Abstract
Background
Scabies is a neglected tropical disease of the skin that can lead to impetigo, serious secondary bacterial infections and immune-mediated diseases. Mass drug administration (MDA) has been reported in several studies to reduce the prevalence of scabies and impetigo. We aimed to assess the efficacy of MDA for scabies on scabies and impetigo.
Methods
We conducted a systematic review and meta-analysis of reports on the impact of MDA on scabies and impetigo. We included randomized control trials and observational evaluations reported from January 1970 to April 2021 and involving human participants. We searched PubMed, Ovid Medline, Embase, and Cochrane. We considered MDA as treatment intended for the whole population, regardless of individual infection status or symptoms. The main outcome assessed was the change in scabies and impetigo prevalence following MDA. This review is registered with PROSPERO (CRD42020169839).
Results
We identified 1110 records, of which 11 met inclusion criteria for the review and 9 were deemed suitable for meta-analysis for scabies and 4 for impetigo. Most studies were in small populations. There was a high degree of heterogeneity between studies (I2 value 96.19%). The overall relative reduction of the impact of MDA on scabies prevalence was 79%. The effect size was comparable for MDA based on ivermectin and permethrin. MDA for scabies also led to a reduction in impetigo prevalence with a relative reduction of 66%.
Conclusions
MDA for scabies is highly effective in reducing the prevalence of scabies and impetigo. Further research is needed to determine the durability of impact, and the effectiveness of MDA regimens in larger populations.
Keywords: scabies, impetigo, mass drug administration, ivermectin, permethrin
This manuscript provides what we believe to be the first systematic-review and meta-analysis on the effectiveness of mass drug administration for scabies. Our findings demonstrate that mass drug administration is highly effective in reducing the prevalence of scabies and impetigo.
Scabies is a skin disease caused by infestation with the mite Sarcoptes scabiei var. hominis. The mite burrows under the skin and causes intense itch and a papular rash. Infestation is often complicated by secondary skin infections with bacteria such as Streptococcus pyogenes and Staphylococcus aureus, which can lead to more severe skin infections, invasive disease, and immune-mediated disease [1]. Scabies also impacts quality of life by disrupting sleep, school or work performance, and by causing stigma [2, 3].
There are an estimated 455 million new cases of scabies globally each year [4]. The mite is transmitted by skin-to-skin contact and is more common in crowded settings. Infestations more frequently occur among people living in resource-poor environments, particularly tropical regions, where prevalence of over 20%, and 50% in children, has been reported [5]. Outbreaks have occurred in institutions such as nursing homes, prisons, and schools. The first line scabies treatment in the majority of guidelines is a topical agent, most commonly permethrin 5% cream or benzyl benzoate 25% lotion [6, 7]. Ivermectin, an oral agent, is also recommended as second line treatment [8]. For scabies, it requires administration of a second dose after 7–14 days because, unlike permethrin or benzyl benzoate, it does not kill mite eggs [9]. Guidelines also recommend treatment for household contacts.
In most high-prevalence settings, individual detection and treatment of scabies is not considered feasible on a large scale, so attention has turned to mass drug administration (MDA), a strategy for infectious disease control that involves treatment of everyone in a population, regardless of whether they have the targeted infection [10]. MDA is a crucial tool in the control of a number of globally important neglected tropical diseases (NTDs) including lymphatic filariasis, onchocerciasis, soil-transmitted helminths, and trachoma [11, 12], and has been used for scabies control in high-prevalence settings, under both programmatic and research frameworks [13]. The first World Health Organization expert consultation on scabies recommended MDA where prevalence is >10% [14].
Investigations of MDA for scabies have been conducted in a range of settings using a variety of treatment regimens. Several also assessed the impact of MDA for scabies on impetigo [5, 15, 16]. The aim of this study is to support the development of public health guidelines by assessing the efficacy of MDA for scabies on scabies and impetigo.
METHODS
We conducted a systematic review and meta-analysis of reports on the impact of MDA for scabies and impetigo. We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and registered with PROSPERO (CRD42020169839).
Search Strategy and Selection Criteria
We searched for randomized controlled trials and observational evaluations of MDA for scabies. We searched Pubmed, Ovid Medline, Embase, and Cochrane from January 1970 to April 2021. We used search terms including “scabies,” “mass-drug-administration,” “preventative-chemotherapy,” “community,” and “population” (full search in Supplementary Materials). We contacted investigators of studies that had been registered but not published and of other studies that we were aware of. We searched gray literature databases, Google Scholar, and reference lists for reports and conference proceedings not published in peer-reviewed journals. No language restrictions were applied.
Study Eligibility
Only human studies were eligible for inclusion. Studies were included if they reported on the prevalence of scabies before and after MDA in the same population, with an agent known to have therapeutic effect against the scabies mite. For the purposes of this review, we consider MDA as treatment intended for the whole population, regardless of individual infection status or symptoms. We included reports of treatment strategies covering at least 80% of the population with an anti-scabies agent. Agents known to have therapeutic activity against scabies include: orally administered ivermectin and topical agents permethrin, benzyl benzoate, lindane, crotamiton, malathion, sulfur and deltamethrin [7]. Reports of mass treatment used in response to outbreaks in institutional settings, or other closed settings such as refugee camps, were excluded. Studies were excluded if prevalence was not assessed before MDA (baseline) and at least one month after MDA. Studies were eligible for inclusion in meta-analysis if they reported scabies prevalence, across all age groups before and after MDA.
The MDA regimen was considered to be “ivermectin-based” even if medications other than ivermectin were offered to people in whom ivermectin was contraindicated (eg, young children or pregnant women) [17].
Quality Assessment
We used the Cochrane Collaboration’s tool for assessing risk of bias [18]. Studies were assessed independently by two investigators (S.J.L. and L.R.) in multiple domains of bias, including selection, performance, detection, attrition, and reporting. We also assessed the response or participation rate in the study, the quality of the scabies assessment (as described in the study report), and the statistical analysis of the results.
Data Extraction
All titles and abstracts identified were screened for relevance by S.J.L. with referral to L.R. and J.M.K. to resolve queries. Full texts of papers assessed to be relevant were reviewed by S.J.L. and L.R. independently of each other with referral to J.M.K. in the case of discordant opinions. The following variables were extracted: population (including location, years, demographic characteristics), study design (comparison groups, randomization, inclusion and exclusion criteria, sample size), scabies assessment method, intervention (MDA agent, dosing regimen), and outcomes (scabies and impetigo prevalence before and after MDA).
Data Analysis
For each study, we calculated the relative and absolute change in scabies prevalence between the pre-MDA and post-MDA assessments. If multiple time points of assessment were reported post-MDA, we calculated the change for the timepoint closest to 12 months. If a study had multiple arms, we considered them separately for analysis. We examined the change in scabies prevalence visually by plotting prevalence for each study over time. We compared outcomes by agent and dosing regimens as well as longer terms outcomes when reported.
For impetigo, we calculated the relative and absolute change in prevalence between the pre- and post-MDA assessments. We visually compared the change in impetigo prevalence for each study as well as comparing the change in scabies and impetigo prevalence for each study.
We used Stata (version 17.0, StataCorp) to perform the meta-analysis. We used a random effects model to calculate the risk-ratio of the change in scabies and impetigo prevalence between baseline and the primary endpoint of each study. We conducted a sub-analysis on studies where scabies prevalence at baseline was greater than 10% and by agent. We calculated the overall I2 [2] statistic to assess heterogeneity of studies.
RESULTS
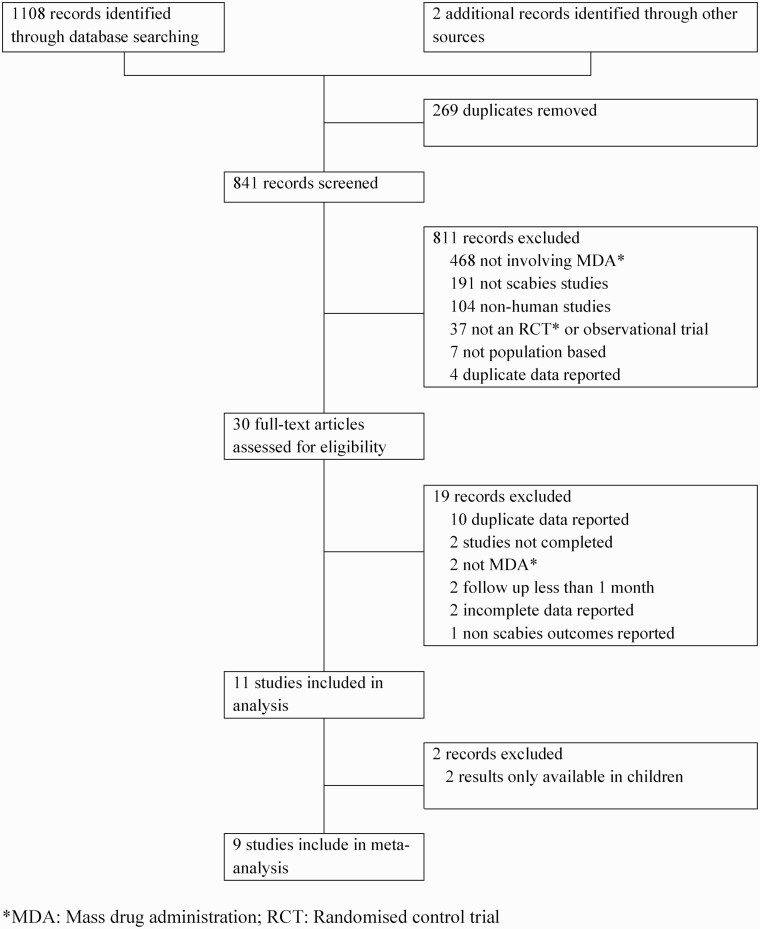
We identified 1108 records through database searches and an additional two records through other sources (Figure 1). After duplicates were removed, 841 records were screened by title and abstract. A further 811 were excluded leaving 30 full-text manuscripts, which were reviewed for eligibility. Following full-text assessment, a further 19 were excluded, leaving 11 studies, of which 9 were included in the meta-analysis for scabies and 4 for the meta-analysis for impetigo. We excluded 2 studies from the meta-analysis for both scabies and impetigo because only prevalence in children was available at the 12-month time point [19, 20].
Figure 1.
Study selection. Abbreviations: MDA, mass drug administration; RCT, randomized control trial.
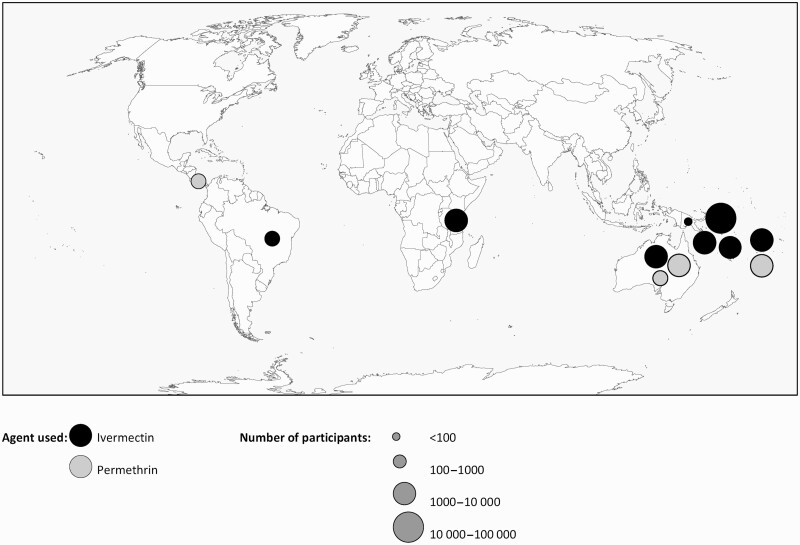
Studies were reported from 3 geographic regions with the majority (n = 9) conducted in Pacific Island countries (Figure 2). Approximately 36 000 individuals received MDA specifically for scabies, and a further 2269 received MDA with an anti-scabetic for lymphatic filariasis and were assessed for the impact on scabies.
Figure 2.
Map showing the location, agent used, and number of participants of mass drug administration for scabies.
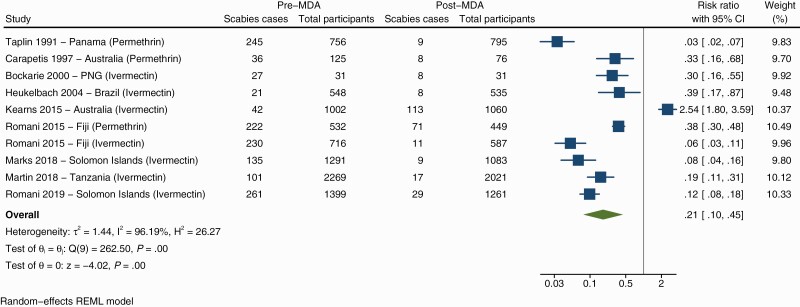
Of the included studies, all but 1 found a decrease in scabies prevalence after MDA (Figure 3). The overall risk-ratio of the change in prevalence following MDA was 0.21 (95% confidence interval [CI] .1–.45) indicating that MDA is favored with a relative reduction of 79%. The I2 value was 96.19%, indicating a high degree of heterogeneity between studies. There was a greater reduction in scabies prevalence when only studies with a baseline prevalence of more than 10% were included (Supplementary Figure 1). In these studies the overall risk-ratio was 0.15 (95% CI .08–.29) equating to a relative reduction of 85%.
Figure 3.
Forest plot showing the association of scabies prevalence before and after mass drug administration. Abbreviations: CI, confidence interval; MDA, mass drug administration; PNG, Papua New Guinea.
Of the 11 studies, 8 were designed to assess interventions specifically for scabies. The other three studies investigated the impact of MDA on scabies alongside other diseases including lymphatic filariasis [21], intestinal helminthiases [22], and trachoma [16]. The impact of scabies MDA on impetigo was assessed in 6 studies, and 1 also investigated the impact on hematuria [19]. A range of dosing regimens were used across the studies (Table 1). Ivermectin was the primary MDA agent for 7 studies, 3 used permethrin, and 1 had separate permethrin and ivermectin arms. For participants in whom ivermectin is contraindicated, most studies used permethrin and 1 used deltamethrin [22]. One study defined its intervention as targeted mass treatment, as agents were only provided to participants with parasitic skin disease or intestinal helminths and their households but was included in this review as over 90% of the community received ivermectin [22]. Two studies had control groups [23, 24].
Table 1.
Studies Included in the Review
| Scabies | Impetigo | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Intervention | Scabies Assessment Method | Endpoint Used | Baseline Prevalence | Post-MDA Prevalence | Relative Reduction | Baseline Prevalence | Post-MDA Prevalence | Relative Reduction | |
| Africa | ||||||||||
| Tanzania [21] | 2269 | Ivermectin 1 dose, 4 years | Community health workers trained in clinical diagnosis of scabies | 12 months | 4.4% | 0.8% | 80.9% | … | … | … |
| Central/South America | ||||||||||
| Brazil [22] | 576 | Ivermectin 2 doses in parasitic skin disease/intestinal helminths and households |
Experienced examiner | 9 months | 3.8% | 1.5% | 60.5% | … | … | … |
| Panama [26] | 756 | Permethrin 2 doses | Experienced examiner | 12 months | 33% | 1.1% | 96.7% | … | … | … |
| Pacific | ||||||||||
| Australia [25] | 1013 | Ivermectin 1 dose in non-scabies 2 doses in scabies |
Health workers using clinical guidelines | 12 months | 4% | 9% | 225% (increase) | … | … | … |
| Australia [20] | 2200 | Permethrin 1 dose in non-scabies and mild scabies 2 doses in moderate-severe scabies and house fumigated |
Experienced examiner | 7 months | 35% | 4.1% | 88.3% | 22.5% | 5.3% | 76.4% |
| ∗Only children <5 years examined | ||||||||||
| Australia [29] | 200–250 | Permethrin 1 dose 2 doses if heavy infestation |
Unclear | 10 months | 28.8% | 9.5% | 67% | 49% | 23% | 53.1% |
| Fiji [5] | 2051 | A. Ivermectin B. Permethrin 1 dose in non-scabies 2 doses in scabies |
According to clinical guidelines | 12 months | A. 32.1% B. 41.7% |
A. 1.9% B. 15.8% |
A. 94% B. 62.1% |
A. 24.6% B. 24.6% |
A. 8% B. 11.4% |
A. 67.5% B. 53.7% |
| PNG [23] | 31 | Ivermectin 1 dose | Unclear | 5 months | 87% | 26% | 70.1% | … | … | … |
| Solomon Islands [16] | 26 188 | Ivermectin 2 doses and Azithromycin |
Experienced examiner | 12 months | 18.7% | 2.3% | 87.7% | 24.8% | 6.4% | 74.2% |
| Solomon Islands [28] | 1291 | A. Ivermectin B. Ivermectin and Azithromycin 1 dose in non-scabies 2 doses in scabies |
Experienced examiner | 12 months | 10.5% | 0.8% | 92.4% | A. 10.1% B. 12.1% |
A. 2.5% B. 3.3% |
A. 75.2% B. 72.7% |
| Solomon Islands [19] | 1558 | Ivermectin 2 doses | Experienced examiner | 12 months | 24% | 3% | 87.5% | 40% | 34% | 15% |
Abbreviations: MDA, mass drug administration; PNG, Papua New Guinea.
The quality of studies varied (Supplementary Table 1). All studies used treatment interventions that were unblinded to participants and study personnel and specifically for outcome assessment. Studies that clearly reported participation rates had at least 85% coverage, but in 3 studies participation rates were unclear.
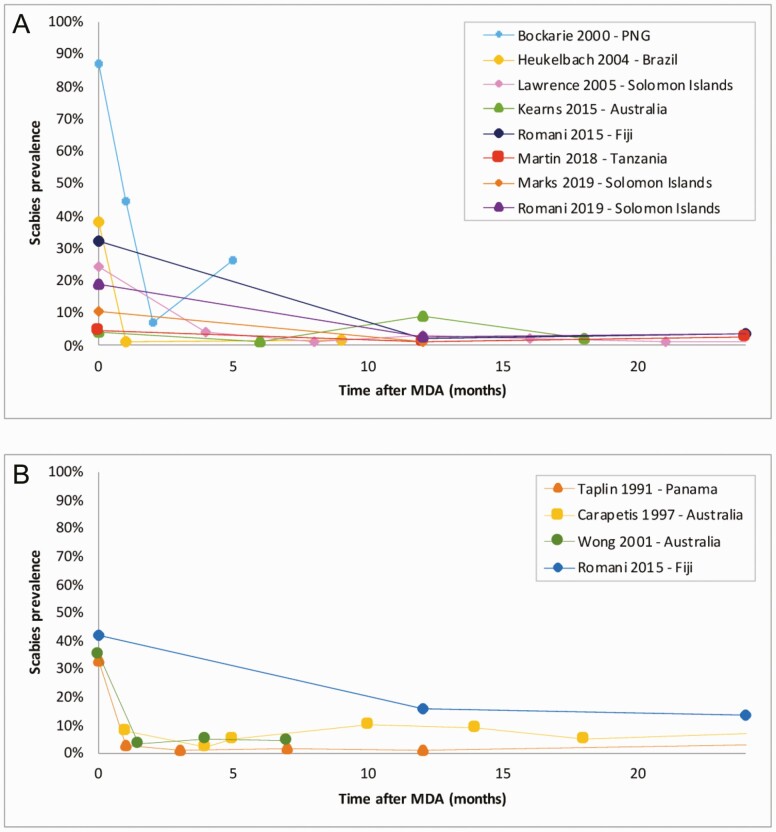
The relative change in scabies prevalence ranged from a reduction of 60.5% to 96.7% (Table 1). The absolute reduction ranged from 2.3% to 61%. There was, however, an increase in scabies prevalence at 12 months after MDA in 1 study but reduced again at 18 months [25].
Seven of the studies using ivermectin reported a decline in scabies prevalence at 12 months after MDA (Figure 4A). The impact was greater in settings with a higher prevalence prior to MDA [19, 24]. The greatest reduction was observed in the ivermectin group of the SHIFT study in Fiji, which had a baseline prevalence of 32.1% [24]. Conversely, the smallest reduction was observed in a study in Brazil, which had a low prevalence at baseline (3.8%) [22].
Figure 4.
A, Scabies prevalence by month following ivermectin-based mass drug administration. B, Scabies prevalence by month following permethrin-based mass drug administration. Abbreviations: MDA, mass drug administration; PNG, Papua New Guinea.
Four studies used permethrin-based MDA (Figure 4B). All reported a reduction in scabies prevalence. The greatest reduction was seen in a study in Panama, which had a prevalence of 33% before MDA [26]. The smallest reduction was in the permethrin group of the SHIFT study in Fiji where there was a prevalence of 41.7% before MDA [24].
The effect size was comparable between ivermectin and permethrin-based MDA. Ivermectin-based MDA had an overall risk-ratio of 0.23 (95% CI .09–.6) and permethrin-based MDA had an overall risk-ratio of 0.17 (95% CI .04–.75) (Supplementary Figures 2 and 3). There were however a small number of studies in the permethrin group and a high degree of heterogeneity in both groups.
For several studies, MDA was supplemented by subsequent monitoring and active management of scabies cases [19, 26]. In Solomon Islands, the program involved skin examination in children under 12 years of age, 3 times a year, with treatment of those with scabies and their household contacts. Returning residents and overnight visitors were offered treatment regardless of whether they had scabies. In Panama, regular skin examinations were conducted, and participants with scabies and their household contacts were treated along with arrivals to the island. These communities maintained a low scabies prevalence (< 5%) for over three years while these interventions were ongoing.
Control groups were used in 2 studies. The SHIFT trial in Fiji included a group in which participants with scabies and their household contacts were referred to their local clinic for treatment and observed a 49% scabies reduction in this group [24]. In Papua New Guinea, participants in a separate village participated in skin examinations but were not treated for scabies, prevalence had increased after 1 month [23].
Six studies reported the impact of MDA beyond 12 months. Scabies prevalence was measured in isolated villages in Solomon Islands, 15 years after MDA and the 3-year active management program. Scabies was found in 0.3% (1 participant) [15]. In Panama, prevalence was measured 40 months after MDA and an active management program, and declined to 1.5% from 33% at baseline [26]. However, following interruption to the supply of medication prevalence rose to 3.6% in 3 weeks, and when the program ceased, prevalence increased to 12% at 4 months. In a study of 1 round of ivermectin-based MDA in Solomon Islands, there was a 74.9% relative reduction in scabies after 36 months [27]. In the ivermectin-based MDA group of SHIFT in Fiji, the prevalence of scabies was 3.6% after 24 months. In the permethrin group of the same trial, the prevalence was 13.5% at 24 months [24]. In a study of 4 annual rounds of single dose, ivermectin-based MDA in Tanzania, scabies prevalence declined from 4.4% to 0.8% by 12 months and increased to 2.9% by 36 months [21].
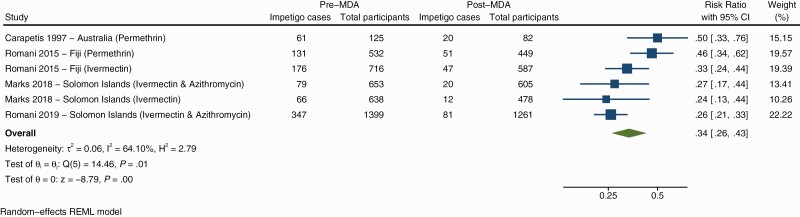
The impact of MDA on impetigo was measured in 6 studies (Supplementary Figure 4). All studies observed reductions in impetigo prevalence after MDA, with relative reduction ranging from 15% to 76.4% [19, 20]. Ivermectin-based MDA was used in 4 studies, and 3 used permethrin. Of the 6 groups included in the meta-analysis (across 4 studies) the overall risk-ratio for impetigo following MDA was 0.34 (0.26–0.43) equating to a relative reduction of 66% (Figure 5).
Figure 5.
Forest plot showing the association of impetigo prevalence before and after mass drug administration. Abbreviations: CI, confidence interval; MDA, mass drug administration.
The SHIFT study in Fiji showed a greater relative reduction in impetigo after ivermectin MDA (67.5%) compared to permethrin MDA (53.7%) [5]. In 2 Solomon Islands studies, azithromycin was simultaneously distributed as MDA, in 1 of the studies, there was a comparison group who received ivermectin only. In this study the relative reduction in impetigo was similar between groups (72.7% compared to 75.2%) [28]. The second study gave all participants both ivermectin and azithromycin MDA and found a relative reduction of 74.2% [16]. A study in Australia demonstrated both a reduction in the severity and prevalence of impetigo, up to 25 months after MDA [29].
DISCUSSION
Our review found that MDA for scabies, across a diverse range of study designs and settings, led to substantial reductions in both scabies and impetigo prevalence. There was a relative reduction of 79% in scabies following MDA and 66% in impetigo. In populations with a prevalence of scabies>10%, there was a greater reduction in scabies indicating that MDA may be more effective in this population. The effect size was similar for ivermectin-based and permethrin MDA.
All reported studies used permethrin for the entire treated population, or ivermectin plus a topical agent for people in whom ivermectin is contra-indicated. Although the overall results for ivermectin-based and permethrin MDA were comparable, in the only study where they were directly compared, there was a substantially greater reduction in scabies following ivermectin MDA [24]. As an oral agent, ivermectin offers a number of practical advantages over permethrin, which requires application to the whole body presenting challenges to use as directly observed treatment, so it is difficult to ensure high levels of adherence [30]. It is also more expensive and logistically challenging to transport cream compared to tablets. Permethrin is often used as an alternative agent to ivermectin due to its favorable safety profile in groups where ivermectin is contraindicated and greater patient acceptability due to less side effects compared to other topical agents, particularly benzyl benzoate [19]. Within the 2 broad groups of MDA, there was no clear relationship between the dose regimen and reduction in scabies. No study directly compared the efficacy of one versus two doses.
Most studies of MDA for scabies have been trials in remote settings. The largest population involved was in Solomon Islands, where 26 000 people received treatment [16]. Several large-scale scabies MDA programs have been conducted in Ethiopia, including 1 reaching over 9 million people, but reporting of these programs did not meet inclusion criteria for this review [31–33]. In these programs, communities with a scabies prevalence over 15% received MDA, although those with a prevalence <15% were offered case and contact treatment.
We found that MDA for scabies led to a reduction in the prevalence of impetigo, although not by as great a magnitude as for scabies (Supplementary Figure 5), and in most studies the reduction was sustained over time. The effect of scabies MDA on impetigo is important because of its potential impact on some of the serious downstream complications [34, 35].
Limitations of our review and meta-analysis was the relatively small number of published reports and the heterogeneity of study quality, sample size, and methods. Despite some geographic diversity across tropical regions, there were no studies from Asia, and few from African and Central and South American countries. Caution is required in generalizing findings beyond small island settings to promote MDA as a public health intervention at a larger scale. There is a need for studies in larger populations and in varied settings, including urban areas.
There was a lack of detail on how scabies and impetigo were diagnosed in many studies, and consensus diagnostic criteria were not used, which may have led to biased reporting of prevalence and directly affect the impact assessments of MDA (Table 1). The International Alliance for the Control of Scabies has published diagnostic criteria that have been used in more recent studies to address the need for standardized diagnosis in surveys, trials, and evaluation of programmatic interventions [36].
Although our data support the role of MDA for scabies control, there remain several key questions to be addressed before it can be adopted as a public health intervention more widely. These research gaps include the relative efficacy of alternate dosing regimens, the number and frequency of MDA rounds, cost-effectiveness, and safety and feasibility of integration with MDA drugs for other NTDs [1]. Although some studies documented the long-term effects of MDA it is unclear if multiple annual or biennial rounds would have a further sustained impact. Some studies showed the impact of active case management after MDA [19, 26], however further research is needed to understand the role of surveillance and treatment once prevalence has been reduced by MDA.
Overall, we demonstrated that although there are a limited number of studies there is substantial evidence to show that MDA for scabies can significantly reduce community prevalence of scabies and impetigo, and these effects can be long lasting. The impact of MDA on scabies prevalence is likely to be multifactorial, dependent on the baseline prevalence of scabies, MDA coverage, movement and social dynamics of the population, as well as the agent and dose regimen used. Further research is required to determine what prevalence of scabies should trigger MDA to control the hyperendemicity of scabies within communities, and the MDA regimen that is most clinical and cost effective for extensive roll out.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Financial support. D. E., L. R., J. M. K., and A. C. S. are supported by fellowships from the National Health and Medical Research Council of Australia. A. C. S. is also supported by the National Heart Foundation of Australia and the Viertel Charitable Foundation.
Contributor Information
Susanna J Lake, Tropical Diseases Research Group, Murdoch Children’s Research Institute, Melbourne, Australia; Department of Paediatrics, University of Melbourne, Melbourne, Australia; Melbourne Children’s Global Health, Melbourne, Australia.
John M Kaldor, Kirby Institute, University of New South Wales, Sydney, Australia.
Myra Hardy, Tropical Diseases Research Group, Murdoch Children’s Research Institute, Melbourne, Australia; Department of Paediatrics, University of Melbourne, Melbourne, Australia; Melbourne Children’s Global Health, Melbourne, Australia.
Daniel Engelman, Tropical Diseases Research Group, Murdoch Children’s Research Institute, Melbourne, Australia; Department of Paediatrics, University of Melbourne, Melbourne, Australia; Melbourne Children’s Global Health, Melbourne, Australia.
Andrew C Steer, Tropical Diseases Research Group, Murdoch Children’s Research Institute, Melbourne, Australia; Department of Paediatrics, University of Melbourne, Melbourne, Australia; Melbourne Children’s Global Health, Melbourne, Australia.
Lucia Romani, Tropical Diseases Research Group, Murdoch Children’s Research Institute, Melbourne, Australia; Kirby Institute, University of New South Wales, Sydney, Australia.
References
- 1. Engelman D, Cantey PT, Marks M, et al. The public health control of scabies: priorities for research and action. The Lancet 2019; 394:81–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Van Der Linden N, Van Gool K, Gardner K, et al. A systematic review of scabies transmission models and data to evaluate the cost-effectiveness of scabies interventions. PLoS NeglTrop Dis 2019; 13:e0007182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mitchell E, Bell S, Thean LJ, et al. Community perspectives on scabies, impetigo and mass drug administration in Fiji: a qualitative study. PLoS NeglTrop Dis 2020; 14:e0008825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vos T, Abajobir AA, Abate KH, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. The Lancet 2017; 390:1211–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Romani L, Steer AC, Whitfeld MJ, Kaldor JM.. Prevalence of scabies and impetigo worldwide: a systematic review. Lancet Infect Dis 2015; 15:960–7. [DOI] [PubMed] [Google Scholar]
- 6. eTG complete. Dermatology - Insects and Mites. Melbourne, Victoria: Therapeutic Guidelines Ltd. [Google Scholar]
- 7. Hay RJ, Steer AC, Engelman D, Walton S.. Scabies in the developing world–-its prevalence, complications, and management. Clin Microbiol Infect 2012; 18:313–23. [DOI] [PubMed] [Google Scholar]
- 8. WHO. World Health Organization Model List of Essential Medicines, 21st List, 2019. Geneva: World Health Organization; 2019. [Google Scholar]
- 9. Currie BJ, McCarthy JS.. Permethrin and ivermectin for scabies. N Engl J Med 2010; 362:717–25. [DOI] [PubMed] [Google Scholar]
- 10. Hotez PJ, Molyneux DH, Fenwick A, et al. Control of neglected tropical diseases. N Engl J Med 2007; 357:1018–27. [DOI] [PubMed] [Google Scholar]
- 11. Hotez P. Mass drug administration and integrated control for the world’s high-prevalence neglected tropical diseases. Clin Pharm Therap 2009; 85:659–64. [DOI] [PubMed] [Google Scholar]
- 12. Amato VS, Tuon FF.. Mass drug administration for the control of Strongyloides stercoralis infection: progress and challenges. Clin Infect Dis 2020; 71:3229–31. [DOI] [PubMed] [Google Scholar]
- 13. Engelman D, Steer AC.. Control strategies for scabies. Trop Med Infect Dis 2018; 3:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Engelman D, Marks M, Steer AC, et al. A framework for scabies control. PLoS Negl Trop Dis 2021; 15:e0009661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Marks M, Taotao-Wini B, Satorara L, et al. Long term control of scabies fifteen years after an intensive treatment programme. PLoS Negl Trop Dis 2015; 9:e0004246–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Romani L, Marks M, Sokana O, et al. Efficacy of mass drug administration with ivermectin for control of scabies and impetigo, with coadministration of azithromycin: a single-arm community intervention trial. Lancet Infect Dis 2019; 19:510–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hardy M, Engelman D, Steer A.. Scabies: a clinical update. Aust Fam Physician 2017; 46:264–8. [PubMed] [Google Scholar]
- 18. Higgins JPT, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011; 343:d5928–d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lawrence G, Leafasia J, Sheridan J, et al. Control of scabies, skin sores and haematuria in children in the Solomon Islands: another role for ivermectin Lutte contre la gale, les lésions cutanées et l’ hématurie chez les enfants des îles Salomon: un autre rôle pour l’ ivermectine: résu. Bull World Health Organ 2005; 83:34–42. [PMC free article] [PubMed] [Google Scholar]
- 20. Wong LCF, Amega B, Connors C, Barker R, Dulla ME, Currie BJ.. Outcome of an interventional program for scabies in an Indigenous community. Med J Aust 2001; 175:367–70. [DOI] [PubMed] [Google Scholar]
- 21. Martin D, Wiegand R, Goodhew B, Lammie P, Mkocha H, Kasubi M.. Impact of ivermectin mass drug administration for lymphatic filariasis on scabies in eight villages in Kongwa District, Tanzania. Am J Trop Med Hyg 2018; 99:937–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Heukelbac J Jr, Winter B, Wilcke T, et al. Selective mass treatment with ivermectin to control intestinal helminthiases and parasitic skin diseases in a severely affected population. Traitement de masse sélectif par l’ ivermectine contre les helminthiases intestinales et les dermatoses parasitaires dans une population séve 2004; 82:563–71. [PMC free article] [PubMed] [Google Scholar]
- 23. Bockarie MJ, Alexander NDE, Kazura JW, Bockarie F, Griffin L, Alpers MP.. Treatment with ivermectin reduces the high prevalence of scabies in a village in Papua New Guinea. Acta Trop 2000; 75:127–30. [DOI] [PubMed] [Google Scholar]
- 24. Romani L, Whitfeld MJ, Koroivueta J, et al. Mass drug administration for scabies control in a population with endemic disease. N Engl J Med 2015; 373:2305–13. [DOI] [PubMed] [Google Scholar]
- 25. Kearns TM, Speare R, Cheng AC, et al. Impact of an ivermectin mass drug administration on scabies prevalence in a remote Australian aboriginal community. PLoS Negl Trop Dis 2015; 9:e0004151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Taplin D, Meinking TL, Porcelain SL, et al. Community control of scabies: a model based on use of permethrin cream. Lancet 1991; 337:1016–8. [DOI] [PubMed] [Google Scholar]
- 27. Marks M, Romani L, Sokana O, et al. Prevalence of scabies and impetigo 3 years after mass drug administration with ivermectin and azithromycin. Clin Infect Dis 2020; 70:1591–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Marks M, Toloka H, Baker C, et al. Randomized trial of community treatment with azithromycin and ivermectin mass drug administration for control of scabies and impetigo. Clin Infect Dis 2019; 68:927–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Carapetis J, Connors CM, Yarmirr D, Krause VL, Currie BJ.. Success of a scabies control program in an Australian aboriginal community. Pediatr Infect Dis J 1997; 16 5:494–9. [DOI] [PubMed] [Google Scholar]
- 30. La Vincente S, Kearns T, Connors C, Cameron S, Carapetis J, Andrews R.. Community management of endemic scabies in remote aboriginal communities of Northern Australia: low treatment uptake and high ongoing acquisition. PLoS NeglTrop Dis 2009; 3:e444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Enbiale W, Ayalew A, Gebrehiwot T, et al. Does mass drug administration for community-based scabies control works? The experience in Ethiopia. J Infect Dev Ctries 2020; 14:78S–85S. [DOI] [PubMed] [Google Scholar]
- 32. Enbiale W, Baynie TB, Ayalew A, et al. “Stopping the itch”: mass drug administration for scabies outbreak control covered for over nine million people in Ethiopia. J Infect Dev Ctries 2020; 14:28S–35S. [DOI] [PubMed] [Google Scholar]
- 33. Enbiale W, Ayalew A.. Investigation of a scabies outbreak in drought-affected areas in Ethiopia. Trop Med Infect Dis 2018; 3:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Thornley S, Marshall R, Jarrett P, Sundborn G, Reynolds E, Schofield G.. Scabies is strongly associated with acute rheumatic fever in a cohort study of Auckland children. J Paediatr Child Health 2018; 54:625–32. [DOI] [PubMed] [Google Scholar]
- 35. Thean LJ, Jenney A, Engelman D, et al. Prospective surveillance for invasive Staphylococcus aureus and group A Streptococcus infections in a setting with high community burden of scabies and impetigo. Int J Infect Dis 2021; 108:333–9. [DOI] [PubMed] [Google Scholar]
- 36. Engelman D, Yoshizumi J, Hay RJ, et al. The 2020 international alliance for the control of scabies consensus criteria for the diagnosis of scabies. Br J Dermatol 2020; 183:808–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.