Abstract
Purpose:
Children with single-sided deafness (SSD) experience difficulty understanding speech in multisource listening situations. Case reports and retrospective studies have indicated that a cochlear implant (CI) may improve masked speech recognition in children with SSD. This prospective study was conducted to determine whether providing a CI to children with SSD supports spatial release from masking (SRM), an improvement in speech recognition associated with separating the target and masker sources.
Method:
Twenty children with at least a moderate-to-profound hearing loss in one ear and normal hearing in the contralateral ear underwent cochlear implantation. The average age of implantation was 5.5 years (range: 3.5–12.7). After 12 months of CI use, subjects completed a sentence recognition task in multitalker masker with and without the CI. The target was presented from the front, and the masker was either colocated with the target (0°) or from the side (+90° or −90°). A two-way repeated-measures analysis of variance was completed to investigate SRM with and without the CI.
Results:
Pediatric CI recipients experienced significant SRM when the masker was directed to the normal-hearing ear or to the affected ear.
Conclusions
The results indicate that cochlear implantation in children with SSD supports binaural skills required for speech recognition in noise. These results are consistent with improved functional communication in multisource environments, like classrooms.
Children with single-sided deafness (SSD) have normal hearing in one ear and contralateral hearing loss that is too severe to be fit with traditional amplification. One of the primary obstacles faced by children with SSD is spatial hearing, particularly with respect to listening in multisource environments. Children with SSD require greater signal-to-noise ratios to recognize masked speech than children with normal hearing in both ears (Griffin et al., 2019; Lieu et al., 2013; Reeder et al., 2015; Ruscetta et al., 2005). These effects are even more pronounced when the target and masker are spatially separated and the masker is itself composed of speech (Corbin et al., 2017; Johnstone & Litovsky, 2006; Litovsky, 2005). These effects could negatively impact access to spoken communication for children with SSD in multisource listening environments, such as a classroom.
Spatial hearing relies in part on integration of inputs from the two ears. When a sound source is offset to the left or right of the listener, the head creates a physical barrier and the level of the sound is reduced at the ear farthest from the source via head shadow, causing an interaural level difference (ILD). Similarly, sound takes longer to arrive at the ear farthest from the sound source, introducing an interaural time difference (ITD). Use of ITD cues is restricted to predominantly low frequencies, due to limits of temporal resolution, and ILDs are larger at high frequencies (Blauert, 1997). The brain begins interpreting ILDs and ITDs at the level of the brainstem (Akeroyd, 2006). Binaural difference cues are integral for spatial hearing, such as sound source localization and masked speech recognition. Listeners with SSD do not have access to interaural differences cues of either type, due to the lack of binaural input.
Masked speech recognition is typically better when the target and masker are spatially separated on the horizontal plane compared to when they are colocated (Bronkhorst & Plomp, 1988). This benefit is described as spatial release from masking (SRM). While speech recognition in the presence of a competing masker in general is impacted by age and development (Buss et al., 2019; Garadat & Litovsky, 2007; Johnstone & Litovsky, 2006; Leibold & Buss, 2019; Schafer et al., 2012; Van Deun et al., 2010), data on the development of SRM are conflicting. Some data suggest that SRM continues to increase throughout childhood (Vaillancourt et al., 2008; Van Deun et al., 2010; Yuen & Yuan, 2014), and others indicate mature performance early in childhood and a positive SRM in children as young as 2–4 years old (Garadat & Litovsky, 2007; Hess et al., 2018; Litovsky, 2005; Lovett et al., 2012). SRM is not observed in children with SSD when the masker is offset to the normal-hearing ear (Reeder et al., 2015), a condition for which the listener cannot benefit from head shadow.
Current treatment options for children with SSD include technology that reroutes the signal from the affected ear to the normal-hearing ear using traditional Contralateral Routing of the Signal (CROS) hearing aid systems or via bone-conduction systems (e.g., Baha or Ponto Softbands). While these technologies can be beneficial under some listening conditions, they can also be detrimental. For instance, the earpiece at the normal-hearing ear may lead to occlusion, reducing access to sound from that side (Bagatto et al., 2019). In cases of SSD, rerouting the signal can cause poorer speech recognition when masking is presented to the poorer ear with these devices (Bagatto et al., 2019; Lin et al., 2006; Updike, 1994), although more recent work has suggested that this effect may not be as pronounced among older children listening in diffuse noise (Picou, Davis, et al., 2020; Picou, Lewis, et al., 2020). As young children are not able to adapt their own technology or adjust their listening environments to avoid situations where the use of a CROS device may be detrimental, these devices are generally not recommended for children (Bagatto et al., 2019; McKay et al., 2008).
Cochlear implantation may support better spatial hearing for patients with SSD. A cochlear implant (CI) could provide binaural input by stimulating the affected auditory pathway as opposed to monaural stimulation when unaided or listening with CROS or bone-conduction devices. One factor that could affect performance of CI users with SSD is whether the listener can integrate acoustic information from the normal-hearing ear with electric stimulation from the CI for improved spatial hearing. Previous studies have shown that pediatric CI recipients can integrate and benefit from acoustic and electric signals presented ipsilaterally via electric–acoustic stimulation (Gladden et al., 2015; Park et al., 2019; Skarzynski & Lorens, 2009; Wolfe et al., 2017) and contralaterally via bimodal listening (Carlson et al., 2015; Davidson et al., 2019; Dhondt et al., 2018; King et al., 2020; Park et al., 2012; Polonenko et al., 2017, 2018, 2019). It is unknown whether children with SSD also experience improved speech recognition when listening with a CI plus their normal-hearing ear.
When compared to alternative technologies or an unaided condition, adult CI recipients with SSD recognize speech in spatially separated noise with similar or greater accuracy when the CI is on as compared to off (Arndt et al., 2011; Buss et al., 2018; Firszt et al., 2012). The benefits of a CI in this population have most often been observed when the target is from the front and the masker is positioned on the side of the normal-hearing ear (Buss et al., 2018; Friedmann et al., 2016; Grossmann et al., 2016; Mertens et al., 2017; Plant & Babic, 2016; Tavora-Vieira et al., 2013). Some studies have also found benefit when a masker is presented to the side of the CI (Friedmann et al., 2016; Grossmann et al., 2016), but most often, there is no significant difference noted with or without the CI under this condition (Buss et al., 2018; Mertens et al., 2017).
While studies investigating CI use in adults with SSD have been largely positive, these results may not directly translate to children. Congenital deafness or hearing loss early in life could impact the ability to integrate binaural signals. However, the literature currently available in children receiving CIs for unilateral hearing loss, consisting mostly of retrospective reviews or case studies, indicates that outcomes similar to adults may be possible (Deep et al., 2020; Friedmann et al., 2016; Hassepass et al., 2012; Plontke et al., 2013; Sladen et al., 2017; Tavora-Vieira & Rajan, 2015; Zeitler et al., 2019). Hassepass et al. (2012) were among the first to describe benefits of CI use for children with SSD in a small case study (n = 2). The children showed improvement after 12 months of CI listening experience on a speech recognition task in spatially separated noise. In contrast, Sladen et al. (2017) did not find significant improvements in speech-in-noise recognition in their multicenter trial with six pediatric CI recipients. Significant effects may not have been observed since the sample had wide variability in both age at implantation (5–15 years) and duration of hearing loss (1–9.5 years). Also, speech recognition was assessed at 6 months postactivation, when other studies have reported improvement after 12 months of CI use (Mertens et al., 2017). In addition, the target and masker used by Sladen et al. were colocated in front of the subjects, so that protocol did not evaluate spatial hearing where benefit would be more likely. More recently, Deep et al. (2020) reviewed the performance of five children with SSD and a CI on a speech recognition in spatially separated noise task, and described their results as indicating that subjects performed the same or better with the CI on versus off. Ceiling effects were observed in that study, however, limiting interpretation.
The present preliminary investigation assessed the SRM of children with SSD 12 months after CI activation. We hypothesized that children with SSD would experience SRM when the masker was presented to the CI or the normal-hearing ear when the device was on. When the device was off, we hypothesized that subjects would experience SRM when the masker was presented to the affected ear, due to head shadow, but not when the masker was presented to the normal-hearing ear.
Method
Study Design
Subjects were evaluated as part of a prospective clinical trial assessing the effectiveness of CI use in pediatric recipients with SSD. The study procedures were approved by the institutional review board at the University of North Carolina at Chapel Hill and received an Investigational Device Exemption from the Food and Drug Administration. A within-subject design was chosen, with each subject serving as his or her own control.
Subjects
Twenty subjects met the inclusion criteria, underwent cochlear implantation, and completed the 12-month study interval at the time of data analysis. Demographic information is listed in Table 1. Subjects presented with a normal pure-tone average (PTA; 500, 1000, and 2000 Hz) in one ear (mean PTA = 9.6 dB HL, SD = 6.3) and at least a moderate-to-profound sensorineural hearing loss in the affected ear (mean PTA = 108.1 dB, SD = 14.9). Potential subjects were between 3.5 and 6.5 years of age at the time of implantation. There were two exceptions to this criterion: Two subjects were older than 6.5 years (7.0 and 12.7 years), but both had short durations of deafness (2.3 years each). The mean age at implantation was 5.5 years (SD = 2.0 years). Estimates of duration of deafness were based on both the medical record and parent report. The mean length of unilateral deafness at the time of initial CI activation was estimated at 3.3 years (SD = 1.7). Etiologies were largely unknown (n = 12). All subjects had typical cognition based on testing with the Leiter-R Brief IQ subscale (Roid & Miller, 1997). Potential subjects were excluded if there was evidence of cochlear nerve deficiency, ossification, a cochlear malformation more significant than an incomplete partition Type II (IP-II; Sennaroğlu & Bajin, 2017), or an inability to complete the study protocol.
Table 1.
Participant demographics.
| Subject | Age at surgery (years) | Length of deafness (years) | Age at HA fit (years) | Age at Dx (years) | Hearing loss onset | NBHS result | Etiology | Affected ear | Pre-op PTA CI (dB HL) | Pre-op PTA Contra (dB HL) |
|---|---|---|---|---|---|---|---|---|---|---|
| 01 | 6.4 | 1.9 | 4.0 | 4.0 | Known sudden | pass | Infection | Right | 112 | 2 |
| 02 | 6.3 | 1.4 | Never aided | 5.0 | Reported sudden | pass | Unknown | Right | 88 | 15 |
| 03 | 4.5 | 1.4 | Never aided | 3.0 | Known sudden | pass | Trauma | Right | 120 | 3 |
| 04 | 6.1 | 6.1 | 2.0 | 2.0 | Suspected congenital | fail, pass on rescreen | Malformation | Left | 85 | 20 |
| 05 | 4.7 | 4.7 | Never aided | 0.2 | Known congenital | failed unilateral | Waardenburg | Right | 118 | 20 |
| 06 | 12.7 | 2.3 | Never aided | 10.0 | Reported sudden | pass | Unknown | Left | 97 | 8 |
| 07 | 4.0 | 4.0 | 1.8 | 0.7 | Known congenital | failed unilateral | Malformation | Right | 82 | 10 |
| 08 | 6.5 | 4.6 | Never aided | 3.0 | Progressive suspected | pass | Unknown | Right | 120 | 7 |
| 09 | 6.5 | 6.5 | Never aided | 6.0 | Suspected congenital | fail, pass on rescreen | Unknown | Left | 110 | 8 |
| 10 | 7.1 | 2.3 | Never aided | 4.0 | Reported sudden | pass | cCMV | Left | 95 | 7 |
| 11 | 6.1 | 3.5 | Never aided | 5.0 | Progressive suspected | pass | Unknown | Right | 120 | 2 |
| 12 | 3.9 | 1.8 | Never aided | 0.2 | Known progressive | failed unilateral | cCMV | Left | 113 | 22 |
| 13 | 4.8 | 4.2 | Never aided | 2.0 | Known progressive | failed unilateral | Unknown | Right | 120 | 8 |
| 14 | 5.4 | 1.3 | Never aided | 4.0 | Reported sudden | pass | Unknown | Left | 118 | 2 |
| 15 | 5.5 | 0.8 | 4.0 | 3.0 | Known progressive | Was not tested | Unknown | Left | 77 | 8 |
| 16 | 3.7 | 3.8 | Never aided | 2.0 | Suspected congenital | fail, pass on rescreen | Unknown | Right | 120 | 8 |
| 17 | 5.4 | 5.4 | Never aided | 0.1 | Known congenital | failed unilateral | Unknown | Left | 117 | 8 |
| 18 | 3.5 | 3.6 | 0.2 | 0.2 | Known congenital | failed unilateral | Unknown | Left | 120 | 15 |
| 19 | 3.9 | 3.9 | Never aided | 3.0 | Suspected congenital | fail, pass on rescreen | Unknown | Left | 110 | 15 |
| 20 | 3.6 | 3.6 | 3.0 | 0.1 | Known congenital | failed unilateral | cCMV | Right | 120 | 3 |
Note. HA = hearing aid; Dx = diagnosis; NBHS = newborn hearing screen; CI = cochlear implant; PTA = pure-tone average (500, 1000, and 2000 Hz); cCMV = congenital cytomegalovirus.
Devices and Mapping
All but one of the subjects received a MED-EL SYNCHRONY device with a FLEX28 electrode array. One subject with an IP-II malformation received a FLEX24 array, with a goal of providing full coverage of the cochlea and a maximal number of stimulation sites. A full insertion of the array was achieved in all cases apart from one FLEX28 recipient with an IP-II malformation who had two extracochlear electrode contacts.
All subjects were fit with the SONNET processor approximately 2 weeks postoperatively. The processors were programmed with omnidirectional microphone settings and filter frequencies of 100–8500 Hz. Most comfortable levels (MCLs) were set to “comfortable but loud” using behavioral methods and/or electrical stapedial reflex thresholds (ESRTs), depending on subject reliability. All subjects were able to scale MCLs reliably by the 12-month interval. Four of the youngest subjects were mapped primarily using ESRTs at the initial intervals. ESRTs were used to confirm behaviorally measured MCLs for an additional 10 subjects. For those who were able (n = 14), MCL levels were loudness balanced with the normal-hearing ear. Thresholds levels in the CI map were set below the subject's behavioral threshold.
Habilitation
Subjects received auditory-based therapy from speech-language pathologists with Listening and Spoken Language Specialist certification. Therapy occurred every 2 weeks for the first 6 months postactivation and was reduced to monthly for the following 6 months. Input was provided to the subject's processor by a computer via a direct connect cable. Sessions were offered in person or virtually. When therapy was conducted in the clinic, the therapist would sit in a separate room from the subject to ensure the subject only heard the therapist via the computer stream.
Masked Speech Recognition
Masked speech recognition was assessed at the 12-month interval in a single-walled sound-attenuating booth. Speech stimuli were Bamford-Kowal-Bench Speech-in-Noise test (Bench et al., 1979) sentences, presented using a laptop computer (Dell Latitude) and an audiometer (Grason-Stadler GSI-61). The target sentence was presented at 60 dBA, and the level of the 4-talker masker increased in 3-dB steps, decreasing the signal-to-noise ratio (SNR) from +21 to −6 dB SNR. Subjects were tested with their device on and off in three spatial conditions: (a) target and masker colocated at 0° azimuth, (b) target in front and masker separated by 90° on the side of the normal-hearing ear, and (c) target in front and masker separated by 90° on the side of the implanted ear. An SNR-50, the SNR required to obtain 50% correct, was computed for each of the six test conditions. One list pair was used for each condition, for a total of 20 sentences per condition. Lists and test order were randomized using a computer-generated random number, so that no test lists were repeated. For younger children and those who had difficulty focusing, a test assistant sat inside the booth to help keep the subject engaged, focused, and facing the front speaker. Testing took approximately 30 min, and breaks were provided when needed.
Statistical Analysis
Statistics were carried out using IBM SPSS Statistics Version 26. A two-way repeated-measures analysis of variance was completed to determine the effect of device in the presence of a spatially separated masker. The dependent variable was SNR-50, and independent variables were listening condition (device on vs. off) and spatial condition. Spatial condition included three levels; masker colocated with the target (in front of the subject), masker to the normal-hearing ear, and masker to the implanted ear. Post hoc testing was carried out using two-tailed t tests with Bonferroni adjustments for multiple comparisons.
Results
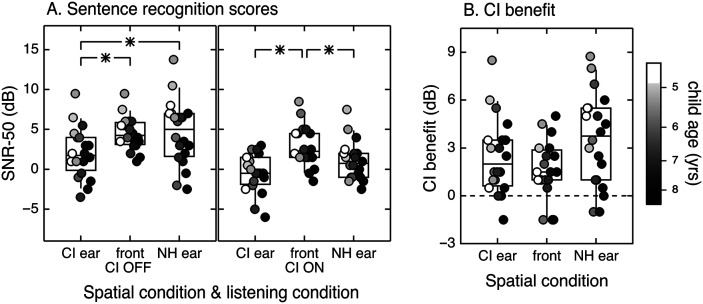
The distributions of scores are shown in Figure 1 for each condition. The two-way repeated-measures analysis of variance indicated statistically significant effects of listening condition, F(1, 19) = 37.80, p < .001, and spatial condition, F(2, 38) = 15.70, p < .001, and a significant interaction between the two variables, F(2, 38) = 5.28, p = .01. Simple main effects were analyzed post hoc.
Figure 1.
Spatial release from masking. Distribution of SNR-50 plotted by spatial and listening conditions (A) and CI benefit in decibels plotted by spatial condition (B). Testing was completed with the device on and the device off, for three spatial configurations. Individual data are indicated with circles, and fill reflects age at testing, as defined in the legend. The center horizontal lines indicate the median, boxes span the 25th and 75th percentiles, and whiskers span the 10th and 90th percentiles. Significant differences are bracketed and indicated with an asterisk. CI = cochlear implant; NH = normal hearing; SNR = signal-to-noise ratio; yrs = years.
Simple main effects of listening condition in each spatial condition indicated that SNR-50 scores were significantly lower when the device was on compared to off in ear spatial condition. The mean benefit of the CI was 1.6 dB when the target and masker were colocated (95% CI [0.75, 2.45], p = .001), 2.5 dB when the masker was on the side of implanted ear (95% CI [1.36, 3.60], p < .001), and 3.5 dB when the masker was on the side of normal-hearing ear (95% CI [2.19, 4.89], p < .001). These scores indicate that the use of a CI was beneficial in diffuse noise conditions, did not introduce interference, and may have promoted true binaural hearing.
Simple main effects of spatial condition with the device on versus off were also analyzed. Descriptive statistics are presented in Table 2. Recall that SRM is defined as the difference in scores for colocated versus spatially separated conditions. As predicted, SRM was significantly greater than zero when the device was off and the masker was on the side of the implanted ear (M = 2.5 dB, 95% CI [1.27, 3.64], p < .001), but not when it was on the side of the normal-hearing ear (M = −0.09 dB, 95% CI [−1.90, 1.73], p = 1.0). With the device off, performance was significantly better when the masker was offset to the side of the implanted ear than the normal-hearing ear (M = 2.5 dB, 95% CI [.70, 4.38], p = .006). With the device on, SRM was significantly greater than zero when the masker was on the side of the implanted ear (M = 3.3 dB, 95% CI [1.89, 4.76], p < .001) and when it was on the side of the normal-hearing ear (M = 1.9 dB, 95% CI [0.25, 3.46], p = .021). Performance with the device on was not significantly different when the masker was offset to the side of the implanted ear or the normal-hearing ear (M = 1.5 dB, 95% CI [−0.10, 3.05], p = .072). These results support the initial prediction that SRM would be observed with the device on or off when the masker was on the side of the implanted ear, but that SRM with the masker on the side of the normal-hearing ear would only be observed when listening with the CI.
Table 2.
Signal-to-noise ratio-50 descriptive statistics.
| Statistic | Device on |
Device off |
Device benefit |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Implanted ear | Front | NH ear | Implanted ear | Front | NH ear | Implanted ear | Front | NH ear | |
| M (dB) | −0.4 | 2.9 | 1.1 | 2.1 | 4.5 | 4.6 | 2.5 | 1.6 | 3.5 |
| SD (dB) | 2.4 | 2.5 | 2.4 | 3.1 | 2.0 | 4.1 | 2.4 | 1.8 | 2.9 |
| Range (dB) | −6.0 to 3.0 | −1.5 to 8.5 | −2.5 to 7.5 | −3.5 to 9.5 | 1.0 to 9.5 | −2.5 to 13.8 | −1.5 to 8.5 | −1.5 to 5.0 | −1.0 to 8.8 |
| 95% CI | — | — | — | — | — | — | 1.36 to 3.60 | 0.75 to 2.45 | 2.19 to 4.89 |
Note. Mean, range, and standard deviations of signal-to-noise ratio 50 scores obtained with the Bamford-Kowal-Bench Speech-in-Noise test. The target was presented from the front at 0° azimuth. Results are described by masker location with the device on and off, as well as device benefit. NH = normal hearing; CI = confidence interval.
While results were significant at the group level, significant differences are not uniformly observed for individual subjects. This could be due to the relatively large confidence intervals for data obtained with only one list pair, especially for young subjects (Bench et al., 1979). The data in the current experiment were collected in a controlled environment with test assistants and breaks provided when needed to help increase reliability. Using the age-based 80% confidence intervals reported in the test manual (Etymotic Research, 2005), seven subjects had significant differences between the colocated and masker on the implanted side conditions and six between the colocated and masker on the normal-hearing side conditions. In device on versus off comparisons, seven subjects exhibited significance with the masker on the implanted side, four when the masker and target were colocated, and 10 when the masker was at the normal-hearing ear. Most importantly, none of the subjects in the current study had a reduction in performance with the device on that would be considered significant using 80% clinical confidence intervals, indicating that there were no significantly negative effects of CI use in this cohort. Individual benefit is illustrated in Figure 1B. Benefits in device use of 1.6–3.5 dB may convey advantages clinically. Adult and pediatric studies have indicated that the psychometric function for the Bamford-Kowal-Bench Speech-in-Noise test is rather steep. A 2 dB SNR advantage for an individual with an SNR-50 of 4–5 dB would result in a 15–percentage point increase in key words correct (Neuman et al., 2010; Wilson et al., 2007).
As some studies have suggested a developmental component to SRM, data were also evaluated with respect to age. At the 12-month interval, subjects were relatively uniformly distributed with respect to age from 4.5 to 8.1 years of age with the exception of the oldest child, who was 13.7 years old. Correlations between scores and age therefore omitted the oldest subject to guard against disproportionate effects associated with this outlier. There was a significant negative correlation between age and SNR-50 scores in the baseline colocated condition for both the device on (r = −.54, p = .017) and device off (r = −.61, p = .005); in both cases, scores improved by 1.1–1.2 dB per year over the range evaluated. Based on data from normal-hearing subjects (Vaillancourt et al., 2008; Van Deun et al., 2010; Yuen & Yuan, 2014), we might expect a correlation between age and SRM; however, a significant correlation was not observed for any of the four spatially separated conditions (r = −.45 to .35).
Discussion
The results of this study indicate that a CI can improve SRM in children with SSD when a masker is moved from a colocated position in front of the subject to either the side of the normal-hearing ear or to the side of the implanted ear. These findings are similar to results of studies in adults with SSD where a CI was found to improve masked speech recognition when the target was in front and the masker was directed to the normal-hearing ear (Buss et al., 2018; Friedmann et al., 2016; Grossmann et al., 2016; Mertens et al., 2017; Plant & Babic, 2016; Tavora-Vieira et al., 2013). This study and some adult studies have also found improvement when a masker is directed to the side of the CI (Friedmann et al., 2016; Grossmann et al., 2016), but this effect is not consistently observed (Buss et al., 2018; Mertens et al., 2017). It could be that greater neural plasticity in children supports more efficient use of spatial hearing cues than in adults with SSD, but further research is needed to investigate these effects.
The children in this study experienced SRM when the masker was offset on the side of the implanted ear even when their device was off, presumably because the head–shadow effect created a better target-to-masker ratio at the ear with normal hearing. This result is consistent with the findings of Reeder et al. (2015) who compared masked speech recognition for children with SSD to that of children with normal hearing bilaterally. In that data set, moving the masker to the side of the affected ear for the children with SSD produced comparable SRM as observed in children with normal hearing bilaterally.
While this is the first prospective study to look specifically at SRM and the use of a CI in children with SSD, results are consistent with published case studies and retrospective reports that included testing with a masker at either ear and the target in front, which tend to indicate a benefit of using a CI. For example, Plontke et al. (2013) presented a case study of a child with a temporal bone fracture who received a CI. This subject experienced improvement in speech recognition associated with CI use when the masker was presented to either ear. Tavora-Vieira and Rajan (2015) noted similar benefits in a child with a short duration of deafness. While the data of Deep et al. (2020) were limited by ceiling effects, those data support the conclusion that there is no significant decrement in performance with CI use. Friedmann et al. (2016) demonstrated varied outcomes in their case series; however, they noted that CI use resulted in similar or better performance when compared to conditions when the CI was off.
The present results support the effectiveness of CI use for improved SRM in children with SSD; however, there are limitations to consider. Unfortunately, the present results cannot be directly compared to those of children with SSD who listen with CROS or bone-conduction technologies due to sparsity of published data regarding SRM with these devices. What is known is that individuals using CROS and bone-conduction technologies experience poorer speech recognition with the device on versus off when there is a masker directed to the affected ear. This has been observed in children in a classroom environment (Updike, 1994) and in adults in a laboratory environment (Lin et al., 2006). This disadvantage has led to recommendations against CROS and bone-conduction devices for young children with SSD, including the age group in this study (Bagatto et al., 2019; McKay et al., 2008). This study subjects experienced better speech recognition with the CI was on versus off in all conditions, including when a masker was directed to the affected ear. Another limitation of this study is that all subjects were recipients of a MED-EL device, and results may not generalize to other devices. It is possible that subjects experienced improved performance with binaural input due to a close alignment between the default frequency filters and the cochlear place frequency as a function of the length of the electrode array (Hochmair et al., 2015). This could limit interaural frequency mismatches that have been shown to negatively influence binaural abilities in other CI recipient cohorts (Kan et al., 2019, 2013; Svirsky et al., 2015). Ongoing work is assessing the influence of frequency-to-place mismatch on binaural abilities in this patient population. Finally, all children received aural habilitation from Listening and Spoken Language Specialist speech-language pathologists, which may have positively influenced outcomes with the CI. Future research is needed regarding effective habilitation methods for children with SSD who undergo cochlear implantation.
Subjects in the present report were part of a prospective, repeated-measures clinical trial assessing the effectiveness of CI use in pediatric cases of SSD on multiple tasks, including speech recognition, spatial hearing, and subjective benefit. With the recent expansion of indications for cochlear implantation to pediatric cases of SSD, there is a need to understand performance outcomes on these tasks and how to implement revised test procedures clinically. Future work will be needed to investigate results in children implanted under 3.5 years of age, which was the minimum age criterion in this study. The present findings support the practice of cochlear implantation in children with SSD to promote binaural hearing skills (Gordon et al., 2013; Polonenko et al., 2017), specifically improved speech recognition in multisource environments.
Author Contributions
Lisa R. Park: Data curation (Lead), Formal analysis (Lead), Funding acquisition (Supporting), Project administration (Lead), Writing – original draft (Lead), Writing – review & editing (Equal). Margaret T. Dillon: Conceptualization (Equal), Funding acquisition (Equal), Methodology (Equal), Validation (Equal), Writing – review & editing (Equal). Emily Buss: Conceptualization (Equal), Formal analysis (Supporting), Methodology (Supporting), Supervision (Supporting), Writing – review & editing (Lead). Brendan P. O'Connell: Data curation (Supporting), Writing – review & editing (Equal). Kevin D. Brown: Conceptualization (Lead), Data curation (Supporting), Funding acquisition (Lead), Investigation (Lead), Supervision (Equal), Writing – review & editing (Equal).
Acknowledgments
This study was funded by MED-EL Corporation. The authors thank Holly Teagle for her work developing the protocol for this study as well as the clinicians and researchers, particularly Meredith Rooth and Kaylene King, who assisted in data collection. The authors also wish to thank Maegan Evans and Sandra Hancock for their expertise in providing habilitative care to the subjects.
Funding Statement
This study was funded by MED-EL Corporation.
References
- Akeroyd, M. A. (2006). The psychoacoustics of binaural hearing. International Journal of Audiology, 45(Suppl. 1), 25–34. https://doi.org/10.1080/14992020600782626 [DOI] [PubMed] [Google Scholar]
- Arndt, S. , Aschendorff, A. , Laszig, R. , Beck, R. , Schild, C. , Kroeger, S. , Ihorst, G. , & Wesarg, T. (2011). Comparison of pseudobinaural hearing to real binaural hearing rehabilitation after cochlear implantation in patients with unilateral deafness and tinnitus. Otology & Neurotology, 32(1), 39–47. https://doi.org/10.1097/MAO.0b013e3181fcf271 [DOI] [PubMed] [Google Scholar]
- Bagatto, M. , DesGeorges, J. , King, A. , Kitterick, P. , Laurnagaray, D. , Lewis, D. , Roush, P. , Sladen, D. P. , & Marie Tharpe, A. (2019). Consensus practice parameter: Audiological assessment and management of unilateral hearing loss in children. International Journal of Audiology, 58(12), 1–11. https://doi.org/10.1080/14992027.2019.1654620 [DOI] [PubMed] [Google Scholar]
- Bench, J. , Kowal, A. , & Bamford, J. (1979). The BKB (Bamford-Kowal-Bench) sentence lists for partially-hearing children. British Journal of Audiology, 13(3), 108–112. https://doi.org/10.3109/03005367909078884 [DOI] [PubMed] [Google Scholar]
- Blauert, J. (1997). Spatial hearing: The psychophysics of human sound localization (2nd Enlarg). The MIT Press. https://doi.org/10.7551/mitpress/6391.001.0001 [Google Scholar]
- Bronkhorst, A. W. , & Plomp, R. (1988). The effect of head-induced interaural time and level differences on speech intelligibility in noise. Journal of the Acoustical Society of America, 83(4), 1508–1516. https://doi.org/10.1121/1.395906 [DOI] [PubMed] [Google Scholar]
- Buss, E. , Dillon, M. T. , Rooth, M. A. , King, E. R. , Deres, E. J. , Buchman, C. A. , Pillsbury, H. C. , & Brown, K. D. (2018). Effects of cochlear implantation on binaural hearing in adults with unilateral hearing loss. Trends in Hearing, 22, 1–15. https://doi.org/10.1177/2331216518771173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss, E. , Hodge, S. E. , Calandruccio, L. , Leibold, L. J. , & Grose, J. H. (2019). Masked sentence recognition in children, young adults, and older adults: Age-dependent effects of semantic context and masker type. Ear and Hearing, 40(5), 1117–1126. https://doi.org/10.1097/AUD.0000000000000692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson, M. L. , Sladen, D. P. , Haynes, D. S. , Driscoll, C. L. , DeJong, M. D. , Erickson, H. C. , Sunderhaus, L. W. , Hedley-Williams, A. , Rosenzweig, E. A. , Davis, T. J. , & Gifford, R. H. (2015). Evidence for the expansion of pediatric cochlear implant candidacy. Otology & Neurotology, 36(1), 43–50. https://doi.org/10.1097/mao.0000000000000607 [DOI] [PubMed] [Google Scholar]
- Corbin, N. E. , Buss, E. , & Leibold, L. J. (2017). Spatial release from masking in children: Effects of simulated unilateral hearing loss. Ear and Hearing, 38(2), 223–235. https://doi.org/10.1097/AUD.0000000000000376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson, L. S. , Geers, A. E. , Uchanski, R. M. , & Firszt, J. B. (2019). Effects of early acoustic hearing on speech perception and language for pediatric cochlear implant recipients. Journal of Speech, Language, and Hearing Research, 62(9), 3620–3637. https://doi.org/10.1044/2019_JSLHR-H-18-0255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deep, N. L. , Gordon, S. A. , Shapiro, W. H. , Waltzman, S. B. , Roland, J. T. , & Friedmann, D. R. (2020). Cochlear implantation in children with single-sided deafness. The Laryngoscope, 131(1), E271–E277. https://doi.org/10.1002/lary.28561 [DOI] [PubMed] [Google Scholar]
- Dhondt, C. M. C. , Swinnen, F. K. R. , & Dhooge, I. J. M. (2018). Bilateral cochlear implantation or bimodal listening in the paediatric population: Retrospective analysis of decisive criteria. International Journal of Pediatric Otorhinolaryngology, 104, 170–177. https://doi.org/10.1016/j.ijporl.2017.10.043 [DOI] [PubMed] [Google Scholar]
- Etymōtic Research. (2005). BKB-SIN test. Speech-in-Noise Test Version, 1, 03. http://www.etymotic.com [Google Scholar]
- Firszt, J. B. , Holden, L. K. , Reeder, R. M. , Waltzman, S. B. , & Arndt, S. (2012). Auditory abilities after cochlear implantation in adults with unilateral deafness: A pilot study. Otology & Neurotology, 33(8), 1339–1346. https://doi.org/10.1097/MAO.0b013e318268d52d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedmann, D. R. , Ahmed, O. H. , McMenomey, S. O. , Shapiro, W. H. , Waltzman, S. B. , & Thomas Roland, J. (2016). Single-sided deafness cochlear implantation: Candidacy, evaluation, and outcomes in children and adults. Otology & Neurotology, 37(2), e154–e160. https://doi.org/10.1097/MAO.0000000000000951 [DOI] [PubMed] [Google Scholar]
- Garadat, S. N. , & Litovsky, R. Y. (2007). Speech intelligibility in free field: Spatial unmasking in preschool children. The Journal of the Acoustical Society of America, 121(2), 1047–1055. https://doi.org/10.1121/1.2409863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladden, C. , Beckf, L. , & Chandlerf, D. (2015). Tutorial tele-audiology: Expanding access to hearing care and enhancing patient connectivity. Journal of the American Academy of Audiology, 26(9), 792–799. https://doi.org/10.3766/jaaa.14107 [DOI] [PubMed] [Google Scholar]
- Gordon, K. A. , Wong, D. D. E. , & Papsin, B. C. (2013). Bilateral input protects the cortex from unilaterally-driven reorganization in children who are deaf. Brain, 136(5), 1609–1625. https://doi.org/10.1093/brain/awt052 [DOI] [PubMed] [Google Scholar]
- Griffin, A. M. , Poissant, S. F. , & Freyman, R. L. (2019). Speech-in-noise and quality-of-life measures in school-aged children with normal hearing and with unilateral hearing loss. Ear and Hearing, 40(4), 887–904. https://doi.org/10.1097/AUD.0000000000000667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossmann, W. , Brill, S. , Moeltner, A. , Mlynski, R. , Hagen, R. , & Radeloff, A. (2016). Cochlear implantation improves spatial release from masking and restores localization abilities in single-sided deaf patients. Otology & Neurotology, 37(6), 658–664. https://doi.org/10.1097/MAO.0000000000001043 [DOI] [PubMed] [Google Scholar]
- Hassepass, F. , Achendorff, A. , Wesarag, T. , Kroger, S. , Laszig, R. , Beck, R. L. , Schild, C. , & Arndt, S. (2012). Unilateral deafness in children: Audiologic and subjective assessment of hearing ability after cochlear implantation. Otology & Neurotology, 34(1), 53–60. https://doi.org/10.1097/MAO.0b013e31827850f0 [DOI] [PubMed] [Google Scholar]
- Hess, C. L. , Misurelli, S. M. , & Litovsky, R. Y. (2018). Spatial release from masking in 2-year-olds with normal hearing and with bilateral cochlear implants. Trends in Hearing, 22, 1–13. https://doi.org/10.1177/2331216518775567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochmair, I. , Hochmair, E. , Nopp, P. , Waller, M. , & Jolly, C. (2015). Deep electrode insertion and sound coding in cochlear implants. Hearing Research, 322, 14–23. https://doi.org/10.1016/j.heares.2014.10.006 [DOI] [PubMed] [Google Scholar]
- Johnstone, P. M. , & Litovsky, R. Y. (2006). Effect of masker type and age on speech intelligibility and spatial release from masking in children and adults. The Journal of the Acoustical Society of America, 120(4), 2177–2189. https://doi.org/10.1121/1.2225416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan, A. , Goupell, M. J. , & Litovsky, R. Y. (2019). Effect of channel separation and interaural mismatch on fusion and lateralization in normal-hearing and cochlear-implant listeners. The Journal of the Acoustical Society of America, 146(2), 1448–1463. https://doi.org/10.1121/1.5123464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan, A. , Stoelb, C. , Litovsky, R. Y. , & Goupell, M. J. (2013). Effect of mismatched place-of-stimulation on binaural fusion and lateralization in bilateral cochlear-implant users. The Journal of the Acoustical Society of America, 134(4), 2923–2936. https://doi.org/10.1121/1.4820889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King, K. , Dillon, M. T. , O'Connell, B. P. , Brown, K. D. , & Park, L. R. (2020). Spatial release from masking in bimodal and bilateral pediatric cochlear implant recipients. American Journal of Audiology. Advanced online publication. https://doi.org/10.1044/2020_AJA-20-00051 [DOI] [PubMed] [Google Scholar]
- Leibold, L. J. , & Buss, E. (2019). Masked speech recognition in school-age children. Frontiers in Psychology, 10, 1–8. https://doi.org/10.3389/fpsyg.2019.01981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieu, J. E. C. , Karzon, R. K. , Ead, B. , & Tye-Murray, N. (2013). Do audiologic characteristics predict outcomes in children with unilateral hearing loss? Otology & Neurotology, 34(9), 1703–1710. https://doi.org/10.1097/MAO.0000000000000190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, L. M. , Bowditch, S. , Anderson, M. J. , May, B. , Cox, K. M. , & Niparko, J. K. (2006). Amplification in the rehabilitation of unilateral deafness: Speech in noise and directional hearing effects with bone-anchored hearing and contralateral routing of signal amplification. Otology & Neurotology, 27(2), 172–182. https://doi.org/10.1097/01.mao.0000196421.30275.73 [DOI] [PubMed] [Google Scholar]
- Litovsky, R. Y. (2005). Speech intelligibility and spatial release from masking in young children. The Journal of the Acoustical Society of America, 117(5), 3091–3099. https://doi.org/10.1121/1.1873913 [DOI] [PubMed] [Google Scholar]
- Lovett, R. E. S. , Kitterick, P. T. , Huang, S. , & Summerfield, A. Q. (2012). The developmental trajectory of spatial listening skills in normal-hearing children. Journal of Speech, Language, and Hearing Research, 55(3), 865–878. https://doi.org/10.1044/1092-4388(2011/11-0096) [DOI] [PubMed] [Google Scholar]
- McKay, S. , Gravel, J. S. , & Tharpe, A. M. (2008). Amplification considerations for children with minimal or mild bilateral hearing loss and unilateral hearing loss. Trends in Amplification, 12(1), 43–54. https://doi.org/10.1177/1084713807313570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertens, G. , De Bodt, M. , & Van De Heyning, P. (2017). Evaluation of long-term cochlear implant use in subjects with acquired unilateral profound hearing loss: Focus on binaural auditory outcomes. Ear and Hearing, 38(1), 117–125. https://doi.org/10.1097/AUD.0000000000000359 [DOI] [PubMed] [Google Scholar]
- Neuman, A. C. , Wroblewski, M. , Hajicek, J. , & Rubinstein, A. (2010). Combined effects of noise and reverberation on speech recognition performance of normal-hearing children and adults. Ear and Hearing, 31(3), 336–344. https://doi.org/10.1097/AUD.0b013e3181d3d514 [DOI] [PubMed] [Google Scholar]
- Park, L. R. , Teagle, H. F. B. , Buss, E. , Roush, P. A. , & Buchman, C. A. (2012). Effects of frequency compression hearing aids for unilaterally implanted children with acoustically amplified residual hearing in the nonimplanted ear. Ear and Hearing, 33(4), e1–e12. https://doi.org/10.1097/AUD.0b013e31824a3b97 [DOI] [PubMed] [Google Scholar]
- Park, L. R. , Teagle, H. F. B. , Gagnon, E. , Woodard, J. , & Brown, K. D. (2019). Electric–acoustic stimulation outcomes in children. Ear and Hearing, 40(4), 849–857. https://doi.org/10.1097/AUD.0000000000000658 [DOI] [PubMed] [Google Scholar]
- Picou, E. M. , Davis, H. , Lewis, D. , & Tharpe, A. M. (2020). Contralateral routing of signal systems can improve speech recognition and comprehension in dynamic classrooms. Journal of Speech, Language, and Hearing Research, 63(7), 2468–2482. https://doi.org/10.1044/2020_JSLHR-19-00411 [DOI] [PubMed] [Google Scholar]
- Picou, E. M. , Lewis, D. , Angley, G. , & Tharpe, A. M. (2020). Rerouting hearing aid systems for overcoming simulated unilateral hearing in dynamic listening situations. Ear and Hearing, 41(4), 790–803. https://doi.org/10.1097/AUD.0000000000000800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant, K. , & Babic, L. (2016). Utility of bilateral acoustic hearing in combination with electrical stimulation provided by the cochlear implant. International Journal of Audiology, 55(Suppl. 2), S31–S38. https://doi.org/10.3109/14992027.2016.1150609 [DOI] [PubMed] [Google Scholar]
- Plontke, S. K. , Heider, C. , Koesling, S. , Hess, S. , Bieseke, L. , Goetze, G. , & Rahne, T. (2013). Cochlear implantation in a child with posttraumatic single-sided deafness. European Archives of Oto-Rhino-Laryngology, 270(5), 1757–1761. https://doi.org/10.1007/s00405-013-2350-2 [DOI] [PubMed] [Google Scholar]
- Polonenko, M. J. , Gordon, K. A. , Cushing, S. L. , & Papsin, B. C. (2017). Cortical organization restored by cochlear implantation in young children with single sided deafness. Scientific Reports, 7(1). Article number, 16900. https://doi.org/10.1038/s41598-017-17129-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polonenko, M. J. , Papsin, B. C. , & Gordon, K. A. (2018). Limiting asymmetric hearing improves benefits of bilateral hearing in children using cochlear implants. Scientific Reports, 8(1). Article number, 13201. https://doi.org/10.1038/s41598-018-31546-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polonenko, M. J. , Papsin, B. , & Gordon, K. A. (2019). Cortical plasticity with bimodal hearing in children with asymmetric hearing loss. Hearing Research, 372, 88–98. https://doi.org/10.1016/j.heares.2018.02.003 [DOI] [PubMed] [Google Scholar]
- Reeder, R. M. , Cadieux, J. , & Firszt, J. B. (2015). Quantification of speech-in-noise and sound localisation abilities in children with unilateral hearing loss and comparison to normal hearing peers. Audiology and Neurotology, 20(Suppl 1), 31–37. https://doi.org/10.1159/000380745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roid, G. H. , & Miller, L. J. (1997). Leiter International Performance Scale–Revised: Examiner's manual. Stoelting Co. https://doi.org/10.1037/t05120-000 [Google Scholar]
- Ruscetta, M. N. , Arjmand, E. M. , & Pratt, S. R. (2005). Speech recognition abilities in noise for children with severe-to-profound unilateral hearing impairment. International Journal of Pediatric Otorhinolaryngology, 69(6), 771–779. https://doi.org/10.1016/j.ijporl.2005.01.010 [DOI] [PubMed] [Google Scholar]
- Schafer, E. C. , Beeler, S. , Ramos, H. , Morais, M. , Monzingo, J. , & Algier, K. (2012). Developmental effects and spatial hearing in young children with normal-hearing sensitivity. Ear and Hearing, 33(6), e32–e43. https://doi.org/10.1097/AUD.0b013e318258c616 [DOI] [PubMed] [Google Scholar]
- Sennaroğlu, L. , & Bajin, M. D. (2017). Classification and current management of inner ear malformations. Balkan Medical Journal, 34(5), 397–411. https://doi.org/10.4274/balkanmedj.2017.0367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skarzynski, H. , & Lorens, A. (2009). Electric acoustic stimulation in children. Cochlear Implants and Hearing Preservation, 67, 135–143. https://doi.org/10.1159/000262605 [DOI] [PubMed] [Google Scholar]
- Sladen, D. P. , Frisch, C. D. , Carlson, M. L. , Driscoll, C. L. W. , Torres, J. H. , & Zeitler, D. M. (2017). Cochlear implantation for single-sided deafness: A multicenter study. The Laryngoscope, 127(1), 223–228. https://doi.org/10.1002/lary.26102 [DOI] [PubMed] [Google Scholar]
- Svirsky, M. A. , Fitzgerald, M. B. , Sagi, E. , & Glassman, E. K. (2015). Bilateral cochlear implants with large asymmetries in electrode insertion depth: Implications for the study of auditory plasticity. Acta Oto-Laryngologica, 135(4), 354–363. https://doi.org/10.3109/00016489.2014.1002052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavora-Vieira, D. , Marino, R. , Krishnaswamy, J. , Kuthbutheen, J. , & Rajan, G. P. (2013). Cochlear implantation for unilateral deafness with and without tinnitus: A case series. The Laryngoscope, 123(5), 1251–1255. https://doi.org/10.1002/lary.23764 [DOI] [PubMed] [Google Scholar]
- Tavora-Vieira, D. , & Rajan, G. P. (2015). Cochlear implantation in children with congenital and noncongenital unilateral deafness: A case series. Otology & Neurotology, 36(2), 235–239. https://doi.org/10.1097/MAO.0000000000000677 [DOI] [PubMed] [Google Scholar]
- Updike, C. (1994). Comparison of FM auditory trainers, CROS aids, and personal amplification in unilaterally hearing impaired children. Journal of the American Academy of Audiology, 5(3), 204–209. [PubMed] [Google Scholar]
- Vaillancourt, V. , Laroche, C. , Giguere, C. , & Soli, S. (2008). Establishment of age-specific normative data for the Canadian French version of the hearing in noise test for children. Ear and Hearing, 29(3), 453–466. https://doi.org/10.1097/01.aud.0000310792.55221.0c [DOI] [PubMed] [Google Scholar]
- Van Deun, L. , Van Wieringen, A. , & Wouters, J. (2010). Spatial speech perception benefits in young children with normal hearing and cochlear implants. Ear and Hearing, 31(5), 702–713. https://doi.org/10.1097/AUD.0b013e3181e40dfe [DOI] [PubMed] [Google Scholar]
- Wilson, R. H. , McArdle, R. A. , & Smith, S. L. (2007). An evaluation of the BKB-SIN, HINT, QuickSIN, and WIN materials on listeners with normal hearing and listeners with hearing loss. Journal of Speech, Language, and Hearing Research, 50(4), 844–856. https://doi.org/10.1044/1092-4388(2007/059) [DOI] [PubMed] [Google Scholar]
- Wolfe, J. , Neumann, S. , Schafer, E. , Marsh, M. , Wood, M. , & Baker, R. (2017). Potential benefits of an integrated electric–acoustic sound processor with children: A preliminary report. Journal of the American Academy of Audiology, 28(2), 127–140. https://doi.org/10.3766/jaaa.15133 [DOI] [PubMed] [Google Scholar]
- Yuen, K. C. P. , & Yuan, M. (2014). Development of spatial release from masking in Mandarin-speaking children with normal hearing. Journal of Speech, Language, and Hearing Research, 57(5), 2005–2023. https://doi.org/10.1044/2014_JSLHR-H-13-0060 [DOI] [PubMed] [Google Scholar]
- Zeitler, D. M. , Sladen, D. P. , DeJong, M. D. , Torres, J. H. , Dorman, M. F. , & Carlson, M. L. (2019). Cochlear implantation for single-sided deafness in children and adolescents. International Journal of Pediatric Otorhinolaryngology, 118, 128–133. https://doi.org/10.1016/j.ijporl.2018.12.037 [DOI] [PubMed] [Google Scholar]