Acute myeloid leukemia (AML) remains incurable in most cases, despite decades of clinical trials and new drug entries.1,2 The standard induction chemotherapy in AML consists of an anthracycline, including daunorubicin (DNR) or idarubicin (IDA), and cytarabine given together, typically for 3 and 7 consecutive days, respectively. However, the choice of anthracycline (DNR vs. IDA) and dose of DNR (45-90 mg/m2) are not standardized and have been the subject of multiple clinical trials and meta-analyses.3-5 The pivotal study regarding DNR dosing suggested the superiority of DNR 90 mg/m2 versus 45 mg/m2 for young (age <60 years) AML patients in terms of both complete remission (CR) rate (71% vs. 57%) and overall survival (median 24 months vs. 16 months);6 follow-up information on the particular study suggested the advantage from DNR-90 to extend across younger age groups, cytogenetic and mutational risk categories, including NPM1, DNMT3A, and FLT3-ITD.7, 8 These observations were confirmed in a similar study from South Korea.9 In another randomized trial of AML patients 60 years or older, DNR-90, compared to DNR-45, induced a higher CR rate but did not improve survival, except in those ages 60-65 years.10 On the other hand, comparison of DNR-90 to DNR-60 did not show differences in CR or survival, save for a subset of FLT3-mutated cases.11 Many other studies have also compared IDA to DNR;4 in one such study, IDA-12 was compared to DNR-90 in AML patients age <60 years with no difference in CR rate, survival or toxicity; however, DNR-90 resulted in superior overall and event-free survival in FLT3-mutated patients.12 Over the last several decades, we at the Mayo Clinic have serially utilized IDA and DNR at different doses for the treatment of AML based on information from existing literature at the time of diagnosis; the objective for the current study was to retrospectively review these cases and compare outcome in terms of CR and survival. The current study population was recruited from Mayo Clinic institutional databases, after Institutional Review Board approval and based on documentation of newly diagnosed AML and induction chemotherapy with DNR or IDA, in combination with cytarabine. Patients were typically prescribed “7+3” induction chemotherapy that included 3 days of DNR at a daily dose of either 60 mg/m2 (DNR-60) or 90 mg/m2 (DNR-90), or IDA 12 mg/m2 (IDA-12). All patients were treated in the context of participation in clinical trials or routine clinical practice; for the purposes of the current study, patients receiving DNR at <60 mg/m2, those receiving a third drug (e.g., midostaurin) for induction, and patients with myeloid sarcoma were excluded. Treatment period spanned from January 2004 through May 2021 and follow-up information was updated as of March 2022. Conventional criteria were used to diagnose AML, assign cytogenetic risk category, and classify treatment responses.13 CR was assessed after the completion of one or two induction courses, as indicated from day 14 bone marrow assessment, which might have required re-induction for presence of residual leukemic blasts. Patients receiving allogeneic hematopoietic stem cell transplant (AHSCT) were censored at time of AHSCT, during survival analysis. The current study focused on treatment efficacy and survival and details on treatment toxicity were not abstracted. Conventional methods were used for cytogenetic and molecular studies, including next- generation sequencing. Statistical analysis was performed using JMP Pro 14.0.0 software package, SAS Institute, Cary, NC.
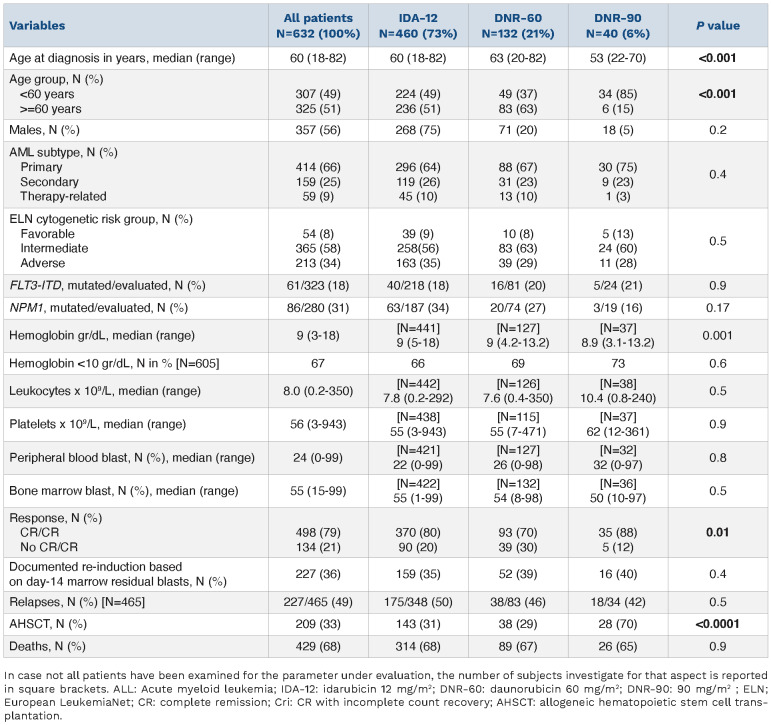
The current study included 632 newly diagnosed AML patients (median age 60 years; range, 18-82 years; 56% males): 460 (73%) patients received IDA-12, 132 (21%) DNR-60, and 40 (6%) DNR-90. All patients in addition received 7 days of continuous intravenous cytarabine (100-200 mg/m2/day). Consolidation chemotherapy utilized high-dose cytarabine 3 gm/m2 days 1, 3, and 5 for patients age <60 years and 1.5 g/m2 days 1, 3, and 5 every 28 days for three to four cycles, for patients age ≥60 years. Table 1 outlines baseline disease features stratified by the three treatment groups and highlights significant differences only in age distribution (P<0.001); median (range) age was 60 (18-82), 63 (20-82), and 53 (22-70) years, for IDA-12, DNR-60, and DNR-90 treatment groups, respectively. Primary, secondary, and therapy-related AML accounted for 66%, 25% and 9% of all study patients: 64%, 26%, and 10% of IDA-12; 67%, 23% and 10% of DNR-60; and 75%, 23%, 3% of DNR-90 (P=0.4). The corresponding frequencies for adverse karyotype were 35%, 29% and 28% (P=0.5) whereas FLT3-ITD (P=0.9) and NPM1 (P=0.17) mutation frequencies were similar between the treatment groups.
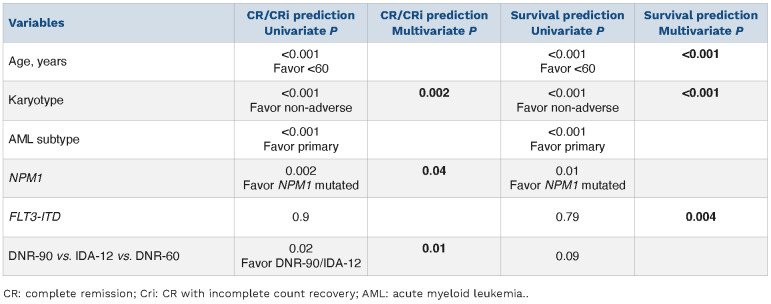
CR/CRi was documented in 79% (498/632) of all evaluable patients: IDA-12 80% (370/460), DNR-60 70% (93/132), and DNR-90 88% (35/40) (P=0.01); there was no difference in the proportion of patients in each treatment group with documentation of a second induction course based on day 14 bone marrow assessment of residual disease (48% vs. 56% vs. 57%, respectively; P=0.38). In univariate analysis (Table 2), significant predictors of CR/CRi were age <60 years (85% vs. 73%; P<0.001); absence of ELN adverse karyotype (96% in favorable and 85% in intermediate vs. 63% in adverse risk; P<0.001), primary (84%) versus secondary (67%) versus therapy-related (75%) (P=0.01), presence of NPM1 mutation (93% vs. 79%; P=0.002), and treatment group other than DNR-60 (88% for DNR-90, 80% for IDA-12 vs. 70% for DNR-60; P=0.01); no significant interaction was noted between FLT3-ITD mutation and CR/CRi (P=0.9). In multivariable analysis of CR/CRi prediction (Table 2), IDA-12 vs. DNR-60 (P=0.005), adverse versus favorable karyotype (P=0.03), adverse versus intermediaterisk karyotype (P=0.005), and NPM1 mutation (P=0.04) remained significant while DNR-90 versus DNR-60 became borderline significant (P=0.09); there was no significant difference in the rate of CR/CRi between IDA-12 and DNR-90 (P=0.64) or between favorable and intermediate risk karyotype (P=0.23).
Table 1.
Presenting features and response patterns among 632 consecutive Mayo Clinic patients with newly diagnosed acute myeloid leukemia (AML), stratified by anthracycline choice for induction chemotherapy: daunorubicin 60 mg/m2 (DNR-60) versus idarubicin 12 mg/m2 (IDA-12) versus daunorubicin 90 mg/m2 (DNR-90), each given for 3 days along with 7-day course of cytarabine.
Table 2.
Predictors of response and survival among 632 Mayo Clinic patients with newly diagnosed acute myeloid leukemia (AML), including the impact of anthracycline choice for induction chemotherapy: daunorubicin 60 mg/m2 (DNR-60) vs. idarubicin 12 mg/m2 (IDA-12) vs. daunorubicin 90 mg/m2 (DNR-90), each given for 3 days along with 7-day course of cytarabine.
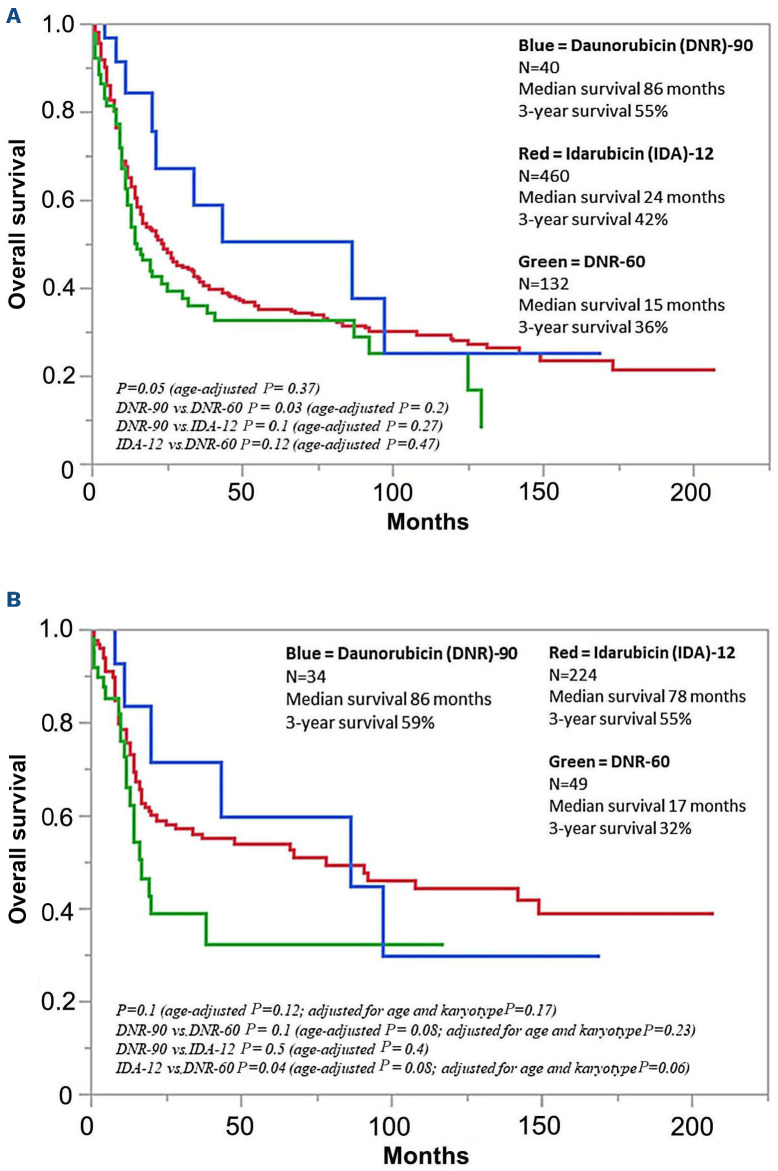
After a median follow-up of 22 months (71 months for alive patients; range 3.6-212), 227 (49%) relapses among 465 evaluable cases were documented and similarly distributed among IDA-12 (n=175/348; 50%), DNR-60 (n=38/83; 46%), and DNR-90 (n=18/34; 42%) treatment groups (P=0.5). During the same period, AHSCT was documented in 209 (33%) patients, including 143 (31%), 38 (29%) and 28 (70%) patients, in the IDA-12, DNR-60 and DNR-90 treatment groups, respectively (P<0.001). At the time of this report, 429 (68%) deaths were documented and equally distributed among IDA-12 (68%), DNR-60 (67%), and DNR-90 (65%) treatment groups; median survivals for IDA-12, DNR-60 and DNR-90 treatment groups were 24, 15, and 86 months, respectively (P=0.05; Figure 1A with survival data that is censored for AHSCT); 36 early deaths (i.e., within 60 days of induction) were documented, including 21 (4.6%) in IDA-12, 15 (11.4%) in DNR-60, and zero in DNR-90 groups (P=0.002); the difference remained significant after adjusting for age (P=0.007) but might have been influenced by the difference in level of fitness that was not systematically measured in the current retrospective study. The respective 3-year OS rates were 42%, 36%, and 55%; the apparent survival advantage favoring DNR-90 was no longer apparent during age-adjusted analysis (P=0.37), including individual comparisons of one treatment group with another (Figure 1A). Similar results were obtained during analysis restricted to patients 60 years or younger (Figure 1B with survival data that is censored for AHSCT), or when survival analysis was performed without censoring patients undergoing AHSCT (all age groups P=0.17, age-adjusted P=0.8; for patients <60 years old P=0.14, age-adjusted P=0.2). We did note, however, the near-significant survival advantage for DNR-90 and IDA-12, in the younger age group, even after adjusting for age and karyotype (Figure 1B). In transplant-censored multivariable analysis that did not include mutations (n=632), independent risk factors for survival included younger age and non-adverse cytogenetic risk profile (Table 2); in the subset of patients in whom FLT3-ITD and NPM1 mutation information for both was available (n=280), FLT3-ITD (P=0.004), but not NPM1 (P=0.4) provided additional prognostic information (Table 2); the type or dose of anthracycline used during induction chemotherapy had no additional impact on survival, regardless of mutational or cytogenetic status; specifically, there was no interaction between FLT3-ITD mutation status and the impact of anthracycline choice on survival (P=0.27 in FLT3-ITD-mutated and 0.69 in unmutated). Among the 280 patients who were informative for FLT3-ITD/NPM1 co-mutation profile: 30 were FLT3-ITD+/NPM1+; 176 FLT3-ITD-/NPM1-; 18 FLT3-ITD+/NPM1-; and 56 FLT3-ITD-/NPM1+; the respective CR/CRi rates were 97%, 91%, 81%, and 61%. Survival comparisons between the three anthracycline induction groups, analyzed separately in each FLT3-ITD/NPM1 profile did not reveal significant differences with respective P values of 0.83, 0.42, 0.28, and 0.64.
Mayo Clinic AML practice over the last two decades included patients who participated in cooperative group trials and those managed according to institutional practice protocols that were influenced by changing views over time. Accordingly, the anthracycline component of our “7+3” induction regimens typically included DNR at 45-90 mg/m2 doses or IDA at 12 mg/m2. Current information strongly supports the superiority of DNR-90 over DNR-45, in young patients with AML,6,9 but the choice between DNR-90, DNR-60 and IDA-12 remains in flux.11,12 In the current study, we retrospectively reviewed CR/CRi rates and overall survival corresponding to adult patients, across all age groups, whose induction regimen included one of three anthracycline choices: DNR-90, DNR-60, and IDA-12. We documented significantly higher CR/CRi rates with both DNR-90 and IDA-12, compared to DNR-60, even after accounting for other independent predictors of CR/CRi, including younger age, non-adverse karyotype and NPM1 mutation. However, overall survival was not significantly different between the three treatment groups, especially after accounting for the younger age distribution in patients treated with DNR-90, although we cannot discard the favorable survival trend for DNR-90 and IDA-12, compared to DNR-60. At the same time, it should be noted that patients treated on the higher potency anthracycline choices (i.e., DNR-90 or IDA-12) might have been not only younger but also fitter in their performance score, thus partly explaining their apparent survival advantage. We compared our findings with those of two other previously published clinical trials that employed similar induction regimens.11,12 In the study by Lee et al.,12 the authors compared IDA-12 with DNR-90 in AML patients 65 years of age or younger and, as was the case in our study, reported similar CR/CRi rates and overall survival. Unlike the particular study,12 however, we did not find a survival advantage for DNR-90, in FLT3-ITD-mutated cases. The second study by Burnett et al.11 compared DNR-90 with DNR-60 and reported similar CR/CRi rates and overall survival, and with inconclusive interaction between DNR dose and FLT3-ITD mutation; however, it should be noted that the particular study employed a different re-induction and consolidation schedule that might have had an impact on overall outcome.
Figure 1.
Overall Survival of Mayo Clinic patients with newly diagnosed acute myeloid leukemia (A) Overall survival of 632 Mayo Clinic patients (median age 60 years; range 18-82) with newly diagnosed acute myeloid leukemia stratified by type and dose (mg/m2) of anthracycline received during induction chemotherapy. (B) Overall survival of 307 Mayo Clinic patients age 60 years or younger with newly diagnosed acute myeloid leukemia stratified by type and dose (mg/m2) of anthracycline received during induction chemotherapy.
We are acutely aware of the substantial limitations to our retrospective study, including the lack of additional information that might have influenced treatment choices and thus indirectly impacted overall survival. The marked imbalance in the number of patients in each treatment group and the significant difference in age distribution should also be noted. The objective for our communication is simply to share experience and not influence current thinking on the subject matter, which is best inferred from properly designed controlled studies.6,11,12 Similarly, we acknowledge the possibility of benefit from a specific anthracycline choice/dose for narrower molecular subsets that might not be accounted for by the ELN-2017 genetic risk stratification. Regardless, we are encouraged by the fact that the findings from the current study are mostly in line with those of previously published work and suggest equivalent value for DNR-60, DNR-90 and IDA-12, in terms of survival in older patients with AML, while confirming superior performance for higher intensity anthracycline in inducing CR/CRi, and possibly survival in younger patients. In our practice, individual treatment decision is often based on patient frailty and recognition of the higher gastrointestinal, but not necessarily cardiac, toxicity associated with DNR-90 and IDA-12;11,14 among the 40 cases that received DNR-90 in our patient cohort, we were able to document four cases (10%) with echocardiogram evidence for cardiomyopathy, all of which were late incidents occurring at 12 months, 15 months, 19 months, and 7 years, after induction. Going forward, the ever-changing treatment landscape in mutation-specified patient groups makes it that much harder to further clarify the optimal anthracycline choice or DNR dose for induction chemotherapy in AML.15,16
References
- 1.Kantarjian H, Kadia T, DiNardo C, et al. Acute myeloid leukemia: current progress and future directions. Blood Cancer J. 2021;11(2):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Estey EH. Acute myeloid leukemia: 2021 update on risk-stratification and management. Am J Hematol. 2020;95(11):1368-1398. [DOI] [PubMed] [Google Scholar]
- 3.McCurdy SR, Luger SM. Dose intensity for induction in acute myeloid leukemia: what, when, and for whom? Haematologica. 2021;106(10):2544-2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang H, Xiao X, Xiao Q, Lu Y, Wu Y. The efficacy and safety of daunorubicin versus idarubicin combined with cytarabine for induction therapy in acute myeloid leukemia: a meta-analysis of randomized clinical trials. Medicine (Baltimore). 2020;99(24):e20094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sekine L, Morais VD, Lima KM, Onsten TGH, Klarmann Ziegelmann P, Antonini Ribeiro R. Conventional and high-dose daunorubicin and idarubicin in acute myeloid leukaemia remission induction treatment: a mixed treatment comparison meta-analysis of 7258 patients. Hematol Oncol. 2015;33(4):212-219. [DOI] [PubMed] [Google Scholar]
- 6.Fernandez HF, Sun Z, Yao X, et al. Anthracycline dose intensification in acute myeloid leukemia. N Engl J Med. 2009;361(13):1249-1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel JP, Gonen M, Figueroa ME, et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N Engl J Med. 2012;366(12):1079-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luskin MR, Lee JW, Fernandez HF, et al. Benefit of high-dose daunorubicin in AML induction extends across cytogenetic and molecular groups. Blood. 2016;127(12):1551-1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee JH, Joo YD, Kim H, et al. A randomized trial comparing standard versus high-dose daunorubicin induction in patients with acute myeloid leukemia. Blood. 2011;118(14):3832-3841. [DOI] [PubMed] [Google Scholar]
- 10.Lowenberg B, Ossenkoppele GJ, van Putten W, et al. High-dose daunorubicin in older patients with acute myeloid leukemia. N Engl J Med. 2009;361(13):1235-1248. [DOI] [PubMed] [Google Scholar]
- 11.Burnett AK, Russell NH, Hills RK, et al. A randomized comparison of daunorubicin 90 mg/m2 vs 60 mg/m2 in AML induction: results from the UK NCRI AML17 trial in 1206 patients. Blood. 2015;125(25):3878-3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee JH, Kim H, Joo YD, et al. Prospective randomized comparison of idarubicin and high-dose daunorubicin in induction chemotherapy for newly diagnosed acute myeloid leukemia. J Clin Oncol. 2017;35(24):2754-2763. [DOI] [PubMed] [Google Scholar]
- 13.Dohner H, Estey EH, Amadori S, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115(3):453-474. [DOI] [PubMed] [Google Scholar]
- 14.Hogan WJ, Letendre L, Litzow MR, et al. Neutropenic colitis after treatment of acute myelogenous leukemia with idarubicin and cytosine arabinoside. Mayo Clin Proc. 2002;77(8):760-762. [DOI] [PubMed] [Google Scholar]
- 15.Stone RM, Larson RA, Dohner H. Midostaurin in FLT3-mutated acute myeloid leukemia. N Engl J Med. 2017;377(19):1903. [DOI] [PubMed] [Google Scholar]
- 16.Daver N, Venugopal S, Ravandi F. FLT3 mutated acute myeloid leukemia: 2021 treatment algorithm. Blood Cancer J. 2021;11(5):104. [DOI] [PMC free article] [PubMed] [Google Scholar]