Abstract
In order to improve the safety of COVID-19 vaccines, there is an urgent need to unravel the pathogenesis of vaccine-induced immune thrombotic thrombocytopenia (VITT), a severe complication of recombinant adenoviral vector vaccines used to prevent COVID-19, and likely due to anti-platelet factor 4 (PF4) IgG antibodies. In this study, we demonstrated that 1E12, a chimeric anti-PF4 antibody with a human Fc fragment, fully mimics the effects of human VITT antibodies, as it activates platelets to a similar level in the presence of platelet factor 4 (PF4). Incubated with neutrophils, platelets and PF4, 1E12 also strongly induces NETosis, and in a microfluidic model of whole blood thrombosis, it triggers the formation of large platelet/leukocyte thrombi containing fibrin(ogen). In addition, a deglycosylated form of 1E12 (DG-1E12), which still binds PF4 but no longer interacts with Fcγ receptors, inhibits platelet, granulocyte and clotting activation induced by human anti-PF4 VITT antibodies. This strongly supports that 1E12 and VITT antibodies recognize overlapping epitopes on PF4. In conclusion, 1E12 is a potentially important tool to study the pathophysiology of VITT, and for establishing mouse models. On the other hand, DG-1E12 may help the development of a new drug that specifically neutralizes the pathogenic effect of autoimmune anti-PF4 antibodies, such as those associated with VITT.
Introduction
Vaccine-induced immune thrombotic thrombocytopenia (VITT) is a rare but very serious complication of recombinant adenoviral vector vaccines used to prevent COVID-19 induced by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). VITT is associated with a high mortality rate, approaching 20-50% of affected patients,1-3 and due to multiple and atypical thrombotic events, particularly cerebral venous sinus thrombosis (CVST) and splanchnic thrombosis (SVT), sometimes accompanied by disseminated intravascular coagulation.1,3-8 Highly reactive anti-platelet factor 4 (PF4) immunoglobulin G (IgG) antibodies were detected in almost all reported patients, although they had not received heparin and likely play a central role in the pathogenesis of VITT.9,10 These antibodies were found to activate platelets in the absence of heparin, by interacting with FcγRIIA receptors via their Fc part.1,6 Although their characteristics strikingly resemble those of antibodies in "autoimmune" HIT,11,12 they remain to be further investigated, especially to better understand the predisposition of VITT patients to cerebral and splanchnic vein thrombosis.
Adenoviral vector-based vaccines are effective tools and were first used to fight the Ebola pandemic in Africa, and, unlike mRNA-based vaccines, they are currently affordable in many countries around the world.13,14 VITT emerged after vaccination with ChadOx1 nCoV19 (AstraZeneca) and Ad26.COV2-S (Janssen, Johnson & Johnson), and other adenoviral vector-based vaccines might therefore trigger similar adverse events in the future. Thus, understanding the mechanisms of VITT has major implications beyond the current pandemic. On the other hand, VITT is an exceptional side effect of COVID-19 vaccination, and patient samples are rare and limited to a few milliliters. This severely limits mechanistic studies or the provision of positive controls for laboratory assays. All these reasons explain the urgent need to provide the scientific community with a monoclonal antibody to study the pathophysiology of VITT and to standardize in vitro diagnostic tests.
In this regard, we have recently developed a chimeric IgG1 anti-PF4 antibody named 1E12 with a human Fc fragment, which exhibits strong similarities to autoimmune HIT antibodies in terms of cellular effects.15 Here we show that this antibody mimics VITT antibodies by comparing cellular activation of platelets and granulocytes induced by 1E12 and human VITT antibodies. Then, we evaluated the capability of deglycosylated 1E12, an inactivated form of the antibody, which is unable to bind FcγR, to inhibit cellular activation induced by VITT antibodies.
Methods
Material and antibodies
The chimeric anti-PF4 monoclonal IgG1, 1E12, has been co-developed with B-cell design (Limoges) as previously described.15,16 The deglycosylated forms of 1E12 (DG-1E12) and cetuximab (Merck) tested as control antibody (DG-Ctrl Ab), were obtained after incubation overnight of each antibody (1 mg/mL) with 40 U of N-glycosidase F (Sigma-Aldrich), followed by removal of the enzyme using a Vivaspin 50 kDa column (Sartorius).
VITT samples (plasma or serum) and HIT plasma samples were obtained from patients diagnosed in Tours or Greifswald. The study of serum and plasma from patients was approved by the local Ethics Board and conducted in accordance with the Declaration of Helsinki.
PF4-modified serotonin release assay
PF4-modified serotonin release assay (PF4-SRA) was performed as previously described.6 Briefly, 14C-serotonin labeled platelets were incubated with PF4 (10 μg/mL), plasma samples or 1E12 for 1 hour at room temperature. Then, the radioactivity was measured in supernatants. For competitive assays, washed platelets and PF4 (10 μg/mL) were pre-incubated for 10 minutes (min) with DG-1E12 or DG-Ctrl Ab before stimulation with either HIT or VITT plasma samples. For more details, see the Online Supplementary Appendix.
Flow cytometry analysis
The flow cytometric assay was performed as a modified version of the PF4-induced flow cytometry-based platelet activation test (PIFPA), which was recently described.17 For more details, see the Online Supplementary Appendix.
Enzyme-linked immunosorbant competition assay
The ability of 1E12 Fab’2 fragment to inhibit binding of VITT or HIT Ab to modified PF4 was performed by using PF4 IgG assay (Immucor). For more details, see the Online Supplementary Appendix.
Microfluidic whole blood thrombosis model
Whole blood (WB) collected on 0.129 M sodium citrate from healthy donors was incubated with VITT plasma, 1E12 or ALB6 Ab (Beckman Coulter). Then, blood samples were recalcified to 5 mM CaCl2 and perfused at a shear rate of 20 μL/min (500 s-1 ) in microfluidic channels (Vena8 Fluo1, Cellix) precoated overnight at 4°C with 160 μg/mL purified human von Willebrand factor (LFB). For competitive assays, WB was pre-incubated for 10 min with DG-1E12 or DG-Ctrl Ab before adding VITT plasma samples or ALB6. For more details, see the Online Supplementary Appendix.
Effect of antibodies on NETosis
The formation of neutrophilic extracellular traps (NET) (“NETosis”) induced by VITT sera or 1E12 (10 μg/mL) was measured in a microplate assay in the presence of purified neutrophils, platelets and PF4 (10 μg/mL). In addition, the inhibitory effect of DG-1E12 and DG-Ctrl Ab (100 μg/mL) on NETosis was evaluated. For more details, see the Online Supplementary Appendix.
Statistical analysis
Statistical analyses were performed with GraphPad Prism version 8.0.1 software. Mann-Whitney U test and Wilcoxon signed-rank test were performed to compare data obtained after DG-1E12 or DG-Ctlr Ab treatment under different conditions. P<0.05 was considered statistically significant.
Results
1E12 mimics vaccine-induced immune thrombotic thrombocytopenia antibody-induced platelet activation
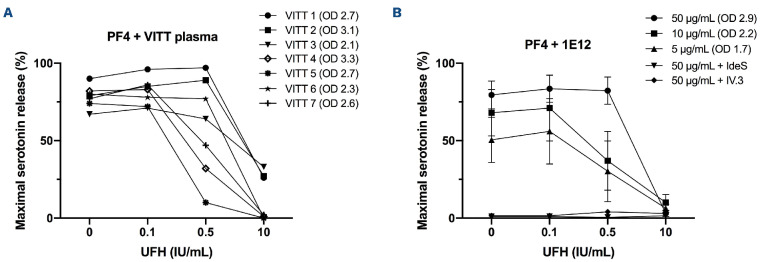
Using a PF4-SRA, we demonstrate that 1E12 strongly activates platelets, with a pattern very similar to that of human VITT plasma samples, i.e., in a PF4-dependent manner and without the addition of heparin (Figure 1). Moreover, platelet activation induced by 1E12 (5 and 10 µg/mL) was inhibited by a low heparin concentration (0.5 IU/mL), an effect we also observed with several VITT samples. Moreover, platelet activation induced by 1E12 was fully inhibited by IV.3, a monoclonal antibody blocking FcγRIIa receptors, and IdeS (IgG-degrading enzyme derived from Streptococcus pyogenes), a bacterial protease that cleaves the hinge region of IgG and thus suppresses the binding of pathogenic IgG antibodies to FcγRIIa receptors (Figure 1B). This inhibitory effect of IV.3 and IdeS, which we previously demonstrated with VITT samples,6 strongly supports that interaction between pathogenic anti-PF4 IgG and FcγRIIa plays a central role in the pathogenesis of VITT.
Figure 1.
1E12 mimics human vaccine-induced immune thrombotic thrombocytopenia antibodies to activate platelets. (A) Platelet activation induced by vaccine-induced immune thrombotic thrombocytopenia (VITT) plasma samples (n=7) and (B) 1E12 (5, 10, 50 μg/mL) in serotonin release assay performed in the presence of exogenous human PF4 (10 μg/mL) and increasing concentrations of heparin. The effects of the immunoglobulin G (IgG) cleaving protease IdeS and the monoclonal FcγRIIa blocking antibody IV.3 (10 μg/mL) on platelet activation induced by 1E12 (50 μg/mL) are also presented in (B). Data are the percentage of platelet activation, with mean (+/- standard error of the mean) of 4 independent experiments for (B). Optical density (OD) values measured in enzyme-linked immunsorbant assay (Immucor) and reflecting the levels of IgG antibodies to PF4 in all samples tested are indicated in brackets.
1E12 mimics vaccine-induced immune thrombotic thrombocytopenia antibody-induced prothrombotic cellular effects
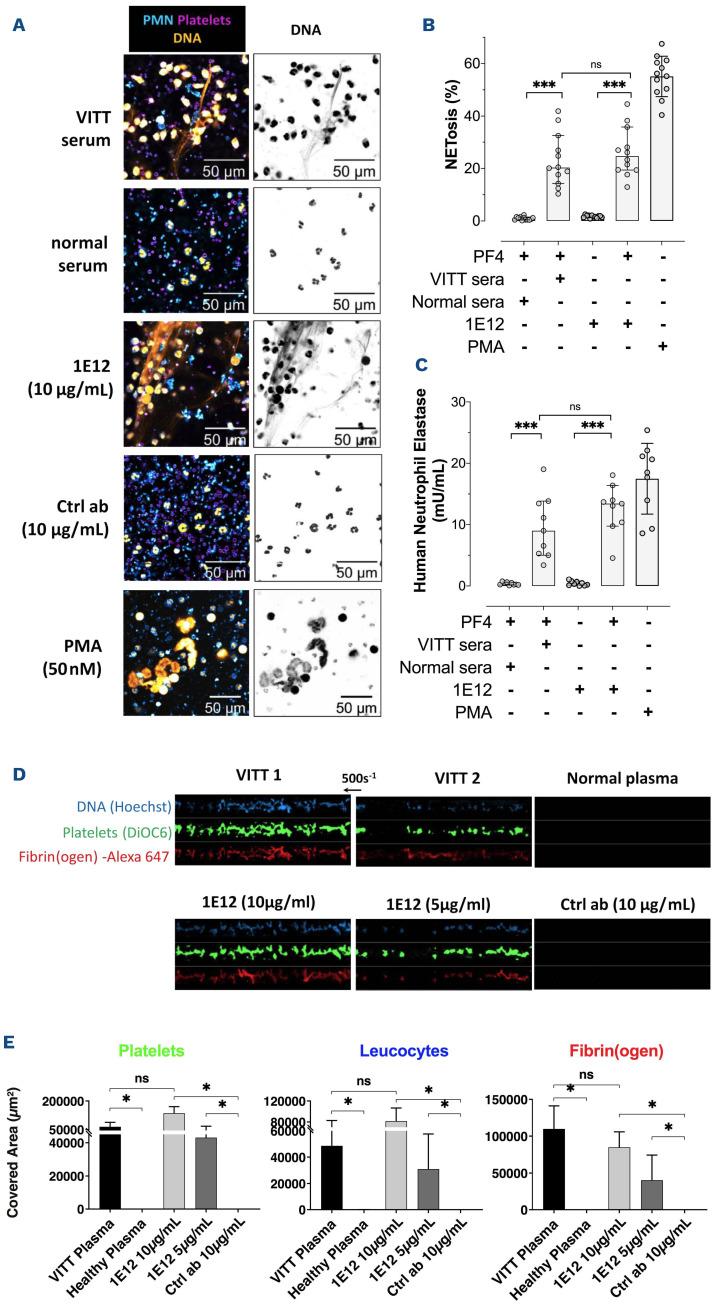
The thrombotic complications of VITT probably result from complex multicellular activation processes involving platelets and neutrophils. It has been recently shown that granulocytes produce large amounts of procoagulant NET in response to VITT antibodies.18,19 Similarly, 1E12, incubated with granulocytes and isolated platelets in the presence of PF4 (10 μg/mL), induced NETosis with the release of elastase associated with DNA, at a level similar to that measured with VITT samples (Figure 2A to C). In addition, the presence of platelets was required for 1E12-induced NETosis (Online Supplementary Figure S1). When whole blood from healthy donors was incubated with 1E12 or VITT plasma and perfused it into capillaries coated with von Willebrand factor under shear stress (500 s-1 ) mimicking venous conditions, numerous large fibrin(ogen)-containing platelet/leukocyte aggregates were observed (Figure 2D and E). As expected, thrombus formation induced by 1E12 was completely prevented by the addition of IdeS (0.1 U), IV.3 (10 μg/mL), or high concentrations of unfractionated heparin (UFH, 100 IU/mL) (Online Supplementary Figure S2). This in vitro thrombus formation did not require exogenous UFH or PF4, in contrast to thrombus induction by HIT antibodies.20,21 These results strongly support that the specificity and affinity of VITT antibodies are different from those of HIT antibodies since they recognize PF4 alone and activate cells without heparin.
Deglycosylated 1E12 inhibits vaccine-induced immune thrombotic thrombocytopenia antibody-induced platelet activation
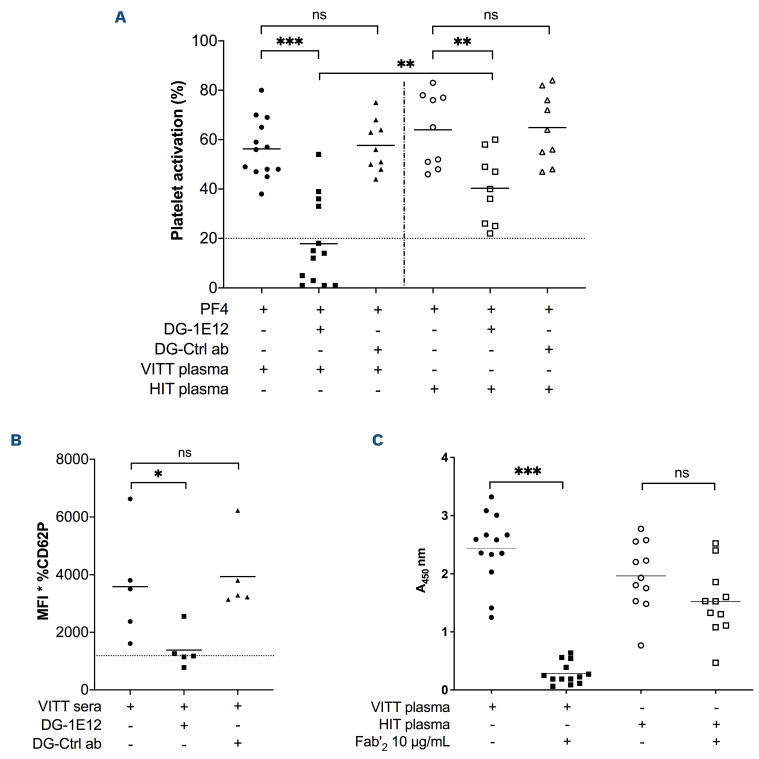
Based on the assumption that 1E12 and VITT antibodies recognize overlapping epitopes on PF4, we performed competitive assays between VITT antibodies and an inactivated of 1E12 (DG-1E12) obtained after N-deglycosylation of its Fc fragment. We first confirmed that DG-1E12 was fully able to bind PF4 but no longer to activate platelets (Online Supplementary Figure S3) since the removal of the Fc glycan abolishes IgG binding to FcγR.22 By incubating washed platelets with DG-1E12 (50 μg/mL), platelet activation induced by VITT antibodies we strongly reduced in PF4-SRA, and fully abrogated with nine of the 13 VITT samples tested (maximal serotonin release < 20%; Figure 3A). In contrast, DG-1E12 could only partially inhibit HIT antibody-induced platelet activation, with an effect less pronounced than those obtained with VITT plasma samples, since the serotonin release remained higher than 20% with all HIT plasma samples tested. In addition, the same inhibitory effect of DG-1E12 on VITT antibodies was demonstrated in whole blood using the recently described PF4-enhanced flow cytometry assay17 (Figure 3B). As expected, no inhibitory effect of deglycosylated control antibody (DG-Ctrl Ab) on VITT or HIT antibody-induced platelet activation could be evidenced (Figure 2A and B). We then investigated whether the competitive effect of DG-1E12 was dependent on its Fab part, by inhibiting the binding of VITT antibodies to PF4. This hypothesis is likely since pre-incubation of the Fab'2 fragment of 1E12 almost fully suppressed the binding of VITT antibodies to PF4/PVS (median A450 2.5 vs. 0.22). In contrast, it did not significantly modify the results obtained with all HIT samples tested (median A450 1.9 vs. 1.5) (Figure 3C). In addition, the Fab'2 fragment of 1E12 efficiently displaced all VITT IgG antibodies pre-bound to PF4/PVS complexes (Online Supplementary Figure S4).
Figure 2.
1E12 activates neutrophils and induces thrombosis in vitro similarly to human vaccine-induced immune thrombotic thrombocytopenia antibodies. (A) Evaluation of in vitro NETosis by Confocal laser scanning microscopy following neutrophils stimulation by vaccine-induced immune thrombotic thrombocytopenia (VITT) patient serum, normal serum, 1E12 10 μg/mL or the control antibody (Ctlr Ab, 10 μg/mL), in the presence of platelets and human PF4 (10 μg/mL). Nuclear and extracellular DNA is shown in orange, platelets in purple, and polymorphonuclear neutrophils (PMN) in blue. (B and C) Quantification of NETosis by analysing 12 individual microscopy images (B) and by measuring levels of DNA-associated elastase activity in the supernatant after neutrophils stimulation (C). Four independent experiments were performed for each experimental condition. (D and E) Thrombus formation in von Willebrand factor (vWF)-coated microfluidic channels perfused with recalcified whole blood incubated for 10 minutes in the presence of VITT plasma samples (n=2), normal plasma, 1E12 (10 and 5 μg/mL) or control antibody (Ctrl Ab; 10 μg/mL). Images corresponding to areas of 0.1 mm2 are shown in (D) with platelets in green (DiOC6), fibrin(ogen) in red (Alexa Fluor 647– labeled fibrinogen), and leukocytes in blue (Hoechst 33342, DNA dye). The mean areas covered (E) by platelets (left, E), leukocytes (middle) or fibrin(ogen) (right) were calculated for each condition (n=4 independent experiments), by measuring using ImageJ software the surface covered by large aggregates (> 100 μm2) in 20 different areas. Wilcoxon signed-rank test and Mann-Whitney U test, respectively, were performed to compare the different conditions tested. *P<0.05, **P<0.01, ***P<0.001.
Deglycosylated 1E12 inhibits vaccine-induced immune thrombotic thrombocytopenia antibody-induced prothrombotic cellular effects
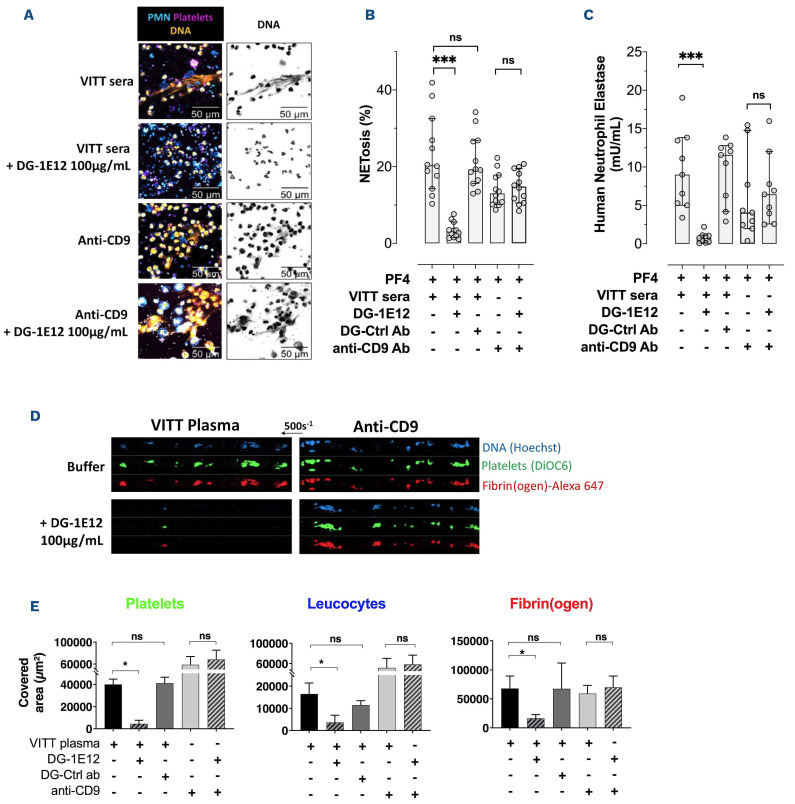
When platelets and neutrophils were pre-incubated with DG-1E12, NETosis induced by VITT antibodies was completely inhibited (NETosis 3% vs. 23%, with or without DG-1E12, respectively) as well as the release of DNA-associated elastase (activity level 0.7 vs. 9 mU/mL) (Figure 4A to C). Using similar experimental conditions, DG-1E12 did not inhibit NETosis induced by ALB6, a murine monoclonal anti-CD9 antibody that also strongly activates platelets in an FcγRIIa-dependent manner (NETosis 15% vs. 14% and elastase activity 6.4 vs. 4 mU/mL, with or without DG-1E12, respectively) (Figure 4A to C).
These data strongly suggested that DG-1E12 could prevent multicellular activation induced by VITT antibodies. This concept was reinforced by demonstrating that DG-1E12 fully abrogated VITT antibody-mediated thrombus formation in whole blood in vitro under vein flow conditions (Figure 4D and E). As expected and in accordance with the data obtained in NETosis experiments, DG-1E12 did not modify the size of platelet/leukocyte thrombi containing fibrin(ogen) induced by anti-CD9 antibodies. In addition, when the deglycosylated form of the irrelevant antibody (DG-Ctlr Ab) was tested in place of DG-1E12, no inhibitory effect on NETosis and thrombus formation induced by VITT antibody was observed (Figure 4B, C and E).
Discussion
In this study, we demonstrated that 1E12, a chimeric anti-PF4 antibody with a human Fc fragment, fully mimics the effects of human VITT antibodies, as it activates platelets to a similar level in the presence of PF4. Incubated with neutrophils, platelets, and PF4, 1E12 also strongly induces NETosis, and in a microfluidic model of whole blood thrombosis, it triggers the formation of large platelet/leukocyte thrombi containing fibrin(ogen). In addition, a deglycosylated form of 1E12 (DG-1E12), which still binds PF4 but no longer interacts with Fcγ receptors, inhibits platelet, granulocyte and clotting activation induced by human anti-PF4 VITT antibodies.
Using an alanine-scanning mutagenesis strategy, Huyhn et al. recently showed that amino acid residues involved in the epitopes recognized by VITT antibodies on PF4 are different from those required for HIT antibody binding to PF4/heparin complexes.23 In addition, it was recently demonstrated that anti-PF4 antibodies in VITT are oligoclonal while those developed in HIT are polyclonal.24 Our results obtained with an enzyme-linked ummunsorbant competitive assay strengthen this restricted specificity of VITT antibodies since the Fab’2 part of 1E12 strongly inhibits the binding of VITT IgG to PF4 without any significant effect on HIT antibody/PF4 interaction. Importantly, VITT antibody binding to PF4 was shown to be restricted to eight amino acids (R22, H23, E28, K46, N47, K50, K62, and K66), all of which are located in the heparin-binding site of PF4.23 In this regard, we also showed by using a predictive docking model that six of these eight residues likely participate in the binding of 1E12 to PF4 (Online Supplementary Figure S5).15 These results likely explain why we could inhibit binding of VITT antibodies or 1E12 to PF4, as well as cellular activation, by adding relatively low concentrations of heparin. In contrast, when low concentrations of heparin were added to HIT plasma samples or incubated with 5B9, a chimeric monoclonal IgG1 anti-PF4/H antibody mimicking human antibodies, HIT antibody binding and related cell activation were always enhanced.16
We had also previously provided evidence that 1E12 likely exhibits a much higher affinity for PF4 and PF4/H complexes than the HIT monoclonal antibody 5B9.15 This also parallels the characteristics of VITT antibodies recently obtained by Huynh et al., who showed that VITT antibodies bind stronger to PF4 and PF4/H complexes than HIT anti-bodies.23 Under our conditions, VITT antibody-induced thrombus formation in flow did not require the addition of exogenous PF4, suggesting that a sufficient amount of this chemokine was released and present on cell surfaces. However, VITT is a thromboinflammatory syndrome with high PF4 plasma levels, and our study model probably does not entirely replicate the complex pathophysiological conditions present in most VITT patients.
Figure 3.
Deglycosylated 1E12 inhibits platelet activation induced by human vaccine-induced immune thrombotic thrombocytopenia antibodies. (A) Platelet activation induced by vaccine-induced immune thrombotic thrombocytopenia (VITT) (n=13) and HIT plasma samples (n=9) in PF4-modified serotonin release assay (PF4-SRA) with or without DG-1E12 or DG-Ctrl antibody (ab) (50 μg/mL). The maximal serotonin release values that were measured are expressed in percentages of (%), with means (horizontal lines). Experiments using DG-Ctrl ab (deglycosylated form of control antibody) was performed with all available VITT plasma samples (n=9) and HIT plasmas (n=9). All SRA experiments were performed in the presence of human PF4 (10 μg/mL). Platelet activation induced by VITT samples (n=5) added in whole blood with or without DG-1E12 or DG-Ctrl antibody (100 μg/mL), and measured by flow cytometry (B). CD62P expression is shown as the mean fluorescence intensity (MFI) of the gated events multiplied by the percentage of gated events. Each data point represents the averaged result from 3 different tests with 1 sample. Horizontal lines indicate the mean values obtained with the five VITT samples. Effects of Fab’2 fragment of 1E12 (10 μg/mL) on VITT (n=13) or HIT (n=11) antibody binding to PF4/PVS evaluated by competitive enzyme immunoassays (C).
Our data also support that the beneficial impact of DG-1E12 in preventing blood cell activation by VITT antibodies and thrombus formation likely depends on the competitive effect of its Fab part on antibody binding to PF4, without inhibiting FcγRIIa-mediated signaling. Hence, our results confirm that 1E12 has features very similar to those of human VITT regarding their specificity and cellular effects.
Figure 4.
Deglycosylated 1E12 inhibits NETosis and thrombus formation induced by human vaccine-induced immune thrombotic thrombocytopenia antibodies. (A) Evaluation of in vitro NETosis by confocal laser scanning microscopy following neutrophils stimulation by vaccine-induced immune thrombotic thrombocytopenia (VITT) serum or anti-CD9 antibody (20 μg/mL), with or without DG-1E12 (100 μg/mL). Nuclear and extracellular DNA is shown in orange, platelets in purple, and polymorphonuclear neutrophils (PMN) in blue. (B and C) Quantification of NETosis by analysing 12 individual microscopy images (B) and by measuring levels of DNA-associated elastase activity in the supernatant after neutrophils stimulation (C). Four independent experiments were performed for each experimental condition. (D and E) Thrombus formation in von Willebrand factor (vWF)-coated microfluidic channels perfused with recalcified whole blood incubated for 10 minutes with VITT plasma or ALB6 (20 μg/mL), with or without DG-1E12 (100 μg/mL). Images corresponding to areas of 0.1 mm2 are shown in (D) with platelets in green (DiOC6), fibrin(ogen) in red (Alexa Fluor 647–labeled fibrinogen), and leukocytes in blue (Hoechst 33342, DNA dye). The mean areas covered (E) by platelets (left), leukocytes (middle) or fibrin(ogen) (right) were calculated for each condition (n=5 independent experiments with 5 VITT plasmas), by measuring using ImageJ software the surface covered by large aggregates (>100 μm2) in 20 different areas. Deglycosylated form of cetuximab was used as control antibody (DG-Ctrl ab). Wilcoxon signed-rank test was performed to compare the different conditions tested. *P<0.05, **P< 0.01, ***P<0.001.
The therapeutic management of VITT patients is currently based on therapeutic dose anticoagulation with non-heparin anticoagulants, combined with high dose intravenous immunoglobulins (IVIG), which inhibit cell activation likely by competing with VITT antibodies.25 Apart from being expensive and limited, IVIG is also problematic for managing severe CVST with intracranial hypertension as it may induce intracranial pressure, and the efficacy of this therapy may be transient in some VITT patients with persistently high levels of activating anti-PF4 IgG anti-bodies.26 In this context, DG-1E12 combined with a non-heparin antithrombotic drug could be a new therapeutic approach for efficiently and safely treating the more severe cases of VITT. Interestingly, a similar strategy based on the use of an epitope-specific deglycosylated antibody was first successfully evaluated in a mouse model of fetal/neonatal alloimmune thrombocytopenia (FNAIT),27 and then more recently in HIT.28 In addition, we recently demonstrated that DG-1E12 completely inhibited cellular activation induced by a spontaneously synthesized anti-PF4 IgG antibody from a patient with monoclonal gammopathy whose paraprotein showed VITT-antibody-like activity and caused recurrend venous and arterial thrombosis.29
Recent guidelines clearly recommended performing functional assays for the diagnosis of VITT.30 SRA and HIPA must be done in the presence of exogenous PF4,1,6 but the diagnosis of VITT remains a challenge for many laboratories. In this context, the monoclonal antibody 1E12 could be useful as a positive control and external quality control to standardize the different functional assays usable for VITT diagnosis.
In conclusion, we demonstrate that 1E12 and VITT antibodies exhibit a similar capacity to activate platelets and neutrophils and to induce thrombus formation. Therefore, 1E12 is likely an excellent model antibody to further study the pathophysiology of VITT and in diagnostic assays.
Our data also support that DG-1E12 may allow the development of a new drug neutralizing the pathogenic effect of autoimmune anti-PF4 antibodies, such as those associated with VITT.
Supplementary Material
Acknowledgements
We are grateful to all biologists (Philippe Cauchie-Charleroi; Emmanuel De Maistre-Dijon; Dorothée Faille-Paris; Isabelle Gouin-Thibault-Rennes; Raphael Marlu-Grenoble; Guillaume Mourey-Besançon; François Mullier-Namur; Alain Stépanian-Paris; Marie Tufgo-Angers; Sophie Voisin-Toulouse) who sent us the plasma samples analyzed in this study. We also thank the technical staf Mrs Séverine Augereau and Mrs Merveille Atsouawe. The work of the technologists Ulrike Strobel, Carmen Freyer, Katrin Stein, Ines Warnig, Ricarda Raschke, Jessica Fuhrmann, Nicole Lembke, Transfusion Medicine Greifswald, is highly appreciated. We thank B Cell design/ArkAb who generously provided the 1E12 antibody.
Funding Statement
Funding: This study was supported by the Institut pour la Recherche sur la Thrombose et l’Hémostase, the program “Investissements d'Avenir” (grant agreement no. LabEx MAbImprove ANR-10-LABX-53-01), the Région Centre-Val de Loire (APR IR 2020_DOMINO) and Force Hémato. The study has been funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – project number 374031971 - TRR 240.
References
- 1.Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021;384(22):2092-2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pavord S, Scully M, Hunt BJ, et al. Clinical features of vaccine-induced immune thrombocytopenia and thrombosis. N Engl J Med. 2021;385(18):1680-1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schultz NH, Sørvoll IH, Michelsen AE, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384(22):2124-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perry RJ, Tamborska A, Singh B, et al. Cerebral venous thrombosis after vaccination against COVID-19 in the UK: a multicentre cohort study. Lancet. 2021;398(10306):1147-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scully M, Singh D, Lown R, et al. Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384(23):2202-2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vayne C, Rollin J, Gruel Y, et al. PF4 Immunoassays in vaccine-induced thrombotic thrombocytopenia. N Engl J Med. 2021;385(4):376-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gresele P, Momi S, Marcucci R, Ramundo F, De Stefano V, Tripodi A. Interactions of adenoviruses with platelets and coagulation and the vaccine-induced immune thrombotic thrombocytopenia syndrome. Haematologica. 2021;106(12):3034-3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pomara C, Sessa F, Ciaccio M, et al. Post-mortem findings in vaccine-induced thrombotic thombocytopenia. Haematologica. 2021;106(8):2291-2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Althaus K, Möller P, Uzun G, et al. Antibody-mediated procoagulant platelets in SARS-CoV-2-vaccination associated immune thrombotic thrombocytopenia. Haematologica. 2021;106(8):2170-2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greinacher A, Selleng K, Palankar R, et al. Insights in ChAdOx1 nCov-19 vaccine-induced immune thrombotic thrombocytopenia (VITT). Blood. 2021;138(22):2256-2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greinacher A, Selleng K, Warkentin TE. Autoimmune heparin-induced thrombocytopenia. J Thromb Haemost. 2017;15(11):2099-2114. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen TH, Medvedev N, Delcea M, Greinacher A. Anti-platelet factor 4/polyanion antibodies mediate a new mechanism of autoimmunity. Nat Commun. 2017;8:14945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kennedy SB, Bolay F, Kieh M, et al. Phase 2 placebo-controlled trial of two vaccines to prevent Ebola in Liberia. N Engl J Med. 2017;377(15):1438-1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Milligan ID, Gibani MM, Sewell R, et al. Safety and immunogenicity of novel Adenovirus type 26- and modified Vaccinia Ankara-vectored Ebola vaccines: a randomized clinical trial. JAMA. 2016;315(15):1610-1623. [DOI] [PubMed] [Google Scholar]
- 15.Vayne C, Nguyen TH, Rollin J, et al. Characterization of new monoclonal PF4-specific antibodies as useful tools for studies on typical and autoimmune heparin-induced thrombocytopenia. Thromb Haemost. 2021;121(3):322-331. [DOI] [PubMed] [Google Scholar]
- 16.Kizlik-Masson C, Vayne C, McKenzie SE, et al. 5B9, a monoclonal antiplatelet factor 4/heparin IgG with a human Fc fragment that mimics heparin-induced thrombocytopenia antibodies. J Thromb Haemost. 2017;15(10):2065-2075. [DOI] [PubMed] [Google Scholar]
- 17.Handtke S, Wolff M, Zaninetti C, et al. A flow cytometric assay to detect platelet-activating antibodies in VITT after ChAdOx1 nCov-19 vaccination. Blood. 2021;137(26):3656-3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greinacher A, Selleng K, Palankar R, et al. Insights in ChAdOx1 nCoV-19 vaccine-induced immune thrombotic thrombocytopenia. Blood. 2021;138(22):2256-2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holm S, Kared H, Michelsen AE, et al. Immune complexes, innate immunity, and NETosis in ChAdOx1 vaccine-induced thrombocytopenia. Eur Heart J. 2021;42(39):4064-4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gollomp K, Kim M, Johnston I, et al. Neutrophil accumulation and NET release contribute to thrombosis in HIT. JCI Insight. 2018;3(18):e99445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kizlik-Masson C, Deveuve Q, Zhou Y, et al. Cleavage of anti-PF4/heparin IgG by a bacterial protease and potential benefit in heparin-induced thrombocytopenia. Blood. 2019;133(22):2427-2435. [DOI] [PubMed] [Google Scholar]
- 22.Radaev S, Sun PD. Recognition of IgG by Fcgamma receptor. The role of Fc glycosylation and the binding of peptide inhibitors. J Biol Chem. 2001;276(19):16478-16483. [DOI] [PubMed] [Google Scholar]
- 23.Huynh A, Kelton JG, Arnold DM, Daka M, Nazy I. Antibody epitopes in vaccine-induced immune thrombotic thrombocytopaenia. Nature. 2021;596(7873):565-569. [DOI] [PubMed] [Google Scholar]
- 24.Singh B, Kanack A, Bayas A, et al. Anti-PF4 VITT antibodies are oligoclonal and variably inhibited by heparin. medRxiv. 2021. Sept 24. doi: 10.1101/2021.09.23.21263047. [preprint, not peer reviewed] [Google Scholar]
- 25.Bourguignon A, Arnold DM, Warkentin TE, et al. Adjunct immune globulin for vaccine-induced immune thrombotic thrombocytopenia. N Engl J Med. 2021;385(8):720-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Douxfils J, Vayne C, Pouplard C, et al. Fatal exacerbation of ChadOx1-nCoV-19-induced thrombotic thrombocytopenia syndrome after initial successful therapy with intravenous immunoglobulins - a rational for monitoring immunoglobulin G levels. Haematologica. 2021;106(12):3249-3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bakchoul T, Greinacher A, Sachs UJ, et al. Inhibition of HPA-1a alloantibody-mediated platelet destruction by a deglycosylated anti-HPA-1a monoclonal antibody in mice: toward targeted treatment of fetal-alloimmune thrombocytopenia. Blood. 2013;122(3):321-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sarkar A, Khandelwal S, Yarovoi S, et al. Fc-modified Kko: a novel therapeutic for heparin-induced thrombocytopenia (HIT), reversing both the thrombocytopenia and thrombosis. Blood. 2021;138(Suppl 1):S581. [Google Scholar]
- 29.Greinacher A, Langer F, Schonborn L, et al. Platelet-activating anti-PF4 antibodies mimicking VITT antibodies in an unvaccinated patient with monoclonal gammopathy. Haematologica. 2022;107(5):1219-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nazy I, Sachs UJ, Arnold DM, et al. Recommendations for the clinical and laboratory diagnosis of VITT against COVID-19: communication from the ISTH SSC Subcommittee on Platelet Immunology. J Thromb Haemost. 2021;19(6):1585-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.