Abstract
Brain metastasis (BM) is the leading cause of mortality in lung cancer patients. The process of BM (from initial primary tumor development, migration and intravasation, dissemination and survival in the bloodstream, extravasation, to colonization and growth to metastases) is a complex process for which few tumor cells complete the entire process. Recent research on BM of lung cancer has recently stressed the essential role of tumor microenvironment (TME) in assisting tumor cells in the completion of each BM step. This review summarizes recent studies regarding the effects of TME on tumor cells in the entire process of BM derived from lung cancer. The identification of vulnerable targets in the TME and their prospects to provide novel therapeutic opportunities are also discussed.
Keywords: Lung cancer, Brain metastasis, Tumor microenvironment
Introduction
Lung cancer has posed a severe burden on human health with a high morbidity and mortality in China and has an increasing incidence over the past decades.[1] Metastasis is the leading cause of the high mortality and brain is the predominant secondary metastasis site in lung cancer. Brain metastasis (BM) occurs in approximately 40% to 50% of patients suffering from lung cancer. The occurrence of BM event indicates a poor prognosis with an average median survival period of 3 to 6 months, posing remarkable clinical challenges.[2,3] BM arises from a series of complex pathophysiological cascade from the original lung site, through the circulation, and finally to the brain. During the entire process, the central and vital role of tumor microenvironment (TME), whether in situ or in the secondary site, has been widely investigated and recognized in recent years. The TME, which is complex and dynamically evolving, comprises a heterogeneous collection of infiltrating and resident host cells (including stromal cells, fibroblasts, endothelial cells, innate and adaptive immune cells), secreted factors, and extracellular matrix (ECM), all of which infiltrate and interact with tumor cells.[4] Increasing evidence indicates that there are cross-talks between tumor cells and TME where TME is reprogrammed by various factors derived from tumor cells, allowing TME to play a decisive role in tumor survival and progression.[5,6] In this review, we focus on recent advances regarding the contribution of TME to lung cancer brain metastasis (LCBM) and related aberrant molecular mechanism. We also discuss the specific targets to TME and their prospects to provide novel diagnostic and therapeutic opportunities.
Metastasis Cascades and “Seed and Soil” Hypothesis
Metastasis is a multistep process where tumor cells from primary sites spread to distant organs or other regions within the same organ.[7,8] The trajectory of lung cancer brain metastatic cells can be generally divided into three major links: (1) primary tumor cells develop and invade the lung in situ via epithelial–mesenchymal transition (EMT)[9]; (2) aggressive tumor cells intravasate, survive, and disseminate in the circulatory system and become circulating tumor cells (CTCs)[10]; (3) CTCs home and adhere to brain microvessels, extravasate through the blood–brain barrier (BBB), and colonize to form micro- and then macroscopic metastases in parenchyma.[11,12]
More than 100 years ago, the “seed and soil” hypothesis was proposed by Paget,[13] which pointed out the important role of TME (soil) in the growth and metastasis behavior of tumor cells (seed). For years, several studies have given solid evidence for this hypothesis, driving the development and approval of new therapeutic strategies that target the TME (eg, vascular endothelial growth factor [VEGF], aromatase and immune checkpoint inhibitors[14]). Here we summarize the interactions between tumor cells and the surrounding stroma in key events of BM process.
TME in BM Process
In situ process
The TME is composed of many complex components, which regulate behaviors of cancer cells from tumorigenesis to metastasis. It includes mainly stromal cells and ECM in an environment characterized by physical and chemical factors such as hypoxia, low pH, and high pressure. Within this environment, mesenchymal cells, such as fibroblasts, endothelial and immune cells, are reprogrammed into tumor-associated types that provide favorable conditions for the development, invasion, and metastasis of primary lung tumor.
Cancer-associated fibroblasts
Cancer-associated fibroblasts (CAFs), as the predominant stromal cells in lung TME, contribute to the local invasion and metastasis of primary lung tumor. It is recognized that CAFs are the major cells involved in the degradation of the ECM,[15,16] which is the first step in the process of tumor invasion and metastasis. CAFs also facilitate the metastasis by promoting the EMT of tumor cells since EMT is shown to drive metastasis during cancer progression. CAFs promote the EMT of lung cancer cells by delivering proteins (Snail 1[17]) or non-coding RNAs (micro RNA [miR]-210, miR-224)[18,19] through exosomes[20] or other approaches; or by secreting soluble factors (interleukin [IL]-6[21,22]; hepatocyte growth factor; insulin-like growth factor 1[23]; transforming growth factor-β [TGF-β][24]; stromal cell-derived factor-1[25]). Upregulated proteins (T-cell lymphoma invasion and metastasis 2, CD34, human mammalian ENA [hMENA]) expressed in CAFs compared with normal fibroblasts are investigated for their roles in the promotion of EMT in lung cancer.[26–28] Further, the stemness of tumor cells, which is regarded as another main axis of metastasis,[29] has been shown to be regulated by CAFs. It is revealed that CD10+ G protein-coupled receptor 77 (GPR77)+ CAFs and CD44+ CAFs subsets are important for the sustainability of tumor stemness in lung cancer[30,31]; CAFs also increased cancer cell stemness by upregulating Netrin-1[32] and paracrine insulin-like growth factor-II/insulin-like growth factor 1 receptor signaling.[33]
Immune populations
Innate and adaptive immune cells (myeloid and lymphoid cell populations), which play an important role in all stages of tumor immune response, surround lung cancer cells in the TME.[34] Amongst these cells, TAMs are the most dominant population in the immune infiltration, accounting for about 30%. TAMs exhibit high plasticity manifested by differentiation into phenotypes such as M1 (pro-inflammatory with anti-tumor activity) and M2 (immunosuppressive with protumor activity), which is driven by specific conditions.[35,36] M2 type TAMs are believed to promote the tumor with more aggressive phenotypes, including the facilitation of invasion and metastasis: lung cancer cell lines (A549 and H1299) co-cultured with M2 subset macrophages display enhanced migration capabilities, with the elevated expression of VEGF, matrix metalloproteinases (MMP)-9 and MMP2, which degrade the ECM and promote tumor cell invasion and migration into blood vessels[37,38]; the presence of M2 TAMs affects the expression of programmed cell death-ligand 1 (PD-L1) both on tumor cells and on tumor-infiltrating immune cells, which advances malignant behavior in non-small cell lung cancer (NSCLC) patients.[39–41] The underlying mechanisms of TAMs promoting the metastasis process have been investigated. Yang et al[42] demonstrated that TAMs promote cancer stem cell-like properties of NSCLC cells by releasing IL-10; Guo et al[43] showed M2 macrophages promote NSCLC metastasis by upregulating αB-crystallin while Lei et al[44] identified an exosomes-related mechanism where M2 macrophages-derived exosomal miR-501-3p promotes the progression of lung cancer. Recently, other research investigated the signaling mechanisms important for the formation of pro-tumoral TAM. Li et al[45] identified a novel Mincle/Syk/nuclear factor kappa-B (NF-κB) signaling pathway in TAM needed for executing their pro-tumoral activities; Liu et al[46] discovered that the transcription factor c-Maf controls many M2-related genes and promotes M2-like macrophage-mediated T cell suppression and tumor progression; Sarode et al[47] showed that β-catenin-mediated transcriptional activation of Fos-like antigen 2 and repression of the AT-rich interaction domain 5A drive gene regulatory switch from M1-like TAMs to M2-like TAMs, thereby promoting tumor progression and metastasis.
Additionally, some studies have revealed the role played by other immune cells in the progression of lung cancer. Schneider et al[48] identified the immunosuppressive role of dendritic cells (DCs) in NSCLC by upregulating B7-H3, which is an independent predictor of poor prognosis in NSCLC patients,[49] while Dumitriu et al[50] found DCs, which are exposed to lung tumor cells, produce increased TGF-β protein and enhance the ability of CD4(+)CD25(+) forkhead box P3(+) regulatory T cells that suppress the proliferation of T lymphocytes, thereby contributing to the immune evasion. Neutrophils are also thought to take an important part in the tumor progression of lung cancer, especially in smoking-associated lung cancer. Similar to macrophages, neutrophils respond to the TME and differentiate into different phenotypes, anti-tumor N1 or pro-tumor N2 type. N1-type tumor associated neutrophils (TANs) predominate in the early stages of tumor development while N2-type TANs accumulate as the tumor progresses.[51] The accumulation of N2-type TANs promotes the process of angiogenesis, tumor cell proliferation, ECM remodeling, and immune evasion by producing pro-inflammatory, proliferative, pro-angiogenic, and immunoregulatory cytokines.[52] Evidence also indicates that tumor-infiltrating B lymphocytes (TIBs) exist in the entire process of lung cancer and play important roles in accelerating tumor development. TIBs are dynamically transformed by modulation of the tumor immune microenvironment and then regulate the immune response. TIBs can suppress anti-tumor immune responses through regulatory B cells which produce immunosuppressive cytokines to regulate other immune effector cells (such as T cells, natural killer cells) or facilitate angiogenesis.[53]
Endothelial cells
Endothelial cell proliferation and migration are prerequisites for angiogenesis in TME which is a key hallmark of cancers,[11] resulting in the generation of new capillaries to support tumor progression and metastasis. On the one hand, lung cancer cells activate endothelial cells and promote angiogenesis by releasing factors (eg, VEGF[54]; hepatoma-derived growth factor[55]) into the TME or upregulating key mediators (eg, profilin 2[56]; long non-coding RNA minichromosome maintenance complex component 3 associated protein antisense RNA 1[57]; miR-494[58]). On the other hand, the activated endothelial cells facilitate tumor metastasis by epigenetic mechanisms that remain incompletely defined. For example, endothelial derived microRNAs contribute to tumor metastasis. Korde et al[59] demonstrated that endothelial growth factor down-regulates miR-1 in the lung endothelium and plays a critical role in tumor progression and angiogenesis while Dimitrova et al[60] identified miR-143/145 expressed in the endothelial cells stimulates angiogenesis and supports tumor expansion in the lung. In addition, in a study performed by Xie et al,[61] the nicotinamide adenine dinucleotide 1-dependent deacetylase sirtuin 1 is confirmed to function as an intrinsic negative modulator of Delta-like ligand 4/Notch signaling in Lewis lung carcinoma xenograft-derived vascular endothelial cells.
Lung microbiota
There is growing evidence that the lung microbiota may play a key role in promoting lung cancer metastasis by influencing the metabolic reprogramming, inflammatory and immune response of tumor cells. Recently, the effects of non-typeable Haemophilus influenzae (NTHi), Streptococcus, and Veillonella on lung cancer cells have been revealed. Ochoa et al[62,63] found that NTHi activates the signal transducer and activator of transcription (STAT) 3 and NF-κB pathways, which are considered as powerful promoters of tumor cell proliferation and angiogenesis, through the release of IL-6 and tumor necrosis factor (TNF); NTHi was also shown to provide an inflammatory microenvironment that favors the acceleration of tumor progression in lung cancer[64–66]; Streptococcus and Veillonella, which were found to be abundant in the lower airways of lung cancer patient, have a close relationship with the upregulation of the extracellular regulated protein kinase (ERK) and phosphatidylinositol 3-kinase (PI3K) signaling pathways that are important for tumor metastasis including BM.[12,67] Besides, it was found that the genus Thermus is more abundant in tissue from advanced stage patients while Legionella is high in lung cancer patients with metastasis; however, the direct evidence for the influence of them on the metastasis cascades is lacking.[68]
“CTC” stage
Although current advances in enriching and analyzing rare cells in the bloodstream have allowed the characteristics of CTCs to be further analyzed,[69] the mechanisms by which CTCs escape immune surveillance and survive or remain dormant in the circulation remain unclear; as only a few CTCs can survive for the next metastasis step. Upon entering the circulation, CTCs derived from primary lung tumor are exposed to tremendous physical and biochemical stresses (eg, high oxygen tension, detachment from ECM, fluid shear stress) which result in extreme oxidative stress characterized by increased reactive oxygen species (ROS) in CTCs, hindering the survival of the vast majority of CTCs. Zheng et al[70] revealed the underlying survival mechanism by performing single-cell RNA-Seq profiles of CTCs from breast, prostate, and lung cancers. They showed that consistent induction of β-globin, which is triggered by increased intracellular ROS in CTCs, efficiently suppresses the level of ROS and mediates the survival and metastasis of CTCs. CTCs are also exposed to the components of the bloodstream, among which the platelets are known to play a vital role in promoting survival, escape from immune surveillance, and ultimate metastasis of CTCs.[71–73] Platelets interact with tumor cells through direct interaction, secretion of platelet microvesicles, or release of platelet granules, all of which are associated with anti-apoptosis behaviors of CTCs.[74] In 1968, Gasic et al[75] showed a correlation between thrombocytopenia and cancer metastasis in mice, indicating the potential effects of platelets in metastasis. Recent studies give insight into the mechanism of the increased metastatic potential mediated by platelets. It is thought that platelets adhere to the surface of CTCs, preventing recognition by the immune system and potentially reducing the shear stress experienced by CTCs.[76,77] However, the exact mechanism of platelet action in LCBM remains less-studied. Beck et al[78] identified platelet-associated genes in metastatic lung cancer using CTC and cell-free RNA capture and expression analysis, suggesting the important role that platelets played in the metastatic lung cancer, which is worthy of further study in the future.
In secondary site
When metastatic lung cancer cells reach the brain, they encounter a complex microenvironment that is different from that of the primary site. Since the brain microenvironment is a vital aspect that drives the progression of brain metastases, dissecting the mechanisms of that tumor cells penetrate through the BBB and interact with different components of the brain microenvironment will facilitate the understanding of the biological behavior of brain metastases.
Blood–brain barrier
BBB is the first structure that tumor cells come across before entering the brain parenchyma. Whether tumor cells can break through the BBB, which is a highly specific neurovascular unit evolved to maintain brain homeostasis, is a key rate-limiting step for BM. Different from the peripheral capillaries, BBB is composed of tight junctions (TJs) formed between endothelial cells, the basement membrane, the peripheral foot of astrocytes, and pericytes.[79] Studies have shown that metastatic tumor cells conquer the BBB by destroying the TJs which are recognized as a key structure to maintain the barrier function of the BBB, suggesting that the destruction of TJs is a key event in extravasation. As yet, the underlying molecular mechanisms remain unclear. In small cell lung cancer (SCLC), activated Rho GTPases promote trans-BBB migration by increasing actomyosin contractility and thereby breaking down intercellular junctions. Further inhibition of endothelial Rho kinase (ROCK) with Y27632 and overexpression of ROCK dominant-negative mutant prevents NCI-H209 SCLC cells from penetrating the endothelium by modifications of TJs, suggesting Rho/ROCK pathway is required for the extravasation through BBB of SCLC cells.[80] Another study that screened the levels of candidate-soluble factors in the serum of SCLC patients showed that SCLC patients with high levels of placental growth factor (PLGF) are prone to BM. Elevated PLGF derived from SCLC cells triggers vascular endothelial growth factor receptor (VEGFR)-1-ERK 1/2 signaling axis activation, leading to disassembly of TJs in brain endothelial cells and promoting the penetration through BBB.[81] Recently, the role of aldehyde ketone reductase 1B10 (AKR1B10) in BM of NSCLC has been elucidated. AKR1B10 is overexpressed both in NSCLC BM patients and cell lines, and it regulates the expression of MMP2/MMP9 by activating the mitogen-activated protein kinase (MAPK) signaling pathway, inducing the destruction of BBB TJs and thereby promoting BM of NSCLC.[12] As the role of non-coding RNAs in tumor progression is further identified, miR-143-3p has been shown to be upregulated in the paired BM tissues compared with primary cancer tissues and it can enhance the invasion ability of in vitro BBB model and angiogenesis of lung cancer by targeting the three binding sites of 3′-untranslated region of vasohibin-1.[82] Vascular endothelial cells are another major component of the BBB that plays an important role in LCBM by facilitating adhesion of CTCs to the brain microvascular wall before the step of trans-BBB. Studies have shown that some adhesion molecules (such as very late antigen-4/vascular cell adhesion molecule-1, activated leukocyte cell adhesion molecule [ALCAM] and leucocyte function-associated antigen-1/intercellular adhesion molecule-1, etc) act as a bridge to mediate adhesion.[83] What's more, CD15 is highly expressed in metastatic NSCLC cells which promotes tumor cells adhesion in interaction with TNF-α-activated brain endothelial cells.[84]
Astrocytes
Astrocytes are the most abundant mesenchymal cell type in the brain microenvironment and possess the biological function of support and maintenance of homeostasis within the central nervous system (CNS). As an important component of the brain metastatic microenvironment that first comes into contact with tumor cells after extravasation, astrocytes play an important role in the formation of brain metastases from lung cancer.[85] Reactive astrocytes can release plasminogen activator (PA) which converts plasminogen into plasmin and thereby mobilizes the pro-apoptotic cytokine Fas ligand to kill the exudative metastatic cancer cells. In addition, converted fibrinolytic enzymes inactivate L1 cell adhesion molecules secreted by tumor cells, thus preventing tumor cells from spreading along blood vessels. Recently, it has been shown that metastatic tumor cells from lung adenocarcinoma produce anti-PA serine protease inhibitors, including neuroserpin and serpin B2 to help tumor cells avoid Fas-mediated apoptosis and allow them to proliferate along intracerebral capillaries.[86] In addition, lung cancer cells interacting with astrocytes upregulate endothelin 1 (ET-1), which activates intracellular signaling pathways including PI3K/AKT and mitogen-activated protein kinase through ET-1 receptors on tumor cells, ultimately contributing to colonization and chemoresistance of metastatic lung cancer cells.[87] The interaction between tumor cells and astrocytes can also be mediated by intracellular pro-calmodulin 7, the expression of which promotes the assembly of connexin 43 gap junctions between tumor cells and astrocytes. After gap junction formation, tumor cells deliver the second messenger protein cyclic guanosine monophosphate-adenosine monophosphate to astrocytes, activating the innate immune response. In turn, these inflammatory cytokines act as paracrine signals to tumor cells, which activate the intracellular STAT1 and NF-κB pathways, leading to the promotion of growth and chemoresistance of BM.[88] Seike et al[89] demonstrate that astrocytes are activated by tumor cell-oriented factors such as macrophage migration inhibitory factor, IL-8, and PA inhibitor-1. Activated astrocytes produce IL-6, TNF-α, and IL-1β, which in turn promote tumor cell proliferation. What's more, recent studies provide solid evidence that the phosphorylated STAT3 (pSTAT3)+ subpopulation of reactive astrocytes is found to be essential for BM due to the abilities of altering the TME and promoting the process.[90]
Immune cells
Microglia are the main resident and unique immune cells in the brain microenvironment, taking a major part in the immune response of CNS. They respond quickly to environmental changes and show plasticity and heterogeneity. Microglia can polarize into two phenotypes with distinct immune effects, M1-like and M2-like phenotypes guided by the microenvironment; these two subtypes present distinct effects, playing a double-edged role in the formation of brain metastases. On the one hand, microglia exercise anti-tumor immune function by secreting nitric oxide and lysing tumor cells, protecting the brain from colonization by metastatic cells.[91] Also, M1-like microglia can act as antigen-presenting cells that stimulate activated CD8+ T cells and adaptive immune responses that kill tumor cells. On the other hand, microglia have been shown to have a pro-tumor role in BM. It is shown that the distribution of tightly clustered activated microglia is localized around brain metastases of lung cancer. Those microglia react quickly to metastatic lung cancer cells in the brain and can foster migration and proliferation.[92,93] At present, there are still many gaps in the phenotypic transformation of microglia and the mechanism of interaction with tumor cells in BMs, which need to be further explored and clarified. There are also a small number of monocytes in the secondary brain TME which are from a small number of chemokine (C-C motif) receptor (CCR) 1+/CCR5+ monocytes in the bloodstream and activated and detained in the CNS. Additionally, Kivisäkk et al[94] showed that cerebrospinal fluid contains activated memory T cells that retain the capacity to either initiate local immune reactions or return to secondary lymphoid organs, indicating that they might be involved in CNS immune surveillance.
Microvessels
Cerebral vessels are involved in the entire process from tumor cells reaching the brain microenvironment to developing into brain metastases, including adherence and extravasation through BBB, vascular co-option, and angiogenesis. Vascular co-option refers to metastatic cells that interact physically with pre-existing vessels without any sign of angiogenesis while angiogenesis is defined as the formation of new blood vessels from pre-existing vessels.[95–97] Studies display that vascular co-option and angiogenesis by lung cancer-derived BM-initiating cells have been regarded an important step in organ colonization. Kienast et al[98] apply multiphoton laser-scanning video-microscopy to experimental lung and melanoma BM and characterize the early stages of colonization in vivo. It is found that the way cancer cells interact with vessels is dependent on the primary tumor type that they originated from. Melanoma cells grow via vascular co-option while lung cancer cells growth is mainly dependent upon angiogenesis. VEGF-A inhibition induces long-term dormancy of lung cancer micrometastases by preventing angiogenic growth to macrometastases. Of note, studies also reveal the involvement of vascular co-option in LCBM. Lung cancer-derived metastatic cells release serpins or activate Yes-associated protein and myocardin-related transcription factor to promote survival and vascular co-option.[86,99]
TME-Targeted Therapies and Immunotherapies
With a growing number of studies, which reflect important cellular and molecular pathways and indicate potential strategies, demonstrating the impact of TME in driving brain metastases from lung cancer, anti-tumor therapies targeting TME have rapidly evolved in recent years [Table 1].
Table 1.
Recent clinical trials for TME-targeted therapy of LCBM.
| Agents | Histology | Phase | Purpose | Status/outcome | Reference or http://clinicaltrials.gov identifier |
| Durvalumab | NSCLC | 2 | To assess the efficacy of durvalumab combined with radiation therapy in LCBM | No yet recruiting | NCT04889066 |
| Pembrolizumab | NSCLC | 2 | To determine the safety of three different stereotactic radiosurgery (SRS) radiation arms in combination with pembrolizumab in melanoma or NSCLC-associated BMs | Recruiting | NCT02858869 |
| GRN1005 | NSCLC | 2 | To assess the efficacy and safety of GRN1005 in LCBM | CR or PR: ≥30% decrease in the largest diameter of target lesions | NCT01497665 |
| Atezolizumab | SCLC | 2 | To assess the efficacy of chemotherapy and atezolizumab in LCBM | Recruiting | NCT04610684 |
| Pembrolizumab | NSCLC | 2 | To evaluate whether pembrolizumab prolongs survival and preserves quality of life in LCBM patients | Not yet recruiting | NCT04964960 |
| Bevacizumab | Non-squamous NSCLC | 2 | To assess the efficacy of bevacizumab in combination with first- or second-line therapy in LCBM | OS: 12.1 (10.3–14.9) months | NCT00312728 |
| Pembrolizumab+bevacizumab | NSCLC | 2 | To study the activity of pembrolizumab in combination with bevacizumab in LCBM | Recruiting | NCT02681549 |
| MK-3475 | NSCLC | 2 | To assess the activity of MK-3475 in untreated melanoma or NSCLC-associated BMs | 11 (11/42) patients showed better response | NCT02085070 |
| Pembrolizumab | NSCLC | 2 | To determine the activity of PD-1 blockade in the CNS | Pembrolizumab has activity in brain metastases from NSCLC with PD-L1 expression at least 1% | [94,95] |
| Nivolumab | Non-squamous NSCLC patients | EAP | To evaluate nivolumab efficacy and safety in LCBM | Nivolumab is active in non-squamous NSCLC patients with BM | [96] |
| Immune checkpoint inhibitor | NSCLC | Retrospective | To evaluate outcomes of LCBM patients treated with checkpoint inhibitors | In multivariate analysis, BMs are not associated with a poorer survival in patients with ICI-treated NSCLC | [97] |
BM: Brain metastasis; CNS: Central nervous system; CR: Complete response; EAP: Expanded access programs; ICI: Immune checkpoint inhibitor; LCBM: Lung cancer brain metastasis; NSCLC: Non-small cell lung cancer; OS: Overall survival; PD-1: Programmed cell death-1; PD-L1: Programmed cell death-Ligand 1; PR: Partial response; SCLC: Small cell lung cancer; SRS: Stereotactic radiosurgery; TME: Tumor microenvironment.
Anti-angiogenesis therapies
The VEGF pathway includes the VEGF ligand and its receptor, as well as downstream signaling molecules. Because activation of the pathway is critical for tumor angiogenesis, inhibitors targeting each participant of this pathway have been developed and tested in advanced NSCLC including LCBM.[100] Several drugs have been approved by the US Food and Drug Administration (FDA). Bevacizumab, the first VEGF inhibitor approved by the FDA (2004), has been approved in 2006 for use in combination with carboplatin and paclitaxel chemotherapy as a first-line treatment for advanced, non-squamous NSCLC.[100,101] Ramucirumab, an antibody targeting VEGFR2, has been approved for use in combination with docetaxel for metastatic NSCLC patients during or following platinum-based chemotherapy.[102] In addition, nintedanib which targets the tyrosine kinases VEGFR1, VEGFR2, VEGFR3, platelet-derived growth factor receptor (PDGFR)-alpha, PDGFRβ, fibroblast growth factor receptor (FGFR) 1, FGFR2, and FGFR3, has been approved by the European Union in combination with docetaxel for advanced NSCLC patients whose disease has progressed after first-line platinum-based chemotherapy.[103] A number of anti-angiogenic agents, including VEGF inhibitor aflibercept and the tyrosine kinase receptor inhibitors sunitinib, sorafenib, motesanib, cediranib, and vandetanib, are currently being evaluated in clinical trials.[34,104]
Targeting astrocytes
There are also pre-clinical and clinical studies that investigate possible therapies targeting astrocytes. As gap junctions between astrocytes and BM tumor cells have been confirmed to promote the formation of metastases,[88] pre-clinical studies have been carried out in breast and lung derived BM animal models in which gap junctions were blocked by meclofenamate, which targets the gate of gap junction, and tonabersat which inhibits gap junctions by specifically binding to astrocytes. Administration of meclofenamate or tonabersat increases tumor chemosensitivity.[105] Another clinical trial testing meclofenamate for recurrent/progressing BM is under way (NCT02429570).[106] In addition, since the efficiency of STAT3 inhibition has been demonstrated to suppress the growth of brain metastases in pre-clinical models, a clinical trial where 18 lung cancer patients suffering from BM are treated with Legasil (STAT3 inhibitor) has been performed.[107] Patients are administered with the inhibitor alone or in combination with other therapies. Results display an overall response rate in the brain of 75%, including three complete responses (20%) and ten partial responses (55%). What's more, the survival of Legasil-treated patients is longer compared to those who were treated with whole-brain radiation and standard chemotherapy (n = 38), suggesting that STAT3 inhibition may be a viable and effective treatment for LCBM.
Immunotherapies
Single-agent pembrolizumab has become the standard first-line treatment for patients with PD-L1 expression.[108–110] Although there are limited data on immunotherapies in lung cancer patients with BM, immune checkpoint inhibitors alone or in combination with chemotherapy have shown promising efficacy and safety results. Data from phrase I/II trials,[111,112] expanded access programs,[113] pre-planned analyses of phase III clinical trials, and retrospective series[114] evidence the therapeutic effectiveness of PD-1/PD-L1 axis inhibitors, including pembrolizumab and nivolumab, for lung cancer patients with BM. Although these studies display similar effects of immunotherapy in overall survival in subgroups of patients with and without BM, there are still considerable limitations to the available evidence. In order to fully develop targeted therapies against the PD-1/PD-L1 axis and other immune factors in this setting, further in-depth studies of immune-related mechanism in the pathogenesis of LCBM are needed.
Research Models for TME in LCBM
For decades, research on TME in lung cancer has utilized in vitro 2D cell cultures, and in vivo xenografts or genetically engineered animal models. However, these traditional models remain limited. In vitro 2D cell cultures usually use the Transwell insert to achieve the co-culture of tumor cells and stromal cells,[115] which lacks other components of microenvironment and the physiologically dynamic state, while animal models that are costly and time consuming, are difficult to visualize and dynamically monitor the precise cell metastasis process and microenvironmental alterations. Recently, more precise models such as microfluidic chips (also as “organ chips”) have been developed, aiding by mimicking physiological environment and allowing investigation of the interaction between tumor and TME. Microfluidic devices consist of transparent plastic, glass, or flexible polymers such as polydimethylsiloxane containing perfused hollow microchannels filled with living cells that reproduce in vivo organ-level physiology and pathophysiology by reconstructing structure and function at the tissue and organ level in vitro.[116] Our team has developed microfluidic chips to study the involvement and effects of TME in LCBM. A microfluidic-based co-culture device has been developed to mimic the TME to assess CAF or TAM effects on invasion and metastasis in NSCLC.[43,117] Further, a multi-organ microfluidic chip mimicking the in vivo microenvironment of lung cancer metastasis has been designed and constructed. The model includes an upstream “lung” and three downstream “distant organs—brain, bone, liver” where bronchial epithelial, lung cancer, microvascular endothelial, mononuclear, and fibroblast cells are grown separated by the bio-membrane in upstream “lung”, while astrocytes, osteocytes, and hepatocytes are grown in distant chambers, to mimic lung cancer cell metastasis to secondary target organs.[118] Based on this multi-organ microfluidic chip, recently we developed a BM chip as a new methodological platform to study BM. The chip consisted of two bionic organ units—an upstream “lung” and a downstream “brain” characterized by a functional “blood-brain barrier” structure, allowing real-time visual monitoring of the entire BM process, from the growth of primary tumor to its breaking through the BBB, and finally reaching the brain parenchyma. The chip was then applied for the BM research where we first demonstrated that the protein expression of AKR1B10 was significantly elevated in LCBM. Silencing AKR1B10 in brain metastatic tumor cells suppressed their extravasation through the BBB in the in vitro Transwell model, in our ex vivo microfluidic chip, as well as the in vivo model of BM in nude mice.[12] With the combined application of our new model and traditional research platform, it is clear that our multi-organ microfluidic chip is a practical and valuable approach to study BM pathogenesis. These studies indicate that microfluidic chips are promising models to study the interaction between TME and tumor cells in the process of BM.
Conclusion and Perspective
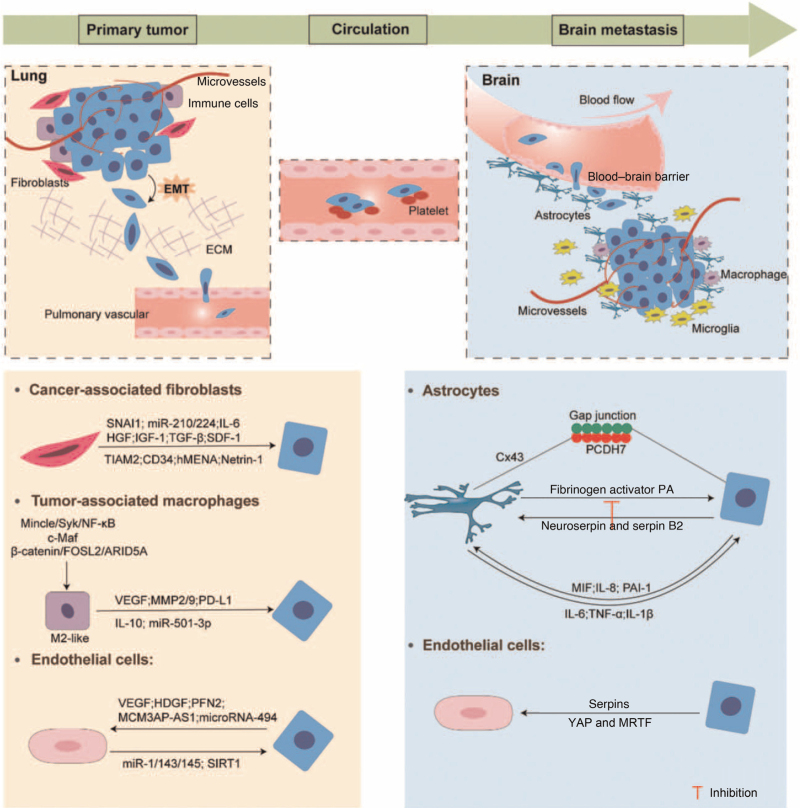
BM is the leading cause of mortality in lung cancer patients. It involves a complex series of pathological cascade processes, which can be divided into three major stages according to the location of incident: primary tumor stage, CTCs stage, and brain metastases stage. With the identification of the important role of the TME in the development of tumorigenesis, we review the involvements of TME (including primary lung microenvironment, factors in circulation, and secondary BM) in each stage of the BM process and the possible mechanisms involved [Figure 1], in an attempt to provide a clear enough description of the interaction between lung cancer cells and microenvironment in LCBM. The complex microenvironment whether in primary or secondary site favors the progress of BM and provides an important resource of targets for therapeutic options. The description and exploration of the tumor reprogrammed microenvironmental landscape, promoted by the concept of precision era, has motivated new ideas to develop precision therapeutic drugs that specifically target the contents of the landscape. This perspective has been particularly facilitated by the recent success of anti-angiogenic therapy and immunotherapy which is a most promising molecular therapeutic strategy thus far. Hence, further understanding of the exact molecular mechanisms involved in LCBM will lead to new prospects for targeted therapies through the design of small molecule drugs and/or monoclonal antibodies. However, it has to be addressed that it is extremely challenging to target the interactions between cancer cells and TME in clinical practice. Patients with brain metastases are often accompanied by extracranial lesions, which must be addressed along with intracranial disease, but existing clinical trials assume that patients with metastasis only have the intracranial lesions and do not reflect the current reality because those untargeted treatments for extracranial lesions will have a negative impact on patient survival. The potential strategies can be foreseen that those TME-targeted therapies can be jointed with tumor-directed agents that can pass through the BBB (eg, osimertinib). The combining multiple orthogonal approaches will impair the toxic systemic treatments, improving the survival and the quality of life in LCBM patients.
Figure 1.
Schematic representation of the stages of the formation of LCBM and interactions between TME and tumor cells. ARID5A: AT-rich interaction domain 5A; Cx43: Connexin 43; ECM: Extracellular matrix; EMT: Epithelial-mesenchymal transition; FOSL2: Fos-like antigen 2; HDGF: Hepatoma-derived growth factor; HGF: Hepatocyte growth factor; hMENA: human mammalian ENA; IGF-1: Insulin-like growth factor 1; IL: Interleukin; LCBM: Lung cancer brain metastasis; MCM3AP-AS1: lncRNA MCM3AP antisense RNA 1; MIF: Migration inhibitory factor; miR: MicroRNA; MMP: Matrix metalloproteinases; MRTF: Myocardin-related transcription factor; NF-κB: Nuclear factor kappa-B; PA: Plasminogen activator; PAI-1: Plasminogen activator inhibitor-1; PCDH7: Pro-calmodulin 7; PD-L1: Programmed cell death-ligand 1; PFN2: Profilin 2; SDF-1: Stromal cell-derived factor-1; SIRT1: Sirtuin 1; SNAI1: Snail 1; TGF-β: Transforming growth factor-β; TIAM2: T-cell lymphoma invasion and metastasis 2; TME: Tumor microenvironment; TNF-α: Tumor necrosis factor-α; VEGF: Vascular endothelial growth factor; YAP: Yes-associated protein.
Funding
This work was supported by grants from the National Natural Science Foundation of China (Nos. 81972916 and 82103054).
Conflicts of interest
None.
Footnotes
How to cite this article: Liu W, Powell CA, Wang Q. Tumor microenvironment in lung cancer-derived brain metastasis. Chin Med J 2022;135:1781–1791. doi: 10.1097/CM9.0000000000002127
References
- 1.Gao S, Li N, Wang S, Zhang F, Wei W, Li N, et al. Lung cancer in People's Republic of China. J Thorac Oncol 2020; 15:1567–1576. doi: 10.1016/j.jtho.2020.04.028. [DOI] [PubMed] [Google Scholar]
- 2.Cheng H, Perez-Soler R. Leptomeningeal metastases in non-small-cell lung cancer. Lancet Oncol 2018; 19:e43–e55. doi: 10.1016/S1470-2045(17)30689-7. [DOI] [PubMed] [Google Scholar]
- 3.Steeg PS, Camphausen KA, Smith QR. Brain metastases as preventive and therapeutic targets. Nat Rev Cancer 2011; 11:352–363. doi: 10.1038/nrc3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hinshaw DC, Shevde LA. The tumor microenvironment innately modulates cancer progression. Cancer Res 2019; 79:4557–4566. doi: 10.1158/0008-5472.CAN-18-3962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ma L, Zhu LJ, Xu ZJ, Shu YQ. Co-mutations in tumor immune microenvironment and immunotherapy. Chin Med J 2021; 134:1055–1057. doi: 10.1097/CM9.0000000000001455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Q, Bao JM, Li XL, Zhang T, Shen XH. Inhibiting effect of Astragalus polysaccharides on the functions of CD4+CD25 highTreg cells in the tumor microenvironment of human hepatocellular carcinoma. Chin Med J 2012; 125:786–793. doi: 10.3760/cma.j.issn.0366-6999.2012.05.012. [PubMed] [Google Scholar]
- 7.Fidler IJ, Balasubramanian K, Lin Q, Kim SW, Kim SJ. The brain microenvironment and cancer metastasis. Mol Cells 2010; 30:93–98. doi: 10.1007/s10059-010-0133-9. [DOI] [PubMed] [Google Scholar]
- 8.Yousefi M, Bahrami T, Salmaninejad A, Nosrati R, Ghaffari P, Ghaffari SH. Lung cancer-associated brain metastasis: molecular mechanisms and therapeutic options. Cell Oncol (Dordr) 2017; 40:419–441. doi: 10.1007/s13402-017-0345-5. [DOI] [PubMed] [Google Scholar]
- 9.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008; 133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Massagué J, Obenauf AC. Metastatic colonization by circulating tumour cells. Nature 2016; 529:298–306. doi: 10.1038/nature17038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 12.Liu W, Song J, Du X, Zhou Y, Li Y, Li R, et al. AKR1B10 (Aldo-keto reductase family 1 B10) promotes brain metastasis of lung cancer cells in a multi-organ microfluidic chip model. Acta Biomater 2019; 91:195–208. doi: 10.1016/j.actbio.2019.04.053. [DOI] [PubMed] [Google Scholar]
- 13.Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev 1989; 8:98–101. [PubMed] [Google Scholar]
- 14.Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature 2018; 553:446–454. doi: 10.1038/nature25183. [DOI] [PubMed] [Google Scholar]
- 15.Chaudhry SI, Hooper S, Nye E, Williamson P, Harrington K, Sahai E. Autocrine IL-1β-TRAF6 signalling promotes squamous cell carcinoma invasion through paracrine TNFα signalling to carcinoma-associated fibroblasts. Oncogene 2013; 32:747–758. doi: 10.1038/onc.2012.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu CQ, Popova SN, Brown ERS, Barsyte-Lovejoy D, Navab R, Shih W, et al. Integrin alpha 11 regulates IGF2 expression in fibroblasts to enhance tumorigenicity of human non-small-cell lung cancer cells. Proc Natl Acad Sci U S A 2007; 104:11754–11759. doi: 10.1073/pnas.0703040104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.You J, Li M, Cao LM, Gu QH, Deng PB, Tan Y, et al. Snail1-dependent cancer-associated fibroblasts induce epithelial-mesenchymal transition in lung cancer cells via exosomes. QJM 2019; 112:581–590. doi: 10.1093/qjmed/hcz093. [DOI] [PubMed] [Google Scholar]
- 18.Yang F, Yan Y, Yang Y, Hong X, Wang M, Yang Z, et al. MiR-210 in exosomes derived from CAFs promotes non-small cell lung cancer migration and invasion through PTEN/PI3K/AKT pathway. Cell Signal 2020; 73:1096752.doi: 10.1016/j.cellsig.2020.109675. [DOI] [PubMed] [Google Scholar]
- 19.Zhang J, Han L, Yu J, Li H, Li Q. miR-224 aggravates cancer-associated fibroblast-induced progression of non-small cell lung cancer by modulating a positive loop of the SIRT3/AMPK/mTOR/HIF-1α axis. Aging (Albany NY) 2021; 13:10431–10449. doi: 10.18632/aging.202803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang S, Cui J, Zhang K, Gu J, Zheng Y, Zhang B, et al. SP13786 inhibits the migration and invasion of lung adenocarcinoma cell A549 by supressing Stat3-EMT via CAFs exosomes (in Chinese). Zhongguo Fei Ai Za Zhi 2021; 24:384–393. doi: 10.3779/j.issn.1009-3419.2021.104.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang L, Cao L, Wang H, Liu B, Zhang Q, Meng Z, et al. Cancer-associated fibroblasts enhance metastatic potential of lung cancer cells through IL-6/STAT3 signaling pathway. Oncotarget 2017; 8:76116–76128. doi: 10.18632/oncotarget.18814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shintani Y, Fujiwara A, Kimura T, Kawamura T, Funaki S, Minami M, et al. IL-6 secreted from cancer-associated fibroblasts mediates chemoresistance in NSCLC by increasing epithelial-mesenchymal transition signaling. J Thorac Oncol 2016; 11:1482–1492. doi: 10.1016/j.jtho.2016.05.025. [DOI] [PubMed] [Google Scholar]
- 23.Yi Y, Zeng S, Wang Z, Wu M, Ma Y, Ye X, et al. Cancer-associated fibroblasts promote epithelial-mesenchymal transition and EGFR-TKI resistance of non-small cell lung cancers via HGF/IGF-1/ANXA2 signaling. Biochim Biophys Acta Mol Basis Dis 2018; 1864:793–803. doi: 10.1016/j.bbadis.2017.12.021. [DOI] [PubMed] [Google Scholar]
- 24.An J, Enomoto A, Weng L, Kato T, Iwakoshi A, Ushida K, et al. Significance of cancer-associated fibroblasts in the regulation of gene expression in the leading cells of invasive lung cancer. J Cancer Res Clin Oncol 2013; 139:379–388. doi: 10.1007/s00432-012-1328-6. [DOI] [PubMed] [Google Scholar]
- 25.Wang Y, Lan W, Xu M, Song J, Mao J, Li C, et al. Cancer-associated fibroblast-derived SDF-1 induces epithelial-mesenchymal transition of lung adenocarcinoma via CXCR4/β-catenin/PPARδ signalling. Cell Death Dis 2021; 12:2142.doi: 10.1038/s41419-021-03509-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li S, Ou Y, Liu S, Yin J, Zhuo W, Huang M, et al. The fibroblast TIAM2 promotes lung cancer cell invasion and metastasis. J Cancer 2019; 10:1879–1889. doi: 10.7150/jca.30477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schulze AB, Schmidt LH, Heitkötter B, Huss S, Mohr M, Marra A, et al. Prognostic impact of CD34 and SMA in cancer-associated fibroblasts in stage I-III NSCLC. Thorac Cancer 2020; 11:120–129. doi: 10.1111/1759-7714.13248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Melchionna R, Spada S, Di Modugno F, D’Andrea D, Di Carlo A, Panetta M, et al. The actin modulator hMENA regulates GAS6-AXL axis and pro-tumor cancer/stromal cell cooperation. EMBO Rep 2020; 21:e50078.doi: 10.15252/embr.202050078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Babaei G, Aziz SGG, Jaghi NZZ. EMT, cancer stem cells and autophagy; the three main axes of metastasis. Biomed Pharmacother 2021; 133:1109092.doi: 10.1016/j.biopha.2020.110909. [DOI] [PubMed] [Google Scholar]
- 30.Su S, Chen J, Yao H, Liu J, Yu S, Lao L, et al. CD10+GPR77+ cancer-associated fibroblasts promote cancer formation and chemoresistance by sustaining cancer stemness. Cell 2018; 172:841–856.e16. doi: 10.1016/j.cell.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 31.Kinugasa Y, Matsui T, Takakura N. CD44 expressed on cancer-associated fibroblasts is a functional molecule supporting the stemness and drug resistance of malignant cancer cells in the tumor microenvironment. Stem Cells 2014; 32:145–156. doi: 10.1002/stem.1556. [DOI] [PubMed] [Google Scholar]
- 32.Sung PJ, Rama N, Imbach J, Fiore S, Ducarouge B, Neves D, et al. Cancer-associated fibroblasts produce netrin-1 to control cancer cell plasticity. Cancer Res 2019; 79:3651–3661. doi: 10.1158/0008-5472.CAN-18-2952. [DOI] [PubMed] [Google Scholar]
- 33.Chen WJ, Ho CC, Chang YL, Chen HY, Lin CA, Ling TY, et al. Cancer-associated fibroblasts regulate the plasticity of lung cancer stemness via paracrine signalling. Nat Commun 2014; 5:34722.doi: 10.1038/ncomms4472. [DOI] [PubMed] [Google Scholar]
- 34.Altorki NK, Markowitz GJ, Gao D, Port JL, Saxena A, Stiles B, et al. The lung microenvironment: an important regulator of tumour growth and metastasis. Nat Rev Cancer 2019; 19:9–31. doi: 10.1038/s41568-018-0081-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Almatroodi SA, McDonald CF, Darby IA, Pouniotis DS. Characterization of M1/M2 tumour-associated macrophages (TAMs) and Th1/Th2 cytokine profiles in patients with NSCLC. Cancer Microenviron 2016; 9:1–11. doi: 10.1007/s12307-015-0174-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ohri CM, Shikotra A, Green RH, Waller DA, Bradding P. Macrophages within NSCLC tumour islets are predominantly of a cytotoxic M1 phenotype associated with extended survival. Eur Respir J 2009; 33:118–126. doi: 10.1183/09031936.00065708. [DOI] [PubMed] [Google Scholar]
- 37.Li H, Huang N, Zhu W, Wu J, Yang X, Teng W, et al. Modulation the crosstalk between tumor-associated macrophages and non-small cell lung cancer to inhibit tumor migration and invasion by ginsenoside Rh2. BMC Cancer 2018; 18:5792.doi: 10.1186/s12885-018-4299-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shay G, Lynch CC, Fingleton B. Moving targets: emerging roles for MMPs in cancer progression and metastasis. Matrix Biol 2015; 44-46:200–206. doi: 10.1016/j.matbio.2015.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singhal S, Stadanlick J, Annunziata MJ, Rao AS, Bhojnagarwala PS, O’Brien S, et al. Human tumor-associated monocytes/macrophages and their regulation of T cell responses in early-stage lung cancer. Sci Transl Med 2019; 11:eaat1500.doi: 10.1126/scitranslmed.aat1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hwang I, Kim JW, Ylaya K, Chung EJ, Kitano H, Perry C, et al. Tumor-associated macrophage, angiogenesis and lymphangiogenesis markers predict prognosis of non-small cell lung cancer patients. J Transl Med 2020; 18:4432.doi: 10.1186/s12967-020-02618-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shima T, Shimoda M, Shigenobu T, Ohtsuka T, Nishimura T, Emoto K, et al. Infiltration of tumor-associated macrophages is involved in tumor programmed death-ligand 1 expression in early lung adenocarcinoma. Cancer Sci 2020; 111:727–738. doi: 10.1111/cas.14272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang L, Dong Y, Li Y, Wang D, Liu S, Wang D, et al. IL-10 derived from M2 macrophage promotes cancer stemness via JAK1/STAT1/NF-κB/Notch1 pathway in non-small cell lung cancer. Int J Cancer 2019; 145:1099–1110. doi: 10.1002/ijc.32151. [DOI] [PubMed] [Google Scholar]
- 43.Guo Z, Song J, Hao J, Zhao H, Du X, Li E, et al. M2 macrophages promote NSCLC metastasis by upregulating CRYAB. Cell Death Dis 2019; 10:3772.doi: 10.1038/s41419-019-1618-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lei J, Chen P, Zhang F, Zhang N, Zhu J, Wang X, et al. M2 macrophages-derived exosomal microRNA-501-3p promotes the progression of lung cancer via targeting WD repeat domain 82. Cancer Cell Int 2021; 21:912.doi: 10.1186/s12935-021-01783-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li C, Xue VW, Wang QM, Lian GY, Huang XR, Lee TL, et al. The Mincle/Syk/NF-κB signaling circuit is essential for maintaining the protumoral activities of tumor-associated macrophages. Cancer Immunol Res 2020; 8:1004–1017. doi: 10.1158/2326-6066.CIR-19-0782. [DOI] [PubMed] [Google Scholar]
- 46.Liu M, Tong Z, Ding C, Luo F, Wu S, Wu C, et al. Transcription factor c-Maf is a checkpoint that programs macrophages in lung cancer. J Clin Invest 2020; 130:2081–2096. doi: 10.1172/JCI131335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sarode P, Zheng X, Giotopoulou GA, Weigert A, Kuenne C, Günther S, et al. Reprogramming of tumor-associated macrophages by targeting β-catenin/FOSL2/ARID5A signaling: a potential treatment of lung cancer. Sci Adv 2020; 6:eaaz6105.doi: 10.1126/sciadv.aaz6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schneider T, Hoffmann H, Dienemann H, Schnabel PA, Enk AH, Ring S, et al. Non-small cell lung cancer induces an immunosuppressive phenotype of dendritic cells in tumor microenvironment by upregulating B7-H3. J Thorac Oncol 2011; 6:1162–1168. doi: 10.1097/JTO.0b013e31821c421d. [DOI] [PubMed] [Google Scholar]
- 49.Mao Y, Li W, Chen K, Xie Y, Liu Q, Yao M, et al. B7-H1 and B7-H3 are independent predictors of poor prognosis in patients with non-small cell lung cancer. Oncotarget 2015; 6:3452–3461. doi: 10.18632/oncotarget.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dumitriu IE, Dunbar DR, Howie SE, Sethi T, Gregory CD. Human dendritic cells produce TGF-beta 1 under the influence of lung carcinoma cells and prime the differentiation of CD4+CD25+Foxp3+ regulatory T cells. J Immunol 2009; 182:2795–2807. doi: 10.4049/jimmunol.0712671. [DOI] [PubMed] [Google Scholar]
- 51.Aloe C, Wang H, Vlahos R, Irving L, Steinfort D, Bozinovski S. Emerging and multifaceted role of neutrophils in lung cancer. Transl Lung Cancer Res 2021; 10:2806–2818. doi: 10.21037/tlcr-20-760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tecchio C, Micheletti A, Cassatella MA. Neutrophil-derived cytokines: facts beyond expression. Front Immunol 2014; 5:5082.doi: 10.3389/fimmu.2014.00508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang SS, Liu W, Ly D, Xu H, Qu L, Zhang L. Tumor-infiltrating B cells: their role and application in anti-tumor immunity in lung cancer. Cell Mol Immunol 2019; 16:6–18. doi: 10.1038/s41423-018-0027-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang Y, Han D, Pan L, Sun J. The positive feedback between lncRNA TNK2-AS1 and STAT3 enhances angiogenesis in non-small cell lung cancer. Biochem Biophys Res Commun 2018; 507:185–192. doi: 10.1016/j.bbrc.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 55.Eguchi R, Wakabayashi I. HDGF enhances VEGF-dependent angiogenesis and FGF-2 is a VEGF-independent angiogenic factor in non-small cell lung cancer. Oncol Rep 2020; 44:14–28. doi: 10.3892/or.2020.7580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cao Q, Liu Y, Wu Y, Hu C, Sun L, Wang J, et al. Profilin 2 promotes growth, metastasis, and angiogenesis of small cell lung cancer through cancer-derived exosomes. Aging (Albany NY) 2020; 12:25981–25999. doi: 10.18632/aging.202213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li X, Yu M, Yang C. YY1-mediated overexpression of long noncoding RNA MCM3AP-AS1 accelerates angiogenesis and progression in lung cancer by targeting miR-340-5p/KPNA4 axis. J Cell Biochem 2020; 121:2258–2267. doi: 10.1002/jcb.29448. [DOI] [PubMed] [Google Scholar]
- 58.Mao G, Liu Y, Fang X, Liu Y, Fang L, Lin L, et al. Tumor-derived microRNA-494 promotes angiogenesis in non-small cell lung cancer. Angiogenesis 2015; 18:373–382. doi: 10.1007/s10456-015-9474-5. [DOI] [PubMed] [Google Scholar]
- 59.Korde A, Jin L, Zhang JG, Ramaswamy A, Hu B, Kolahian S, et al. Lung endothelial microRNA-1 regulates tumor growth and angiogenesis. Am J Respir Crit Care Med 2017; 196:1443–1455. doi: 10.1164/rccm.201610-2157OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dimitrova N, Gocheva V, Bhutkar A, Resnick R, Jong RM, Miller KM, et al. Stromal expression of miR-143/145 promotes neoangiogenesis in lung cancer development. Cancer Discov 2016; 6:188–201. doi: 10.1158/2159-8290.CD-15-0854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xie M, Liu M, He CS. SIRT1 regulates endothelial Notch signaling in lung cancer. PLoS One 2012; 7:e45331.doi: 10.1371/journal.pone.0045331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ochoa CE, Mirabolfathinejad SG, Ruiz VA, Evans SE, Gagea M, Evans CM, et al. Interleukin 6, but not T helper 2 cytokines, promotes lung carcinogenesis. Cancer Prev Res (Phila) 2011; 4:51–64. doi: 10.1158/1940-6207.CAPR-10-0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Caetano MS, Zhang H, Cumpian AM, Gong L, Unver N, Ostrin EJ, et al. IL6 blockade reprograms the lung tumor microenvironment to limit the development and progression of K-ras-Mutant lung cancer. Cancer Res 2016; 76:3189–3199. doi: 10.1158/0008-5472.CAN-15-2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jungnickel C, Schmidt LH, Bittigkoffer L, Wolf L, Wolf A, Ritzmann F, et al. IL-17C mediates the recruitment of tumor-associated neutrophils and lung tumor growth. Oncogene 2017; 36:4182–4190. doi: 10.1038/onc.2017.28. [DOI] [PubMed] [Google Scholar]
- 65.King PT, Sharma R. The lung immune response to nontypeable haemophilus influenzae (lung immunity to NTHi). J Immunol Res 2015; 2015:7063762.doi: 10.1155/2015/706376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sriram KB, Cox AJ, Sivakumaran P, Singh M, Watts AM, West NP, et al. Non-typeable Haemophilus Influenzae detection in the lower airways of patients with lung cancer and chronic obstructive pulmonary disease. Multidiscip Respir Med 2018; 13:112.doi: 10.1186/s40248-018-0123-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tsay JJ, Wu BG, Badri MH, Clemente JC, Shen N, Meyn P, et al. Airway microbiota is associated with upregulation of the PI3K pathway in lung cancer. Am J Respir Crit Care Med 2018; 198:1188–1198. doi: 10.1164/rccm.201710-2118OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yu G, Gail MH, Consonni D, Carugno M, Humphrys M, Pesatori AC, et al. Characterizing human lung tissue microbiota and its relationship to epidemiological and clinical features. Genome Biol 2016; 17:1632.doi: 10.1186/s13059-016-1021-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ruan H, Zhou Y, Shen J, Zhai Y, Xu Y, Pi L, et al. Circulating tumor cell characterization of lung cancer brain metastases in the cerebrospinal fluid through single-cell transcriptome analysis. Clin Transl Med 2020; 10:e246.doi: 10.1002/ctm2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zheng Y, Miyamoto DT, Wittner BS, Sullivan JP, Aceto N, Jordan NV, et al. Expression of β-globin by cancer cells promotes cell survival during blood-borne dissemination. Nat Commun 2017; 8:143442.doi: 10.1038/ncomms14344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Micalizzi DS, Maheswaran S, Haber DA. A conduit to metastasis: circulating tumor cell biology. Genes Dev 2017; 31:1827–1840. doi: 10.1101/gad.305805.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Labelle M, Begum S, Hynes RO. Platelets guide the formation of early metastatic niches. Proc Natl Acad Sci U S A 2014; 111:E3053–E3061. doi: 10.1073/pnas.1411082111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell 2011; 20:576–590. doi: 10.1016/j.ccr.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Velez J, Enciso LJ, Suarez M, Fiegl M, Grismaldo A, López C, et al. Platelets promote mitochondrial uncoupling and resistance to apoptosis in leukemia cells: a novel paradigm for the bone marrow microenvironment. Cancer Microenviron 2014; 7:79–90. doi: 10.1007/s12307-014-0149-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gasic GJ, Gasic TB, Stewart CC. Antimetastatic effects associated with platelet reduction. Proc Natl Acad Sci U S A 1968; 61:46–52. doi: 10.1073/pnas.61.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nieswandt B, Hafner M, Echtenacher B, Männel DN. Lysis of tumor cells by natural killer cells in mice is impeded by platelets. Cancer Res 1999; 59:1295–1300. [PubMed] [Google Scholar]
- 77.Franco AT, Corken A, Ware J. Platelets at the interface of thrombosis, inflammation, and cancer. Blood 2015; 126:582–588. doi: 10.1182/blood-2014-08-531582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Beck TN, Boumber YA, Aggarwal C, Pei J, Thrash-Bingham C, Fittipaldi P, et al. Circulating tumor cell and cell-free RNA capture and expression analysis identify platelet-associated genes in metastatic lung cancer. BMC Cancer 2019; 19:6032.doi: 10.1186/s12885-019-5795-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Arvanitis CD, Ferraro GB, Jain RK. The blood-brain barrier and blood-tumour barrier in brain tumours and metastases. Nat Rev Cancer 2020; 20:26–41. doi: 10.1038/s41568-019-0205-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li B, Zhao WD, Tan ZM, Fang WG, Zhu L, Chen YH. Involvement of Rho/ROCK signalling in small cell lung cancer migration through human brain microvascular endothelial cells. FEBS Lett 2006; 580:4252–4260. doi: 10.1016/j.febslet.2006.06.056. [DOI] [PubMed] [Google Scholar]
- 81.Li B, Wang C, Zhang Y, Zhao XY, Huang B, Wu PF, et al. Elevated PLGF contributes to small-cell lung cancer brain metastasis. Oncogene 2013; 32:2952–2962. doi: 10.1038/onc.2012.313. [DOI] [PubMed] [Google Scholar]
- 82.Wang H, Deng Q, Lv Z, Ling Y, Hou X, Chen Z, et al. N6-methyladenosine induced miR-143-3p promotes the brain metastasis of lung cancer via regulation of VASH1. Mol Cancer 2019; 18:1812.doi: 10.1186/s12943-019-1108-x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 83.Soto MS, Serres S, Anthony DC, Sibson NR. Functional role of endothelial adhesion molecules in the early stages of brain metastasis. Neuro Oncol 2014; 16:540–551. doi: 10.1093/neuonc/not222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jassam SA, Maherally Z, Smith JR, Ashkan K, Roncaroli F, Fillmore HL, et al. TNF-α enhancement of CD62E mediates adhesion of non-small cell lung cancer cells to brain endothelium via CD15 in lung-brain metastasis. Neuro Oncol 2016; 18:679–690. doi: 10.1093/neuonc/nov248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Langley RR, Fidler IJ. The biology of brain metastasis. Clin Chem 2013; 59:180–189. doi: 10.1373/clinchem.2012.193342. [DOI] [PubMed] [Google Scholar]
- 86.Valiente M, Obenauf AC, Jin X, Chen Q, Zhang XHF, Lee DJ, et al. Serpins promote cancer cell survival and vascular co-option in brain metastasis. Cell 2014; 156:1002–1016. doi: 10.1016/j.cell.2014.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim SW, Choi HJ, Lee HJ, He J, Wu Q, Langley RR, et al. Role of the endothelin axis in astrocyte- and endothelial cell-mediated chemoprotection of cancer cells. Neuro Oncol 2014; 16:1585–1598. doi: 10.1093/neuonc/nou128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen Q, Boire A, Jin X, Valiente M, Er EE, Lopez-Soto A, et al. Carcinoma-astrocyte gap junctions promote brain metastasis by cGAMP transfer. Nature 2016; 533:493–498. doi: 10.1038/nature18268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Seike T, Fujita K, Yamakawa Y, Kido MA, Takiguchi S, Teramoto N, et al. Interaction between lung cancer cells and astrocytes via specific inflammatory cytokines in the microenvironment of brain metastasis. Clin Exp Metastasis 2011; 28:13–25. doi: 10.1007/s10585-010-9354-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sarmiento Soto M, Larkin JR, Martin C, Khrapitchev AA, Maczka M, Economopoulos V, et al. STAT3-Mediated Astrocyte Reactivity Associated with Brain Metastasis Contributes to Neurovascular Dysfunction. Cancer Res 2020; 80:5642–5655. doi: 10.1158/0008-5472.CAN-20-2251. [DOI] [PubMed] [Google Scholar]
- 91.Brantley EC, Guo L, Zhang C, Lin Q, Yokoi K, Langley RR, et al. Nitric oxide-mediated tumoricidal activity of murine microglial cells. Transl Oncol 2010; 3:380–388. doi: 10.1593/tlo.10208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Prodinger C, Bunse J, Krüger M, Schiefenhövel F, Brandt C, Laman JD, et al. CD11c-expressing cells reside in the juxtavascular parenchyma and extend processes into the glia limitans of the mouse nervous system. Acta Neuropathol 2011; 121:445–458. doi: 10.1007/s00401-010-0774-y. [DOI] [PubMed] [Google Scholar]
- 93.Soffietti R, Ahluwalia M, Lin N, Rudà R. Management of brain metastases according to molecular subtypes. Nat Rev Neurol 2020; 16:557–574. doi: 10.1038/s41582-020-0391-x. [DOI] [PubMed] [Google Scholar]
- 94.Kivisäkk P, Mahad DJ, Callahan MK, Trebst C, Tucky B, Wei T, et al. Human cerebrospinal fluid central memory CD4+ T cells: evidence for trafficking through choroid plexus and meninges via P-selectin. Proc Natl Acad Sci U S A 2003; 100:8389–8394. doi: 10.1073/pnas.1433000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.García-Gómez P, Valiente M. Vascular co-option in brain metastasis. Angiogenesis 2020; 23:3–8. doi: 10.1007/s10456-019-09693-x. [DOI] [PubMed] [Google Scholar]
- 96.Holash J, Maisonpierre PC, Compton D, Boland P, Alexander CR, Zagzag D, et al. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science 1999; 284:1994–1998. doi: 10.1126/science.284.5422.1994. [DOI] [PubMed] [Google Scholar]
- 97.Ramjiawan RR, Griffioen AW, Duda DG. Anti-angiogenesis for cancer revisited: is there a role for combinations with immunotherapy? Angiogenesis 2017; 20:185–204. doi: 10.1007/s10456-017-9552-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kienast Y, von Baumgarten L, Fuhrmann M, Klinkert WEF, Goldbrunner R, Herms J, et al. Real-time imaging reveals the single steps of brain metastasis formation. Nat Med 2010; 16:116–122. doi: 10.1038/nm.2072. [DOI] [PubMed] [Google Scholar]
- 99.Er EE, Valiente M, Ganesh K, Zou Y, Agrawal S, Hu J, et al. Pericyte-like spreading by disseminated cancer cells activates YAP and MRTF for metastatic colonization. Nat Cell Biol 2018; 20:966–978. doi: 10.1038/s41556-018-0138-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 2006; 355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 101.Chang DB, Yang PC, Luh KT, Kuo SH, Hong RL, Lee LN. Late survival of non-small cell lung cancer patients with brain metastases. Influence of treatment. Chest 1992; 101:1293–1297. doi: 10.1378/chest.101.5.1293. [DOI] [PubMed] [Google Scholar]
- 102.Garon EB, Ciuleanu TE, Arrieta O, Prabhash K, Syrigos KN, Goksel T, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet 2014; 384:665–673. doi: 10.1016/S0140-6736(14)60845-X. [DOI] [PubMed] [Google Scholar]
- 103.Reck M, Kaiser R, Mellemgaard A, Douillard JY, Orlov S, Krzakowski M, et al. Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1): a phase 3, double-blind, randomised controlled trial. Lancet Oncol 2014; 15:143–155. doi: 10.1016/S1470-2045(13)70586-2. [DOI] [PubMed] [Google Scholar]
- 104.Tabchi S, Blais N. Antiangiogenesis for advanced non-small-cell lung cancer in the era of immunotherapy and personalized medicine. Front Oncol 2017; 7:522.doi: 10.3389/fonc.2017.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Harks EG, de Roos AD, Peters PH, de Haan LH, Brouwer A, Ypey DL, et al. Fenamates: a novel class of reversible gap junction blockers. J Pharmacol Exp Ther 2001; 298:1033–1041. [PubMed] [Google Scholar]
- 106.Giridharan N, Oliva ICG, O’Brien BJ, Kerrigan BCP, Heimberger AB, Ferguson SD. Targeting the tumor microenvironment in brain metastasis. Neurosurg Clin N Am 2020; 31:641–649. doi: 10.1016/j.nec.2020.06.011. [DOI] [PubMed] [Google Scholar]
- 107.Priego N, Zhu L, Monteiro C, Mulders M, Wasilewski D, Bindeman W, et al. STAT3 labels a subpopulation of reactive astrocytes required for brain metastasis. Nat Med 2018; 24:1024–1035. doi: 10.1038/s41591-018-0044-4. [DOI] [PubMed] [Google Scholar]
- 108.Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 2016; 375:1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 109.Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 2018; 378:2078–2092. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 110.Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gümüş M, Mazières J, et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med 2018; 379:2040–2051. doi: 10.1056/NEJMoa1810865. [DOI] [PubMed] [Google Scholar]
- 111.Goldberg SB, Gettinger SN, Mahajan A, Chiang AC, Herbst RS, Sznol M, et al. Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol 2016; 17:976–983. doi: 10.1016/S1470-2045(16)30053-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Goldberg SB, Schalper KA, Gettinger SN, Mahajan A, Herbst RS, Chiang AC, et al. Pembrolizumab for management of patients with NSCLC and brain metastases: long-term results and biomarker analysis from a non-randomised, open-label, phase 2 trial. Lancet Oncol 2020; 21:655–663. doi: 10.1016/S1470-2045(20)30111-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Crinò L, Bronte G, Bidoli P, Cravero P, Minenza E, Cortesi E, et al. Nivolumab and brain metastases in patients with advanced non-squamous non-small cell lung cancer. Lung Cancer 2019; 129:35–40. doi: 10.1016/j.lungcan.2018.12.025. [DOI] [PubMed] [Google Scholar]
- 114.Hendriks LEL, Henon C, Auclin E, Mezquita L, Ferrara R, Audigier-Valette C, et al. Outcome of patients with non-small cell lung cancer and brain metastases treated with checkpoint inhibitors. J Thorac Oncol 2019; 14:1244–1254. doi: 10.1016/j.jtho.2019.02.009. [DOI] [PubMed] [Google Scholar]
- 115.Song J, Wang W, Wang Y, Qin Y, Wang Y, Zhou J, et al. Epithelial-mesenchymal transition markers screened in a cell-based model and validated in lung adenocarcinoma. BMC Cancer 2019; 19:6802.doi: 10.1186/s12885-019-5885-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sontheimer-Phelps A, Hassell BA, Ingber DE. Modelling cancer in microfluidic human organs-on-chips. Nat Rev Cancer 2019; 19:65–81. doi: 10.1038/s41568-018-0104-6. [DOI] [PubMed] [Google Scholar]
- 117.Yu T, Guo Z, Fan H, Song J, Liu Y, Gao Z, et al. Cancer-associated fibroblasts promote non-small cell lung cancer cell invasion by upregulation of glucose-regulated protein 78 (GRP78) expression in an integrated bionic microfluidic device. Oncotarget 2016; 7:25593–25603. doi: 10.18632/oncotarget.8232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Xu Z, Li E, Guo Z, Yu R, Hao H, Xu Y, et al. Design and construction of a multi-organ microfluidic chip mimicking the in vivo microenvironment of lung cancer metastasis. ACS Appl Mater Interfaces 2016; 8:25840–25847. doi: 10.1021/acsami.6b08746. [DOI] [PubMed] [Google Scholar]