Abstract
Background
Children and adolescents living with HIV (CALHIV) face unique challenges, including poorer treatment outcomes, risk for drug-resistance mutations (HIVDRMs), and limited drug formulations. We estimated viral suppression (VS) prevalence and evaluated predictors of VS and HIVDRMs in Kenya.
Methods
From 2018–2020, CALHIV 1–19 years on antiretroviral therapy (ART) >6 months were enrolled in this cross-sectional study. Participants underwent viral load (VL) testing; those with VL ≥1000 copies/mL had HIVDRM testing. Sociodemographic questionnaires and medical record abstraction were completed. VS prevalence (VL <1000 copies/mL) was estimated; robust Poisson regression models were used to estimate prevalence ratios (PRs) and 95% CIs for associations between potential predictors of VS.
Results
Nine hundred and sixty-nine participants were enrolled. VS prevalence was .80 (95% CI: .78–.83). Being on ART >24 months (adjusted PR [aPR]: 1.22; 95% CI: 1.06–1.41), an integrase strand transfer inhibitor–containing regimen (1.13; 1.02–1.26), and attending a level 3 health facility (1.23; 1.11–1.36) were associated with VS. Missing ≥3 doses of ART in the past month (aPR: .73; 95% CI: .58–.92), having a viremic mother with HIV (.72; .53–.98), and having 3–7 (.90; .83–.97), 8–13 (.89; .82–.97), or ≥14 (.84; .77–.92) compared with <2 adherence counseling referrals were inversely associated with VS. A high proportion (n = 119, 81.5%) of unsuppressed participants had evidence of any major HIVDRM.
Conclusions
HIV treatment programs should target interventions for pediatric patients at risk for treatment failure—namely, those with a caregiver with failed VS and those struggling with adherence.
Keywords: HIV, Kenya, viral load, drug resistance
Viral suppression prevalence was 80% among children/adolescents living with HIV. Missing ART doses, unsuppressed maternal viral load, and adherence counseling referrals were inversely associated with viral suppression, while on ART ≥2 years and an INSTI-containing regimen was associated with suppression.
Human immunodeficiency virus (HIV)/AIDS is a leading cause of morbidity and mortality in pediatric populations [1]. With over 90% of children and adolescents with HIV (CAHIV) under age 15 living in sub-Saharan Africa (SSA), the region carries the global burden of pediatric HIV/AIDS [2]. An estimated 110 000 CALHIV under 15 years and 180 000 youth aged 15 to 24 years are living with HIV in Kenya, with approximately 70 090 CALHIV younger than 15 years on antiretroviral therapy (ART) [2].
ART has reduced HIV-related mortality and morbidities, increasing life expectancy for people living with HIV [3]. However, in 2020, the Joint United Nations Program on HIV/AIDS (UNAIDS) reported that only 41% of children in need have access to treatment globally, lagging behind adults in terms of ART uptake [2]. Long-term treatment success is further complicated by adherence challenges, limited pediatric drug formulations, variable pharmacokinetics, body-weight changes necessitating vigilant dose adjustments, and a high rate of CALHIV who have not gone through the disclosure process [4–6]. The shift towards routine viral load monitoring as a measure of treatment efficacy at individual and program levels has become a quality benchmark for HIV programs in support of UNAIDS’ 95-95-95 targets by 2030 [7–9]. The third component of the 95 target is to achieve viral load (VL) suppression in 95% of patients on ART [10].
In 2016, Kenya adopted VL monitoring to assess treatment effectiveness per national ART guidelines [11]. However, significant challenges in achieving optimized pediatric VL persist. Additionally, adolescents frequently find consistent, long-term adherence difficult [12]. Sustaining high levels of ART adherence is critical to treatment success, prevention of drug resistance and disease progression, and decreasing risk of onward transmission once sexual debut occurs [13]. Poor adherence during childhood and adolescence, along with a multitude of individual- and program-level factors, could contribute to viral nonsuppression and the emergence of HIV drug-resistance mutations (HIVDRMs).
Viral suppression (VS) among children and adolescents in SSA has not been well characterized, particularly in Kenya which has one of the highest burdens of CAHIV [13–15]. There remains a need to assess VS rates and to understand individual- and program-level correlates of VS in this age group.
We estimated the prevalence of VS among participants aged 1–19 years in Kenya to evaluate the effectiveness of treatment interventions at a program level. We also identified participant-level factors associated with VS and described HIVDRM patterns among participants with high VL.
METHODS
Study Population
Between December 2018 and March 2020, participants were invited to enroll in this cross-sectional study based on random selection from 13 treatment clinics supported by the Military HIV Research Program (MHRP) and the US President’s Emergency Plan for AIDS Relief (PEPFAR) in Western Kenya. The random sample was approximately weighted by clinic and stratified by time on ART and age. Randomization was conducted in Stata (StataCorp, College Station, TX) using listings of current clients from each clinic and is representative of the general population of children and adolescents on ART. Individuals were eligible for enrollment if they were at least 1 year to 19 years of age, had been on first- or second-line ART for at least 6 months, and had attended at least 1 follow-up ART clinic visit in the last 6 months. Participants aged 13–17 years were required to have been informed of their HIV status.
Data Collection
Study staff administered sociodemographic questionnaires to participants aged 18 years and older, caregivers if the participant was younger than 13 years, and both caregivers and participants if the participant was between 13 and 17 years. Responses captured demographics, self- or parent-reported ART adherence, side effects, and support group participation. Participants underwent a blood draw for VL unless results of a VL drawn for routine clinical care within 1 month of study enrollment were available.
Medical and pharmacy record abstraction was completed within 3 weeks of the participant’s visit, including duration on ART, ART regimen, referral history, nutritional status assessments, World Health Organization (WHO) staging, maternal VL where available, and tuberculosis (TB) treatment history. Data from medical record abstraction captured information and events prior to enrollment. Referral history captured documented referrals for adherence counselling and other services; however, evidence of completion of the referred services was unavailable.
Data were transcribed onto case report forms and entered in the Clinplus platform (Anju Software, Tempe, AZ).
Viral Load Monitoring
HIV VL was measured via nucleic acid amplification methods on the Abbott m2000sp/rt RealTime System testing platform with a 1-mL plasma sample volume and a lower limit of detection of 40 copies/mL. All testing was performed according to the manufacturer’s instructions.
HIV Genotyping and Subtyping
Plasma samples from participants with a VL greater than 1000 copies/mL underwent sequencing of the Pol region using a laboratory-validated modification to the ViroSeq HIV-1 Genotyping System v2.0 (Abbott Molecular, Chicago, IL). Sequences were evaluated for major mutations conferring resistance to nucleoside reverse transcriptase inhibitors (NRTIs), non-nucleoside reverse transcriptase inhibitors (NNRTIs), and protease inhibitors (PIs) using the SmartGene Integrated Database Network System (SmartGene, Zug, Switzerland) to access mutation lists from the Stanford HIV Drug Resistance Database, version 8.8.0 (Stanford University, Stanford, CA) [16]. The Kericho laboratory is accredited by the College of American Pathologists (CAP) and runs CAP EQA for HIVDRM testing.
HIV-1 subtype was inferred from the consensus evolutionary tree from SmartGene Integrated Database Network System, which utilizes the neighbor-joining method in MEGA4 software version 4 (Tamura, Dudley, Nei, and Kumar 2007). Evolutionary distances were computed using the maximum composite likelihood method in units of the number of base substitutions per site. The tree was then generated by the neighbor-joining method from a nucleotide alignment.
Statistical Analyses
Viral suppression was defined using WHO criteria as a VL of less than 1000 copies/mL. The prevalence of VS was estimated using the Wilson score method and reported with 95% confidence intervals (CIs) in the overall sample, and by age group, duration on ART, and first- versus second-line ART.
Bivariate analyses were conducted using Pearson’s chi-square and Wilcoxon rank-sum tests. Generalized linear models with a Poisson distribution and robust standard errors were used to estimate unadjusted and adjusted prevalence ratios (aPRs) and 95% CIs for associations between sociodemographic and clinical factors and VS. Factors significant (α = .05) in the unadjusted models and those identified based on a priori and clinical knowledge of the study setting were included in the adjusted model. ART regimen was dropped from the adjusted model due to redundancy with ART class. We tested for multicollinearity using the variable inflation factor. Analyses were restricted to complete cases after creating separate categories for unknown data from participant medical records.
For participants with a VL of 1000 copies/mL or higher, the prevalence of specific HIVDRMs and categories of HIVDRMs were calculated by dividing the number of participants with 1 or more mutations by the total number of participants genotyped. For the drug-resistance analyses, we included all participants with available HIVDRM data and did not restrict to complete cases.
Analyses were performed in SAS version 9.4 (SAS Institute, Cary, NC) and Stata version 16.1 (StataCorp) software.
Ethical Assurance
The study was approved by institutional review boards of the Walter Reed Army Institute of Research and the Kenya Medical Research Institute Scientific and Ethics Review Unit (KEMRI SERU). All participants provided informed consent and assent, as applicable.
RESULTS
Cohort Characteristics
From 973 participants screened, 969 (99.6%) participants were enrolled; of these, 935 (96.5%) had complete case data and were included in further analyses. The median age was 12 (interquartile range [IQR]: 8–15) years and 482 (51.6%) were female (Table 1). Twenty (2.1%) had been on ART for less than 1 year, 56 (6.0%) for 1–2 years, and 859 (91.9%) for more than 2 years. The median duration on current ART regimen was 2.3 (IQR: 0.7–4.5) years. A total of 442 (47.3%) were on an ART regimen with an abacavir (ABC)/lamivudine (3TC) backbone, 223 (23.9%) were on a zidovudine (AZT)/3TC backbone regimen, 136 (14.5%) were on tenofovir (TDF)/3TC/efavirenz (EFV) (TLE), 70 (7.5%) were on a non-TLE regimen with a TDF/3TC backbone, and 64 (6.8%) were on tenofovir/lamivudine/dolutegravir (DTG) (TLD). History of referral for other services was common, with a median of 10 (IQR: 4–23) referrals for other services (eg, nutrition support) and a median of 7 (IQR: 2–13) referrals for adherence counseling.
Table 1.
Characteristics of Children and Adolescents Attending MHRP/PEPFAR-Supported Antiretroviral Treatment Programs in Kenya by Viral Suppression Status
| Viral Load <1000 Copies/mL (n = 750) | Viral Load >1000 Copies/mL (n = 185) | P | |
|---|---|---|---|
| Sex | .92 | ||
| Male | 364 (80.4%) | 89 (19.6%) | |
| Female | 386 (80.1%) | 96 (19.9%) | |
| Age | .48 | ||
| 1–9 years | 245 (81.7%) | 55 (18.3%) | |
| 10–14 years | 276 (78.2%) | 77 (21.8%) | |
| 15–19 | 229 (81.2%) | 53 (18.8%) | |
| Duration on ART | .11 | ||
| 6–12 months | 14 (70.0%) | 6 (30.0%) | |
| 13–24 months | 40 (71.4%) | 16 (28.6%) | |
| >24 months | 696 (81.0%) | 163 (19.0%) | |
| Current ART regimena | <.001 | ||
| ABC/3TC backboneb | 360 (81.4%) | 82 (18.6%) | |
| AZT/3TC backbonec | 160 (71.7%) | 63 (28.3%) | |
| TDF/3TC backbone | 49 (70.0%) | 21 (30.0%) | |
| TLD | 62 (96.9%) | 2 (3.1%) | |
| TLE | 119 (87.5%) | 17 (12.5%) | |
| Duration on current regimen >6 months | .43 | ||
| No | 136 (82.4%) | 29 (17.6%) | |
| Yes | 614 (79.7%) | 156 (20.3%) | |
| Current ART classd | <.001 | ||
| NRTI/NNRTI | 439 (81.9%) | 97 (18.1%) | |
| NRTI/PI | 246 (74.8%) | 83 (25.2%) | |
| INSTI-containing | 65 (92.9%) | 5 (7.1%) | |
| First- vs second-line ART | <.01 | ||
| First line | 505 (83.1%) | 103 (16.9%) | |
| Second line | 245 (74.9%) | 82 (25.1%) | |
| Number of referrals (quartiles)e | .13 | ||
| 0–4 | 201 (82.4%) | 43 (17.6%) | |
| 5–10 | 224 (80.9%) | 53 (19.1%) | |
| 11–23 | 151 (83.0%) | 31 (17.0%) | |
| 24+ | 174 (75.0%) | 58 (25.0%) | |
| Number of referrals for adherence counseling (quartiles)f | .02 | ||
| 0–2 | 229 (85.8%) | 38 (14.2%) | |
| 3–7 | 191 (78.3%) | 53 (21.7%) | |
| 8–13 | 164 (80.8%) | 39 (19.2%) | |
| 14+ | 166 (75.1%) | 55 (24.9%) | |
| Current WHO clinical stageg | .50 | ||
| I | 248 (83.2%) | 50 (16.8%) | |
| II | 257 (78.6%) | 70 (21.4%) | |
| III | 190 (80.2%) | 47 (19.8%) | |
| IV | 15 (75.0%) | 5 (25.0%) | |
| Unknown | 40 (75.5%) | 13 (24.5%) | |
| Current nutritional statush | .02 | ||
| No malnutrition | 692 (81.3%) | 159 (18.7%) | |
| Moderate/severe malnutrition | 23 (74.2%) | 8 (25.8%) | |
| Unknown | 35 (66.0%) | 18 (34.0%) | |
| History of tuberculosis treatmenti | .30 | ||
| No | 635 (80.9%) | 150 (19.1%) | |
| Yes | 94 (75.2%) | 31 (24.8%) | |
| Unknown | 21 (84.0%) | 4 (16.0%) | |
| Maternal viral loadj | <.01 | ||
| Mother living with HIV, viral load <1000 copies/mL | 270 (83.9%) | 52 (16.1%) | |
| Mother living with HIV, viral load >1000 copies/mL | 16 (59.3%) | 11 (40.7%) | |
| Mother HIV status or viral load unknown | 464 (79.2%) | 122 (20.8%) | |
| Child/adolescent experienced side effectsk | .62 | ||
| No | 579 (79.9%) | 146 (20.1%) | |
| Yes | 171 (81.4%) | 39 (18.6%) | |
| Doses of ART missed in past monthl | <.001 | ||
| None | 670 (81.6%) | 151 (18.4%) | |
| 1–2 | 53 (77.9%) | 15 (22.1%) | |
| 3+ | 27 (58.7%) | 19 (41.3%) | |
| Clinic support group participationm | .26 | ||
| Child <13 years | 419 (80.7%) | 100 (19.3%) | |
| Attends | 224 (77.5%) | 65 (22.5%) | |
| Does not attend | 107 (84.3%) | 20 (15.7%) | |
| Community support group participationn | .68 | ||
| Child <13 years | 419 (80.7%) | 100 (19.3%) | |
| Attends | 23 (85.2%) | 4 (14.8%) | |
| Does not attend | 308 (79.2%) | 81 (20.8%) | |
| Engaged in revenue-generating activityo | .59 | ||
| No | 193 (81.4%) | 44 (18.6%) | |
| Yes | 557 (79.8%) | 141 (20.2%) | |
| Household in urban or rural areap | .13 | ||
| Urban | 233 (81.2%) | 54 (18.8%) | |
| Peri-urban | 55 (71.4%) | 22 (28.6%) | |
| Rural | 462 (80.9%) | 109 (19.1%) | |
| Level of care deliveryq | <.01 | ||
| Level 1 health facility | 124 (71.3%) | 50 (28.7%) | |
| Level 2 health facility | 97 (77.6%) | 28 (22.4%) | |
| Level 3 health facility | 529 (83.2%) | 107 (16.8%) |
Data are presented as n (row %). Bold indicates significance at P < .05. Pearson’s chi-square and Wilcoxon rank-sum tests were used to compare participants with viral load <1000 copies/mL and those with viral load >1000 copies/mL. P values were not corrected for multiple-hypothesis testing. Facilities participating in this study include Kombewa, Manuyanda, Rodi, Kericho District Hospital, Bomet Health Centre, Sotik Health Centre, Kapkangani Health Centre, Kurangurik Dispensary, Sosiot Health Centre, Enoosaen Health Centre, Ndanai, Kipketer Dispensary, and Kabiyet Health Centre. Abbreviations: ABC, abacavir; ART, antiretroviral therapy; AZT, azidothymidine (zidovudine); DTG, dolutegravir; HIV, human immunodeficiency virus; INSTI, integrase strand transfer inhibitor; MHRP, Military HIV Research Program; NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PEPFAR, US President’s Emergency Plan for AIDS Relief; PI, protease inhibitor; TB, tuberculosis; TDF, tenofovir; TLD, tenofovir/lamivudine/dolutegravir; TLE, tenofovir/lamivudine/efavirenz; WHO, World Health Organization; 3TC, lamivudine.
Current ART regimen: ART regimen was obtained through medical and pharmacy record abstraction and grouped by common regimen/regimen backbone.
n = 4 on ABC/3TC/DTG.
n = 2 on AZT/3TC/DTG.
ART class: ART class was derived from ART regimen data obtained through medical and pharmacy record abstraction.
Number of referrals: Participants’ referral history was obtained through medical record abstraction of referrals for services including TB treatment, adherence counseling, nutrition support, inpatient care, mental services, psychosocial support, social support services, disclosure counseling, or other services not otherwise specified; the number of any referral services were totaled by participant and categorized by quartile.
Number of referrals for adherence counseling: Adherence counseling referrals were obtained through medical record abstraction; the number of adherence counseling referrals were totaled by participant and categorized by quartile.
WHO clinical stage: WHO staging was obtained through medical record abstraction at the most recent clinical care visit.
Nutritional status: Nutritional status was obtained through medical record abstraction at most recent clinical care visit and categorized according to the WHO classification scheme for malnutrition.
History of TB treatment: History of having ever received TB treatment was obtained through medical record abstraction.
Maternal viral load: Most recent maternal viral load was obtained through medical record abstraction where available; maternal viral load was categorized as viral load <1000 copies/mL, viral load >1000 copies/mL, or maternal HIV status or viral load unknown if maternal viral load was unknown/unavailable.
Ever experienced side effects: Participants 18–19 years, caregivers if the participant was younger than 13 years, and both caregivers and participants if the participant was between 13 and 17 years old were asked if they/the child had ever experienced side effects as a result of ART treatment; for participants 13–17 years, the caregiver response was taken as the primary response; however, if the caregiver response was missing, the participant response was utilized.
Doses of ART missed in the past month: Adolescents aged 18–19 years, caregivers if the participant was younger than 13 years, and both caregivers and participants if the participant was between 13 and 17 years were asked how many self-reported doses of ART medication they/the child had missed over the last month; responses were categorized into none, 1–2, or 3 or more missed ART doses in the past month; for participants aged 13–17 years, the caregiver response was taken as the primary response; however, if the caregiver response was missing, the participant response was utilized.
Clinic support group participation: Children and adolescents 13–19 years were asked if they participate in a support group at the clinic; children under age 13 were considered too young to participate as disclosure in this age group was not required for enrollment.
Community support group participation: Children and adolescents 13–19 years were asked if they participate in an HIV treatment support group in their community; children under age 13 were considered too young to participate as disclosure in this age group was not required for enrollment.
Engaged in revenue-generating activity: Adolescents 18–19 years, caregivers if the participant was younger than 13 years, and both caregivers and participants if the participant was between 13 and 17 years were asked whether they are currently involved in revenue generating activity; for participants aged 18–19 years old, the participant’s revenue-generating activity status was utilized while for participants younger than 18, the caregiver’s revenue generating activity status was utilized.
Household in urban or rural area: Adolescents 18–19 years and caregivers if the participant was younger than 18 years were asked if their household was in an urban, peri-urban, or rural area.
Level 1 health facility: Community facilities run by certified healthcare providers. Major roles are to treat minor ailments and issue referral letters to other facilities. Examples of activities are TB screening, contact tracing of TB patients and TB defaulters, screening for malnutrition, malaria rapid test, blood pressure and blood sugar testing, HIV testing services, and health talks. Level 2 health facility: Health dispensaries run by clinical officers (those in the cities also act as level 3 health facilities). Services include general outpatient as well as antenatal and postnatal services, no in-patient services. Level 3 health facility: Health centers (smaller hospitals) led by a nurse, a clinical officer, or at least with 1 medical officer. Services include maternity and in-patient services, antenatal and postnatal services, curative, laboratory, dental, and pharmacy. Level 4 health facility: County hospitals run by a director who is a medic, a doctor by profession. They offer more holistic services—as in level 3.
Prevalence of Viral Suppression
The overall prevalence of VS in the cohort was .80 (95% CI: .78–.83) (Table 2). The prevalence of VS was lower in the 10- to 14-year age group compared with the 1- to 9-year and 15- to 19-year age groups, and trended upwards with increasing duration on ART. The prevalence of VS was lower among participants on a second-line ART regimen (.75; 95% CI: .70–.79) as compared with those on first-line ART (.83; 95% CI: .80–.86).
Table 2.
Prevalence of Viral Suppression Among Children and Adolescents Attending MHRP/PEPFAR-Supported Antiretroviral Treatment Programs in Kenya
| Prevalence of Viral Load <1000 Copies/mL (95% CI) | |
|---|---|
| Overall | .80 (.78–.83) |
| Age group | |
| 1–9 years | .82 (.77–.86) |
| 10–14 years | .78 (.74–.82) |
| 15–19 years | .81 (.76–.85) |
| Duration on ART | |
| 6–12 months | .70 (.48–.85) |
| 13–24 months | .71 (.59–.82) |
| >24 months | .81 (.78–.84) |
| First- vs second-line ART | |
| First line | .83 (.80–.86) |
| Second line | .75 (.70–.79) |
Viral suppression was defined using WHO criteria as a viral load <1000 copies/mL. The prevalence of viral suppression was estimated using the Wilson score method and reported with 95% CIs in the overall sample, and by age group and duration on ART. Abbreviations: ART, antiretroviral therapy; CI, confidence interval; MHRP, Military HIV Research Program; PEPFAR, US President’s Emergency Plan for AIDS Relief; WHO, World Health Organization.
Factors Associated With Viral Suppression
Factors independently associated with VS included duration on ART over 24 months compared to those on ART for 24 months or less (aPR: 1.22; 95% CI: 1.06–1.41), being on an integrase strand transfer inhibitor (INSTI)–containing regimen as compared with an NRTI/NNRTI-based regimen (aPR: 1.13; 95% CI: 1.02–1.26), and attending a level 3 health facility (aPR: 1.23; 95% CI: 1.11–1.36) (Table 3). Factors inversely associated with VS included unknown malnutrition status compared with no malnutrition (aPR: .79; 95% CI: .66–.95), missing 3 or more doses of ART in the past month compared with missing none (aPR: .73; 95% CI: .58–.92), and having a mother living with HIV with a viral load of more than 1000 copies/mL (aPR: .72; 95% CI: .53–0.98) or a mother of unknown HIV or VL status (aPR: .93; 95% CI: .87–.99) as compared with a mother living with HIV with a VL of less than 1000 copies/mL. Additionally, as compared with having 2 or fewer adherence counseling referrals documented, having been referred for adherence counseling 3–7 times (aPR: .90; 95% CI: .83–.97), 8–13 times (aPR: .89; 95% CI: .82–.97), or 14 or more times (aPR: .84; 95% CI: .77–0.92) was inversely associated with VS.
Table 3.
Factors Associated With Viral Suppression (Viral Load <1000 Copies/mL) Among Children and Adolescents Attending MHRP/PEPFAR-Supported Antiretroviral Treatment Programs in Kenya
| PR (95% CI) | aPR (95% CI) | |
|---|---|---|
| Sex | ||
| Male | Ref | Ref |
| Female | .99 (.94–1.06) | .99 (.93–1.06) |
| Age | ||
| 1–9 years | Ref | Ref |
| 10–14 years | .96 (.89–1.03) | .94 (.87–1.02) |
| 15–19 | .99 (.92–1.08) | .96 (.88–1.05) |
| Duration on ART | ||
| 24 months or less | Ref | Ref |
| >24 months | 1.14 (.98–1.32) | 1.22 (1.06–1.41) |
| Current ART regimen | ||
| ABC/3TC backbone | Ref | … |
| AZT/3TC backbone | .88 (.80–.97) | … |
| TDF/3TC backbone | .86 (.73–1.01) | … |
| TLD | 1.19 (1.12–1.27) | … |
| TLE | 1.07 (.99–1.16) | … |
| Duration on current regimen >6 months | ||
| No | Ref | … |
| Yes | .97 (.89–1.05) | … |
| Current ART class | ||
| NRTI/NNRTI | Ref | Ref |
| NRTI/PI | .91 (.85–.98) | .91 (.84–.98) |
| INSTI-containing | 1.13 (1.05–1.22) | 1.13 (1.02–1.26) |
| First- vs second-line ART | ||
| First line | Ref | … |
| Second line | .90 (.84–.97) | … |
| Number of adherence counseling referrals (quartiles) | ||
| 0–2 | Ref | Ref |
| 3–7 | .91 (.84–.99) | .90 (.83–.97) |
| 8–13 | .94 (.87–1.02) | .89 (.82–.97) |
| 14+ | .88 (.80–.96) | .84 (.77–.92) |
| Current WHO clinical stage | ||
| I | Ref | … |
| II | .94 (.88–1.02) | … |
| III | .96 (.89–1.04) | … |
| IV | .90 (.70–1.17) | … |
| Unknown | .91 (.77–1.07) | … |
| Current nutritional status | ||
| No malnutrition | Ref | Ref |
| Moderate/severe malnutrition | .91 (.74–1.13) | .87 (.71–1.06) |
| Unknown | .81 (.67–.99) | .79 (.66–.95) |
| History of TB treatment | ||
| No | Ref | … |
| Yes | .93 (.84–1.03) | … |
| Unknown | 1.04 (.87–1.24) | … |
| Maternal viral load (copies/mL) | ||
| Mother living with HIV, viral load <1000 | Ref | Ref |
| Mother living with HIV, viral load >1000 | .71 (.52–.97) | .72 (.53–.98) |
| Mother HIV status or viral load unknown | .94 (.89–1.01) | .93 (.87–.99) |
| Ever experienced side effects | ||
| No | Ref | … |
| Yes | 1.02 (.95–1.10) | … |
| Doses of ART missed in the past month | ||
| None | Ref | Ref |
| 1–2 | .96 (.84–1.09) | .96 (.84–1.09) |
| 3+ | .72 (.56–.92) | .73 (.58–.92) |
| Clinic support group participation | ||
| Child <13 years | Ref | … |
| Attends | .96 (.89–1.03) | … |
| Does not attend | 1.04 (.96–1.14) | … |
| Community support group participation | ||
| Child <13 years | Ref | … |
| Attends | 1.06 (.90–1.24) | … |
| Does not attend | .98 (.92–1.05) | … |
| Engaged in revenue generating activity | ||
| No | Ref | … |
| Yes | .98 (.91–1.05) | … |
| Household in urban or rural area | ||
| Urban | Ref | … |
| Peri-urban | .88 (.76–1.02) | … |
| Rural | 1.00 (.93–1.07) | … |
| Level of care delivery | ||
| Level 1 health facility | Ref | Ref |
| Level 2 health facility | 1.09 (.95–1.24) | 1.08 (.94–1.23) |
| Level 3 health facility | 1.17 (1.06–1.29) | 1.23 (1.11–1.36) |
Bold indicates significance at P < .05. P values were not corrected for multiple-hypothesis testing. Generalized linear models with a Poisson distribution and robust standard errors were used to estimate unadjusted and aPRs and 95% CIs for associations between sociodemographic and clinical factors and viral suppression. Unknown categories were retained for inclusion in the models; then, observations were restricted to those with complete case data. Individual-level predictors determined to be significant (α = .05) in the unadjusted models along with predictors identified based on a priori and clinical knowledge of the study setting were included in the fully adjusted model. Predictors identified a priori included sex, age, duration on ART, and current nutritional status. Additional predictors identified in bivariate analyses included current ART regimen and class, number of adherence counseling referrals, maternal viral load, number of ART doses missed in the past month, and level of care. Current ART regimen was left out of the adjusted model due to redundancy with current ART class. Engagement in revenue-generating activity was assessed as a potential confounder between nutritional status and viral suppression. Using a threshold of a 10% change in coefficient, engagement in revenue-generating activity was determined to not be confounding the relationship between nutritional status and viral suppression and was left out of the adjusted model. Abbreviations: ABC, abacavir; aPR, adjusted prevalence ratio; ART, antiretroviral therapy; AZT, azidothymidine (zidovudine); CI, confidence interval; HIV, human immunodeficiency virus; INSTI, integrase strand transfer inhibitor; MHRP, Military HIV Research Program; NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PEPFAR, US President’s Emergency Plan for AIDS Relief; PI, protease inhibitor; PR, prevalence ratio; Ref, reference; TB, tuberculosis; TDF, tenofovir; TLD, tenofovir/lamivudine/dolutegravir; TLE, tenofovir/lamivudine/efavirenz; WHO, World Health Organization; 3TC, lamivudine.
HIV Drug-Resistance Patterns
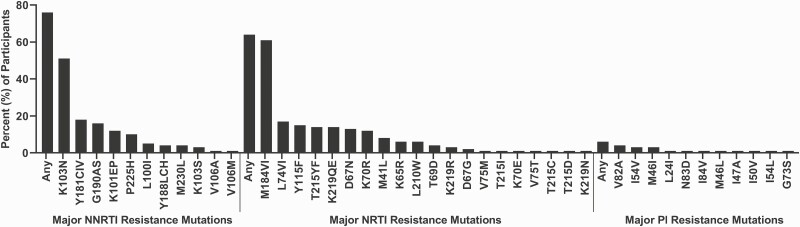
Of the 198 children and adolescents who were unsuppressed, samples from 146 (73.7%) were successfully sequenced. Class-specific HIVDRM prevalence was 76.0% (n = 111) for NNRTIs, 64.4% (n = 94) for NRTIs, and 6.2% (n = 9) for PIs. There were 11 HIVDRMs identified in the protease (PR) gene and 31 in the reverse transcriptase (RT) gene. The dominant HIVDRMs in the RT gene were K103N (51%, n = 75) and M184V (61%, n = 89), while in the PR gene, V82A was dominant (4%, n = 6) (Figure 1).
Figure 1.
Frequency of NNRTI, NRTI, and PI resistance mutations among participants with viral load >1000 copies/mL. Plasma samples from participants with viral load >1000 copies/mL underwent sequencing of the Pol region using a laboratory-validated modification to the ViroSeq HIV-1 Genotyping System v2.0 (Abbott Molecular, Chicago, IL). Sequences were evaluated for major mutations conferring resistance to NRTIs, NNRTIs, and PIs using the SmartGene Integrated Database Network System (SmartGene, Zug, Switzerland) to access mutation lists from the Stanford HIV Drug Resistance Database version 8.8.0 (Stanford University, Stanford, CA). The prevalence of specific drug-resistance mutations and categories of drug-resistance mutations were calculated by dividing the number of participants with 1 or more mutations by the total number of participants genotyped. Abbreviations: NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
HIV Genetic Diversity
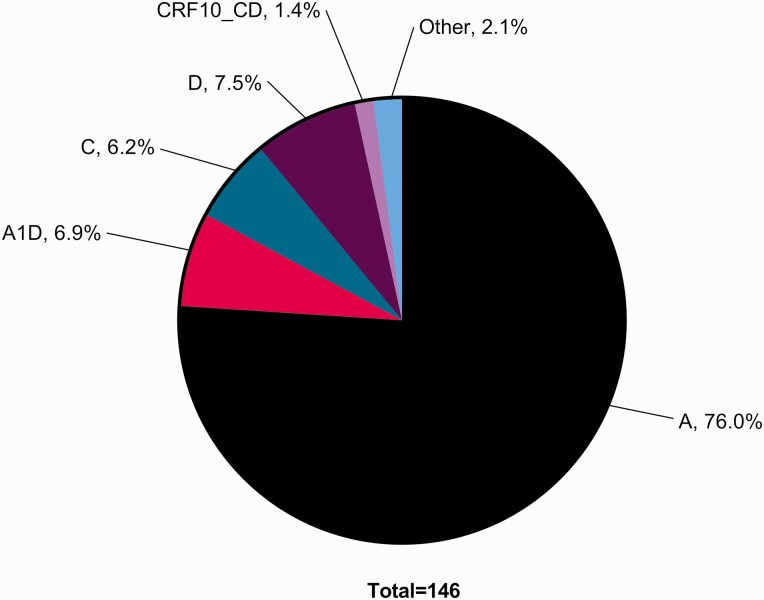
The subtype distribution was heterogeneous, with subtype A predominating (76%, n = 111). Other HIV-1 variants included D (8%, n = 11), A1D (7%, n = 10), C (6%, n = 9), and circulating recombinant forms (CRFs) that comprised CD (1%, n = 2) (Figure 2).
Figure 2.
HIV-1 subtypes. HIV-1 subtype was inferred from the consensus evolutionary tree from SmartGene Integrated Database Network System, which utilizes the neighbor-joining method in MEGA4 software. The evolutionary distances were computed using the maximum composite likelihood method in units of the number of base substitutions per site. The tree was then generated by the neighbor-joining method from a nucleotide alignment. Abbreviations: CRF, circulating recombinant form; HIV, human immunodeficiency virus.
DISCUSSION
This study showed a failure of VS in 20% of this pediatric cohort, reflecting a broader challenge of achieving targets for VS in this demographic group [17–20]. A study of Kenyan adolescents and young adults showed a similar viral nonsuppression rate of 27% [15]. Results from this study stress the importance of optimizing regimens to include INSTIs and evaluating the effectiveness of adherence counseling programs across all levels of healthcare settings. In addition, nonsuppressed caregiver VL has been identified as a prominent risk factor for pediatric nonsuppression [21]. In this study, 60% of the children who were virally unsuppressed also had caregivers with a nonsuppressed maternal VL; however, failed maternal VS represented only 3% of the study population, limiting impactful conclusions. Family-centered, program-level interventions to improve parent–child VS are currently being implemented to eliminate this discordance.
This study demonstrates high compliance with recommended first-line regimens. In the unadjusted model, TLE and TLD were associated with successful VS, and in the adjusted model, INSTI-based regimens were associated with VS [17, 21, 22]. The small number on INSTI-containing regimens, often in newly treated patients, likely reflects an emerging effort to optimize regimens for adolescents, as described in the new clinical guidelines [23]. As DTG use increases in ART experienced and naive CAHIV in SSA, more data will be available on its effectiveness and safety in this population, but these findings offer a positive perspective.
A high proportion of unsuppressed participants had evidence of HIVDRM. In a similar study examining adults enrolled at PEPFAR sites in Uganda, Kenya, Tanzania, and Nigeria, 82.5% of ART-experienced participants with a VL of more than 1000 copies/mL demonstrated at least 1 HIVDRM [16]. The class and individual drug mutations were similar between the 2 studies, with mutations associated with NNRTI resistance being the most common, followed by mutations associated with NRTI and PI resistance [16]. The concern that children are at higher risk for mutations was not apparent when comparing these 2 populations with similar available healthcare resources. Interestingly, although resistance rates were similar, the adult population had a lower rate of failed VS at 12% versus 20% [16]. Disparities in pediatric suppression rates compared with adults in low- to middle-income countries were identified in a 2016 meta-analysis [24]. The acquired drug-resistance pattern in our study was similar to a WHO literature review in children around the world [25]. No discernable pattern in NNRTI or NRTI resistance emerged from the small number of participants on INSTI regimens with high VL. Additional analyses of the HIVDRMs in this population will be the subject of future analyses.
The correlation between ART adherence, resistance, and VS is well documented [26]. This study showed a correlation between failed VS and number of missed doses of ART per month. Traditionally, the benchmark for ART adherence to provide the greatest chance for VS is 95%. This has been challenged within the last 10 years given new classes of drugs, newer generations of PIs and NNRTIs, and novel drug combinations [27]. Although 94% of caregivers in our population reported that their child had received at least 93% of their required doses, self- or caregiver reports of adherence tend to overestimate actual adherence [28, 29].
Factors related to nonadherence to ART are complex and multifactorial. The child’s behavioral and cognitive maturity; clinical status; the child’s primary caregivers’ physical and psychosocial health, biological relationship, cultural beliefs, and financial status; and the child’s surrounding cultural and social climate impact adherence [30]. Other factors related to ART nonadherence include conduct, learning and hyperactivity problems, length of time on ART, food insecurity, religion, drug formulation, and age of the patient [31–33]. These factors can persistently affect adherence despite interventions from trained professionals, and participants who had multiple referrals to adherence counseling due to nonadherence were less likely to have VS. Lack of benefit from adherence counseling has been shown in other studies [5]. Adherence counseling techniques and strategies may require modification to identify the root cause of nonadherence. Current Kenyan guidelines on adherence counseling for those who fail therapy describe a thorough review on identifying barriers to adherence, mental health screening, case management, home visits, and directly observed therapy [23]. It is unclear whether each site has the resources for such extensive adherence counseling and is potentially alluded to by the association of VS with those who have access to more resource-rich level 3 healthcare facilities. Differentiated care models in specialized adolescent clinics have, in some circumstances, been shown to improve VS by utilizing a client-centered approach and addressing gaps and unmet needs specific to adolescents [20]. In a study by Zanoni et al [20], a specialized weekend clinic only for adolescents aimed at de-stigmatizing, increasing peer support, and reducing absences from school showed higher retention and VS rates. The high number of adherence counseling sessions in those who fail therapy may not reflect a lack of adherence counseling effectiveness, as Kenya requires 3 adherence counseling sessions for anyone prior to ART regimen switching [23]. Given the high rate of HIVDRMs among treatment failures seen in this study, it is reasonable to be concerned that, in a setting that lacks routine access to HIVDRM testing, these mandatory counseling sessions may delay transition to a more optimized therapy, in the absence of clearly identified root causes and evidence that these sessions will improve adherence. Particularly for those adolescents for whom adherence remains challenging, further study of the effectiveness of long-acting injectable ART may ultimately provide a useful alternative [34].
Interventional trials that investigate ways to improve adherence within Africa are lacking. This observational study provides inconclusive evidence of program or community interventions, such as support groups or adherence counseling, positively affecting VS, and well-designed interventional trials are needed for more definitive conclusions. Nonetheless, these results are in line with a meta-analysis of different types of adherence interventions in pediatric patients with chronic disease showing that educational interventions had a very small effect size (d = 0.16) [35].
Limitations of this study include its cross-sectional nature and the inability to follow VS, resistance patterns, and ART regimens over time; thus, findings are associations and not causal. Additional longitudinal data are needed to determine the direction of the associations observed. This study lacked an assessment of weight-based dosing that can lead to failed VS or poor adherence if not routinely addressed at regular clinic visits. Although the number of participants on INSTI-based regimens was relatively small, integrase resistance testing would have been informative and should become part of standardized testing now that DTG use is becoming widespread. Measurements of adherence were based solely on caregiver or participant recall rather than more objective measures such as pill count. The exclusion of adolescents who were unaware of their HIV status may have excluded a population at high risk for nonadherence. Last, this population was drawn from those attending MHRP/PEPFAR-supported ART programs and may not represent the larger local or national population.
Conclusions
This study demonstrated 80% VS prevalence among CALHIV in Kenya. While it is hopeful that increased use of INSTI-containing regimens will improve suppression at the population level, the continued interplay between HIV resistance and adherence observed in this study merits close program monitoring. Current HIV treatment programs should develop targeted interventions for pediatric patients at high risk for treatment failure, such as those with a caregiver with failed VS and those struggling with adherence. By identifying those at higher risk, better patient-centered assistance can be provided. Further research into the effectiveness of adherence counseling and the policies mandating its use should be explored. In addition, resources across healthcare facilities should be examined to ensure care can be provided that matches current guideline recommendations. This study also serves as a baseline to demonstrate impact on VS as Kenya continues to transition children to INSTI-containing first-line regimens.
Notes
Acknowledgments. The authors thank the study participants, local implementing partners, and leadership at Kombewa Hospital, Manuyanda, Rodi, Kericho District Hospital, Bomet Health Centre, Sotik Health Centre, Kapkangani Health Centre, Kurangurik Dispensary, Sosiot Health Centre, Enoosaen Health Centre, Ndanai Health Centre, Kipketer Dispensary, and Kabiyet Health Centre who made this work possible. They also thank the RV518 Study Team from the US Military HIV Research Program Headquarters team: Ayesha Rashid, Ryan Mumma, Ying Fan, Daniel Choi, Mark Milazzo, Leilani Francisco, Steven Schech, Tsedal Mebrahtu, and Kimberly Bohince; from the RV518 South Rift Valley, Kenya team: Bornes Ngtech, Grace Engoke, Irene Metet, Alice Airo, Raphael Langat, and Margaret Biomdo; and from the RV518 Kisumu, Kenya team: John Owuoth, June Otieno, Zachaeus Osuo, Oliver Nyapiedho, Adam Mohammed, Ludia Badhi, Dorothy Okello, Annette Onyamasi, and Eunice Oyola.
Disclaimer. The views expressed are those of the authors and should not be construed to represent the positions of the US Army, the Department of Defense, the Uniformed Services University, the Henry M. Jackson Foundation for the Advancement of Military Medicine, or the National Institutes of Health. The investigators have adhered to the policies for protection of human subjects as prescribed in Army Regulation 70-25.
Financial support. This work was supported by the President’s Emergency Plan for AIDS Relief/Office of the Global AIDS Coordinator (funded the project in whole, payments made to the institution), the State Department via a cooperative agreement between the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc, and the US Department of Defense (grant number W81XWH-11-2-0174). Research reported in this publication was also supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award number T32AI114398.
Contributor Information
Isaac Tsikhutsu, US Army Medical Research Directorate–Africa, Nairobi, Kenya; HJF Medical Research International, Kericho, Kenya.
Margaret Bii, US Army Medical Research Directorate–Africa, Nairobi, Kenya; HJF Medical Research International, Kericho, Kenya.
Nicole Dear, US Military HIV Research Program, Walter Reed Army Institute of Research, Silver Spring, Maryland, USA; Henry M. Jackson Foundation for the Advancement of Military Medicine, Bethesda, Maryland, USA.
Kavitha Ganesan, US Military HIV Research Program, Walter Reed Army Institute of Research, Silver Spring, Maryland, USA; Henry M. Jackson Foundation for the Advancement of Military Medicine, Bethesda, Maryland, USA.
Alex Kasembeli, US Army Medical Research Directorate–Africa, Nairobi, Kenya; HJF Medical Research International, Kericho, Kenya.
Valentine Sing’oei, US Army Medical Research Directorate–Africa, Nairobi, Kenya; HJF Medical Research International, Kisumu, Kenya.
Kevin Rombosia, Ministry of Health, Kisumu County, Kisumu, Kenya.
Christopher Ochieng, US Army Medical Research Directorate–Africa, Nairobi, Kenya; HJF Medical Research International, Kericho, Kenya.
Priyanka Desai, US Military HIV Research Program, Walter Reed Army Institute of Research, Silver Spring, Maryland, USA; Henry M. Jackson Foundation for the Advancement of Military Medicine, Bethesda, Maryland, USA.
Vanessa Wolfman, US Military HIV Research Program, Walter Reed Army Institute of Research, Silver Spring, Maryland, USA; Henry M. Jackson Foundation for the Advancement of Military Medicine, Bethesda, Maryland, USA.
Peter Coakley, US Military HIV Research Program, Walter Reed Army Institute of Research, Silver Spring, Maryland, USA; Henry M. Jackson Foundation for the Advancement of Military Medicine, Bethesda, Maryland, USA.
Elizabeth H Lee, US Military HIV Research Program, Walter Reed Army Institute of Research, Silver Spring, Maryland, USA; The Uniformed Services University of the Health Sciences, Bethesda, Maryland, USA.
Patrick W Hickey, The Uniformed Services University of the Health Sciences, Bethesda, Maryland, USA.
Jeffrey Livezey, The Uniformed Services University of the Health Sciences, Bethesda, Maryland, USA.
Patricia Agaba, US Military HIV Research Program, Walter Reed Army Institute of Research, Silver Spring, Maryland, USA; Henry M. Jackson Foundation for the Advancement of Military Medicine, Bethesda, Maryland, USA.
References
- 1. Ahmed I, Lemma S.. Mortality among pediatric patients on HIV treatment in sub-Saharan African countries: a systematic review and meta-analysis. BMC Public Health 2019; 19:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. UNAIDS; UNICEF; World Health Organization. AIDSInfo. 2020. Available at: http://aidsinfo.unaids.org/. Accessed 3 September 2021.
- 3. Patel K, Hernán MA, Williams PL, et al. Long-term effects of highly active antiretroviral therapy on CD4+ cell evolution among children and adolescents infected with HIV: 5 years and counting. Clin Infect Dis 2008; 46:1751–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bernheimer JM, Patten G, Makeleni T, et al. Paediatric HIV treatment failure: a silent epidemic. J Int AIDS Soc 2015; 18:20090. Available at: https://pubmed.ncbi.nlm.nih.gov/26208630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jobanputra K, Parker LA, Azih C, et al. Factors associated with virological failure and suppression after enhanced adherence counselling, in children, adolescents and adults on antiretroviral therapy for HIV in Swaziland. PLoS One 2015; 10:e0116144. Available at: https://pubmed.ncbi.nlm.nih.gov/25695494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. van Rossum AMC, Fraaij PLA, de Groot R.. Efficacy of highly active antiretroviral therapy in HIV-1 infected children. Lancet Infect Dis 2002; 2:93–102. [DOI] [PubMed] [Google Scholar]
- 7. Calmy A, Ford N, Hirschel B, et al. HIV viral load monitoring in resource-limited regions: optional or necessary? Clin Infect Dis 2007; 44:128–34. [DOI] [PubMed] [Google Scholar]
- 8. Erb P, Battegay M, Zimmerli W, Rickenbach M, Egger M.. Effect of antiretroviral therapy on viral load, CD4 cell count, and progression to acquired immunodeficiency syndrome in a community human immunodeficiency virus-infected cohort. Swiss HIV cohort study. Arch Intern Med 2000; 160:1134–40. [DOI] [PubMed] [Google Scholar]
- 9. Ivers L, Kendrick D, Doucette K.. Efficacy of antiretroviral therapy programs in resource-poor settings: a meta-analysis of the published literature. Clin Infect Dis 2005; 41:217–24. [DOI] [PubMed] [Google Scholar]
- 10. UNAIDS. Understanding fast-track accelerating action to end the AIDS epidemic by 2030. 2015. Available at: https://www.unaids.org/sites/default/files/media_asset/201506_JC2743_Understanding_FastTrack_en.pdf. Accessed 3 September 2021.
- 11. World Health. Organization. . 2016.Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. 2nd ed. Geneva, Switzerland: World Health Organization. Available at: https://apps.who.int/iris/handle/10665/208825. Accessed 3 September 2021. [Google Scholar]
- 12. MacDonell K, Naar-King S, Huszti H, Belzer M.. Barriers to medication adherence in behaviorally and perinatally infected youth living with HIV. AIDS Behav 2013; 17:86–93. Available at: https://pubmed.ncbi.nlm.nih.gov/23142855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ferrand RA, Briggs D, Ferguson J, et al. Viral suppression in adolescents on antiretroviral treatment: review of the literature and critical appraisal of methodological challenges. Trop Med Int Health 2016; 21:325–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mwau M, Syeunda CA, Adhiambo M, et al. Scale-up of Kenya’s national HIV viral load program: Findings and lessons learned. PLoS One 2018; 13:e0190659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Njuguna I, Neary J, Mburu C, et al. Clinic-level and individual-level factors that influence HIV viral suppression in adolescents and young adults: a national survey in Kenya. AIDS 2020; 34:1065–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Crowell TA, Danboise B, Parikh A, et al. Pretreatment and acquired antiretroviral drug resistance among persons living with HIV in four African Countries. Clin Infect Dis 2020; 12:e0190260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chouraya C, Ashburn K, Khumalo P, et al. Association of antiretroviral drug regimen with viral suppression in HIV-positive children on antiretroviral therapy in Eswatini. Pediatr Infect Dis J 2019; 38:835–9. [DOI] [PubMed] [Google Scholar]
- 18. Jiamsakul A, Kariminia A, Althoff KN, et al. HIV viral load suppression in adults and children receiving antiretroviral therapy-results from the IeDEA collaboration. J Acquir Immune Defic Syndr 2017; 76:319–29. Available at: https://pubmed.ncbi.nlm.nih.gov/28708808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nsanzimana S, McArdle F, Remera E, et al. Viral suppression in a nationwide sample of HIV-infected children on antiretroviral therapy in Rwanda. Pediatr Infect Dis J 2019; 38:149–51. [DOI] [PubMed] [Google Scholar]
- 20. Zanoni BC, Sibaya T, Cairns C, Lammert S, Haberer JE.. Higher retention and viral suppression with adolescent-focused HIV clinic in South Africa. PLoS One 2017; 12:e0190260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Humphrey JM, Genberg BL, Keter A, et al. Viral suppression among children and their caregivers living with HIV in western Kenya. J Int AIDS Soc 2019; 22:e25272. Available at: https://pubmed.ncbi.nlm.nih.gov/30983148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Osman F, Yizengaw M.. Virological failure and associated risk factors among HIV/AIDS pediatric patients at the ART clinic of Jimma University Medical Center, Southwest Ethiopia. Open AIDS J 2020; 14:61–7. [Google Scholar]
- 23. Division of National AIDS and STI Control Program, Ministry of Health of Kenya. Guidelines on use of antiretroviral drugs for treating and preventing HIV infection in Kenya. 2018 edition. 2018. Available at: https://www.nascop.or.ke/new-guidelines/. Accessed 3 September 2021.
- 24. Boerma RS, Boender TS, Bussink AP, et al. Suboptimal viral suppression rates among HIV-infected children in low- and middle-income countries: a meta-analysis. Clin Infect Dis 2016; 63:1645–54. [DOI] [PubMed] [Google Scholar]
- 25. World Health Organization. HIV drug resistance report 2017. 2017. Available at: https://www.who.int/hiv/pub/drugresistance/hivdr-report-2017/en/. Accessed 3 September 2021.
- 26. Nachega JB, Hislop M, Nguyen H, et al. Antiretroviral therapy adherence, virologic and immunologic outcomes in adolescents compared with adults in southern Africa. J Acquir Immune Defic Syndr 2009; 51:65–71. Available at: https://pubmed.ncbi.nlm.nih.gov/19282780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Martin M, Cacho E D, Codina C, et al. Relationship between adherence level, type of the antiretroviral regimen, and plasma HIV type 1 RNA viral load: a prospective cohort study. AIDS Res Hum Retroviruses 2008; 24:1263–8. [DOI] [PubMed] [Google Scholar]
- 28. Alili M E, Vrijens B, Demonceau J, Evers SM, Hiligsmann M.. A scoping review of studies comparing the Medication Event Monitoring System (MEMS) with alternative methods for measuring medication adherence. Br J Clin Pharmacol 2016; 82:268–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Martin S, Elliott-DeSorbo DK, Calabrese S, et al. A comparison of adherence assessment methods utilized in the United States: perspectives of researchers, HIV-infected children, and their caregivers. AIDS Patient Care STDS 2009; 23:593–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Haberer J, Mellins C.. Pediatric adherence to HIV antiretroviral therapy. Curr HIV/AIDS Rep 2009; 6:194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Malee K, Williams P, Montepiedra G, et al. Medication adherence in children and adolescents with HIV infection: associations with behavioral impairment. AIDS Patient Care STDS 2011; 25:191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shah CA. Adherence to high activity antiretrovial therapy (HAART) in pediatric patients infected with HIV: issues and interventions. Indian J Pediatr 2007; 74:55–60. [DOI] [PubMed] [Google Scholar]
- 33. Young S, Wheeler AC, McCoy SI, Weiser SD.. A review of the role of food insecurity in adherence to care and treatment among adult and pediatric populations living with HIV and AIDS. AIDS Behav 2014; 18:S505–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Culhane J, Sharma M, Wilson K, et al. Modeling the health impact and cost threshold of long-acting ART for adolescents and young adults in Kenya. EClinicalMedicine 2020; 25:100453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kahana S, Drotar D, Frazier T.. Meta-analysis of psychological interventions to promote adherence to treatment in pediatric chronic health conditions. J Pediatr Psychol 2008; 33:590–611. [DOI] [PubMed] [Google Scholar]