Abstract
In search of novel suicide gene candidates we have cloned and characterized thymidine kinases from three viruses; vaccinia virus TK (VVTK), feline herpesvirus TK (FHV-TK), and canine herpesvirus TK (CHV-TK). Our studies showed that VVTK primarily is a thymidine kinase, with a substrate specificity mainly restricted to dThd and only minor affinity for dCyd. VVTK also is related closely to mammalian thymidine kinase 1 (TK1), with 66% identity and 75% general homology. Although CHV-TK and FHV-TK are sequence related to herpes simplex virus types 1 thymidine kinase (HSV1-TK), with 31% and 35% identity and a general similarity of 54%, the substrate specificity of these enzymes was restricted to dThd and thymidine analogs.
Keywords: Nucleoside analog, Gene therapy, Suicide gene, Nucleoside kinases
INTRODUCTION
The prototype for combined gene/chemotherapy of malignant tumors is the expression of herpes simplex virus thymidine kinase (HSV-TK) in tumor cells and subsequent systemic chemotherapy with the nucleoside analog ganciclovir. In search of novel suicide gene candidates we have cloned and characterized thymidine kinases from three other viruses; vaccinia virus TK (VVTK), feline herpesvirus TK (FHV-TK), and canine herpesvirus TK (CHV-TK).
MATERIAL AND METHODS
Protein expression and purification. We expressed the three kinases in E. coli as poly-histidine fusion proteins in the pET-16b vector (Clontech, Mountain View, CA, USA). The expressed proteins were purified with TALON metal affinity resin (Clontech, Mountain View, CA, USA).
Enzyme assays. The activity of the purified recombinant enzymes was assayed in a 50 μl reaction mixture containing: 50 mM Tris-HCl pH 7.6, 0.1 mg/ml BSA, 2.5 mM ATP, 5 mM MgCl2, 5 mM dithiothreitol, and [3H] analogs (Amersham Biosciences, Uppsala, Sweden). The samples were incubated 30 minutes at 37°C and every 10 minutes 10 μl aliquots were spotted on Whatman DE-81 filter paper disks. The filter bound nucleoside monophosphates were eluted in 500 μl of 0.1 M HCl and 0.1 M KCl and the radioactivity quantified by scintillation counting. The substrate specificity of the purified enzymes was assayed by thin layer chromatography.
RESULTS
VVTK had the ability to recognize both dCyd and dThd among the natural substrates although the efficiency of dCyd phosphorylation was much less compared with dThd phosphorylation (Table 1). VVTK also efficiently converted araT, AZT and BVDU (data not shown) to their corresponding monophosphates (Figure 1). The substrate specificity of both FHV-TK and CHV-TK was limited to dThd and thymidine analogs, with poor affinity for dCyd. Neither the purines dAdo and dGuo nor several purine- and deoxycytidine analogs tested were recognized by FHV-TK or CHV-TK (Table 2, Figure 2).
TABLE 1 Kinetic Properties of VVTK (kcat was Calculated Using a Molecular Mass of 22.5 kDa).
| Substrate | Km(μM) | Vmax(μmol/mg/min) | kcat(s−1) | kcat/Km(s−1 M−1) |
|---|---|---|---|---|
| dThd | 330 | 585 | 220 | 665 × 103 |
| dCyd | >10,000 | |||
| AZT | 600 | 1,100 | 412 | 687 × 103 |
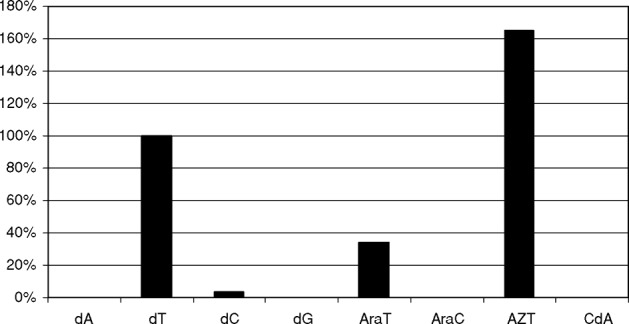
FIGURE 1 Nucleoside and nucleoside analog phosphorylation by VVTK. Relative levels of phosphorylation expressed in relation to percentage dThd phosphorylation. (AraT = 1-β-D-arabinofuranosylthymine; AraC = 1-β-D-arabinofuranosylcytosine; AZT = 3′-azido-2′,3′-dideoxythymidine; CdA = 2-chloro-2′-deoxyadenosine).
TABLE 2 Kinetic Properties of FHV-TK and CHV-TK (kcat was Calculated Using a Molecular Mass of 41.8 kDa for FHV-TK and 40.9 kDa for CHV-TK).
| FHV-TK |
CHV-TK |
|||||||
|---|---|---|---|---|---|---|---|---|
| Substrate | Km(μM) | Vmax(μmol/ mg/min) | kcat(s−1) | kcat/Km (s−1 M−1) | Km(μM) | Vmax(μmol/ mg/min) | kcat(s−1) | kcat/Km (s−1 M−1) |
| dThd | 41 | 33 | 23 | 560 × 103 | 16 | 9.5 | 6.47 | 405 × 103 |
| AZT | 15 | 1.4 | 0.97 | 65 × 103 | 50 | 0.78 | 0.53 | 10 × 103 |
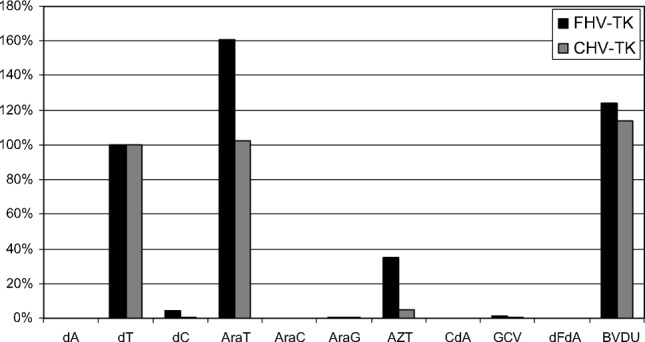
FIGURE 2 Nucleoside and nucleoside analog phosphorylation by FHV-TK and CHV-TK. Relative levels of phosphorylation expressed in relation to percentage dThd phosphorylation. (AraG = 9-β-D-arabinofuranosylguanine; GCV = Ganciclovir; dFdA = 2′,2′-difluorodeoxyadenosine BVDU = (E)-5-(2-bromovinyl)-2′-deoxyuridine).
DISCUSSION
Deoxyribonucleoside kinases are a family of enzymes with high sequence similarity between different species. Although this high degree of conservation a thorough characterization often reveal differences in substrate specificity and unique properties for several of the nucleoside kinases. The multisubstrate nucleoside kinase from Drosophila melanogaster and the herpes viral thymidine kinases all show a broad substrate specificity and are therefore used as suicide genes in gene/chemotherapy protocols. In search of even more efficient suicide gene candidates, we have investigated three viral nucleoside kinases with regard to their substrates specificities and kinetic properties. All of the enzymes studied in this report were strictly pyrimidine nucleoside kinases that most efficiently phosphorylated dThd among the natural substrates. The VVTK also recognized dCyd with low efficiency. VVTK also efficiently convert the pyrimidine analogs araT, AZT, and BVDU to their corresponding mono-phosphates. The feline and canine herpes virus TKs showed a sequence similarity of approximately 54% with the herpes simplex virus TK. However, the substrate specificity of FHV-TK and CHV-TK was limited to dThd and thymidine analogs.
We conclude that the substrate specificity of VVTK, FHV-TK and CHV-TK is limited to thymidine and thymidine analogs, although VVTK also recognized dCyd with low efficiency. None of the three investigated thymidine kinases in our study demonstrate the broad substrate specificity characteristic for HSV-TK.
Acknowledgements
This work was supported by grants from the Swedish Cancer Society, the Swedish Research Council and the Medical Faculty of the Karolinska Institute.
REFERENCES
- Remond, M., Sheldrick, P., Lebreton, F., and Foulon, T., 1995. Sequence of the canine herpesvirus thymidine kinase gene: taxon-preferred amino acid residues in the alphaherpesviral thymidine kinases, Virus Res. 39 (1995), pp. 341–354, [INFOTRIEVE][CSA]. [DOI] [PubMed] [Google Scholar]
- Nunberg, J. H., Wright, D. K., Cole, G. E., Petrovskis, E. A., Post, L. E., Compton, T., and Gilbert, J. H., 1989. Identification of the thymidine kinase gene of feline herpesvirus: use of degenerate oligonucleotides in the polymerase chain reaction to isolate herpesvirus gene homologs, J. Virol. 63 (1989), pp. 3240–3249, [INFOTRIEVE][CSA]. [DOI] [PMC free article] [PubMed] [Google Scholar]


