Abstract
The rise in environmental pollutant levels in recent years is mostly attributable to anthropogenic activities such as industrial, agricultural and other activities. Additionally, these activities may produce excessive levels of dangerous toxicants such as heavy metals, organic pollutants including pesticide and herbicide chemicals, and sewage discharges from residential and commercial sources. With a focus on environmentally friendly, sustainable technology, new technologies such as combined process of nanotechnology and bioremediation are urgently needed to accelerate the cost-effective remediation process to alleviate toxic contaminants than the conventional remediation methods. Numerous studies have shown that nanoparticles possess special qualities including improved catalysis and adsorption as well as increased reactivity. Currently, microorganisms and their extracts are being used as promising, environmentally friendly catalysts for engineered nanomaterial. In the long term, this combination of both technologies called nano-bioremediation may significantly alter the field of environmental remediation since it is more intelligent, safe, environmentally friendly, economical and green. This review provides an overview of soil and water remediation techniques as well as the use of nano-bioremediation, which is made from various living organisms. Additionally, current developments related to the mechanism, model and kinetic studies for remediation of agricultural contaminants have been discussed.
Keywords: Biosynthesis, Environmental contaminants, Nanoparticles, Persistent organic pollutants
Introduction
Agricultural pollution is a global environmental concern that is mainly caused by the function of many farming inputs (such as pesticides and fertilizers) and numerous practices, such as excessive soil tillage and runoff (El-Ramady et al. 2020). Agricultural pollutants may encompass unsustainable use of agrochemicals resulting in accumulation of heavy metals, halogenated compounds, phenols and organic compounds such as pesticides, insecticides which are one of the most pressing issues confronting agriculture around the world (Singh et al. 2020a). These agro-pollutants are extremely toxic and can have a negative impact on soils, water and air (Khan et al. 2020; Singh et al. 2020a; Ken and Sinha 2020). Organic pollutants primarily consist of phenols, chlorinated phenols, azo dyes, phthalic esters, pesticides, persistent organic pollutants (POPs), and other chemicals. Inorganic pollutants, on the other hand, include heavy metals such as arsenic, nickel, chromium, lead, mercury, and cadmium. These agro-pollutants have been linked to significant negative affects on soil and water pollution, as well as severe toxicity in living creatures (Saxena et al. 2019).
One of the most pressing challenges facing agriculture today is the need to monitor and reduce the use of agrochemicals associated with certain toxic contaminants. It is well understood that agrochemical-derived pollutants can have a negative impact on the agro-ecosystem (Mall et al. 2018). Indeed, injudicious use of excessive amount of pesticides may result in volatilization, oxidation and photolysis and only about 0.1 percent of pesticides reach the targeted species (Liang et al. 2017). Toxic pollutants have increased to alarming levels in the environment, deteriorating environmental quality, disrupting ecosystems, and negatively impacting human health (Singh et al. 2020a). As a result, developing innovative techniques capable of eliminating agro-pollutants in trace amounts is critical. Nanotechnological approaches have been rapidly used in every field of science and environmental application owing to their novel characteristics such high surface area and small nano-size in a wide range of application (Hidangmayum and Dwivedi 2021). Several remediation methods have been developed for both in situ and ex situ applications. However, nanobioemediation techniques have received much attention in recent times due to their sustainability and minimizing environmental pollutants efficiently (Rajput et al. 2021). Bioremediation, according to the United States Environmental Protection Agency, is a method that transforms harmful compounds into less dangerous or benign ones using naturally existing organisms (Singh et al. 2020b). Bioremediation can primarily be achieved by applying microorganisms to the remediation of pollutants found in water and soil (Saxena et al. 2019; Halecký and Kozliak 2020; Bhojiya et al. 2021). It is worth mentioning that various modern bioremediation approaches, such as the application of biosurfactants, emulsifiers, enzymes, biopesticides, and Genetically Modified Organisms (GMOs), could be used (Halecký and Kozliak 2020). Combining nanotechnology and bioremediation could increase the efficiency, time efficiency and environmental friendliness of remediation, increasing benefits (El-Ramady et al. 2020; Ghani et al. 2022). Nano-bioremediation (NBR) is one of these methods that have attracted the attention of many scientists in recent years due to its unique combination of nanomaterials and bioremediation. NBR is the process of removing environmental pollutants from contaminated areas by utilizing nanoparticles derived from prokaryotes (Gram-negative rods, actinobacteria) and eukaryotes (fungi, algae, and plants) with the aid of nanotechnology. (Mallikarjuna et al. 2011; Rajput et al. 2021). For instance, dechlorination and biodegradation of biphenyls was reported using zinc nanoparticle and the bacterium, Burkholderia xenovorans (Le et al. 2015). Also, nanomaterials derived from plants, for example, Noaea mucronata, were applied for the bioremediation of heavy metal contaminants from rivers, streams and groundwater (Mohsenzadeh and Rad 2012).
Regarding the benefits of NBR, nanotechnology and bioremediation are integrated for a number of reasons. Since NPs have a large surface area per unit mass, a greater number of particles can be introduced into the environment, increasing remediation (Kaur et al. 2018). In this way, NBR works to reduce secondary environmental impacts while minimizing pollutant concentrations. Furthermore, nanotechnology and bioremediation are combined with each other to create an improved, faster, and more environmentally benign remediation method (Kumar et al. 2021).
There are, however, specific merits as well as demerits associated with each advancement in the remediation process. As a result of the extensive literature survey, it can be concluded that integrating bioremediation and nanotechnology would be a viable alternative to conventional remediation technologies. Nevertheless, more research and development are needed to bring these types of sustainable technology to the market for full implementation. This current review paper makes an effort to integrate and summarise findings on the application of nano-bioremediation to alleviate the environmental agro-pollutants. Furthermore, it includes an in-depth discussion of the synthesis and mechanism/kinetics model, interaction with nanomaterials with other microorganisms, as well as their role in environmental remediation.
Mechanism and action of nano-bioremediation in soil
Nano-bioremediation is the process of employing nanoparticles/nanomaterial generated by plants, fungus and bacteria to remove environmental toxins (such as organic and inorganic pollutants) from polluted locations with the aid of nanotechnology. Apart from the many methods available for contaminated site remediation (such as chemical and physical remediation), bioremediation now provides an ecologically acceptable and cost-effective option for removing toxins from the environment (Yadav et al. 2017). Bioremediation encompasses bioaccumulation, biotransformation, biosorption, and biological stabilization, among others with the use of microbes, plants, and enzymes or combinations of them (Fernández et al. 2018). In one technique, nanoparticles can be used to treat soils contaminated with pollutants, while in another, they can be employed by combining phytoremediation with enzyme-based bioremediation (Sunanda et al. 2021). This challenge might be overcome by merging nanotechnology and biotechnology, in which nano-encapsulated enzymes transform complex organic molecules into simpler ones, which are subsequently quickly eliminated by bacteria and plants working together (Prasad et al. 2017). Figure 1 shows aspects of nano-bioremediation in agriculture soil in terms of applications. In case of agricultural soil, it works as remedial approach to remove inorganic, organic, and emerging pollutants. The same way it helps in soil health, growth of plants and also works to improve the degraded land. Bacteria have the ability to mobilize and immobilize metals, and in some cases, the microorganism that can decrease metal ions may also precipitate metals at nanoscale size. Bacteria are being investigated as a latent "biofactory" for the production of nanoparticles such as gold, silver, iron, zero valent iron, platinum, palladium, titanium dioxide, cadmium sulfide, gold nanowire, titanium, selenium, magnetite, and zinc sulfide (Yadav et al. 2017; Ramezani et al. 2021). The utilization of microorganisms can act as catalyst because of their enzyme present for specific reaction to inorganic nanoparticle which is a new coherent biosynthesis approach for production of nanoparticles, which comprises application of polysaccharides, biodegradable polymers, microorganisms, enzymes, microbial enzymes, vitamins, and organic structures (Ramezani et al. 2021).
Fig. 1.
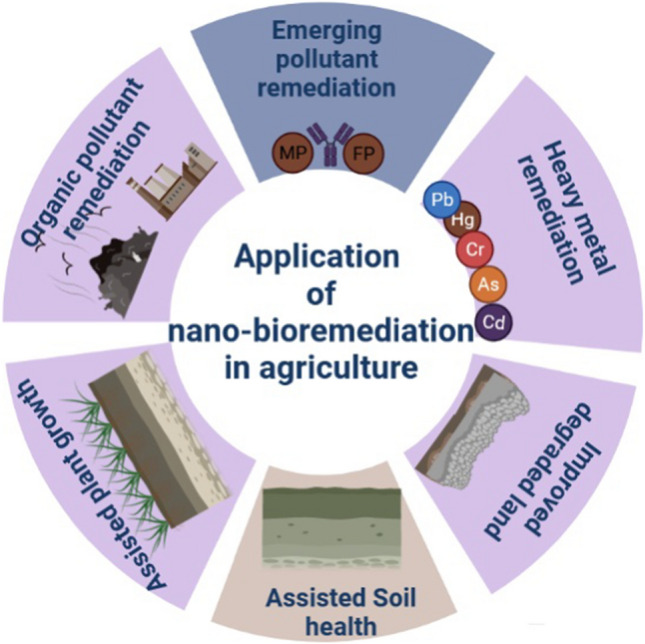
Application of nano-bioremediation in agriculture soil
Preparation of nanomaterial
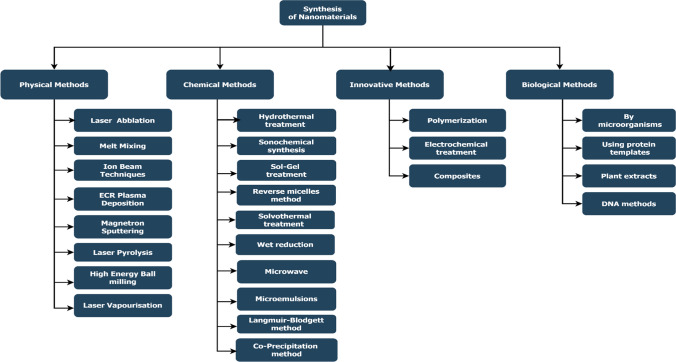
Only physical and chemical processes were previously used to create nanoparticles. In the search for more cost-effective and sustainable production of nanoparticle, microorganism and plants extracts were utilized. Table 1 represents the synthesis methods or procedures of different types of nanomaterial with suitable examples. Synthesis of nanomaterials from different methods of preparation is shown in Fig. 2. The biogenesis of nanoparticles follow a bottom-up process involving the primary reaction of reduction/oxidation. The reduction in metal compounds into their respective nanoparticles is usually mediated by microbial enzymes or phytochemicals with antioxidant or reducing properties. Nanomaterials are being produced in the millions of tons throughout the world, and this number is likely to skyrocket in the near future. The word "nanomaterial" refers to specially designed materials with at least one dimension between 1 and 100 nm (European Commission, 2011). Microorganisms such as bacteria, filamentous fungi, yeasts, algae, and actinobacteria can be employed for synthesis of biogenic nanoparticle in addition to vascular plants.
Table 1.
Synthesis of nanomaterial with different methods
| Nanomaterials | Methods | Metals/metalloids/composites/microorganisms/polymers | References |
|---|---|---|---|
| Metal nanoparticles (NPs) | Photochemical | Pt, Rh, Pd, Ir, Ag, Au, Cu, Co, Ni, FeNi, Cu3Au, CoNi, CdTe, CdSe, ZnS | Das and Ansari (2009), Koul and Taak (2018) |
| Electrochemical | |||
| Biochemical | |||
| Thermochemical | |||
| Carbon NMs | Arc-discharge | Cylindrical nanotube | Rizwan et al. (2014), Prasad et al. (2017), Singh et al. (2020a, b) |
| Laser ablation | |||
| Chemical vapor deposition | |||
| Metal oxide NPs | Sol–gel | ZnO, Fe2O3, Fe3O4, MgO, BaCO3, BaSO4, TiO2 | Newkome et al. (1985), Chauhan et al. (2012), Koul and Taak (2018) |
| Hydrothermal | |||
| Reverse micelles method | |||
| Solvothermal | |||
| Electrochemical deposition | |||
| Polymer NMs | Electrochemical | Nanowire of polypyrrole, polyaniline, poly (3,4-ethylenedioxythiophane) dendrimers (PAMAM) | Rizwan et al. (2014), Prasad et al. (2017), K and V (2017) |
| Polymerization | |||
| Nanocomposite | Innovative | CNTs (fluoropolymers polycarbonates, polyethylene, glycol, polyester polyamides); epoxy composites | Prasad et al. (2017), Yadav et al. (2017), Benjamin et al. (2019), Singh et al. (2020a, b), Vázquez-Núñez et al. (2020) |
| Bio-nanomaterials | Biological | Viruses, plasmids, and protein NPs | Koul and Taak (2018), Bahrulolum et al. (2021) |
Fig. 2.
Methods for synthesizing nanomaterials (Rizwan et al. 2014; Koul and Taak 2018; Singh et al. 2020b)
Botanical synthesis
Biosynthesis of nanoparticles by plants namely Azadirachta indica, Catharanthus roseus, Aloe barbadensis, etc., is gaining wide acceptance due to the single-step biosynthesis process, the unavailability of toxic elements, the presence of natural capping agents, and the presence of a wide variety of metabolites that may assist in reduction. Plants require a shorter incubation period for the reduction in metal ions than fungi and bacteria; hence, they are regarded superior candidates for nanoparticle synthesis (Table 2). In case of synthesis on industrial scale, plant tissue culture and downstream processing techniques generally used, though it depends on the metabolic status of the plant (Mura et al. 2013). Though effect of nanoparticles on contaminants diverges from plant to plant, but it depends on the physiological, biochemical, molecular mechanisms of the plant, and their interactions with contaminants and living organisms (Yadav et al. 2017).
Table 2.
Biosynthesis of nanomaterials with plants, bacteria, yeast, and filamentous fungi
| Nanomaterials | Plant | Bacteria | Yeast and filamentous fungi | References |
|---|---|---|---|---|
| Gold and silver | Hibiscus rosasinensis, Coriandrum sativum, Emblica officinalis, Diopyros kaki (persimmon), Citrus sinensis, Pelargonium graveolens, Phyllanthium Mushroom extract | Verticillium sp.,Fusarium oxysporum | Shankar et al. (2003b, 2004), Ankamwar et al. (2005), Narayanan and Sakthivel (2008), Song and Kim (2008), Kasthuri et al. (2009), Philip (2009), Popescu et al. (2010), Song et al. (2010) | |
| Silicon-geranium | Stauroneis sp. Freshwater diatom | MubarakAli et al. (2011) | ||
| Silver, nickel, cobalt, zinc and copper | Helianthus annuus, Medicago sativa, Brassica mjuncea | Streptomyces griseus, Endophytic actinomycetes Ca-1 | Fusarium culmorum, Alternaria alternate, Pleurotus sajor-caju, Trichoderma harzianum, Volvariella volvaceae | Bali et al. (2006), Bharde et al. (2006), Gajbhiye et al. (2009), Philip (2009), Ponmurugan et al. (2016), Hassan et al. (2018), Chaturvedi et al. (2020) |
| Gold | Syzygium aromaticum L., Medicago sativa, Camellia sinensis L., Chenopodium album L., Magnolia kobus, Juniperus communis (Zimbora tea), banana peel, Azadirachta indica, Mentha piperita L., Allium cepa L., Justicia gendarussa L., Sesbania grandiflora L., Mirabilis jalapa L., Macrotyloma uniflorum, Cinnamomum zeylanicum, Terminalia catappa, Terminalia catappa L., Dyopiros kaki., Eucommia ulmoides, Mucuna pruriens, Amaranthus spinosus | Pseudomonas aeruginosa, Yarrowia lipolytica NCIM3589 Thermomonospora sp., Actinobacter spp., Bacillus subtilis, Escherichia coli k12, Lacticaseibacillus casei (strain JCM1134), Haloferax volcanii, Deinococcus radiodurans, Lactobacillus strain, Alkalothermophilic actinomycete | Gardea-Torresdey et al. (2002), Sastry et al. (2003), Ankamwar et al. (2005), Bharde et al. (2007), Sintubin et al. (2009), Smitha et al. (2009), Song et al. (2009), Pimprikar et al. (2009), Ankamwar (2010), Raghunandan et al. (2010), Thirumurugan et al. (2010), Vankar and Bajpai (2010), Arulkumar and Sabesan (2010), Bankar et al. (2010), Mubarak Ali et al. (2011), Parida et al. (2011), Dwivedi and Gopal (2011), Fazaludeen et al. (2012), Konwar Boruah et al. (2012), Aromal et al. (2012), Das et al. (2012), Das and Velusamy (2014), Guo et al. (2015), Geraldes et al. (2016), Kikuchi et al. (2016), Li et al. (2016), Markus et al. (2016), Wadhwani et al. (2016), Nadhe et al. (2020), Tariq et al. (2020), Costa et al. (2020), Wang et al. (2020) | |
| Silver | Elettaria cardamomom, Nerium indicum, Ficus indica, Parthenium hysterophorus, Clerodendrum serratum, Pelargoneum graveolens, Azadirachta indica, Ocimum sp., Desmodium triflorum, Pterocarpus santalinus, Brassica juncea, Clerodendrum inerme, Pongamia pinnata, Euphorbia hirta, Rumex hymenosepalus, Grewia flaviscences, Carica papaya (fruit), Gliricidia sepium, Aloe vera extract, Capsicum annum, Coriandrum sativum, Tephrosia tinctorial, Avicennia marina, Ceriops tagal, Rhizophora mucronata, Opuntia, Sonchus asper | Bacillus cereus, B. subtilis, Oscillatory willei NTDMO1, Pseudomonis stuzeri, Corynebacterium, Escherichia coli, B. thuringiensis Ureibacillus thermosphaericus, Lactobacillus strain, Staphylococcus aureus L. acidophilus, L. acidophilus 58p, Brevibacterium casei, Bacillus licheniformis Dahb1, Pseudomonas deceptionensis DC5, Pseudomonas fluorescens CA 417, Sporosarcina koreensis DC4, Pseudomonas rhodesiae, Pseudomonas poae CO, Streptomyces capillispiralis Ca-1, Haloalkaliphilic Streptomyces sp. | Yeast strains MKY3, Coriolus versicolor, Phaenerochaete chrysosporium, Cladosporium cladosporioides, Aspergillus flavus, Fusarium semitectum, F. oxysporum, Extremophillic yeast, Pleurotus sajor-caju, Aspergillus niger, A. oryzae, Trichoderma viride, Fusarium solani, P. nagiovense AJ12 | Dameron et al. (1989), Klaus et al. (1999), Joerger et al. (2000), Klaus-Joerger et al. (2001), Ahmad et al. (2003), (2011), Shankar et al. (2003a, b), Kowshik et al. (2003), Zhang et al. (2005), Vigneshwaran et al. (2006), (2007), Chandran et al. (2006), Li et al. (2007), Basavaraja et al. (2008), Mohanpuria et al. (2008), Gade et al. (2008), (2010), Ganesh Babu and Gunasekaran (2009), Gurunathan et al. (2009), Ingle et al. (2009), Balaji et al. (2009), Jain et al. (2009, 2010), Nanda and Saravanan (2009), Nithya and Ragunathan (2009), Parashar et al. (2009), Pugazhenthiran et al. (2009), Saifuddin et al. (2009), Sanghi and Verma (2009), Shekhawat and Arya (2009), Sintubin et al. (2009), Kalishwaralal et al. (2010), Binupriya et al. (2010), Philip (2010), Sathyavathi et al. (2010), Thakkar et al. (2010), Thirumurugan et al. (2010), Tripathy et al. (2010), Raut et al. (2010), Juibari et al. (2011), Mallikarjuna et al. (2011), Mano Priya et al. (2011), Mourato et al. (2011), Mubarak Ali et al. (2011), George et al. (2012), Gnanadesigan et al. (2012), Gnanajobitha et al. (2012), Selvi and Sivakumar (2012), Sunkar and Nachiyar (2012), Umashankari et al. (2012), Gopinath et al. (2013), Priya Dhas et al. (2013), Rodríguez-León et al. (2013), Verma et al. (2013), Maliszewska et al. (2014), Priyadharshini Raman et al. (2015), Rajaram et al. (2015), Sana et al. (2015), Garmasheva et al. (2016), Jo et al. (2016), Shanthi et al. (2016), Singh et al. (2016), Hossain et al. (2019), Ibrahim et al. (2020), Fouda et al. (2020), Marathe et al. (2021) |
| Gold nanowires | Rhodopseudomonas capsulate | He et al. (2008) | ||
| Palladium | Cinnamomum camphora L., Gardenia jasminoides Ellis., Glycine Max L., Chlorella vulgaris, Origanum vulgare L., Anogeissus latifolia, Cinnamomum zeylanicum Blume | Desulfovibrio desulfuricans NCIMB 8307 | Nair and Pradeep (2002), Jia et al. (2009), Sathishkumar et al. (2009), Yang et al. (2010), Kumar Petla et al. (2012), Arsiya et al. (2017), Rafi Shaik et al. (2017), Kora and Rastogi (2018) | |
| As-S nanotubes | Shewanella sp. | Jiang et al. (2009) | ||
| ZnS | Sulfate reducing Bacteria, Desulfobacteriaceae | Labrenz et al. (2000), Mandal et al. (2006), Murugadoss et al. (2009), Sharma et al. (2009a, b), Popescu et al. (2010), Chauhan et al. (2012) | ||
| Iron, Iron oxide | Psidium guajava, Vaccinium corymbosum, Eichhornia crassipes, L. Alston, Ramalina sinensis, Vaccinium Floribundum, Aloe vera, Mentha spicata L., | Shewanella oneidensis, G. sulfurreducens, Geobacter sp., | Perez-Gonzalez et al. (2010), Iwahori et al. (2014), Prasad et al. (2014), Watts et al. (2015), Xiao et al. (2016), Mukherjee et al. (2016), Wei et al. (2017), Manquián-Cerda et al. (2017), Abril et al. (2018), Kamath et al. (2020), Arjaghi et al. (2021, Shukla et al. (2022) | |
| nZVI, nZVAg | Eucalyptus globules, Ficus benjamina, Oak, mulberry | Pseudomonas, E. Coli, B. Subtillis, B. Subtilis-niger, P. Fluorences, Aspergillus versicolor, B. Cereus, Sphingomonas sp. | Aspergillus versicolor | Diao and Yao (2009), Chen et al. (2011), Kim et al. (2012), Fajardo et al. (2013), Madhavi et al. (2013), Chaithawiwat et al. (2016), Poguberović et al. (2016), Al-Qahtani (2017), Slijepčević et al. (2021) |
| CdS | Klebsiella aerogens, Clostridicum thermoaceticum, Escherichia coli, Lactobacillus acidophilus DSMZ 20079 T, Shewanella oneidensis MR -1, Pseudomonas aeruginosa JP-11, Rhodopseudomonas palustris | Schizosaccharomyce pombe, Candida glabrata, Fusarium oxysporum, | Dameron et al. (1989), Holmes et al. (1995), Ahmad et al. (2002), Sweeney et al. (2004), Mandal et al. (2006), Bai et al. (2009), Raj et al. (2016), Xiao et al. (2017), Riaz et al. (2020) | |
| PbS | Rhodospiridium Dibovatum, Torilopsis sp. | Kowshik et al. (2003), Seshadri et al. (2011) | ||
| Selenium | Anaerobic granular sludge, Citrobacter freundii Y9, Shewanella loihica PV‐4 | Jain et al. (2015, 2016a, b), Wang et al. (2017, 2018) | ||
| Platinum | Quercus glauca, Azadirachta indica, Diopyros kaki, Ocimum sanctum L | Song et al. (2010), Soundarrajan et al. (2012), Karthik et al. (2016), Thirumurugan et al. (2016) | ||
| CdS quantum dots | Schizosacharomyces pombe | Kowshik et al. (2003) | ||
| Stable silver | A. terreus, A. furnigatus, A. nidulans Aspergillus flavus | Bhainsa and D’Souza (2006), Neveen (2013) | ||
| Magnetite | Aloe vera | Magnetosirillium magneticum, Sulfate reducing bacteria | Gericke and Pinches (2006), Mohanpuria et al. (2008), Phumying et al. (2013) | |
| Bioactive | Lichen fungi (Usneea longissimia) | Shahi and Patra (2003) |
Bacterial synthesis
Bacteria are being investigated as a latent "biofactory" for the production of nanoparticles such as gold (Au), silver (Ag), iron (Fe), zero valent iron (ZVI), platinum (Pt), palladium (Pd), gold (Au) nanowire, titanium (Ti), titanium dioxide (TiO2), selenium (Se), magnetite {Fe2+(Fe3+)2(O2−)4}, cadmium sulfide (CdS), zinc sulfide (ZnS), and they can immobilize metals at the nanometer scale (Chauhan et al. 2012; Phumying et al. 2013; Ramezani et al. 2021; Wang et al. 2018; Xiao et al. 2017). It uses bacteria as a source of enzymes, microbial enzymes, vitamins, and polysaccharides for the formation of nanoparticles, and it follows an innovative rational biosynthesis approach that does not involve any toxic, harmful, or expensive chemical for stabilizing operations (Benjamin et al. 2019). Although it is devoid of other cellular proteins, the NPs produced by enzyme secretion have an edge in metal binding capacities, making them valuable in nano-bioremediation. The production of nanoparticles is influenced by a number of parameters, including microbe growth circumstances, chemical composition, cellular activity, and enzymatic activities. More in-depth research is needed to fully comprehend the chemical pathways and identify the enzymes and proteins involved in biosynthesis of nanoparticles.
Fungal synthesis
Yeast and filamentous fungi have long been utilized for nanoparticle synthesis, and they are a great source of extracellular enzymes that influence nanoparticle synthesis. In case of production of larger amounts of nanoparticles, filamentous fungi could be employed as a source since it secretes higher volume of proteins which translates into better nanoparticle production efficiency (Koul and Taak 2018). One of the key disadvantages of employing filamentous fungi is that it produces nanoparticles at a significantly slower rate than plant synthesis, owing to the creation of enzymes that catalytically decrease salt metallic solid nanoparticles. In terms of nanoparticle synthesis, filamentous fungi offer an advantage over other biological systems owing to an environmentally acceptable approach with a large variety of species, simple culture methods, reduced time, and increased cost-effectiveness (Sunanda et al. 2021). Yeast strains also have certain advantages over bacteria in terms of synthesis, such as quick growth with the use of basic nutrients and enzymes, and the use of yeast in the manufacture of metallic nanoparticles is being studied. Table 2 contains a list of diverse plants, bacteria, yeast and filamentous fungi that are employed in the processing and manufacture of nanomaterials.
Kinetics and models for nano-bioremediation of contaminants
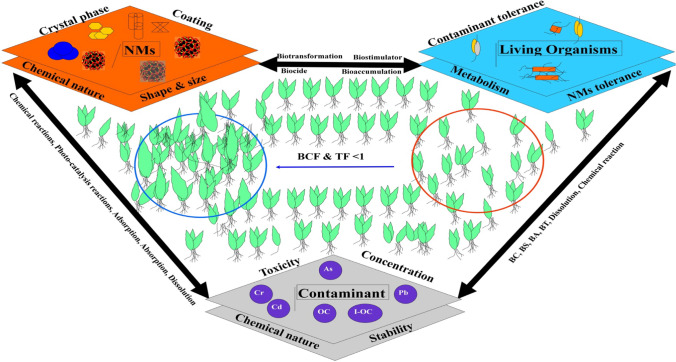
A proper interaction between nanomaterials (NMs), living organisms and contaminants takes place during whole nano-bioremediation process. A set of parameters influence on each of the component which have physical, chemical, and biochemical interactions among them (Fig. 3) (Taylor et al. 2012; Tang et al. 2016; Tan et al. 2018; Vázquez-Núñez et al. 2020). In this complex interaction phenomena nanomaterial depends on size and shape, surface coating of material, and its chemical nature; contaminants depends upon its concentration in soil, level of toxicity, and its compounds stability. Due to nanomaterial and contaminant interactions from both side, living organisms undergo biocidal (BC) or biostimulation (BS) effect, which impacts its remedial performance in bioremediation process. Sorption is a critical step in the nano-bioremediation process. Sorption is the process of a pollutant interacting with a sorbent at the surface, and the pollutant then penetrating deeper layers of the sorbent to produce a solution (Vieira and Volesky 2000). To understand the nature of the adsorption processes employing NMs, a substantial amount of studies have been conducted (Hu et al. 2006; Wang et al. 2011; Sebeia et al. 2020). On other side, contaminants get adsorbed through adsorption process with the influence of temperature, pH, media. Mechanistic, thermodynamic and kinetic investigations are, therefore, required to describe the behavior of the nanomaterial when it comes into contact with contaminants. The Langmuir, Freundlich and Temkin isotherms, as well as the Dubinin–Radushkevich models, are some of the models that describe the nature of involving the biological material in remediation processes (1–12) (A.O.D 2012; Olalekan et al. 2013; Dada et al. 2013; Pathak et al. 2015; Matouq et al. 2015).
Fig. 3.
Schematic diagram depicting the nano-bioremediation of contaminants (Taylor et al. 2012; Tang et al. 2016; Tan et al. 2018; Vázquez-Núñez et al. 2020); BCF (Bioconcentration factor), TF (Translocation factor)
According to the Langmuir isotherm, a monolayer adsorption occurs on a uniform surface that has a finite number of adsorption sites, and the adsorbate cannot transmigrate along the surface plane. This isotherm is presented by Eq. (1) (Langmuir 1916, 1918)
| 1 |
The linear model is expression as (Eq. 2):
| 2 |
where qe—quantity of contaminants absorbed (mg/g), Ce—equilibrium liquid-concentration (mg/g), b0—Langmuir isotherm constant (L/mg), qm—maximum adsorption capacity (mg/g).
Langmuir model can also be used as equilibrium parameter or separation factor “RL” for interpreting the adsorption behavior of the nanoparticles system (Eq. 3) (Weber and Chakravorti 1974).
| 3 |
where Co is the initial concentration of adsorbate (mg/L). The irreversibility (RL = 0), linearity (RL = 1) and viability (0 < RL < 1) of the sorption process is determined by the values of RL.
A Freindlich isotherm achieves multilayer coverage of an adsorbent by means of transmigration interaction. It suggests that model is applied to the absorption characteristics on heterogeneous surfaces. This isotherm is presented by the following empirical equation as follows (Eq. 4) (Freundlich 1906).
| 4 |
where KF and n are the freundlich constant and heterogeneity factor, respectively.
Linearized illustration of the isotherm provided as (Eq. 5):
| 5 |
Temkin model assumes that the heat energy of sorption process decreases with the coverage of the adsorbent surface and its interaction with adsorbate molecules. This isotherm equation and linear form of the model expressed in following Eqs. (Eqs. 6, 7) (Temkin and Pyzhev 1940):
| 6 |
| 7 |
| 8 |
where B is the Temkin isotherm constant (Eq. 8), KT is the Temkin equilibrium binding constant (L/mg), R = universal gas constant (8.314 J/mol.K), T = Temperature at 298 K.
D-R isotherm gives an idea about the nature of the adsorption process either physical or chemical and it does not hypothesize a homogenous surface or constant absorption potential (Hutson and Yang 1997). Dubinin–Radushkevich isotherm can be expressed as (Eq. 9)
| 9 |
where qe signifies the quantity of adsorbate species adsorbed in adsorbent dose (mg/g), qm is the theoretical isotherm saturation capacity (mg/g), represents D-R coefficient (mol2/kJ2).
ε is the polyani potential which is obtained from Eq. (Eq.10):
| 10 |
where R signifies the gas constant (0.008314 kJ/mol/K), T is the absolute temperature in Kelvin,.
Linear for of D–R isotherm can be expressed as (Eq. 11):
| 11 |
However, as discussed above that the D–R isotherm can be use in defining the nature of the adsorption process through the relationship with apparent energy (E) parameter (Eq. 12) (Igwe and Abia 2006).
| 12 |
Adsorption is regarded as following a chemisorption mechanism when the parametric value of E is between 8 and 16 kJ/mol while a value below 8 kJ/mol is indicative of a dominance of physisorption.
The photocatalytic activity of nanomaterials is mostly determined by light absorption, charge transport, and separation on the catalyst surface (Bhattacharjee et al. 2022). Photocatalysts reaction with light results formation of anionic electron-oxidative hole pairs, which later gets separated (Eqs. 13, 14). As h+ (hole) further reacts with water molecules redox active species and subsequently hydroxyl radicals have been formed (Eq. 15). On the other side superoxide radical anion is formed with the reaction with anionic electron (Eq. 16).
| 13 |
| 14 |
| 15 |
| 16 |
| 17 |
| 18 |
| 19 |
Furthermore, strong oxidants like hydroxyl (·OH) and perhydroxyl (HOO·) radicals are formed with the reaction of water and superoxide anion (O2·‒) radicals (Eqs. 17, 18) (Fujishima et al. 2008). Aqueous medium contains organic contaminants which are effectively degraded by these radicals (Rana et al. 2018). The reactions that follow show a mechanism that is similar to the photoelectronic breakdown of organic contaminants (Eq. 19). Contaminants may be reduced by photocatalytic reactions, depending on the type of the NMs. The resulting compounds may be bio-transformed by biotic systems, by lowering contaminants concentrations in the environment. In addition, certain enzymes produced by living organisms can degrade many types of pollutants (Peixoto et al. 2011). Apart from other entities, NPs can reach contaminated zones due to their small size, hence expanding the application areas of nano-bioremediation technologies, which is an advantage over conventional remediation strategies (Sohail et al. 2019). Several contaminants which are removed by the application of nano-bioremediation (nanoparticles and bioagents) are shown in Table 3. Other factors to consider are the standardization of techniques for testing nanomaterials toxicity in soil and water, the explication of their relationships with biotic and abiotic components, and the applicable regulatory context in which these materials might be employed (Ramírez-García et al. 2018). Finally, in regard to synergistic applications of nanomaterials over the long-term, collateral effects of nanomaterials on microorganisms, and trophic transfer of nanomaterials in the food supply chain, future research should focus on the selection of NPs and living organisms (Vázquez-Núñez et al. 2020).
Table 3.
Removal of contaminants through nano-bioremediation techniques
| Nanoparticle | Bioagent | Contaminant | Remark | References |
|---|---|---|---|---|
| Magnetic Fe3O4 nanoparticles | Pseudomonas delafieldii | Dibenzothiophene | The bio-desulfurization of dibenzothiophene was higher in the magnetic nanoparticle-coated microbial cells than in the uncoated or celite-coated cells. It has also been discovered that it may be reused up to five times | Shan et al. (2005) |
| Pd (0) nanoparticles | Shewanella oneidensis MR-1 | PCBs | Around 90% of PCBs were efficiently dechlorinated by the bio-Pd produced by the microbial reduction, resulting in less hazardous by-products | Windt et al. (2005) |
| Fe3O4 nanoparticles/gellan- gum gel beads | Sphingomonas sp. strain XLDN2–5 cells | Carbazole | Microbial cells immobilized in Fe3O4 nanoparticles/gellan gum gel beads decomposed carbazole more efficiently than free cells or cells that were not magnetically immobilized. When this integrated system was recycled, it revealed signs of increasing deterioration | Wang et al. (2007) |
| Pd/nFe | Laccase derived from Trametes versicolor | Triclosan | The remediation of triclosan was accomplished entirely by the use of Fe nanoparticles. The laccase released by the T. versicolor strain, on the other hand, transformed the degraded by-products into harmless compounds | Bokare et al. (2010) |
| Bio-Pd nanoparticle | C. pasteurianum BC1 | Cr (VI) | Clostridium pasteurianum converted Pd (II) ions to Pd nanoparticles, which persisted in the form of bio-Pd in the organism's cell membrane and cytoplasm. The Cr (VI) reduction process was effectively catalyzed, and hydrogen gas was created as well | Chidambaram et al. (2010) |
| nZVI | Dehalococcoides sp. | TCE | This research found that nZVI increased methanogen metabolic activity while deactivating dechlorinating bacteria; yet, after a lag period, the dechlorinating bacteria were able to eliminate TCE and produce ethene as a by-product | Xiu et al. (2010) |
| Pd/nFe | Sphingomonas wittichii RW1 (DSM 6014) | 2,3,7,8- tetrachlorodibenzo- p-dioxin (2,3,7,8-TeCDD) | The very lethal dioxin isomer is naturally refractory, and it could not be readily degraded by a single approach. The degradation was achieved by progressively utilizing Pd/nFe nanoparticles and the Sphingomonas strain | Bokare et al. (2012) |
| nZVI | Sphingomonas sp. PH-07 | Polybrominated diphenyl ethers (PBDEs) | Effective for PBDEs breakdown via reductive debromination and biological oxidation. This technology might lead to a remediation strategy for highly halogenated contaminants in the environment | Kim et al. (2012) |
| Carbon nanotubes | Shewanella oneidensis MR-1 | Cr (VI) | The MR-1 strain that was immobilized by CNT infused CA beads was able to remove four times more Cr (VI) than free cells, CNTs, or CA beads | Yan et al. (2013) |
| Fe3O4 | Sphingomonas sp. XLDN2-5 cells | Carbazole | The Fe3O4 nanoparticles linked to the bacterial strain's surface, degraded at the same rate as free cells, yet they were very reusable. Another benefit of employing magnetic nanoparticles is that they may be isolated from microorganisms with the help of external magnet sources | Li et al. (2013) |
| Nano sponge | Two organo-clays (Dellite 67G and Dellite 43 B) | Triclopyr (3,5,6-Trichloro-2-pyridinyloxyacetic acid) | Removal capacity of Cyclodextrin-based, highly cross-linked polymers is around 92% in Triclopyr contaminated soil | Baglieri et al. 2013) |
| nZVI | Paracoccus sp. strain YF1 | Nitrate | Lower concentrations of nZVI (50 mg/L) accelerated denitrification while generating little microbial toxicity, but larger concentrations (1000 mg/L) considerably retarded denitrification | Liu et al. (2014) |
| Nanotubes | Enzyme organophosphate hydrolase–MWNTs paper | Organophosphates and heavy metals Triclopyr | CNT, single-walled CNT, and multi-walled CNT shows low removal efficiency (~ 22%) | Fosso-Kankeu et al. (2014), Mechrez et al. (2014) |
| nZVI | Oak and mulberry leaf extracts | Cu and Ni | Mulberry-nZVI and Oak-nZVI were effective in transforming labile metals (Cu, Ni) bound to Danube river sediments to stable fractions | Slijepčević et al. (2021) |
| Pd/nFe | Burkholderia xenovorans LB400 | Polychlorinated biphenyl (PCB) Aroclor 1248 | Bi-, tri-, tetra-, penta-, and hexa-chlorinated biphenyls were efficiently dechlorinated into biodegradable intermediates by Pd/nFe nanoparticles, which were then quickly degraded by Burkholderia xenovorans | Le et al. (2015) |
| nZVI-C-A beads | Bacillus subtilis, E. coli, and Acinetobacter junii | Cr (VI) | Removal efficiency is around 92% of Cr (VI) with the application of nZVI entrapped calcium alginate beads | Ravikumar et al. (2016) |
| TiNPs | Dead yeast biomass | Cr (VI) | High remediation efficiency (99.92%) with removal capacity of 162.07 mg/g. It follows langmuir adsorption process with pseudo-second order kinetics | |
| nZVI | nZVI combination with a second metal or microorganisms | PCB | High removal efficiency (78–99%) of PCB with rapid reaction time | Jing et al. (2018) |
| Multi-walled carbon nanotubes Immobilized | Saccharomyces cerevisiae Rhizobium sp. | Cr (VI) | Removal capacity Cr (VI) is around 24.82–31.6 mg/g. Sorption experiment follows langmuir adsorption process with pseudo-second order kinetics | Sathvika et al. (2018) |
| Bimetallic iron-based NPs | Tobacco plants | hexabromocyclododecane (HBCD) | Removal efficiency is around 27% in case of HBCD contaminated soil | Le et al. (2019) |
| Polyvinylpyrrolidone (PVP)-coated iron oxide NPs | Halomonas sp. | PB, Cd | Due to this integrated strategy, metal removal was enhanced, and metal remediation durations were also reduced (approx. 100% removal of Pb after 24 h, of Cd after 48 h) | Cao et al. (2020) |
Nano-bioremediation for heavy metals and other pollutants
In recent years, carbon nanotubes (CNTs) have been utilized for remediation of wastewater contaminated with heavy metals. There are mainly two types of carbon nanotubes: single-walled and multi-walled (Martel et al. 1998). CNTs are able to treat heavy metal wastewater much better than other treatment methods due to their rapid adsorption kinetics associated with high specific surface area and high adsorption capacity which is more prominent in heavy metal ions such as Mn7+,Ti+, Cu2+, Pb2+, and Cr7+ (Pu et al. 2013; Yadav and Srivastava 2017). CNT surfaces may be modified by heat treatment and chemical modification or endohedral filling to increase their adsorption properties toward heavy metals through introduction of functional groups such as COOH, NH, OH (Kumar et al. 2021).
A study was conducted that examined the use of a graphene oxide (rGO) modified electrode that contains Au nanoparticles (rGO-Au NPs). The nanoparticles were synthesized using a vegetable extract from Abemoschus esculentus as a reducing agent and sensing of toxic metal ions such as Cd2+, Pb2+, Cu2+, and Hg2+ were detected simultaneously. Several bacteria were introduced such as Pseudomonas aeruginosa, Bacillus subtilis, Rizobium gallium, and Staphylococcus aureus for their high adsorptive potential for bioremediation of the toxic metal ions and these were reported to scavenge heavy metal ions effectively (Gnanaprakasam et al. 2016). Cao and his team demonstrated in a seminal paper that polyvinylpyrrolidone-coated iron oxide nanoparticles were useful for metal removal, specifically Cd2+ and Pb2+, through bacterial interaction with Halomonas sp. The treatments included a bacteria and nanoparticle both individually and in combination. After 24 h of treatment, Cd was removed with the removal efficiency of 100% in the combined treatment. After 48 h, the percentage remained the same. The removal of Cd and Pb was found to be 60% and 80% for treatments with only NPs, respectively. Whereas, by Halomonas sp., the removal of Cd rose to 80%, while treatment of Pb showed the same removal as for NPs, i.e., 80% (Cao et al. 2020).
The possibility of making iron nanoparticles from natural consortia that can adsorb arsenic, copper, zinc, and chromium from wastewaters was reported in a recent study (Castro et al. 2018). Biogenic Fe–Mn oxides (BFMO) synthesis was carried out using the soil bacterium Pseudomonas sp. QJX-1 isolated from manganese mines. These oxides were found to oxidize and adsorb arsenic. This allows for adequate As(III) and As(V) adsorption and oxidation (Bai et al. 2016). The use of sulfate-reducing bacteria (SRB) to treat wastewater containing chromium has also been demonstrated. The bacteria rely on organic compounds as a carbon source to be able to remove sulfate and COD from wastewater. As a result of experiments conducted at optimized conditions on synthetic wastewater, 81.9% of COD, 89.2% of Cr7+, and 95.3% of sulfate were removed (Verma et al. 2015). Biogenic manganese oxide (BMO) derived from Pseudomonas putida MnB1 has been used to remove heavy metals from the environment. With regard to adsorbing heavy metals, BMO have shown superior performance compared with chemically synthesized manganese oxide (birnessite). It is amorphous, small size, and the large surface area makes BMO an excellent adsorbent. Compared to birnessite, BMO adsorbs zinc, lead, and cadmium at a rate 7–8 times higher, and when the pH and temperature are changed, BMO adsorbs heavy metals even more powerfully (Zhou et al. 2015). Recent reports have found that nanoparticle synthesis through biogenic method utilizing Citrobacter freundii Y9 are effective in remediating Hg contaminated soil (Wang et al. 2017). Combined elements of mercury and selenium form HgSe, an inert and chemically inert compound that is less toxic than mercury and selenium alone. Furthermore, nano-selenium has the ability to capture mercury in anaerobic and aerobic conditions. Immobilizing mercury using nano-selenium is an effective way to remediate soils that are contaminated with mercury.
Nano-bioremediation for organic contaminants (including pesticides and herbicides) in soils
Le et al. (2015) investigated the degradation of Aroclor 1248-containing solutions which is a polychlorinated biphenyls (PCB) with nZVI (1000 mg/L) followed by biodegradation with Burkholderia xenovorans. Following the application of nZVI, the researchers obtained an 89% degradation of the congeners. Following that, Biphenyls formed by bacterial metabolism after PCB dechlorination showed a biodegradation rate of 90%. The nZVI was found to have no toxic effects on microorganisms in this study. Also, Bokare and his team investigated the feasibility of combining bioremediation and reductive processes using nanoparticles in a triclosan (5 g/L) contaminated solution. A sequential degradation was promoted by exposing the contaminant to anaerobic dechlorination using Pd/Fe nanoparticles. Following that, a further remediation step involves oxidizing the by-products with Trametes versicolor laccase. As a result, triclosan was completely dechlorinated and its by-products completely oxidized 20 min after application (Bokare et al. 2010). Another experiment conducted by Bokare and his team combined the use of Pd/Fe nanoparticles and Sphingomonas wittichii to remediate a 2,3,7,8-tetrachlorodibenzo-p-dioxin-contaminated solution. A dechlorination process was carried out in an anaerobic environment with 2,3,7,8-tetrachlorodibenzo-p-dioxin via Pd/Fe nanoparticles, yielding 100 percent dechlorination in just 10 h. As a result of dechlorination, the residual by-product (dibenzo-p-dioxin) could not be remedied by nanoparticles, but was degraded completely through microbial metabolism (Bokare et al. 2012). Singh and his team investigated the integrated remediation of Lindane-contaminated soil. Using CMC-Pd/Fe nanoparticle the researchers evaluated how Sphingomonas sp. behaved when exposed to different concentrations. The researchers discovered that nanoparticle suspensions containing 40 mg/L of nanoparticles produced the best results for microbial growth. Combining the two systems (biotic and abiotic) resulted in a contaminant degradation rate of 99 percent in 6 days, a result that was far superior to either treatment alone (Singh et al. 2013).
Kim and her team investigated the effect of combining nZVI and diphenyl ether with the bacteria Sphingomonas sp. PH-07 on the biodegradation of polybrominateddiphenyl ethers (PBDEs). They found that PH-07 is capable of growing at 5 g/L nZVI concentrations and has the ability to biodegrade PBDEs (Kim et al. 2012). Furthermore, utilization of nZVI nanomaterials as part of bioremediation, electrokinetic remediation and chemical oxidation has assisted in the cleanup of heavily polluted sites (Fan et al. 2016). In their report, nZVI-assisted dechlorination was followed by a biosurfactant-based soil washing procedure to eliminate polychlorinated biphenyls (PCBs) from soil contaminated by transformer oil. Aside from direct dechlorination, it was discovered that nZVI greatly improved efficiency of soil washing in oil and soil phases by lowering the interfacial tension between them, and 90 percent of PCBs were removed. A combination of treatments with nZVI, surfactants, electrokinetic treatments has previously been reported to pretreat sites of contaminated with heavy metals, polychlorinated biphenyls (PCBs), pesticides and chlorinated volatile organic compounds (cVOCs) (de Lima et al. 2012; Bhattacharya et al. 2016). The reactivity of nZVI is affected by natural organic matter (NOM), such as fulvic acids and humic acids, as they compete with pollutants for the reactive sites on the surface of nZVI where reactions occur. However, some have argued that NMs are ineffective in bioaugmentation because they inhibit microbes living in polluted environments (Nzila et al. 2016; Amoatey and Baawain 2019). However, it should be noted that NMs can reduce microbial diversity and abundance after a few days, but they can return again after a few weeks. Furthermore, although NMs decreased enzyme concentrations involved in ecological processes, they began to increase again after the first few days of the experiment. There is an indication that NMs have a priming effect initially, but because of their resilience, ecological balance returns shortly thereafter. It has been reported, however, that high CNT concentrations inhibit bacterial growth and microbial activity, while low CNT concentrations lead to greater biodegradation by causing bacteria to grow and overexpress degradation genes (Zhang et al. 2015). Because of its large surface area, NM could help to overcome limitations in microorganism immobilization and entrapment during bioaugmentation strategies (Nzila et al. 2016).
With the introduction of the basic idea of how nanoparticles interact with microbes, different nanoparticles have been fabricated using biological entities. It has become the focus of "green nanotechnology" to immobilize microbes on nanoparticles with desirable characteristics, and their use in environmental remediation combines both technologies into an innovative, hybrid approach that could open up new approaches to sustainable pesticide mitigation. Different nanoscale structures are used here to enhance the degradation of pesticides from polluted sites by simultaneously or sequentially combining with microbes. Reátegui and his team employed whole cell immobilization techniques to degrade pesticides. To remove the pesticide, they used recombinant Escherichia coli cells that expressed the long-lasting atrazine-dechlorinating enzyme. To encapsulate cells in a porous gel, siliconized silica nanoparticles (SiNPs) were used. By silanizing nanoparticles with methyltrimethoxysilane (MTMS) and polyethylene glycol, a porous gel was created which served as a cross-linker. Stability testing was carried out at 23 and 45 °C, respectively. In the higher temperature range, gel displayed a good structural rigidity. The degradation of the pesticide by encapsulated cells was three times more efficient than that of free cells (Reátegui et al. 2012).
Zhuang et al. (2015) used Streptomyces sp. N01 immobilized on bamboo-carbon supported Fe3O4 nanoparticles (Fe3O4/BC) to break down the heterocyclic aromatic organic compound quinoline. It was found to be effective at quinoline concentrations of 100–400 mg L−1, pH levels of 5–10, and temperatures of 20–45 °C. When temperature and pH were changed, Fe3O4/BC immobilized cells demonstrated greater pesticide concentration tolerance. In the ninth cycle, pesticides were removed at an 85.3 percent rate. Hou and his team observed that aniline-degrading Rhodococcus rhodochrous cells immobilized on Fe3O4 nanoparticles were capable of degrading chlorophenols (CPs) which is an organochloride of phenol utilized for a variety of purposes such as antiseptics, disinfectants, pesticides and wood preservatives. Immobilization of Rhodococcus rhodochrous DSM6263 strain was carried out with k-carrageenan. In this study, 2,3-dichlorophenol, 4-chlorophenol and their mixture were efficiently degraded by cells immobilized by k-carrageenan in combination with 9 g L−1 Fe3O4 nanoparticles, indicating a higher degradative efficiency than free cells. With these immobilized cells, the process could be repeated for a minimum of six times (Hou et al. 2016). Khataon and Rai (2018) isolated two Bacillus sp. capable of degrading atrazine, B. badius and B. ensimensis. Atrazine's biodegradation in various environmental conditions was studied using free and α-Fe2O3 immobilized cells. In order to immobilize these bacterial isolates, α-Fe2O3 magnetic nanoparticles were developed because free cells of microbes were rarely able to degrade atrazine. SEM images revealed that immobilized bacterial cells were completely held on to and spread across the surface of α-Fe2O3 magnetic nanoparticles. In comparison with free cells, immobilized α -Fe2O3 cells degraded by 90.56 percent after 20 days.
Advances in nano-bioremediation and crop productivity
Nano-bioremediation is defined as the method wherein environmental contaminants including heavy metals, organic and inorganic pollutants are removed from polluted sites by using nanoparticles/nanomaterials produced by plants or microorganisms such as fungi, bacteria through nanotechnology. Nano-bioremediation is gaining popularity as a flexible utility for long-term environmental cleanup (Koul and Taak 2018). Currently, bioremediation provides an environmentally useful and economically feasible option to remove pollutants from the environment, following latest advancements (Ken and Sinha 2020). Microbes, plants and enzymes-mediated remediation are the three fundamental methods used in bioremediation. Nano-bioremediation is one such technology that employs physiochemical and biological approaches and is now being researched in several polluted areas. Nanomaterials are utilized in the nano-bioremediation process to break down pollutants to a level that is suitable for biodegradation, and then the contaminants are biodegraded. NPs that are biologically produced from plant extracts or microbes are utilized in nano-bioremediation to clear away polluted water and land locations. Nanomaterials have developed as a viable alternative to conventional methods of treatment in the last two decades due to their high efficacy, cost-effectiveness, and environmental friendliness (Dastjerdi and Montazer 2010). The first nanoparticles to be utilized for environmental cleanup were iron NPs (Tratnyek and Johnson 2006). For the treatment of polluted soil or groundwater remediation, several potential iron-based solutions are available. Zero-valent iron NPs have been demonstrated to successfully remediate acidic water polluted with heavy metals by solubilizing the heavy metal contaminants on their interface, making them a viable and necessary technique of nano-remediation (Iravani 2011; Saif et al. 2016). Because of their exceptional ability to destroy organic dyes, Zn NPs have been widely studied and examined by scientists all over the world. Because Zn NPs are semiconductor photocatalysts, they may completely degrade a broad range of substances, including dyes, phenols, and pharmaceuticals (El-Kemary et al. 2010). Noble metallic nanoparticles, such as gold and silver, offer a broad range of uses, the most notable of which is the decomposition of organic dyes (Rajeshkumar and Santhiyaa 2018). Copper nanoparticles have also been demonstrated to be effective at degrading organic pigments (Marcelo et al. 2018).
Nanotechnology also improves the effectiveness of phytoremediation (Zhu et al. 2019). Organic contaminants like atrazine, chlorpyrifos and molinate can be eliminated by nano-sized zero-valent ions, as reported by (Ghormade et al. 2011) Ghormade and his team. In enzymatic bioremediation, nanoparticles can also be employed in combination with phytoremediation (Singh 2010). Microbial and plant degradation are especially resistant to some organic substances that are complex in nature such as organochlorines and long-chain hydrocarbons. This limitation might be overcome by linking nanotechnology and biotechnology, for example, complex organic substances would be reduced into simpler compounds by nano-encapsulated enzymes, which would then be promptly broken down by microorganisms and plants functioning together. Carbon nanotubes have also been shown to be particularly successful in the treatment of polluted water due to their high affinity and adsorption properties for pollutants (Kitching et al. 2014). Excellent thermal and chemical stability of carbon nanotubes makes them a viable alternative to activated carbon for the detoxification of various organic and inorganic pollutants such as zinc, lead, and chromium (Hu and Xia 2018). Even though nano-remediation helps in treating the mine water, there are still a number of safety problems that need to be resolved. Various eco-remediation nano-applications are rapidly progressing from trial to full-scale success in addressing ecologically hazardous chlorinated areas. Nanoscale TiO2, carbon nanotubes, dendrimers, swellable organically modified silica (SOMS), and metallo-porphyrinogens are nanoproducts for in situ and ex situ remediation of pollution (Shi et al. 2015; Wang et al. 2017). During photo-catalysis, TiO2 nanoparticles can treat a variety of chemical fertilizers, herbicides, insecticides, and pesticides, and are being explored for ex situ handling of diseased groundwater reservoirs (Pandey 2018). The kinetics of the redox reaction is increased by biologically produced iron, copper and titanium nanomaterials in conjunction with a metal precursor including gold, nickel, Pt, and Pd. Pd NPs have the ability to catalyze the conversion of trichloroethene to ethane while avoiding the formation of an intermediate by-product, vinyl chloride. Palladium-Osorb, a parallel metal–glass fused material, has been experimentally verified and employed for ex situ cleanup of chlorinated volatile organic compounds (Song et al. 2009). Silica NPs are used for lead remediation (Ma et al. 2018), zinc NPs for carbon disulfide removal from the air, and nano-crystalline hydroxylapatite for the removal of lead and cadmium, zero-valent nano-iron, carbon nanotubes, fullerenes, TiO2 and ZnO NPs, and bimetallic nano-metals for DDT, carbamates, and heavy metals such as chromium, lead, arsenic, and cadmium from soil (Lárez Velásquez 2018). The incorporation of biologically generated Iron NPs and Iron-Pd NPs in the remediation of dyes, hydrocarbons, 2,3,7,8-tetrachloro dibenzo-p-dioxin, pesticides, trichloroethylene, polychlorinated biphenyls, and Lindane, among other things, has become more widespread (Asmel et al. 2017). Biogenic uranite nanoparticles were utilized for the bioremediation of uranium (Bargar et al. 2008). Heavy metals, namely Cu, Zn, Pb, and Ni, were deposited in biologically produced nanoparticles from Gundelia tournefortii, Centaurea virgata, Reseda lutea, Scariola orientalis, Eleagnum angustifolia, Bacillus sp., and Noaea mucronata (Sinha et al. 2011; Ingle et al. 2014).
A few chemicals in the agricultural system, such as pesticides, degrade slowly or are chemically stable in nature, so they remain in the environment for longer periods of time and lead to serious consequences. However, by using nanoscience, these hazardous and toxic derivatives can be degraded under certain circumstances. They may penetrate the food chain if they are not decomposed, posing a major health risk. Agriculture nanotechnology has taken a positive stride in this regard in recent years. For example, in polluted soil, a nanoparticle-water slurry can be combined, and over time, these particles will diminish the toxicity of pesticides that are slow to degrade or resistant. Plant-derived nanometric lignocellulosic materials have led to a new market place for creative and value-added nanoscale materials and products, such as nano-sized cellulose crystals utilized as ultralight augmentation in polymeric matrixes (Dasgupta et al. 2016). These can be implemented in food as well as other packaging, manufacturing and body components of transport vehicles. Because we can extract nano-lignocellulosic components from lignin- and cellulose-based agricultural residues, nano-lignocellulosic materials are the ideal solution to manage agricultural wastes (Brinchi et al. 2013).
Commercialization of nano-materials for restoration of contaminated agricultural land
The "Environmental Remediation Market with COVID-19 Impact Analysis by environmental medium (soil & groundwater), Technology (Bioremediation, Pump & Treat, Soil Vapor Extraction, Chemical Treatment), Site Type, Application, and Region—Global Forecast to 2026" reported that the market for environmental remediation is predictable to reach USD 158.8 billion by 2026, up from USD 104.6 billion in 2021, at an 8.7 percent CAGR over the forecast horizon. By 2026, bioremediation technology is expected to rationalize the majority of the environmental remediation market. Due to the growing demand for this methodology for the remediation of both soil and groundwater, the bioremediation section is supposed to prompt the market for environmental remediation during the baseline period. Increasing government initiatives for environmental safety; a rising emphasis on the development of environmental friendly enterprises, and rapid population growth and industrialization in developing countries are key elements which drive this market's growth. In the midst of COVID-19, the development of sophisticated remediation technologies and the steady expansion of the oil and gas industry caused a heavy demand for environmental remediation for productive industrial activities.
The global agricultural land is projected to produce nearly 99 percent of our food, as well as textiles, timber, and raw materials of industrial significance. However, anthropogenic activities have led to the contamination of agricultural land due to the release of organic pollutants such as pesticides, fertilizers, plastics, bisphenols; inorganic pollutants like heavy metals and metalloids, and other pollutants including personal care products, pharmaceuticals, surfactants, nanoplastics, etc. (Marziali et al. 2021). Engineered nanoparticles (ENMs) are now being explored as a way to enhance agricultural soil quality and, as a result, promote sustainable agriculture. ENMs are particularly reactive due to their tiny size and wide surface area, and they exhibit a range of features such as improved cation exchange capacity, sustained release of nutrient in the soil that make them useful in soils application (Thul et al. 2013). Chemical reductant-based nanoparticles such as nano-zerovalent iron (nZVI), manganese (Mn), thiosulfate (S2O3−2), molybdenum sulfides (MoS2), zinc (Zn), hydrogen peroxide (H2O2), and iron sulfide (FeS) are effective at transforming, degrading, and detoxifying organic and inorganic soil pollutants. Adsorption, immobilization, ion exchange, complex formation, reduced dehalogenation, and SN2 nucleophilic substitution were the basic mechanisms (Wang et al. 2017; Chen et al. 2020; Zhou et al. 2021). nZVI is one of the most effective technologies that has been used to create permeable barriers or restore soil in situ by removing several pollutants. Nano form of ZVI has also been shown to be very successful in eradicating organic chemicals from the soil, particularly chlorinated solvents (e.g., TCE), in real-world applications at military-based locations across the United States and Europe (Viskutyte et al. 2014). The utilization of flower-like MoS2 nano-hybrid has been proved to be effective in removing Hg(II) and Pb(II) from soil (Wang et al. 2020). Other interesting nanoscale materials including the self-assembled monolayers on mesoporous supports (SAMMS) have been reported to remove non-polar pollutants like polyaromatic hydrocarbons (PAHs) from soil (Brandl et al. 2015). Metal oxides-based nanoparticles such as oxides of Mn, Zn have greater capabilities to detoxify contaminated soil. Mn oxide is being applied for the detoxification of contaminated land due to its interesting properties such as large specific surface and low pH. However, Mn oxide-based nanomaterials are not desirable for the pollutants with reduced species (e.g., Cr3+ versus Cr6+; their application is restricted only to the pollutants with oxidized chemical species, e.g., As3+ versus As5+) since Mn oxides have strong oxidative characteristics (Villalobos et al. 2014). In comparison with Mn oxides, biogenic nanostructured Mn oxides are more productive, cost-effective, and environmental-friendly materials. They have been reported to be successful in restoring arsenic-contaminated soil (Wang et al. 2020). Zn oxide nanoparticles have been reported to reduce both cadmium and arsenic contamination in paddy (Ma et al. 2020). With adsorption and degradation efficiencies of up to 80% and 99%, respectively, carbon-based materials such as biochar, graphene, activated carbon, single and multi-walled CNTs have been proven to be effective in extracting both organic and inorganic contaminants from soil (Gopinath et al. 2021). Biochar/Fe components have been utilized in soil restoration via three different mechanisms—adsorption, reduction and oxidation (Lyu et al. 2020). For the treatment of Cd2+ and Pb2+ polluted soils, a new thiol-modified rice straw biochar was produced by esterification with-mercaptoethanol. Rice straw preferentially adsorbed Cd2+ over Pb2+, lowering Cd availability by up to 40% while allowing Pb to be immobilized to a limited extent (Fan et al. 2020). Nano-sized biopolymers have been described as a flexible category of materials that may be employed in a variety of applications, particularly soil conditioners. However, there’s limited information on the application of nanotechnology in soil restoration and hence this deserves further investigation.
Conclusion
The biosynthesis of nanoparticles is a slow process compared with physical and chemical processes. Further studies should focus on reducing the reaction time for the synthesis of nanoparticles, which will enhance the attractiveness of the process. In addition, it is crucial to determine and isolate the compound(s) that results in metal reduction during synthesis so that the process can be more efficient. Nanobioremediation-based approaches have been established as an effective treatment for a number of environmental contaminants, including micropollutants. Nevertheless, these studies are confined to laboratory conditions, so their efficacy in soil, wastewater treatment plants remains to be further explored. The creation of intermediate complexes led to toxicity in aqueous systems when nanoparticles are utilized to remediate environmental contaminants. Therefore, it is mandatory to check the genesis and fate of these compounds at miscellaneous points during the treatment procedure, as well as to take adequate steps to remove them.
Acknowledgments
The authors are thankful to UGC-BHU fellowship to AH.
Authors’ contributions
AH, PD contributed to conceptualization and design of the study; AH, AD, AG contributed to investigation, resources, writing original draft; SKU contributed to preparation of figures; PD, BNS contributed to editing, reviewing, and revised the manuscript critically for important intellectual content.
Funding
The authors declare that no funding was received for this work that could have influenced its outcome.
Declarations
Conflict of interests
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Contributor Information
A. Hidangmayum, Email: akashhidang@gmail.com
A. Debnath, Email: abhijit1732@gmail.com
P. Dwivedi, Email: pdwivedi25@rediffmail.com
References
- A.O D, Langmuir, Freundlich, Temkin and Dubinin-Radushkevich isotherms studies of equilibrium sorption of Zn2+ unto phosphoric acid modified rice husk. IOSR J Appl Chem. 2012 doi: 10.9790/5736-0313845. [DOI] [Google Scholar]
- Abril M, Ruiz H, Cumbal LH. Biosynthesis of multicomponent nanoparticles with extract of mortiño (Vaccinium floribundum Kunth) berry: application on heavy metals removal from water and immobilization in soils. J Nanotechnol. 2018 doi: 10.1155/2018/9504807. [DOI] [Google Scholar]
- Ahmad A, Mukherjee P, Mandal D, et al (2002) Enzyme mediated extracellular synthesis of CdS nanoparticles by the fungus, Fusarium oxysporum. J Am Chem Soc. 10.1021/ja027296o [DOI] [PubMed]
- Ahmad A, Mukherjee P, Senapati S, et al. Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium oxysporum. Coll Surf B Biointerfaces. 2003 doi: 10.1016/S0927-7765(02)00174-1. [DOI] [PubMed] [Google Scholar]
- Ahmad N, Sharma S, Singh VN, et al. Biosynthesis of silver nanoparticles from Desmodium triflorum: A novel approach towards weed utilization. Biotechnol Res Int. 2011 doi: 10.4061/2011/454090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Qahtani KM. Cadmium removal from aqueous solution by green synthesis zero valent silver nanoparticles with Benjamina leaves extract. Egypt J Aquat Res. 2017 doi: 10.1016/j.ejar.2017.10.003. [DOI] [Google Scholar]
- Amoatey P, Baawain MS. Effects of pollution on freshwater aquatic organisms. Water Environ Res. 2019;91:1272–1287. doi: 10.1002/wer.1221. [DOI] [PubMed] [Google Scholar]
- Ankamwar B. Biosynthesis of gold nanoparticles (Green-Gold) using leaf extract of Terminalia Catappa. E-Journal Chem. 2010 doi: 10.1155/2010/745120. [DOI] [Google Scholar]
- Ankamwar B, Chaudhary M, Sastry M. Gold nanotriangles biologically synthesized using tamarind leaf extract and potential application in vapor sensing. Synth React Inorganic, Met Nano-Metal Chem. 2005 doi: 10.1081/SIM-200047527. [DOI] [Google Scholar]
- Arjaghi SK, Alasl MK, Sajjadi N, et al. Green synthesis of iron oxide nanoparticles by RS Lichen extract and its application in removing heavy metals of lead and cadmium. Biol Trace Elem Res. 2021 doi: 10.1007/s12011-020-02170-3. [DOI] [PubMed] [Google Scholar]
- Aromal SA, Vidhu VK, Philip D. Green synthesis of well-dispersed gold nanoparticles using Macrotyloma uniflorum. Spectrochim Acta-Part A Mol Biomol Spectrosc. 2012 doi: 10.1016/j.saa.2011.09.035. [DOI] [PubMed] [Google Scholar]
- Arsiya F, Sayadi MH, Sobhani S (2017) Green synthesis of palladium nanoparticles using Chlorella vulgaris. Mater Lett
- Arulkumar S, Sabesan M (2010) Biosynthesis and characterization of gold nanoparticle using antiparkinsonian drug Mucuna pruriens plant extract. Int J Res Pharm Sci [DOI] [PMC free article] [PubMed]
- Asmel NK, Yusoff ARM, Sivarama Krishna L, et al. High concentration arsenic removal from aqueous solution using nano-iron ion enrich material (NIIEM) super adsorbent. Chem Eng J. 2017;317:343–355. doi: 10.1016/j.cej.2017.02.039. [DOI] [Google Scholar]
- Baglieri A, Nègre M, Trotta F, et al. Organo-clays and nanosponges for acquifer bioremediation: adsorption and degradation of triclopyr. J Environ Sci Heal - Part B Pestic Food Contam Agric Wastes. 2013 doi: 10.1080/03601234.2013.780943. [DOI] [PubMed] [Google Scholar]
- Bahrulolum H, Nooraei S, Javanshir N, et al. Green synthesis of metal nanoparticles using microorganisms and their application in the agrifood sector. J Nanobiotechnology. 2021;19:1–26. doi: 10.1186/s12951-021-00834-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai HJ, Zhang ZM, Guo Y, Yang GE. Biosynthesis of cadmium sulfide nanoparticles by photosynthetic bacteria Rhodopseudomonas palustris. Coll Surf B Biointerfaces. 2009 doi: 10.1016/j.colsurfb.2008.12.025. [DOI] [PubMed] [Google Scholar]
- Bai Y, Yang T, Liang J, Qu J. The role of biogenic Fe-Mn oxides formed in situ for arsenic oxidation and adsorption in aquatic ecosystems. Water Res. 2016;98:119–127. doi: 10.1016/j.watres.2016.03.068. [DOI] [PubMed] [Google Scholar]
- Balaji DS, Basavaraja S, Deshpande R, et al. Extracellular biosynthesis of functionalized silver nanoparticles by strains of Cladosporium cladosporioides fungus. Coll Surf B Biointerfaces. 2009 doi: 10.1016/j.colsurfb.2008.09.022. [DOI] [PubMed] [Google Scholar]
- Bali R, Razak N, Lumb A, Harris AT (2006) The synthesis of metallic nanoparticles inside live plants. In: Proceedings of the 2006 international conference on nanoscience and nanotechnology, ICONN
- Bankar A, Joshi B, Ravi Kumar A, Zinjarde S. Banana peel extract mediated synthesis of gold nanoparticles. Coll Surf B Biointerfaces. 2010 doi: 10.1016/j.colsurfb.2010.05.029. [DOI] [PubMed] [Google Scholar]
- Bargar JR, Bernier-Latmani R, Giammar DE, Tebo BM. Biogenic uraninite nanoparticles and their importance for uranium remediation. Elements. 2008;4:407–412. doi: 10.2113/gselements.4.6.407. [DOI] [Google Scholar]
- Basavaraja S, Balaji SD, Lagashetty A, et al. Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium semitectum. Mater Res Bull. 2008 doi: 10.1016/j.materresbull.2007.06.020. [DOI] [Google Scholar]
- Benjamin SR, de Lima F, Florean EOPT, Guedes MIF. Current trends in nanotechnology for bioremediation. Int J Environ Pollut. 2019;66:19–40. doi: 10.1504/IJEP.2019.104526. [DOI] [Google Scholar]
- Bhainsa KC, D’Souza SF. Extracellular biosynthesis of silver nanoparticles using the fungus Aspergillus fumigatus. Coll Surf B Biointerfaces. 2006 doi: 10.1016/j.colsurfb.2005.11.026. [DOI] [PubMed] [Google Scholar]
- Bharde A, Kulkarni A, Rao M, et al. Bacterial enzyme mediated biosynthesis of gold nanoparticles. J Nanosci Nanotechnol. 2007 doi: 10.1166/jnn.2007.891. [DOI] [PubMed] [Google Scholar]
- Bharde A, Rautaray D, Bansal V, et al. Extracellular biosynthesis of magnetite using fungi. Small. 2006 doi: 10.1002/smll.200500180. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee N, Som I, Saha R, Mondal S. A critical review on novel eco-friendly green approach to synthesize zinc oxide nanoparticles for photocatalytic degradation of water pollutants. Int J Environ Anal Chem. 2022;00:1–28. doi: 10.1080/03067319.2021.2022130. [DOI] [Google Scholar]
- Bhattacharya K, Mukherjee SP, Gallud A, et al. Biological interactions of carbon-based nanomaterials: from coronation to degradation. Nanomed Nanotechnol Biol Med. 2016;12:333–351. doi: 10.1016/j.nano.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhojiya AA, Joshi H, Upadhyay SK, et al. Screening and optimization of zinc removal potential in Pseudomonas aeruginosa-HMR1 and its plant growth-promoting attributes. Bull Environ Contam Toxicol. 2021 doi: 10.1007/s00128-021-03232-5. [DOI] [PubMed] [Google Scholar]
- Binupriya AR, Sathishkumar M, Yun S. Myco-crystallization of silver ions to nanosized particles by live and dead cell filtrates of aspergillus oryzae var. viridis and its bactericidal activity toward staphylococcus aureus KCCM 12256. Ind Eng Chem Res. 2010 doi: 10.1021/ie9014183. [DOI] [Google Scholar]
- Bokare V, Murugesan K, Kim JH, et al. Integrated hybrid treatment for the remediation of 2,3,7,8-tetrachlorodibenzo-p-dioxin. Sci Total Environ. 2012 doi: 10.1016/j.scitotenv.2012.07.079. [DOI] [PubMed] [Google Scholar]
- Bokare V, Murugesan K, Kim YM, et al. Degradation of triclosan by an integrated nano-bio redox process. Bioresour Technol. 2010 doi: 10.1016/j.biortech.2010.03.062. [DOI] [PubMed] [Google Scholar]
- Brandl F, Bertrand N, Lima EM, Langer R. Nanoparticles with photoinduced precipitation for the extraction of pollutants from water and soil. Nat Commun. 2015;6:7765. doi: 10.1038/ncomms8765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinchi L, Cotana F, Fortunati E, Kenny JM. Production of nanocrystalline cellulose from lignocellulosic biomass: technology and applications. Carbohydr Polym. 2013;94:154–169. doi: 10.1016/j.carbpol.2013.01.033. [DOI] [PubMed] [Google Scholar]
- Cao X, Alabresm A, Chen YP, et al. Improved metal remediation using a combined bacterial and nanoscience approach. Sci Total Environ. 2020;704:135378. doi: 10.1016/j.scitotenv.2019.135378. [DOI] [PubMed] [Google Scholar]
- Castro L, Blázquez ML, González F, et al. Heavy metal adsorption using biogenic iron compounds. Hydrometallurgy. 2018;179:44–51. doi: 10.1016/j.hydromet.2018.05.029. [DOI] [Google Scholar]
- Chaithawiwat K, Vangnai A, McEvoy JM, et al. Impact of nanoscale zero valent iron on bacteria is growth phase dependent. Chemosphere. 2016 doi: 10.1016/j.chemosphere.2015.09.025. [DOI] [PubMed] [Google Scholar]
- Chandran SP, Chaudhary M, Pasricha R, et al. Synthesis of gold nanotriangles and silver nanoparticles using Aloe vera plant extract. Biotechnol Prog. 2006;22:577–583. doi: 10.1021/bp0501423. [DOI] [PubMed] [Google Scholar]
- Chaturvedi VK, Yadav N, Rai NK, et al. Pleurotus sajor-caju-mediated synthesis of silver and gold nanoparticles active against colon cancer cell lines: a new era of herbonanoceutics. Molecules. 2020 doi: 10.3390/molecules25133091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan R, Kumar A, Chaudhary RP (2012) Characterization of chemically synthesized Mn doped ZnS nanoparticles. Chalcogenide Lett
- Chen PJ, Su CH, Tseng CY, et al. Toxicity assessments of nanoscale zerovalent iron and its oxidation products in medaka (Oryzias latipes) fish. Mar Pollut Bull. 2011 doi: 10.1016/j.marpolbul.2011.02.045. [DOI] [PubMed] [Google Scholar]
- Chen Y, Liu Y, Li Y, et al. Synthesis, application and mechanisms of ferro-manganese binary oxide in water remediation: a review. Chem Eng J. 2020;388:124313. doi: 10.1016/j.cej.2020.124313. [DOI] [Google Scholar]
- Chidambaram D, Hennebel T, Taghavi S, et al. Concomitant microbial generation of palladium nanoparticles and hydrogen to immobilize chromate. Environ Sci Technol. 2010 doi: 10.1021/es101559r. [DOI] [PubMed] [Google Scholar]
- Costa MI, Álvarez-Cerimedo MS, Urquiza D, et al. Synthesis, characterization and kinetic study of silver and gold nanoparticles produced by the archaeon Haloferax volcanii. J Appl Microbiol. 2020 doi: 10.1111/jam.14726. [DOI] [PubMed] [Google Scholar]
- Dada AO, Ojediran JO, Olalekan AP. Sorption of sorption of Pb2+ from aqueous solution unto modified rice husk: isotherms studies. Adv Phys Chem. 2013;2013:1–6. doi: 10.1155/2013/842425. [DOI] [Google Scholar]
- Dameron CT, Reese RN, Mehra RK, et al. Biosynthesis of cadmium sulphide quantum semiconductor crystallites. Nature. 1989 doi: 10.1038/338596a0. [DOI] [Google Scholar]
- Das I, Ansari SA (2009) Nanomaterials in science and technology. J Sci Ind Res (India)
- Das J, Velusamy P. Catalytic reduction of methylene blue using biogenic gold nanoparticles from Sesbania grandiflora L. J Taiwan Inst Chem Eng. 2014 doi: 10.1016/j.jtice.2014.04.005. [DOI] [Google Scholar]
- Das RK, Gogoi N, Babu PJ, et al. The synthesis of gold nanoparticles using Amaranthus spinosus: leaf extract and study of their optical properties. Adv Mater Phys Chem. 2012;02:275–281. doi: 10.4236/ampc.2012.24040. [DOI] [Google Scholar]
- Dasgupta N, Ranjan S, Chakraborty AR, et al (2016) Nanoagriculture and water quality management. pp 1–42
- Dastjerdi R, Montazer M. A review on the application of inorganic nano-structured materials in the modification of textiles: Focus on anti-microbial properties. Coll Surf B Biointerfaces. 2010;79:5–18. doi: 10.1016/j.colsurfb.2010.03.029. [DOI] [PubMed] [Google Scholar]
- De WW, Aelterman P, Verstraete W. Bioreductive deposition of palladium (0) nanoparticles on Shewanella oneidensis with catalytic activity towards reductive dechlorination of polychlorinated biphenyls. Environ Microbiol. 2005 doi: 10.1111/j.1462-2920.2005.00696.x. [DOI] [PubMed] [Google Scholar]
- de Lima R, Seabra AB, Durán N. Silver nanoparticles: a brief review of cytotoxicity and genotoxicity of chemically and biogenically synthesized nanoparticles. J Appl Toxicol. 2012;32:867–879. doi: 10.1002/jat.2780. [DOI] [PubMed] [Google Scholar]
- Diao M, Yao M. Use of zero-valent iron nanoparticles in inactivating microbes. Water Res. 2009 doi: 10.1016/j.watres.2009.08.051. [DOI] [PubMed] [Google Scholar]
- Dwivedi AD, Gopal K. Plant-mediated biosynthesis of silver and gold nanoparticles. J Biomed Nanotechnol. 2011 doi: 10.1166/jbn.2011.1250. [DOI] [PubMed] [Google Scholar]
- El-Kemary M, El-Shamy H, El-Mehasseb I. Photocatalytic degradation of ciprofloxacin drug in water using ZnO nanoparticles. J Lumin. 2010;130:2327–2331. doi: 10.1016/j.jlumin.2010.07.013. [DOI] [Google Scholar]
- El-Ramady H, El-Henawy A, Amer M, et al (2020) Agro-Pollutants and their nano-remediation from soil and water: a mini-review. Environ Biodivers Soil Secur 0–0. 10.21608/jenvbs.2020.47751.1111
- European Commission (2011) 2011/696/EU Commission Recommendation of 18 October 2011 on the definition of nanomaterial. Off J Eur Union, L275, 54 (2011), p. 38
- Fajardo C, Saccà ML, Martinez-Gomariz M, et al. Transcriptional and proteomic stress responses of a soil bacterium bacillus cereus to nanosized zero-valent iron (nZVI) particles. Chemosphere. 2013 doi: 10.1016/j.chemosphere.2013.05.082. [DOI] [PubMed] [Google Scholar]
- Fan G, Wang Y, Fang G, et al. Review of chemical and electrokinetic remediation of PCBs contaminated soils and sediments. Environ Sci Process Impacts. 2016;18:1140–1156. doi: 10.1039/C6EM00320F. [DOI] [PubMed] [Google Scholar]
- Fan J, Cai C, Chi H, et al. Remediation of cadmium and lead polluted soil using thiol-modified biochar. J Hazard Mater. 2020;388:122037. doi: 10.1016/j.jhazmat.2020.122037. [DOI] [PubMed] [Google Scholar]
- Fazaludeen F, Manickam C, Ashankyty I, et al (2012) Synthesis and characterizations of gold nanoparticles by Justicia gendarussa Burm F leaf extract. J Microbiol Biotechnol Res
- Fernández PM, Viñarta SC, Bernal AR, et al (2018) Bioremediation strategies for chromium removal: current research, scale-up approach and future perspectives. Chemosphere [DOI] [PubMed]
- Fosso-Kankeu E, Mulaba-Bafubiandi AF, Mishra AK (2014) Prospects for immobilization of microbial sorbents on carbon nanotubes for biosorption: bioremediation of heavy metals polluted water. In: Application of nanotechnology in water research
- Fouda A, Hassan SED, Abdo AM, El-Gamal MS. Antimicrobial, antioxidant and larvicidal activities of spherical silver nanoparticles synthesized by Endophytic Streptomyces spp. Biol Trace Elem Res. 2020 doi: 10.1007/s12011-019-01883-4. [DOI] [PubMed] [Google Scholar]
- Freundlich HMF (1906) Uber die adsorption in losungen, Z. Phys Chem
- Fujishima A, Zhang X, Tryk DA (2008) TiO2 photocatalysis and related surface phenomena. Surf Sci Rep
- Gade A, Gaikwad S, Tiwari V, et al. Biofabrication of silver nanoparticles by opuntia ficus-indica: in vitro antibacterial activity and study of the mechanism involved in the synthesis. Curr Nanosci. 2010 doi: 10.2174/157341310791659026. [DOI] [Google Scholar]
- Gade AK, Bonde P, Ingle AP, et al. Exploitation of Aspergillus niger for synthesis of silver nanoparticles. J Biobased Mater Bioenergy. 2008 doi: 10.1166/jbmb.2008.401. [DOI] [Google Scholar]
- Gajbhiye M, Kesharwani J, Ingle A, et al. Fungus-mediated synthesis of silver nanoparticles and their activity against pathogenic fungi in combination with fluconazole. Nanomed Nanotechnol Biol Med. 2009 doi: 10.1016/j.nano.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Ganesh Babu MM, Gunasekaran P. Production and structural characterization of crystalline silver nanoparticles from Bacillus cereus isolate. Coll Surf B Biointerfaces. 2009 doi: 10.1016/j.colsurfb.2009.07.016. [DOI] [PubMed] [Google Scholar]
- Gardea-Torresdey JL, Parsons JG, Gomez E, et al. Formation and growth of au nanoparticles inside live alfalfa plants. Nano Lett. 2002 doi: 10.1021/nl015673+. [DOI] [Google Scholar]
- Garmasheva I, Kovalenko N, Voychuk S, et al (2016) Lactobacillus species mediated synthesis of silver nanoparticles and their antibacterial activity against opportunistic pathogens in vitro. BioImpacts. 10.15171/bi.2016.29 [DOI] [PMC free article] [PubMed]
- George VC, Kumar DN, Suresh PK, Kumar RA. A review on the therapeutic potentials of parthenolide: a sesquiterpene lactone. Int Res J Pharm. 2012;3:69–73. [Google Scholar]
- Geraldes AN, da Silva AA, Leal J, et al. Green nanotechnology from plant extracts: synthesis and characterization of gold nanoparticles. Adv Nanoparticles. 2016 doi: 10.4236/anp.2016.53019. [DOI] [Google Scholar]
- Gericke M, Pinches A. Biological synthesis of metal nanoparticles. Hydrometallurgy. 2006 doi: 10.1016/j.hydromet.2006.03.019. [DOI] [Google Scholar]
- Ghani MI, Saleem S, Rather SA, et al. Foliar application of zinc oxide nanoparticles: an effective strategy to mitigate drought stress in cucumber seedling by modulating antioxidant defense system and osmolytes accumulation. Chemosphere. 2022;289:133202. doi: 10.1016/j.chemosphere.2021.133202. [DOI] [PubMed] [Google Scholar]
- Ghormade V, Deshpande MV, Paknikar KM. Perspectives for nano-biotechnology enabled protection and nutrition of plants. Biotechnol Adv. 2011;29:792–803. doi: 10.1016/j.biotechadv.2011.06.007. [DOI] [PubMed] [Google Scholar]
- Gnanadesigan M, Anand M, Ravikumar S, et al. Antibacterial potential of biosynthesised silver nanoparticles using Avicennia marina mangrove plant. Appl Nanosci. 2012 doi: 10.1007/s13204-011-0048-6. [DOI] [Google Scholar]
- Gnanajobitha G, Annadurai G, Kannan C (2012) Green synthesis of silver nanoparticle using Elettaria cardamomom and assesment of its antimicrobial activity. Int J Pharma Sci Res (IJPSR)
- Gnanaprakasam P, Jeena SE, Premnath D, Selvaraju T. Simple and robust green synthesis of Au NPS on reduced graphene oxide for the simultaneous detection of toxic heavy metal ions and bioremediation using bacterium as the scavenger. Electroanalysis. 2016;28:1885–1893. doi: 10.1002/elan.201600002. [DOI] [Google Scholar]
- Gopinath K, Gowri S, Arumugam A. Phytosynthesis of silver nanoparticles using Pterocarpus santalinus leaf extract and their antibacterial properties. J Nanostruct Chem. 2013 doi: 10.1186/2193-8865-3-68. [DOI] [Google Scholar]
- Gopinath KP, Vo D-VN, Gnana Prakash D, et al. Environmental applications of carbon-based materials: a review. Environ Chem Lett. 2021;19:557–582. doi: 10.1007/s10311-020-01084-9. [DOI] [Google Scholar]
- Guo M, Li W, Yang F, Liu H. Controllable biosynthesis of gold nanoparticles from a Eucommia ulmoides bark aqueous extract. Spectrochim Acta - Part A Mol Biomol Spectrosc. 2015 doi: 10.1016/j.saa.2015.01.109. [DOI] [PubMed] [Google Scholar]
- Gurunathan S, Kalishwaralal K, Vaidyanathan R, et al. Biosynthesis, purification and characterization of silver nanoparticles using Escherichia coli. Coll Surf B Biointerfaces. 2009 doi: 10.1016/j.colsurfb.2009.07.048. [DOI] [PubMed] [Google Scholar]
- Halecký M, Kozliak E (2020) Modern bioremediation approaches: use of biosurfactants, emulsifiers, enzymes, biopesticides, GMOs. pp 495–526
- Hassan SE-D, Salem SS, Fouda A, et al. New approach for antimicrobial activity and bio-control of various pathogens by biosynthesized copper nanoparticles using endophytic actinomycetes. J Radiat Res Appl Sci. 2018 doi: 10.1016/j.jrras.2018.05.003. [DOI] [Google Scholar]
- He S, Zhang Y, Guo Z, Gu N. Biological synthesis of gold nanowires using extract of Rhodopseudomonas capsulata. Biotechnol Prog. 2008 doi: 10.1021/bp0703174. [DOI] [PubMed] [Google Scholar]
- Hidangmayum A, Dwivedi P. Chitosan based nanoformulation for sustainable agriculture with special reference to abiotic stress: a review. J Polym Environ. 2021 doi: 10.1007/s10924-021-02296-y. [DOI] [Google Scholar]
- Holmes JD, Smith PR, Evans-Gowing R, et al. Energy-dispersive X-ray analysis of the extracellular cadmium sulfide crystallites of Klebsiella aerogenes. Arch Microbiol. 1995 doi: 10.1007/BF00381789. [DOI] [PubMed] [Google Scholar]
- Hossain A, Hong X, Ibrahim E, et al. Green synthesis of silver nanoparticles with culture supernatant of a bacterium Pseudomonas rhodesiae and their antibacterial activity against soft rot pathogen Dickeya dadantii. Molecules. 2019 doi: 10.3390/molecules24122303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou J, Liu F, Wu N, et al. Efficient biodegradation of chlorophenols in aqueous phase by magnetically immobilized aniline-degrading Rhodococcus rhodochrous strain. J Nanobiotechnology. 2016;14:5. doi: 10.1186/s12951-016-0158-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L, Xia Z. Application of ozone micro-nano-bubbles to groundwater remediation. J Hazard Mater. 2018;342:446–453. doi: 10.1016/j.jhazmat.2017.08.030. [DOI] [PubMed] [Google Scholar]
- Hu ZG, Zhang J, Chan WL, Szeto YS. The sorption of acid dye onto chitosan nanoparticles. Polymer (guildf) 2006 doi: 10.1016/j.polymer.2006.05.071. [DOI] [Google Scholar]
- Hutson ND, Yang RT. Theoretical basis for the Dubinin-Radushkevitch (D-R) adsorption isotherm equation. Adsorption. 1997 doi: 10.1007/BF01650130. [DOI] [Google Scholar]
- Ibrahim E, Zhang M, Zhang Y, et al. Green-synthesization of silver nanoparticles using endophytic bacteria isolated from garlic and its antifungal activity against wheat fusarium head blight pathogen Fusarium graminearum. Nanomaterials. 2020 doi: 10.3390/nano10020219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igwe JC, Abia AA (2006) A bioseparation process for removing heavy metals from waste water using biosorbents. Afr J Biotechnol
- Ingle A, Rai M, Gade A, Bawaskar M. Fusarium solani: A novel biological agent for the extracellular synthesis of silver nanoparticles. J Nanoparticle Res. 2009 doi: 10.1007/s11051-008-9573-y. [DOI] [Google Scholar]
- Ingle AP, Seabra AB, Duran N, Rai M (2014) Nanoremediation. In: Microbial biodegradation and bioremediation. Elsevier, pp 233–250
- Iravani S. Green synthesis of metal nanoparticles using plants. Green Chem. 2011;13:2638. doi: 10.1039/c1gc15386b. [DOI] [Google Scholar]
- Iwahori K, Watanabe J, Tani Y, et al. Removal of heavy metal cations by biogenic magnetite nanoparticles produced in Fe(III)- reducing microbial enrichment cultures. J Biosci Bioeng. 2014 doi: 10.1016/j.jbiosc.2013.08.013. [DOI] [PubMed] [Google Scholar]
- Jain D, Kachhwaha S, Jain R, et al (2010) Novel microbial route to synthesize silver nanoparticles using spore crystal mixture of Bacillus thuringiensis. Indian J Exp Biol [PubMed]
- Jain D, Kumar Daima H, Kachhwaha S, Kothari SL (2009) Synthesis of plant-mediated silver nanoparticles using papaya fruit extract and evaluation of their anti microbial activities. Dig J Nanomater Biostruct
- Jain R, Dominic D, Jordan N, et al. Preferential adsorption of Cu in a multi-metal mixture onto biogenic elemental selenium nanoparticles. Chem Eng J. 2016 doi: 10.1016/j.cej.2015.08.144. [DOI] [Google Scholar]
- Jain R, Dominic D, Jordan N, et al. Higher Cd adsorption on biogenic elemental selenium nanoparticles. Environ Chem Lett. 2016 doi: 10.1007/s10311-016-0560-8. [DOI] [Google Scholar]
- Jain R, Jordan N, Schild D, et al. Adsorption of zinc by biogenic elemental selenium nanoparticles. Chem Eng J. 2015 doi: 10.1016/j.cej.2014.09.057. [DOI] [Google Scholar]
- Jia L, Zhang Q, Li Q, Song H. The biosynthesis of palladium nanoparticles by antioxidants in Gardenia jasminoides Ellis: Long lifetime nanocatalysts for p-nitrotoluene hydrogenation. Nanotechnology. 2009 doi: 10.1088/0957-4484/20/38/385601. [DOI] [PubMed] [Google Scholar]
- Jiang S, Lee JH, Kim MG, et al. Biogenic formation of As-S nanotubes by diverse Shewanella strains. Appl Environ Microbiol. 2009 doi: 10.1128/AEM.00450-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing R, Fusi S, Kjellerup B V. (2018) Remediation of polychlorinated biphenyls (PCBs) in contaminated soils and sediment: state of knowledge and perspectives. Front Environ Sci
- Jo JH, Singh P, Kim YJ, et al. Pseudomonas deceptionensis DC5-mediated synthesis of extracellular silver nanoparticles. Artif Cells, Nanomed Biotechnol. 2016 doi: 10.3109/21691401.2015.1068792. [DOI] [PubMed] [Google Scholar]
- Joerger R, Klaus T, Granqvist CG. Biologically produced silver-carbon composite materials for optically functional thin-film coatings. Adv Mater. 2000 doi: 10.1002/(SICI)1521-4095(200003)12:6<407::AID-ADMA407>3.0.CO;2-O. [DOI] [Google Scholar]
- Juibari MM, Abbasalizadeh S, Jouzani GS, Noruzi M. Intensified biosynthesis of silver nanoparticles using a native extremophilic Ureibacillus thermosphaericus strain. Mater Lett. 2011 doi: 10.1016/j.matlet.2010.12.056. [DOI] [Google Scholar]
- Kalishwaralal K, Deepak V, Ram Kumar Pandian SB, et al. Biosynthesis of silver and gold nanoparticles using Brevibacterium casei. Coll Surf B Biointerfaces. 2010 doi: 10.1016/j.colsurfb.2010.02.007. [DOI] [PubMed] [Google Scholar]
- Kamath V, Chandra P, Jeppu GP. Comparative study of using five different leaf extracts in the green synthesis of iron oxide nanoparticles for removal of arsenic from water. Int J Phytoremediation. 2020 doi: 10.1080/15226514.2020.1765139. [DOI] [PubMed] [Google Scholar]
- Karthik R, Sasikumar R, Chen SM, et al (2016) Green synthesis of platinum nanoparticles using Quercus glauca extract and its electrochemical oxidation of hydrazine in water samples. Int J Electrochem Sci 10.20964/2016.10.62
- Kasthuri J, Veerapandian S, Rajendiran N. Biological synthesis of silver and gold nanoparticles using apiin as reducing agent. Coll Surf B Biointerfaces. 2009 doi: 10.1016/j.colsurfb.2008.09.021. [DOI] [PubMed] [Google Scholar]
- Kaur R, Bhatti SS, Singh S, Singh J, Singh S. Phytoremediation of heavy metals using cotton plant: A field analysis. Bull Environ Contam Toxicol B Environ Contam Tox. 2018;101:637–643. doi: 10.1007/s00128-018-2472-8. [DOI] [PubMed] [Google Scholar]
- Ken DS, Sinha A. Recent developments in surface modification of nano zero-valent iron (nZVI): Remediation, toxicity and environmental impacts. Environ Nanotechnol Monit Manag. 2020;14:100344. doi: 10.1016/j.enmm.2020.100344. [DOI] [Google Scholar]
- Khan MI, Cheema SA, Anum S, et al (2020) Phytoremediation of agricultural pollutants. pp 27–81
- Khatoon H, Rai JPN. Augmentation of Atrazine biodegradation by two Bacilli immobilized on α-Fe2O3 magnetic nanoparticles. Sci Rep. 2018;8:17831. doi: 10.1038/s41598-018-36296-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi F, Kato Y, Furihata K, et al. Formation of gold nanoparticles by glycolipids of Lactobacillus casei. Sci Rep. 2016 doi: 10.1038/srep34626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YM, Murugesan K, Chang YY, et al. Degradation of polybrominated diphenyl ethers by a sequential treatment with nanoscale zero valent iron and aerobic biodegradation. J Chem Technol Biotechnol. 2012 doi: 10.1002/jctb.2699. [DOI] [Google Scholar]
- Kitching H, Kenyon AJ, Parkin IP. The interaction of gold and silver nanoparticles with a range of anionic and cationic dyes. Phys Chem Chem Phys. 2014;16:6050–6059. doi: 10.1039/C3CP55366C. [DOI] [PubMed] [Google Scholar]
- Klaus-Joerger T, Joerger R, Olsson E, Granqvist CG (2001) Bacteria as workers in the living factory: Metal-accumulating bacteria and their potential for materials science. Trends Biotechnol. [DOI] [PubMed]
- Klaus T, Joerger R, Olsson E, Granqvist CG. Silver-based crystalline nanoparticles, microbially fabricated. Proc Natl Acad Sci U S A. 1999 doi: 10.1073/pnas.96.24.13611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konwar Boruah S, Medhi;Okhil Kumar Medhi C, Kumar Boruah P, Sarma P, Green synthesis of gold nanoparticles using Camellia Sinensis and kinetics of the reaction. Adv Mater Lett. 2012 doi: 10.5185/amlett.2012.icnano.103. [DOI] [Google Scholar]
- Kora AJ, Rastogi L. Green synthesis of palladium nanoparticles using gum ghatti (Anogeissus latifolia) and its application as an antioxidant and catalyst. Arab J Chem. 2018 doi: 10.1016/j.arabjc.2015.06.024. [DOI] [Google Scholar]
- Koul B, Taak P. Biotechnological strategies for effective remediation of polluted soils. Singapore: Springer; 2018. [Google Scholar]
- Kowshik M, Ashtaputre S, Kharrazi S, et al. Extracellular synthesis of silver nanoparticles by a silver-tolerant yeast strain MKY3. Nanotechnology. 2003;14:95–100. doi: 10.1088/0957-4484/14/1/321. [DOI] [Google Scholar]
- Kumar Petla R, Vivekanandhan S, Misra M, et al. Soybean (Glycine Max) leaf extract based green synthesis of palladium nanoparticles. J Biomater Nanobiotechnol. 2012;03:14–19. doi: 10.4236/jbnb.2012.31003. [DOI] [Google Scholar]
- Kumar P, Kumar A, Kumar R. Phytoremediation and nanoremediation. In: Kumar R, Kumar R, Kaur G, editors. New frontiers of nanomaterials in environmental science. Singapore: Springer; 2021. pp. 281–297. [Google Scholar]
- Labrenz M, Druschel GK, Thomsen-Ebert T, et al. Formation of sphalerite (ZnS) deposits in natural biofilms of sulfate-reducing bacteria. Science (80-) 2000 doi: 10.1126/science.290.5497.1744. [DOI] [PubMed] [Google Scholar]
- Langmuir I. The constitution and fundamental properties of solids and liquids. Part I. Solids. J Am Chem Soc. 1916 doi: 10.1021/ja02268a002. [DOI] [Google Scholar]
- Langmuir I. The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc. 1918 doi: 10.1021/ja02242a004. [DOI] [Google Scholar]
- Lárez Velásquez C. Chitosan-based nanomaterials on controlled bioactive agents delivery: a review. J Anal Pharm Res. 2018;7:484–489. doi: 10.15406/japlr.2018.07.00271. [DOI] [Google Scholar]
- Le TT, Nguyen KH, Jeon JR, et al. Nano/bio treatment of polychlorinated biphenyls with evaluation of comparative toxicity. J Hazard Mater. 2015 doi: 10.1016/j.jhazmat.2015.02.001. [DOI] [PubMed] [Google Scholar]
- Le TT, Yoon H, Son MH, et al. Treatability of hexabromocyclododecane using Pd/Fe nanoparticles in the soil-plant system: Effects of humic acids. Sci Total Environ. 2019 doi: 10.1016/j.scitotenv.2019.06.290. [DOI] [PubMed] [Google Scholar]
- Li J, Li Q, Ma X, et al. Biosynthesis of gold nanoparticles by the extreme bacterium Deinococcus radiodurans and an evaluation of their antibacterial properties. Int J Nanomed. 2016 doi: 10.2147/IJN.S119618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Shen Y, Xie A, et al. Rapid, room-temperature synthesis of amorphous selenium/protein composites using Capsicum annuum L extract. Nanotechnology. 2007 doi: 10.1088/0957-4484/18/40/405101. [DOI] [Google Scholar]
- Li Y, Du X, Wu C, et al. An efficient magnetically modified microbial cell biocomposite for carbazole biodegradation. Nanoscale Res Lett. 2013 doi: 10.1186/1556-276X-8-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y, Guo M, Fan C, et al. Development of novel urease-responsive pendimethalin microcapsules using silica-IPTS-PEI As controlled release carrier materials. ACS Sustain Chem Eng. 2017;5:4802–4810. doi: 10.1021/acssuschemeng.7b00208. [DOI] [Google Scholar]
- Liu Y, Li S, Chen Z, et al. Influence of zero-valent iron nanoparticles on nitrate removal by Paracoccus sp. Chemosphere. 2014 doi: 10.1016/j.chemosphere.2014.02.045. [DOI] [PubMed] [Google Scholar]
- Lyu H, Tang J, Cui M, et al. Biochar/iron (BC/Fe) composites for soil and groundwater remediation: synthesis, applications, and mechanisms. Chemosphere. 2020;246:125609. doi: 10.1016/j.chemosphere.2019.125609. [DOI] [PubMed] [Google Scholar]
- Ma X, Sharifan H, Dou F, Sun W. Simultaneous reduction of arsenic (As) and cadmium (Cd) accumulation in rice by zinc oxide nanoparticles. Chem Eng J. 2020;384:123802. doi: 10.1016/j.cej.2019.123802. [DOI] [Google Scholar]
- Madhavi V, Prasad TNVKV, Reddy AVB, et al. Application of phytogenic zerovalent iron nanoparticles in the adsorption of hexavalent chromium. Spectrochim Acta - Part A Mol Biomol Spectrosc. 2013 doi: 10.1016/j.saa.2013.06.045. [DOI] [PubMed] [Google Scholar]
- Maliszewska I, Juraszek A, Bielska K. Green synthesis and characterization of silver nanoparticles using ascomycota fungi Penicillium nalgiovense AJ12. J Clust Sci. 2014 doi: 10.1007/s10876-013-0683-z. [DOI] [Google Scholar]
- Mall D, Larsen A, Martin E. Investigating the (Mis)Match between natural pest control knowledge and the intensity of pesticide use. Insects. 2018;9:2. doi: 10.3390/insects9010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallikarjuna K, Narasimha G, Dillip GR, et al (2011) Green synthesis of silver nanoparticles using Ocimum leaf extract and their characterization. Dig J Nanomater Biostructures
- Mandal D, Bolander ME, Mukhopadhyay D, et al (2006) The use of microorganisms for the formation of metal nanoparticles and their application. Appl Microbiol Biotechnol [DOI] [PubMed]
- Mano Priya M, Karunai Selvi B, John Paul JA (2011) Green synthesis of silver nanoparticles from the leaf extracts of Euphorbia hirta and Nerium indicum. Dig J Nanomater Biostruct
- Manquián-Cerda K, Cruces E, Angélica Rubio M, et al. Preparation of nanoscale iron (oxide, oxyhydroxides and zero-valent) particles derived from blueberries: Reactivity, characterization and removal mechanism of arsenate. Ecotoxicol Environ Saf. 2017 doi: 10.1016/j.ecoenv.2017.07.004. [DOI] [PubMed] [Google Scholar]
- Marathe K, Naik J, Maheshwari V. Biogenic synthesis of silver nanoparticles using Streptomyces spp. and their antifungal activity against Fusarium verticillioides. J Clust Sci. 2021 doi: 10.1007/s10876-020-01894-5. [DOI] [Google Scholar]
- Marcelo CR, Puiatti GA, Nascimento MA, et al. Degradation of the reactive blue 4 Dye in aqueous solution using zero-valent copper nanoparticles. J Nanomater. 2018;2018:1–9. doi: 10.1155/2018/4642038. [DOI] [Google Scholar]
- Markus J, Mathiyalagan R, Kim YJ, et al. Intracellular synthesis of gold nanoparticles with antioxidant activity by probiotic Lactobacillus kimchicus DCY51T isolated from Korean kimchi. Enzyme Microb Technol. 2016 doi: 10.1016/j.enzmictec.2016.08.018. [DOI] [PubMed] [Google Scholar]
- Martel R, Schmidt T, Shea HR, et al. Single- and multi-wall carbon nanotube field-effect transistors. Appl Phys Lett. 1998;73:2447–2449. doi: 10.1063/1.122477. [DOI] [Google Scholar]
- Marziali L, Guzzella L, Salerno F, et al. Twenty-year sediment contamination trends in some tributaries of Lake Maggiore (Northern Italy): relation with anthropogenic factors. Environ Sci Pollut Res. 2021;28:38193–38208. doi: 10.1007/s11356-021-13388-6. [DOI] [PubMed] [Google Scholar]
- Matouq M, Jildeh N, Qtaishat M, et al. The adsorption kinetics and modeling for heavy metals removal from wastewater by Moringa pods. J Environ Chem Eng. 2015;3:775–784. doi: 10.1016/j.jece.2015.03.027. [DOI] [Google Scholar]
- Mechrez G, Krepker MA, Harel Y, et al. Biocatalytic carbon nanotube paper: A “one-pot” route for fabrication of enzyme-immobilized membranes for organophosphate bioremediation. J Mater Chem B. 2014 doi: 10.1039/c3tb21439g. [DOI] [PubMed] [Google Scholar]
- Mohanpuria P, Rana NK, Yadav SK (2008) Biosynthesis of nanoparticles: technological concepts and future applications. J. Nanoparticle Res
- Mohsenzadeh F, Rad AC. Bioremediation of heavy metal pollution by nano-particles of Noaea Mucronata (2012) Int J Biosci Biochem Bioinforma. 2012;2:2. [Google Scholar]
- Mourato A, Gadanho M, Lino AR, Tenreiro R. Biosynthesis of crystalline silver and gold nanoparticles by extremophilic yeasts. Bioinorg Chem Appl. 2011 doi: 10.1155/2011/546074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mubarak Ali D, Sasikala M, Gunasekaran M, Thajuddin N (2011) Biosynthesis and characterization of silver nanoparticles using marine cyanobacterium, oscillatoria willei ntdm01. Dig J Nanomater Biostruct
- MubarakAli D, Thajuddin N, Jeganathan K, Gunasekaran M. Plant extract mediated synthesis of silver and gold nanoparticles and its antibacterial activity against clinically isolated pathogens. Coll Surf B Biointerfaces. 2011 doi: 10.1016/j.colsurfb.2011.03.009. [DOI] [PubMed] [Google Scholar]
- Mukherjee D, Ghosh S, Majumdar S, Annapurna K. Green synthesis of α-Fe2O3 nanoparticles for arsenic(V) remediation with a novel aspect for sludge management. J Environ Chem Eng. 2016 doi: 10.1016/j.jece.2015.12.010. [DOI] [Google Scholar]
- Mura S, Seddaiu G, Bacchini F, et al. Advances of nanotechnology in agro-environmental studies. Ital J Agron. 2013;8:127–140. doi: 10.4081/ija.2013.e18. [DOI] [Google Scholar]
- Murugadoss G, Rajamannan B, Madhusudhanan U (2009) Synthesis and characterization of water-soluble ZnS: Mn2+ nanocrystals. Chalcogenide Lett
- Nadhe SB, Wadhwani SA, Singh R, Chopade BA. Green synthesis of AuNPs by Acinetobacter sp GWRVA25: optimization, characterization, and its antioxidant activity. Front Chem. 2020 doi: 10.3389/fchem.2020.00474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair B, Pradeep T. Coalescence of nanoclusters and formation of submicron crystallites assisted by Lactobacillus strains. Cryst Growth Des. 2002 doi: 10.1021/cg0255164. [DOI] [Google Scholar]
- Nanda A, Saravanan M. Biosynthesis of silver nanoparticles from Staphylococcus aureus and its antimicrobial activity against MRSA and MRSE. Nanomed Nanotechnol Biol Med. 2009 doi: 10.1016/j.nano.2009.01.012. [DOI] [PubMed] [Google Scholar]
- Narayanan KB, Sakthivel N. Coriander leaf mediated biosynthesis of gold nanoparticles. Mater Lett. 2008 doi: 10.1016/j.matlet.2008.08.044. [DOI] [Google Scholar]
- Neveen MK. Biogenic silver nanoparticles by Aspergillus terreus as a powerful nanoweapon against Aspergillus fumigatus. African J Microbiol Res. 2013 doi: 10.5897/ajmr2013.6429. [DOI] [Google Scholar]
- Newkome GR, Yao ZQ, Baker GR, Gupta VK (1985) Cascade molecules: a new approach to micelles.1aA [27]-Arborol. J Org Chem
- Nithya R, Ragunathan R (2009) Synthesis of silver nanoparticle using Pleurotus sajor caju and its antimicrobial study. Dig J Nanomater Biostruct
- Nzila A, Razzak S, Zhu J. Bioaugmentation: an emerging strategy of industrial wastewater treatment for reuse and discharge. Int J Environ Res Public Health. 2016;13:846. doi: 10.3390/ijerph13090846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olalekan A., Dada A., Okewale A. (2013) Comparative adsorption isotherm study of the removal of Pb2+ and Zn2+ onto agricultural waste. Res J Chem Environ Sci
- Pandey G. Prospects of nanobioremediation in environmental cleanup. Orient J Chem. 2018;34:2838–2850. doi: 10.13005/ojc/340622. [DOI] [Google Scholar]
- Parashar V, Parashar R, Sharma B, Pandey AC (2009) Parthenium leaf extract mediated synthesis of silver nanoparticles: a novel approach towards weed utilization. Dig J Nanomater Biostruct
- Parida UK, Bindhani BK, Nayak P. Green synthesis and characterization of gold nanoparticles using onion (Allium cepa) extract. World J Nano Sci Eng. 2011 doi: 10.4236/wjnse.2011.14015. [DOI] [Google Scholar]
- Pathak PD, Mandavgane SA, Kulkarni BD. Fruit peel waste as a novel low-cost bio adsorbent. Rev Chem Eng. 2015 doi: 10.1515/revce-2014-0041. [DOI] [Google Scholar]
- Peixoto RS, Vermelho AB, Rosado AS (2011) Petroleum-degrading enzymes: bioremediation and new prospects. Enzyme Res. [DOI] [PMC free article] [PubMed]
- Perez-Gonzalez T, Jimenez-Lopez C, Neal AL, et al. Magnetite biomineralization induced by Shewanella oneidensis. Geochim Cosmochim Acta. 2010;74:967–979. doi: 10.1016/j.gca.2009.10.035. [DOI] [Google Scholar]
- Philip D (2009) Biosynthesis of Au, Ag and Au-Ag nanoparticles using edible mushroom extract. Spectrochim Acta - Part A Mol Biomol Spectrosc. 10.1016/j.saa.2009.02.037 [DOI] [PubMed]
- Philip D. Green synthesis of gold and silver nanoparticles using Hibiscus rosa sinensis. Phys E Low-Dimensional Syst Nanostruct. 2010 doi: 10.1016/j.physe.2009.11.081. [DOI] [Google Scholar]
- Phumying S, Labuayai S, Thomas C, et al. Aloe vera plant-extracted solution hydrothermal synthesis and magnetic properties of magnetite (Fe3O4) nanoparticles. Appl Phys A Mater Sci Process. 2013 doi: 10.1007/s00339-012-7340-5. [DOI] [Google Scholar]
- Pimprikar PS, Joshi SS, Kumar AR, et al. Influence of biomass and gold salt concentration on nanoparticle synthesis by the tropical marine yeast Yarrowia lipolytica NCIM 3589. Colloids Surf, B. 2009 doi: 10.1016/j.colsurfb.2009.07.040. [DOI] [PubMed] [Google Scholar]
- Poguberović SS, Krčmar DM, Dalmacija BD, et al. Removal of Ni(II) and Cu(II) from aqueous solutions using “green” zero-valent iron nanoparticles produced by oak and mulberry leaf extracts. Water Sci Technol. 2016 doi: 10.2166/wst.2016.387. [DOI] [PubMed] [Google Scholar]
- Ponmurugan P, Manjukarunambika K, Elango V, Gnanamangai BM. Antifungal activity of biosynthesised copper nanoparticles evaluated against red root-rot disease in tea plants. J Exp Nanosci. 2016 doi: 10.1080/17458080.2016.1184766. [DOI] [Google Scholar]
- Popescu M, Velea A, Lorinczi A (2010) Biogenic production of nanoparticles. Dig J Nanomater Biostructures
- Prasad KS, Gandhi P, Selvaraj K. Synthesis of green nano iron particles (GnIP) and their application in adsorptive removal of As(III) and As(V) from aqueous solution. Appl Surf Sci. 2014 doi: 10.1016/j.apsusc.2014.09.042. [DOI] [Google Scholar]
- Prasad R, Bhattacharyya A, Nguyen QD. Nanotechnology in sustainable agriculture: recent developments, challenges, and perspectives. Front Microbiol. 2017;8:1–13. doi: 10.3389/fmicb.2017.01014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priya Dhas S, Mukerjhee A, Chandrasekaran N (2013) Phytosynthesis of silver nanoparticles using Ceriops tagal and its antimicrobial potential against human pathogens. Int J Pharm Pharm Sci
- Priyadharshini Raman R, Parthiban S, Srinithya B, et al. Biogenic silver nanoparticles synthesis using the extract of the medicinal plant Clerodendron serratum and its in-vitro antiproliferative activity. Mater Lett. 2015 doi: 10.1016/j.matlet.2015.08.009. [DOI] [Google Scholar]
- Pu Y, Yang X, Zheng H, et al. Adsorption and desorption of thallium(I) on multiwalled carbon nanotubes. Chem Eng J. 2013;219:403–410. doi: 10.1016/j.cej.2013.01.025. [DOI] [Google Scholar]
- Pugazhenthiran N, Anandan S, Kathiravan G, et al. Microbial synthesis of silver nanoparticles by Bacillus sp. J Nanoparticle Res. 2009 doi: 10.1007/s11051-009-9621-2. [DOI] [Google Scholar]
- Rafi Shaik M, Ali ZJQ, Khan M, et al (2017) Green synthesis and characterization of palladium nanoparticles using Origanum vulgare L. extract and their catalytic activity. Molecules. 10.3390/molecules22010165 [DOI] [PMC free article] [PubMed]
- Raghunandan D, Bedre MD, Basavaraja S, et al. Rapid biosynthesis of irregular shaped gold nanoparticles from macerated aqueous extracellular dried clove buds (Syzygium aromaticum) solution. Coll Surfaces B Biointerfaces. 2010 doi: 10.1016/j.colsurfb.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Raj R, Dalei K, Chakraborty J, Das S. Extracellular polymeric substances of a marine bacterium mediated synthesis of CdS nanoparticles for removal of cadmium from aqueous solution. J Colloid Interface Sci. 2016 doi: 10.1016/j.jcis.2015.10.004. [DOI] [PubMed] [Google Scholar]
- Rajaram K, Aiswarya DC, Sureshkumar P. Green synthesis of silver nanoparticle using Tephrosia tinctoria and its antidiabetic activity. Mater Lett. 2015 doi: 10.1016/j.matlet.2014.10.017. [DOI] [Google Scholar]
- Rajeshkumar S, Santhiyaa RV (2018) Degradation dye using gold and silver nanoparticles synthesized by using green route and its characteristics. pp 221–240
- Rajput VD, Singh A, Minkina T, et al. Nano-enabled products: challenges and opportunities for sustainable agriculture. Plants. 2021;10:2727. doi: 10.3390/plants10122727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramezani M, Rad FA, Ghahari S, et al. Nano-bioremediation application for environment contamination by microorganism. In: Panpatte DG, Jhala YK, et al., editors. Microbial rejuvenation of polluted environment, microorganisms for sustainability. 26. Singapore: Springer; 2021. pp. 349–378. [Google Scholar]
- Ramírez-García R, Gohil N, Singh V (2018) Recent advances, challenges, and opportunities in bioremediation of hazardous materials. In: Phytomanagement of polluted sites: market opportunities in sustainable phytoremediation
- Rana N, Ghosh KS, Chand S, Gathania AK (2018) Investigation of ZnO nanoparticles for their applications in wastewater treatment and antimicrobial activity. Indian J Pure Appl Phys
- Rani K, Sridevi V. An overview on role of nanotechnology in green and clean technology. Austin Environ Sci. 2017;2:1–6. [Google Scholar]
- Raut RW, Kolekar NS, Lakkakula JR, et al. Extracellular synthesis of silver nanoparticles using dried leaves of Pongamia pinnata (L) pierre. Nano-Micro Lett. 2010;2:106–113. doi: 10.1007/BF03353627. [DOI] [Google Scholar]
- Ravikumar KVG, Kumar D, Kumar G, et al. Enhanced Cr(VI) removal by nanozerovalent iron-immobilized alginate beads in the presence of a biofilm in a continuous-flow reactor. Ind Eng Chem Res. 2016 doi: 10.1021/acs.iecr.6b01006. [DOI] [Google Scholar]
- Reátegui E, Reynolds E, Kasinkas L, et al. Silica gel-encapsulated AtzA biocatalyst for atrazine biodegradation. Appl Microbiol Biotechnol. 2012;96:231–240. doi: 10.1007/s00253-011-3821-2. [DOI] [PubMed] [Google Scholar]
- Riaz S, Raza ZA, Majeed MI. Preparation of cadmium sulfide nanoparticles and mediation thereof across poly (hydroxybutyrate) nanocomposite. Polym Bull. 2020 doi: 10.1007/s00289-019-02775-2. [DOI] [Google Scholar]
- Rizwan M, Singh M, Mitra CK, Morve RK. Ecofriendly application of nanomaterials: nanobioremediation. J Nanoparticles. 2014;2014:1–7. doi: 10.1155/2014/431787. [DOI] [Google Scholar]
- Rodríguez-León E, Iñiguez-Palomares R, Navarro RE, et al. Synthesis of silver nanoparticles using reducing agents obtained from natural sources (Rumex hymenosepalus extracts) Nanoscale Res Lett. 2013 doi: 10.1186/1556-276X-8-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saif S, Tahir A, Chen Y. Green synthesis of iron nanoparticles and their environmental applications and implications. Nanomaterials. 2016;6:209. doi: 10.3390/nano6110209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saifuddin N, Wong CW, Yasumira AAN. Rapid biosynthesis of silver nanoparticles using culture supernatant of bacteria with microwave irradiation. E-Journal Chem. 2009 doi: 10.1155/2009/734264. [DOI] [Google Scholar]
- Sana SS, Badineni VR, Arla SK, Naidu Boya VK. Eco-friendly synthesis of silver nanoparticles using leaf extract of Grewia flaviscences and study of their antimicrobial activity. Mater Lett. 2015 doi: 10.1016/j.matlet.2015.01.096. [DOI] [Google Scholar]
- Sanghi R, Verma P. Biomimetic synthesis and characterisation of protein capped silver nanoparticles. Bioresour Technol. 2009 doi: 10.1016/j.biortech.2008.05.048. [DOI] [PubMed] [Google Scholar]
- Sastry M, Ahmad A, Islam Khan M, Kumar R (2003) Biosynthesis of metal nanoparticles using fungi and actinomycete. Curr Sci
- Sathishkumar M, Sneha K, Kwak IS, et al. Phyto-crystallization of palladium through reduction process using Cinnamom zeylanicum bark extract. J Hazard Mater. 2009 doi: 10.1016/j.jhazmat.2009.06.014. [DOI] [PubMed] [Google Scholar]
- Sathvika T, Soni A, Sharma K, et al. Potential application of saccharomyces cerevisiae and rhizobium immobilized in multi walled carbon nanotubes to adsorb hexavalent chromium. Sci Rep. 2018 doi: 10.1038/s41598-018-28067-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathyavathi R, Krishna MB, Rao SV, et al. Biosynthesis of silver nanoparticles using Coriandrum sativum leaf extract and their application in nonlinear optics. Adv Sci Lett. 2010 doi: 10.1166/asl.2010.1099. [DOI] [Google Scholar]
- Saxena G, Purchase D, Mulla SI, et al (2019) Phytoremediation of heavy metal-contaminated sites: eco-environmental concerns, field studies, sustainability issues, and future prospects. pp 71–131 [DOI] [PubMed]
- Sebeia N, Jabli M, Ghith A, Saleh TA. Eco-friendly synthesis of Cynomorium coccineum extract for controlled production of copper nanoparticles for sorption of methylene blue dye. Arab J Chem. 2020 doi: 10.1016/j.arabjc.2019.07.007. [DOI] [Google Scholar]
- Selvi KV, Sivakumar T (2012) Isolation and characterization of silver nanoparticles from Fusarium oxysporum. Int J Curr Microbiol Appl Sci
- Seshadri S, Saranya K, Kowshik M (2011) Green synthesis of lead sulfide nanoparticles by the lead resistant marine yeast, Rhodosporidium diobovatum. Biotechnol Prog. 10.1002/btpr.651 [DOI] [PubMed]
- Shahi SK, Patra M (2003) Microbially synthesized bioactive nanoparticles and their formulation active against human pathogenic fungi. Rev Adv Mater Sci
- Shan GB, Xing JM, Zhang HY, Liu HZ. Biodesulfurization of dibenzothiophene by microbial cells coated with magnetite nanoparticles. Appl Environ Microbiol. 2005 doi: 10.1128/AEM.71.8.4497-4502.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar SS, Ahmad A, Pasricha R, Sastry M. Bioreduction of chloroaurate ions by geranium leaves and its endophytic fungus yields gold nanoparticles of different shapes. J Mater Chem. 2003 doi: 10.1039/b303808b. [DOI] [Google Scholar]
- Shankar SS, Ahmad A, Sastry M. Geranium leaf assisted biosynthesis of silver nanoparticles. Biotechnol Prog. 2003 doi: 10.1021/bp034070w. [DOI] [PubMed] [Google Scholar]
- Shankar SS, Rai A, Ahmad A, Sastry M. Rapid synthesis of Au, Ag, and bimetallic Au core-Ag shell nanoparticles using Neem (Azadirachta indica) leaf broth. J Colloid Interface Sci. 2004 doi: 10.1016/j.jcis.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Shanthi S, David Jayaseelan B, Velusamy P, et al. Biosynthesis of silver nanoparticles using a probiotic Bacillus licheniformis Dahb1 and their antibiofilm activity and toxicity effects in Ceriodaphnia cornuta. Microb Pathog. 2016 doi: 10.1016/j.micpath.2016.01.014. [DOI] [PubMed] [Google Scholar]
- Sharma R, Chandra BP, Bisen DP (2009a) Thermoluminescence and optical absorption spectra of ZnS:Mn nanoparticles. Chalcogenide Lett
- Sharma VK, Yngard RA, Lin Y (2009b) Silver nanoparticles: green synthesis and their antimicrobial activities. Adv Colloid Interface Sci [DOI] [PubMed]
- Shekhawat GS, Arya V (2009) Biological synthesis of Ag nanoparticles through in vitro cultures of Brassica juncea C. zern. In: Advanced materials research
- Shi Z, Fan D, Johnson RL, et al. Methods for characterizing the fate and effects of nano zerovalent iron during groundwater remediation. J Contam Hydrol. 2015;181:17–35. doi: 10.1016/j.jconhyd.2015.03.004. [DOI] [PubMed] [Google Scholar]
- Shukla N, Debnath A, Banerjee S. Sonochemical synthesis of silica supported iron nanoparticles for enhanced removal of Cr(VI) species from aqueous medium. Nanotechnol Environ Eng. 2022;7:2. doi: 10.1007/s41204-021-00171-8. [DOI] [Google Scholar]
- Singh BK. Exploring microbial diversity for biotechnology: the way forward. Trends Biotechnol. 2010;28:111–116. doi: 10.1016/j.tibtech.2009.11.006. [DOI] [PubMed] [Google Scholar]
- Singh BN, Hidangmayum A, Singh A, et al (2020a) Effect of emerging contaminants on crops and mechanism of toxicity. In: Lichtfouse E (eds) Sustainable agriculture reviews 40: 217–241. 10.1007/978-3-030-33281-5_6
- Singh P, Singh H, Kim YJ, et al. Extracellular synthesis of silver and gold nanoparticles by Sporosarcina koreensis DC4 and their biological applications. Enzyme Microb Technol. 2016 doi: 10.1016/j.enzmictec.2016.02.005. [DOI] [PubMed] [Google Scholar]
- Singh R, Behera M, Kumar S. Nano-bioremediation: an innovative remediation technology for treatment and management of contaminated sites. Bioremed Ind Waste Environ Saf. 2020 doi: 10.1007/978-981-13-3426-9_7. [DOI] [Google Scholar]
- Singh R, Manickam N, Mudiam MKR, et al. An integrated (nano-bio) technique for degradation of γ-HCH contaminated soil. J Hazard Mater. 2013;258–259:35–41. doi: 10.1016/j.jhazmat.2013.04.016. [DOI] [PubMed] [Google Scholar]
- Sinha A, Singh VN, Mehta BR, Khare SK. Synthesis and characterization of monodispersed orthorhombic manganese oxide nanoparticles produced by Bacillus sp. cells simultaneous to its bioremediation. J Hazard Mater. 2011;192:620–627. doi: 10.1016/j.jhazmat.2011.05.103. [DOI] [PubMed] [Google Scholar]
- Sintubin L, De Windt W, Dick J, et al. Lactic acid bacteria as reducing and capping agent for the fast and efficient production of silver nanoparticles. Appl Microbiol Biotechnol. 2009 doi: 10.1007/s00253-009-2032-6. [DOI] [PubMed] [Google Scholar]
- Slijepčević N, Pilipović DT, Kerkez Đ, et al. A cost effective method for immobilization of Cu and Ni polluted river sediment with nZVI synthesized from leaf extract. Chemosphere. 2021 doi: 10.1016/j.chemosphere.2020.127816. [DOI] [PubMed] [Google Scholar]
- Smitha SL, Philip D, Gopchandran KG. Green synthesis of gold nanoparticles using Cinnamomum zeylanicum leaf broth. Spectrochim Acta - Part A Mol Biomol Spectrosc. 2009 doi: 10.1016/j.saa.2009.08.007. [DOI] [PubMed] [Google Scholar]
- Sohail MI, Waris AA, Ayub MA, et al (2019) Environmental application of nanomaterials: a promise to sustainable future. In: Comprehensive analytical chemistry
- Song JY, Jang HK, Kim BS. Biological synthesis of gold nanoparticles using Magnolia kobus and Diopyros kaki leaf extracts. Process Biochem. 2009 doi: 10.1016/j.procbio.2009.06.005. [DOI] [Google Scholar]
- Song JY, Kim BS. Biological synthesis of bimetallic Au/Ag nanoparticles using Persimmon (Diopyros kaki) leaf extract. Korean J Chem Eng. 2008 doi: 10.1007/s11814-008-0133-z. [DOI] [PubMed] [Google Scholar]
- Song JY, Kwon EY, Kim BS. Biological synthesis of platinum nanoparticles using Diopyros kaki leaf extract. Bioprocess Biosyst Eng. 2010 doi: 10.1007/s00449-009-0373-2. [DOI] [PubMed] [Google Scholar]
- Soundarrajan C, Sankari A, Dhandapani P, et al. Rapid biological synthesis of platinum nanoparticles using Ocimum sanctum for water electrolysis applications. Bioprocess Biosyst Eng. 2012 doi: 10.1007/s00449-011-0666-0. [DOI] [PubMed] [Google Scholar]
- Sunanda MM, Ghosh Sachan S. Nanobioremediation of heavy metals: perspectives and challenges. J Basic Microbiol. 2021 doi: 10.1002/jobm.202100384. [DOI] [Google Scholar]
- Sunkar S, Nachiyar CV (2012) Synthesis and characterization of biologically synthesized antibacterial silver nanoparticles using the endophytic bacterium Bacillus Cereus: a novel source in the benign synthesis. Glob J Med Res [DOI] [PMC free article] [PubMed]
- Sweeney RY, Mao C, Gao X, et al. Bacterial biosynthesis of cadmium sulfide nanocrystals. Chem Biol. 2004 doi: 10.1016/j.chembiol.2004.08.022. [DOI] [PubMed] [Google Scholar]
- Thul T, S, K Sarangi B, Avatar Pandey R, Nanotechnology in agroecosystem: implications on plant productivity and its soil environment. Exp Opin Environ Biol. 2013 doi: 10.4172/2325-9655.1000101. [DOI] [Google Scholar]
- Tan W, Peralta-Videa JR, Gardea-Torresdey JL (2018) Interaction of titanium dioxide nanoparticles with soil components and plants: current knowledge and future research needs-a critical review. Environ Sci Nano
- Tang H, Chen X, Niu YW, et al. Thermal stability characteristics of in situ nano-particles formed in metal melt. Mater Lett. 2016 doi: 10.1016/j.matlet.2015.09.052. [DOI] [Google Scholar]
- Tariq F, Ahmed N, Afzal M, et al. Synthesis, characterization and antimicrobial activity of bacillus subtilis - derived silver nanoparticles against multidrug-resistant bacteria. Jundishapur J Microbiol. 2020 doi: 10.5812/jjm.91934. [DOI] [Google Scholar]
- Taylor A, Wilson KM, Murray P, et al. Long-term tracking of cells using inorganic nanoparticles as contrast agents: are we there yet? Chem Soc Rev. 2012 doi: 10.1039/c2cs35031a. [DOI] [PubMed] [Google Scholar]
- Temkin MI, Pyzhev V. Kinetics of ammonia synthesis on promoted iron catalyst. Acta Phys Chem. 1940;12:327–356. [Google Scholar]
- Thakkar KN, Mhatre SS, Parikh RY (2010) Biological synthesis of metallic nanoparticles. Nanomed Nanotechnol Biol Med [DOI] [PubMed]
- Thirumurugan A, Aswitha P, Kiruthika C, et al. Green synthesis of platinum nanoparticles using Azadirachta indica - An eco-friendly approach. Mater Lett. 2016 doi: 10.1016/j.matlet.2016.02.026. [DOI] [Google Scholar]
- Thirumurugan A, Jiflin GJ, Rajagomathi G, et al (2010) Biotechnological synthesis of gold nanoparticles of Azadirachta indica leaf extract. Int J Biol Technol
- Tratnyek PG, Johnson RL. Nanotechnologies for environmental cleanup. Nano Today. 2006;1:44–48. doi: 10.1016/S1748-0132(06)70048-2. [DOI] [Google Scholar]
- Tripathy A, Raichur AM, Chandrasekaran N, et al. Process variables in biomimetic synthesis of silver nanoparticles by aqueous extract of Azadirachta indica (Neem) leaves. J Nanoparticle Res. 2010 doi: 10.1007/s11051-009-9602-5. [DOI] [Google Scholar]
- Umashankari J, Inbakandan D, Ajithkumar TT, Balasubramanian T. Mangrove plant, Rhizophora mucronata (Lamk, 1804) mediated one pot green synthesis of silver nanoparticles and its antibacterial activity against aquatic pathogens. Aquat Biosyst. 2012 doi: 10.1186/2046-9063-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vankar PS, Bajpai D (2010) Preparation of gold nanoparticles from Mirabilis jalapa flowers. Indian J Biochem Biophys [PubMed]
- Vázquez-Núñez E, Molina-Guerrero CE, Peña-Castro JM, et al. Use of nanotechnology for the bioremediation of contaminants: a review. Processes. 2020;8:1–17. doi: 10.3390/pr8070826. [DOI] [Google Scholar]
- Verma A, Dua R, Singh A, Bishnoi NR. Biogenic sulfides for sequestration of Cr (VI), COD and sulfate from synthetic wastewater. Water Sci. 2015;29:19–25. doi: 10.1016/j.wsj.2015.03.001. [DOI] [Google Scholar]
- Verma A, Joshi P, Arya A (2013) Synthesis of plant-mediated silver nanoparticles using plant extract of Sonchus asper. Int J Nanotechnol Appl
- Vieira RHSF, Volesky B. Biosorption: a solution to pollution? Int Microbiol. 2000 doi: 10.2436/im.v3i1.9237. [DOI] [PubMed] [Google Scholar]
- Vigneshwaran N, Ashtaputre NM, Varadarajan PV, et al. Biological synthesis of silver nanoparticles using the fungus Aspergillus flavus. Mater Lett. 2007 doi: 10.1016/j.matlet.2006.07.042. [DOI] [Google Scholar]
- Vigneshwaran N, Kathe AA, Varadarajan P V, et al (2006) Biomimetics of silver nanoparticles by white rot fungus, Phaenerochaete chrysosporium. Colloids Surfaces B Biointerfaces. 10.1016/j.colsurfb.2006.07.014 [DOI] [PubMed]
- Villalobos M, Escobar-Quiroz IN, Salazar-Camacho C. The influence of particle size and structure on the sorption and oxidation behavior of birnessite: I. Adsorption of As(V) and oxidation of As(III) Geochim Cosmochim Acta. 2014;125:564–581. doi: 10.1016/j.gca.2013.10.029. [DOI] [Google Scholar]
- Wadhwani SA, Shedbalkar UU, Singh R, et al. Kinetics of Synthesis of Gold Nanoparticles by Acinetobacter sp. SW30 Isolated from environment. Indian J Microbiol. 2016 doi: 10.1007/s12088-016-0598-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Zhang B, Liu Q, et al. Biosynthesis of palladium nanoparticles using: Shewanella loihica PV-4 for excellent catalytic reduction of chromium(VI) Environ Sci Nano. 2018 doi: 10.1039/c7en01167a. [DOI] [Google Scholar]
- Wang X, Gai Z, Yu B, et al. Degradation of carbazole by microbial cells immobilized in magnetic gellan gum gel beads. Appl Environ Microbiol. 2007 doi: 10.1128/AEM.01051-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zhang D, Pan X, et al. Aerobic and anaerobic biosynthesis of nano-selenium for remediation of mercury contaminated soil. Chemosphere. 2017 doi: 10.1016/j.chemosphere.2016.12.020. [DOI] [PubMed] [Google Scholar]
- Wang Y, Morin G, Ona-Nguema G, et al. Distinctive arsenic(V) trapping modes by magnetite nanoparticles induced by different sorption processes. Environ Sci Technol. 2011 doi: 10.1021/es200299f. [DOI] [PubMed] [Google Scholar]
- Wang Z, Zhang J, Wen T, et al. Highly effective remediation of Pb(II) and Hg(II) contaminated wastewater and soil by flower-like magnetic MoS2 nanohybrid. Sci Total Environ. 2020;699:134341. doi: 10.1016/j.scitotenv.2019.134341. [DOI] [PubMed] [Google Scholar]
- Watts MP, Coker VS, Parry SA, et al. Effective treatment of alkaline Cr(VI) contaminated leachate using a novel Pd-bionanocatalyst: impact of electron donor and aqueous geochemistry. Appl Catal B Environ. 2015 doi: 10.1016/j.apcatb.2015.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber TW, Chakravorti RK. Pore and solid diffusion models for fixed-bed adsorbers. AIChE J. 1974 doi: 10.1002/aic.690200204. [DOI] [Google Scholar]
- Wei Y, Fang Z, Zheng L, Tsang EP. Biosynthesized iron nanoparticles in aqueous extracts of Eichhornia crassipes and its mechanism in the hexavalent chromium removal. Appl Surf Sci. 2017 doi: 10.1016/j.apsusc.2016.12.090. [DOI] [Google Scholar]
- De Windt W, Aelterman P, Verstraete W. Bioreductive deposition of palladium (0) nanoparticles on Shewanella oneidensis with catalytic activity towards reductive dechlorination of polychlorinated biphenyls. Environmental Microbiology. 2005 doi: 10.1111/j.1462-2920.2005.00696.x. [DOI] [PubMed] [Google Scholar]
- Xiao X, Liu QY, Lu XR, et al. Self-assembly of complex hollow CuS nano/micro shell by an electrochemically active bacterium Shewanella oneidensis MR-1. Int Biodeterior Biodegrad. 2017 doi: 10.1016/j.ibiod.2016.09.021. [DOI] [Google Scholar]
- Xiao Z, Yuan M, Yang B, et al. Plant-mediated synthesis of highly active iron nanoparticles for Cr (VI) removal: investigation of the leading biomolecules. Chemosphere. 2016 doi: 10.1016/j.chemosphere.2016.02.056. [DOI] [PubMed] [Google Scholar]
- Xiu Z, ming, Jin Z hui, Li T long,, et al. Effects of nano-scale zero-valent iron particles on a mixed culture dechlorinating trichloroethylene. Bioresour Technol. 2010 doi: 10.1016/j.biortech.2009.09.057. [DOI] [PubMed] [Google Scholar]
- Yadav DK, Srivastava S. Carbon nanotubes as adsorbent to remove heavy metal ion (Mn +7) in wastewater treatment. Mater Today Proc. 2017;4:4089–4094. doi: 10.1016/j.matpr.2017.02.312. [DOI] [Google Scholar]
- Yadav KK, Singh JK, Gupta N, Kumar V. A review of nanobioremediation technologies for environmental cleanup: a novel biological approach. J Mater Environ Sci. 2017;8:740–757. [Google Scholar]
- Yan FF, Wu C, Cheng YY, et al. Carbon nanotubes promote Cr(VI) reduction by alginate-immobilized Shewanella oneidensis MR-1. Biochem Eng J. 2013 doi: 10.1016/j.bej.2013.06.009. [DOI] [Google Scholar]
- Yang X, Li Q, Wang H, et al. Green synthesis of palladium nanoparticles using broth of Cinnamomum camphora leaf. J Nanoparticle Res. 2010 doi: 10.1007/s11051-009-9675-1. [DOI] [Google Scholar]
- Zhang C, Li M, Xu X, Liu N. Effects of carbon nanotubes on atrazine biodegradation by Arthrobacter sp. J Hazard Mater. 2015;287:1–6. doi: 10.1016/j.jhazmat.2015.01.039. [DOI] [PubMed] [Google Scholar]
- Zhang H, Li Q, Lu Y, et al. Biosorption and bioreduction of diamine silver complex by Corynebacterium. J Chem Technol Biotechnol. 2005 doi: 10.1002/jctb.1191. [DOI] [Google Scholar]
- Zhou D, Kim D-G, Ko S-O. Heavy metal adsorption with biogenic manganese oxides generated by Pseudomonas putida strain MnB1. J Ind Eng Chem. 2015;24:132–139. doi: 10.1016/j.jiec.2014.09.020. [DOI] [Google Scholar]
- Zhou H, Zhang H, He Y, et al. Critical review of reductant-enhanced peroxide activation processes: trade-off between accelerated Fe3+/Fe2+ cycle and quenching reactions. Appl Catal B Environ. 2021;286:119900. doi: 10.1016/j.apcatb.2021.119900. [DOI] [Google Scholar]
- Zhu Y, Xu F, Liu Q, et al. Nanomaterials and plants: Positive effects, toxicity and the remediation of metal and metalloid pollution in soil. Sci Total Environ. 2019;662:414–421. doi: 10.1016/j.scitotenv.2019.01.234. [DOI] [PubMed] [Google Scholar]
- Zhuang H, Han H, Xu P, et al. Biodegradation of quinoline by Streptomyces sp. N01 immobilized on bamboo carbon supported Fe3O4 nanoparticles. Biochem Eng J. 2015;99:44–47. doi: 10.1016/j.bej.2015.03.004. [DOI] [Google Scholar]