Abstract
Neisseria gonorrhoeae is naturally able to take up exogenous DNA and undergo genetic transformation. This ability correlates with the presence of functional type IV pili, and uptake of DNA is dependent on the presence of a specific 10-bp sequence. Among the known competence factors in N. gonorrhoeae, none has been shown to interact with the incoming DNA. Here we describe ComE, a DNA-binding protein involved in neisserial competence. The gene comE was identified through similarity searches in the gonococcal genome sequence, using as the query ComEA, the DNA receptor in competent Bacillus subtilis. The gene comE is present in four identical copies in the genomes of both N. gonorrhoeae and Neisseria meningitidis, located downstream of each of the rRNA operons. Single-copy deletion of comE in N. gonorrhoeae did not have a measurable effect on competence, whereas serial deletions led to gradual decrease in transformation frequencies, reaching a 4 × 104-fold reduction when all copies were deleted. Transformation deficiency correlated with impaired ability to take up exogenous DNA; however, the mutants presented normal piliation and twitching motility phenotype. The product of comE has 99 amino acids, with a predicted signal peptide; by immunodetection, a 8-kDa protein corresponding to processed ComE was observed in different strains of N. gonorrhoeae and N. meningitidis. Recombinant His-tagged ComE showed DNA binding activity, without any detectable sequence specificity. Thus, we identified a novel gonococcal DNA-binding competence factor which is necessary for DNA uptake and does not affect pilus biogenesis or function.
Transformation plays a major role in the biology of pathogenic Neisseria species: it is their only known way to exchange genetic material, since neither conjugative plasmids capable of mobilizing chromosomal elements nor bacteriophages have been found in these organisms (27). Neisseriae are naturally competent (39), and the high level of horizontal genetic exchange that occurs in these bacteria is reflected in their panmictic population structure, in which gene mosaicism and allelic linkage equilibrium are observed (25, 37).
Transformation can be described as a multistep process, where the first event is binding of exogenous DNA to the bacterial surface. This is followed by DNA uptake, i.e., its transport into a DNase-resistant state. In neisseriae and other gram-negative organisms, DNA uptake is usually regarded as passage across the outer membrane (11). After uptake, the DNA molecule must cross the murein layer and the cytoplasmic membrane in order to gain access to the cytoplasm, where it may undergo recombination and integration into the host chromosome, in a RecA-dependent process (21).
Neisseria binds and takes up DNA in an efficient and yet largely uncharacterized way. Uptake is dependent on the presence of a 10-bp sequence, 5′GCCGTCTGAA3′ (DNA uptake sequence [DUS]) (12, 17). Neisseria has type IV pili (TFP), and it was early observed that piliation is necessary for DNA uptake (3), but it is still not known whether pili themselves participate in the process, since DNA-binding activity has not been detected in pilus components (26). Among the genes known to be necessary for competence are those encoding components of the TFP, such as pilE (pilin) and pilC (tip adhesin) (34), and those encoding proteins involved in pilus biogenesis (pilD, pilG, pilF, and pilQ) or function, such as twitching motility (pilT) (10, 14, 42, 44). Mutations in these genes result in the absence of pili or in nonfunctional pili. Few identified competence factors are not involved in Tfp: comL and tpc products participate in the remodelling of the cell wall (15, 16), whereas comA encodes a protein involved in transport of DNA across the cytoplasmic membrane (13). A gene encoding a pilin-like protein, comP, has been recently described as essential for competence, but not for pilus assembly or function; localization of its product, however, remains unknown (45).
The process of DNA uptake has been examined in several systems, and there is abundant evidence of the conservation of the machinery involved in this process across different species of naturally competent bacteria (for a review, see reference 11). Factors involved in competence in different bacteria show sequence homology; remarkably, orthologs of proteins which participate in TFP structure, biogenesis, or function are necessary for competence, although piliation is not observed in some naturally competent bacteria, such as Bacillus subtilis.
In this work, we decided to explore the similarities between different DNA uptake systems and the availability of genomic sequence data to identify new genes involved in competence in Neisseria gonorrhoeae (gonococcus [GC]). The sequence from GC strain FA1090 is available from the ongoing Gonococcal Genome Sequencing Project at the University of Oklahoma (http://dna1.chem.ou.edu/gono.html). Thus, we searched the neisserial genome database, using as queries competence factors of other bacteria which lacked a described ortholog in Neisseria.
MATERIALS AND METHODS
Bacterial strains and growth.
N. gonorrhoeae MS11 (43) and derivatives used in this work are listed in Table 1. Piliated (P+) and transparent (Opa−) bacteria were grown on gonococcal (GC) agar at 37°C with 5% CO2, with the following antibiotics when necessary: erythromycin (2.5 μg/ml), kanamycin (50 μg/ml), spectinomycin (40 μg/ml), and chloramphenicol (10 μg/ml). Plasmids were hosted routinely in Escherichia coli XL-1 Blue, with exception of pSY6 (40), which was hosted in E. coli DH5α. Antibiotic concentrations used for E. coli were 100 μg of ampicillin, 200 μg of erythromycin, 25 μg of kanamycin, 50 μg of spectinomycin, and 100 μg of chloramphenicol/ml.
TABLE 1.
N. gonorrhoeae strains used in this work
| Strain | Genotype | Source |
|---|---|---|
| MS11 | Wild type | J. Swanson |
| MS11 ΔcomE1::Spc | ΔcomE1::Spcr | This work |
| MS11 ΔcomE1::Erm | ΔcomE1::Ermr | This work |
| MS11 ΔcomE2 | ΔcomE2::Spcr | This work |
| MS11 ΔcomE3 | ΔcomE3::Clmr | This work |
| MS11 ΔcomE4 | ΔcomE4::Kanr | This work |
| MS11 ΔcomE43 | ΔcomE4::Kanr ΔcomE3::Clmr | This work |
| MS11 ΔcomE432 | ΔcomE4::Kanr ΔcomE3::Clmr ΔcomE2::Spcr | This work |
| MS11 ΔcomE-all | ΔcomE4::Kanr ΔcomE3::Clmr ΔcomE1::Ermr ΔcomE2::Spcr | This work |
Construction of deletion mutants.
Plasmid pGCx1 is a derivative of pBluescript II SK(−) (Stratagene) containing one copy of the GC DUS. It was constructed by inserting a pair of annealed complementary 33-bp oligonucleotides (MutIII [5′TCACTGGCCGTCTGAATACAACGTCGTGACTGG3′] and MutIV [5′CCAGTCACGACGTTGTATTCAGACGGCCAGTGA3′]; DUS is underlined) into the EcoRV site of the vector. Chromosomal DNA from GC strain MS11 was used as the template for PCR amplifications.
The sequence corresponding to a 590-bp region upstream of the comE gene was amplified by PCR using primers comE-up1 (5′TAACGCGTGAAGCTAACCCATAC3′) and comE-up2 (5′CCTAAGCTTGCAAACAACAAACCGCATCT3′); this product was digested with HindIII (one site in the 5′ region of the product and the other site in comE-up2, underlined), and the resulting 515-bp fragment was cloned into pGCx1 linearized with HindIII. The plasmid pUP24 contains the fragment oriented with its 5′ end closer to the KpnI site from the vector polylinker, and it was used to generate the other constructs.
For deletion of comE1, a 327-bp fragment containing the 3′ end of comE1 and the sequence downstream was amplified by PCR using the primers comE-dwn (5′CCAGAATTCCGCGCCCGCACCAAAAG3′) and comE-dwn1 (5′CCATAGATCTCCCATTCTGCAGATAAAACT3′). This fragment was digested with BglII and EcoRI (sites underlined in the primer sequences) and ligated to pUP24 digested with BamHI and EcoRI, generating pUPD1. The omega (Ω) element containing the cassette for spectinomycin and streptomycin resistance was excised from pHP45Ω (31) by EcoRI digestion and cloned into pUPD1 linearized with EcoRI, yielding pΔ1Ω.
A second construct was made for comE1 deletion by inserting an erythromycin resistance cassette into pUPD1. The erythromycin resistance cassette, originally from pIM13 (32), was previously subcloned into pBluescript II SK(−) (E. C. Gotschlich, unpublished data), from which it was excised by digestion with NsiI (site immediately downstream of the stop codon of the ermC gene) and EcoRI. This fragment was ligated into pUPD1 digested with EcoRI (from vector polylinker) and NsiI (site in the region downstream of comE1 [see Fig. 4A]), yielding pΔ1Erm.
FIG. 4.
Deletion of comE copies in GC strain MS11 and mutant phenotypes. (A) Deletions of comE, shown by Southern blot analysis. (B) Deletions of comE affect genetic transformation of GC MS11. Frequencies are means ± standard deviations from six independent experiments, performed on three different days. (C) Deletion of comE1 affects comP expression. Total RNA was prepared from the indicated strains and used as a template for RT-PCR. Amplification of tbpA, an iron-regulated gene (6), served as an internal control. Negative controls were performed with heat-inactivated RT, with no detectable product (not shown). (D) Deletions of comE affect GC ability to take up DNA. Results are means ± standard deviations of cell-associated radioactivity from one experiment done in triplicate and are representative of at least five independent assays. (E) Detection of ComE by immunoblotting. Purified rComE (25 ng) and whole-cell lysates from GC MS11 (wild type [wt]) and comE deletion strains (ca. 2 × 108 CFU) were loaded on to a 12.5% polyacrylamide–Tris–Tricine–SDS gel, fractionated by electrophoresis, transferred to nitrocellulose filters, and probed with rabbit anti-rComE serum.
For deletion of comE2, a 362-bp fragment containing the 3′ end of comE2 and the sequence downstream was amplified by PCR using Vent polymerase and the primers comE-dwn and com2 (5′ACACTCGAGCCTAATCTGTACCTGCGTCTGT3′). This product was digested with EcoRI (site inside primer comE-dwn) and ligated to pUP24 digested with SmaI and EcoRI, generating pUPD2. The Ω element was excised from pHP45Ω as a 2-kb EcoRI fragment and cloned into pUPD2 linearized with EcoRI, yielding pΔ2Ω.
For deletion of comE3, a 436-bp fragment containing the 3′ end of comE3 and the sequence downstream was amplified by PCR using Vent polymerase and the primers comE-dwn and com3 (5′TAACTCGAGGCCCGGATGCCTGATTAT3′). This product was digested with EcoRI (site inside primer comE-dwn) and ligated to pUP24 digested with SmaI and EcoRI, generating pUPD3. A 1.1-kb fragment containing the chloramphenicol acetyltransferase gene (cat) was excised from pHSS6-Cat2.9 (a gift from H. S. Seifert) by NotI digestion, treated with the Klenow fragment of E. coli DNA polymerase I, and cloned into pUPD3 linearized with EcoRI and treated with Klenow fragment, yielding pΔ3Clm.
For deletion of comE4, a 631-bp fragment containing the 3′ end of comE4 and the sequence downstream was amplified by PCR using Vent polymerase and the primers comE-dwn and com4 (5′TAACTCGAGTGCTGCCTTTTCCCATTT3′). This product was digested with EcoRI (site inside primer comE-dwn) and ligated to pUP24 digested with SmaI and EcoRI, generating pUPD4. A 1.2-kb fragment containing a kanamycin resistance cassette was excised from pCR2.1-kan/DUS (a gift from Stuart Hill) and cloned into pUPD4 linearized with EcoRI, yielding pΔ4Kan.
The plasmids pΔ1Ω, pΔ1Erm, pΔ2Ω, pΔ3Clm, and pΔ4Kan were used to transform N. gonorrhoeae MS11 and derivatives.
Southern blot analysis.
Total DNA from GC strains was digested with HindIII, subjected to electrophoresis in a 0.8% agarose gel and transferred to a nylon membrane (Hybond N+; AP Biotech) using standard techniques. Probe labeling, hybridization, and detection were performed with AlkPhos system (AP Biotech). A PCR product corresponding to an internal fragment of the comE gene was used as a probe; it was amplified using the oligonucleotides com-S (5′CCATGAAAAAAATGTTTGTATTG3′) and com-RT (5′GCCGGACCGATGCCCTTC3′) as primers and plasmid pGC-comE1 as the template. The latter was constructed by inserting a PCR fragment containing the comE1 gene into plasmid pGCx1; the fragment was amplified with the primers com1 (5′AACAAGCTTTTAGAAAATGACCCGTTTTA3′) and com1-2 (5′AACTCGAGTACAGACAATATCAAGACCACT3′), digested with HindIII and XhoI (sites underlined in primer sequences), and ligated to pGCx1 digested with the same restriction enzymes.
RT-PCR.
Reverse transcription-PCR (RT-PCR) was performed with Ready-To-Go beads (AP Biotech), using as the template 500 ng of total RNA prepared from GC strains with Tri Reagent (Molecular Research Center) and treated with RNase-free DNase I. Two sets of primers were used: (i) comP1 (5′AATCGGGGGTTTACACTGGT3′) and comP2 (5′TCACTACACGAACTGGCGGACTT3′) and (ii) tbpA1 (5′ATTTGCCTTCCGGTTGGTCATAGC3′) and tbpA2 (5′GGCGGTCGGGCGGTAAAATAAA3′). DNA contamination controls were performed by inactivating reverse transcriptase with 10 min of incubation at 95°C. Amplification was carried out for 27 cycles, and products were analyzed by agarose gel electrophoresis.
Transformation assay.
Gonococcal strains grown on GC agar for 12 to 15 h were suspended in Proteose Peptone medium with 10 mM MgCl2 to ca. 108 CFU/ml. A 0.5-ml portion of the suspensions was mixed with 500 ng of plasmid pSY6, which contains a neisserial chromosomal fragment with a mutated form of the gene gyrB that confers resistance to nalidixic acid (40). The suspensions were kept at 37°C for 30 min, after which 100 μg of DNase I was added, and the mixtures were further incubated for 5 to 10 min at room temperature. The bacteria were diluted with 2 ml of Proteose Peptone broth and incubated for 5 h at 37°C with 5% CO2, after which they were serially diluted and plated in triplicates on GC agar, with or without 2 μg of nalidixic acid/ml. Transformation frequencies were calculated relative to the total number of CFU recovered on GC agar.
Uptake assay.
A 350-bp DNA fragment containing the DUS in its central portion was amplified by PCR, using pGCx1 as the template and primers T3 (5′ATTAACCCTCACTAAAGGGA3′) and Blue (5′ATTTCCATTCGCCATTCAGG3′). Radiolabeled [α-32P]dATP and [α-32P]dCTP were added to the reaction mixture and incorporated into the product, which was purified using G-50 spin columns (ProbeQuant; AP Biotech); specific activities obtained were about 5 × 106 cpm/μg.
Bacterial suspensions in Proteose Peptone medium with 10 mM MgCl2 were adjusted to an optical density at 540 nm of 0.25. Assays were performed in triplicate: 200 μl of the suspension (corresponding to ca. 5 × 107 CFU) was mixed with 250 ng of radioactively labeled DNA and allowed to incubate at 37°C for 30 min. The samples were treated with 100 μg of DNase I for 10 min at room temperature in order to degrade exogenous DNA, filtered through 0.45 μM HATF membranes in 96-well plates (MultiScreen-HA; Millipore), and washed 5 times with 250 μl of GC broth. The membranes were suspended in 2 ml of scintillation liquid, and cell-associated radioactivity was measured on a scintillation counter.
Primer extension.
Total RNA (30 μg) from GC strain MS11 was annealed with 0.2 pmol of the oligonucleotide comE-XT (5′ATGTTTACCGCCGCAAGGGAGAAG3′) end labeled with [γ-32P]ATP and T4 polynucleotide kinase. Primer extension reactions were performed with Superscript II enzyme (Gibco BRL), and the product was analyzed in an 8% polyacrylamide gel, together with products of sequencing reactions using comE-XT as the primer and pGC-comE1 as the template.
Expression and purification of rComE.
The primers comE-M (5′GAAGGATCCGCGGTAAACATCAATGCGGC3′) and com1-2 (5′AACTCGAGTACAGACAATATCAAGACCACT3′) were used to amplify the sequence corresponding to the putative mature ComE; the fragment was digested with BamHI and XhoI (sites underlined in primer sequences) and cloned into pQE30 (Qiagen) digested with BamHI and SalI, yielding plasmid pQM1. Expression of mature six-His-tagged ComE (rComE) in E. coli strain M15/pRP4 was induced by adding 0.5 mM IPTG (isopropyl-β-d-thiogalactopyranoside) to cultures in mid-log phase, and the recombinant protein was purified by affinity chromatography with nickel-nitrilotriacetic acid resin under native conditions, as recommended by the manufacturer. The purified protein was used to raise rabbit antiserum.
DNA-binding assays.
Southwestern blots were performed as follows: protein samples were fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose filters. The filters were equilibrated in binding buffer (25 mM HEPES [pH 7.9], 3 mM MgCl2, 4 mM KCl) with 5% skim milk and 0.05% NaN3 for 4 h, probed overnight with radiolabeled DNA, and washed with binding buffer before exposure. Different DNA molecules were radiolabeled by primer extension and used as probes: plasmid pBluescript II SK(−), plasmid pGCx7 [pBluescript II SK(−) containing seven copies of the DUS], and a 0.7-kb PvuII fragment derived from the latter.
For polyacrylamide gel retardation assays, a 96-bp fragment containing one copy of the DUS was excised from pGCx1 as an XbaI-XhoI fragment and end labeled with [γ-32P]ATP and T4 polynucleotide kinase. Approximately 4 × 10−3 pmol (0.25 ng) of the probe was mixed with different amounts of rComE in 15 μl of gel shift buffer (25 mM HEPES [pH 7.9], 3 mM MgCl2, 4 mM KCl, 150 mM NaCl, and 5% glycerol), with or without competing DNA [poly(dI-dC) or calf thymus DNA]. The mixture was incubated for 15 min at room temperature and separated by electrophoresis in an 8% polyacrylamide gel in Tris-borate-EDTA buffer.
For agarose gel retardation assays, 500 ng of pBluescript II SK(−) or pGCx1 was incubated with different amounts of rComE in 10 μl of 25 mM HEPES (pH 7.9)–2.5 mM MgCl2–50 mM NaCl–500 ng bovine serum albumin for 15 min at room temperature. Reaction products were analyzed by electrophoresis in 0.8% agarose gel in Tris-acetate-EDTA buffer.
Immunoblots.
Proteins were fractionated by SDS-PAGE on a 12.5% polyacrylamide gel in Tris-Tricine buffer and transferred to nitrocellulose membrane (Hybond-C; AP Biotech). The membrane was blocked with 3% skim milk–0.05% Triton X-100 in phosphate-buffered saline and probed with rabbit anti-ComE antiserum diluted in the same solution, followed by incubation with protein A conjugated to horseradish peroxidase and detection by chemiluminescence (ECL kit; AP Biotech).
RESULTS
Identification of an ORF with homology to B. subtilis comEA in the Neisseria genome.
ComEA is the DNA receptor in competent B. subtilis (33), being required for both binding and transport of DNA during transformation (20). Using the TBLASTN algorithm (1), the gonococcal genome was queried against ComEA. We initially found one open reading frame (ORF) whose derived amino acid sequence showed homology (46% identity and 69% similarity over a 63-amino-acid stretch) (Fig. 1A) to the C-terminal portion of the B. subtilis protein, which is the domain with DNA-binding activity. The neisserial ORF was named comE.
FIG. 1.
(A) Alignment of B. subtilis ComEA and amino acid sequence derived from the GC FA1090 ORF. Identical residues and conservative substitutions are indicated. The predicted signal peptide cleavage site between residues 19 and 20 of the GC ORF is marked by an arrow. The putative flexible hinge in B. subtilis ComEA is underlined. (B) Multiple alignment of amino acid sequences sharing homology with the GC ComE region containing HhH motifs. Residues conserved in at least 5 of the 13 sequences are shown in bold. The sequences (and their accession numbers in GenBank, for protein or nucleotide) are from B. subtilis ComEA (B. sub, AAC36905), S. pneumoniae CelA (S.pne, AF052208), D. nodosus ORF E (partial) (D.nod, AAB41275), E. coli hypothetical protein (E.col, AAB40198), Pseudomonas aeruginosa (P.aer, AF147795), Haemophilus influenzae hypothetical protein (H.inf, HI1008), Haemophilus ducreyi ORF (H.duc, AF087414), Campylobacter jejuni putative protein (C.jej, CAB72504), Deinococcus radiodurans (D.rad, AAF11406), Mycobacterium tuberculosis hypothetical protein (M.tub, AB03744), Synechocystis sp. protein (Synec, BAA10416), and Thermotoga maritima protein (T.mar, AAD36129).
The 99-amino-acid sequence derived from comE contains a cleavable 19-residue signal peptide (Fig. 1A), as predicted by the SignalP algorithm (28). This would lead to a 8-kDa mature polypeptide, with the high proportion of lysine residues contributing to its basic pI (9.86). Two helix-hairpin-helix (HhH) motifs (8) were predicted in the sequence by SMART/Pfam alignment (36, 38). BLAST searches in GenBank identified similarities with the products of ORFs from different bacteria (Fig. 1B), including the pneumococcal protein CelA, which is necessary for competence (30), and several hypothetical ORFs. The homology is restricted to the region with the predicted HhH motifs.
As more sequence data from the gonococcal genome became available, the presence of four copies of comE in FA1090 was revealed. These copies are identical at the nucleotide level, except for a 6-bp deletion in three of them, which would result in the deletion of two internal residues (positions 41 and 42 of the sequence shown in Fig. 1A). Based on the FA1090 sequence, we designed specific primers and amplified each of the four copies from GC strain MS11, which were found to be identical to each other at the nucleotide and hence amino acid levels (accession numbers AF268314 to 268317); the derived polypeptide is the same as in Fig. 1A.
Four copies of comE are also present in the Neisseria meningitidis (meningococcus [MC]) serogroup A strain Z2491 genome (29), and the derived amino acid sequences are highly similar: they all show a Thr at position 62 (instead of Ile as in GC [Fig. 1A]), and there are two variable residues toward the C terminus (positions 93 and 97) (partial sequences from NMA0211, NMA0423, NMA1915, and NMA2187 at the Sanger Centre database [http://www.sanger.ac.uk/Projects/N_meningitidis/]). The genome of MC serogroup B strain MC58 has also become available recently (41): again, there are four copies of comE (partial sequences from NMB0299, NMB1657, NMB2017, and NMB0057 at The Institute for Genomic Research database [http://www.tigr.org/tigr-scripts/CMR2/GenomePage3.spl?database=gnm]), with Thr at position 62 and substitutions at four positions in the C-terminal region (positions 88, 93, 94, and 97), and one copy contains an in-frame deletion leading to a 30-amino-acid truncation in the middle of the deduced sequence (from position 39 to 68).
Genetic organization of the comE loci in Neisseria.
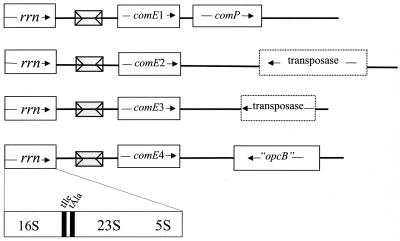
The comE copies are distributed throughout the bacterial chromosome, located downstream of each of the four rRNA operons (rrn) of GC FA1090 (Fig. 2), and were arbitrarily numbered 1 to 4. The sequence between the rrn operons and the comE copies is absolutely conserved in all four locations and contains a copy of the Correia element, a neisserial 152-bp repetitive sequence with 26-bp inverted repeats at its extremities (7). In both MC strains, Z2491 and MC58, the comE copies are also found next to the rRNA operons but the Correia element is absent at all four locations.
FIG. 2.
Genetic organization of the comE copies in GC FA1090. The diagram shows previously characterized genes as solid boxes and putative genes as dotted-line boxes. Correia elements are indicated by gray boxes.
As seen in Fig. 2, the sequences at the 3′ end of the comE copies are not similar. The comP gene, which encodes a pilin-like protein essential for competence in GC (45), is localized downstream of comE1 in GC as well as in MC (NMA0424 in the Sanger Centre database and NMB2016 in The Institute for Genomic Research database). Located ca. 1 kb downstream of comE2 there is an ORF whose derived 217-aa sequence shares homology with the transposase from IS1016. An ORF yielding a 138-amino-acid polypeptide similar to the N-terminal portion of IS1106 transposase is found 400 bp downstream of comE3. The region downstream of comE4 has been recently characterized in GC and MC, and it was suggested to be a DNA island probably imported from unrelated bacteria, with the presence of pseudogenes, deletions, and insertion elements indicating the genome plasticity of the region (46).
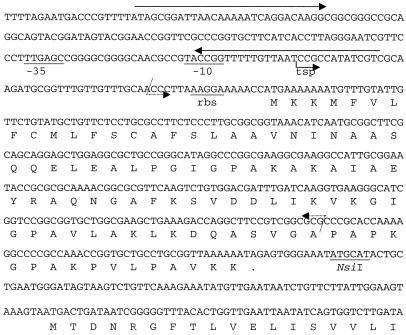
A more detailed view of comE1 in GC is provided in Fig. 3, but it should be noted that in strain MS11 all 4 copies are identical at the nucleotide level up to the stop codon of comE. A potential ribosomal binding site (5′AAGGA3′) is located six bases upstream of the initial ATG. The transcription start point of comE in GC MS11, identified by primer extension analysis (not shown), is located 59 nucleotides upstream of the initial ATG. The derived −10 region (5′TACCGG3′) has only two out of six bases identical to the sigma 70 promoter consensus sequence (5′TATAAT3′), while a potential −35 region (5′TTGAGC3′) can be recognized 18 bp further upstream, with four of six bases of the consensus sequence (5′TTGACA3′). This putative promoter is localized inside the Correia element, with the −10 region and the transcription start point contained in the proximal 26-bp inverted repeat (Fig. 3).
FIG. 3.
Sequence of comE1 and flanking sequences in GC. Solid arrows above the sequence indicate the inverted repeats that constitute the extremities of the Correia element. Tsp, transcription start point. The −10 and −35 elements of the derived promoter are underlined. rbs, putative ribosomal binding site. The limits of the deletion in the construct ΔcomE1::Spc, and also for ΔcomE2, ΔcomE3, and ΔcomE4, are marked by dotted arrows, whereas the 3′ limit of the deletion in the construct ΔcomE1::Erm is indicated by the NsiI site. The ORF downstream of comE1 corresponds to the 5′ region of comP.
Deletion of comE copies affects transformation ability.
In order to determine whether comE is necessary for Neisseria competence, mutants containing deletions of comE were constructed in a GC MS11 background and tested for transformability. This was accomplished by replacing the structural region of each comE copy with an antibiotic resistance cassette, with the use of four different cassettes allowing for the construction of mutants with multiple comE deletions. The deletions were confirmed by PCR (not shown) and Southern blot analysis (Fig. 4A).
Deletion of comE single copies did not result in a measurable effect on competence (Fig. 4B), except for the mutant with the ΔcomE1::Spc genotype, which exhibited a ca. 10-fold reduction in transformation frequency compared to the parental strain. Since the sequence upstream of each of the comE copies and the structural regions themselves are identical in MS11, this phenotype could be due to a polar effect of this particular construct. In fact, comP, the gene located immediately downstream of comE1, is essential for competence, and it was reported that transposon insertions in the 3′-terminal region of comE1 partially reduced the transformation ability of GC (45).
In order to confirm the polar effect of ΔcomE1::Spc, a second strain (the ΔcomE1::Erm mutant) was constructed by replacing comE1 with the Ermr cassette without its transcriptional terminator, inserted in the same orientation as comP and close to its initial ATG (see the NsiI site in Fig. 3). In this way, comP would be expressed as a transcriptional fusion from the Ermr cassette promotor. RT-PCR analysis was performed to assess comP mRNA levels in the strains: as shown in Fig. 4C, a small decrease in the amount of comP transcript could be detected in the ΔcomE1::Spc strain compared to the wild-type strain; in contrast, the ΔcomE1::Erm strain had elevated levels of comP mRNA. Transformation frequencies obtained with the ΔcomE1::Erm strain were consistently higher (ca. threefold) than wild-type frequencies (Fig. 4B), suggesting that ComP may be limiting during the transformation process. This is consistent with the fact that ComP was not detectable by immunoblotting in GC (45), which indicates that this protein is normally present in very small amounts.
Sequential deletion of the comE copies yielded the following results: deletion of copies 3 and 4 did not have a marked effect on transformation frequency (an approximately 50% decrease compared to the parental strain), whereas deletion of copies 4, 3, and 2 diminished transformability to ca. 10 to 15% of the wild-type level. Finally, deletion of all four copies severely impaired transformation capacity, even with comP being overexpressed (Fig. 4C): frequencies were reduced to ca. 0.0025% of wild-type values (Fig. 4B), which corresponds to a 4 × 104-fold decrease. The ΔcomE-all mutant did not show detectable growth deficiency in vitro and exhibited normal piliation, as evaluated by colony morphology; the twitching motility phenotype was also preserved. Thus, we conclude that comE is not necessary for pilus formation, but it is essential for genetic competence in GC.
Effects of comE deletions on transformation correlates with DNA uptake ability.
In order to understand in which step of the transformation process comE participates, we compared the abilities of GC MS11 and the deletion mutants to take up DNA to a DNase-protected state (Fig. 4D). The ΔcomE2, ΔcomE3, and ΔcomE4 mutants were able to internalize DNA at levels similar to that of the wild-type strain. The ΔcomE1::Spc mutant showed impaired ability to take up DNA, whereas the ΔcomE1::Erm strain was up to 10 times more efficient than the parental strain in this assay; these data confirm the role of ComP in DNA uptake (45). Multiple deletions of comE led to progressive reduction in the ability to internalize DNA; the ΔcomE-all mutant protected DNA at the same level as nonpiliated bacteria, which are unable to take up DNA (3). The pattern shown by the comE deletion strains in this assay resembles their phenotypes regarding transformation capacity (Fig. 4B), indicating that the influence of comE mutations on transformability reflects their effect on DNA uptake. Thus, we conclude that comE is necessary for efficient DNA uptake in GC.
The comE gene is expressed as a protein.
Rabbit antiserum was raised against purified rComE and used for immunoblotting analysis of whole-cell extracts from MS11 and comE mutants. As shown in Fig. 4E, a number of bands were recognized by this antiserum, including a strongly immunoreactive band corresponding to a protein of ca. 8 kDa. This band could be observed in lysates from the wild-type and single-copy deletion strains, with similar intensity; a decrease in the signal could be observed in the ΔcomE43 strain, whereas it was very weak in the ΔcomE432 strain. Finally, this band was absent in the mutant ΔcomE-all strain, indicating that it corresponds to the product of comE. The migration rate of this band agrees with the predicted molecular mass of the protein after processing of the putative signal peptide (8 kDa), while the unprocessed protein would be 10.3 kDa. rComE has a calculated molecular mass of 9.5 kDa.
ComE was detected by immunoblotting in total cell lysates from an MS11 nonpiliated variant (data not shown), indicating that piliation is not necessary for expression or stability of this protein. The same band was observed in lysates from other GC strains (FA1090 and F62), as well as MC strains from serogroups A (A1) and B (M1080) (data not shown).
Recombinant ComE binds DNA.
B. subtilis ComEA protein has DNA-binding activity (33). In order to characterize the properties of the neisserial ortholog, we expressed and purified rComE and tested its ability to bind DNA in a number of in vitro assays.
A DNA-binding activity could be observed in a Southwestern assay in whole-cell lysate from E. coli M15/pRP4/pQM1 (which encodes the His-tagged ComE) after IPTG induction (Fig. 5A). The purified recombinant protein showed the same property, indicating that the DNA-binding activity belongs to the His-tagged protein. However, different probes were found to bind to the protein (data not shown), irrespective of the presence or absence of DUS.
FIG. 5.
rComE binds DNA. (A) Southwestern blot with lysates from E. coli strains hosting control plasmid pQE30, pQM1, and pQM1 with IPTG induction. The pQM1/IPTG lysate was loaded on a nickel-nitrilotriacetic acid column, and rComE was eluted with imidazole. After electrophoresis through a 15% polyacrylamide–SDS gel, proteins were transferred to a nitrocellulose filter, which was probed with a radiolabeled 0.7-kb DNA fragment containing seven copies of the DUS. (B) Gel retardation assay. A radiolabeled 96-bp DNA fragment containing a copy of the DUS was incubated with increasing amounts of rComE in the presence of 250 ng of calf thymus DNA and loaded on 8% polyacrylamide gel. (C) Gel retardation assay showing binding of rComE to DNA with or without 1 μg of poly (dI-dC). (D) Agarose gel retardation assay. Plasmid pBluescript II SK(−) was incubated with increasing amounts of rComE and loaded on a 0.8% agarose gel.
Purified rComE was also tested by polyacrylamide gel retardation assays, confirming its ability to bind DNA (Fig. 5B and C). The DNA probe used in these assays contained DUS, but partial inhibition of binding was observed in the presence of 250 ng of calf thymus DNA (compare Fig. 5B and C). DNA binding could also be competitively inhibited by 1 μg of poly(dI-dC) (Fig. 5C). These data indicate that rComE binds to DNA without sequence specificity.
When plasmids were used as ligand, a “ladder” pattern could be observed in agarose gel (Fig. 5D). This could be explained by the presence of multiple binding sites for ComE in the plasmid molecule, confirming the lack of sequence specificity of the binding. In fact, the recombinant protein could bind to different plasmids, regardless of the presence or absence of DUS, with the same apparent affinity (data not shown).
Recombinant ComE inhibits neisserial transformation.
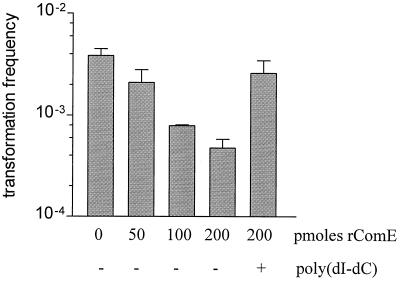
When purified ComE was exogenously added to a transformation mixture, it was able to inhibit transformation of GC MS11 in a dose-dependent manner (Fig. 6). This effect can be attributed to sequestration of the plasmid DNA by rComE, and it could be alleviated by the presence of poly(dI-dC) (Fig. 6), which by itself did not have any effect on transformation (data not shown). These results support the notion that the binding of DNA to ComE is not sequence specific.
FIG. 6.
Exogenous rComE is able to inhibit GC MS11 transformation in a dose-dependent manner. The transformation assay was carried out with 0.5 μg of pSY6. The inhibition by rComE was alleviated by the presence of 5 μg of poly (dI-dC). Results are means ± standard deviations from one experiment done in duplicate and are representative of at least three independent experiments.
Strains with mutations of pilC, whose product is a pilus tip adhesin (35), are impaired in their ability to take up DNA and undergo transformation; competence could be partially restored in these mutants by supplying them with exogenous purified PilC (34). We tested rComE in a similar way and found that exogenously added ComE did not restore the transformation ability of comE mutant bacteria (data not shown).
DISCUSSION
We identified comE, a gene involved in DNA uptake during transformation of N. gonorrhoeae. So far, this is the only gonococcal competence factor known to interact with DNA. Its identification was possible due to the similarity to its ortholog in B. subtilis and to the availability of neisserial genomic sequence information. Given the presence of four copies of this gene in the chromosome and the need to inactivate at least three of them in order to have a clearly noticeable phenotype (Fig. 4B), its identification by classical genetic methods such as mutagenesis would have been unlikely.
Why are there four copies of comE in the Neisseria genome? As shown here, a single copy of this gene in GC is sufficient to achieve efficient transformation, and the complete absence of comE did not affect viability in vitro or piliation, suggesting that there would not be a strong selective pressure to keep multiple copies of comE. In GC, there are Correia elements flanking the comE genes; copies of this repetitive sequence have been found upstream of several members of the opa gene family (2), indicating that it might play a role in gene duplication and rearrangement. Its presence upstream of the comE copies in GC could suggest that a similar process led to the duplication of this gene. However, these elements are not found upstream of comE genes in N. meningitidis.
The localization of comE next to the rRNA operons might provide a hint on how its duplication occurred and how the multiple copies are kept homogeneous. rRNA operons, present in multiple copies in most bacterial species, are subject to concerted evolution by gene conversion (18), and homogenization of flanking regions resulting from coconversion has been described (24). Thus, it is conceivable that comE was initially present downstream of one rrn operon, from where it was propagated to the other locations by gene conversion. This process would also account for the sequence homogeneity among the copies within one strain, whereas the sequence could drift between strains. Interestingly, as seen from MC genomes, the differences among the comE copies within a strain occur mostly in the 3′ end of the gene, where the homogenizing effect of the rrn operon proximity might be less pronounced. The insertion of the Correia element between the rrn operons and the comE copies in GC would have occurred after comE duplication, initially as a single insertion which then spread to the other loci through the same mechanism. A strikingly similar situation is found in Dichelobacter nodosus, where identical copies of the 5′ region of ORF E, whose product is homologous to ComE (Fig. 1B), are located downstream of all three rrn operons (22). Another suggestive analogy can be drawn from Streptococcus pneumoniae, where two identical copies of comX, which encodes a competence-specific transcriptional modulator, are located upstream of two rrn operon copies (23).
In MC, the ORFs containing the comE copies can be extended beyond the ATG start codon for GC shown in Fig. 3. In fact, Zhu et al. (46) described a comEA-like ORF (which corresponds to comE4) in MC and assumed that the corresponding gene in GC would be a pseudogene, interrupted by the Correia element. Furthermore, the annotation of both MC genomes features ComEA-like proteins of 148 amino acids. However, our data show that ComE in GC is functional, and immunoblotting analysis detected a protein in MC with the same migration rate as in GC.
Neisserial ComE shares sequence similarity with the C-terminal portion of the B. subtilis ComEA, which is the domain with DNA-binding activity. The alignment of the two protein sequences shows that the homology starts immediately after the putative signal peptide cleavage site in neisserial ComE and after a flexible region containing a stretch of glycine residues in B. subtilis ComEA, which could function as a hinge that allows the protein to bend and direct the bound DNA to a channel spanning the cytoplasmic membrane (11). B. subtilis ComEA is an integral membrane protein which spans this organism's thick cell wall, in order to make contact with DNA on the cell surface. In contrast, neisserial ComE is much smaller and does not contain any identifiable transmembrane domain, but it does possess a predicted signal peptide. Thus, the protein would presumably be translocated across the cytoplasmic membrane by the Sec machinery; since the signal peptide is apparently cleaved, as suggested by the migration rate of the ComE band (Fig. 4E), the mature protein should be released into the periplasm.
ComE and its B. subtilis ortholog (ComEA) contain predicted HhH motifs, which interact with DNA in a non-sequence-specific manner (8). Indeed, DNA binding and uptake in B. subtilis do not have any sequence requirement, and our data indicate that the DNA-binding activity of ComE lacks sequence specificity. However, DNA uptake in Neisseria is dependent on the presence of the DUS, a motif which is overrepresented in the neisserial genome, frequently appearing as an inverted pair 3′ of ORFs and serving as a transcriptional terminator (17, 29). So, one may question what role ComE plays in the process of DNA uptake in Neisseria.
The first possible scenario is that ComE binds DNA nonspecifically to the cell surface. DNA binding to gonococci has been reported to be nonspecific (9), and different positively charged surface structures may contribute to this interaction, such as the Opa proteins (19). In the same manner, ComE could prolong sequestration of DNA to the cell surface, which would translate into more efficient uptake over time. However, anti-ComE serum did not inhibit transformation (data not shown) and transformability was not restored in comE mutants by supplying exogenous recombinant protein, in contrast to what was observed with PilC (34): these data suggest that ComE is not surface exposed. Besides, this model would also imply a rather accessory role in transformation, as with Opa (19), whereas ComE is essential for efficient Neisseria transformation. A second possibility is that ComE is part of a structure that binds DNA and recognizes the DUS but is not capable of specific binding by itself. But in that case, transformation would be prone to inhibition by nonspecific DNA, which does not happen in Neisseria.
The third possibility is that ComE is involved in binding of DNA molecules which have already been selected for the presence of the DUS; in that case, sequence specificity would not be a requirement anymore. The DUS would be recognized at the cell surface by its specific receptor, whose identity remains unknown, and committed to uptake, maybe through binding to ComE.
The gene comP, whose product is essential for competence, is located directly downstream of comE1, in both GC and MC. So far, ComP and now ComE are the only factors known to be necessary for DNA uptake which are not involved in pilus structure, biogenesis, or function in Neisseria. In B. subtilis, DNA binding during transformation requires the presence of four pilin-like proteins, ComGC, -GD, -GE, and -GG (4, 5). It has been suggested that these ComG proteins form a channel structure which would allow ComEA to traverse the cell wall and gain access to DNA on the surface (33). Since ComP is a pilin-like protein, it is tempting to speculate whether it would play an equivalent role in Neisseria, perhaps forming a composite structure together with PilE (45), with which ComE would interact.
ACKNOWLEDGMENTS
We acknowledge the following genome projects: the Gonococcal Genome Sequencing Project, N. meningitidis Z2491 at the Sanger Centre, and N. meningitidis MC58 at The Institute for Genomic Research. We thank David Dubnau and Roberta Provvedi for sharing unpublished data on B. subtilis ComEA and helpful suggestions; Vijay Pancholi, Daniel Nelson, and Chris Elkins for technical advice; and Ben Mulder for insightful discussion.
This work was supported by Public Health Service grant AI 10615 to E.C.G.
REFERENCES
- 1.Altschul S F, Madden T L, Schaffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belland R J, Morrison S G, Carlson J H, Hogan D M. Promoter strength influences phase variation of neisserial opagenes. Mol Microbiol. 1997;23:123–135. doi: 10.1046/j.1365-2958.1997.1971556.x. [DOI] [PubMed] [Google Scholar]
- 3.Biswas G D, Sox T, Blackman E, Sparling P F. Factors affecting genetic transformation of Neisseria gonorrhoeae. J Bacteriol. 1977;129:983–992. doi: 10.1128/jb.129.2.983-992.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chung Y S, Breidt F, Dubnau D. Cell surface localization and processing of the ComG proteins, required for DNA binding during transformation of Bacillus subtilis. Mol Microbiol. 1998;29:905–913. doi: 10.1046/j.1365-2958.1998.00989.x. [DOI] [PubMed] [Google Scholar]
- 5.Chung Y S, Dubnau D. All seven comG open reading frames are required for DNA binding during transformation of competent Bacillus subtilis. J Bacteriol. 1998;180:41–45. doi: 10.1128/jb.180.1.41-45.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cornelissen C N, Biswas G D, Tsai J, Paruchuri D K, Thompson S A, Sparling P F. Gonococcal transferrin-binding protein 1 is required for transferrin utilization and is homologous to TonB-dependent outer membrane receptors. J Bacteriol. 1992;174:5788–5797. doi: 10.1128/jb.174.18.5788-5797.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Correia F F, Inouye S, Inouye M. A family of small repeated elements with some transposon-like properties in the genome of Neisseria gonorrhoeae. J Biol Chem. 1988;263:12194–12198. [PubMed] [Google Scholar]
- 8.Doherty A J, Serpell L C, Ponting C P. The helix-hairpin-helix DNA-binding motif: a structural basis for non-sequence-specific recognition of DNA. Nucleic Acids Res. 1996;24:2488–2497. doi: 10.1093/nar/24.13.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dougherty T J, Asmus A, Tomasz A. Specificity of DNA uptake in genetic transformation of gonococci. Biochem Biophys Res Commun. 1979;86:97–104. doi: 10.1016/0006-291x(79)90386-3. [DOI] [PubMed] [Google Scholar]
- 10.Drake S L, Koomey M. The product of the pilQ gene is essential for the biogenesis of type IV pili in Neisseria gonorrhoeae. Mol Microbiol. 1995;18:975–986. doi: 10.1111/j.1365-2958.1995.18050975.x. [DOI] [PubMed] [Google Scholar]
- 11.Dubnau D. DNA uptake in bacteria. Annu Rev Microbiol. 1999;53:217–244. doi: 10.1146/annurev.micro.53.1.217. [DOI] [PubMed] [Google Scholar]
- 12.Elkins C, Thomas C E, Seifert H S, Sparling P F. Species-specific uptake of DNA by gonococci is mediated by a 10-base-pair sequence. J Bacteriol. 1991;173:3911–3913. doi: 10.1128/jb.173.12.3911-3913.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Facius D, Meyer T F. A novel determinant (comA) essential for natural transformation competence in Neisseria gonorrhoeae and the effect of a comAdefect on pilin variation. Mol Microbiol. 1993;10:699–712. doi: 10.1111/j.1365-2958.1993.tb00942.x. [DOI] [PubMed] [Google Scholar]
- 14.Freitag N E, Seifert H S, Koomey M. Characterization of the pilF-pilD pilus-assembly locus of Neisseria gonorrhoeae. Mol Microbiol. 1995;16:575–586. doi: 10.1111/j.1365-2958.1995.tb02420.x. [DOI] [PubMed] [Google Scholar]
- 15.Fussenegger M, Facius D, Meier J, Meyer T F. A novel peptidoglycan-linked lipoprotein (ComL) that functions in natural transformation competence of Neisseria gonorrhoeae. Mol Microbiol. 1996;19:1095–1105. doi: 10.1046/j.1365-2958.1996.457984.x. [DOI] [PubMed] [Google Scholar]
- 16.Fussenegger M, Kahrs A F, Facius D, Meyer T F. Tetrapac (tpc), a novel genotype of Neisseria gonorrhoeaeaffecting epithelial cell invasion, natural transformation competence and cell separation. Mol Microbiol. 1996;19:1357–1372. doi: 10.1111/j.1365-2958.1996.tb02479.x. [DOI] [PubMed] [Google Scholar]
- 17.Goodman S D, Scocca J J. Identification and arrangement of the DNA sequence recognized in specific transformation of Neisseria gonorrhoeae. Proc Natl Acad Sci USA. 1988;85:6982–6986. doi: 10.1073/pnas.85.18.6982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gurtler V. The role of recombination and mutation in 16S–23S rDNA spacer rearrangements. Gene. 1999;238:241–252. doi: 10.1016/s0378-1119(99)00224-3. [DOI] [PubMed] [Google Scholar]
- 19.Hill S A. Opa expression correlates with elevated transformation rates in Neisseria gonorrhoeae. J Bacteriol. 2000;182:171–178. doi: 10.1128/jb.182.1.171-178.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inamine G S, Dubnau D. ComEA, a Bacillus subtilisintegral membrane protein required for genetic transformation, is needed for both DNA binding and transport. J Bacteriol. 1995;177:3045–3051. doi: 10.1128/jb.177.11.3045-3051.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koomey J M, Falkow S. Cloning of the recA gene of Neisseria gonorrhoeae and construction of gonococcal recAmutants. J Bacteriol. 1987;169:790–795. doi: 10.1128/jb.169.2.790-795.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.La Fontaine S, Rood J I. Organization of ribosomal RNA genes from the footrot pathogen Dichelobacter nodosus. Microbiology. 1996;142:889–899. doi: 10.1099/00221287-142-4-889. [DOI] [PubMed] [Google Scholar]
- 23.Lee M S, Morrison D A. Identification of a new regulator in Streptococcus pneumoniaelinking quorum sensing to competence for genetic transformation. J Bacteriol. 1999;181:5004–5016. doi: 10.1128/jb.181.16.5004-5016.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liao D. Gene conversion drives within genic sequences: concerted evolution of ribosomal RNA genes in bacteria and archaea. J Mol Evol. 2000;51:305–317. doi: 10.1007/s002390010093. [DOI] [PubMed] [Google Scholar]
- 25.Maiden M C. Population genetics of a transformable bacterium: the influence of horizontal genetic exchange on the biology of Neisseria meningitidis. FEMS Microbiol Lett. 1993;112:243–250. doi: 10.1111/j.1574-6968.1993.tb06457.x. [DOI] [PubMed] [Google Scholar]
- 26.Mathis L S, Scocca J J. On the role of pili in transformation of Neisseria gonorrhoeae. J Gen Microbiol. 1984;130:3165–3173. doi: 10.1099/00221287-130-12-3165. [DOI] [PubMed] [Google Scholar]
- 27.Meyer T F, Pohlner J, van Putten J. Biology of the pathogenic Neisseriae. Curr Top Microbiol Immunol. 1994;192:283–317. doi: 10.1007/978-3-642-78624-2_13. [DOI] [PubMed] [Google Scholar]
- 28.Nielsen H, Engelbrecht J, Brunak S, von Heijne G. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Prot Eng. 1997;10:1–6. doi: 10.1093/protein/10.1.1. [DOI] [PubMed] [Google Scholar]
- 29.Parkhill J, Achtman M, James K D, Bentley S D, Churcher C, Klee S R, Morelli G, Basham D, Brown D, Chillingworth T, Davies R M, Davis P, Devlin K, Feltwell T, Hamlin N, Holroyd S, Jagels K, Leather S, Moule S, Mungall K, Quail M A, Rajandream M A, Rutherford K M, Simmonds M, Skelton J, Whitehead S, Spratt B G, Barrell B G. Complete DNA sequence of a serogroup A strain of Neisseria meningitidisZ2491. Nature. 2000;404:502–506. doi: 10.1038/35006655. [DOI] [PubMed] [Google Scholar]
- 30.Pestova E V, Morrison D A. Isolation and characterization of three Streptococcus pneumoniae transformation-specific loci by use of a lacZreporter insertion vector. J Bacteriol. 1998;180:2701–2710. doi: 10.1128/jb.180.10.2701-2710.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prentki P, Krisch H M. In vitro insertional mutagenesis with a selectable DNA fragment. Gene. 1984;29:303–313. doi: 10.1016/0378-1119(84)90059-3. [DOI] [PubMed] [Google Scholar]
- 32.Projan S J, Monod M, Narayanan C S, Dubnau D. Replication properties of pIM13, a naturally occurring plasmid found in Bacillus subtilis, and of its close relative pE5, a plasmid native to Staphylococcus aureus. J Bacteriol. 1987;169:5131–5139. doi: 10.1128/jb.169.11.5131-5139.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Provvedi R, Dubnau D. ComEA is a DNA receptor for transformation of competent Bacillus subtilis. Mol Microbiol. 1999;31:271–280. doi: 10.1046/j.1365-2958.1999.01170.x. [DOI] [PubMed] [Google Scholar]
- 34.Rudel T, Facius D, Barten R, Scheuerpflug I, Nonnenmacher E, Meyer T F. Role of pili and the phase-variable PilC protein in natural competence for transformation of Neisseria gonorrhoeae. Proc Natl Acad Sci USA. 1995;92:7986–7990. doi: 10.1073/pnas.92.17.7986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rudel T, Scheurerpflug I, Meyer T F. NeisseriaPilC protein identified as type-4 pilus tip-located adhesin. Nature. 1995;373:357–359. doi: 10.1038/373357a0. [DOI] [PubMed] [Google Scholar]
- 36.Schultz J, Copley R R, Doerks T, Ponting C P, Bork P. SMART: a web-based tool for the study of genetically mobile domains. Nucleic Acids Res. 2000;28:231–234. doi: 10.1093/nar/28.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith J M, Smith N H, O'Rourke M, Spratt B G. How clonal are bacteria? Proc Natl Acad Sci USA. 1993;90:4384–4388. doi: 10.1073/pnas.90.10.4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sonnhammer E L, Eddy S R, Birney E, Bateman A, Durbin R. Pfam: multiple sequence alignments and HMM-profiles of protein domains. Nucleic Acids Res. 1998;26:320–322. doi: 10.1093/nar/26.1.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sparling P F. Genetic transformation of Neisseria gonorrhoeaeto streptomycin resistance. J Bacteriol. 1966;92:1364–1371. doi: 10.1128/jb.92.5.1364-1371.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stein D C, Danaher R J, Cook T M. Characterization of a gyrB mutation responsible for low-level nalidixic acid resistance in Neisseria gonorrhoeae. Antimicrob Agents Chemother. 1991;35:622–626. doi: 10.1128/aac.35.4.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tettelin H, Saunders N J, Heidelberg J, Jeffries A C, Nelson K E, Eisen J A, Ketchum K A, Hood D W, Peden J F, Dodson R J, Nelson W C, Gwinn M L, DeBoy R, Peterson J D, Hickey E K, Haft D H, Salzberg S L, White O, Fleischmann R D, Dougherty B A, Mason T, Ciecko A, Parksey D S, Blair E, Cittone H, Clark E B, Cotton M D, Utterback T R, Khouri H, Qin H, Vamathevan J, Gill J, Scarlato V, Masignani V, Pizza M, Grandi G, Sun L, Smith H O, Fraser C M, Moxon E R, Rappuoli R, Venter J C. Complete genome sequence of Neisseria meningitidis serogroup B strain MC58. Science. 2000;287:1809–1815. doi: 10.1126/science.287.5459.1809. [DOI] [PubMed] [Google Scholar]
- 42.Tonjum T, Freitag N E, Namork E, Koomey M. Identification and characterization of pilG, a highly conserved pilus-assembly gene in pathogenic Neisseria. Mol Microbiol. 1995;16:451–464. doi: 10.1111/j.1365-2958.1995.tb02410.x. [DOI] [PubMed] [Google Scholar]
- 43.West S E, Clark V L. Genetic loci and linkage associations in Neisseria gonorrhoeae and Neisseria meningitidis. Clin Microbiol Rev. 1989;2(Suppl.):S92–S103. doi: 10.1128/cmr.2.suppl.s92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wolfgang M, Lauer P, Park H S, Brossay L, Hebert J, Koomey M. PilT mutations lead to simultaneous defects in competence for natural transformation and twitching motility in piliated Neisseria gonorrhoeae. Mol Microbiol. 1998;29:321–330. doi: 10.1046/j.1365-2958.1998.00935.x. [DOI] [PubMed] [Google Scholar]
- 45.Wolfgang M, van Putten J, Hayes S F, Koomey M. The comP locus of Neisseria gonorrhoeaeencodes a type IV prepilin that is dispensable for pilus biogenesis but essential for natural transformation. Mol Microbiol. 1999;31:1345–1357. doi: 10.1046/j.1365-2958.1999.01269.x. [DOI] [PubMed] [Google Scholar]
- 46.Zhu P, Morelli G, Achtman M. The opcA and φopcB regions in Neisseria: genes, pseudogenes, deletions, insertion elements and DNA islands. Mol Microbiol. 1999;33:635–650. doi: 10.1046/j.1365-2958.1999.01514.x. [DOI] [PubMed] [Google Scholar]