Abstract
Background
Collecting information on sustainability of immune responses after infection or vaccination is crucial to inform medical decision-making and vaccination strategies. Data on how long-lasting antibodies against SARS-COV-2 could provide a humoral and protective immunity and prevent reinfection with SARS-CoV-2 or its variants is particularly valuable. This study presents a novel method to quantitatively measure and monitor the diversity of SARS-CoV-2 specific antibody profiles over time.
Methods
Serum samples from two groups were used in this study: Samples from 20 naturally infected subjects (followed for up to 1 year) and samples from 83 subjects vaccinated with one or two doses of the Pfizer BioNtech vaccine (BNT162b2/BNT162b2) (followed for up to 6 months). The Multi-SARS-CoV-2 assay, a multiparameter serology test developed for the serological confirmation of past-infections, was used to determine the reactivity of six different SARS-CoV-2 antigens. For each subject sample, 3 dilutions (1/50, 1/400 and 1/3200) were defined as an optimal set over the six antigens and their respective linear ranges. This allowed accurate quantification of the corresponding six antibodies. Nonlinear mixed-effects modelling was applied to convert intensity readings from 3 determined dilutions to a single quantification value for each antibody.
Results
Median half-life for the 20 naturally infected vs 74 vaccinated subjects (two doses) was 120 vs 50 days for RBD, 127 vs 53 days for S1 and 187 vs 86 days for S2 antibodies respectively.
Conclusion
The newly proposed method, based on a series of a limited number of dilutions, can convert a conventional qualitative assay into a quantitative assay. This conversion helps define the sustainability of specific immune responses against each relevant viral antigen and can help in defining the protection characteristics after an infection or a vaccination.
Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) was first described in December 2019 and has rapidly spread. It continues to cause a major health crisis around the world since then [1]. The virus causes the respiratory illness COVID-19, and the outbreak was declared a pandemic on 11 March 2020 by the World Health Organization (WHO) [2]. The COVID-19 pandemic represents the greatest medical and socio-economic challenge of our time [3].
Collecting information on sustainability of immune response after infection is crucial for medical decision-making and vaccination strategies. It is particularly valuable to determine for how long antibodies against SARS-COV-2 could provide humoral immunity and prevent reinfection with SARS-COV-2 or its variants. With the implementation of large-scale vaccination programs, it is critical to determine the duration of vaccine-induced immunity and to anticipate possible modifications of the vaccination strategies. There is currently no consistent depiction of the humoral immune response after natural SARS-COV-2 infection.
The duration of immunity is a key metric of protection following natural infection or vaccination. In addition to cellular immunity, virus-specific antibodies represent an important component of protection. Large efforts are being made globally to set objective criteria to define the degree and duration of such protective immunity at the individual level [4–7]. So far, studies suggest that antibody levels may decrease rapidly in infected individuals. This rate of decrease depends on the severity of the infection (asymptomatic, mildly symptomatic, or severely symptomatic requiring hospitalization and referral to the ICU) [8–10]. Long et al. described a decline in antibody titers in the convalescent phase of the disease, suggesting that antibodies to SARS-CoV-2 may fade away rapidly [8]. Furthermore, Prévost et al. evaluated 98 infected patients and found that while most individuals developed neutralizing antibodies within the first two weeks of infection, the level of neutralizing activity decreased significantly over time [11]. These studies highlight the importance of characterizing the kinetics of antibody levels.
In this study we first present the Multi-SARS-CoV-2 assay (InfYnity Biomarkers, Lyon, France). When performed on a minimal dilution sequence, the assay provides a novel method to quantify and monitor SARS-CoV-2 specific antibody profiles over time. The assay can detect the presence of 6 different SARS-CoV-2 specific antibodies in a single well of a 96-well microplate thus reducing inter-assay variability. The method provides a quantitative serological profile, in contrast to conventional immunoassay methods which provide single antibody qualitative results. Second, to demonstrate the effectiveness of the assay, we present an investigation on the difference in antibody waning kinetics in naturally infected versus vaccinated subjects. The presented assay offers a simple and universal method that can be implemented in a wide majority of moderate-resource laboratories.
Methods
Study design, population, and origin of the samples
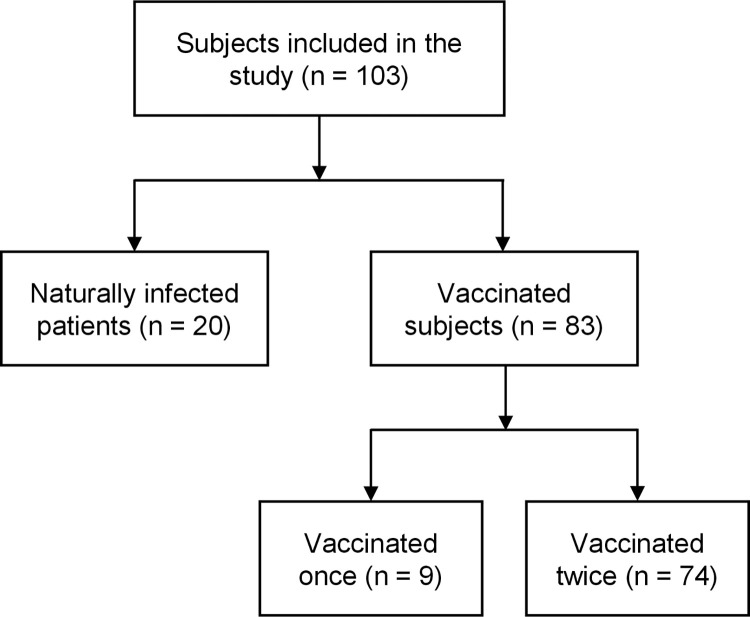
Serum samples were collected from two groups: naturally infected and vaccinated subjects (Fig 1). For the first group 101 serum samples were available, originating from 20 naturally infected patients, out of which 17 were followed up over time, up to 360 days (median = 268). For each patient 2 to 9 longitudinal samples were available (median = 5). The collection time of the first sample after onset of symptoms was between 14 and 41 days (median = 27.5). All patients were symptomatic, mainly with fever, coughing, having shortness of breath, muscle pain and general weakness. Most patients were confined at home with ambulatory treatment, only a few were hospitalized.
Fig 1. Subject groups.
The flowchart indicates the grouping of the subjects in the study.
Serum samples from these 20 patients were collected for up to 360 days and tested with the Multi-SARS-CoV-2 immunoassay to demonstrate the monitoring capabilities of the new assay and to investigate the antibody dynamics of naturally infected patients. In addition, serological investigation was performed on the samples using commercial assays: SCoV-2 DetectTM IgG ELISA kit for IgG detection from InBios, Elecsys® Anti-SARS-CoV-2 test for antibodies detection against the nucleocapsid (N) antigen (including IgG) from Roche and LIAISON® SARS-CoV-2 S1/S2 IgG test from Diasorin.
Samples from the vaccinated group come from a prospective longitudinal cohort study conducted by the laboratory associated with the National Reference Center for Respiratory Viruses (University Hospital of Lyon, France). Eighty-three health care workers vaccinated with 1 or 2 doses of the Pfizer–BioNTech BNT162b2 vaccine were included in this study. Of these 83 subjects, 74 self-reported no prior COVID infection and were vaccinated twice. The 9 other subjects self-reported prior COVID infection and were only vaccinated once. Blood samples were collected at T0: before the first dose, T1: 4 weeks after the first dose, immediately preceding the second dose, T2: 4 weeks, and T3: 6 months after the full vaccination. The pre-vaccination blood samples were only used to document a previous SARS-CoV-2 infection. Subjects who have been previously infected with SARS-CoV-2 (convalescent group, 11%) received only one injection, therefore the second sample was omitted (no data at time-point T1). One subject was infected with SARS-CoV-2 between the 2 doses and therefore got a single dose of the vaccine.
Serological investigation was performed on the 323 vaccinated group samples using the Multi-SARS-CoV-2 immuno-assay as well as commercial assay VIDAS® SARS-COV-2 IgG II (9COG) for the detection of IgG specific for the SARS-CoV-2 receptor-binding domain (RBD) from bioMérieux. Samples at timepoints T0, T1 and T2 were also tested on LIAISON® SARS-CoV-2 TrimericS IgG for the detection of IgG specific to the Spike protein from DiaSorin, IgG SARS-CoV-2 (sCOVG) that detects IgG against SARS-CoV-2 from Siemens and WANTAI SARS-CoV-2 Ab ELISA for the detection of total antibodies against SARS-CoV-2 from Wantai.
Patient characteristics of both subject groups are shown in Table 1.
Table 1. Patient characteristics.
| Characteristic | Naturally infected group | Vaccinated group |
|---|---|---|
| Sex (Male/Female) | 60% / 40% (12 / 8) | 81% / 19% (67 / 16) |
| Age (Years) | 43 ±a 10 (range: 23–60) | 47 ± 11 (range: 22–76) |
| Median # samples | 5 (IQR = 2; range: 2–9) | 4 |
| Follow-up time (days) | 268 (IQR = 89; range: 127–360) | 180 |
a ± indicates the standard deviation.
For each naturally infected patient, 3 dilutions (1/50, 1/400 and 1/3200) at each time-point were assayed. A fourth dilution was available for the vaccinated subjects (1/100), which was used for a qualitative version of the Multi-SARS-CoV-2 assay. In this article, however, we only report the quantitative version of the Multi-SARS-CoV-2 assay.
For the vaccinated subjects, a written informed consent was obtained from all subjects. Ethics approval was obtained from the national review board for biomedical research in April 2020 (Comité de Protection des Personnes Sud Méditerranée I, Marseille, France; ID RCB 2020-A00932-37), and the study was registered on ClinicalTrials.gov (NCT04341142).
For the naturally infected covid patients, immune serum samples collected in 2020 and 2021 were acquired from ABO Pharmaceuticals (ABO Pharmaceuticals, SanDiego, CA, USA) a duly authorized blood banking organization.
None of the subjects were involved in the design or implementation of this study.
Multi-SARS-CoV-2 assay
The Multi-SARS-CoV-2 immunoassay was used to monitor detailed serological profiles. The multiplex technology allows for the combination of multiple parameters in a single well of a 96-well microplate. The printing process is based on non-contact piezo electric impulsion of a defined volume of an antigenic solution. The bioprinting was performed to print six different SARS-CoV-2 specific antigens: 1) MP1, specific epitope for SARS-CoV-2 membrane antigen, enhanced according to disease severity; 2) NP1, full-length nucleocapsid recombinant protein antigen; 3) NP2, specific epitope of the nucleocapsid protein, enhanced according to disease severity; 4) RBD, recombinant Receptor Binding Domain of the spike protein; 5) S1, recombinant spike protein S1; 6) S2, recombinant spike protein S2.
Antigens were printed at the bottom of each well at precise X-Y coordinates, under controlled humidity and temperature conditions. Each antigen spot is printed in duplicate to improve fault tolerance. Positive control spots were printed in quadruplicate to define a precise spatial orientation pattern and validate the correct sequential distribution of all biological and chemical materials as well as operating performance (human serum samples, enzyme conjugate, and substrate).
Prior to this study, assay validation was performed in an independent public health laboratory (SCIENSANO, Belgium) and included an extensive testing of well-characterized positive and negative samples to ensure that all performance attributes were in line with in-vitro diagnostic and quality requirements. In brief, each antigen was analyzed for its intrinsic specificity and sensitivity using a set of seropositive (n = 540; all >14 days post-PCR determination) and seronegative samples (n = 270; including pre-pandemic samples). Optimal concentration for each antigen was determined by Receiver Operating Characteristic curves (ROC).
For performance evaluation an NIBSC calibrator was used (ref. 20/136) to define the lower limit of detection for each antibody specificity.
Each plate was read and analyzed using a colorimetric image analyzer. The software calculates the median pixel intensity for each spot with the background noise subtracted. To establish the net intensity for each antigen, the mean value of duplicated spots was calculated. Mean spot intensities for each antigen were normalized by dividing the value by the average positive control intensity, which showed the maximum attainable intensity in the assay. This was done to reduce assay variability and to have a common pixel intensity range of [0, 100] for all tested antigens.
Dilution sequence
Biomarker reactivity curves that express the relationship between log antibody concentration and intensity typically show a sigmoidal shape that levels off at both ends of the curve. This creates two plateaus one at the low end and one at the high end of the pixel intensity ranges. Consequently, at high and low intensities, a small change in intensity leads to a considerable change in antibody concentration reading. Since minor fluctuations may always be expected due to test variability, the nonlinear plateau-range is ill-suited for an accurate quantification. This is not the case for the linear range that connects the two plateaus and is therefore better suited for quantification.
The Multi-SARS-CoV-2 assay was developed for the serological confirmation of past infections. The assay was therefore optimized to produce strong signals to sera of infected subjects and to show no or non-significant signals to sera from uninfected subjects. Readings in the plateau-ranges are therefore expected.
To ensure a reading of each antigen in the linear range, samples were serially diluted. The dilution factors were chosen in such a way that overlap of linear ranges for all antigens was assured, observed maximal concentrations could be quantified, and the number of dilutions was minimized (to reduce operational cost). This process is explained in more detail in S1 File. Finally, we selected the set of 3 dilutions (1/50, 1/400 and 1/3200) as an optimal set over the 6 antigens. A fourth dilution was available for the vaccinated subjects (1/100).
Statistical methods
A nonlinear mixed-effects model was used to convert intensity readings from the selected dilutions to a single quantification per antibody. The R ‘LME4’ package was used for model fitting [12]. The model is based on the standard 2-parameter logistic curve (sigmoidal standard curve with fixed top = 100 and bottom = 0) and is described in the following Eq 1.
| (1) |
In the above equation, Intensityi,j,k corresponds to the intensity of the ith sample, jth antibody and kth dilution. Intensity values were available for I = 424 samples (coming from 103 naturally infected or vaccinated subjects at different time-points), J = 6 antibodies and K = 3 or 4 dilutions. The Dilution Factor 50 (DF50) value corresponds to the estimated dilution at which an intensity value of 50% is observed. This value summarizes the 3 (or 4) antibody intensities and represents quantitative information of antibody concentration. Although the unit of DF50 is arbitrary, its relationship with concentration is linear. The model fit results into 6 (antibody) DF50 values for each individual patients’ time-point. Note that the location of the sigmoid curve and thus the DF50 value is obtained from the fitted curve for each sample (thus for each subject at each time-point), based on the measured ODs of the 3 tested dilutions. The hillslopes (per marker) are, however, fitted on the combination of all 424 samples.
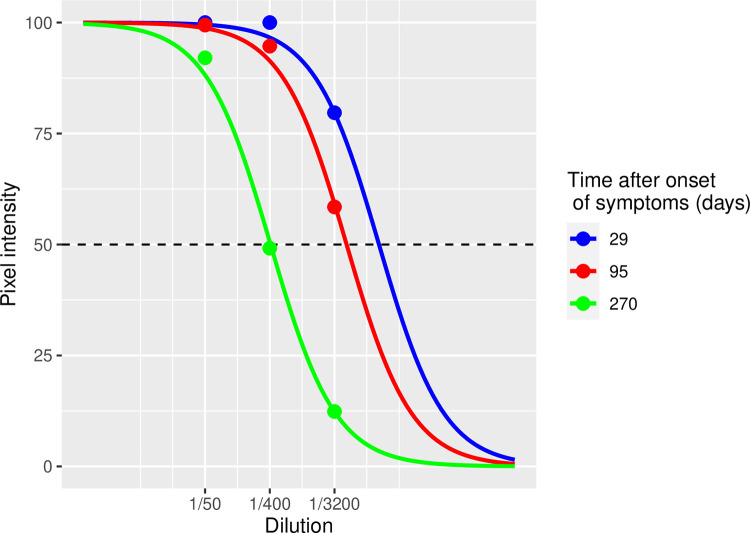
As an example, Fig 2 shows the RBD fits for 3 time-points of one naturally infected subject (subject #6). At dilution 1/50 the 29 days, 95 days and 270 days serum samples show high and saturated pixel intensities (or biomarker reactivities) and are thus unquantifiable without the dilution sequence. The fitted sigmoid curves on the three dilutions shift position over time. The position of the curve is described by the DF50-value, that is, the estimated dilution factor corresponding to a pixel intensity of 50. In Fig 2, the DF50s are located at the points where the dashed line at OD = 50 intersects the curves. In the above example, the complete curve clearly shifts over time from right to left, corresponding to a decreasing DF50. This example clearly illustrates the need to use the dilution approach for quantitative measurements.
Fig 2. Sigmoidal curve fitting example.
Sigmoidal curve fitting of 3 dilutions (1/50, 1/400, 1/3200) for 3 timepoints of the naturally infected subject #6 for the anti-RBD antibodies.
It is also possible to convert the 2-parameter sigmoidal function to a linear equation, allowing to determine hillslope and DF50 for each individual sample (see S2 File). The plot in this supplement shows an example of the DF50 decay over time for naturally infected patient #1, as calculated with the alternative method.
Results
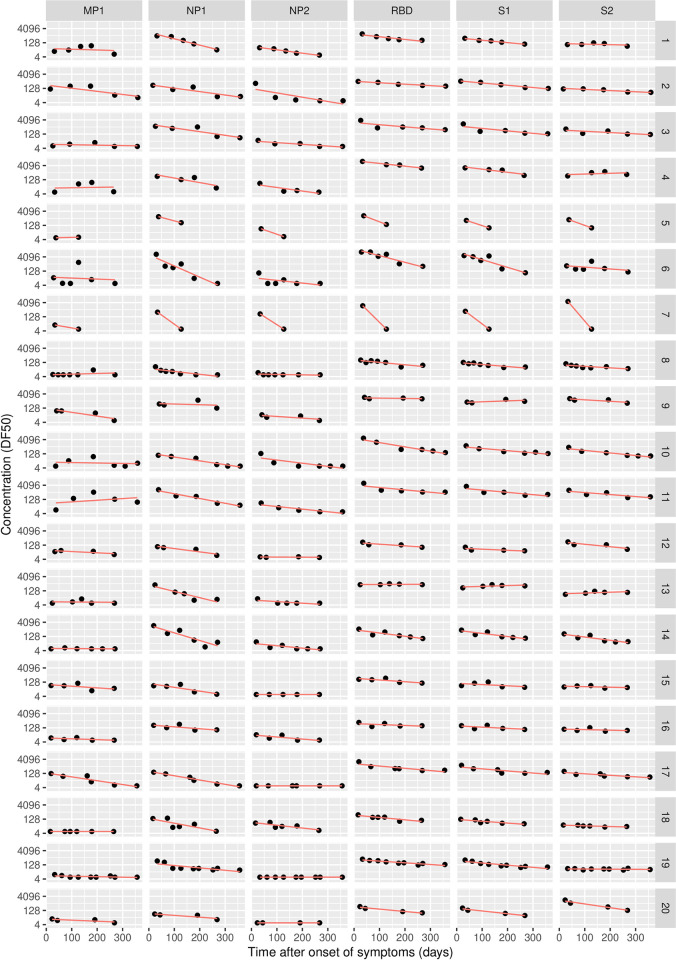
In the naturally infected group, antibody concentration generally declines over time. Fig 3 shows the evolution of each (log-transformed) antibody concentration for the 20 patients and 6 markers of the naturally infected group. The general time-evolution trend displays a log-linear decline of concentration, which supports constant half-life for at least the duration of the study period (Fig 3). The graphs also show that decline rates differ between patients and antibodies. A linear regression model was used to fit the log-linear decline per patient and per marker to model the individual dynamics (S1 Table). Table 2 summarizes the decline dynamics for each antibody for the 20 naturally infected subjects. The DF50-values for the first sample of each subject and the calculated half-lives are listed in S1 Table.
Fig 3. Antibody concentrations over time for 20 naturally infected subjects.
Table 2. Summary of the decline dynamics for 20 naturally infected subjects.
| Antibody | median DF50 (IQR) of the first sample | median half-life T50 in days (IQR) | T90 in days (IQR) |
|---|---|---|---|
| MP1 | 11 (6–19) | NE | NE |
| NP1 | 309 (149–536) | 66 (44–79) | 219 (147–264) |
| NP2 | 31 (10–52) | NE | NE |
| RBD | 935 (474–2240) | 120 (87–164) | 398 (289–545) |
| S1 | 461 (230–897) | 127 (91–153) | 420 (302–510) |
| S2 | 262 (93–518) | 187 (112–206) | 620 (374–684) |
Summary of the decline dynamics for each antibody for the 20 naturally infected subjects. DF50 < 50 of the first sample corresponds to no or little reactivity. NE = Not Estimable because of low reactivity.
In the vaccinated group, antibody concentrations also generally decline over time. The median DF50-values at the different time-points (T0, T1, T2 and T3) for the vaccinated individuals are presented in Table 3. However, among the 74 subjects who were vaccinated twice (Group 1, Table 3), we identified 6 subjects who showed antibody reactivity at baseline (T0). The characteristic times T50 and T90, obtained from the DF50-values at the two time-points T2 and T3 are summarized in Table 4. These characteristic times are estimated from 2 points only, assuming an exponential decay. As this might be prone to error, we also calculated the relative loss in DF50-reactivity between T2 and T3 (assuming a linear decay between these two time-points). Using RBD as the reference, there is a loss of about 90% in antibodies at time T3, which is 6 months after the second vaccine shot.
Table 3. Summary of the dynamics for each antibody for the 83 vaccinated subjects.
| Group | Antibody | T0 | T1 | T2 | T3 |
|---|---|---|---|---|---|
| 1 (n = 74) | RBD | ≤6 (≤6–8) | 261 (122–551) | 3123 (2225–7076) | 460 (277–735) |
| S1 | ≤6 (≤6 – ≤6) | 104 (53–205) | 1530 (873–2727) | 245 (140–453) | |
| S2 | ≤6 (≤6–12) | 32 (18–56) | 109 (51–193) | 29 (20–53) | |
| 2 (n = 9) | RBD | 148 (42–257) | - | 6448 (5650–10316) | 1473 (1264–716) |
| S1 | 60 (20–66) | - | 4773 (3384–5488) | 833 (716–865) | |
| S2 | 45 (27–62) | - | 1501 (936–1626) | 126 (74–164) | |
| 3 (n = 6) | RBD | 38 (22–67) | 3121 (144–5936) | 4018 (3339–8527) | 1211 (527–2910) |
| S1 | 15 (8–33) | 1802 (56–4317) | 2278 (1466–7109) | 708 (375–1890) | |
| S2 | 21 (12–42) | 388 [39–1283) | 327 (118–1028) | 108 (57–208) |
Median DF50-values (IQR) for RBD, S1 and S2 are presented at the different time-points T0 (baseline), T1 (3 weeks after first vaccine), T2 (1 month after second vaccine) and T3 (6 months after second vaccine) for group 1 (n = 74) vaccinated subjects who had no prior COVID infection, for group 2 (n = 9) vaccinated subjects who had a prior COVID infection and for group 3 (n = 6) vaccinated subjects who reported no prior COVID infection, but who showed antibodies reactivity at baseline (T0). The other antibodies (MP1, NP1 and NP2) were not reported as they were not triggered by the vaccines.
Table 4. Characteristic times for the waning antibodies.
| Group | Antibody | T50 in days (IQR) | T90 in days (IQR) | Estimated % relative loss between T2 and T3 |
|---|---|---|---|---|
| 1 (n = 74) | RBD | 50 (42–58) | 168 (140–193) | 87% (82–91) |
| S1 | 53 (45–68) | 177 (149–227) | 86% (77–90) | |
| S2 | 86 (59–123) | 287 (197–309) | 69% (56–81) | |
| 2 (n = 9) | RBD | 55 (48–61) | 182 (160–203) | 84% (76–86) |
| S1 | 49 (46–65) | 164 (153–214) | 84% (71–89) | |
| S2 | 37 (37–51) | 122 (121–168) | 92% (71–94) | |
| 3 (n = 6) | RBD | 84 (62–102) | 278 (205–340) | 71% (64%– 82%) |
| S1 | 82 (78–91) | 272 (258–302) | 72% (68%– 74%) | |
| S2 | 122 (94–155) | 404 (312–514) | 58% (49%– 67%) |
The half-life T50 (T90) equals the number of days at which there is 50% (10%) left from the estimated DF50 at time zero assuming an exponential decay. The estimated % relative loss is calculated as [DF50(T2)—DF50(T3)) / DF50(T2). We compared specific antibodies between groups 1 and 2 using a Wilcoxon rank sum test: RBD, p = 0.330; S1, p = 0.855: S2, p = 0.001.
When comparing the naturally infected and the vaccinated groups, we found that antibody concentration declines more slowly in the naturally infected group than in the vaccinated group (Tables 2 and 4). In addition, we compared median half-lives for the 20 naturally infected vs 74 vaccinated subjects (two doses) using a Wilcoxon rank sum test. Median half-lives were respectively 120 vs 50 days for RBD (p < 0.001), 127 vs 53 days for S1 (p < 0.001), and 187 vs 86 days for S2 (p < 0.001) antibodies.
The raw measurement data of both the naturally infected and vaccinated subjects are available in S3 File. This supplement also contains the results of several commercial assays that were used for clinical screening purposes.
Discussion
Quantitative measurement of humoral immune response provides an easy and robust surrogate marker of protection. If a natural infection or vaccination induces a sustained and protective immunity, or at least, a long-lasting protection, it may enable the establishment of herd immunity. The presence of antibodies is a key indicator of protective humoral immunity. Anti-RBD and anti-S-protein titers may be particularly important because they correlate with neutralizing activity and are typically associated with early virus control [13–16]. Therefore, the accurate determination of the duration of antibody presence is essential.
Waning antibodies operate in a stealth mode and may not be captured by conventional methods that collectively measure a global and consolidated immune response. Such waning can more easily be detected when monitoring each antibody individually. The novel multiparametric method presented in this study can serve as a tool to measure how individual antibodies wane over time. Serum samples from naturally infected and vaccinated subjects were investigated to examine the effectiveness of our method. We were able to determine the median antibody half-lives for each group (Tables 2 and 4).
Both our results (summarized in Table 2) and several other studies show that the humoral response to SARS-CoV-2 declines over time after natural infection. Zhang et al. reported a significant reduction of humoral response to SARS-CoV-2 within 4 months after diagnosis [17]. Studies of Dan et al. and Wu et al. have provided evidence that the circulating immune memory to SARS-CoV-2 appears to reduce over time, but endures for more than 5 months in patients with a previous infection [18, 19]. The observed antibody kinematics for naturally infected subjects in our study correspond well with those reported in literature. Indeed, despite the heterogeneity of immune responses, our limited data indicates sustained (although declining over time) humoral immunity in recovered patients who had symptomatic COVID-19.
More specifically, our estimated RBD half-life can be compared with values previously published in the literature. Dan et al. report that immunoglobulins (Ig)G targeting the SARS-CoV-2 spike protein were found to be relatively stable over time with a half-life of 140 days (95% CI: 20–240 days). Subjects in this study showed mostly mild symptoms [18]. Another study by Kannenberg et al. found an RBD half-life of 158 days (95% CI: 141–181 days) for patients after severe acute respiratory syndrome [20]. Both values are similar to the median RBD half-life found in our study (120 days, IQR: 87–164 days).
It should be noted that the 20 naturally infected patients in our study showed mild to moderate symptoms. Patients who experience high viral replication during COVID-19 infections, typically demonstrate more severe clinical outcomes and show high levels of humoral immunity [21]. However, in this case, our RBD half-lives appear similar to the ones reported by Kannenberg et al. for severe acute respiratory syndrome [20].
In the vaccinated group, we found that our results (summarized in Table 4) correspond well with published studies. Achiron et al. report an estimated half-life of 45 days for the S1 antibody [22]. Doria-Rose estimated a RBD half-life of 52 days (95% CI: 46–58 days) from a cohort vaccinated twice with the mRNA1273 (Spikevax) vaccine [23]. Both these half-life values are comparably close to our estimate of 53 days.
Our results show that the initial humoral response is stronger in the vaccinated group, but declines much faster, than in the naturally infected group (Tables 2 and 4). Half-lives were significantly shorter for every antibody. For example, the RBD median half-life was 50 days in the group that received 2 vaccinations versus 120 days for the naturally infected group. This faster antibody titer decline in vaccinated groups versus naturally infected groups, corresponds with results previously published in literature. For example, larger declines in antibody titers have previously been observed in subjects vaccinated with the Pfizer BioNtech vaccine versus naturally infected subjects [24].
Our results indicate that the Multi-SARS-CoV-2 assay can distinguish the humoral response to natural infections from the response to vaccination. In vaccinated subjects, antibodies targeting MP1, NP1 and NP2 were typically absent, while this is not the case for the naturally infected group. This is in line with the nature of the BNT162b2/Comirnaty vaccine because it is solely based on the spike protein antigens [25].
A potential future area of study is to investigate the role of the individual antibodies measured in the Multi-SARS-CoV-2 assay. While anti-RBD and anti-S antibodies may be related to neutralization activity, the precise role of the membrane protein antigen (MP1) and the nucleocapsid recombinant protein antigens (NP1 and NP2) still need to be determined. Our analyses on the naturally infected group showed that the estimated half-life of the nucleocapsid recombinant protein antigen NP1 was only about half of the estimated half-life of RBD, S1 and S2. In vaccinated subjects who had a prior COVID infection, baseline values of NP1 and NP2 were similar (that is, quite low) to those of the baseline values of subjects who did not declare a prior COVID infection. This suggests that these antibodies disappear much faster after infection than the other antibodies.
Conclusions
The correspondence in antibody kinematics of our results and the data found in the literature, both for naturally infected and vaccinated subjects, supports the validity of our newly presented method. The method, based on a series of a limited number of dilutions, can convert a conventional assay from qualitative testing into a quantitative assay. This enables the quantification of the time decay of antibody response as well as the calculation of the half-life for these antibodies. This new procedure provides information on the sustainability of immune response, helping us to understand and estimate the duration of humoral immunity, after infection or vaccination.
Supporting information
Dilution Factors corresponding to 50% reactivity for the first sample is presented for the 6 different antibodies. First and Last sample* indicates the time lapse in days since first symptoms of infection. N = number of samples for each subject. “≤ 6” means that the DF50-value was estimated below limit of quantification. Half-lives are obtained from the linear regression model of ln(DF50) against time in case the model returned a negative slope. Half-life greater than last sample time or when the model returned zero or positive slopes were annotated as > last sample day. Half-lives were indicated as Non-Estimable (NE) when slopes could not be estimated due to low first sample DF50 values (≤50).
(DOCX)
(DOCX)
(DOCX)
Excel database containing pixel intensities for 424 samples, 6 markers and several dilutions. All the results obtained from commercial assays (VIDAS, DIASORIN, SIEMENS AND WANTAI) correspond to a working dilution as recommended by the corresponding manufacturer. The dilution sequence method has been performed only for the Multi-SARS-CoV2 kits.
(XLSX)
Acknowledgments
The Covid-Ser study group composed of Kahina Saker, Christelle Compagnon, Bouchra Mokdad, Virginie Pitiot, Cecile Barnel, Vanessa Escuret, Florence Morfin, Mary-Anne Trabaud, Laurence Josset, Dulce Alfaiate, Jean-Baptiste Fassier, Alexandre Gaymard, Grégory Destras, Nicolas Guibert, Hélène Lozano and Amélie Massardier Pilonchery Collected the samples and performed experiments.
Group leader and contact: Sophie Trouillet-Assant, email: sophie.assant@chu-lyon.fr
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382: 727–733. doi: 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Organization WH. WHO Director-General’s opening remarks at the media briefing on COVID-19–11 March 2020. In: WHO Director General’s speeches [Internet]. 2020. [cited 3 Jun 2022]. Available: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19—11-march-2020 [Google Scholar]
- 3.Nicola M, Alsafi Z, Sohrabi C, Kerwan A, Al-Jabir A, Iosifidis C, et al. The socio-economic implications of the coronavirus pandemic (COVID-19): A review. Int J Surg. 2020;78: 185–193. doi: 10.1016/j.ijsu.2020.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perreault J, Tremblay T, Fournier MJ, Drouin M, Beaudoin-Bussières G, Prévost J, et al. Waning of SARS-CoV-2 RBD antibodies in longitudinal convalescent plasma samples within 4 months after symptom onset. Blood. American Society of Hematology; 2020. pp. 2588–2591. doi: 10.1182/blood.2020008367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wajnberg A, Amanat F, Firpo A, Altman DR, Bailey MJ, Mansour M, et al. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science (80-). 2020;370: 1227–1230. doi: 10.1126/science.abd7728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choe PG, Kim KH, Kang CK, Suh HJ, Kang EK, Lee SY, et al. Antibody responses 8 months after asymptomatic or mild SARS-CoV-2 infection. Emerg Infect Dis. 2021;27: 928–931. doi: 10.3201/eid2703.204543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khoury J, Najjar-Debbiny R, Hanna A, Jabbour A, Abu Ahmad Y, Saffuri A, et al. COVID-19 vaccine–Long term immune decline and breakthrough infections. Vaccine. 2021;39: 6984–6989. doi: 10.1016/j.vaccine.2021.10.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Long QX, Tang XJ, Shi QL, Li Q, Deng HJ, Yuan J, et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med. 2020;26: 1200–1204. doi: 10.1038/s41591-020-0965-6 [DOI] [PubMed] [Google Scholar]
- 9.Ibarrondo FJ, Fulcher JA, Goodman-Meza D, Elliott J, Hofmann C, Hausner MA, et al. Rapid Decay of Anti–SARS-CoV-2 Antibodies in Persons with Mild Covid-19. N Engl J Med. 2020;383: 1085–1087. doi: 10.1056/NEJMc2025179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu L, To KKW, Chan KH, Wong YC, Zhou R, Kwan KY, et al. High neutralizing antibody titer in intensive care unit patients with COVID-19. Emerg Microbes Infect. 2020; 1664–1670. doi: 10.1080/22221751.2020.1791738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prévost J, Gasser R, Beaudoin-Bussières G, Richard J, Duerr R, Laumaea A, et al. Cross-Sectional Evaluation of Humoral Responses against SARS-CoV-2 Spike. Cell Reports Med. 2020;1: 100126. doi: 10.1016/j.xcrm.2020.100126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bates D, Mächler M, Bolker BM, Walker SC. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67. doi: 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- 13.Yang J, Wang W, Chen Z, Lu S, Yang F, Bi Z, et al. A vaccine targeting the RBD of the S protein of SARS-CoV-2 induces protective immunity. Nat 2020 5867830. 2020;586: 572–577. doi: 10.1038/s41586-020-2599-8 [DOI] [PubMed] [Google Scholar]
- 14.Salazar E, Kuchipudi S V., Christensen PA, Eagar T, Yi X, Zhao P, et al. Convalescent plasma anti–SARS-CoV-2 spike protein ectodomain and receptor-binding domain IgG correlate with virus neutralization. J Clin Invest. 2020;130: 6728–6738. doi: 10.1172/JCI141206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Irsara C, Egger AE, Prokop W, Nairz M, Loacker L, Sahanic S, et al. Clinical validation of the Siemens quantitative SARS-CoV-2 spike IgG assay (sCOVG) reveals improved sensitivity and a good correlation with virus neutralization titers. Clin Chem Lab Med. 2021;59: 1453–1462. doi: 10.1515/cclm-2021-0214 [DOI] [PubMed] [Google Scholar]
- 16.Salazar E, Kuchipudi S V., Christensen PA, Eagar TN, Yi X, Zhao P, et al. Relationship between Anti-Spike Protein Antibody Titers and SARS-CoV-2 In Vitro Virus Neutralization in Convalescent Plasma. bioRxiv. 2020; 2020.06.08.138990. doi: 10.1101/2020.06.08.138990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang K, Ma ZG, Yang L, Kang W, Yin Y, Lau JYN. Significant reduction of humoral response to SARS-CoV-2 4 months after the diagnosis of COVID-19. Precision Clinical Medicine. Oxford University Press; 2021. pp. 73–76. doi: 10.1093/pcmedi/pbaa036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dan JM, Mateus J, Kato Y, Hastie KM, Yu ED, Faliti CE, et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science (80-). 2021;371. doi: 10.1126/science.abf4063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu J, Liang B, Chen C, Wang H, Fang Y, Shen S, et al. SARS-CoV-2 infection induces sustained humoral immune responses in convalescent patients following symptomatic COVID-19. Nat Commun. 2021;12: 1–9. doi: 10.1038/s41467-021-22034-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kannenberg J, Trawinski H, Henschler R, Buhmann R, Hönemann M, Jassoy C. Antibody Course and Memory B-Cell Response in the First Year After Severe Acute Respiratory Syndrome Coronavirus 2 Infection. J Infect Dis. 2022;XX: 1–9. doi: 10.1093/infdis/jiac034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seow J, Graham C, Merrick B, Acors S, Pickering S, Steel KJA, et al. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nat Microbiol. 2020;5: 1598–1607. doi: 10.1038/s41564-020-00813-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Achiron A, Mandel M, Dreyer-Alster S, Harari G, Gurevich M. Humoral SARS-COV-2 IgG decay within 6 months in COVID-19 healthy vaccinees: The need for a booster vaccine dose? European Journal of Internal Medicine. Elsevier; 2021. pp. 105–107. doi: 10.1016/j.ejim.2021.10.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doria-Rose N, Suthar MS, Makowski M, O’Connell S, McDermott AB, Flach B, et al. Antibody Persistence through 6 Months after the Second Dose of mRNA-1273 Vaccine for Covid-19. N Engl J Med. 2021;384: 2259–2261. doi: 10.1056/NEJMc2103916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Israel A, Shenhar Y, Green I, Merzon E, Golan‐cohen A, Schäffer AA, et al. Large‐Scale Study of Antibody Titer Decay Following BNT162b2 mRNA Vaccine or SARS‐CoV‐2 Infection. Vaccines. 2022;10: 64. doi: 10.3390/vaccines10010064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walsh EE, Frenck RW, Falsey AR, Kitchin N, Absalon J, Gurtman A, et al. Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates. N Engl J Med. 2020;383: 2439–2450. doi: 10.1056/NEJMoa2027906 [DOI] [PMC free article] [PubMed] [Google Scholar]