Abstract
Background
Influenza affects approximately a billion people globally, including > 10 million Japanese individuals every year. Baloxavir marboxil (baloxavir [BXM]; a selective cap-dependent endonuclease inhibitor) is approved for influenza treatment in Japan. We compared the incidence of intra-familial transmission of influenza between BXM and oseltamivir (OTV) treatments using a simulation model.
Methods
Using the JMDC Claims Database, we identified index case (IC) as the first family member diagnosed with influenza during the 2018–19 influenza season, and classified the families into BXM or OTV group per the drug dispensed to ICs. Using a novel influenza intra-familial infection model, we simulated the duration of influenza infection in ICs based on agent-specific virus shedding periods. Intra-familial infections were defined as non-IC family members infected during the agent-specific viral shedding period in ICs. The virus incubation periods in the non-IC family members were considered to exclude secondary infections from potentially external exposure. The primary endpoint was proportion of families with intra-familial infections. For between-group comparisons, we used a multivariate logistic regression model.
Results
The median proportion of families with intra-familial transmission was 9.57% and 19.35% in the BXM (N = 84 672) and OTV (N = 62 004) groups, respectively. The multivariate odds ratio of 1.73 (2.5th–97.5th percentiles, 1.68–1.77) indicated a substantially higher incidence of intra-familial infections in the OTV group versus the BXM group. Subgroup analyses by ICs’ age category, virus type, and month of onset revealed similar trends favoring BXM.
Conclusions
BXM treatment of ICs may contribute to a greater reduction in intra-familial influenza transmission than OTV treatment.
Keywords: baloxavir marboxil, intra-familial infection, Japan, influenza virus, oseltamivir
Based on a simulation model factoring in agent-specific infectivity, treating influenza in the first family member with baloxavir marboxil than with oseltamivir, may contribute to a greater reduction in intra-familial influenza transmission.
Seasonal influenza is an acute respiratory infection caused by influenza viruses, resulting in approximately 1 billion cases, 3–5 million cases of severe illness, and up to 0.65 million deaths per year worldwide [1]. Notably, Japan reports the second highest number of cases in the Western Pacific region, after China [2], with approximately 12 million cases reported in the 2018–19 season [3]. This places a substantial clinical and economic burden on the Japanese healthcare system. Although influenza affects people of all age groups, morbidity and mortality can be high, especially among children, elderly, and those with comorbidities [4]. Efficacy of neuraminidase inhibitors (NAIs), such as oseltamivir (OTV), zanamivir, laninamivir, and peramivir, in the treatment of influenza has been abundantly reported [5]. Baloxavir marboxil (BXM), a selective cap-dependent endonuclease inhibitor, was approved in Japan in February 2018 as a single oral dose for the treatment of influenza types A and B [6]. This approval was based on the results from 2 randomized controlled trials (RCTs) in patients aged 12–64 years with uncomplicated influenza (JapicCTI number 153090 [phase 2], and CAPSTONE-1 ClinicalTrials.gov number NCT02954354 [phase 3]), which reported its superior efficacy in the time to alleviation of symptoms and viral load compared with placebo and viral load reduction with OTV [7]. Similar results were observed in an open-label study assessing the safety and effectiveness of BXM in Japanese children (aged 1–11 years) with influenza (JapicCTI number 163417) [8].
Considering that the average household in Japan includes 4 members [9] and secondary transmission to family members represents a significant mode of influenza transmission in Japan [10], it is crucial to not only alleviate patient symptoms for a rapid recovery but also reduce intra-familial transmission. Post-exposure prophylactic treatment of household family members with either NAIs [11] or BXM [9] is effective in reducing the incidence of intra-familial transmission, irrespective of the treatment of the first patient with influenza infection in the family (index case [IC]). Although several studies have suggested that treatment of ICs with an anti-influenza agent may reduce intra-familial transmission of influenza without the need for prophylaxis in non-infected individuals [12–16], the magnitude of the effect is variable and highly dependent on the time [17]. Komeda et al reported a significantly reduced incidence of intra-familial transmission with BXM treatment compared with OTV treatment in ICs by using a Japanese claims database [18]. In many of these studies, including Komeda et al’s [12, 13, 15, 18], intra-familial infection was assumed to have occurred when the first day of influenza onset in the family was considered to be day 1 and when other family members developed influenza on days 3–.8. However, these methodologies did not consider the possibility of an infection from a source other than the IC (external exposure) or the differential impact of anti-influenza agents on the duration of viral shedding by the IC; this consideration may change the probability and window of transmission to others and, consequently, the incidence of intra-familial transmission among ICs treated with different anti-influenza agents.
We applied the previously described influenza intra-familial infection model [19] and the viral shedding period of BXM and OTV described in a clinical study [7] to the data set previously analyzed by Komeda et al [18]. This enabled simulation of the duration of influenza virus infection for both anti-influenza agents in ICs and the incubation period of influenza virus (a period when a person is infected but does not show symptoms) in other family members infected with influenza, thereby overcoming the limitation of the previously published studies. We aimed to compare the incidence of intra-familial transmission of influenza between BXM and OTV treatments using a simulation model.
METHODS
Database
Patients’ medical information was derived from the JMDC Claims Database (JMDC-DB). A unique family code assigned to patients facilitated identification of family relationships. Data were retrieved for 1 October 2018 to 30 April 30 2019, covering most patients from the 2018–2019 influenza season in Japan.
Study Design and Population
The overall methodology of this retrospective cohort study has been described previously [18]. This study was registered at the University Hospital Medical Information Network Clinical Trials Registry as UMIN000038155 and conducted in compliance with the Ethical Guidelines for Medical Research Involving Human Subjects and the Guidance for Medical Research Involving Human Subjects. Informed consent was not required because the presented data are deidentified. The first patient in a unique family diagnosed with influenza infection (International Classification of Diseases (ICD)-10 codes: J09, J10, and J11) in the JMDC-DB was considered the IC, and the day when the IC was diagnosed with influenza infection was defined as day 1.
Families meeting the following criteria were included in the simulation: (1) all family members were enrolled in the health insurance association from October 2018 to April 2019; (2) day 1 of IC occurred between 1 October 2018 and 23 April 23 2019; (3) IC received BXM or OTV on day 1; and (4) IC was not hospitalized on days 1–2. Exclusion criteria included families with no member other than the IC, ≥2 ICs within the same month of Day 1, or multiple anti-influenza agents dispensed to ICs on days 1–2. The included families were grouped based on the anti-influenza agent (BXM or OTV) dispensed to the IC on day 1.
Intra-Familial Infection Simulation Model
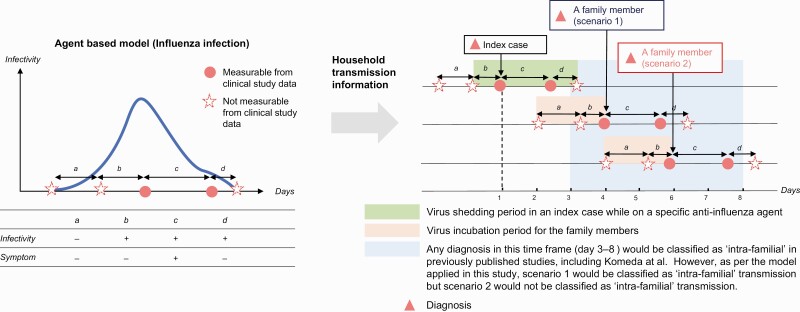
Saito et al (2021) [19] described a natural course of influenza infection using an agent-based model that divides the period from the first influenza infection to the disappearance of infectivity into four distinct periods (Figure 1). We introduced a distribution for the variation in each period for data on infection within families and a method for maximum likelihood estimation of the parameters of each distribution, providing distributions for a, c, and d under the assumption that the disease develops at the point of infectivity, that is, b = 0.
Figure 1.
Modified model of agent-based intra-familial transmission of influenza. Parameters a and b are based on the results by Saito et al [19], whereas parameters c and d are based on the results by Hayden et al [7]. The beginning of period c is available from JMDC-DB as the date of a positive rapid influenza diagnostic test in most cases. Patient status: (a) asymptomatic and noninfectious; (b) asymptomatic and infectious; (c) symptomatic and infectious; (d) asymptomatic (post-recovery) and infectious. Scenarios included in the figure are for illustrative purposes only and do not encompass all possibilities of intra-familial transmission. Scenario 1, intra-familial infection. Scenario 2, secondary infection from external sources. Left panel describes the treatment course of influenza infection as described by Saito et al [19], whereas the right panel describes how intra-familial infections were defined in JMDC-DB. Abbreviation: JMDC-DB, JMDC Claims Database.
For each family in the JMDC-DB, if a family member other than the IC had a diagnosis of influenza, eligibility for intra-familial infection was determined based on the simulation model. The day when IC was diagnosed with influenza infection (day 1) corresponded to the first time point of period c. We assumed that c + d corresponds to the virus shedding period in the CAPSTONE-1 RCT comparing BXM and OTV [7] and generated it as a random number following a lognormal distribution. The time to cessation of virus shedding determined by the infectious virus titer was defined as the time between initiation of anti-influenza treatment and when the infectious virus titer was below the limit of detection (0.7 log10 of the 50% tissue culture infective dose [TCID50/mL]) the first time [7]. Specifically, the [log-scale parameter, shape parameter] of the BXM and OTV groups were estimated as [0.54, 0.64] and [1.17, 0.57], respectively, based on the CAPSTONE-1 RCT, such that the median of the log-normal distribution is 1.72 days and 3.23 days for the BXM and OTV groups, respectively. Based on the results of the CAPSTONE-1 RCT [7] and the pediatric open-label study on BXM [8], we assumed that there is no significant difference in the duration of viral shedding between children and adults. If a family member other than the IC had a diagnosis of influenza infection, we estimated the date of influenza infection for that family member by generating a random number corresponding to periods a-b that follows a gamma distribution (shape parameter, 0.99; scale parameter, 1.46) with a mean of 1.43 days, as described by Saito et al [19]. Consequently, we defined the presence of intra-familial infection when the duration of influenza virus infectivity (shedding period) for each anti-influenza agent in IC (periods b–d) overlapped with the virus incubation period of other family members infected with influenza (periods a–b). In Figure 1, both scenarios would have been classified as intra-familial infections from the IC in the previously published studies because of their diagnoses during days 3–8 in the respective studies. Using the agent-specific intra-familial infection simulation model, we compared the incidence of intra-familial transmission of influenza virus infection between BXM and OTV during the 2018–2019 influenza season.
Statistical Analyses
Primary endpoints were the number and proportion of families with intra-familial infections (Supplementary Material).
We generated 1000 simulated samples, repeated the odds ratio (OR) computation, and calculated the median (50th percentile) of ORs as a measure of central tendency, and 2.5th and 97.5th percentiles to describe variability. Similarly, subgroup analyses were conducted to assess the effectiveness of BXM vs OTV in reducing intra-familial influenza transmission in subpopulations where ICs were stratified by age group, virus type, and infection onset. Methods for secondary analyses are described in Supplementary Material. SAS® 9.4 (SAS Institute, Cary, North Carolina, USA) was used for all statistical analyses.
RESULTS
Analysis Population
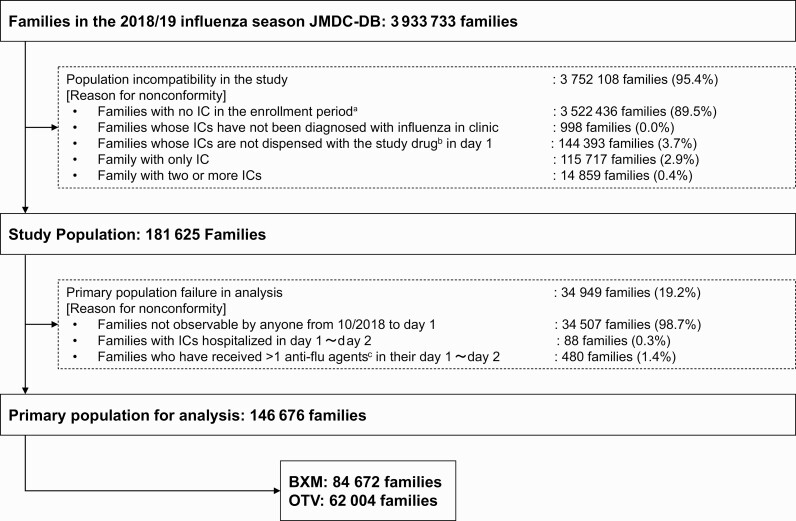
Of the 3 933 733 families in the 2018–2019 influenza season in the JMDC-DB, 146 676 were included in the analysis (BXM, 84 672; OTV, 62 004; Figure 2). ICs were predominantly aged < 12 years in the overall and OTV groups, whereas most ICs in BXM group were adults (Table 1). In all groups, most ICs were male, with January 2019 being the most common month of onset. Influenza type A infection was far more common compared with type B in the overall population (71.0% vs 0.9%), whereas virus type information was unknown for many ICs (28.0%; Table 1). The overall concordance rate of influenza virus types between the IC and the family member with secondary infection was > 99%, with a concordance of 99.5% for type A and 85.5% for type B, when individuals with unknown virus type were excluded (Table 2).
Figure 2.
Flow chart of the study cohort. Abbreviations: BXM, baloxavir marboxil; IC, index case; OTV, oseltamivir. aA 1 October 2018 to 23 April 2019. bBXM or OTV. cAnti-influenza agents: BXM and OTV.
Table 1.
Background Characteristics of Index Cases
| BXM (N = 84 672) | OTV (N = 62 004) | Overall (N = 146 676) | ||
|---|---|---|---|---|
| Age | <12 years | 24 157 (28.5) | 42 001 (67.7) | 66 158 (45.1) |
| ≥12 years to <19 years | 18 909 (22.3) | 3231 (5.2) | 22 140 (15.1) | |
| ≥19 years to <65 years | 40 254 (47.5) | 16 082 (25.9) | 56 336 (38.4) | |
| ≥65 years | 1352 (1.6) | 690 (1.1) | 2042 (1.4) | |
| Sex | Male | 48 399 (57.2) | 34 202 (55.2) | 82 601 (56.3) |
| Female | 36 273 (42.8) | 27 802 (44.8) | 64 075 (43.7) | |
| Influenza virus type | Type A | 61 246 (72.3) | 42 883 (69.2) | 104 129 (71.0) |
| Type B | 818 (1.0) | 570 (0.9) | 1388 (0.9) | |
| Types A and B | 24 (0.0)a | 15 (0.0)a | 39 (0.0)a | |
| Unknown | 22 584 (26.7) | 18 536 (29.9) | 41 120 (28.0) | |
| Time of onset | 2018 October | 146 (0.2) | 172 (0.3) | 318 (0.2) |
| 2018 November | 613 (0.7) | 421 (0.7) | 1034 (0.7) | |
| 2018 December | 11 167 (13.2) | 6165 (9.9) | 17 332 (11.8) | |
| 2019 January | 58 548 (69.1) | 40 211 (64.9) | 98 759 (67.3) | |
| 2019 February | 11 248 (13.3) | 12 165 (19.6) | 23 413 (16.0) | |
| 2019 March | 1952 (2.3) | 1889 (3.0) | 3841 (2.6) | |
| 2019 April | 998 (1.2) | 981 (1.6) | 1979 (1.3) |
All data are presented as no. (%).
Abbreviations: BXM, baloxavir marboxil; OSV, oseltamivir.
Percentages are too low and appear 0.0 due to rounding off to one decimal value.
Table 2.
Concordance Rate of Influenza Type Between Index Cases (IC) and Family Members (Simulation)
| IC | |||||
|---|---|---|---|---|---|
| Simulation Number | N | Influenza Virus Type | Type A | Type B | |
| 1 | 24 560 | Infected persons within family, except IC | Type A | 13 644 (99.6) | 12 (15.0) |
| Type B | 53 (0.4) | 68 (85.0) | |||
| Overall | 13 697 (100.0)a | 80 (100.0)a | |||
| 2 | 24 589 | Infected persons within family, except IC | Type A | 13 701 (99.5) | 12 (14.5) |
| Type B | 69 (0.5) | 71 (85.5) | |||
| Overall | 13 770 (100.0)b | 83 (100.0)b | |||
Data are presented only for infected individuals (IC and intra-familial infections) whose virus type was known as either A or B. Individuals with unknown or coinfection with both virus types (A and B) are excluded from the table.
All values are presented as no. (%) of overall data.
Of the infected individuals, 2 had coinfection with types A and B; virus type was not known for 11 324 infected individuals in simulation 1.
Of the infected individuals, 3 had coinfection with types A and B; virus type was not known for 11 309 infected individuals in simulation 2.
Incidence of Intra-Familial Transmission
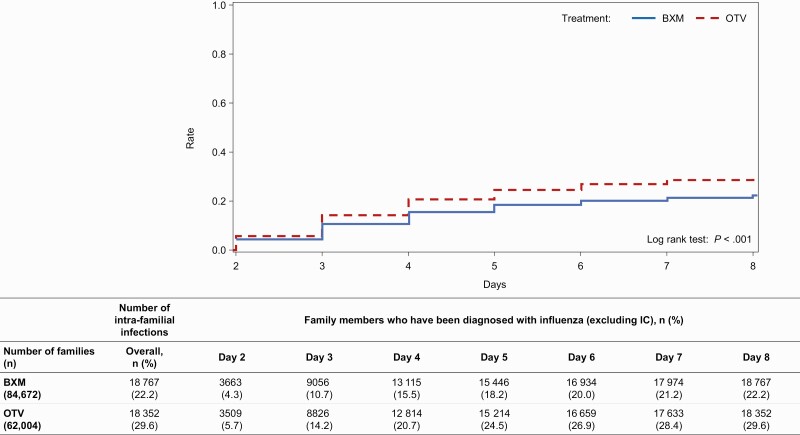
The median proportion of families with intra-familial transmission was 9.57% and 19.35% in the BXM and OTV groups, respectively (Table 3). The median OR (2.5th and 97.5th percentiles), with BXM as control, from the multivariate regression model was 1.73 (1.68, 1.77), indicating substantially higher odds of developing an intra-familial infection when the IC is treated with OTV compared with BXM (Table 3). The subgroup analysis by IC’s age group showed that the incidence of intra-familial infection was highest in the age groups of < 7 and ≥ 7 to < 13 years. Moreover, OTV treatment resulted in substantially higher intra-familial transmission compared with BXM treatment, with median multivariate ORs ranging from 1.43 to 1.93 across age groups (Table 4). Regarding the viral type of ICs, median multivariate ORs (2.5th and 97.5th percentiles) for OTV for influenza A and B were 1.78 (1.73, 1.83) and 1.65 (1.16, 2.37), respectively, showing a tendency for the incidence of intra-familial infection to be higher in the OTV group compared with the BXM group across both virus types (Table 5). Although the incidence of intra-familial infections varied by month, the proportion of families with intra-familial transmission was highest in December 2018 for ICs treated with BXM (10.13%) and January 2019 for ICs treated with OTV (20.67%; Table 6). The median multivariate ORs ranged from 1.61 to 1.99, indicating the higher odds of intra-familial transmission with OTV compared with BXM irrespective of the month of onset of illness (Table 6). The simulated cumulative percentages of intra-familial infections were 10.7% and 14.2% on day 3 in the BXM and OTV groups, respectively, and the difference increased on day 6 (20.0% and 26.9% in the BXM and OTV groups, respectively; Figure 3).
Table 3.
Proportion of Families With and Odds Ratios (ORs) for Intra-Familial Infection Based on the Simulation Results
| Drug (Number of Families) | Proportion of Families With an Intra-Familial Infection | Univariate Logistic Regression Modela | Multivariate Logistic Regression Modelb |
|---|---|---|---|
| Median (2.5th–97.5th percentile) | Median OR (2.5th–97.5th percentile) | Median OR (2.5th–97.5th percentile) | |
| BXM (84 672) | 9.57 (9.42, 9.72) | 2.27 (2.22, 2.32) | 1.73 (1.68, 1.77) |
| OTV (62 004) | 19.35 (19.16, 19.54) |
BXM was used as a reference against OTV for the calculation of ORs.
Families with no missing data on age, sex, relationship, size of medical facility, time of onset, and influenza virus type for the included family members (corresponding to the IC) were included in the analysis.
Number of simulations was set to 1000.
Abbreviations: BXM, baloxavir marboxil; IC, index case; OTV, oseltamivir.
Logistic regression model with the presence or absence of an intra-familial infection as the outcome variable and drug group as the exposure variable.
Logistic regression model as explained above further adjusted for covariates including age, sex, relationship, size of medical facility, time of onset, and influenza virus type.
Table 4.
Proportion of Families With and Odds Ratios (ORs) for Intra-Familial Infection According to Age of the Index Case (IC) Based on Simulation Results
| Age (years) | Drug (Number of Families) | Proportion of Families With an Intra-Familial Infection | Univariate Logistic Regression Modela | Multivariate Logistic Regression Modelb |
|---|---|---|---|---|
| Median (2.5th–97.5th percentile) | Median OR (2.5th–97.5th percentile) | Median OR (2.5th–97.5th percentile | ||
| <7 | BXM (5493) | 16.35 (15.62, 17.09) | 1.56 (1.47, 1.65) | 1.71 (1.61, 1.81) |
| OTV (32 278) | 23.38 (23.10, 23.69) | |||
| ≥7 to <13 | BXM (22 293) | 12.48 (12.16, 12.81) | 1.85 (1.78, 1.93) | 1.93 (1.85, 2.01) |
| OTV (10 280) | 20.89 (20.46, 21.43) | |||
| ≥13 to <19 | BXM (15 280) | 6.58 (6.27, 6.88) | 1.61 (1.47, 1.76) | 1.72 (1.57, 1.89) |
| OTV (2674) | 10.17 (9.46, 10.92) | |||
| ≥19 to <65 | BXM (40 254) | 8.25 (8.05, 8.45) | 1.54 (1.48, 1.60) | 1.60 (1.53, 1.66) |
| OTV (16 082) | 12.19 (11.88, 12.50) | |||
| ≥65 | BXM (1352) | 7.25 (6.14, 8.21) | 1.35 (1.08, 1.69) | 1.43 (1.15, 1.81) |
| OTV (690) | 9.57 (8.12, 10.94) |
BXM was used as a reference against OTV for the calculation of ORs.
Families with no missing data on age, sex, relationship, size of medical facility, time of onset, and influenza virus type for the included family members (corresponding to the IC) were included in the analysis. Number of simulations was set to 1000.
Abbreviations: BXM, baloxavir marboxil; OTV, oseltamivir.
Logistic regression model with the presence or absence of an intra-familial infection as the outcome variable and drug group as the exposure variable.
Logistic regression model as explained above further adjusted for covariates including sex, relationship, size of medical facility, time of onset, and influenza virus type.
Table 5.
Proportion of Families With and Odds Ratios (ORs) for Intra-Familial Infection According to Viral Type of the Index Case (IC) Infection Based on the Simulation Results
| Viral Type | Drug (Number of Families) | Proportion of Families With an Intra-Familial Infection | Univariate Logistic Regression Modela | Multivariate Logistic Regression Modelb |
|---|---|---|---|---|
| Median (2.5th–97.5th percentile) | Median OR (2.5th–97.5th percentile) | Median OR (2.5th–97.5th percentile) | ||
| Type A | BXM (61 246) | 9.67 (9.50, 9.85) | 2.34 (2.29, 2.40) | 1.78 (1.73, 1.83) |
| OTV (42 883) | 20.05 (19.83, 20.29) | |||
| Type B | BXM (818) | 5.26 (4.16, 6.36) | 1.70 (1.29, 2.28) | 1.65 (1.16, 2.37) |
| OTV (570) | 8.77 (7.19, 10.18) | |||
| Unknown | BXM (66 775) | 7.54 (7.38, 7.68) | 2.21 (2.15, 2.27) | 1.67 (1.62, 1.72) |
| OTV (47 438) | 15.24 (15.03, 15.45) |
BXM was used as a reference against OTV for the calculation of ORs.
Families with no missing data on age, sex, relationship, size of medical facility, time of onset, and influenza virus type for the included family members (corresponding to the IC) were included in the analysis. Number of simulations was set to 1000.
Abbreviations: BXM, baloxavir marboxil; OTV, oseltamivir.
Logistic regression model with the presence or absence of an intra-familial infection as the outcome variable and drug group as the exposure variable.
Logistic regression model as explained above further adjusted for covariates including age, sex, relationship, size of medical facility, and time of onset.
Table 6.
Proportion of Families With and Odds Ratio (ORs) for Intra-Familial Infection According to Month of Onset of the Index Case (IC) Infection Based on the Simulation Results
| Onset of Illness | Drug (Number of Families) | Proportion of Families With an Intra-Familial Infection | Univariate Logistic Regression Modela | Multivariate Logistic Regression Modelb |
|---|---|---|---|---|
| Median (2.5th–97.5th percentile) | Median OR (2.5th–97.5th percentile) | Median OR (2.5th–97.5th percentile) | ||
| October to November 2018 | BXM (759) | 7.77(6.46, 9.35) | 2.43(1.93, 3.09) | 1.99(1.52, 2.66) |
| OTV (593) | 17.03(15.18, 18.72) | |||
| December 2018 | BXM (11 167) | 10.13(9.73, 10.53) | 2.02(1.90, 2.15) | 1.75(1.63, 1.87) |
| OTV (6165) | 18.53(17.94, 19.12) | |||
| January 2019 | BXM (58 548) | 9.95(9.77, 10.13) | 2.36(2.30, 2.42) | 1.75(1.70, 1.81) |
| OTV (40 211) | 20.67(20.42, 20.92) | |||
| February 2019 | BXM (11 248) | 7.68(7.31, 8.03) | 2.35(2.21, 2.50) | 1.61(1.51, 1.73) |
| OTV (12 165) | 16.35(15.96, 16.75) | |||
| March 2019 | BXM (1952) | 8.25(7.38, 9.14) | 2.19(1.92, 2.50) | 1.70(1.46, 1.99) |
| OTV (1889) | 16.41 (15.40, 17.42) | |||
| April 2019 | BXM (998) | 6.71 (5.61, 7.82) | 2.32 (1.91, 2.88) | 1.68 (1.31, 2.12) |
| OTV (981) | 14.37 (12.90, 15.60) |
BXM was used as a reference against OTV for the calculation of ORs.
Families with no missing data on age, sex, relationship, size of medical facility, time of onset, and influenza virus type for the included family members (corresponding to the IC) were included in the analysis.
Number of simulations was set to 1000.
Abbreviations: BXM, baloxavir marboxil; OTV, oseltamivir.
Logistic regression model with the presence or absence of an intra-familial infection as the outcome variable and drug group as the exposure variable.
Logistic regression model as explained above further adjusted for covariates including age, sex, relationship, size of medical facility, and influenza virus type.
Figure 3.
Kaplan-Meier curve presenting the cumulative percentage of intra-familial transmission. This figure shows the percentage of households infected, where the denominator is the number of households and the numerator is the number of households in which infections occurred. In the case of a household with more than 1 infected member, the date of the last infection among the household members was used as the date of infection for the household. Abbreviations: BXM, baloxavir marboxil; IC, index case; OTV, oseltamivir.
DISCUSSION
In this study, the odds of developing intra-familial infection were substantially higher in the OTV group compared with the BXM group, supporting the possibility that BXM, compared with OTV, is more effective in suppressing influenza intra-familial infections. This finding aligns with published literature reporting better efficacy and effectiveness of BXM in terms of time to cessation of viral shedding compared with OTV [7, 18, 20–22].
Although influenza symptoms are self-limiting and can subside within a week in most patients, the infection can result in severe illness or death in high-risk patients [1], thereby negatively affecting the quality of life of patients, families, and caregivers [23]. Furthermore, because most secondary infections are acquired from a household member [24], curbing intra-familial infections is a key goal of influenza prevention globally. Using a simulation model that linked viral load dynamics from clinical trial data to inter-host transmission, a study estimated that approximately 22 million infections and > 6000 deaths could have been prevented in the 2017–2018 epidemic in the United States by administering BXM to 30% of the infected cases within 48 hours after symptom onset, whereas treatment within 24 hours would have doubled the positive impact [25]. Using viral shedding as a proxy measure of influenza infectivity, as done previously [26, 27], our comparative findings for BXM and OTV support the hypothesis that treatment of ICs with BXM may contribute more to prevention of intra-familial influenza than OTV, possibly by reducing time to cessation of viral shedding and more effectively suppressing viral load [21]. The cumulative incidence of intra-familial infections differed between the BXM and OTV groups on day 3, and the difference widened on day 6. This can be attributed to the earlier suppression of viral shedding by BXM than OTV, which was confirmed in an RCT [7] and an observational study [18].
Compared with the results from Komeda et al [18]., our results showed a relatively low proportion of intra-familial infections for both BXM (9.57% vs 17.98%) and OTV (19.35% vs 24.16%) groups, despite having the same JMDC-DB population spanning across the same influenza season. This variation is probably owing to the novel intra-familial infection simulation model applied in our study: the model likely provided a more accurate representation of intra-familial infections by factoring in the agent-specific viral shedding period, which was not accounted for in the previous publication, and by effectively excluding any familial infections from external sources. This is further reinforced by a very high concordance rate of 99.5% and 85.5% among ICs and their family members affected by influenza types A and B, respectively.
Patients < 12 years of age appeared to have a higher rate of infections with influenza virus variants with reduced BXM susceptibility compared with adults in previous clinical trials [7, 8]. Although the present study was not aimed to confirm whether low-susceptibility strains developed or whether they were transmitted within families, our results did not indicate that presence of treatment-emergent low-susceptibility strains might have increased intra-familial transmission in the < 12-year age group. However, it is crucial to monitor the susceptibility of circulating viruses to antiviral agents in clinical settings and understand how community transmission is affected by presence of antiviral agents. To that effect, CENTERSTONE (NCT03969212) [28], an ongoing placebo-controlled trial, assessing the efficacy of BXM in reducing household transmission of Influenza A/B virus, will provide valuable clinical data.
Our findings should be interpreted in light of the limitations of this study. First, the results may not reflect the actual family composition or shared household living, as data are based only on the shared family code without any confirmation whether the family members were actually living in the same household. Future studies should assess the external validity of this study in evaluating transmission outside the household (schools, long-term care facilities, etc.) and potential application to countries other than Japan. Second, the possibility of any pre-existing infection in a non-IC family member before IC treatment and prescriptions of anti-influenza agents for prophylaxis among non-IC family members cannot be discounted; notably, only OTV was approved for post-exposure prophylaxis of influenza in Japan at the time of data collection. Third, our results are based on data from a single season with limited cases of type B virus; multi-season studies may shed more light on the impact of yearly variation in virus subtypes, onsets, and infectivity on intra-familial transmission. Fourth, JMDC database may have included patients infected with viruses having reduced susceptibility selected during the treatment with anti-influenza agents. Indeed, in Japanese 2018/19 influenza season, both AH3 (56%) and AH1 (36%) pandemics were observed, and viruses with reduced susceptibility to BXM or OTV were detected [29]. However, the percentage of patients infected with reduced susceptibility viruses and their impact on household transmission with anti-influenza agents were not assessed because information on virus subtype and/or altered susceptibility was unavailable in the database. Moreover, potential treatment-emergent antiviral resistance with BXM vs OTV use in IC may develop differently. Although the median viral shedding period was the same in children and adults treated with BXM in the 2 studies [7, 8], this assumption may not be true in patients treated with OTV. Finally, we applied the model described by Saito et al [19], in which the asymptomatic period with virus shedding (b) was determined as 0; however, because period b was common regardless of treatment, it did not affect the comparison of the transmission rates after anti-influenza treatment. Nonetheless, our findings provide valuable epidemiological data with multiple strengths. The 2018–2019 season data from the JMDC-DB provided a large sample size for robust analysis. Notably, use of the diagnosis of influenza [18] instead of administration of anti-influenza agents [13] to define onset of influenza (day 1) enabled adjustment for the viral type infecting the IC and subgroup analyses. In addition, application of the influenza intra-familial infection model to the database on a simulation basis allowed incorporation of the differences in viral shedding periods between BXM and OTV [7] and the period of infectivity after onset in order to mimic plausible real-world transmission dynamics with different anti-influenza agents. With the consideration of an “asymptomatic but infective” phase of the IC and an “asymptomatic and noninfective” phase of the family member (Figure 1), the model allows accurate identification of intra-familial infections and potentially excludes any cases from external exposure.
CONCLUSION
Using the influenza intra-familial infection model, a national insurance claims database, and shared family codes, we derived that treatment with BXM substantially reduced the odds of intra-familial infection from an IC compared with treatment with OTV on a simulation basis. Our results provide more accurate clinical information by considering the impact of individual anti-influenza agents on viral shedding time.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank Satoshi Kojima and Masahiro Kinoshita of Shionogi & Co, Ltd for their scientific reviews and operational assistance during the development of this manuscript. The authors thank Dr Vidula Bhole, MD, MHSc, and Mr Ivan D’Souza, MS, of MedPro Clinical Research for providing medical writing support for this manuscript.
Financial support. This work was supported by Shionogi & Co, Ltd.
Contributor Information
Shogo Miyazawa, Data Science Department, Shionogi & Co, Ltd, Osaka, Japan.
Takahiro Takazono, Department of Respiratory Medicine, Nagasaki University Hospital, Nagasaki, Japan; Department of Infectious Diseases, Nagasaki University Graduate School of Biomedical Sciences, Nagasaki, Japan.
Naoki Hosogaya, Department of Respiratory Medicine, Nagasaki University Hospital, Nagasaki, Japan; Clinical Research Center, Nagasaki University Hospital, Nagasaki, Japan.
Kazuko Yamamoto, Department of Respiratory Medicine, Nagasaki University Hospital, Nagasaki, Japan; Department of Respiratory Medicine, Nagasaki University Graduate School of Biomedical Sciences, Nagasaki, Japan.
Hideaki Watanabe, Biostatistics Center, Shionogi & Co, Ltd, Osaka, Japan.
Masakazu Fujiwara, Data Science Department, Shionogi & Co, Ltd, Osaka, Japan.
Satoki Fujita, Data Science Department, Shionogi & Co, Ltd, Osaka, Japan.
Hiroshi Mukae, Department of Respiratory Medicine, Nagasaki University Hospital, Nagasaki, Japan; Department of Respiratory Medicine, Nagasaki University Graduate School of Biomedical Sciences, Nagasaki, Japan.
References
- 1. World Health Organization. WHO launches new global influenza strategy. Available at: https://www.who.int/news/item/11-03-2019-who-launches-new-global-influenza-strategy. Accessed 14 January 2022.
- 2. Western Pacific Region Global Influenza Surveillance and Response System. Epidemiological and virological characteristics of influenza in the Western Pacific Region of the World Health Organization, 2006–2010. PLoS One 2012; 7:e37568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Taniguchi K, Ikeda S, Hagiwara Y, et al. Epidemiology and burden of illness of seasonal influenza among the elderly in Japan: a systematic literature review and vaccine effectiveness meta-analysis. Influenza Other Respir Viruses 2021; 15:293–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Matias G, Taylor R, Haguinet F, Schuck-Paim C, Lustig R, Shinde V.. Estimates of mortality attributable to influenza and RSV in the United States during 1997–2009 by influenza type or subtype, age, cause of death, and risk status. Influenza Other Respir Viruses 2014; 8:507–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Heneghan CJ, Onakpoya I, Jones MA, et al. Neuraminidase inhibitors for influenza: a systematic review and meta-analysis of regulatory and mortality data. Health Technol Assess 2016; 20:1–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Heo Y-A. Baloxavir: first global approval. Drugs 2018; 78:693–7. [DOI] [PubMed] [Google Scholar]
- 7. Hayden FG, Sugaya N, Hirotsu N, et al. Baloxavir marboxil for uncomplicated influenza in adults and adolescents. N Engl J Med 2018; 379:913–23. [DOI] [PubMed] [Google Scholar]
- 8. Hirotsu N, Sakaguchi H, Sato C, et al. Baloxavir marboxil in Japanese pediatric patients with influenza: safety and clinical and virologic outcomes. Clin Infect Dis 2020; 71:971–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ikematsu H, Hayden FG, Kawaguchi K, et al. Baloxavir marboxil for prophylaxis against influenza in household contacts. N Engl J Med 2020; 383:309–20. [DOI] [PubMed] [Google Scholar]
- 10. Endo A, Uchida M, Kucharski AJ, Funk S.. Fine-scale family structure shapes influenza transmission risk in households: insights from primary schools in Matsumoto city, 2014/15. PLoS Comput Biol 2019; 15:e1007589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Okoli GN, Otete HE, Beck CR, Nguyen-Van-Tam JS.. Use of neuraminidase inhibitors for rapid containment of influenza: a systematic review and meta-analysis of individual and household transmission studies. PLoS One 2014; 9:e113633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fry AM, Goswami D, Nahar K, et al. Effects of oseltamivir treatment of index patients with influenza on secondary household illness in an urban setting in Bangladesh: secondary analysis of a randomised, placebo-controlled trial. Lancet Infect Dis 2015; 15:654–62. [DOI] [PubMed] [Google Scholar]
- 13. Nakano T, Shiosakai K.. Spread of viral infection to family members from influenza patients treated with a neuraminidase inhibitor. J Infect Chemother 2014; 20:401–6. [DOI] [PubMed] [Google Scholar]
- 14. Halloran ME, Hayden FG, Yang Y, Longini IM Jr, Monto AS.. Antiviral effects on influenza viral transmission and pathogenicity: observations from household-based trials. Am J Epidemiol 2007; 165:212–21. [DOI] [PubMed] [Google Scholar]
- 15. Nishiura H, Oshitani H.. Household transmission of influenza (H1N1-2009) in Japan: age-specificity and reduction of household transmission risk by zanamivir treatment. J Int Med Res 2011; 39:619–28. [DOI] [PubMed] [Google Scholar]
- 16. Ng S, Cowling BJ, Fang VJ, et al. Effects of oseltamivir treatment on duration of clinical illness and viral shedding, and household transmission of influenza virus. Clin Infect Dis 2010; 50:707–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hayden FG, Asher J, Cowling BJ, et al. Reducing influenza virus transmission: the potential value of antiviral treatment. Clin Infect Dis 2022; 74:532–40. doi: 10.1093/cid/ciab625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Komeda T, Takazono T, Hosogaya N, et al. Comparison of household transmission of influenza virus from index patients treated with baloxavir barboxil or neuraminidase inhibitors: a health insurance claims database study. Clin Infect Dis 2021; 72:e859–67. [DOI] [PubMed] [Google Scholar]
- 19. Saito MM, Hirotsu N, Hamada H, et al. Reconstructing the household transmission of influenza in the suburbs of Tokyo based on clinical cases. Theor Biol Med Model 2021; 18:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yoshii N, Tochino Y, Fujioka M, et al. The comparison of the efficacy of baloxavir and neuraminidase inhibitors for patients with influenza A in clinical practice. Intern Med 2020; 59:1509–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Taieb V, Ikeoka H, Ma F-F, et al. A network meta-analysis of the efficacy and safety of baloxavir marboxil versus neuraminidase inhibitors for the treatment of influenza in otherwise healthy patients. Curr Med Res Opin 2019; 35:1355–64. [DOI] [PubMed] [Google Scholar]
- 22. Taieb V, Ikeoka H, Wojciechowski P, et al. Efficacy and safety of baloxavir marboxil versus neuraminidase inhibitors in the treatment of influenza virus infection in high-risk and uncomplicated patients—a Bayesian network meta-analysis. Curr Med Res Opin 2021; 37:225–44. [DOI] [PubMed] [Google Scholar]
- 23. Chow MYK, Morrow AM, Booy R, Leask J.. Impact of children’s influenza-like illnesses on parental quality of life: a qualitative study. J Paediatr Child Health 2013; 49:664–70. [DOI] [PubMed] [Google Scholar]
- 24. Tsang TK, Cowling BJ, Fang VJ, et al. Influenza A virus shedding and infectivity in households. J Infect Dis 2015; 212:1420–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Du Z, Nugen C, Galvani AP, Krug RM, Meyers LA.. Modeling mitigation of influenza epidemics by baloxavir. Nat Commun 2020; 11:2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lao LLH, Ip DKM, Nishiura H, et al. Heterogeneity in viral shedding among individuals with medically attended influenza A virus infection. J Infect Dis 2013; 207:1281–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fielding JE, Kelly HA, Mercer GN, Glass K.. Systematic review of influenza A(H1N1)pdm09 virus shedding: duration is affected by severity, but not age. Influenza Other Respir Viruses 2014; 8:142–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. National Institute of Health. U.S. National Library of Medicine. Study to assess the efficacy of baloxavir marboxil versus placebo to reduce onward transmission of influenza A or B in households. ClinicalTrials.gov Identifier: NCT03969212. Available at: https://clinicaltrials.gov/ct2/show/NCT03969212. Accessed 8 October 2021.
- 29. National Institute of Infectious Diseases. Influenza 2018/19 season, Japan. IASR 2020; 40:177–9. Available at: https://www.niid.go.jp/niid/en/865-iasr/9288-477te.html. Accessed 14 January 2022. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.