Significance
Spontaneous brain activity remains a mysterious quantity. It is unknown whether they are driven by those events outside the brain or the brain is playing an active role in generating them. Investigating their coordination is crucial to understanding principles of brain function in an embodied system. The application of functional ultrasound imaging technology to the marmoset monkey brain allowed us to image a large section of medial cortical and subcortical areas in behaving animals. Using simultaneous measurements of brain, motor, and cardiac activities together with the multivariate inference of information flow, we directly linked high-dimensional brain activity to the dynamics of behavioral and physiological observables. Our study reveals the shifting brain dynamics involved in coordinating motor behavior with internal states.
Keywords: functional ultrasound imaging, information flow, spontaneous behavior, arousal, brain fluctuation
Abstract
The brain continuously coordinates skeletomuscular movements with internal physiological states like arousal, but how is this coordination achieved? One possibility is that the brain simply reacts to changes in external and/or internal signals. Another possibility is that it is actively coordinating both external and internal activities. We used functional ultrasound imaging to capture a large medial section of the brain, including multiple cortical and subcortical areas, in marmoset monkeys while monitoring their spontaneous movements and cardiac activity. By analyzing the causal ordering of these different time series, we found that information flowing from the brain to movements and heart-rate fluctuations were significantly greater than in the opposite direction. The brain areas involved in this external versus internal coordination were spatially distinct, but also extensively interconnected. Temporally, the brain alternated between network states for this regulation. These findings suggest that the brain’s dynamics actively and efficiently coordinate motor behavior with internal physiology.
Animals and humans continuously regulate physiology and behavior to maintain stability—i.e., keep physiological variables within a tenable range (1). This regulation not only involves triggering autonomic reflexes that directly adjust physiological processes, such as heart rate (HR), glucose level, and body temperature, but also directs skeletomotor behaviors to interact with the external world that affect physiological states (e.g., feeding, locomotion, and social interaction) (2, 3). The changing internal states of the body remodel sensorimotor interactions with the external environment on various timescales (4). For example, the phase of the cardiac cycle influences the emotional processing of faces (5); vocal interactions are correlated with autonomic oscillations (6–8); and hunger can modulate the switch between sleep versus foraging behaviors (9). In all cases, the internal physiological states must be coordinated with motor behaviors through the dynamics of large-scale networks of cortical and subcortical regions, but how?
Based on the spectrum of cytoarchitectonic differentiation, one proposal is that the mammalian brain follows a “centrifugal” cortical organization from the outer side (the primary sensory and motor areas) to the inside. Inside areas include the heteromodal, paralimbic, and limbic regions that, in humans, overlap substantially with the default mode network (DMN) (10–12). The outside areas directly regulate the interactions with the external environment, while the inside areas are associated with autonomic functions regulating the internal milieu through subcortical areas, primarily the hypothalamus (12–14). A more recent proposal, based on the meta-analyses of rat cortical neuroanatomy, is a topologic core–shell arrangement with two sensory-motor modules (core) and two limbic modules (shell) corresponding to this external–internal dichotomy (15). In humans, functional imaging and gene-expression analyses also reveal a division of the brain into two cortical networks (16, 17). Such a brain architecture entails an embodied account of perception, emotion, and decision making (18–23).
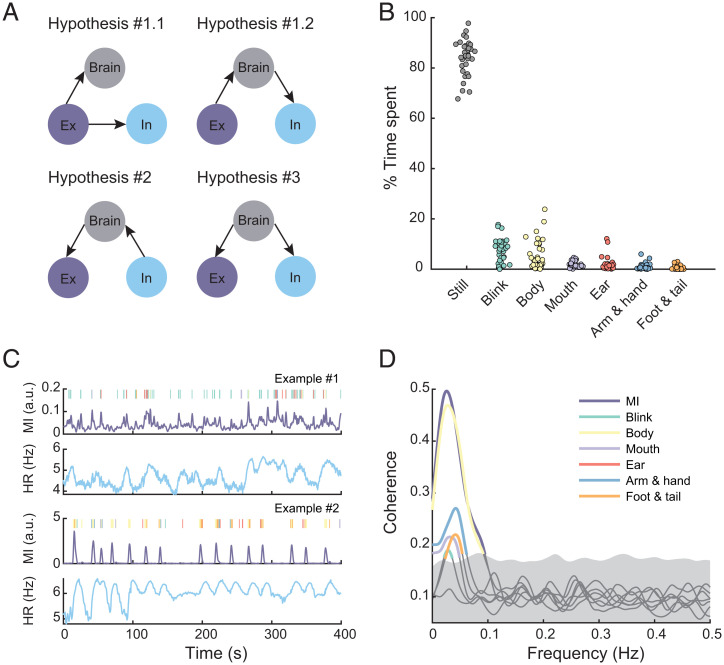
However, within such a framework, how are motor behaviors and the internal state of the body organized with the brain’s activity in space and time? If we assume a context in which there are no overt external sensory signals, and the animal coordinates interactions with external and internal environments by its intrinsic dynamics, then there are currently three hypotheses for the interrelationships between brain activity, motor behavior, and internal physiology (Fig. 1A). The first hypothesis postulates that spontaneous motor behaviors—like twitches, facial movements, and fidgets—drive the large-scale activity of the brain (24–27). The behavior-related widespread brain activity could originate from reafferent sensory input and may facilitate contextualized signal processing (28). Under this hypothesis, there are two possibilities for the internal physiological state: It is either a follower of movements through peripheral regulation (Hypothesis 1.1) or centrally regulated (Hypothesis 1.2). In the second hypothesis, brain dynamics are passive responses to ongoing physiological states and provide signals—including those to produce behaviors—to maintain homeostasis of the body (Hypothesis 2) (29–31). The third hypothesis proposes that the brain not only actively predicts signals of the external world (32, 33), but also the interoceptive signals of the body for homeostatic control (Hypothesis 3) (34–36).
Fig. 1.
Organization of external and internal activities. (A) Candidate hypotheses on the interrelationships between brain activity, movement, and HR fluctuation. (B) Percentage of time spent on different types of movements. Each point is a session. Jitters are added to help visualize. (C) Two exemplar data of movement type, MI, and HR. Types of movements are color-coded similarly to B. Note that small MI variations correspond to facial movements, such as blinks, and large MIs correspond to movements of the body parts. A.u., arbitrary units. (D) Coherence between each HR-movement type pair and the HR–MI pair. Highlighted segments are significantly higher than the 95% CI of the phase-randomized surrogates.
To test these hypotheses, we studied the interrelationships between the brain, external (spontaneous movement), and internal (cardiac) activities in marmoset monkeys (Callithrix jacchus) under a task-absent context. These various activities are prevalent on the timescale of tens of seconds (24, 37–41) and, thus, can be tested in experimental sessions lasting for 10 to 20 min. We simultaneously used functional ultrasound (fUS) imaging of the midsagittal plane of the marmoset brain, along with videos of behaviors and measures of HR (electrocardiograms [ECGs]) to establish 1) the direction of information flow between the three components, and 2) the spatial and temporal organizations of brain activity as a function of spontaneous movements and cardiac changes.
Results
Subjects (n = 3) were placed in a partial-restraint device to allow for both stable neural imaging and movements of most of the body. Using behavioral videography analysis (35 sessions, 27,259 s of recording), we detected occasional movements of the limbs, tail, and body, as well as facial movements, including blinks and movements of the ears and mouth (Fig. 1B). Movements were also quantified by the motion intensity (MI), regardless of the type. In parallel, we carried out ECGs for continuous heart-rate recording (Fig. 1C). The occurrence of different behaviors varied coherently with HR to different degrees, with all, except ear movements, showing a peak around 0.03 Hz (test against the 95% CI of phase-randomized surrogates; Fig. 1D). Using MI alone, the coherence between movement and HR rate could also be well-captured (Fig. 1D), and there was no temporal difference between MI and elaborated movements with respect to HR (SI Appendix, Fig. S1). Thus, for ease of presentation, we used MI to represent all kinds of movements in the following analyses.
Meanwhile, we measured brain activity in a large portion of the midsagittal plane, including cortical and subcortical areas, using fUS (35 sessions; Fig. 2A). fUS measures cerebral blood volume (CBV) dynamics in the microvasculature, an indirect measure of neuronal activity (42–44), with a large field of view (FOV) (16 mm [anteroposterior; AP] × 20 mm [dorsoventral; DV]), a spatial resolution of 130 µm (AP) × 125 µm (DV), and a frame rate of 2 Hz. The FOV covered primary sensory and motor areas: the medial motor (M1) and somatosensory (SS); motor associate areas: presupplementary motor area (preSMA) and supplemental motor area (SMA); and high-heteromodal and paralimbic areas: prefrontal cortex (PFC) and cingulate motor area (CMA); as well as cortices constituting the DMN: the ventromedial PFC (vmPFC) and posterior cingulate cortex (45). It also covered subcortical areas relevant to autonomic functions (46), including the dorsal and ventral lateral septal nuclei (LSD and LSV), preoptic area (POA), ventromedial hypothalamus (VMH), posterior hypothalamus (PH), mediodorsal nucleus of the thalamus (MD), habenula nuclei, and part of the reticular formation (RF) (Fig. 2A). Thus, the simultaneous activities of many brain areas (though not all of them) highly relevant to motor behavior and internal physiology were incorporated in the measurement.
Fig. 2.
The brain plays an active role in coordinating movement and HR. (A) fUS setup and brain regions covered by imaging. (B) Exemplar pixel signals from different areas of the brain. Vertical bar indicates 2 SD. (C) Within-subject and group-level brain parcellation. We use SMA to represent the ROI covering SS, M1, and SMA. (D) Illustration of bPDC. (E) bPDC of each direction. Red means significantly higher than phase-randomized surrogate distribution. Note that the lines in “HR to MI” and “MI to HR” cells are very low. (F) Estimated distribution of information (info) flow of each direction, sorted from high to low. Outward flow from brain is greater than the corresponding inward flow (P < 0.05, Bonferroni-corrected). (G) Information-flow graph. The thickness of the arrows is proportional to the information flow.
To evaluate the relationship between brain and peripheral activities, we first parcellated the image into regions of interest (ROIs) based on fUS signals. We estimated a group-level parcellation using all available fUS data by concatenating temporally reduced data using principal component analysis (PCA; 4 subjects, 60 sessions, 85,427 frames; Methods) and segregating the brain section into 19 ROIs by spectral clustering (Fig. 2 B and C). Among the 19 ROIs, 14 were identified as nonvessel ROIs and were named based on corresponding anatomical annotations after aligning to a reference brain atlas (Methods; Fig. 2C and SI Appendix, Fig. S2) (47, 48). The brain-parcellation results were consistent across subjects (Fig. 2C), and, thus, we concatenated the ROI signals across all sessions.
The information flow between time series of brain activity, movements (MI), and HR was assessed by using block partial directed coherence (bPDC), a measure of information flow in the frequency domain (49) (Methods) and invariant to causal filtering like the hemodynamic response function (SI Appendix, SI Text) (50). The bPDC treated the ROI signals as one multivariate time-series network node and the movement and HR as the other two nodes (Fig. 2D). We found that the bPDC was significant in both directions between brain and movement and between brain and HR, while the bPDC between movement and HR was not significant, suggesting that their coordination was via the brain (test against the 95% CIs of phase-randomized surrogates, Bonferroni-corrected; Fig. 2E). Hence, we could reject Hypothesis 1.1, as the information flow between movement and HR was indirect. We then tested if the brain activity was driven more by the periphery or vice versa by comparing the total information flow in each direction, which was calculated by integrating the bPDC over frequencies (Methods). On average, information flowing from the brain to the other two components was significantly higher than the inward flow (bootstrap and paired z test, P < 0.05, Bonferroni-corrected; Fig. 2 F and G; regardless of whether filters were applied [SI Appendix, Fig. S3]). Consistently, brain activity led the other two activities temporally, and the causal direction was due to 0- to 1-s leading of the brain (SI Appendix, Fig. S4). The higher outward information flow was observed for all types of movements as well (SI Appendix, Fig. S5). Thus, the results favored Hypothesis 3, that the brain activity was mostly predictive of motor output and fluctuations of internal physiology.
A caveat is that brain hemodynamics is naturally linked to cardiac activity, and one may expect to observe information flow solely from HR to brain (51). While the influence from HR to CBV could be added up by the effect of blood flow, we argue here that information flow in the opposite direction can be a conservative estimation of neural regulation of HR. First, for a reality check, we randomly selected patches within the superior sagittal sinus, an area outside the neural content, as a control and assessed the information flow within a brain–sinus–HR network. The information was significantly greater flowing from HR to sinus than the opposite direction (n = 30, P = 0.0036, paired t test), verifying that blood flow not driven by neural activity was influenced by HR. The information flow from CBV to HR can be realized via hidden neural activity in the neighborhood of the CBV signal (52). Consider a network consisting of the CBV of an ROI, the neuronal activity of this ROI, and HR, where the neural activity causes an immediate CBV change via neurovascular coupling, as well as an HR change with a relatively longer latency via a signaling pathway to the heart. Meanwhile, the HR influences brain hemodynamics (SI Appendix, Fig. S6A). If we only measure the CBV and HR, it is possible to observe information flow from CBV to HR, as long as the weight from neuron to CBV is greater than that from HR (SI Appendix, Fig. S6B). Thus, using CBV as a surrogate for neural activity, it is possible to reveal the neural influence on HR, and our results should indicate this.
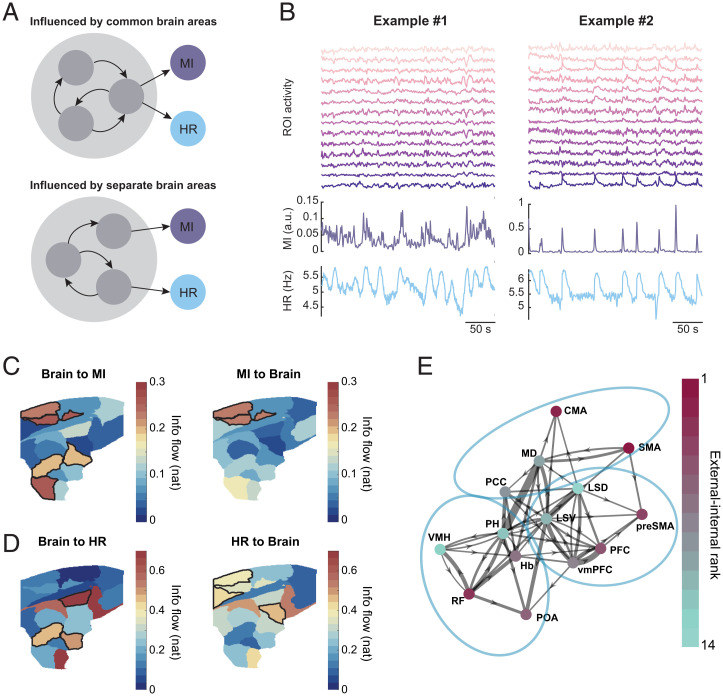
One possible mechanism coupling external and internal activities is that they could be driven by a common set of brain regions; alternatively, they could be influenced by separate regions that are themselves temporally coordinated (Fig. 3A). To test this, we first established the network composed of individual ROIs, movement, and HR using partial directed coherence (PDC) analysis for univariate time series (Fig. 3B) (53). The strength of connection in each direction between two nodes was quantified as integrated information flow. Two different subsets of ROIs were involved in strong information exchange with movement and HR (Fig. 3C and D). For example, the premotor areas SMA and CMA are known for motor sequence and planning (54, 55); the PDC analysis revealed their information exchange with movement. Another area exhibiting highly predictive activity of movement was the RF, whose function of coordinating crucial somatic movements, as well as relaying descending signals to motor neurons, has been well documented (55). The areas that sent significant information to HR, including the LSD and VMH, are also known to regulate motivation in general and cardiovascular functions (56). Furthermore, the estimated information flow between the brain and HR was not driven by different regional density of vascularization; in both directions, the information flow was not dependent on the vascular density (P > 0.05; SI Appendix, Fig. S7). To further verify our results based on functional parcellation, we aligned the fUS image to a reference atlas (Methods) (48) and conducted PDC analysis with anatomical segmentation. The significant information flow from SMA, CMA, and RF to movement (MI) and from LSD and VMH to HR was robust against parcellation method (SI Appendix, Fig. S8). Thus, movement and HR activities were coordinated by spatially separate brain regions, with the exceptions of PH and SMA, which coordinated both types of activities.
Fig. 3.
Separate brain regions regulate internal and external activities. (A) Hypotheses of spatial distribution for different brain areas involved in movement and HR regulation. (B) Exemplars showing individual ROI signals with MI and HR. Each time series is used as a node for PDC analysis. A.u., arbitrary units. (C and D) Brain regions sending or receiving information flow to MI and HR. Outlined areas are the ones above the 75th percentile of the information flow within the category. (E) Brain-network summary. Circled areas are communities determined by spectral clustering. Node color corresponds to the preference of control, with purple toward external activity and green toward internal activity. Note that low-level subcortical nodes are separated from higher-level nodes, which are further separated into two groups, but opposite control preferences can exist in the same community.
We next investigated the connectivity between these two widespread groups of regions. One hypothesis is that the functional connectivity, defined as information flow between ROIs, exhibits two network communities, corresponding to the external and internal control. Conversely, these two groups of regions might be so coupled that the network cannot be cleanly separated by the degree of participation in external and internal control. As the outward information flow was dominant (and also because the heart-to-brain direction was confounded by vascular information flow), we formulated the ROI function as the difference between information flowing to movement and to HR (termed control preference). The graph revealed that ROIs with different control preference were highly interconnected and could not be separated by their functional connectivity (spectral clustering for directed graph; Fig. 3E). This suggested that the coordination between movement and HR was due to strongly coupled brain areas. The functional connectivity established by using fUS was backed by structural connectivity from fiber tractography mapped to the same areas (functional and structural connectivity correlation r = 0.46, P = 5.0 × 10−6; SI Appendix, Fig. S9), comparable to measurements in other modalities and species (57–59). This internally connected network, ranging from the primary sensorimotor cortex for external control to the limbic areas (here, mainly the lateral septum) for internal control, is consistent with the idea of “centrifugal” brain organization (12).
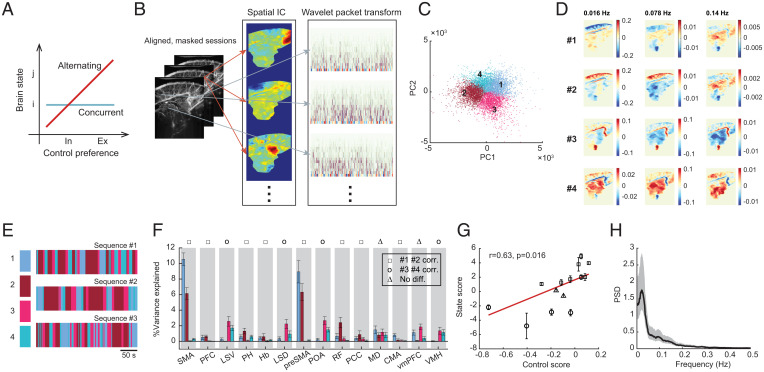
We then investigated how the control of ongoing behavior and internal physiology were coordinated temporally. Brain regions collectively transition into different states (39, 60), so the regulation of external and internal activities may occur concurrently during certain states or alternatively between states. In the first case, brain regions activated during the same brain state can have different control preferences, while in the second case, areas with different control preferences are correlated with different brain states (Fig. 4A). To test these possibilities, we evaluated the fluctuation of brain states by first transforming the fUS images into a spatial independent component (IC; of dimensions)–wavelet (of dimensions) representation (Fig. 4B). Briefly, each frame was reduced to dimensions containing spatial and spectral information of the fUS signal (61). Before identifying brain states, we conducted data harmonization to mitigate the session-dependent variance, such that the subsequent clustering would be driven by brain-activity patterns instead of experiments (Methods; SI Appendix, Fig. S10). Using k-means clustering with each time point as a sample, we clustered the reduced data into four states (n = 85,427 frames; Fig. 4C). The four clusters represented distinct spectral and spatial activation patterns (Fig. 4D). The fUS signals were then converted to sequences of brain states (Fig. 4E).
Fig. 4.
Brain alternates the control for external and internal activities. (A) Two possibilities for external and internal regulation relative to brain states: concurrent or alternating. Ex, external; in, internal. (B) Illustration of dimensionality reduction onto ICA-wavelet space. (C) K-means clustering of the reduced data into four clusters, plotted in the space of the first two PCs for visualization. (D) Maps of mean wavelet amplitude for each cluster at different frequency bands. (E) Exemplar sequences of brain states. (F) Percentage variance explained for each ROI by different brain states. Most ROIs are correlated (corr.) with either states 1 and 2 or states 3 and 4. Error bars are SDs. (G) Correlation between state score and control preference. Error bars are SDs. The line is fitted by weighted linear regression. is weight correlation coefficient, and is the P value of the slope. (H) PSD of the alternation between state 1 plus state 2 and state 3 plus state 4. Shaded area is 95% CI. Peak around 0.02 Hz.
We then evaluated the ROIs’ participation in brain states by calculating the percentage variance of an ROI signal explained solely by the occurrence of a brain state. Most of the ROIs were significantly explained by either only states 1 and 2 or only states 3 and 4, except the MD and vmPFC (z test, P < 0.05; Fig. 4F). Based on this characteristic, we quantified a state score as the log-ratio between the total variance explained by states 1 and 2 and by states 3 and 4. The state score correlated significantly with control preference (r = 0.63, P < 0.05; Fig. 4G), suggesting that external and internal regulation occurred during different brain states, with states 1 and 2 for external regulation and states 3 and 4 for internal regulation. To verify this result, we also carried out spatial-spectral decomposition of fUS images using an IC analysis (ICA)–multitaper approach (62) and obtained a significant correlation between state score and control score, demonstrating that the finding was not method-specific (SI Appendix, Fig. S11). By calculating the power spectral density (PSD) of the alternation between external (state 1 plus 2) and internal (state 3 plus 4) brain states, we observed a peak around 0.02 Hz (Fig. 4H), close to the frequency of the peak coherence between movement and HR (Fig. 1D). Therefore, the coordinated external and internal activities were due to the slow oscillations between these two brain states.
Discussion
Our findings that brain dynamics are predictive of motor activities and HR fluctuations support a view of the brain as being actively coordinating behavior with internal states (2, 3, 34). The regulation of these two aspects is linked to spatially separate, but functionally and structurally interconnected, brain regions. This organization of behavior and internal physiology with brain dynamics is analogous to the blood circulatory system: Different chambers of the heart are involved in the systemic (analogous to the external interaction) and pulmonary (analogous to the internal interaction) circulations, but are also synchronized by the muscle contractions of the heart. Failure of any loop will lead to the breakdown of the entire system. Although the brain is a much more complex organ, it may drive coupled interactions with the external and internal environments in a similar way. We also found that the transitions of brain states signal the switches between external and internal control. As the brain is metabolically expensive, multifunctionality in a task-free context would be less economical (63, 64); functional alternation might be a solution for lowering the running cost of the brain (65).
Our findings suggest that in the low-frequency band (<0.5 Hz), information flow from brain to movement is significantly greater than in the opposite direction. In contrast to previous studies, which suggest that brain-wide fluctuation is driven by spontaneous movements (24, 26, 27), our study proposes that the fluctuation, at least in the low-frequency component, is more of an intrinsically organized pattern. Whereas there is no report in vertebrates thus far, a study in Caenorhabditis elegans observed a distributed motor-command network activated even in the immobilized worm, suggesting brain-wide behavior-related activities independent of reafferent sensory input (66). If this applies also to the vertebrates, a possible mechanism unifying the seemingly conflicting findings is that the medial brain (the focus of this study) may provide the motivation that guides behaviors (67, 68). This motor guidance is broadcast to other brain areas and gets manifested in detail by interacting with local neural activities. The movement-driven neural activity pattern has been mainly observed in the visual cortex (24); it would be interesting to investigate the temporal coordination between the medial brain and those sensory areas for spontaneous behaviors to understand how these activities are organized. Another reason for the seemingly different conclusion could be attributed to our different data-analysis approach. Previous studies adopted multivariate linear models or cross-correlation estimating the variance explained by movements. Our adoption of the PDC method allows us to distinguish direct and indirect flows in the frequency domain; this is not possible with correlation methods (50, 69).
Despite the incompleteness of the brain network that could be measured in this study, the general functional division into the external and the internal agrees with modular divisions of other, more exhaustive connectomes. In the rat cerebral cortex, for example, four modules are identified from a graph of directed synaptic connections (15). The four modules are organized into a topologic core–shell structure with two core modules: M1, containing the visual and auditory areas, and M2, including mainly the somatic and visceral sensory-motor areas; and two shell modules: M3, including most of the cingulate cortex and hippocampal formation, and M4, containing the olfactory system, infralimbic and prelimbic areas, and amygdala. Our study partially overlapped M2–4 under this framework with additional subcortical areas measured. The external state (1 and 2 in Fig. 4D) was mainly associated with the activation and deactivation of the motor areas (SMA and preSMA), corresponding to activity belonging to the M2 network. These premotor areas are usually linked to motor planning, and, for marmosets, the SMA has coevolved with their exceptional volitional vocal control ability compared to other nonhuman primate species (70). The internal state (3 and 4) was strongly correlated with activity of the ventral subcortical areas, including the lateral septum, POA, and hypothalamus. Although these areas are not included in the four modules, the lateral septum, for example, strongly connects with the hippocampus (71), which belongs to the core modules M3 and M4. Another area, vmPFC, was also correlated with the internal state (but not statistically greater than the correlation with the external one). This area, more precisely area 25, is a major player in M4 along the middle wall and connects strongly with the amygdala (72). Thus, one hypothesis yet to be examined is that the internal state may more extensively recruit brain areas within the M4. In the rat-connectome study, M2 and M4 exhibit strong reciprocal connections. This may account for the overall highly coupled functional network (Fig. 3E) and the dominating transitions between external and internal brain states.
Brain fluctuation correlated with physiological signals has been found universally (51, 73–76). However, when using the hemodynamic signal as a surrogate for neural activity, such as in functional MRI (fMRI) and fUS, the cardiovascular and respiratory signals have been treated as confounds and regressed out in fMRI studies (77). In addition, due to signal distortion and CO2-concentration change in the brain brought by respiration, it is also a general practice to regress out global fMRI signals approximating the removal of respiration (78). The fUS method does not have the signal-distortion issue, but could be influenced by CO2 concentration. Although we did not explicitly remove potential global influences in our analysis, the PDC estimate automatically treated independent external sources as noise. Thus, the global effect should be discounted, at least to the extent of removing the global average. Another technical difference is that the CBV of the arterioles and capillaries measured with fUS (42) are more similar to CBV-fMRI than blood-oxygen-level-dependent (BOLD)-fMRI (44). CBV signals exhibit a shorter onset time and time-to-peak than BOLD signals in marmosets (79) and correlate linearly with neural activity for a wide range of physiological regimes (80–82). The higher sensitivity of the fUS signal may have contributed to our inference of information flow. However, we do realize that the cerebral blood flow is not necessarily driven by local neural activity, but may share common input with the electrophysiological activity from, for example, the rostral ventrolateral medulla (83). How faithfully the fUS signals can be translated into neuronal activity at different locations in the brain requires further investigation. Besides the entangled brain-physiological signal owing to the imaging technique, there is also a neurobiological basis for the correlation between brain and physiological states (73). Multiple cortical and subcortical areas participate in autonomic regulations on respiratory, cardiovascular, digestive, and other systems (74, 76, 84). The temporal relationships between brain activity and the physiological signal are frequency-dependent. For example, in mice, theta oscillations precede and are Granger-causal for the variation of respiration rate, whereas it is the opposite for the gamma oscillations (85). Our results support that at frequencies < 0.5 Hz, the information mainly flows from brain to HR, outweighing the opposite direction. However, we did not exclude that HR and respiratory sinus arrhythmia can influence CBV at higher frequencies around blood pulsation (∼5 Hz) and respiration rate (∼1 Hz) in marmosets.
Intrinsic functional architecture revealed in slow, spontaneous fluctuations across the brain is not unique to humans (86) and has been observed across various animal species (87–92). With technologies allowing for higher spatial and temporal resolutions, we are now able to better understand the neural and hemodynamic accounts for behavior on a large scale (93). Using fUS, we revealed how HR, a measure of the moment-to-moment variation of energy supply to the body, is coordinated with motor activity, which consumes this energy—via a complex brain network. The brain is at the nexus between internal and external environments. Its functional division into external and internal regulations and the inseparable nature of these two dynamics support a view of the DMN as a dynamic sense-making network that actively integrates external and internal life events (94). This coordination may be a consequence of context-dependent energy optimization. Modern robotics design also follows such an architecture with a “task planning” layer for optimizing objectives and a “motor planning and control” layer for taking actions (95). Questions for future studies are how the brain exploits and reconfigures its intrinsic networks in the face of different internal and external challenges in contexts such as social interaction and learning (96, 97) and how life experiences can shape the energy landscape of network dynamics and give rise to the diversity of behavioral phenotypes (94).
Methods
Subjects.
All experiments were performed in compliance with the Princeton University Institutional Animal Care and Use Committee guidelines. The subjects used in the study were four adult common marmosets (>2 y old, one female and three males) housed at Princeton University. All four subjects participated in the fUS recording; three of them were also used for ECGs and video recording. The colony room was maintained at 27 °C temperature and 50 to 60% humidity with a 12-h light:12-h dark light cycle. Marmosets had ad libitum access to water and were fed with a standard diet. All subjects were acclimated to the experimental environment and positioning at least a month before the formal experiments.
Surgery.
For preoperative procedures, the animal was placed on a warmed blanket with temperature, pulse, respiration, and oxygen saturation being monitored and blood glucose measured. Dexamethasone (1 mg/kg) and Baytril (5 mg/kg) were administered intramuscularly (IM). The animal was induced with alfaxalone 10 mg/kg IM before intubation. The intubated animal was then connected to the anesthesia machine and moved into the operating room. The surgical site was clipped, and the remaining hair was removed with Nair. The surgical site was cleaned with betadine and covered with surgical drape (3M). Lidocaine was injected into the scalp at multiple sites. An incision (∼20 mm, rostral/caudal orientation) was made to expose the skull along the top of the head through the scalp. Tissue was reflected with a retractor to expose the skull. Sterilized miniature titanium screws were inserted into the bone at several locations to anchor the head post and the head plate. A customized head post and a head plate were immobilized on the skull with adhesive cement (C&B Metabond Quick Adhesive Cement System, Parkell). The head plate allowed the ultrasound probe to be aligned with the midline of the brain. Within the head plate, a cranial window of ∼8 mm × 16 mm was created. During off-experiment time, a stainless-steel cover was attached to the head plate to protect the head plate and the craniotomy.
fUS Imaging.
Our custom ultrasound probe allowed a wide FOV (20 mm depth, 16 mm width), a temporal resolution of 2 Hz, and a spatial resolution of 125 µm in width, 130 µm in depth, and 200 to 800 µm in thickness, depending on the depth (200 µm at 12-mm depth). The probe was connected to an ultrasound scanner (Vantage 128, Verasonics) controlled by a PC. fUS signals were acquired at the midline sagittal plane. The image acquisition method was described (44).
Experimental Protocol for fUS Recording.
Each subject participated in experiments once per day around the same time of the day. The subject was brought to the experimental room from their home cage. The walls of the room (3.2 m × 5.5 m) were covered with sound-attenuating foam. The subject was placed in a custom-designed partial restraint device with the head fixed by the head post. The cover was removed to expose the cranial window, which was then flushed with sterile water and covered by sterile ultrasound gel (Sterile Aquasonic 100 Ultrasound Gel). A customized probe holder was then attached to the top of the head plate with screws, and the ultrasound probe was placed inside the holder. The head post was released before the experiment to allow head movement. An initial image was acquired to examine the imaging position and quality. Each task-absent trial lasted 10 to 20 min. After the experiment, the recording surface was cleaned with 0.05% chlorhexidine and sterile water and was closed with the cover. The animal was then returned to their home cage.
Image Processing.
We first aligned image frames using a rigid-body transformation within each session to eliminate any slow drift of image position within the FOV. To achieve this, we first calculated an averaged image across the session and saved it as a reference. Then, we aligned each frame to this reference using Elastix software (98). To align all sessions to a common reference, we next consecutively performed rigid-body, affine, and B-spline transformations using Elastix. We then created a mask for the within-brain area shared across all sessions with the sagittal sinus excluded.
To remove time points contaminated by motion artifacts, we used the averaged signal of an area outside the brain as control. We set a criterion for a time point to be an outlier if the control signal was above 1.5(Q3 − Q1) + Q3, where Qi stands for the ith quartile of the control signal. Once the noise was removed, we band-pass-filtered the image data between 0.005 Hz and 0.5 Hz and standardized the signal of each pixel. Finally, we spatially smoothed the images using a two-dimensional (2D) Gaussian filter with sigma = 2 pixels in each dimension.
Brain Parcellation.
We first vectorized the image data such that each column was the masked pixels within a frame. To perform group parcellation across all sessions, we calculated PCA to reduce the temporal dimensionality (no. of columns) to 30 for each session and concatenated all sessions by the reduced dimension (99). Suppose we have the vectorized and centered fUS data for the i-th session , where is the number of pixels (the same across sessions) and is the number of frames in session . Conducting PCA decomposition, we have , where is the number of PCs, is the set of spatial eigenvectors, and is the temporal ones. Projecting onto the first temporal eigenvectors, we have reduced data of the i-th session . Concatenating of all sessions, we have a group-level PCA . Brain parcellation was carried out on , where the reduced dimensions were features. Pixels with similar patterns were identified by using spectral clustering. Briefly, we calculated the pairwise Spearman’s rank correlation coefficient for the rows of , based on which we established an similarity matrix with diagonal elements 0 and off-diagonals , where is the Spearman’s correlation between pixel and . We then performed spectral clustering (100) using the similarity matrix, for which we calculated the symmetric normalized Laplacian matrix , where is the degree matrix. We calculated the eigenvectors corresponding to the largest eigenvalues of and performed k-means clustering on the eigenvectors. This yielded the spectral clustering results. A similar procedure was carried out for the subject specific parcellation.
Cardiac Data Acquisition.
To record ECGs, we put on an elastic band to the chest of the marmosets with a pair of Ag–AgCl surface electrodes (Grass Technology) sewed on. The data were resampled into 1 kHz and band-pass-filtered between 15 and 100 Hz. To identify heartbeats, we calculated sliding correlation coefficients between session-specific templates and the ECG signal, and these locations were marked as one if the correlation coefficient was above a threshold. This binary signal was convolved with a Gaussian window to estimate the momentary HR.
Behavioral Videography.
In a lighted room, the subject was placed in a customized chair with its front facing a camera (Logitech C920). Videos of the subjects were recorded at 24-Hz framerate and synchronized with fUS acquisition. Essentially, a 1-µs pulse signaling the end of image acquisition (400 ms long per 500-ms cycle) was passed to the interrupt channel of an Arduino board, which converted the signal to a 200-µs transistor–transistor logic (TTL) pulse to be recorded. The sound was also recorded from a microphone placed next to the subject (Zoom H4n Pro). Audio, TTL pulse, and ECG signal were simultaneously recorded through a data-acquisition box (Muscle SpikerBox Pro) at a sample rate of 44.1 kHz. This audio signal was aligned with the audio channel of the video through a brief playback of white noise. The of the ECG and video was aligned to the onset of the first fUS frame’s TTL pulse before downsampling to 2 Hz, where the first sample was kept as the one corresponding to before the downsampling. We created masks for the subject based on pixel variance contrasting the static background and aligned the masked images across sessions through rigid-body transformation and affine transformation using Elastix. Video analysis was carried out for the aligned videos. We defined MI as the absolute value of the total intensity difference between two adjacent frames, scaled by the mean across the session.
To extract different types of motor behaviors, we performed a group ICA. First, we carried out group PCA by concatenating the first 100 PCs across 35 video sessions. Next, we performed ICA on the group PCs and identified 34 spatial modes. By inspecting the spatial patterns, we categorized the behaviors into six types: blink, mouth movement, ear movement, arm and hand movement, foot and tail movement, and body movement. We calculated the momentary behavioral index as the inner product between each IC and the video frame, then took the maximal value at each time point across the subset of ICs belonging to the same behavioral category. To estimate the fractional time spent on each type of behavior, we calculated the proportion of time during which behavior indices were above a threshold of the session. Time not spent on any movements was determined as being still.
Coherence.
The coherence between MI and HR was calculated by using the MATLAB function cmtm. We concatenated the time series of all 35 sessions to minimize the edge effect. To remove the baseline difference in HR by sessions, we used the z score of HR. Statistical significance was determined as being greater than the 95% CI of the null coherence, which was estimated by phase-randomized surrogates of the time series repeated 1,000 times. We separated periods of substantial movements and subtle movements using a threshold of MI. If a substantial movement was detected, we also included the time points 10 s before and after. The rest was considered to contain only subtle movements. The coherence analyses were repeated for concatenated segments of substantial and subtle movements.
PDC.
PDC infers causal relationships based on the vector autoregressive (VAR) model coefficients in the frequency domain. In the following, we first introduce the definition of PDC and then modify it to bPDC. Let be a K-dimension vector time series. In this study, each dimension was the signal of an ROI, the MI, or the HR. The VAR model of signal is given by
where is the multivariate zero-mean white-noise process with covariance matrix , and is a matrix. Here, we assume that signals are zero mean and standardized. To choose the order for the VAR(p) model, we used the Akaike’s information criterion (AIC)
where is the estimated covariance matrix with model order , and is the number of observations. The maximum-likelihood estimator for with order is . With the optimal selected by AIC, the VAR coefficients were estimated by using the Nuttall–Strand method.
Denoting the elements of by , we define elements of in the frequency domain
where . The PDC from to in the frequency domain is then defined as
where denotes the complex conjugate. We used the squared PDC measure , which relates to the mutual information rate (MIR) via . In this work, we used a similar measure as MIR for total information flow between two nodes , where the cutoff frequency Hz (1/4 unit frequency with a sampling rate of 2 Hz).
To consider the multivariate brain activity as a whole subset of the brain-motor-physiology network, we adopted the bPDC. If , where and , the total dimension of is a vector consisting of subsets. Following the VAR representation above, we now divide into blocks , each an dimensional matrix, and, hence, now is defined in the block sense, i.e., the block. Subsequently, the inverse spectral density matrix of the multivariate time series is
where is the block of the inverse covariance matrix . The bPDC from to block is then defined as
where is the determinant. The calculation of the information flow between blocks is the same as the PDC case.
In this study, we concatenated the time series across all sessions to reduce potential edge effects and increase statistical power. Before the concatenation, we normalized ROI signals and HR, but not MI, within each session. The PDC was calculated by using a package available at https://www.lcs.poli.usp.br/~baccala/pdc/. The bPDC expansion is available at https://www.lcs.poli.usp.br/~baccala/pdc/canon/. To test the significance of bPDC values, we phase-randomized the time series 1,000 times and constructed the 95% CI of the null distribution. We set the criteria for statistical significance with Bonferroni correction for multiple comparisons , as there were six directional relationships to examine. To test the difference between and , we bootstrapped the data by resampling sessions with replacement and calculated the bPDC for each resampled time series. This procedure was repeated 1,000 times to estimate the distribution of information flow in each direction. We then compared each pair of directions using paired z tests with a Bonferroni-corrected criterion .
We defined information flow above the 75th percentile within each category as strongly influencing directions. The categories were 1) for , 2) for , and 3) for .
Brain-Image Registration to Atlas.
We adopted a two-step registration using the volumetric brain vasculature images as a nexus linking the fUS images to a standard brain atlas. More details of this procedure (applied to a rat brain) have been described in ref. 101; here, we briefly introduce the steps and the adaptation for marmoset brains.
Perfusion.
After the endpoint of each subject, the brain was perfused with 50 mL of fluorescence-tagged albumin hydrogel. The hydrogel was made of 5 mg of AF647-albumin (catalog no. A34785, Invitrogen) mixed in 50 mL of 2% (weight/volume) gelatin (catalog no. G6144, Sigma-Aldrich) in phosphate-buffered saline. The brain was extracted and further submerged in 4% paraformaldehyde at 4 °C overnight and then was processed by using the iDISCO clearing protocol (102).
Light-sheet microscopy.
The brain was cut along the sagittal direction into six pieces of equal thickness (∼4 mm) with the cerebellum excluded. Each chunk was imaged with the medial side facing up under a light-sheet microscope (LaVision Biotech) using a 1.3x objective. We used the 488-nm channel for autofluorescence imaging and the 647-nm for vasculature imaging. The sample was scanned with a 1.3x objective, which yielded a 5-μm × 5-μm pixel size and with a z step of 5 μm.
Registration.
The registration procedure took two steps: One registered the autofluorescence channel of light-sheet images to a standard brain atlas (step 1), and one registered a reference fUS image to a plane of vasculature light-sheet image from the same subject (step 2).
Step 1: Light sheet to the brain atlas.
The light-sheet image stacks were first down-sampled to 50 μm isotropic. Using the autofluorescence images, the surfaces between two adjacent brain pieces were extracted and matched by using the iterative closest-point algorithm (MATLAB routine icp). The chunks were translated and rotated numerically to be coarsely assembled. We then registered this assembly to a brain atlas (103) by applying rigid and affine transformations using Elastix (98). After this coarse registration, we refined the alignment to the atlas for each piece iteratively by applying rigid, affine, and B-spline transformations. The same transformations were applied to the vasculature channel, thereby registering the brain vasculature to the atlas.
Step 2: fUS to light sheet.
To localize the fUS position in the brain, we created a subject-specific local 2D vascular atlas by taking the max-intensity projection over 300-μm thickness along the midline of the registered vasculature volume. The fUS image of the same subject was aligned with this vasculature reference by using a landmark-based 2D registration procedure [ImageJ plugin bUnwarpJ (104)]. The fUS images were hence registered to the standard atlas.
Directed Graph Clustering.
To detect clusters (or communities) within a directed graph, we adopted the weighted-cuts algorithm for spectral clustering (105). Here, the similarity matrix is the asymmetric directed information flow with zero diagonal between each pair of nodes in the network, i.e., the ROIs. Essentially, we normalized the Laplacian matrix with node weights ; here, we used , which is simply the out-degree. We next found the eigenvectors corresponding to the smallest eigenvalues of and then performed the k-means clustering on the eigenvectors. The clustering result identifies the nodes belonging to the same cluster. The MATLAB package can be found at https://sites.stat.washington.edu/mmp/software.html.
Brain-State Clustering.
We followed ref. 61 to decompose imaging data into a spectral-spatial space. Using the group-PCA calculated above, we further extracted 25 group ICs (group-ICA) representing the spatial distribution of potential source signals. We filtered the brain images into spectral bands using maximal overlap discrete wavelet packet transformation with a Daubechies wavelet of two vanishing moments. Then, we multiplied the ICs and each of the frequency bands of the brain image , where is the ICs of size and is the wavelet packet transform of frequency with size of . Thus, the dimension of a time point was reduced to . For the multitaper approach, we decomposed each pixel into time and frequency by calculating the multitaper spectrum over a moving window. The spectrogram was estimated by using the chronux MATLAB package (106, 107) function mtspecgramc, with TW = 0.5, K = 1, and 4.5-s window size.
One problem with group-level clustering is that variations created by experiments due to slight differences in, e.g., probe positioning and signal quality and the difference in subjects’ brain morphology may become dominant and drive the clustering results. To deal with this, we minimized the dependence on experimental sessions using an expectation-maximization procedure with penalization on session dependence in the objective function of K-means clustering. Then, we transformed the data iteratively to a common space across sessions. This algorithm was based on the Harmony method developed for high-dimensional biological data like RNA sequencing (108). In this study, we modified this algorithm to deal with time series with the temporal structure maintained during the optimization by applying ridge regularization (the elaborated method will appear in a separate article). The transformed data yielded clustering with session-dependence largely reduced (SI Appendix, Fig. S10). Data used for the correction were the dimensionality-reduced data described above. We further performed k-means clustering with correlation measure for distance to identify the brain state for each time point.
State Score.
To evaluate to what extent the ROI activity was attributed to changes in brain state, we estimated the percentage variance of ROI activity explained by the occurrence of each brain state using cross-validation. We randomly stratified the data into 10 folds, trained linear models of , where is the activity of the ith ROI, is the one-hot encoding of brain state , and is a random noise, using the 9 folds of the data, and calculated test variance explained as using the remaining fold. We estimated the mean and SD of variance explained across the 10-time cross-validations for each ROI and each brain state. Assuming the variances explained were normally distributed, we tested whether the variance explained was equal to zero. The null hypothesis would be rejected if the mean variance explained was greater than 1.96 times the estimated SD. Our results showed that states 1 and 4 covaried and 2 and 3 covaried. We thus defined a state score as , where is the variance explained for ROI i by the j-th state. Thus, if a signal were more strongly correlated with states 1 and 2 than states 3 and 4, the state score would be positive, and vice versa. To estimate the variance of the state scores, we applied chain rule .
State Correlation with Control Preference.
We defined the control preference as the difference between the information flow from ROI to movement and from ROI to HR: . To test the dependence of control preference on brain state, using each ROI as a sample, we performed a weighted linear regression with the weight as (a similar result was obtained with ordinary least squares). The criterion for statistical significance was set at .
PSD.
To estimate the temporal profile of the alternation between the two groups of states, we converted the sequence of brain states into a binary sequence with states 1 and 2 labeled 1 and states 3 and 4 labeled 0. We used the chronux routine mtspectrumc_unequal_length_trials to estimate the PSD and the 95% CI of the PSD with taper TW = 10.5 and K = 20.
Supplementary Material
Acknowledgments
We thank Uri Hasson and Samuel Nastase for comments on the manuscript. This work was supported by NIH–National Institute of Neurological Disorders and Stroke Grant R01NS054898 (to A.A.G.).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2201194119/-/DCSupplemental.
Data, Materials, and Software Availability
Data are available from the Dryad Digital Repository (DOI: 10.5061/dryad.jwstqjq9x) (109).
References
- 1.Cannon W. B., Organization for physiological homeostasis. Physiol. Rev. 9, 399–431 (1929). [Google Scholar]
- 2.Pezzulo G., Rigoli F., Friston K., Active inference, homeostatic regulation and adaptive behavioural control. Prog. Neurobiol. 134, 17–35 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seth A. K., Interoceptive inference, emotion, and the embodied self. Trends Cogn. Sci. 17, 565–573 (2013). [DOI] [PubMed] [Google Scholar]
- 4.Anderson D. J., Circuit modules linking internal states and social behaviour in flies and mice. Nat. Rev. Neurosci. 17, 692–704 (2016). [DOI] [PubMed] [Google Scholar]
- 5.Gray M. A., et al. , Emotional appraisal is influenced by cardiac afferent information. Emotion 12, 180–191 (2012). [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y. S., Ghazanfar A. A., Perinatally influenced autonomic system fluctuations drive infant vocal sequences. Curr. Biol. 26, 1249–1260 (2016). [DOI] [PubMed] [Google Scholar]
- 7.Borjon J. I., Takahashi D. Y., Cervantes D. C., Ghazanfar A. A., Arousal dynamics drive vocal production in marmoset monkeys. J. Neurophysiol. 116, 753–764 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liao D. A., Zhang Y. S., Cai L. X., Ghazanfar A. A., Internal states and extrinsic factors both determine monkey vocal production. Proc. Natl. Acad. Sci. U.S.A. 115, 3978–3983 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Skora S., Mende F., Zimmer M., Energy scarcity promotes a brain-wide sleep state modulated by insulin signaling in C. elegans. Cell Rep. 22, 953–966 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Margulies D. S., et al. , Situating the default-mode network along a principal gradient of macroscale cortical organization. Proc. Natl. Acad. Sci. U.S.A. 113, 12574–12579 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huntenburg J. M., Bazin P.-L., Margulies D. S., Large-scale gradients in human cortical organization. Trends Cogn. Sci. 22, 21–31 (2018). [DOI] [PubMed] [Google Scholar]
- 12.Mesulam M. M., From sensation to cognition. Brain 121, 1013–1052 (1998). [DOI] [PubMed] [Google Scholar]
- 13.Pollatos O., Schandry R., Auer D. P., Kaufmann C., Brain structures mediating cardiovascular arousal and interoceptive awareness. Brain Res. 1141, 178–187 (2007). [DOI] [PubMed] [Google Scholar]
- 14.Andrews-Hanna J. R., The brain’s default network and its adaptive role in internal mentation. Neuroscientist 18, 251–270 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bota M., Sporns O., Swanson L. W., Architecture of the cerebral cortical association connectome underlying cognition. Proc. Natl. Acad. Sci. U.S.A. 112, E2093–E2101 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cioli C., Abdi H., Beaton D., Burnod Y., Mesmoudi S., Differences in human cortical gene expression match the temporal properties of large-scale functional networks. PLoS One 9, e115913 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Golland Y., Golland P., Bentin S., Malach R., Data-driven clustering reveals a fundamental subdivision of the human cortex into two global systems. Neuropsychologia 46, 540–553 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boly M., et al. , Baseline brain activity fluctuations predict somatosensory perception in humans. Proc. Natl. Acad. Sci. U.S.A. 104, 12187–12192 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vinck M., Batista-Brito R., Knoblich U., Cardin J. A., Arousal and locomotion make distinct contributions to cortical activity patterns and visual encoding. Neuron 86, 740–754 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niell C. M., Stryker M. P., Modulation of visual responses by behavioral state in mouse visual cortex. Neuron 65, 472–479 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Damasio A. R., et al. , Subcortical and cortical brain activity during the feeling of self-generated emotions. Nat. Neurosci. 3, 1049–1056 (2000). [DOI] [PubMed] [Google Scholar]
- 22.de Gee J. W., et al. , Dynamic modulation of decision biases by brainstem arousal systems. eLife 6, e23232 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Allen W. E., et al. , Thirst regulates motivated behavior through modulation of brainwide neural population dynamics. Science 364, 253 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stringer C., et al. , Spontaneous behaviors drive multidimensional, brainwide activity. Science 364, 255 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Drew P. J., Winder A. T., Zhang Q., Twitches, blinks, and fidgets: Important generators of ongoing neural activity. Neuroscientist 25, 298–313 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Musall S., Kaufman M. T., Juavinett A. L., Gluf S., Churchland A. K., Single-trial neural dynamics are dominated by richly varied movements. Nat. Neurosci. 22, 1677–1686 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salkoff D. B., Zagha E., McCarthy E., McCormick D. A., Movement and performance explain widespread cortical activity in a visual detection task. Cereb. Cortex 30, 421–437 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaplan H. S., Zimmer M., Brain-wide representations of ongoing behavior: A universal principle? Curr. Opin. Neurobiol. 64, 60–69 (2020). [DOI] [PubMed] [Google Scholar]
- 29.Damasio A., Carvalho G. B., The nature of feelings: Evolutionary and neurobiological origins. Nat. Rev. Neurosci. 14, 143–152 (2013). [DOI] [PubMed] [Google Scholar]
- 30.McGinley M. J., et al. , Waking state: Rapid variations modulate neural and behavioral responses. Neuron 87, 1143–1161 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raut R. V., et al. , Global waves synchronize the brain’s functional systems with fluctuating arousal. Sci. Adv. 7, eabf2709 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adams R. A., Shipp S., Friston K. J., Predictions not commands: Active inference in the motor system. Brain Struct. Funct. 218, 611–643 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keller G. B., Mrsic-Flogel T. D., Predictive processing: A canonical cortical computation. Neuron 100, 424–435 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barrett L. F., Simmons W. K., Interoceptive predictions in the brain. Nat. Rev. Neurosci. 16, 419–429 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sterling P., Allostasis: A model of predictive regulation. Physiol. Behav. 106, 5–15 (2012). [DOI] [PubMed] [Google Scholar]
- 36.Seth A. K., Friston K. J., Active interoceptive inference and the emotional brain. Philos. Trans. R. Soc. B: Biol. Sci. 371, 20160007 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Winder A. T., Echagarruga C., Zhang Q., Drew P. J., Weak correlations between hemodynamic signals and ongoing neural activity during the resting state. Nat. Neurosci. 20, 1761–1769 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Julien C., The enigma of Mayer waves: Facts and models. Cardiovasc. Res. 70, 12–21 (2006). [DOI] [PubMed] [Google Scholar]
- 39.Gutierrez-Barragan D., Basson M. A., Panzeri S., Gozzi A., Infraslow state fluctuations govern spontaneous fMRI network dynamics. Curr. Biol. 29, 2295–2306.e5 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Palva J. M., Palva S., Infra-slow fluctuations in electrophysiological recordings, blood-oxygenation-level-dependent signals, and psychophysical time series. Neuroimage 62, 2201–2211 (2012). [DOI] [PubMed] [Google Scholar]
- 41.Drew P. J., Mateo C., Turner K. L., Yu X., Kleinfeld D., Ultra-slow oscillations in fMRI and resting-state connectivity: Neuronal and vascular contributions and technical confounds. Neuron 107, 782–804 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Macé E., et al. , Functional ultrasound imaging of the brain. Nat. Methods 8, 662–664 (2011). [DOI] [PubMed] [Google Scholar]
- 43.Urban A., et al. , Real-time imaging of brain activity in freely moving rats using functional ultrasound. Nat. Methods 12, 873–878 (2015). [DOI] [PubMed] [Google Scholar]
- 44.Macé É., et al. , Whole-brain functional ultrasound imaging reveals brain modules for visuomotor integration. Neuron 100, 1241–1251.e7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Raichle M. E., The brain’s default mode network. Annu. Rev. Neurosci. 38, 433–447 (2015). [DOI] [PubMed] [Google Scholar]
- 46.Napadow V., et al. , Brain correlates of autonomic modulation: Combining heart rate variability with fMRI. Neuroimage 42, 169–177 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Woodward A., et al. , The Brain/MINDS 3D digital marmoset brain atlas. Sci. Data 5, 180009 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hardman C. D., Ashwell K. W., Stereotaxic and Chemoarchitectural Atlas of the Brain of the Common Marmoset (Callithrix jacchus) (CRC Press, Boca Raton, FL, 2012). [Google Scholar]
- 49.Takahashi D. Y., Baccalá L. A., Sameshima K., Canonical information flow decomposition among neural structure subsets. Front. Neuroinform. 8, 49 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baccalá L. A., Sameshima K., Partial directed coherence: A new concept in neural structure determination. Biol. Cybern. 84, 463–474 (2001). [DOI] [PubMed] [Google Scholar]
- 51.Chang C., Cunningham J. P., Glover G. H., Influence of heart rate on the BOLD signal: The cardiac response function. Neuroimage 44, 857–869 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mateo C., Knutsen P. M., Tsai P. S., Shih A. Y., Kleinfeld D., Entrainment of arteriole vasomotor fluctuations by neural activity is a basis of blood-oxygenation-level-dependent “resting-state” connectivity. Neuron 96, 936–948.e3 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Takahashi D. Y., Baccalá L. A., Sameshima K., Information theoretic interpretation of frequency domain connectivity measures. Biol. Cybern. 103, 463–469 (2010). [DOI] [PubMed] [Google Scholar]
- 54.Penfield W., Welch K., The supplementary motor area of the cerebral cortex; a clinical and experimental study. AMA Arch. Neurol. Psychiatry 66, 289–317 (1951). [DOI] [PubMed] [Google Scholar]
- 55.Kandel E. R., et al. , Principles of Neural Science (McGraw-Hill, New York, 2000). [Google Scholar]
- 56.Sheehan T. P., Chambers R. A., Russell D. S., Regulation of affect by the lateral septum: Implications for neuropsychiatry. Brain Res. Brain Res. Rev. 46, 71–117 (2004). [DOI] [PubMed] [Google Scholar]
- 57.Díaz-Parra A., Osborn Z., Canals S., Moratal D., Sporns O., Structural and functional, empirical and modeled connectivity in the cerebral cortex of the rat. Neuroimage 159, 170–184 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hori Y., et al. , Comparison of resting-state functional connectivity in marmosets with tracer-based cellular connectivity. Neuroimage 204, 116241 (2020). [DOI] [PubMed] [Google Scholar]
- 59.Messé A., Parcellation influence on the connectivity-based structure-function relationship in the human brain. Hum. Brain Mapp. 41, 1167–1180 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chang C., Glover G. H., Time-frequency dynamics of resting-state brain connectivity measured with fMRI. Neuroimage 50, 81–98 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Billings J. C. W., et al. , Instantaneous brain dynamics mapped to a continuous state space. Neuroimage 162, 344–352 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mitra P. P., Pesaran B., Analysis of dynamic brain imaging data. Biophys. J. 76, 691–708 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rocks J. W., Ronellenfitsch H., Liu A. J., Nagel S. R., Katifori E., Limits of multifunctionality in tunable networks. Proc. Natl. Acad. Sci. U.S.A. 116, 2506–2511 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bullmore E., Sporns O., The economy of brain network organization. Nat. Rev. Neurosci. 13, 336–349 (2012). [DOI] [PubMed] [Google Scholar]
- 65.Attwell D., Laughlin S. B., An energy budget for signaling in the grey matter of the brain. J. Cereb. Blood Flow Metab. 21, 1133–1145 (2001). [DOI] [PubMed] [Google Scholar]
- 66.Kato S., et al. , Global brain dynamics embed the motor command sequence of Caenorhabditis elegans. Cell 163, 656–669 (2015). [DOI] [PubMed] [Google Scholar]
- 67.Stawarczyk D., Bezdek M. A., Zacks J. M., Event representations and predictive processing: The role of the midline default network core. Top. Cogn. Sci. 13, 164–186 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Raichle M. E., Gusnard D. A., Intrinsic brain activity sets the stage for expression of motivated behavior. J. Comp. Neurol. 493, 167–176 (2005). [DOI] [PubMed] [Google Scholar]
- 69.Baccalá L. A., Sameshima K., “Overcoming the limitations of correlation analysis for many simultaneously processed neural structures” in Advances in Neural Population Coding, Nicolelis M. A. L., Ed. (Progress in Brain Research, Elsevier, Amsterdam, 2001), vol. 130, pp. 33–47. [DOI] [PubMed] [Google Scholar]
- 70.Cerkevich C. M., Rathelot J.-A., Strick P. L., Cortical basis for skilled vocalization. Proc. Natl. Acad. Sci. U.S.A. 119, e2122345119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Swanson L., “The limbic region. I: The septohippocampal system” in Methods in Chemical Neuroanatomy, Björklund A., Ed. (Handbook of Chemical Neuroanatomy, Elsevier, Amsterdam, 1987), vol. 5, pp. 125–277. [Google Scholar]
- 72.Alexander L., Clarke H. F., Roberts A. C., A focus on the functions of area 25. Brain Sci. 9, 129 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chang C., et al. , Association between heart rate variability and fluctuations in resting-state functional connectivity. Neuroimage 68, 93–104 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rebollo I., Devauchelle A.-D., Béranger B., Tallon-Baudry C., Stomach-brain synchrony reveals a novel, delayed-connectivity resting-state network in humans. eLife 7, e33321 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim K., et al. , Resting-state neural firing rate is linked to cardiac-cycle duration in the human cingulate and parahippocampal cortices. J. Neurosci. 39, 3676–3686 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Beissner F., Meissner K., Bär K. J., Napadow V., The autonomic brain: An activation likelihood estimation meta-analysis for central processing of autonomic function. J. Neurosci. 33, 10503–10511 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Murphy K., Birn R. M., Bandettini P. A., Resting-state fMRI confounds and cleanup. Neuroimage 80, 349–359 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Power J. D., et al. , Ridding fMRI data of motion-related influences: Removal of signals with distinct spatial and physical bases in multiecho data. Proc. Natl. Acad. Sci. U.S.A. 115, E2105–E2114 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hirano Y., et al. , Investigation of the BOLD and CBV fMRI responses to somatosensory stimulation in awake marmosets (Callithrix jacchus). NMR Biomed. 31, e3864 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nunez-Elizalde A. O., et al. , Neural correlates of blood flow measured by ultrasound. Neuron. 110, 1631–1640 (2022). [DOI] [PMC free article] [PubMed]
- 81.Aydin A.-K., et al. , Transfer functions linking neural calcium to single voxel functional ultrasound signal. Nat. Commun. 11, 2954 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Boido D., et al. , Mesoscopic and microscopic imaging of sensory responses in the same animal. Nat. Commun. 10, 1110 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Golanov E. V., Yamamoto S., Reis D. J., Spontaneous waves of cerebral blood flow associated with a pattern of electrocortical activity. Am. J. Physiol. 266, R204–R214 (1994). [DOI] [PubMed] [Google Scholar]
- 84.Cechetto D. F., Cortical control of the autonomic nervous system. Exp. Physiol. 99, 326–331 (2014). [DOI] [PubMed] [Google Scholar]
- 85.Tort A. B. L., Hammer M., Zhang J., Brankačk J., Draguhn A., Temporal relations between cortical network oscillations and breathing frequency during REM sleep. J. Neurosci. 41, 5229–5242 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fox M. D., Raichle M. E., Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci. 8, 700–711 (2007). [DOI] [PubMed] [Google Scholar]
- 87.Li J. M., Bentley W. J., Snyder L. H., Functional connectivity arises from a slow rhythmic mechanism. Proc. Natl. Acad. Sci. U.S.A. 112, E2527–E2535 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vanni M. P., Chan A. W., Balbi M., Silasi G., Murphy T. H., Mesoscale mapping of mouse cortex reveals frequency-dependent cycling between distinct macroscale functional modules. J. Neurosci. 37, 7513–7533 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mitra A., et al. , Spontaneous infra-slow brain activity has unique spatiotemporal dynamics and laminar structure. Neuron 98, 297–305.e6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Belcher A. M., et al. , Large-scale brain networks in the awake, truly resting marmoset monkey. J. Neurosci. 33, 16796–16804 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schroeder M. P., Weiss C., Procissi D., Disterhoft J. F., Wang L., Intrinsic connectivity of neural networks in the awake rabbit. Neuroimage 129, 260–267 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pawela C. P., et al. , Resting-state functional connectivity of the rat brain. Magn. Reson. Med. 59, 1021–1029 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pais-Roldán P., et al. , Contribution of animal models toward understanding resting state functional connectivity. Neuroimage 245, 118630 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yeshurun Y., Nguyen M., Hasson U., The default mode network: Where the idiosyncratic self meets the shared social world. Nat. Rev. Neurosci. 22, 181–192 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Russell S. J., Artificial Intelligence a Modern Approach (Pearson Education, Inc., Harlow, UK, 2010). [Google Scholar]
- 96.Bassett D. S., et al. , Dynamic reconfiguration of human brain networks during learning. Proc. Natl. Acad. Sci. U.S.A. 108, 7641–7646 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schmälzle R., et al. , Brain connectivity dynamics during social interaction reflect social network structure. Proc. Natl. Acad. Sci. U.S.A. 114, 5153–5158 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Klein S., Staring M., Murphy K., Viergever M. A., Pluim J. P., Elastix: A toolbox for intensity-based medical image registration. IEEE Trans. Med. Imaging 29, 196–205 (2010). [DOI] [PubMed] [Google Scholar]
- 99.Smith S. M., Hyvärinen A., Varoquaux G., Miller K. L., Beckmann C. F., Group-PCA for very large fMRI datasets. Neuroimage 101, 738–749 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ng A. Y., Jordan M. I., Weiss Y., On spectral clustering: Analysis and an algorithm. Adv. Neural Inf. Process. Syst. 14, 849–856 (2002). [Google Scholar]
- 101.El Hady A., et al. , Chronic brain functional ultrasound imaging in freely moving rodents performing cognitive tasks. bioRxiv [Preprint] (2022). https://www.biorxiv.org/content/10.1101/2022.01.29.478327v2. Accessed 7 September 2022. [DOI] [PMC free article] [PubMed]
- 102.Renier N., et al. , iDISCO: A simple, rapid method to immunolabel large tissue samples for volume imaging. Cell 159, 896–910 (2014). [DOI] [PubMed] [Google Scholar]
- 103.Liu C., et al. , A digital 3D atlas of the marmoset brain based on multi-modal MRI. Neuroimage 169, 106–116 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Arganda-Carreras I., et al. , "Consistent and elastic registration of histological sections using vector-spline regularization" in Computer Vision Approaches to Medical Image Analysis. R. R. Beichel, M. Sonka, Eds. (CVAMIA, 2006). 4241, pp. 85–95 . [Google Scholar]
- 105.Meilă M., Pentney W. (2007) “Clustering by weighted cuts in directed graphs” in Proceedings of the 2007 SIAM International Conference on Data Mining (SDM), Apte C., Skillicorn D., Liu B., Parthasarathy S., Eds. (Society for Industrial and Applied Mathematics, Philadelphia: ), 135–144. [Google Scholar]
- 106.Mitra P., Observed Brain Dynamics (Oxford University Press, Oxford, UK, 2007). [Google Scholar]
- 107.Bokil H., Andrews P., Kulkarni J. E., Mehta S., Mitra P. P., Chronux: A platform for analyzing neural signals. J. Neurosci. Methods 192, 146–151 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Korsunsky I., et al. , Fast, sensitive and accurate integration of single-cell data with Harmony. Nat. Methods 16, 1289–1296 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhang Y., Takahashi D., El Hady A., Ghazanfar A., Active neural coordination of motor behaviors with internal states. Dryad. 10.5061/dryad.jwstqjq9x. Deposited 17 May 2022. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available from the Dryad Digital Repository (DOI: 10.5061/dryad.jwstqjq9x) (109).