Introduction
Superficial fungal infections of the skin, hair, and nails are the fourth most common cause of human disease affecting 20% to 25% of the world’s population [1]. Dermatophytosis is a superficial fungal infection caused by dermatophytic fungi that affect skin and the keratinized structures (hair and nails) arising from it. Dermatophytes, especially from the genera Trichophyton, cause the majority of superficial mycoses. These infections result in considerable morbidity and economic burden on the healthcare system [2]. In recent years, an alarming increase in the frequency of recalcitrant superficial fungal infections caused by novel species of Trichophyton, i.e., Trichophyton indotineae has been witnessed worldwide [3–24]. Importantly, the majority of the T. indotineae strains exhibit alteration in the squalene epoxidase (SQLE) gene that confers high terbinafine (TRB) resistance [3–5,7,10,11,13–17,19,21,22,25–28]. TRB is a first-line drug for treatment of moderate to severe dermatophytosis, and patients with T. indotineae infections typically show decreased effectiveness of oral therapy with this antifungal [29]. T. indotineae has been designated recently in the year 2020 as a distinct species independent of Trichophyton interdigitale and Trichophyton mentagrophytes on the basis of internal transcribed spacer (ITS) region sequencing of 2 highly TRB-resistant Trichophyton strains from a Nepali patient and an Indian patient [5]. On ITS phylogenetic analysis, TRB-resistant Indian strains cluster independently of the clusters of the T. interdigitale and T. mentagrophytes strains and differ in 2 to 3 single-nucleotide polymorphisms (SNPs) from T. mentagrophytes/T. interdigitale strains [5]. Subsequently, multigene polyphasic analysis of a larger data set of T. indotineae strains showed that these strains have distinct sequences of the high mobility group (HMG) gene as compared to T. mentagrophytes s. str. and T. interdigitale s. str [30]. Unlike the infections caused by T. mentagrophytes and T. interdigitale, T. indotineae often presents with extensive skin lesions and a chronic relapsing course. The whole genome sequencing analysis of 20 T. indotineae strains demonstrate that this new species is distinct clonal offshoot of T. mentagrophytes/T. interdigitale spp. complex. Thus, naming of this emerging antifungal-resistant species was essential as it could not be unambiguously identified as either T. mentagrophytes or T. interdigitale based on ITS sequencing, mycological and physiological characteristics.
In the last few years, dermatophytosis due to T. indotineae has not been limited to the Indian subcontinent but has also spread to Europe, Middle East, and North America related to travel and migration [7–24]. Further, reports of increasing treatment failure and acquisition of drug resistance in these difficult to treat T. indotineae infections have brought this entity to forefront due to limited alternative therapies. Although the significance of this problem has not gained global attention, it is just a matter of time when recalcitrant superficial dermatophytosis will be a potential public health threat worldwide. In this update, we apprise the emergence of T. indotineae in the Indian subcontinent and its rapid worldwide migration. Further, we highlight the challenges in the mycological identification and impact of the drug resistant T. indotineae strains on treatment of dermatophytosis.
A unique terbinafine-resistant Trichophyton species, T. indotineae, is causing alarming, difficult-to-treat dermatophytosis in India
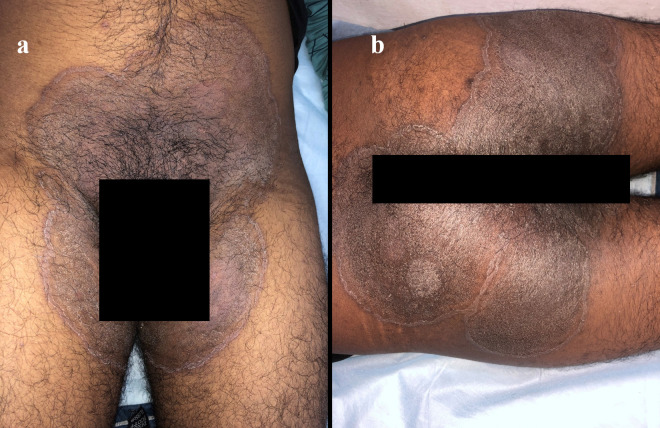
The ongoing outbreak of dermatophytosis in India is characterized by extensive and difficult—to-treat chronic and chronic relapsing infection of the body (tinea corporis) and the groins (tinea cruris) (Fig 1) [31]. The possible underlying factors driving the outbreak of recalcitrant infections in India are multiple but not limited to over-the-counter availability and use of combination steroid–antifungal–antibiotic creams, suboptimal and irrational regimens of prescribed antifungals, and brands with low efficacy. In 2014 to 2017, highly TRB-resistant T. interdigitale strains causing tinea cruris and tinea corporis infections were identified in North India [3,4,25]. TRB is an allylamine antifungal used orally and topically as a first-line drug in the therapy of dermatophyte infections. TRB resistance has been predominantly attributed to point alterations in the SQLE target gene, a key enzyme in the ergosterol biosynthetic pathway leading to single amino acid substitutions. In 2018, Singh and colleagues reported that TRB-resistant T. interdigitale isolates from cases of tinea corporis/cruris in 3 hospitals in Delhi, India exhibited elevated minimum inhibitory concentrations (MICs range 1 to ≥32 mg/L) to TRB and had single amino acid substitutions Leu393Phe or Phe397Leu in the SQLE. Remarkably, a considerably high TRB resistance rate of 32% was recorded using CLSI broth microdilution method [3]. Further, whole genome sequence analysis of Trichophyton species causing severe superficial dermatophytosis in North India confirmed a unique Trichophyton strain related to an early diverging clade of the T. mentagrophytes/interdigitale complex. The study pointed out that a new population of Trichophyton with highly related isolates (42 SNPs difference between any 2 isolates) exhibiting high rates of in vitro antifungal resistance was driving an ongoing outbreak of dermatophytosis in India [4]. Followed by this report, in 2020, Japanese investigators Kano and colleagues identified these highly TRB-resistant T. interdigitale-like strains isolated from a Nepali patient and an Indian patient with tinea corporis in Japan as a new species, i.e., T. indotineae. The rDNA ITS region sequences of their study isolates were 100% identical to TRB-resistant strains of T. interdigitale, which were isolated in Delhi, India, and harbored alterations in SQLE. Similar to Indian strains, the isolates exhibited high MICs (32 mg/L) for TRB and contained an amino acid substitution (Phe397Leu) in SQLE. To avoid confusion in the taxonomy of the T. mentagrophytes/interdigitale complex, the highly TRB-resistant Indian strains were designated as a new species independent of T. interdigitale/T. mentagrophytes, according to clinical and mycological features [5].
Fig 1.
Annular plaques with raised edges and scaly central region over groins (a) and buttocks (b). Note the prominent pigmentation, a common feature seen in T. indotineae infections in Indian patients.
Molecular identification of T. indotineae based on the internal transcribed spacer (ITS) region sequences
The identification of T. indotineae is challenging in the routine microbiology laboratories due to the marked morphological similarities of the species with T. mentagrophytes and T. interdigitale [5,30]. These species cannot be distinguished by phenotypic tests, although colony reverse of T. indotineae is most often pale-brown to yellow-orange in colour and isolates are less often positive in Tween-80 opacity, urea hydrolysis, and hair perforation tests than T. mentagrophytes and T. interdigitale [30]. In fact, in the last 5 years before labelling T. indotineae as a species de novo, studies based on rDNA ITS region sequencing identified Indian Trichophyton strains as T. mentagrophytes/interdigitale; further, Nenoff and colleagues grouped the strains as T. mentagrophytes Type VIII [3,4,6,26,32]. Currently, T. indotineae is unequivocally identified by the ITS sequences, which differ only at 2 and 3 nucleotide positions from ITS sequences of T. mentagrophytes and T. interdigitale, respectively. The BLAST searches of ITS sequences of T. indotineae on NCBI database still show ≥99% sequence similarity with T. mentagrophytes, T. interdigitale, and T. indotineae. Therefore, to obtain accurate identification (i.e., sequence similarity of 100% with T. indotineae), ITS sequences of well-defined reference strains described by Tang and colleagues [30], importantly, primary T. indotineae strains (NUBS19006 and NUBS19007), should be included in the analysis. Further, based on HMG gene sequences, T. indotineae can be differentiated from T. interdigitale and T. mentagrophytes, which differ at 4 and 1 nucleotide positions, respectively [30]. It is important to draw attention that incorrect nomenclature of several CBS reference and neotype strains of Trichophyton spp. in the public database results in misidentification warranting updating of the database [32].
Global spread of terbinafine-resistant T. indotineae strains
The spread of T. indotineae in Europe was noticed as early as in 2011 in Germany and most recently in 2019 to 2020, several cases have been reported from other European countries related to travel and migration [11–17,22,23,33]. Notably, 2 cases of tinea corporis and tinea cruris due to T. indotineae reported recently from Germany occurred in 2011 and 2013, even before the outbreak of dermatophytosis in India was recognised. Both the patients had travel links to India [12]. However, the strains had low TRB MICs and no SQLE alterations associated with TRB resistance was observed, suggesting that the early outbreak strains were probably not resistant to TRB. These findings correlate with absence of reports of TRB resistance until 2017 in India. Another report from Germany highlights that 29 patients with T. indotineae infections occurring during 2016 to 2020 had a history of travel to India, Pakistan, Bangladesh, Iraq, Bahrain, Libya, and other unspecified countries [15]. Notably, TRB-resistant T. indotineae strains isolated from cases in Germany, Denmark, and Switzerland during 2016 to 2020 exhibited Phe397Leu and Leu393Phe amino acid substitutions that confer resistance to TRB [11,15,22,33]. Since 2018, several cases of clinically resistant tinea corporis with extensive lesions that do not respond to TRB have been reported from France [13,14]. These patients were either recent immigrants or born in a country on the Indian subcontinent. The first case series of TRB-resistant T. indotineae infection in Canadian patients also emphasise travel or immigration from northern India as the source of T. indotineae [18]. The global reports of T. indotineae are listed in Table 1, which predominantly spread from 2016 onwards from the Indian subcontinent. Interestingly, Jabet and colleagues screened rDNA ITS sequences (in GenBank) of T. indotineae through March 2021 and observed widespread dissemination of the Indian strain with 12.8% of known sequences in the GenBank were from the Middle East and 9.6% from Europe. Remarkably, 98.8% of the sequences were of human origin and 6 sequences indicated an animal origin [13].
Table 1. Details of T. indotineae infections outside India.
| Countries reporting T. indotineae: Year of publication | Year of collection of strains | T. indotineae strains/total number of Trichophyton spp. strains investigated | Travel history or patients origin | Patients age group; clinical details | Treatment and outcome | TRB MICs (mg/L) / substitution in SQLE gene | Azole MICs (mg/L) | References |
|---|---|---|---|---|---|---|---|---|
| East and South East Asia | ||||||||
| Japan: 2020 | 2019 | 2/2 | Nepal and India | 27 and 47-years; tinea faciei/ corporis/ cruris |
Oral TRB→ITC/RVZ→ITR Topical BFN, TRB, KTC, LUZ Outcome Complete cure with ITC and LUZ |
>32 /Phe397Leu | ITC: 0.03 CTZ: 0.06–4 MCZ: 0.125–8 LUZ: <0.03 RVZ: 0.03–0.5 |
[5] |
| Vietnam: 2022 | 2020 | 1/1 | Autochthonous | 27 years; tinea corporis |
Oral ITC Topical KTC Outcome Complete cure |
0.25 | ITC: 0.125 VRC: 0.25 |
[8] |
| Cambodia: 2019 | 2019 | 1/4 | NM | 26 years; tinea corporis | NM | ND | ND | [9] |
| Middle East Asia | ||||||||
| Iran: 2019 | 2016–2018* | 116/1003* | Australia/ India/ Iran/Oman | NM; tinea corporis/ cruris/ faciei | NM | 0.003- ≥32/ ND | ND | [20] |
| Iran: 2020 | NM | 4/4 | Autochthonous | 4–64 years; tinea corporis/ cruris/ pedis |
Oral FLU→ prednisolone+methotrexate→ TRB→ITC→VRC/ FLU→ITC→VRC/ TRB→ITC Topical TRB, CTZ and SER. Outcome Complete cure with oral VRC or ITC |
≥8/Phe397Leu | ITC: ≥4 FLU: ≥16 VRC: 0.2–0.5 PSZ: 0.06–0.313 |
[21] |
| Iran: 2020 | 2016–2018 | 28/141 | Autochthonous | NM; tinea corporis/ cruris | NM | >32/ Phe397Leu, Leu393Ser |
ITC: 0.062–2 EFN: 0.001–0.125 CTZ: 0.5–32 LUZ: 0.0004–0.015 GRE: 0.25–4 AMO: 0.125–4 CPO: 0.062–1 |
[19] |
| Iraq: 2021 | 2016–2021 | 18/48 | Autochthonous | 4 months-70 years; tinea corporis/ faciei/ manuum/ capitis / pedis/ cruris/ unguium/ barbae |
Topical Steroids Outcome lesions either enlarged or flared again |
ND | ND | [24] |
| Iran: 2022 | 2018–2019 | 10/82 | Autochthonous | 11–60 years; tinea corporis/ cruris/ pedis/ capitis/ faciei/ manuum | NM | 0.015–32/ Phe397Leu |
ITC 0.06–16 FLU 0.125–16 VRC 0.125–8 KTC: 0.125–16 PSZ: 0.125–16 AMB: 0.125–16 |
[7] |
| Europe | ||||||||
| Germany: 2020 | 2011–2020 | 5/5 | Autochthonous/ India/Yemen | 20–38 years; tinea corporis/ cruris/ manuum/ faciei |
Oral ITC →TRB/ Several cycle of TRB and ITC Topical CTZ, CPO, TRB and MCZ Outcome Majority of patients experienced recurrence. |
≤ 0.06 | FLU: 16–64 ITC: 0.03–0.06 VRC: 0.125–0.5 |
[12] |
| Germany: 2019 | 2019 | 1/1 | Bahrain | 6 months; tinea corporis/ cruris |
Topical TRB→ MCZ and CPO Outcome Complete cure with MCZ and CPO |
>0.2/ Phe397Leu | ND | [10] |
| Germany: 2020 | 2016–2020 | 29/29 | Autochthonous/ India/Pakistan/ Bangladesh/ Iraq/Bahrain/ Libya | 6 months–58 years; tinea cruris/ corporis/ faciei/ manuum/ unguium/ pedis | Treatment mentioned for 4 patients only. Oral FLU and TRB→ITC Topical TRB, MCZ, CPO, CTZ, SER Outcome Complete cure with oral ITC |
0.2-16/ Phe397Leu, Leu393Phe |
ITC: 0.03–0.5 VRC: 0.03–0.5 |
[15] |
| Switzerland: 2021 | 2009–2019 | 11/162 | India/Bangladesh/ Thailand | 31–41 years; tinea cruris/ corporis/ faciei/ pedis |
Oral TRB or ITC or FLU Topical TRB, KTC, CLT, AMO, MCZ, ECZ, ISA Outcome No follow up |
≥4/ Phe397Leu |
MIC values not mentioned | [22] |
| France: 2022 | 2017–2021 | 7/10 | France/ India/ Bangladesh/ Myanmar | 16–53 years; tinea cruris/ corporis |
Oral TRB/ TRB→GRE/ TRB→GRE→ITC Topical TRB, ECZ, BFN, OMC, MCZ, CPO Outcome Treatment failed in patients harbouring strains of TRB MICs 2->8 mg/L |
0.06 ->8/ Phe397Leu, Leu393Ser |
ITC: 0.016–0.25 VRC: 0.03–0.5 AMO: 0.01–0.125 |
[13] |
| France: 2022 | 2018–2019 | 7/350 | India/ Bangladesh/ Sri Lanka | 20–57 years; tinea corporis/ cruris |
Oral TRB/ TRB→ITC/ TRB→GRE→ITC/ FLU Topical BFN, CPO, TRB Outcome Cure with oral ITC and oral ITC with topical BFN |
0.014-4/ Phe397Leu, Leu393Ser |
ITC: 015–16 VRC: 0.125–2 PSZ 0.03–0.5 ISA: 0.125–4 |
[14] |
| Belgium: 2020 | 2018 | 1/182 | Autochthonous | 25 years; tinea cruris/corporis/capitis |
Oral TRB Topical Sulconazole nitrate and KTC Outcome No follow up |
4/ Phe397Leu |
ITC: 0.016 VRC: 0.5 AMO: 0.06 |
[16] |
| Greece: 2019 | 2010–2019 | 9/112 | Greece/Syria/Iran | 9 months to 90 years; tinea cruris/corporis |
Oral TRB or ITC or FLU Topical TRB and azole ointment Outcome Improvement with topical azoles and oral ITC |
0.25-8/ Phe397Leu, Leu393Ser |
ITC: 0.016–0.125 VRC: 0.03–0.5 AMO: 0.125–0.25 |
[17] |
| Switzerland, Greece, Estonia, Finland: 2021 | 2020 | 11/96 | India/Bangladesh/ Pakistan/The United Arab Emirates | NM; tinea/ corporis/ capitis |
Oral TRB→ ITC/ TRB→ FLU/ TRB→GRE Outcome NM |
ND | ND | [23] |
| Denmark: 2022 | 2019–2020 | 7/63 | Autochthonous | NM | NM | 2- ≥4 Phe397Leu, Leu393Phe |
ITC: ≤ 0.016–0.06 VRC: 0.06–0.5 ISA: 0.06–0.5 PSZ: 0.008–0.125 OLO: 0.008–0.03 |
[11] |
| Denmark: 2019 | 2013–2018 | 1/14 | Autochthonous | 25 years; tinea cruris/corporis/faciei/ pedis |
Oral TRB/ TRB, ITC/ TRB, ITC, FLU/ TRB, ITC, GRE/ TRB, ITC, FLU, GRE Topical TRB, AMO, CPO, KTC and MCZ-hydrocortisone combination Outcome NM |
≥4 Phe397Leu |
ND | [33] |
| North America | ||||||||
| Canada: 2022 | 2021 | 8/8 | India/Thailand | 26–78 years; tinea cruris/corporis/faciei/ pedis |
Oral TRB/ TRB→FLU/ ITC/ ITC→ FLU Topical TRB, KTC, CTZ, clobetasol, betamethasone, betamethasone dipropionate and fluocinonide Outcome Complete cure with oral ITC in 1 patient |
ND | ND | [18] |
AMO, amorolfine; AMB, Amphotericin B; BFN, bifonazole; CPO, ciclopirox olamine; CTZ, clotrimazole; ECZ, econazole; EFN, efinaconazole; FLU, fluconazole; GRE, griseofulvin; ISA, isavuconazole; ITC, itraconazole; KTC, ketoconazole; LUZ, luliconazole; MCZ, miconazole; ND, not determined; NM, not mentioned; OLO, olorofim; OMC, omoconazole; PSZ, posaconazole; RVZ, ravuconazole; SER, sertaconazole; TRB, terbinafine; VRC, voriconazole.
*Out of 1,003, 397 were strains from patient samples (collected during 2016–2018) and the remaining were ITS (internal transcribed spacer) region sequences retrieved from GenBank.
→ denotes “followed by”.
Developments in the antifungal susceptibility testing (AFST) and mechanism of resistance in T. indotineae
AFST of dermatophytes is not routinely performed as both the reference CLSI and EUCAST methods are time consuming, and technical constraints related to slow growth and bacterial contamination remain a challenge [34,35]. The EUCAST method recommends chloramphenicol and cycloheximide supplemented growth media (to inhibit bacterial contamination) with a spectrophotometric endpoint reading using 50% growth inhibition [34]. EUCAST tentative epidemiological cutoff values (ECOFFS) for T. indotineae successfully demarcate isolates with and without SQLE resistance alterations [11]. No clinical breakpoints for TRB have been established by CLSI, and several reports have adopted variable MIC values (0.25 and ≥2 mg/L) for identification of TRB-resistant isolates. [3,4,15,26]. Further, for determination of MICs against azole drugs, variable criteria have been adopted by CLSI and EUCAST. For example, in itraconazole (ITC) testing, trailing growth may impact the observed resistance rate particularly when the MICs are determined using a stringent endpoint (90% inhibition) as adopted by CLSI in comparison with the 50% endpoint adopted by EUCAST [11,34,35]. The susceptibility testing by both the reference methods warrant further harmonization and standardization.
With the increasing number of resistant and recalcitrant T. indotineae cases, understanding the mechanism of resistance remains vital. In TRB-resistant Trichophyton species, alterations in the SQLE gene leading to amino acid substitutions at one of the 4 positions (Leu393, Phe397, Phe415, and His440) have been linked to resistance [36]. The most common substitution in T. indotineae strains reported worldwide is Phe397Leu in 95% of the studies leading to high TRB MICs (range: 1 to >32 mg/L) followed by Leu393Phe (MIC range: 1 to 64 mg/L) and Leu393Ser (MIC range: 0.5 to 1 mg/L) [3–5,7,10,11,13–17,19,21,22,25–28]. Notably, Phe397Leu or Leu393Phe substitutions confer high-level TRB resistance in T. mentagrophytes, T. interdigitale, T. indotineae, and T. rubrum [37]. Yamada and colleagues introduced the 2 common abovementioned amino acid substitutions into the endogenous SQLE gene of a TRB-sensitive Arthroderma vanbreuseghemii (formerly T. mentagrophytes) strain and showed that resistance to TRB in A. vanbreuseghemii transformants was due to the respective point alterations [36]. A newly developed DermaGenius Resistance real-time PCR assay is found to be highly efficacious in differentiation of SQLE wild type (T. indotineae susceptible) from mutant genotypes harbouring Phe397Leu or Leu393Phe substitution. However, the significance and clinical utility of such assays in patient management needs to be investigated [38]. Importantly, azole resistance in T. indotineae has been observed in one-third of reports from India and Europe [3,4,12,14,25–27]. T. indotineae strains with the double substitutions in the SQLE gene, i.e., Phe397Leu and Ala448Thr, exhibit increased MIC values of fluconazole, ITC, and voriconazole (VRC) [27]. However, the speculation that these double mutants lead to FLU, ITC, and VRC resistance in T. indotineae need to be experimentally investigated. A recent study highlighted that azole resistance in T. indotineae is due to overexpression of the TinCYP51B gene encoding sterol 14α-demethylases enzyme [39].
Multidrug-resistant T. indotineae and its impact on the treatment of dermatophytosis
TRB resistance rates ranging 17% to 75% and varying levels of ITC resistance up to 25% have been reported in T. indotineae strains from India [3,25,26]. In addition, several studies have reported high in vitro MIC values of VRC (range: 2 to >16 mg/L) and griseofulvin (range: 4 to 128 mg/L) [3,4,26]. It is important to emphasise that the treatment options for dermatophytosis are restricted and resistance to existing antifungals leaves no options for clinicians to treat severe persistent skin infections [40]. ITC remains the most effective antifungal for dermatophyte infections, with rising resistance to TRB. However, oral formulations of ITC have erratic absorption patterns leading to wide fluctuations in its serum concentrations. Although, monitoring of serum levels of ITC as in systemic mycoses is important for effective treatment outcome [41,42]. However, the high cost burden associated with regular therapeutic drug monitoring for this extremely prevalent infection, especially in lower-middle countries, is not practical. A single centre-based study reported that higher doses and longer durations of TRB therapy could overcome treatment failure associated with TRB-resistant strains [29]. Thus, appropriate dosage of TRB in treatment of dermatophytosis could prevent the usage of azole drugs and development of resistance against azole-based antifungal drugs. Recently, in vitro synergistic interactions with varying combinations of ITC, TRB, ketoconazole, and luliconazole (LUZ) have been observed in TRB-resistant Indian strains [43,44]. However, effectiveness of TRB and ITC in combination for treatment of tinea infections in a recent randomised trial showed no added beneficial effect over treatment with ITC alone [45]. Thus, combination treatment consisting of 2 systemic antifungals has no proven clinical benefit and must be avoided as it not only adds to cost of treatment, but also exposes patients to a greater array of adverse effects. It is more useful and rationale instead to combine oral drugs with topical antifungals with high susceptibility against the prevalent strain (e.g., LUZ) especially as topical antifungals achieve much higher levels in the skin. However, extensive skin involvement, as often seen with T. indotineae, makes the option economically unfeasible in many patients. Therefore, it’s important to develop newer highly potent antifungals for oral use and also gear up the clinical application of drugs like olorofim, which have high potent in vitro activity against T. indotineae [3,4,46,47]. Finally, T. indotineae has become widespread due to travel, immigration, and subsequent local transmission in the countries warranting urgent collective efforts at the global level to prevent its further dissemination.
Funding Statement
A.Ka. received research fellowship from the Science and Engineering Research Board [SERB File No. CRG/2020/001735] Department of Science and Technology, Government of India. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Ameen M. Epidemiology of superficial fungal infections. Clin Dermatol. 2010;28:197–201. doi: 10.1016/j.clindermatol.2009.12.005 [DOI] [PubMed] [Google Scholar]
- 2.Benedict K, Jackson BR, Chiller T, Beer KD. Estimation of direct healthcare costs of fungal diseases in the United States. Clin Infect Dis. 2019;68:1791–1797. doi: 10.1093/cid/ciy776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh A, Masih A, Khurana A, Singh PK, Gupta M, Hagen F, et al. High terbinafine resistance in Trichophyton interdigitale isolates in Delhi, India harbouring mutations in the squalene epoxidase gene. Mycoses. 2018;61:477–484. [DOI] [PubMed] [Google Scholar]
- 4.Singh A, Masih A, Monroy-Nieto J, Singh PK, Bowers J, Travis J, et al. A unique multidrug-resistant clonal Trichophyton population distinct from Trichophyton mentagrophytes/Trichophyton interdigitale complex causing an ongoing alarming dermatophytosis outbreak in India: Genomic insights and resistance profile. Fungal Genet Biol. 2019;133:103266. [DOI] [PubMed] [Google Scholar]
- 5.Kano R, Kimura U, Kakurai M, Hiruma J, Kamata H, Suga Y, et al. Trichophyton indotineae sp. nov.: A new highly terbinafine-resistant anthropophilic dermatophyte species. Mycopathologia. 2020;185:947–958. [DOI] [PubMed] [Google Scholar]
- 6.Nenoff P, Verma SB, Vasani R, Burmester A, Hipler UC, Wittig F, et al. The current Indian epidemic of superficial dermatophytosis due to Trichophyton mentagrophytes-A molecular study. Mycoses. 2019;62:336–356. [DOI] [PubMed] [Google Scholar]
- 7.Pashootan N, Shams-Ghahfarokhi M, Chaichi Nusrati A, Salehi Z, Asmar M, Razzaghi-Abyaneh M. Phylogeny, antifungal susceptibility, and point mutations of SQLE gene in major pathogenic dermatophytes isolated from clinical dermatophytosis. Front Cell Infect Microbiol. 2022;12:851769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ngo TMC, Ton Nu PA, Le CC, Ha TNT, Do TBT, Tran TG. First detection of Trichophyton indotineae causing tinea corporis in central Vietnam. Med Mycol Case Rep. 2022;36:37–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uhrlass S, Sithach M, Koch D, Wittig F, Muetze H, Krueger CNP. Trichophyton mentagrophytes—A new genotype in Cambodia. J. Fungi. 2019;5:460. [Google Scholar]
- 10.Süß A, Uhrlaß S, Ludes A, Verma SB, Monod M, Krüger C, et al. Ausgeprägte tinea corporis durch ein terbinafin resistentes Trichophyton-mentagrophytes-isolat vom indischen genotyp bei einem säugling aus Bahrain in Deutschland. Der Hautarzt. 2019;70:888–896. [DOI] [PubMed] [Google Scholar]
- 11.Astvad KMT, Hare RK, Jørgensen KM, Saunte DML, Thomsen PK, et al. Increasing terbinafine resistance in Danish Trichophyton isolates 2019–2020. J Fungi (Basel). 2022;8:150. doi: 10.3390/jof8020150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brasch J, Gräser Y, Beck-Jendroscheck V, Voss K, Torz K, Walther G, et al. "Indian" strains of Trichophyton mentagrophytes with reduced itraconazole susceptibility in Germany. J Dtsch Dermatol Ges. 2021;19:1723–1727. [DOI] [PubMed] [Google Scholar]
- 13.Jabet A, Brun S, Normand AC, Imbert S, Akhoundi M, Dannaoui E, et al. Extensive dermatophytosis caused by terbinafine-resistant Trichophyton indotineae, France, Emerg Infect Dis. 2022;28:229–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dellière S, Joannard B, Benderdouche M, Mingui A, Gits-Muselli M, Hamane S, et al. Emergence of difficult-to-treat tinea corporis caused by Trichophyton mentagrophytes complex isolates, Paris. France. Emerg Infect Dis. 2022;28:224–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nenoff P, Verma SB, Ebert A, Süß A, Fischer E, Auerswald E, et al. Spread of terbinafine-resistant Trichophyton mentagrophytes type VIII (India) in Germany-"the tip of the iceberg?". J Fungi (Basel). 2020;6:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sacheli R, Harag S, Dehavay F, Evrard S, Rousseaux D, Adjetey A, et al. Belgian national survey on tinea capitis: epidemiological considerations and highlight of terbinafine-resistant T. mentagrophytes with a mutation on SQLE gene. J Fungi (Basel). 2020;6:195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siopi M, Efstathiou I, Theodoropoulos K, Pournaras S, Meletiadis J. Molecular epidemiology and antifungal susceptibility of Trichophyton isolates in Greece: emergence of terbinafine-resistant Trichophyton mentagrophytes type VIII locally and globally. J Fungi (Basel). 2021;7:419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Posso-De Los Rios CJ, Tadros E, Summerbell RC, Scott JA. Terbinafine resistant Trichophyton indotineae isolated in patients with superficial dermatophyte infection in Canadian patients. J Cutan Med Surg. 2022;12034754221077891. doi: 10.1177/12034754221077891 [DOI] [PubMed] [Google Scholar]
- 19.Taghipour S, Shamsizadeh F, Pchelin IM, Rezaei-Matehhkolaei A, Zarei Mahmoudabadi A, Valadan R, et al. Emergence of terbinafine resistant Trichophyton mentagrophytes in Iran, harboring mutations in the squalene epoxidase (SQLE) gene. Infect Drug Resist. 2020;13:845–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taghipour S, Pchelin IM, Zarei Mahmoudabadi A, Ansari S, Katiraee F, Rafiei A, et al. Trichophyton mentagrophytes and T interdigitale genotypes are associated with particular geographic areas and clinical manifestations. Mycoses. 2019;62:1084–1091. [DOI] [PubMed] [Google Scholar]
- 21.Fattahi A, Shirvani F, Ayatollahi A, Rezaei-Matehkolaei A, Badali H, Lotfali E, et al. Multidrug-resistant Trichophyton mentagrophytes genotype VIII in an Iranian family with generalized dermatophytosis: report of four cases and review of literature. Int J Dermatol. 2021;60:686–692. [DOI] [PubMed] [Google Scholar]
- 22.Klinger M, Theiler M, Bosshard PP. Epidemiological and clinical aspects of Trichophyton mentagrophytes/Trichophyton interdigitale infections in the Zurich area: a retrospective study using genotyping. J Eur Acad Dermatol Venereol. 2021;35:1017–1025. [DOI] [PubMed] [Google Scholar]
- 23.Saunte DML, Pereiro-Ferreirós M, Rodríguez-Cerdeira C, Sergeev AY, Arabatzis M, Prohić A, et al. Emerging antifungal treatment failure of dermatophytosis in Europe: take care or it may become endemic. J Eur Acad Dermatol Venereol. 2021;35:1582–1586. doi: 10.1111/jdv.17241 [DOI] [PubMed] [Google Scholar]
- 24.Sharquie KE, Jabbar RI. Major Outbreak of dermatophyte infections leading into imitation of different skin diseases: Trichophyton mentagrophytes is the main criminal fungus. J Turk Acad Dermatol. 2021;15:91–100. [Google Scholar]
- 25.Rudramurthy SM, Shankarnarayan SA, Dogra S, Shaw D, Mushtaq K, Paul RA, et al. Mutation in the squalene epoxidase gene of Trichophyton interdigitale and Trichophyton rubrum associated with allylamine resistance. Antimicrob Agents Chemother. 2018;62:e02522–e02517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ebert A, Monod M, Salamin K, Burmester A, Uhrlaß S, Wiegand C, et al. Alarming India-wide phenomenon of antifungal resistance in dermatophytes: A multicentre study. Mycoses. 2020;63:717–728. doi: 10.1111/myc.13091 [DOI] [PubMed] [Google Scholar]
- 27.Kong X, Tang C, Singh A, Ahmed SA, Al-Hatmi AMS, Chowdhary A, et al. Antifungal susceptibility and mutations in the squalene epoxidase gene in dermatophytes of the Trichophyton mentagrophytes species complex. Antimicrob Agents Chemother. 2021;65:e0005621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burmester A, Hipler UC, Uhrlaß S, Nenoff P, Singal A, Verma SB, et al. Indian Trichophyton mentagrophytes squalene epoxidase erg1 double mutants show high proportion of combined fluconazole and terbinafine resistance. Mycoses. 2020. Jul 29. doi: 10.1111/myc.13150 Epub ahead of print . [DOI] [PubMed] [Google Scholar]
- 29.Khurana A, Masih A, Chowdhary A, Sardana K, Borker S, Gupta A, et al. Correlation of in vitro susceptibility based on MICs and SQLE mutations with clinical response to terbinafine in patients with tinea corporis/cruris. Antimicrob Agents Chemother. 2018. pii: AAC.01038-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang C, Kong X, Ahmed SA, Thakur R, Chowdhary A, Nenoff P, et al. Taxonomy of the Trichophyton mentagrophytes/T. interdigitale species complex harboring the highly virulent, multiresistant genotype T. indotineae. Mycopathologia. 2021;186:315–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verma SB, Panda S, Nenoff P, Singal A, Rudramurthy SM, Uhrlass S, et al. The unprecedented epidemic-like scenario of dermatophytosis in India: Epidemiology, risk factors and clinical features. Indian J Dermatol Venereol Leprol. 2021;87:154–175. [DOI] [PubMed] [Google Scholar]
- 32.Chowdhary A, Singh A, Singh PK, Khurana A, Meis JF. Perspectives on misidentification of Trichophyton interdigitale/Trichophyton mentagrophytes using internal transcribed spacer region sequencing: Urgent need to update the sequence database. Mycoses. 2019;62:11–15. [DOI] [PubMed] [Google Scholar]
- 33.Saunte DML, Hare RK, Jørgensen KM, Jørgensen R, Deleuran M, Zachariae CO, et al. Emerging terbinafine resistance in Trichophyton: Clinical characteristics, squalene epoxidase gene mutations, and a reliable EUCAST method for detection. Antimicrob Agents Chemother. 2019;63:e01126–e01119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.The European Committee on Antimicrobial Susceptibility Testing. Overview of antifungal ECOFFs and clinical breakpoints for yeasts, moulds and dermatophytes using the EUCAST E.Def 7.3, E.Def 9.4 and E.Def 11.0 procedures. Version 3, 2022. http://www.eucast.org."
- 35.CLSI. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi. 3rd ed. CLSI standard M38. Wayne, PA: Clinical and Laboratory Standards Institute; 2017. [Google Scholar]
- 36.Yamada T, Maeda M, Alshahni MM, Tanaka R, Yaguchi T, Bontems O, et al. Terbinafine resistance of Trichophyton clinical isolates caused by specific point mutations in the squalene epoxidase gene. Antimicrob Agents Chemother. 2017;61:e00115–e00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rogers TR, Verweij PE, Castanheira M, Dannaoui E, White PL, Arendrup MC; Subcommittee on Antifungal Susceptibility Testing (AFST) of the ESCMID European Committee for Antimicrobial Susceptibility Testing (EUCAST). Molecular mechanisms of acquired antifungal drug resistance in principal fungal pathogens and EUCAST guidance for their laboratory detection and clinical implications. J Antimicrob Chemother. 2022; dkac161. [Epub ahead of print. doi: 10.1093/jac/dkac161 ]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singh A, Singh P, Dingemans G, Meis JF, Chowdhary A. Evaluation of DermaGenius resistance real-time polymerase chain reaction for rapid detection of terbinafine-resistant Trichophyton species. Mycoses. 2021;64:721–726. [DOI] [PubMed] [Google Scholar]
- 39.Yamada T, Yaguchi T, Maeda M, Alshahni MM, Salamin K, Guenova E, et al. Gene amplification of CYP51B: a new mechanism of resistance to azole compounds in Trichophyton indotineae. Antimicrob Agents Chemother. 2022:e0005922. doi: 10.1128/aac.00059-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khurana A, Sardana K, Chowdhary A. Antifungal resistance in dermatophytes: Recent trends and therapeutic implications. Fungal Genet Biol. 2019;132:103255. doi: 10.1016/j.fgb.2019.103255 [DOI] [PubMed] [Google Scholar]
- 41.Singh S, Chandra U, Anchan VN, Verma P, Tilak R. Limited effectiveness of four oral antifungal drugs (fluconazole, griseofulvin, itraconazole and terbinafine) in the current epidemic of altered dermatophytosis in India: results of a randomized pragmatic trial. Br J Dermatol. 2020;183:840–846. doi: 10.1111/bjd.19146 [DOI] [PubMed] [Google Scholar]
- 42.Khurana A, Agarwal A, Singh A, Sardana K, Ghadlinge M, Agrawal D, et al. Predicting a therapeutic cut-off serum level of itraconazole in recalcitrant tinea corporis and cruris- A prospective trial. Mycoses. 2021;64:1480–1488. doi: 10.1111/myc.13367 [DOI] [PubMed] [Google Scholar]
- 43.Bidaud AL, Schwarz P, Chowdhary A, Dannaoui E. In Vitro antifungal combination of terbinafine with itraconazole against isolates of Trichophyton species. Antimicrob Agents Chemother. 2022;66:e0144921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sardana K, Gupta A, Sadhasivam S, Gautam RK, Khurana A, Saini S, et al. Checkerboard analysis to evaluate synergistic combinations of existing antifungal drugs and propylene glycol monocaprylate in isolates from recalcitrant tinea corporis and cruris patients harboring squalene epoxidase gene mutation. Antimicrob Agents Chemother. 2021;65:e0032121. doi: 10.1128/AAC.00321-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singh SK, Subba N, Tilak R. Efficacy of terbinafine and itraconazole in different doses and in combination in the treatment of tinea infection: a randomized controlled parallel group open labeled trial with clinico-mycological correlation. Indian J Dermatol. 2020;65:284–289. doi: 10.4103/ijd.IJD_548_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Singh A, Singh P, Meis JF, Chowdhary A. In vitro activity of the novel antifungal olorofim against dermatophytes and opportunistic moulds including Penicillium and Talaromyces species. J Antimicrob Chemother. 2021;76:1229–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Astvad KMT, Jørgensen KM, Hare RK, Datcu R, Arendrup MC. Olorofim susceptibility testing of 1,423 Danish mold isolates obtained in 2018–2019 confirms uniform and broad-spectrum activity. Antimicrob Agents Chemother. 2020;65:e01527–e01520. doi: 10.1128/AAC.01527-20 [DOI] [PMC free article] [PubMed] [Google Scholar]