Evidence of complement activation, endothelial injury, and thrombotic microangiopathy (TMA) is becoming increasingly recognized in patients with acute SARS-CoV-2 infection and multisystem inflammatory syndrome in children (MIS-C) and adults (MIS-A). Recently, Diorio et al. found elevated lactate dehydrogenase (LDH) and soluble C5b-9 (sC5b-9), anemia, thrombocytopenia, schistocytes, renal damage, and hypertension in a subset of pediatric patients with active SARS-CoV-2 infection and MIS-C, consistent with complement-mediated TMA (CM-TMA).1 Eculizumab, an anti-C5 monoclonal antibody that inhibits terminal complement activation, has been used to treat hematopoietic cell transplant-associated TMA (TA-TMA), with a response rate of up to 66%.2 Given reported findings consistent with TMA in both acute SARS-CoV-2 infection and MIS-C, eculizumab may provide therapeutic benefit in this setting. However, there is a paucity of data describing its use. Here we present three patients diagnosed with TMA during acute COVID-19/MIS-C who were treated with eculizumab and propose a “multiple-hit” hypothesis for pediatric patients with an increased risk of SARS-CoV-2 associated TMA.
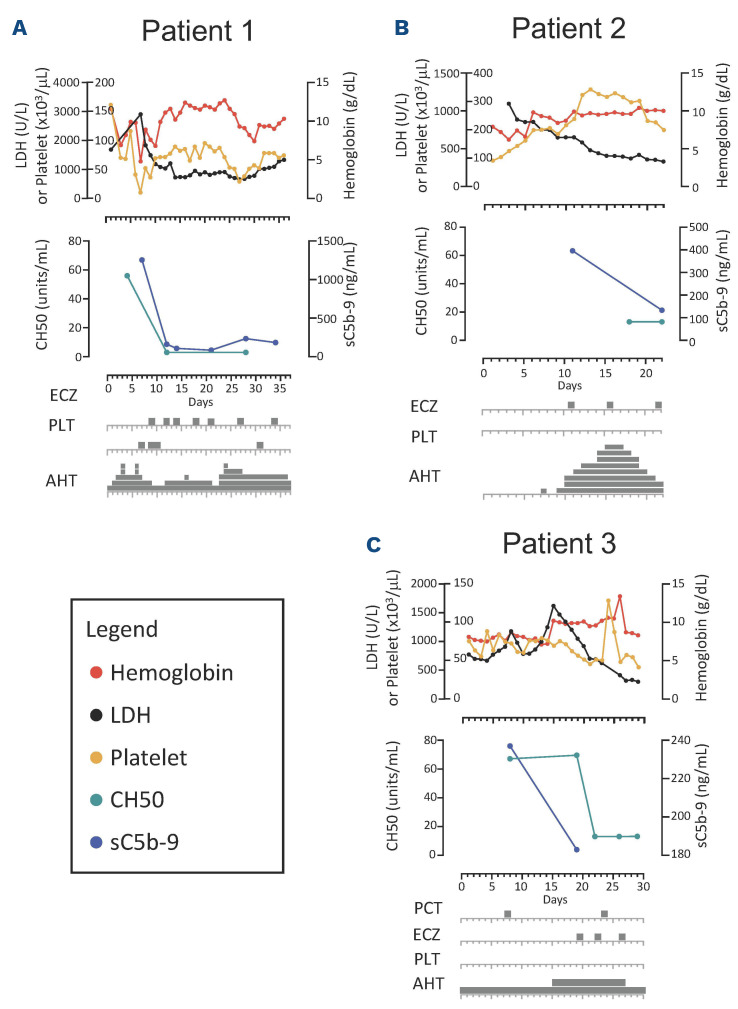
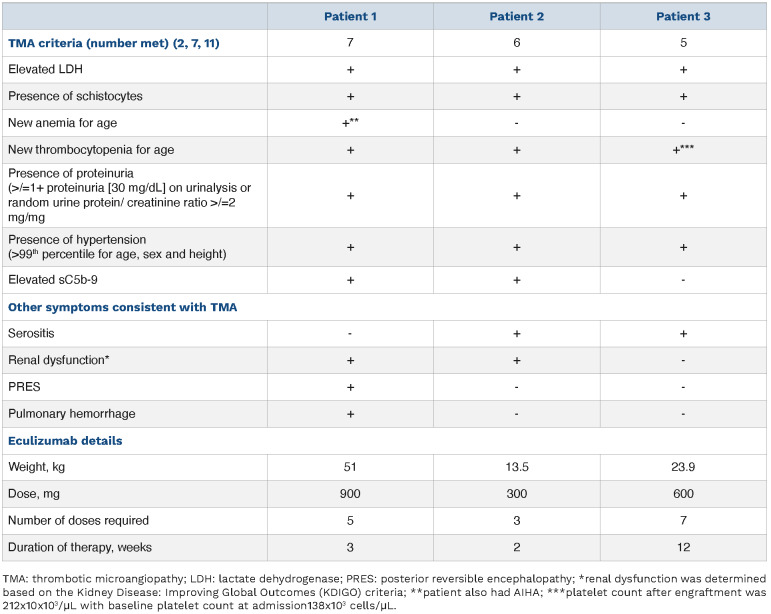
Patient 1 (Figure 1A) is an 11-year-old Black female with sickle cell disease (HbS β thalassemia) who was initially hospitalized with positive SARS-CoV-2 PCR and acute chest syndrome. While hospitalized, she developed warm autoimmune hemolytic anemia (AIHA) with initial response to IVIG and methylprednisolone. She was discharged on prednisone (1 mg/kg every 12 hours). Five days after discharge she presented in status epilepticus and was notably hypertensive and intermittently febrile. Schistocytes and an elevated LDH were noted. Brain magnetic resonance imaging with angiography (MRI/MRA) was most consistent with posterior reversible encephalopathy syndrome (PRES). Despite continuation of prednisone for AIHA, she developed worsening anemia and thrombocytopenia, both requiring transfusions, and worsening refractory hypertension requiring five antihypertensive medications. Elevated temperature, C-reactive protein (CRP; peak 27.3 mg/dL), ferritin (peak 34,053 ng/mL), and D-Dimer (peak >20.0 µg/mL) also suggested a hyperinflammatory state (Figure 1A). This prompted, on hospital day (HD) 7-8, initiation of high-dose methylprednisolone (1 g/day divided every 6 hours), rituximab (560 mg), and IVIG (1 g/kg) for aggressive AIHA treatment, and anakinra (150 mg every 6 hours) and tocilizumab (400 mg twice) for treatment of hyperinflammation (Figure 1A). Despite these therapies, on HD 9 she developed new hypoxic respiratory failure and bronchoscopy revealed diffuse alveolar hemorrhage (DAH). She also demonstrated pulmonary hypertension on transthoracic echocardiogram (TEE), proteinuria (peak 5.15 mg/mg creatinine), elevated LDH (peak 2,905 units/L), elevated sC5b-9 (peak 1,254 ng/mL), thrombocytopenia refractory to platelet transfusions, schistocytes, and a low C4, consistent with CM-TMA (Table 1). Following the diagnosis of CM-TMA, she received her first dose of eculizumab (HD 9), after which both her CH50 and sC5b9 levels normalized, and her DAH and pulmonary hypertension all rapidly improved. Prior to eculizumab she required platelets and blood almost daily. Following her second dose of eculizumab (HD 12) she did not require any transfusions for 3 weeks. Following her third dose of eculizumab (HD 14), she was transferred out of the intensive care unit. She was able to be discharged on HD 36.
Patient 2 (Figure 1B) is a 2-year-old Black female with sickle cell (Hb SC) disease who presented with 1 day of fever and vaso-occlusive crisis. She had no respiratory symptoms or known history of COVID-19. After hospital admission, she was persistently febrile with rising inflammatory markers despite antibiotic therapy. Infectious disease work-up was negative including blood cultures, urine cultures, and chest X-ray (CXR) were negative for pathology, but revealed anti-SARS-CoV-2 antibodies in blood with a negative SARS-CoV-2 PCR. Further evaluation demonstrated elevated CRP (19.9 mg/dL), ferritin (2,913 ng/mL), B-type natriuretic peptide (NT-proBNP; 39,972 pg/mL), and D-dimer (14.59 µg/mL) with a normal troponin T. TTE revealed mildly reduced left ventricle ejection fraction (LVEF) of 50-55%, diastolic dysfunction, and a moderate pericardial effusion. Based on these findings and presence of thrombocytopenia, a diagnosis of MIS-C was made. IVIG (2 g/kg), methylprednisolone (1 mg/kg every 12 hours) and enoxaparin were initiated. Though she initially had no evidence of renal dysfunction, her creatinine gradually increased during her hospitalization (peak HD 5), and she developed severe hypertension requiring initiation of five antihypertensives. Repeat CXR demonstrated bilateral pleural effusions. Given the concern for uncontrolled inflammation and serositis, anakinra (10 mg/kg/day, divided every 6 hours) and ibuprofen were started on HD 10. Based on lack of improvement of severe LDH elevation (peak 885 units/L), anemia (nadir 6.3 g/dL), thrombocytopenia, presence of schistocytes, proteinuria (2+), severe hypertension, serositis, and elevated soluble C5b-9 (396 ng/mL) despite intensification of MIS-C therapy, the patient was diagnosed with CM-TMA and was treated with eculizumab 600 mg (Table 1). Two days following the first dose of eculizumab, her blood pressure improved and three of her five antihypertensives were discontinued. After her second dose of eculizumab 600 mg, she was transferred out of the intensive care unit and only required one antihypertensive agent. Her inflammatory markers, renal function, and thrombocytopenia all returned to baseline following her second dose. Subsequent TTE showed improvement in LVEF and resolution of the pericardial effusion. By the end of her hospital stay, her sC5b-9 level normalized, and she was discharged on one cardioprotective antihypertensive medication.
Figure 1.
Temporal association of clinical and laboratory improvement with eculizumab dosing. (A) In patient 1, lactate dehydrogenase (LDH) drastically decreased after the first dose of eculizumab. Terminal complement (C5b-9) and total complement (CH50) notably decreased after the first dose of eculizumab illustrating adequate inhibition of the complement cascade. Hemoglobin and platelets count stabilized after 2 doses of eculizumab with significant reduction in need for platelet transfusions. Systemic hypertension fluctuated during the hospital course with no temporal association with eculizumab. (B) In patient 2, initial improvement in LDH and hemoglobin as hemolysis related to improvement of underlying vaso-occlusive crisis can be seen; however, thrombocytopenia persists despite use of steroids, anakinra and IVIG for treatment of multisystem inflammatory syndrome in children (MIS-C). Peak creatinine occurred on hopital day (HD) 5 with improvement to baseline by HD 8. There was continued improvement of LDH and hemoglobin and resolution of thrombocytopenia after the initiation of eculizumab. Adequate complement inhibition was achieved based on decreased C5b-9 and CH50 levels. Systolic and diastolic blood pressure increased on HD 6. Hypertension was progressive and refractory to multiple antihypertensives. After the second dose of eculizumab, blood pressure improved, and antihypertensive agents were aggressively weaned, and steroids were titrated down with the improvement of inflammation. (C) In patient 3, there was improvement in hemoglobin and C5b-9 with cyclosporine discontinuation. Adequate complement inhibition was achieved based on decreased CH50 levels. LDH showed rapid and progressive reduction after initiation of eculizumab. PCT: pericardiocentesis; PLT: platelet transfusion; ECZ: eculizumab; AHT: hypertensive medications.
Patient 3 (Figure 1C) is a 6-year-old Hispanic male with relapsed B-cell acute lymphoblastic leukemia admitted with fever and hypoxemia on day +45 following a matched sibling donor hematopoietic cell transplant. Work-up revealed a positive SARS-CoV-2 PCR on HD 1. Elevated Creactive protein (CRP) (3.9 mg/dL), ferritin (680 ng/mL), D-dimer (0.83 µg/mL) and NT-proBNP; 1,740 pg/mL were noted without elevation of troponin-T. A TTE on HD 1 showed a pericardial effusion without tamponade physiology and preserved biventricular function. Remdesivir (125 mg daily for 10 days) and a single infusion of convalescent plasma were given with improvement of respiratory status. However, on HD 12 repeat CXR and TTE demonstrated worsening serositis with bilateral pleural effusions and enlargement of the pericardial effusion with tamponade physiology. Emergent pericardial drainage was performed, and dexamethasone (0.15 mg/kg/day for 10 days) was started. Additionally, the patient was noted to have rising LDH, schistocytes, thrombocytopenia, proteinuria (4.51 mg/mg creatinine), and hypertension which was suggestive of evolving TMA (Table 1; Figure 1C). On HD 15, cyclosporine for graft-versus-host disease (GVHD) prophylaxis was discontinued with some improvement in anemia and sCb-9 but without resolution of serositis or significant improvement in other laboratory findings (proteinuria [11.45 mg/mg creatinine], LDH, schistocyte count [7.1 OIF schistocytes/100 X objective]). The patient underwent a pericardial window procedure for persistent pericardial effusion and eculizumab was started on HD 19 for TA-TMA (Table 1). After initiation of eculizumab, LDH and schistocyte count improved (3.2 OIF) and hemoglobin stabilized (Figure 1C). After the second dose of eculizumab, proteinuria resolved, and antihypertensive therapy was weaned. He was discharged after a 4-week hospitalization and eculizumab was continued 8 weeks after discharge. His platelet count, creatinine, and hemoglobin normalized with this therapy.
Table 1.
Signs and symptoms of complement-mediated thrombotic microangiopathy in patient series.
There has been increasing attention given to the contribution of complement pathway activation in the pathogenesis of SARS-CoV-2 infection (Figure 2). Indeed, elevated soluble C5b-9 has been found to correlate with SARS-CoV-2 disease severity.1,3,4 Reported efficacy of terminal complement inhibition for treatment of severe acute COVID-19 has been mixed. Initially, there was evidence of improved outcomes in a proof-of-concept study;5 however, a phase III trial of ravulizumab (mAb for C5a; clinicaltrials gov. Identifier: NCT 04369469) was stopped early due to a lack of efficacy. However, these studies did not select for patients with clear evidence of TMA or endothelial dysfunction, which leaves open the possibility of efficacy for the subset of patients with these findings in the setting of COVID-19.
Figure 2.
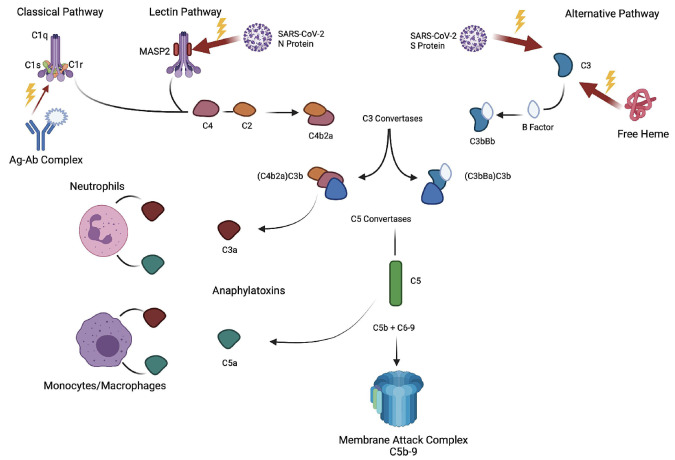
Multiple-hit hypothesis of complement activation. SARS-CoV-2 infection leads to activation of the complement cascade through all three known pathways - classical, lectin, and alternative. The classical pathway is activated by binding of the C1 complex with antigen-antibody (Ag-Ab) complexes. The robust formation and continued presence of Ag-Ab complexes may act as a driver of complement activation in the setting of patient with multisystem inflammatory syndrome in children (MIS-C). SARS-CoV-2 N protein is thought to interact with the MASP2-MBL complex leading to activation of the lectin pathway.8 SARS-CoV-2 S protein interacts and activates the C3 within the alternative pathway.10,12 All 3 pathways converge with the production of C3 and C5 convertases and ultimately the cleavage and production of both C3a and C5a, which are anaphylatoxins, and C5b, leading to the development of the membrane attach complex (MAC). C3a and C5a propagate inflammation by recruiting neutrophils and monocytes/macrophages, which release proinflammatory cytokines, leading to damage to surrounding tissue and endothelium. Additionally, in the setting of complement-mediated thrombotic microangiopathy (CM-TMA), the MAC is thought to lead to endothelial damage by forming in the membrane of endothelial cells, endotheliitis, and the formation of microthrombi.2,7 In our patient series, there was likely additional activation of the complement system in the setting of sickle cell disease (patients 1 and 2) or autoimmune hemolytic anemia due to free heme that is known to interact with C3 (alternative pathway), which lead to increased due to free heme that is known to cause C3 (alternative pathway) hydrolysis with production of downstream complement (C3a, C3b, C5a, C5b).9 Patient 3 was day +45 after hematopoietic cell transplant and on a calcineurin inhibitor for graft-versus-host disease prophylaxis that is known to lead to increased complement activation through the alternative pathway.2,7 An additional risk factor that was unknown in our patients could be the presence of complement mutations leading to issues with homeostatic balance of complement activity. The mechanism of action of eculizumab is the binding of C5, thereby preventing the formation of C5a and the MAC preventing downstream effects. All patients in this series either showed evidence of or had presumed increased level of MAC with clinical response with initiation of eculizumab.
Here we discuss three patients with diagnoses of acute COVID-19/MIS-C and complement-mediated TMA with progressive end organ dysfunction, refractory hypertension, acute kidney injury, and serositis and/or DAH, despite appropriate COVID-19/MIS-C directed therapies (Table 1). Interestingly, all patients had a biphasic clinical course with initial improvement upon starting COVID-19/MIS-C directed therapies followed by clinical worsening (median 9 days after admission). After treatment with eculizumab, all patients had appropriate laboratory response and improvement in serositis, hypertension, and organ function. The diagnosis of TMA in these patients was difficult due to possible alternative etiologies for the corresponding abnormal findings, such as elevated LDH in a patient with sickle cell disease. However, the presence of multiple specific TMA-related findings along with clinical response to eculizumab are suggestive of CM-TMA. All three patients had known causes of underlying endothelial injury and complement activation, including AIHA, TA-TMA, and sickle cell disease.6 We hypothesize that the additional complement activation associated with SARS-CoV-2 infection contributed to a multiple-hit pathogenesis that resulted in uncontrolled complement activation and severe organ dysfunction in these at-risk patients (Figure 2).2,6-10 None of these patients experienced recurrent TMA, consistent with previous reports of resolution of increased terminal complement levels by 30 days after initial SARS-CoV-2 infection.3
CM-TMA should be considered in patients with acute COVID-19/MIS-C who have known underlying endothelial injury and evidence of worsening end organ dysfunction, particularly if previously showing improvement or failing standard therapy. In these situations, terminal complement inhibition with eculizumab may offer therapeutic benefit. Further investigation is warranted to determine efficacy and length of therapy of eculizumab in patients with evidence of TMA and end organ dysfunction in the setting of acute COVID-19/MIS-C.
Funding Statement
Funding: GM receives research funding from Astellas Inc and SymBio Pharmaceuticals Limited. MH receives research funding from Incyte. AS is the site principal investigator of clinical trials for genome editing of sickle cell disease sponsored by Vertex Pharmaceuticals/CRISPR Therapeutics (clinicaltrials gov. Identifier: NCT03745287) and Novartis (clinicaltrials gov. Identifier: NCT04443907). The industry sponsors provide funding for the clinical trial, which includes salary support paid to AS institution. AS has received consultant fee from Spotlight Therapeutics, Medexus Inc. and Vertex Pharmaceuticals. AS has also received research funding from CRISPR Therapeutics and honoraria from Vindico Medical Education. JH receives research funding from Global Blood Therapeutics and consultancy fees from Global Blood Therapeutics, Forma Therapeutics and bluebird bio. SB receives grant support from the American Society of Hematology.
References
- 1.Diorio C, Henrickson SE, Vella LA, et al. Multisystem inflammatory syndrome in children and COVID-19 are distinct presentations of SARS-CoV-2. J Clin Invest. 2020;130(11):5967-5975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jodele S, Dandoy CE, Lane A, et al. Complement blockade for TA-TMA: lessons learned from a large pediatric cohort treated with eculizumab. Blood. 2020;135(13):1049-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cugno M, Meroni PL, Gualtierotti R, et al. Complement activation and endothelial perturbation parallel COVID-19 severity and activity. J Autoimmun. 2021;116:102560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Nooijer AH, Grondman I, Janssen NAF, et al. Complement activation in the disease course of coronavirus disease 2019 and its effects on clinical outcomes. J Infect Dis. 2021;223(2):214-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Annane D, Heming N, Grimaldi-Bensouda L, et al. Eculizumab as an emergency treatment for adult patients with severe COVID-19 in the intensive care unit: a proof-of-concept study. EClinicalMedicine. 2020;28:100590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frimat M, Tabarin F, Dimitrov JD, et al. Complement activation by heme as a secondary hit for atypical hemolytic uremic syndrome. Blood. 2013;122(2):282-292. [DOI] [PubMed] [Google Scholar]
- 7.Jodele S, Laskin BL, Dandoy CE, et al. A new paradigm: diagnosis and management of HSCT-associated thrombotic microangiopathy as multi-system endothelial injury. Blood Rev. 2015;29(3):191-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chouaki Benmansour N, Carvelli J, Vivier E. Complement cascade in severe forms of COVID-19: recent advances in therapy. Eur J Immunol. 2021;51(7):1652-1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Merle NS, Grunenwald A, Rajaratnam H, et al. Intravascular hemolysis activates complement via cell-free heme and hemeloaded microvesicles. JCI Insight. 2018;3(12):e96910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu J, Yuan X, Chen H, et al. Direct activation of the alternative complement pathway by SARS-CoV-2 spike proteins is blocked by factor D inhibition. Blood. 2020;136(18):2080-2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gloude NJ, Dandoy CE, Davies SM, et al. Thinking beyond HLH: clinical features of patients with concurrent presentation of hemophagocytic lymphohistiocytosis and thrombotic microangiopathy. J Clin Immunol. 2020;40(5):699-707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yan B, Freiwald T, Chauss D, et al. SARS-CoV-2 drives JAK1/2-dependent local complement hyperactivation. Sci Immunol. 2021;6(58):eabg0833. [DOI] [PMC free article] [PubMed] [Google Scholar]