Abstract
Background
Radiation exposure to the thyroid gland seems unavoidable in breast cancer (BC) patients receiving radiation therapy (RT) to the supraclavicular (SC) region. Hence, this study aimed to evaluate the effects of SC region RT on thyroid function and the prevalence of radiation-induced hypothyroidism (RIHT) in BC patients at regular intervals post-treatment.
Materials and methods
Twenty-one patients with BC were enrolled in this analytical cross-sectional study by simple and convenient sampling, from March 2019 to March 2020. Thyroid function and the prevalence of RIHT were evaluated and compared by measuring the serum of thyroid-stimulating hormone (TSH) and free thyroxine hormone (fT4) levels before radiation therapy (pre-RT) and 3 and 6 months after radiation therapy (post-RT). The patients underwent 3 dimensional conformal. radiation therapy (3D CRT) of breast/chest wall, axillary, and supraclavicular lymph nodes with 50 Gy/25 fractions/5 weeks. The collected data were analyzed using SPSS software (version 20).
Results
Serum levels of TSH increased at 3 and 6 months post-RT, this increase was not statistically significant (p > 0.05). Nevertheless, serum levels of fT4 were significantly elevated at 3 and 6 months post-RT (p < 0.01). A correlation was observed between the follow-up period and the incidence of RIHT, where it was 0% at 3 months and 9.5% at 6 months post-RT. RIHT was not significantly associated with any factors, including patient’s age, type of surgery, thyroid gland dose, and thyroid gland volume.
Conclusions
It seems that SC region RT does not have a significant adverse effect on the thyroid function among BC patients at 3 and 6 months post-treatment. Hence, a long-term follow-up with a larger sample size is suggested.
Keywords: breast cancer, radiation therapy, thyroid function, radiation-induced hypothyroidism
Introduction
Breast cancer (BC), which is the most common malignancy among women, is known as the main cause of cancer mortality in females throughout the world [1–4]. Breast cancer detection at an early stage with screening mammography significantly reduces the risk of death from the disease, when treatment strategies will be most likely to be successful [5]. Radiation therapy (RT) has frequently been employed as adjuvant therapy following surgery for locally advanced BC or as palliative therapy for local recurrence in the supraclavicular nodes [6]. The routine RT for BC patients involves irradiation of the breast or chest wall [7, 8], ipsilateral supraclavicular, and internal mammary nodes with 50 Gy/25 fractions/5 weeks [6, 8]. RT to the supraclavicular field includes parts of the thyroid gland; therefore, there is a concern about the effect on thyroid function, including the incidence of hypothyroidism (HT) [6, 9, 10]. Subclinical HT followed by clinical HT has been known as the most common type of radiation-induced thyroid dysfunction [11]. Radiation-induced hypothyroidism (RIHT) is a potential complication after RT when the treatment field includes the thyroid gland and develops at a median interval of 1.4–1.8 years, but it has been reported even 3 months or 20 years after RT [6, 10, 12, 13]. Subclinical HT is defined as a normal free thyroxine hormone (fT4) level in the presence of a high thyroid-stimulating hormone (TSH) level, with no clinical symptoms, whereas clinical HT is described by a low serum fT4 level and a high TSH level, in which patients may present with clinical symptoms like weight gain, cold intolerance, fatigue, and slow mentation [11]. Evidence indicates a dose-dependent risk of RIHT, but the dose-response relationship is not well founded. Although RIHT is relatively common and treatable, regarding the symptoms influencing the quality of life, it seems important to estimate the risk of RIHT.
RT to the supraclavicular field includes a part of the thyroid gland; therefore, radiation exposure to this gland seems unavoidable in BC patients receiving RT to the supraclavicular region [14]. However, thyroid dysfunction is usually underestimated in patients with BC who had supraclavicular RT. Nevertheless, the relationship between RT and thyroid function in BC patients has been examined only in a few studies. To our knowledge, no prior study has clearly defined whether applying RT in BC patients has a risk of thyroid dysfunction according to patient’s characteristics and dose-volume factors. Quantifying the magnitude of risk in these patients seems essential to help determine whether regular monitoring of thyroid function would be helpful. Therefore, the present study aimed to evaluate the effects of supraclavicular region RT on thyroid function and the prevalence of RIHT in BC patients at regular intervals post-treatment. Determining changes in thyroid hormone levels pre-RT and at regular intervals post-RT, defining the correlation between thyroid hormone levels and mean thyroid dose, as well as evaluating the relationship between HT post-RT and some factors, including patient’s age, thyroid volume, type of surgery, and prescribed dose were the other aims of this study.
Materials and methods
Twenty-one patients with BC were enrolled in this analytical cross-sectional study by simple and convenient sampling, from March 2019 to March 2020. Cases with thyroid dysfunction, primary thyroid disease, previous surgery, and/or radiotherapy were excluded. A checklist was employed to record patients’ personal and clinical data, and all the data was kept confidential. This study was approved by the institutional ethics committee (No: IR.KUMS.REC.1399.995). Participation in this study was absolutely voluntary and non-cooperation did not induce any problems in the treatment procedure. The informed consent form was signed by all the enrolled patients in compliance with the principles of the Declaration of Helsinki.
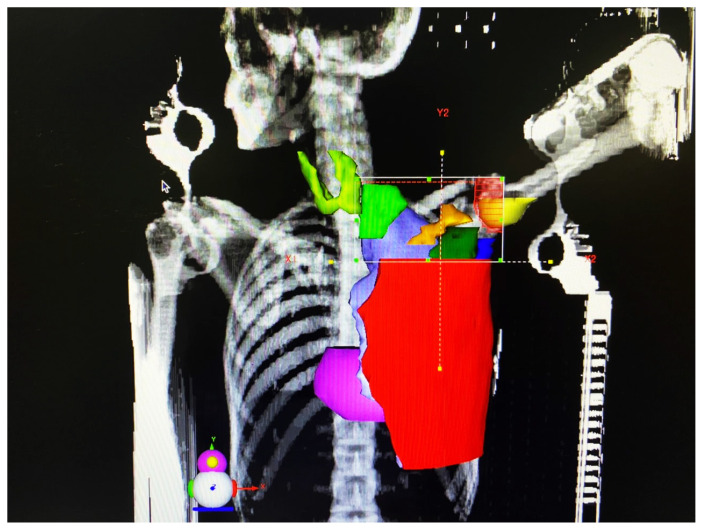
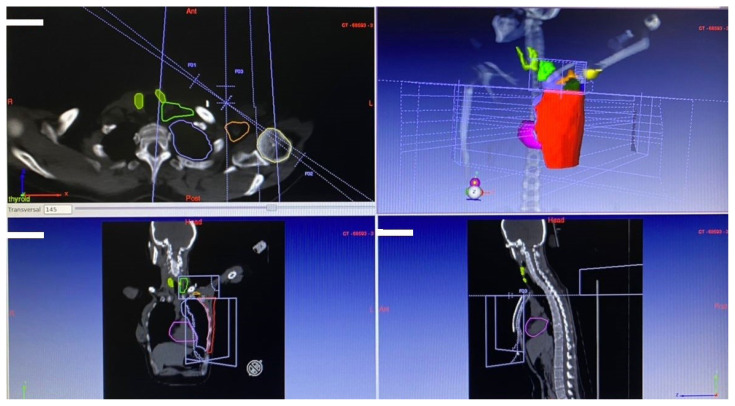
Treatment planning CT simulation was done using a multi-slice CT scanner (Aquilion 16 Slice; Toshiba, Japan). After CT simulation, CT images were transferred to the treatment planning system (TPS; DosiSoft, France) for contouring [15, 16]. Both thyroid lobes of each patient were delineated and contoured separately and also together by one person. In addition, the contouring of the other organs was performed by the same person according to the Radiation Therapy Oncology Group (RTOG) criteria (Fig. 1). All plans were generated in the TPS which was adjusted to the linear accelerator (ELEKTA, England). After treatment planning, the patients underwent Three-Dimensional Conformal Radiation Therapy (3D CRT) of breast/chest wall, axillary, and supraclavicular lymph nodes (Fig. 2) with 50 Gy/25 fractions/5 weeks. The mean and maximum doses of the thyroid and its lobes, as well as thyroid V5, V10, V20, V30, V40, and V50 (percentage of thyroid volume receiving ≥ 5 Gy, ≥ 10 Gy, ≥ 20 Gy, ≥ 30 Gy, ≥ 40 Gy, and ≥ 50 Gy, respectively) were calculated from each patient’s tissue-specific dose-volume histogram (DVH) based on the TPS [17, 18]. DVH shows a graphical representation of the radiation dose delivered to any defined volume. The mean volume of the thyroid gland and its lobes was estimated using ultrasonography.
Figure 1.
Contouring and delineation of both thyroid lobes and other organs
Figure 2.
The supraclavicular field includes parts of the thyroid gland
Patients’ information and test results were recorded on a specific checklist that was specifically designed for the current study. The collected data were analyzed using SPSS software (version 20). Mean and standard deviation (SD) was applied for quantitative data description. Pre-RT serum TSH and fT4 levels were compared with the corresponding values obtained 3 and 6 months post-RT by the Wilcoxon test and/or paired t-test with repeated measures. Unadjusted association between RIHT and categorical variables was calculated by Chi-square and/or Fisher-Exact test. Selecting a statistical test was dependent on data normality. The meaningless level was considered as p < 0.05.
Results
A total of twenty-one women with BC were enrolled in the study with a mean age of 49.24 ± 10.31 years. The subjects’ age ranged from 33 to 76 years. The patients’ characteristics, volumes of the thyroid gland, right-sided, left-sided, and isthmus lobes are shown in Table 1. In this study, invasive ductal carcinoma was reported as the most common type of carcinoma (76.2%). Invasive lobular carcinoma and the other types of BC were in the next categories. The mean volume of the thyroid gland was 9.14 ± 2.48 cc (3–13 cc). The mean volume of the right-sided, left-sided, and isthmus thyroid lobes were 4.33 ± 1.37 cc (1.4–6.2 cc), 4.18 ± 1.15 cc (1.2–6.3 cc), and 0.93 ± 0.17 cc (0.6–1.3 cc), respectively.
Table 1.
Patients’ characteristics and volumes of the thyroid gland, right-sided, left-sided, and isthmus lobes (n = 21)
| Patients’ characteristics Volumes of thyroid gland and its lobes |
Number of patients (n) | Percentage (%) |
|---|---|---|
|
| ||
| Age (years) | ||
|
| ||
| < 40 | 3 | 14.3 |
| 40–50 | 10 | 47.5 |
| 50–60 | 4 | 19.1 |
| ≥ 60 | 4 | 19.1 |
|
| ||
| Menopausal status | ||
|
| ||
| Perimenopause | 8 | 38.1 |
| Menopause | 7 | 33.3 |
| Postmenopause | 6 | 28.6 |
|
| ||
| ECOG Performance Status | ||
|
| ||
| 0 | 19 | 90.5 |
| 1 | 2 | 9.5 |
|
| ||
| Breast cancer histology | ||
|
| ||
| Ductal | 16 | 76.2 |
| Lobular | 2 | 9.5 |
| Other | 3 | 14.3 |
|
| ||
| Treatment side | ||
|
| ||
| Right | 14 | 66.7 |
| Left | 7 | 33.3 |
|
| ||
| Type of surgery | ||
|
| ||
| BCS | 12 | 57.1 |
| MRM | 9 | 42.9 |
|
| ||
| Adjuvant therapy | ||
|
| ||
| Yes | 19 | 90.5 |
| No | 2 | 9.5 |
|
| ||
| Thyroid gland volume [cc] | ||
|
| ||
| < 5 | 1 | 4.8 |
| 5–10 | 10 | 47.6 |
| ≥ 10 | 10 | 47.6 |
|
| ||
| Volume of right-sided thyroid lobe [cc] | ||
|
| ||
| < 3 | 3 | 14.3 |
| 3–5 | 10 | 47.7 |
| ≥ 5 | 8 | 38 |
|
| ||
| Volume of left-sided thyroid lobe [cc] | ||
|
| ||
| < 3 | 3 | 14.3 |
| 3–5 | 14 | 66.7 |
| ≥ 5 | 4 | 19 |
|
| ||
| Volume of Isthmus Thyroid Lobe [cc] | ||
|
| ||
| < 0.75 | 4 | 19 |
| 0.75–1 | 4 | 19 |
| ≥ 1 | 13 | 62 |
ECOG — Eastern Cooperative Oncology Group; BCS — breast-conserving surgery; MRM — modified radical mastectomy
Dosimetric parameters of the thyroid gland, right-sided, left-sided, and isthmus thyroid lobes are summarized in Table 2. The mean thyroid gland dose was 9.41 ± 7.21 Gy (0.11–22.23 Gy). The mean absorbed dose received by the right-sided, left-sided, and isthmus thyroid lobes were 7.52 ± 12.62 Gy (0.12–41.16 Gy), 12.8 ± 14.4 Gy (0.1–40.86 Gy), and 4.47± 3.72 Gy (0.1–11.2 Gy), respectively. The maximum thyroid gland dose was 31.82 ± 22.8 Gy (0.19–56.04 Gy). The maximum absorbed dose received by the right-sided, left-sided, and isthmus thyroid lobes were 13.72 ± 18.39 Gy (0.19–55.75 Gy), 23.68 ± 24.13 Gy (0.17–56.54 Gy), and 7.36 ± 6.09 Gy (0.1–18.6 Gy), respectively.
Table 2.
Dosimetric parameters of the thyroid gland, right-sided, left-sided, and isthmus lobes (n = 21)
| Dosimetric parameters | Number of patients (n) | Percentage (%) |
|---|---|---|
|
| ||
| Irradiated lobe of the thyroid gland | ||
|
| ||
| Right | 7 | 33.3 |
| Left | 14 | 66.7 |
|
| ||
| Mean Thyroid Gland Dose [Gy] | ||
|
| ||
| < 1 | 5 | 23.8 |
| 1–10 | 11 | 52.4 |
| ≥ 10 | 5 | 23.8 |
|
| ||
| Mean dose of right-sided thyroid lobe [Gy] | ||
|
| ||
| < 1 | 4 | 19 |
| 1–10 | 13 | 62 |
| ≥ 10 | 4 | 19 |
|
| ||
| Mean Dose of Left-Sided Thyroid Lobe [Gy] | ||
|
| ||
| < 1 | 5 | 23.8 |
| 1–10 | 7 | 33.3 |
| ≥ 10 | 9 | 42.9 |
|
| ||
| Mean dose of isthmus thyroid lobe [Gy] | ||
|
| ||
| < 1 | 5 | 23.8 |
| 1–10 | 14 | 66.7 |
| ≥ 10 | 2 | 9.5 |
|
| ||
| Maximum thyroid gland dose [Gy] | ||
|
| ||
| < 25 | 7 | 33.7 |
| 25–50 | 7 | 33.7 |
| ≥ 50 | 7 | 33.7 |
|
| ||
| Maximum dose of right-sided thyroid lobe [Gy] | ||
|
| ||
| < 25 | 12 | 57.2 |
| 25–50 | 4 | 19 |
| ≥ 50 | 5 | 23.8 |
|
| ||
| Maximum dose of left-sided thyroid lobe [Gy] | ||
|
| ||
| < 25 | 5 | 23.8 |
| 25–50 | 7 | 33.3 |
| ≥ 50 | 9 | 42.9 |
|
| ||
| Maximum dose of isthmus thyroid lobe [Gy] | ||
|
| ||
| < 1 | 4 | 19 |
| 1–10 | 12 | 57.2 |
| ≥ 10 | 5 | 23.8 |
Thyroid V5, V10, V20, V30, V40, and V50 (percentage of thyroid volume receiving ≥ 5 Gy, ≥ 10 Gy, ≥ 20 Gy, ≥ 30 Gy, ≥ 40 Gy, and ≥ 50 Gy, respectively) is presented in Table 3. Thyroid hormones level, pre-and post-RT, are listed in Table 4. Although, 3 and 6 months post-RT, the mean serum levels of TSH were increased, this increase was not statistically significant (p > 0.05). However, the mean serum levels of fT4 were significantly elevated at 3 and 6 months post-RT (p < 0.01).
Table 3.
Thyroid V5, V10, V20, V30, V40, and V50
| Thyroid volume receiving % | Minimum | Maximum | Median | Mean ± SD |
|---|---|---|---|---|
| V5 | 10 | 100 | 57 | 60.19 ± 29.84 |
| V10 | 5 | 100 | 50 | 53 ± 29.24 |
| V20 | 0 | 100 | 35 | 32.33 ± 23.92 |
| V30 | 0 | 48 | 12 | 17.71 ± 17.33 |
| V40 | 0 | 36 | 0 | 9.57 ± 12.49 |
| V50 | 0 | 17 | 0 | 2.42 ± 4.82 |
SD — standard deviation
Table 4.
Thyroid hormones level pre-radiotherapy (Pre-RT) and post-radiotherapy (Post-RT)
| Thyroid hormone levels | Pre-RT Mean ± SD |
Post-RT | p-value | |
|---|---|---|---|---|
| 3 months Mean ± SD |
6 months Mean ± SD |
|||
| TSH [μIU/mL] | 1.87 ± 0.82 | 2.03 ± 0.93 | 2.25 ± 1.38 | > 0.05 |
| fT4 [ng/dL] | 1.08 ± 0.18 | 1.19 ± 0.21 | 1.21 ± 0.24 | < 0.01 |
SD — standard deviation; TSH — thyroid-stimulating hormone; fT4 — free thyroxine hormone
The incidence of HT at 3 and 6 months post-RT was observed in 0% and 9.5% of patients, respectively (Tab. 5). In the incidence of HT, no significant difference was reported between 3 and 6 months post-RT. The chi-squared test showed that RIHT was not significantly associated with studied parameters, including patient’s age, type of surgery, thyroid gland volume, and thyroid gland dose (p > 0.05) (Tab. 6).
Table 5.
The incidence of radiation-induced hypothyroidism (RIHT)
| Post-RT | Number of patients (%) | p-value | |
|---|---|---|---|
| Euthyroid | Hypothyroid | ||
| 3 months | 21 (100) | 0 (0) | > 0.05 |
| 6 months | 19 (90.5) | 2 (9.5) | |
RT — radiation therapy
Table 6.
Association between radiation-induced hypothyroidism (RIHT) incidence and studied parameters
| RIHT | Parameters Number of Patients (%) |
|||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Age [years] | Type of surgery | Thyroid gland volume [cc] | Thyroid gland dose [Gy] | |||||
|
| ||||||||
| < 50 | ≥ 50 | BCS | MRM | < 10 | ≥ 10 | < 10 | ≥ 10 | |
|
| ||||||||
| No | 13 (100) | 6 (75) | 10 (83.3) | 9 (100) | 11 (84.6) | 8 (100) | 11 (100) | 8 (80) |
| Yes | 0 (0) | 2 (25) | 2 (16.7) | 0 (0) | 2 (15.4) | 0 (0) | 0 (0) | 2 (20) |
|
| ||||||||
| p-value | 0.133 | 0.486 | 0.505 | 0.214 | ||||
Discussion
In RT procedures, peripheral radiation can injure the out-of-field organs; hence, the minimization of radiation-induced injuries is a major concern in treatment planning [19, 20]. Since treatment plans for BC patients are along with RT to the supraclavicular field [21, 22]; there is a concern about the effect on thyroid function, including the incidence of RIHT. RIHT is a potential complication after RT when the treatment field includes the thyroid gland [6, 10, 12, 13].
The first case of HT following RT for malignancy was reported in the literature in 1961 [23, 24]. Various studies have been conducted to evaluate the effects of supraclavicular region RT on thyroid function among BC patients, which different results reported. The true prevalence of RIHT in these patients is not known because thyroid function tests are not routinely assessed post-RT in clinics [25].
In agreement with the study of Alhosainy et al. [6], our results showed that the mean serum levels of TSH increased at 3 and 6 months post-RT compared with the baseline but this increase was not statistically significant. Laway et al. [24] observed that TSH increased significantly after 3 months in patients who had received RT to the neck region. In this regard, Yoden et al. reported that V30 Gy had a significant effect on the peak level of TSH, and this risk is possible for thyroid dose ranges between 10 and 30 Gy. Since the mean thyroid gland dose was 9.41 ± 7.21 Gy in our study, there is a potential risk of RIHT [26]. Tunio et al. estimated the dose distribution at the thyroid gland in BC patients treated by supraclavicular RT procedure. They showed that the risk of HT depends on the thyroid gland volume and follow-up duration and can be minimized using a thyroid shield [25]. Nevertheless, the results of this study showed that the mean serum levels of fT4 were significantly elevated at 3 and 6 months post-RT.
Thyroid dysfunction develops slowly, up to 15% of patients show dysfunction, and a maximum of 66% is affected within 6 years [27, 28]. According to the literature, RIHT develops at a median interval of 1.4–1.8 years, but it has been reported even 3 months or 20 years post-RT. In the current study, a correlation was observed between the follow-up period and the incidence of HT, where it was 0% at 3 months and 9.5% at 6 months post-RT. This is while the difference between these periods, 3 and 6 months post-RT, was not significant. As mentioned, 6 months post-RT to the supraclavicular region, the incidence of RIHT was observed in 9.5% of patients with BC, which was consistent with that previously reported (6–18%) [25, 28–30]. In this regard, Cutuli et al. reported that 6.2% of BC patients who had undergone RT, chemotherapy, and surgery had clinically symptomatic HT on short-term follow-up after primary treatment [31]. RIHT was not significantly associated with any factors, including patient’s age, type of surgery, thyroid gland dose, and thyroid gland volume. Hence, in the current study, no threshold was reported for RIHT. In confirmation of our results, the other studies reported that dose-volume parameters, including thyroid V10–60 as well as thyroid gland volume, were not associated with the development of RIHT [6, 32, 33]. Nevertheless, in disagreement with our results, Kikawa et al. [10] reported that thyroid gland volume < 8 cm3 can be a predictive factor of HT in patients with BC who had received RT to the supraclavicular field. In this respect, Johansen et al. [14] reported that in BC patients with small thyroid glands, the risk of RIHT depends on the thyroid gland volume. According to the literature, both patient’s age and radiation dose can be associated with the development of RIHT, and radiosensitivity of the thyroid gland is assumed to decrease with increasing age [14]. This discrepancy between our results and previous findings might be due to the differences in the selected samples, radiation dose, and RT procedures.
Radiation as a risk factor for the development of HT remains controversial. Based on the previous reports, radiation-induced injury to small thyroid vessels, atherosclerosis of larger vessels, parenchymal thyroid cell injury, and capsular fibrosis secondary, also immune-mediated injury may be involved as mechanisms in the development of HT [9, 29].
National Comprehensive Cancer Network (NCCN) guidelines suggest routine screening of thyroid function within the first year post-RT in head and neck cancer patients and Hodgkin’s disease if the radiation treatment field includes the neck region. Given the paucity of prior studies of RIHT risk for BC patients, it is not surprising that no formal recommendations exist regarding post-RT screening for HT in this group. Hence, further studies on the efficacy and cost-effectiveness of routine post-RT thyroid function screening in patients with BC seem necessary [29].
Conclusion
Regarding the results of this study, it seems that supraclavicular region RT does not have a significant adverse effect on thyroid function among BC patients at 3 and 6 months post-treatment. Since the short-term follow-up with a small sample size can be considered as the limitation of the current study, a long-term follow-up with a larger sample size is suggested.
Acknowledgements
The authors gratefully acknowledge the Research Council of Kermanshah University of Medical Sciences and Clinical Research Development Center, Imam Reza Hospital for the financial support and cooperation. This work (approved research plan No: 990885) was performed in partial fulfillment of the requirements for Med. D. of Mohammad Hosein Saiedian Azar, Faculty of Medicine, Kermanshah University of Medical Sciences, Kermanshah, Iran.
Footnotes
Conflict of interests
None declared.
Funding
None declared.
References
- 1.Heydarheydari S, Haghparast A. Diagnostic Value of PET/CT in Comparison with Other Conventional Imaging Modalities for the Evaluation of Breast Cancer Recurrence: A Systematic Review of the Literature. Arch Breast Cancer. 2016:77–82. [Google Scholar]
- 2.Heydarheydari S, Rezaeijo SM, Cheki M, et al. Diagnostic Efficacy of Technetium-99m-Sestamibi Scintimammography in Comparison with Mammography to Detect Breast Lesions: A Systematic Review. Arch Breast Cancer. 2018:98–105. [Google Scholar]
- 3.Rezaeijo SM, Ghorvei M, Mofid B. Predicting breast cancer response to neoadjuvant chemotherapy using ensemble deep transfer learning based on CT images. J Xray Sci Technol. 2021;29(5):835–850. doi: 10.3233/XST-210910. [DOI] [PubMed] [Google Scholar]
- 4.Sadeghi S. The relationship between anxiety and depression with breast cancer screening in women referring to the mammography clinics in Kermanshah, 2013–2014. J Clin Res Paramed Sci. 2015;4(3) [Google Scholar]
- 5.Niell BL, Freer PE, Weinfurtner RJ, et al. Screening for Breast Cancer. Radiol Clin North Am. 2017;55(6):1145–1162. doi: 10.1016/j.rcl.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 6.Alhosainy A, Mohamed A, Elattar A. The impact of supraclavicular irradiation on thyroid function and size in postoperative breast cancer patients by comparing 2D versus 3D-CRT. Life Sci J. 2015;12:57–62. [Google Scholar]
- 7.Fosnot J, Fischer JP, Smartt JM, et al. Does previous chest wall irradiation increase vascular complications in free autologous breast reconstruction? Plast Reconstr Surg. 2011;127(2):496–504. doi: 10.1097/PRS.0b013e3181fed560. [DOI] [PubMed] [Google Scholar]
- 8.Ryu WG, Oh KiK, Kim EK, et al. The effect of supraclavicular lymph node irradiation upon the thyroid gland in the post-operative breast carcinoma patients. Yonsei Med J. 2003;44(5):828–835. doi: 10.3349/ymj.2003.44.5.828. [DOI] [PubMed] [Google Scholar]
- 9.Jereczek-Fossa BA, Alterio D, Jassem J, et al. Radiotherapy-induced thyroid disorders. Cancer Treat Rev. 2004;30(4):369–384. doi: 10.1016/j.ctrv.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 10.Kikawa Y, Kosaka Y, Hashimoto K, et al. Prevalence of hypothyroidism among patients with breast cancer treated with radiation to the supraclavicular field: a single-centre survey. ESMO Open. 2017;2(1):e000161. doi: 10.1136/esmoopen-2017-000161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akyurek S. Thyroid Dysfunction Following Supraclavicular Irradiation in The Management of Carcinoma of the Breast. Int J Hematol Oncol. 2014;24(1):1–6. doi: 10.4999/uhod.14234. [DOI] [Google Scholar]
- 12.Mercado G, Adelstein DJ, Saxton JP, et al. Hypothyroidism: a frequent event after radiotherapy and after radiotherapy with chemotherapy for patients with head and neck carcinoma. Cancer. 2001;92(11):2892–2897. doi: 10.1002/1097-0142(20011201)92:11<2892::aid-cncr10134>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 13.Tell R, Lundell G, Nilsson Bo, et al. Long-term incidence of hypothyroidism after radiotherapy in patients with head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2004;60(2):395–400. doi: 10.1016/j.ijrobp.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 14.Johansen S, Reinertsen KV, Knutstad K, et al. Dose distribution in the thyroid gland following radiation therapy of breast cancer — a retrospective study. Radiat Oncol. 2011;6:68. doi: 10.1186/1748-717X-6-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heydarheydari S, Farshchian N, Haghparast A, et al. Influence of the contrast agents on dose-volume histograms in radiotherapy treatment planning based on CT-scan. Tehran University Medical Journal TUMS Publications. 2018;75(11):805–12. [Google Scholar]
- 16.Heydarheydari S, Farshchian N, Haghparast A. Influence of the contrast agents on treatment planning dose calculations of prostate and rectal cancers. Rep Pract Oncol Radiother. 2016;21(5):441–446. doi: 10.1016/j.rpor.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rezaeijo SM, Hashemi B, Mofid B, et al. Comparison of various common whole pelvic radiotherapy (WPRT) and local radiotherapy (LRT) procedures to treat prostate cancer based on dosimetric parameters and radiobiological models. Int J Radiat Res. 2021;19(4):843–852. doi: 10.52547/ijrr.19.4.10. [DOI] [Google Scholar]
- 18.Rezaeijo SM, Hashemi B, Mofid B, et al. The feasibility of a dose painting procedure to treat prostate cancer based on mpMR images and hierarchical clustering. Radiat Oncol. 2021;16(1):182. doi: 10.1186/s13014-021-01906-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schonfeld SJ, Lee C, Berrington de González A. Medical exposure to radiation and thyroid cancer. Clin Oncol (R Coll Radiol) 2011;23(4):244–250. doi: 10.1016/j.clon.2011.01.159. [DOI] [PubMed] [Google Scholar]
- 20.Ansari L, Nasiri N, Aminolroayaei F, et al. The Measurement of Thyroid Absorbed dose by Gafchromic™ EBT2 Film and Changes in Thyroid Hormone Levels Following Radiotherapy in Patients with Breast Cancer. J Med Signals Sens. 2020;10(1):42–47. doi: 10.4103/jmss.JMSS_10_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kourinou KM, Mazonakis M, Lyraraki E, et al. Scattered dose to radiosensitive organs and associated risk for cancer development from head and neck radiotherapy in pediatric patients. Phys Med. 2013;29(6):650–655. doi: 10.1016/j.ejmp.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 22.Lee B, Lee S, Sung J, et al. Radiotherapy-induced secondary cancer risk for breast cancer: 3D conformal therapy versus IMRT versus VMAT. J Radiol Prot. 2014;34(2):325–331. doi: 10.1088/0952-4746/34/2/325. [DOI] [PubMed] [Google Scholar]
- 23.Felix H, Dupre N, Drape M, et al. [Long-term influence of radiotherapy for cancer of larynx on the appearance of myxedema]. Lyon Med. 1961;93:1043–1050. [PubMed] [Google Scholar]
- 24.Laway BA, Shafi KM, Majid S, et al. Incidence of primary hypothyroidism in patients exposed to therapeutic external beam radiation, where radiation portals include a part or whole of the thyroid gland. Indian J Endocrinol Metab. 2012;16(Suppl 2):S329–S331. doi: 10.4103/2230-8210.104078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tunio MA, Al Asiri M, Bayoumi Y, et al. Is thyroid gland an organ at risk in breast cancer patients treated with locoregional radiotherapy? Results of a pilot study. J Cancer Res Ther. 2015;11(4):684–689. doi: 10.4103/0973-1482.167613. [DOI] [PubMed] [Google Scholar]
- 26.Yoden E, Maruta T, Soejima T, et al. Hypothyroidism after radiotherapy to the neck. Int J Radiat Oncol Biol Phys. 2001;51(3):337–338. doi: 10.1016/s0360-3016(01)02445-2. [DOI] [Google Scholar]
- 27.Schimpff SC, Diggs CH, Wiswell JG, et al. Radiation-related thyroid dysfunction: implications for the treatment of Hodgkin’s disease. Ann Intern Med. 1980;92(1):91–98. doi: 10.7326/0003-4819-92-1-91. [DOI] [PubMed] [Google Scholar]
- 28.Wolny-Rokicka E, Tukiendorf A, Wydmański J, et al. Thyroid Function after Postoperative Radiation Therapy in Patients with Breast Cancer. Asian Pac J Cancer Prev. 2016;17(10):4577–4581. doi: 10.22034/apjcp.2016.17.10.4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith GL, Smith BD, Giordano SH, et al. Risk of hypothyroidism in older breast cancer patients treated with radiation. Cancer. 2008;112(6):1371–1379. doi: 10.1002/cncr.23307. [DOI] [PubMed] [Google Scholar]
- 30.Reinertsen KV, Cvancarova M, Wist E, et al. Thyroid function in women after multimodal treatment for breast cancer stage II/III: comparison with controls from a population sample. Int J Radiat Oncol Biol Phys. 2009;75(3):764–770. doi: 10.1016/j.ijrobp.2008.11.037. [DOI] [PubMed] [Google Scholar]
- 31.Cutuli B, Quentin P, Rodier JF, et al. Severe hypothyroidism after chemotherapy and locoregional irradiation for breast cancer. Radiother Oncol. 2000;57(1):103–105. doi: 10.1016/s0167-8140(00)00183-3. [DOI] [PubMed] [Google Scholar]
- 32.Diaz R, Jaboin JJ, Morales-Paliza M, et al. Hypothyroidism as a consequence of intensity-modulated radiotherapy with concurrent taxane-based chemotherapy for locally advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2010;77(2):468–476. doi: 10.1016/j.ijrobp.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 33.Alterio D, Jereczek-Fossa BA, Franchi B, et al. Thyroid disorders in patients treated with radiotherapy for head-and-neck cancer: a retrospective analysis of seventy-three patients. Int J Radiat Oncol Biol Phys. 2007;67(1):144–150. doi: 10.1016/j.ijrobp.2006.08.051. [DOI] [PubMed] [Google Scholar]