Abstract
STIP1-homologous U-Box containing protein 1 (STUB1) is involved in the development of immune pathologies and the regulation of T cell. However, the potential role of STUB1 in the pathogenesis of rheumatoid arthritis (RA), especially in the regulation of T cells, remains elusive. Here we show that STUB1 promotes the imbalance of Th17/Treg cells through non-degradative ubiquitination of aryl hydrocarbon receptor (AHR). Using Western blot and flow cytometry analysis, we observe that the level of STUB1 was increased in RA patients compared with healthy controls. In particular, the expression of STUB1 protein was different in Th17 cells and Treg cells of RA patients. We also demonstrated that STUB1 facilitates Th17/Treg imbalance by up- or downregulating the expression of STUB1. In a subsequent series of in vitro experiments, we revealed that STUB1 promoted the imbalance of Th17 and Treg cells through non-degradative ubiquitination of AHR. Both knockdown of the AHR expression by siRNA and assays of CYP1A1 enzymatic activity by ethoxyresorufin-O-deethylase (EROD) supported this conclusion. Furthermore, we explored the ubiquitination sites of AHR responsible for STUB1-mediated ubiquitination and revealed that STUB1 promotes ubiquitination of AHR via K63 chains. Together, STUB1 may induce the imbalance of Th17/Treg cells via ubiquitination of AHR and serve as a potential therapeutic target for RA.
Keywords: STUB1, Th17/Treg imbalance, aryl hydrocarbon receptor, ubiquitination, rheumatoid arthritis
CHIP level was upregulated in Th17 cells and downregulated in Treg cells in RA patients. - CHIP induced Th17/Treg imbalance via AHR pathway. - AHR was modified by CHIP for K63-linked ubiquitination and involved in the Th17/Treg imbalance.
Introduction
Rheumatoid arthritis (RA) is a typical chronic inflammatory autoimmune disease with persistent inflammatory cell infiltration of synovium leading to the progressive destruction of cartilage and bone [1]. Although the exact immunopathological effects and underlying intracellular regulatory mechanisms of RA is incompletely understood, lines of the evidence have validated the dysregulation of various molecules in immune cell including CD4+ T cell as well as the cooperation and activation of multiple cytokines and chemokines work in the pathogenesis of the disease [2, 3]. Particularly, disturbed Th17/Treg cell balance plays a critical role in the early onset and the progression of RA [4, 5]. It has been confirmed that T helper (Th)17 cells was overactivated in the development of RA [6]. However, regulatory T (Treg) cells, which maintain the immune homeostasis and mediate the anti-inflammatory response, was suppressed in this process [7]. Patients with RA, especially those in the active phase, had a significantly higher Th17/Treg ratio compared with healthy individuals [8]. Rectifying the Th17/Treg imbalance, therefore, is critical for the remission and treatment of RA.
Ubiquitination has long been acknowledged as a post-translational modification involved in the inflammatory signaling cascade [9]. Recent studies confirmed and further revealed the critical role of STIP1-homologous U-Box containing protein 1 (STUB1, also known as CHIP), an E3 ubiquitin ligase, in proinflammatory pathways and the development of various types of immune pathologies, especially in the regulation of T cell [10–12]. Consistently, the earlier study of our group indicated that STUB1 was implicated in the pathology of inflammatory bone loss in RA [13]. Although STUB1 is known to perform important roles in immune regulation, whether STUB1 participates in the imbalance of Th17/Treg cells in RA has not been described in any of the previously studies.
Available evidence supports a central role for aryl hydrocarbon receptor (AHR) in immune regulation [14]. AHR, a ligand-activated transcription factor, mediates the adaptive responses to exogenous or endogenous compounds including 2, 3, 7, 8-Tetrachlorodibenzo-p-dioxin (TCDD) and 6-formylindolo [3,2-b] carbazole (FICZ) [15, 16]. AHR is activated by various ligands and involved in the generation of Th17 cells and regulatory T cells during immune responses [17, 18]. As reported in previous studies, E3 ubiquitin ligase STUB1 is associated with the regulation of AHR complexes via hsp90 (a part of the AHR complex). STUB1 can promote the ubiquitination of mature unliganded AHR complexes cooperating with unidentified factors [15], suggesting that STUB1 may act as a novel ligand to activate the AHR pathway.
Based upon these studies, we speculated that STUB1 might regulate the imbalance of Th17/Treg cells by targeting AHR in RA. In this project, our group set out to investigate a novel aspect of the molecular mechanism that mediates the Th17/Treg imbalance in RA. We reported that E3 ligase STUB1 as a potent factor induces the imbalance of Th17/Treg cells, which is dependent on the ubiquitination of AHR. Our study may provide a new theoretical basis for the investigation of the pathogenesis of RA, as well as a basis for subsequent studies on the signaling pathways involved in the immune imbalance between STUB1 and Th17/Treg cells.
Materials and methods
Subjects
Peripheral blood samples were obtained from 22 RA patients (in the active stage) and 20 healthy donors. All bloods were collected in tubes with sodium citrate. The synovial fluid (SF) samples were collected from 8 patients with RA and 8 osteoarthritis patients (OA) included as a control group. RA was diagnosed according to the American College of Rheumatology/European League Against Rheumatism 2010 diagnostic criteria [19]. Also, the protein level of STUB1 in Th17 and Treg cells as well as ubiquitination of AHR were determined among RA patients and normal control. Studies were performed with approval from the ethics committee of Nanjing Medical University and informed written consent was obtained from every donor. Patients and controls were age and sex matched in all experiments.
Cell separation and culture
Peripheral blood mononuclear cells (PBMCs) and synovial fluid mononuclear cells (SFMCs) were isolated by Ficoll density gradient centrifugation (Sigma-Aldrich). CD4+ T cells were isolated using the CD4+T cell magnetic isolation kit (Miltenyi). CD4+T cells were cultured in RPMI-1640 medium (Gibco) supplemented with 10% FBS (Gibco) in 5% CO2 at 37 °C. Th17 and Treg cells were purified from peripheral blood mononuclear cells (PBMCs) of RA patients and healthy subjects using a fluorescence-activated cell sorting (FACS) flow cytometer (BD Biosciences, San Jose, CA, USA). The HEK293T cells and Jurkat T cells were maintained in DMEM and RPMI-1640 medium (Gibco), respectively.
Transfection of plasmids and siRNAs
The transfection of HEK293T cells, Jurkat T cells and CD4+ T cells were performed using lipofectamine 2000 (Invitrongen) according to the manufacturer’s instructions. The cells were cultured in the presence or absence of anti-CD3 and anti-CD28 mAbs for 48 h after transfection and then collected for future experiments. The expression constructs and siRNA include Flag-AHR, Myc-STUB1, HA-Ub, His-Ub (WT), K63R, K48R, and siSTUB1.
Isolated CD4+T cells were transfected with lentivirus vector or siRNA was also performed. For up- or downregulation of STUB1, the CD4+ T cells were transfected with LV-STUB1 (ID: NM_005861) or LV-sh-STUB1 (target sequence: 5ʹ-GGGACGACATCCCCAGCGCTCT-3ʹ) or with either negative control lentiviral vector (LV-NC) according to previously described procedures (Genechem, Shanghai, China) [20]. CD4+T cells also transfected with AHR siRNA (siAHR) or control siRNA (si-NC) according to the manufacturer’ s instructions. Both STUB1 and AHR siRNAs were purchased from Invitrogen. The transfection efficiency was detected by qRT-PCR after 48 h and Western blot analysis after 72 h (Supplementary Fig. S1).
Co-immunoprecipitation (co-IP) and Western blotting
HEK293 T cells were lysed in lysis buffer and the lysates were collected. Protein G PLUS Agarose Immunoprecipitation Reagent (Santa Cruz) was used for co-immunoprecipitation (co-IP) analysis according to the manufacturer’s indicated procedures. Cell lysates were incubated with anti-Flag or anti-Myc as well as protein G-Sepharose at 4 °C for overnight and the immunoprecipitates were analyzed by Western blotting with primary antibodies.
Western blot analysis was executed as delineated in previous studies [21]. Antibodies used are as follows: anti-STUB1, anti-AHR, anti-β-actin (Abcam), anti-ubiquitin (Cell Signaling), anti-CYP1A1, anti-HA (F-7), anti-His (H-3), anti-Flag (D-8), and anti-Myc (9E10) (Santa Cruz). Quantification of band density indicating protein amount was performed using Image J software.
Ubiquitination assays
Ubiquitination assay was performed as previously described [22]. Transfected HEK293T and Jurkat T cells as well as CD4+ T cells collected from PBMCs were harvested and lysed. Western blotting was used to detect the degree of ubiquitination and measure precipitated proteins with various antibodies.
CD4+ T-cell differentiation in vitro
The transfected CD4+ T cells (1 × 106 cells/ml) were harvested and stimulated with plate-bound anti-CD3 (5 mg/ml) and anti-CD28 (2 mg/ml) mAbs for 5 days. Where indicated, FICZ was added to cells. The cells were cultured under different skewing conditions: Th17 cells: IL-1β (10 ng/ml), IL-6 (10 ng/ml), IL-23 (10 ng/ml), and TGF-β (10 ng/ml); Treg cells: IL-2 (10 ng/ml) and TGF-β (10 ng/ml). The levels of IL-17A, IL-6, TNF-α, IL-10, and TGF-β in supernatant were measured using ELISA kits (Biolegend, USA) according to the manufacturer’s instructions.
Quantitative real-time polymerase chain reaction
Transfected CD4+T cells were harvested, and RNA was isolated and reverse transcribed as described previously [23]. Results were normalized to the corresponding β-actin level and the expression of detected genes was presented as the fold change relative to that of the control.
Flow cytometry
For the assessment of T-cell differentiation, flow cytometry was performed as described previously [1]. Transfected CD4+T cells were harvested and then were stimulated with PMA/ionomycin for 5 h. Golgi-Stop (BD Phar-Mingen) was added for the final 2hrs. Cells were surface stained with FITC conjugated anti-CD4 and PE conjugated anti-CD25. Subsequently, Cytofix/Cytoperm (BD PharMingen) was used to fix and permeabilize cells before intracellular staining with APC-Cy7 conjugated anti-IL-17 and APC-conjugated anti-IFN-γ. For Foxp3 staining, cells were stained with APC-conjugated anti-FoxP3 intracellularly after fixing and permeabilizing. Th1 cells, Th17 cells and Treg cells were identified as CD4+ IFN-γ+, CD4+ IL-17+ and CD4+ CD25+ Foxp3+, respectively. The corresponding representative plots of the gating strategies was shown in Supplementary Fig. S2. For the detection of STUB1 level in Th1, Th17, or Treg cells, STUB1 antibody (JG38-22) was purchased from Novus Biologicals. Flow cytometric analysis was performed with Cytomics FC500 flow cytometer (Beckman Coulter) and data were analyzed using FlowJo software (Three Star Inc.).
Ethoxyresorufin-O-deethylase (EROD) assay
Incubated isolated CD4+ T cells with or without FICZ for 24 h. CD4+ T cells overexpressing STUB1 also transfected with AHR siRNA (siAHR) or control siRNA (si-NC). Ethoxyresorufin-O-deethylase (EROD) assay was performed to measure CYP1A1 activity, as described in our previous studies [24].
Statistical analysis
All of the statistical analyses were performed with Prism v.9 (GraphPad Software, La Jolla, CA). The data are presented as mean ± SD. To assess the statistical significance, the P-values were calculated using unpaired two-tailed Student’s t-test. P-values less than 0.05 was considered statistically significant.
Results
The expression of STUB1 in Th17 and Treg cells of RA patients
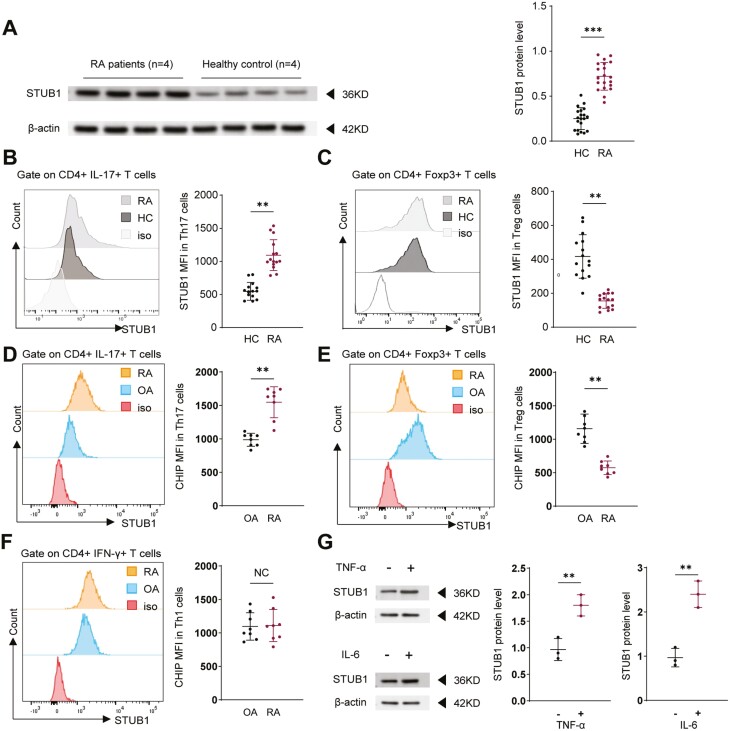
Given the potential role of STUB1 in the pathogenesis of autoimmune diseases as well as its effect on T-cell activation and signaling pathway [11, 25], we investigated whether STUB1 is also dysregulated in RA. The protein level of STUB1 was measured in CD4+ T cells from RA patients and healthy controls (HC). We found that protein level of STUB1 in CD4+ T cells was significantly increased in patients with RA compared with normal subjects (Fig. 1A). We then evaluated the expression of STUB1 on or in Th17 and Treg cells from the peripheral blood (Fig. 1B,C) and SF (Fig. 1D,E). Strikingly, the expression of STUB1 protein in Th17 (CD4+IL-17+) cells was also higher in RA patients comparing with healthy populations (Fig. 1B,D). However, the lower level of STUB1 was observed in Treg (CD4+Foxp3+) cells of patients with RA (Fig. 1C,E). We also investigated the expression of STUB1 on Th1 (CD4+IFN-γ+) cells in RA versus HC. There was no significant difference in the expression level of STUB1 in Th1 cells between RA patients and HC (Fig. 1F), which revealed that the increased expression of STUB1 is unique to Th17 cells. Considering RA is a chronic autoimmune disease characterized by a failure of spontaneous resolution of inflammation. We further investigated whether the presence of pro-inflammatory cytokines (such as TNF-α or IL-6) induced STUB1 expression in RA patients. As expected, the expression of STUB1 was elevated in the presence of inflammatory stimulation (Fig. 1G). The clinical characteristics of RA patients and HC are summarized in Table. Taken together, our results indicated the potential role of STUB1 in modulating CD4 + T cell differentiation.
Figure 1.
: The STUB1 protein level in patients with rheumatoid arthritis (RA) and control groups. (A) CD4+ T cells were isolated from RA patients (n = 4) and healthy controls (n = 4). The expressive protein levels of STUB1 were performed by Western blot. The STUB1 protein levels were quantified by band intensity and normalized to β-actin levels. STUB1 protein expression in CD4+IL-17+ T (Th17) cells (B) and CD4+Foxp3+ T (Treg) cells (C) from RA PB (n = 15) and HCs PB(n = 15). (D-F) STUB1 levels in the SF of RA patients were detected, and osteoarthritis patients (OA) were included as a control group. The expression of STUB1 in Th17 cells (D) and Treg cells (E) from RA SF (n = 8) and controls (OA) SF(n = 8). (F) The expression of STUB1 in Th1 cells from SF of RA patients (n = 8) and controls (OA) (n = 8). (G) CD4+ T cells were stimulated with or without TNF-α and IL-6, respectively. The levels of STUB1 were performed by Western blot and data are representative of three independent experiments. ** P <.01 and *** P <.001 vs. healthy controls (Student’s t test). Error bars show mean ± SEM. STUB1, STIP1-homologous U-Box containing protein 1.
STUB1 promotes the imbalance of Th17/Treg cells in RA patients
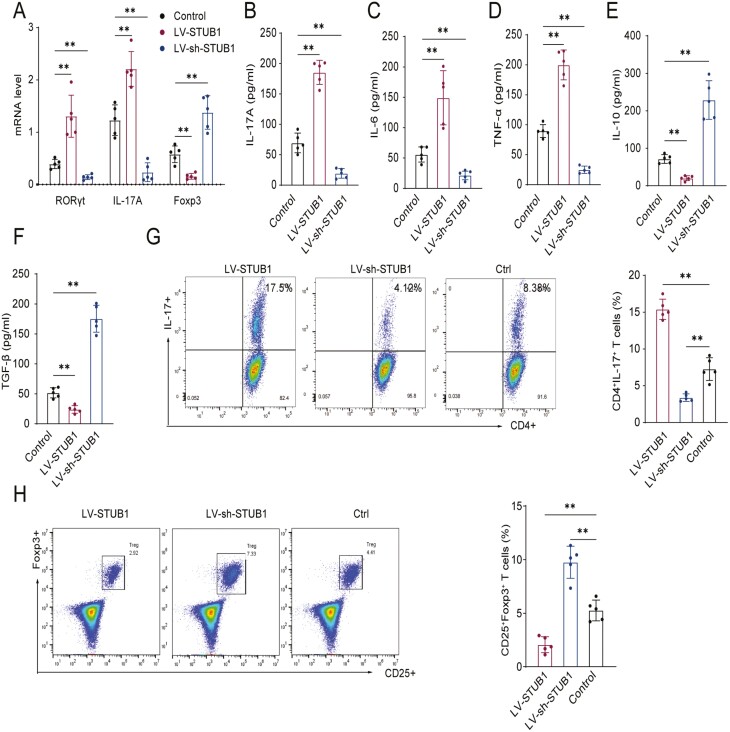
Because the broken Th17/Treg balance including overactivated Th17 cells has been recognized as one of the critical pathological cells in pathogenesis of RA [8, 26, 27], we determined to explore whether STUB1 regulated CD4+ T cell differentiation in pathogenesis of RA. To this end, we sorted CD4+ T cell from peripheral blood of RA patients and normal subjects, and then up-regulated or down-regulated the expression of STUB1 by transfection of lentivirus-expressing STUB1 (LV-STUB1) and LV-sh-STUB1, respectively. It was manifested that mRNA expressions of RORγt and IL-17A were elevated but the expression of Foxp3 was reduced while STUB1 was up-regulated in LV-STUB1-transfected cells versus control group (Fig. 2A). Conversely, STUB1 down-regulation decreased the mRNA levels of RORγt and IL-17A but increased the level of Foxp3 (Fig. 2A). Moreover, the concentrations of functional cytokines of Th17 cell (IL-17A, IL-6, TNF-α) and Treg cell (IL-10, TGF-β) in the supernatants were also examined after stimulation. Compared with LV-sh-STUB1–transfected cells, the levels of the cytokines such as IL-17A, IL-6, and TNF-α in the supernatant of LV-STUB1–transfected cells were markedly elevated (Fig. 2B-D). In contrast, the levels of IL-10 and TGF-β were decreased after the upregulation of STUB1 (Fig. 2E, F).
Figure 2:
STUB1 affectes Th17 and Treg cell polarization from naive CD4+ T cell. Transfected the lentivirus-expressing STUB1 (LV-STUB1) and LV-sh-STUB1 in isolated CD4+ T cell, stimulated with plate-bound anti-CD3 (5 mg/mL) and anti-CD28 (2 mg/mL) mAbs, and cultured under specific conditions for 5 days. (A) The expression of RORγt, IL-17A and Foxp3 mRNA was evaluated by qRT-PCR in control, LV-STUB1–transfected and LV-sh-STUB1-transfected cells. (B-F) The concentration of IL-17A, IL-6, TNF-α, IL-10 and TGF-β in cell supernatant was detected by ELISA. (G, H) Transfected CD4+ T cells were stimulated with anti-CD3 (5 mg/mL) and anti-CD28 (2 mg/mL) mAbs with Th17 and Treg-polarizing condition, respectively. The proportion of Th17 (CD4+IL-17+) and Treg (CD25+Foxp3+) cells was detected by flow cytometry. Percentages of Th17 cells and Treg cells are shown in the bar. ** P <.01 vs. control groups (Student’s t test). Data are representative of three independent experiments. Error bars show mean ± SEM. IL, interleukin; TNF-α, tumor necrosis factor-α; TGF-β, transforming growth factor-β; qRT-PCR, real-time reverse transcription-polymerase chain reaction.
Flow cytometry analysis was performed to further confirm the alteration of Th17/Treg cell balance. Consistently, flow cytometry analysis got similar results with qRT-PCR assays. It was noticed that with respect to the LV-sh-STUB1–transfected cells, the percentage of Th17 (CD4+IL-17+) cells was increased (Fig. 2G) while that of Treg (CD25+Foxp3+) cells was decreased in CD4+ T cells transfected with LV-STUB1 (Fig. 2H). However, the lower percentage of Th17 cells (Fig. 2G) while the higher percentage of Treg cells was observed in LV-sh-STUB1-transfected cells comparing with control group (Fig. 2H). Collectively, the results shown above suggested that STUB1 had a regulatory effect on the differentiation and balance of Th17/Treg cells.
STUB1 can physically interact with AHR
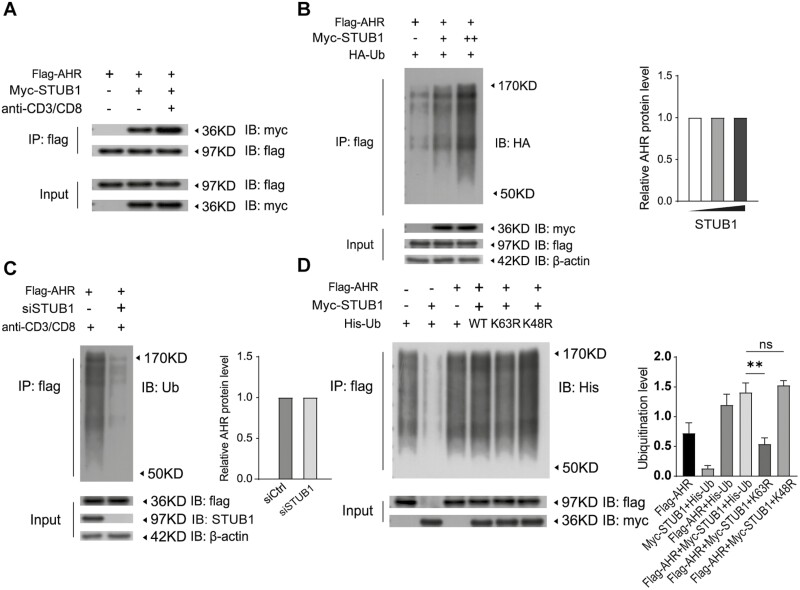
Accumulating evidence suggests that AHR was participated in the differentiation of Th17 and Treg cell as a regulator [1, 28, 29]. Based on our aforementioned results, therefore, we hypothesized that STUB1 might regulate Th17/Treg balance by targeting AHR. Therefore, to investigate whether AHR acts as one of the targets of STUB1 and participates in the STUB1-mediated the Th17 and Treg cell differentiation, coimmunoprecipitation (co-IP) study was performed. As could be seen from co-IP results, AHR coprecipitated with STUB1 in HEK293T cells as well as in CD4+ T cells co-transfected with tagged AHR or STUB1 expression constructs (Fig. 3A), suggesting that STUB1 physically associated with AHR. Intriguingly, this association was substantially enhanced in CD4+ T cells stimulated with anti-CD3 and anti-CD28 mAbs.
Figure 3:
STUB1 physically associates with AHR and promotes the ubiquitination of AHR. (A) Interaction between STUB1 and AHR in the lysates of CD4+ T cells that had been transfected with the indicated plasmids and then were stimulated without or with anti-CD3/anti-CD8. Samples were subjected to immunoprecipitation (IP) with anti-Flag antibody. The immunoprecipitates were immunoblotted (IB) with indicated antibody. (B) Flag-AHR and HA-Ub were co-transfected with different amounts of Myc-STUB1 into HEK293T cells. Polyubiquitination of AHR was detected with the indicated antibody. Cell extracts were immunoblotted with antibody to Flag or Myc tag. β-actin served as a loading control. The AHR protein levels are shown in the bar. (C) Ubiquitination assay for AHR in Jurkat T cells cotransfected with or without STUB1 siRNA and the indicated plasmids. Cell lysates were immunoprecipitated with antibody against Flag and the complex were immunoblotted with antibody to Ubiquitin. Immunoblotting with the indicated antibodies was used to detect the protein level of AHR, STUB1, or β-actin in cell lysates. The AHR protein levels are shown in the bar. (D) HEK293T cells were transfected with Myc-STUB1 and Flag-AHR together with plasmid encoding His-Ub (WT) or the ubiquitin mutants K48R or K63R. The cells were lysed for Co-IP as indicated. The ubiquitination levels are shown in the bar. Data are representative of three independent experiments.
STUB1 catalyzes the non-degradative ubiquitination of AHR
Since STUB1 is an E3 ubiquitin ligase, we next ascertained whether STUB1 plausibly has potential to induce the ubiquitination of AHR. To this end, Myc-STUB1 and Flag-AHR expression plasmids were then co-transfected with HA-Ubiquitin to check whether the ubiquitination status of AHR was affected by STUB1 or not. We found that AHR polyubiquitination was significantly enhanced in HEK293T cells when it was co-transfected with STUB1 (Fig. 3B). Further, STUB1 promoted AHR polyubiquitination in a dose-dependent manner (Fig. 3B). Moreover, co-IP and immunoblotting data revealed that knockdown of STUB1 by siRNA significantly impaired the ubiquitination of AHR in Jurkat T cell with anti-CD3 and anti-CD28 stimulation (Fig. 3C). Of note, the alteration in ubiquitination status of AHR, either in HEK293T cells or Jurkat T cells, without any changes in its protein levels (Fig. 3B, C). Together, these findings indicated that STUB1 catalyzes the AHR via a non-degradative ubiquitin modification.
STUB1 promotes ubiquitination of AHR via K63 chains
Further, we sought to gain insight into the kind of ubiquitination of AHR might be affected by STUB1 and identify the lysine residues of AHR responsible for STUB1-mediated ubiquitination. HEK293T cells were transfected with Myc-STUB1 and Flag-AHR together with or without His-Ubi (WT) or the ubiquitin mutants (K48R and K63R) and then ubiquitination of AHR was assessed by co-IP. As shown in Fig. 3D, the ubiquitination of AHR mediated by STUB1 was diminished in K63R mutant but not in K48R mutant or WT control. Moreover, there is no marked alteration on expression levels of AHR was observed in the ubiquitination modification of STUB1. Hence, these observations demonstrated that the STUB1 promoted the non-degradative ubiquitination of AHR through K63-linked polyubiquitin chains.
The STUB1-mediated imbalance of Th17/Treg cells in RA is an AHR-dependent manner
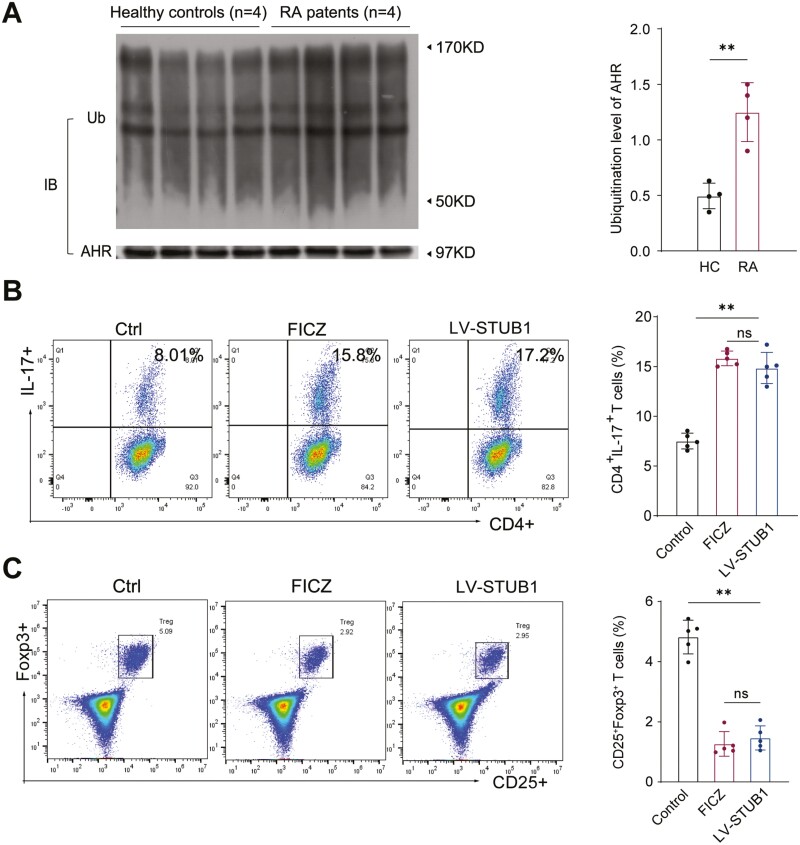
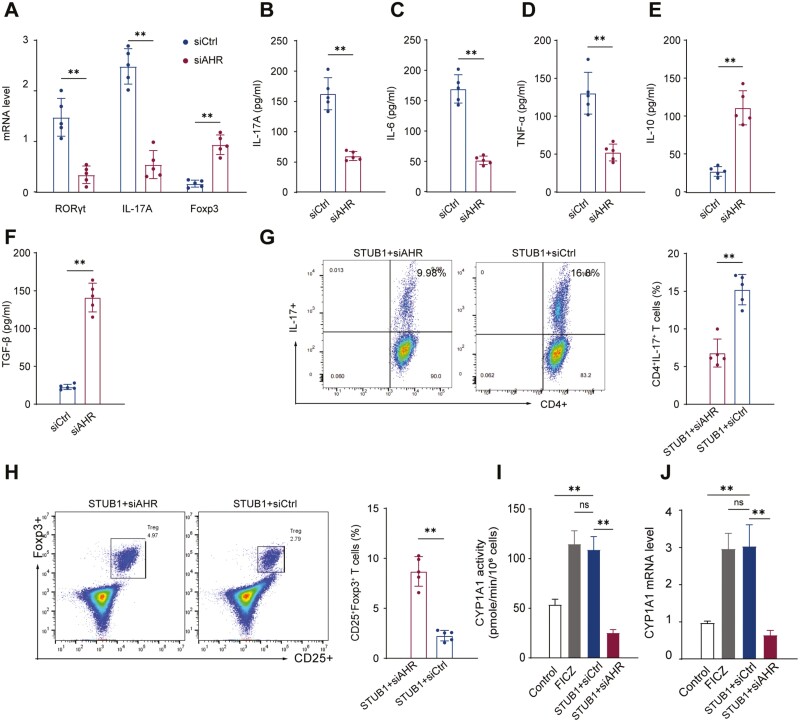
We further explored the regulatory mechanism of STUB1-mediated Th17/Treg cells balance. Given our previous findings, we inferred that AHR might involve in the STUB1-mediated balance of Th17 cells and Tregs. We initially examined the ubiquitination level of AHR in patients with RA and we found that AHR ubiquitination status was more pronounced in RA patients compared with HC according to the densitometric quantitation of ubiquitinated AHR normalized to total AHR from lysates of CD4+ T cells (Fig. 4A). FICZ, an endogenous agonist of AHR, can activate AHR signaling as a dynamic mediator [30]. Therefore, we compared effect of STUB1 on Th17/Treg cells with that of FICZ. As illustrated in Fig. 4B, C, both STUB1 overexpression and FICZ stimulation increased the proportion of Th17 cells and decreased the proportion of Treg cells in CD4 + T cells. Moreover, we transfected siAHR in CD4+ T cells overexpressing STUB1. Under the Th17- or Treg-polarizing conditions with anti-CD3/CD28 antibodies treatment in vitro, the expression of RORγt and IL-17A mRNA were markedly downregulated while the expression of Foxp3 mRNA was upregulated in conditions of AHR interference (Fig. 5A). Parallelly, the interference of AHR decreased IL-17A, IL-6 and TNF-α (Fig. 5B-D) but increased the IL-10 and TGF-β levels in the culture supernatant (Fig. 5E, F). Flow cytometry analysis confirmed that the proportion of Th17 (CD4+IL-17+) cells was reduced (Fig. 5G) while that of Treg (CD25+Foxp3+) cells was elevated following the knockdown of AHR (Fig. 5H), suggesting that the effect of STUB1 on Th17/Treg cells imbalance was disappeared. The cytochrome P450, family 1, subfamily A, and polypeptide 1 (CYP1A1), a canonical gene targets of AHR, is considered to be a functional bio-marker of activation of the AHR affecting the genomic signaling of AHR [31, 32]. Consistent with these findings, we found that the activity and expression of CYP1A1 was also increased during the dysregulation of Th17/Treg cells balance caused by STUB1 (Fig. 5I, J). All the results revealed that STUB1 promotes the imbalance of Th17/Treg cells in AHR-dependent manner.
Figure 4.
: STUB1 improves the imbalance of Th17/Treg cells in AHR-dependent manner. (A) Ubiquitination of AHR was increased in RA patients compared with healthy controls. Purified CD4+T cells from peripheral blood of RA patients (n = 4) and healthy controls (n = 4). AHR ubiquitination was detected with the indicated antibody. Densitometry was performed and quantitation of ubiquitinated AHR was normalized to total AHR from lysates. (B, C) Compared effect of STUB1 on Th17/Treg cells with that of FICZ. The proportion of Th17 (CD4+IL-17+) and Treg (CD25+Foxp3+) cells was detected by flow cytometry. Percentages of Th17 cells and Treg cells are shown in the bar. ** P < .01 vs. control groups. NS, no significant (Student’s t-test). Data are pooled from three independent experiments. Error bars show mean ± SEM.
Figure 5.
: AHR pathway involves in STUB1-mediated Th17/Treg cell imbalance. CD4+ T cells overexpressing STUB1 were transfected with siAHR or control siRNA and cultured under Th17 or Treg cells polarizing-conditions with anti-CD3/CD28 antibodies treatment. (A) RORγt, IL-17A and Foxp3 gene expression levels were determined by RT-qPCR. (B-F) The concentration of IL-17A, IL-6, TNF-α, IL-10 and TGF-β in supernatant was detected by ELISA. (G, H) The proportion of Th17 (CD4+IL-17+) cells and Treg (CD25+Foxp3+) cells was detected by flow cytometry. Percentages of Th17 cells and Treg cells are shown in the bar. (I, J) The mRNA levels and enzymatic activity of CYP1A1 were evaluated by qRT-PCR and EROD, respectively. ** P < .01 vs. control groups (Student’s t-test). Data are representative of three independent experiments. Error bars show mean ± SEM.
Discussion
The imbalance of CD4+ T lymphocyte subsets, especially Th17/Treg cells, is one of the key mechanisms underlying the pathogenesis of RA [4, 33, 34]. Accumulating evidences have highlighted the role of STUB1 in modulation of T cell [35–37]. Further, a number of studies have previously reported the role of STUB1 in autoimmune diseases such as systemic lupus erythematosus (SLE) [25]. However, how STUB1 engages in the regulation of T cell populations especially in RA remained elusive. Our group have demonstrated that STUB1, as a molecular mediator, was involved in the effect of TNF-α on osteoblast inhibition in RA [13], indicating a relationship between STUB1 and RA. In the present study, we found that STUB1 protein level is elevated in RA patients comparing with HC. Perhaps strikingly, our results showed that STUB1 protein is increased in Th17 cells from peripheral blood and SF of RA patients compared to normal subjects while it is reduced in their Treg cells, STUB1 was suggested as a possible contributor to aberrant balance of Th17/Treg cells in RA. Here, we illustrate the role of STUB1 in Th17/Treg cells imbalance by up- or downregulating the expression of STUB1 in CD4+ T cells.
The upregulation of STUB1 promoted Th17 cell differentiation while suppressed Treg differentiation as reflected in the qRT-PCR, ELISA and flow cytometry data. But the opposite results were obtained after downregulating the expression of STUB1. Although much is known about the imbalance of Th17 and Treg cells in RA [34, 38], the certain molecular regulations are still not well characterized. Our observation suggesting that STUB1 is a pivotal factor in the development of RA, and the rise of STUB1 in RA patients may play an important role in the imbalance of Th17/Treg cells during RA development. Previous researches have confirmed that AHR is necessary for Th17 and Treg cell differentiation [16, 39]. AHR activation by FICZ boosted Th17 cell differentiation, but interfered with Treg cell differentiation [29]. Interestingly, our results also shown that the upregulation of STUB1 in the peripheral blood of RA patients accompanied by enhanced ubiquitination level of AHR. Moreover, STUB1 overexpression can exert the same effect as FICZ, i.e., to activate the AHR pathway. These findings provide insights into the pathway of regulation of STUB1 on Th17 cells and Treg cells and suggests that STUB1 and AHR may be simultaneously implicated in the pathogenesis of RA.
We herein hypothesize that STUB1 may target AHR to regulate CD4+T cell differentiation as well as induce the imbalance of Th17/Treg cells. As expected, the results showed that the effect of STUB1 on Th17/Treg cells imbalance was disappeared in STUB1 over-expressed CD4+ T cells transfected with siAHR and the modulation of STUB1 on CD4+ T cell plasticity was also impaired after knocking down AHR, suggesting that the AHR pathway may be involved in STUB1-mediated Th17/Treg cell imbalance. In addition, both EROD and qRT-PCR analysis showed that the expression and activity of CYP1A1 (the central to the regulation of the AHR pathway) was also increased during the dysregulation of Th17/Treg cells balance caused by STUB1, which further suggesting that STUB1 induces the Th17/Treg imbalance via the AHR pathway. Thus, we decided to further verify the modification of AHR by STUB1 in regulating CD4+ T cell differentiation and have a more complete understanding of the specific mechanism by which STUB1 targets AHR to promote the imbalance of Th17/Treg cells. Of note, a vitro study previously indicated that STUB1 can interact with the AHR at cellular levels and promote the ubiquitination of AHR [15]. Consistent with this, co-immunoprecipitations of STUB1 and AHR in HEK293T cells and CD4+ T cells in our present study established the interaction between STUB1 and AHR at endogenous cellular levels.
STUB1 is a well-known cochaperone E3 ligase that targets its substrates and undergo regulatory ubiquitination or proteasomal degradation [40]. Therefore, we conducted the ubiquitination assays in vitro to probe whether STUB1 modifies AHR as previously described. The results indicated that STUB1 could promoted the polyubiquitination of AHR. Strikingly, we found that STUB1 catalyzes the ubiquitination of AHR without altering AHR protein levels, which is not consistent with previous reports that STUB1 degrades target substrates in a ubiquitination and proteasome dependent manner.
As described above, our present study revealed that AHR is a downstream target mediating the modification of STUB1 on Th17/Treg balance in patients with RA. Ubiquitination exerts diverse functions depending on the type of ubiquitin linkage on the substrates. Consistently, K48-linked polyubiquitination often targets proteins for degradation by the proteasomes, whereas K63-linked polyubiquitination often helps cellular signal transduction via non proteasomal mechanisms [35, 41]. In this context, we further explore the ubiquitination sites of AHR mediating the regulation of CD4+ T cell differentiation by STUB1. We demonstrated that K63-linked ubiquitination of AHR catalyzed by STUB1 may contribute to differentiation of Th17/Treg cells as well as Th17/Treg imbalance, which also supports the unchanged AHR protein levels that we have observed following the ubiquitination modification through STUB1. These findings provide the possibility that may account for the mechanism by which STUB1 promotes Th17/Treg imbalance during the process of RA.
Cumulatively, we have revealed that STUB1 acts as a critical factor of the imbalance of Th17/Treg cells highlighting the role of STUB1 in the pathogenesis of RA. Furthermore, STUB1 promoted the imbalance of Th17 and Treg cells through non-degradative ubiquitination of AHR. The current study provides new insight into the mechanism responsible for regulation of balance of Th17/Treg cells in RA. STUB1, therefore, may cause inflammatory reaction via post-transcriptional modification of AHR and serve as a potential therapeutic target for RA in the future.
Supplementary Material
Acknowledgements
We are grateful to all the patients who took part in this study as well as our colleagues at the Institute of Rheumatology for their help in recruiting patients for the study.
Glossary
Abbreviations
- AHR
aryl hydrocarbon receptor
- CHIP
carboxyl-terminus of Hsc70-interacting protein
- co-IP
co-immunoprecipitation
- CYP1A1
cytochrome P450, family 1, subfamily A, and polypeptide 1
- EROD
ethoxyresorufin-O-deethylase
- FACS
fluorescence-activated cell sorting
- FICZ
6-formylindolo [3,2-b] carbazole
- HC
healthy controls
- PBMCs
: peripheral blood mononuclear cells
- qRT-PCR
quantitative real-time polymerase chain reaction
- RA
rheumatoid arthritis
- SLE
systemic lupus erythematosus
- TCDD
2, 3, 7, 8-tetrachlorodibenzo-p-dioxin
Contributor Information
Wen Wang, Department of Rheumatology, the Second Affiliated Hospital of Nanjing Medical University, Nanjing, China.
Ting Xiang, Department of Rheumatology, the Second Affiliated Hospital of Nanjing Medical University, Nanjing, China.
Yachen Yang, Department of Rheumatology, the Second Affiliated Hospital of Nanjing Medical University, Nanjing, China.
Zitao Wang, Department of Rheumatology, the Second Affiliated Hospital of Nanjing Medical University, Nanjing, China.
Jianmin Xie, Department of Rheumatology, the Second Affiliated Hospital of Nanjing Medical University, Nanjing, China.
Funding
This study was supported by grants from the National Natural Science Foundation of China (No. 81871279), The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest disclosure
The authors declare that they have no competing interests.
Ethics approval and consent to participate
All patients gave written informed consent before any study-related procedures were performed. The study protocol was approved by the local institutional ethics committee of Nanjing Medical University ([2018] KY030).
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1. Nakahama T, Kimura A, Nguyen NT, Chinen I, Hanieh H, Nohara K, et al.. Aryl hydrocarbon receptor deficiency in T cells suppresses the development of collagen-induced arthritis. Proc Natl Acad Sci USA 2011, 108(34),14222–7. Epub 2011/08/10. doi: 10.1073/pnas.1111786108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Weyand CM, Goronzy JJ.. The immunology of rheumatoid arthritis. Nat Immunol 2021, 22(1),10–18. Epub 2020/12/02. doi: 10.1038/s41590-020-00816-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen Z, Bozec A, Ramming A, Schett G.. Anti-inflammatory and immune-regulatory cytokines in rheumatoid arthritis. Nat Rev Rheumatol 2019, 15(1),9–17. Epub 2018/10/21. doi: 10.1038/s41584-018-0109-2 [DOI] [PubMed] [Google Scholar]
- 4. Niu Q, Cai B, Huang ZC, Shi YY, Wang LL.. Disturbed Th17/Treg balance in patients with rheumatoid arthritis. Rheumatol Int 2012, 32(9),2731–6. Epub 2011/08/03. doi: 10.1007/s00296-011-1984-x [DOI] [PubMed] [Google Scholar]
- 5. Paradowska-Gorycka A, Wajda A, Romanowska-Próchnicka K, Walczuk E, Kuca-Warnawin E, Kmiolek T, et al.. Th17/Treg-related transcriptional factor expression and cytokine profile in patients with rheumatoid arthritis. Front Immunol 2020, 11:572858. Epub 2020/12/29. doi: 10.3389/fimmu.2020.572858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yang P, Qian FY, Zhang MF, Xu AL, Wang X, Jiang BP, et al.. Th17 cell pathogenicity and plasticity in rheumatoid arthritis. J Leukoc Biol 2019, 106(6),1233–1240. Epub 2019/09/10. doi: 10.1002/JLB.4RU0619-197R [DOI] [PubMed] [Google Scholar]
- 7. Kanjana K, Chevaisrakul P, Matangkasombut P, Paisooksantivatana K, Lumjiaktase P.. Inhibitory activity of Foxp3+ regulatory T Cells Reveals High Specificity for Displaying Immune Tolerance in Remission State Rheumatoid Arthritis. Sci Rep 2020, 10(1),19789. Epub 2020/11/15. doi: 10.1038/s41598-020-76168-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang W, Shao S, Jiao Z, Guo M, Xu H, Wang S.. The Th17/Treg imbalance and cytokine environment in peripheral blood of patients with rheumatoid arthritis. Rheumatol Int 2012, 32(4),887–93. Epub 2011/01/12. doi: 10.1007/s00296-010-1710-0 [DOI] [PubMed] [Google Scholar]
- 9. Lopez-Castejon G. Control of the inflammasome by the ubiquitin system. FEBS J 2020,287(1),11–26. Epub 2019/11/05. doi: 10.1111/febs.15118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhu L, Li Y, Zhou L, Yang G, Wang Y, Han J, et al.. Role of ring-type E3 ubiquitin ligases in inflammatory signalling and inflammatory bowel disease. Mediators Inflamm 2020, 2020:5310180. Epub 2020/08/28. doi: 10.1155/2020/5310180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gao SF, Zhong B, Lin D.. Regulation of T helper cell differentiation by E3 ubiquitin ligases and deubiquitinating enzymes. Int Immunopharmacol 2017, 42:150–156. Epub 2016/12/04. doi: 10.1016/j.intimp.2016.11.013 [DOI] [PubMed] [Google Scholar]
- 12. Chen Z, Barbi J, Bu S, Yang HY, Li Z, Gao Y, et al.. The ubiquitin ligase Stub1 negatively modulates regulatory T cell suppressive activity by promoting degradation of the transcription factor Foxp3. Immunity 2013, 39(2),272–85. Epub 2013/08/27. doi: 10.1016/j.immuni.2013.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Xie J, Gu J.. Identification of C-terminal Hsp70-interacting protein as a mediator of tumour necrosis factor action in osteoblast differentiation by targeting Osterix for degradation. J Cell Mol Med 2015, 19(8),1814–24. Epub 2015/03/31. doi: 10.1111/jcmm.12553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cheng L, Qian L, Tan Y, Wang GS, Li XM, Li XP, et al.. Unbalanced expression of aryl hydrocarbon receptor in peripheral blood Ccr6(+)Cd4(+) and Cd4(+)Cd25(+)T cells of rheumatoid arthritis. Revista brasileira de reumatologia 2017, 57(3),190–196. Epub 2017/05/26. doi: 10.1016/j.rbre.2016.07.002 [DOI] [PubMed] [Google Scholar]
- 15. Morales JL, Perdew GH.. Carboxyl terminus of Hsc70-interacting protein (Chip) can remodel mature aryl hydrocarbon receptor (Ahr) complexes and mediate ubiquitination of both the Ahr and the 90 KDa heat-shock protein (Hsp90) in vitro. Biochemistry 2007, 46(2),610–21. Epub 2007/01/11. doi: 10.1021/bi062165b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Abdulla OA, Neamah W, Sultan M, Chatterjee S, Singh N, Nagarkatti M, et al.. Ahr ligands differentially regulate Mirna-132 which targets Hmgb1 and to control the differentiation of Tregs and Th-17 cells during delayed-type hypersensitivity response. Front Immunol 2021, 12:635903. Epub 2021/03/09. doi: 10.3389/fimmu.2021.635903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Neavin D, Liu D, Ray B, Weinshilboum R.. The role of the aryl hydrocarbon receptor (Ahr) in immune and inflammatory diseases. Int J Mol Sci 2018, 19(12),3851. 10.3390/ijms19123851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bock KW. Aryl hydrocarbon receptor (Ahr), integrating energy metabolism and microbial or obesity-mediated inflammation. Biochem Pharmacol 2021, 184:114346. Epub 2020/11/24. doi: 10.1016/j.bcp.2020.114346 [DOI] [PubMed] [Google Scholar]
- 19. Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, 3rd, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League against Rheumatism Collaborative Initiative. Arthritis Rheum 2010, 62(9),2569–81. Epub 2010/09/28. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 20. Zhou G, Wu W, Yu L, Yu T, Yang W, Wang P, et al.. Tripartite motif-containing (Trim) 21 negatively regulates intestinal mucosal inflammation through inhibiting T(H)1/T(H)17 cell differentiation in patients with inflammatory bowel diseases. J Allergy Clin Immunol 2018, 142(4),1218–1228.e12.e12. Epub 2017/11/09. doi: 10.1016/j.jaci.2017.09.038 [DOI] [PubMed] [Google Scholar]
- 21. Xiao XY, Li YT, Jiang X, Ji X, Lu X, Yang B, et al.. Ezh2 deficiency attenuates Treg differentiation in rheumatoid arthritis. J Autoimmun 2020, 108:102404. Epub 2020/01/19. doi: 10.1016/j.jaut.2020.102404 [DOI] [PubMed] [Google Scholar]
- 22. Tanaka T, Yamamoto Y, Muromoto R, Ikeda O, Sekine Y, Grusby MJ, et al.. Pdlim2 inhibits T helper 17 cell development and granulomatous inflammation through degradation of Stat3. Sci Signal 2011, 4(202),ra85. Epub 2011/12/14. doi: 10.1126/scisignal.2001637 [DOI] [PubMed] [Google Scholar]
- 23. Wei Q, Sha Y, Bhattacharya A, Abdel Fattah E, Bonilla D, Jyothula SS, et al.. Regulation of Il-4 receptor signaling by Stub1 in lung inflammation. Am J Respir Crit Care Med 2014, 189(1),16–29. Epub 2013/11/21. doi: 10.1164/rccm.201305-0874OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Xie J, Wang Z, Wang W.. Semaphorin 4d induces an imbalance of Th17/Treg cells by activating the aryl hydrocarbon receptor in Ankylosing spondylitis. Front Immunol 2020, 11:2151. doi: 10.3389/fimmu.2020.02151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Guo Y, Zhao M, Lu Q.. Transcription factor Rfx1 is ubiquitinated by E3 ligase Stub1 in systemic lupus erythematosus. Clin Immunol 2016, 169:1–7. Epub 2016/06/11. doi: 10.1016/j.clim.2016.06.003 [DOI] [PubMed] [Google Scholar]
- 26. Lubberts E. Th17 cytokines and arthritis. Semin Immunopathol 2010, 32, 43–53. doi: 10.1007/s00281-009-0189-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kondo Y, Yokosawa M, Kaneko S, Furuyama K, Segawa S, Tsuboi H, et al.. Review: transcriptional regulation of Cd4+ T cell differentiation in experimentally induced arthritis and rheumatoid arthritis. Arthritis Rheumatol 2018, 70(5),653–661. Epub 2017/12/16. doi: 10.1002/art.40398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nguyen NT, Nakahama T, Kishimoto T.. Aryl hydrocarbon receptor and experimental autoimmune arthritis. Semin Immunopathol 2013, 35(6),637–44. Epub 2013/08/29. doi: 10.1007/s00281-013-0392-6 [DOI] [PubMed] [Google Scholar]
- 29. Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, et al.. Control of T(Reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature 2008, 453(7191),65–71. Epub 2008/03/26. doi: 10.1038/nature06880 [DOI] [PubMed] [Google Scholar]
- 30. Rannug A, Rannug U.. The tryptophan derivative 6-formylindolo[3,2-B]carbazole, Ficz, a dynamic mediator of endogenous aryl hydrocarbon receptor signaling, balances cell growth and differentiation. Crit Rev Toxicol 2018, 48(7),555–574. Epub 2018/09/19. doi: 10.1080/10408444.2018.1493086 [DOI] [PubMed] [Google Scholar]
- 31. Liu H, Shi L, Giesy JP, Yu H.. Polychlorinated diphenyl sulfides can induce Ros and genotoxicity via the Ahr-Cyp1a1 pathway. Chemosphere 2019, 223:165–170. Epub 2019/02/19. doi: 10.1016/j.chemosphere.2019.01.169 [DOI] [PubMed] [Google Scholar]
- 32. Wang XS, Cao F, Zhang Y, Pan HF.. Therapeutic potential of aryl hydrocarbon receptor in autoimmunity. Inflammopharmacology 2020, 28(1),63–81. Epub 2019/10/17. doi: 10.1007/s10787-019-00651-z [DOI] [PubMed] [Google Scholar]
- 33. Wu R, Li N, Zhao X, Ding T, Xue H, Gao C, et al.. Low-dose interleukin-2: biology and therapeutic prospects in rheumatoid arthritis. Autoimmunity Rev 2020, 19(10),102645. Epub 2020/08/18. doi: 10.1016/j.autrev.2020.102645 [DOI] [PubMed] [Google Scholar]
- 34. Li M, Yang C, Wang Y, Song W, Jia L, Peng X, et al.. The expression of P2x7 receptor on Th1, Th17, and regulatory T cells in patients with systemic lupus erythematosus or rheumatoid arthritis and its correlations with active disease. J Immunol 2020, 205(7),1752–1762. Epub 2020/09/02. doi: 10.4049/jimmunol.2000222 [DOI] [PubMed] [Google Scholar]
- 35. Wang S, Li Y, Hu YH, Song R, Gao Y, Liu HY, et al.. Stub1 is essential for T-cell activation by ubiquitinating Carma1. Eur J Immunol 2013, 43(4),1034–41. Epub 2013/01/17. doi: 10.1002/eji.201242554 [DOI] [PubMed] [Google Scholar]
- 36. Godoy GJ, Olivera C, Paira DA, Salazar FC, Ana Y, Stempin CC, et al.. T regulatory cells from non-obese diabetic mice show low responsiveness to Il-2 stimulation and exhibit differential expression of anergy-related and ubiquitination factors. Front Immunol 2019, 10:2665. Epub 2019/12/12. doi: 10.3389/fimmu.2019.02665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhang Y, Chen Z, Luo X, Wu B, Li B, Wang B.. Cimetidine down-regulates stability of Foxp3 Protein Via Stub1 in Treg Cells. Hum Vaccin Immunother 2016, 12(10),2512–2518. Epub 2016/06/22. doi: 10.1080/21645515.2016.1191719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jin S, Chen H, Li Y, Zhong H, Sun W, Wang J, et al.. Maresin 1 improves the Treg/Th17 imbalance in rheumatoid arthritis through Mir-21. Ann Rheum Dis 2018, 77(11),1644–1652. Epub 2018/07/27. doi: 10.1136/annrheumdis-2018-213511 [DOI] [PubMed] [Google Scholar]
- 39. Liu C, Li Y, Chen Y, Huang S, Wang X, Luo S, et al.. Baicalein restores the balance of Th17/Treg cells via aryl hydrocarbon receptor to attenuate colitis. Mediators Inflamm 2020, 2020:5918587. Epub 2020/10/22. doi: 10.1155/2020/5918587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Paul I, Ghosh MK.. The E3 ligase chip: insights into its structure and regulation. BioMed Res Int 2014, 2014:918183. Epub 2014/05/29. doi: 10.1155/2014/918183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Malynn BA, Ma A.. Ubiquitin makes its mark on immune regulation. Immunity 2010, 33(6),843–52. Epub 2010/12/21. doi: 10.1016/j.immuni.2010.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.