Abstract
BACKGROUND
Early identification of metabolic-associated fatty liver disease (MAFLD) is urgent. Atherogenic index of plasma (AIP) is a reference predictor of obesity-related diseases, but its predictive value for MAFLD remains unclear. No studies have reported whether its combination with waist circumference (WC) and body mass index (BMI) can improve the predictive performance for MAFLD.
AIM
To systematically explore the relationship between AIP and MAFLD and evaluate its predictive value for MAFLD and to pioneer a novel noninvasive predictive model combining AIP, WC, and BMI while validating its predictive performance for MAFLD.
METHODS
This cross-sectional study consecutively enrolled 864 participants. Multivariate logistic regression analysis and receiver operating characteristic curve were used to evaluate the relationship between AIP and MAFLD and its predictive power for MAFLD. The novel prediction model A-W-B combining AIP, WC, and BMI to predict MAFLD was established, and internal verification was completed by magnetic resonance imaging diagnosis.
RESULTS
Subjects with higher AIP exhibited a significantly increased risk of MAFLD, with an odds ratio of 12.420 (6.008-25.675) for AIP after adjusting for various confounding factors. The area under receiver operating characteristic curve of the A-W-B model was 0.833 (0.807-0.858), which was significantly higher than that of AIP, WC, and BMI (all P < 0.05). Subgroup analysis illustrated that the A-W-B model had significantly higher area under receiver operating characteristic curves in female, young and nonobese subgroups (all P < 0.05). The best cutoff values for the A-W-B model to predict MAFLD in males and females were 0.5932 and 0.4105, respectively. Additionally, in the validation set, the area under receiver operating characteristic curve of the A-W-B model to predict MAFLD was 0.862 (0.791-0.916). The A-W-B level was strongly and positively associated with the liver proton density fat fraction (r = 0.630, P < 0.001) and significantly increased with the severity of MAFLD (P < 0.05).
CONCLUSION
AIP was strongly and positively associated with the risk of MAFLD and can be a reference predictor for MAFLD. The novel prediction model A-W-B combining AIP, WC, and BMI can significantly improve the predictive ability of MAFLD and provide better services for clinical prediction and screening of MAFLD.
Keywords: Atherogenic index of plasma, Metabolic-associated fatty liver disease, Receiver operating characteristic curve, Predictor
Core Tip: Metabolic-associated fatty liver disease (MAFLD) is the most common chronic liver disease, and early identification of MAFLD is urgent. This study demonstrated that the atherogenic index of plasma was strongly and positively associated with the risk of MAFLD, and it can be a reference predictor for MAFLD. Then, we pioneered a novel noninvasive prediction model, A-W-B, combining atherogenic index of plasma, waist circumference, and body mass index and validated its excellent predictive performance for MAFLD. Furthermore, we also pointed out the optimal cutoff values of the A-W-B model to predict MAFLD in males and females, which will facilitate early clinical identification of MAFLD in different sex populations. This study is highly innovative, and the noninvasive prediction model, A-W-B, is convenient, affordable, and easy to obtain, which can provide better services for clinical prediction and screening of MAFLD and metabolic-related diseases.
INTRODUCTION
Metabolic-associated fatty liver disease (MAFLD), formerly known as nonalcoholic fatty liver disease, is a common disease closely related to genetic, obesity, and metabolic abnormalities and has become a global public health problem[1,2]. In recent decades, obesity has become increasingly widespread owing to huge changes in dietary structure and living habits[3,4]. The prevalence of MAFLD has risen rapidly, and patients tend to be younger[5]. Notably, MAFLD can not only progress to hepatitis, liver cirrhosis, and liver cancer[6] but also increase the occurrence and development of diabetes[7] and cardiovascular diseases[8,9], which seriously endangers individual health and increases the social and medical economic burden[10]. Therefore, it is necessary to predict and screen MAFLD at an early stage to intervene in a timely manner.
The occurrence and development of MAFLD are closely related to lipid metabolism disorders and dyslipidemia caused by the accumulation of visceral fat[11]. Patients with fatty liver usually have elevated triglycerides (TG) and generally lower high-density lipoprotein cholesterol (HDL-C) than healthy people[12]. Waist circumference (WC) and body mass index (BMI) are commonly used as indicators to assess obesity, and it has been shown that elevated WC and BMI can significantly increase the risk of fatty liver disease. However, they have certain limitations in accurately reflecting the accumulation of visceral fat[13].
Recently, the atherogenic index of plasma (AIP), calculated from the logarithm of the ratio of TG to HDL-C[14], has been proven to be closely related to abdominal obesity, and it can sensitively reflect the accumulation of visceral fat and effectively predict the risk of atherosclerosis and cardiovascular disease[15,16]. Previous studies indicate that AIP is significantly higher in patients with fatty liver and may be a potential indicator for identifying fatty liver disease[17]. However, few studies have systematically reported the predictive value of AIP for MAFLD and whether AIP combined with WC and BMI can improve the predictive ability for MAFLD is unclear.
Therefore, the two main objectives of this study were: (1) To systematically assess the relationship between AIP and MAFLD and evaluate its predictive value for MAFLD; and (2) To establish a novel noninvasive prediction model combining AIP, WC and BMI and validate its predictive performance for MAFLD.
MATERIALS AND METHODS
Study design and participants
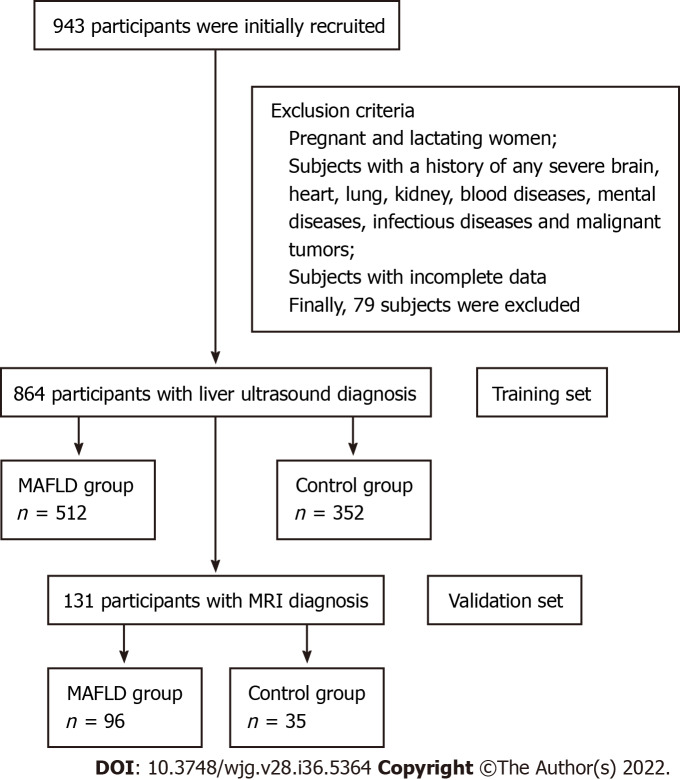
This study was conducted in Beijing, China. Among the adults who underwent a physical examination for health at China-Japan Friendship Hospital in Beijing from September 2018 to October 2021, we consecutively recruited 943 participants who completed the standardized questionnaire, finished anthropometric and laboratory tests, and underwent liver ultrasonography. All subjects agreed to participate in this study voluntarily and submitted informed consent forms.
According to quality control, after excluding pregnant and lactating women, subjects who had a history of any severe brain, heart, lung, kidney, or blood diseases, mental illness, infectious diseases, malignant tumors, etc as well as the subjects with incomplete data, a total of 864 subjects were finally included (Figure 1), with 624 males and 240 females, aged from 20-years-old to 78-years-old.
Figure 1.
Flow chart of the study subjects. MAFLD: Metabolic-associated fatty liver disease; MRI: Magnetic resonance imaging.
This study was approved by the Clinical Research Ethics Committee of China-Japan Friendship Hospital (2018-110-K79-1).
Data collection and definition
The physical examination was performed in the morning in a fasting state. Anthropometric indicators were measured by professionally trained doctors. Height, weight, and waist circumference were measured while subjects were naturally standing barefoot with lightweight clothes. After 10 min of rest, the blood pressure was measured with an upper arm electronic sphygmomanometer. Peripheral blood was drawn into an EDTA-containing tube and subjected to biochemical experiments within 2 h. The relevant laboratory indicators were obtained through the electronic database of this physical examination center, including alanine aminotransferase (ALT), aspartate aminotransferase (AST), total cholesterol, TG, HDL-C, low-density lipoprotein cholesterol, fasting blood glucose (FBG), serum uric acid (SUA) and so on.
BMI was calculated as the body weight (in kilograms) divided by the square of the height (in meters). WC referred to the waist circumference at the level of the flat navel. AIP was calculated as the logarithmic transformation of the ratio of TG to HDL-C [log (TG/HDL-C)].
Diagnostic criteria and detection methods of MAFLD
The diagnostic criteria of MAFLD refer to the consensus of international experts in 2020 that in addition to the evidence of hepatic steatosis[18], one of the following three criteria, namely, overweight/obesity, type 2 diabetes, or metabolic dysregulation, needs to be met[19,20]. Among them, metabolic dysregulation refer to the existence of at least two of the following metabolic risk criteria: (1) Waist circumference ≥ 102/88 cm in Caucasian males and females, respectively, or ≥ 90/80 cm in Asian males and females; (2) Blood pressure ≥ 130/85 mmHg or specific drug treatment; (3) Plasma TG ≥ 1.70 mmol/L or specific drug treatment; (4) Plasma HDL-C < 1.0 mmol/L for males and < 1.3 mmol/L for females or specific drug treatment; (5) Prediabetes (i.e. fasting glucose levels 5.6 to 6.9 mmol/L or 2-h post load glucose levels 7.8 to 11.0 mmol or glycated hemoglobin 5.7% to 6.4%; (6) Homeostasis model assessment-insulin resistance score ≥ 2.5; and (7) Plasma high-sensitivity C-reactive protein level > 2 mg/L.
In the training set, fatty liver (hepatic steatosis) was defined by liver ultrasound examination based on at least two of the following three abnormal findings: (1) Diffusely increased echogenicity of the liver relative to the kidney or spleen; (2) Ultrasound beam attenuation; and (3) Poor visualization of intrahepatic structures.
In the validation set, the proton density fat fraction (PDFF) based on magnetic resonance spectroscopy and magnetic resonance imaging (MRI) was used to diagnose fatty liver and to evaluate the severity of MAFLD[21]. PDFF < 5% was defined as no fatty liver, PDFF 5.0%-14.0% was defined as mild MAFLD, and PDFF > 14.0% was defined as moderate to severe MAFLD.
Statistical analysis
First, the baseline characteristics of the MAFLD and non-MAFLD groups were compared. The independent samples t test was used for comparing normally or approximately normally distributed quantitative data between groups, expressed as the mean and standard deviation (SD). The Mann-Whitney U test was used for comparing nonnormally distributed quantitative data between groups, represented as medians and quartiles. The χ2 test was used for comparing categorical data between groups, represented as numbers and percentages.
Then, multivariate logistic regression analysis was conducted to calculate the odds ratios (ORs) and 95% confidence intervals of AIP for MAFLD under different adjustment conditions. The logistic regression prediction model A-W-B combining AIP, WC, and BMI was established. The Hosmer-Lemeshow test and the receiver operating characteristic (ROC) curve were used to evaluate the calibration and discrimination of this model. The DeLong test was used to compare the predictive ability of the AIP, WC, BMI, and A-W-B model for MAFLD. Spearman’s correlation analysis was used to explore the correlation between parameters. Finally, the internal verification was completed with MRI as the diagnostic standard.
All statistical tests were two-tailed and were considered significant for P less than 0.05 (P < 0.05). Statistical analyses were performed using Statistical Package for the Sciences (SPSS, version 25.0) and MedCalc statistical software (version 19.6.4).
RESULTS
Characteristics of participants
The demographics, anthropometrics, and laboratory test characteristics of 864 subjects are presented in Table 1. The prevalence of male and young patients (age < 40-years-old) and the percentage of smoking history, drinking history, overweight, obesity, elevated ALT, and elevated ALT in MAFLD subjects were significantly higher than those in the non-MAFLD subjects (all P < 0.05). Participants with MAFLD had dramatically higher WC, BMI, SBP, DBP, ALT, AST, total cholesterol, TG, low-density lipoprotein cholesterol, FBG, and SUA and significantly lower AST/ALT and HDL-C (all P < 0.05). In addition, a significant association between AIP and MAFLD was initially demonstrated.
Table 1.
Baseline characteristics of the study subjects
|
Variable
|
Non-MAFLD, n = 352
|
MAFLD, n = 512
|
Statistics1
|
P
value
|
| Demographics | ||||
| Sex | χ2 = 14.020 | < 0.001 | ||
| Male | 230 (65.3) | 394 (77.0) | ||
| Female | 122 (34.7) | 118 (23.0) | ||
| Age in yr | 37.750 ± 10.736 | 41.240 ± 10.852 | t = -4.634 | < 0.001 |
| Age ≥ 40 yr | 115 (32.7) | 254 (49.6) | χ2 = 24.461 | < 0.001 |
| Age < 40 yr | 237 (67.3) | 258 (40.4) | ||
| Smoking history | 81 (23.0) | 174 (34.0) | χ2= 12.073 | < 0.001 |
| Drinking history | 76 (21.6) | 145 (28.3) | χ2= 4.962 | 0.027 |
| Anthropometrics | ||||
| WC in cm | 86.240 ± 9.893 | 96.790 ± 8.618 | t = -16.219 | < 0.001 |
| BMI in kg/m2 | 24.436 ± 3.169 | 28.028 ± 3.221 | t = -16.228 | < 0.001 |
| SBP in mmHg | 125.880 ± 14.883 | 133.610 ± 16.859 | t = -6.908 | < 0.001 |
| DBP in mmHg | 77.500 ± 11.658 | 82.950 ± 12.525 | t = -6.461 | < 0.001 |
| Laboratory tests | ||||
| ALT in U/L | 21.0 (15.0, 30.0) | 34.0 (24.0, 54.0) | Z = -12.276 | < 0.001 |
| AST in U/L | 19.0 (17.0, 23.0) | 23.0 (19.0, 29.0) | Z = -9.058 | < 0.001 |
| TC in mmol/L | 4.539 ± 0.847 | 4.783 ± 0.902 | t = -4.104 | < 0.001 |
| TG in mmol/L | 1.1 (0.7, 1.5) | 1.8 (1.3, 2.6) | Z = -13.650 | < 0.001 |
| HDL-C in mmol/L | 1.351 ± 0.283 | 1.191 ± 0.256 | t = 8.321 | < 0.001 |
| LDL-C in mmol/L | 2.645 ± 0.708 | 2.906 ± 0.884 | t = -4.568 | < 0.001 |
| FBG in mmol/L | 5.2 (4.9, 5.4) | 5.4 (5.1, 5.9) | Z = -7.637 | < 0.001 |
| SUA in μmol/L | 332.722 ± 86.289 | 377.836 ± 85.859 | t = -7.590 | < 0.001 |
| AIP | -0.11 (-0.30, 0.08) | 0.20 (0.03, 0.39) | Z = -14.006 | < 0.001 |
Comparison of significant differences between the two groups; χ2 value calculated by the χ2 test; Z value calculated by the Mann-Whitney U test; t value calculated by the independent samples t test. Data are presented as median and interquartile range, mean and standard deviation or frequency (percentage). MAFLD: Metabolic-associated fatty liver disease; WC: Waist circumference; BMI: Body mass index; SBP: Systolic blood pressure; DBP: Diastolic blood pressure; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; TC: Total cholesterol; TG: Triglyceride; HDL-C: High-density lipoprotein cholesterol; LDL-C: Low-density lipoprotein cholesterol; FBG: Fasting blood glucose; SUA: Serum uric acid; AIP: Atherogenic index of plasma.
Multivariate logistic regression analysis of AIP on the risk of MAFLD
Multivariate logistic regression analyses were conducted to further explore the relationship between AIP and MAFLD, and the results are shown in Table 2. AIP had a strong association with the risk of MAFLD, and the OR for a 1-SD increase in AIP was 50.286 (26.953-93.819) without adjustment (Model 1). After adjusting for sex and age, the OR for a 1-SD increase in AIP was 48.874 (26.087-91.569) (Model 2). After further adjusting for smoking history, drinking history, WC, and BMI, the degree of this association changed but was still strong; the OR for a 1-SD increase in AIP was 16.184 (7.961-32.902) (Model 3). Further adjusting for SBP, DBP, ALT, AST, total cholesterol, TG, HDL-C, low-density lipoprotein cholesterol, FBG, and SUA attenuated the association but only slightly; there was still a 12.420-fold (6.008-25.675) higher risk for MAFLD with a 1-SD increase in AIP (Model 4).
Table 2.
Multivariate logistic regression of atherogenic index of plasma for the risk of metabolic-associated fatty liver disease
|
Variable
|
β
|
SE
|
Wald χ2
|
P
value
|
OR (95%CI)
|
|
| Model 1 | AIP level (per change in SD) | 3.918 | 0.318 | 151.599 | < 0.001 | 50.286 (26.953-93.819) |
| Quartiles of AIP | ||||||
| A1 (≤ -0.1265) | - | - | - | - | 1 (Ref) | |
| A2 (-0.1265-0.0947) | 1.241 | 0.209 | 35.108 | < 0.001 | 3.460 (2.295-5.217) | |
| A3 (0.0947-0.3023) | 2.323 | 0.226 | 105.794 | < 0.001 | 10.204 (6.554-15.885) | |
| A4 (> 0.3022) | 2.826 | 0.245 | 132.611 | < 0.001 | 16.882 (10.436-27.311) | |
| Model 2 | AIP level (per change in SD) | 3.889 | 0.32 | 147.414 | < 0.001 | 48.874 (26.087-91.569) |
| Quartiles of AIP | ||||||
| A1 (≤ -0.1265) | - | - | - | - | 1 (Ref) | |
| A2 (-0.1265-0.0947) | 0.847 | 0.241 | 12.347 | < 0.001 | 3.218 (2.124-4.876) | |
| A3 (0.0947-0.3023) | 1.690 | 0.260 | 42.107 | < 0.001 | 9.774 (6.256-15.270) | |
| A4 (> 0.3022) | 2.103 | 0.281 | 55.913 | < 0.001 | 16.514 (10.176-26.799) | |
| Model 3 | AIP level (per change in SD) | 2.784 | 0.362 | 59.147 | < 0.001 | 16.184 (7.961-32.902) |
| Quartiles of AIP | ||||||
| A1 (≤ -0.1265) | - | - | - | - | 1 (Ref) | |
| A2 (-0.1265-0.0947) | 0.847 | 0.241 | 12.347 | < 0.001 | 2.334 (1.455-3.744) | |
| A3 (0.0947-0.3023) | 1.690 | 0.260 | 42.107 | < 0.001 | 5.421 (3.253-9.032) | |
| A4 (> 0.3022) | 2.103 | 0.281 | 55.913 | < 0.001 | 8.194 (4.721-14.22) | |
| Model 4 | AIP level (per change in SD) | 2.519 | 0.371 | 46.230 | < 0.001 | 12.420 (6.008-25.675) |
| Quartiles of AIP | ||||||
| A1 (≤ -0.1265) | - | - | - | - | 1 (Ref) | |
| A2 (-0.1265-0.0947) | 0.828 | 0.249 | 11.088 | 0.001 | 2.288 (1.4063-725) | |
| A3 (0.0947-0.3023) | 1.642 | 0.268 | 37.636 | < 0.001 | 5.167 (3.058-8.732) | |
| A4 (> 0.3022) | 1.969 | 0.289 | 46.343 | < 0.001 | 7.160 (4.062-12.62) | |
Model 1: Unadjusted. Model 2: Adjusted for age and sex. Model 3: Adjusted for age, sex, smoking history, drinking history, waist circumference and body mass index. Model 4: Adjusted for age, sex, smoking history, drinking history, waist circumference, body mass index, systolic blood pressure, diastolic blood pressure, alanine aminotransferase, aspartate aminotransferase, total cholesterol, triglyceride, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, fasting blood glucose, and serum uric acid. AIP: Atherogenic index of plasma; SE: Standard error; OR: Odds ratio; CI: Confidence interval; SD: Standard deviation.
After dividing AIP into quartiles, the risk of MAFLD increased robustly with higher AIP quartiles. When comparing the top quartiles with the bottom categories, the risk of MAFLD increased 16.882-fold to 7.160-fold from Model 1 to Model 4. The P values for the linear trend were less than 0.01, signifying that linear trends from the lowest to the highest quartiles were eminent.
Predictive ability of AIP for MAFLD in different subgroups
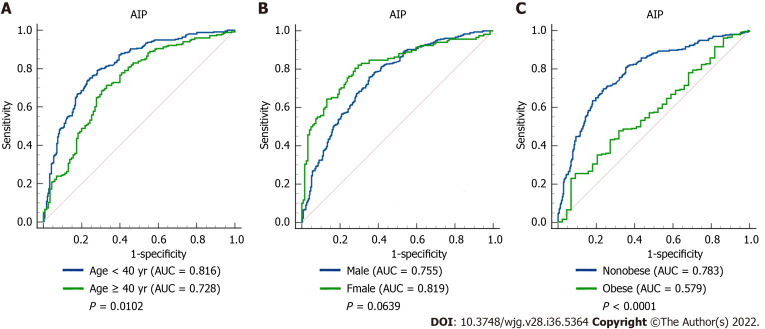
The ROC curve of AIP for predicting MAFLD in different sex, age, and weight subgroups was plotted, and the DeLong test was used to compare the area under the ROC curve (AUC) between the subgroups. As shown in Figure 2, the AUC of AIP for MAFLD in the young was significantly higher than that in middle-age and elderly subjects [0.816 (0.779-0.849) vs 0.726 (0.678-0.771), P < 0.05]. The AUC of AIP for MAFLD in nonobese subjects was significantly higher than that in obese subjects [0.783 (0.747-0.816) vs 0.579 (0.519-0.638), P < 0.0001]. However, there was no significant difference between males and females (P = 0.0639).
Figure 2.
Receiver operating characteristic curves of atherogenic index of plasma in predicting metabolic-associated fatty liver disease in different subgroups. A: Comparison between the young (age < 40 years) and middle-age and elderly (age ≥ 40 years) subjects (P = 0.0102); B: Comparison between male and female subjects (P = 0.0639); C: Comparison between nonobese and obese subjects (P < 0.0001). AIP: Atherogenic index of plasma; AUC: Area under the receiver operating characteristic curve.
In addition, the best cutoff values for AIP to predict MAFLD in males and females were 0.0821 and -0.1390, respectively. The AUCs of AIP, WC, and BMI for predicting total MAFLD were 0.780 (0.751-0.807), 0.790 (0.761-0.817), and 0.788 (0.759-0.814), respectively, with no significant difference among the three (Table 3).
Table 3.
Results analysis of the receiver operating characteristic curves
|
Variable
|
AUC (95%CI)
|
P
value
|
Sensitivity (%)
|
Specificity (%)
|
Youden index
|
Cutoff value
|
| Total | ||||||
| AIP | 0.780 (0.751-0.807) | < 0.0001 | 75.20 | 69.89 | 0.4508 | 0.0340 |
| WC in cm | 0.790 (0.761-0.817) | < 0.0001 | 85.94 | 58.52 | 0.4446 | 88.00 |
| BMI in kg/m2 | 0.788 (0.759-0.814) | < 0.0001 | 83.20 | 59.09 | 0.4229 | 25.10 |
| A-W-B | 0.833 (0.807-0.858)1,2,3 | < 0.0001 | 86.13 | 68.47 | 0.5460 | 0.5019 |
| Male | ||||||
| AIP | 0.755 (0.719-0.788) | < 0.0001 | 75.89 | 64.78 | 0.4067 | 0.0821 |
| WC in cm | 0.770 (0.735-0.803) | < 0.0001 | 72.59 | 67.39 | 0.3998 | 92.50 |
| BMI in kg/m2 | 0.773 (0.738-0.805) | < 0.0001 | 72.34 | 68.70 | 0.4103 | 26.32 |
| A-W-B | 0.814 (0.781-0.843)1,2,3 | < 0.0001 | 82.99 | 65.65 | 0.4865 | 0.5932 |
| Female | ||||||
| AIP | 0.819 (0.764-0.865) | < 0.0001 | 82.2 | 70.49 | 0.5270 | -0.1390 |
| WC in cm | 0.820 (0.765-0.866) | < 0.0001 | 81.36 | 69.67 | 0.5103 | 85.50 |
| BMI in kg/m2 | 0.804 (0.748-0.852) | < 0.0001 | 88.98 | 58.20 | 0.4718 | 23.34 |
| A-W-B | 0.874 (0.826-0.914)1,2,3 | < 0.0001 | 78.81 | 83.61 | 0.6242 | 0.4105 |
| Age ≥ 40 yr | ||||||
| AIP | 0.726 (0.678-0.771) | < 0.0001 | 71.26 | 66.96 | 0.3822 | 0.0367 |
| WC in cm | 0.736 (0.688-0.780) | < 0.0001 | 82.68 | 52.17 | 0.3485 | 88.00 |
| BMI in kg/m2 | 0.736 (0.688-0.780) | < 0.0001 | 71.26 | 66.96 | 0.3822 | 26.03 |
| A-W-B | 0.787 (0.742-0.828)1,2,3 | < 0.0001 | 77.56 | 69.57 | 0.4712 | 0.5574 |
| Age < 40 yr | ||||||
| AIP | 0.816 (0.779-0.849) | < 0.0001 | 73.64 | 77.22 | 0.5086 | 0.0814 |
| WC in cm | 0.824 (0.787-0.856) | < 0.0001 | 89.15 | 61.60 | 0.5075 | 88.00 |
| BMI in kg/m2 | 0.820 (0.783-0.853) | < 0.0001 | 83.72 | 66.24 | 0.4997 | 25.31 |
| A-W-B | 0.863 (0.830-0.892)1,2,3 | < 0.0001 | 89.92 | 71.73 | 0.6165 | 0.4947 |
| BMI ≥ 28 kg/m2 | ||||||
| AIP | 0.579 (0.519-0.638) | 0.0849 | 25.63 | 90.91 | 0.1654 | 0.4140 |
| WC in cm | 0.597 (0.688-0.780) | 0.0303 | 36.97 | 79.55 | 0.1652 | 104.50 |
| BMI in kg/m2 | 0.648 (0.589-0.704) | 0.0005 | 65.13 | 68.18 | 0.3331 | 29.38 |
| A-W-B | 0.644 (0.585-0.700)1 | 0.0007 | 43.28 | 84.09 | 0.2737 | 0.9009 |
| BMI < 28 kg/m2 | ||||||
| AIP | 0.783 (0.747-0.816)3 | < 0.0001 | 69.71 | 76.30 | 0.4601 | 0.0340 |
| WC in cm | 0.754 (0.717-0.789) | < 0.0001 | 78.1 | 63.31 | 0.4141 | 87.50 |
| BMI in kg/m2 | 0.733 (0.695-0.768) | < 0.0001 | 77.37 | 59.42 | 0.3679 | 24.56 |
| A-W-B | 0.822 (0.789-0.852)1,2,3 | < 0.0001 | 80.66 | 72.08 | 0.5273 | 0.4531 |
Indicates significantly larger compared with atherogenic index of plasma.
Indicates significantly larger as compared with waist circumference.
Indicates significantly larger as compared with body mass index.
AUC: Area under the receiver operating characteristic curve; AIP: Atherogenic index of plasma; WC: Waist circumference; BMI: Body mass index; A-W-B: Prediction model combining atherogenic index of plasma, waist circumference, and body mass index; CI: Confidence interval.
Establishment of the A-W-B model for better predicting MAFLD
To further improve the predictive ability of MAFLD, we combined AIP, WC, and BMI and put them into the binary logistic regression model to construct a new logistic regression prediction model, A-W-B. The regression equation was logit (A-W-B) = -8.782 + 2.560 × AIP + 0.049 × WC + 0.170 × BMI (Table 4).
Table 4.
Establishment of the logistic regression prediction model A-W-B combined with the atherogenic index of plasma, waist circumference, and body mass index
|
Variable
|
β
|
SE
|
Wald χ2
|
P
value
|
OR (95%CI)
|
| AIP | 2.560 | 0.345 | 55.017 | < 0.001 | 12.939 (6.578-25.45) |
| WC | 0.049 | 0.017 | 7.951 | 0.005 | 1.050 (1.015-1.087) |
| BMI | 0.170 | 0.051 | 11.087 | 0.001 | 1.186 (1.073-10311) |
| Constant | -8.782 | 1.003 | 76.689 |
AIP: Atherogenic index of plasma; WC: Waist circumference; BMI: Body mass index; SE: Standard error; OR: Odds ratio; CI: Confidence interval.
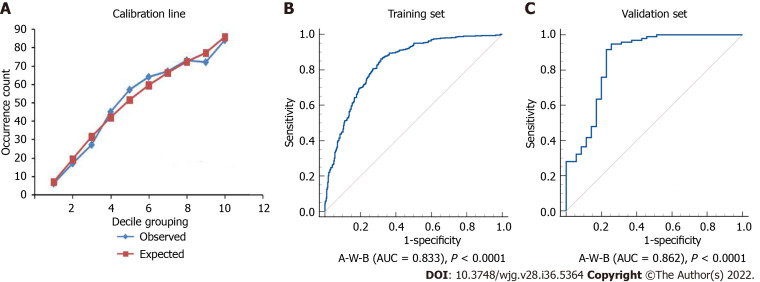
The Hosmer-Lemeshow test and ROC curve analysis were used to evaluate the calibration and discrimination of the A-W-B model. Figure 3A illustrated the calibration line graph of the A-W-B model, and the result of the Hosmer-Lemeshow test showed that χ2 = 8.5901, P = 0.3780 > 0.05, indicating that the A-W-B model had a good calibration ability for MAFLD. Figure 3B illustrated the ROC curve of the A-W-B model, with the AUC of 0.833 (0.807-0.858), indicating that the A-W-B model had a good discrimination ability for MAFLD.
Figure 3.
Calibration line and receiver operating characteristic curves of the A-W-B model. A: Calibration line of the prediction model combining atherogenic index of plasma, waist circumference, and body mass index (A-W-B) model (Hosmer-Lemeshow test: χ2 = 8.5901, P = 0.3780); B: Receiver operating characteristic curve of the A-W-B model in the training set (area under the receiver operating characteristic curve [AUC] = 0.833); C: Receiver operating characteristic curve of the A-W-B model in the validation set (AUC = 0.862).
Predictive ability of the A-W-B model for MAFLD in different subgroups
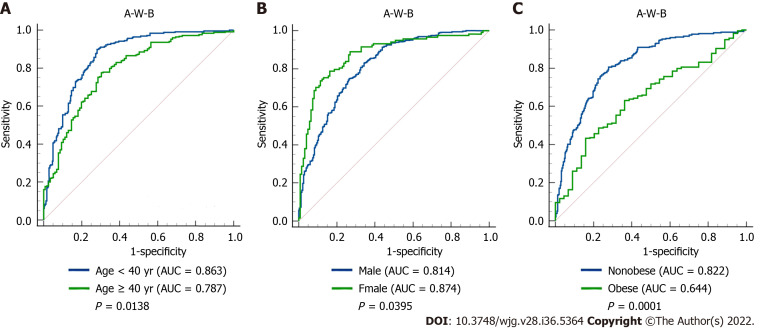
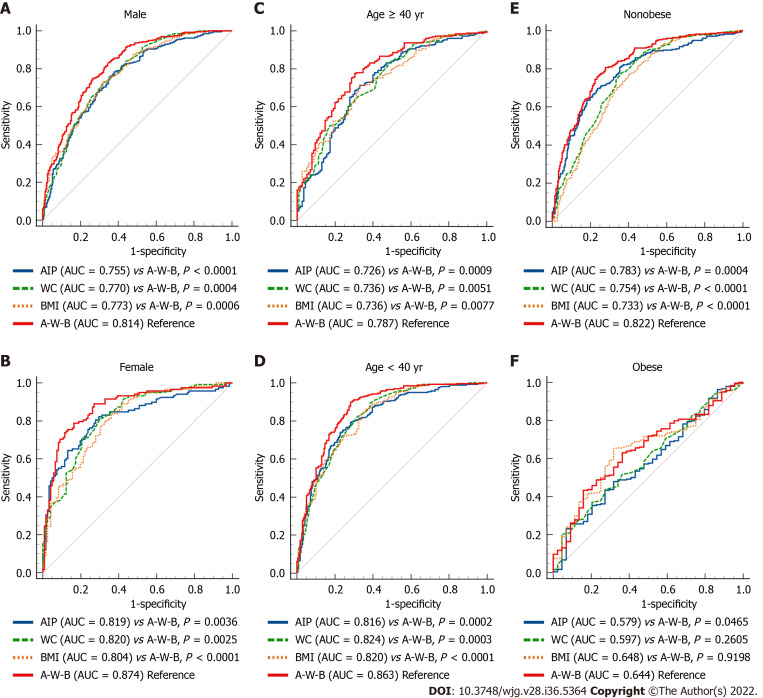
To further evaluate the predictive power of the A-W-B model for MAFLD in different populations, we performed a subgroup analysis. As shown in Figure 4, the AUCs of A-W-B for MAFLD in female, young, and nonobese subjects were 0.874 (0.826-0.914), 0.863 (0.830-0.892), and 0.822 (0.789-0.852), respectively, which were significantly higher than those in male [0.814 (0.781-0.843)], middle-aged and elderly [0.787 (0.742-0.828)], and obese subjects [0.644 (0.585-0.700)] (all P < 0.05). These results indicated that the A-W-B model had stronger predictive power for MAFLD in female, young, and nonobese subjects. Furthermore, the results in Table 3 showed that the best cutoff values for A-W-B to predict MAFLD in males and females were 0.5932 and 0.4105, respectively.
Figure 4.
Receiver operating characteristic curves of the A-W-B model to predict metabolic-associated fatty liver disease in different subgroups. A: Comparison between young (age < 40 years) and middle-age and elderly (age ≥ 40 years) subjects (P = 0.0138); B: Comparison between male and female subjects (P = 0.0395); C: Comparison between nonobese and obese subjects (P = 0.0001); AUC: Area under the receiver operating characteristic curve; A-W-B: Prediction model combining atherogenic index of plasma, waist circumference, and body mass index.
Comparison of the A-W-B model and AIP, WC, and BMI for predicting MAFLD
The DeLong test was used to compare the predictive ability of the A-W-B model and AIP, WC, and BMI. The AUC of the A-W-B model for MAFLD was 0.833 (0.807-0.858), which was significantly higher than that of AIP, WC, and BMI (P < 0.05). The sensitivity, specificity, Youden index, and cutoff value of the A-W-B model were 86.13%, 68.47%, 0.5460, and 0.5019, respectively (Table 3).
As shown in Figure 5A-E, subgroup analysis showed that the AUCs of the A-W-B model were significantly higher than those of AIP, WC, and BMI in male, female, young, middle-aged and elderly, and nonobese subjects (all P < 0.01), demonstrating that the A-W-B model has a higher ability to predict MAFLD than AIP, WC, and BMI in different age, sex, and nonobese subjects. However, as shown in Figure 5F, among obese subjects, the predictive ability of the A-W-B model for MAFLD was only better than that of AIP. In addition, the Z values between the AIP, WC, BMI, and the A-W-B model in different subgroups were illustrated in Supplementary Table 1.
Figure 5.
Receiver operating characteristic curves of atherogenic index of plasma, waist circumference, body mass index, and A-W-B model to predict metabolic-associated fatty liver disease in different subgroups. A-E: The area under the receiver operating characteristic curve (AUC) of the prediction model combining atherogenic index of plasma, waist circumference (WC), and body mass index (BMI) (A-W-B) model was significantly higher than that of A-W-B in male, female, young, middle-aged and elderly, and nonobese subjects (all P < 0.01); F: The AUC of the A-W-B model was only better than atherogenic index of plasma (AIP) in obese subjects (P = 0.0465).
The concrete ROC curve results of the AIP, WC, BMI, and A-W-B model for predicting MAFLD in different subgroups were illustrated in Table 3. Compared to AIP, WC, and BMI, the A-W-B model had the best sensitivity in male, young and nonobese subjects, which may be more conducive to identifying patients with positive MAFLD to reduce the missed diagnosis rate. Meanwhile, the A-W-B model had the best specificity in female and middle-aged and elderly subjects, which may be more beneficial to identifying people without MAFLD and reducing the misdiagnosis rate.
Moreover, Spearman’s correlations between A-W-B, AIP, WC, BMI, and various physical and chemical indicators are illustrated in Table 5. Compared with AIP, WC, and BMI, the A-W-B model had a higher positive association with SBP, DBP, ALT, AST, FBG, and SUA.
Table 5.
Spearman’s correlation analysis results between various indicators (r value)
|
Variable
|
AIP
|
WC
|
BMI
|
A-W-B
|
| SBP in mmHg | 0.299b | 0.358b | 0.362b | 0.392b |
| DBP in mmHg | 0.327b | 0.325b | 0.315b | 0.375b |
| ALT in U/L | 0.470b | 0.455b | 0.451b | 0.530b |
| AST in U/L | 0.306b | 0.307b | 0.304b | 0.351b |
| TC in mmol/L | 0.193b | 0.070a | 0.056 | 0.132b |
| TG in mmol/L | 0.954b | 0.455b | 0.430b | 0.763b |
| HDL-C in mmol/L | -0.617b | -0.365b | -0.373b | -0.543b |
| LDL-C in mmol/L | 0.253b | 0.128b | 0.105b | 0.190b |
| FBG in mmol/L | 0.294b | 0.280b | 0.274b | 0.334b |
| SUA in μmol/L | 0.462b | 0.375b | 0.358b | 0.469b |
P < 0.05.
P < 0.01.
SBP: Systolic blood pressure; DBP: Diastolic blood pressure; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; TC: Total cholesterol; TG: Triglyceride; HDL-C: High-density lipoprotein cholesterol; LDL-C: Low-density lipoprotein cholesterol; FBG: Fasting blood glucose; SUA: Serum uric acid; AIP: Atherogenic index of plasma; WC: Waist circumference; BMI: Body mass index; A-W-B: Prediction model combining atherogenic index of plasma, waist circumference, and body mass index.
Validation of the A-W-B model
To further validate the diagnostic performance of the A-W-B model for MAFLD, we randomly selected approximately 15% of the subjects (131 cases) from the overall subjects as the validation set and used MRI to diagnose MAFLD. Among them, 35 cases were in the control group, 67 cases were mild MAFLD, and 29 cases were moderate to severe MAFLD. The data comparison between the training set and validation set was shown in Table 6. There was no statistically significant difference in age, sex, and various indicators between the two groups (all P > 0.05).
Table 6.
Data comparison between training set and validation set
|
Variable
|
Training set, n = 864
|
Validation set, n = 131
|
P
value
|
| Sex, female | 240 (27.8) | 38 (29.0) | 0.770 |
| Age in yr | 39.820 ± 10.934 | 39.660 ± 10.861 | 0.885 |
| Smoking history | 255 (29.5) | 38 (29.0) | 0.906 |
| Drinking history | 221 (25.6) | 24 (18.3) | 0.072 |
| WC in cm | 92.490 ± 10.522 | 93.690 ± 10.759 | 0.057 |
| BMI in kg/m2 | 26.563 ± 3.653 | 27.055 ± 3.772 | 0.155 |
| SBP in mmHg | 130.460 ± 16.517 | 129.090 ± 16.822 | 0.386 |
| DBP in mmHg | 80.730 ± 12.464 | 78.560 ± 12.192 | 0.063 |
| ALT in U/L | 28.0 (19.0, 42.0) | 28.0 (19.0, 43.0) | 0.837 |
| AST in U/L | 21.0 (18.0, 26.0) | 22.0 (18.0, 26.0) | 0.612 |
| TC in mmol/L | 4.683 ± 0.888 | 4.607 ± 0.865 | 0.349 |
| TG in mmol/L | 1.5 (1.0, 2.2) | 1.6 (1.0, 2.5) | 0.307 |
| HDL-C in mmol/L | 1.256 ± 0.279 | 1.241 ± 0.293 | 0.533 |
| LDL-C in mmol/L | 2.800 ± 0.826 | 2.763 ± 0.769 | 0.651 |
| FBG in mmol/L | 5.3 (5.0, 5.7) | 5.2 (4.9, 5.8) | 0.326 |
| SUA in μmol/L | 359.434 ± 88.800 | 342.901 ± 88.448 | 0.052 |
| AIP | 0.09 (-0.13, 0.30) | 0.12 (-0.13, 0.33) | 0.306 |
Data are presented as median and interquartile range, mean ± SD, or n (%). WC: Waist circumference; BMI: Body mass index; SBP: Systolic blood pressure; DBP: Diastolic blood pressure; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; TC: Total cholesterol; TG: Triglyceride; HDL-C: High-density lipoprotein cholesterol; LDL-C: Low-density lipoprotein cholesterol; FBG: Fasting blood glucose; SUA: Serum uric acid; AIP: Atherogenic index of plasma.
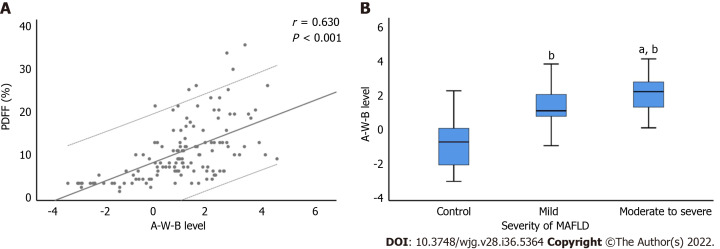
Figure 3C illustrated the ROC curve of the A-W-B model in the validation set, with the AUC of 0.862 (0.791-0.916), indicating that the A-W-B model also exhibited outstanding discrimination for MAFLD in the validation set. In addition, Figure 6 shows that the A-W-B level was strongly and positively associated with the PDFF (r = 0.630, P < 0.001). With the degree of severity of MAFLD increased, the A-W-B level also increased significantly (P < 0.05).
Figure 6.
Correlation between A-W-B levels with liver proton density fat fraction and the severity of metabolic-associated fatty liver disease. A: The prediction model combining atherogenic index of plasma, waist circumference, and body mass index (A-W-B) level was strongly and positively associated with liver proton density fat fraction (PDFF) (r = 0.630, P < 0.001); B: Compared with the control group, bP < 0.001; compared with the mild group, aP < 0.01. MAFLD: Metabolic-associated fatty liver disease.
DISCUSSION
In this study, the relationship between AIP and MAFLD was systematically assessed, and it was confirmed that AIP had a strong and positive association with the risk of MAFLD and can be used as a reference predictor for MAFLD. Then, to further improve the predictive power for MAFLD, we combined AIP, WC, and BMI to pioneer a novel noninvasive prediction model, A-W-B, and confirmed that it had a better predictive value for MAFLD than AIP, WC, and BMI. At the same time, we also pointed out the optimal cutoff values of the A-W-B model to predict MAFLD in males and females, which will facilitate early clinical identification of MAFLD in different sex populations. Finally, we internally validated the model with MRI as the diagnostic standard, further affirming its outstanding predictive performance for MAFLD, which provided a new tool for the early prevention and screening of MAFLD.
As a new type of body fat index calculated as the logarithmic transformation of the ratio of TG to HDL-C, AIP is more sensitive to visceral fat accumulation than WC and BMI and has shown predictive potential for fatty liver in previous studies[15]. Xie et al[17] found that a higher AIP level was positively associated with fatty liver, which might be a novel and strong predictor associated with fatty liver in the Chinese Han population. In this study, the multivariate logistic regression results showed that the subjects with higher AIP still exhibited a significantly increased risk of MAFLD after adjusting for age, sex, smoking history, drinking history, WC, BMI, and various physical and chemical indicators, indicating that AIP was strongly and positively associated with the risk of MAFLD.
Visceral fat accumulation has been proven to increase the prevalence of a variety of cardiovascular risk factors, including insulin resistance and dyslipidemia, which also play a tremendously crucial role in the pathogenesis of fatty liver disease[16]. It is widely accepted that the increased TG caused by liver lipid accumulation is a prerequisite for MAFLD and that insulin resistance is a key factor during its development. Previous studies demonstrated that increasing levels of TG and decreasing concentrations of HDL-C could reduce sensitivity to insulin, and higher TG/HDL-C usually indicates insulin resistance[22], which may explain the close relationship between AIP and MAFLD. In addition, this study also pointed out the optimal cutoff values of AIP for predicting MAFLD in males and females, which provided a new idea for the early prevention of MAFLD. People with AIP levels above this cutoff point may be at higher risk for MAFLD and require more attention to liver conditions.
Xie et al[17] indicated that the ORs of AIP on the risk of fatty liver disease in women and young adults increased faster. This study showed that the AIP had a better predictive ability for MAFLD in young subjects, which may be related to the higher excessive fat accumulation of young people caused by dietary irregularities and insufficient exercise. However, there was no significant difference between males and females. Wang et al[23] and Dong et al[24] studied the predictive value of AIP for nonalcoholic fatty liver disease in obese and nonobese populations separately, with AUCs of 0.718 and 0.803, respectively. It seems that the predictive ability of AIP in the nonobese population might be better, but no studies have directly compared the ability of AIP in identifying MAFLD between different weights. Fortunately, our study filled this gap, and confirmed that the AIP had a remarkably higher predictive ability for MAFLD in nonobese subjects. A cohort study demonstrated that visceral obesity was dose-dependently associated with nonalcoholic fatty liver disease[25]. It is worth noting that lean people with unhealthy metabolism may have a greater accumulation of visceral fat[26], and nonobese MAFLD patients with unhealthy metabolism usually exhibit higher liver damage and cardiovascular risks[27]. AIP, as a sensitive indicator that reflects the accumulation of visceral fat, might have a stronger association with nonobese MAFLD patients and thus might have a better predictive ability for MAFLD in nonobese populations.
WC and BMI are common indicators for obesity evaluation and have been proven to be good predictors of fatty liver[17]. This study confirmed that they also had good predictive ability for MAFLD, with no significant difference compared to AIP. However, no previous study has assessed the predictive power of AIP combined with WC and BMI for MAFLD. Therefore, another focus of this study was to explore whether AIP combined with WC and BMI can improve the ability to identify MAFLD. The results showed that the logistic regression prediction Model A-W-B established by AIP combined with WC and BMI had excellent calibration and discrimination for MAFLD and had a significantly better ability to identify MAFLD. At the same time, it also had an outstanding ability to identify MAFLD in the validation, further affirming its outstanding predictive performance for MAFLD. Interestingly, we also found that the level of A-W-B was positively correlated with the liver fat content and the degree of severity of MAFLD in the validation, which may be objective evidence explaining the positive association and excellent predictive ability of the A-W-B model for MAFLD.
The Spearman’s correlation analysis showed that AIP, WC, and BMI were all positively associated with SBP, DBP, ALT, AST, FBG, and SUA. Notably, these physical and chemical indicators were also risk factors for MAFLD, and compared with AIP, WC, and BMI, the A-W-B model had a higher correlation with SBP, DBP, ALT, AST, FBG, and SUA, which may indirectly explain the better correlation and predictive ability of the A-W-B model for MAFLD. Furthermore, this study also pointed out that the optimal cutoff values of the A-W-B model to predict MAFLD in males and females were 0.5932 and 0.4105, respectively, which will facilitate early clinical identification of MAFLD in different sex populations. When the A-W-B level of the subject is above the cutoff point, it can be preliminarily identified as MAFLD.
In summary, compared with other studies, this study has the following advantages. First, this study confirmed that AIP was strongly and positively associated with the risk of MAFLD, and it can be a reference predictor for MAFLD and determined the optimal cutoff values of AIP for predicting MAFLD in males and females, providing a new idea for early prevention of MAFLD. Then, we pioneered a novel noninvasive prediction model A-W-B combining AIP, WC, and BMI that can significantly improve the predictive ability for MAFLD and determined its optimal cutoff values of the A-W-B model to predict MAFLD in males and females. Furthermore, we also validated the model with MRI as the diagnostic standard, further affirming its outstanding predictive performance for MAFLD. This study is highly innovative, and this noninvasive prediction model A-W-B is convenient, affordable, and easy to obtain, which can provide better services for clinical prediction and screening of MAFLD and metabolic-related diseases.
However, there are still some limitations. First, to avoid subject recall bias, this study did not collect confounding factors, such as specific dietary structure and physical activity, and mainly focused on objective laboratory indicators and basic demographic indicators, which may slightly affect the results of multiple logistic regression analysis. Second, the subjects in this study were limited to a single physical examination center, which may cause selection bias. Third, fatty liver in the training set of this study was diagnosed by abdominal ultrasonography, and we did not use ultrasonography to accurately classify the severity of MAFLD. Therefore, the relationship between AIP, A-W-B model, and the severity of fatty liver by ultrasonography was unclear. Notably, we found that the A-W-B levels were positively correlated with MRI-diagnosed MAFLD severity in the validation set. However, due to limited funds, the number of validation sets using MRI as the diagnostic standard was relatively small. Therefore, further multicenter, large-sample prospective cohort studies are needed in the future to verify and explore the predictive value of AIP and A-W-B for MAFLD and differential severity.
CONCLUSION
AIP was strongly and positively associated with MAFLD, and it can be a reference predictor for MAFLD. The novel noninvasive prediction model A-W-B combining AIP, WC, and BMI can significantly improve the predictive ability for MAFLD and provide better services for clinical prediction and screening of MAFLD.
ARTICLE HIGHLIGHTS
Research background
Metabolic-associated fatty liver disease (MAFLD) is the most common chronic liver disease and poses great harm to people’s health. Early identification of MAFLD is imminent.
Research motivation
Atherogenic index of plasma (AIP) is a reference predictor of obesity-related diseases, but its predictive value for MAFLD remains unclear. No studies have reported whether its combination with waist circumference (WC) and body mass index (BMI) can improve the predictive performance for MAFLD.
Research objectives
This study had two main objectives: (1) To systematically explore the relationship between AIP and MAFLD and evaluate its predictive value for MAFLD; and (2) To pioneer a novel prediction model combining AIP, WC, and BMI and validate its predictive performance for MAFLD.
Research methods
This cross-sectional study consecutively enrolled 864 participants. Multivariate logistic regression analysis and receiver operating characteristic curve were used to evaluate the relationship between AIP and MAFLD and its predictive power for MAFLD. The novel prediction model A-W-B combining AIP, WC, and BMI to predict MAFLD was established, and internal verification was completed by magnetic resonance imaging diagnosis.
Research results
Subjects with higher AIP exhibited a significantly increased risk of MAFLD, with an odds ratio of 12.420 (6.008-25.675) for AIP after adjusting for various confounding factors. The area under receiver operating characteristic curve of the A-W-B model was 0.833 (0.807-0.858), which was significantly higher than that of AIP, WC, and BMI (all P <0.05). The best cutoff values for the A-W-B model to predict MAFLD in males and females were 0.5932 and 0.4105, respectively. Additionally, in the validation set the area under receiver operating characteristic curve of A-W-B model to predict MAFLD was 0.862 (0.791-0.916). The A-W-B level was strongly and positively associated with the liver proton density fat fraction (r = 0.630, P < 0.001) and significantly increased with the severity of MAFLD (P < 0.05).
Research conclusions
AIP was strongly and positively associated with MAFLD and can be a reference predictor for MAFLD. The novel noninvasive prediction model A-W-B combining AIP, WC, and BMI can significantly improve the predictive ability for MAFLD and provide better services for clinical prediction and screening of MAFLD.
Research perspectives
Studies that may be conducted in the future should further explore the predictive value of AIP and the A-W-B model for different severities of MAFLD and other related metabolic diseases.
ACKNOWLEDGEMENTS
We thank all staff for helping recruit the subjects. We thank English language expert Liang JT for the English language revision.
Footnotes
Institutional review board statement: This study was approved by the Clinical Research Ethics Committee of China-Japan Friendship Hospital (2018-110-K79-1).
Informed consent statement: All study participants provided informed written consent prior to study enrollment.
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
STROBE statement: The authors have read the STROBE Statement checklist of items, and the manuscript was prepared and revised according to the STROBE Statement checklist of items.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: June 26, 2022
First decision: August 1, 2022
Article in press: September 8, 2022
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cumhur Cure M, Turkey; Lin WR, Taiwan S-Editor: Gong ZM L-Editor: Filipodia P-Editor: Gong ZM
Contributor Information
Shao-Jie Duan, Graduate School, Beijing University of Chinese Medicine, Beijing 100029, China.
Zhi-Ying Ren, Graduate School, Beijing University of Chinese Medicine, Beijing 100029, China.
Tao Zheng, Graduate School, Beijing University of Chinese Medicine, Beijing 100029, China.
Hong-Ye Peng, Graduate School, Beijing University of Chinese Medicine, Beijing 100029, China.
Zuo-Hu Niu, Graduate School, Beijing University of Chinese Medicine, Beijing 100029, China.
Hui Xia, Graduate School, Beijing University of Chinese Medicine, Beijing 100029, China.
Jia-Liang Chen, Center of Integrative Medicine, Beijing Ditan Hospital, Capital Medical University, Beijing 100015, China.
Yuan-Chen Zhou, Graduate school, Peking University China-Japan Friendship School of Clinical Medicine, Beijing 100029, China.
Rong-Rui Wang, Graduate School, Beijing University of Chinese Medicine, Beijing 100029, China.
Shu-Kun Yao, Department of Gastroenterology, China-Japan Friendship Hospital, Beijing 100029, China. shukunyao@126.com.
Data sharing statement
No additional data are available.
References
- 1.Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol. 2013;10:686–690. doi: 10.1038/nrgastro.2013.171. [DOI] [PubMed] [Google Scholar]
- 2.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 3.Caballero B. Humans against Obesity: Who Will Win? Adv Nutr. 2019;10:S4–S9. doi: 10.1093/advances/nmy055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ogden CL, Fakhouri TH, Carroll MD, Hales CM, Fryar CD, Li X, Freedman DS. Prevalence of Obesity Among Adults, by Household Income and Education - United States, 2011-2014. MMWR Morb Mortal Wkly Rep. 2017;66:1369–1373. doi: 10.15585/mmwr.mm6650a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sarin SK, Kumar M, Eslam M, George J, Al Mahtab M, Akbar SMF, Jia J, Tian Q, Aggarwal R, Muljono DH, Omata M, Ooka Y, Han KH, Lee HW, Jafri W, Butt AS, Chong CH, Lim SG, Pwu RF, Chen DS. Liver diseases in the Asia-Pacific region: a Lancet Gastroenterology & Hepatology Commission. Lancet Gastroenterol Hepatol. 2020;5:167–228. doi: 10.1016/S2468-1253(19)30342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dulai PS, Singh S, Patel J, Soni M, Prokop LJ, Younossi Z, Sebastiani G, Ekstedt M, Hagstrom H, Nasr P, Stal P, Wong VW, Kechagias S, Hultcrantz R, Loomba R. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: Systematic review and meta-analysis. Hepatology. 2017;65:1557–1565. doi: 10.1002/hep.29085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dewidar B, Kahl S, Pafili K, Roden M. Metabolic liver disease in diabetes - From mechanisms to clinical trials. Metabolism. 2020;111S:154299. doi: 10.1016/j.metabol.2020.154299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee H, Lee YH, Kim SU, Kim HC. Metabolic Dysfunction-Associated Fatty Liver Disease and Incident Cardiovascular Disease Risk: A Nationwide Cohort Study. Clin Gastroenterol Hepatol. 2021;19:2138–2147.e10. doi: 10.1016/j.cgh.2020.12.022. [DOI] [PubMed] [Google Scholar]
- 9.Stahl EP, Dhindsa DS, Lee SK, Sandesara PB, Chalasani NP, Sperling LS. Nonalcoholic Fatty Liver Disease and the Heart: JACC State-of-the-Art Review. J Am Coll Cardiol. 2019;73:948–963. doi: 10.1016/j.jacc.2018.11.050. [DOI] [PubMed] [Google Scholar]
- 10.Wong MCS, Huang JLW, George J, Huang J, Leung C, Eslam M, Chan HLY, Ng SC. The changing epidemiology of liver diseases in the Asia-Pacific region. Nat Rev Gastroenterol Hepatol. 2019;16:57–73. doi: 10.1038/s41575-018-0055-0. [DOI] [PubMed] [Google Scholar]
- 11.Ipsen DH, Lykkesfeldt J, Tveden-Nyborg P. Molecular mechanisms of hepatic lipid accumulation in non-alcoholic fatty liver disease. Cell Mol Life Sci. 2018;75:3313–3327. doi: 10.1007/s00018-018-2860-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katsiki N, Mikhailidis DP, Mantzoros CS. Non-alcoholic fatty liver disease and dyslipidemia: An update. Metabolism. 2016;65:1109–1123. doi: 10.1016/j.metabol.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 13.Eslam M, Sarin SK, Wong VW, Fan JG, Kawaguchi T, Ahn SH, Zheng MH, Shiha G, Yilmaz Y, Gani R, Alam S, Dan YY, Kao JH, Hamid S, Cua IH, Chan WK, Payawal D, Tan SS, Tanwandee T, Adams LA, Kumar M, Omata M, George J. The Asian Pacific Association for the Study of the Liver clinical practice guidelines for the diagnosis and management of metabolic associated fatty liver disease. Hepatol Int. 2020;14:889–919. doi: 10.1007/s12072-020-10094-2. [DOI] [PubMed] [Google Scholar]
- 14.Dobiásová M. Atherogenic index of plasma [log(triglycerides/HDL-cholesterol)]: theoretical and practical implications. Clin Chem. 2004;50:1113–1115. doi: 10.1373/clinchem.2004.033175. [DOI] [PubMed] [Google Scholar]
- 15.Zhu X, Yu L, Zhou H, Ma Q, Zhou X, Lei T, Hu J, Xu W, Yi N, Lei S. Atherogenic index of plasma is a novel and better biomarker associated with obesity: a population-based cross-sectional study in China. Lipids Health Dis. 2018;17:37. doi: 10.1186/s12944-018-0686-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shen SW, Lu Y, Li F, Yang CJ, Feng YB, Li HW, Yao WF, Shen ZH. Atherogenic index of plasma is an effective index for estimating abdominal obesity. Lipids Health Dis. 2018;17:11. doi: 10.1186/s12944-018-0656-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xie F, Zhou H, Wang Y. Atherogenic index of plasma is a novel and strong predictor associated with fatty liver: a cross-sectional study in the Chinese Han population. Lipids Health Dis. 2019;18:170. doi: 10.1186/s12944-019-1112-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eslam M, Sanyal AJ, George J International Consensus Panel. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology. 2020;158:1999–2014.e1. doi: 10.1053/j.gastro.2019.11.312. [DOI] [PubMed] [Google Scholar]
- 19.Shiha G, Korenjak M, Eskridge W, Casanovas T, Velez-Moller P, Högström S, Richardson B, Munoz C, Sigurðardóttir S, Coulibaly A, Milan M, Bautista F, Leung NWY, Mooney V, Obekpa S, Bech E, Polavarapu N, Hamed AE, Radiani T, Purwanto E, Bright B, Ali M, Dovia CK, McColaugh L, Koulla Y, Dufour JF, Soliman R, Eslam M. Redefining fatty liver disease: an international patient perspective. Lancet Gastroenterol Hepatol. 2021;6:73–79. doi: 10.1016/S2468-1253(20)30294-6. [DOI] [PubMed] [Google Scholar]
- 20.Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, Zelber-Sagi S, Wai-Sun Wong V, Dufour JF, Schattenberg JM, Kawaguchi T, Arrese M, Valenti L, Shiha G, Tiribelli C, Yki-Järvinen H, Fan JG, Grønbæk H, Yilmaz Y, Cortez-Pinto H, Oliveira CP, Bedossa P, Adams LA, Zheng MH, Fouad Y, Chan WK, Mendez-Sanchez N, Ahn SH, Castera L, Bugianesi E, Ratziu V, George J. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J Hepatol. 2020;73:202–209. doi: 10.1016/j.jhep.2020.03.039. [DOI] [PubMed] [Google Scholar]
- 21.Kromrey ML, Ittermann T, Berning M, Kolb C, Hoffmann RT, Lerch MM, Völzke H, Kühn JP. Accuracy of ultrasonography in the assessment of liver fat compared with MRI. Clin Radiol. 2019;74:539–546. doi: 10.1016/j.crad.2019.02.014. [DOI] [PubMed] [Google Scholar]
- 22.Li YW, Kao TW, Chang PK, Chen WL, Wu LW. Atherogenic index of plasma as predictors for metabolic syndrome, hypertension and diabetes mellitus in Taiwan citizens: a 9-year longitudinal study. Sci Rep. 2021;11:9900. doi: 10.1038/s41598-021-89307-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Q, Zheng D, Liu J, Fang L, Li Q. Atherogenic index of plasma is a novel predictor of non-alcoholic fatty liver disease in obese participants: a cross-sectional study. Lipids Health Dis. 2018;17:284. doi: 10.1186/s12944-018-0932-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dong BY, Mao YQ, Li ZY, Yu FJ. The value of the atherogenic index of plasma in non-obese people with non-alcoholic fatty liver disease: a secondary analysis based on a cross-sectional study. Lipids Health Dis. 2020;19:148. doi: 10.1186/s12944-020-01319-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim D, Chung GE, Kwak MS, Seo HB, Kang JH, Kim W, Kim YJ, Yoon JH, Lee HS, Kim CY. Body Fat Distribution and Risk of Incident and Regressed Nonalcoholic Fatty Liver Disease. Clin Gastroenterol Hepatol. 2016;14:132–8.e4. doi: 10.1016/j.cgh.2015.07.024. [DOI] [PubMed] [Google Scholar]
- 26.Stefan N, Schick F, Häring HU. Causes, Characteristics, and Consequences of Metabolically Unhealthy Normal Weight in Humans. Cell Metab. 2017;26:292–300. doi: 10.1016/j.cmet.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 27.Fracanzani AL, Petta S, Lombardi R, Pisano G, Russello M, Consonni D, Di Marco V, Cammà C, Mensi L, Dongiovanni P, Valenti L, Craxì A, Fargion S. Liver and Cardiovascular Damage in Patients With Lean Nonalcoholic Fatty Liver Disease, and Association With Visceral Obesity. Clin Gastroenterol Hepatol. 2017;15:1604–1611.e1. doi: 10.1016/j.cgh.2017.04.045. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data are available.