Background.
Solid organ transplant recipients are at high risk for fatal forms of coronavirus disease 2019 (COVID-19). We conducted a cohort study among kidney transplant (KT) recipients from the French Solid Organ Transplant COVID-19 Registry to investigate the association between maintenance immunosuppressive drugs and 60-d mortality.
Methods.
Data from all KT recipients with COVID-19 included in the French Solid Organ Transplant COVID-19 Registry between February 28, 2020, and December 30, 2020, were retrieved. We evaluated associations between immunosuppressive drugs and death within 60 d using logistic regression, with all baseline characteristics considered to influence outcome or immunosuppressive regimen. The Benjamini-Hochberg correction was used for controlling false positive rate; 40 multiple imputations were performed. Adjusted P value <0.05 was considered statistically significant.
Results.
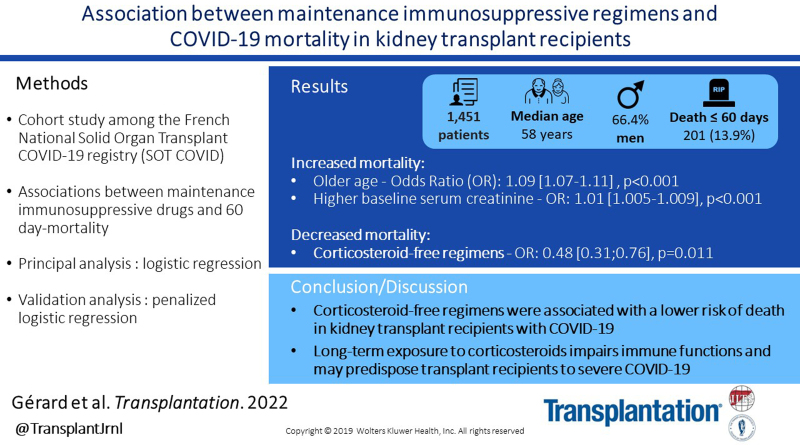
There were 1451 KT recipients included. Median age was 58 y, and 66.4% were men. Most frequent comorbidities were hypertension (81.9%), diabetes (34.5%), and cardiovascular disease (29.5%). Median time since transplant was 71 mo. Maintenance immunosuppression regimens included calcineurin inhibitors (1295, 89.2%), antimetabolites (1205, 83%), corticosteroids (1094, 75.4%), mammalian target of rapamycin inhibitors (144, 9.9%), and belatacept (58, 4.0%). Among 1451 transplant recipients, 201 (13.9%) died within 60 d. Older age and higher baseline serum creatinine were associated with mortality (odds ratios, 1.09 [1.07-1.11] and 1.01 [1.005-1.009], P < 0.001). Corticosteroid-free regimens were associated with a significantly lower risk of death (odds ratio, 0.48 [0.31-0.76]; P = 0.011).
Conclusions.
Corticosteroid-free regimens were associated with a lower risk of death in KT recipients with COVID-19. Long-term exposure to corticosteroids impairs immune functions and may predispose solid organ transplant recipients to severe forms of COVID-19.
INTRODUCTION
Since December 2019, the coronavirus disease 2019 (COVID-19) pandemic has had a large and unprecedented impact on societies and healthcare systems. Severe forms of the disease are characterized by respiratory failure and systemic complications, with a death toll of >6 million people. Solid organ transplant recipients receive immunosuppressive drugs, increasing their risk for fatal forms of COVID-19.1-4 These patients are also less likely to mount effective immune responses to vaccination.5 Since curative treatments are not readily available, all strategies aiming to mitigate the impact of COVID-19 on solid organ transplant recipients are worth considering. Yet, the impact of the different maintenance immunosuppressive regimens on COVID-19 mortality remains poorly known, and there is still no specific evidence to guide how best to adjust maintenance immunosuppression in solid organ transplant recipients.6,7 We conducted a cohort study among kidney transplant (KT) recipients from the French Solid Organ Transplant COVID-19 Registry to investigate the association between maintenance immunosuppressive drugs and 60-d mortality in KT patients with COVID-19.
MATERIALS AND METHODS
Data from all KT recipients with COVID-19 included in the French Solid Organ Transplant COVID-19 Registry between February 28 and December 30, 2020, were retrieved. Inclusion criteria were age >18 y at diagnosis of COVID-19 and the presence of a functioning kidney graft. Among the 1567 KT recipients included in the Registry during this period, 116 were not included because data on maintenance immunosuppressive therapy were missing. Clinical and laboratory variables were extracted from medical records.
Descriptive statistics were expressed as median (interquartile range [IQR]). We evaluated the associations between immunosuppressive drugs and death within 60 d of first symptoms using logistic regression. Baseline characteristics of patients considered to influence the outcome or the immunosuppressive regimen, which can constitute a source of confounding, were included in the multivariate analysis: immunosuppressive drugs, blood group, donor type (deceased or living), time from transplantation to diagnosis of COVID-19, sex, number of previous transplantations, induction therapy, baseline serum creatinine, cardiac disease, respiratory disease, diabetes, cancer, smoking, hypertension, and body mass index. To account for multiple testing, the Benjamini-Hochberg correction was used to help controlling the false positive rate. We performed 40 multiple imputations for missing data, using the package mice in the R software (R Core Team, 2020). An adjusted P value <0.05 was considered statistically significant.
A validation analysis, based on a penalized logistic regression, was conducted to corroborate the findings of the primary analysis. This analysis was performed on the same 40 multiple imputed data sets as the primary analysis but additionally on 500 bootstraps per multiple imputation. The bootstrap is a resampling method that enables assessment of the accuracy of an estimator by random sampling with replacement from an original dataset. It allows to compute a variable inclusion probability (VIP), which weights the importance of each variable to predict the outcome (death, <60 d). The significance threshold for VIP is >80%. The penalized logistic regression considered the same variables as the primary multivariate analysis. A supplementary penalized logistic regression was also performed to analyze the impact of the immunosuppressive protocols.
The French Solid Organ Transplant COVID-19 Registry was approved by the Institutional Review Board of the Strasbourg University (approval number 02.26) and registered at clinicaltrials.gov (NCT04360707). All patients were duly informed about their inclusion in the registry.
RESULTS
There were 1451 KT recipients with COVID-19 included in the study (Table 1). The median age of patients was 58 (IQR, 48–67) y, and 963 (66.4%) were men. The most frequent comorbidities were hypertension (n = 1188, 81.9%), diabetes (n = 501, 34.5%), and cardiovascular disease (n = 428, 29.5%). The median time since transplant was 71 (IQR, 29–144) mo.
TABLE 1.
Baseline characteristics of kidney transplant recipients with COVID-19 (n = 1451)
| Variable | Median (IQR) or n (%) |
|---|---|
| Age, y | 58 (48–67) |
| Gender, male | 963 (66.4) |
| Blood group | |
| A | 624 (43.0) |
| B | 163 (11.2) |
| AB | 77 (5.3) |
| O | 560 (38.6) |
| Comorbidities | |
| Hypertension | 1188 (81.9) |
| Diabetes | 501 (34.5) |
| Cardiovascular disease | 428 (29.5) |
| Cancer | 197 (13.6) |
| Smoking | 193 (13.3) |
| Respiratory disease | 162 (11.2) |
| BMI, kg/m2 | 26 (23–29) |
| ≥2 transplantations | 164 (11.3) |
| Donor type | |
| Deceased donor | 1254 (86.4) |
| Living donor | 185 (12.7) |
| Time from transplantation to COVID-19, mo | 71 (29–144) |
| Baseline serum creatinine, µmol/L | 131 (102–173) |
| Induction therapy | |
| Anti-CD25 | 611 (42.1) |
| Thymoglobulins | 740 (51.0) |
| No induction | 56 (3.9) |
| Maintenance immunosuppression | |
| Calcineurin inhibitorsa | 1295 (89.2) |
| Antimetabolitesb | 1205 (83.0) |
| Corticosteroids | 1094 (75.4) |
| mTOR inhibitors | 144 (9.9) |
| Belatacept | 58 (4.0) |
Data are expressed as median (IQR) or count (%), as appropriate.
aTacrolimus or ciclosporine.
bMycophenolate mofetil, mycophenolate acid, or azathioprine.
BMI, body mass index; COVID-19, coronavirus disease 2019; IQR, interquartile range; mTOR, mammalian target of rapamycin.
Maintenance immunosuppression included calcineurin inhibitors (CNIs; n = 1295, 89.2%), antimetabolites (n = 1205, 83%), corticosteroids (n = 1094, 75.4%), mammalian target of rapamycin inhibitors (n = 144, 9.9%), and belatacept (n = 58, 4.0%). The most common regimens were combination of CNI, antimetabolites, and steroids (n = 718, 49.5%); combination of CNI and antimetabolites (n = 284, 19.6%); and combination of CNI and steroids (n = 104, 7.2%).
Among 1451 patients, 201 (13.9%) died within 60 d of first symptoms. In the primary multivariate analysis, older age (odds ratio [OR], 1.09 [1.07-1.11]; P < 0.001) and baseline serum creatinine (OR, 1.01 [1.005-1.009] for each 1 µmol/L increase in serum creatinine; P < 0.001) were significantly associated with 60-d mortality. The median age of deceased patients was 69 (IQR, 61–75) y, as compared with 56 (IQR, 46–65) y in patients who survived. Median baseline serum creatinine was 160 (IQR, 120–209) µmol/L in patients who died and 130 (IQR, 100–164) µmol/L in patients who survived. As for maintenance immunosuppression, corticosteroid-free regimens were associated with a significantly lower risk of death (OR, 0.48 [0.31-0.76]; P = 0.011). All other variables yielded nonsignificant adjusted P values in the logistic regression (Table 2).
TABLE 2.
Logistic regression of risk factors for 60-d mortality in kidney transplant recipients with COVID-19 (n = 1451)
| OR | 95% CI | Adjusted P | |
|---|---|---|---|
| Age, y | 1.089 | 1.07-1.11 | <0.001a |
| Gender | |||
| Female | Reference | ||
| Male | 0.636 | 0.44-0.92 | 0.06 |
| Blood group | |||
| A | Reference | ||
| B | 1.905 | 1.11-3.27 | 0.06 |
| AB | 1.082 | 0.48-2.42 | 0.875 |
| O | 1.044 | 0.72-1.52 | 0.875 |
| Comorbidities | |||
| Hypertension | 1.051 | 0.57-1.94 | 0.875 |
| Diabetes | 1.524 | 1.07-2.17 | 0.06 |
| Cardiovascular disease | 1.078 | 0.75-1.55 | 0.868 |
| Cancer | 1.708 | 1.10-2.65 | 0.06 |
| Smoking | 1.178 | 0.72-1.91 | 0.754 |
| Respiratory disease | 0.745 | 0.45-1.23 | 0.466 |
| BMI, kg/m2 | 1.04 | 1.01-1.08 | 0.063 |
| ≥2 transplantations | 1.171 | 0.68-2.01 | 0.754 |
| Living donor | 0.738 | 0.37-1.48 | 0.628 |
| Time from transplantation to COVID-19, mo | 1.000 | 0.10-1.00 | 0.874 |
| Baseline serum creatinine, µmol/L | 1.006b | 1.005-1.009b | <0.001a |
| Induction therapy | |||
| Anti-CD25 | Reference | ||
| Thymoglobulins | 0.959 | 0.67-1.38 | 0.875 |
| No induction | 0.305 | 0.09-1.06 | 0.149 |
| Maintenance immunosuppression | |||
| Calcineurin inhibitorsc: yes | Reference | ||
| Calcineurin inhibitors: no | 1.792 | 0.90-3.55 | 0.208 |
| Antimetabolitesd: yes | Reference | ||
| Antimetabolites: no | 0.773 | 0.48-1.24 | 0.497 |
| Corticosteroids: yes | Reference | ||
| Corticosteroids: no | 0.483 | 0.31-0.76 | 0.011a |
| mTOR inhibitors: yes | Reference | ||
| mTOR inhibitors: no | 0.824 | 0.44-1.55 | 0.754 |
| Belatacept: yes | Reference | ||
| Belatacept: no | 1.89 | 0.68-5.24 | 0.441 |
ORs, 95% CIs, and multiple corrected (false discovery rate) adjusted P values from the logistic regression are shown.
aStatistically significant.
bFor each 1 µmol/L increase in serum creatinine.
cTacrolimus or ciclosporine.
dMycophenolate mofetil, mycophenolate acid, or azathioprine.
BMI, body mass index; CD, Cluster of Differentiation; COVID-19, coronavirus disease 2019; mTOR, mammalian target of rapamycin; OR, odds ratio.
In the validation analysis (penalized logistic regression), long-term treatment with corticosteroids was the only one to be associated with a higher risk of death (OR, 1.008 [1.00-1.44]; VIP = 83.6%; Table S1, SDC, http://links.lww.com/TP/C487), confirming the results of the primary analysis. Consistently, combination of CNI and antimetabolites without corticosteroids was the only regimen associated with a lower OR for death (OR, 0.625 [0.28-0.92]; VIP = 99.4%; Table S2, SDC, http://links.lww.com/TP/C487).
DISCUSSION
In this evaluation of the association between immunosuppressive regimens and COVID-19 mortality in KT recipients, corticosteroid-free regimens were associated with a significantly lower risk of death. This finding in a large cohort of 1445 KT recipients with COVID-19 is in accordance with independent studies performed with a lower number of patients.8,9
Although a short course of high-dose corticosteroids is beneficial in severely ill COVID-19 patients with respiratory failure,10 prolonged maintenance corticosteroid therapy exposes to chronic metabolic and immune disorders that may predispose solid organ transplant patients to severe forms of COVID-19. The timing, dose, and duration of the exposure to corticosteroids may be determinant. Indeed, high-dose pulse corticosteroids and low-dose chronic corticotherapy have different immunological effects. For instance, corticosteroids administered at high dose interfere on leukocyte aggregation and phagocytic function,11,12 rapidly deplete most circulating T cells, and inhibit interleukin-2 signaling, which is not the case with low-dose steroids.13-15 For these reasons, high-dose corticosteroids might rapidly mitigate the inflammatory storm or lung inflammation and are, therefore, clearly beneficial in patients with severe COVID-19 pneumonia. As for low-dose corticosteroids, they profoundly alter innate and adaptive immune function through a range of direct effects on gene transcription and posttranslational events. Most of these effects are known to be dependent on the cumulative dose of corticosteroids, especially regarding the infectious risk.16 Long-term preexisting corticosteroid exposure may, therefore, impair the establishment of an efficient immune response at the initial stages of the infection. This may underpin the increased COVID-19 mortality observed in patients long-term treated with corticosteroids. Consistently, chronic corticosteroid exposition has also been associated with increased COVID-19 severity in an array of other conditions such as inflammatory bowel and rheumatic diseases, as well as myasthenia gravis.17-19 Corticosteroid-free KT recipients were also recently shown to respond better to mRNA COVID-19 vaccines.5 Yet, a relevant bias is still possible, and our findings warrant further confirmation. We may not have considered all potential confounding factors (eg, primary renal disease) despite all the variables included in the analysis. Besides, we did not include in the analysis the specific treatments given for COVID-19. We reasoned that this analysis would have been biased by the fact that most of these treatments were administered to the most severe patients at higher risk of death, especially during the first months of the pandemic.
Tapering or withdrawal of corticosteroids is increasingly attempted to minimize metabolic disorders and decrease overall immunosuppression. Corticosteroid-free maintenance immunosuppression might provide noninferior outcomes in long-term patient and graft survival in KT recipients,20-22 as recently confirmed.23 At the time of the COVID-19 pandemic, long-term safety and reduced COVID-19 mortality rate may support corticosteroid-free regimen in KT recipients with low-to-intermediate immune risk. However, corticosteroid withdrawal must be balanced against the risk of rejection and can only be envisaged by the clinician in charge of the patient on a case-by-case basis.
ACKNOWLEDGMENTS
We thank all collaborators of the French SOT COVID Registry: Sophie Caillard and Bruno Moulin, Service de Néphrologie et Transplantation, Hôpitaux Universitaires de Strasbourg, Strasbourg; Samira Fafi-Kremer, Laboratoire de Virologie, Hôpitaux Universitaires de Strasbourg, Strasbourg; Marc Hazzan, Service de Néphrologie, Hôpital Huriez, Lille; Dany Anglicheau, Service de Néphrologie et Transplantation Adultes, AP-HP, Hôpital Necker, Paris; Alexandre Hertig, Jérôme Tourret, and Benoit Barrou, Service de Néphrologie, AP-HP, Hôpital La Pitié Salpétrière, Paris; Emmanuel Morelon and Olivier Thaunat, Service de Néphrologie, Hôpital Edouard Herriot, Lyon; Lionel Couzi and Pierre Merville, Service de Néphrologie–Transplantation–Dialyse, Hôpital Pellegrin, Bordeaux; Valérie Moal and Tristan Legris, Service de Néphrologie et Transplantation, AP-HM, Hôpital de la Conception, Marseille; Pierre-François Westeel and Maïté Jaureguy, Service de Néphrologie, CHU Amiens Picardie, Amiens; Luc Frimat, Service de Néphrologie, CHRU Nancy, Vandoeuvre; Didier Ducloux and Jamal Bamoulid, Service de Néphrologie, Hôpital Jean-Minjoz, Besancon; Dominique Bertrand, Service de Néphrologie, CHU de Rouen, Rouen; Michel Tsimaratos and Florentine Garaix-Gilardo, Service de Pédiatrie Multidisciplinaire, Hôpital La Timone, Marseille; Jérôme Dumortier, Service d’Hépato-Gastroentérologie, Hôpital Edouard Herriot, Lyon; Sacha Mussot and Antoine Roux, Centre Chirurgical Marie Lannelongue, Le Plessis Robinson; Laurent Sebbag, Service d’insuffisance Cardiaque, Hôpital Louis Pradel, Bron; Yannick Le Meur, Service de Néphrologie, Hôpital de la Cavale Blanche, Brest; GillesBlancho and Christophe Masset, Service de Néphrologie– Transplantation, Hôtel Dieu, Nantes; Nassim Kamar, Service de Néphrologie et Transplantation, Hôpital Rangueil, Toulouse; Hélène Francois and Eric Rondeau, Service de Néphrologie, Dialyse et Transplantation, AP-HP, Hôpital Tenon, Paris; Nicolas Bouvier, Service de Néphrologie, Dialyse, Transplantation Rénale, CHU, Caen; Christiane Mousson, Service de Néphrologie, Dijon; Matthias Buchler and Philippe Gatault, Service de Néphrologie, Tours; Jean-François Augusto and Agnès Duveau, Service de Néphrologie, Dialyse, Transplantation, CHU Angers, Angers; Cécile Vigneau, Marie-Christine Morin, Jonathan Chemouny, and Leonard Golbin, Service de Néphrologie, CHU de Rennes, Rennes; Philippe Grimbert, Marie Matignon, and Antoine Durrbach, Service de Néphrologie, Hôpital Henri-Mondor, Creteil; Clarisse Greze, Service de Néphrologie, AP-HP, Hôpital Bichat Claude Bernard, Paris; Renaud Snanoudj, Service de Néphrologie, Hôpital Foch, Service de Néphrologie et Transplantation Hôpital du Kremlin Bicêtre, Le Kremlin Bicetre; Charlotte Colosio and Betoul Schvartz, Service de Néphrologie, Hôpital Maison Blanche, Reims; Paolo Malvezzi, Service de Néphrologie, Hémodialyse, Transplantation Rénale, Hôpital La Tronche, Grenoble; Christophe Mariat, Service de Néphrologie, CHU de Saint Etienne, Saint Etienne; Antoine Thierry, Service de Néphrologie, Hémodialyse et Transplantation Rénale, Hôpital Jean Bernard, Poitiers; Moglie Le Quintrec, Service de Néphrologie-Transplantation-Dialyse, CHU Lapeyronie, Montpellier; Antoine Sicard, Service de Néphrologie, Hôpital Pasteur, Nice; Jean Philippe Rerolle, Service de Néphrologie, CHU Dupuytren, Limoges; Anne-Élisabeth Heng and Cyril Garrouste, Service de Néphrologie, CHU Gabriel Montpied, Clermont- Ferrand; Henri Vacher Coponat, Service de Néphrologie, CHU de La Réunion, Saint Denis; Éric Epailly, Service de Cardiologie, Hôpitaux Universitaires de Strasbourg, Strasbourg; Olivier Brugiere, Service d’hépatologie, Hôpital Foch, Suresnes; Sébastien Dharancy, Service d’hépatologie, Hôpital Huriez, Lille; Éphrem Salame, Service de Chirurgie Hépatique, Hôpital Universitaire de Tours, Tours; and Faouzi Saliba, Service d’Hépatologie, Centre Hépato-Biliaire Paul Brousse, Villejuif.
Supplementary Material
Footnotes
S.C. and A.S. contributed equally to this article.
Collaborators of the French SOT COVID Registry have been listed in the Acknowledgments.
The authors declare no funding or conflicts of interest.
D.A., L.C., M.H., O.T., G.B., S.C., and A.S. participated in research design. A.O.G., S.B., S.C., and A.S. participated in the writing of the paper. A.O.G., S.B., and A.S. participated in data analysis.
French SOT COVID Registry: approved by the Institutional Review Board of Strasbourg University (approval number 02.26), registered at clinicaltrials.gov (NCT04360707).
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantjournal.com).
Contributor Information
Collaborators: Sophie Caillard, Bruno Moulin, Samira Fafi-Kremer, Marc Hazzan, Dany Anglicheau, Alexandre Hertig, Jérôme Tourret, Benoit Barrou, Emmanuel Morelon, Olivier Thaunat, Lionel Couzi, Pierre Merville, Valérie Moal, Tristan Legris, Pierre-François Westeel, Maïté Jaureguy, Luc Frimat, Didier Ducloux, Jamal Bamoulid, Dominique Bertr, Michel Tsimaratos, Florentine Garaix-Gilardo, Jérôme Dumortier, Sacha Mussot, Antoine Roux, Laurent Sebbag, Yannick Le Meur, Gilles Blancho, Christophe Masset, Nassim Kamar, Hélène Francois, Eric Rondeau, Nicolas Bouvier, Christiane Mousson, Matthias Buchler, Philippe Gatault, Jean-François Augusto, Agnès Duveau, Cécile Vigneau, Marie-Christine Morin, Jonathan Chemouny, Leonard Golbin, Philippe Grimbert, Marie Matignon, Antoine Durrbach, Clarisse Greze, Renaud Snanoudj, Charlotte Colosio, Betoul Schvartz, Paolo Malvezzi, Christophe Mariat, Antoine Thierry, Moglie Le Quintrec, Antoine Sicard, Jean Philippe Rerolle, Anne-Élisabeth Heng, Cyril Garrouste, Henri Vacher Coponat, Éric Epailly, Olivier Brugiere, Sébastien Dharancy, Éphrem Salame, and Faouzi Saliba
REFERENCES
- 1.Akalin E, Azzi Y, Bartash R, et al. COVID-19 and kidney transplantation. N Engl J Med. 2020;382:2475–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caillard S, Anglicheau D, Matignon M, et al. ; French SOT COVID Registry. An initial report from the French SOT COVID registry suggests high mortality due to COVID-19 in recipients of kidney transplants. Kidney Int. 2020;98:1549–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cristelli MP, Viana LA, Dantas MTC, et al. The full spectrum of COVID-19 development and recovery among kidney transplant recipients. Transplantation. 2021;105:1433–1444. [DOI] [PubMed] [Google Scholar]
- 5.Caillard S, Thaunat O, Benotmane I, et al. Antibody response to a fourth messenger RNA COVID-19 vaccine dose in kidney transplant recipients: a case series. Ann Intern Med. 2022;175:455–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zaidan M, Legendre C. Solid organ transplantation in the era of COVID-19: lessons from France. Transplantation. 2021;105:61–66. [DOI] [PubMed] [Google Scholar]
- 7.Bae S, McAdams-DeMarco MA, Massie AB, et al. Early changes in kidney transplant immunosuppression regimens during the COVID-19 pandemic. Transplantation. 2021;105:170–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marinelli T, Ferreira VH, Ierullo M, et al. Prospective clinical, virologic, and immunologic assessment of COVID-19 in transplant recipients. Transplantation. 2021;105:2175–2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Willicombe M, Gleeson S, Clarke C, et al. ; ICHNT Renal COVID Group. Identification of patient characteristics associated with SARS-CoV-2 infection and outcome in kidney transplant patients using serological screening. Transplantation. 2021;105:151–157. [DOI] [PubMed] [Google Scholar]
- 10.Cano EJ, Fonseca Fuentes X, Corsini Campioli C, et al. Impact of corticosteroids in coronavirus disease 2019 outcomes: systematic review and meta-analysis. Chest. 2021;159:1019–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hammerschmidt DE, White JG, Craddock PR, et al. Corticosteroids inhibit complement-induced granulocyte aggregation. A possible mechanism for their efficacy in shock states. J Clin Invest. 1979;63:798–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Youssef P, Roberts-Thomson P, Ahern M, et al. Pulse methylprednisolone in rheumatoid arthritis: effects on peripheral blood and synovial fluid neutrophil surface phenotype. J Rheumatol. 1995;22:2065–2071. [PubMed] [Google Scholar]
- 13.Paliogianni F, Ahuja SS, Balow JP, et al. Novel mechanism for inhibition of human T cells by glucocorticoids. Glucocorticoids inhibit signal transduction through IL-2 receptor. J Immunol. 1993;151:4081–4089. [PubMed] [Google Scholar]
- 14.Hanson JA, Sohaib SA, Newell-Price J, et al. Computed tomography appearance of the thymus and anterior mediastinum in active Cushing’s syndrome. J Clin Endocrinol Metab. 1999;84:602–605. [DOI] [PubMed] [Google Scholar]
- 15.Cohen JJ, Duke RC. Glucocorticoid activation of a calcium-dependent endonuclease in thymocyte nuclei leads to cell death. J Immunol. 1984;132:38–42. [PubMed] [Google Scholar]
- 16.George MD, Baker JF, Winthrop K, et al. Risk for serious infection with low-dose glucocorticoids in patients with rheumatoid arthritis: a cohort study. Ann Intern Med. 2020;173:870–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brenner EJ, Ungaro RC, Gearry RB, et al. Corticosteroids, but not TNF antagonists, are associated with adverse COVID-19 outcomes in patients with inflammatory bowel diseases: results from an international registry. Gastroenterology. 2020;159:481–491.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gianfrancesco M, Hyrich KL, Al-Adely S, et al. ; COVID-19 Global Rheumatology Alliance. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis. 2020;79:859–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jakubíková M, Týblová M, Tesař A, et al. Predictive factors for a severe course of COVID-19 infection in myasthenia gravis patients with an overall impact on myasthenic outcome status and survival. Eur J Neurol. 2021;28:3418–3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rama I, Cruzado JM, Gil-Vernet S, et al. Steroids can be safely withdrawn from cyclosporine and mycophenolate mofetil-treated renal allograft recipients: long-term results. Transplantation. 2005;80:164–168. [DOI] [PubMed] [Google Scholar]
- 21.Woodle ES, First MR, Pirsch J, et al. ; Astellas Corticosteroid Withdrawal Study Group. A prospective, randomized, double-blind, placebo-controlled multicenter trial comparing early (7 day) corticosteroid cessation versus long-term, low-dose corticosteroid therapy. Ann Surg. 2008;248:564–577. [DOI] [PubMed] [Google Scholar]
- 22.Vazquez T, Alonso-Titos J, Gamez JP, et al. Effect of steroid withdrawal on the appearance of de novo donor-specific HLA antibodies in kidney transplant recipients: a prospective, randomized, controlled, parallel group study. Preliminary results. Transplantation. 2018;102:S203. [Google Scholar]
- 23.Woodle ES, Gill JS, Clark S, et al. Early corticosteroid cessation vs long-term corticosteroid therapy in kidney transplant recipients: long-term outcomes of a randomized clinical trial. JAMA Surg. 2021;156:307–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.