Dear Editor,
The impact of corticosteroids on the outcomes of acute respiratory distress syndrome (ARDS) is controversial. This study was performed as a large-scale analysis of Korean health insurance claims data to determine whether ARDS etiology, baseline characteristics, or dosage of, duration of treatment with, and type of steroids modified the association between corticosteroid use and mortality among patients with ARDS.
Adult (age ≥ 18 years) patients with 2009 influenza A (H1N1) (May 2009–April 2010), non-viral (January 2015–April 2019), or coronavirus disease 2019 (COVID-19) ARDS (January–December 2020) who received mechanical ventilation were included. Corticosteroid use was defined as at least one dose for intravenous dexamethasone, hydrocortisone, or methylprednisolone during the hospitalization. Logistic regression analyses were performed for 30- and 180-day mortality with corticosteroid use as an independent variable and age, sex, Charlson Comorbidity Index (CCI), immunosuppression, hospital type, organ dysfunction, and extracorporeal membrane oxygenation as covariates. Separate analyses were performed to ascertain the associations of dosage of, duration of treatment with, and type of steroids. The associations between corticosteroid use and mortality were also assessed with stratification according to age, CCI, number of organ dysfunctions, and immunosuppression. All statistical analyses were performed using SAS software (version 9.4; SAS Institute, Cary, NC, USA).
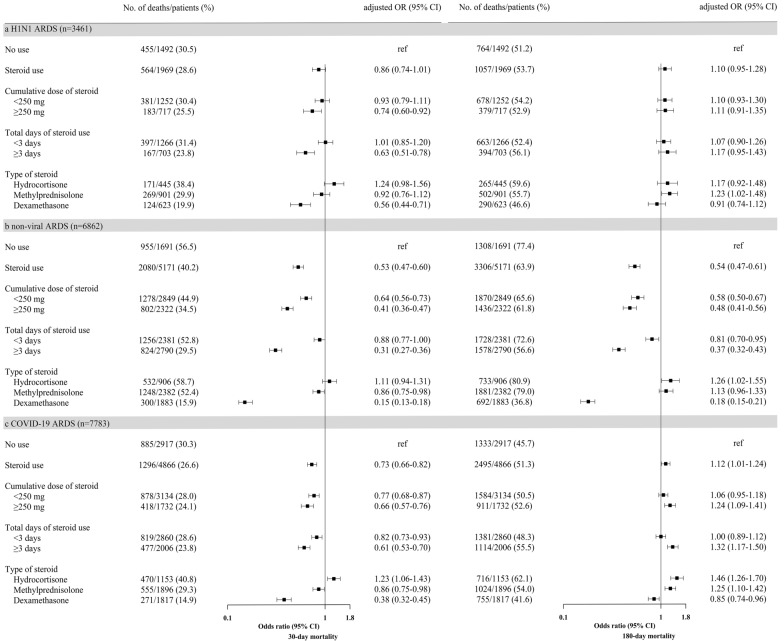
Among 18,106 included patients (mean [standard deviation] age, 67.4 [14.7] years; men, 63%), 3461 (19%), 6862 (38%), and 7783 (43%) were diagnosed with H1N1, non-viral, and COVID-19 ARDS, respectively (eFig. 1). The baseline characteristics and clinical outcomes are shown in eTables 1 and 2. Corticosteroids were associated with decreased 30- and 180-day mortality in non-viral ARDS, but were not associated with decreased 180-day mortality in H1N1 or COVID-19 ARDS (Fig. 1). Furthermore, corticosteroids for ≥ 3 days were associated with decreased 30- and 180-day mortality in non-viral ARDS. However, these findings were not observed for 180-day mortality in H1N1 ARDS, and an even higher risk of 180-day mortality was observed in COVID-19 ARDS. Recent studies have shown that patients with COVID-19 ARDS who received long-term corticosteroids had higher rates of survival [1, 2]; however, none of them evaluated long-term mortality. As corticosteroids can delay viral clearance [3], it might be necessary to assess the outcomes after 28 days for viral ARDS.
Fig. 1.
Associations between corticosteroid use or dosage of, duration of treatment with, and type of steroids and 30- and 180-day mortality in a H1N1, b non-viral, or c COVID-19 ARDS. The numbers and percentages of patients who died according to each risk factor and the resulting odds ratios. For steroid use, the odds ratios were adjusted for age, sex, Charlson Comorbidity Index, immunosuppression, hospital type, organ dysfunction, steroid use, neuromuscular blocking agent use, and extracorporeal membrane oxygenation. For cumulative dose of steroid, total days of steroid use, and type of steroids, the odds ratios were adjusted for age, sex, Charlson Comorbidity Index, immunosuppression, hospital type, organ dysfunction, neuromuscular blocking agent use, and extracorporeal membrane oxygenation. ARDS acute respiratory distress syndrome; COVID-19 coronavirus disease 2019; H1N1 2009 influenza A
Dexamethasone was associated with decreased 30- and 180-day mortality among all patients with ARDS, while methylprednisolone was associated with increased 180-day mortality in H1N1 or COVID-19 ARDS (Fig. 1). Previous meta-analyses among patients with ARDS revealed no differences in survival benefit between steroid types [1, 4]; however, long-term mortality was not assessed. There may be a strong association between methylprednisolone and muscle weakness in patients receiving mechanical ventilation [5]. In H1N1 ARDS, corticosteroids were associated with decreased 30-day mortality in older patients (≥ 65 years) (eFig. 4). In COVID-19 ARDS, corticosteroids were associated with increased 180-day mortality in patients with less organ dysfunction (< 3) (eFig. 6).
Corticosteroid use was disproportionately associated with long-term mortality in viral and non-viral ARDS. Additional studies evaluating its use other than dexamethasone for long-term mortality in COVID-19 ARDS are warranted.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
K-EK participated in the data acquisition, data analysis, and draft of the manuscript. S-YJ participated in the data acquisition, data analysis and interpretation, and helped to revise the manuscript for important intellectual content. MSB participated in the data interpretation and helped to revise the manuscript for important intellectual content. W-YK participated in the conception and design of study, data interpretation, and draft of the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the research grant from Biomedical Research Institute, Chung-Ang University Hospital (2020) and the National Research Foundation of Korea grant funded by the Korea government (Ministry of Science, ICT & Future Planning) (2022R1F1A1067609). The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval
The study protocol for analysis of de-identified patient data was exempted from review by the Institutional Review Board of Chung-Ang University (1041078–202007-HR-180–01).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Kyoung-Eun Kwon and Sun-Young Jung contributed equally to this work.
References
- 1.Chaudhuri D, Sasaki K, Karkar A, et al. Corticosteroids in COVID-19 and non-COVID-19 ARDS: a systematic review and meta-analysis. Intensive Care Med. 2021;47:521–537. doi: 10.1007/s00134-021-06394-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Torres A, Motos A, Cillóniz C, et al. Major candidate variables to guide personalised treatment with steroids in critically ill patients with COVID-19: CIBERESUCICOVID study. Intensive Care Med. 2022;48:850–864. doi: 10.1007/s00134-022-06726-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giannella M, Alonso M, Garcia de Viedma D, et al. Prolonged viral shedding in pandemic influenza A(H1N1): clinical significance and viral load analysis in hospitalized patients. Clin Microbiol Infect. 2011;17:1160–1165. doi: 10.1111/j.1469-0691.2010.03399.x. [DOI] [PubMed] [Google Scholar]
- 4.WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group. Sterne JAC, Murthy S, et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020;324:1330–1341. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herridge MS, Cheung AM, Tansey CM, et al. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348:683–693. doi: 10.1056/NEJMoa022450. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.