Abstract
Oxidative stress, inflammation, and gut microbiota impairments have been implicated in the development and maintenance of diabetes mellitus. Strategies capable of recovering the community of commensal gut microbiota and controlling diabetes mellitus have increased in recent years. Some lactobacilli strains have an antioxidant and anti-inflammatory system capable of protecting against oxidative stress, inflammation, and diabetes mellitus. Experimental studies and some clinical trials have demonstrated that Limosilactobacillus fermentum strains can beneficially modulate the host antioxidant and anti-inflammatory system, resulting in the amelioration of glucose homeostasis in diabetic conditions. This review presents and discusses the currently available studies on the identification of Limosilactobacillus fermentum strains with anti-diabetic properties, their sources, range of dosage, and the intervention time in experiments with animals and clinical trials. This review strives to serve as a relevant and well-cataloged reference of Limosilactobacillus fermentum strains capable of inducing anti-diabetic effects and promoting health benefits.
Keywords: Diabetes Mellitus, Gut dysbiosis, Oxidative stress, Probiotics, Limosi-lactobacillus fermentum
Core Tip: This review strives to serve as a relevant and well-cataloged reference of L. fermentum strains with aptitudes of inducing anti-diabetic effects and health-promoting benefits to the host envisaging their wide applicability to diabetes control.
INTRODUCTION
Diabetes mellitus (DM) is a chronic non-communicable disease that affects millions of people and has become one of the leading causes of death worldwide[1,2]. The Diabetes Atlas published by the International Diabetes Federation estimated that 537 million adults worldwide had type DM in 2021[3]. Associated to this prevalence, clinical management of DM has elevated the costs of the health system, increasing by 316% in the last 15 years[3,4]. One of the etiological factors of this metabolic disorder includes long-term inappropriate diet such as regular consumption of sugary drinks, red meat, and low consumption of whole grains and fiber. In addition, smoking, physical inactivity, history of gestational diabetes or delivery of newborns > 4 kg weight, medications such as statins, thiazides, and beta-blockers, psychosocial stress, and depression have been described as risk factors for DM[5-7].
Clinical features and laboratory findings of DM include changes in body weight, increased blood glucose, insulin resistance, development of lipid metabolism disorder, polyuria, polydipsia, visual disturbances, ketoacidosis, and hyperosmolar non-ketoacidotic syndrome with risk of coma[8,9]. When uncontrolled, diabetes can induce grave complications, including death[10]. Insulin resistance in sensitive tissues such as liver, muscle, and adipose tissue and β-cell dysfunctions are the main factors involved in initiating and progressing the pathophysiology of type 2 DM[5,11]. Moreover, it has been reported that gut microbiota (GM) impairment plays a crucial role in developing DM[12].
DM patients show an altered intestinal microbiota resulting from an increase in opportunistic bacteria and Gram-negative toxin-producing bacteria that alter metabolism energetic[13]. Furthermore, the accumulation of gut-derived pro-inflammatory molecules, including lipopolysaccharide (LPS), peptidoglycans, and flagellin, appear to accelerate the inflammatory response in patients with DM[14]. Deregulation of the GM, also called dysbiosis, promotes intestinal permeability and energy homeostasis changes, causing metabolic endotoxemia, inflammation, hyperglycemia, and hyperlipidemia[15,16]. Dysbiosis impairs the integrity of the intestinal wall and allows the translocation of toxins from the intestinal lumen into the systemic circulation, promoting inflammation, autoimmunity, and oxidative stress that can lead to β-cell destruction or insulin resistance[17,18].
The findings involving the association between gut dysbiosis and DM reinforce the importance of gut-targeting approaches in the treatment of DM[19-21] Strategies capable of recovering the community of commensal GM and controlling DM have recently increased. Probiotic therapy has begun to be used to improve GM composition and management of DM[22,23]. Given this scenario, the identification of new potentially-probiotic strains with anti-diabetic properties is essential for the development of new probiotic products and testing in well-controlled trials.
Among Lactobacillus species, strains of Limosilactobacillus fermentum (L. fermentum) has been reported to exert probiotic properties due to its ability to improve GM composition, reduce blood cholesterol, modulate the intestinal immune system, stimulate the release of immunoglobulin A, reduce intestinal inflammation, and increase the activity of antioxidant enzymes[24-27]. Although early studies have identified anti-diabetic properties in some L. fermentum strains, an in-depth review focusing on L. fermentum strains as a potential anti-diabetic has not been found in the available literature to the time of this writing[28,29].
This present literature review focuses on the emerging findings of experimental and clinical studies that have used L. fermentum supplementation to prevent or treat complications of DM. To investigate the effectiveness of L. fermentum more thoroughly, we focus on the type of strain, source of probiotics, dosage, duration of treatment, and the primary outcomes reported.
PROBIOTIC THERAPY IN THE TREATMENT OF METABOLIC DISORDERS
Probiotics are live microorganisms that confer host health benefits when administrated adequately. Probiotics have significant importance in the industrial economy and are among the most consumed food supplements worldwide[30]. Experimental studies and clinical trials have documented that probiotics can modulate the GM, inducing beneficial effects and increasing overall wellness[28,31,32]. Over the last few years, studies on probiotics have been growing sharply due to their beneficial health effects, which have been used as adjuvant therapy for metabolic disorders[33]. A number of preclinical and clinical studies have investigated the effectiveness of probiotics by evaluating the intestinal microbiota after probiotics use, showing promising results in treating metabolic diseases[31].
Impairment in commensal homeostasis of GM and intestinal functional capacity, called gut dysbiosis, is associated with the development of metabolic diseases such as colitis, obesity, liver, obesity, and DM[34]. Thus, a probiotic may be able to relieve GM dysbiosis, through various mechanisms including improvement in the composition and diversity of the GM, induction of immunomodulation, protection against physiological stress, and pathogen suppression[35]. Probiotics also promote health benefits to the host through other mechanisms of action, such as the production of organic acids, including lactic acid and short-chain fatty acids (SCFA) (mainly acetate, propionate, and butyrate)[36,37]. Another mechanism reported is the capacity of probiotics to protect the integrity of the intestinal wall by stimulating mucin production and upregulating tight-junction claudin, occludin, and zonulin protein expression[37]. Furthermore, probiotics are also responsible for producing small molecules with systemic effects essential for maintaining vital functions, such as cortisol, serotonin, gamma-aminobutyric acid (GABA), tryptophan, histamine derivatives, satiety hormones, and conjugated linoleic acid[37].
Coupled with the mechanisms mentioned above, some experimental and clinical evidence has demonstrated that probiotics have anti-inflammatory and antioxidant properties[27,37,38]. This antioxidant capacity results from signaling pathways that produce antioxidant enzymes and molecules, reducing serum and tissues levels of oxidative stress[38,39]. Concerning their anti-inflammatory properties, probiotics have been reported to reduce inflammatory markers, including LPS, tumor necrosis factor alpha (TNF-α), interleukin (IL)-6, as well as to promote an increase of anti-inflammatory markers, such as IL- 10.
LIMOSILACTOBACILLUS FERMENTUM, LEAKY GUT AND DIABETES MELLITUS
Probiotics have shown satisfactory results as an adjunct treatment in DM[40,42]. Both single strain, combined with other foods, and multiple strain probiotics can be used as supplements. Among more effective probiotic strains, the therapeutic potential of L. fermentum has been investigated for adjuvant management of DM[40,48,55].
L. fermentum is a Gram-positive, rod- or coccoid-shaped, heterofermentative, and anaerobic or aerotolerant bacteria found in fermented cereals and other fermenting plant materials, dairy products, manure sewage and feces, and the human vagina[41]. The Lactobacillus genus is widely used as an intestinal modulator due to its safety and probiotic activity[40]. Among these bacterial groups, L. fermentum is a well-studied species, mainly due to its action in improving metabolic function and oxidative stress, which may be considered for DM management[26,27,42].
DM is one of the main metabolic diseases related to leaky gut, oxidative stress, and chronic inflammation. GM impairment has been described in the pathogenesis of DM and metabolic syndrome. Due to the high mortality rate of patients with of DM and this direct relationship with intestinal health, the number of studies involving probiotic therapy has increased in recent years. L. fermentum has been proven to alleviate metabolic disorder-related symptoms, including improvement in glucose and insulin levels, control of the lipid profile, to decrease in pro-inflammatory cytokines and to increase antioxidant capacity[27,41,43]. However, these protective responses need to be further investigated in clinical studies to elucidate the responsiveness of L. fermentum therapy in DM patients.
Most diabetes treatments, particularly drug therapies, use agents that act directly on signaling pathways to regulate glucose. Because of this, it is pertinent to explore therapies that adjunctively attenuate deregulation of GM, such as probiotics[44]. Among the main harmful effects in GM induced by DM, gram-negative bacteria in the colon increase the concentration of LPS in the lumen. LPS causes high production of free radicals, increasing intestinal permeability and generating a systemic chronic inflammatory process. This pro-inflammatory state is a critical mechanism in the genesis of chronic diseases, such as DM[38,40]. Additionally, GM imbalances observed in DM patients are characterized by changes in the composition of SCFAs, including increasing acetate levels and decreasing butyrate production. As a consequence, there may be acetate excess and reduction of butyrate, caused by dysbiosis, and impaired blood glucose homeostasis[45].
On the other hand, L. fermentum manipulation could attenuate GM imbalance, which may decrease DM complications. Considering the inversely proportional relationship between butyrate and acetate levels and the effects of excess acetate on the worsening of DM, keeping these fatty acids in balance becomes an important way to assist glycemic control[45]. Increased butyrate production by L. fermentum regulates acetate production, preventing increased hepatic gluconeogenesis and insulin resistance. Additionally, the increase in butyrate production resulting from L. fermentum supplementation may repair enterocyte tight junctions and improve intestinal permeability[46]. Experimental evidence has revealed that increasing levels of SCFA, especially acetate and succinate, decreases cellular damage of enterocytes, leading to a reduction in inflammation state, oxidative stress, and leaky gut in DM-induced rodents[28].
Another antidiabetic property of L. fermentum is to maintain normal levels of the intestinal hormone GLP-1[47]. GLP-1 has been shown to stimulate proliferation and prevent apoptosis of pancreatic beta cells, upregulating insulin synthesis and promoting a reasonable glycemic control[29,36]. In the liver, GLP1 decreases gluconeogenesis and stimulates glycolysis, contributing to reducing glycemic levels in individuals with DM. The main consequence of reducing these peptides is the exacerbation of hunger, the search for palatable food, and the preference for hypercaloric foods, which can be a predisposing factor for developing obesity and insulin resistance[30]. Leaky gut also generates chronic low-grade inflammation in organs such as the liver, skeletal muscle, and adipose tissue, causing metabolic changes such as hyperglycemia and dyslipidemias. L. fermentum also promotes benefits in these organs because it stimulates the synthesis of the fasting-induced adipose factor, a protein that regulates the function of the LPL enzyme and prevents hepatic steatosis and dyslipidemia, common in diabetic subjects[48]. Therefore, it is suggested that L. fermentum may improve intestinal permeability, normalize GLP-1 Levels, and reduce DM complications.
Another important action of L. fermentum is to reduce oxidative stress and glycation. Studies indicate that pathophysiological findings of DM, including macular degeneration, vascular endothelial injury, hepatic fibrosis, renal failure, are related to the glycation process. This process occurs when circulating glucose binds to proteins, inactivating them and increasing inflammatory cytokines such as interferon-gamma (IFN-γ), IL-6, and IL-4. The main biochemical marker for glycation is glycated hemoglobin (HB1ac), but this process can occur with any protein, including antioxidant enzymes. When glycation events occur more expressively, oxidative stress is even higher due to the increase in reactive oxygen species (ROS) and inactivation of the enzymatic antioxidant systems, such as superoxide dismutase (SOD) and glutathione peroxidase[40,49].
Conversely, the administration of L. fermentum decreased the glycation events and oxidative stress through the increasing production of ferulic acid (FA). This potent antioxidant metabolite can significantly reduce ROS formation and prevent glycation events. This mechanism is related to decreasing inflammatory markers, Hb1ac, and serum glucose. High levels of FA are also related to lower cardiometabolic risk in diabetic individuals[40,44].
To evaluate the effectiveness of L. fermentum, the following sections refer to the findings on the antidiabetic properties of different strains of L. fermentum, investigated in preclinical and clinical studies.
ANTI-DIABETIC PROPERTIES OF DIFFERENT STRAINS OF LIMOSILACTOBACILLUS FERMENTUM
We investigated studies that analyzed the role of L. fermentum administered singly or combined with other therapies to alleviate DM complications. Among ten of the studies included, nine evaluated anti-diabetic properties in experimental studies using rats or mice. Only one clinical study assessed the anti-diabetic potential of probiotic intervention in women with gestational DM. Since the majority of beneficial effects following administration of L. fermentum come from animal studies, this present review investigated emerging findings of their potential role in DM management. The characteristics of the studies and the primary outcomes are summarized in Table 1 and Table 2, respectively.
Table 1.
Characteristics of the studies testing the anti-diabetic effect of Limosilactobacillus fermentum strains
|
Ref.
|
Type of study
|
Experimental groups
|
Source of probiotics
|
Dosage of probiotic
|
Duration of treatment
|
| Hartajanie et al[50], 2020 | Experimental: 24 male Sprague-Dawley rats at 8 wk and weighing 170-200 g | Diabetic group; Diabetic group + acarbose; Diabetic group + bitter melon; Diabetic group + fermented bitter melon | L. fermentum LLB3 was isolated from the bamboo shoot pickle | 1 × 107 CFU L. fermentum LLB3 | 4 wk |
| Hu et al[35], 2019 | Experimental: 4-wk-old male Kunming mice (18 ± 2 g) were used | Normal control group; Diabetic group; Positive drug control group; Diabetic group + fructose 1 6-bisphosphatase (low dose); Diabetic group + 1-Deoxynojirimycin (middle dose); Diabetic group + 1-Deoxynojirimycin (high dose) | All probiotics were purchased from the Guangdong culture collection center | 5 × 104 CFU/mL of each activated strain (L. plantarum + L. fermentum, L. plantarum + L. mesenteroides, L. plantarum + S. cerevisiae, L. fermentum + L. mesenteroides, L. fermentum + S. cerevisiae, and L. mesenteroides + S. cerevisiae) | 4 wk |
| Chaiyasut et al[51], 2018 | Experimental: male Wistar rats | Control group; Control group + L. fermentum; Control group + fermented H. erinaceus juice; Diabetic group; Diabetic group pretreatment and posttreatment treated with fermented H. erinaceus juice, L. fermentum, and insulin | L. fermentum HP3 was isolated from fermented Thai foods | L. fermentum HP3 in a concentration of 109 CFU/mL. L. fermentum HP3 was used with H. Erinaceus Juice | 12 wk |
| Guilbaud et al[52], 2020 | Experimental: 30 mice with 6 wk of age | Wild-type group;Wild-type group + L. fermentum;Diabetic group;Diabetic group + L. fermentum | Isolated from a fecal sample of one-year-old healthyEstonian child | L. fermentum ME-3 in a concentration of 1010 CFU per 400 μL H2O | 12 wk |
| Archer et al[48], 2021 | Experimental: 40 female Wistar rats | Control group; Diabetic group + high-fat diet; Diabetic group + high-fat diet + L. fermentum. MCC2759; Diabetic group + high-fat diet + L. fermentum. MCC2760 | Isolated from fecal (L. fermentum. MCC2759) and from curd (L. fermentum. MCC2760) | Both isolated probiotics were offered in a concentration of 1 × 109 CFU/mL | 4 wk |
| Ai et al[31], 2021 | Experimental: 160 Male C57BL/6J mice with 6 wk of age | Control group; Diabetic group + high-fat diet; Diabetic group + defatted rice bran unfermented extracts; Diabetic group + pioglitazone; Diabetic group + high-dose of defatted rice bran fermentation extracts; Diabetic group + low-dose of defatted rice bran fermentation extracts | Isolated from Chinese rice noodle wastewater | The study evaluated the role of L. fermentum MF423. Dose of 100 μg/mL of defatted rice bran unfermented extracts | 8 wk |
| Yadav et al[54], 2018 | Experimental: 70 male Wistar rats with 8 ws old | Normal control group; Diabetic control group; Diabetic + normal diet supplemented with milk; Diabetic + L. rhamnosus MTCC5957; Diabetic + L. rhamnosus MTCC5897; Diabetic + L. fermentum MTCC 5898; Diabetic + L.rhamnosus 5957 and 5958 and L. fermentum MTCC 5898 | The probiotics L. rhamnosus MTCC: 5957 and L. rhamnosus MTCC: 5897 were isolated from household curds. The probiotic L. fermentum MTCC: 5898 was isolated from the feces of breastfed human infants | All probiotic strains were offered in a dosage of 1 × 109 CFU | 6 wk |
| Yousaf et al[55], 2016 | Experimental: female mice of 6-8 wk, with an initial body weight of 21-23 g | Normal healthy mice; Diabetic mice; Diabetic mice + Momordica charanti; Diabetic mice + Eugenia Jambolana; Diabetic mice + L. Fermentum; Diabetic mice + L. Fermentum + Momordicacharanti + Eugenia Jambolana; Diabetic mice + Glucophage | L. fermentum fruit extracts of Eugenia Jambolana and Momordica charantia were isolated from local yogurt samples (Lahore, Pakistan) | Momordica charantia 200 mg/kg, and Eugenia Jambolana 100 mg/kg. The authors did not inform the concentration of L. fermentum (Gene Bank Accession KJ754019) | 3 wk |
| Balakumar et at[49], 2018 | Experimental: adult male C57BL/6J mice (age 8-10 wk) | Normal pellet diet; High-fat diet; High-fat diet + L. rhamnosus; High-fat diet + L. plantarum MTCC5690; High-fat diet + L. fermentum MTCC5689; High-fat diet + metformim; High-fat diet + vildagliptin | Isolated from Indian gut (Karnal, India) | Lactobacillus MTCC 5690 and MTCC 5689 in a concentration of 1.5 × 109 colonies/mouse/d | 24 wk |
| Babadi et al[30], 2018 | Clinical: primigravid women aged between 18 and 40 years, between the 24th and 28th week of gestation, diagnosed with gestational diabetes mellitus | Placebo group; Probiotic group | Probiotic supplements were produced by LactoCare®, Zisttakhmir Company (Tehran, Iran) | Probiotic capsule containing Lactobacillus acidophilus, Lactobacillus casei, Bifidobacterium bifidum and L. fermentum in a dosage of 2 × 109 CFU/g | 6 wk |
L. fermentum: Lactobacillus fermentum; L. rhamnosus: Lactobacillus rhamnosus; L. plantarum: Lactobacillus plantarum; S. cerevisiae: Saccharomyce cerevisae; L. mesenteroides: Leuconostoc mesenteroides; H. erinaceus: Hericium erinaceus.
Table 2.
Primary outcomes of the studies testing the anti-diabetic effect of Limosilactobacillus fermentum strains
|
Ref.
|
Primary end-points
|
| Hartajanie et al[50], 2020 | ↓ The fasting blood glucose; ↓ Postprandial blood glucose; ↑ In SOD concentrations |
| Hu et al[35], 2019 | ↓ Blood glucose levels; ↓ Insulin levels; Reversed insulin resistance; Improved serum lipid levels; Relieved gut dysbiosis |
| Chaiyasut et al[51], 2018 | ↓ Weight Gain; Improved insulin levels (↑ insulin); Recovery progress of hyperglycemia; ↓ HbA1c level (only with cointerventions); ↓ Inflammatory cytokines level |
| Guilbaud et al[52], 2020 | ↓ Weight Gain; ↓ Glycemic response 60-120 min; ↑ In HbA1c; ↓Weight of liver; ↓ FL-furosine levels in kidney ↓The expression of TNF-α; ↓The TG concentrations in liver; ↓ HDL and Non-HDL; Lower lipid droplets in liver. |
| Archer et al[48], 2021 | ↓ Blood glucose levels; Improved insulin levels (↑ insulin); ↓ levels of cholesterol, triglycerides, and LDL-C; ↓ The expression levels of TNF-α, and ↑ expression of IL-10; ↓ Expression of the TLR4 receptor, ↑ Expression of tight junction protein ZO-1, endocannabinoid receptor CB2 and GLP1, and ↑ Expression of GLUT4 in MAT and muscle tissue; Showed accumulation of neutrophils around the portal tracts in liver tissue, and reduction in the glomerular injury in kidney sections |
| Ai et al[31], 2021 | Inhibit the degree of weight loss; ↓ The fasting blood glucose; ↓ Blood glucose levels; ↓ Levels of total cholesterol and LDL and ↑ HDL levels; Ameliorate the damage to liver cells and significantly reduced the accumulation of lipid droplets; Upregulated the levels of SOD, T-AOC and GSH-PX, and reversed elevation of MDA; ↓ Damage in composition of gut microbiota1 |
| Yadav et al[54], 2018 | Inhibit the degree of weight loss; ↓ The fasting blood glucose; ↓ Consumption of food and liquids; ↑ In oral glucose tolerance; ↑ In liver weight; Improved insulin levels (↑ Insulin); ↓ HbA1c level; ↑ CAT, SOD activity in kidney and liver; ↓ Serum levels of total cholesterol, LDL-C, VLDL-C and triglycerides; ↓ The serum inflammatory index, cytokine levels (IL-6 and TNF-α); ↓ In the expression of the genes G6Pase and pepck in the liver |
| Yousaf et al[55], 2016 | ↑ Body weight; ↓ Blood glucose levels; Lipid profile: no effect on cholesterol, ↓ tryglyceride, LDL, slight increase in the level of HDL |
| Balakumar et al[49], 2018 | ↓ Body weight; ↓ Blood glucose levels; ↑ In oral glucose tolerance; ↓ HbA1c level; Improved insulin levels (↓ Insulin); ↑ levels of GLP-1; ↓ Cholesterol, triglyceride and LDL levels; ↑ HDL level; ↓ Plasma DX-4000–FITC; ↑ mRNA expression of epithelial tight junction occludin and ZO-1; ↓ Serum levels of LPS; ↓ Proinflammatory gene expression profiles (IL6 and TNFα), ↑ adiponectin gene expression; ↓ Gene expression profiles of endoplasmic reticulum stress |
| Babadi et al[30], 2018 | Downregulated gene expression of TNF-α; ↓ The fasting blood glucose; ↓ Serum insulin level; ↓ Insulin resistance; ↑ Insulin sensitivity; ↓ Levels of triglycerides, VLDL-cholesterol and total / HDL-cholesterol ratio, and ↑ levels of HDL-cholesterol; ↓ In plasma MDA; ↑ In plasma NO and total antioxidant capacity |
These results were obtained by rice bran fermented with Lactobacillus fermentum MF423.
SOD: Superoxide dismutase; HbA1c: Glycayed hemoglobin A; TNF-α: Tumor necrosis factor-alpha; TG: Triglyceride; HDL- C: High-density lipoprotein cholesterol; LDL-C: Low-density lipoprotein cholesterol; VLDL-C: Very-low-density lipoprotein cholesterol; IL-6: Interleukin-6; IL-10: Interleukin-10; TLR4: Toll-like receptor 4; ZO-1: Zonula occludens-1; CB2: Cannabinoid receptor type 2; GLP1: Glucagon-like peptide-1; GLUT4: Glucose transporter type 4; MAT: Mesenteric adipose tissue; T-AOC: Total antioxidant capacity; GSH-PX: Glutathione peroxidase; MDA: Malondialdehyde; CAT: Catalase; G6Pase: Glucose 6-phosphatase; Pepck: Phosphoenolpyruvate carboxykinase; FITC: Fluorescein isothiocyanate-dextran; LPS: Lipopolysaccharide; NO: Nitric oxide.
L. fermentum LLB3
An experimental study revealed that treatment with L. fermentum LLB3 isolated from the bamboo shoot pickle and offered in fermented bitter melon (Momordica charantia), in a concentration of 1 × 107 CFU during 4 wk, reduced fasting glucose and postprandial blood glucose levels and increased SOD enzyme activity in rats subjected to type 2 DM induced by streptozotocin (STZ)[50]. This suggests that L. fermentum LLB3 might be considered an adjuvant therapy to attenuate type 2 DM-related symptoms[50].
L. fermentum HP3
Administration of a fermented Hericium erinaceus juice containing 109 CFU/mL of L. fermentum HP3 for 12 wk reduced weight gain, increased insulin level, and reduced hyperglycemia in diabetic mice induced by STZ[51]. In addition, treated mice showed lower levels of inflammatory cytokines, including IL-6, IL-17, and IFN-γ[51], suggesting that fermented Hericium erinaceus juice can be used as nutritional manipulation in the treatment of type 2 DM.
L. fermentum ME-3
A previous preclinical study investigated the anti-diabetic effect of L. fermentum ME-3 in genetically diabetic mice[52]. L. fermentum ME-3 was administered in mice at 6 wk of age, in a concentration of 1010 CFU, for 12 wk[52]. The treatment with L. fermentum ME-3 reduced body weight, inhibited expression of TNF-α, but did not improve glycemic control[52]. In addition, supplementation with L. fermentum ME-3 reduced the formation of glycation products, including FL-furosine levels in the kidney. However, the researchers found an increase in HbA1c, another marker of early glycation. The authors noted that while HbA1c reflects early glycation mainly in red blood cells, FL-furosine provides information on the extent of early glycation in fluids, tissues, and organs and offers a broader view of the early glycation status of the whole organism[52]. In summary, L. fermentum ME-3 has the therapeutic potential to reduce the formation of some glycation products in kidneys and attenuate some typical type 2 DM-related symptoms.
L. fermentum MCC2759 and MCC2760
Recently, an Indian research group analyzed the activity of the probiotic L. fermentum MCC2759 and MCC2760 on intestinal markers of inflammation using a high-fat diet model associated with the STZ-induced diabetic model[48]. Both L. fermentum strains were administered in a concentration of 1 × 109 CFU/mL for 4 wk. The main findings of the study revealed that diabetic female rats treated with L. fermentum MCC2759 and MCC2760 reduced blood glucose levels, increased insulin levels, and improved the lipid profile[48]. Coupled with biochemical changes, L. fermentum administration downregulated TNF-α mRNA and up-regulated mRNA IL-10 in the intestine, liver, mesenteric adipose tissue, and muscle, suggesting that the anti-diabetic effect promoted by L. fermentum MCC2759 and MCC2760 can be associated with a decrease in inflammatory markers[48]. In addition, L. fermentum MCC2759 and MCC2760 administration modulated other gene expressions, such as reduced expression of Toll-like receptor 4, enhanced expression of tight junction protein ZO-1, endocannabinoid receptor CB2 and GLP1, glucose transporter type 4 in mesenteric adipose tissue and muscle tissue. The results demonstrated that L. fermentum MCC2759 and MCC2760 might be a potential probiotic in treating type 2 DM.
L. fermentum MF423
L. fermentum MF423 is a strain isolated from Chinese rice noodle wastewater[31]. The authors analyzed adverse effects triggered by an experimental model of type 2 DM induced by STZ and tested the effectiveness of different therapies, including supplementation with unfermented extracts of defatted rice bran, high and low doses of defatted rice bran fermented by L. fermentum MF423, and drug intervention (pioglitazone)[31]. Mice receiving a high dose (1 g/kg) of defatted rice bran fermentation extracts containing L. fermentum MF423 for 8 wk evidenced weight loss and reduced fasting blood glucose, lipid accumulation, and liver cells damage[31]. Moreover, probiotic groups intensified antioxidant activity in diabetic mice through up-regulation levels of SOD, total antioxidant capacity (T-AOC), and reversed elevation of malondialdehyde (MDA) in the liver[31]. It is important to mention that no effects were found in animals treated only by unfermented extracts of rice bran, highlighting the antioxidant activity of L. fermentum MF423.
To complete the evaluation of the therapeutic potential of L. fermentum MF423, the authors investigated the role of this probiotic in the modulation of GM. Diabetic rats treated either to high dose of defatted rice fermented by L. fermentum or pioglizatone showed GM composition similar to the control group, compared to untreated diabetic animals[31]. The relative abundances of Bacteroidetes (20%) and Firmicutes (40%) were increased in both mentioned groups compared to a diabetic group without treatment[31]. A decreased abundance of Firmicutes can be found in diabetic patients compared to their non-diabetic counterparts[53]. These two major phyla may play an essential role in hyperglycemia, hyperlipidemia, and inflammation. Moreover, probiotic treatment increased the relative abundance of SCFA-producing bacteria in diabetic mice, including Lactobacillus, Parabacteroides, norank_f_Ruminococcaceae, Ruminococcus_torques_group, and Alloprevotella. Interestingly, a decrease in the genus Lactobacillus was significant in diabetic mice, while treatment with defatted rice bran fermented by L. fermentum MF423 increased its abundance, similar to control mice[31]. L. fermentum is known for its probiotic role in food consumption, which could modify abnormalities in intestinal microbes and retard hyperglycemia. In conclusion, defatted rice bran fermentation by L. fermentum MF423 Lessened damage to the structure and function of GM induced by type 2 DM.
L. fermentum MTCC: 5898
Probiotic fermented milk prepared using different probiotic strains, including L. rhamnosus MTCC: 5957, L. rhamnosus MTCC: 5897, and L. fermentum MTCC: 5898, were evaluated in an experimental study[54]. Probiotic strains were offered independently or in combination for treating STZ induced type 1 DM in male Wistar rats. All probiotic strains were provided in a dosage of 1 × 109 CFU for 6 wk. The study demonstrated that the diabetic rats who received fermented milk containing L. fermentum MTCC: 5898 had less weight loss, improved glucose metabolism by reducing fasting blood glucose, HbA1c associated with increased insulin level, reduced diabetic dyslipidemia, and attenuated inflammation status through reduction of IL-6 and TNF-α[54]. In addition, supplementation with L. fermentum MTCC: 5898 showed antioxidant properties by increasing catalase (CAT) and SOD activities in the kidney and liver[54]. Moreover, administration of probiotics reduced mRNA expression of phosphoenolpyruvate carboxykinase and Glucose 6-phosphatase genes that code the key enzymes of the gluconeogenesis pathway[54]. Compared to other lactobacilli strains, rats receiving L. fermentum MTCC: 5898 displayed the most effective responses including oral glucose tolerance, serum insulin, serum, liver CAT, serum triglycerides, VLDL[54]. Therefore, it is suggested that daily consumption of probiotic fermented milk, especially L. fermentum MTCC: 5898, may be effective in attenuating complications of type 1 DM.
L. fermentum MTCC 5690 and MTCC 5689
L. fermentum MTCC 5690 and MTCC 5689 were isolated from the Indian gut and used to treat high-fat diet-induced type 2 DM mice[49]. The present study compared the anti-diabetic effect of L. fermentum MTCC 5690 and MTCC 5689 to other probiotics (Lactobacillus rhamnosus, Lactobacillus plantarum MTCC5690) and drug intervention (metformin, vildagliptin). L. fermentum MTCC 5690 and MTCC 5689 were administered in a concentration of 1.5 × 109 colonies/mouse/day for 24 wk[49]. Both probiotics and drugs groups reduced body weight, improved oral glucose tolerance, and reduced fasting glucose and Hb1Ac levels in diabetic mice[49]. Concerning insulin levels, probiotic groups contributed to normalizing levels of this hormone, which approximated the levels observed in the control group[49]. In addition, a significant reduction of insulin levels was found in the vildagliptin group compared with other groups, which may be considered a possible adverse mild effect of this drug. Furthermore, all treatment groups improved lipid profile by reduction of levels of cholesterol, triglycerides, LDL, associated with an increase in HDL levels.
Additionally, the study evaluated the gut integrity after 24 wk of treatment. Probiotic treatment and drug therapy were able to reduce damage in gut integrity, which may contribute to normalizing gut permeability[49]. The authors quantified mRNA expression of epithelial tight junction occludin and ZO-1 and LPS levels. All the probiotic and anti-diabetic drugs increased gene expression of the intestinal tight junction occludin and ZO-1[49]. To evaluate endotoxemia state and intestinal barrier integrity, probiotic treatments decreased LPS levels and pro-inflammatory cytokines IL-6 and TNF-α in mice subjected to type 2 DM[49]. In addition, the authors verified the effectiveness of treatments in attenuating endoplasmic reticulum stress of skeletal muscle of diabetic mice. Results showed that both probiotic and drug therapies reduced endoplasmic reticulum stress markers[49]. The findings suggested that L. fermentum MTCC 5690 and MTCC 5689 act like anti-diabetic drugs, highlighting the therapeutic potential of these probiotic strains in alleviating type 2 DM complications.
Non mentioned strain of L. fermentum
The role of L. fermentum fruit extracts of Syzygium cumini and Momordica charantia, isolated from yogurt samples (Pakistan) on STZ induced DM mice, was previously investigated[55]. L. fermentum and the extracts were administered individually as well as in combination with DM-induced mice for 3 wk. Results were compared with mice that received drug intervention (Glucophage). Administration of probiotics and natural extracts improved body weight, and reduced blood glucose levels and both results were similar with the Glucophage group[55]. Concerning lipid profile, L. fermentum and natural extracts improved almost all lipid profile parameters, including reduced triglycerides, LDL, and increased HDL serum levels[55]. The study demonstrated that Glucophage treatment might affect some parameters, such as increased total cholesterol, triglycerides, and LDL concentration. These findings showed that L. fermentum and natural extracts have hypoglycemic and hypolipidemic activity, which may reduce DM complications.
Another experimental study demonstrated that mixed probiotics containing L. fermentum could reverse insulin resistance, reduce blood glucose levels, and improve lipid profile in STZ induced DM in old male Kunming mice after 4 wk of treatment[35]. In addition, the authors showed a significant impact of the supplementation of L. fermentum on the relief of gut dysbiosis, lowering the damage in the composition of GM[35]. However, the authors did not specify which strain of L. fermentum was administered, limiting the understanding of these effects.
Regarding clinical data, we found only one randomized, double-blind, placebo-controlled trial that evaluated the effectiveness of L. fermentum in attenuating complications of DM[30]. This study was carried out to assess the effects of probiotic supplementation on genetic and metabolic profiles in patients with gestational DM, aged 18-40 years (at weeks 24-28 of gestation)[30]. Participants were randomly divided into two groups: a control group and a probiotic group, made up of women who received a probiotic capsule containing Lactobacillus acidophilus, Lactobacillus casei, Bifidobacterium bifidum, L. fermentum (2 × 109 CFU/g each) for 6 wk[30]. However, the authors did not inform the specific strain of L. fermentum used, limiting the interpretation of results. The probiotic group showed lower levels of fasting blood glucose serum insulin, reduced insulin resistance, and significantly increased insulin sensitivity compared with the control group[30]. In addition, probiotic supplementation decreased triglycerides, VLDL, and increased HDL levels compared with the control group. Additionally, probiotic administration reduced plasma MDA, and an elevation in plasma nitric oxide and T-AOC was found compared with the control group. Therefore, the probiotic treatment showed great therapeutic potential in alleviating complications found in women with gestational DM. Future clinical studies are needed to investigate further the specific strains of L. fermentum to elucidate which strains are more effective in attenuated DM.
CONCLUSION
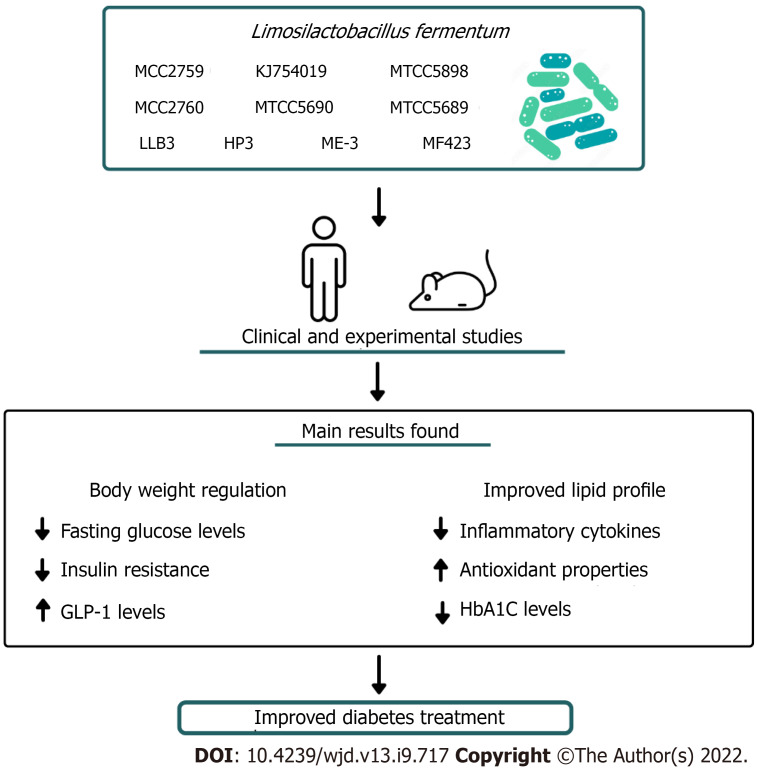
This literature review showed that L. fermentum is a promising strain for the management of DM (Figure 1). Evidence from experimental and clinical study verified that L. fermentum supplementation contributed to normalizing body weight, reduced blood glucose and fasting blood glucose levels, reduced insulin resistance, and improved lipid profile. Coupled with these biochemical changes, L. fermentum therapy showed anti-oxidant and anti-inflammatory properties, which contributed to alleviating related symptoms of DM. However, the heterogeneities of studies, including variations in dosage, and duration of treatment, limit the elucidation of the most effective way to use L. fermentum as adjuvant therapy of DM. Moreover, it is relevant to explore the effectiveness of co-intervention with L. fermentum associated with bioactive compounds with antioxidant and anti-inflammatory properties, such as quercetin and resveratrol [15]. We also highlight that most of the available data came from preclinical studies, hence, therapeutic potential of different strains of L. fermentum in minimizing complications of DM needs to be further investigated in randomized, double-blind, placebo-controlled trials to confirm these findings in human studies.
Figure 1.
Schematic drawing showing that Limosilactobacillus fermentum exert an anti-diabetic effect.
ACKNOWLEDGEMENTS
The authors are grateful to the Fundação de Apoio à Pesquisa do Estado da Paraíba (FAPESQ, Brazil) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for the scholarships awarded to Lacerda DC, Trindade da Costa PC, Pontes PB, Carneiro dos Santos LA, Cruz Neto JPR, Silva Luis CC. Additionally, the authors give thanks for the research productivity fellowship granted by the Brazilian National Council for Scientifc and Technological Development (CNPq) to de Brito Alves, JL.
Footnotes
Conflict-of-interest statement: The authors declare no competing interests.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: March 2, 2022
First decision: April 17, 2022
Article in press: August 14, 2022
Specialty type: Microbiology
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bala C, Romania; Shi J S-Editor: Chang KL L-Editor: A P-Editor: Chang KL
Contributor Information
Diego Cabral Lacerda, Department of Nutrition, Health Sciences Center, Federal University of Paraíba, João Pessoa, 58051-900, Paraíba, Brazil.
Paulo César Trindade da Costa, Department of Nutrition, Health Sciences Center, Federal University of Paraíba, João Pessoa, 58051-900, Paraíba, Brazil.
Paula Brielle Pontes, Department of Neuropsychiatry, Health Sciences Center, Federal University of Pernambuco, Recife, 50670-901, Pernambuco, Brazil.
Lucas Alves Carneiro dos Santos, Department of Nutrition, Health Sciences Center, Federal University of Paraíba, João Pessoa, 58051-900, Paraíba, Brazil.
José Patrocínio Ribeiro Cruz Neto, Department of Nutrition, Health Sciences Center, Federal University of Paraíba, João Pessoa, 58051-900, Paraíba, Brazil.
Cristiane Cosmo Silva Luis, Department of Nutrition, Health Sciences Center, Federal University of Paraíba, João Pessoa, 58051-900, Paraíba, Brazil.
Vanessa Polyana de Sousa Brito, Department of Nutrition, Health Sciences Center, Federal University of Paraíba, João Pessoa, 58051-900, Paraíba, Brazil.
José Luiz de Brito Alves, Department of Nutrition, Federal University of Paraíba, João Pessoa, 58045-190, Paraíba, Brazil. jose_luiz_61@hotmail.com.
References
- 1.Khan MAB, Hashim MJ, King JK, Govender RD, Mustafa H, Al Kaabi J. Epidemiology of Type 2 Diabetes - Global Burden of Disease and Forecasted Trends. J Epidemiol Glob Health . 2020;10:107–111. doi: 10.2991/jegh.k.191028.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tinajero MG, Malik VS. An Update on the Epidemiology of Type 2 Diabetes: A Global Perspective. Endocrinol Metab Clin North Am . 2021;50:337–355. doi: 10.1016/j.ecl.2021.05.013. [DOI] [PubMed] [Google Scholar]
- 3.Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, Stein C, Basit A, Chan JCN, Mbanya JC, Pavkov ME, Ramachandaran A, Wild SH, James S, Herman WH, Zhang P, Bommer C, Kuo S, Boyko EJ, Magliano DJ. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119. doi: 10.1016/j.diabres.2021.109119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jin Q, Ma RCW. Metabolomics in Diabetes and Diabetic Complications: Insights from Epidemiological Studies. Cells . 2021;10 doi: 10.3390/cells10112832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol . 2018;14:88–98. doi: 10.1038/nrendo.2017.151. [DOI] [PubMed] [Google Scholar]
- 6.Taylor R. Type 2 diabetes: etiology and reversibility. Diabetes Care . 2013;36:1047–1055. doi: 10.2337/dc12-1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee E. Diabetes: Screening, Diagnosis, and Prevention of Type 2 Diabetes. FP Essent . 2021;504:16–21. [PubMed] [Google Scholar]
- 8.Roden M. [Diabetes mellitus: Definition, classification and diagnosis] Wien Klin Wochenschr . 2012;124 Suppl 2:1–3. doi: 10.1007/s00508-012-0269-z. [DOI] [PubMed] [Google Scholar]
- 9.Cloete L. Diabetes mellitus: an overview of the types, symptoms, complications and management. Nurs Stand . 2022;37:61–66. doi: 10.7748/ns.2021.e11709. [DOI] [PubMed] [Google Scholar]
- 10.Glovaci D, Fan W, Wong ND. Epidemiology of Diabetes Mellitus and Cardiovascular Disease. Curr Cardiol Rep . 2019;21:21. doi: 10.1007/s11886-019-1107-y. [DOI] [PubMed] [Google Scholar]
- 11.Ceriello A, Prattichizzo F. Variability of risk factors and diabetes complications. Cardiovasc Diabetol. 2021;20:101. doi: 10.1186/s12933-021-01289-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Festi D, Schiumerini R, Eusebi LH, Marasco G, Taddia M, Colecchia A. Gut microbiota and metabolic syndrome. World J Gastroenterol . 2014;20:16079–16094. doi: 10.3748/wjg.v20.i43.16079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chakaroun RM, Massier L, Kovacs P. Gut Microbiome, Intestinal Permeability, and Tissue Bacteria in Metabolic Disease: Perpetrators or Bystanders? Nutrients . 2020;12 doi: 10.3390/nu12041082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woldeamlak B, Yirdaw K, Biadgo B. Role of Gut Microbiota in Type 2 Diabetes Mellitus and Its Complications: Novel Insights and Potential Intervention Strategies. Korean J Gastroenterol. 2019;74:314–320. doi: 10.4166/kjg.2019.74.6.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sampaio KB, do Nascimento YM, Tavares JF, Cavalcanti MT, de Brito Alves JL, Garcia EF, de Souza EL. Development and in vitro evaluation of novel nutraceutical formulations composed of Limosilactobacillus fermentum, quercetin and/or resveratrol. Food Chem . 2021;342:128264. doi: 10.1016/j.foodchem.2020.128264. [DOI] [PubMed] [Google Scholar]
- 16.Slingerland AE, Schwabkey Z, Wiesnoski DH, Jenq RR. Clinical Evidence for the Microbiome in Inflammatory Diseases. Front Immunol . 2017;8:400. doi: 10.3389/fimmu.2017.00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arora T, Bäckhed F. The gut microbiota and metabolic disease: current understanding and future perspectives. J Intern Med . 2016;280:339–349. doi: 10.1111/joim.12508. [DOI] [PubMed] [Google Scholar]
- 18.Sohail MU, Althani A, Anwar H, Rizzi R, Marei HE. Role of the Gastrointestinal Tract Microbiome in the Pathophysiology of Diabetes Mellitus. J Diabetes Res . 2017;2017:9631435. doi: 10.1155/2017/9631435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cabello-Olmo M, Araña M, Urtasun R, Encio IJ, Barajas M. Role of Postbiotics in Diabetes Mellitus: Current Knowledge and Future Perspectives. Foods . 2021;10 doi: 10.3390/foods10071590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bastos RMC, Rangel ÉB. Gut microbiota-derived metabolites are novel targets for improving insulin resistance. World J Diabetes . 2022;13:65–69. doi: 10.4239/wjd.v13.i1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xia F, Wen LP, Ge BC, Li YX, Li FP, Zhou BJ. Gut microbiota as a target for prevention and treatment of type 2 diabetes: Mechanisms and dietary natural products. World J Diabetes . 2021;12:1146–1163. doi: 10.4239/wjd.v12.i8.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xi Y, Xu PF. Diabetes and gut microbiota. World J Diabetes . 2021;12:1693–1703. doi: 10.4239/wjd.v12.i10.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patterson E, Ryan PM, Cryan JF, Dinan TG, Ross RP, Fitzgerald GF, Stanton C. Gut microbiota, obesity and diabetes. Postgrad Med J. 92:286–300. doi: 10.1136/postgradmedj-2015-133285. [DOI] [PubMed] [Google Scholar]
- 24.Liang T, Wu L, Xi Y, Li Y, Xie X, Fan C, Yang L, Yang S, Chen X, Zhang J, Wu Q. Probiotics supplementation improves hyperglycemia, hypercholesterolemia, and hypertension in type 2 diabetes mellitus: An update of meta-analysis. Crit Rev Food Sci Nutr . 2021;61:1670–1688. doi: 10.1080/10408398.2020.1764488. [DOI] [PubMed] [Google Scholar]
- 25.Rittiphairoj T, Pongpirul K, Janchot K, Mueller NT, Li T. Probiotics Contribute to Glycemic Control in Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Adv Nutr . 2021;12:722–734. doi: 10.1093/advances/nmaa133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jang YJ, Kim WK, Han DH, Lee K, Ko G. Lactobacillus fermentum species ameliorate dextran sulfate sodium-induced colitis by regulating the immune response and altering gut microbiota. Gut Microbes . 2019;10:696–711. doi: 10.1080/19490976.2019.1589281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yadav R, Khan SH, Mada SB, Meena S, Kapila R, Kapila S. Consumption of Probiotic Lactobacillus fermentum MTCC: 5898-Fermented Milk Attenuates Dyslipidemia, Oxidative Stress, and Inflammation in Male Rats Fed on Cholesterol-Enriched Diet. Probiotics Antimicrob Proteins. 2019;11:509–518. doi: 10.1007/s12602-018-9429-4. [DOI] [PubMed] [Google Scholar]
- 28.de Luna Freire MO, do Nascimento LCP, de Oliveira KÁR, de Oliveira AM, Dos Santos Lima M, Napoleão TH, da Costa Silva JH, Lagranha CJ, de Souza EL, de Brito Alves JL. Limosilactobacillus fermentum Strains with Claimed Probiotic Properties Exert Anti-oxidant and Anti-inflammatory Properties and Prevent Cardiometabolic Disorder in Female Rats Fed a High-Fat Diet. Probiotics Antimicrob Proteins . 2021 doi: 10.1007/s12602-021-09878-1. [DOI] [PubMed] [Google Scholar]
- 29.Kim JE, Lee JY, Kang CH. Limosilactobacillus fermentum MG4295 Improves Hyperglycemia in High-Fat Diet-Induced Mice. Foods . 2022;11 doi: 10.3390/foods11020231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Babadi M, Khorshidi A, Aghadavood E, Samimi M, Kavossian E, Bahmani F, Mafi A, Shafabakhsh R, Satari M, Asemi Z. The Effects of Probiotic Supplementation on Genetic and Metabolic Profiles in Patients with Gestational Diabetes Mellitus: a Randomized, Double-Blind, Placebo-Controlled Trial. Probiotics Antimicrob Proteins . 2019;11:1227–1235. doi: 10.1007/s12602-018-9490-z. [DOI] [PubMed] [Google Scholar]
- 31.Ai X, Wu C, Yin T, Zhur O, Liu C, Yan X, Yi C, Liu D, Xiao L, Li W, Xie B, He H. Antidiabetic Function of Lactobacillus fermentum MF423-Fermented Rice Bran and Its Effect on Gut Microbiota Structure in Type 2 Diabetic Mice. Front Microbiol . 2021;12:682290. doi: 10.3389/fmicb.2021.682290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clarke TC, Black LI, Stussman BJ, Barnes PM, Nahin RL. Trends in the use of complementary health approaches among adults: United States, 2002-2012. Natl Health Stat Report . 2015:1–16. [PMC free article] [PubMed] [Google Scholar]
- 33.Kim SK, Guevarra RB, Kim YT, Kwon J, Kim H, Cho JH, Kim HB, Lee JH. Role of Probiotics in Human Gut Microbiome-Associated Diseases. J Microbiol Biotechnol . 2019;29:1335–1340. doi: 10.4014/jmb.1906.06064. [DOI] [PubMed] [Google Scholar]
- 34.Chang CJ, Lin TL, Tsai YL, Wu TR, Lai WF, Lu CC, Lai HC. Next generation probiotics in disease amelioration. J Food Drug Anal . 2019;27:615–622. doi: 10.1016/j.jfda.2018.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. 35 Hu TG, Wen P, Shen WZ, Liu F, Li Q, Li EN, Liao ST, Wu H, Zou YX. Effect of 1-Deoxynojirimycin Isolated from Mulberry Leaves on Glucose Metabolism and Gut Microbiota in a Streptozotocin-Induced Diabetic Mouse Model. J Nat Prod . 2019;82:2189–2200. doi: 10.1021/acs.jnatprod.9b00205. [DOI] [PubMed] [Google Scholar]
- 36.Aziz G, Zaidi A, Tariq M. Compositional Quality and Possible Gastrointestinal Performance of Marketed Probiotic Supplements. Probiotics Antimicrob Proteins . 2022;14:288–312. doi: 10.1007/s12602-022-09931-7. [DOI] [PubMed] [Google Scholar]
- 37.Sanders ME, Merenstein DJ, Reid G, Gibson GR, Rastall RA. Probiotics and prebiotics in intestinal health and disease: from biology to the clinic. Nat Rev Gastroenterol Hepatol . 2019;16:605–616. doi: 10.1038/s41575-019-0173-3. [DOI] [PubMed] [Google Scholar]
- 38.Li HY, Zhou DD, Gan RY, Huang SY, Zhao CN, Shang A, Xu XY, Li HB. Effects and Mechanisms of Probiotics, Prebiotics, Synbiotics, and Postbiotics on Metabolic Diseases Targeting Gut Microbiota: A Narrative Review. Nutrients. 2021;13 doi: 10.3390/nu13093211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mañé J, Lorén V, Pedrosa E, Ojanguren I, Xaus J, Cabré E, Domènech E, Gassull MA. Lactobacillus fermentum CECT 5716 prevents and reverts intestinal damage on TNBS-induced colitis in mice. Inflamm Bowel Dis . 2009;15:1155–1163. doi: 10.1002/ibd.20908. [DOI] [PubMed] [Google Scholar]
- 40.Feng T, Wang J. Oxidative stress tolerance and antioxidant capacity of lactic acid bacteria as probiotic: a systematic review. Gut Microbes . 2020;12:1801944. doi: 10.1080/19490976.2020.1801944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dowarah R, Verma AK, Agarwal N, Singh P, Singh BR. Selection and characterization of probiotic lactic acid bacteria and its impact on growth, nutrient digestibility, health and antioxidant status in weaned piglets. PLoS One. 2018;13:e0192978. doi: 10.1371/journal.pone.0192978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun Z, Sun X, Li J, Li Z, Hu Q, Li L, Hao X, Song M, Li C. Using probiotics for type 2 diabetes mellitus intervention: Advances, questions, and potential. Crit Rev Food Sci Nutr . 2020;60:670–683. doi: 10.1080/10408398.2018.1547268. [DOI] [PubMed] [Google Scholar]
- 43.Zheng J, Wittouck S, Salvetti E, Franz CMAP, Harris HMB, Mattarelli P, O'Toole PW, Pot B, Vandamme P, Walter J, Watanabe K, Wuyts S, Felis GE, Gänzle MG, Lebeer S. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int J Syst Evol Microbiol. 2020;70:2782–2858. doi: 10.1099/ijsem.0.004107. [DOI] [PubMed] [Google Scholar]
- 44.Paulino do Nascimento LC, Lacerda DC, Ferreira DJS, de Souza EL, de Brito Alves JL. Limosilactobacillus fermentum, Current Evidence on the Antioxidant Properties and Opportunities to be Exploited as a Probiotic Microorganism. Probiotics Antimicrob Proteins. 2022 doi: 10.1007/s12602-022-09943-3. [DOI] [PubMed] [Google Scholar]
- 45.de Luna Freire MO, do Nascimento LCP, de Oliveira KÁR, de Oliveira AM, Napoleão TH, Lima MDS, Lagranha CJ, de Souza EL, de Brito Alves JL. Effects of a Mixed Limosilactobacillus fermentum Formulation with Claimed Probiotic Properties on Cardiometabolic Variables, Biomarkers of Inflammation and Oxidative Stress in Male Rats Fed a High-Fat Diet. Foods. 2021;10 doi: 10.3390/foods10092202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cavalcante RGS, de Albuquerque TMR, de Luna Freire MO, Ferreira GAH, Carneiro Dos Santos LA, Magnani M, Cruz JC, Braga VA, de Souza EL, de Brito Alves JL. The probiotic Lactobacillus fermentum 296 attenuates cardiometabolic disorders in high fat diet-treated rats. Nutr Metab Cardiovasc Dis. 2019;29:1408–1417. doi: 10.1016/j.numecd.2019.08.003. [DOI] [PubMed] [Google Scholar]
- 47.Adeshirlarijaney A, Gewirtz AT. Considering gut microbiota in treatment of type 2 diabetes mellitus. Gut Microbes . 2020;11:253–264. doi: 10.1080/19490976.2020.1717719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Archer AC, Muthukumar SP, Halami PM. Lactobacillus fermentum MCC2759 and MCC2760 Alleviate Inflammation and Intestinal Function in High-Fat Diet-Fed and Streptozotocin-Induced Diabetic Rats. Probiotics Antimicrob Proteins . 2021;13:1068–1080. doi: 10.1007/s12602-021-09744-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Balakumar M, Prabhu D, Sathishkumar C, Prabu P, Rokana N, Kumar R, Raghavan S, Soundarajan A, Grover S, Batish VK, Mohan V, Balasubramanyam M. Improvement in glucose tolerance and insulin sensitivity by probiotic strains of Indian gut origin in high-fat diet-fed C57BL/6J mice. Eur J Nutr . 2018;57:279–295. doi: 10.1007/s00394-016-1317-7. [DOI] [PubMed] [Google Scholar]
- 50.Hartajanie L, Fatimah-Muis S, Heri-Nugroho Hs K, Riwanto I, Sulchan M. Probiotics Fermented Bitter Melon Juice as Promising Complementary Agent for Diabetes Type 2: Study on Animal Model. J Nutr Metab . 2020;2020:6369873. doi: 10.1155/2020/6369873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chaiyasut C, Woraharn S, Sivamaruthi BS, Lailerd N, Kesika P, Peerajan S. Lactobacillus fermentum HP3-Mediated Fermented Hericium erinaceus Juice as a Health Promoting Food Supplement to Manage Diabetes Mellitus. J Evid Based Integr Med . 2018;23:2515690X18765699. doi: 10.1177/2515690X18765699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guilbaud A, Howsam M, Niquet-Léridon C, Delguste F, Fremont M, Lestavel S, Maboudou P, Garat A, Schraen S, Onraed B, Foligné B, Boulanger É, Tessier FJ. The Effect of Lactobacillus fermentum ME-3 Treatment on Glycation and Diabetes Complications. Mol Nutr Food Res . 2020;64:e1901018. doi: 10.1002/mnfr.201901018. [DOI] [PubMed] [Google Scholar]
- 53.Larsen N, Vogensen FK, van den Berg FW, Nielsen DS, Andreasen AS, Pedersen BK, Al-Soud WA, Sørensen SJ, Hansen LH, Jakobsen M. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS One . 2010;5:e9085. doi: 10.1371/journal.pone.0009085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yadav R, Dey DK, Vij R, Meena S, Kapila R, Kapila S. Evaluation of anti-diabetic attributes of Lactobacillus rhamnosus MTCC: 5957, Lactobacillus rhamnosus MTCC: 5897 and Lactobacillus fermentum MTCC: 5898 in streptozotocin induced diabetic rats. Microb Pathog. 2018;125:454–462. doi: 10.1016/j.micpath.2018.10.015. [DOI] [PubMed] [Google Scholar]
- 55.Yousaf S, Hussain A, Rehman S, Aslam MS, Abbas Z. Hypoglycemic and hypolipidemic effects of Lactobacillus fermentum, fruit extracts of Syzygium cumini and Momordica charantia on diabetes induced mice. Pak J Pharm Sci. 2016;29:1535–1540. [PubMed] [Google Scholar]