Abstract
Hemorrhage is the leading cause of trauma-related deaths, in hospital and prehospital settings. Hemostasis is a complex mechanism that involves a cascade of clotting factors and proteins that result in the formation of a strong clot. In certain surgical and emergency situations, hemostatic agents are needed to achieve faster blood coagulation to prevent the patient from experiencing a severe hemorrhagic shock. Therefore, it is critical to consider appropriate materials and designs for hemostatic agents. Many materials have been fabricated as hemostatic agents, including synthetic and naturally derived polymers. Compared to synthetic polymers, natural polymers or biopolymers, which include polysaccharides and polypeptides, have greater biocompatibility, biodegradability and processibility. Thus, in this review, we focus on biopolymer-based hemostatic agents of different forms, such as powder, particles, sponges and hydrogels. Finally, we discuss biopolymer-based hemostatic materials currently in clinical trials and offer insight into next-generation hemostats for clinical translation.
Keywords: hemostasis, coagulation, polysaccharides, polypeptides, biomaterials
Graphical Abstract
Introduction
Hemorrhage is the loss of blood from blood vessels due to traumatic injuries and is responsible for 30–40% of trauma-related mortality [1–3]. Hemostasis is the first step in the wound healing process and is the body’s natural mechanism to stop bleeding. In response to an injury, blood vessels vasoconstrict to restrict blood flow, and the damaged endothelial cells release von Willebrand factor to trigger a complex clotting factor cascade to form a soft platelet plug, followed by a stronger fibrin clot [4]. Often, the process of hemostasis starts within seconds of an injury, and it can take up to several minutes for the fibrin clot to be formed [5]. Depending on the type of injury, the individual may lose excessive amounts of blood and experience hemorrhagic shock due to inadequate oxygen and nutrient delivery to tissue [2]. To avoid excessive blood loss, hemostatic agents are needed to facilitate coagulation.
An ideal hemostat for emergency settings, battlefield injuries and premedical treatment facility hemorrhage control should have the following characteristics [6–9]: (i) quick and adequate hemostasis in a wide range of injuries and wounds; (ii) sustained hemostasis for several hours with the ability to deliver antibiotics in situations of delayed evacuation; (iii) easy removal without any residues left in the injury or wound; (iv) ready-to-use and ease-of-administration by a layperson with little to no training; (v) ease-of-manufacturing and sterilization with low cost; (vi) easy storage with prolonged stability even under austere conditions; (vii) good biocompatibility with low/no adverse body immune-response. In emergency medicine, several types of hemostatic agents have been employed, including chemical or topical hemostats [10, 11], direct pressure or pressure dressings [12], sutures and ties [13, 14], as well as physical agents [15], and they sometimes need to be combined to achieve effective hemostasis [16, 17].
The field of hemorrhage control is expansive and quickly evolving. This review summarizes the recent advances in biopolymer-based materials for hemostatic applications. At first, we describe the coagulation cascade, the various pathways and clotting factors involved in the clot formation. Then, we provide insights into different strategies to achieve coagulation and stop bleeding, focusing on using fibrin, thrombin, clotting factors and molecular drugs. Notably, we explore a great range of polysaccharide- and polypeptide/protein-based materials and their composites for bleeding management. Specifically, biopolymer-based hemostats of several forms, including powder and particles, sponges and foams, sheets and films, and hydrogels, and their importance for specific hemostatic applications have been reviewed. Finally, we discuss biopolymer-based hemostats currently in clinical trials and provide an outlook on where the field of hemostasis is headed.
Hemostasis coagulation cascade
Hemostasis is the natural mechanism in response to vascular endothelial impairment. It involves a series of events supporting the production of a clot to preserve blood within the vasculature [18]. There are two types of hemostasis: primary and secondary. Vasoconstriction and platelet adhesion, activation and aggregation are all involved in primary hemostasis, which leads to the creation of a soft platelet plug. This process is then followed by secondary hemostasis, which results in the transformation of a soft platelet plug into a hard, insoluble fibrin clot achieved by the conversion of fibrinogen to fibrin [19].
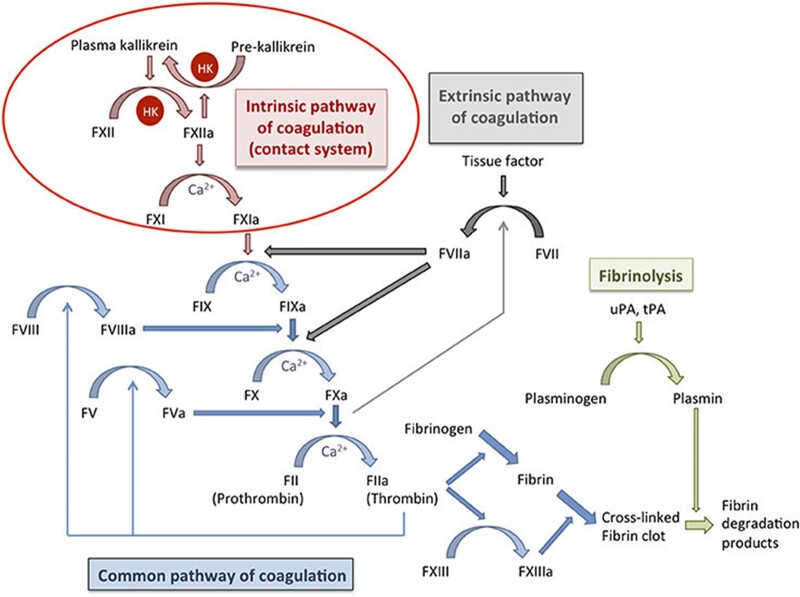
The mechanism of hemostasis coagulation is a complicated process involving many clotting factors, such as fibrinogen, prothrombin, etc., that activate each other and create a cascade (Fig. 1). Blood clotting factors are critical elements of hemostasis. They circulate in the blood in an inactivated form known as zymogens or proenzymes [20]. The coagulation process highly depends upon the amount of these proteins in the blood [21]. Majority of the coagulants are produced in the liver except for a few [22]. Most clotting factors are produced primarily through hepatocytes, which play a major role in delivering a variety of clotting factors (XIII, XII, XI, X, IX, VII, V, II and I). On the other hand, clotting factors VIII (antihemophilic factor A) and III (tissue factor) are produced by endothelial cells, whereas clotting factor IV (calcium ion) is found freely in plasma. Megakaryocytes are responsible for the development of platelets as well as component V [23, 24].
Figure 1.
The schematic representation of the coagulation cascade, which includes the intrinsic pathway, extrinsic pathway and common pathway. Both the intrinsic and extrinsic pathways converge at the common pathway and lead to factor X activation, which helps convert prothrombin to thrombin. Thrombin is necessary to convert fibrinogen to fibrin and activate factor XIII, which eventually leads to a crosslinked fibrin clot. Fibrinolysis helps regulate hemostasis and helps degrade the fibrin network via plasmin. HK, high molecular weight kininogen; uPA, urokinase plasminogen activator; tPA, tissue plasminogen activator. Image adapted with permission from Loof et al. [29].
Traditionally, the coagulation cascade is categorized into two major pathways based on activation mechanism: intrinsic and extrinsic. The intrinsic pathway is engaged in response to internal vascular endothelial injury, whereas the extrinsic pathway is triggered in response to extrinsic trauma. These two pathways converge into a common pathway at the fibrin clotting step with factor X (FX) activation. Activated clotting factors are denoted by adding ‘a’ at the end of the name.
Intrinsic pathway
The intrinsic pathway is considered as the initiation of the coagulation reaction process, involving factors I (fibrinogen), II (prothrombin), IX (Christmas factor), X (Stuart-Power factor), XI (plasma thromboplastin) and XII (Hageman factor) (Fig. 1). The intrinsic pathway is initiated by the autoactivation of FXII (factor XII), which changes its conformation. Negatively charged molecules or surfaces can activate FXII [22]. After hemorrhage, FXII is activated by either negatively charged collagen fibrils in damaged tissue or negatively charged phospholipids from endothelial cells. Activated FXII (FXIIa) converts factor XI to FXIa. Next, downstream, FXIa further activates factor IX to FIXa. FIXa subsequently activates factor X by combining with its cofactor VIIIa, which together forms a complex on a phospholipid surface. FXa binds to FVa and converts prothrombin to thrombin. In addition, FXIIa converts prekallikrein to kallikrein, which in turn activates FXII and maintains a feedback loop to amplify the production of FXIIa.
Extrinsic pathway
The extrinsic pathway is shorter compared to the intrinsic pathway. This pathway involves factors I, II (Prothrombin), VII (Stable factor) and X (Fig. 1). It is activated by vascular injury when endothelial cell-released tissue factors (TFs) located in the extracellular matrix bind to factor VII to form FVIIa and initiate the extrinsic pathway cascade [25]. FVIIa and TF bind to FX (FXa), which binds to factor V and activates (Va). FXa binds to FVa, and calcium (Ca2+) produces a prothrombinase complex entering the common pathway [22].
Common pathway
The common pathway involves factors I, II, V, VII and X (Fig. 1). It initiates after activating factor X (FXa) via the intrinsic pathway form a complex with factors VIII, IXa, a phospholipid and Ca2+ or via the extrinsic pathway involving factors VII, TF and Ca2+ [26]. After activating factor Xa, it converts factor II to factor IIa with cofactor V. This complex formation occurs at the cell membrane of the platelet with phosphatidylserine. Factor IIa activates other factors and cofactors such as FXI, V, VIII and XIII, creating a feedback loop. Factor IIa further converts fibrinogen to fibrin leading to fibrin polymerization. This reaction is catalyzed by factor XIIIa. Polymerized fibrin further creates a network structure by crosslinking with FXIIIa, activating lysine and glutamic acid. This reaction further stabilizes the platelet plug leading to activation and aggregation, creating a thrombus. This fibrin network structure is the primary structure of the blood clot [18].
The coagulation cascade is controlled by other factors that encounter the coagulation activity. For example, antithrombin (AT III) inactivates IIa and FXa by blocking the enzyme (i.e. serine protease) activity at the injured site, forming a complex structure. The process is slower and can be counteracted by using heparin [27]. Moreover, in specific clinical cases, limitations of the coagulation cascade are observed. Patients deficient in coagulation factor FXII, which is the initiation factor of intrinsic pathways, can have thromboplastin partially without bleeding tendency. In contrast, a patient deficient in factors VII, VIII and IX, shows bleeding tendency while extrinsic pathways remain unaffected [28]. This shows that the intrinsic and extrinsic pathways are dependent [21].
Targeting strategies for achieving hemostasis
As pointed out in the previous section, hemostasis is a tightly regulated process that results in coagulation, owing to the synchronized action of enzymes and clotting factors. Here, it is worth mentioning that in instances of severe blood loss, adopting adjuvant measures for bleeding control becomes necessary since the body's natural mechanism cannot control hemostasis on its own. Therefore, understanding the natural hemostasis process is essential to developing practical hemostatic devices that can mimic and stimulate the natural process [30].
Fibrin is one of the most popular natural hemostatic agents. It is formed from fibrinogen under the action of thrombin at a wound site or a site of tissue damage in the presence of factor XIII and calcium and, in combination with platelets, creates a blood clot [31]. However, during the shortage of clotting factors, either due to high blood loss, genetic diseases or excessive use of anticoagulants, thrombin activation is inhibited, which leads to inhibition of fibrin formation [6]. The thickness of the layer of fibrin fibers solely depends on the thrombin concentration. Moreover, the weak and thin fibrin fibers are more prone to fibrinolysis, hindering hemostasis [32]. Therefore, there are several strategies to use biomaterials to create fibrin fibers by mimicking clotting factors and inducing mechanically strong clot formation for hemostasis applications [33].
The synthetic peptide mimicking knob A, such as double-headed ligand bis(Gly-Pro-Arg-Pro-amido)polyethylene-glycol, was first reported to be the replacement for thrombin-modified-E-nodule for fibrinogen–fibrinogen or D–D crosslinking (non-covalent) by the interaction between the two-hole a’s in the γ-chain in the vicinity [34]. As a result, the peptide was found to crosslink the fibrin by showing the biphasic behaviors (productive and non-productive) depending on the peptide concentration [35]. Later, cysteine-terminated knob-A-peptide mimics (GPRPAAC) were conjugated with four-arm and two-arm maleimide-functionalized polyethylene glycols (PEGs) to synthesize GPRP4-PEG. At a lower concentration of GPRP4-PEG to fibrinogen, the clotting showed higher density and larger fiber diameter [36]. On the contrary, PEGylated knob peptides were recognized as anticoagulants [37]. In addition, the linear polymer hemostats (PolySTAT), composed of poly(hydroxyethyl)methacrylate conjugated to N-hydroxysuccinimidyl ester methacrylate, were also found to crosslink fibrin. The PolySTAT-crosslinked fibrin was denser and less porous with higher elastic moduli than the control [38–40]. For the rat model, the intravenous injection of PolySTAT showed a significantly higher survival rate for the injury at the femoral artery [38].
Apart from that, engineered platelet-like-particles (PLPs) composed of ultra-low-crosslinked poly(N-isopropyl acrylamide-co-acrylic acid) microgel particles with single domain variable fragments (sdFv’s) were developed for use in intravenous hemorrhage in the case of injury in a rat model. The PLPs deformed like platelets during fibrin formation due to the low density of crosslinking [41]. Besides, the nanoparticles created by cholic-acid-mediated self-assembly of polyethyleneimine release various soluble biomolecules, including growth factors, coagulant factors and extracellular vesicles. Platelets can also evade the immune system, adhere to the subendothelial layer and interact with pathogens [42, 43]. Due to these properties, numerous platelet membrane-coated nanostructures have been developed as drug delivery methods. Numerous scientific groups around the world demonstrated that drug-containing silica or copolymer nanoparticles could be targeted with membranes using platelets extracted from the whole blood of humans. These platelet-like particles could be fabricated in the form of biomimetic nanocarriers [44, 45].
Drugs, polymers and technological advances that inhibit fibrinolysis or improve fibrin strength (and stability) can be of considerable therapeutic benefit. Tranexamic acid (TXA) is an US Food and Drug Administration (FDA) approved synthetic derivative of the amino acid lysine. TXA is responsible for downregulation of upregulated tissue plasminogen activator, fibrinolysis by inhibiting the lysine-binding sites on plasminogen, treats severe menstrual and postpartum bleeding, and trauma therapy. Several clinical research findings suggest that TXA is also linked to off-target systemic coagulopathy, thromboembolic consequences and neuropathy [46–50]. Nanomedicine-based platforms can resolve these obstacles by either (i) encapsulating TXA within the liposomal nanovesicles, (ii) actively targeted surface functionalization with a cyclo-CNPRGDY(OEt)RC peptide and (iii) using fibrinogen-mimetic peptide targeted on integrin GPIIb-IIIa for anchoring to active platelets within trauma-associated clots. Off-target effects can be avoided by utilizing targeted delivery of TXA to the traumatic injury site via liposomes, enabling its clot-stabilizing action to improve hemostasis and survival [32, 46].
Researchers recently recommended that super-hydrophobic or super-hydrophilic materials be used for hemostatic reasons. It has been observed that a super-hydrophobic graphene sponge quickly absorbs water from the blood, generating a thick layer of blood cells and platelets and thus increasing coagulation [51]. Spray-coated chitosan on the nanopores hollow-fibrous matting may also produce a hydrophilic hemostatic product, and the hydrophilic chitosan coating can improve blood surface properties and coagulation. Moreover, the fundamental operation of these techniques has been predicated on blood-absorbing (super-hydrophilic) hemostatic substances that reduce internal and peripheral bleeding or blood-repelling (super-hydrophobic) materials that simply resist blood but do not effectively trigger clot formation. Super-hydrophobic carbon nanotubes fibrous gauze were developed to overcome these problems, achieving fast clotting without blood loss [51]. These hemostatic material surfaces tend to detach from the clot following clot maturation, driven by the contractile tension in the clot contraction phase, allowing a natural and unforced removal of the hemostatic dressing without inducing subsequent hemorrhage. Topical patches or gauzes fabricated by micro/nanoparticles or fibers within bounded polymer networks prevent loose micro/nanoparticles or fibers from entering the vascular system [51]. These technologies have proven to be safe and compatible, can minimize microbial contamination and lower the chance of infection.
Hemophilia A is a bleeding disorder caused by hereditary coagulation factor VIII deficiency (FVIII). The conventional injectable clotting factor augmentation was formerly used two or three times per week to treat hemophilia, with low patient compliance, high costs and the formation of inhibitory antibodies following long-term treatment. Novel gene-based treatment has previously been explored and found to be a promising treatment for hemophilia. For example, recombinant FVIII (N8-GP) has an extended half-life, lesser reactivity and better hemostasis effectiveness. Some of those are appealing, new techniques with promising clinical uses, but they have certain efficacy and safety limitations. Because of individual variances in FVIII activity, the typical dose regimen of recombinant FVIII must be further formed to ensure the regulation of supplemental proteins in plasma for efficiently minimizing bleeding. However, in a recent study, asialoglycoprotein receptor ligand (N-acetylgalactosamine [GalNAc]-heparin cofactor II [HCII]) was conjugated to small interfering RNA, allowing for active targeting to the liver targeting the heparin cofactor II [52].
Polysaccharide materials for hemostasis
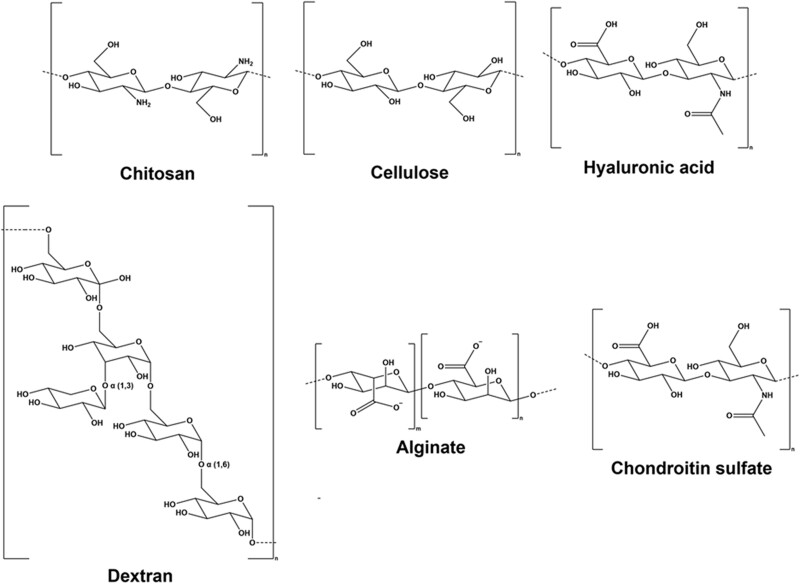
Polysaccharides are long-chain polymeric carbohydrates abundantly found in food and are an essential component of living matter. Various biomedical and tissue engineering applications utilize polysaccharides because of their structural and biochemical similarity to the extracellular matrix. They are known to be biodegradable, biocompatible and easily tunable to achieve desired mechanical properties and tissue response [53, 54]. The following section details various polysaccharides and their use as hemostatic materials. Figure 2 depicts the chemical structures of the polysaccharides discussed in this review, while Table 1 summarizes and highlights key references of polysaccharide and polypeptide-based hemostatic materials discussed in this review.
Figure 2.
The chemical structures of various polysaccharides discussed in this review article.
Table 1.
Summary of biopolymer-based hemostatic materials and their hemostasis time and blood loss in various in vivo animal models
| Biopolymer | Form | Animal model | Treatment | Hemostasis time | Blood loss | References |
|---|---|---|---|---|---|---|
| Chitosan (CTS) | Particle | Rat Liver Injury Model | PECs w/10 wt% CTS | 48 s | [67] | |
| PECs w/20 wt% CTS | 67 s | |||||
| PECs w/30 wt% CTS | 82 s | |||||
| Control: Gelatin sponge in powder form | 121 s | |||||
| Film | Rabbit Femoral Artery and Vein Model | CTS/Kaolin Composite | 4.9 ± 1.6 min | 3.5 ± 1.9 g | [68] | |
| Control: QuikClot Gauze | 26.1 ± 22.8 min | 25.1 ± 6.1 g | ||||
| Control: Gauze | 55.1 ± 13.8 min | 31.1 ± 9.7 g | ||||
| Rat Femoral Artery and Vein Model | CTS/Kaolin Composite | 3.8 ± 1.4 min | 1.3 ± 0.4 g | |||
| Control: QuikClot Gauze | 8 ± 1.9 min | 3.3 ± 0.7 g | ||||
| Control: Gauze | >10 min | 5.5 ± 1.4 g | ||||
| Particle | Rat Tail Amputation Model | CTS Microspheres | 214 ± 25 s | [69] | ||
| CTS Microspheres w/thin layer of Calcium Alginate | 161 ± 17 s | |||||
| Control: Gauze | 725 ± 21 s | |||||
| Rat Liver Laceration Model | CTS Microspheres | 107 ± 9 s | ||||
| CTS Microspheres w/thin layer of Calcium Alginate | 53 ± 10 s | |||||
| Control: Gauze | 238 ± 12 s | |||||
| Cellulose | Film | Rabbit Liver Model | Oxygen Regenerated Cellulose (ORC) film (oxidized 16 h) | 192 ± 30 s | 3.48 ± 0.82 g | [72] |
| ORC film 44 h | 178 ± 25 s | 2.46 ± 0.66 g | ||||
| ORC film 64 h | 205 ± 40 s | 3.06 ± 0.22 g | ||||
| ORC film 88 h | 233 ± 38 s | 3.66 ± 0.54 g | ||||
| Control: Gauze | 300 ± 40 s | 4.78 ± 0.53 g | ||||
| Sponge | Mouse Tail Amputation Model | 1 wt% Chitosan-Cellulose (CTS-Cel) Sponge | 67 s | 95 mg* | [80] | |
| Control: Gelatin Sponge | 159 s | 170 mg* | ||||
| Control: Gauze | 168 s | 150 mg* | ||||
| Control: No treatment | 320* | 210 mg* | ||||
| Rat Liver Trauma Model | 1 wt% CTS-Cel Sponge | 89 s | 400 mg* | |||
| Control: Gelatin Sponge | 131 s | 600 mg* | ||||
| Control: Gauze | 172 s | 700 mg* | ||||
| Control: No treatment | 225 s* | 900 mg* | ||||
| Rat Leg Artery Model | 1 wt% CTS-Cel Sponge | 105 s | ||||
| Control: Gelatin Sponge | 372 s | |||||
| Control: Gauze | 486 s | |||||
| Control: No treatment | 840 s* | |||||
| Dextran | Sponge | NZ White Rabbit Marginal Vein Cut | Dextran-derived PDA Sponge | 54 ± 6.0 s | 0.11 ± 0.14 g | [84] |
| Control: Celox | >420 s | 2.65 ± 0.36 g | ||||
| NZ White Rabbit Femoral Artery Cut | Dextran-derived PDA Sponge | <120 s | 4.6 ± 0.55 g | |||
| Control: Celox | >180 s | 0.1 ± 0.24 g | ||||
| Hydrogel | Rat Liver Hemorrhaging Model | 0.5 wt% Chitosan/Oxidized Dextran | 53 mg | [87] | ||
| 1.0 wt% Chitosan/Oxidized Dextran | 15 mg | |||||
| 0.5 wt% Chitosan | 136 mg | |||||
| Sponge | Rat Liver Hemorrhaging Model | Dextran-derived sponge | 59.2 ± 3.8 s | 0.52 g | [89] | |
| Control: Celox | 108.7 ± 8.0 s | 3.8 g | ||||
| Control: Gauze | 128 ± 8.0 s | 4.7 g | ||||
| Alginate | Microspheres | Porcine Liver Punch | Thrombin-loaded Alginate-Calcium Microspheres | 1.5 ± 1 min | [96] | |
| Thrombin-loaded Whole Blood | >10 min | |||||
| Hydrogel | Rat Liver Hemorrhaging Model | Pept-1 (cell adhesive peptide) and Alginate | 38 mg | [97] | ||
| Control: No treatment | 208 mg | |||||
| Sponge | Mice Liver Hemorrhaging Model | Compressed Bi-Layer Alginate Sponge | 18.3 mg | [95] | ||
| Uncompressed Bi-Layer Alginate Sponge | 232.4 mg | |||||
| Control: TachoSil® | 10.6 mg | |||||
| Control: Surgicel® | 19.0 mg | |||||
| HA | Hydrogel | Rat Liver Hemorrhaging Model | Self-crosslinking Gelatin Hydrogel | 194.7 ± 140.5 mg | [100] | |
| HA/Gelatin Hydrogel | 120.4 ± 149.5 mg | |||||
| Control: Fibrin Glue | 119.1 ± 77.6 mg | |||||
| Control: No Treatment | 233.8 ± 181.4 mg | |||||
| Chondroitin Sulfate (CS) | Powder | Porcine Liver Punch | CS, Collagen and Thrombin | 0.46 g/min | [108] | |
| Gelatin-Thrombin matrix with smooth particles | 0.14 g/min | |||||
| Hydrogel | Mouse Liver Hemorrhage Model | CS–Serotonin Hydrogel | 14.2 ± 0.8 mg | [109] | ||
| Control: Chitosan-gelatin hemostatic agent | 31.0 ± 7.7 mg | |||||
| Control: No treatment | 69.2 ± 11 mg | |||||
| Gelatin | Sponge | Dog spleen bleeding model | 2-layer Gelatin sheet | 104 ± 115 s | [116] | |
| Control: TachoSil® | 277 ± 117 s | |||||
| Sponge | NZ white rabbit ear bleeding model | Gelatin nanofiber sponge | 112 ± 19 s | 199 ± 32 mg | [148] | |
| Control: TachoSil® | 133 ± 12 s | 285 ± 34 mg | ||||
| Gelatin nanofiber membrane | 169 ± 20 s | 318 ± 41 mg | ||||
| Control: Gauze | 266 ± 25 s | 668 ± 108 mg | ||||
| NZ white rabbit liver bleeding model | Gelatin nanofiber sponge | 99 ± 11 s | 131 ± 21 mg | |||
| Control: TachoSil® | 128 ± 10 s | 226 ± 27 mg | ||||
| Gelatin nanofiber membrane | 147 ± 13 s | 238 ± 40 mg | ||||
| Control: Gauze | 237 ± 25 s | 420 ± 91 mg | ||||
| Cryogel | Rabbit liver defect hemorrhage | 25 wt% Gelatin, 8 wt% Dopamine | 83 s | 1.2 g | [149] | |
| Control: Hemostatic sponge | 222 s | 4 g ± 1.5 g | ||||
| Swine subclavian artery transection | 25 wt% Gelatin, 8 wt% Dopamine | 5.8 ± 1.4 min | 193 ± 81 ml | |||
| Control: Gauze | 25.4 ± 7.7 min | 487 ± 142 ml | ||||
| Particle | Rabbit Liver incision | Porous Gelatin microspheres (PGMs) | 95.7 s | [118] | ||
| Control: Chitosan Hemostasis Powder (CHP) | 113 s | |||||
| Control: Yunnan Baiyao | 130 s | |||||
| Rabbit Ear incision | Gelatin microspheres w/surgical gauze | 45.3 s | ||||
| Control: CHP | 52.3 s | |||||
| Control: Yunnan Baiyao | 86.3 s | |||||
| Fibrin | Hydrogel | Human Vascular Surgery | 3 ml fibrin sealant | 3 min: 46.4% of patients; 4 min: 62.7%; | [121] | |
| 5 min: 74.5%; | ||||||
| 7 min: 100% | ||||||
| Control: Manual compression | 3 min: 26.3% of patients; 4 min: 31.6%; | |||||
| 5 min: 49.1%; | ||||||
| 10 min: 100% | ||||||
| Foam | Rabbit Liver Partial Resection | Fibrin Foam | 50 ± 10 ml | [150] | ||
| Control: No treatment | 122 ± 11.5 ml | |||||
| Film | Swine Spleen Incision | Fibrin patch | 3 min: 86% success rate; 10 min: 100% | [124] | ||
| Control: TachoSil® | 3 min: 0% success rate; 10 min: 4% | |||||
| Keratin | Hydrogel | Rat Intracranial hemorrhage model (rebleeding) | 0.2 U collagenase, then Keratin hydrogel | 23.05 mm3 ± 9.67 mm3 | [151] | |
| Control: 0.2 U collagenase | 122.09 mm3 ± 25.25 mm3 | |||||
| 0.4 U collagenase, then keratin hydrogel | 42.09 mm3 ± 7.81 mm3 | |||||
| Control: 0.4 U collagenase | 170.46 mm3 ± 25.25 mm3 | |||||
| 0.6 U collagenase, then keratin hydrogel | 60.87 mm3 ± 16.43 mm3 | |||||
| Control: 0.6 U collagenase | 231.86 mm3 ± 32.28 mm3 | |||||
| Film | Rat liver puncture model | Full-length Keratin + PCL sheet | 69 s | 459 mg | [127] | |
| Rod domain Keratin + PCL sheet | 60 s | 368 mg | ||||
| Alpha helical Keratin + PCL sheet | 41 s | 308 mg | ||||
| PCL nanofiber sheet | 155 s | 671 mg | ||||
| Control: Gauze | 168 s | 856 mg | ||||
| Silk Fibroin (SF) | Powder | Murine hepatic injury model | LMSF powder | 120.6 ± 23.7 s | 0.39 ± 0.04 g | [133] |
| Control: No treatment | 300 s | 0.73 ± 0.04 g | ||||
| Control: Arista® | 110.5 ± 30.1 s | 0.37 ± 0.05 g | ||||
| Control: Surgicel® | 102.8 ± 22.5 s | 0.32 ± 0.04 g | ||||
| Sponge | Rabbit liver trauma model | SF-PEG sponge | 136.17 ± 62.27 s | 2.16 ± 1.27 g | [134] | |
| Control: No treatment | 557.75 ± 42.38 s | 7.92 ± 0.8 g | ||||
| Control: Gelatin sponge | 249.83 ± 29.18 s | 4.97 ± 1.44 g | ||||
| 3D scaffold | Rabbit ear artery hemorrhage model | TEMPO-oxidized cellulose nanofiber-5 wt% SF scaffolds with thrombin (TOCN-SF5-Th) | 110 ± 5 s* | [135] | ||
| TEMPO-oxidized cellulose nanofiber with thrombin (TOCN-Th) | 225 ± 5 s* | |||||
| Rat-tail amputation model | TOCN-SF5-Th | 133 ± 14 s | 0.59 ± 0.01 g | |||
| TOCN-Th | 300 ± 6 s | 2.3 ± 0.08 g | ||||
| Control: Floseal® | 120 ± 6 s | 0.45 ± 0.08 g | ||||
| Rat liver avulsion model | TOCN-SF5-Th | 140 ± 5 s | 0.84 ± 0.08 g | |||
| TOCN-Th | 370 ± 6 s | 2.0 ± 0.08 g | ||||
| Control: Floseal® | 140 ± 6 s | 0.75 ± 0.08 g | ||||
| 3D scaffold | Rat tail truncation model | Tannic acid-SF with diclofenac potassium | 160 ± 72 s | 0.06 ± 0.03 g | [132] | |
| Control: Gauze | 680 ± 60 s | 0.58 ± 0.10 g | ||||
| Engineered Polypeptides | Spongy film | Murine liver trauma model | Fusion protein 96R | 15.85 ± 1.21 s | [136] | |
| Control: RADA-16 lyophilized on gauze | 14.44 ± 1.33 s | |||||
| Control: No treatment | 37.00 ± 1.75 s | |||||
| Hydrogel | Rat liver trauma model | RADA16-1 | <20 s | [137] | ||
| Control: No treatment | >180 s | |||||
| Hydrogel | Mice Liver Hemorrhaging Model | R-Gel-4 | 14 ± 4 s | [139] | ||
| V-Gel-4 | 18 ± 2 s | |||||
| Control: Fibrin glue | 64 ± 10 s | |||||
| Control: No treatment | 170 ± 10 s | |||||
| Bio-glue | Pig heart model | Cationic supercharged polypeptides (SUP) glue | 75 ± 5 s* | [141] | ||
| Control: Histoacryl® | 110 ± 40 s* | |||||
| Pig liver model | SUP glue | 45 ± 10 s* | ||||
| Control: Histoacryl® | 100 ± 25 s* | |||||
| Pig kidney model | SUP glue | 30 ± 12 s* | ||||
| Control: Histoacryl® | 35 ± 10 s* | |||||
| Hydrogel | Mouse liver bleeding model | Methacrylated elastin-like polypeptides | 55.3 mg | [140] | ||
| Control: No treatment | 214.5 mg | |||||
| L-DOPA | High viscosity solution | Murine liver trauma model | PPDAC-PPDAL | 0.36 ± 0.13 g | [142] | |
| Control: No treatment | 1.72 ± 0.63 g | |||||
| High viscosity solution | Hemorrhaging liver rat model | BPEDAC-BPEDAL | 0.54 ± 0.11 g | [143] | ||
| Control: No treatment | 1.76 ± 0.56 g |
Data extrapolated from figures.
Chitosan-based materials
Chitin deacetylation is the primary source of chitosan synthesis. Strong alkali solutions are employed at both room and increased temperatures to remove N-acetyl groups during the deacetylation process. Chitosan is a heteropolymer chain with β (1 → 4) linked D-glucosamine and N-acetyl-D-glucosamine residues with <50% of N-acetyl-D-glucosamine units. It is a natural, non-immunogenic, biodegradable, non-toxic and mucoadhesive polysaccharide that has been investigated for use in a variety of biomedical applications [55, 56]. Chitosan is accessible for several recognized processing procedures due to its solubility in dilute acids and has been utilized to make films, gels and porous sponge-like scaffolds. Chitosan is insoluble in water at pH values >6.5 because it is a cationic polyelectrolyte with a pKa of 6.5. When coupled with a weak base-like glycerol phosphate, chitosan can generate an injectable thermogel [57]. At room temperature and physiological pH, this system stayed in solution. But when heated to physiological temperatures, it transforms into a gel, resulting in heat-induced gelation.
Due to its hemostatic and antibacterial qualities, chitosan has shown promise as a wound dressing material. The hemostasis activity is determined by a dynamic balance between anticoagulant and coagulating substances in the blood and blood vessels [58]. Recently, a chitin-based hemostatic agent was employed by Jorgensen et al. [59] for critical treatment during hemorrhage in an open wound. It has been reported that chitosan can shorten in vitro blood clotting time by 40% compared to blood alone [60]. Cationic chitosan contains coagulant characteristics, allowing red blood cells to have more active sites and activating platelets, which aids in developing a fibrin clot to stop bleeding [61]. Chitosan causes clotting due to the positive charge of the amino groups on the molecule, which interacts with the negative charge of red blood cell membranes [62]. A higher level of deacetylation results in a higher positive charge, which positively promotes coagulation [63]. The amount of coagulation has also been influenced by the type of chitosan employed, whether a solid or a solution. There was a dose-related clotting response, with increasing chitosan concentrations causing more coagulation [64]. Since 2003, chitosan-based hemostatic dressings have been utilized to treat injuries in military and civilian emergency response contexts, with encouraging results [65, 66]. In 27 of 34 cases in an emergency medical context, a chitosan dressing reduced hemorrhage within 3 min of administration. Twenty-one percent (7 of 34 incidents) of the failures were due to user error, which may have been avoided with better training and product design [65].
More recently researchers have combined the advantages of chitosan with other materials to enhance their hemostatic properties. For example, Chen et al. [67] prepared polyelectrolyte complexes (PECs) made up of chitosan oligosaccharide (COS) and carboxymethyl starch as absorbable hemostatic agents. They observed that PECs made up of 10 wt% COS improved hemostasis in a rat liver bleeding model, but the hemostasis efficacy reduced as the COS content increased. In a recent study, Elsabahy and Hamad [68] designed and evaluated chitosan/kaolin hemostatic dressings and utilized a surfactant to enhance the even distribution of kaolin throughout the chitosan fibers. They demonstrated their chitosan/kaolin dressings improved hemostasis and survival rates compared to QuikClot® in both a rat and rabbit bleeding models. Wu et al. [69] prepared microspheres with a core made up of porous chitosan and a compact shell made up of alginate and demonstrated that this combinational microsphere promoted blood clot formation and was more effective as a hemostat compared to porous chitosan microspheres alone in both a rat tail amputation model and a rat liver bleeding model.
Cellulose-based materials
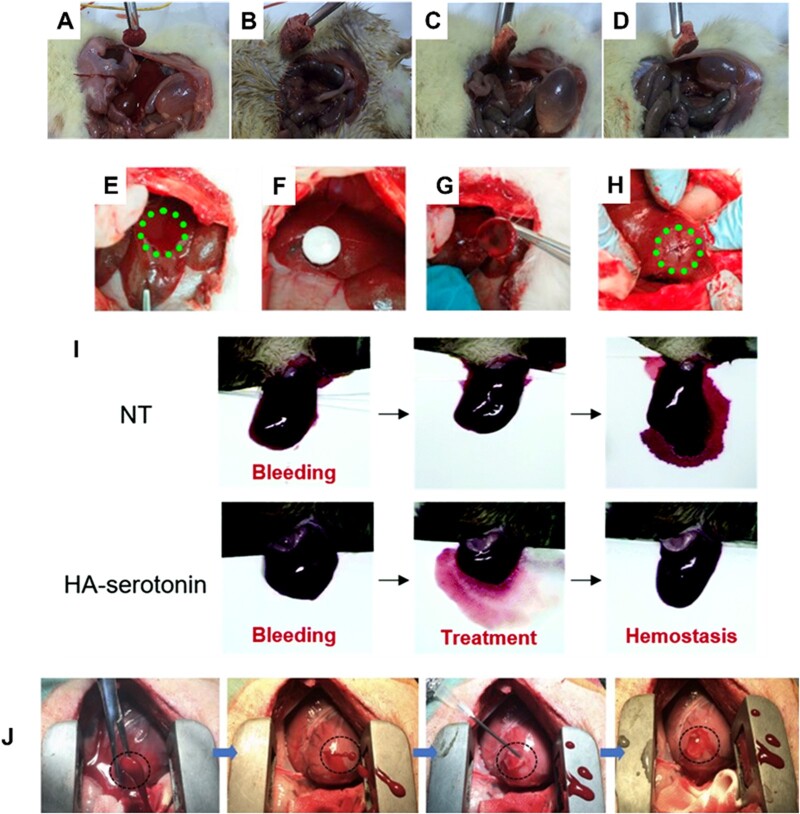
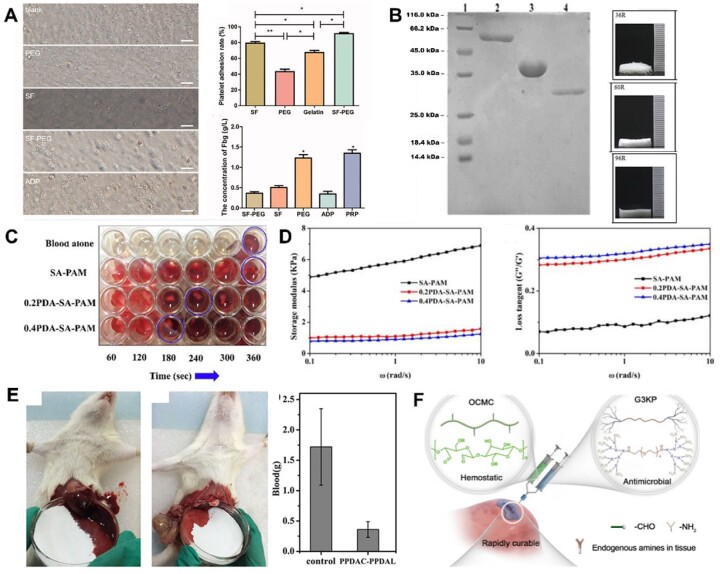
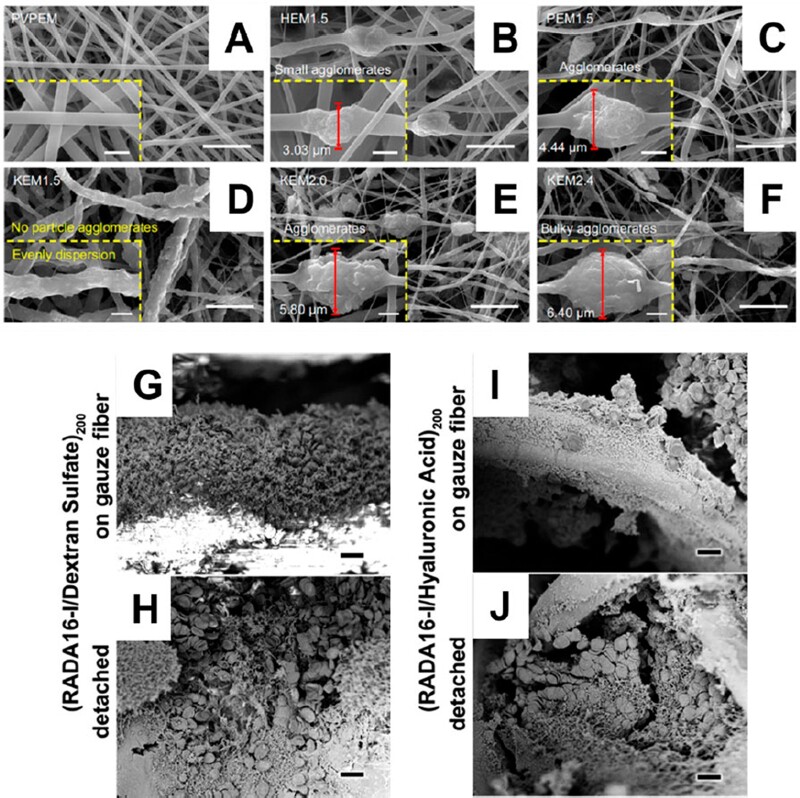
Cellulose is a D-glucopyranose homopolysaccharide that contains a linear chain of D-glucose and is the most common organic polymer on Earth. Structurally, it is an essential part of the primary cell wall of green plants and many forms of algae, oomycetes and some bacteria biofilms [70, 71]. Due to its excellent biocompatibility, biodegradability, low costs and abundance, cellulose and its derivatives are commonly used as absorbable devices in wound dressings and hemostatic products [72–75]. Cellulose oxide (OC) is a cellulose derivative that is mainly investigated for hemostatic applications due to its ability to absorb liquids and traps platelets and erythrocytes quickly, which leads to an increase in the concentration of clotting factors, accelerates the clotting process when applied at the bleeding sites and facilitates the fibrin clots formation and block blood flow [76]. In the meantime, the carboxyl groups of OC triggers the coagulation cascade by self-activation of coagulation factor XII. It should be noted that, although OC has been extensively studied in hemostasis, its clinical application is significantly limited due to the acidic pH of various carboxyl groups [77]. To address such limitations, researchers have modified the structure of OC to improve its hemostatic applications. One of the popular methods to modify OC is adding other polysaccharides to enhance the hemostatic and improve the limitations of OC. For example, He et al. [78] coated chitosan on the surface of OC gauze and showed a significant improvement in OC hemostatic properties compared to traditional OC gauze. In another study, a bilayer wound dressing was prepared by Karahaliloğlu et al. [79] using chitosan and bacterial cellulose in the top layer, and (SF) on the bottom of the dressing. Their results indicated that applying such a platform on top of a bleeding area can quickly prevent bleeding by absorbing a lot of liquid from blood due owing to the bacterial cellulose and chitosan parts. Moreover, the top layer of SF can promptly cause platelet adhesion, which effectively showed a hemostatic effect in vitro and in vivo (Fig. 3A–D). In a more recent study, Fan et al. [80] prepared cellulose-based porous hemostatic sponges using surfactants and pore-forming agents. In comparison to traditional gauze and gelatin sponge, their cellulose-chitosan sponges showed improved hemostasis in several in vivo animal models, including mouse tail amputation model, as well as a rat liver trauma model and rat leg artery trauma model.
Figure 3.
In vivo hemostasis studies using polysaccharide-based hemostats. (A) Standard gauze, (B) SF-coated bacterial cellulose/chitosan, (C) SF-coated Vit K/bacterial cellulose/chitosan, (D) SF-coated protamine sulfate/bacterial cellulose/chitosan applied to the bleeding site in a diabetic rat femoral artery model. Images adapted with permission from Karahaliloglu et al. [79], (E) creation of liver injury in the exposed left medial lobe of a New Zealand rabbit, (F) treatment of the injury site with aldehyde dextran (PDA) sponges. Hemostasis was maintained after removing the PDA sponge (G) and even after squeezing the wound (H). Images adapted with permission from Liu et al. [84]. (I) Liver hemorrhage model of factor VIII-deficient hemophilia mice with no treatment (NT), and HA-serotonin hemostatic adhesives. Image adapted with permission from An et al. [99]. (J) Six-millimeter cardiac puncture injury in pig hearts followed by treatment with methacrylated HA show rapid hemostasis and sealing following UV-induced polymerization. Image adapted with permission from Hong et al. [101].
Dextran-based materials
Dextran is another polysaccharide made up of a 1,6-linked D-glucopyranose residue. Dextran has many hydroxyl groups in its structure, facilitating chemical modification and the ability to add other functional groups. In addition, it has a high capacity to absorb water, making dextran a promising candidate for hemostatic application, specifically as a tissue adhesive agent [81]. Usually, sodium periodate (NaIO4) is used to oxidize the hydroxyl groups of dextran to change them to aldehyde groups. Amino groups of tissue proteins and other biomaterials can chemically crosslink with the aldehyde groups in dextran. This phenomenon produces strong adhesion to tissues, making them useful as tissue sealants [82]. However, the number and density of aldehyde groups in oxidized dextran are significant. It can affect local inflammation and systemic tissue toxicity when the oxidized dextran binds to tissues due to the tissue-material adhesion force [83]. Thus, the oxidation degree of dextran should be tuned based on its toxicity and adhesion. For example, Liu et al. [84] proposed a new aldehyde dextran sponge with improved water absorption and adhesive properties (Fig. 3E–H). The sponge showed quick blood absorption, powerful tissue adhesion and efficient hemostasis in a rabbit model. Furthermore, the aldehyde dextran sponge could facilitate wound blocking and cell aggregation and easily trigger blood coagulation without needing coagulation cascade activation.
In another study, Artzi et al. [85, 86] synthesized a sealant composed of star-shaped PEG-NH2 and dextran aldehyde with various molecular weights and degrees of aldehyde oxidation. Their results indicated that such materials could efficiently attach to tissue and prevent bleeding. Similarly, Du et al. [87] used modified chitosan and oxidized dextran to develop a novel hydrogel dressing, which demonstrated multifunctional activities to improve hemorrhagic and infected wound therapy in a rat hemorrhaging liver. Although oxidized dextran has been extensively investigated as sealants and tissue adhesives, the imine bond formation is an unstable equilibrium reaction in aqueous solutions. To address this limitation, Wang et al. [88] prepared a tissue glue made up of aldehyde dextran and gelatin, and incorporated 2-isocyanoethyl methacrylate into the backbone of dextran hydrogel to make the imine binding more stable. This modification improved the mechanical strength and stability of the hydrogels by significantly increasing the degree of crosslinking, which formed a dense intermolecular network. Most recently, Liu et al. [89] developed poly aldehyde dextran sponges loaded with montmorillonite powder (a natural silicate) for hemorrhage control. Their results suggest that their dextran-based hemostatic sponge exhibited good tissue adhesion, antibacterial activity against Escherichia coli and improved wound healing as well.
Alginate-based materials
Alginate is a naturally derived polysaccharide from alga made up of L-glucuronic acid and D-mannuronic acid monomers. Alginate has been used for various biomedical applications due to its good biocompatibility, biodegradability and ability to quickly form a hydrogel when exposed to calcium or divalent ions [90–92]. However, when calcium alginate hydrogel comes in contact with blood, the calcium ions can exchange with sodium ions, making the gel lose. This exchange speeds up platelet aggregation and triggers the coagulation process, as the calcium ion works as a cofactor in the coagulation cascade [93]. In addition, calcium alginate can absorb high percentages of water, making it possible for calcium alginate gels to attach to the injury site and show an excellent hemostatic property. Shi et al. [94] synthesized carboxymethyl chitosan, sodium alginate and collagen composite microspheres and showed that their composites are biodegradable and facilitate platelet adherence, aggregation and activation in vitro. A systematic study by Singh Chandel et al. [95] evaluated the effect of sponge compression on bilayer alginate sponges prepared by lyophilization on hemostasis and antiadhesion. Their results indicated that their 100 µm compressed sponge demonstrated improved hemostasis in a mice liver bleeding model, while their 200 µm compressed sponge showed enhanced antiadhesion in a hepatectomy-induced adhesion model in rats.
In addition to fast gelation, alginate's easy drug loading capability has attracted researchers to investigate alginate in various fields. For example, Rong et al. [96] prepared thrombin-loaded calcium alginate microspheres prepared via emulsion/crosslinking technique and investigated the delivery of their hemostatic agent in an in vivo bleeding model. In another study, Zhai et al. [97] investigated a coassembly peptide and alginate system, which showed attractive cell adhesions and effective hemostasis. The proposed peptide-alginate hydrogel efficiently prevented bleeding in an in vivo mice model and reduced blood volume loss by 18% compared to the untreated group. Besides, the histology from a mouse full-thickness skin defect model indicated that the proposed peptide-alginate enhanced fibroblast migration to the injury site and facilitated wound healing.
Hyaluronic acid-based materials
Hyaluronic acid (HA), commonly found throughout connective, epithelial and neural tissues, is another naturally derived polysaccharide made up of D-glucuronic acid and N-acetyl-D-glucosamine units. Due to its outstanding water maintenance and inherent swelling property, HA can facilitate cell adhesion and migration, making it one of the most suitable polysaccharides for wound healing as it facilities collagen production from wound surfaces via the fibroblast proliferation effect [98]. A study by An et al. [99] proposed serotonin-conjugated HA hydrogel systems as a new class of hemostatic adhesives. They determined that serotonin can boost hemostasis, and their hydrogels showed improved hemostatic ability in regular and hemophilic lesions in a rat model compared to commercially available fibrinolytic, making them good candidates for hemorrhage control (Fig. 3I) [99].
In another study, Luo et al. [100] synthesized two types of injectable self-crosslinking gels using gelatin and HA, and investigated them for use in hemorrhage control. Their HA-gelatin hydrogels had excellent stability, low cytotoxicity, beneficial burst strength and remarkable hemostatic ability compared to commercial fibrin glue. Hong et al. [101] developed methacrylated HA hemostatic hydrogels as a strongly adhesive for arterial and cardiac injury applications (Fig. 3J). The proposed material can rapidly gel upon UV light irradiation and form hydrogels that can adhere to and seal the bleeding arteries and heart walls, withstanding up to 290 mmHg blood pressure. Meanwhile, their results indicated that the treated heart pig model could survive for several days after incision, suggesting that their hemostatic hydrogel would be suitable for use as a traumatic wound sealant.
Chondroitin sulfate-based materials
Chondroitin sulfate (CS) is the most prevalent glycosaminoglycan in the body, composed of repeating units of glucuronic acid and galactosamine. It is present in various structural proteoglycans in multiple tissues, including skin, cartilage, tendons, heart valves and the central nervous system. CS is a biodegradable, anti-inflammatory, antioxidant, anticancer, anticoagulant and anti-thrombogenic anionic heteropolysaccharide [102]. CS has several critical functions, including resistance to compression forces and activation of crucial pathways involved in vascular healing. CS can regulate cellular processes, such as cell motility and receptor binding. It also has anti-apoptotic and antioxidant properties and plays a crucial role in immunological responses, such as growth factor activity modulation and leukocyte recruitment. Furthermore, CS-based hydrogels have a high wound healing potential, and cellular biological activity [103, 104].
Moreover, due to its affinity for CD 44 receptors and glycosylation enzymes on the surface of tumor cells and intracellular organelles, it also inherits the potential to target active and subcellular targets [105, 106]. CS degrades in the presence of physiological stimuli; it could be a viable biomaterial for the delivery of biopharmaceuticals and stimuli-sensitive delivery systems like tumor-targeted delivery [107]. For individuals with a high risk of bleeding, CS has the potential to be a successful treatment [105, 106].
Slezak et al. [108] developed an active powdered hemostatic product of swine collagen, bovine CS and plasma-derived human thrombin that can be applied directly to the bleeding site. Compared to flowable agents, powdered hemostatic agents can be applied directly over large surface areas when the source of bleeding is unknown. However, their efficacy is limited to little bleeds. Zhang et al. [109] developed a highly biocompatible serotonin-conjugated CS hydrogel and demonstrated improved hemostasis and wound healing abilities in an in vivo model.
Polypeptide materials for hemostasis
Polypeptides are continuous, unbranched chains of amino acids linked by peptide bonds that connect amine and carboxyl groups of adjacent amino acids to make an amide bond. Polypeptides are crucial building blocks for designing biomaterials because of their capacity to form well-defined secondary structures (i.e. -helix and -sheets). These secondary structures play an essential role in polypeptide chain self-assembly, resulting in unique supramolecular structures [110]. Furthermore, they can have a range of reactive functional groups (carboxylic acids, hydroxyl, amino and thiol groups) on their side chains, which can be easily chemically modified [111]. The most significant limitation of peptide-based polymers is their limited number of building blocks. They are limited to the 20 natural amino acids compared to synthetic polymers, which can be made from many monomers [112]. On the other hand, these biomaterials are in high demand due to several advantages. Short peptide motifs like RGD, which are abundant ligands for cell receptors and regulate various cell activities, such as attachment and spreading, may be attached to or embedded more easily in polypeptide materials than synthetic materials [113].
Furthermore, because many peptide-based polymers are readily degradable by the body, they are good candidates as biomaterials. Moreover, peptide self-assembly has recently attracted scientific attention as a potential method for producing functional biomaterials [114]. Due to their adhesive properties, polypeptide biomaterials have also been reported as hemostatic materials, tissue adhesives and wound healing applications. This section highlights the advantageous properties of various adhesive materials derived from polypeptide compounds in hemostasis.
Gelatin-based materials
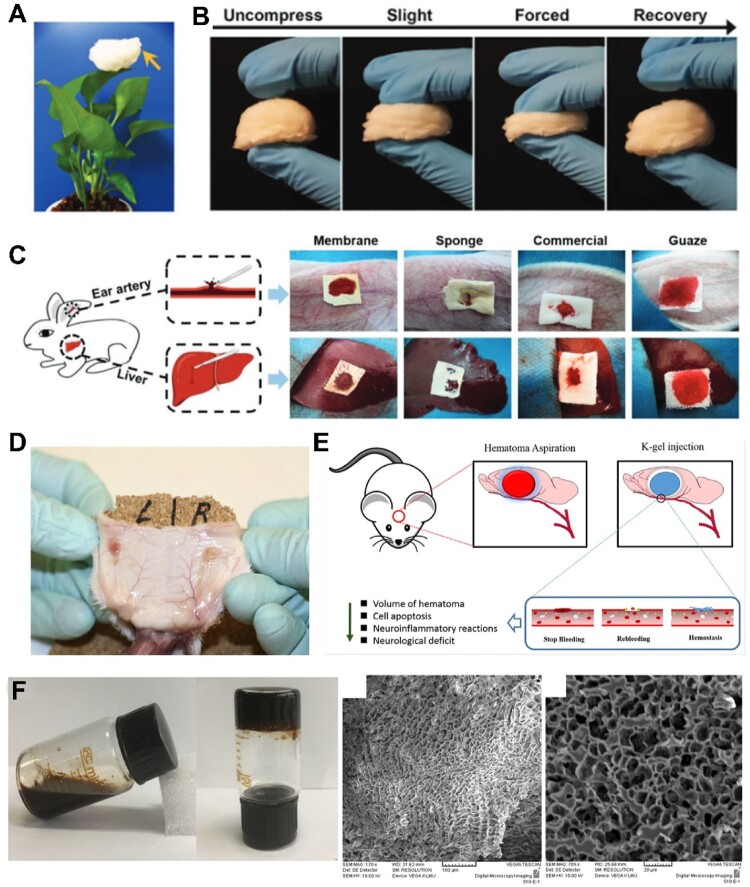
Gelatin is a denatured form of collagen and is known to possess optimal properties as a material for various biomedical applications, including hemorrhage control [115]. The hemostatic effect of gelatin is attributed to the swelling of the biopolymer upon contact with blood as well as activation and aggregation of platelets which in turn accelerates blood coagulation [116]. However, gelatin suffers from poor mechanical properties at physiological temperatures. Therefore, it is typically combined with other materials, such as levodopa (L-DOPA), or nanosilicates, such as Laponite. Xie et al. [117] developed an electrospinning method to create an ultralight nanofiber gelatin sponge with a porous topology and large surface area (Fig. 4A–C). According to the researchers, the nanofiber sponge, made up of gelatin aggregates, activated many platelets, promoting platelet embolism and escalated coagulation pathways. Furthermore, in vivo studies demonstrated the ability of these gelatin sponges to generate stable blood clots in a short amount of time and exhibited negligible blood loss. Li et al. [118] prepared porous gelatin microspheres using a water-in-oil emulsion method, followed by glutaraldehyde crosslinking, and lyophilizing after freezing in liquid nitrogen. Their experiments demonstrated the use of their microspheres as hemostatic agents in both in vitro and in vivo studies compared to hemostatic powder controls.
Figure 4.
(A) Ultralight gelatin nanofiber sponge. (B) Compressibility and recovery properties of the gelatin nanofiber sponge. (C) In vivo rabbit model evaluation of the hemostatic capacity of the ultralight gelatin sponge in comparison to sponge, gauze and commercial product. Image adapted with permission from Xie et al. [117]. (D) Fibrin pad (subcutaneously implanted; on the left), shows good vascularization after implantation day 14 compared to the control on the right. Image adapted with permission from Harmon et al. [123]. (E) Human hair hemostatic keratin hydrogels for the treatment of intracerebral hemorrhage were tested in the rabbit model. (F) Keratin solution and hydrogel showing gelation properties, scanning electron microscope (SEM) images of the gels. Images adapted from He et al. [128].
Fibrin-based materials
After sustaining an injury, the blood coagulation pathway gets activated, and a fibrin clot forms to prevent bleeding. Fibrin generates a fibrous matrix with important biomechanical characteristics, such as extensibility, stiffness, resistance to rupture, and optimal elastic and viscous characteristics, making it suitable for hemostatic applications [119, 120]. Fibrin sealants are typically composed of human-derived fibrinogen and thrombin, which help to increase coagulation factors and activate blood clotting [24, 121, 122]. To prevent blood loss, the sealants also create a barrier for sealing. Several commercially available fibrin sealants are FDA approved for use in surgical procedures to achieve hemostasis. These include Tisseel and Artiss from Baxter, Evicel and Vistaseal from Johnson & Johnson, and Vitagel from Orthovita [31]. Harmon et al. [123] developed a fibrin pad for hemostasis for surgical interventions with good healing properties (Fig. 4D). Their fibrin pad, composed of fibrinogen and thrombin-loaded matrix component, served as a hemostatic patch by delivering fibrin to the injury site. In another study, Matonick and Hammond [124] compared two FDA-approved fibrin-based sealant patches, EVAREST™ and TachoSil®, in a heparinized swine spleen incision model. Their results indicated that EVARREST™ outperformed TachoSil® in both hemostasis success rates and adhesion to wound tissue.
Keratin-based materials
Keratin is a family of proteins containing cysteine amino acids rich in sulfur. It is a fibrous biomaterial commonly found in hair, nails, wool, etc. Keratins are categorized as either hard or soft keratins, where the sulfur crosslinking contributes to the hardness of the polymer. They can also be classified as alpha and beta keratins, depending on the source of the keratin and the tertiary structure of the polymer (alpha helices or beta sheets) [125]. Keratin biomaterials are often used for drug delivery, bone regeneration and wound dressing. However, they recently attracted attention as hemostatic materials because they reduce clotting times and blood loss [126]. In addition, they cause polymerization of fibrinogen into fibrin which also contributes to hemostasis. In a comparative study by Wang et al. [127], they observed that the α-helical segment contents of keratin are directionally proportional to its hemostatic activity, while the Tyr, Phe and Gln amino acids at the N-termini of the α-helices in keratins play an important role in fibrin polymerization. He et al. [128] fabricated keratin-based hydrogels extracted from human hair, named K-gels (Fig. 4E and F). Their gels showed excellent biocompatibility, promising hemostatic efficacy and demonstrated the potential to relieve brain damage in vivo by preventing bleeding that may happen postoperatively.
Silk-based materials
SF is most commonly obtained from Bombyx mori cocoons [129] and was approved for sutures and surgical materials more than two decades ago by the FDA [130]. SF is made up of a heavy and a light chain bound together with disulfide bonds and can assume either an α-helical amorphous structure or more crystalline β sheets [131]. It can be processed into mats, foams, fibers or hydrogels, although it requires longer and more complicated processes than previously mentioned biopolymers. As a result of this, SF by itself is not of great interest for hemostasis and is often combined with other materials. Zhu et al. [132] demonstrated improved hemostasis and biocompatibility of SF hydrogels with tannic acid and diclofenac potassium in in vitro studies and a rat tail truncation model. Lei et al. [133], however, modified SF by hydrolysis to obtain low molecular weight SF (LMSF) and showed that LMSF activates the intrinsic pathway and decreases bleeding time and blood loss. On the other hand, Wei et al. [134] developed an SF-polyethylene (SF-PE) sponge gellable in aqueous physiological environments. While SF showed superior platelet adhesive properties, SF-PE sponges shortened hemostatic time and decreased blood loss compared to SF alone or gelatin commercial sponges (Fig. 5A). In another study, Shefa et al. [135] combined SF with nanocellulose and enhanced the hemostatic properties by loading sponges with thrombin. The addition of SF improved the sponge’s biocompatibility and blood absorption capacity, while thrombin enhanced its hemostasis.
Figure 5.
(A) Platelet adhesion and fibrinogen concentration in response to silk fibroin and SF-PEG sponges. Image adapted from Wei et al. [134]. (B) SDS-PAGE showing three hemostatic peptides and their sponges after purification. Image adapted from Yang et al. [136]. (C) PDA–sodium alginate–polyacrylamide (PDA–SA–PAM) hydrogel network showing accelerated clotting time [144]. (D) The addition of polyDOPA to SA–PAM structure improves rheological properties drastically. Images adapted from Suneetha et al. [144]. (E) Hemostatic capacity of the triblock DOPA peptide. Image adapted from Lu et al. [142]. (F) Hemostatic OCMC and antimicrobial G3KP polysaccharide-peptide dendrimers for rapid curing tissue adhesive-hemostat development. Image adapted from Zhu et al. [147].
Engineered polypeptides
In recent years, engineered self-assembling peptide materials have evoked interest in hemostatic biomaterials research. Yang et al. [136] developed RADA-16-based biomolecules with hemostatic effects. It was reported that the peptide can quickly self-assemble to form a layer of fibers that acts as a barrier to block hemorrhage in <15 s [137]. It has amphipathic properties that prevent bleeding wounds in the brain, liver, skin and spinal cord (Fig. 5B) [138]. Teng et al. [139] engineered glycopolypeptides using a poly-L-lysine backbone grafted with glucose and catechol groups crosslinked to form covalent hydrogels. These gels exhibited strong tissue adhesion and showed efficient hemostatic properties. In addition, they reported that their gels regenerated dermis and epidermis tissues holding potential as high-performance hemostats and wound dressings. Guo et al. [140] synthesized methacrylated lysine-rich elastin-like peptides (ELP-MA) with lower transition temperature and the ability to photocrosslink. Furthermore, their ELP-MA showed improved hemostasis in a mice liver bleeding model and functioned as elastic adhesives. Ma et al. [141] designed a family of supercharged polypeptide-based adhesives demonstrating strong adhesion to soft tissues, which outperformed some commercially available adhesive products. The supramolecular interactions between cationic supercharged polypeptides and anionic aromatic surfactant with lysine resulted in strong adhesion to tissues. In addition, their bio-glue showed robust in vitro and in vivo performance for hemostasis application and wound healing compared to surgical wound closures.
Mussel-inspired l-3,4-dihydroxyphenylalanine (L-DOPA) materials
When exposed to high humidity, marine mussels show strong adherence to surfaces [142]. These adhesive properties can be attributed to the amino-peptide compound L-DOPA containing catechol functional groups capable of forming strong covalent and non-covalent interactions and lysine near the interface [143]. As a result, the L-DOPA motif has been incorporated into many polymeric backbones to develop novel materials with adhesive and hemostatic properties. Furthermore, the amino and phenolic hydroxyl groups of DOPA also activate the coagulation system. Suneetha et al. [144] developed polydopamine–sodium alginate–polyacrylamide (PDA–SA–PAM) hydrogel networks with high porosity. The polydopamine chains in this hydrogel significantly improved the scaffold’s mechanical properties and contributed to faster coagulation (Fig. 5C and D).
Lu et al. [143] investigated a series of novel biomedical adhesive gels derived from L-DOPA. Their polymers demonstrated biocompatibility and good biodegradability as well as thermoresponsiveness. Furthermore, these polymers excelled in mechanical properties and in vitro adhesion tests without showing cytotoxicity. They also performed in vivo antibleeding studies where their polypeptide materials demonstrated superior hemostatic properties. In another study, Lu et al. [142] developed thermoresponsive polypeptide-pluronic-polypeptide triblock copolymers. These triblock copolymers had different functional side groups showing good biodegradability and biocompatibility and excellent hemostatic properties. The hemostatic properties were due to rapid polymerization and solidification of the polymer (Fig. 5E). Furthermore, they demonstrated that these glues had excellent wet adhesive properties. These results demonstrate the potential for these mussel-inspired materials to act as high-performance hemostatic materials.
Polysaccharide and polypeptide composite materials for hemostasis
Several studies have combined polysaccharides and polypeptides to mimic the natural extracellular matrix structure. These combinations have the advantage of adding the properties of both materials to improve their adhesiveness. For example, poly-lysine, which occurs naturally, is biodegradable and non-toxic. It also shows tissue adhesive properties stemming from the ionic interaction between the polymer and target tissues. Nie et al. [145] combined chitosan and poly-lysine, which showed high functionality at adhesion sites, excellent hemostatic properties when applied to a rat liver defect and proved to be a promising novel hemostatic material also functioning as a tissue sealant. Lu et al. [146] also took advantage of the properties of both polysaccharides and polypeptides by combining chitosan and the marine mussel-inspired L-DOPA peptide. They generated a series of chitosan-polypeptide polymers having different functional groups. These materials exhibited high adhesion strength and showed good hemostatic performance. Zhu et al. [147] described a fast and high-strength bioadhesive hydrogel based on polysaccharide and peptide dendrimers named OCMC/G3KP (Fig. 5F). Their hydrogels demonstrated excellent hemostatic properties with a 5-fold increase in adhesion strength compared to CoSeal, a commercially available bioadhesive.
Various forms of hemostat devices from biopolymers
Hemostasis is a biological process ensured by closely controlled coagulation pathways, including coordinated enzyme activities. In cases of extreme blood loss, the body's natural hemostasis mechanism cannot control bleeding; hence adjuvant methods for bleeding control are required. Generally, these hemostatic devices should be readily available, easily transportable, storable and applicable even in austere environments. Understanding the natural hemostasis process is critical in developing viable hemostat devices that mimic or stimulate the natural process [30]. During World War II, biopolymer-based hemostat devices received significant attention. They were immediately accepted by the medical community and were shown to be entirely safe for human use [152]. The ability of hemostatic materials to limit blood loss and biofilm formation is crucial to their clinical value. Materials including hydrogels, sponges, woven and non-woven fiber sheets, and powders have been widely used [153–155].
The primary objective of hemorrhage treatment is to stop bleeding and restore blood volume that is circulating within the body [156]. The likelihood of survival could very well be determined by the severity of the bleeding. Patients experiencing moderate hypotension due to bleeding may benefit from delayed massive fluid restoration until they reach a medical treatment facility that provides definitive care [157]. Generally, hemorrhage or bleeding can be categorized into three classes: arterial bleeding, venous bleeding and capillary bleeding [158]. Arterial bleeding occurs when there is a wound or incision to a major artery, resulting in rapid reduction of blood volume. Venous bleeding occurs when there is a wound or incision to a vein. Like arterial bleeding, blood loss from venous bleeding can be substantial and occur quickly without intervention. Finally, capillary bleeding occurs due to an incision or wound to capillaries with minimal blood loss which can be easily controlled. The key first aid treatment for all these types of bleeding is direct pressure over the wound followed by using different hemostasis agents to stop the bleeding [159].
In addition, hemorrhages can also be categorized into four classes based on various parameters, such as, blood loss, pulse rate, blood pressure, respiratory rate, urine output and central nervous system symptoms. The primary observation of the parameters, as seen in Table 2, may be helpful to medical personnel in providing the most appropriate treatment and medication to stop bleeding and increase the patient's chance of survival [156].
Table 2.
Classification of hemorrhage
| Parameter | Class |
|||
|---|---|---|---|---|
| I | II | III | IV | |
| Blood loss (ml) | <750 | 750–1500 | 1500–2000 | >2000 |
| Blood loss (%) | <15% | 15–30% | 30–40% | >40% |
| Pulse rate (beats/min) | <100 | >100 | >120 | >140 |
| Blood pressure | Normal | Decreased | Decreased | Decreased |
| Respiratory rate (breaths/min) | 14–20 | 20–30 | 30–40 | >35 |
| Urine output (ml/hour) | >30 | 20–30 | 5–15 | Negligible |
| CNS symptoms | Normal | Anxious | Confused | Lethargic |
The first biological-based hemostat devices, also called first-generation hemostat devices, were single-component products using thrombin, fibrin, collagen and cellulose. Among them, thrombin and fibrin were the first materials to be surveyed using the pioneering study developed by Hong and Loughlin [160] The most significant developments in the use of naturally occurring and synthetic polymers as hemostatic devices were recently reviewed by di Lena [161] While, Biswal [162] have surveyed the applications of biopolymers for tissue engineering, especially in hemostatic devices. Besides, functional tuning associated with specific hemostatic components (thrombin or fibrin) is mainly produced to achieve better hemostatic performance, particularly in malfunctioning the natural coagulation process [163].
Researchers have demonstrated a high interest in naturally generated biomaterials for various biomedical applications. Bose et al. [164] describe that biopolymers made up of proteins and cellulose have been used to stop bleeding for a long time. The primary goal of biopolymer hemostat devices is to reduce surgical complications by providing easy handling and optimal hemostatic capability [152, 165]. Furthermore, they should be non-stick and possess enough mechanical strength to withstand the bleeding pressure [166, 116]. The following section highlights various forms of biopolymer-based hemostatic devices.
Powder and particles
Powders and particles are a beneficial and practical form of hemostatic materials. They can halt blood loss rapidly, can be easily applied, have a long shelf life, can be stored in various temperature settings, and pose no risk of disease transmission to patients [167]. Generally, microparticulate hemostats, such as ChitoHem [168], Arista [169] and Quickclot [24], were developed because of their superior hemostatic activity and simplicity. Recently, some researchers have proposed nanoparticles and beads as hemostatic devices for rapid blood coagulation in several in vivo studies [170–172].
In terms of hemostatic material development, many materials show positive results due to their biocompatibility and simplicity [173]. Oxidized regenerated cellulose (ORC) is a natural-based polymer derived from chemical modification of cellulose. Due to its biocompatibility, biodegradability, low toxicity and low cost, among other characteristics, it has been widely used for many biomedical applications [163]. Hutchinson et al. [76] described that ORC could be utilized as a hemostat due to its exceptional behavior in stopping bleeding. Two different ORC powders with sodium or potassium were tested in vitro for bactericidal action by Basagaoglu Demirekin et al. [163]. Furthermore, the chemical characterization of ORC was carried out using the textile form of the compound and regenerated cellulose. However, due to the differences in material form, they were unable to be used in in vivo research due to safety concerns. The study aimed to determine whether they were effective as a hemostatic substance. These materials had bactericidal action because of the acidic features of ORC and biocompatibility and efficiently halt bleeding.
A study on QuikClot (a zeolite-based granular hemostatic substance) was undertaken by Pusateri et al. [9] in a pig liver laceration model to determine its effectiveness. Jegatheeswaran et al. [174] reported a swine liver laceration model was used to test the feasibility of a chitosan-based powder hemostat. They found no indication of an inflammatory response or heat damage but did find some evidence of sinusoidal congestion. Yang et al. [173] developed polysaccharide-based hemostatic materials with antimicrobial and healing properties. Sakoda et al. [175] presented a hydrogel hemostat consisting of hyaluronan (HA) coupled with inorganic polyphosphate as a treatment option for hemorrhage control. Aldehyde-modified HA and hydrazide-modified HA coupled with PolyP quickly generated HAX-PolyP. In a mouse liver bleeding model, HAX-PolyP had the same hemostatic effect as fibrin glue in accelerating the coagulation rate of human plasma ex vivo.
Panwar et al. [176] developed a biodegradable and biocompatible hemostatic device by conjugating carboxymethyl moiety to starch (CM-starch). They altered it with calcium ions (CaCM-starch) to generate free-flowing microparticles, which were injected into wounds. In vitro and in vivo research on hemostatic efficacy indicated CaCM-starch as a promising candidate for further clinical evaluation as a topical hemostat. Zhu et al. [177] reported a crosslinked starch microparticle to improve hemostasis during irregular surgical procedures.
Sponges and foams
Sponges are highly absorbent porous structures holding great promise in tissue engineering and regeneration [178–182]. Especially, their unique swelling/shrinkage properties render them desirable for hemostatic applications, where they can fill the wound cavity and absorb lots of blood [183]. Various studies have reported processing synthetic and naturally derived materials into sponges for hemorrhage control [164, 184–185]. Naturally derived materials have been increasingly attractive compared to synthetic materials due to their better biocompatibility, biodegradability and low/no toxicity [186, 187]. A great range of biopolymers and derivatives have been processed into hemostatic sponges, such as gelatin [148, 188], chitosan [185, 189, 190], cellulose [154, 183] and alginate [191].
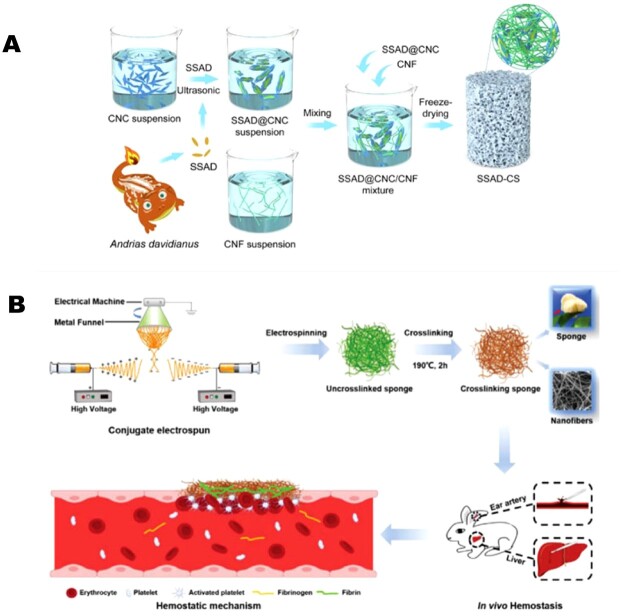
Zheng et al. [183] used skin secretion of Andrias davidianus (Chinese giant salamander), combined with cellulose nanocrystals and cellulose nanofibers to fabricate injectable hemostatic sponges that were shape-recoverable, elastic and had a high blood absorption ratio (Fig. 6A). In vitro performance of as-prepared sponges was assessed, with the results showing significant volumetric expansion (>11.54) and high water absorption ratios (up to 6276 ± 398%). The sponges outperformed the hemostatic effects of pure cellulose and gelatin sponges in vivo [183]. In another study, porous and hydrophilic composite sponges comprising cellulose and chitosan were fabricated with incredible mechanical resilience [184]. Incorporating chitosan in the sponges resulted in antibacterial efficacy against both gram-positive and gram-negative bacteria. These composite sponges with high water absorption capability could better facilitate coagulation compared to commercial gauze and gelatin sponges, in vivo, and showed rapid hemostasis in the rat liver bleeding model.
Figure 6.
Examples of biopolymer-based hemostatic sponges. (A) SSAD (skin secretion of Andrias davidianus)-enabled cellulose hemostatic sponges. Image adapted with permission from Zheng et al. [183] Copyright 2021, Elsevier. (B) Demonstration of fabrication, blood clotting and the hemostatic mechanism of 3D gelatin nanofiber-based sponges. Image adapted with permission from Xie et al. [148] Copyright 2021, Wiley.
SF has attracted much attention recently because of its tunable mechanical properties and great processibility [192–195]. As a result, SF-PEG sponges have been explored [134]. The authors proposed that SF itself could impact the hemostatic process by activating platelet aggregation and adhesion. Mixing with PEG could further accelerate the hemostatic process because PEG-SF structural networks serve as a physical barrier to the bleeding area [134]. Another example was reported by Teuschl et al. [196] where they used SF as a carrier for delivering fibrinogen and thrombin to control blood loss. The silk sponges showed excellent mechanical robustness as the physical barrier. The delivery of these coagulation proteins facilitated the clotting of hemorrhaging wounds under dysregulated thrombin and fibrinogen levels and illustrated the promising role of SF as the carrier for various bioactive agents for biomedical applications [196].
Gelatin has been adopted for hemostatic applications due to its ability to cause platelet aggregation [149]. Xie et al. [148] reported a conjugate electrospinning method to engineer 3D gelatin-based hemostatic sponges (Fig. 6B). The resulting sponges have interconnected nanofiber networks, porous structure and a high surface area, with excellent hemostatic capability. In vitro assessments of blood coagulation were performed and revealed that the 3D microstructured nanofibers could trap blood to shorten the bleeding time. Compared to traditional gauze and commercial hemostatic agents and membranes, the proposed sponges demonstrated great in vitro blood-clotting ability with a blood-clotting index of 21.9 ± 4.4% and blood-clotting time of about 180 ± 15 s. Compared to the commercial gelatin sponge (133 ± 12 s), the membrane (169 ± 20 s) and gauze (266 ± 25 s), the sponge showed a faster clotting efficiency of 112 ± 19 s in a rabbit ear artery injury model. The mechanism can result from a great surface area and highly porous structure inside the sponges, and these factors benefit the quick absorbance of wound exudes, blood cells and platelets [148].
Regarding clinical translation, sponges for the control of bleeding during surgery are commercially available and accepted for medical usage [187]. Pfizer has marketed Gelfoam®, a hemostatic sponge made from porcine skin gelatin and used as an FDA-approved medical device for bleeding surfaces [197]. Similarly, Spongostan™ gelatin sponge features resorbable, malleable and water-insoluble properties, which prevent blood loss from the wound cavity [198]. Another example is developed by Ethicon, Surgifoam® for medical applications of stopping the bleeding with the sponge-like highly absorbable properties [199].
Although there has been a great selection of sponge/foam-like hemostatic materials for bleeding control, some shortcomings associated with their in vivo applications need to be addressed in the fabrication of the next generation of hemostatic sponges. For instance, the degradation time of some of the commercially available absorbable sponges, such as those fibrin-based and gelatin-based hemostats, is very long-ranging from a few weeks to 12 months. Such a long biodegradation time may interfere with wound healing, resulting in scar formation or enhanced inflammatory responses [200]. In addition, some hemostatic sponges cannot withstand blood flow and may relocate, resulting in unwanted off-target adhesion to the surrounding tissues [201]. Furthermore, several pathological abnormalities, such as fibrotic and necrotic tissues associated with long-term implantation of hemostatic sponges, are reported in the literature [201, 202]. Another common observation regarding hemostatic sponges is infection after implantation [200]. Therefore, engineering of hemostatic sponges with antibacterial properties is preferred. According to the shortcomings mentioned above, their usage conditions should be well-understood to avoid unnecessary danger in clinical applications.
Films and sheets
Increasing demands for innovative approaches are necessary to avoid excessive bleeding in different cases, including surgery, battlefield injuries and accident cases [1, 203–206]. Most successful hemostasis depends on patients' clotting capabilities. As a result, the medication efficacy may vary, or hemostatic medications sometimes fail [207]. While considering the different forms of hemostats or coagulant-loaded hemostats like hydrogel, foams, particles and sheets to stop the bleeding in the injury site [116, 150, 208, 209]. Hemostatic sheets or pads are a great candidate for ease of use and large-scale preparation without any laborious procedures [116]. In this section, we summarize the significance of hemostatic pads or sheets for hemorrhage control in different ways. Generally, hemostatic sheets are prepared with different types of natural or synthetic polymers [210]. They can be engineered in various forms, such as film sheets, nanofiber sheets and layer-by-layer approaches [211–213]. Topical hemostats like hemostatic sheets offer an advantage over other types of hemostats when controlling bleeding with vessel occlusion pressure or ligature methods is complicated, as is the case with wounds from which blood continuously seeps [211–213].
Due to their unique features, like absorption and adhesiveness, gelatin, chitosan and other naturally abundant biopolymers have been widely employed as hemostatic dressings [214]. Li et al. [211] prepared chitosan and gelatin films by solvent casting to create ibuprofen-loaded composite films to maintain sterility and control the bleeding in the surgical site. Tensile strength and elongation at break were influenced by the amount of chitosan in the composite films. The glutaraldehyde crosslinking presumably increased moisture vapor transfer and composite film swelling. Ibuprofen-loaded chitosan/gelatin composite films outperformed the control in antibacterial activity tests against E. coli and Staphylococcus aureus. Furthermore, according to the assessment of the hemostatic effects, they could reduce bleeding in a surgical operation with low pressure and had good absorption properties.
Alginate has gained popularity as a hemostatic material due to its exceptional properties, such as wound adherence and water absorption [215, 216]. Yunnan Baiyao, a well-known herbal prescription used in oriental countries for over a century, has been shown to be safe and effective as a surgical sealant and hemostat [217]. Lu et al. [218] recently reported Yunnan Baiyao incorporated chitosan/alginate composite films for rapid coagulation in a rat liver hemorrhage model. Their results suggested that compared to controls, Yunnan Baiyao functioned synergistically with chitosan and sodium alginate to induce stronger hemostasis.
Recently, bioactive materials have gained much attention in bone regeneration and wound healing [219–221]. Among them, mesoporous bioactive glass (MBG) is a new class of material that offers consistent nanoscale mesoporous structure with high-specific surface areas and good biological activity [221]. Recently, Jia et al. [222] prepared an MBG/chitosan hemostatic film via solvent casting, followed by freeze-drying. The MBG/chitosan films showed good water adsorption and were tuned by changing the MBG/chitosan composite ratio during preparation. Different MBG/chitosan ratio composite films demonstrated varying hemostatic effects in a rat hepatic hemorrhage model. The hemostatic time and volume of bleeding decreased with MBG. The composite films were easily degradable in vitro, were biocompatible and non-cytotoxic, indicating that MBG/chitosan composite porous films function as a novel hemostatic material compared to previous hemostatic materials.
Sometimes technically improved hemostats are necessary to enhance the efficacy and functions [116, 223]. For example, Takagi et al. [116] recently reported a gelatin-based two-layer sheet composed of gelatin sponge and gelatin sheet as a topical agent to stop bleeding. They compared their material with commercially available hemostats TachoSil® made by collagen and loaded with clotting molecules like thrombin and fibrinogen. Their results showed that the two-layer gelatin sheet was a better topical hemostatic agent than TachoSil® with a lesser danger of viral transmission and inflammation than topical treatments containing fibrin components and/or thrombin. Their two-layer gelatin sheets showed excellent hemostatic efficacy in bleeding dog spleens (an organ where bleeding is difficult to control) and other organs, such as the liver, kidney, lung and arteries. Lately, microneedle technology has shown great promise in various applications, including drug and cell delivery [224, 225]. Inspired by that, Yokoyama et al. [223] developed a hemostatic sheet based on their microneedle technology. In addition, their microneedle hemostatic sheet can be potentially used in intracorporeal topical hemostasis for parenchymatous organs, major arteries and the heart wall following trauma or surgery.
Nanofibrous sheets exhibit similar morphology to the natural extracellular matrix, with varying pore size distribution and large surface areas [212, 62]. Therefore, nanofibrous sheet hemostats can be a great candidate compared to film-based hemostats. Generally, nanofibrous sheets are prepared using different techniques, like electrospinning, and self-assembly [226, 227]. Among them, electrospun nanofibrous sheets have gained much attention due to their simplicity and large-scale production [226]. For example, Cui et al. [228] recently reported a nanoclay-based polyvinyl pyrrolidine (PVP) hemostatic nanofiber membranes (Fig. 7A–F). The nanoclay electrospun membrane (NEM) with Kaolinite (KEM) showed 60% higher hemostatic performance than other NEMs in both in vitro and in vivo models. The influence of extracellular matrix microstructure and nanoclay (60%) played a pivotal role in reducing clotting time, suggesting that KEM bandages are a good candidate for compressible hemorrhage management. In another study, Gu et al. [62] reported electrospun-chitosan/gelatin nanofibrous sheets with improved pore size and surface area via ultrasonic treatment. Both hydrophilic gelatin and ultrasonication had synergetic effects on hemostatic activities, such as quick blood absorption and coagulation in vitro. Furthermore, it also encouraged active cell growth, migration and invasion. These results suggested that sonicated chitosan/gelatin nanofiber mats could be used as a hemostatic dressing and tissue engineering scaffold.
Figure 7.
SEM images of fiber sheet and film hemostats. (A) PVP electrospun membranes, (B) halloysite electrospun membranes (1.5 g nanoclay), (C) palygorskite electrospun membranes (1.5 g nanoclay), (D) kaolinite electrospun membrane (1.5 g nanoclay), (E) kaolinite electrospun membrane (2.0 g nanoclay) and (F) kaolinite electrospun membrane (2.4 g nanoclay). The insets are the corresponding 5× magnification of the images (scale bar = 2 µm). Images adapted with permission from Cui et al. [228]. SEM images of anticoagulated whole blood in contact with (RADA16-I/dextran sulfate) 200 films (G and H) and (RADA16/HA) 200 films (I and J) show interaction of the films with the blood components (scale bar = 10 µm). Images adapted with permission from Hsu et al. [213].
In severe cases, hemostatic substances in the form of fibers and non-woven sheets can be applied and withdrawn quickly from the bleeding site [229]. Fiber and non-woven dressings have excellent permeability and an extremely porous microstructure and have been widely used for wiping blood from surgical sites. Interestingly, a biodegradable and biocompatible polycaprolactone (PCL) with chitosan fibrous sheet was prepared using electrospinning and modified drop-casting [212]. The chitosan/PCL nanofibrous sheets were stronger than chitosan/PCL blended nanofibers and just the chitosan membrane alone. Moreover, they reported that adding tree tea oil to the chitosan overlayer of chitosan/PCL dressing increased platelet aggregation, antibacterial and anti-inflammatory characteristics.
ORC is an absorbable hemostatic material used in surgery to control low-pressure and diffuse bleeding [230]. He et al. [78] developed a water-soluble chitosan/ORC composite gauze produced using an N, O-carboxymethyl chitosan (N, O-chitosan)-modified version of ORC gauze. N, O-chitosan/ORC composite gauze had excellent hemostatic properties in a rabbit liver bleeding model. Furthermore, its antibacterial effectiveness increased as the N, O-chitosan percentage increased. These findings demonstrated that N, O-chitosan/ORC composite gauze could be an effective hemostatic device and adhesion prevention tool after surgery.
In another study, Jaiswal et al. [231] reported the hemostatic efficacy of PCL/gelatin nanofibrous matrix in rat livers. The time to stop bleeding, prothrombin time and fibrinogen concentration were all measured, and showed that the electrospun sheets caused fast hemostasis in all test animals. They also examined the livers of both treated and untreated mice and found that the test group's liver wounds healed faster than the control group. The nanofibrous matrix showed potential for liver regeneration and safety and efficacy as a hemostat.
Similarly, Li et al. [232] reported curcumin-loaded mesoporous silica incorporated antibacterial PVP nanofibers. The engineered mats were tested for structure, biocompatibility, antibacterial activity and hemostatic impact in an in vivo liver injury model. A loading ratio of <8% CCM-MSN in PVP electrospun nanofibers was found to be homogenous. The hybrid nanofiber mats were non-cytotoxic and more effective against methicillin-resistant S. aureus (MRSA) in vitro, which was validated via in vivo tests. When exposed to blood, the hybrid nanofiber mats quickly change into hydrogels, activating the clotting system to stop the wound from bleeding. With their excellent biocompatibility and antibacterial activity, CCM-MSN integrated PVP nanofiber mats are a suitable option as a hemostatic material.
The Hammond group has developed thin-film coatings on gauze and gelatin sponges using layer-by-layer technology to function as hemostatic devices [213, 233]. These nanofibers elute RADA-16 peptide upon hydration and form nanofiber-based clots when they come in contact with blood (Fig. 7G–J). Moreover, compared to ordinary gauze, these nanofiber-based films from gauze bandages accelerated hemostasis in porcine skin lesions. Due to RADA-16 peptide's potent hemostatic activity, biocompatibility, biodegradability and low production costs, their strategy is a promising cheap yet effective hemostatic bandage.
Conclusions and future perspectives
In this review, we first describe the coagulation cascade, which involves numerous pathways to stop bleeding, followed by a discussion of the effects of clotting factors in hemostasis. Next, we summarize the biopolymer-based hemostats covering major polysaccharides- and polypeptides-based materials for hemostatic applications fabricated into various engineered forms, such as powder, sponges, sheets and hydrogels.
From a materials design point of view, chemically modified biopolymer-based hemostats with strong adhesion to tissue will need to be developed for passive hemostasis, without activating the coagulation process or causing systemic embolism and thrombosis. Compared to synthetic polymers, the mechanical properties of biopolymer-based hemostatic agents are weak. Therefore, developing advanced biofabrication techniques will be necessary to engineer biopolymers into clinically effective and mechanically robust hemostatic materials. For example, structurally reinforced foams or aerogels could be designed to help pressure dissipation at the wound site for non-compressible hemorrhages. Moreover, the addition of bioactive hemostatic additives, such as silica nanoparticles, into the biopolymer-based substrates has also been reported to trigger the coagulation cascade to improve the hemostatic ability of materials.
In terms of clinical translation, surgeons are demanding the development of high-quality hemostats with rapid blood clotting capacity with ease of use under any conditions. Therefore, researchers and developers in the field of hemostats and sealants, as well as adhesives, are continually motivated by the desires of surgeons for improved tools that will make it easier for them to perform technically challenging operations. In the case of topical injuries, several research efforts have been reported on various natural and synthetic biomaterials. Specifically, most hemostats exhibit different forms, including adhesive, band power, foams and gels. Table 3 shows the biopolymer-based hemostats on the market and are currently in National Institutes of Health (NIH) clinical trials.
Table 3.
Biopolymer-based hemostats currently on the market and in National Institutes of Health (NIH) clinical trials
| Product name | Main component(s) | Form of hemostat | Company/sponsor | Phase | References |
|---|---|---|---|---|---|
| Floseal® | Gelatin matrix with thrombin | Gel Sealant | Baxter | On-market | [234,235] |
| Surgicel® | ORC | Powder | Ethicon | On-market | [236] |
| Surgifoam® | Gelatin | Sponge/powder | Johnson and Johnson | On-market | [237, 238] |
| Celox Granules | Chitosan | Powder | Celox Medical | On-market | [239, 220] |
| Spongostan™ | Gelatin | Sponge-like dressing | Ethicon | On-market | [240] |
| BaneCel® | Oxidized cellulose | Gel Sealant | Unicare Biomedical | On-market | [241] |
| HemCon® Bandage PRO | Chitosan | Sponge-like dressing | Tricol Biomedical | On-market | [242] |
| Avitene™ | Collagen | Sheet/sponge | BD | On-market | [243] |
| Gelfoam® | Gelatin | Sponge | Pfizer | On-market | [244] |
| Tachosil® | Fibrin | Patch | Baxter | On-market | [245, 246] |
| Surgiflo® | Gelatin matrix with thrombin | Gel Sealant | Ethicon | On-market | [234, 247] |
| PeproStat | Recombinant human albumin (rHA) conjugated with fibrinogen-binding peptides | Liquid | Haemostatix Ltd | Phase 2 (NCT03131336) | [248] |
| Evarrest® | Fibrin | Patch | Ethicon | Phase 3 (NCT03255174) | [249] |
| Thrombi-Gel® | Thrombin-JMI (bovine-derived), carboxylmethylcellulose | Sponge/pad | Vascular Solutions LLC | Phase 4 (NCT00652314) | [250, 251] |
| Gelatin | |||||
| HEMOCOLLAGENE® | Collagen | Sponge | Septodont | N/A | [252] |
| (NCT05171231) |
Overall, challenges remain in developing next-generation biopolymer-based hemostatic agents. Scientists with a background in materials sciences, engineering, biology, clinics and even electronics engineering, need to join forces in exerting the potential to advance naturally derived materials into desired hemostats. Furthermore, intelligent skin-interfaced electronics is another emerging topic that should be explored to achieve smart monitoring of the coagulation process and responsive therapeutics to the wound sites at the same time.
Acknowledgements
The authors would like to acknowledge funding from the National Institute of Health (5R01HL137193). They would also like to thank Emily Torres and Ramon Morales for assisting with the graphical abstract, and Junjie Chen and Pierce Newman with organizing our literature review.
Conflicts of interest statement. None declared.
Contributor Information
Marvin Mecwan, Terasaki Institute for Biomedical Innovation, Los Angeles, CA 90064, USA.
Jinghang Li, Terasaki Institute for Biomedical Innovation, Los Angeles, CA 90064, USA.
Natashya Falcone, Terasaki Institute for Biomedical Innovation, Los Angeles, CA 90064, USA.
Menekse Ermis, Terasaki Institute for Biomedical Innovation, Los Angeles, CA 90064, USA.
Emily Torres, Terasaki Institute for Biomedical Innovation, Los Angeles, CA 90064, USA; Department of Molecular and Cell Biology, University of California, Berkeley, CA 94720, USA.
Ramon Morales, Terasaki Institute for Biomedical Innovation, Los Angeles, CA 90064, USA; Department of Biomedical Engineering, University of Southern California, Los Angeles, CA 90089, USA.
Alireza Hassani, Terasaki Institute for Biomedical Innovation, Los Angeles, CA 90064, USA.
Reihaneh Haghniaz, Terasaki Institute for Biomedical Innovation, Los Angeles, CA 90064, USA.
Kalpana Mandal, Terasaki Institute for Biomedical Innovation, Los Angeles, CA 90064, USA.
Saurabh Sharma, Terasaki Institute for Biomedical Innovation, Los Angeles, CA 90064, USA.
Surjendu Maity, Terasaki Institute for Biomedical Innovation, Los Angeles, CA 90064, USA.
Fatemeh Zehtabi, Terasaki Institute for Biomedical Innovation, Los Angeles, CA 90064, USA.
Behnam Zamanian, Terasaki Institute for Biomedical Innovation, Los Angeles, CA 90064, USA.
Rondinelli Herculano, Terasaki Institute for Biomedical Innovation, Los Angeles, CA 90064, USA; Bioengineering & Biomaterials Group, School of Pharmaceutical Sciences, São Paulo State University (UNESP), Araraquara, SP 14800-903, Brazil.
Mohsen Akbari, Terasaki Institute for Biomedical Innovation, Los Angeles, CA 90064, USA; Department of Mechanical Engineering, University of Victoria, Victoria, BC V8P 5C2, Canada; Biotechnology Center, Silesian University of Technology, Gliwice 44-100, Poland.
Johnson V. John, Terasaki Institute for Biomedical Innovation, Los Angeles, CA 90064, USA.
Ali Khademhosseini, Terasaki Institute for Biomedical Innovation, Los Angeles, CA 90064, USA.
References
- 1. Kauvar DS, Lefering R, Wade CE.. Impact of hemorrhage on trauma outcome: an overview of epidemiology, clinical presentations, and therapeutic considerations. J Trauma 2006;60:S3–11. [DOI] [PubMed] [Google Scholar]
- 2. Donley ER, Loyd JW.. Hemorrhage Control. In: StatPearls. Treasure Island, FL: StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC, 2022. https://www.ncbi.nlm.nih.gov/books/NBK535393/ (15 August 2022, date last accessed). [Google Scholar]
- 3. Hu Z, Lu S, Cheng Y, Kong S, Li S, Li C, Yang L.. Investigation of the effects of molecular parameters on the hemostatic properties of chitosan. Molecules 2018;23:3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sung YK, Lee DR, Chung DJ.. Advances in the development of hemostatic biomaterials for medical application. Biomater Res 2021;25:37. doi: 10.1186/s40824-021-00239-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boon GD. An overview of hemostasis. Toxicol Pathol 1993;21:170–9. [DOI] [PubMed] [Google Scholar]
- 6. Peng HT. Hemostatic agents for prehospital hemorrhage control: a narrative review. Mil Med Res 2020;7:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Peng T. Biomaterials for hemorrhage control. Trends Biomater Artif Organs 2010;24:27–68. [Google Scholar]
- 8. Neuffer MC, McDivitt J, Rose D, King K, Cloonan CC, Vayer JS.. Hemostatic dressings for the first responder: a review. Mil Med 2004;169:716–20. [DOI] [PubMed] [Google Scholar]
- 9. Pusateri AE, Delgado AV, Dick EJJ, Martinez RS, Holcomb JB, Ryan KL.. Application of a granular mineral-based hemostatic agent (QuikClot) to reduce blood loss after grade V liver injury in swine. J Trauma 2004;57:555–62. [DOI] [PubMed] [Google Scholar]
- 10. Chiara O, Cimbanassi S, Bellanova G, Chiarugi M, Mingoli A, Olivero G, Ribaldi S, Tugnoli G, Basilicò S, Bindi F.. A systematic review on the use of topical hemostats in trauma and emergency surgery. BMC Surg 2018;18:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Grissom TE, Fang R.. Topical hemostatic agents and dressings in the prehospital setting. Curr Opin Anaesthesiol 2015;28:210–6. [DOI] [PubMed] [Google Scholar]
- 12. Salibi A, Barabas A.. The ‘pole and tent’ pressure dressing. Emerg Med J 2015;32:254. [DOI] [PubMed] [Google Scholar]
- 13. Ozer MT, Eryilmaz M, Coskun K, Demirbas S, Uzar AI, Kozak O.. A new method for hepatic resection and hemostasis: absorbable plaque and suture. Eurasian J Med 2010;42:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vinjirayer A, Jefferson P, Ball D.. Securing central venous catheters: a comparison of sutures with staples. Emerg Med J 2004;21:582–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kabiri M, Emami SH, Rafinia M, Tahriri M.. Preparation and characterization of absorbable hemostat crosslinked gelatin sponges for surgical applications. Curr Appl Phys 2011;11:457–61. [Google Scholar]
- 16. Khoshmohabat H, Paydar S, Kazemi HM, Dalfardi B.. Overview of agents used for emergency hemostasis. Trauma Mon 2016;21:e26023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shkatula YV, Badion YO, Novikov M.. Efficiency of different methods of temporary external hemostasis at the pre-hospital stage of emergency medical care. Новости хирургии 2020;28:688–93. [Google Scholar]
- 18. LaPelusa A, Dave HD.. Physiology, hemostasis. In: StatPearls. Treasure Island, FL: StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC, 2022. https://www.ncbi.nlm.nih.gov/books/NBK545263/ (15 August 2022, date last accessed). [Google Scholar]
- 19. Garmo C, Bajwa T, Burns B.. Physiology, clotting mechanism. In: StatPearls. Treasure Island, FL: StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC, 2022. https://www.ncbi.nlm.nih.gov/books/NBK507795/ (15 August 2022, date last accessed). [PubMed] [Google Scholar]
- 20. Stroud RM, Kossiakoff AA, Chambers JL.. Mechanisms of zymogen activation. Annu Rev Biophys Bioeng 1977;6:177–93. [DOI] [PubMed] [Google Scholar]
- 21. Monroe DM, Hoffman M.. What does it take to make the perfect clot? Arterioscler Thromb Vasc Biol 2006;26:41–8. [DOI] [PubMed] [Google Scholar]
- 22. Palta S, Saroa R, Palta A.. Overview of the coagulation system. Indian J Anaesth 2014;58:515–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Barmore W, Bajwa T, Burns B.. Biochemistry, clotting factors. In: StatPearls. Treasure Island, FL: StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC, 2022. https://www.ncbi.nlm.nih.gov/books/NBK507850/ (15 August 2022, date last accessed). [PubMed] [Google Scholar]
- 24. Zhong Y, Hu H, Min N, Wei Y, Li X, Li X.. Application and outlook of topical hemostatic materials: a narrative review. Ann Transl Med 2021;9:577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Owens AP 3rd, Mackman N.. Tissue factor and thrombosis: the clot starts here. Thromb Haemost 2010;104:432–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chaudhry R, Usama SM, Babiker HM.. Physiology, coagulation pathways. In: StatPearls. Treasure Island, FL: StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC, 2022. https://www.ncbi.nlm.nih.gov/books/NBK482253/ (15 August 2022, date last accessed). [PubMed] [Google Scholar]
- 27. Kuchinka J, Willems C, Telyshev DV, Groth T.. Control of blood coagulation by hemocompatible material surfaces—a review. Bioengineering 2021;8:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bombeli T, Spahn DR.. Updates in perioperative coagulation: physiology and management of thromboembolism and haemorrhage. Br J Anaesth 2004;93:275–87. [DOI] [PubMed] [Google Scholar]
- 29. Loof T, Deicke C, Medina E.. The role of coagulation/fibrinolysis during Streptococcus pyogenes infection. Front Cell Infect Microbiol 2014;4:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang L, Zhong Y, Qian C, Yang D, Nie J, Ma G.. A natural polymer-based porous sponge with capillary-mimicking microchannels for rapid hemostasis. Acta Biomater 2020;114:193–205. [DOI] [PubMed] [Google Scholar]
- 31. Spotnitz WD. Fibrin sealant: past, present, and future: a brief review. World J Surg 2010;34:632–4. [DOI] [PubMed] [Google Scholar]
- 32. Chan LW, White NJ, Pun SH.. Synthetic strategies for engineering intravenous hemostats. Bioconjug Chem 2015;26:1224–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Blombäck B, Carlsson K, Fatah K, Hessel B, Procyk R.. Fibrin in human plasma: gel architectures governed by rate and nature of fibrinogen activation. Thromb Res 1994;75:521–38. [DOI] [PubMed] [Google Scholar]
- 34. Lorand L, Parameswaran Kumarapuram N, Murthy SNP.. A double-headed Gly-Pro-Arg-Pro ligand mimics the functions of the E domain of fibrin for promoting the end-to-end crosslinking of γ chains by factor XIIIa. Proc Natl Acad Sci USA 1998;95:537–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Everse SJ, Spraggon G, Veerapandian L, Riley M, Doolittle RF.. Crystal structure of fragment double-D from human fibrin with two different bound ligands. Biochemistry 1998;37:8637–42. [DOI] [PubMed] [Google Scholar]
- 36. Soon AS, Lee CS, Barker TH.. Modulation of fibrin matrix properties via knob: hole affinity interactions using peptide-PEG conjugates. Biomaterials 2011;32:4406–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Stabenfeldt SE, Aboujamous NM, Soon AS, Barker TH.. A new direction for anticoagulants: inhibiting fibrin assembly with PEGylated fibrin knob mimics. Biotechnol Bioeng 2011;108:2424–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chan LW, Wang X, Wei H, Pozzo LD, White NJ, Pun SH.. A synthetic fibrin cross-linking polymer for modulating clot properties and inducing hemostasis. Sci Transl Med 2015;7:277ra29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kolodziej AF, Nair SA, Graham P, McMurry TJ, Ladner RC, Wescott C, Sexton DJ, Caravan P.. Fibrin specific peptides derived by phage display: characterization of peptides and conjugates for imaging. Bioconjug Chem 2012;23:548–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kolodziej AF, Zhang Z, Overoye-Chan K, Jacques V, Caravan P.. Peptide optimization and conjugation strategies in the development of molecularly targeted magnetic resonance imaging contrast agents. Methods Mol Biol 2014;1088:185–211. [DOI] [PubMed] [Google Scholar]
- 41. Brown AC, Stabenfeldt SE, Ahn B, Hannan RT, Dhada KS, Herman ES, Stefanelli V, Guzzetta N, Alexeev A, Lam WA, Lyon LA, Barker TH.. Ultrasoft microgels displaying emergent platelet-like behaviours. Nat Mater 2014;13:1108–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Estevez B, Du X.. New concepts and mechanisms of platelet activation signaling. Physiology (Bethesda) 2017;32:162–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nenna A, Nappi F, Lusini M, Satriano UM, Schilirò D, Spadaccio C, Chello M.. Effect of statins on platelet activation and function: from molecular pathways to clinical effects. Biomed Res Int 2021;2021:6661847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, Langer R.. Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov 2021;20:101–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sun Y, Su J, Liu G, Chen J, Zhang X, Zhang R, Jiang M, Qiu M.. Advances of blood cell-based drug delivery systems. Eur J Pharm Sci 2017;96:115–28. [DOI] [PubMed] [Google Scholar]
- 46. Girish A, Hickman DA, Banerjee A, Luc N, Ma Y, Miyazawa K, Sekhon UDS, Sun M, Huang S, Sen Gupta A.. Trauma-targeted delivery of tranexamic acid improves hemostasis and survival in rat liver hemorrhage model. J Thromb Haemost 2019;17:1632–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Roberts I, Shakur H, Coats T, Hunt B, Balogun E, Barnetson L, Cook L, Kawahara T, Perel P, Prieto-Merino D, Ramos M, Cairns J, Guerriero C.. The CRASH-2 trial: a randomised controlled trial and economic evaluation of the effects of tranexamic acid on death, vascular occlusive events and transfusion requirement in bleeding trauma patients. Health Technol Assess 2013;17:1–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Morrison JJ, Dubose JJ, Rasmussen TE, Midwinter MJ.. Military Application of Tranexamic Acid in Trauma Emergency Resuscitation (MATTERs) study. Arch Surg 2012;147:113–9. [DOI] [PubMed] [Google Scholar]
- 49. Brown JB, Neal MD, Guyette FX, Peitzman AB, Billiar TR, Zuckerbraun BS, Sperry JL.. Design of the study of tranexamic acid during air medical prehospital transport (STAAMP) trial: addressing the knowledge gaps. Prehosp Emerg Care 2015;19:79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ng W, Jerath A, Wąsowicz M.. Tranexamic acid: a clinical review. Anaesthesiol Intensive Ther 2015;47:339–50. [DOI] [PubMed] [Google Scholar]
- 51. Li Z, Milionis A, Zheng Y, Yee M, Codispoti L, Tan F, Poulikakos D, Yap CH.. Superhydrophobic hemostatic nanofiber composites for fast clotting and minimal adhesion. Nat Commun 2019;10:5562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lin W-Y, Zhu R, Zhang Z, Lu X, Wang H, He W, Hu Y, Tang L.. RNAi targeting heparin cofactor II promotes hemostasis in hemophilia A. Mol Ther Nucleic Acids 2021;24:658–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wasupalli GK, Verma D.. 3 - Polysaccharides as biomaterials. In: Thomas S, Balakrishnan P, Sreekala MS (eds). Fundamental Biomaterials: Polymers. Sawston, Cambridge: Woodhead Publishing, 2018, 37–70. [Google Scholar]
- 54. Jin M, Shi J, Zhu W, Yao H, Wang DA.. Polysaccharide-based biomaterials in tissue engineering: a review. Tissue Eng Part B Rev 2021;27:604–26. [DOI] [PubMed] [Google Scholar]
- 55. Wang G, Li R, Parseh B, Du G.. Prospects and challenges of anticancer agents’ delivery via chitosan-based drug carriers to combat breast cancer: a review. Carbohydr Polym 2021;268:118192. [DOI] [PubMed] [Google Scholar]
- 56. Tsvetkov YE, Paulovičová E, Paulovičová L, Farkaš P, Nifantiev NE.. Synthesis of biotin-tagged chitosan oligosaccharides and assessment of their immunomodulatory activity. Front Chem 2020;8:554732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zehtabi F, Dumont-Mackay V, Fatimi A, Bertrand-Grenier A, Héon H, Soulez G, Lerouge S.. Chitosan-sodium tetradecyl sulfate hydrogel: characterization and preclinical evaluation of a novel sclerosing embolizing agent for the treatment of endoleaks. Cardiovasc Intervent Radiol 2017;40:576–84. [DOI] [PubMed] [Google Scholar]
- 58. Olson ST, Chuang YJ.. Heparin activates antithrombin anticoagulant function by generating new interaction sites (exosites) for blood clotting proteinases. Trends Cardiovasc Med 2002;12:331–8. [DOI] [PubMed] [Google Scholar]
- 59. Jorgensen MR, Räägel H, Rollins TS.. Advances in biocompatibility: a prerequisite for biomedical application of biopolymers. In: Biopolymers for Biomedical and Biotechnological Applications. Germany: Wiley-VCH: Weinheim, 2021, 1–17. [Google Scholar]
- 60. Lord MS, Cheng B, McCarthy SJ, Jung M, Whitelock JM.. The modulation of platelet adhesion and activation by chitosan through plasma and extracellular matrix proteins. Biomaterials 2011;32:6655–62. [DOI] [PubMed] [Google Scholar]
- 61. Kalsoom Khan A, Saba AU, Nawazish S, Akhtar F, Rashid R, Mir S, Nasir B, Iqbal F, Afzal S, Pervaiz F, Murtaza G.. Carrageenan based bionanocomposites as drug delivery tool with special emphasis on the influence of ferromagnetic nanoparticles. Oxid Med Cell Longev 2017;2017:8158315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Gu BK, Park SJ, Kim MS, Lee YJ, Kim JI, Kim CH.. Gelatin blending and sonication of chitosan nanofiber mats produce synergistic effects on hemostatic functions. Int J Biol Macromol 2016;82:89–96. [DOI] [PubMed] [Google Scholar]
- 63. Sorlier P, Denuzière A, Viton C, Domard A.. Relation between the degree of acetylation and the electrostatic properties of chitin and chitosan. Biomacromolecules 2001;2:765–72. [DOI] [PubMed] [Google Scholar]
- 64. Denzinger M, Hinkel H, Kurz J, Hierlemann T, Schlensak C, Wendel HP, Krajewski S.. Hemostyptic property of chitosan: opportunities and pitfalls. Biomed Mater Eng 2016;27:353–64. [DOI] [PubMed] [Google Scholar]
- 65. Brown MA, Daya MR, Worley JA.. Experience with chitosan dressings in a civilian EMS system. J Emerg Med 2009;37:1–7. [DOI] [PubMed] [Google Scholar]
- 66. Granville-Chapman J, Jacobs N, Midwinter MJ.. Pre-hospital haemostatic dressings: a systematic review. Injury 2011;42:447–59. [DOI] [PubMed] [Google Scholar]
- 67. Chen X, Yan Y, Li H, Wang X, Tang S, Li Q, Wei J, Su J.. Evaluation of absorbable hemostatic agents of polyelectrolyte complexes using carboxymethyl starch and chitosan oligosaccharide both in vitro and in vivo. Biomater Sci 2018;6:3332–44. [DOI] [PubMed] [Google Scholar]
- 68. Elsabahy M, Hamad MA.. Design and preclinical evaluation of chitosan/kaolin nanocomposites with enhanced hemostatic efficiency. Mar Drugs 2021;19:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wu X, Tang Z, Liao X, Wang Z, Liu H.. Fabrication of chitosan@calcium alginate microspheres with porous core and compact shell, and application as a quick traumatic hemostat. Carbohydr Polym 2020;247:116669. [DOI] [PubMed] [Google Scholar]
- 70. Nishiyama Y, Langan P, Chanzy H.. Crystal structure and hydrogen-bonding system in cellulose Iβ from synchrotron X-ray and neutron fiber diffraction. J Am Chem Soc 2002;124:9074–82. [DOI] [PubMed] [Google Scholar]
- 71. Klemm D, Heublein B, Fink HP, Bohn A.. Cellulose: fascinating biopolymer and sustainable raw material. Angew Chem Int Ed Engl 2005;44:3358–93. [DOI] [PubMed] [Google Scholar]
- 72. Cheng W, He J, Wu Y, Song C, Xie S, Huang Y, Fu B.. Preparation and characterization of oxidized regenerated cellulose film for hemostasis and the effect of blood on its surface. Cellulose 2013;20:2547–58. [Google Scholar]
- 73. Mertaniemi H, Escobedo-Lucea C, Sanz-Garcia A, Gandía C, Mäkitie A, Partanen J, Ikkala O, Yliperttula M.. Human stem cell decorated nanocellulose threads for biomedical applications. Biomaterials 2016;82:208–20. [DOI] [PubMed] [Google Scholar]
- 74. Metaxa A-F, Efthimiadou EK, Kordas G.. Cellulose-based drug carriers for cancer therapy: cytotoxic evaluation in cancer and healthy cells. Mater Lett 2014;132:432–5. [Google Scholar]
- 75. Kwak MH, Kim JE, Go J, Koh EK, Song SH, Son HJ, Kim HS, Yun YH, Jung YJ, Hwang DY.. Bacterial cellulose membrane produced by Acetobacter sp. A10 for burn wound dressing applications. Carbohydr Polym 2015;122:387–98. [DOI] [PubMed] [Google Scholar]
- 76. Hutchinson RW, George K, Johns D, Craven L, Zhang G, Shnoda P.. Hemostatic efficacy and tissue reaction of oxidized regenerated cellulose hemostats. Cellulose 2013;20:537–45. [Google Scholar]
- 77. Ohta S, Nishiyama T, Sakoda M, Machioka K, Fuke M, Ichimura S, Inagaki F, Shimizu A, Hasegawa K, Kokudo N.. Development of carboxymethyl cellulose nonwoven sheet as a novel hemostatic agent. J Biosci Bioeng 2015;119:718–23. [DOI] [PubMed] [Google Scholar]
- 78. He JM, Wu YD, Wang FW, Cheng WL, Huang YD, Fu B.. Hemostatic, antibacterial and degradable performance of the water-soluble chitosan-coated oxidized regenerated cellulose gauze. Fibers Polym 2014;15:504–9. [Google Scholar]
- 79. Karahaliloğlu Z, Demirbilek M, Ulusoy İ, Gümüşkaya B, Denkbaş EB.. Active nano/microbilayer hemostatic agents for diabetic rat bleeding model. J Biomed Mater Res B Appl Biomater 2017;105:1573–85. [DOI] [PubMed] [Google Scholar]
- 80. Fan X, Li M, Yang Q, Wan G, Li Y, Li N, Tang K.. Morphology-controllable cellulose/chitosan sponge for deep wound hemostasis with surfactant and pore-foaming agent. Mater Sci Eng C Mater Biol Appl 2021;118:111408. [DOI] [PubMed] [Google Scholar]
- 81. Yan C, Yang T, Zhu S, Wu H.. Synthesis and properties of poly (DEX-GMA/AAc) microgel particle as a hemostatic agent. J Mater Chem B 2017;5:3697–705. [DOI] [PubMed] [Google Scholar]
- 82. Chen F, Huang G, Huang H.. Preparation and application of dextran and its derivatives as carriers. Int J Biol Macromol 2020;145:827–34. [DOI] [PubMed] [Google Scholar]
- 83. Bhatia SK, Arthur SD, Chenault HK, Figuly GD, Kodokian GK.. Polysaccharide-based tissue adhesives for sealing corneal incisions. Curr Eye Res 2007;32:1045–50. [DOI] [PubMed] [Google Scholar]
- 84. Liu C, Liu X, Liu C, Wang N, Chen H, Yao W, Sun G, Song Q, Qiao W.. A highly efficient, in situ wet-adhesive dextran derivative sponge for rapid hemostasis. Biomaterials 2019;205:23–37. [DOI] [PubMed] [Google Scholar]
- 85. Artzi N, Shazly T, Baker AB, Bon A, Edelman ER.. Aldehyde‐amine chemistry enables modulated biosealants with tissue‐specific adhesion. Adv Mater 2009;21:3399–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Artzi N, Zeiger A, Boehning F, bon Ramos A, Van Vliet K, Edelman ER.. Tuning adhesion failure strength for tissue-specific applications. Acta Biomater 2011;7:67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Du X, Liu Y, Wang X, Yan H, Wang L, Qu L, Kong D, Qiao M, Wang L.. Injectable hydrogel composed of hydrophobically modified chitosan/oxidized-dextran for wound healing. Mater Sci Eng C Mater Biol Appl 2019;104:109930. [DOI] [PubMed] [Google Scholar]
- 88. Wang T, Nie J, Yang D.. Dextran and gelatin based photocrosslinkable tissue adhesive. Carbohydr Polym 2012;90:1428–36. [DOI] [PubMed] [Google Scholar]
- 89. Liu C, Liu C, Yu S, Wang N, Yao W, Liu X, Sun G, Song Q, Qiao W.. Efficient antibacterial dextran-montmorillonite composite sponge for rapid hemostasis with wound healing. Int J Biol Macromol 2020;160:1130–43. [DOI] [PubMed] [Google Scholar]
- 90. Akbari M, Tamayol A, Laforte V, Annabi N, Najafabadi AH, Khademhosseini A, Juncker D.. Composite living fibers for creating tissue constructs using textile techniques. Adv Funct Mater 2014;24:4060–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Ghahramanpoor MK, Hassani Najafabadi SA, Abdouss M, Bagheri F, Baghaban Eslaminejad M.. A hydrophobically-modified alginate gel system: utility in the repair of articular cartilage defects. J Mater Sci Mater Med 2011;22:2365–75. [DOI] [PubMed] [Google Scholar]
- 92. Tamayol A, Najafabadi AH, Aliakbarian B, Arab Tehrany E, Akbari M, Annabi N, Juncker D, Khademhosseini A.. Hydrogel templates for rapid manufacturing of bioactive fibers and 3D constructs. Adv Healthc Mater 2015;4:2146–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Xie Y, Gao P, He F, Zhang C.. Application of alginate-based hydrogels in hemostasis. Gels 2022;8:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Shi X, Fang Q, Ding M, Wu J, Ye F, Lv Z, Jin J.. Microspheres of carboxymethyl chitosan, sodium alginate and collagen for a novel hemostatic in vitro study. J Biomater Appl 2016;30:1092–102. [DOI] [PubMed] [Google Scholar]
- 95. Singh Chandel AK, Ohta S, Taniguchi M, Yoshida H, Tanaka D, Omichi K, Shimizu A, Isaji M, Hasegawa K, Ito T.. Balance of antiperitoneal adhesion, hemostasis, and operability of compressed bilayer ultrapure alginate sponges. Biomater Adv 2022;137:212825. [DOI] [PubMed] [Google Scholar]
- 96. Rong J-J, Liang M, Xuan F-Q, Sun J-Y, Zhao L-J, Zhen H-Z, Tian X-X, Liu D, Zhang Q-Y, Peng C-F, Yao T-M, Li F, Wang X-Z, Han Y-L, Yu W-T.. Alginate-calcium microsphere loaded with thrombin: a new composite biomaterial for hemostatic embolization. Int J Biol Macromol 2015;75:479–88. [DOI] [PubMed] [Google Scholar]
- 97. Zhai Z, Xu K, Mei L, Wu C, Liu J, Liu Z, Wan L, Zhong W.. Co-assembled supramolecular hydrogels of cell adhesive peptide and alginate for rapid hemostasis and efficacious wound healing. Soft Matter 2019;15:8603–10. [DOI] [PubMed] [Google Scholar]
- 98. Neuman MG, Nanau RM, Oruña-Sanchez L, Coto G.. Hyaluronic acid and wound healing. J Pharm Pharm Sci 2015;18:53–60. [DOI] [PubMed] [Google Scholar]
- 99. An S, Jeon EJ, Jeon J, Cho S-W.. A serotonin-modified hyaluronic acid hydrogel for multifunctional hemostatic adhesives inspired by a platelet coagulation mediator. Mater Horiz 2019;6:1169–78. [Google Scholar]
- 100. Luo J-W, Liu C, Wu J-H, Lin L-X, Fan H-M, Zhao D-H, Zhuang Y-Q, Sun Y-L.. In situ injectable hyaluronic acid/gelatin hydrogel for hemorrhage control. Mater Sci Eng C Mater Biol Appl 2019;98:628–34. [DOI] [PubMed] [Google Scholar]
- 101. Hong Y, Zhou F, Hua Y, Zhang X, Ni C, Pan D, Zhang Y, Jiang D, Yang L, Lin Q.. A strongly adhesive hemostatic hydrogel for the repair of arterial and heart bleeds. Nat Commun 2019;10:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Medeiros LHC, Vasconcelos BMF, Silva MB, Souza-Junior AA, Chavante SF, Andrade GPV.. Chondroitin sulfate from fish waste exhibits strong intracellular antioxidant potential. Braz J Med Biol Res 2021;54:e10730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Garnjanagoonchorn W, Wongekalak L, Engkagul A.. Determination of chondroitin sulfate from different sources of cartilage. Chem Eng Process Process Intensif 2007;46:465–71. [Google Scholar]
- 104. Kim HD, Lee EA, An YH, Kim SL, Lee SS, Yu SJ, Jang HL, Nam KT, Im SG, Hwang NS.. Chondroitin sulfate-based biomineralizing surface hydrogels for bone tissue engineering. ACS Appl Mater Interfaces 2017;9:21639–50. [DOI] [PubMed] [Google Scholar]
- 105. Bourin MC, Lindahl U.. Glycosaminoglycans and the regulation of blood coagulation. Biochem J 1993;289(Pt 2):313–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Scully MF, Ellis V, Seno N, Kakkar VV.. The anticoagulant properties of mast cell product, chondroitin sulphate E. Biochem Biophys Res Commun 1986;137:15–22. [DOI] [PubMed] [Google Scholar]
- 107. Sharma R, Kuche K, Thakor P, Bhavana V, Srivastava S, Mehra NK, Jain S.. Chondroitin sulfate: emerging biomaterial for biopharmaceutical purpose and tissue engineering. Carbohydr Polym 2022;286:119305. [DOI] [PubMed] [Google Scholar]
- 108. Slezak P, Keibl C, Redl H, Labahn D, Gulle H.. An efficacy comparison of two hemostatic agents in a porcine liver bleeding model: gelatin/thrombin flowable matrix versus collagen/thrombin powder. J Invest Surg 2020;33:828–38. [DOI] [PubMed] [Google Scholar]
- 109. Zhang X, Ma Z, Ke Y, Xia Y, Xu X, Liu J, Gong Y, Shi Q, Yin J.. An injectable serotonin-chondroitin sulfate hydrogel for bio-inspired hemostatic adhesives with high wound healing capability. Mater Adv 2021;2:5150–9. [Google Scholar]
- 110. Duncan R. The dawning era of polymer therapeutics. Nat Rev Drug Discov 2003;2:347–60. [DOI] [PubMed] [Google Scholar]
- 111. González-Aramundiz JV, Lozano MV, Sousa-Herves A, Fernandez-Megia E, Csaba N.. Polypeptides and polyaminoacids in drug delivery. Expert Opin Drug Deliv 2012;9:183–201. [DOI] [PubMed] [Google Scholar]
- 112. Connor RE, Tirrell DA.. Non‐canonical amino acids in protein polymer design. Polymer Rev 2007;47:9–28. [Google Scholar]
- 113. Hersel U, Dahmen C, Kessler H.. RGD modified polymers: biomaterials for stimulated cell adhesion and beyond. Biomaterials 2003;24:4385–415. [DOI] [PubMed] [Google Scholar]
- 114. Chow DC, Nunalee ML, Lim DW, Simnick AJ, Chilkoti A.. Peptide-based biopolymers in biomedicine and biotechnology. Mater Sci Eng R Rep 2008;62:125–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Lynn AK, Yannas IV, Bonfield W.. Antigenicity and immunogenicity of collagen. J Biomed Mater Res B Appl Biomater 2004;71:343–54. [DOI] [PubMed] [Google Scholar]
- 116. Takagi T, Tsujimoto H, Torii H, Ozamoto Y, Hagiwara A.. Two-layer sheet of gelatin: a new topical hemostatic agent. Asian J Surg 2018;41:124–30. [DOI] [PubMed] [Google Scholar]
- 117. Xie X, Li D, Chen Y, Shen Y, Yu F, Wang W, Yuan Z, Morsi Y, Wu J, Mo X.. Conjugate electrospun 3D gelatin nanofiber sponge for rapid hemostasis. Adv Healthc Mater 2021;10:e2100918. [DOI] [PubMed] [Google Scholar]
- 118. Li L, Du Y, Yin Z, Li L, Peng H, Zheng H, Yang A, Li H, Lv G.. Preparation and the hemostatic property study of porous gelatin microspheres both in vitro and in vivo. Colloids and Surf B Biointerfaces 2020;187:110641. [DOI] [PubMed] [Google Scholar]
- 119. Litvinov RI, Weisel JW.. Fibrin mechanical properties and their structural origins. Matrix Biol 2017;60–61:110–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Feller T, Connell SDA, Ariёns RAS.. Why fibrin biomechanical properties matter for hemostasis and thrombosis. J Thromb Haemost 2022;20:6–16. [DOI] [PubMed] [Google Scholar]
- 121. Chetter I, Stansby G, Sarralde JA, Riambau V, Giménez-Gaibar A, MacKenzie K, Acín F, Navarro-Puerto J.. A prospective, randomized, multicenter clinical trial on the safety and efficacy of a ready-to-use fibrin sealant as an adjunct to hemostasis during vascular surgery. Ann Vasc Surg 2017;45:127–37. [DOI] [PubMed] [Google Scholar]
- 122. Verra WC, van Hilten JA, Honohan Á, van Zwet EW, van der Bom JG, Nelissen R; FIRST-research group. The effect of a fibrin sealant on knee function after total knee replacement surgery. Results from the FIRST trial. A multicenter randomized controlled trial. PLoS One 2018;13:e0200804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Harmon AM, Kong W, Buensuceso CS, Gorman AJ, Muench TR.. Effects of fibrin pad hemostat on the wound healing process in vivo and in vitro. Biomaterials 2011;32:9594–601. [DOI] [PubMed] [Google Scholar]
- 124. Matonick JP, Hammond J.. Hemostatic efficacy of EVARREST™, fibrin sealant patch vs. TachoSil® in a heparinized swine spleen incision model. J Invest Surg 2014;27:360–5. [DOI] [PubMed] [Google Scholar]
- 125. Feroz S, Muhammad N, Ratnayake J, Dias G.. Keratin-based materials for biomedical applications. Bioact Mater 2020;5:496–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Rahmany MB, Hantgan RR, Van Dyke M.. A mechanistic investigation of the effect of keratin-based hemostatic agents on coagulation. Biomaterials 2013;34:2492–500. [DOI] [PubMed] [Google Scholar]
- 127. Wang Y, Xu Y, Zhang Z, He Y, Hou Z, Zhao Z, Deng J, Qing R, Wang B, Hao S.. Rational design of high-performance keratin-based hemostatic agents. Adv Healthc Mater 2022;11:e2200290. [DOI] [PubMed] [Google Scholar]
- 128. He Y, Qu Q, Luo T, Gong Y, Hou Z, Deng J, Xu Y, Wang B, Hao S.. Human hair keratin hydrogels alleviate rebleeding after intracerebral hemorrhage in a rat model. ACS Biomater Sci Eng 2019;5:1113–25. [DOI] [PubMed] [Google Scholar]
- 129. Fang G, Sapru S, Behera S, Yao J, Shao Z, Kundu SC, Chen X.. Exploration of the tight structural-mechanical relationship in mulberry and non-mulberry silkworm silks. J Mater Chem B 2016;4:4337–47. [DOI] [PubMed] [Google Scholar]
- 130. Panilaitis B, Altman GH, Chen J, Jin HJ, Karageorgiou V, Kaplan DL.. Macrophage responses to silk. Biomaterials 2003;24:3079–85. [DOI] [PubMed] [Google Scholar]
- 131. Sun W, Gregory DA, Tomeh MA, Zhao X.. Silk fibroin as a functional biomaterial for tissue engineering. Int J Mol Sci 2021;22:1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Zhu J, Zhong K, Zong Y, Wang S, Yang H, Zhen L, Tao S, Sun L, Yang J, Li J.. A mussel-inspired wet-adhesion hydrogel with hemostasis and local anti-inflammation for managing the development of acute wounds. Mater Des 2022;213:110347. [Google Scholar]
- 133. Lei C, Zhu H, Li J, Feng X, Chen J.. Preparation and hemostatic property of low molecular weight silk fibroin. J Biomater Sci Polym Ed 2016;27:403–18. [DOI] [PubMed] [Google Scholar]
- 134. Wei W, Liu J, Peng Z, Liang M, Wang Y, Wang X.. Gellable silk fibroin-polyethylene sponge for hemostasis. Artif Cells Nanomed Biotechnol 2020;48:28–36. [DOI] [PubMed] [Google Scholar]
- 135. Shefa AA, Taz M, Lee SY, Lee BT.. Enhancement of hemostatic property of plant derived oxidized nanocellulose-silk fibroin based scaffolds by thrombin loading. Carbohydr Polym 2019;208:168–79. [DOI] [PubMed] [Google Scholar]
- 136. Yang S, Wei S, Mao Y, Zheng H, Feng J, Cui J, Xie X, Chen F, Li H.. Novel hemostatic biomolecules based on elastin-like polypeptides and the self-assembling peptide RADA-16. BMC Biotechnol 2018;18:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Wang T, Zhong X, Wang S, Lv F, Zhao X.. Molecular mechanisms of RADA16-1 peptide on fast stop bleeding in rat models. Int J Mol Sci 2012;13:15279–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Wang R, Wang Z, Guo Y, Li H, Chen Z.. Design of a RADA16-based self-assembling peptide nanofiber scaffold for biomedical applications. J Biomater Sci Polym Ed 2019;30:713–36. [DOI] [PubMed] [Google Scholar]
- 139. Teng L, Shao Z, Bai Q, Zhang X, He Y-S, Lu J, Zou D, Feng C, Dong CM.. Biomimetic glycopolypeptide hydrogels with tunable adhesion and microporous structure for fast hemostasis and highly efficient wound healing. Adv Funct Mater 2021;31:2105628. [Google Scholar]
- 140. Guo Z, Xu Y, Dong L, Desai MS, Xia J, Liang M, Lee S-W, Mi S, Sun W.. Design of functional hydrogels using smart polymer based on elastin-like polypeptides. Chem Eng J 2022;435:135155. [Google Scholar]
- 141. Ma C, Sun J, Li B, Feng Y, Sun Y, Xiang L, Wu B, Xiao L, Liu B, Petrovskii VS, Bin L, Zhang J, Wang Z, Li H, Zhang L, Li J, Wang F, Göstl R, Potemkin II, Chen D, Zeng H, Zhang H, Liu K, Herrmann A.. Ultra-strong bio-glue from genetically engineered polypeptides. Nat Commun 2021;12:3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Lu D, Wang H, Li T, Li Y, Dou F, Sun S, Guo H, Liao S, Yang Z, Wei Q, Lei Z.. Mussel-inspired thermoresponsive polypeptide–pluronic copolymers for versatile surgical adhesives and hemostasis. ACS Appl Mater Interfaces 2017;9:16756–66. [DOI] [PubMed] [Google Scholar]
- 143. Lu D, Wang H, Li T, Li Y, Wang X, Niu P, Guo H, Sun S, Wang X, Guan X, Ma H, Lei Z.. Versatile surgical adhesive and hemostatic materials: synthesis, properties, and application of thermoresponsive polypeptides. Chem Mater 2017;29:5493–503. [Google Scholar]
- 144. Suneetha M, Rao KM, Han SS.. Mussel-inspired cell/tissue-adhesive, hemostatic hydrogels for tissue engineering applications. ACS Omega 2019;4:12647–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Nie W, Yuan X, Zhao J, Zhou Y, Bao H.. Rapidly in situ forming chitosan/ε-polylysine hydrogels for adhesive sealants and hemostatic materials. Carbohydr Polym 2013;96:342–8. [DOI] [PubMed] [Google Scholar]
- 146. Lu D, Wang H, Wang X, Li Y, Guo H, Sun S, Zhao X, Yang Z, Lei Z.. Biomimetic chitosan-graft-polypeptides for improved adhesion in tissue and metal. Carbohydr Polym 2019;215:20–8. [DOI] [PubMed] [Google Scholar]
- 147. Zhu H, Mei X, He Y, Mao H, Tang W, Liu R, Yang J, Luo K, Gu Z, Zhou L.. Fast and high strength soft tissue bioadhesives based on a peptide dendrimer with antimicrobial properties and hemostatic ability. ACS Appl Mater Interfaces 2020;12:4241–53. [DOI] [PubMed] [Google Scholar]
- 148. Xie X, Li D, Chen Y, Shen Y, Yu F, Wang W, Yuan Z, Morsi Y, Wu J, Mo X.. Conjugate electrospun 3D gelatin nanofiber sponge for rapid hemostasis. Adv Healthc Mater 2021;10:2100918. [DOI] [PubMed] [Google Scholar]
- 149. Huang Y, Zhao X, Zhang Z, Liang Y, Yin Z, Chen B, Bai L, Han Y, Guo B.. Degradable Gelatin-Based IPN cryogel hemostat for rapidly stopping deep noncompressible hemorrhage and simultaneously improving wound healing. Chem Mater 2020;32:6595–610. [Google Scholar]
- 150. Kheirabadi BS, Sieber J, Bukhari T, Rudnicka K, Murcin LA, Tuthill D.. High-pressure fibrin sealant foam: an effective hemostatic agent for treating severe parenchymal hemorrhage. J Surg Res 2008;144:145–50. [DOI] [PubMed] [Google Scholar]
- 151. He Y, Qu Q, Luo T, Gong Y, Hou Z, Deng J, Xu Y, Wang B, Hao S.. Human hair keratin hydrogels alleviate rebleeding after intracerebral hemorrhage in a rat model. ACS Biomater Sci Eng 2019;5:1113–22. [DOI] [PubMed] [Google Scholar]
- 152. Pacheco M, Barros AA, Aroso IM, Autorino R, Lima E, Silva JM, Reis RL.. Use of hemostatic agents for surgical bleeding in laparoscopic partial nephrectomy: biomaterials perspective. J Biomed Mater Res 2020;108:3099–123. [DOI] [PubMed] [Google Scholar]
- 153. Wang D, Zhang N, Meng G, He J, Wu F.. The effect of form of carboxymethyl-chitosan dressings on biological properties in wound healing. Colloids Surf B Biointerfaces 2020;194:111191. [DOI] [PubMed] [Google Scholar]
- 154. Zhang S, Li J, Chen S, Zhang X, Ma J, He J.. Oxidized cellulose-based hemostatic materials. Carbohydr Polym 2020;230:115585. [DOI] [PubMed] [Google Scholar]
- 155. Liu J, Zhou X, Zhang Y, Zhu W, Wang A, Xu M, Zhuang S.. Rapid hemostasis and excellent antibacterial cerium-containing mesoporous bioactive glass/chitosan composite sponge for hemostatic material. Mater Today Chem 2022;23:100735. [Google Scholar]
- 156. Gutierrez G, Reines H, Wulf-Gutierrez ME.. Clinical review: hemorrhagic shock. Crit Care 2004;8:373–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Fülöp A, Turóczi Z, Garbaisz D, Harsányi L, Szijártó A.. Experimental models of hemorrhagic shock: a review. Eur Surg Res 2013;50:57–70. [DOI] [PubMed] [Google Scholar]
- 158. Nichols R, Horstman J.. Recommendations for improving stop the bleed: a systematic review. Mil Med 2022; usac019. [DOI] [PubMed] [Google Scholar]
- 159. Kenny T. The Three Types of Bleeding and How to Stop Them. https://www.northwestcareercollege.edu/blog/the-three-types-of-bleeding-and-how-to-stop-them/ (15 August 2022, date last accessed).
- 160. Hong YM, Loughlin KR.. The use of hemostatic agents and sealants in urology. J Urol 2006;176:2367–74. [DOI] [PubMed] [Google Scholar]
- 161. di Lena F. Hemostatic polymers: the concept, state of the art and perspectives. J Mater Chem B 2014;2:3567–77. [DOI] [PubMed] [Google Scholar]
- 162. Biswal T. Biopolymers for tissue engineering applications: a review. Mater Today Proc 2021;41:397–402. [Google Scholar]
- 163. Basagaoglu Demirekin Z, Aydemir Sezer U, Ulusoy Karatopuk D, Sezer S.. Development of metal ion binded oxidized regenerated cellulose powder as hemostatic agent: a comparative study with in vivo performance. Ind Eng Chem Res 2015;54:4906–14. [Google Scholar]
- 164. Bose S, Koski C, Vu AA.. Additive manufacturing of natural biopolymers and composites for bone tissue engineering. Mater Horiz 2020;7:2011–27. [Google Scholar]
- 165. Maleksabet N, Masoumian M, Aramvash A.. Evaluating the safety and toxicity of a modified dextran-based biopolymer as a hemostat. Curr Biotechnol 2021;9:290–6. [Google Scholar]
- 166. Lewis KM, McKee J, Schiviz A, Bauer A, Wolfsegger M, Goppelt A.. Randomized, controlled comparison of advanced hemostatic pads in hepatic surgical models. ISRN Surg 2014;2014:930803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Malik A, Rehman FU, Shah KU, Naz SS, Qaisar S.. Hemostatic strategies for uncontrolled bleeding: a comprehensive update. J Biomed Mater Res 2021;109:1465–77. [DOI] [PubMed] [Google Scholar]
- 168. Kordestani SS, Noohi F, Azarnik H, Basiri H, Hashemi M, Abdi S, Mohebi A, Madani M, NayebHabib F.. A randomized controlled trial on the hemostasis of femoral artery using topical hemostatic agent. Clin Appl Thromb Hemost 2012;18:501–5. [DOI] [PubMed] [Google Scholar]
- 169. Zheng C, Zeng Q, Pimpi S, Wu W, Han K, Dong K, Lu T.. Research status and development potential of composite hemostatic materials. J Mater Chem B 2020;8:5395–410. [DOI] [PubMed] [Google Scholar]
- 170. Chen J, Cheng W, Chen S, Xu W, Lin J, Liu H, Chen Q.. Urushiol-functionalized mesoporous silica nanoparticles and their self-assembly into a Janus membrane as a highly efficient hemostatic material. Nanoscale 2018;10:22818–29. [DOI] [PubMed] [Google Scholar]
- 171. de la Harpe KM, Kondiah PP, Choonara YE, Marimuthu T, du Toit LC, Pillay V.. The hemocompatibility of nanoparticles: a review of cell–nanoparticle interactions and hemostasis. Cells 2019;8:1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Luo T, Hao S, Chen X, Wang J, Yang Q, Wang Y, Weng Y, Wei H, Zhou J, Wang B.. Development and assessment of kerateine nanoparticles for use as a hemostatic agent. Mater Sci Eng C Mater Biol Appl 2016;63:352–8. [DOI] [PubMed] [Google Scholar]
- 173. Yang X, Liu W, L N, Wang M, Liang B, Ullah I, Neve AL, Feng Y, Chen H, Shi C.. Design and development of polysaccharide hemostatic materials and their hemostatic mechanism. Biomater Sci 2017;5:2357–68. [DOI] [PubMed] [Google Scholar]
- 174. Jegatheeswaran S, Bhanot U, Siriwardena A.. In vivo evaluation of the chitosan-based haemostatic agent omni-stat® in porcine liver resection and in liver injury. Eur Surg Res 2012;49:73–9. [DOI] [PubMed] [Google Scholar]
- 175. Sakoda M, Kaneko M, Ohta S, Qi P, Ichimura S, Yatomi Y, Ito T.. Injectable hemostat composed of a polyphosphate-conjugated hyaluronan hydrogel. Biomacromolecules 2018;19:3280–90. [DOI] [PubMed] [Google Scholar]
- 176. Panwar V, Sharma A, Thomas J, Chopra V, Kaushik S, Kumar A, Ghosh D.. In-vitro and in-vivo evaluation of biocompatible and biodegradable calcium-modified carboxymethyl starch as a topical hemostat. Materialia 2019;7:100373. [Google Scholar]
- 177. Zhu J, Wu Z, Sun W, Meng Z, Zhu X, Gan H, Gu R, Guo X, Dou G.. Hemostatic efficacy and biocompatibility evaluation of a novel absorbable porous starch hemostat. Surg Innov 2021;29:367–77. [DOI] [PubMed] [Google Scholar]
- 178. Chen G, Ushida T, Tateishi T.. Hybrid biomaterials for tissue engineering: a preparative method for PLA or PLGA–collagen hybrid sponges. Adv Mater 2000;12:455–7. [Google Scholar]
- 179. Rnjak-Kovacina J, Wray LS, Burke KA, Torregrosa T, Golinski JM, Huang W, Kaplan DL.. Lyophilized silk sponges: a versatile biomaterial platform for soft tissue engineering. ACS Biomater Sci Eng 2015;1:260–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Seol Y-J, Lee J-Y, Park Y-J, Lee Y-M, Ku Y, Rhyu I-C, Lee S-J, Han S-B, Chung C-P.. Chitosan sponges as tissue engineering scaffolds for bone formation. Biotechnol Lett 2004;26:1037–41. [DOI] [PubMed] [Google Scholar]
- 181. Szymkowiak S, Sandler N, Kaplan DL.. Aligned silk sponge fabrication and perfusion culture for scalable proximal tubule tissue engineering. ACS Appl Mater Interfaces 2021;13:10768–77. [DOI] [PubMed] [Google Scholar]
- 182. Giannelli M, Barbalinardo M, Riminucci A, Belvedere K, Boccalon E, Sotgiu G, Corticelli F, Ruani G, Zamboni R, Aluigi A.. Magnetic keratin/hydrotalcites sponges as potential scaffolds for tissue regeneration. Appl Clay Sci 2021;207:106090. [Google Scholar]
- 183. Zheng L, Wang Q, Zhang YS, Zhang H, Tang Y, Zhang Y, Zhang W, Zhang X.. A hemostatic sponge derived from skin secretion of Andrias davidianus and nanocellulose. Chem Eng J 2021;416:129136. [Google Scholar]
- 184. Fan X, Li M, Yang Q, Wan G, Li Y, Li N, Tang K.. Morphology-controllable cellulose/chitosan sponge for deep wound hemostasis with surfactant and pore-foaming agent. Mater Sci Eng C Mater Biol Appl 2021;118:111408. [DOI] [PubMed] [Google Scholar]
- 185. Wei X, Ding S, Liu S, Yang K, Cai J, Li F, Wang C, Lin S, Tian F.. Polysaccharides-modified chitosan as improved and rapid hemostasis foam sponges. Carbohydr Polym 2021;264:118028. [DOI] [PubMed] [Google Scholar]
- 186. Udayakumar GP, Muthusamy S, Selvaganesh B, Sivarajasekar N, Rambabu K, Sivamani S, Sivakumar N, Maran JP, Hosseini-Bandegharaei A.. Ecofriendly biopolymers and composites: preparation and their applications in water-treatment. Biotechnol Adv 2021;52:107815. [DOI] [PubMed] [Google Scholar]
- 187. Li J, Yu X, Martinez EE, Zhu J, Wang T, Shi S, Shin SR, Hassan S, Guo C.. Emerging biopolymer‐based bioadhesives. Macromol Biosci 2022;22:2100340. [DOI] [PubMed] [Google Scholar]
- 188. Kuo Z-K, Lai P-L, Toh EK-W, Weng C-H, Tseng H-W, Chang P-Z, Chen C-C, Cheng C-M.. Osteogenic differentiation of preosteoblasts on a hemostatic gelatin sponge. Sci Rep 2016;6:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Huang X, Sun Y, Nie J, Lu W, Yang L, Zhang Z, Yin H, Wang Z, Hu Q.. Using absorbable chitosan hemostatic sponges as a promising surgical dressing. Int J Biol Macromol 2015;75:322–9. [DOI] [PubMed] [Google Scholar]
- 190. Wu Z, Zhou W, Deng W, Xu C, Cai Y, Wang X.. Antibacterial and hemostatic thiol-modified chitosan-immobilized AgNPs composite sponges. ACS Appl Mater Interfaces 2020;12:20307–20. [DOI] [PubMed] [Google Scholar]
- 191. Li P, Cao L, Sang F, Zhang B, Meng Z, Pan L, Hao J, Yang X, Ma Z, Shi C.. Polyvinyl alcohol/sodium alginate composite sponge with 3D ordered/disordered porous structure for rapidly controlling noncompressible hemorrhage. Biomater Adv 2022;134:112698. [DOI] [PubMed] [Google Scholar]
- 192. Lyu H, Sun Z, Liu Y, Yu X, Guo C.. Processing-structure-properties relationships of glycerol-plasticized silk films. Molecules 2022;27:1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Guo C, Li C, Mu X, Kaplan DL.. Engineering silk materials: from natural spinning to artificial processing. Appl Phys Rev 2020;7:011313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Li C, Guo C, Fitzpatrick V, Ibrahim A, Zwierstra MJ, Hanna P, Lechtig A, Nazarian A, Lin SJ, Kaplan DL.. Design of biodegradable, implantable devices towards clinical translation. Nat Rev Mater 2020;5:61–81. [Google Scholar]
- 195. Guo C, Li C, Vu HV, Hanna P, Lechtig A, Qiu Y, Mu X, Ling S, Nazarian A, Lin SJ.. Thermoplastic moulding of regenerated silk. Nat Mater 2020;19:102–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Teuschl AH, Zipperle J, Huber Gries C, Kaplan DL.. Silk fibroin based carrier system for delivery of fibrinogen and thrombin as coagulant supplements. J Biomed Mater Res A 2017;105:687–96. [DOI] [PubMed] [Google Scholar]
- 197. Hu J, Hou Y, Park H, Choi B, Hou S, Chung A, Lee M.. Visible light crosslinkable chitosan hydrogels for tissue engineering. Acta Biomater 2012;8:1730–8. [DOI] [PubMed] [Google Scholar]
- 198. Petersen JK, Krogsgaard J, Nielsen KM, Nørgaard EB.. A comparison between 2 absorbable hemostatic agents: gelatin sponge (Spongostan®) and oxidized regenerated cellulose (Surgicel®). Int J Oral Maxillofac Surg 1984;13:406–10. [DOI] [PubMed] [Google Scholar]
- 199. Kita AE, Bradbury DW, Taylor ZD, Kamei DT, St. John MA.. Point-of-care cerebrospinal fluid detection. Otolaryngol Head Neck Surg 2018;159:824–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Lindstrom PA. Complications from the use of absorbable hemostatic sponges. AMA Arch Surg 1956;73:133–41. [DOI] [PubMed] [Google Scholar]
- 201. Özer A, Köstü B.. Use of gelatin sponge affects postoperative morbidity in cesarean section patients. Med Sci Monit 2017;23:1141–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Blaine G. Absorbable gelatin sponge in experimental surgery. Lancet 1951;2:427–9. [DOI] [PubMed] [Google Scholar]
- 203. Eastridge BJ, Mabry RL, Seguin P, Cantrell J, Tops T, Uribe P, Mallett O, Zubko T, Oetjen-Gerdes L, Rasmussen TE, Butler FK, Kotwal RS, Holcomb JB, Wade C, Champion H, Lawnick M, Moores L, Blackbourne LH.. Death on the battlefield (2001-2011): implications for the future of combat casualty care. J Trauma Acute Care Surg 2012;73(6 Suppl 5):S431–7. [DOI] [PubMed] [Google Scholar]
- 204. Holcomb JB, McMullin NR, Pearse L, Caruso J, Wade CE, Oetjen-Gerdes L, Champion HR, Lawnick M, Farr W, Rodriguez S, Butler FK.. Causes of death in U.S. Special operations forces in the global war on terrorism: 2001-2004. Ann Surg 2007;245:986–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Adams D, McDonald PL, Sullo E, Merkle AB, Nunez T, Sarani B, Shackelford SA, Bowyer MW, van der Wees P.. Management of non-compressible torso hemorrhage of the abdomen in civilian and military austere/remote environments: protocol for a scoping review. Trauma Surg Acute Care Open 2021;6:e000811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Zhang ZY, Zhang HY, Talmy T, Guo Y, Zhou SR, Zhang LY, Li Y.. Management of non-compressible torso hemorrhage: an update. Chin J Traumatol 2021;24:125–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. MacDonald MH, Zhang G, Tasse L, Wang D, De Leon H, Kocharian R.. Hemostatic efficacy of two topical adjunctive hemostats in a porcine spleen biopsy punch model of moderate bleeding. J Mater Sci Mater Med 2021;32:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Hong Y, Zhou F, Hua Y, Zhang X, Ni C, Pan D, Zhang Y, Jiang D, Yang L, Lin Q, Zou Y, Yu D, Arnot DE, Zou X, Zhu L, Zhang S, Ouyang H.. A strongly adhesive hemostatic hydrogel for the repair of arterial and heart bleeds. Nat Commun 2019;10:2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Bruckner BA, Blau LN, Rodriguez L, Suarez EE, Ngo UQ, Reardon MJ, Loebe M.. Microporous polysaccharide hemosphere absorbable hemostat use in cardiothoracic surgical procedures. J Cardiothorac Surg 2014;9:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Ghimire S, Sarkar P, Rigby K, Maan A, Mukherjee S, Crawford KE, Mukhopadhyay K.. Polymeric materials for hemostatic wound healing. Pharmaceutics 2021;13:2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Li H, Cheng F, Gao S, Wu Z, Dong L, Lin S, Luo Z, Li X.. Preparation, characterization, antibacterial properties, and hemostatic evaluation of ibuprofen-loaded chitosan/gelatin composite films. J Appl Polymer Sci 2017;134:45441. [Google Scholar]
- 212. Bai M-Y, Chou T-C, Tsai J-C, Yang H-C.. Active ingredient-containing chitosan/polycaprolactone nonwoven mats: characterizations and their functional assays. Mater Sci Eng C Mater Biol Appl 2013;33:224–33. [DOI] [PubMed] [Google Scholar]
- 213. Hsu BB, Conway W, Tschabrunn CM, Mehta M, Perez-Cuevas MB, Zhang S, Hammond PT.. Clotting mimicry from robust hemostatic bandages based on self-assembling peptides. ACS Nano 2015;9:9394–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Pulieri E, Chiono V, Ciardelli G, Vozzi G, Ahluwalia A, Domenici C, Vozzi F, Giusti P.. Chitosan/gelatin blends for biomedical applications. J Biomed Mater Res A 2008;86:311–22. [DOI] [PubMed] [Google Scholar]
- 215. Sutar T, Bangde P, Dandekar P, Adivarekar R.. Herbal hemostatic biopolymeric dressings of alginate/pectin coated with Croton oblongifolius extract. Carbohydr Polym Technol Appl 2021;2:100025. [Google Scholar]
- 216. Bidarra SJ, Barrias CC, Granja PL.. Injectable alginate hydrogels for cell delivery in tissue engineering. Acta Biomater 2014;10:1646–62. [DOI] [PubMed] [Google Scholar]
- 217. Ladas EJ, Karlik JB, Rooney D, Taromina K, Ndao DH, Granowetter L, Kelly KM.. Topical Yunnan Baiyao administration as an adjunctive therapy for bleeding complications in adolescents with advanced cancer. Support Care Cancer 2012;20:3379–83. [DOI] [PubMed] [Google Scholar]
- 218. Lu B, Hu S-s, Zhu X, Zhang L, Zhang Z.. Experimental study of hemostasis effect of chitosan/sodium-alginate-yunnanbaiyao composite film. Health Res 2011;31:94–6. [Google Scholar]
- 219. Rahaman MN, Day DE, Sonny Bal B, Fu Q, Jung SB, Bonewald LF, Tomsia AP.. Bioactive glass in tissue engineering. Acta Biomater 2011;7:2355–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Pajares-Chamorro N, Chatzistavrou X.. Bioactive glass nanoparticles for tissue regeneration. ACS Omega 2020;5:12716–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Pourshahrestani S, Kadri NA, Zeimaran E, Gargiulo N, Samuel S, Naveen SV, Hasikin K, Kamarul T, Towler MR.. Comparative efficacy of hemorrhage control of a novel mesoporous bioactive glass versus two commercial hemostats. Biomed Mater 2018;13:025020. [DOI] [PubMed] [Google Scholar]
- 222. Jia T-b, Chen J.-y, Feng X.-x, Chang J.. Fabrication and characterization of chitosan/mesoporous bioactive glasses porous films. J Clin Rehabil Tissue Eng Res 2011;15:7877–80. [Google Scholar]
- 223. Yokoyama M, Chihara N, Tanaka A, Katayama Y, Taruya A, Ishida Y, Yuzaki M, Honda K, Nishimura Y, Kondo T, Akasaka T, Kato N.. A biodegradable microneedle sheet for intracorporeal topical hemostasis. Sci Rep 2020;10:18831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Nagarkar R, Singh M, Nguyen HX, Jonnalagadda S.. A review of recent advances in microneedle technology for transdermal drug delivery. J Drug Delivery Sci Technol 2020;59:101923. [Google Scholar]
- 225. Gupta J, Gupta R, Vanshita .. Microneedle technology: an insight into recent advancements and future trends in drug and vaccine delivery. Assay Drug Dev Technol 2020;19:97–114. [DOI] [PubMed] [Google Scholar]
- 226. Xue J, Wu T, Dai Y, Xia Y.. Electrospinning and electrospun nanofibers: methods, materials, and applications. Chem Rev 2019;119:5298–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Liao H-S, Lin J, Liu Y, Huang P, Jin AJ, Chen X.. Self-assembly mechanisms of nanofibers from peptide amphiphiles in solution and on substrate surfaces. Nanoscale 2016;8:14814–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Cui Y, Huang Z, Lei L, Li Q, Jiang J, Zeng Q, Tang A, Yang H, Zhang Y.. Robust hemostatic bandages based on nanoclay electrospun membranes. Nat Commun 2021;12:5922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Yan D, Hu S, Zhou Z, Zeenat S, Cheng F, Li Y, Feng C, Cheng X, Chen X.. Different chemical groups modification on the surface of chitosan nonwoven dressing and the hemostatic properties. Int J Biol Macromol 2018;107:463–9. [DOI] [PubMed] [Google Scholar]
- 230. He J, Wang FW, Wu Y, Huang Y, Zhang H.. Preparation of the water-soluble chitosan-coated oxidized regenerated cellulose gauze. Cellulose 2011;18:1651–9. [Google Scholar]
- 231. Jaiswal AK, Chhabra H, Narwane S, Rege N, Bellare JR.. Hemostatic efficacy of nanofibrous matrix in rat liver injury model. Surg Innov 2017;24:23–8. [DOI] [PubMed] [Google Scholar]
- 232. Li D, Nie W, Chen L, Miao Y, Zhang X, Fancheng C, Yu B, Ao R, Yu B, He C.. Fabrication of curcumin-loaded mesoporous silica incorporated polyvinyl pyrrolidone nanofibers for rapid hemostasis and antibacterial treatment. RSC Adv 2017;7:7973–82. [Google Scholar]
- 233. Hsu BB, Hagerman SR, Jamieson K, Castleberry SA, Wang W, Holler E, Ljubimova JY, Hammond PT.. Multifunctional self-assembled films for rapid hemostat and sustained anti-infective delivery. ACS Biomater Sci Eng 2015;1:148–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Echave M, Oyagüez I, Casado MA.. Use of Floseal®, a human gelatine-thrombin matrix sealant, in surgery: a systematic review. BMC Surg 2014;14:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Floseal Hemostatic Matrix. Baxter. https://www.baxter.com/healthcare-professionals/surgical-care/floseal-hemostatic-matrix-surgical-care (15 August 2022, date last accessed).
- 236.SURGICEL® Powder Absorbable Hemostat. Johnson&Johnson MEDTECH. https://www.jnjmedtech.com/en-US/product/ethicon-surgicel-powder (15 August 2022, date last accessed).
- 237.SURGIFOAM* Sponge (Sterile) (absorbable gelatin sponge USP). https://www.accessdata.fda.gov/cdrh_docs/pdf/P990004c.pdf (August 15, 2022).
- 238.SURGIFOAM Absorbable Gelatin Powder. Johnson&Johnson MEDTECH. https://www.jnjmedtech.com/sites/default/files/user_uploaded_assets/pdf_assets/2020-03/110658-190329%20Surgifoam%201%20pager%20v7_FINAL.pdf (15 August 2022, date last accessed).
- 239.Celox Granules. Celox Medical. https://www.celoxmedical.com/cx-product/celox-granules/ (15 August 2022, date last accessed).
- 240.SPONGOSTAN Absorbable Haemostatic Gelatin Sponge. Johnson&Johnson MEDTECH. https://www.jnjmedtech.com/en-EMEA/product/spongostan-absorbable-haemostatic-gelatin-sponge (15 August 2022, date last accessed).
- 241.BenaCel® Dental Dressing. Unicare Biomedical. https://www.unicarebiomedical.com/dental-supplies/benacel.html (15 August 2022, date last accessed).
- 242.HemCon® Bandage PRO. Tricol Biomedical Inc. https://tricolbiomedical.com/product/hemcon-bandage-pro/ (15 August 2022, date last accessed).
- 243.Avitene™ Sheets. BD. https://www.bd.com/en-us/products-and-solutions/products/product-families/avitene-sheets (15 August 2022, date last accessed).
- 244.Gelfoam® absorbable gelatin sponge, USP. Pfizer. https://labeling.pfizer.com/ShowLabeling.aspx?id=624 (15 August 2022, date last accessed).
- 245.TACHOSIL® Fibrin Sealant Patch. Baxter. https://advancedsurgery.baxter.com/tachosil (15 August 2022, date last accessed).
- 246.TachoSil. US Food and Drug Administration. https://www.fda.gov/vaccines-blood-biologics/approved-blood-products/tachosil (15 August 2022, date last accessed).
- 247.SURGIFLO® Hemostatic Matrix Kit. Johnson&Johnson MEDTECH. https://www.jnjmedtech.com/sites/default/files/user_uploaded_assets/pdf_assets/2022-03/135173-220210_ETH_SURGIFLO%20and%20Go%20Sales%20Aid_External_2_22_0051_CR.pdf (15 August 2022, date last accessed).
- 248.PeproStat as a Topical Agent Used to Stop Bleeding in Patients Undergoing Surgery. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT03131336?term=sponge&cond=Hemorrhage&draw=2&rank=3 (15 August 2022, date last accessed).
- 249.The EVARREST® Pediatric Mild or Moderate Liver and Soft Tissue Bleeding Study. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT03255174?term=ADHESIVE&cond=Bleeding&draw=2&rank=8 (15 August 2022, date last accessed).
- 250. Thrombi-Gel® (thrombin/gelatin foam hemostat). Pfizer. https://cdn.pfizer.com/pfizercom/products/Thrombi-Gel_Pfizer_US.PDF (15 August 2022, date last accessed).
- 251.Evaluation of an Absorbable Surgical Hemostatic Agent: Thrombi-Gel® Versus Gelfoam-Thrombin (Control). ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT00652314?term=sponge&cond=Bleeding&draw=2&rank=8 (15 August 2022, date last accessed).
- 252.Performance and Safety of the Surgical Hemostatic Agent “HEMOCOLLAGENE®” in Patients Requiring Oral Surgery. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT05171231?term=sponge&cond=Bleeding&draw=2&rank=9 (15 August 2022, date last accessed).