; 95% CI: 1.04, 2.4, respectively). Adequate use of PPE was associated with decreased odds of irritation (; 95% CI: 0.4, 0.9). Use of endosulfan was associated with increased odds of hospitalization (; 95% CI: 0.9, 8.1). |
| 49. Cezar-Vaz et al.268 | 2016/Brazil | 331 workers from two rural municipalities | Cross-sectional | Multiple pesticide classes | Questionnaire (residential and occupational exposure history) | Not applicable | Health status related to work: mental and nervous system, respiratory, gastric, circulatory, and dermatological outcomes | Rural workers who used pesticides had a 90% higher prevalence of dermatologic disorders than those who did not (; 95% CI: 1.4, 2.6). No differences in prevalence of mental and nervous system, respiratory, gastric, and circulatory diseases between rural workers who used and did not use pesticides. |
| 50. Muñoz-Quezada et al.277 | 2017/Chile | 114 farmworkers/93 controls | Cross-sectional | OPs | Questionnaire (occupational and residential exposure history) | Not applicable | Health status: self-reported skin allergies, anemia, asthma, cancer, liver damage, depression, diabetes mellitus, epilepsy, hypertension, kidney failure, heart problems, anxiety, symptoms of recent OP pesticide poisoning, and hospitalization for pesticide poisoning | An increasing number of years of OP pesticide use was associated with increased odds of symptoms of recent OP pesticide poisoning (; 95% CI: 1.0, 1.1), but not with other health problems. |
| 51. Cupul-Uicab et al.263 | 2017/Mexico | 448 urban newborn boys/299 rural newborn boys | Cohort | OCs | Questionnaire (residential exposure history) Maternal serum DDE and DDT at birth |
Median (IQR) (): Urban residents: ; Rural residents: ; |
Gastrointestinal infection: diarrhea | Among boys living in the urban area, those with the highest DDE ( serum lipid) had higher incidence rate of diarrhea than those in the lowest category ( serum lipid) (; 95% CI: 1.1, 1.8). Among boys from a rural area, higher DDE and DDT were not associated with a higher incidence rate of diarrhea. |
| 52. Silvério et al.57 | 2017/Brazila | 94 farmworkers exposed to pesticides including OPs/94 farmworkers exposed to pesticides not including OPs/50 controls | Cross-sectional | OPs | Questionnaire (occupational exposure history) Urinary DAPsb Blood AchE, BchEb |
(): Occupationally exposed to complex mixtures with OPs: ; ; occupationally exposed to complex mixtures without OPs: ; ; control group: DETP ; |
Clinical changes in systems: cardiovascular, CNS, digestive, respiratory, auditory, urinary, and skin and mucous membranes. | High prevalence of changes in CNS, respiratory, and auditory systems in both exposed groups compared with controls (). |
| 53. Suarez-Lopez et al.261 | 2018/Ecuador | 310 children (4–9 years of age) living near flower plantations | Cross-sectional | Multiple pesticide classes | Questionnaire (parental and residential exposure histories) Tertiles of residential distance to the nearest plantation Blood AchEb |
Not applicable | Blood pressure (SBP and DBP) | For every greater proximity to a plantation, SBP increased by (95% CI: 0.5, 5.8), and SBP -score increased by 0.3 SD (95% CI: 0.1, 0.5). For every of flower plantation areas, SBP increased by 0.4 mmHg (95% CI: 0.03, 0.7) and SBP -score increased by 0.03 (95% CI: 0.00, 0.06). |
| 54. Mejia-Sanchez et al.265 | 2018/Mexico | 169 floriculturists/96 controls | Cross-sectional | Multiple pesticide classes | Questionnaire (occupational exposure history) | Not applicable | Blood GST and GSTT1 activities | Higher total GST and GSTT1 enzymatic activity in exposed floriculturists than controls (). Significant difference in total GST, but not GSTT1 activity, between those who applied pesticides in the past 0–10 d and those who applied pesticides in the past 11–20 d (). |
| 55. Machado et al.266 | 2018/Brazil | 1,421 subjects living in rural areas | Cross-sectional | Multiple pesticide classes | Questionnaire (residential exposure history) | Not applicable | Sleep problems (MSQ score) | Having had a pesticide intoxication, but not living in a rural area, was associated with more sleep problems (; 95% CI: 1.1, 4.1). |
| 56. Barrón Cuenca et al.223 | 2019/Bolivia | 297 men and women living in three agricultural communities | Cross-sectional | Fungicides, OPs, pyrethroids, herbicides | Questionnaire (occupational history) Urinary metabolites of tebuconazole, chlorpyrifos, pyrethroids, 2,4-D, MCPA, thiabendazole, pyrimethanilb |
Mean (range) () ; ; ; ; ; ; ; ; ; |
Self-reported symptoms of pesticide poisoning (headache, dizziness, fatigue), respiratory outcomes (dyspnea, cough), muscular outcomes (cramps, fasciculation), digestive outcomes (abdominal pain, nausea, vomiting, red skin, itchy skin, eyes burning, red eyes), reproductive outcomes (miscarriages, stillbirths, congenital malformations) | No differences in self-reported miscarriages, congenital malformations, and stillbirths between female farmers and nonfarmers. No report on differences in other symptoms between farmers and nonfarmers. |
| 57. Suarez-Lopez et al.262 | 2019/Ecuador | 310 children (4–9 years of age) living near flower plantations | Cross-sectional | OPs, CBs | Questionnaire (parental and residential exposure histories) Blood AchEb |
Not applicable | Blood pressure (SBP and DBP), resting heart rate | Inverse relationship of time after the spray season with percentiles of SBP ( per 10.9 d after the harvest ; 95% CI: , ) and DBP (; 95% CI: , ). For every 10.9 d that a child was examined sooner after the harvest, the OR of elevated BP/hypertension doubled (; 95% CI: 1.3, 3.1). |
| 58. Butinof et al.62 | 2019/Argentinaa | 47 pesticide applicators/52 unexposed controls | Cross-sectional | OPs, CBs | Questionnaire (occupational exposure history) Blood AchEb |
Not applicable | Health status by perception of general, neurological, cardiorespiratory, dermatological, gastric, ocular, and urinary symptoms | Higher presence of general, cardiorespiratory, and dermatological symptoms among pesticide applicators compared with controls ( for each). |
| 59. Buralli et al.138 | 2020/Brazilf | 42 pesticide applicators/36 farmworkers who did not apply pesticides | Cross-sectional | OPs | Questionnaire (occupational exposure history) Blood AChE, BChEb |
Not applicable | Self-reported symptoms of pesticide poisoning | Farmworkers who did not spray pesticides had a higher prevalence of headache (; 95% CI: 1.1, 4.0), dyspnea (; 95% CI: 1.5, 9.5), wheezing (; 95% CI: 2.4, 108.8), cough (; 95% CI: 1.1, 6.5), and poor digestion (; 95% CI: 1.17, 52.89) compared with pesticide applicators. |
| 60. Okuyama et al.274 | 2020/Brazil | 3,826 patients with pesticide poisoning | Case–control | Multiple pesticide classes | Pesticide poisoning reported to surveillance system | Not applicable | Death by pesticide poisoning | Farmworkers had increased odds of death from pesticide poisoning (; 95% CI: 1.2, 4.2) compared with those not working in the agricultural sector. Those poisoned by pesticides classified as having “extreme” toxicity had increased odds of death compared with those poisoned by pesticides categorized as having “high/moderate/low” toxicity (; 95% CI: 1.8, 4.2). |
| 61. Schneider Medeiros et al.273 | 2020/Brazil | 150 patients with idiopathic PD | Prospective cohort | Multiple pesticide classes | Questionnaire (occupational exposure history) | Not applicable | Mortality risk from PD | Exposed patients with PD had increased risk of death compared with unexposed patients with PD (; 95% CI: 1.2, 4.7). Occupational pesticide exposure was associated with elevated mortality rate (; 95% CI: 1.1, 4.6). Evidence of dose-dependent relationship between occupational pesticide exposure and mortality rate; patients with y of occupational pesticide exposure had significantly elevated mortality rate compared with patients with y of exposure (; 95% CI: 1.2, 6.7). |
| 62. de Carvalho et al.269 | 2020/Brazil | 2,649 tobacco farmworkers | Cross-sectional | Multiple pesticide classes | Questionnaire (occupational exposure history) | Not applicable | Thoracic spine pain (NQMS) | Lifetime history of pesticide poisoning was associated with increased prevalence of thoracic spine pain in the previous year among females (; 95% CI: 1.3, 2.5). Effect of pesticide poisoning on prevalence of thoracic spine pain was not reported among men. |
| 63. Fassa et al.270 | 2020/Brazil | 2,649 tobacco farmworkers | Cross-sectional | Multiple pesticide classes | Questionnaire (occupational exposure history) | Not applicable | Neck pain (NQMS) | Pesticide poisoning in the past year was associated with increased prevalence of neck pain among males (; 95% CI: 1.1, 14.1), but not females. |
| 64. Campos et al.272 | 2020/Brazil | 354 small tobacco farmers | Cross-sectional | Multiple pesticide classes | Questionnaire (occupational exposure history) | Not applicable | Green tobacco sickness (defined as urinary cotinine levels , contact with tobacco leaves in up to 48 h before sample collection and report of headache, dizziness, nausea, vomiting, weakness, or bellyache) | Farmers with current exposure to pesticides had increased odds of green tobacco sickness compared with those without current exposure to pesticides (; 95% CI: 1.4, 9.3). |
| 65. Luce et al.275 | 2020/Guadeloupe | 11,112 farm owners and farmworkers who worked on banana plantations between 1973 and 1993 | Retrospective cohort | Multiple pesticide classes | Agricultural census | Not applicable | All-cause mortality, all-cancer mortality, cancer-specific mortality | Lower all-cause mortality in male (; 95% CI: 0.9, 1.0), but not female (; 95% CI: 0.9, 1.0), farm owners and farmworkers compared with the general population. All-cancer mortality did not differ from that of the general population. Excess of deaths from stomach (; 95% CI: 1.3, 4.5) and pancreatic (; 95% CI 1.1, 4.4) cancer in female, but not male (; 95% CI: 0.7, 1.3 and ; 95% CI: 0.5, 1.2, respectively) farm owners. |
| 66. Lovison Sasso et al.102 | 2021/Brazila | 50 male exposed farmworkers/50 unexposed male controls | Cross-sectional | Multiple pesticide classes | Questionnaire (occupational history) Blood BChEb |
Not applicable | Inflammatory response (IL-6, ), chemoattractant (IL-8), and anti-inflammatory (IL-10) interleukins | The exposed group had higher IL-8 and IL-10 concentrations than the control group. |
| 67. Hutter et al.319 | 2021/Ecuador | 34 male farmworkers engaged in conventional farming/37 male unexposed controls engaged in ecological farming from 5 different communities | Cross-sectional | Multiple pesticide classes | Questionnaire (occupational exposure history) | Not applicable | Self-reported conditions: headache, vision problems, dizziness, nausea, vomiting, excess salivation, strong fatigue, exhaustion, stomach pain, diarrhea, sleeplessness, burning eyes, skin irritation, runny nose, breathing difficulties, irregular heartbeat, watering eyes, skin rash, cough, twitches/trembling | Compared with controls working in ecological farms, participants exposed to pesticides in conventional farms had increased odds of dizziness (; 95% CI: 1.6, 14.9), nausea/vomiting (; 95% CI: 1.8, 31.8), strong fatigue (; 95% CI: 1.7, 14.9), diarrhea (; 95% CI: 1.1, 39.0), sleeplessness (; 95% CI: 1.2, 9.9), burning eyes (; 95% CI: 1.4, 12.3), skin irritation (; 95% CI: 1.1, 11.7), and irregular heartbeat (; 95% CI: 1.1, 30.7). |
| 68. Fillippi et al.103 | 2021/Argentinaa | 47 pesticide applicators/53 unexposed controls | Cross-sectional | Multiple pesticide classes | Questionnaire (occupational exposure history) Blood HCB, HCH, endosulfan, DDE, DDT, AChE, BChEb |
Not applicable | Perceived health symptoms (general, dermatological, neurological, ocular, cardiorespiratory, urinary) | Higher prevalence of perceived general (), dermatological (), neurological (), and cardiorespiratory () health symptoms among pesticide applicators compared with controls. |
| 69. de Araújo et al.271 | 2021/Brazil | 122 farmworkers and their family members | Cross-sectional | Multiple pesticide classes | Questionnaire (occupational exposure history) | Not applicable | Height, weight, waist circumference Overweight (BMI ) Abdominal obesity (waist circumference in women and in men) |
Long-term use of insecticides in general ( y) (; 95% CI: 1.0, 2.1), particularly OP pesticides (; 95% CI: 1.0, 2.1), was associated with a higher prevalence of overweight but not of abdominal obesity. |
| 70. Ayhan et al.189 | 2021/Guadeloupec,g | 285 mother–child (7 years of age) pairs | Prospective cohort | OCs | Cord and child blood chlordecone, cord blood DDE | Median (P25–P75) (): Cord blood chlordecone: , ; child chlordecone: , ; cord blood DDE: , |
Serum IGF-1, adiponectin, leptin | Null associations of cord blood chlordecone with IGF-1, adiponectin, and leptin. No report on associations of child chlordecone and cord blood DDE with these hormones. |
Note: 2,4-D, 2,4-dichlorophenoxy acetic acid; 3-PBA, 3-phenoxybenzoic acid; 4F3BPA, 4-fluoro-3-phenoxybenzoic acid; 5-OH-TBZ, 5-hydroxythiabendazole; AChE, acetylcholinesterase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BAEP, brainstem auditory evoked potentials; BMI, body mass index; BChE, butyrylcholinesterase; BP, blood pressure; CB, carbamate; CFCA, chloro-3,3,3-trifluoro-1-propen-1-yl]-2,2-dimethylcyclopropanecarboxylic acid; CI, confidence interval; CKD, chronic kidney disease; CNS, central nervous system; DAP, dialkyl phosphate; DBP, diastolic blood pressure; DCCA, 3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylic acid; DDE, dichlorodiphenyldichloroethylene; DDT, dichlorodiphenyltrichloroethane; DETP, diethylthiophosphate; DPOAE, Distortion Product Otoacoustic Emissions; DPT, Duration Pattern test; eGFR, estimated glomerular filtration rate; ETU, ethylenethiourea; , forced expiratory flow between 25% and 75%; , forced expiratory volume in the first second; FRAP, ferric-reducing ability of plasma; FVC, forced vital capacity; GGT, gamma glutamyl transpeptidase; GIN, Gaps-in-Noise test; GST, glutathione -transferases; GSTT1, glutathione -transferases theta 1; Hb, hemoglobin; HCB, hexa-chlorobenzene; HCH, hexa-chlorocyclohexane; HDL, high-density lipoprotein; HL, hearing loss; HR, hazard ratio; Hz, Hertz; ICAM-1, Intercellular Adhesion Molecule 1; Ig, immunoglobulin; IGF-1, insulin growth factor-1; IL, interleukin; IQR, interquartile range; IRR, incidence rate ratios; ISAAC, International Study of Asthma and Allergies in Childhood; LDL, low-density lipoproteins; LFA-1, Lymphocyte Function-Associated Antigen 1; LOD, limit of detection; LRTI, lower respiratory tract infection; max, maximum; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; MCPA, 4-chloro-2-methylphenoxy acetic acid; MCV, mean corpuscular volume; mHA, methylhippuric; min, minimum; MSQ, Mini Sleep Questionnaire; NAG, ; NQMS, Nordic Questionnaire for Musculoskeletal Symptoms; OC, organochlorine; OP, organophosphate; OR, odds ratio; OH-PYR, hydroxypyrimethanil; PD, Parkinson’s disease; PON1, paraoxonase 1; PPE, personal protective equipment; PR, prevalence ratio; RDW, red cell distribution width; RBC, red blood cells; Ref, reference group; SBP, systolic blood pressure; SD, standard deviation; SF, short format; SMR, standardized mortality ratio; SPG, ; SRPI, speech recognition percentage index; SRT, speech reception threshold; TCPy, 3,5,6-trichloro-2-pyridinol; TEOAE, transient evoked otoacoustic emissions; , tumor necrosis factor-alpha; TRZ, atrazine; VENG, vector electronystagmography; VLDL, very low-density lipoproteins; WBC, white blood cells.
Also included in Table 2 (genotoxicity).
Investigators did not use exposure biomarker concentrations in multivariate analyses.
Also included in Table 7 (reproductive outcomes).
Also included in Table 4 (placental outcomes and teratogenicity).
Also included in Table 8 (birth outcomes and child growth).
Also included in Table 3 (neurobehavioral outcomes).
Also included in Table 6 (thyroid function).
Respiratory and allergic outcomes.
Seven publications from three cross-sectional studies, two prospective cohort studies, and one case–control study reported on the associations of pesticide exposure with respiratory and allergic outcomes (Table 9). Publications from all three cross-sectional studies focused on occupational exposure to pesticides,232–234 but only one examined exposure–outcome associations using direct methods of pesticide exposure assessment.234 One of the publications reported increased odds of wheeze and shortness of breath among Costa Rican female farmworkers exposed to chlorpyrifos and terbufos compared with the control group (organic farmworkers/unexposed women) but found no differences in lung function between groups.232 In contrast, a cross-sectional study of farmworkers and their relatives living in rural areas in Brazil observed associations between years of working with pesticides and pesticide handling frequency with decreased pulmonary function.233 A publication from a study of Colombian farmworkers reported that those exposed to mixtures of pesticides containing paraquat—assessed via urinary biomarkers—and profenofos or glyphosate—assessed via questionnaire—had an increased prevalence of allergic rhinitis.234 This publication also reported that farmworkers chronically exposed to paraquat had an increased prevalence of self-reported asthma.
Four studies examined the potential effects of pesticide exposure on respiratory and allergic outcomes among mothers and their children. For instance, a publication from a prospective cohort study in Costa Rica reported that self-reported current pesticide use near the home (yes/no) and higher urinary concentrations of 5-hydroxytiabendazole (5-OH-TBZ)—a metabolite of the fungicide thiabendazole—were associated with increased odds of asthma among mothers, whereas previous work in agriculture was associated with decreased odds of rhinitis but increased odds of eczema.235 A publication from this same cohort study in Costa Rica reported an association between high urinary ETU concentrations during the first half of pregnancy and increased odds of lower respiratory tract infections (LRTIs) in the first year of life.236 This publication also reported that high ETU concentrations during the second half of pregnancy were associated with decreased odds of wheezing in the first year of life. Notably, a publication from a prospective cohort study in Mexico reported null associations of prenatal DDT or DDE exposure with LRTIs months among boys assessed up to 30 months of age.237 At last, a publication from a case–control study of school-age children in Brazil reported that factors such as living close to agricultural activity, and aerial pesticide spraying near the home were associated with increased odds of uncontrolled asthma at 6–7 and 13–14 years of age.235
Liver injury.
Eight publications reported on the association of pesticide exposure with markers of liver injury (Table 9). Six of the eight publications were from studies that ascertained pesticide exposure only via questionnaire,81,88,206,238–240 whereas two studies measured blood ChE241 or blood activity.242 A publication from a cross-sectional study in Mexico reported that a higher activity of —a sensitive biomarker of OP pesticide exposure243,244—was associated with increased aspartate aminotransferase (AST), alanine aminotransferase (ALT), and gamma-glutamyl transpeptidase (GGT) levels.242 Notably, a publication from a cross-sectional study conducted in Brazil reported null associations of AChE and BChE activities with markers of liver injury, but it also reported that farmworkers had lower AST and ALT levels than controls.241 In contrast, a publication from a second study in Brazil reported higher AST levels in farmworkers than in controls during the high pesticide exposure period, but it also reported lower ALT levels in farmworkers during both the high and low pesticide exposure periods.239 A publication from a prospective cohort study of rural pregnant women environmentally exposed to OP pesticides in Argentina reported higher AST, but not ALT, levels during the spraying period compared with the prespraying period.206 Likewise, a publication from a cross-sectional study conducted in Brazil reported higher AST and ALT levels in Brazilian female, but not male, farmworkers occupationally exposed to multiple classes of pesticides than in controls.238 A published cross-sectional study conducted in Ecuador also found that women living in one agricultural community, but not women living in another agricultural area, had a greater percentage of ALT and AST levels exceeding normal levels compared with controls.81 A publication from a separate cross-sectional study in Brazil reported lower alkaline phosphatase levels in farmworkers who had worked with pesticides than in those who had not.88 Last, a publication from a cross-sectional study of individuals living close to an uncontrolled contaminated site containing the residues and leftovers of a deactivated OC pesticide factory in Brazil reported null associations of pesticide exposure with markers of liver injury.240
Hematological parameters and lipid profiles.
Fourteen publications reported on the associations of pesticide exposure with hematological parameters (Table 9). Twelve of the 14 publications relied on questionnaires to assess environmental or occupational pesticide exposure52,54,73,81,94,96,98,238,245–248; only 2 ascertained exposure via direct exposure assessment and used these measurements in their exposure–outcome analyses.242,249 Four publications reported null associations with hematological parameters.54,94,98,238 Conversely, 2 publications from cross-sectional studies conducted in Mexico96 and Ecuador81 reported higher hemoglobin and hematocrit levels in those occupationally or environmentally exposed to pesticides than in those unexposed. In addition, a publication from a different cross-sectional study in Mexico (mentioned above) found that higher activity was associated with higher hemoglobin and hematocrit levels.242 A case–control study conducted in Brazil, Argentina, and Mexico found increased odds of aplastic anemia among adults exposed to pesticides.245
A publication from a cross-sectional study in Brazil reported decreased neutrophils and monocytes among tobacco farmworkers exposed to multiple pesticide classes, but not among controls.73 Notably, a publication from a prospective cohort study of school-age children from a tobacco-producing region in Brazil (mentioned above) also reported lower numbers of neutrophils, monocytes, and basophils, but higher hemoglobin levels and lymphocytes, at the beginning of the pesticide application period compared with the leaf harvest period.52 Similarly, a publication from a cross-sectional study of Brazilian farmworkers and their families reported that detectable serum concentrations of various OC pesticides [i.e., hexachlorocyclohexane (HCH), aldrin, heptachlor, trans-nonachlor, endosulfan, endrin, DDT, DDE, and methoxychlor] were associated with lower numbers of white blood cells, particularly monocytes and eosinophils.249 This publication also reported that detectable serum concentrations of were associated with lower hemoglobin levels. Last, cross-sectional studies conducted in Brazil246,247 and Colombia248 reported associations between farm work, length of pesticide exposure (i.e., y), and exposure during the harvest period with alterations in various hematological parameters, including hemoglobin levels and number of leukocytes, platelets, and monocytes.
Eight publications reported on associations between pesticide exposure and lipid profiles in LAC populations (Table 9); three of them reported null associations.94,96,98 Two publications from cross-sectional studies conducted in Ecuador81 and Mexico250 reported lower cholesterol or low-density lipoprotein levels among individuals with high pesticide exposure compared with those with moderate or no exposure. The study in Mexico also found associations of higher blood BChE activity with higher cholesterol, triglyceride, very low-density lipoprotein, or total lipid levels, but these associations varied by BMI.250 A publication from another cross-sectional study in Mexico (mentioned above) found that higher activity was associated with higher cholesterol, triglyceride, and total lipid levels, but also with lower high-density lipoprotein levels.242 A publication from a cross-sectional study of coffee harvesters in Colombia exposed to OP pesticides reported that higher blood BChE activity was associated with hypercholesterolemia (defined as total cholesterol ).251 Conversely, a cross-sectional study in Brazil reported higher total cholesterol levels among farmworkers who had not worked with pesticides.88
Acoustic damage.
Eight publications from cross-sectional studies conducted in Brazil evaluated the association of exposure to either the OP pesticide malathion or several pesticide classes—assessed via questionnaire—with hearing problems (Table 9). Seven of the eight publications reported that elevated pesticide exposure was associated with acoustic damage, as indicated by poorer performance in tests such as the Distortion Product Otoacoustic Emissions (DPOAE) test, the Transient Stimulus Otoacoustic Emissions (TSOAE) test, the Duration Pattern test (DPT), and the Gaps-in-Noise test (GIN).252–258 In contrast, a publication from a small cross-sectional study of rural workers in Brazil reported null associations between AChE activity and hearing loss.259
Other outcomes.
Single publications reported on the associations of pesticide exposure with various health outcomes (Table 9). Overall, these publications—which were primarily from cross-sectional studies that ascertained exposure solely through questionnaires—reported associations of exposure to several classes of pesticides with a variety of outcomes, including changes in blood pressure,251,260–262 diarrhea,263 rheumatoid arthritis,264 high blood glucose levels242 and glutathione -transferase activity,265 sleep disorders,266 skin problems,62,84,103,267,268 thoracic spine and neck pain,269,270 changes in interleukin expression,102 overweight/obesity,271 green tobacco sickness,272 and death273–275; however, results should be interpreted with caution given the limited weight of evidence. In addition, some publications reported that pesticide exposure was associated with poorer general health status or symptoms of APP (e.g., fatigue/tiredness, nervousness, headache, anxiety, and depression).57,62,84,103,138,159,223,267,268,276,277
Overall, publications from studies conducted to date provide somewhat consistent evidence of the associations between pesticide exposure with acoustic damage and changes in markers of liver injury (e.g., when comparing exposed with unexposed or when comparing exposed during the spraying and prespraying season). Conversely, published studies that have examined the associations of pesticide exposure with kidney function, respiratory/allergic outcomes, and hematological parameters and lipid profiles in LAC populations have reported mixed findings. All these reported associations need to be interpreted with caution given that most published studies were relatively small, cross-sectional in design, and assessed exposure to multiple classes of pesticides via questionnaire.
Discussion
The results of our scoping review provide some evidence that exposure to pesticides may adversely impact the health of LAC populations. For instance, we observed that occupational and residential exposure to OP pesticides or several pesticide classes was consistently associated with higher levels of increased chromosomal aberration frequency, nuclear buds, oxidative stress, or cell death. We also observed relatively consistent evidence of the adverse neurobehavioral effects of elevated OP pesticide and carbamate exposure levels, particularly among children and farmworkers. The latter finding is in line with those of previous systematic reviews on the neurobehavioral effects of OP pesticide exposure.23,36,37,40,278,279 Published studies on teratogenicity and placental outcomes, cancer, thyroid function, reproductive outcomes, and birth outcomes and child growth were largely heterogeneous in terms of pesticide exposure and outcome assessment methods and their results were mixed. Findings on other health outcomes, including respiratory and allergic effects, were too sparse to discern the directionality of an effect, if any.
To our knowledge, only one literature review besides ours has focused on the health effects of pesticide exposure in different populations from a specific region of the world.280 This systematic literature review of all research on environmental and human health issues associated with pesticide exposure in sub-Saharan Africa published between 2006 and 2021 reported some findings consistent with ours.280 For example, the review of sub-Saharan Africa literature found that OC and OP pesticides were the pesticides classes most frequently studied in the region. In our scoping review, we found that OC and OP pesticides such as DDT, endosulfan, and chlordane—pesticides that have been banned by countries in the European Union and the United States281–283—were among the pesticides classes most frequently examined in the LAC region. Both reviews identified that published studies were primarily cross-sectional in design and relied largely on indirect pesticide exposure assessment methods (e.g., questionnaire, job status ascertainment). Notably, the most frequently examined health effects in sub-Saharan Africa studies were signs and symptoms of APP (self-reported and doctor-diagnosed), whereas genotoxicity and neurobehavioral outcomes were the most frequently assessed among LAC populations.
As more research on the health effects of pesticide exposure is conducted in LAC countries, we believe that it is critical to address three fundamental limitations to the current body of literature. First, there must be a more widespread investment in research capacities across the LAC region. In our scoping review, we identified studies from 16 of the 43 LAC countries and territories, and 2 countries—Brazil and Mexico—accounted for nearly 60% of the included studies. Central American countries (except for Costa Rica) and Caribbean territories were among those with the lowest research outputs, and evidence suggests that efforts to increase research capacities often focus on the countries with some existing capacity,284 perpetuating health inequities in countries with the lowest levels of research and support. Second, future research must address limitations in study design and data collection to increase the rigor and robustness of epidemiological findings. Given the limited funding to develop infrastructure and conduct research in most LAC countries,285 most studies included in this review were small cross-sectional studies—which are important in terms of hypothesis generation but have limited causal inference. In addition, nearly half of the studies included in this review relied on indirect exposure assessment methods (e.g., questionnaires or exposure classification based on self-report, job title, or area of residence), which may result in exposure misclassification that could bias epidemiological findings toward the null286–288 and potentially account for conflicting study findings.289 Self-reported pesticide exposure may be particularly prone to recall bias288,289 and may be worsened under certain conditions, including studies of participants with low educational attainment or high residential mobility.290 Furthermore, pesticide use in LAC countries varies by crop and season—which causes significant exposure variation, both in terms of intensity and chemical composition291—and farmworkers or pesticide applicators are often not informed of the specific pesticide active ingredients used in their farms.233,277,292 In our scoping review, most of the studies that assessed pesticide exposure via biomonitoring relied on analysis of a single sample and may have not accurately captured chronic exposure to pesticides with short biological half-lives and high inter- and intra-individual variability,293,294 which are frequently used in LAC countries. This potential exposure misclassification due to single time point sampling may have biased study findings toward or away from the null, depending on the time in which the exposure was captured. Last, studies included in our review employed a wide range of health outcome assessment methods, which were often not validated nor considered gold standards, hindering comparisons of study findings across populations within and outside of the LAC region. Third, studies should employ more robust statistical analyses and more systematic reporting of methods and results to facilitate comparisons across study populations. We found that many studies lacked clear presentation of key information, such as the covariates used in multivariable analyses or the specific pesticide(s) being examined (e.g., some publications solely indicated they collected samples to be analyzed for AChE activity, and we inferred they were examining OPs and carbamates). In addition, multiple studies did not report effect estimates and simply reported the prevalence of the outcome among exposed and unexposed groups. Strengthening research capacity in the LAC region is needed to increase the rigor of epidemiological studies and generate robust evidence regarding associations between pesticide exposure and its health effects.
In addition to addressing the limitations raised above, several knowledge gaps remain regarding the health effects of pesticides in LAC populations. As an example, a limited number of studies included in our review have assessed exposure to current-use pesticides that are applied widely in the LAC region and the rest of the world, such as pyrethroids, glyphosate, neonicotinoids, and fungicides.20,295–297 Similarly, few studies have examined the health effects of early-life exposure to pesticides—a critical period of brain298,299 and lung300,301 development—or the effects of pesticides on common chronic diseases, such as cardiometabolic disorders and neurodegenerative diseases.302,303 Although farmworkers and those living in agricultural areas are simultaneously exposed to numerous pesticides,304 only three studies have examined the health effects of exposure to pesticide mixtures using statistical methods that accounted for copollutant confounding.82,106,234 More studies are needed to understand the true independent and aggregate effect of exposure to mixtures of pesticides,305,306 which may require more widespread training of researchers in environmental mixtures methods. Finally, it is increasingly understood that the health effects of environmental chemicals may be due in part to interactions with nonchemical exposures, such as poverty, neighborhood violence, and malnutrition.307–310 Socioeconomically disadvantaged populations in LAC countries, such as immigrants or indigenous people, have less access to legal protections and are frequent victims of unregulated work arrangements, leading to disproportionately high levels of pesticide exposure292 and potentially more adverse health outcomes. Nevertheless, few of the studies included in our scoping review examined the joint effects of pesticides and unique psychosocial stressors experienced by populations in the region.
Recommendations for Future Research
In LAC countries and territories, generating robust evidence on the health effects of pesticide exposure is essential to inform agricultural policies and public health surveillance programs aimed at post-registration control of pesticides and the development and implementation of pesticide safety guidelines. Given the resource limitations and sociocultural context of agricultural populations across the LAC region, potential areas of prioritization for future work include the following:
-
Increasing funding for research and capacity building. The Pan American Health Organization (PAHO), a regional office of the World Health Organization (WHO) for the Americas, has called for strengthening research in each member country to promote health equity and socioeconomic development.311 Given the widespread use of pesticides across the LAC region, it is imperative to strengthen institutional capacities to produce research and generate robust evidence that could be used to inform national and regional health policies. For example, difficulties associated with pesticide biomonitoring may be amplified in studies conducted in LAC countries owing to limited laboratory capacity and availability of analytical techniques to measure biomarkers of exposure. In addition, insufficient funding and infrastructure limit the ability to carry out large-scale epidemiological studies, which may contribute to the widespread reliance on small cross-sectional studies.
To improve the quality and quantity of health research in the LAC region, capacity building must become a key component of global research funding, with a focus on countries where the infrastructure and capacity do not currently exist.285 Although some models have proposed increased “North–South” collaborations, these projects often align with the priorities of the funders, rather than the countries’ needs, and few projects have resulted in sustainable long-term partnerships that are equitable to the investigators in the home countries where the research was conducted.312 We recommend that any collaborations with institutions outside of the LAC region explicitly include local researchers in the design and implementation of the study,313 focus on capacity development in the country, and critically examine power dynamics to ensure more equitable partnerships where the research is tailored to the needs of the local populations.314
-
Increasing collaboration within the LAC region. Beyond collaborations outside of the LAC region, we recommend increasing research synergies and the development of more interdisciplinary research teams across LAC countries. For example, the creation of networks of researchers within the region could contribute to the homogenization of exposure and health outcomes assessments (e.g., specific test or scale employed, age of assessment) and the systematization of reporting methods and results in publications, improving the ability to compare and synthesize results across studies. Previous literature discussing the need for increased research synergies in the LAC region have specifically focused on supporting early career researchers through initiatives such as in the development of national and regional graduate programs that strengthen regional collaborations, enable sustainable research careers, and decrease the high mobility of doctoral students and early career researchers outside of the region.315
Although farming systems and ecological conditions vary across the LAC region,316 increased homogenization of research within the region could potentially contribute to the homogenization of regulatory decisions, such as banning particular hazardous pesticides that are subject of international conventions and agreements, improving management and control of pesticides, restricting dispersive pesticide applications methods (e.g., light aircrafts, spray-booms), implementing pesticide-free buffer zones, and promoting sustainable agriculture and alternatives to pesticide use, which could result in more protective policies at both the national and regional levels.
-
Increasing rigor of epidemiological studies. Studies that can incorporate biomonitoring should consider the use of biomarkers that reflect exposure to specific pesticides, including current-use pesticides (e.g., glyphosate, neonicotinoids, pyrethroids), and should assess exposure at multiple time points, if possible. In studies where biomonitoring is cost prohibitive or logistically infeasible, indirect exposure assessment may be improved by incorporating additional methods that are less prone to bias, including purchasing/inventory records, personal exposure monitoring (e.g., breathing zone air sampling, dermal wipes), environmental sampling data (e.g., ambient air monitoring, drinking water),9,11,12 and development of surrogate exposure estimates based on nearby pesticide use assessed via Geographic Information Systems.33 In addition, rather than dichotomously classifying participants as farmworkers vs. non-farmworkers, studies could employ more detailed occupational assessments and job-exposure matrices examining factors such as job titles and tasks, specific crops and active ingredients, and more complete occupational history that may decrease error due to exposure misclassification.288 Studies should also use standardized and validated outcome assessment methods across population subgroups from different LAC countries and territories to improve researchers’ ability to compare findings across studies inside and outside the region.
In addition to increasing the rigor when designing epidemiological studies, we recommend the inclusion of more robust statistical analyses and a shift away from the presentation of bivariate results alone. We also recommend the systematization of the presentation of key information in the methods and results of publications, including the specific pesticides being assessed, statistical methods used, and study results to facilitate comparisons across studies and better support causal inference.
Strengths and Limitations of This Scoping Review
Given the methodological differences in study design, populations studied, and exposure and health outcome assessments employed across the studies included in this review, we were not able to summarize the evidence on health effects of pesticide exposure in LAC populations using a quantitative synthesis or meta-analysis. In addition, our search strategy was focused on the use of the word “pesticides” plus Latin America or “pesticides” plus each of the names of the 43 LAC countries and territories. This strategy may have led to missed information because some studies could have used more specific keywords such as the pesticide’s nature (e.g., herbicides, fungicides, insecticides) or the names of pesticide active ingredients (e.g., mancozeb, chlordecone). Our literature search also focused solely on PubMed and SciELO, and it is possible that other common databases in the LAC region, such as Latindex and Latin American and Caribbean Health Sciences Literature (LILACS), could have yielded additional publications. Despite its limitations, we believe that this scoping review provides a useful overview of the status of the research regarding the health effects of pesticide exposure and gives insight into existing data gaps and research capacity building needs in the region.
Conclusions
Our scoping review provides some evidence that exposure to pesticides may adversely impact the health of LAC populations. Nevertheless, methodological limitations such as reliance on cross-sectional study designs and indirect exposure assessment methods, as well as heterogeneity in the assessment of health outcomes and presentation of study findings, undermine the strength of the conclusions. We recommend increasing capacity building, integrating research initiatives, and conducting more rigorous epidemiological studies that can address these limitations, better inform public health surveillance systems, and increase the impact of research on public policies.
Supplementary Material
Acknowledgments
We acknowledge the leadership of the Latin American and the Caribbean (LAC) Chapter of the International Society of Environmental Epidemiology (ISEE) for their support of this article. Agnes Soares da Silva is a staff member of the Pan American Health Organization. The contents are the sole responsibility of the authors and do not necessarily reflect the official views, decisions, or policies of the Pan American Health Organization or ISEE LAC. For more information on ISEE LAC Chapter, please visit https://isee-lac.org/.
L.A.Z.V., M.T.M.Q., M.B., G.C., S.C., and A.M.M. conceived the scoping review. Literature search and screening were carried out by L.A.Z.V., C.H., and M.T.M.Q. Full-text review and information extraction were conducted by L.A.Z.V., C.H., M.T.M.Q., L.Q.A., M.B., R.B., A.C., R.A.F., C.F., N.G., J.P.G.J., B.A.L., M.P.M., M.R.S., A.R.S., N.T., B.vW.dJ., G.M.C., A.J.H., A.S.daS., S.C., and A.M.M. The first draft of the manuscript was written by L.A.Z.V. and M.T.M.Q. and critically reviewed by C.H., L.Q.A., A.J.H., A.S.daS., S.C., and A.M.M. Figures and tables were elaborated by L.A.Z.V., C.H., and A.M.M. All authors read and approved the final manuscript.
References
- 1.OECD/FAO (Organization for Economic Cooperation and Development, Food and Agriculture Organization). 2019. AOCED-FAO Agricultural Outlook 2019–2028. Special Focus: Latin America. Rome, Italy: Paris/Food and Agriculture Organization of the United Nations. https://reliefweb.int/attachments/22af2430-3306-308b-9f79-174616ac4148/CA4076EN.pdf [accessed 30 June 2022]. [Google Scholar]
- 2.Jepson PC, Murray K, Bach O, Bonilla MA, Neumeister L. 2020. Selection of pesticides to reduce human and environmental health risks: a global guideline and minimum pesticides list. Lancet Planet Health 4(2):e56–e63, PMID: , 10.1016/S2542-5196(19)30266-9. [DOI] [PubMed] [Google Scholar]
- 3.FAO (Food and Agriculture Organization). 2019. FAOSTAT food and agricultural data, pesticide use data 2019. http://www.fao.org/faostat/en/#data/RP [accessed 16 January 2022].
- 4.Winkler MS, Atuhaire A, Fuhrimann S, Mora A, Niqagaba C, Oltramare C, et al. 2019. Environmental exposures, health effects and institutional determinants of pesticide use in two tropical settings. DORA Eawag. https://www.dora.lib4ri.ch/eawag/islandora/object/eawag:19081 [accessed 16 February 2022].
- 5.Wesseling C, Corriols M, Bravo V. 2005. Acute pesticide poisoning and pesticide registration in Central America. Toxicol Appl Pharmacol 207(suppl 2):697–705, PMID: , 10.1016/j.taap.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 6.Caldas ED. 2016. Pesticide poisoning in Brazil. In: Reference Module in Earth Systems and Environmental Sciences. pp. 419–427. New York, NY: Elsevier. [Google Scholar]
- 7.World Bank. 2020. Population, total. https://data.worldbank.org/indicator/SP.POP.TOTL?end=2020&start=1960&view=chart [accessed 17 January 2022].
- 8.Bardach AE, García-Perdomo HA, Alcaraz A, Tapia López E, Gándara RAR, Ruvnsky S, et al. 2019. Interventions for the control of Aedes aegypti in Latin America and the Caribbean: systematic review and meta-analysis. Trop Med Int Health 24(5):530–552, PMID: , 10.1111/tmi.13217. [DOI] [PubMed] [Google Scholar]
- 9.Córdoba Gamboa L, Solano Diaz K, Ruepert C, van Wendel de Joode B. 2020. Passive monitoring techniques to evaluate environmental pesticide exposure: results from the Infant’s Environmental Health study (ISA). Environ Res 184:109243, PMID: , 10.1016/j.envres.2020.109243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dereumeaux C, Fillol C, Quenel P, Denys S. 2020. Pesticide exposures for residents living close to agricultural lands: a review. Environ Int 134:105210, PMID: , 10.1016/j.envint.2019.105210. [DOI] [PubMed] [Google Scholar]
- 11.Pozo K, Llanos Y, Estellano VH, Cortés S, Jorquera H, Gerli L, et al. 2016. Occurrence of chlorpyrifos in the atmosphere of the Araucanía Region in Chile using polyurethane foam-based passive air samplers. Atmos Pollut Res 7(4):706–710, 10.1016/j.apr.2016.03.003. [DOI] [Google Scholar]
- 12.Cortes S, Pozo K, Llanos Y, Martinez N, Foerster C, Leiva C, et al. 2020. First measurement of human exposure to current use pesticides (CUPs) in the atmosphere of central Chile: the case study of Mauco cohort. Atmos Pollut Res 11(4):776–784, 10.1016/j.apr.2019.12.023. [DOI] [Google Scholar]
- 13.López-Gálvez N, Wagoner R, Quirós-Alcalá L, Ornelas Van Horne Y, Furlong M, Avila E, et al. 2019. Systematic literature review of the take-home route of pesticide exposure via biomonitoring and environmental monitoring. Int J Environ Res Public Health 16(12):2177, PMID: , 10.3390/ijerph16122177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caldas ED, Boon PE, Tressou J. 2006. Probabilistic assessment of the cumulative acute exposure to organophosphorus and carbamate insecticides in the Brazilian diet. Toxicology 222(1–2):132–142, PMID: , 10.1016/j.tox.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 15.Caldas ED, Souza LCKR. 2004. Chronic dietary risk for pesticide residues in food in Brazil: an update. Food Addit Contam 21(11):1057–1064, PMID: , 10.1080/02652030400009225. [DOI] [PubMed] [Google Scholar]
- 16.Benítez-Diaz P, Miranda-Contreras L. 2013. Surface water pollution by residues in Venezuela and other Latin American countries. Rev Int de Contam Ambient 29(1):7–23. [Google Scholar]
- 17.de Carvalho Dores EFG, De-Lamonica-Freire EM. 2001. Aquatic environment contamination by pesticides. Case study: water used for human consumption in Primavera do Leste, Mato Grosso—preliminary analyses [in Portuguese]. Quim Nova 24:27–36, 10.1590/S0100-40422001000100007. [DOI] [Google Scholar]
- 18.González-Andrade F, López-Pulles R, Estévez E. 2010. Acute pesticide poisoning in Ecuador: a short epidemiological report. J Public Health 18(5):437–442, 10.1007/s10389-010-0333-y. [DOI] [Google Scholar]
- 19.Corriols M, Marín J, Berroteran J, Lozano LM, Lundberg I. 2009. Incidence of acute pesticide poisonings in Nicaragua: a public health concern. Occup Environ Med 66(3):205–210, PMID: , 10.1136/oem.2008.040840. [DOI] [PubMed] [Google Scholar]
- 20.Benbrook CM. 2016. Trends in glyphosate herbicide use in the United States and globally. Environ Sci Eur 28(1):3, PMID: , 10.1186/s12302-016-0070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gullino ML, Tinivella F, Garibaldi A, Kemmitt GM, Bacci L, Sheppard B. 2010. Mancozeb: past, present, and future. Plant Dis 94(9):1076–1087, PMID: , 10.1094/PDIS-94-9-1076. [DOI] [PubMed] [Google Scholar]
- 22.Kim KH, Kabir E, Jahan SA. 2017. Exposure to pesticides and the associated human health effects. Sci Total Environ 575: 525–535, PMID: , 10.1016/j.scitotenv.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 23.Sapbamrer R, Hongsibsong S. 2019. Effects of prenatal and postnatal exposure to organophosphate pesticides on child neurodevelopment in different age groups: a systematic review. Environ Sci Pollut Res Int 26(18):18267–18290, PMID: , 10.1007/s11356-019-05126-w. [DOI] [PubMed] [Google Scholar]
- 24.Staudacher P, Fuhrimann S, Farnham A, Mora AM, Atuhaire A, Niwagaba C, et al. 2020. Comparative analysis of pesticide use determinants among smallholder farmers From Costa Rica and Uganda. Environ Health Insights 14:1178630220972417, PMID: , 10.1177/1178630220972417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thundiyil JG, Stober J, Besbelli N, Pronczuk J. 2008. Acute pesticide poisoning: a proposed classification tool. Bull World Health Organ 86(3):205–209, PMID: , 10.2471/blt.08.041814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Corriols M, Marín J, Berroteran J, Lozano LM, Lundberg I, Thörn Å. 2008. The Nicaraguan Pesticide Poisoning Register: constant underreporting. Int J Health Serv 38(4):773–787, PMID: , 10.2190/HS.38.4.k. [DOI] [PubMed] [Google Scholar]
- 27.Cervantes Morant R. 2010. Plaguicidas en Bolivia: sus implicaciones en la salud, agricultura y medio ambiente. Rev virtual REDESMA 4(1). [Google Scholar]
- 28.Kesavachandran CN, Fareed M, Pathak MK, Bihari V, Mathur N, Srivastava AK. 2009. Adverse health effects of pesticides in agrarian populations of developing countries. Rev Environ Contam Toxicol 200:33–52, PMID: , 10.1007/978-1-4419-0028-9_2. [DOI] [PubMed] [Google Scholar]
- 29.Bravo V, Rodríguez T, van Wendel de Joode B, Canto N, Calderón GR, et al. 2011. Monitoring pesticide use and associated health hazards in Central America. Int J Occup Environ Health 17(3):258–269, PMID: , 10.1179/107735211799041896. [DOI] [PubMed] [Google Scholar]
- 30.Ruiz-Guzmán JA, Gómez-Corrales P, Cruz-Esquivel Á, Marrugo-Negrete JL. 2017. Cytogenetic damage in peripheral blood lymphocytes of children exposed to pesticides in agricultural areas of the department of Cordoba, Colombia. Mutat Res Genet Toxicol Environ Mutagen 824:25–31, PMID: , 10.1016/j.mrgentox.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 31.Noyes PD, McElwee MK, Miller HD, Clark BW, Van Tiem LA, Walcott KC, et al. 2009. The toxicology of climate change: environmental contaminants in a warming world. Environ Int 35(6):971–986, PMID: , 10.1016/j.envint.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 32.Weiss FT, Leuzinger M, Zurbrügg C, Eggen HIL. 2016. Chemical Pollution in Low- and Middle-Income Countries. Dübendorf, Switzerland: Swiss Federal Institute of Aquatic Science and Technology (Eawag). [Google Scholar]
- 33.Goodman JE, Prueitt RL, Boffetta P, Halsall C, Sweetman A. 2020. “Good epidemiology practice” guidelines for pesticide exposure assessment. Int J Environ Res Public Health 17(14):5114, PMID: , 10.3390/ijerph17145114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ntzani EE, Ntritsos G CM, Evangelou E, Tzoulaki I. 2013. Literature review on epidemiological studies linking exposure to pesticides and health effects. EFSA Support Publ 10(10):497E, 10.2903/sp.efsa.2013.EN-497. [DOI] [Google Scholar]
- 35.EFSA PPR (EFSA Panel on Plant Protection Products and their Residues), Ockleford C, Adriaanse P, Berny P, Brock T, Duquesne S, et al. 2017. Scientific Opinion of the PPR Panel on the follow-up of the findings of the External Scientific Report ‘Literature review of epidemiological studies linking exposure to pesticides and health effects’. EFSA J 15(10):e05007, PMID: , 10.2903/j.efsa.2017.5007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muñoz-Quezada MT, Lucero BA, Iglesias VP, Muñoz MP, Cornejo CA, Achu E, et al. 2016. Chronic exposure to organophosphate (OP) pesticides and neuropsychological functioning in farm workers: a review. Int J Occup Environ Health 22(1):68–79, PMID: , 10.1080/10773525.2015.1123848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muñoz-Quezada MT, Lucero BA, Barr DB, Steenland K, Levy K, Ryan PB, et al. 2013. Neurodevelopmental effects in children associated with exposure to organophosphate pesticides: a systematic review. Neurotoxicology 39:158–168, PMID: , 10.1016/j.neuro.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lucero B, Muñoz-Quezada MT. 2021. Neurobehavioral, neuromotor, and neurocognitive effects in agricultural workers and their children exposed to pyrethroid pesticides: a review. Front Hum Neurosci 15:648171, PMID: , 10.3389/fnhum.2021.648171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chang ET, Odo NU, Acquavella JF. 2022. Systematic literature review of the epidemiology of glyphosate and neurological outcomes. Int Arch Occup Environ Health Preprint posted online 23 May 2022, PMID: , 10.1007/s00420-022-01878-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dórea JG. 2021. Exposure to environmental neurotoxic substances and neurodevelopment in children from Latin America and the Caribbean. Environ Res 192:110199, PMID: , 10.1016/j.envres.2020.110199. [DOI] [PubMed] [Google Scholar]
- 41.Buralli RJ, Dultra AF, Ribeiro H. 2020. Respiratory and allergic effects in children exposed to pesticides—a systematic review. Int J Environ Res Public Health 17(8):2740, PMID: , 10.3390/ijerph17082740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rozas ME. 2021. Revisión de Estudios Epídemiológicos sobre Efects de los Plaguicidas en Niñas, Niños e Inantes de América Latina. Buenos Aires, Argentina: Red de Acción en Plaguicidas y sus Alternativas de América Latina (RAP-AL). https://reduas.com.ar/wp-content/uploads/2021/12/Revision-de-Estudios-epidemiologicos_ni%C3%B1os_plaguicidas_Maria-Elena-Rozas-071221.doc-1.pdf [accessed 30 June 2022]. [Google Scholar]
- 43.Sánchez-Alarcón J, Milić M, Kašuba V, Tenorio-Arvide MG, Montiel-González JMR, Bonassi S, et al. 2021. A systematic review of studies on genotoxicity and related biomarkers in populations exposed to pesticides in Mexico. Toxics 9(11):272, PMID: , 10.3390/toxics9110272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. 2018. PRISMA Extension for Scoping Reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med 169(7):467–473, PMID: , 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- 45.Latin American and the Caribbean Chapter of the International Society of Environmental Epidemiology. 2018. Bylaws: International Society for Environmental Epidemiology Latin American and the Caribbean (LAC) Chapter. https://iseepi.org/docs/ISEE_LAC_Chapter_Bylaws_FINAL.pdf [accessed 8 August 2022].
- 46.Alvarado-Hernandez DL, Montero-Montoya R, Serrano-García L, Arellano-Aguilar O, Jasso-Pineda Y, Yáñez-Estrada L. 2013. Assessment of exposure to organochlorine pesticides and levels of DNA damage in mother–infant pairs of an agrarian community. Environ Mol Mutagen 54(2):99–111, PMID: , 10.1002/em.21753. [DOI] [PubMed] [Google Scholar]
- 47.Jasso-Pineda Y, Díaz-Barriga F, Yáñez-Estrada L, Pérez-Vázquez FJ, Pérez-Maldonado IN. 2015. DNA damage in Mexican children living in high-risk contaminated scenarios. Sci Total Environ 518–519:38–48, PMID: , 10.1016/j.scitotenv.2015.02.073. [DOI] [PubMed] [Google Scholar]
- 48.Anguiano-Vega GA, Cazares-Ramirez LH, Rendon-Von Osten J, Santillan-Sidon AP, Vazquez-Boucard CG. 2020. Risk of genotoxic damage in schoolchildren exposed to organochloride pesticides. Sci Rep 10(1):17584, PMID: , 10.1038/s41598-020-74620-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gómez-Arroyo S, Martínez-Valenzuela C, Calvo-González S, Villalobos-Pietrini R, Waliszewski SM, Calderón-Segura ME, et al. 2013. Evaluación del riesgo genotóxico de niños mexicanos que viven cerca de zonas agrícolas con aspersión aérea de plaguicidas. Rev Int Contam Ambie 29:217–225. [Google Scholar]
- 50.Bernardi N, Gentile N, Mañas F, Méndez A, Gorla N, Aiassa D. 2015. Assessment of the level of damage to the genetic material of children exposed to pesticides in the province of Córdoba. Arch Argent Pediatr 113(2):126–131, PMID: , 10.5546/aap.2015.eng.126. [DOI] [PubMed] [Google Scholar]
- 51.Castañeda-Yslas IJ, Arellano-García ME, García-Zarate MA, Ruíz-Ruíz B, Zavala-Cerna MG, Torres-Bugarín O. 2016. Biomonitoring with micronuclei test in buccal cells of female farmers and children exposed to pesticides of Maneadero Agricultural Valley, Baja California, Mexico. J Toxicol 2016:7934257, PMID: , 10.1155/2016/7934257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nascimento SN, Göethel G, Baierle M, Barth A, Brucker N, Charão MF, et al. 2017. Environmental exposure and effects on health of children from a tobacco-producing region. Environ Sci Pollut Res Int 24(3):2851–2865, PMID: , 10.1007/s11356-016-8071-5. [DOI] [PubMed] [Google Scholar]
- 53.Leite SB, Franco de Diana DM, Segovia Abreu JA, Avalos DS, Denis MA, Ovelar CC, et al. 2019. DNA damage induced by exposure to pesticides in children of rural areas in Paraguay. Indian J Med Res 150(3):290–296, PMID: , 10.4103/ijmr.IJMR_1497_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Quintana MM, Vera B, Magnarelli G, Guiñazú N, Rovedatti MG. 2017. Neonatal, placental, and umbilical cord blood parameters in pregnant women residing in areas with intensive pesticide application. Environ Sci Pollut Res Int 24(25):20736–20746, PMID: , 10.1007/s11356-017-9642-9. [DOI] [PubMed] [Google Scholar]
- 55.Barron Cuenca J, Aguilar Mercado X, Navia Bueno P. 2015. Exposición a plaguicidas, desnutrición crónica y daño genotóxico en menores de tres años. Luribay. Cuad Hosp Clín 56(2):9–17. [Google Scholar]
- 56.Franco FC, Alves AA, Godoy FR, Avelar JB, Rodrigues DD, Pedroso TMA, et al. 2016. Evaluating genotoxic risks in Brazilian public health agents occupationally exposed to pesticides: a multi-biomarker approach. Environ Sci Pollut Res Int 23(19):19723–19734, PMID: , 10.1007/s11356-016-7179-y. [DOI] [PubMed] [Google Scholar]
- 57.Silvério ACP, Machado SC, Azevedo L, Nogueira DA, de Castro Graciano MM, Simões JS, et al. 2017. Assessment of exposure to pesticides in rural workers in southern of Minas Gerais, Brazil. Environ Toxicol Pharmacol 55:99–106, PMID: , 10.1016/j.etap.2017.08.013. [DOI] [PubMed] [Google Scholar]
- 58.Zepeda-Arce R, Rojas-García AE, Benitez-Trinidad A, Herrera-Moreno JF, Medina-Díaz IM, Barrón-Vivanco BS, et al. 2017. Oxidative stress and genetic damage among workers exposed primarily to organophosphate and pyrethroid pesticides. Environ Toxicol 32(6):1754–1764, PMID: , 10.1002/tox.22398. [DOI] [PubMed] [Google Scholar]
- 59.Benitez-Trinidad AB, Medina-Díaz IM, Bernal-Hernández YY, Barrón-Vivanco BS, González-Aria CA, Herrera-Moreno JF, et al. 2018. Relationship between LINE-1 methylation pattern and pesticide exposure in urban sprayers. Food Chem Toxicol 113:125–133, PMID: , 10.1016/j.fct.2018.01.035. [DOI] [PubMed] [Google Scholar]
- 60.Xotlanihua-Gervacio MDC, Guerrero-Flores MC, Herrera-Moreno JF, Medina-Díaz IM, Bernal-Hernández YY, Barrón-Vivanco BS, et al. 2018. Micronucleus frequency is correlated with antioxidant enzyme levels in workers occupationally exposed to pesticides. Environ Sci Pollut Res Int 25(31):31558–31568, PMID: , 10.1007/s11356-018-3130-8. [DOI] [PubMed] [Google Scholar]
- 61.Herrera-Moreno JF, Medina-Díaz IM, Bernal-Hernández YY, Ramos KS, Alvarado-Cruz I, Quintanilla-Vega B, et al. 2019. Modified CDKN2B (p15) and CDKN2A (p16) DNA methylation profiles in urban pesticide applicators. Environ Sci Pollut Res Int 26(15):15124–15135, PMID: , 10.1007/s11356-019-04658-5. [DOI] [PubMed] [Google Scholar]
- 62.Butinof M, Fernández RA, Lerda D, Lantieri MJ, Filippi I, Díaz MDP. 2019. Biomonitoreo en exposición a plaguicidas y su aporte en vigilancia epidemiológica en agroaplicadores en Córdoba, Argentina. Gac Sanit 33(3):216–221, PMID: , 10.1016/j.gaceta.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 63.Bernieri T, Moraes MF, Ardenghi PG, Basso da Silva L. 2020. Assessment of DNA damage and cholinesterase activity in soybean farmers in southern Brazil: high versus low pesticide exposure. J Environ Sci Health B 55(4):355–360, PMID: , 10.1080/03601234.2019.1704608. [DOI] [PubMed] [Google Scholar]
- 64.Valencia-Quintana R, López-Durán RM, Milić M, Bonassi S, Ochoa-Ocaña MA, Uriostegui-Acosta MO, et al. 2021. Assessment of cytogenetic damage and cholinesterases’ activity in workers occupationally exposed to pesticides in Zamora-Jacona, Michoacan, Mexico. Int J Environ Res Public Health 18(12):6269, PMID: , 10.3390/ijerph18126269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Aiassa DE, Mañas FJ, Gentile NE, Bosch B, Salinero MC, Gorla NBM. 2019. Evaluation of genetic damage in pesticides applicators from the province of Córdoba, Argentina. Environ Sci Pollut Res Int 26(20):20981–20988, PMID: , 10.1007/s11356-019-05344-2. [DOI] [PubMed] [Google Scholar]
- 66.Simoniello MF, Contini L, Benavente E, Mastandrea C, Roverano S, Paira S. 2017. Different end-points to assess effects in systemic lupus erythematosus patients exposed to pesticide mixtures. Toxicology 376:23–29, PMID: , 10.1016/j.tox.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 67.Martinez LN, Mastandrea C, Benavente E, Roverano S, Paira S, Poletta GL, et al. 2016. Evaluación de estrés oxidativo en pacientes con Lupus Eritematoso Sistémico y su posible relación con la exposición ambiental a agroquímicos. Acta Toxicol Argent 24(1):10–20. [Google Scholar]
- 68.Paredes-Céspedes DM, Herrera-Moreno JF, Bernal-Hernández YY, Medina-Díaz IM, Salazar AM, Ostrosky-Wegman P, et al. 2019. Pesticide exposure modifies DNA methylation of coding region of WRAP53α, an antisense sequence of p53, in a Mexican population. Chem Res Toxicol 32(7):1441–1448, PMID: , 10.1021/acs.chemrestox.9b00153. [DOI] [PubMed] [Google Scholar]
- 69.Jørs E, Gonzáles AR, Ascarrunz ME, Tirado N, Takahashi C, Lafuente E, et al. 2007. Genetic alterations in pesticide exposed Bolivian farmers: an evaluation by analysis of chromosomal aberrations and the comet assay. Biomark Insights 2:439–445, PMID: , 10.1177/117727190700200017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kehdy FSG, Cerqueira EMM, Bonjardim MB, Camelo RM, Castro MCL. 2007. Study of the cytogenetic effects of occupational exposure to pesticides on sanitation workers in Belo Horizonte, Brazil. Genet Mol Res 6(3):581–593, PMID: . [PubMed] [Google Scholar]
- 71.Khayat CB, Costa EOA, Gonçalves MW, da Cruz e Cunha DM, da Cruz AS, de Araújo Melo CO, et al. 2013. Assessment of DNA damage in Brazilian workers occupationally exposed to pesticides: a study from Central Brazil. Environ Sci Pollut Res Int 20(10):7334–7340, PMID: , 10.1007/s11356-013-1747-1. [DOI] [PubMed] [Google Scholar]
- 72.Wilhelm CM, Calsing AK, da Silva LB. 2015. Assessment of DNA damage in floriculturists in southern Brazil. Environ Sci Pollut Res Int 22(11):8182–8189, PMID: , 10.1007/s11356-014-3959-4. [DOI] [PubMed] [Google Scholar]
- 73.Alves JS, da Silva FR, da Silva GF, Salvador M, Kvitko K, Rohr P, et al. 2016. Investigation of potential biomarkers for the early diagnosis of cellular stability after the exposure of agricultural workers to pesticides. An Acad Bras Cienc 88(1):349–360, PMID: , 10.1590/0001-3765201520150181. [DOI] [PubMed] [Google Scholar]
- 74.Kahl VFS, Simon D, Salvador M, Branco CdosS, Dias JF, da Silva FR, et al. 2016. Telomere measurement in individuals occupationally exposed to pesticide mixtures in tobacco fields. Environ Mol Mutagen 57(1):74–84, PMID: , 10.1002/em.21984. [DOI] [PubMed] [Google Scholar]
- 75.Chaves TVS, Islam MT, de Moraes MO, de Alencar MVOB, Gomes DCV, de Carvalho RM, et al. 2017. Occupational and life-style factors-acquired mutagenicity in agric-workers of northeastern Brazil. Environ Sci Pollut Res Int 24(18):15454–15461, PMID: , 10.1007/s11356-017-9150-y. [DOI] [PubMed] [Google Scholar]
- 76.Tomiazzi JS, Judai MA, Nai GA, Pereira DR, Antunes PA, Favareto APA. 2018. Evaluation of genotoxic effects in Brazilian agricultural workers exposed to pesticides and cigarette smoke using machine-learning algorithms. Environ Sci Pollut Res Int 25(2):1259–1269, PMID: , 10.1007/s11356-017-0496-y. [DOI] [PubMed] [Google Scholar]
- 77.Vazquez Boucard C, Lee-Cruz L, Mercier L, Ramírez Orozco M, Serrano Pinto V, Anguiano G, et al. 2017. A study of DNA damage in buccal cells of consumers of well- and/or tap-water using the comet assay: assessment of occupational exposure to genotoxicants. Environ Mol Mutagen 58(8):619–627, PMID: , 10.1002/em.22111. [DOI] [PubMed] [Google Scholar]
- 78.Hutter HP, Khan AW, Lemmerer K, Wallner P, Kundi M, Moshammer H. 2018. Cytotoxic and genotoxic effects of pesticide exposure in male coffee farmworkers of the Jarabacoa Region, Dominican Republic. Int J Environ Res Public Health 15(8):1641, PMID: , 10.3390/ijerph15081641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Claudio SR, Simas JMM, Souza ACF, do Carmo Baracho de Alencar M, Yamauchi LY, Ribeiro DA. 2019. Genomic instability and cytotoxicity in buccal mucosal cells of workers in banana farming evaluated by micronucleus test. Anticancer Res 39(3):1283–1286, PMID: , 10.21873/anticanres.13239. [DOI] [PubMed] [Google Scholar]
- 80.Kahl VFS, Dhillon VS, Simon D, da Silva FR, Salvador M, Branco CDS, et al. 2018. Chronic occupational exposure endured by tobacco farmers from Brazil and association with DNA damage. Mutagenesis 33(2):119–128, PMID: , 10.1093/mutage/gex045. [DOI] [PubMed] [Google Scholar]
- 81.Arévalo-Jaramillo P, Idrobo A, Salcedo L, Cabrera A, Vintimilla A, Carrión M, et al. 2019. Biochemical and genotoxic effects in women exposed to pesticides in southern Ecuador. Environ Sci Pollut Res Int 26(24):24911–24921, PMID: , 10.1007/s11356-019-05725-7. [DOI] [PubMed] [Google Scholar]
- 82.Barrón Cuenca J, Tirado N, Barral J, Ali I, Levi M, Stenius U, et al. 2019. Increased levels of genotoxic damage in a Bolivian agricultural population exposed to mixtures of pesticides. Sci Total Environ 695:133942, PMID: , 10.1016/j.scitotenv.2019.133942. [DOI] [PubMed] [Google Scholar]
- 83.Cepeda S, Forero-Castro M, Cárdenas-Nieto D, Martínez-Agüero M, Rondón-Lagos M. 2020. Chromosomal instability in farmers exposed to pesticides: high prevalence of clonal and non-clonal chromosomal alterations. Risk Manag Healthc Policy 13:97–110, PMID: , 10.2147/RMHP.S230953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hutter HP, Poteser M, Lemmerer K, Wallner P, Sanavi SS, Kundi M, et al. 2020. Indicators of genotoxicity in farmers and laborers of ecological and conventional banana plantations in Ecuador. Int J Environ Res Public Health 17(4):1435, PMID: , 10.3390/ijerph17041435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Salazar-Flores J, Pacheco-Moisés FP, Ortiz GG, Torres-Jasso JH, Romero-Rentería O, Briones-Torres AL, et al. 2020. Occupational exposure to organophosphorus and carbamates in farmers in La Cienega, Jalisco, Mexico: oxidative stress and membrane fluidity markers. J Occup Med Toxicol 15(1):32, PMID: , 10.1186/s12995-020-00283-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.da Silva J, Moraes CR, Heuser VD, Andrade VM, Silva FR, Kvitko K, et al. 2008. Evaluation of genetic damage in a Brazilian population occupationally exposed to pesticides and its correlation with polymorphisms in metabolizing genes. Mutagenesis 23(5):415–422, PMID: , 10.1093/mutage/gen031. [DOI] [PubMed] [Google Scholar]
- 87.de Souza Espindola Santos A, Parks CG, Senna MM, de Carvalho LVB, Meyer A. 2021. Exposure to pesticides and oxidative stress in Brazilian agricultural communities. Biomarkers 26(6):539–547, PMID: , 10.1080/1354750X.2021.1933593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cattelan MDP, Maurer F, Garcia F, Berro LF, Machado MM, Manfredini V, et al. 2018. Occupational exposure to pesticides in family agriculture and the oxidative, biochemical and hematological profile in this agricultural model. Life Sci 203:177–183, PMID: , 10.1016/j.lfs.2018.04.038. [DOI] [PubMed] [Google Scholar]
- 89.Kahl VFS, Dhillon V, Fenech M, de Souza MR, da Silva FN, Marroni NAP, et al. 2018. Occupational exposure to pesticides in tobacco fields: the integrated evaluation of nutritional intake and susceptibility on genomic and epigenetic instability. Oxid Med Cell Longev 2018:7017423, PMID: , 10.1155/2018/7017423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Marcelino AF, Wachtel CC, Ghisi NdeC. 2019. Are our farm workers in danger? Genetic damage in farmers exposed to pesticides. Int J Environ Res Public Health 16(3):358, PMID: , 10.3390/ijerph16030358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Simoniello MF, Kleinsorge EC, Scagnetti JA, Grigolato RA, Poletta GL, Carballo MA. 2008. DNA damage in workers occupationally exposed to pesticide mixtures. J Appl Toxicol 28(8):957–965, PMID: , 10.1002/jat.1361. [DOI] [PubMed] [Google Scholar]
- 92.de Bortoli GM, de Azevedo MB, da Silva LB. 2009. Cytogenetic biomonitoring of Brazilian workers exposed to pesticides: micronucleus analysis in buccal epithelial cells of soybean growers. Mutat Res 675(1–2):1–4, PMID: , 10.1016/j.mrgentox.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 93.Martínez-Valenzuela C, Gómez-Arroyo S, Villalobos-Pietrini R, Waliszewski S, Calderón-Segura ME, Félix-Gastélum R, et al. 2009. Genotoxic biomonitoring of agricultural workers exposed to pesticides in the north of Sinaloa State, Mexico. Environ Int 35(8):1155–1159, PMID: , 10.1016/j.envint.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 94.Remor AP, Totti CC, Moreira DA, Dutra GP, Heuser VD, Boeira JM. 2009. Occupational exposure of farm workers to pesticides: biochemical parameters and evaluation of genotoxicity. Environ Int 35(2):273–278, PMID: , 10.1016/j.envint.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 95.Simoniello MF, Kleinsorge EC, Carballo MA. 2010. Evaluación bioquímica de trabajadores rurales expuestos a pesticidas. Medicina (B Aires) 70:489–498, PMID: . [PubMed] [Google Scholar]
- 96.Payán-Rentería R, Garibay-Chávez G, Rangel-Ascencio R, Preciado-Martínez V, Muñoz-Islas L, Beltrán-Miranda C, et al. 2012. Effect of chronic pesticide exposure in farm workers of a Mexico community. Arch Environ Occup Health 67(1):22–30, PMID: , 10.1080/19338244.2011.564230. [DOI] [PubMed] [Google Scholar]
- 97.Benedetti D, Nunes E, Sarmento M, Porto C, Dos Santos CEI, Dias JF, et al. 2013. Genetic damage in soybean workers exposed to pesticides: evaluation with the comet and buccal micronucleus cytome assays. Mutat Res 752(1–2):28–33, PMID: , 10.1016/j.mrgentox.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 98.Adad LMDM, de Andrade HH, Kvitko K, Lehmann M, Calcante AADCM, Dihl RR. 2015. Occupational exposure of workers to pesticides: toxicogenetics and susceptibility gene polymorphisms. Genet Mol Biol 38(3):308–315, PMID: , 10.1590/S1415-475738320140336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bianco GE, Suarez E, Cazon L, de la Puente TB, Ahrendts MRB, De Luca JC. 2017. Prevalence of chromosomal aberrations in Argentinean agricultural workers. Environ Sci Pollut Res Int 24(26):21146–21152, PMID: , 10.1007/s11356-017-9664-3. [DOI] [PubMed] [Google Scholar]
- 100.Hilgert Jacobsen-Pereira C, Dos Santos CR, Troina Maraslis F, Pimental L, Feigó AJL, Silva CI, et al. 2018. Markers of genotoxicity and oxidative stress in farmers exposed to pesticides. Ecotoxicol Environ Saf 148:177–183, PMID: , 10.1016/j.ecoenv.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 101.de Oliveira AFB, de Souza MR, Benedetti D, Scotti AS, Piazza LS, Garcia ALH, et al. 2019. Investigation of pesticide exposure by genotoxicological, biochemical, genetic polymorphic and in silico analysis. Ecotoxicol Environ Saf 179:135–142, PMID: , 10.1016/j.ecoenv.2019.04.023. [DOI] [PubMed] [Google Scholar]
- 102.Lovison Sasso E, Cattaneo R, Rosso Storck T, Spanamberg Mayer M, Sant’Anna V, Clasen B. 2021. Occupational exposure of rural workers to pesticides in a vegetable-producing region in Brazil. Environ Sci Pollut Res Int 28(20):25758–25769, PMID: , 10.1007/s11356-021-12444-5. [DOI] [PubMed] [Google Scholar]
- 103.Filippi I, Lucero P, Bonansea RI, Lerda D, Butinof M, Fernandez RA, et al. 2021. Validation of exposure indexes to pesticides through the analysis of exposure and effect biomarkers in ground pesticide applicators from Argentina. Heliyon 7(9):e07921, PMID: , 10.1016/j.heliyon.2021.e07921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mañas F, Agost L, Salinero MC, Méndez Á, Aiassa D. 2021. Cytogenetic markers and their spatial distribution in a population living in proximity to areas sprayed with pesticides. Environ Toxicol Pharmacol 88:103736, PMID: , 10.1016/j.etap.2021.103736. [DOI] [PubMed] [Google Scholar]
- 105.Paz-y-Miño C, Muñoz MJ, Maldonado A, Valladares C, Cumbal N, Herrera C, et al. 2011. Baseline determination in social, health, and genetic areas in communities affected by glyphosate aerial spraying on the northeastern Ecuadorian border. Rev Environ Health 26(1):45–51, PMID: , 10.1515/reveh.2011.007. [DOI] [PubMed] [Google Scholar]
- 106.Varona-Uribe ME, Torres-Rey CH, Díaz-Criollo S, Palma-Parra RM, Narváez DM, Carmona SP, et al. 2016. Exposure to pesticide mixtures and DNA damage among rice field workers. Arch Environ Occup Health 71(1):3–9, PMID: , 10.1080/19338244.2014.910489. [DOI] [PubMed] [Google Scholar]
- 107.Torres-Sánchez L, Rothenberg SJ, Schnaas L, Cebrián ME, Osoria E, Del Carmen Hernández M, et al. 2007. In utero p,p′-DDE exposure and infant neurodevelopment: a perinatal cohort in Mexico. Environ Health Perspect 115(3):435–439, PMID: , 10.1289/ehp.9566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Torres-Sánchez L, Schnaas L, Cebrián ME, Hernández MdelC, Valencia EO, García Hernández RM, et al. 2009. Prenatal dichlorodiphenyldichloroethylene (DDE) exposure and neurodevelopment: a follow-up from 12 to 30 months of age. Neurotoxicology 30(6):1162–1165, PMID: , 10.1016/j.neuro.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bahena-Medina LA, Torres-Sánchez L, Schnaas L, Cebrián ME, Chávez CH, Osorio-Valencia E, et al. 2011. Neonatal neurodevelopment and prenatal exposure to dichlorodiphenyldichloroethylene (DDE): a cohort study in Mexico. J Expo Sci Environ Epidemiol 21(6):609–614, PMID: , 10.1038/jes.2011.25. [DOI] [PubMed] [Google Scholar]
- 110.Dallaire R, Muckle G, Rouget F, Kadhel P, Bataille H, Guldner L, et al. 2012. Cognitive, visual, and motor development of 7-month-old Guadeloupean infants exposed to chlordecone. Environ Res 118:79–85, PMID: , 10.1016/j.envres.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 111.Boucher O, Simard MN, Muckle G, Rouget F, Kadhel P, Bataille H, et al. 2013. Exposure to an organochlorine pesticide (chlordecone) and development of 18-month-old infants. Neurotoxicology 35:162–168, PMID: , 10.1016/j.neuro.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 112.Torres-Sánchez L, Schnaas L, Rothenberg SJ, Cebrián ME, Osorio-Valencia E, Hernández MdelC, et al. 2013. Prenatal p,p′-DDE exposure and neurodevelopment among children 3.5–5 years of age. Environ Health Perspect 121(2):263–268, PMID: , 10.1289/ehp.1205034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Osorio-Valencia E, Torres-Sánchez L, López-Carrillo L, Cebrián ME, Rothenberg SJ, Hernández Chávez MdelC, et al. 2015. Prenatal p,p′-DDE exposure and establishment of lateralization and spatial orientation in Mexican preschooler. Neurotoxicology 47:1–7, PMID: , 10.1016/j.neuro.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ogaz-González R, Mérida-Ortega Á, Torres-Sánchez L, Schnaas L, Hernández-Alcaraz C, Cebrián ME, et al. 2018. Maternal dietary intake of polyunsaturated fatty acids modifies association between prenatal DDT exposure and child neurodevelopment: a cohort study. Environ Pollut 238:698–705, PMID: , 10.1016/j.envpol.2018.03.100. [DOI] [PubMed] [Google Scholar]
- 115.Saint-Amour D, Muckle G, Gagnon-Chauvin A, Rouget F, Monfort C, Michineau L, et al. 2020. Visual contrast sensitivity in school-age Guadeloupean children exposed to chlordecone. Neurotoxicology 78:195–201, PMID: , 10.1016/j.neuro.2020.02.012. [DOI] [PubMed] [Google Scholar]
- 116.Cordier S, Forget-Dubois N, Desrochers-Couture M, Rouget F, Michineau L, Monfort C, et al. 2020. Prenatal and childhood exposure to chlordecone and sex-typed toy preference of 7-year-old Guadeloupean children. Environ Sci Pollut Res Int 27(33):40971–40979, PMID: , 10.1007/s11356-019-05686-x. [DOI] [PubMed] [Google Scholar]
- 117.Cordier S, Bouquet E, Warembourg C, Massart C, Rouget F, Kadhel P, et al. 2015. Perinatal exposure to chlordecone, thyroid hormone status and neurodevelopment in infants: the Timoun cohort study in Guadeloupe (French West Indies). Environ Res 138:271–278, PMID: , 10.1016/j.envres.2015.02.021. [DOI] [PubMed] [Google Scholar]
- 118.Campos É, Freire C, Novaes CdeO, Koifman RJ, Koifman S. 2015. Exposure to organochloride pesticides and the cognitive development of children and adolescents living in a contaminated area in Brazil. Rev Bras Saude Matern Infant 15(1):105–120, 10.1590/S1519-38292015000100009. [DOI] [Google Scholar]
- 119.Steenland K, Mora AM, Barr DB, Juncos J, Roman N, Wesseling C. 2014. Organochlorine chemicals and neurodegeneration among elderly subjects in Costa Rica. Environ Res 134:205–209, PMID: , 10.1016/j.envres.2014.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Handal AJ, Harlow SD, Breilh J, Lozoff B. 2008. Occupational exposure to pesticides during pregnancy and neurobehavioral development of infants and toddlers. Epidemiology 19(6):851–859, PMID: , 10.1097/EDE.0b013e318187cc5d. [DOI] [PubMed] [Google Scholar]
- 121.Handal AJ, Lozoff B, Breilh J, Harlow SD. 2007. Neurobehavioral development in children with potential exposure to pesticides. Epidemiology 18(3):312–320, PMID: , 10.1097/01.ede.0000259983.55716.bb. [DOI] [PubMed] [Google Scholar]
- 122.Suarez-Lopez JR, Himes JH, Jacobs DR Jr, Alexander BH, Gunnar MR. 2013. Acetylcholinesterase activity and neurodevelopment in boys and girls. Pediatrics 132(6):e1649–e1658, PMID: , 10.1542/peds.2013-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Harari R, Julvez J, Murata K, Barr D, Bellinger DC, Debes F, et al. 2010. Neurobehavioral deficits and increased blood pressure in school-age children prenatally exposed to pesticides. Environ Health Perspect 118(6):890–896, PMID: , 10.1289/ehp.0901582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Suarez-Lopez JR, Hood N, Suárez-Torres J, Gahagan S, Gunnar MR, López-Paredes D. 2019. Associations of acetylcholinesterase activity with depression and anxiety symptoms among adolescents growing up near pesticide spray sites. Int J Hyg Environ Health 222(7):981–990, PMID: , 10.1016/j.ijheh.2019.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Suarez-Lopez JR, Nguyen A, Klas J, Gahagan S, Checkoway H, Lopez-Paredes D, et al. 2021. Associations of acetylcholinesterase inhibition between pesticide spray seasons with depression and anxiety symptoms in adolescents, and the role of sex and adrenal hormones on gender moderation. Expo Health 13(1):51–64, PMID: , 10.1007/s12403-020-00361-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Suarez-Lopez JR, Checkoway H, Jacobs DR Jr, Al-Delaimy WK, Gahagan S. 2017. Potential short-term neurobehavioral alterations in children associated with a peak pesticide spray season: the Mother’s Day flower harvest in Ecuador. Neurotoxicology 60:125–133, PMID: , 10.1016/j.neuro.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Muñoz-Quezada MT, Iglesias V, Lucero-Mondaca B. 2011. Exposición a organofosforados y desempeño cognitivo en escolares rurales chilenos: un estudio exploratorio. Rev Fac Nac Salud Pública 29(3):256–263. [Google Scholar]
- 128.Fortenberry GZ, Meeker JD, Sánchez BN, Barr DB, Panuwet P, Bellinger D, et al. 2014. Urinary 3,5,6-trichloro-2-pyridinol (TCPY) in pregnant women from Mexico City: distribution, temporal variability, and relationship with child attention and hyperactivity. Int J Hyg Environ Health 217(2–3):405–412, PMID: , 10.1016/j.ijheh.2013.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Martos-Mula AJ, Saavedra ON, Wierna NR, Ruggeri MA, Tschambler JA, Carreras A, et al. 2013. Afectación de las funciones cognitivas y motoras en niños residentes de zonas rurales de Jujuy y su relación con plaguicidas inhibidores de la colinesterasa: un estudio piloto. Acta Toxicol Argent 21(1):15–25. [Google Scholar]
- 130.Handal AJ, Lozoff B, Breilh J, Harlow SD. 2007. Effect of community of residence on neurobehavioral development in infants and young children in a flower-growing region of Ecuador. Environ Health Perspect 115(1):128–133, PMID: , 10.1289/ehp.9261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Corral SA, de Angel V, Salas N, Zúñiga-Venegas L, Gaspar PA, Pancetti F. 2017. Cognitive impairment in agricultural workers and nearby residents exposed to pesticides in the Coquimbo Region of Chile. Neurotoxicol Teratol 62:13–19, PMID: , 10.1016/j.ntt.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 132.Muñoz-Quezada MT, Lucero B, Iglesias V, Muñoz MP, Achú E, Cornejo C, et al. 2016. Organophosphate pesticides and neuropsychological and motor effects in the Maule Region, Chile [in Spanish]. Gac Sanit 30(3):227–231, PMID: , 10.1016/j.gaceta.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 133.Ramírez-Santana M, Zúñiga-Venegas L, Corral S, Roeleveld N, Groenewoud H, Van der Velden K, et al. 2020. Reduced neurobehavioral functioning in agricultural workers and rural inhabitants exposed to pesticides in northern Chile and its association with blood biomarkers inhibition. Environ Health 19(1):84, PMID: , 10.1186/s12940-020-00634-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ramírez-Santana M, Zúñiga-Venegas L, Corral S, Roeleveld N, Groenewoud H, van der Velden K, et al. 2020. Association between cholinesterase’s inhibition and cognitive impairment: a basis for prevention policies of environmental pollution by organophosphate and carbamate pesticides in Chile. Environ Res 186:109539, PMID: , 10.1016/j.envres.2020.109539. [DOI] [PubMed] [Google Scholar]
- 135.Grillo Pizarro Á, Achú Peralta E, Muñoz-Quezada MT, Lucero Mondaca B. 2018. Exposure to organophosphate pesticides and peripheral polyneuropathy in workers from Maule Region, Chile [in Spanish]. Rev Esp Salud Publica 92:e201803006, PMID: . [PMC free article] [PubMed] [Google Scholar]
- 136.Wesseling C, van Wendel de Joode B, Keifer M, London L, Mergler D, Stallones L. 2010. Symptoms of psychological distress and suicidal ideation among banana workers with a history of poisoning by organophosphate or n-methyl carbamate pesticides. Occup Environ Med 67(11):778–784, PMID: , 10.1136/oem.2009.047266. [DOI] [PubMed] [Google Scholar]
- 137.Serrano-Medina A, Ugalde-Lizárraga A, Bojorquez-Cuevas MS, Garnica-Ruiz J, González-Corral MA, García-Ledezma A, et al. 2019. Neuropsychiatric disorders in farmers associated with organophosphorus pesticide exposure in a rural village of northwest México. Int J Environ Res Public Health 16(5):689, PMID: , 10.3390/ijerph16050689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Buralli RJ, Ribeiro H, Iglesias V, Muñoz-Quezada MT, Leão RS, Marques RC, et al. 2020. Occupational exposure to pesticides and health symptoms among family farmers in Brazil. Rev Saude Publica 54:133, PMID: , 10.11606/s1518-8787.2020054002263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lu C, Essig C, Root C, Rohlman DS, McDonald T, Sulzbacher S. 2009. Assessing the association between pesticide exposure and cognitive development in rural Costa Rican children living in organic and conventional coffee farms. Int J Adolesc Med Health 21(4):609–621, PMID: , 10.1515/ijamh.2009.21.4.609. [DOI] [PubMed] [Google Scholar]
- 140.van Wendel de Joode B, Mora AM, Lindh CH, Hernández-Bonilla D, Córdoba L, Wesseling C, et al. 2016. Pesticide exposure and neurodevelopment in children aged 6–9 years from Talamanca, Costa Rica. Cortex 85:137–150, PMID: , 10.1016/j.cortex.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 141.Mora AM, Córdoba L, Cano JC, Hernandez-Bonilla D, Pardo L, Schnaas L, et al. 2018. Prenatal mancozeb exposure, excess manganese, and neurodevelopment at 1 year of age in the Infants’ Environmental Health (ISA) study. Environ Health Perspect 126(5):057007, PMID: , 10.1289/EHP1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Watkins DJ, Fortenberry GZ, Sánchez BN, Barr DB, Panuwet P, Schnaas L, et al. 2016. Urinary 3-phenoxybenzoic acid (3-PBA) levels among pregnant women in Mexico City: distribution and relationships with child neurodevelopment. Environ Res 147:307–313, PMID: , 10.1016/j.envres.2016.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Eckerman DA, Gimenes LS, de Souza RC, Galvão PR, Sarcinelli PN, Chrisman JR. 2007. Age related effects of pesticide exposure on neurobehavioral performance of adolescent farm workers in Brazil. Neurotoxicol Teratol 29(1):164–175, PMID: , 10.1016/j.ntt.2006.09.028. [DOI] [PubMed] [Google Scholar]
- 144.Friedman E, Hazlehurst MF, Loftus C, Karr C, McDonald KN, Suarez-Lopez JR. 2020. Residential proximity to greenhouse agriculture and neurobehavioral performance in Ecuadorian children. Int J Hyg Environ Health 223(1):220–227, PMID: , 10.1016/j.ijheh.2019.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Christian MA, Samms-Vaughan M, Lee M, Bressler J, Hessabi M, Grove ML, et al. 2018. Maternal exposures associated with autism spectrum disorder in Jamaican children. J Autism Dev Disord 48(8):2766–2778, PMID: , 10.1007/s10803-018-3537-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Steenland K, Wesseling C, Román N, Quirós I, Juncos JL. 2013. Occupational pesticide exposure and screening tests for neurodegenerative disease among an elderly population in Costa Rica. Environ Res 120:96–101, PMID: , 10.1016/j.envres.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 147.Hansen MRH, Jørs E, Lander F, Condarco G, Debes F, Bustillos NT, et al. 2017. Neurological deficits after long-term pyrethroid exposure. Environ Health Insights 11:1178630217700628, PMID: , 10.1177/1178630217700628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Conti CL, Barbosa WM, Simão JBP, Álvares-da-Silva AM. 2018. Pesticide exposure, tobacco use, poor self-perceived health and presence of chronic disease are determinants of depressive symptoms among coffee growers from Southeast Brazil. Psychiatry Res 260:187–192, PMID: , 10.1016/j.psychres.2017.11.063. [DOI] [PubMed] [Google Scholar]
- 149.Campos Ÿ, dos Santos Pinto da Silva V, Sarpa Campos de Mello M, Barros Otero U. 2016. Exposure to pesticides and mental disorders in a rural population of southern Brazil. Neurotoxicology 56:7–16, PMID: , 10.1016/j.neuro.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 150.Conti CL, Borçoi AR, Almança CCJ, Barbosa WM, Archanjo AB, de Assis Pinheiro J, et al. 2020. Factors associated with depressive symptoms among rural residents from remote areas. Community Ment Health J 56(7):1292–1297, PMID: , 10.1007/s10597-020-00637-0. [DOI] [PubMed] [Google Scholar]
- 151.Farnham A, Fuhrimann S, Staudacher P, Quirós-Lépiz M, Hyland C, Winkler MS, et al. 2021. Long-term neurological and psychological distress symptoms among smallholder farmers in Costa Rica with a history of acute pesticide poisoning. Int J Environ Res Public Health 18(17):9021, PMID: , 10.3390/ijerph18179021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Faria NM, Fassa AG, Meucci RD, Fiori NS, Miranda VI. 2014. Occupational exposure to pesticides, nicotine and minor psychiatric disorders among tobacco farmers in southern Brazil. Neurotoxicology 45:347–354, PMID: , 10.1016/j.neuro.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 153.Cruzeiro Szortyka ALS, Faria NMX, Carvalho MP, Feijó FR, Meucci RD, Flesch BD, et al. 2021. Suicidality among South Brazilian tobacco growers. Neurotoxicology 86:52–58, PMID: , 10.1016/j.neuro.2021.06.005. [DOI] [PubMed] [Google Scholar]
- 154.Gonzaga CWP, Baldo MP, Caldeira AP. 2021. Exposure to pesticides or agroecological practices: suicidal ideation among peasant farmers in Brazil’s semi-arid region. Cien Saude Colet 26(9):4243–4252, PMID: , 10.1590/1413-81232021269.09052020. [DOI] [PubMed] [Google Scholar]
- 155.Portilla-Portilla Á, Pinilla-Monsalve GD, Caballero-Carvajal AJ, Gómez-Rodrígues E, Márin-Hernández LR, et al. 2014. Prevalencia de signos y síntomas asociados a la exposición directa a plaguicidas neurotóxicos en una población rural colombiana en 2013. Rev Medicas UIS 27(2):41–49. [Google Scholar]
- 156.Vasconcellos PRO, Rizzotto MLF, Obregón PL, Alonzo HGA. 2020. Exposição a agrotóxicos na agricultura e doença de Parkinson em usuários de um serviço público de saúde do Paraná, Brasil. Cad Saude Colet 28:567–578, 10.1590/1414-462x202028040109. [DOI] [Google Scholar]
- 157.Silvestre GCSB, Ferreira MJM, Figueiredo SEFMR, Silva CALD, Siqueira HH, Silva AMCD. 2020. Parkinson disease and occupational and environmental exposure to pesticides in a region of intense agribusiness activity in Brazil: a case–control study. J Occup Environ Med 62(12):e732–e737, PMID: , 10.1097/JOM.0000000000002043. [DOI] [PubMed] [Google Scholar]
- 158.de Azevedo MFA, Meyer A. 2017. Essential tremor in endemic disease control agents exposed to pesticides: a case–control study [in Portuguese]. Cad Saude Publica 33(8):e00194915, PMID: , 10.1590/0102-311X00194915. [DOI] [PubMed] [Google Scholar]
- 159.de Araújo AJ, de Lima JS, Moreira JC, Jacob SdoC, Soares MdeO, Monteiro MCM, et al. 2007. Exposição múltipla a agrotóxicos e efeitos à saúde: estudo transversal em amostra de 102 trabalhadores rurais, Nova Friburgo, RJ. Cien Saude Colet 12(1):115–130, PMID: , 10.1590/S1413-81232007000100015. [DOI] [PubMed] [Google Scholar]
- 160.Palzes VA, Sagiv SK, Baker JM, Rojas-Valverde D, Gutiérrez-Vargas R, Winkler MS, et al. 2019. Manganese exposure and working memory-related brain activity in smallholder farmworkers in Costa Rica: results from a pilot study. Environ Res 173:539–548, PMID: , 10.1016/j.envres.2019.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bustamante Montes LP, Waliszewski S, Hernández-Valero M, Sanín-Aguirre L, Infanzón-Ruiz RM, Jañas AG. 2010. Prenatal exposure to organochlorine pesticides and cryptorchidism [in Spanish]. Cien Saude Colet 15(suppl 1):1169–1174, PMID: , 10.1590/S1413-81232010000700025. [DOI] [PubMed] [Google Scholar]
- 162.Oliveira NP, Moi GP, Atanaka-Santos M, Silva AM, Pignati WA. 2014. Congenital defects in the cities with high use of pesticides in the state of Mato Grosso, Brazil [in Portuguese]. Cien Saude Colet 19(10):4123–4130, PMID: , 10.1590/1413-812320141910.08512014. [DOI] [PubMed] [Google Scholar]
- 163.Ueker ME, Silva VM, Moi GP, Pignati WA, Mattos IE, Silva AMC. 2016. Parenteral exposure to pesticides and occurence of congenital malformations: hospital-based case–control study. BMC Pediatr 16(1):125, PMID: , 10.1186/s12887-016-0667-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Gaspari L, Sampaio DR, Paris F, Audran F, Orsini M, Neto JB, et al. 2012. High prevalence of micropenis in 2710 male newborns from an intensive-use pesticide area of Northeastern Brazil. Int J Androl 35(3):253–264, PMID: , 10.1111/j.1365-2605.2011.01241.x. [DOI] [PubMed] [Google Scholar]
- 165.Castillo-Cadena J, Mejia-Sanchez F, López-Arriaga JA. 2017. Congenital malformations according to etiology in newborns from the floricultural zone of Mexico state. Environ Sci Pollut Res Int 24(8):7662–7667, PMID: , 10.1007/s11356-017-8429-3. [DOI] [PubMed] [Google Scholar]
- 166.Silva SR, Martins JL, Seixas S, Silva DC, Lemos SP, Lemos PV. 2011. Congenital defects and exposure to pesticides in São Francisco Valley [in Portuguese]. Rev Bras Ginecol Obstet 33(1):20–26, PMID: . [PubMed] [Google Scholar]
- 167.Rouget F, Kadhel P, Monfort C, Viel JF, Thome JP, Cordier S, et al. 2020. Chlordecone exposure and risk of congenital anomalies: the Timoun Mother–Child Cohort Study in Guadeloupe (French West Indies). Environ Sci Pollut Res Int 27(33):40992–40998, PMID: , 10.1007/s11356-019-06031-y. [DOI] [PubMed] [Google Scholar]
- 168.Vera B, Santa Cruz S, Magnarelli G. 2012. Plasma cholinesterase and carboxylesterase activities and nuclear and mitochondrial lipid composition of human placenta associated with maternal exposure to pesticides. Reprod Toxicol 34(3):402–407, PMID: , 10.1016/j.reprotox.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 169.Rivero Osimani VL, Valdez SR, Guiñazú N, Magnarelli G. 2016. Alteration of syncytiotrophoblast mitochondria function and endothelial nitric oxide synthase expression in the placenta of rural residents. Reprod Toxicol 61:47–57, PMID: , 10.1016/j.reprotox.2016.02.018. [DOI] [PubMed] [Google Scholar]
- 170.Bulgaroni V, Lombardo P, Rivero-Osimani V, Vera B, Dulgerian L, Cerbán F, et al. 2013. Environmental pesticide exposure modulates cytokines, arginase and ornithine decarboxylase expression in human placenta. Reprod Toxicol 39:23–32, PMID: , 10.1016/j.reprotox.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 171.Acosta-Maldonado B, Sánchez-Ramírez B, Reza-López S, Levario-Carrillo M. 2009. Effects of exposure to pesticides during pregnancy on placental maturity and weight of newborns: a cross-sectional pilot study in women from the Chihuahua State, Mexico. Hum Exp Toxicol 28(8):451–459, PMID: , 10.1177/0960327109107045. [DOI] [PubMed] [Google Scholar]
- 172.Chiapella G, Genti-Raimondi S, Magnarelli G. 2014. Placental oxidative status in rural residents environmentally exposed to organophosphates. Environ Toxicol Pharmacol 38(1):220–229, PMID: , 10.1016/j.etap.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 173.Brureau L, Emeville E, Helissey C, Thome JP, Multigner L, Blanchet P. 2020. Endocrine disrupting-chemicals and biochemical recurrence of prostate cancer after prostatectomy: a cohort study in Guadeloupe (French West Indies). Int J Cancer 146(3):657–663, PMID: , 10.1002/ijc.32287. [DOI] [PubMed] [Google Scholar]
- 174.Emeville E, Giusti A, Coumoul X, Thomé JP, Blanchet P, Multigner L. 2015. Associations of plasma concentrations of dichlorodiphenyldichloroethylene and polychlorinated biphenyls with prostate cancer: a case–control study in Guadeloupe (French West Indies). Environ Health Perspect 123(4):317–323, PMID: , 10.1289/ehp.1408407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hyland C, Gunier RB, Metayer C, Bates MN, Wesseling C, Mora AM. 2018. Maternal residential pesticide use and risk of childhood leukemia in Costa Rica. Int J Cancer 143(6):1295–1304, PMID: , 10.1002/ijc.31522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Monge P, Wesseling C, Guardado J, Lundberg I, Ahlbom A, Cantor KP, et al. 2007. Parental occupational exposure to pesticides and the risk of childhood leukemia in Costa Rica. Scand J Work Environ Health 33(4):293–303, PMID: , 10.5271/sjweh.1146. [DOI] [PubMed] [Google Scholar]
- 177.Hernández-Morales AL, Zonana-Nacach A, Zaragoza-Sandoval VM. 2009. Associated risk factors in acute leukemia in children. A cases and controls study [in Spanish]. Rev Med Inst Mex Seguro Soc 47(5):497–503, PMID: . [PubMed] [Google Scholar]
- 178.Ferreira JD, Couto AC, Pombo-de-Oliveira MS, Koifman S, Brazilian Collaborative Study Group of Infant Acute Leukemia. 2013. In utero pesticide exposure and leukemia in Brazilian children < 2 years of age. Environ Health Perspect 121(2):269–275, PMID: , 10.1289/ehp.1103942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ferreira JD, Couto AC, Alves LC, Pombo de Oliveira MdoS, Koifman S. 2012. Exposições ambientais e leucemias na infância no Brasil: uma análise exploratória de Sua associação. Rev Bras Estud Popul 29:477–492, 10.1590/S0102-30982012000200014. [DOI] [Google Scholar]
- 180.Ortega Jacome GP, Koifman RJ, Rego Monteiro GT, Koifman S. 2010. Environmental exposure and breast cancer among young women in Rio de Janeiro, Brazil. J Toxicol Environ Health A 73(13–14):858–865, PMID: , 10.1080/15287391003744773. [DOI] [PubMed] [Google Scholar]
- 181.Silva AMC, Campos PHN, Mattos IE, Hajat S, Lacerda EM, Ferreira MJM. 2019. Environmental exposure to pesticides and breast cancer in a region of intensive agribusiness activity in Brazil: a case–control study. Int J Environ Res Public Health 16(20):3951, PMID: , 10.3390/ijerph16203951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Segatto MM, Bonamigo RR, Hohmann CB, Müller KR, Bakos L, Mastroeni S, et al. 2015. Residential and occupational exposure to pesticides may increase risk for cutaneous melanoma: a case–control study conducted in the south of Brazil. Int J Dermatol 54(12):e527–e538, PMID: , 10.1111/ijd.12826. [DOI] [PubMed] [Google Scholar]
- 183.Boccolini PM, Boccolini CS, Chrisman JR, Koifman RJ, Meyer A. 2017. Non-Hodgkin lymphoma among Brazilian agricultural workers: a death certificate case-control study. Arch Environ Occup Health 72(3):139–144, PMID: , 10.1080/19338244.2016.1179167. [DOI] [PubMed] [Google Scholar]
- 184.Meyer A, Alexandre PC, Chrisman JdeR, Markowitz SB, Koifman RJ, Koifman S. 2011. Esophageal cancer among Brazilian agricultural workers: case–control study based on death certificates. Int J Hyg Environ Health 214(2):151–155, PMID: , 10.1016/j.ijheh.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 185.Miranda-Filho AL, Monteiro GT, Meyer A. 2012. Brain cancer mortality among farm workers of the State of Rio de Janeiro, Brazil: a population-based case–control study, 1996–2005. Int J Hyg Environ Health 215(5):496–501, PMID: , 10.1016/j.ijheh.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 186.Boccolini PdeMM, Asmus CIRF, Chrisman JdeR, Câmara VdeM, Markowitz SB, Meyer A. 2014. Stomach cancer mortality among agricultural workers: results from a death certificate-based case–control study. Cad Saude Colet 22:86–92, 10.1590/1414-462X201400010013. [DOI] [Google Scholar]
- 187.Freire C, Koifman RJ, Sarcinelli P, Rosa AC, Clapauch R, Koifman S. 2012. Long term exposure to organochlorine pesticides and thyroid function in children from Cidade dos Meninos, Rio de Janeiro, Brazil. Environ Res 117:68–74, PMID: , 10.1016/j.envres.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 188.Arrebola JP, Cuellar M, Bonde JP, González-Alzaga B, Mercado LA. 2016. Associations of maternal o,p′-DDT and p,p′-DDE levels with birth outcomes in a Bolivian cohort. Environ Res 151:469–477, PMID: , 10.1016/j.envres.2016.08.008. [DOI] [PubMed] [Google Scholar]
- 189.Ayhan G, Rouget F, Giton F, Costet N, Michineau L, Monfort C, et al. 2021. In utero chlordecone exposure and thyroid, metabolic, and sex-steroid hormones at the age of seven years: a study from the TIMOUN Mother–Child Cohort in Guadeloupe. Front Endocrinol 12:771641, PMID: , 10.3389/fendo.2021.771641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Freire C, Koifman RJ, Sarcinelli PN, Simões Rosa AC, Clapauch R, Koifman S. 2013. Long-term exposure to organochlorine pesticides and thyroid status in adults in a heavily contaminated area in Brazil. Environ Res 127:7–15, PMID: , 10.1016/j.envres.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 191.Piccoli C, Cremonese C, Koifman RJ, Koifman S, Freire C. 2016. Pesticide exposure and thyroid function in an agricultural population in Brazil. Environ Res 151:389–398, PMID: , 10.1016/j.envres.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 192.Blanco-Muñoz J, Lacasaña M, López-Flores I, Rodríguez-Barranco M, González-Alzaga B, Bassol S, et al. 2016. Association between organochlorine pesticide exposure and thyroid hormones in floriculture workers. Environ Res 150:357–363, PMID: , 10.1016/j.envres.2016.05.054. [DOI] [PubMed] [Google Scholar]
- 193.Hernández-Mariano JÁ, Torres-Sánchez L, Bassol-Mayagoitia S, Escamilla-Nuñez MC, Cebrian ME, Villeda-Gutiérrez ÉA, et al. 2017. Effect of exposure to p,p′-DDE during the first half of pregnancy in the maternal thyroid profile of female residents in a Mexican floriculture area. Environ Res 156:597–604, PMID: , 10.1016/j.envres.2017.04.013. [DOI] [PubMed] [Google Scholar]
- 194.Londoño ÁL, Restrepo B, Sánchez JF, García-Ríos A, Bayona A, Landázuri P. 2018. Pesticides and hypothyroidism in farmers of plantain and coffee growing areas in Quindio, Colombia [in Spanish]. Rev Salud Publ (Bogota) 20(2):215–220, PMID: , 10.15446/rsap.v20n2.57694. [DOI] [PubMed] [Google Scholar]
- 195.Phillips S, Suarez-Torres J, Checkoway H, Lopez-Paredes D, Gahagan S, Suarez-Lopez JR. 2021. Acetylcholinesterase activity and thyroid hormone levels in Ecuadorian adolescents living in agricultural settings where organophosphate pesticides are used. Int J Hyg Environ Health 233:113691, PMID: , 10.1016/j.ijheh.2021.113691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Lacasaña M, López-Flores I, Rodríguez-Barranco M, Aguilar-Garduño C, Blanco-Muñoz J, Pérez-Méndez O, et al. 2010. Association between organophosphate pesticides exposure and thyroid hormones in floriculture workers. Toxicol Appl Pharmacol 243(1):19–26, PMID: , 10.1016/j.taap.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 197.Lacasaña M, López-Flores I, Rodríguez-Barranco M, Aguilar-Garduño C, Blanco-Muñoz J, Pérez-Méndez O, et al. 2010. Interaction between organophosphate pesticide exposure and PON1 activity on thyroid function. Toxicol Appl Pharmacol 249(1):16–24, PMID: , 10.1016/j.taap.2010.07.024. [DOI] [PubMed] [Google Scholar]
- 198.Bernieri T, Rodrigues D, Barbosa IR, Ardenghi PG, Basso da Silva L. 2019. Occupational exposure to pesticides and thyroid function in Brazilian soybean farmers. Chemosphere 218:425–429, PMID: , 10.1016/j.chemosphere.2018.11.124. [DOI] [PubMed] [Google Scholar]
- 199.Torres-Sánchez L, Gamboa R, Bassol-Mayagoitia S, Huesca-Gómez C, Nava MP, Vázquez-Potisek JI, et al. 2019. Para-occupational exposure to pesticides, PON1 polymorphisms and hypothyroxinemia during the first half of pregnancy in women living in a Mexican floricultural area. Environ Health 18(1):33, PMID: , 10.1186/s12940-019-0470-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Miranda-Contreras L, Gómez-Pérez R, Rojas G, Cruz I, Berrueta L, Salmen S, et al. 2013. Occupational exposure to organophosphate and carbamate pesticides affects sperm chromatin integrity and reproductive hormone levels among Venezuelan farm workers. J Occup Health 55(3):195–203, PMID: , 10.1539/joh.12-0144-fs. [DOI] [PubMed] [Google Scholar]
- 201.Santos R, Piccoli C, Cremonese C, Freire C. 2019. Thyroid and reproductive hormones in relation to pesticide use in an agricultural population in Southern Brazil. Environ Res 173:221–231, PMID: , 10.1016/j.envres.2019.03.050. [DOI] [PubMed] [Google Scholar]
- 202.Blanco-Muñoz J, Lacasaña M, Aguilar-Garduño C, Rodríguez-Barranco M, Bassol S, Cebrián ME, et al. 2012. Effect of exposure to p,p′-DDE on male hormone profile in Mexican flower growers. Occup Environ Med 69(1):5–11, PMID: , 10.1136/oem.2010.059667. [DOI] [PubMed] [Google Scholar]
- 203.Freire C, Koifman RJ, Sarcinelli PN, Rosa AC, Clapauch R, Koifman S. 2014. Association between serum levels of organochlorine pesticides and sex hormones in adults living in a heavily contaminated area in Brazil. Int J Hyg Environ Health 217(2–3):370–378, PMID: , 10.1016/j.ijheh.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 204.Bastos AM, Souza MdoC, Almeida Filho GL, Krauss TM, Pavesi T, Silva LE. 2013. Organochlorine compound levels in fertile and infertile women from Rio de Janeiro, Brazil. Arq Bras Endocrinol Metabol 57(5):346–353, PMID: , 10.1590/s0004-27302013000500003. [DOI] [PubMed] [Google Scholar]
- 205.Aguilar-Garduño C, Lacasaña M, Blanco-Muñoz J, Rodríguez-Barranco M, Hernández AF, Bassol S, et al. 2013. Changes in male hormone profile after occupational organophosphate exposure. A longitudinal study. Toxicology 307:55–65, PMID: , 10.1016/j.tox.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 206.Cecchi A, Rovedatti MG, Sabino G, Magnarelli GG. 2012. Environmental exposure to organophosphate pesticides: assessment of endocrine disruption and hepatotoxicity in pregnant women. Ecotoxicol Environ Saf 80:280–287, PMID: , 10.1016/j.ecoenv.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 207.Blanco-Muñoz J, Morales MM, Lacasaña M, Aguilar-Garduño C, Bassol S, Cebrián ME. 2010. Exposure to organophosphate pesticides and male hormone profile in floriculturist of the state of Morelos, Mexico. Hum Reprod 25(7):1787–1795, PMID: , 10.1093/humrep/deq082. [DOI] [PubMed] [Google Scholar]
- 208.Yucra S, Gasco M, Rubio J, Gonzales GF. 2008. Semen quality in Peruvian pesticide applicators: association between urinary organophosphate metabolites and semen parameters. Environ Health 7:59, PMID: , 10.1186/1476-069X-7-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Recio-Vega R, Ocampo-Gómez G, Borja-Aburto VH, Moran-Martínez J, Cebrian-Garcia ME. 2008. Organophosphorus pesticide exposure decreases sperm quality: association between sperm parameters and urinary pesticide levels. J Appl Toxicol 28(5):674–680, PMID: , 10.1002/jat.1321. [DOI] [PubMed] [Google Scholar]
- 210.Silvia SC, Magnarelli G, Rovedatti MG. 2020. Evaluation of endocrine disruption and gestational disorders in women residing in areas with intensive pesticide application: an exploratory study. Environ Toxicol Pharmacol 73:103280, PMID: , 10.1016/j.etap.2019.103280. [DOI] [PubMed] [Google Scholar]
- 211.Sanin LH, Carrasquilla G, Solomon KR, Cole DC, Marshall EJ. 2009. Regional differences in time to pregnancy among fertile women from five Colombian regions with different use of glyphosate. J Toxicol Environ Health A 72(15–16):949–960, PMID: , 10.1080/15287390902929691. [DOI] [PubMed] [Google Scholar]
- 212.Rojas M, Guevara H. 2014. Estudio preliminar sobre ocupación y estilos de vida como factores condicionantes del ciclo menstrual en mujeres de una región de Venezuela. Rev Cienc Salud 12(3):385–400, 10.12804/revsalud12.03.2014.07. [DOI] [Google Scholar]
- 213.Miranda-Contreras L, Cruz I, Osuna J, Gómez-Pérez R, Berrueta L, Salmen S, et al. 2015. Efectos de la exposición ocupacional a plaguicidas sobre la calidad del semen en trabajadores de una comunidad agrícola del estado Mérida, Venezuela. Invest Clín 56(2):123–136, PMID: . [PubMed] [Google Scholar]
- 214.Cremonese C, Piccoli C, Pasqualotto F, Clapauch R, Koifman RJ, Koifman S, et al. 2017. Occupational exposure to pesticides, reproductive hormone levels and sperm quality in young Brazilian men. Reprod Toxicol 67:174–185, PMID: , 10.1016/j.reprotox.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 215.Cupul-Uicab LA, Hernández-Avila M, Terrazas-Medina EA, Pennell ML, Longnecker MP. 2010. Prenatal exposure to the major DDT metabolite 1,1-dichloro-2,2-bis(p-chlorophenyl)ethylene (DDE) and growth in boys from Mexico. Environ Res 110(6):595–603, PMID: , 10.1016/j.envres.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Garced S, Torres-Sánchez L, Cebrián ME, Claudio L, López-Carrillo L. 2012. Prenatal dichlorodiphenyldichloroethylene (DDE) exposure and child growth during the first year of life. Environ Res 113:58–62, PMID: , 10.1016/j.envres.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Motta IS, Volpato GT, Damasceno DC, Sinzato YK, Vesentini G, Rudge CV, et al. 2016. Contamination index. A novel parameter for metal and pesticide analyses in maternal blood and umbilical cord. Acta Cir Bras 31(7):490–497, PMID: , 10.1590/S0102-865020160070000010. [DOI] [PubMed] [Google Scholar]
- 218.Mora AM, van Wendel de Joode B, Mergler D, Córdoba L, Cano C, Quesada R, et al. 2015. Maternal blood and hair manganese concentrations, fetal growth, and length of gestation in the ISA cohort in Costa Rica. Environ Res 136:47–56, PMID: , 10.1016/j.envres.2014.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Cecchi A, Alvarez G, Quidel N, Bertone MC, Anderle S, Sabino G, et al. 2021. Residential proximity to pesticide applications in Argentine Patagonia: impact on pregnancy and newborn parameters. Environ Sci Pollut Res Int 28(40):56565–56579, PMID: , 10.1007/s11356-021-14574-2. [DOI] [PubMed] [Google Scholar]
- 220.Kadhel P, Monfort C, Costet N, Rouget F, Thomé JP, Multigner L, et al. 2014. Chlordecone exposure, length of gestation, and risk of preterm birth. Am J Epidemiol 179(5):536–544, PMID: , 10.1093/aje/kwt313. [DOI] [PubMed] [Google Scholar]
- 221.Hervé D, Costet N, Kadhel P, Rouget F, Monfort C, Thomé JP, et al. 2016. Prenatal exposure to chlordecone, gestational weight gain, and birth weight in a Guadeloupean birth cohort. Environ Res 151:436–444, PMID: , 10.1016/j.envres.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 222.Costet N, Pelé F, Comets E, Rouget F, Monfort C, Bodeau-Livinec F, et al. 2015. Perinatal exposure to chlordecone and infant growth. Environ Res 142:123–134, PMID: , 10.1016/j.envres.2015.06.023. [DOI] [PubMed] [Google Scholar]
- 223.Barrón Cuenca J, Tirado N, Vikström M, Lindh CH, Stenius U, Leander K, et al. 2020. Pesticide exposure among Bolivian farmers: associations between worker protection and exposure biomarkers. J Expo Sci Environ Epidemiol 30(4):730–742, PMID: , 10.1038/s41370-019-0128-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Ruiz-Alejos A, Caplin B, Miranda JJ, Pearce N, Bernabé-Ortiz A. 2021. CKD and CKDu in northern Peru: a cross-sectional analysis under the DEGREE protocol. BMC Nephrol 22(1):37, PMID: , 10.1186/s12882-021-02239-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Vela XF, Henríquez DO, Zelaya SM, Granados DV, Hernández MX, Orantes CM. 2014. Chronic kidney disease and associated risk factors in two Salvadoran farming communities, 2012. MEDICC Rev 16(2):55–60, PMID: , 10.37757/MR2014.V16.N2.9. [DOI] [PubMed] [Google Scholar]
- 226.Wesseling C, Aragón A, González M, Weiss I, Glaser J, Rivard CJ, et al. 2016. Heat stress, hydration and uric acid: a cross-sectional study in workers of three occupations in a hotspot of Mesoamerican nephropathy in Nicaragua. BMJ Open 6(12):e011034, PMID: , 10.1136/bmjopen-2016-011034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Smpokou ET, González-Quiroz M, Martins C, Alvito P, Le Blond J, Glaser J, et al. 2019. Environmental exposures in young adults with declining kidney function in a population at risk of Mesoamerican nephropathy. Occup Environ Med 76(12):920–926, PMID: , 10.1136/oemed-2019-105772. [DOI] [PubMed] [Google Scholar]
- 228.Sanoff SL, Callejas L, Alonso CD, Hu Y, Colindres RE, Chin H, et al. 2010. Positive association of renal insufficiency with agriculture employment and unregulated alcohol consumption in Nicaragua. Ren Fail 32(7):766–777, PMID: , 10.3109/0886022X.2010.494333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Prudente IRG, Souza BRS, Nascimento LC, Gonçalves VSDS, Silva DSD, Rabelo TK, et al. 2021. Nephrotoxic effects caused by occupational exposure to agrochemicals in a region of northeastern Brazil: a cross-sectional study. Environ Toxicol Chem 40(4):1132–1138, PMID: , 10.1002/etc.4962. [DOI] [PubMed] [Google Scholar]
- 230.Raines N, González M, Wyatt C, Kurzrok M, Pool C, Lemma T, et al. 2014. Risk factors for reduced glomerular filtration rate in a Nicaraguan community affected by Mesoamerican nephropathy. MEDICC Rev 16(2):16–22, PMID: , 10.37757/MR2014.V16.N2.4. [DOI] [PubMed] [Google Scholar]
- 231.López-Gálvez N, Wagoner R, Canales RA, Ernst K, Burgess JL, de Zapien J, et al. 2021. Longitudinal assessment of kidney function in migrant farm workers. Environ Res 202:111686, PMID: , 10.1016/j.envres.2021.111686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Fieten KB, Kromhout H, Heederik D, van Wendel de Joode B. 2009. Pesticide exposure and respiratory health of indigenous women in Costa Rica. Am J Epidemiol 169(12):1500–1506, PMID: , 10.1093/aje/kwp060. [DOI] [PubMed] [Google Scholar]
- 233.Buralli RJ, Ribeiro H, Mauad T, Amato-Lourenço LF, Salge JM, Diaz-Quijano FA, et al. 2018. Respiratory condition of family farmers exposed to pesticides in the state of Rio de Janeiro, Brazil. Int J Environ Res Public Health 15(6):1203, PMID: , 10.3390/ijerph15061203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Díaz-Criollo S, Palma M, Monroy-García AA, Idrovo AJ, Combariza D, Varona-Uribe ME. 2020. Chronic pesticide mixture exposure including paraquat and respiratory outcomes among Colombian farmers. Ind Health 58(1):15–21, PMID: , 10.2486/indhealth.2018-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Alhanti B, van Wendel de Joode B, Soto Martinez M, Mora AM, Córdoba Gamboa L, Reich B, et al. 2022. Environmental exposures contribute to respiratory and allergic symptoms among women living in the banana growing regions of Costa Rica. Occup Environ Med 79(7):469–476, PMID: , 10.1136/oemed-2021-107611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Mora AM, Hoppin JA, Córdoba L, Cano JC, Soto-Martínez M, Eskenazi B, et al. 2020. Prenatal pesticide exposure and respiratory health outcomes in the first year of life: results from the Infants’ Environmental Health (ISA) study. Int J Hyg Environ Health 225:113474, PMID: , 10.1016/j.ijheh.2020.113474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Cupul-Uicab LA, Terrazas-Medina EA, Hernández-Ávila M, Longnecker MP. 2014. Prenatal exposure to p,p′-DDE and p,p′-DDT in relation to lower respiratory tract infections in boys from a highly exposed area of Mexico. Environ Res 132:19–23, PMID: , 10.1016/j.envres.2014.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Lermen J, Bernieri T, Rodrigues IS, Suyenaga ES, Ardenghi PG. 2018. Pesticide exposure and health conditions among orange growers in Southern Brazil. J Environ Sci Health B 53(4):215–221, PMID: , 10.1080/03601234.2017.1421823. [DOI] [PubMed] [Google Scholar]
- 239.Bernieri T, Rodrigues D, Randon Barbosa I, Perassolo MS, Grolli Ardenghi P, Basso da Silva L. 2021. Effect of pesticide exposure on total antioxidant capacity and biochemical parameters in Brazilian soybean farmers. Drug Chem Toxicol 44(2):170–176, PMID: , 10.1080/01480545.2019.1566353. [DOI] [PubMed] [Google Scholar]
- 240.Bahia CA, Guimarães RM, Asmus CIRF. 2014. Alterações nos marcadores hepáticos decorrentes da exposição ambiental a organoclorados no Brasil. Cad Saude Colet 22(2):133–141, 10.1590/1414-462X201400020005. [DOI] [Google Scholar]
- 241.Cestonaro LV, Garcia SC, Nascimento S, Gauer B, Sauer E, Göethel G, et al. 2020. Biochemical, hematological and immunological parameters and relationship with occupational exposure to pesticides and metals. Environ Sci Pollut Res Int 27(23):29291–29302, PMID: , 10.1007/s11356-020-09203-3. [DOI] [PubMed] [Google Scholar]
- 242.Ruíz-Arias MA, Herrera-Moreno JF, Medina-Díaz IM, Bernal-Hernández YY, González-Arias CA, Rojas-García AE. 2018. β-Glucuronidase and its relationship with clinical parameters and biomarkers of pesticide exposure. J Occup Environ Med 60(11):e602–e609, PMID: , 10.1097/JOM.0000000000001460. [DOI] [PubMed] [Google Scholar]
- 243.Hernández A, Gómez MA, Pena G, Gil F, Rodrigo L, Villanueva E, et al. 2004. Effect of long-term exposure to pesticides on plasma esterases from plastic greenhouse workers. J Toxicol Environ Health A 67(14):1095–1108, PMID: , 10.1080/15287390490452371. [DOI] [PubMed] [Google Scholar]
- 244.Ueyama J, Satoh T, Kondo T, Takagi K, Shibata E, Goto M, et al. 2010. β-Glucuronidase activity is a sensitive biomarker to assess low-level organophosphorus insecticide exposure. Toxicol Lett 193(1):115–119, PMID: , 10.1016/j.toxlet.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 245.Maluf E, Hamerschlak N, Cavalcanti AB, Júnior AA, Eluf-Neto J, Falcão RP, et al. 2009. Incidence and risk factors of aplastic anemia in Latin American countries: the LATIN case–control study. Haematologica 94(9):1220–1226, PMID: , 10.3324/haematol.2008.002642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Jacobsen-Pereira CH, Cardoso CC, Gehlen TC, Regina Dos Santos C, Santos-Silva MC. 2020. Immune response of Brazilian farmers exposed to multiple pesticides. Ecotoxicol Environ Saf 202:110912, PMID: , 10.1016/j.ecoenv.2020.110912. [DOI] [PubMed] [Google Scholar]
- 247.Dalbó J, Filgueiras LA, Mendes AN. 2019. Effects of pesticides on rural workers: haematological parameters and symptomalogical reports. Cien Saude Colet 24(7):2569–2582, PMID: , 10.1590/1413-81232018247.19282017. [DOI] [PubMed] [Google Scholar]
- 248.Cortés-Iza SC, Rodríguez AI, Prieto-Suarez E. 2017. Assessment of hematological parameters in workers exposed to organophosphorus pesticides, carbamates and pyrethroids in Cundinamarca 2016–2017. Rev Salud Publica (Bogota) 19(4):468–474, PMID: , 10.15446/rsap.v19n4.68092. [DOI] [PubMed] [Google Scholar]
- 249.Piccoli C, Cremonese C, Koifman R, Koifman S, Freire C. 2019. Occupational exposure to pesticides and hematological alterations: a survey of farm residents in the South of Brazil. Cien Saude Colet 24:2325–2340, PMID: , 10.1590/1413-81232018246.13142017. [DOI] [PubMed] [Google Scholar]
- 250.Molina-Pintor IB, Rojas-García AE, Bernal-Hernández YY, Medina-Díaz IM, González-Arias CA, Barrón-Vivanco BS. 2020. Relationship between butyrylcholinesterase activity and lipid parameters in workers occupationally exposed to pesticides. Environ Sci Pollut Res Int 27(31):39365–39374, PMID: , 10.1007/s11356-020-08197-2. [DOI] [PubMed] [Google Scholar]
- 251.Siller-López F, Garzón-Castaño S, Ramos-Márquez ME, Hernández-Cañaveral I. 2017. Association of paraoxonase-1 Q192R (rs662) single nucleotide variation with cardiovascular risk in coffee harvesters of central Colombia. J Toxicol 2017:6913106, PMID: , 10.1155/2017/6913106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Guida HL, Morini RG, Cardoso ACV. 2010. Avaliação audiológica em trabalhadores expostos a ruído e praguicida. Braz J Otorhinolaryngol 76(4):423–427, PMID: , 10.1590/S1808-86942010000400003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Bazilio MM, Frota S, Chrisman JR, Meyer A, Asmus CI, Camara VdeM. 2012. Temporal auditory processing in rural workers exposed to pesticide. J Soc Bras Fonoaudiol 24(2):174–180, PMID: , 10.1590/s2179-64912012000200015. [DOI] [PubMed] [Google Scholar]
- 254.Alcarás PAdeS, Larcerda ABM, Marques JM. 2013. Study of evoked otoacoustic emissions and suppression effect on workers exposed to pesticides and noise. Codas 25(6):527–533, PMID: , https://pdfs.semanticscholar.org/a0b6/451a8a094fc9bc9b654131be07073d347c66.pdf [accessed 30 June 2022]. [DOI] [PubMed] [Google Scholar]
- 255.de Sena TR, Vargas MM, Oliveira CC. 2013. Hearing care and quality of life among workers exposed to pesticides [in Portuguese]. Cien Saude Colet 18(6):1753–1761, PMID: . [PubMed] [Google Scholar]
- 256.Garcia TR, de Andrade MIKP, Frota SM, Miranda MF, Guimarães RM, Meyer A. 2017. Função coclear em escolares expostos aos agrotóxicos. Codas 29(3):e20160078, PMID: , 10.1590/2317-1782/20172016078. [DOI] [PubMed] [Google Scholar]
- 257.Tomiazzi JS, Pereira DR, Judai MA, Antunes PA, Favareto APA. 2019. Performance of machine-learning algorithms to pattern recognition and classification of hearing impairment in Brazilian farmers exposed to pesticide and/or cigarette smoke. Environ Sci Pollut Res Int 26(7):6481–6491, PMID: , 10.1007/s11356-018-04106-w. [DOI] [PubMed] [Google Scholar]
- 258.de Souza Alcarás PA, Zeigelboim BS, Corazza MCA, Lüders D, Marques JM, de Lacerda ABM. 2021. Findings on the central auditory functions of endemic disease control agents. Int J Environ Res Public Health 18(13):7051, PMID: , 10.3390/ijerph18137051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Mattiazzi ÂL, Caye JL, Frank JG, Endruweit Battisti ID. 2020. Hearing screening and cholinesterase activity among rural workers exposed to pesticides. Rev Bras Med Trab 17(2):239–246, PMID: , 10.5327/Z1679443520190374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Suarez-Lopez JR, Jacobs DR Jr, Himes JH, Alexander BH. 2013. Acetylcholinesterase activity, cohabitation with floricultural workers, and blood pressure in Ecuadorian children. Environ Health Perspect 121(5):619–624, PMID: , 10.1289/ehp.1205431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Suarez-Lopez JR, Hong V, McDonald KN, Suarez-Torres J, López D, De La Cruz F. 2018. Home proximity to flower plantations and higher systolic blood pressure among children. Int J Hyg Environ Health 221(8):1077–1084, PMID: , 10.1016/j.ijheh.2018.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Suarez-Lopez JR, Amchich F, Murillo J, Denenberg J. 2019. Blood pressure after a heightened pesticide spray period among children living in agricultural communities in Ecuador. Environ Res 175:335–342, PMID: , 10.1016/j.envres.2019.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Cupul-Uicab LA, Terrazas-Medina EA, Hernández-Ávila M, Longnecker MP. 2017. In utero exposure to DDT and incidence of diarrhea among boys from tropical Mexico. Environ Res 159:331–337, PMID: , 10.1016/j.envres.2017.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Meyer A, Sandler DP, Beane Freeman LE, Hofmann JN, Parks CG. 2017. Pesticide exposure and risk of rheumatoid arthritis among licensed male pesticide applicators in the Agricultural Health Study. Environ Health Perspect 125(7):077010, PMID: , 10.1289/EHP1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Mejia-Sanchez F, Montenegro-Morales LP, Castillo-Cadena J. 2018. Enzymatic activity induction of GST-family isoenzymes from pesticide mixture used in floriculture. Environ Sci Pollut Res Int 25(1):601–606, PMID: , 10.1007/s11356-017-0410-7. [DOI] [PubMed] [Google Scholar]
- 266.Machado AKF, Wendt A, Wehrmeister FC. 2018. Sleep problems and associated factors in a rural population of a southern Brazilian city. Rev Saude Publica 52(1):5s, 10.11606/S1518-8787.2018052000260. [DOI] [Google Scholar]
- 267.Butinof M, Fernandez RA, Stimolo MI, Lantieri MJ, Blanco M, Machado AL, et al. 2015. Pesticide exposure and health conditions of terrestrial pesticide applicators in Córdoba Province, Argentina. Cad Saude Publica 31(3):633–646, PMID: , 10.1590/0102-311x00218313. [DOI] [PubMed] [Google Scholar]
- 268.Cezar-Vaz MR, Bonow CA, de Mello MCVA, da Silva MRS. 2016. Socio-environmental approach in nursing: focusing on rural labor and the use of pesticides. Rev Bras Enferm 69(6):1179–1187, PMID: , 10.1590/0034-7167-2016-0364. [DOI] [PubMed] [Google Scholar]
- 269.de Carvalho MP, Fiori NS, Meucci RD, Faria NMX, Fassa AG. 2020. Thoracic spine pain and associated factors among tobacco farmers. Rev Bras Saude Ocup 45:e33, 10.1590/2317-6369000002019. [DOI] [Google Scholar]
- 270.Fassa AG, Spada Fiori N, Dalke Meucci R, Müller Xavier Faria N, Peres de Carvalho M. 2020. Neck pain among tobacco farm workers in southern Brazil [in Spanish]. Salud Colect 16:e2307, PMID: , 10.18294/sc.2020.2307. [DOI] [PubMed] [Google Scholar]
- 271.Araújo RAL, Cremonese C, Santos R, Piccoli C, Carvalho G, Freire C, et al. 2021. Association of occupational exposure to pesticides with overweight and abdominal obesity in family farmers in southern Brazil. Int J Environ Health Res 2021:1–12, PMID: , 10.1080/09603123.2021.1991284. [DOI] [PubMed] [Google Scholar]
- 272.Campos É, Costa VIDB, Alves SR, Rosa ACS, Geraldino BR, Meira BDC, et al. 2020. Occurrence of green tobacco sickness and associated factors in farmers residing in Dom Feliciano Municipality, Rio Grande do Sul State, Southern Region of Brazil. Cad Saude Publica 36(8):e00122719, PMID: , 10.1590/0102-311x00122719. [DOI] [PubMed] [Google Scholar]
- 273.Schneider Medeiros M, Reddy SP, Socal MP, Schumacher-Schuh AF, Mello Rieder CR. 2020. Occupational pesticide exposure and the risk of death in patients with Parkinson’s disease: an observational study in southern Brazil. Environ Health 19(1):68, PMID: , 10.1186/s12940-020-00624-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Okuyama JHH, Galvão TF, Silva MT, Grupo Datatox. 2020. Poisoning and associated factors to death from pesticides: case–control study, Brazil, 2017. Rev Bras Epidemiol 23:e200024, PMID: , 10.1590/1980-549720200024. [DOI] [PubMed] [Google Scholar]
- 275.Luce D, Dugas J, Vaidie A, Michineau L, El-Yamani M, Multigner L. 2020. A cohort study of banana plantation workers in the French West Indies: first mortality analysis (2000–2015). Environ Sci Pollut Res Int 27(33):41014–41022, PMID: , 10.1007/s11356-019-06481-4. [DOI] [PubMed] [Google Scholar]
- 276.de Souza A, Medeiros Ados R, de Souza AC, Wink M, Siqueira IR, Ferreira MB, et al. 2011. Evaluation of the impact of exposure to pesticides on the health of the rural population: Vale do Taquari, State of Rio Grande do Sul (Brazil) [in Portugeuse]. Cien Saude Colet 16(8):3519–3528, PMID: , 10.1590/s1413-81232011000900020. [DOI] [PubMed] [Google Scholar]
- 277.Muñoz-Quezada MT, Lucero B, Iglesias V, Levy K, Muñoz MP, Achú E, et al. 2017. Exposure to organophosphate (OP) pesticides and health conditions in agricultural and non-agricultural workers from Maule, Chile. Int J Environ Health Res 27(1):82–93, PMID: , 10.1080/09603123.2016.1268679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.González-Alzaga B, Lacasaña M, Aguilar-Garduño C, Rodríguez-Barranco M, Ballester F, Rebagliato M, et al. 2014. A systematic review of neurodevelopmental effects of prenatal and postnatal organophosphate pesticide exposure. Toxicol Lett 230(2):104–121, PMID: , 10.1016/j.toxlet.2013.11.019. [DOI] [PubMed] [Google Scholar]
- 279.Takahashi N, Hashizume M. 2014. A systematic review of the influence of occupational organophosphate pesticides exposure on neurological impairment. BMJ Open 4(6):e004798, PMID: , 10.1136/bmjopen-2014-004798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Fuhrimann S, Wan C, Blouzard E, Veludo A, Holtman Z, Chetty-Mhlanga S, et al. 2022. Pesticide research on environmental and human exposure and risks in sub-Saharan Africa: a systematic literature review. Int J Environ Res Public Health 19(1):259, PMID: , 10.3390/ijerph19010259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Muñoz-Piña C, Avila Forcada S. 2004. Effects of an environmental tax on pesticides in Mexico. Ind Environ 27(2):33–36. [Google Scholar]
- 282.Sharma A, Kumar V, Shahzad B, Tanveer M, Sidhu GPS, Handa N, et al. 2019. Worldwide pesticide usage and its impacts on ecosystem. SN Appl Sci 1(11):1446, 10.1007/s42452-019-1485-1. [DOI] [Google Scholar]
- 283.Directorate General for External Policies of the Union. 2021. The Use of Pesticides in Developing Countries and Their Impact on Health and the Right to Food. PE 653.622. Brussels, Belgium: European Commission, European Parliament Policy Department. https://www.europarl.europa.eu/RegData/etudes/STUD/2021/653622/EXPO_STU(2021)653622_EN.pdf [accessed 30 June 2022]. [Google Scholar]
- 284.McKee M, Stuckler D, Basu S. 2012. Where there is no health research: what can be done to fill the global gaps in health research? PLoS Med 9(4):e1001209, PMID: , 10.1371/journal.pmed.1001209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Tulloch-Reid MK, Saravia NG, Dennis RJ, Jaramillo A, Cuervo LG, Walker SP, et al. 2018. Strengthening institutional capacity for equitable health research: lessons from Latin America and the Caribbean. BMJ 362:k2456, PMID: , 10.1136/bmj.k2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Blair A, Thomas K, Coble J, Sandler DP, Hines CJ, Lynch CF, et al. 2011. Impact of pesticide exposure misclassification on estimates of relative risks in the Agricultural Health Study. Occup Environ Med 68(7):537–541, PMID: , 10.1136/oem.2010.059469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Bradman A, Kogut K, Eisen EA, Jewell NP, Quirós-Alcalá L, Castorina R, et al. 2013. Variability of organophosphorous pesticide metabolite levels in spot and 24-hr urine samples collected from young children during 1 week. Environ Health Perspect 121(1):118–124, PMID: , 10.1289/ehp.1104808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Carles C, Bouvier G, Lebailly P, Baldi I. 2017. Use of job-exposure matrices to estimate occupational exposure to pesticides: a review. J Expo Sci Environ Epidemiol 27(2):125–140, PMID: , 10.1038/jes.2016.25. [DOI] [PubMed] [Google Scholar]
- 289.Ohlander J, Fuhrimann S, Basinas I, Cherrie JW, Galea KS, Povey AC, et al. 2020. Systematic review of methods used to assess exposure to pesticides in occupational epidemiology studies, 1993–2017. Occup Environ Med 77(6):357–367, PMID: , 10.1136/oemed-2019-105880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Mueller W, Atuhaire A, Mubeezi R, van den Brenk I, Kromhout H, Basinas I, et al. 2022. Evaluation of two-year recall of self-reported pesticide exposure among Ugandan smallholder farmers. Int J Hyg Environ Health 240:113911, PMID: , 10.1016/j.ijheh.2021.113911. [DOI] [PubMed] [Google Scholar]
- 291.Tielemans E, Bretveld R, Schinkel J, van Wendel de Joode B, Kromhout H, Gerritsen-Ebben R, et al. 2007. Exposure profiles of pesticides among greenhouse workers: implications for epidemiological studies. J Expo Sci Environ Epidemiol 17(6):501–509, PMID: , 10.1038/sj.jes.7500544. [DOI] [PubMed] [Google Scholar]
- 292.López-Gálvez N, Wagoner R, Beamer P, de Zapien J, Rosales C. 2018. Migrant farmworkers’ exposure to pesticides in Sonora, Mexico. Int J Environ Res Public Health 15(12):2651, PMID: , 10.3390/ijerph15122651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Barr DB, Bravo R, Weerasekera G, Caltabiano LM, Whitehead RD Jr, Olsson AO, et al. 2004. Concentrations of dialkyl phosphate metabolites of organophosphorus pesticides in the U.S. population. Environ Health Perspect 112(2):186–200, PMID: , 10.1289/ehp.6503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Bradman A, Castorina R, Barr DB, Chevrier J, Harnly ME, Eisen EA, et al. 2011. Determinants of organophosphorus pesticide urinary metabolite levels in young children living in an agricultural community. Int J Environ Res Public Health 8(4):1061–1083, PMID: , 10.3390/ijerph8041061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Buszewski B, Bukowska M, Ligor M, Staneczko-Baranowska I. 2019. A holistic study of neonicotinoids neuroactive insecticides—properties, applications, occurrence, and analysis. Environ Sci Pollut Res Int 26(34):34723–34740, PMID: , 10.1007/s11356-019-06114-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Jeschke P, Nauen R, Schindler M, Elbert A. 2011. Overview of the status and global strategy for neonicotinoids. J Agric Food Chem 59(7):2897–2908, PMID: , 10.1021/jf101303g. [DOI] [PubMed] [Google Scholar]
- 297.Li HZ, Cheng F, Wei Y, Lydy MJ, You J. 2017. Global occurrence of pyrethroid insecticides in sediment and the associated toxicological effects on benthic invertebrates: an overview. J Hazard Mater 324(pt B):258–271, PMID: , 10.1016/j.jhazmat.2016.10.056. [DOI] [PubMed] [Google Scholar]
- 298.Rauh VA, Margolis AE. 2016. Research review: environmental exposures, neurodevelopment, and child mental health—new paradigms for the study of brain and behavioral effects. J Child Psychol Psychiatry 57(7):775–793, PMID: , 10.1111/jcpp.12537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Hensch TK. 2004. Critical period regulation. Ann Rev Neurosci 27(1):549–579, PMID: , 10.1146/annurev.neuro.27.070203.144327. [DOI] [PubMed] [Google Scholar]
- 300.De Luca G, Olivieri F, Melotti G, Aiello G, Lubrano L, Boner AL. 2010. Fetal and early postnatal life roots of asthma. J Matern Fetal Neonatal Med 23(suppl 3):80–83, PMID: , 10.3109/14767058.2010.509931. [DOI] [PubMed] [Google Scholar]
- 301.Raanan R, Harley KG, Balmes JR, Bradman A, Lipsett M, Eskenazi B. 2015. Early-life exposure to organophosphate pesticides and pediatric respiratory symptoms in the CHAMACOS cohort. Environ Health Perspect 123(2):179–185, PMID: , 10.1289/ehp.1408235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Baygi F, Herttua K, Jensen OC, Djalalinia S, Mahdavi Ghorabi A, Asayesh H, et al. 2020. Global prevalence of cardiometabolic risk factors in the military population: a systematic review and meta-analysis. BMC Endocr Disord 20(1):8, PMID: , 10.1186/s12902-020-0489-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Gitler AD, Dhillon P, Shorter J. 2017. Neurodegenerative disease: models, mechanisms, and a new hope. Dis Models Mech 10(5):499–502, PMID: , 10.1242/dmm.030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Damalas CA, Koutroubas SD. 2016. Farmers’ exposure to pesticides: toxicity types and ways of prevention. Toxics 4(1):1, PMID: , 10.3390/toxics4010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Hamra GB, Buckley JP. 2018. Environmental exposure mixtures: questions and methods to address them. Curr Epidemiol Rep 5(2):160–165, PMID: , 10.1007/s40471-018-0145-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Gibson EA, Nunez Y, Abuawad A, Zota AR, Renzetti S, Devick KL, et al. 2019. An overview of methods to address distinct research questions on environmental mixtures: an application to persistent organic pollutants and leukocyte telomere length. Environ Health 18(1):76, PMID: , 10.1186/s12940-019-0515-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Appleton AA, Holdsworth EA, Kubzansky LD. 2016. A systematic review of the interplay between social determinants and environmental exposures for early-life outcomes. Curr Environ Health Rep 3(3):287–301, PMID: , 10.1007/s40572-016-0099-7. [DOI] [PubMed] [Google Scholar]
- 308.Cory-Slechta DA. 2005. Studying toxicants as single chemicals: does this strategy adequately identify neurotoxic risk? Neurotoxicology 26(4):491–510, PMID: , 10.1016/j.neuro.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 309.Clougherty JE, Shmool JLC, Kubzansky LD. 2014. The role of non-chemical stressors in mediating socioeconomic susceptibility to environmental chemicals. Curr Environ Health Rep 1(4):302–313, 10.1007/s40572-014-0031-y. [DOI] [Google Scholar]
- 310.Kordas K, Lönnerdal B, Stoltzfus RJ. 2007. Interactions between nutrition and environmental exposures: effects on health outcomes in women and children. J Nutr 137(12):2794–2797, PMID: , 10.1093/jn/137.12.2794. [DOI] [PubMed] [Google Scholar]
- 311.Pan American Health Organization/World Health Organization. 2009. Policy on Research for Health. Document CD49/10 of the 49th Directing Council, 61st Session of the Regional Committee. Washington, DC: Pan American Health Organization/World Health Organization. https://www.paho.org/hq/dmdocuments/2009/CD49-10-e.pdf [accessed 15 June 2022]. [Google Scholar]
- 312.Franzen SRP, Chandler C, Lang T. 2017. Health research capacity development in low and middle income countries: reality or rhetoric? A systematic meta-narrative review of the qualitative literature. BMJ Open 7(1):e012332, PMID: , 10.1136/bmjopen-2016-012332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Ali R, Finlayson A, Indox Cancer Research Network. 2012. Building capacity for clinical research in developing countries: the INDOX Cancer Research Network experience. Glob Health Action 5(1):17288, PMID: , 10.3402/gha.v5i0.17288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Barreto ML. 2009. Health research in developing countries. BMJ 339:b4846, PMID: , 10.1136/bmj.b4846. [DOI] [PubMed] [Google Scholar]
- 315.Lopez-Verges S, Valiente-Echeverría F, Godoy-Faúndez A, Fernandez Rivas D, Urbani B, Berger JJ, et al. 2021. Call to action: supporting Latin American early career researchers on the quest for sustainable development in the region. Front Res Metr Anal 6:657120, PMID: , 10.3389/frma.2021.657120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Vryzas Z, Ramwell C, Sans C. 2020. Pesticide prioritization approaches and limitations in environmental monitoring studies: from Europe to Latin America and the Caribbean. Environ Int 143:105917, PMID: , 10.1016/j.envint.2020.105917. [DOI] [PubMed] [Google Scholar]
- 317.Rocha CBD, Nascimento APC, da Silva AMC, Botelho C. 2021. Uncontrolled asthma in children and adolescents exposed to pesticides in an area of intense agribusiness activity [in Portuguese]. Cad Saude Publica 37(5):e00072220, PMID: , 10.1590/0102-311x00072220. [DOI] [PubMed] [Google Scholar]
- 318.Saunders L, Kadhel P, Costet N, Rouget F, Monfort C, Thomé JP, et al. 2014. Hypertensive disorders of pregnancy and gestational diabetes mellitus among French Caribbean women chronically exposed to chlordecone. Environ Int 68:171–176, PMID: , 10.1016/j.envint.2014.03.024. [DOI] [PubMed] [Google Scholar]
- 319.Hutter HP, Poteser M, Lemmerer K, Wallner P, Kundi M, Moshammer H, et al. 2021. Health symptoms related to pesticide use in farmers and laborers of ecological and conventional banana plantations in Ecuador. Int J Environ Res Public Health 18(3):1126, PMID: , 10.3390/ijerph18031126. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.