Significance
Populations of large-bodied mammals are declining at an alarming rate. Their decline has serious ecosystem consequences because they have important functional roles that are not replicated by smaller-bodied animals. Understanding how their decline or potential extinction will influence ecosystem processes is critically important and time-sensitive. Here, we use the paleorecord as an analogy for modern biodiversity loss. We examine consequences of the terminal-Pleistocene megafauna extinction on a mammal community from the Edwards Plateau, Texas by characterizing changes in animal body size and dietary isotopic niche before and after the event. We find significant reorganization of the community, particularly among carnivores, a loss of ecological complexity, and many vacant niches. The loss of complexity likely meant a reduction in ecosystem resilience.
Keywords: megafauna, biodiversity loss, functional diversity, mesocarnivore release, stable isotopes
Abstract
The conservation status of large-bodied mammals is dire. Their decline has serious consequences because they have unique ecological roles not replicated by smaller-bodied animals. Here, we use the fossil record of the megafauna extinction at the terminal Pleistocene to explore the consequences of past biodiversity loss. We characterize the isotopic and body-size niche of a mammal community in Texas before and after the event to assess the influence on the ecology and ecological interactions of surviving species (>1 kg). Preextinction, a variety of C4 grazers, C3 browsers, and mixed feeders existed, similar to modern African savannas, with likely specialization among the two sabertooth species for juvenile grazers. Postextinction, body size and isotopic niche space were lost, and the δ13C and δ15N values of some survivors shifted. We see mesocarnivore release within the Felidae: the jaguar, now an apex carnivore, moved into the specialized isotopic niche previously occupied by extinct cats. Puma, previously absent, became common and lynx shifted toward consuming more C4-based resources. Lagomorphs were the only herbivores to shift toward C4 resources. Body size changes from the Pleistocene to Holocene were species-specific, with some animals (deer, hare) becoming significantly larger and others smaller (bison, rabbits) or exhibiting no change to climate shifts or biodiversity loss. Overall, the Holocene body-size-isotopic niche was drastically reduced and considerable ecological complexity lost. We conclude biodiversity loss led to reorganization of survivors and many “missing pieces” within our community; without intervention, the loss of Earth’s remaining ecosystems that support megafauna will likely suffer the same fate.
Across the globe today, populations of large-bodied mammals are in rapid decline (1–5). This is a continuation of an extinction event that has been ongoing for more than 100 ky. As humans became increasingly abundant and dispersed around the globe over the late Quaternary, a temporally and spatially transgressive extinction of large-bodied mammals followed (6–9); the degree of size selectivity was unprecedented in the Cenozoic fossil record (8). Moreover, extinction rates in the modern have accelerated and are now 10 to 100 times greater than the normal background rates recorded in the geologic record (10–13). Indeed, more than 40% of mammals have lost at least 80% of their historical range (5) and domesticated animals now make up most of contemporary vertebrate biomass on Earth (14, 15). Predictions for the future are sobering (13). For example, if all North American mammals currently at risk eventually do go extinct, ∼30% of species will have been lost relative to the late Pleistocene (8).
The transition of the biosphere from a natural to heavily human-modified system has important implications for ecosystem function (1–4, 9, 11, 15, 16). Widespread biodiversity loss is more than species extinction; it also leads to the loss of ecological function, which may be realized at local, regional, or even global scales. Large-bodied wild mammals, in particular, play an important role within ecosystems through the transport of nutrients and biogeochemical cycling, modification of vegetation composition and structure, ecological interactions with other animals, and even feedbacks with climate (3, 17–23). Hence, there is growing concern that the ongoing loss of large-bodied wild mammals may lead to the unraveling of ecosystems because these complex ecological roles are not generally replicated by domesticated or smaller-bodied animals (1–4, 11). Because mammals interact with their environments in both direct and indirect ways, it can take decades before the full impact of extirpations is revealed. Yet, waiting for the results of long-term studies is at odds with the time-critical nature of extinction risk (1–5). One promising strategy is to integrate paleoecology into conservation biology (15, 16, 23); by quantifying the ecological consequences of earlier extinctions on taxa and community structure and function, we can gain insights relevant to the ongoing biodiversity crisis. Thus, we focus on the terminal Pleistocene of North America, where the arrival of early humans ∼13 ky precipitated a massive size-selective extinction (24–26), continuing the long-term shift from a world dominated by wild animals to one largely composed of humans and their livestock (8, 14, 15).
At the terminal Pleistocene, the mammal assemblage of North America was diverse and included large-bodied species such as camelids (Camelops and Palaeolama), mammoth (Mammuthus), ground sloth (Paramylodon and Megalonyx), mastodon (Mammut), glyptodont (Glyptotherium), bison (Bison spp.), and numerous horses (Equus spp.). While there has been a long and rancorous debate about the culpability of humans in this extinction (7, 24–27), we do not focus on causation. What is not in debate is that the extinction of >65 species of large-bodied mammals in North America—and another >70 species in South America—led to a massive reorganization of ecosystems in the New World, which has only recently begun to be investigated (9, 15–23, 28, 29).
Here, we examine the ecological consequences of the extinction for a nonvolant terrestrial mammal community in the Edward’s Plateau of central Texas with an abundant and well-resolved fossil record. We hypothesized that loss of large-bodied mammals at the terminal Pleistocene profoundly altered the ecology of surviving mammals, disrupting predator–prey pairs and causing shifts in resource use and morphology. Hence, we addressed two fundamental questions: 1) How unique was the functional role played by large-bodied mammals in this terrestrial ecosystem? Are there “pieces” of functional space that remain unfilled today? and 2) What was the ecological legacy of this extinction? Did the loss of large-bodied congeners result in dietary or body-size shifts among the surviving mammals to exploit newly accessible resources? Were predator–prey relationships altered in the wake of the loss of top consumers? We anticipated that surviving herbivores would move toward C4-based resources after the loss of almost all grazers in the community and might also increase in body size as a physiological adaptation to better exploit fibrous vegetation (30). Finally, we expected to find that smaller-bodied carnivores experienced ecological release after the extinction of the top predators in the system. We included all mammals >1 kg in our analyses; while smaller-bodied mammals were also likely to have demonstrated sensitivity to the community and vegetation shifts at the terminal Pleistocene, they were less likely to directly compete or interact with the extinct large-bodied species.
To address these questions, we focused on metrics that are readily measured with fossil materials: body size and the isotopic niche that primarily reflects the functional role(s) of organisms. Body size is a fundamental attribute of an organism, which influences most life processes, ecology, and interactions with both the abiotic and biotic environment (30). Stable isotope analysis provides information about resource and habitat use that is independent of morphology; it has been extensively used to examine the ecology of ancient mammals and to reconstruct Pleistocene food webs (e.g., refs. 31–38). At the base of the food web, variation in carbon isotope (δ13C) values is largely driven by different photosynthetic pathways used by primary producers. Trees, most shrubs, and grasses in cool-growing seasons employ the C3 pathway, while most tropical and subtropical grasses that grow in warmer environments use the C4 pathway (34, 39) Thus, measuring δ13C of consumers allows differentiation between different herbivore guilds (i.e., browsers versus grazers) in ecosystems where both photosynthetic pathways contribute to primary production. In contrast, δ15N values are positively correlated with trophic level (40). By measuring δ13C and δ15N values of consumers and their resources, we can characterize isotopic niches at different levels of ecological organization from the individual to the community and quantify the relative consumption of plant and prey functional groups across the food chain (41).
The use of both body mass and stable isotopes in our analysis provides powerful information for recreating ancient ecosystems. For example, while stable isotopes quantify the use of C3- vs. C4-based resources and trophic level, they provide incomplete information about how species coexist in diverse ecosystems. Body size mediates how an herbivore uses a C3 or C4 resource. While a mammoth or mastodon can forage at the level of an entire plant, bush, or tree, smaller-bodied herbivores feed on parts of the plant (i.e., flowers, seeds, grass heads, or leaves), and the smallest herbivores can selectively feed on the cell contents of the leaves themselves, avoiding fibrous portions (42). While there can be isotopic differences between photosynthetic and nonphotosynthetic tissue types in C3 plants, these differences are relatively small (1 to 3 per mil), highly variable, and may not be found in C4 plants (43). Thus, despite feeding on different parts of a plant, herbivores may have only slightly different isotopic values and resource partitioning could be difficult to detect without the additional information gained by using body size. Such fine-scale resource partitioning has been shown to support a wide diversity of herbivores in the savannahs of Africa today (44, 45) and may have been important in ancient ecosystems as well.
Results
Herbivore Isotopic and Body Size Changes.
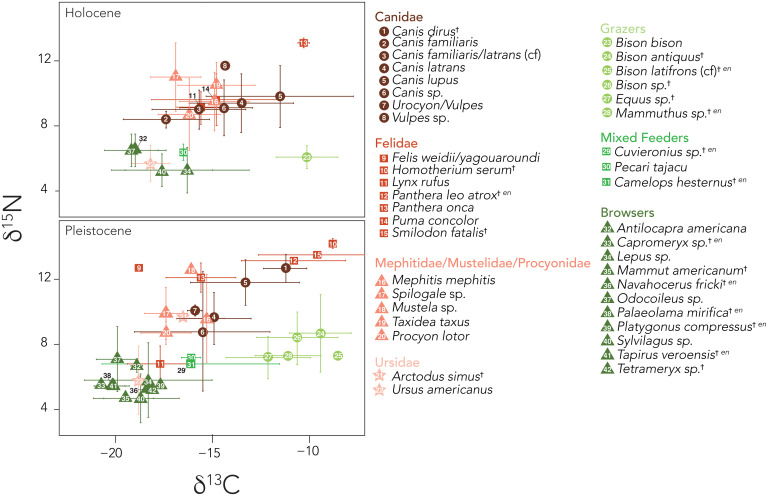
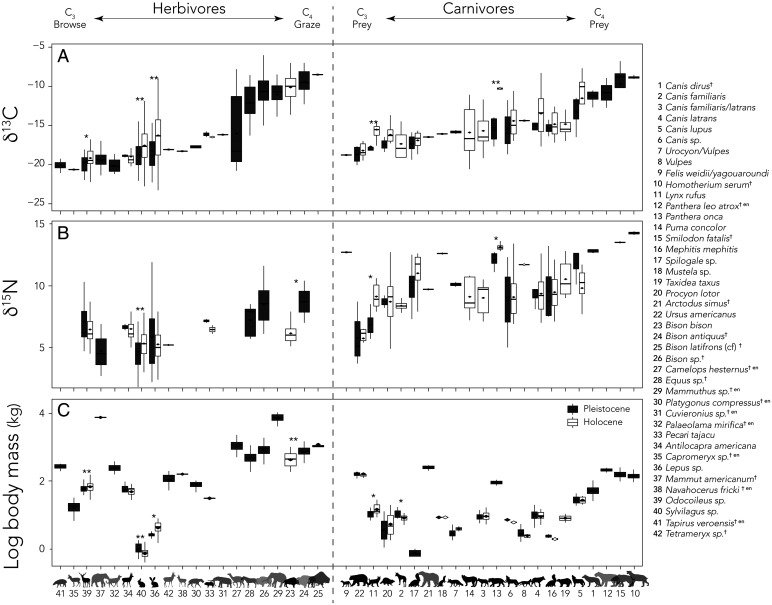
Preextinction, our community contained a diversity of C4 grazers, C3 browsers, and mixed feeders, occupying the entire spectrum of C3 to C4 resources (Fig. 1A and SI Appendix, Table S1). The browser guild included mastodon, deer, antelopes, llama, tapir, and lagomorphs (Mammut, Odocoileus, and Navahoceros, Antilocapra, Tetrameryx, and Capromeryx, Palaeolama, Tapirus, Sylvilagus, and Lepus), while the grazers included multiple species of bison, mammoth, and horses (Bison, Mammuthus, and Equus); mixed feeders such as peccary, camels, and gomphothere (Pecari and Platygonus, Camelops, and Cuvieronius) were also present. We were unable to obtain isotopic data for several extinct taxa (Megalonyx, Glyptotherium, and Bootherium). Large-bodied mammals occupied all trophic niches; thus, the size-selective terminal Pleistocene megafauna extinction influenced diversity across the entire carbon isotopic spectrum. Indeed, 71.4% of browsers, 89% of grazers, and 80% of mixed feeders >1 kg were extirpated (SI Appendix, Table S1 and Fig. 1B). Fewer carnivores and omnivores were lost, 27.7% and 17%, respectively. Despite including specimens from multiple sites, the carbon isotopic composition of taxa did not significantly differ among locations. Body size was also constrained across sites with the exception of deer, where Holocene animals from Sheep Shelter were significantly larger than at some of the other Holocene locations (ANOVA degrees of freedom [df] = 8/114, F = 6.98, P < 0.001).
Fig. 1.
Mean δ13C and δ15N values of species within the mammalian community at Hall’s Cave. (A) Holocene and (B) Pleistocene. Error bars represent SD. All mammals >1 kg were included. For species where mean δ13C values were determined based on enamel (indicated by superscript “en”), the mean δ15N value for the functional group (e.g., browser, mixed feeder, grazer) or guild (Felidae) was used as a surrogate for the nitrogen isotope composition. Enamel δ13C values were corrected to corresponding collagen values using standardized offsets (SI Appendix). See SI Appendix, Tables S1 and S5 for isotope data.
The mean (± SD) δ13C value of the single surviving Holocene grazer, Bison bison (−10.3 ± 1.4‰), was not different from Pleistocene bison (−10.8 ± 2.1‰; SI Appendix, Tables S1 and S7 and Fig. 2A) despite a highly significant difference in average body mass between these species (Pleistocene bison = 779 kg vs. Holocene bison = 477 kg; two-sample t test df = 119.0, t = −6.93, P < 0.001). The two surviving large-bodied browsers, deer (Odocoileus) and antelope (Antilocapra), also remained within the same narrow isotopic space over time (Pleistocene pooled enamel/collagen δ13C: −19.9 ± 1.1‰, −18.9 ± 0.3‰; Holocene pooled δ13C: −19.2 ± 1.4‰, −19.0 ± 1.6‰, respectively) but varied in their body mass response. While deer became significantly larger in the Holocene (73 kg vs. 61 kg; two-sample t test df = 137.3, t = 3.01, P < 0.01), pronghorn became slightly but not significantly smaller (52 kg vs. 58 kg; two-sample t test df = 14.5, t = −0.64, P > 0.05). After the extinction, the mean δ13C value of both the jackrabbit (Lepus) and cottontail (Sylvilagus) significantly increased in the Holocene (two-sample t test df = 71.4, t = 3.53, P < 0.001; Fig. 2A). This was accompanied by an increase in size among jackrabbits (4.5 kg vs. 2.8 kg; two-sample t test df = 24.1, t = 3.07, P < 0.01; Fig. 2C) and a decrease in size among cottontails (0.8 kg vs. 1.0 kg; two-sample t test df = 134.9, t = −3.55, P < 0.001). Interestingly, the jackrabbit was the only taxon to significantly diminish in mass from the Holocene to the modern (4.5 kg vs. 2.6 kg; two-sample t test df = 20.7, t = 4.42, P < 0.001). Although there were slight mass changes in cottontails between the Holocene and modern time periods (Sylvilagus Holocene = 0.8 kg, modern = 1.3 kg; two-sample t test df = 10.4, t = −2.26, P < 0.05), this was not significant after a Bonferroni correction (SI Appendix, Table S7). Morphological changes in herbivorous taxa were unlikely to be driven largely by climate; only bison and cottontails significantly decreased in body size as predicted by Bergmann’s rule.
Fig. 2.
Change in the dietary isotopic space and body size of mammals at Hall’s Cave across the Pleistocene to Holocene. (A) Change in δ13C, (B) δ15N, and (C) body mass in log kilograms. In all panels, taxa were arranged in rank order of increasing δ13C values. Data were plotted separately for herbivores (Left) and carnivores (Right). Extinct species are indicated with gray silhouettes. Values for Pleistocene are in black; values for surviving mammals in the Holocene are shown in white. Asterisks indicate significance levels: *marginal, that is, i.e., not significant after Bonferroni corrections, **remains significant after correction (SI Appendix, Table S7). Enamel values were corrected to corresponding collagen values using standardized offsets (SI Appendix, Table S6). Legend is the same as Fig. 1.
Carnivore Isotopic and Body-Size Changes.
In the Pleistocene, carnivore families occupied distinct isotopic niches, which reflected different types of C3 vs. C4 prey and differing levels of carnivory vs. omnivory (SI Appendix, Table S1 and Fig. 1). Among families, Ursidae had the lowest mean δ15N value (7.8 ± 1.8‰), while Mephitidae (9.8 ± 2.2‰) and Procyonidae (8.7 ± 0.7‰) had slightly but not significantly higher nitrogen isotope values. The δ15N values of Canidae (10.6 ± 1.6‰) were higher than these groups, but this was driven largely by wolves, who had a significantly higher mean nitrogen isotope value (12.3 ± 0.6‰) than other canids. Felidae had the highest mean δ15N value (11.9 ± 0.8‰) among carnivore families, with the extinct felid species exhibiting the highest values found within the community (13.9 ± 0.5‰).
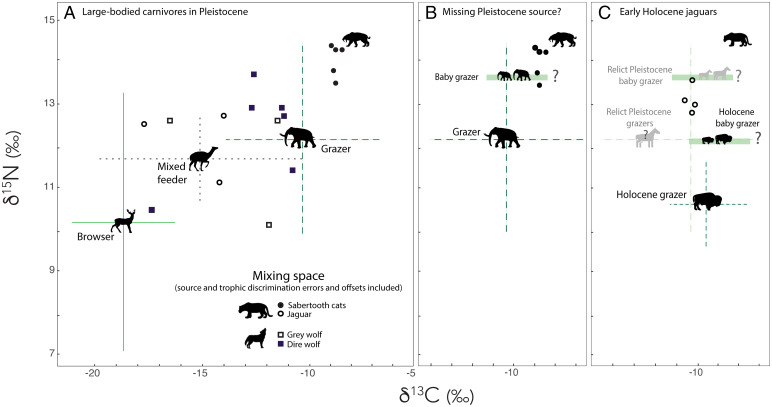
The two species of extinct sabertooth cats (the dirk-toothed Smilodon and the scimitar-toothed Homotherium) occupied distinct, and in the case of Homotherium very narrow, isotopic niches, suggesting a high level of prey specialization. Their δ13C values indicated a diet composed exclusively of grazers, likely bison and mammoth (Fig. 1B). They also had particularly high δ15N values. In this community, grazers had significantly higher mean (± SD) δ15N values than did browsers (8.1 ± 1.8‰ vs. 5.7 ± 1.6‰), reflecting differences in the nitrogen isotope composition of C3 vs. C4 vegetation (47, 48). However, both Smilodon and Homotherium had δ15N values that were elevated by ∼6‰ over that of grazers. While this may have been driven by large trophic discrimination values, which can be 4 to 5‰ for terrestrial hypercarnivores (47, 48), it may also have reflected consumption of juvenile nursing herbivores (49). Because nursing mammals essentially consume their lactating mothers, their δ15N values are enriched relative to adults (49). Indeed, when we examined the mixing space of extinct cats, it was clear an important source was missing (Fig. 3A); adding imaginary nursing juveniles to the mixing space resolved the issue (Fig. 3B). Browsers and mixed feeders were likely a very minor component of the diet (Fig. 3). In contrast, the wider carbon isotopic niche of the American cave lion (Panthera leo atrox) suggested a more generalist diet including both grazers and mixed feeders. However, the low sample size for this taxon (n = 2) and lack of collagen-derived δ15N data mean this interpretation is tentative (Fig. 1B and SI Appendix, Fig. S1). The two other felids present, jaguar (Panthera onca) and lynx (Lynx rufus), likely fed on mixed feeders and/or browsers (Figs. 1 and 3). However, after the extinction, the jaguar became an apex carnivore and moved from this generalized diet (Fig. 3A) into the specialized isotopic niche focused on consumption of C4 grazers previously occupied by the sabertooth cats (Fig. 3B). Jaguar mean pooled δ13C values shifted from −15.6 ± 1.8‰ before the extinction to −10.3 ± 0.3‰, with a smaller but marginally significant increase in δ15N (Figs. 1–3). The puma (Puma concolor), previously absent in the community, became widespread with a broad isotopic niche, while lynx (L. rufus) had significantly higher δ13C and δ15N values after the extinction (Figs. 1 and 2) Unfortunately, a lack of samples precluded analyzing body-size changes for these species from the Pleistocene to the Holocene.
Fig. 3.
Potential prey space for large-bodied carnivores at Hall’s Cave. (A) All large-bodied carnivores in Pleistocene. (B) The two extinct sabertooth cats with potential juvenile grazers added. (C) Early Holocene jaguars. In C, we show the Holocene grazer isotopic space (i.e., the sole remaining grazer, B. bison), the location of potential juvenile bison, and Pleistocene herbivore isotopic values (in gray). In all panels, carnivore data are from bone collagen; we subtracted 1.0‰ and 4.5‰ from the measured δ13C and δ15N values, respectively, of carnivores to account for trophic discrimination. Data points for browsers, mixed feeders, and grazers are the weighted average of all herbivores within each dietary group. The error bars around each prey group represent propagated SD, calculated as a combination of variability in raw herbivore isotope values and uncertainty in trophic discrimination (i.e., 1.0‰ for both δ13C and δ15N), where prey error bars = (σ2herbivore + σ2discrimation)1/2. Isotope values for juveniles were estimated by adding 0.5‰ and 1.5‰ to the δ13C and δ15N values of the adult herbivores, respectively.
Ursids had a mean (± SD) δ13C value indicative of reliance on C3-based resources (−17.7 ± 1.1‰). The isotopic niche of black bear completely overlapped with those of browsing herbivores (i.e., pronghorn, deer, and cottontail), suggesting a diet composed largely of vegetation (Fig. 1). The single short-faced bear (Arctodus simus) in our dataset had an intermediate δ15N value (9.7‰) similar to those of striped skunks (Mephitis mephitis; 9.6‰). Interestingly, new insights from Rancho La Brea also suggest this extinct bear might have been more omnivorous than previously recognized and/or at least more flexible in diet (46). The single ursid species that survived the extinction, the black bear, exhibited no change in either body size or isotopic niche over time (Figs. 1 and 2).
Central Texas in the late Pleistocene supported a diversity of canids, although not all could be reliably identified to species. In particular, it was not always possible to differentiate dogs (Canis familiaris), which accompanied early humans and were certainly present by the terminal Pleistocene, from similar-sized coyotes (Canis latrans), leading to a “Canis latrans/familiaris” category (SI Appendix, Table S1). Nonetheless, in the Pleistocene there was clear isotopic separation between foxes, coyote/domestic dogs, and wolves, with a trajectory toward more meat consumption and greater reliance on C4-based resources with increasing body mass in this family (e.g., C. latrans to Canis lupus to Canis dirus, Fig. 1). Broadly, Canidae were generalists, with a wider carbon isotopic niche than most other carnivores (Figs. 1 and 3 and SI Appendix, Fig. S1). An exception were the dire wolves, whose isotopic niche was more constrained and overlapped in δ13C values with several of the now-extinct cats (Fig. 1). Unlike other canids, grazers likely made up a substantial portion of the diet of dire wolves (Fig. 3). After the extinction of the much larger dire wolf, both the coyote and surviving gray wolf shifted slightly toward consumption of C4-based resources, although not significantly so, and neither changed significantly in body mass from the Pleistocene to Holocene (Fig. 2 and SI Appendix, Table S7).
Mesocarnivores all shifted slightly toward consumption of C4-based resources during the Holocene, perhaps tracking lagomorphs or other prey that had shifted in a similar fashion, but mesocarnivore δ15N values did not significantly differ over time (Figs. 1 and 2 and SI Appendix, Tables S1 and S7). The raccoon (Procyon lotor) was larger in the Holocene than in the Pleistocene (7.1 kg vs. 5.8 kg, respectively), but this mass difference was not significant after Bonferroni correction. Other mesocarnivores did not vary significantly in body mass over time; note data were lacking to compare body mass or isotopic niche of the badger (Taxidea taxus) or ringtailed cat (Bassariscus astutus) over time.
Community-Scale Body Size and Isotopic Niche Space.
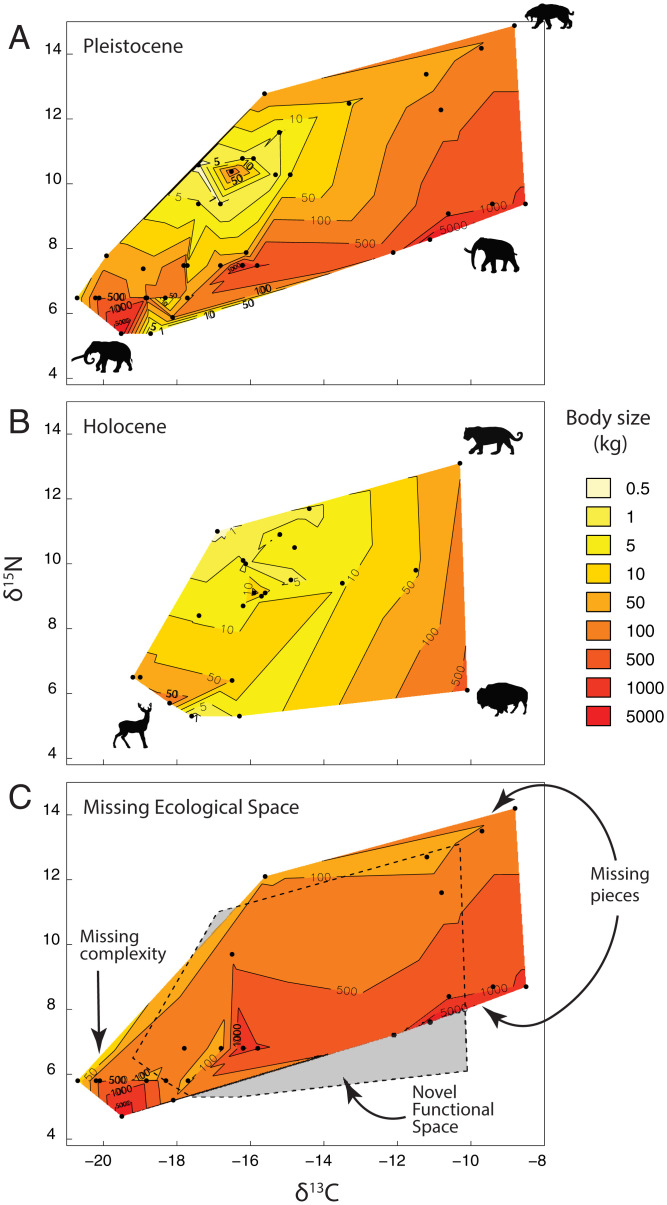
The community-scale relationship between body size and isotopic niche space changed over time (Fig. 4). The Pleistocene contour plot exhibited a complex topography of body size across δ13C and δ15N isotopic niche space (Fig. 4A). Notably, C4 specialists were both larger and consumed resources that varied more in their nitrogen isotope composition than consumers that specialized on C3-based resources. The contours were steepest among C3 specialists, suggesting a diverse array of body sizes were packed into a relatively small isotopic niche space. In comparison to the Pleistocene, the Holocene was substantially truncated in both isotopic niche space and body-size topography (Fig. 4B). The starkest difference between the Holocene and Pleistocene was found on the C4 end of the isotopic spectrum, although topographical complexity was lost throughout. Most of the “missing pieces” of the body-size isotope space consisted of large C4-based specialists across multiple trophic levels (primary and secondary consumers) (Fig. 4C).
Fig. 4.
Mammal body size, δ13C, and δ15N isotopic niche over time for meso- and mega-mammals within the Hall’s Cave community. (A) Pleistocene. (B) Holocene. (C) The “missing ecological pieces.” Mean species values were used for all plots instead of individual specimens because isotopic and body size data were not always matched and moreover we lack a method for measuring abundance. Species average body masses are interpolated across the maximum convex hull of δ13C and δ15N isotope space. Contours thus represent the relationship of body mass to δ13C and δ15N composition within the Texas mammal community. Contours located close together (i.e., “peaks”) represent portions of isotopic space where animals of divergent body size were found together; although they had very similar δ13C and δ15N values, they were likely partitioning resources in some fashion—structurally, temporally, or spatially. For example, note the C3 peak (∼−19‰) where multiple browsers of different body size cooccurred. C includes only the species that went extinct at the terminal Pleistocene and thus represents all the functional space lost with the extinction—both the complexity of the body size contours as well as the truncation of isotopic niche space. The convex hull from the Holocene is superimposed on C (indicated by the dashed outline). Interestingly, the Holocene displays a small slice of novel isotopic space relative to the Pleistocene because of slight isotopic or body size shifts of surviving carnivores or lagomorphs.
Discussion
Our analysis clearly demonstrated that the Holocene mammal community was vastly different from that present before the terminal Pleistocene megafauna extinction. This should not be surprising given the magnitude and size-selective nature of the extinction event (8, 25). However, what our combined body-size and isotopic analysis revealed was that the community was also functionally distinct (Fig. 4). We found little evidence of compensation in the response of herbivores to the loss of their larger-bodied congeners. Rather, the ecological legacy of the extinction was a strikingly truncated mammal community lacking much of its former ecological complexity; the Holocene had many “missing pieces,” each representing absent body size and isotopic niche space (Fig. 4C). For example, the Pleistocene community contained a rich grazer guild of mammoth and multiple horse and bison species, well-differentiated in combined body size and isotopic niche space (Fig. 1A). Nearly all of these taxa went extinct with only a single grazer, B. bison, occupying this isotopic niche space in the Holocene (Fig. 1B). The Pleistocene browser guild was particularly diverse with many species overlapping substantially in isotopic space (Fig. 1A). However, when they overlapped isotopically, they tended to diverge in body mass (i.e., overlap of mastodon and cottontails, mountain deer and jackrabbits, and tapirs and dwarf pronghorn), leading to considerable ecological topographical relief (Fig. 4A). This suggests a strong role for body size in partitioning resources within the Pleistocene community, like that found in modern African savannahs (44, 45). In contrast, surviving browsers in the Holocene were much more similar in body mass (Fig. 1B and SI Appendix, Table S1). By targeting both C3- and C4-dependent large-bodied herbivores and most of their predators, the size-selective terminal Pleistocene extinction removed much of the functional complexity and redundancy present in the mammal community (50), which is also a key trait of modern mammal ecosystems in Africa. A loss of functional redundancy is of concern as it has been implicated as a key determinant of ecosystem resilience (51).
We had anticipated that the extirpation of virtually all large-bodied herbivores at the terminal Pleistocene would lead to isotopic niche shifts by the survivors to exploit newly available resources. Yet, strikingly, we see essentially no difference in the average δ13C values of the largest surviving herbivores—deer, pronghorn, or bison—after the extinction (Fig. 2). Deer and pronghorn had largely invariant δ13C values over time and moreover overlapped almost completely in the Holocene (SI Appendix, Table S1 and Figs. 1 and 2). Bison, the sole surviving grazer, had the same isotopic niche space as did earlier species of bison in the Pleistocene (SI Appendix, Table S1 and Fig. 1). While the isotopic niche did not alter, the body size of these animals shifted: Bison and pronghorn became smaller, while deer became larger (Fig. 2). The significantly larger body mass of deer, which is in the opposite direction as that expected from thermal selection in accordance with Bergmann’s rule (52), may have facilitated resource partitioning and ameliorated interspecific competition between deer and the other remaining browser, pronghorn. The smaller body size of bison was unexpected, because large body size facilitates digestion of fibrous materials (42) and there was no longer any competition for C4 (grass) resources. We suspect this may well reflect adaptation to warming conditions (53). Another likely response of surviving herbivores was numerical: Taxa may have increased in abundance to exploit newly available food resources. Certainly, there is good evidence that bison herds were extensive during the late Holocene, which may have been a response to resource availability (54). However, abundance is notoriously difficult to characterize in the fossil record, and we have no current way of assessing this as a likely response to terminal Pleistocene biodiversity loss at our site.
In sharp contrast to the large-bodied herbivores, there was a highly significant shift in the δ13C isotopic niche of the surviving meso-herbivores. Both cottontails (Sylvilagus) and jackrabbits (Lepus) shifted significantly toward C4-based resources. This was accompanied by body size changes in both taxa: Lepus became ∼60% larger and Sylvilagus, 20% smaller (Fig. 2). Indeed, by the early Holocene, the lagomorphs at our site had transitioned from browsers into mixed feeders and were more divergent in body mass than they had been in the Pleistocene. Only for cottontails was this shift in the direction predicted by Bergmann’s rule. The early Holocene changes in both diet and body mass suggest greater flexibility in resource selection or sensitivity to environmental or ecological factors among meso-herbivores than in their larger-bodied congeners. Interestingly, for Sylvilagus this may have been driven by changes in species abundance and/or composition and not by in situ adaptation. Because of difficulties in accurately identifying lagomorph fossils, our analyses were necessarily conducted at the generic level. Today, two species of Sylvilagus are found in sympatry within a ∼600-km vicinity of Hall’s Cave: the desert cottontail, Sylvilagus audubonii (∼0.9 kg) and the eastern cottontail, Sylvilagus floridanus (∼1.2 kg). The similarities between the average body mass of these taxa and the size changes we report from the Pleistocene to Holocene—a small but significant shift in average mass from 1.0 to 0.8 kg—may reflect changes in the relative abundance of S. floridanus and S. audubonii over time. However, the only large-bodied leporid reported from the vicinity of Hall’s Cave is Lepus californicus (55); thus, the isotopic and body size changes we observed in Lepus likely do reflect in situ ecological and morphological adaptation.
Many of the most striking changes from the Pleistocene to Holocene occurred among the particularly diverse carnivore guild at the site. Prior to the terminal Pleistocene extinction, there was a clear hierarchy in δ13C and δ15N isotopic niche space among the different carnivore families (Fig. 1). Felidae had the highest δ15N values, followed by Canidae and Mustelidae, while Ursids were largely indistinguishable from herbivores. Moreover, the degree of carnivory and specialization appeared to be related to body size within families (45, 56, 57). For example, Homotherium had an exceptionally narrow isotopic niche, suggesting a high degree of specialization. Indeed, at our site, both sabertooth species appeared to largely consume juvenile grazers, particularly mammoth and bison (Figs. 1 and 3). This interpretation is consistent with paleontological studies of fossil remains from cave sites in the region (58, 59). For example, recent work at Friesenhahn Cave employing dental microwear texture analysis along with stable isotopes, concluded that Homotherium preferentially consumed juvenile mammoths (59). Certainly, 15N-enriched juvenile herbivores were probably consistently abundant because lactation continued for at least 3 y in megaherbivores such as mammoth (60). Moreover, trackway analysis suggests mammoth herds were a mix of adults, subadults, and nursing juveniles (61).
Similarly, among the Canidae the extinct dire wolf had a narrower isotopic niche than did other smaller-bodied canids, although all consumed a broader variety of prey than did most felids. We did find evidence of similar prey types shared between dire and gray wolves, although the relative proportions of each likely differed substantially (Fig. 3). There was overlap in isotopic values, and thus presumably diet, between dire wolves and the extinct cats, particularly Smilodon and perhaps the cave lion (Fig. 1). This suggests some level of intraguild competition between dire wolves and the extinct cats, as has been reported elsewhere (47, 62). The varying results found among studies (e.g., refs. 47 and 62–64) highlight the dietary flexibility of carnivores, which responded to both local resource availability and the presence of conspecifics.
Ecologists have argued for a strong role of apex predators in structuring ecosystems (e.g., refs. 3, 65, and 66). The extirpation of large-bodied predators can result in the population expansion of medium-bodied competitors; a phenomenon known as “mesocarnivore release” (66, 67). How common this pattern is, or indeed how important top-down control is within ecosystems, remains an ongoing area of research (3, 65, 66, 68–70). Here, we find strong evidence of mesocarnivore release among the Felidae following the extinction of the three largest cats at the terminal Pleistocene. The smaller-bodied jaguar (P. onca, ∼92 kg) significantly shifted its diet by more than 5‰ and moreover went from consuming a broad variety of prey to a narrow range of grazers (Fig. 3 A and C), essentially moving into the same isotopic niche space once occupied by extinct felids. While earlier cats had a variety of large-bodied grazer species to consume, the jaguar had only one (i.e., bison) in the Holocene. Intriguingly, the isotopic values of Holocene jaguars were higher in δ15N and lower in δ13C than we would expect if only bison and their calves were consumed (Fig. 3C), suggesting another contributing source. This was perplexing because there were no other C4-based sources during this time frame. However, we noted that our Holocene jaguar samples—all from our main Hall’s Cave site—dated to a narrow temporal window right at the terminal Pleistocene/Holocene boundary. This raised the possibility that relict populations of some Pleistocene grazers were still present, although Smilodon and Homotherium had already been extirpated in the community. Regardless of what exactly jaguars were eating, our results indicate the absence of larger felids led to a rapid competitive release.
The smaller-bodied mountain lion (P. concolor, ∼52 kg), which was scarce in Pleistocene assemblages, became widespread during the Holocene and moved into the generalized isotopic niche space previously occupied by the jaguar in the Pleistocene. The habitats and dietary niche occupied by mountain lions during the Pleistocene in Texas remain unclear; however, dental microwear texture analysis from the La Brea Tar Pits in southern California confirms a generalized diet with moderate levels of durophagy (71). The other surviving felid in the community, the bobcat (L. rufus) also responded significantly. Bobcats transitioned from δ13C values reflecting consumption of mostly browsers in the Pleistocene to those indicating a greater reliance on mixed feeders in the Holocene. In addition, the mean δ15N values of bobcats increased significantly, as did body size (Figs. 1 and 2). While the various isotopic and body-size shifts observed in surviving felids during the Holocene suggest exploitation of newly vacated ecological space, their much smaller body sizes led to different functional roles than those of the extinct large-bodied sabertooth cats (56, 72, 73). Studies of modern ecosystems suggest that medium-bodied carnivores pose less of a perceived threat to potential prey and/or competitors (72, 73).
The evidence for mesocarnivore release among canids was inconclusive, at least partially because of low sample sizes for the Pleistocene. While surviving canids shifted toward C4-based resources after the extinction of the dire wolf (C. dirus), their δ15N values either did not change or decreased. The isotopic shift by coyotes may have been facilitated by the movement of surviving wolves toward increased consumption of C4-based resources. Other studies have found that competition between coyotes and wolves influenced space use, diet composition, and even morphology in the late Quaternary (63, 64, 74, 75); our site-specific body masses diverge from the Pleistocene to Holocene, but not significantly so (SI Appendix, Table S1). In the Holocene, gray wolf δ13C values increased by 2‰, which was accompanied by a decrease of similar magnitude in δ15N (Figs. 1 and 2). The lower δ15N value of wolves in the Holocene was unlikely to have been solely the result of a baseline shift; while mean δ15N values of some surviving herbivores decreased (deer, hare), others increased (cottontails; SI Appendix, Table S1). Moreover, the decrease in δ15N values of canids was of higher magnitude than changes in herbivores, suggesting greater reliance on plant matter in the Holocene. Alternatively, it is possible that scavenging and/or the availability of large-bodied herbivore carcasses from kills by extinct felids—perhaps including 15N-enriched juvenile herbivores—were important dietary inputs in the Pleistocene for wolves (76). High frequencies of tooth breakage, found among many Pleistocene carnivores, has been interpreted to mean more extensive use of carcasses and bones (62, 70, 77). Foxes appeared to have exhibited a slight niche release, increasing in body size in the Holocene, consistent with the conclusions of other studies (75). Overall, the Holocene community no longer demonstrated strong isotopic differentiation among carnivore families; only the jaguar exhibited a dietary isotopic niche distinct from canids or other carnivores (Fig. 1A). A similar Holocene homogenization of carnivore diet has been reported elsewhere (64).
Note that with the possible exception of wolves we were unlikely to have included all of the potential dietary inputs for the smaller-bodied carnivores in our analyses. Rodents become increasingly important as a food resource as carnivore body size decreases. For example, coyote coprolites preserved at Hall’s Cave have revealed the consumption of woodrats (Neotoma spp.), gophers (Geomys spp.), pocket mice (Chaetodipus), and rabbits [Sylvilagus spp. (55)]; only the latter taxa were included in our analyses. However, rodents in this community tend to fall on the C3 or mixed C3/C4 portion of the δ13C spectrum. For example, Neotoma were browser/mixed C3/C4 foragers with δ13C values ranging from −19.5‰ to −17.8‰ during the Pleistocene and early Holocene (78). Even Sigmodon, which is generally assumed to be tightly associated with grassland habitats, had δ13C ranging from −15.6‰ to −14.1‰ over this time frame (79). Thus, directional shifts by wolves or coyotes toward C4-based resources were unlikely to be driven by increased rodent consumption. Indeed, given the location of wolves along the C3–C4 spectrum, the most parsimonious explanation is greater consumption of bison, the sole surviving grazer in the Holocene ecosystem.
We largely attribute the changes in body size and isotopic niche from the Pleistocene to the Holocene to biodiversity loss and not climate change. While climate change did occur over this time interval, our data are temporally averaged for both periods and thus unlikely to reflect specific climatic events. In general, Texas became progressively warmer and drier during the early to mid-Holocene (80), which should have selected for a general pattern of body size dwarfing (52). We did not find this in our community; while temperature influenced the body size of some taxa, especially small-bodied mammals (e.g., refs. 78 and 79), we found no consistent pattern among the larger-bodied species examined here. Indeed, only bison and jackrabbits changed morphologically in the direction predicted by Bergmann’s rule, suggesting that biotic interactions were a stronger driver than temperature for most taxa. Isotopic shifts seen in browsers and mixed feeders may have been influenced by changing climate and associated shifts in vegetation. Generally, aridity is associated with higher carbon isotope values in C3 plants, which should also be reflected in the δ13C values of browsers and mixed feeders (81). While some herbivores showed a slight (0.1‰ to 0.5‰) positive shift in mean δ13C values over time (SI Appendix, Table S1), most species were invariant across the extinction. This suggests that climate-driven vegetation changes from the terminal Pleistocene to early Holocene were relatively modest at this site, consistent with previous work from Texas (82). Alternatively, the species we examined may have shifted microhabitats to track preferred vegetation types and/or consumed different vegetation but maintained the same general location along the C3–C4 spectrum. By extension, the large ∼2 to 5‰ shifts in δ13C values observed in many carnivore species over time are unlikely to have been driven by climate.
Our study provides an important baseline for modern conservation efforts by quantifying the significant changes in ecological function and organization that occurred with the terminal Pleistocene biodiversity loss. We find that the functional role played by large-bodied mammals was unique and that there are pieces of unfilled ecological space (e.g., 83–94). Moreover, the ecological legacy of the extinction is still evident today (95). For example, we found that surviving large-bodied herbivores did not systematically increase in body size with the loss of larger-bodied congeners, nor did they expand or shift their isotopic niche to exploit newly available resources. Instead, the functional role of the extinct herbivores simply went “missing” (Fig. 4C); the reduced complexity we found in the Holocene (Fig. 4B) represented lost ecological function in the Hall’s Cave community across the isotopic-body-size spectrum. In contrast, the loss of apex consumers led to significant ecological reorganization of the remaining carnivore guild and greater isotopic overlap among surviving taxa. Indeed, the Holocene community no longer demonstrated strong isotopic differentiation among carnivore families. Thus, the loss of large-bodied mammals at the terminal Pleistocene profoundly altered the behavior of surviving carnivores, disrupting predator–prey pairs and causing shifts in resource use. Our findings are consistent with continental-level studies demonstrating a decline in the importance of biotic interactions after the megafauna extinction and a homogenization of ecosystems (28, 29).
With the exception of a few locations, such as African savannah ecosystems, the world has continued to lose much of its large animal biodiversity over the Holocene (4, 10, 94, 96, 97). Wildlife have been largely replaced with domesticated animals (14, 19), and remaining natural areas are under threat. Our study suggests that even after thousands of years, surviving small-bodied species do not compensate for lost large-bodied ones. Moreover, we can expect that ecological interactions between surviving species will be disrupted in the future. Thus, continued biodiversity loss will not only truncate the range of ecological function within communities but will also further reduce the complexity of natural ecosystems (84–96). To the extent that complexity equals resilience (51), continued body size, functional, and trophic downgrading may lead to further unanticipated and unwelcome effects. By incorporating a deeper understanding of ecological interactions over millennia, our work highlights the critical importance of maintaining body size and dietary complexity within natural communities as wildlife managers and conservationists strive to preserve ecosystems.
Materials and Methods
Study Region and Mammal Communities.
We reconstructed the Pleistocene and Holocene mammal communities on the Edwards Plateau based on the faunal lists from the Hall’s Cave fossil site (Texas Memorial Museum site 41229) and surrounding paleontological localities. These materials are all housed at the Texas Memorial Museum (TMM) in Austin, TX. The initial faunal list for Hall’s Cave was compiled by Toomey (55); our ongoing efforts over the past 4 y with the unidentified fossil materials and recent ancient DNA analysis of sediment (98) from this site have resulted in additional species being recovered (SI Appendix, Table S1) (20).
Our reconstructed Pleistocene and Holocene mammal communities contained 52 and 24 mammal taxa, respectively (SI Appendix, Table S1). This was a conservative estimate of the true diversity because several taxa were only analyzed at the generic level. For example, although multiple species of Equus were present in the Edward’s Plateau during the Pleistocene, their identification and taxonomic status remain unsettled. Here, we combined all equid species and considered them at the generic level; this was unlikely to conflate our analyses because of the considerable overlap in body size and diet among the individual species. We focused on the medium- and large-bodied mammals, which we defined as larger than 1 kg. This included the lagomorphs (Lepus and Sylvilagus), large-bodied herbivores, and most skunks (Spilogale and Mephitis spp.), even though some species within these genera may have mean masses slightly less than the ∼1-kg threshold.
Estimation of Body Mass from Fossils.
Site-specific body size was quantified using fossils housed at TMM (SI Appendix). We measured 2,124 molar and postcranial elements and were able to obtain temporally and spatially constrained estimates of body mass for 2,086 animals within our community (SI Appendix, Table S2). Elements measured included those commonly used for estimating size, such as the length and/or width of molars, tooth rows, astragali, calcanea, humeri, radii, and other postcranial material. For animals the size of lagomorphs or smaller, we photographed specimens using a Dinoscope or high-resolution DSLR camera (20.4 MP Canon EOS 70D or 50.6MP Canon EOS 5DS R), and measured elements using ImageJ (https://imagej.nih.gov/ij/download.html) software; larger specimens were measured using digital calipers, or as in the case of mammoth long bones, a calibrated string. We used allometric regressions developed on extant species to translate these various measures into estimates of body mass; all data, equations, and sources are provided in SI Appendix, Tables S2–S7. Because allometric regressions were not available for all elements to translate into body size, we developed our own as necessary using modern museum specimens (SI Appendix).
Isotopic Analyses.
We measured the carbon (δ13C), nitrogen (δ15N), and/or oxygen (δ18O) isotope values of 1,006 fossils from Hall’s Cave and the surrounding sites (SI Appendix, Table S5). We also included 251 isotopic measurements of large-bodied mammals reported in the literature (31, 82, 99), for a total initial dataset of isotope values for 1,257 fossil specimens. Most samples were bone collagen, which provides an integrated multiyear estimate of diet (34, 36). For some taxa and sites with poor preservation of bone collagen, we extracted apatite from tooth enamel (100), which records a shorter time window than does collagen. We computed a “collagen equivalent” for each enamel-derived value by first calculating the apatite to collagen δ13C spacing for ruminants, nonruminants, and carnivore families (canids and felids) using data from Codron et al. (45) and then applying these mean (± SD) offsets to our data (SI Appendix, Table S6): ruminants (8.8 ± 2.2‰), nonruminants (8.8 ± 1.8‰), canids (5.0 ± 1.2‰), and felids (4.4 ± 1.2‰). A series of ANOVAs with Bonferroni corrections were used to check for potential biases introduced by sampling from different bone and tooth elements and/or from using corrected δ13C values from tooth enamel vs. bone collagen. We found no difference in the average δ13C or δ15N values for taxa when using different elements or even molar positions, nor did we find a difference between collagen equivalents and measured collagen values when we had both for a taxon (SI Appendix, Tables S9 and S10). Detailed information on collagen extraction, preparation, and analytical protocols can be found in SI Appendix.
Isotopic results are reported in delta (δ) notation using the equation δ13C or δ15N = 1,000*[(Rsample/Rstandard) − 1], where Rsample and Rstandard are the 13C:12C or 15N:14N ratios of the sample and standard, respectively. The internationally accepted standards for δ13C and δ15N analysis are respectively Vienna-Pee Dee Belemnite limestone (V-PDB) and atmospheric N2; δ18O data were calibrated using the V-SMOW international standard. The units are expressed as parts per thousand, or per mil (‰). As a control for the quality of our ancient collagen samples, we calculated the atomic percent [C]:[N] ratio from measured weight percent [C]:[N] values. See SI Appendix for further details.
Data Analysis.
We calculated the mean, median, and SD of δ13C and δ15N values for each species (SI Appendix, Table S1) and the change in values (if any) from the Pleistocene to the Holocene (SI Appendix, Table S7). For species with only enamel data, we used the δ15N values for the guild when presenting data in figures (Fig. 1). Significant differences in mean δ13C and δ15N among taxa, functional group (e.g., grazer vs. browser), and time (Pleistocene vs. Holocene) were assessed using ANOVA and two-sided Welch two-sample t tests with Bonferroni corrections for multiple comparisons (SI Appendix). We characterized the dietary space for Pleistocene felids and canids and Holocene jaguars to explore patterns in prey preferences among carnivore guilds. We compared isotopic data of carnivores with that of large-bodied herbivores, grouped as grazers, mixed-feeders, or browsers; data were corrected for trophic discrimination (SI Appendix). Because juvenile grazers have been suggested as an important food source for felids, we included them by adding +0.5‰ for δ13C, and 1.5‰ for δ15N to the mean isotope values of adult grazers (49) (SI Appendix). While grazers and browsers were isotopically distinct, mixed feeders overlapped with both groups; this was driven largely by the huge variability among camels (Camelops hesternus) that spanned the entire isotopic range from browser to grazer even within the same location (SI Appendix, Table S5).
To characterize community-scale changes through time, we used contour plots to illustrate the relationship between body mass and isotopic niche space. Mean δ13C, δ15N, and body mass were calculated for each species during the Pleistocene and Holocene. The contour plots used a linear interpolated distribution of body mass within the maximum convex hull of δ13C and δ15N space for the Pleistocene and Holocene communities. Contours thus represented the relationship of body mass to δ13C and δ15N composition within the Texas mammal communities for each period. Finally, we plotted the body mass and isotopic niche space of just the extinct species, which allowed us to not only represent the “space” lost but also the reduced complexity in occupied body size-isotopic space. Plots were created using base R or package ggplot2 (101).
Supplementary Material
Acknowledgments
We are extremely grateful to Drs. Chris Sagebiel and Ernie Lundelius at the Texas Memorial Museum for access to collections and their able assistance in locating references and specimens or gathering information about the fossil sites. We also thank Dr. Rickard Toomey for his foundational work at Hall’s Cave, without which our project would not have been possible, and the Hall family for having graciously allowed generations of paleontologists to work at this very important late Quaternary site. Dr. Thomas W. Stafford, Jr. was instrumental in helping us conduct this work; we very much appreciate his enthusiasm, interest, and sense of humor. We thank the Museum of Southwest Biology at the University of New Mexico (UNM) for access to modern specimens used to derive body size allometries and the members of the F.A.S. laboratory at UNM (especially J. Keller and C. Hedberg) for their help with this project. This work was supported by the NSF Division of Environmental Biology (DEB 1555525; F.A.S. Principal Investigator; S.K.L. and S.D.N., co-Principal Investigators).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2115015119/-/DCSupplemental.
Data, Materials, and Sofrware Availability
All study data are included in the article and/or SI Appendix.
References
- 1.Cardillo M., et al. , Multiple causes of high extinction risk in large mammal species. Science 309, 1239–1241 (2005). [DOI] [PubMed] [Google Scholar]
- 2.Schipper J., et al. , The status of the world’s land and marine mammals: Diversity, threat, and knowledge. Science 322, 225–230 (2008). [DOI] [PubMed] [Google Scholar]
- 3.Estes J. A., et al. , Trophic downgrading of planet Earth. Science 333, 301–306 (2011). [DOI] [PubMed] [Google Scholar]
- 4.Dirzo R., et al. , Defaunation in the Anthropocene. Science 345, 401–406 (2014). [DOI] [PubMed] [Google Scholar]
- 5.Ceballos G., Ehrlich P. R., Dirzo R., Biological annihilation via the ongoing sixth mass extinction signaled by vertebrate population losses and declines. Proc. Natl. Acad. Sci. U.S.A. 114, E6089–E6096 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barnosky A. D., Koch P. L., Feranec R. S., Wing S. L., Shabel A. B., Assessing the causes of late Pleistocene extinctions on the continents. Science 306, 70–75 (2004). [DOI] [PubMed] [Google Scholar]
- 7.Koch P. L., Barnosky A. D., Late quaternary extinctions: State of the debate. Annu. Rev. Ecol. Evol. Syst. 37, 215–250 (2006). [Google Scholar]
- 8.Smith F. A., Elliott Smith R. E., Lyons S. K., Payne J. L., Body size downgrading of mammals over the late Quaternary. Science 360, 310–313 (2018). [DOI] [PubMed] [Google Scholar]
- 9.Smith F. A., Elliott Smith R. E., Lyons S. K., Payne J. L., Villaseñor A., The accelerating influence of humans on mammalian macroecological patterns over the late Quaternary. Quat. Sci. Rev. 211, 1–16 (2019). [Google Scholar]
- 10.Ceballos G., et al. , Accelerated modern human-induced species losses: Entering the sixth mass extinction. Sci. Adv. 1, e1400253 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barnosky A. D., et al. , Has the Earth’s sixth mass extinction already arrived? Nature 471, 51–57 (2011). [DOI] [PubMed] [Google Scholar]
- 12.Pimm S. L., et al. , The biodiversity of species and their rates of extinction, distribution, and protection. Science 344, 1246752 (2014). [DOI] [PubMed] [Google Scholar]
- 13.McCallum M. L., Vertebrate biodiversity losses point to a sixth mass extinction. Biodivers. Conserv. 24, 2497–2519 (2015). [Google Scholar]
- 14.Barnosky A. D., Colloquium paper: Megafauna biomass tradeoff as a driver of Quaternary and future extinctions. Proc. Natl. Acad. Sci. U.S.A. 105 (suppl. 1), 11543–11548 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith F. A., Doughty C. E., Malhi Y., Svenning J.-C., Terborgh J., Megafauna in the Earth system. Ecography 39, 99–108 (2016). [Google Scholar]
- 16.Malhi Y., et al. , Megafauna and ecosystem function from the Pleistocene to the Anthropocene. Proc. Natl. Acad. Sci. U.S.A. 113, 838–846 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith F. A., Elliott S. M., Lyons S. K., Methane emissions from extinct megafauna. Nat. Geosci. 3, 374–375 (2010). [Google Scholar]
- 18.Gill J. L., Ecological impacts of the late Quaternary megaherbivore extinctions. New Phytol. 201, 1163–1169 (2014). [DOI] [PubMed] [Google Scholar]
- 19.Smith F. A., et al. , Exploring the influence of ancient and historic megaherbivore extirpations on the global methane budget. Proc. Natl. Acad. Sci. U.S.A. 113, 874–879 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith F. A., et al. , Unraveling the consequences of the terminal Pleistocene megafauna extinction on mammal community assembly. Ecography 39, 223–239 (2016). [Google Scholar]
- 21.Doughty C. E., et al. , Global nutrient transport in a world of giants. Proc. Natl. Acad. Sci. U.S.A. 113, 868–873 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doughty C. E., Wolf A., Baraloto C., Malhi Y., Interdependency of plants and animals in controlling the sodium balance of ecosystems and the impacts of global defaunation. Ecography 39, 204–212 (2016). [Google Scholar]
- 23.Galetti M., et al. , Ecological and evolutionary legacy of megafauna extinctions. Biol. Rev. Camb. Philos. Soc. 93, 845–862 (2018). [DOI] [PubMed] [Google Scholar]
- 24.Martin P. S., Klein R. G., Quaternary Extinctions: A Prehistoric Revolution (University of Arizona Press, 1984). [Google Scholar]
- 25.Lyons S. K., Smith F. A., Brown J. H., Of mice, mastodons and men: Human-mediated extinctions on four continents. Evol. Ecol. Res. 6, 339–358 (2004). [Google Scholar]
- 26.Sandom C., Faurby S., Sandel B., Svenning J.-C., Global late Quaternary megafauna extinctions linked to humans, not climate change. Proc. Biol. Sci. 281, 20133254 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grayson D. K., Deciphering North American Pleistocene extinctions. J. Anthropol. Res. 63, 185–213 (2007). [Google Scholar]
- 28.Lyons S. K., et al. , Holocene shifts in the assembly of plant and animal communities implicate human impacts. Nature 529, 80–83 (2016). [DOI] [PubMed] [Google Scholar]
- 29.Tóth A. B., et al. , Reorganization of surviving mammal communities after the end-Pleistocene megafaunal extinction. Science 365, 1305–1308 (2019). [DOI] [PubMed] [Google Scholar]
- 30.Peters R. H., The Ecological Implications of Body Size (Cambridge University Press, 1983). [Google Scholar]
- 31.Cerling T. E., Harris J. M., Leakey M. G., Browsing and grazing in elephants: The isotope record of modern and fossil proboscideans. Oecologia 120, 364–374 (1999). [DOI] [PubMed] [Google Scholar]
- 32.Koch P. L., Hoppe K. A., Webb S. D., The isotopic ecology of late Pleistocene mammals in North America Part 1. Florida. Chem. Geol. 152, 119–138 (1998). [Google Scholar]
- 33.Koch P. L., Fox-Dobbs K., Newsome S. D., “The isotopic ecology of fossil vertebrates and conservation paleobiology” in Conservation Paleobiology: Science and Practice, Dietl G. P., Flessa K. W., Eds. (University of Chicago Press, 2009), pp. 95–112. [Google Scholar]
- 34.Koch P. L., “Isotopic study of the biology of modern and fossil vertebrates” in Stable Isotopes in Ecology and Environmental Science, Michener R., Lajtha K., Eds. (John Wiley & Sons, 2007), pp. 99–154. [Google Scholar]
- 35.Newsome S. D., et al. , Pleistocene to historic shifts in bald eagle diets on the Channel Islands, California. Proc. Natl. Acad. Sci. U.S.A. 107, 9246–9251 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clementz M. T., New insight from old bones: Stable isotope analysis of fossil mammals. J. Mammal. 93, 368–380 (2012). [Google Scholar]
- 37.Bocherens H., et al. , Reconstruction of the Gravettian food-web at Předmostí I using multi-isotopic tracking (13C, 15N, 34S) of bone collagen. Quat. Int. 359–360, 211–228 (2015). [Google Scholar]
- 38.Elliott Smith E. A., et al. , Reductions in the dietary niche of southern sea otters (Enhydra lutris nereis) from the Holocene to the Anthropocene. Ecol. Evol. 10, 3318–3329 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ehleringer J., Björkman O., Quantum yields for CO(2) uptake in C(3) and C(4) plants: Dependence on temperature, CO(2), and O(2) concentration. Plant Physiol. 59, 86–90 (1977). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vanderklift M. A., Ponsard S., Sources of variation in consumer-diet delta 15N enrichment: A meta-analysis. Oecologia 136, 169–182 (2003). [DOI] [PubMed] [Google Scholar]
- 41.Newsome S. D., del Rio C. M., Bearhop S., Phillips D. L., A niche for isotopic ecology. Front. Ecol. Environ. 5, 429–436 (2007). [Google Scholar]
- 42.Smith F. A., Scaling of digestive efficiency with body size in Neotoma. Funct. Ecol. 9, 299–305 (1995). [Google Scholar]
- 43.Cernusak L. A., et al. , Why are non-photosynthetic tissues generally 13C enriched compared with leaves in C3 plants? Review and synthesis of current hypotheses. Funct. Plant Biol. 36, 199–213 (2009). [DOI] [PubMed] [Google Scholar]
- 44.De Iongh H. H., et al. , Resource partitioning among African savanna herbivores in North Cameroon: The importance of diet composition, food quality and body mass. J. Trop. Ecol. 27, 503–513 (2011). [Google Scholar]
- 45.Codron D., Clauss M., Codron J., Tütken T., Within trophic level shifts in collagen-carbonate stable carbon isotope spacing are propagated by diet and digestive physiology in large mammal herbivores. Ecol. Evol. 8, 3983–3995 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Figueirido B., et al. , Dental caries in the fossil record: A window to the evolution of dietary plasticity in an extinct bear. Sci. Rep. 7, 17813 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Coltrain J. B., et al. , Rancho La Brea stable isotope biogeochemistry and its implications for the palaeoecology of late Pleistocene, coastal southern California. Palaeogeogr. Palaeoclimatol. Palaeoecol. 205, 199–219 (2004). [Google Scholar]
- 48.Fox-Dobbs K., Bump J. K., Peterson R. O., Fox D. L., Koch P. L., Carnivore-specific stable isotope variables and variation in the foraging ecology of modern and ancient wolf populations: Case studies from Isle Royale, Minnesota, and La Brea. Can. J. Zool. 85, 458–471 (2007). [Google Scholar]
- 49.Fogel M. L., Tuross N., Owsley D. W., Nitrogen isotope tracers of human lactation in modern and archaeological populations. Carnegie Inst. Washington Yearbook 88, 111–117 (1989). [Google Scholar]
- 50.Hedberg C., Lyons S. K., Smith F. A., The hidden legacy of megafaunal extinction: Loss of functional diversity and resilience over the late Quaternary at Hall’s Cave. Glob. Ecol. Biogeogr. 31, 294–307 (2022). [Google Scholar]
- 51.Mori A. S., Furukawa T., Sasaki T., Response diversity determines the resilience of ecosystems to environmental change. Biol. Rev. Camb. Philos. Soc. 88, 349–364 (2013). [DOI] [PubMed] [Google Scholar]
- 52.Millien V., et al. , Ecotypic variation in the context of global climate change: Revisiting the rules. Ecol. Lett. 9, 853–869 (2006). [DOI] [PubMed] [Google Scholar]
- 53.Martin J. M., Mead J. I., Barboza P. S., Bison body size and climate change. Ecol. Evol. 8, 4564–4574 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lott D.F., American Bison: A Natural History (University of California Press, 2002). [Google Scholar]
- 55.R. S. Toomey, III, “Late Pleistocene and Holocene faunal and environmental changes at Hall’s Cave, Kerr County, Texas,” PhD dissertation, University of Texas at Austin, Austin, TX (1993).
- 56.Gittleman J. L., Carnivore body size: Ecological and taxonomic correlates. Oecologia 67, 540–554 (1985). [DOI] [PubMed] [Google Scholar]
- 57.Van Valkenburgh B., Wang X., Damuth J., Cope’s rule, hypercarnivory, and extinction in North American canids. Science 306, 101–104 (2004). [DOI] [PubMed] [Google Scholar]
- 58.Graham R. W., Lundelius E. L., Meissner L., “Friesenhahn Cave: Late Pleistocene paleoecology and predator-prey relationships of mammoths with an extinct scimitar cat” in Late Cretaceous to Quaternary Strata and Fossils of Texas: Field Excursions Celebrating 125 Years of GSA and Texas Geology, GSA South-Central Section Meeting, Austin, Texas, April 2013 (Geological Society of America, 2013), pp. 15–31.
- 59.DeSantis L. R. G., Feranec R. S., Antón M., E. L. Lundelius, Jr, Dietary ecology of the scimitar-toothed cat Homotherium serum. Curr. Biol. 31, 2674–2681.e3 (2021). [DOI] [PubMed] [Google Scholar]
- 60.Metcalfe J. Z., Longstaffe F. J., Zazula G. D., Nursing, weaning, and tooth development in woolly mammoths from Old Crow, Yukon, Canada: Implications for Pleistocene extinctions. Palaeogeogr. Palaeoclimatol. Palaeoecol. 298, 257–270 (2010). [Google Scholar]
- 61.McNeil P., Hills L. V., Kooyman B., Tolman S. M., Mammoth tracks indicate a declining Late Pleistocene population in southwestern Alberta, Canada. Quat. Sci. Rev. 24, 1253–1259 (2005). [Google Scholar]
- 62.Vanvalkenburgh B., Hertel F., Tough times at la brea: Tooth breakage in large carnivores of the late pleistocene. Science 261, 456–459 (1993). [DOI] [PubMed] [Google Scholar]
- 63.Meachen J. A., Janowicz A. C., Avery J. E., Sadleir R. W., Ecological changes in Coyotes (Canis latrans) in response to the ice age megafaunal extinctions. PLoS One 9, e116041 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.DeSantis L. R. G., et al. , Causes and consequences of Pleistocene megafaunal extinctions as revealed from Rancho La Brea mammals. Curr. Biol. 29, 2488–2495.e2 (2019). [DOI] [PubMed] [Google Scholar]
- 65.Berger K. M., Gese E. M., Berger J., Indirect effects and traditional trophic cascades: A test involving wolves, coyotes, and pronghorn. Ecology 89, 818–828 (2008). [DOI] [PubMed] [Google Scholar]
- 66.Ritchie E. G., Johnson C. N., Predator interactions, mesopredator release and biodiversity conservation. Ecol. Lett. 12, 982–998 (2009). [DOI] [PubMed] [Google Scholar]
- 67.Soulé M. E., et al. , Reconstructed dynamics of rapid extinctions of chaparral-requiring birds in Urban Habitat Islands. Conserv. Biol. 2, 75–92 (1988). [Google Scholar]
- 68.Elmhagen B., Rushton S. P., Trophic control of mesopredators in terrestrial ecosystems: Top-down or bottom-up? Ecol. Lett. 10, 197–206 (2007). [DOI] [PubMed] [Google Scholar]
- 69.Ritchie E. G., et al. , Ecosystem restoration with teeth: What role for predators? Trends Ecol. Evol. 27, 265–271 (2012). [DOI] [PubMed] [Google Scholar]
- 70.Van Valkenburgh B., Hayward M. W., Ripple W. J., Meloro C., Roth V. L., The impact of large terrestrial carnivores on Pleistocene ecosystems. Proc. Natl. Acad. Sci. U.S.A. 113, 862–867 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Desantis L. R. G., Haupt R. J., Cougars’ key to survival through the Late Pleistocene extinction: Insights from dental microwear texture analysis. Biol. Lett. 10, 20140203 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Prugh L. R., et al. , The rise of the mesopredator. Bioscience 59, 779–791 (2009). [Google Scholar]
- 73.Roemer G. W., Gompper M. E., Van Valkenburgh B., The ecological role of the mammalian Mesocarnivore. Bioscience 59, 165–173 (2009). [Google Scholar]
- 74.Meachen J. A., Samuels J. X., Evolution in coyotes (Canis latrans) in response to the megafaunal extinctions. Proc. Natl. Acad. Sci. U.S.A. 109, 4191–4196 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pardi M. I., Smith F. A., Biotic responses of canids to the terminal Pleistocene megafauna extinction. Ecography 39, 141–151 (2016). [Google Scholar]
- 76.Pereira L. M., Owen-Smith N., Moleón M., Facultative predation and scavenging by mammalian carnivores: Seasonal, regional and intra-guild comparisons. Mammal Rev. 44, 44–55 (2014). [Google Scholar]
- 77.Binder W. J., Van Valkenburgh B., A comparison of tooth wear and breakage in Rancho La Brea sabertooth cats and dire wolves across time. J. Vertebr. Paleontol. 30, 255–261 (2010). [Google Scholar]
- 78.Tomé C. P., Lyons S. K., Newsome S. D., Smith F. A., The sensitivity of Neotoma to climate change and biodiversity loss over the late Quaternary. Quat. Res. 105, 49–63 (2021). [Google Scholar]
- 79.Tomé C. P., Elliott Smith E. A., Lyons S. K., Newsome S. D., Smith F. A., Changes in the diet and body size of a small herbivorous mammal (hispid cotton rat, Sigmodon hispidus) following the late Pleistocene megafauna extinction. Ecography 43, 604–619 (2020). [Google Scholar]
- 80.Wong C. I., Banner J. L., Musgrove M., Holocene climate variability in Texas, USA: An integration of existing paleoclimate data and modeling with a new, high-resolution speleothem record. Quat. Sci. Rev. 127, 155–173 (2015). [Google Scholar]
- 81.Kohn M. J., Carbon isotope compositions of terrestrial C3 plants as indicators of (paleo)ecology and (paleo)climate. Proc. Natl. Acad. Sci. U.S.A. 107, 19691–19695 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Koch P. L., Diffenbaugh N. S., Hoppe K. A., The effects of late Quaternary climate and pCO2 change on C4 plant abundance in the south-central United States. Palaeogeogr. Palaeoclimatol. Palaeoecol. 207, 331–357 (2004). [Google Scholar]
- 83.Owen-Smith R. N., Megaherbivores: The Influence of Very Large Body Size on Ecology (Cambridge University Press, 1988). [Google Scholar]
- 84.Terborgh J., Estes J. A., Trophic Cascades: Predators, Prey, and the Changing Dynamics of Nature (Island Press, 2010). [Google Scholar]
- 85.Asner G. P., Levick S. R., Landscape-scale effects of herbivores on treefall in African savannas. Ecol. Lett. 15, 1211–1217 (2012). [DOI] [PubMed] [Google Scholar]
- 86.Doughty C. E., Wolf A., Malhi Y., The legacy of the Pleistocene megafauna extinctions on nutrient availability in Amazonia. Nat. Geosci. 6, 761–764 (2013). [Google Scholar]
- 87.Bakker E. S., et al. , Combining paleo-data and modern exclosure experiments to assess the impact of megafauna extinctions on woody vegetation. Proc. Natl. Acad. Sci. U.S.A. 113, 847–855 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Johnson C. N., et al. , Geographic variation in the ecological effects of extinction of Australia’s Pleistocene megafauna. Ecography 39, 109–116 (2016). [Google Scholar]
- 89.Asner G. P., Vaughn N., Smit I. P. J., Levick S., Ecosystem-scale effects of megafauna in African savannas. Ecography 39, 240–252 (2016). [Google Scholar]
- 90.Knapp A. K., et al. , The Keystone Role of Bison in North American Tallgrass Prairie: Bison increase habitat heterogeneity and alter a broad array of plant, community, and ecosystem processes. Bioscience 49, 39–50 (1999). [Google Scholar]
- 91.Whyte I. J., “Kruger’s elephant population: Its size and consequences for ecosystem heterogeneity” in The Kruger Experience: Ecology and Management of Savanna Heterogeneity, du Toit J. T., Rogers K. H., Biggs H. C., Eds. (Island Press, 2003), pp. 332–348. [Google Scholar]
- 92.Western D., Maitumo D., Woodland loss and restoration in a Savanna Park: A 20-year experiment. Afr. J. Ecol. 42, 111–121 (2004). [Google Scholar]
- 93.Terborgh J., et al. , “The role of top carnivores in regulating terrestrial ecosystems” in Continental Conservation—Scientific Foundations of Regional Reserve Networks, Soulé M. E., Terborgh J., Eds. (Island Press, 1999), pp. 39–64. [Google Scholar]
- 94.Ripple W. J., et al. , Collapse of the world’s largest herbivores. Sci. Adv. 1, e1400103 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pineda-Munoz S., Wang Y., Lyons S. K., Tóth A. B., McGuire J. L., Mammal species occupy different climates following the expansion of human impacts. Proc. Natl. Acad. Sci. U.S.A. 118, e1922859118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Young H. S., McCauley D. J., Galetti M., Dirzo R., Patterns, causes, and consequences of Anthropocene Defaunation. Annu. Rev. Ecol. Evol. Syst. 47, 333–358 (2016). [Google Scholar]
- 97.Ripple W. J., et al. , Extinction risk is most acute for the world’s largest and smallest vertebrates. Proc. Natl. Acad. Sci. U.S.A. 114, 10678–10683 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Seersholm F. V., et al. , Rapid range shifts and megafaunal extinctions associated with late Pleistocene climate change. Nat. Commun. 11, 2770 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yann L. T., DeSantis L. R. G., Koch P. L., Lundelius E. L., Dietary ecology of Pleistocene camelids: Influences of climate, environment, and sympatric taxa. Palaeogeogr. Palaeoclimatol. Palaeoecol. 461, 389–400 (2016). [Google Scholar]
- 100.Ambrose S. H., Preparation and characterization of bone and tooth collagen for isotopic analysis. J. Archaeol. Sci. 17, 431–451 (1990). [Google Scholar]
- 101.R Core Team, R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2019). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or SI Appendix.