Background.
Comparative studies of third heterologous doses following the CoronaVac vaccine against coronavirus disease 2019 (COVID-19) in kidney transplant recipients are lacking.
Methods.
This prospective, single-center cohort study included kidney transplant recipients without previous COVID-19. Patients received a third heterologous (BNT162b2 mRNA) or homologous dose at least 4 wk after 2 doses of the CoronaVac vaccine. Immunoglobulin G antibody response and seroprevalence for neutralizing anti–severe acute respiratory syndrome coronavirus 2 antibodies immediately before and 28 d after third doses were compared between the groups.
Results.
There were 307 patients in the heterologous group and 777 in the homologous group. Patients in the heterologous group were older (54 versus 50 y; P < 0.0001), with a longer time since transplant (11 versus 6 y; P < 0.0001). Immediately before the third dose, immunoglobulin G seroprevalence (36% versus 34%; P = 0.597) and antibody titers (246 versus 268 AU/mL; P = 0.279) were similar. After booster, seroconversion was higher in the heterologous group (49% versus 32%; P < 0.0001), resulting in a higher seroprevalence (67% versus 55%; P = 0.0003); however, 42% of all patients remained seronegative. Antibody titers after booster in seropositive patients were higher in the heterologous group (7771 versus 599 AU/mL; P < 0.0001). These results persisted after adjusting for confounding variables. Lastly, a similar proportion of patients became seropositive for neutralizing antibodies (98% versus 94%; P = 0.098).
Conclusions.
In kidney transplant recipients fully vaccinated with CoronaVac, a third dose with an mRNA vaccine produced a higher seroconversion rate and antibody titers than a third homologous dose. However, both boosters achieved equivalent seroprevalence for neutralizing antibodies. The high proportion of still seronegative patients indicates the need for alternative strategies of protection.
INTRODUCTION
Kidney transplant recipients achieve seroprevalence rates between 3% and 59% 4 wk after 2 doses of mRNA, viral vector, or inactivated whole-virion vaccines,1-4 which is lower than the general population.5,6 Developing a humoral immune response is associated with protection against breakthrough infections because more severe disease and deaths concentrate among seronegative patients.3,4,7 Thus, it seemed reasonable to apply additional doses of vaccine to achieve immunization in transplanted patients, who are at higher risk for coronavirus disease 2019 (COVID-19)–related mortality.8
The first attempts were to administer homologous boosters to seronegative patients after the full 2 doses of vaccination. However, in a French cohort, only 44% of the 59 solid organ transplant recipients who had been seronegative developed antibodies after a third homologous dose of the BNT162b2 mRNA vaccine.9 Similarly, we have observed a low seroconversion rate of 20.3% after a third homologous dose of the CoronaVac vaccine in those seronegative kidney transplant recipients.10
In the general population, initiated from safety considerations associated with the Oxford-AstraZeneca ChAdOx1-S (AZD1222) vaccine, mixing with mRNA vaccines ultimately has produced evidence in favor of a similar or even superior humoral and cellular response and clinical effectiveness compared to those with the homologous prime boosters.11-15 In the transplant population, a small German study including 40 kidney transplant recipients and 70 immunocompetent controls found that heterologous boosting with mRNA after vector vaccine priming was the regimen that produced the most robust humoral and cellular response.16 The little experience on heterologous boosters in kidney transplant recipients is restricted to endogenous antigen platform vaccines, either viral vector or mRNA. Theoretically, exposure to multiple viral antigens from an inactivated virus vaccine could trigger a stronger induction of neutralizing antibodies. Additionally, there are few studies systematically comparing the effect of a heterologous versus a homologous third dose.
In this analysis, we compared the seroconversion rates after a heterologous third dose of the BNT262b2 mRNA vaccine versus a homologous third dose of the CoronaVac inactivated whole-virion vaccine after the former 2-dose schedule of CoronaVac. Additionally, we evaluated the increased rate in antibody titers and neutralizing activity after heterologous BNT162b2 versus homologous CoronaVac third doses. We also described the occurrence and severity of breakthrough infections up to 3 mo after the third dose. Finally, we investigated independent factors for seroconversion after the third dose, high antibody values, and the occurrence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection after vaccination.
MATERIALS AND METHODS
Study Design
This is a prospective single-center cohort study derived from the previously published original trial in that kidney transplant recipients received 2 doses of the inactivated CoronaVac vaccine.17 The conception of the present study was driven by the urge of giving to the largest number of patients as soon as possible an additional vaccine dose against coronavirus, following the National Guidelines. Therefore, no prior hypothesis was conceived, and no sample size was determined on advance. This study protocol was approved by the local ethics committee, and the patients signed an updated informed consent form.
Inclusion criteria consisted of kidney transplant recipients aged 30–69 y, with >30 d of transplant, who had received the standard schedule of 2 doses of the CoronaVac vaccine at the transplant center between March 20 and April 25, 2021, and had been included in the original study. Exclusion criteria were having a confirmed COVID-19 after vaccination, allograft loss, death, or loss of follow-up. Additionally, to avoid large displacements and increased exposure to SARS-CoV-2 infection, and considering that the additional vaccine doses were already available in the public health system, only patients living in the city of Sao Paulo were approached. Those who lived abroad were excluded from this extension and instructed to be vaccinated in the health center of their municipality of residence.
The decision to vaccinate with one or the other platform was purely driven by the availability of the different vaccines. The patients were not advised to wait for any specific platform, once the center’s policy was to vaccinate the largest number of eligible patients as soon as possible, in accordance with the National Guidelines. The homologous booster of CoronaVac vaccine was readily available at the time the study was approved; thus, all patients who met the inclusion/exclusion criteria were invited to receive the third dose at the transplant center. As of September 17, 2021, the use of a third heterologous booster dose for patients at higher risk for worse COVID-19 outcomes, among them kidney transplant recipients, was approved by the Brazilian Ministry of Health.18 Thus, those patients who were eligible to participate in the study but who had been unable to attend in the first call were recalled and received the heterologous additional dose.
For the purpose of this analysis, all patients who received a third vaccine dose (either homologous or a 30-μg dose of the BNT162b2 mRNA vaccine) in the center were included and had blood samples collected for immunogenicity analysis, as described below.
The primary end point was the seroconversion rate after the third heterologous versus homologous dose. The secondary end points were the final seroprevalence rate, the magnitude of the difference in antibody titers before and after the third dose, the percentage of seropositive patients testing positive for neutralizing antibodies, the incidence rate of confirmed COVID-19 and associated lethality and risk factors for seroconversion, high antibody values, and occurrence of SARS-CoV-2 infection.
Immunogenicity Analysis
Antibody response immediately before and 28 d after the third dose was assessed using the Architect SARS-CoV-2 IgG II assay (Abbot Laboratories, IL) that measures quantitatively the immunoglobulin G (IgG) antibodies against the spike receptor-binding domain (RBD) of SARS-CoV-2. Values >50 AUs/mL were considered positive.19 Antibody values >840 AU/mL were labeled as high antibody values, based on the Food and Drug Administration guideline criterion for the therapeutic use convalescent plasma.20
Only for those IgG-positive patients before the third dose, neutralizing anti-SARS-CoV-2 antibodies were assessed through the cPass SARS-CoV-2 Neutralization Antibody Detection Kit, a test based on antibody-mediated blockage of the interaction between the angiotensin-converting enzyme 2 receptor protein and the RBD in the 2 laboratory evaluations; those results showing ≥30% signal inhibition were considered positive.21
Occurrence of Breakthrough Infections
Patients were instructed to seek the tele-assistance center, which had been in operation since the beginning of the pandemic, in case of any symptoms, any time after vaccination. Reverse transcription–polymerase chain reaction and rapid antigen test were readily available at the center for the symptomatic patients. A dedicated group followed those with the confirmed diagnosis and were referred to local hospitals for COVID-19 treatment when indicated.
The occurrence of new cases of symptomatic SARS-CoV-2 infection, confirmed through the reverse transcription–polymerase chain reaction or antigen test, and the COVID-19–associated lethality rate were registered during 3 mo after the third dose of vaccination.
Statistical Analysis
Categorical data were presented in absolute numbers and percentages, and continuous variables were shown in median and interquartile range (IQR). Differences between the 2 vaccine groups were tested using the Mann-Whitney U test and Pearson χ2 test. Differences were considered significant with P < 0.05. Binary logistic regression model was used to perform the multivariate analysis for independent risk factors for seroconversion, presence of high antibody titers, and occurrence of symptomatic COVID-19 after vaccination. Statistical analysis was performed using the SPSS program v. 22.0 (SPSS Inc., Chicago, IL) and GraphPad Prism program v.9.2.0 (Prisma Inc., CA).
RESULTS
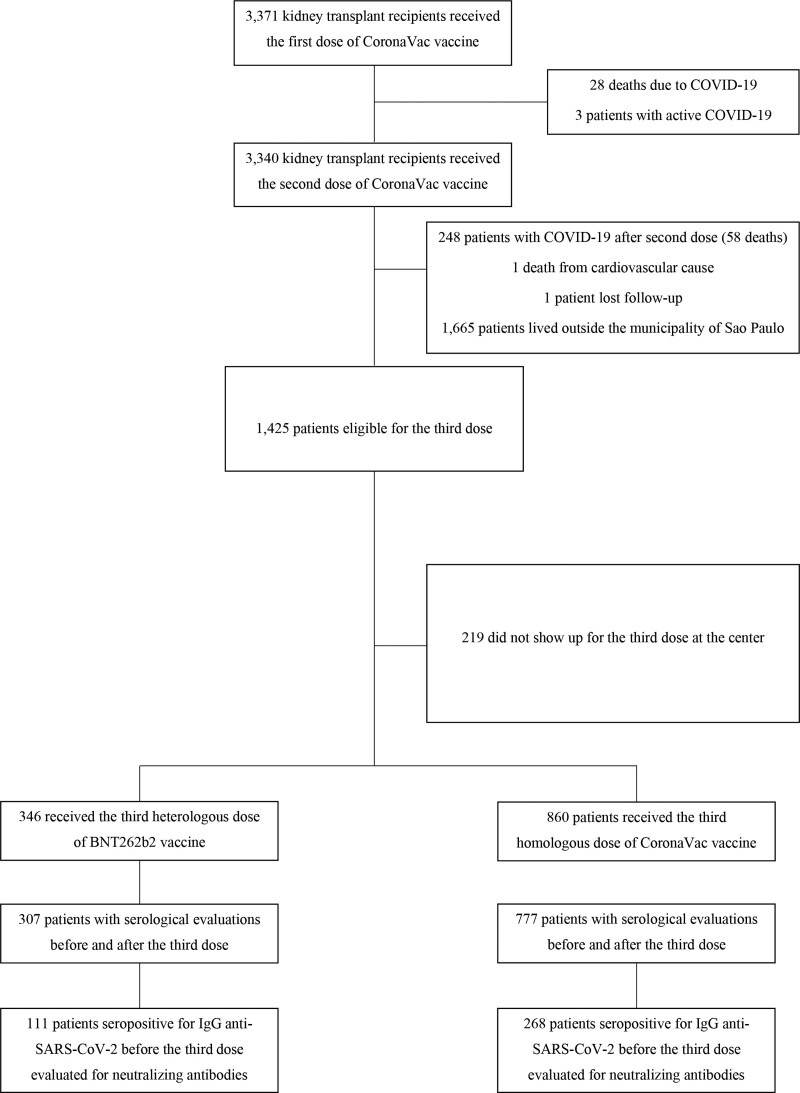
Among the 3340 patients fully vaccinated with 2 doses of CoronaVac vaccine in the original study, 1 subject died of a cardiovascular cause, another was lost to follow-up, 248 had a confirmed COVID-19 diagnosis after vaccination, and 1665 subjects lived outside the municipality of São Paulo, being instructed to vaccinate in their city of residence as mentioned above. Of the 1425 eligible patients, 219 did not show up for the third dose. Thus, as mentioned earlier, after vaccine doses were available, 346 patients were vaccinated at the center with the third heterologous BNT262b2 dose between September 20, 2021, and October 1, 2021, and 860 with the third CoronaVac homologous dose between July 2, 2021, and September, 27, 2021. From these, 307 patients in the heterologous group and 777 patients in the homologous group had laboratory evaluations before and after the booster, therefore being included for the present analysis (Figure 1).
FIGURE 1.
Disposition of the entire cohort and of the patients included in the study, according to homologous vs heterologous third vaccine dose. COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Baseline demographic characteristics are detailed in Table 1. Mainly, patients who received the heterologous BNT262b2 third dose were older (median age, 54 versus 50.3 y; P < 0.0001; patients >60 y, 29.6% versus 12.3%; P < 0.001), with a lower prevalence of diabetes (6.8% versus 11.1%; P = 0.032), lower percentage of deceased donors (59.6% versus 68.4%; P = 0.006), and a longer time since transplant (median, 11.2 versus 6.0 y; P < 0.0001). Regarding immunosuppression, less patients in the heterologous group were in use of tacrolimus (75.5% versus 87.2%; P < 0.0001) and of mycophenolate (37.8% versus 45.9% in the homologous group; P = 0.0154). All patients were on steroids.
TABLE 1.
Baseline characteristics of the studied population, according to homologous CoronaVac vs heterologous BNT162b2 third dose
| Parameters | Third heterologous BNT162b2 | Third homologous CoronaVac | P |
|---|---|---|---|
| (n = 307) | (n = 777) | ||
| Median age, y (IQR) | 54.0 (47.6–61.1) | 50.3 (43.5–56.7) | <0.0001 |
| 30–60 y, n (%) | 216 (70.3) | 681 (87.7) | <0.0001 |
| >60 y, n (%) | 91 (29.6) | 96 (12.3) | |
| Male gender, n (%) | 179 (58.3) | 449 (57.7) | 0.857 |
| Diabetes, n (%) | 21 (6.8) | 86 (11.1) | 0.032 |
| Deceased donor transplant, n (%) | 183 (59.6) | 532 (68.4) | 0.006 |
| Organ, n (%) | |||
| Kidney | 289 (94.1) | 749 (96.3) | 0.109 |
| Simultaneous pancreas-kidney | 18 (5.9) | 28 (3.7) | |
| Median length of transplant, y (IQR) | 11.2 (6.8–17.2) | 6.0 (2.8–10.8) | <0.0001 |
| Maintenance immunosuppressive regimen, n (%) | |||
| TAC-Pred-AZA | 100 (32.6) | 238 (30.7) | 0.543 |
| TAC-Pred-MPA | 108 (35.1) | 345 (44.4) | 0.005 |
| CSA-Pred-AZA | 62 (20.2) | 80 (10.3) | <0.0001 |
| TAC-Pred-mTORi | 21 (6.8) | 91 (11.7) | 0.017 |
| Other | 16 (5.3) | 23 (2.9) | 0.055 |
| Maintenance regimen by immunosuppressive drug, n (%) | |||
| Calcineurin inhibitors | |||
| TAC | 232 (75.5) | 677 (87.2) | <0.0001 |
| CSA | 70 (22.8) | 84 (10.8) | <0.0001 |
| Pred | 307 (100) | 777 (100) | – |
| Antimetabolite | |||
| MPA | 116 (37.8) | 357 (45.9) | 0.015 |
| AZA | 165 (53.7) | 321 (41.3) | 0.0002 |
| mTORi | 23 (7.5) | 100 (12.9) | 0.012 |
| Median baseline creatinine, mg/dL | 1.38 (1.14–1.81) | 1.49 (1.19–1.88) | 0.077 |
| Median time from 2nd to 3rd dose, d (IQR) | 156 (152–158) | 139 (80–146) | <0.0001 |
| Median time after the 3rd dose to laboratory evaluation, d (IQR) | 25 (23–26) | 35 (29–35) | <0.0001 |
AZA, azathioprine; CSA, cyclosporine; IQR, interquartile range; MPA, mycophenolic acid; mTORi, mammalian target of rapamycin inhibitor; Pred, prednisone; TAC, tacrolimus.
No patients had been treated for acute rejection within the past 3 mo before the third dose of vaccines, and no difference was observed in the baseline creatinine.
The median time from second dose to third dose was longer in the heterologous group (156 versus 139 d; P < 0.001).
SARS-CoV-2 Spikes RBD-specific IgG Antibody Response
The analysis for IgG antibody response included 307 patients in the heterologous BNT262b2 group and 777 in the homologous CoronaVac group. Immediately before the third dose, no significant difference was observed either in the seroprevalence for IgG anti-RBD-SARS-CoV-2 antibodies (36.2% versus 34.5%; P = 0.597) or in the median antibody titers among those seropositive individuals (246 versus 268 AU/mL; P = 0.279; Table 2).
TABLE 2.
Immunogenicity analysis of the studied population, according to homologous CoronaVac vs heterologous BNT162b2 third dose
| Parameters | Heterologous BNT162b2 | Homologous CoronaVac | P | ||
|---|---|---|---|---|---|
| (n = 307) | (n = 777) | ||||
| Serological status immediately before the third dose | |||||
| IgG-anti-RBD-negative, n (%) | 196 (63.8) | 509 (65.5) | 0.597 | ||
| IgG-anti-RBD-positive, n (%) | 111 (36.2) | 268 (34.5) | |||
| Median antibody values, AU/mL (IQR) | 246 (109–792) | 268 (118–1158) | 0.279 | ||
| Serological status after the third dose | |||||
| IgG-anti-RBD-negative, n (%) | 100 (32.6) | 346 (44.5) | 0.0003 | ||
| IgG-anti-RBD positive, n (%) | 207 (67.4) | 431 (55.5) | |||
| Median antibody values, AU/mL (IQR) | 7771 (1295–20 158) | 599 (195–1661) | <0.0001 | ||
| Serologic status combinations | Before third dose (D1) | After third dose (D2) | Before third dose (D1) | After third dose (D2) | |
| D1 (+) D2 (+), n (%) | 111 (36.2) | 268 (34.5) | |||
| Median antibody values, AU/mL (IQR) | 246 (109–792) a,b | 16 468 (6414–28 020) a | 288 (118–1158)a,b | 1094 (512–2730)a | |
| D1 (−) D2 (+), n (%) | 96 (31.2) | 165 (21.2) | |||
| Median antibody values, AU/mL (IQR) | NA | 1768 (438–7657) a | NA | 189 (98–417)a | |
| D1 (−) D2 (−), n (%) | 100 (32.6) | 344 (44.2) | |||
aP < 0.0001 for comparisons between D1 and D2 and between heterologous and homologous subgroups.
bP = 0.277 for comparisons between heterologous and homologous D1 within the D1(+) D2(+) subgroup.
D1, serological status immediately before the third dose; D2, serological status after the third dose; IgG, immunoglobulin G; IQR, interquartile range; NA, not applicable; RBD, receptor domain binding.
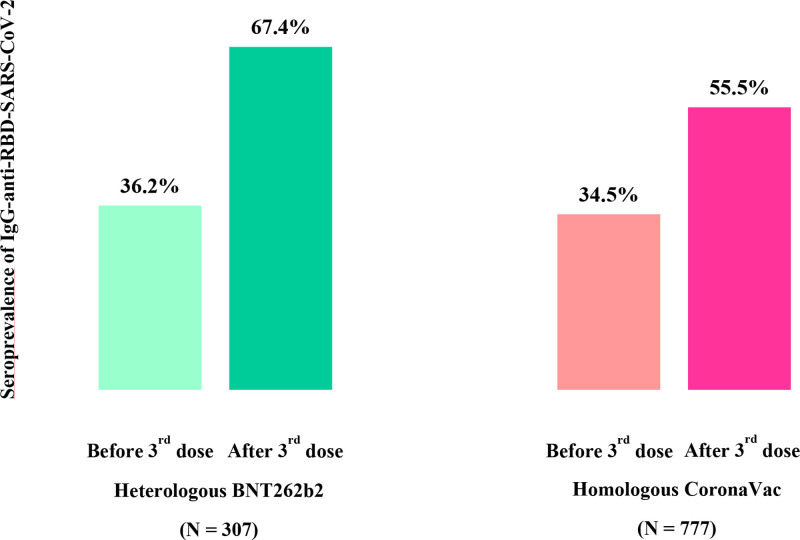
At a median of 25 d after the heterologous BNT262b2 and 35 d after the homologous CoronaVac third-dose vaccine, the seroconversion rate was significantly higher in the heterologous group (48.9% versus 32.4%; P < 0.0001), resulting in a significantly higher seroprevalence rate (67.4% versus 55.5%; P = 0.0003; Table 2; Figure 2). Furthermore, after the booster, the median antibody titers among seropositive patients were higher in those to whom the third heterologous BNT262b2 was administered (7771 versus 599 AU/mL; P < 0.0001; Table 2). However, 444 individuals or 40.1% of the entire cohort (32.6% in the heterologous BNT262b2 and 44.2% in the homologous CoronaVac groups; P = 0.0003) remained seronegative even after 3 doses.
FIGURE 2.
Comparative analysis of seroprevalence and seroconversion rates between the heterologous BNT262b2 and homologous CoronaVac vaccine groups. IgG, immunoglobulin G; RBD, receptor-binding domain; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
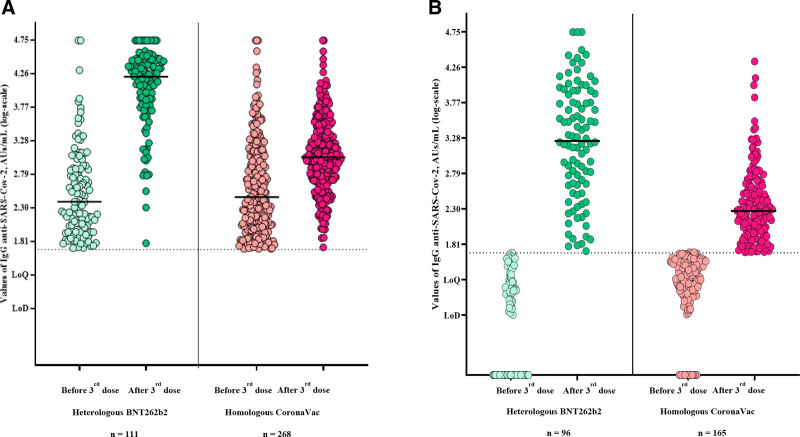
When stratifying the immunogenicity analysis by serological status before the third dose, a 66-fold increase in antibody titers was observed in the heterologous group, compared to a 4-fold increase after the third homologous dose (P < 0.0001; Figure 3A). Finally, among patients who seroconverted, median titers were approximately 10× higher after the third heterologous compared to homologous dose (1768 versus 189 AU/mL; P < 0.0001; Figure 3B).
FIGURE 3.
Comparative analysis of the IgG anti-SARS-CoV-2 values before and after the heterologous vs homologous vaccination (A) among those patients who were already seropositive before the third dose and (B) among those patients who were seronegative before the third dose, but presented Seroconversion afterwards. IgG, immunoglobulin G; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Subanalysis of SARS-CoV-2 Surrogate Virus Neutralization Testing
The surrogate virus neutralization test was performed in those patients who were seropositive for IgG anti-SARS-CoV-2 before the booster. In this condition, there were 111 patients in the heterologous BNT262b2 group and 268 in the homologous CoronaVac group.
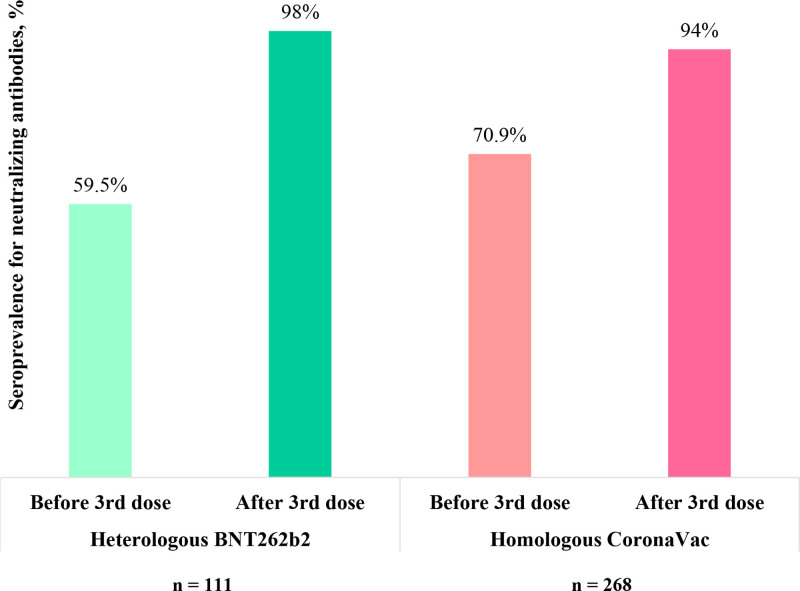
Immediately before the third dose, 66 of 111 (59.5%) patients in the heterologous group and 190 of 268 (70.9%) in the homologous group (P = 0.035) had a positive surrogate virus neutralization test.
A higher rate of seroconversion for neutralizing antibodies was observed after BNT262b2 heterologous versus CoronaVac homologous vaccination (38% versus 24%; P = 0.0059), resulting in a similar final seroprevalence rate (98% versus 94%; P = 0.098; Figure 4).
FIGURE 4.
Comparative analysis of the seroprevalence for neutralizing antibody values before and after the heterologous vs homologous vaccination among those patients who had a positive test for IgG–anti–SARS-CoV-2 before the third dose. IgG, immunoglobulin G; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Multivariate Analysis for Serologic Vaccine Response
After multivariate analysis, receiving a heterologous third dose was an independent factor for both seroconversion and high titers of antibodies. Other factors for seroconversion were younger age and living donor transplantation. The additional independent factor for high antibody titers was the absence of diabetes (Tables 3 and 4).
TABLE 3.
Univariate and multivariate analysis for seroconversion in the 705 patients who were seronegative immediately before the third dose
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| Factors | P | OR (95% CI) | P | OR (95% CI) |
| Age at third dose | 0.006 | 0.98 (0.96-0.99) | 0.01 | 0.98 (0.96-0.99) |
| Male gender | 0.20 | 1.14 (0.94-1.39) | – | |
| Diabetes | 0.28 | 0.97 (0.91-1.02) | – | |
| Time from transplantation to third dose | <0.0001 | 1.05 (1.02-1.07) | 0.22 | 1.02 (0.99-1.05) |
| Deceased donor (vs living donor) | <0.0001 | 0.49 (0.39-0.63) | <0.0001 | 0.44 (0.30-0.65) |
| Tacrolimus (vs cyclosporine) | 0.11 | 0.74 (0.53-1.05) | – | |
| Mycophenolate (vs AZA/mTORi, in patients on calcineurin inhibitors) | 0.39 | 0.94 (0.82-1.07) | – | |
| mTOR inhibitors (vs AZA/MPA, in patients on calcineurin inhibitors) | 0.71 | 0.99 (0.94-1.04) | – | |
| Time from second to third dose | 0.39 | 1.00 (1.00-1.00) | – | |
| Heterologous third dose (vs homologous) | <0.0001 | 1.22 (1.10-1.36) | <0.0001 | 2.00 (1.36-2.93) |
AZA, azathioprine; CI, confidence interval; MPA, mycophenolate; mTORi, inhibitors of the mammalian target of rapamycin; OR, odds ratio.
TABLE 4.
Univariate and multivariate analysis for presenting high antibody values (>840 AU/mL)a after the third dose, independently on the titers before the third dose
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| Factors | P | OR (95% CI) | P | OR (95% CI) |
| Age at third dose | 0.15 | 0.99 (0.98-1.00) | – | |
| Male gender | 0.60 | 0.96 (0.83-1.11) | – | |
| Diabetes | 0.001 | 0.93 (0.90-0.97) | 0.04 | 0.57 (0.34-0.97) |
| Time from transplantation to third dose | <0.0001 | 1.05 (1.03-1.07) | 0.54 | 1.01 (0.98-1.03) |
| Deceased donor (vs living donor) | <0.0001 | 0.67 (0.56-0.80) | 0.006 | 0.65 (0.47-0.88) |
| Tacrolimus (vs cyclosporine) | 0.02 | 0.71 (0.53-0.93) | 0.89 | 0.97 (0.66-1.44) |
| Mycophenolate (vs AZA/mTORi, in patients on calcineurin inhibitors) | 0.11 | 0.91 (0.82-1.02) | – | |
| mTOR inhibitors (vs AZA/MPA, in patients on calcineurin inhibitors) | 0.54 | 0.98 (0.94-1.03) | – | |
| Time from second to third dose | <0.0001 | 1.04 (1.04-1.05) | 0.14 | 1.00 (1.00-1.00) |
| Heterologous third dose (vs homologous) | <0.0001 | 1.54 (1.39-1.72) | <0.0001 | 3.69 (2.72-5.01) |
aAntibody values higher than 840 AU/mL were labeled as high antibody values, based on the Food and Drug Administration guideline criterion for the therapeutic use convalescent plasma.20
AZA, azathioprine; CI, confidence interval; MPA, mycophenolate; mTORi, inhibitors of the mammalian target of rapamycin; OR, odds ratio.
Breakthrough Infections
Within 3 mo after the third dose, there were 31 patients in the global cohort with confirmed COVID-19. From these, 5 patients received a BNT262b2 heterologous third dose (incidence rate of 1.59 cases/10 000 patients-day) and 26 patients received homologous CoronaVac (incidence rate of 3.07 cases/10 000 patients-day; P = 0.171). The median time from vaccination to SARS-CoV-2 infection in the heterologous group was 99 (IQR, 94–99) versus 56 (IQR, 36–92) d in the homologous group (P = 0.375). Neither demographic nor vaccine characteristics were independent factors for acquiring SARS-CoV-2 infection after vaccination (Table 5).
TABLE 5.
Univariate and multivariate analysis for acquiring SARS-CoV-2 infection after the third dose
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| Factors | P | OR (95% CI) | ||
| Age at third dose | 0.42 | 0.98 (0.95-1.03) | ||
| Male gender | 0.46 | 0.86 (0.59-1.25) | ||
| Diabetes | 1.00 | 0.99 (0.89-1.12) | ||
| Time from transplantation to third dose | 0.25 | 0.97 (0.91-1.02) | ||
| Deceased donor (vs living donor) | 0.43 | 1.34 (0.69-2.59) | ||
| Tacrolimus (vs cyclosporine) | 0.04 | 0.86 (0.81-0.93) | ||
| Mycophenolate (vs AZA/mTORi, in patients on calcineurin inhibitors) | 0.56 | 0.91 (0.69-1.21) | ||
| mTOR inhibitors (vs AZA/MPA, in patients on calcineurin inhibitors) | 0.07 | 1.15 (0.95-1.39) | ||
| Heterologous third dose (vs homologous) | 0.16 | 0.85 (0.72-0.99) | ||
AZA, azathioprine; CI, confidence interval; MPA, mycophenolate; mTORi, inhibitors of the mammalian target of rapamycin; OR, odds ratio.
One of the 2 patients who died among the 5 COVID-19 patients in the BNT262B2 heterologous group and all 6 dead among the 26 individuals in the CoronaVac homologous group had not developed a humoral response after the third dose. When patients who developed COVID-19 were analyzed independently of the vaccination platform, the lethality rate among the seropositives was 1 of 16 (6.3%) versus 7 of 15 (46.7%) among the seronegatives after the booster dose (P = 0.012).
DISCUSSION
In this prospective single-center study including kidney transplant recipients with no previous COVID-19 and 2 doses primed with the inactivated whole-virion CoronaVac vaccine, the heterologous booster with BNT262b2 was associated with higher seroprevalence, seroconversion, and IgG antibody values than the homologous regimen. Among the patients seropositive for IgG antibodies at baseline, the increment of the seropositivity for neutralizing antibodies was higher in the heterologous group, even after controlling the confounding variables through a multivariate analysis; however, the final proportion of patients with a positive surrogate virus neutralization testing was high and similar in both groups.
In the general population, the large CovBoost study, which included subjects fully vaccinated with mRNA or viral vector vaccines, confirmed that the third dose improved all immunological parameters but suggested that heterologous regimens would induce a better cellular response than a homologous booster.22 Two studies evaluating the CoronaVac vaccine found that the homologous third dose also achieved increased immunogenicity but that a heterologous booster produced a response of greater magnitude in terms of antibody titer and neutralizing antibody concentrations.23,24 Such results were reproduced even against the Omicron variant.25 Among kidney transplant recipients, 2 studies found similar increments in seroconversion and antibody titers when comparing homologous versus heterologous regimens. However, as the authors themselves acknowledge, they were limited to a small number of patients, and one of the studies included only people who had not seroconverted after the traditional 2-dose vaccine regimen.26,27 Our results confirm the utility of the booster in increasing seroconversion and antibody titers but question the superiority of a heterologous versus a homologous regimen in ultimately obtaining antibodies with neutralizing activity.
There are still no data evaluating the effects of different booster doses on clinical efficacy against COVID-19 in kidney transplant recipients. Our study did not observe differences in the occurrence of COVID-19 after vaccination. However, the patients in the heterologous group received the third dose after the patients in the homologous group, in a period of decreased virus circulation in Brazil, preventing drawing conclusions from these results.28
It is noteworthy that among the 8 deaths from COVID-19, 7 occurred in individuals who did not seroconvert independently of the third dose. Although the number of cases was small and did not allow for comparisons between platforms, the COVID-19–related lethality rate among patients with no immune response after booster, irrespectively of what type of vaccine, was 6× higher than those who developed antibodies and similar to the prevaccination era.29
The strengths of our study are the large number of patients and the homogeneity of analytical methods. We recognize the fact that data were obtained at a single center, precluding extrapolation to other populations. Furthermore, all patients received the first 2 doses of CoronaVac, precluding comparison with other vaccine platforms. Finally, we did not evaluate cellular immunity, and because vaccination and follow-up of patients occurred in the pre-Omicron era, the data found should be interpreted with caution after the emergence of new variants.
In summary, in kidney transplant recipients initiated with 2 doses of an inactivated vaccine, combining different vaccine platforms elicited a stronger humoral immune response, compared to administering a homologous booster dose. Both resulted in a high and comparable rate of patients with positive neutralizing antibodies. Although it was impossible to compare clinical effectiveness, our data reinforce the concept that achieving an immune response is crucial to reducing lethality from COVID-19. The fact that 40% of patients did not demonstrate seroconversion with any regimen cries out for additional protective strategies and the maintenance of nonpharmacological preventive measures against coronavirus infection in this population, even in the face of a more flexible new-normal scenario.
Footnotes
The authors declare no funding or conflicts of interest.
J.M.-P., L.A.V., C.F.H.G., M.P.C., L.R.R.-M., and H.T.-S. conceptualized the study; M.R.N., E.F.L., Y.C.D., L.V.P.A., C.Y.Z.C., and R.D.F. obtained the data; M.P.C., L.A.V., C.F.H.G., and H.T.-S. analyzed the data and drafted and revised the paper; all authors approved the final version of the manuscript.
References
- 1.Carr EJ, Kronbichler A, Graham-Brown M, et al. Review of early immune response to SARS-CoV-2 vaccination among patients with CKD. Kidney Int Rep. 2021;6:2292–2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Watcharananan SP, Jaru-Ampornpan P, Sahawongcharoen S, et al. ; Praram 9-BIOTEC-Ramathibodi COVID-19 Vaccine Study Group. Comparison of the immunogenicity of ChAdOx1 nCoV-19 vaccine against the wild-type and delta variants in kidney transplant recipients and healthy volunteers. Am J Transplant. 2022;22:1459–1466. [DOI] [PubMed] [Google Scholar]
- 3.Medina-Pestana J, Covas DT, Viana LA, et al. Inactivated whole-virus vaccine triggers low response against SARS-CoV-2 infection among renal transplant patients: prospective phase 4 study results. Transplantation. 2022;106:853–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asderakis A, Khalid U, Koimtzis G, et al. An analysis of serological response and infection outcomes following Oxford-AstraZeneca (AZD1222) and Pfizer-BioNTech (mRNA BNT162b2) SARS-CoV-2 vaccines in kidney and kidney pancreas transplants. Transplantation 2022;106:1421–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sauré D, O’Ryan M, Torres JP, et al. Dynamic IgG seropositivity after rollout of CoronaVac and BNT162b2 COVID-19 vaccines in Chile: a sentinel surveillance study. Lancet Infect Dis. 2022;22:56– 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wei J, Stoesser N, Matthews PC, et al. ; COVID-19 Infection Survey Team. Antibody responses to SARS-CoV-2 vaccines in 45,965 adults from the general population of the United Kingdom. Nat Microbiol. 2021;6:1140–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feng S, Phillips DJ, White T, et al. ; Oxford COVID Vaccine Trial Group. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat Med. 2021;27:2032–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Medina-Pestana J, Cristelli MP, Foresto RD, et al. The higher COVID-19 fatality rate among kidney transplant recipients calls for further action. Transplantation. 2022;106:908–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kamar N, Abravanel F, Marion O, et al. Three doses of an mRNA Covid-19 vaccine in solid-organ transplant recipients. N Engl J Med. 2021;385:661–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Medina-Pestana J, Covas DT, Viana LA, et al. Homologous third dose of inactivated whole-virion vaccine fails to elicit a robust immune response among kidney seronegative transplant recipients. Transplantation. 2022;106:e284–e285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fabricius D, Ludwig C, Scholz J, et al. mRNA vaccines enhance neutralizing immunity against SARS-CoV-2 variants in convalescent and ChAdOx1-primed subjects. Vaccines (Basel). 2021;9:918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nordström P, Ballin M, Nordström A. Effectiveness of heterologous ChAdOx1 nCoV-19 and mRNA prime-boost vaccination against symptomatic COVID-19 infection in Sweden: a nationwide cohort study. Lancet Reg Health Eur. 2021;11:100249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borobia AM, Carcas AJ, Pérez-Olmeda M, et al. ; CombiVacS Study Group. Immunogenicity and reactogenicity of BNT162b2 booster in ChAdOx1-S-primed participants (CombiVacS): a multicentre, open-label, randomised, controlled, phase 2 trial. Lancet. 2021;398:121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Normark J, Vikström L, Gwon YD, et al. Heterologous ChAdOx1 nCoV-19 and mRNA-1273 vaccination. N Engl J Med. 2021;385:1049–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barros-Martins J, Hammerschmidt SI, Cossmann A, et al. Immune responses against SARS-CoV-2 variants after heterologous and homologous ChAdOx1 nCoV-19/BNT162b2 vaccination. Nat Med. 2021;27:1525–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmidt T, Klemis V, Schub D, et al. Cellular immunity predominates over humoral immunity after homologous and heterologous mRNA and vector-based COVID-19 vaccine regimens in solid organ transplant recipients. Am J Transplant. 2021;21:3990–4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Medina-Pestana J, Cristelli MP, Viana LA, et al. Clinical impact, reactogenicity, and immunogenicity after the first CoronaVac dose in kidney transplant recipients. Transplantation. 2022;106:e95–e97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ministério da Saúde. Secretaria Extraordinária de Enfrentamento à COVID-19. Nota Técnica Nº 27/2021-SECOVID/GAB/SECOVID/MS. August 26, 2021. Available at https://www.gov.br/saude/pt-br/coronavirus/vacinas/plano-nacional-de-operacionalizacao-da-vacina-contra-a-covid-19/notas-tecnicas/nota-tecnica-no-27-2021-secovid-gab-secovid-ms.pdf. Accessed November 4, 2021.
- 19.Abbott. AdviseDx SARS-CoV-2 IgG II instructions for use H18575R01. 2021. Available at https://www.fda.gov/media/146371/download.
- 20.Hinton DM. Emergency Use Authorization Declaration. Washington, DC: US Food and Drug Administration; 2021. [Google Scholar]
- 21.GenScript USA Inc. cPass SARS-CoV-2 neutralization antibody detection kit. Version 4.0. 2020. Available at https://fda.report/media/143583/EUA-GenScript-cpass-ifu.pdf.
- 22.Munro APS, Janani L, Cornelius V, et al. ; COV-BOOST Study Group. Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): a blinded, multicentre, randomised, controlled, phase 2 trial. Lancet. 2021;398:2258–2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Costa Clemens SA, Weckx L, Clemens R, et al. ; RHH-001 Study Team. Heterologous versus homologous COVID-19 booster vaccination in previous recipients of two doses of CoronaVac COVID-19 vaccine in Brazil (RHH-001): a phase 4, non-inferiority, single blind, randomised study. Lancet. 2022;399:521–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Çağlayan D, Süner AF, Şiyve N, et al. An analysis of antibody response following the second dose of CoronaVac and humoral response after booster dose with BNT162b2 or CoronaVac among healthcare workers in Turkey. J Med Virol. 2022;94:2212–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng SMS, Mok CKP, Leung YWY, et al. Neutralizing antibodies against the SARS-CoV-2 Omicron variant BA.1 following homologous and heterologous CoronaVac or BNT162b2 vaccination. Nat Med. 2022;28:486–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Masset C, Ville S, Garandeau C, et al. Observations on improving COVID-19 vaccination responses in kidney transplant recipients: heterologous vaccination and immunosuppression modulation. Kidney Int. 2022;101:642–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reindl-Schwaighofer R, Heinzel A, Mayrdorfer M, et al. Comparison of SARS-CoV-2 antibody response 4 weeks after homologous vs heterologous third vaccine dose in kidney transplant recipients: a randomized clinical trial. JAMA Intern Med. 2022;182:165–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.World Health Organization. WHO Coronavirus (COVID-19) Dashboard. Available at https://covid19.who.int/. Accessed April 4, 2022.
- 29.Cristelli MP, Viana LA, Dantas MTC, et al. The full spectrum of COVID-19 development and recovery among kidney transplant recipients. Transplantation. 2021;105:1433–1444. [DOI] [PubMed] [Google Scholar]