Abstract
Background
Syphilis diagnosis may be challenging, especially in the asymptomatic and early clinical stages. We evaluated the presence of Treponema pallidum DNA (TP-DNA) in various sample types to elucidate transmissibility during various syphilis stages.
Methods
The study was conducted at the Amsterdam Centre for Sexual Health. We included adult men who have sex with men (MSM), who were suspected of having syphilis. The 2020 European guidelines definitions were followed for the diagnosis and staging of syphilis. Using a polymerase chain reaction (PCR) targeting the polA gene of Treponema pallidum (TP-PCR), we tested the following study samples on TP-DNA: peripheral blood, oropharyngeal swab, ano-rectal swab, and urine.
Results
From November 2018 to December 2019 we included 293 MSM. Seventy clients had primary syphilis, 73 secondary syphilis, 86 early latent syphilis, 14 late latent syphilis, 23 treated syphilis, and 27 had no syphilis. TP-DNA was detected in at least 1 study sample in 35/70 clients with primary syphilis (2/70 peripheral blood, 7/70 oropharynx, 13/70 ano-rectum, and 24/70 urine); in 62/73 clients with secondary syphilis (15/73 peripheral blood, 47/73 oropharynx, 37/73 ano-rectum, and 26/73 urine); and in 29/86 clients with early latent syphilis (5/86 peripheral blood, 21/86 oropharynx, 11/86 ano-rectum, and 6/86 urine). TP-DNA was not detected in clients with late latent syphilis or treated syphilis, nor in clients without syphilis.
Conclusions
TP-DNA was frequently detected in various sample types in the absence of lesions. This is in line with the high transmission rate of syphilis and opens diagnostic opportunities for early presymptomatic syphilis stages.
Keywords: Treponema pallidum, syphilis, homosexuality, male
DNA of Treponema pallidumis frequently detected in oral and anal mucosa and urine of men with early syphilis, even in the absences of lesions. This may explain the high contagiousness of early syphilis.
Syphilis is a multistage sexually transmitted disease (STD) caused by the bacterium Treponema pallidum subspecies pallidum (TP) [1]. Worldwide syphilis rates are on the rise [2]. Like in many countries in the Western world, in the Netherlands syphilis is predominantly found in men who have sex with men (MSM), and especially in MSM living with human immunodeficiency virus (HIV) [3, 4].
Early syphilis is defined as the infectious stage within the first year after acquisition. Early syphilis can be subdivided in primary syphilis, characterized by the presence of a chancre, secondary syphilis, which is clinically polymorphous, and early latent syphilis, which is symptomless [5]. After the first year, spirochetes can persist in untreated patients and cause late symptoms of syphilis. However, the spirochete burden at this late stage is considered to be very low, and patients are regarded as not sexually infectious [6]. Sexual syphilis acquisition is believed to occur via exposure to infectious genital lesions but also via lesions in body orifices involved in sexual contact such as the anus and mouth. These lesions are considered highly infectious, with an efficiency of transmission estimated at approximately 30% per sexual act [7]. The high incidence of early syphilis among MSM emphasizes the need to detect infected individuals as early as possible to prevent ongoing transmission.
The diagnosis of syphilis remains challenging. A combination of clinical findings, direct demonstration of TP by dark field microscopy or polymerase chain reaction (PCR) tests, or indirect serological tests form the basis for a syphilis diagnosis [8]. However, in primary syphilis, serological tests may be negative due to the window period between transmission and seroconversion. Therefore, PCR tests for the detection of T. pallidum have been introduced as routine diagnostics in several medical microbiology laboratories worldwide [9–12], including in our public health laboratory in Amsterdam, The Netherlands [13, 14].
The sensitivity of T. pallidum PCR (TP-PCR) tests is high in primary syphilitic chancres and secondary stage condyloma lata but low when used on samples from stage 2 roseoles and peripheral blood samples [9, 14–18]. Positive TP-DNA results have also been seen in anal [19] and pharyngeal swabs in patients with primary ulcers on these locations [20, 21]. In addition, TP-DNA has been found in blood and urine samples from patients with syphilis [22–26]. A recent study performed in Australia showed that TP-DNA was frequently found in lesional samples but less often in nonlesional samples [27].
Although it is known that primary and secondary syphilis are highly infectious, it is not well understood how transmission to other patients occurs [28]. So far, the extent of secondary spirochetal spread to distant mucosal locations has not been systematically evaluated in patients. The aim of this study is to evaluate the presence of TP-DNA in peripheral blood and body orifices such as the oropharynx, ano-rectum, and urethra (urine used as a proxy for urethra) in patients with early syphilis. This to improve syphilis diagnostic options and elucidate transmissibility during various syphilis stages.
METHODS
Study Population and Study Design
The Center for Sexual Health of the Amsterdam Public Health Service, The Netherlands, is a low-threshold sexually transmitted infection (STI) clinic performing approximately 50 000 consultations annually. Consultations are at the client’s own initiative, anonymous and free of charge. From November 2018 through December 2019 participants were recruited among clients aged 18 years and older visiting the STI clinic.
Clients were eligible for participation if they were MSM and (1) had signs and symptoms suggestive of primary syphilis or secondary syphilis or (2) were diagnosed with early latent or late latent syphilis. Clients belonging to the first group were invited on their first visit (Supplementary Figure 1). Patients belonging to the second group were invited at their follow-up clinic visit.
Upon consent, we collected peripheral blood, client-obtained oropharyngeal and ano-rectal swab, and urine (as proxy for urethral mucosa) for TP-DNA analysis, in addition to the routine diagnostic samples, often including a genital ulcer swab. According to the 2020 European guideline for syphilis [29], participants were allocated into groups based on clinical signs and symptoms, and serological and routine molecular test results using the following algorithm:
Primary syphilis: oro- or ano-genital ulcerative disease and a positive dark field microscopy (DFM) or a positive PCR result of the ulcer swab.
Secondary syphilis: a rash with or without lymphadenopathy, or mucosal lesions such as condylomata lata, and a rapid plasma reagin (RPR) ≥ 1:4.
Early latent syphilis: no symptoms and a seroconversion of the chemoluminence immunoassay (CLIA), or a RPR ≥ 1:32, or a 4-fold or higher RPR titer rise.
Late latent syphilis/latent syphilis of unknown duration: no symptoms and no previous syphilis diagnosis and a positive CLIA and a RPR with a titer of <1:32.
Findings compatible with treated syphilis (hereafter: treated syphilis): history of previous diagnosis and treatment for syphilis, and not meeting criteria for groups 1–4.
No syphilis: signs and symptoms compatible with syphilis, but CLIA negative and not meeting criteria for groups 1–5.
In the event of a symptomatic participant with a negative initial DFM, negative PCR ulcer swabs, and negative serology but 1 or more positive PCR results in the study samples, such patients were followed up to exclude incubating syphilis. Follow-up would in that case be at 3, 6, and 12 weeks after inclusion with tests at each visit by PCR on peripheral blood, oropharyngeal swab, ano-rectal swab, urine, and by serology.
Every client included in this study was examined by a physician for the staging of syphilis. The oral cavity was examined under direct visualization. External examination of the anus was done. If clinically indicated, proctoscopy was done.
Treponema Pallidum Diagnostics
In patients with suspected primary syphilis, DFM on ulcer exudate was performed. Moreover, an ulcer swab for DNA extraction and real-time PCR targeting the polA gene was routinely collected [13, 14]. This TP-PCR was considered positive when the fluorescent signal was clearly visible, and the cycle threshold (Ct) was <36. For a Ct value between 36 and 40 the DNA extraction was repeated, and both the first and the second DNA extract were (re)tested in the TP-DNA assay. When the Ct value was ≤40, the result was positive for Treponema pallidum DNA detection. A Ct value >40 was considered a negative result.
Routine diagnostics at the Public Health Laboratory in Amsterdam include 3 routine serological tests: a treponemal antibody test (CLIA; Diasorin) used for screening and 2 confirmatory tests: the non-treponemal quantitative rapid plasma reagin (RPR) flocculation test (RPR-Nosticon II; bioMérieux) and the immunoblot (Inno-LIA). The immunoblot was only done if the CLIA value was ≤ 30. A previous analysis in our department showed that immunoblots were always positive in serum samples with a CLIA value >30 (unpublished data). All serological tests were performed according to the specifications of the manufacturers.
DNA Isolation and PCR Testing
DNA was extracted from oropharyngeal and ano-rectal dry swab samples using isopropanol precipitation after which the DNA pellet was dissolved in 50 µL of T10 buffer (10 mM Tris-HCl, pH 8.0) [30]. From March 2019, the DNA from the swabs was isolated using the MagNA Pure 24 (Roche Molecular Systems), according to the specifications of the manufacturers, after in-house validation to ensure fully comparable results.
DNA from urine and peripheral blood samples was extracted within 24 hours of collection. From the urine samples 1ml was centrifuged at 14 000 rpm for 10 minutes. The residual fluid was removed and the pellet resuspended with 200 μL PBS and 500 μL easyMAG Lysisbuffer (BioMerieux) containing 0.2% glycogen after which isopropanol precipitation was performed [31]. DNA was extracted from peripheral blood using the QIAamp Blood Mini Kit following the protocol from the manufacturer (Qiagen).
All extracted DNA samples were tested using the TP-DNA PCR assay. Simultaneously, all samples were spiked with phocine herpesvirus 1 (PhHV) and tested as inhibition control in the PCR. PCR on DNA extracted from anal swabs frequently showed inhibition and was therefore always simultaneously tested in a 1:10 dilution.
Statistical Analysis
We described categorical variables using count data and proportions. Continuous variables were described using their median and interquartile range (IQR). The Fisher exact test was used to compare proportions within categorical variables. The Wilcoxon rank-sum test was used to compare the distribution of continuous variables between groups. We estimated odds ratios (OR) and their 95% confidence intervals (CI) using univariable and multivariable logistic regression to assess associations between determinants and TP-DNA detection in at least 1 sample type.
We carried out analyses using Stata (v15.1, StataCorp, College Station, Texas, USA).
Ethical Statement
This study was approved by the Medical Ethics Committee of the Amsterdam University Medical Center (NL66419.018.18, 2018_236).
RESULTS
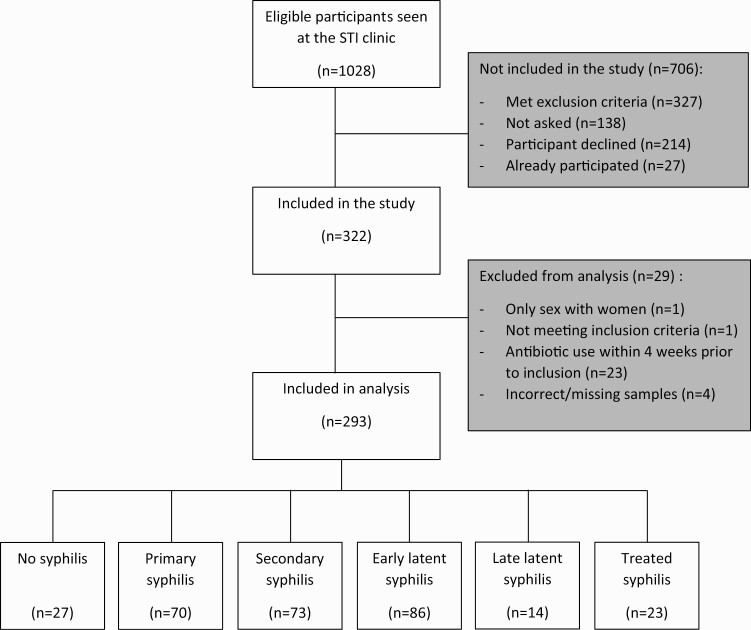
Between November 2018 and December 2019, we included 322 participants in the study, of whom 29 participants did not meet the inclusion criteria (Figure 1). Thus, 293 MSM were included in the analysis. Among these participants 27 (9%) had no syphilis, 70 (24%) were diagnosed with primary syphilis, 73 (25%) with secondary syphilis, 86 (29%) with early latent syphilis, 14 (5%) with late latent syphilis, and 23 (8%) with treated syphilis. There were no participants with incubating syphilis. Median age was 40 years (IQR 31–48) (Table 1). Of the 293 participants, 167 (57%) were born in the Netherlands. Among the 103 men living with HIV (35%), 93 (90%) used antiretroviral therapy. Of the 190 HIV-negative men, 27 (14%) had used pre-exposure prophylaxis (PrEP) in the preceding year. Thirty-one (11%) of the 293 participants had been notified for syphilis by a sexual partner.
Figure 1.
Flow chart of the inclusion and exclusion of MSM included in the study, Amsterdam, The Netherlands, November 2018 to December 2019. Abbreviations: MSM, men who have sex with men; STI, sexually transmitted infection.
Table 1.
Sociodemographic and Clinical Characteristics of Male Patients Included in the Trepoli study (n = 293) by Syphilis Stage, Amsterdam, The Netherlands, November 2018 to December 2019
| Syphilis Stage | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No Syphilis (n = 27) |
Primary (n = 70) |
Secondary (n = 73) |
Early Latent (n = 86) |
Late Latent (n = 14) |
Treated Syphilis (n = 23) |
|||||||
| na | %a | na | %a | na | %a | na | %a | na | %a | na | %a | |
| Demographics | ||||||||||||
| Age, y | ||||||||||||
| Median [IQR] | 35 | [27–43] | 41 | [33–50] | 39 | [33–48] | 40 | [30–52] | 35 | [26–42] | 41 | [35–48] |
| <35 y | 13 | 48% | 22 | 31% | 24 | 33% | 30 | 35% | 7 | 50% | 5 | 22% |
| 35–44 y | 8 | 30% | 21 | 30% | 25 | 34% | 21 | 24% | 6 | 43% | 9 | 39% |
| ≥45 y | 6 | 22% | 27 | 39% | 24 | 33% | 35 | 41% | 1 | 7% | 9 | 39% |
| Country of origin | ||||||||||||
| The Netherlands | 16 | 59% | 46 | 66% | 36 | 50% | 51 | 59% | 5 | 36% | 13 | 57% |
| Other | 11 | 41% | 24 | 34% | 36 | 50% | 35 | 41% | 9 | 64% | 10 | 43% |
| Education | ||||||||||||
| None/primary/secondary | 2 | 8% | 15 | 23% | 16 | 27% | 20 | 25% | 4 | 3% | 3 | 15% |
| College/university | 24 | 92% | 49 | 75% | 41 | 68% | 60 | 74% | 8 | 67% | 16 | 80% |
| Other | 0 | 0% | 1 | 2% | 3 | 5% | 1 | 1% | 0 | 0% | 1 | 5% |
| Sexual behavior | ||||||||||||
| Gender of sex partners | ||||||||||||
| Men | 26 | 96% | 68 | 97% | 73 | 100% | 85 | 99% | 13 | 93% | 22 | 96% |
| Men and women | 1 | 4% | 2 | 3% | 0 | 0% | 1 | 1% | 1 | 7% | 1 | 4% |
| No. of sexual partners (6 mo)b | ||||||||||||
| Median [IQR] | 4 | [3–15] | 8 | [4–20] | 6 | [4–15] | 10 | [5–20] | 8 | [4–12] | 8 | [5–15] |
| <5 | 14 | 52% | 19 | 27% | 23 | 32% | 16 | 20% | 5 | 36% | 5 | 22% |
| 5–9 | 4 | 15% | 18 | 26% | 18 | 25% | 21 | 26% | 2 | 14% | 7 | 30% |
| 10–14 | 2 | 7% | 11 | 16% | 11 | 15% | 14 | 18% | 4 | 29% | 3 | 13% |
| ≥15 | 7 | 26% | 22 | 31% | 21 | 29% | 29 | 36% | 3 | 21% | 8 | 35% |
| Health and biometrics | ||||||||||||
| HIV status | ||||||||||||
| Negative | 21 | 78% | 52 | 74% | 44 | 60% | 49 | 57% | 13 | 93% | 11 | 48% |
| Positive | 6 | 22% | 18 | 26% | 29 | 40% | 37 | 43% | 1 | 7% | 12 | 52% |
| cART usec | ||||||||||||
| No | 1 | 17% | 0 | 0% | 3 | 11% | 2 | 6% | 0 | 0% | 0 | 0% |
| Yes | 5 | 83% | 18 | 100% | 25 | 89% | 33 | 94% | 1 | 100% | 11 | 100% |
| Most recent CD4 count (cells/µL)c | ||||||||||||
| <350 | 0 | 0% | 0 | 0% | 1 | 5% | 0 | 0% | 0 | 0% | 0 | 0% |
| 350–499 | 0 | 0% | 0 | 0% | 2 | 10% | 1 | 3% | 0 | 0% | 0 | 0% |
| ≥500 | 4 | 100% | 14 | 100% | 17 | 85% | 29 | 97% | 1 | 100% | 8 | 100% |
| PrEP used | ||||||||||||
| No | 20 | 95% | 44 | 85% | 37 | 84% | 42 | 86% | 11 | 85% | 9 | 82% |
| In the past 3 months | 1 | 8% | 8 | 15% | 7 | 16% | 6 | 12% | 1 | 8% | 2 | 18% |
| In the past 4–12 months | 0 | 0% | 0 | 0% | 0 | 0% | 1 | 2% | 1 | 8% | 0 | 0% |
| Chlamydia diagnosise | ||||||||||||
| No | 23 | 85% | 65 | 93% | 69 | 95% | 86 | 100% | 13 | 93% | 19 | 83% |
| Yes | 4 | 15% | 6 | 7% | 4 | 5% | 0 | 0% | 1 | 7% | 4 | 17% |
| LGV diagnosise | ||||||||||||
| No | 24 | 89% | 69 | 99% | 73 | 100% | 86 | 100% | 14 | 100% | 22 | 96% |
| Yes | 3 | 11% | 1 | 1% | 0 | 0% | 0 | 0% | 0 | 0% | 1 | 4% |
| Gonorrhea diagnosise | ||||||||||||
| No | 22 | 81% | 64 | 91% | 67 | 92% | 84 | 98% | 13 | 93% | 19 | 83% |
| Yes | 5 | 19% | 6 | 9% | 6 | 8% | 2 | 2% | 1 | 7% | 4 | 17% |
| Herpes simplex diagnosise | ||||||||||||
| No | 21 | 78% | 70 | 100% | 72 | 99% | 84 | 98% | 14 | 100% | 18 | 78% |
| Yes | 6 | 22% | 0 | 0% | 1 | 1% | 2 | 2% | 0 | 0% | 5 | 22% |
| Notified for syphilis | ||||||||||||
| No | 23 | 85% | 58 | 83% | 62 | 85% | 86 | 100% | 14 | 100% | 19 | 83% |
| Yes | 4 | 15% | 12 | 17% | 11 | 15% | 0 | 0% | 0 | 0% | 4 | 17% |
| RPR | ||||||||||||
| Median [IQR] | NA | NA | 2 | [0–8] | 16 | [16–32] | 8 | [4–32] | 6 | [0–8] | 0 | [0–1] |
| Negative | 27 | 100% | 21 | 30% | 0 | 0% | 10 | 12% | 4 | 29% | 17 | 74% |
| Low (1:1–1:4) | NA | NA | 24 | 34% | 4 | 5% | 13 | 15% | 3 | 21% | 5 | 22% |
| Middle (1:8–1:16) | NA | NA | 15 | 21% | 38 | 52% | 39 | 45% | 7 | 50% | 1 | 4% |
| High (1:32–1:128) | NA | NA | 10 | 14% | 31 | 42% | 24 | 28% | 0 | 0% | 0 | 0% |
Abbreviations: cART, combination antiretroviral therapy; HIV, human immunodeficiency virus; IQR, interquartile range; LGV, lymphogranuloma venereum; NA, not applicable; no., number; PrEP, pre-exposure prophylaxis; RPR, rapid plasma reagin.
Data missing for: country of origin (n = 1), education level (n = 29), number of sexual partners (n = 6), ART use (n = 4), and most recent CD4 count (n = 26).
Unless otherwise stated.
In the 6 months before the consultation.
In patients with HIV.
In HIV negative men only.
At current visit.
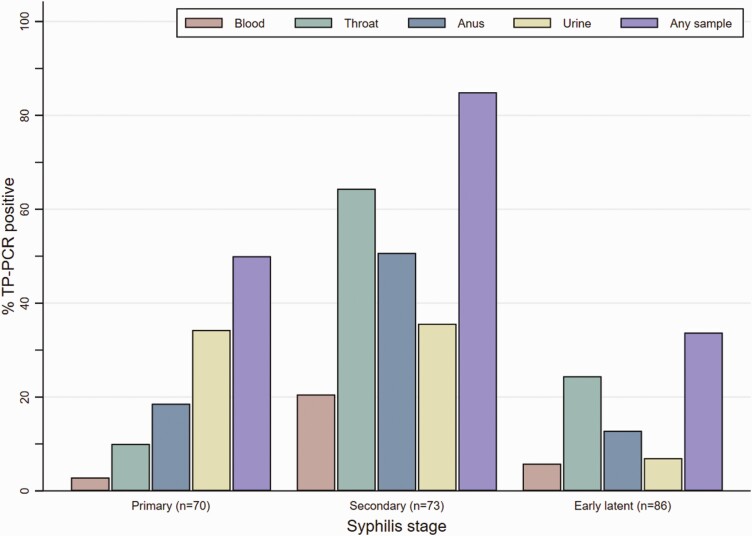
For 35/70 (50%) participants with primary syphilis, TP-DNA was detected in at least one study sample: 2 (3%) peripheral blood, 7 (10%) pharyngeal, 13 (19%) anal, and 24 (34%) urine samples (Table 2, Figure 2). For 62/73 (85%) participants with secondary syphilis, TP-DNA was detected in at least 1 study sample: 15 (21%) peripheral blood, 47 (64%) pharyngeal, 37 (51%) anal, and 26 (36%) urine samples. For 29/86 (34%) participants with early latent syphilis, TP-DNA was detected in at least 1 study sample: 5 (6%) peripheral blood, 21 (24%) pharyngeal, 11 (13%) anal, and 6 (7%) urine samples. No TP-DNA was detected in participants with late latent syphilis or treated syphilis, nor those without syphilis.TP-DNA was detected in 2 or more sample types in 7 (10%) participants with primary syphilis, in 39 (53%) participants with secondary syphilis, and in 9 (11%) participants with early latent syphilis (Supplementary Figure 2). Irrespective of syphilis stage, TP-DNA was less frequently detected in peripheral blood samples than in pharyngeal, anal, or urine samples (Figure 2). In primary and early latent syphilis the median RPR titer in TP-DNA positive participants was higher than in TP-DNA negative participants, but this difference was significant only in participants with early latent syphilis (P = .006) (Supplementary Figure 3).
Table 2.
TP-DNA Detection in Peripheral Blood, Throat and Anal Swabs and Urine, by Syphilis Stage, in MSM with Early Syphilis, Amsterdam, The Netherlands, November 2018 to December 2019
| Syphilis Stage | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No Syphilis (n = 27) |
Primary (n = 70) |
Secondary (n = 73) |
Early Latent (n = 86) |
Late Syphilis (n = 14) |
Treated Syphilis (n = 23) |
|||||||
| n | % | n | % | n | % | n | % | n | % | n | % | |
| Anatomical location | ||||||||||||
| Peripheral blood | 0 | 0% | 2 | 3% | 15 | 21% | 5 | 6% | 0 | 0% | 0 | 0% |
| Throat | 0 | 0% | 7 | 10% | 47 | 64% | 21 | 24% | 0 | 0% | 0 | 0% |
| Anus | 0 | 0% | 13 | 19% | 37 | 51% | 11 | 13% | 0 | 0% | 0 | 0% |
| Urine | 0 | 0% | 24 | 34% | 26 | 36% | 6 | 7% | 0 | 0% | 0 | 0% |
| No. of locations positive | ||||||||||||
| 0 | 27 | 100% | 35 | 50% | 11 | 15% | 57 | 66% | 14 | 100% | 23 | 100% |
| 1 | 0 | 0% | 28 | 40% | 23 | 32% | 20 | 23% | 0 | 0% | 0 | 0% |
| 2 | 0 | 0% | 4 | 6% | 21 | 29% | 6 | 7% | 0 | 0% | 0 | 0% |
| 3 | 0 | 0% | 2 | 3% | 12 | 16% | 1 | 1% | 0 | 0% | 0 | 0% |
| 4 | 0 | 0% | 1 | 1% | 6 | 8% | 2 | 2% | 0 | 0% | 0 | 0% |
| ≥1 location positive | 0 | 0% | 35 | 50% | 62 | 85% | 29 | 34% | 0 | 0% | 0 | 0% |
Abbreviations: MSM, men who have sex with men; No., number; TP-DNA, Treponema pallidum DNA.
Figure 2.
TP-DNA detection in peripheral blood, throat and anal swabs, and urine in MSM with early syphilis, by stage, Amsterdam, The Netherlands, November 2018—December 2019. Abbreviations: MSM, men who have sex with men; TP-DNA, Treponema pallidum DNA; TP-PCR, Treponema pallidum polymerase chain reaction.
To assess whether TP-DNA positivity in oropharyngeal and ano-rectal swabs and in urine was due to TP-DNA from lesions in patients with ulcerative disease at the site of collection, we separately analyzed those with and without localized ulcerative disease. Among primary syphilis patients without a penile ulcer, 8/29 (28%) urine samples were TP-DNA positive; among secondary syphilis patients without a penile ulcer this was 22/68 (39%) (Table 3, Supplementary Figure 4). Among primary syphilis patients without an anal ulcer, 7/60 (12%) ano-rectal samples were TP-DNA positive; among secondary syphilis patients without an anal ulcer this was 32/68 (47%). Among primary syphilis patients without a pharyngeal ulcer, 6/70 (9%) pharyngeal samples were TP-DNA positive; among secondary syphilis patients without a pharyngeal ulcer this was 46/71 (65%).
Table 3.
Urine, Anal and Pharyngeal TP-DNA Positivity in the Presence or Absence of Ulcers in MSM with Early Syphilis, Amsterdam, The Netherlands, November 2018 to December 2019
| Penile Ulcer Present | Penile Ulcer Absent | |||
|---|---|---|---|---|
| TP-DNA positive | TP-DNA positive | |||
| N | n (%) | N | n (%) | |
| Urine | ||||
| Primary syphilis | 41 | 16 (39%) | 29 | 8 (28%) |
| Secondary syphilis | 5 | 4 (80%) | 68 | 22 (33%) |
| Early latent syphilis | 0 | 0 (0%) | 86 | 6 (7%) |
| Anal Ulcer Present | Anal Ulcer Absent | |||
| TP-DNA positive | TP-DNA positive | |||
| N | n (%) | N | n (%) | |
| Anus | ||||
| Primary syphilis | 10 | 6 (60%) | 60 | 7 (12%) |
| Secondary syphilis | 5 | 5 (100%) | 68 | 32 (47%) |
| Early latent syphilis | 1 | 0 (0%) | 85 | 11 (13%) |
| Pharyngeal Ulcer Present | Pharyngeal Ulcer Absent | |||
| TP-DNA positive | TP-DNA positive | |||
| N | n (%) | N | n (%) | |
| Pharyngeal | ||||
| Primary syphilis | 1 | 1 (100%) | 69 | 6 (9%) |
| Secondary syphilis | 2 | 1 (50%) | 71 | 46 (65%) |
| Early latent syphilis | 2 | 1 (50%) | 84 | 20 (24%) |
Abbreviations: MSM, men who have sex with men; No., number; TP-DNA, Treponema pallidum DNA.
We assessed the determinants of TP-DNA detection in one or more sample types. In the multivariable model secondary syphilis was significantly associated with TP-DNA positivity (adjusted odds ratio [aOR] 6.83 (95% CI 2.94–15.90, Table 4). Also, fewer sex partners and a negative HIV status were significantly associated with TP-DNA positivity. There were no significant associations between the Ct value (as a measure of sample TP-DNA content) of the various sample types and syphilis stage. The TP-DNA content of the peripheral blood samples however was markedly lower (higher Ct values) than those of the other sample types (Supplementary Table 1). We assessed study sample TP-DNA positivity by duration of symptoms (dichotomized into ≤2 weeks or >2 weeks) in the primary and secondary syphilis subgroups. In almost all combinations of stage and sample type TP-PCR positivity was higher in participants with symptoms lasting >2 weeks, reaching statistical significance for anal TP-PCR positivity in participants with primary syphilis (P = .042) and throat TP-PCR positivity in participants with secondary syphilis (P = .038, Supplementary Table 2).
Table 4.
Univariable and Multivariable Analysis of Determinants of TP-DNA Detection in one or more Sample types in MSM With Early Syphilis, Amsterdam, The Netherlands, November 2018 to December 2019
| TP-DNA Detected ≥ 1 Sample Type | Univariable Model | Multivariable Model | |||||
|---|---|---|---|---|---|---|---|
| n/N (%)a | OR | 95% CI | P-Value | aOR | 95% CI | P-Value | |
| Syphilis stage | |||||||
| Primary | 35/70 (50%) | Ref | <.001 | Ref | <.001 | ||
| Secondary | 62/73 (85%) | 5.64 | 2.55–12.47 | 6.83 | 2.94–15.90 | ||
| Early latent | 29/86 (34%) | 0.51 | .27–.97 | 0.55 | .27–1.12 | ||
| Age, median [IQR]b | 38 [31–48] | 0.81 | .03–1.36 | .084 | 0.86 | .65–1.14 | .304 |
| Age | |||||||
| <35 y | 48/76 (63%) | Ref | .140 | ||||
| 35–44 y | 37/67 (55%) | 0.72 | .37–1.41 | ||||
| ≥45 y | 41/86 (48%) | 0.53 | .28–1.00 | ||||
| Country of birthc | |||||||
| The Netherlands | 74/133 (56%) | Ref | .770 | Ref | .246 | ||
| Other | 51/95 (54%) | 0.92 | .54–1.57 | 0.68 | .36–1.31 | ||
| No. of sex partners, median [IQR] d | 6 [4–15] | 0.65 | .47–.89 | .008 | 0.69 | .48–1.00 | .046 |
| No. of sex partners | |||||||
| <5 | 39/58 (67%) | Ref | .105 | ||||
| 5–9 | 32/57 (56%) | 0.62 | .29–1.33 | ||||
| 10–14 | 19/36 (53%) | 0.54 | .23–1.28 | ||||
| ≥15 | 33/72 (46%) | 0.41 | .20–.85 | ||||
| HIV status | |||||||
| Negative | 88/145 (61%) | Ref | .024 | Ref | .041 | ||
| Positive | 38/84 (45%) | 0.54 | .31–.92 | 0.50 | .25–.98 | ||
| Any C. trachomatis infection | |||||||
| No | 120/220 (55%) | Ref | .468 | Ref | .657 | ||
| Yes | 6/9 (67%) | 1.67 | .41–6.83 | 1.46 | .27–7.85 | ||
| Any N. gonorrhoeae infection | |||||||
| No | 118/215 (55%) | Ref | .869 | Ref | .433 | ||
| Yes | 8/14 (57%) | 1.09 | .37–3.27 | 0.59 | .16–2.19 | ||
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval; HIV, human immunodeficiency virus; IQR, interquartile range ; MSM, men who have sex with men; No., number; OR, odds ratio; Ref, reference; TP-DNA, Treponema pallidum DNA.
Unless otherwise indicated.
OR per 10 year increase in age.
1 missing.
Odds ratio (OR) for each (log + 1) increase in number of partners.
DISCUSSION
In this study we detected TP-DNA in anal and pharyngeal swabs, in urine samples (as a proxy of urethral mucosa) and in peripheral blood in patients with early stages of syphilis (primary, secondary and early latent syphilis). These findings support the following assumptions about the nature of syphilis: (1) The presence of TP-DNA in mucosal tissue and body fluids of patients with early latent syphilis supports the notion that syphilis is transmissible in the absence of signs or symptoms [2]. In contrast, the absence of TP-DNA in patients with late latent syphilis is in line with the assumption that the late stages are considered not infectious [3, 5]. The presence of TP-DNA in peripheral blood samples in all early syphilis stages is in line with the assumption that hematogenous dissemination of the infection occurs soon after inoculation [4, 31, 32]. In comparison to patients with either primary or early latent syphilis, patients with secondary syphilis significantly more often had at least 1 or more TP-DNA positive study sample, significantly more often a TP-DNA positive oropharyngeal and peripheral blood sample. This finding supports the notion that secondary syphilis is the most contagious stage of syphilis [5]. Finally, the absence of TP-DNA in patients with treated syphilis and those without syphilis confirms the specificity of the test used to detect TP-DNA [14]. In none of the study participants an extra diagnosis of syphilis was found based on TP-DNA positivity of study samples, compared to routine diagnostics.
Several studies have shown that PCR can be used to detect TP-DNA in whole blood at various syphilis stages [15–17, 24, 25]. Here, we used peripheral blood and confirmed TP-PCR positivity, although this was infrequently detected. The presence of TP-DNA in pharyngeal and anal samples in the presence of a lesion has been reported before [19–21]. In this study, we also detected TP-DNA in the absence of a lesion. Oral and anal shedding could therefore play a role in the transmission of syphilis. We also detected TP-DNA in urine samples as was reported previously [23, 27]. In both Dubourg et al and Towns et al, urine samples were positive for TP-DNA in 4/25 (16%) and 12/198 (6%) samples, respectively. Although urine samples are easy to obtain, they are hardly ever used for syphilis diagnosis, as judged by the low number of studies. Whether TP-DNA in urine originates from uro-genital tract lesions, such as penile ulcers, or from renal filtration or asymptomatic mucosal lesions is unknown.
Towns et al [27]. reported the presence of TP-DNA in both lesion and nonlesional samples. In their study, oral rinses, urine and anal swabs were also analyzed, as well as semen from some participants. However, they did not analyze peripheral blood samples. Similar to our study, they frequently detected TP-DNA in the oral (44%) and anal (29%) cavity of patients with secondary syphilis, although we found higher proportions (64% and 51%, respectively). We also more frequently detected TP-DNA in extra-lesional samples of participants with primary syphilis (50% in our study vs 30% by Towns et al) and early latent syphilis (34% in our study vs 8% by Towns et al). We could not confirm a relation between RPR titer and extra-lesional TP-DNA as found by Towns et al. They did not include late latent and treated infections nor persons with a negative syphilis diagnosis. In addition, compared to Towns et al we diagnosed fewer patients with extra-genital ulcers which may explain their lower positivity rates.
The strength of this study was that we systematically screened peripheral blood, oropharynx and ano-rectum samples, and urine which may play a role in the transmission of Treponema pallidum. This study also has several limitations. The detection of TP-DNA does not prove the presence of viable and potentially infectious bacteria. Future studies should investigate the viability of TP-DNA positive samples, especially found in the oral and anal cavity. Presently this has not been possible because TP culture of direct patient samples has proven to be very difficult. Furthermore, we found that fewer sex partners and a negative HIV status were associated with TP-DNA positivity. This counterintuitive finding could possibly be explained assuming that those with fewer sex partners and/or a negative HIV status seek care at an earlier (ie, more infectious) stage and respond better to partner notification calls. In patients with early syphilis, we might have missed lesions. Proctoscopy was only done if clinically indicated (eg, proctitis or intra-anal lesion) and during throat examination ulcers might have been overlooked. Furthermore, the number of patients without syphilis was small, so the demonstrated precision of the specificity of the test is limited. In addition, this study was conducted among an MSM population; it would be worthwhile to examine whether women may have a different pattern of TP-DNA shedding. Finally, we do not know if patients with 2 or more positive TP-DNA samples acquired this from the same sexual partner. However, we found intra-patient homogeneity in an investigation of the T. pallidum molecular variation within patients [33].
Performing TP-PCR on extra-genital samples could be useful as an additional diagnostic tool, especially to identify early incubating infections within the serological window phase. Blood samples are rarely PCR positive and thus may not be useful in routine screening. Urine samples, and anal and pharyngeal swabs are frequently routinely collected for STI screening for molecular diagnosis of C. trachomatis and N. gonorrhoeae infections. An additional TP-NAAT assay could possibly help to diagnose early syphilis infections in patients with non-reactive serological test results, who are nevertheless suspected of having syphilis [34]. The diagnostic value of TP-DNA assays in routine STI screening of asymptomatic persons at increased risk for syphilis should be further evaluated in larger cohorts and other routine clinic settings.
In conclusion, we frequently found TP-DNA in peripheral blood, oropharyngeal, and ano-rectal swabs, and urine collected from MSM diagnosed with early syphilis. Detection of TP-DNA in these sample types provide a better understanding of the infectious nature of early syphilis stages.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors would like to acknowledge Michelle Kroone and Ertan Ersan for creating the database used in this study. Furthermore, they would like to thank Belle Toussaint and Michelle Himschoot for their technical assistance.
Disclaimer. A current study on syphilis at the Public Health Service has received research use only Treponema pallidum Transcription Mediated Assay kits by the company HOLOGIC.
Patient consent. Patient consent was obtained for every individual participating in this study
Financial support. This work was supported by the Public Health Service of Amsterdam
Contributor Information
S A Nieuwenburg, Department of Infectious Diseases, Public Health Service Amsterdam, Amsterdam, The Netherlands.
H C A Zondag, Department of Infectious Diseases, Public Health Service Amsterdam, Amsterdam, The Netherlands.
S M Bruisten, Department of Infectious Diseases, Public Health Service Amsterdam, Amsterdam, The Netherlands; Amsterdam institute for Infection and Immunity (AII), Amsterdam UMC, University of Amsterdam, Amsterdam, The Netherlands.
V W Jongen, Department of Infectious Diseases, Public Health Service Amsterdam, Amsterdam, The Netherlands.
M F Schim van der Loeff, Department of Infectious Diseases, Public Health Service Amsterdam, Amsterdam, The Netherlands; Amsterdam institute for Infection and Immunity (AII), Amsterdam UMC, University of Amsterdam, Amsterdam, The Netherlands; Department of Internal Medicine, Division of Infectious Diseases, Amsterdam UMC, University of Amsterdam, Amsterdam, The Netherlands.
A P van Dam, Department of Infectious Diseases, Public Health Service Amsterdam, Amsterdam, The Netherlands; Department of Medical Microbiology, Amsterdam UMC, University of Amsterdam, Amsterdam, The Netherlands.
H J C de Vries, Department of Infectious Diseases, Public Health Service Amsterdam, Amsterdam, The Netherlands; Amsterdam institute for Infection and Immunity (AII), Amsterdam UMC, University of Amsterdam, Amsterdam, The Netherlands; Department of Dermatology, Amsterdam UMC, University of Amsterdam, Amsterdam, The Netherlands.
References
- 1. Golden MR, Marra CM, Holmes KK.. Update on syphilis: resurgence of an old problem. JAMA 2003; 290:1510–4. [DOI] [PubMed] [Google Scholar]
- 2. Stamm LV. Syphilis: re-emergence of an old foe. Microb Cell 2016; 3:363–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Unemo M, Bradshaw CS, Hocking JS, et al. Sexually transmitted infections: challenges ahead. Lancet Infect Dis 2017; 17:e235–e79. [DOI] [PubMed] [Google Scholar]
- 4. Staritsky LE, Visser M, van Aar M, et al. Sexually transmitted infections in the Netherlands in 2020, RIVM. Available at: https://rivm.openrepository.com/bitstream/handle/10029/625007/2021-0052.pdf?sequence=1&isAllowed=y.
- 5. Ghanem KG, S R, Rice PA.. The modern epidemic of syphilis. N Engl J Med 2020; 382:845–54. [DOI] [PubMed] [Google Scholar]
- 6. Lafond RE, Lukehart SA.. Biological basis for syphilis. Clin Microbiol Rev 2006; 19:29–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schmidt R, Carson PJ, Jansen RJ.. Resurgence of syphilis in the United States: an assessment of contributing factors. Infect Dis (Auckl) 2019; 12:1178633719883282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Henao-Martinez AF, Johnson SC.. Diagnostic tests for syphilis: new tests and new algorithms. Neurol Clin Pract 2014; 4:114–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gayet-Ageron A, Lautenschlager S, Ninet B, Perneger TV, Combescure C.. Sensitivity, specificity and likelihood ratios of PCR in the diagnosis of syphilis: a systematic review and meta-analysis. Sex Transm Infect 2013; 89:251–6. [DOI] [PubMed] [Google Scholar]
- 10. Brischetto A, Gassiep I, Whiley D, Norton R.. Retrospective review of treponema pallidum PCR and serology results: are both tests necessary? J Clin Microbiol 2018; 56:e01782–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Leslie DE, Azzato F, Karapanagiotidis T, Leydon J, Fyfe J.. Development of a real-time PCR assay to detect Treponema pallidum in clinical specimens and assessment of the assay’s performance by comparison with serological testing. J Clin Microbiol 2007; 45:93–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Scott LJ, Gunson RN, Carman WF, Winter AJ.. A new multiplex real-time PCR test for HSV1/2 and syphilis: an evaluation of its impact in the laboratory and clinical setting. Sex Transm Infect 2010; 86:537–9. [DOI] [PubMed] [Google Scholar]
- 13. Koek AG, Bruisten SM, Dierdorp M, van Dam AP, Templeton K.. Specific and sensitive diagnosis of syphilis using a real-time PCR for Treponema pallidum. Clin Microbiol Infect 2006; 12:1233–6. [DOI] [PubMed] [Google Scholar]
- 14. Heymans R, van der Helm J, J, et al. Clinical value of Treponema pallidum real-time PCR for diagnosis of syphilis. J Clin Microbiol 2010; 48:497–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gayet-Ageron A, Ninet B, Toutous-Trellu L, et al. Assessment of a real-time PCR test to diagnose syphilis from diverse biological samples. Sex Transm Infect 2009; 85:264–9. [DOI] [PubMed] [Google Scholar]
- 16. Castro R, Prieto E, Aguas MJ, Manata MJ, Botas J, Pereira FM.. Molecular subtyping of Treponema pallidum subsp. pallidum in Lisbon, Portugal. J Clin Microbiol 2009; 47:2510–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Martin IE, Tsang RS, Sutherland K, et al. Molecular characterization of syphilis in patients in Canada: azithromycin resistance and detection of Treponema pallidum DNA in whole-blood samples versus ulcerative swabs. J Clin Microbiol 2009; 47:1668–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shields M, Guy RJ, Jeoffreys NJ, Finlayson RJ, Donovan B.. A longitudinal evaluation of Treponema pallidum PCR testing in early syphilis. BMC Infect Dis 2012; 12:353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Towns JM, Leslie DE, Denham I, Azzato F, Fairley CK, Chen M.. Painful and multiple anogenital lesions are common in men with Treponema pallidum PCR-positive primary syphilis without herpes simplex virus coinfection: a cross-sectional clinic-based study. Sex Transm Infect 2016; 92:110–5. [DOI] [PubMed] [Google Scholar]
- 20. Gayet-Ageron A, Sednaoui P, Lautenschlager S, et al. Use of Treponema pallidum PCR in testing of ulcers for diagnosis of primary syphilis. Emerg Infect Dis 2015; 21:127–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Glatz M, Juricevic N, Altwegg M, et al. A multicenter prospective trial to assess a new real-time polymerase chain reaction for detection of Treponema pallidum, herpes simplex-1/2 and Haemophilus ducreyi in genital, anal and oropharyngeal ulcers. Clin Microbiol Infect 2014; 20:O1020–7. [DOI] [PubMed] [Google Scholar]
- 22. Castro R, Prieto E, Aguas MJ, et al. Detection of Treponema pallidum sp. pallidum DNA in latent syphilis. Int J STD AIDS 2007; 18:842–5. [DOI] [PubMed] [Google Scholar]
- 23. Dubourg G, Edouard S, Prudent E, Fournier PE, Raoult D.. Incidental syphilis diagnosed by real-time PCR screening of urine samples. J Clin Microbiol 2015; 53:3707–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Grange PA, Gressier L, Dion PL, et al. Evaluation of a PCR test for detection of Treponema pallidum in swabs and blood. J Clin Microbiol 2012; 50:546–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang C, Cheng Y, B L, et al. Sensitive detection of Treponema pallidum DNA from the whole blood of patients with syphilis by the nested PCR assay. Emerg Microbes Infect 2018; 7:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhou C, Zhang X, Zhang W, Duan J, Zhao F.. PCR detection for syphilis diagnosis: status and prospects. J Clin Lab Anal 2019; 33:e22890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Towns JM, Leslie DE, Denham I, et al. Treponema pallidum detection in lesion and non-lesion sites in men who have sex with men with early syphilis: a prospective, cross-sectional study. Lancet Infect Dis 2021; 21:1324–31. [DOI] [PubMed] [Google Scholar]
- 28. Stoltey JE, Cohen SE.. Syphilis transmission: a review of the current evidence. Sex Health 2015; 12:103–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Janier M, Unemo M, Dupin N, Tiplica GS, Potocnik M, Patel R.. 2020 European guideline on the management of syphilis. J Eur Acad Dermatol Venereol 2021; 35:574–88. [DOI] [PubMed] [Google Scholar]
- 30. Bom RJ, Christerson L, Schim van der Loeff MF, Coutinho RA, Herrmann B, Bruisten SM.. Evaluation of high-resolution typing methods for Chlamydia trachomatis in samples from heterosexual couples. J Clin Microbiol 2011; 49:2844–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Salazar JC, Rathi A, Michael NL, Radolf JD, Jagodzinski LL.. Assessment of the kinetics of Treponema pallidum dissemination into blood and tissues in experimental syphilis by real-time quantitative PCR. Infect Immun 2007; 75:2954–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Church B, Wall E, Webb JR, Cameron CE.. Interaction of Treponema pallidum, the syphilis spirochete, with human platelets. PLoS One 2019; 14:e0210902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zondag H, Nieuwenburg S, van Dam A, et al. Treponema pallidum intra-patient homogeneity between various body locations in patients with infectious syphilis. Presented at: ISSTDR & HIV 2021 Conference; Amsterdam, The Netherlands. 14–17 July 2021. [Google Scholar]
- 34. Getman D, M L, Barakat N, et al. Analytical performance characteristics of a new transcription-mediated amplification assay for Treponema pallidum. J Clin Microbiol 2021; 59:e0051121. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.