Abstract
The Bacillus subtilis ResDE two-component system plays a positive role in global regulation of genes involved in aerobic and anaerobic respiration. ctaA is one of the several genes involved in aerobic respiration that requires ResD for in vivo expression. The ctaAB-divergent promoter regulatory region has three ResD binding sites; A1, A2, and A3. The A2 site is essential for in vivo promoter activity, while binding sites A2 and A3 are required for full ctaA promoter activity. In this study, we demonstrate the role of ResD∼P in the activation of the ctaA promoter using an in vitro transcription system. The results indicate that the ctaA promoter (binding sites A2 and A3) has two transcriptional start sites. Binding site A2 was sufficient for weak transcription of the upstream promoter (Pv) by EςA, transcription which was enhanced approximately 1.5-fold by ResD and 5-fold by ResD∼P. The downstream promoter (Ps) required both binding sites A2 and A3 and was not transcribed by EςA with or without ResD∼P. RNA polymerase (RNAP) isolated from B. subtilis when cells were at the end of exponential growth (T0) or 3, 4, or 5 h into the stationary phase (T3, T4, or T 5, respectively) was used in in vitro transcription assays. Maximal transcription from Ps required T4 RNAP plus ResD∼P. RNAP isolated from a spo0A or a sigE mutant strain was not capable of Ps transcription. Comparison of the Ps promoter sequence with the SigE binding consensus suggests that the ctaA Ps promoter may be a SigE promoter. The collective data from ResD footprinting, in vivo promoter deletion analysis, and in vitro transcription assays suggest that ctaA is transcribed during late exponential to early stationary phases of growth from the Pv promoter, which requires ResD binding site A2, EςA, and ResD∼P, and during later stationary phase from Ps, which requires binding sites A2 and A3, ResD∼P, and EςE or a sigma factor whose transcription is dependent on SigE.
The Bacillus subtilis two-component regulatory pair, designated ResD and ResE, has a positive role in global regulation of both aerobic and anaerobic respiration (18, 23). The ResDE system is required for transcription of the following genes and operons involved in aerobic respiration; the resABCDE operon (23), encoding proteins similar to those involved in cytochrome c biogenesis (resABC) (6) and ResD-ResE (resDE) (23); the petCBD operon, encoding subunits of the cytochrome bf complex; the ctaBCDEF operon (12), encoding CtaB, which is required for the synthesis of heme O from heme B (ctaB) (25) and structural genes for cytochrome caa3 (ctaCDEF) (22) and ctaA (23); and a gene required for heme A biogenesis (24, 25) and hence for the synthesis of the heme A-containing terminal cytochrome oxidases aa3 and caa3. Recognition of phenotypic traits shared by resD and ctaA mutants (15) led to a study that revealed that ResD has an essential role in the activation of in vivo expression of the ctaA promoter (23). Phenotypic similarities shared by resD and ctaA mutants, among others, included a sporulation defect and the absence of the heme A-containing terminal oxidases aa3 and caa3. A recent study has shown that either one of these two terminal oxidases is sufficient for sporulation since a qoxABCD (structural genes for aa3) ctaCD (structural genes for caa3) double mutant is sporulation deficient but a single mutant with either mutation is not (28). Thus, the sporulation defect in a resD mutant may be explained by the role of ResD in ctaA and/or ctaB regulation.
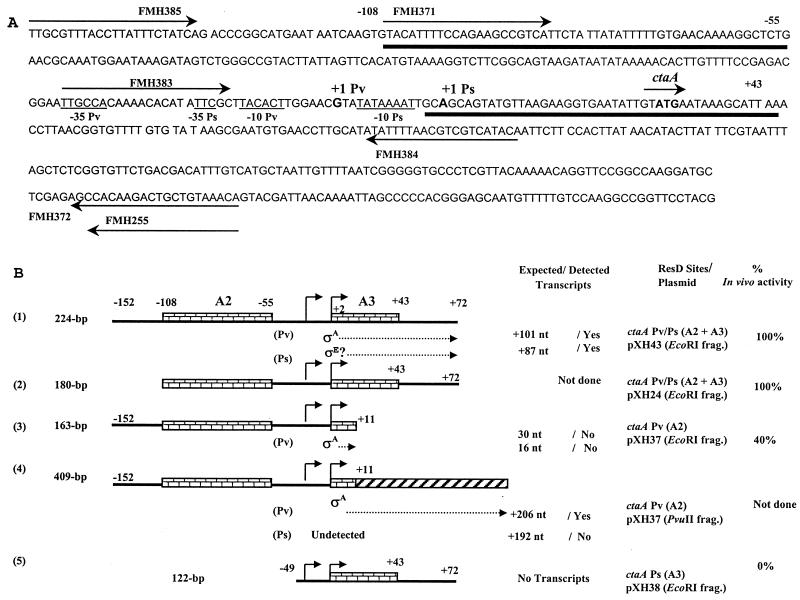
A direct role for ResD in ctaA promoter activation was suggested in a recent study which showed that there are three ResD binding sites (A1, A2, and A3) in the intercistronic ctaAB promoter region to which either unphosphorylated or phosphorylated ResD binds (29). A1 and A2 are situated upstream of the −35 promoter region, and A3 is downstream of the −10 region of the ctaA promoter previously identified (15). Deletion experiments revealed that binding site A1 did not influence the in vivo expression of the ctaA gene (29), suggesting that site A1 may be involved in the regulation of the divergent ctaB promoter, which also requires ResD for expression (12). ctaA-lacZ fusion experiments showed that ResD binding site A2 was essential for ctaA promoter expression in vivo but that both A2 and A3 were required for full ctaA expression. Enhanced binding affinity of ResD to site A2 in the presence of site A3 on the same DNA fragment was considered important for full ctaA promoter activity (29).
Similar in vivo expression patterns have been observed for ctaA and the resA operon, the operon encoding ResD and ResE (23). The levels of expression of both promoters are low during exponential growth, increase significantly during the late exponential stage, reach maximum levels after 4 h into stationary phase (termed T4), and thereafter decrease sharply 4 to 6 h into the stationary phase (23). The decrease in ctaA promoter activity correlates with a decrease in resA transcription, suggesting that decreasing intercellular ResD-ResE protein concentrations may account for the turnoff of ctaA transcription. Expression in promoter deletion constructs containing only the ctaA A2 binding site was induced at the same time as that in the construct with the complete ctaA promoter fusion, reached nearly 40% of the level of the full promoter within 1 h, but failed to increase significantly during stationary growth (29).
The in vitro transcription studies reported here were designed to explore the role of ResD and ResD∼P in ctaA promoter activation. The changing composition of the RNA polymerase (RNAP) holoenzyme during growth has been well established, both during the transition from vegetative growth to stationary growth (1a, 9, 17) and during sporulation (3). Bacterial RNAP has four subunits (α2ββ′) in the core enzyme that are capable of polymerization activity in vitro but requires the specific factor (ς) to initiate transcription from a promoter (14, 27). In Bacillus subtilis, 17 genes are known or believed to encode RNAP sigma subunits (4). The primary sigma factor in a growing Bacillus subtilis cell, ςA, is homologous to ς70 of Escherichia coli (13). Although the ςA protein is present throughout sporulation, its activity decreases markedly during the first 2 h of sporulation (8), a decrease which may result from competition for RNAP by other ς factors (2) or by additional factors affecting the RNAP holoenzyme composition (9, 10). In this study, we demonstrate that ResD∼P is required for maximal transcription of ctaA from a ςA-dependent promoter during exponential growth but that, during stationary phase, ResD is required for transcription from a second ResD-activated promoter using a developmental RNAP holoenzyme, possibly EςE. The contribution to ctaA expression from each promoter depends on stage of growth, since the ςA-dependent in vitro transcript decreases while the transcript from the second promoter increases when RNAP from progressively older cultures is used. ctaA promoter fragments containing only ResD binding site A2 are sufficient for in vitro transcription from the ςA promoter; ctaA promoter fragments containing both ResD binding sites, A2 and A3, are required for in vitro activation of the second promoter.
MATERIALS AND METHODS
Bacterial strains and plasmids.
Bacterial strains used in this work are listed in Table 1. B. subtilis MH5654 was constructed by transforming chromosomal DNA from EU8701 (spoIIG::Ermr) into MH5636 with selection for Emrr. pXH43 containing the ctaA promoter region was constructed by amplifying a 224-bp fragment from B. subtilis JH642 chromosomal DNA by PCR using primers FMH385 (5′-TTG CGT TTA CCT TAT TTC TAT CA-3′) and FMH372 (5′-GGA TCC ACA AAT GTC GTC AGA ACA CCG A-3′). The amplified product was cloned into pCR2.1 and sequenced. Plasmids containing deletions of the ctaA promoter were made by using the same method and primers whose sequences are identified in Fig. 3A. We constructed pXH43 (primers FMH385 and FMH372), pXH24 (primers FMH371 and FMH372), pXH37 (primers FMH385 and FMH384), and pXH38 (primers FMH383 and FMH372). Primers FMH372 and FMH384 contained a BamHI site added at the 5′ end, GGATCC, which is not homologous to adjacent DNA in the ctaA promoter.
TABLE 1.
Bacterial strains and plasmids
| B. subtilis strain or plasmid | Relevant characteristics | Source or reference |
|---|---|---|
| Strains | ||
| JH642 | pheA1 trpC2 | J. A. Hoch |
| EU8701 | pheA1 trpC2 ΔsigE::Ermr | C. P. Moran |
| MH5636 | pheA1, trpC2, and rpoCΩpYQ52, Cmr | 20 |
| MH5654 | pheA1, trpC2, and rpoCΩpYQ52, Cmr ΔsigE::Ermr | Y. Qi |
| Plasmids | ||
| pCR2.1 | Vector for cloning PCR products (Ampr Kanr) | Invitrogen |
| pXH24 | Ampr Kanr (4.0 kb), ctaA promoters A2 and A3 (180 bp) in pCR2.1 | 29 |
| pXH37 | Ampr Kanr (4.0 kb), ctaA promoter A2 (163 bp) in pCR2.1 | 29 |
| pXH38 | Ampr Kanr (3.9 kb), ctaA promoter A3 (122 bp) in pCR2.1 | 29 |
| pXH43 | Ampr Kanr (4.1 kb), ctaA promoters A2 and A3 (224 bp) in pCR2.1 | 29 |
FIG. 3.
ResD binding sites on the ctaA promoter sequence and diagrams of various promoter clones. (A) ctaA promoter sequence and 5′ coding sequence of ctaA showing the ResD and ResD∼P binding sites. The coding and the noncoding sequence of the fragment are shown. The transcriptional start sites are shown in bold and are labeled. The binding sites for both ResD and ResD∼P are represented by bold solid lines below the sequence of the coding strand, and the base pair position of the binding site relative to position +1 of the downstream promoter is marked above the sequence. Primers used for amplification of the ctaA promoter or the various ctaA promoter deletions are shown by arrows (pXH43, FMH385 and FMH372; pXH24, FMH371 and FMH372; pXH37, FMH385 and FMH384; pXH38, FMH383 and FMH372). (B) Diagrams of various ctaA promoter DNA template fragments, pCR2.1 plasmids containing each fragment, expected transcript size from each promoter (Pv or Ps), and in vivo promoter-lacZ fusion expression from each promoter fragment (29). The ResD binding sites (A2 and A3) are marked as brick walls, and the addition of vector DNA is shown as a black candy stripe. The total number of the base pairs in each DNA fragment, the expected and detected in vitro transcription products, and the percentage of maximal in vivo expression are indicated for each promoter fragment. The + or − base pair position used is based on the transcription start site determined for Ps, the downstream promoter. frag., fragment.
Purification of ResD and ResE.
E. coli BL21(DE3) (Novagen) was used as a host for overexpressing ResD or ResE protein. Overexpression and purification of ResD and ∗ResE were performed according to a previously published method (29). ∗ResE is a soluble, N-terminally truncated ResE protein missing its 230 N-terminal amino acids but retaining much of its extended cytoplasmic domain and the complete C-terminal catalytic domain.
Template DNAs for in vitro transcription reactions.
All linear templates used in in vitro transcription assays were DNA fragments digested by standard methods and purified from an agarose gel with a QIAquick gel extraction kit (Qiagen) according to the manufacturer's directions. We used the following templates: a 224-bp linear DNA fragment from pXH43 digested by EcoRI containing binding sites A2 and A3 (see Fig. 3B, section 1), a 163-bp linear DNA fragment from pXH37 digested by EcoRI containing binding site A2, (see Fig. 3B, section 3), and a 122-bp linear DNA fragment purified from pXH38 digested by EcoRI containing binding site A3 (see Fig. 3B, section 5). A2 binding site template DNA was extended by the digestion of pXH37 with the enzyme PvuII (see Fig. 3B, section 4).
Primer extensions.
RNA templates for primer extension experiments were prepared with the buffer and temperature used for in vitro transcription but with 10-fold-greater amounts of template DNA, RNAP, ResD, ResE, and ATP in a 100-μl volume. The reaction mixture was incubated at 37°C for 15 min. ATP, GTP, CTP, and UTP (250 μM each) were added to a final volume of 125 μl. After additional incubation for 15 min at 37°C, the reactions were stopped by the addition of 5 U of RNase-free DNase I (Boehringer Mannheim) and incubation was continued for 20 min. The in vitro-generated RNA templates were extracted with phenol-chloroform. The primer extension reaction mixtures were the same as described previously by Chesnut et al. (1). A sequencing ladder was produced by end labeling the primer FMH255 (5′-ACAAATGTCGTCAGAACACC-3′) with [α-32 P]dATP, annealing it to pXH43, and using Sequenase (United States Biochemical Corp.) according to the instructions of the manufacturer.
Phosphorylation and stability.
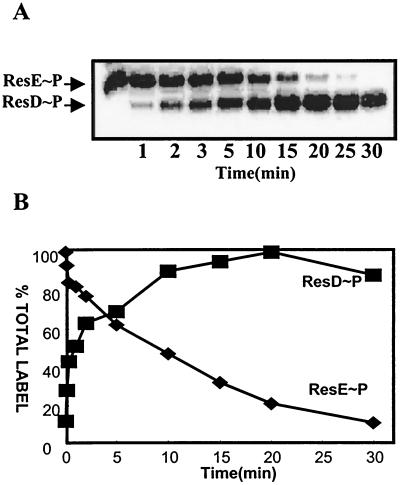
ResE phosphorylation conditions and phosphorylated ResE purification were as described previously (11). In the phosphotransfer reaction mixtures the purified ∗ResE∼P (5 μM) was mixed with an equimolar concentration of ResD in a 180-μl reaction mixture containing P buffer (50 mM HEPES, 50 mM KCl, 5 mM MgCl2 [pH 8.0]). Twenty microliters of each reaction mixture was taken at 0, 1, 2, 3, 5, 10, 15, 20, 25, and 30 min, as indicated in Fig. 1, and the reaction was stopped by addition of 6× sodium dodecyl sulfate (SDS) sample buffer. The phosphoproteins were separated by SDS-polyacrylamide gel electrophoresis (PAGE). To assess the stability of ResD∼P, ResD was phosphorylated by glutathione S-transferase (GST)–ResE∼P bound to glutathione beads and separated from the GST-ResE by a procedure described previously for phosphorylation of PhoP by GST-PhoR∼P (11). ∗ResE∼P or ResD∼P was individually separated by SDS-PAGE on 10% polyacrylamide gels (5), dried, and exposed to PhosphorImaging screens. Products were analyzed using a PhosphorImager.
FIG. 1.
Time course of phosphorylation of ResD by ResE∼P. (A) ∗ResE∼P free from unbound ATP was purified according to the experimental procedures. ∗ResE∼P (5 μM) was mixed with an equimolar concentration of ResD in a 180-μl reaction mixture containing P buffer. The 20-μl aliquots of each reaction mixture were taken at 0, 1, 2, 3, 5, 10, 15, 20, 25, and 30 min, as indicated, and reactions were stopped by addition of 6× SDS sample buffer. The phosphoproteins were separated by SDS-PAGE. The gel was dried and exposed to X-ray film. (B) Quantitation of radioactivity in ResE∼P and ResD∼P by PhosphorImaging. The activities of ResE∼P (⧫) and ResD∼P (■) are shown.
Purification of RNAP and core polymerase.
B. subtilis MH5636 (20) or MH5654 cells which contain a sequence encoding a 10-amino-acid His tag fused to rpoC (gene encoding the β′ subunit of RNAP), were grown in SSG medium (7). Cells were harvested during vegetative growth 2 h before the end of exponential growth (T−2), at the end of exponential growth (T0), or at the T3, T4, or T5 stage of stationary growth by centrifugation (4,000 × g, 30 min). RNAP holoenzyme was purified as previously described (21). To prepare the core enzyme, the holoenzyme (1.2 mg) was applied to a phosphocellulose column (1 by 3 cm) preequilibrated with equilibration buffer (50 mM Tris [pH 8.0], 1 mM EDTA, 0.3 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride and 20% glycerol) containing 100 mM KCl. The column was washed with 20 column volumes of the above-described equilibration buffer. The ςA was released in the flowthrough. The core enzyme was eluted with equilibration buffer containing 600 mM KCl and then dialyzed against storage buffer (10 mM Tris [pH 8.0], 10 mM MgCl2, 100 mM KCl, 0.1 mM EDTA, and 50% glycerol). SDS analysis of purified core polymerase showed two major bands, ββ′ and α. A light band just under ββ′ was judged to be a breakdown product of β subunits. By comparing equal amounts of core and whole polymerase, the bands judged be sigma factors and the δ subunit in the RNAP were not detected in the core preparation. Core enzyme (0.5 pmol) and ςA (15 pmol) were preincubated for 30 min at 4°C before in vitro transcription assays were performed. Purified ςA was provided by John Helmann, Cornell University.
In vitro transcription.
The transcription reaction mixture (20-μl final volume) consisted of 0.08 pmol of template, various concentrations of ResD or ∗ResE, ATP, and 0.4 pmol of purified B. subtilis RNAP (21). The transcription buffer contained 100 mM potassium glutamate, 10 mM Tris (pH 8.0), 0.1 mM EDTA, 50 mM KCl, 1 mM CaCl2, 5 mM MgCl2, 10 μg of bovine serum albumin per ml, 1 mM dithiothreitol, and 5% glycerol. Either ResD alone or ResD-ResE (equimolar concentrations) plus ATP (50 μM) was preincubated with the template at 37°C for 10 min. RNAP or the core polymerase plus ςA was then added to the reaction mixture, and incubation continued at 37°C for 15 min. A single round of transcription was initiated by the addition of 5 μl of transcription buffer containing ATP, GTP, and CTP at 100 μM each, 10 μM UTP, 5 μCi of [α-32P]UTP (Amersham), and 50 μg of heparin per ml. After incubation at 37°C for 15 min, reactions were stopped by the addition of 10 μl of loading dye (7 M urea, 100 mM EDTA, 5% glycerol, 0.05% xylene cyanol, and 0.05% [wt/vol] bromophenol blue). Samples were subjected to electrophoresis on 8 M urea–6% polyacrylamide gels. Dried gels were analyzed with a PhosphorImager.
RESULTS
ResDE phosphotransfer and stability of phosphorylated proteins.
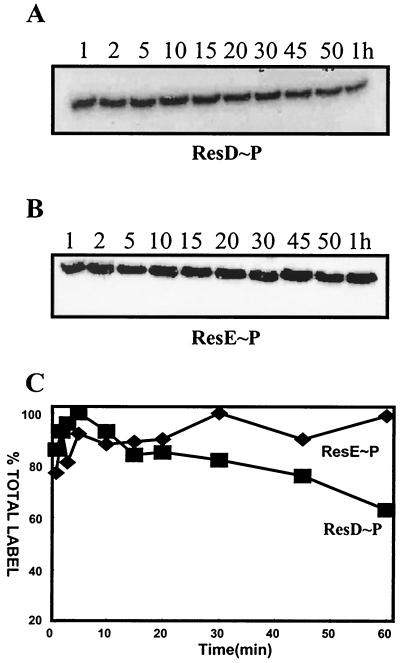
It was recently shown that ResD could be phosphorylated by ∗ResE (29), the soluble catalytic domain of ResE. To examine the time course of transfer of phosphate from ResE∼P to ResD, we incubated ResD with purified ResE∼P protein isolated free from ATP by gel filtration. The result indicates (Fig. 1B) that the phosphorylation of ResD by ResE occurred slowly, requiring approximately 3 min for 50% transfer of phosphate from ResE to ResD. ResD∼P and ∗ResE∼P were isolated to examine the stability of each phosphorylated protein. The level of ResD∼P phosphate (Fig. 2A) decreased slightly after 60 min of incubation, whereas the level of ResE∼P phosphate (Fig. 2B) was stable over 1 h of incubation. The half-life of ResD∼P was calculated to be approximately 2 h (Fig. 2C). These data were incorporated into the ResD∼P in vitro transcription assay design.
FIG. 2.
Stability of ResD∼P and ResE∼P. (A) ResD∼P (3 μM) was purified free from ResE∼P and unbound ATP according to the experimental procedures. The 20-μl aliquots of ResD∼P were taken at 1, 2, 5, 10, 15, 20, 30, 45, 50, and 60 min, as indicated, denatured by addition of 6× SDS sample buffer, and subjected to SDS-PAGE. The dried gel was exposed to a PhosphorImager. (B) Stability of phosphorylated ∗ResE∼P. ∗ResE∼P (3 μM), free from unbound ATP, was treated as described above for ResD∼P. (C) Quantitation of results from panels A and B by PhosphorImaging. The activities of ∗ResE∼P (⧫) and ResD∼P (■) are shown.
ResD∼P enhances in vitro transcription of the ctaA promoter.
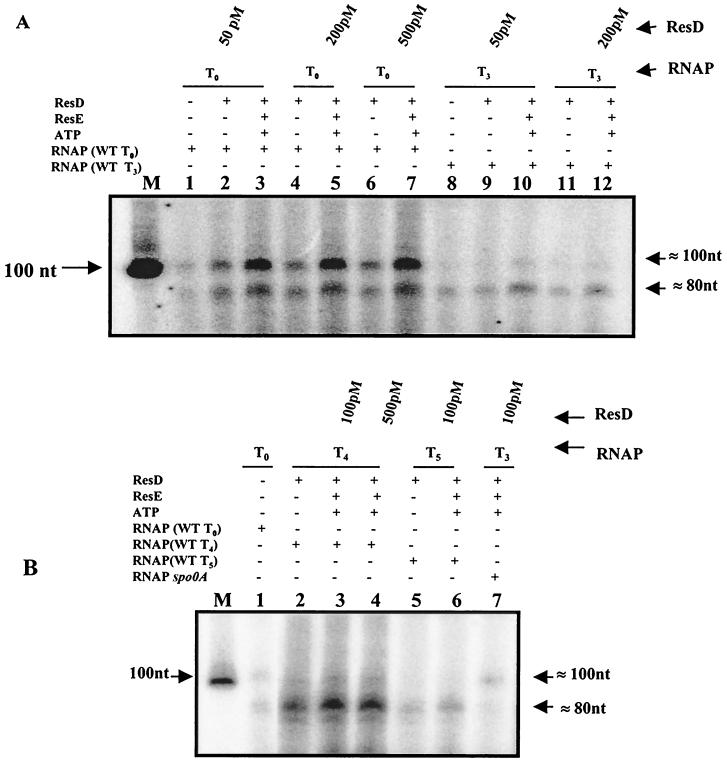
ResD and to a lesser extent ResE are required for ctaA promoter activity in vivo (23). Expression of the ctaA promoter in vivo was initiated during late exponential growth and increased until T4 to T5, after which it is turned off (29). Both ResD and ResD∼P bind to ResD-regulated promoters (16, 29), including the ctaA promoter (Fig. 3A). To study the role of ResD and ResD∼P in ctaA promoter activation, we performed in vitro transcription experiments using purified B. subtilis RNAP isolated at different stages of growth (T0, T3, T4, and T5) in SSG medium. A 224-bp template (EcoRI fragment of pXH43) (Fig. 3B, section 1) shown to be sufficient for full ctaA promoter activity (29) was used as a template. The reactions were performed in the presence of either unphosphorylated ResD or ResD∼P. The results indicate that the transcription of ctaA is controlled by two promoters. (Fig. 4A, lanes 1 to 7). The RNAP isolated from T0-stage cells produced a weak transcript from both the promoters (Fig. 4A, lane 1). The longer transcript (≈100 nucleotides [nt]) was enhanced approximately fivefold (Fig. 4A, lane 3) with 50 pM ResD∼P and did not increase in amount with increasing ResD∼P (Fig. 4A, lanes 5 and 7). The shorter transcript (≈80 nt) was enhanced approximately threefold. Both transcripts showed some enhancement with unphosphorylated ResD. In vitro transcription assays using RNAP from stage T3 with or without ResD resulted in transcription from the downstream promoter that produces only the ≈80-nt transcript (Fig. 4A, lanes 8, 9, and 11). Expression of the shorter transcript was enhanced by ResD∼P, while the ≈100-nt transcript was barely detectable (Fig. 4A, lanes 10 and 12).
FIG. 4.
In vitro transcription of the ctaA promoter with RNAP from stage T0, T3, T4, or T5 cells and with ResD or ResD∼P suggests two promoters. (A) In vitro transcription of the ctaA promoter (224-bp EcoRI fragment from pXH43) with B. subtilis RNAP isolated at stage T0 (lanes 1 to 7) or T3 (lanes 8 to 12) from cells cultured in SSG. Phosphorylation of ResD and in vitro transcription reactions were carried out as described in Materials and Methods. Samples were separated electrophoretically on 8 M urea–6% polyacrylamide gels. WT, wild type; M, 100-nt RNA marker; −, absent; +, present. ResD concentrations are given above the sample lanes. (B) Enhancement of ctaA promoter expression from RNAP isolated from cells at the T4 (lanes 2 to 4) or T5 (lanes 5 and 6) stage of growth in SSG medium and with ResD or ResD∼P. Arrows at the right indicate the ctaA upstream (≈ 100-nt) and downstream (≈ 80-nt) promoter transcripts. In vitro expression of the upstream ctaA promoter (lane 7) using RNAP isolated from a Spo0A mutant strain is shown. All the reaction procedures were the same as those mentioned above.
In vitro transcription using RNAP isolated at stage T4 or T5 showed that the level of the ≈80-nt transcript was further increased in the T4 RNAP reaction (Fig. 4B, lanes 2 to 4) compared to that in the T3 RNAP in Fig. 4A but was significantly decreased in the T5 RNAP reaction (Fig. 4B, lanes 5 and 6). ResD∼P increased transcription especially with the RNAP isolated from stage T4 cells. The longer (≈100-nt) transcript observed using early-transition-stage RNAP from T0 (Fig. 4A, lane 1) was absent in the T4 and T5 reactions. RNAP isolated from a spo0A mutant strain failed to give any transcript from the downstream promoter (Fig. 4B, lane 7), suggesting that the stage T3 to T5 RNAP isolated from the wild-type strain contains a ς factor dependent on Spo0A which is required for expression of the downstream ctaA promoter.
Determination of the ctaA transcription start sites.
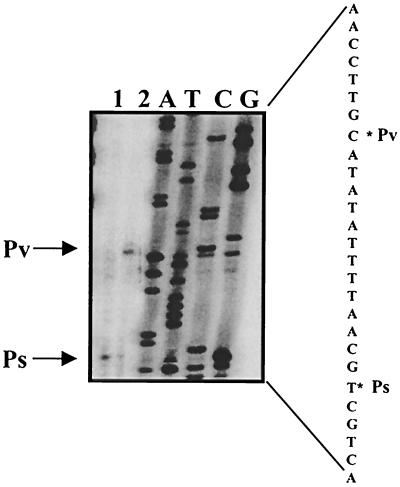
To determine the transcription initiation sites within the ctaA promoter, we extracted RNA from in vitro transcription assays. Primer FMH255 (5′-ACAAATGTCGTCAGAACACC-3′), shown in Fig. 3A, was used for mapping the start site(s). Two transcriptional start sites were determined using in vitro-derived mRNA from T0 RNAP (Fig. 5, lane 2). The product from the upstream promoter was more abundant, as was predicted from the results shown in Fig. 4A (lanes 3, 5, and 7). ctaA mRNA generated using RNAP isolated from stage T4 clearly identified the downstream transcription start site (Fig. 5, lane 1). The two transcriptional start sites observed here and whose products are shown in Fig. 4A (lanes 2 to 7) correspond to the start sites previously proposed (15) in a study that mapped the downstream promoter start site by high-resolution S1 nuclease mapping using RNA from cells at stage T2.
FIG. 5.
Primer extension analysis of the ctaA promoter determines two transcriptional start sites. The end-labeled primer (FMH255) was annealed to RNA and then extended with reverse transcriptase. In lane 1, mRNA was synthesized in an in vitro transcription reaction mixture containing ResD∼P, the DNA template (EcoRI fragment of pXH43) (Fig. 3B, section 1), and RNAP isolated from cells at stage T4 in SSG medium. In lane 2, mRNA was synthesized in an in vitro transcription reaction mixture containing ResD∼P, the DNA template (EcoRI fragment of pXH43) (Fig. 3B), and RNAP isolated from cells at stage T0 in SSG medium. Lanes A, T, C, and G contain sequencing ladders generated by annealing the same end-labeled primer to a plasmid (pXH43) containing the 5′ end of ctaA and extending it with Sequenase (United States Biochemical Corp.). The sequence of the region is indicated at the right. The asterisks indicate the base to which the primer extension products map.
ResD∼P, the RNAP core enzyme, and ςA are sufficient for enhanced expression from the ctaA upstream (Pv) promoter in vitro, while the downstream promoter (Ps) requires EςE or a sigma factor dependent on EςE.
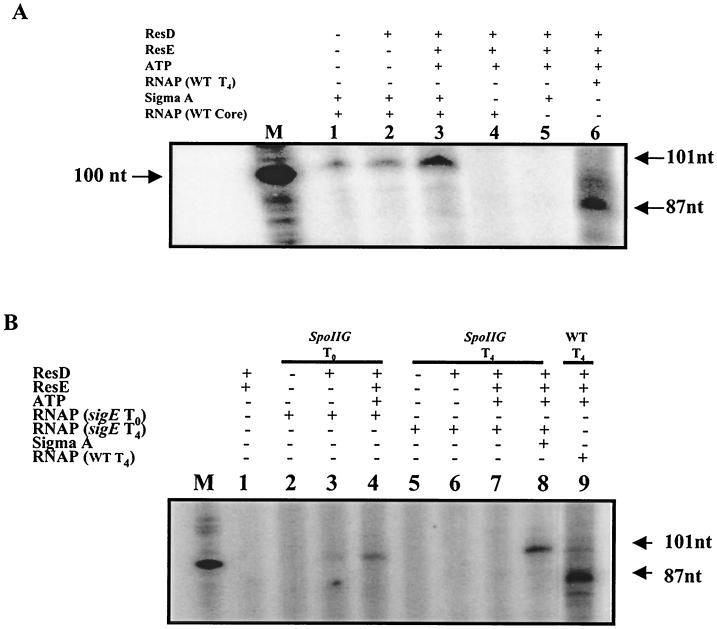
In vitro transcription experiments using purified B. subtilis RNAP core enzyme and/or purified ςA identified the upstream ctaA promoter as a ςA promoter. The addition of core RNAP plus ςA resulted in a transcript (Fig. 6A, lane 1) similar in size to that observed using RNAP isolated from T0 (101-nt transcript) (Fig. 4A, lanes 1 to 7), which was enhanced by ResD∼P (Fig. 6A, lane 3) but only slightly by unphosphorylated ResD (Fig. 6A, lane 2). Addition of core enzyme or ςA alone (Fig. 6A, lanes 4 and 5) gave no transcript from the upstream promoter.
FIG. 6.
ResD∼P plus the RNAP core enzyme with ςA is sufficient for in vitro expression from the upstream ctaA (Pv) but not for expression of the downstream (Ps) promoter. (A) Lane 1, core RNAP plus ςA; lane 2; core RNAP plus ςA and ResD; lane 3, core RNAP plus ςA and ResD∼P; lane 4, core RNAP alone; lane 5, ςA alone; lane 6, T4 stage RNAP plus ResD∼P. (B) Lane 1, no RNAP; lane 2, T0 sigE mutant RNAP; lane 3, T0 sigE mutant RNAP plus ResD; lane 4, T0 sigE mutant RNAP plus ResD∼P; lane 5, T4 sigE mutant RNAP; lane 6, T4 sigE mutant RNAP plus ResD; lane 7, T4 sigE mutant RNAP plus ResD∼P; lane 8, T4 sigE mutant RNAP plus ResD∼P and ςA; lane 9, T4-stage RNAP plus ResD∼P. The DNA template, reaction mixture, and sample analysis were as described for Fig. 4. WT, wild type; −, absent; +, present; M, 100-nt RNA marker. ResD was added at 100 pMol. ResE was added at 100 pMol.
It has been reported that there is a sharp decrease in ςA activity during the first 2 h after the onset of sporulation in B. subtilis (8, 26). Using the same RNA template with T4 RNAP and ResD∼P resulted in a transcription product (Fig. 6A, lane 6) similar in size to that observed in Fig. 4A, lanes 8 to 12.
The ctaA downstream promoter contains sequences similar to those of SigE-regulated promoters. To determine if SigE or a sigma factor whose synthesis depends on SigE was required for transcription from the downstream promoter, we isolated RNAP from a sigE mutant strain, MH5654, at stage T0 and at T4. SDS-gel comparison of T4 RNAP from the parental stain (JH642) with that from the sigE mutant strain showed the absence of SigE protein in RNAP from the sigE mutant (data not shown). In vitro transcription experiments using the same template and RNAP isolated from the sigE mutant strain (T0 or T4) resulted in no transcript from the downstream promoter (Fig. 6B, lanes 2 through 8) with or without ResD∼P. Using T0 RNAP from the sigE strain, a transcript from the upstream ctaA ςA promoter was obtained in the presence of ResD∼P (Fig. 6B, lane 4), a transcript that could be obtained with the T4 RNAP only when ςA was added to the reaction (Fig. 6B, lane 8), indicating that the T4 RNAP from the sigE mutant was functional but lacked sigma factors required for either ctaA promoter.
These data suggest that during vegetative growth, the expression of the ctaA promoter is from EςA polymerase initiated at the upstream Pv (P vegetative) promoter and that, at the onset of stationary phase, ςA is replaced by another ς factor, possibly SigE, resulting in ctaA transcription from the downstream Ps (P stationary) promoter. Expression of both Pv and Ps is enhanced by ResD∼P.
ResD binding site 2 is sufficient for in vitro expression of the ctaA Pv promoter, and binding sites 2 and 3 are required for in vitro expression of the ctaA Ps promoter.
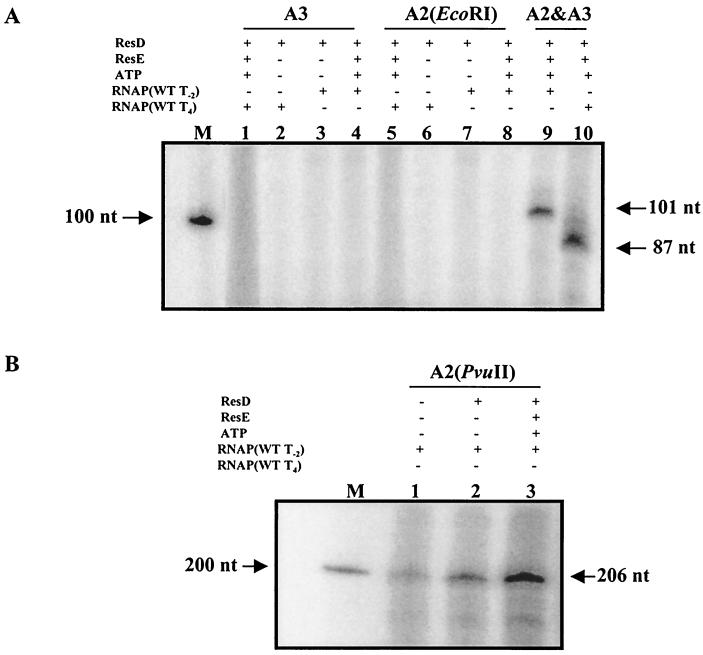
The ctaA-ctaB-divergent promoter region has three ResD binding sites (A1, A2, and A3). Binding site A2 is essential for ctaA promoter activity in vivo, and A2 and A3 are required for full promoter activity (29). To study the role of these binding sites with ResD in vitro, we used the same promoter fragments used in the lacZ promoter fusions in vivo, which are illustrated in Fig. 3B. RNAP isolated from vegetative T−2 or stationary-stage T4 cells gave no transcript (Fig. 7A, lanes 1 to 4) from the promoter region containing only binding site A3 and the −10 and −35 sequences of both promoters, Pv and Ps (pXH38, EcoRI digestion) (Fig. 3B, section 5). This result corroborates in vivo data which showed that this promoter-lacZ fusion containing the A3 site alone was not functional in vivo (29). The 224-bp template (pXH43, EcoRI digestion) (Fig. 3B, section 1) including sites A2 and A3, which retained full ctaA promoter-lacZ expression in vivo, was transcribed from the Pv promoter by vegetative RNAP (T−2) and ResD∼P (Fig. 7A, lane 9) and from Ps by the stage T4 RNAP and ResD∼P (Fig. 7A, lane 10). The 163-bp template including binding site A2 alone (from pXH37 with EcoRI digestion) (Fig. 3B, section 3) did not show a transcript (Fig. 7A, lanes 5 to 8), indicating that either there was no transcript or the expected 30-nt transcript could not be resolved in this gel system. Digestion of pXH37 with PvuII placed vector DNA adjacent to the ctaA promoter fragment, extending the sizes of the expected runoff transcripts from Pv to 206 nt and from Ps to 192 nt (Fig. 3B, section 4). Using this template and vegetative RNAP alone, a weak transcript was visible (Fig. 7B, lane 1). ResD∼P in the in vitro transcription reaction increased the level of transcription significantly (Fig. 7B, lane 3). No transcription resulted using the stage T4 RNAP with this A2 extended template (data not shown), suggesting that both the A2 and A3 ResD binding sites are required for Ps activation. Together, these data suggest that the in vivo transcription study (29) that showed that the A2 ResD binding site was sufficient for ctaA promoter function reported ctaA Pv promoter function and that the promoter fusion containing A2 and A3 required for full promoter expression reported both ctaA Pv and Ps promoter functions.
FIG. 7.
The upstream promoter (Pv) requires only ResD binding site A2, while the downstream promoter (Ps) requires both A2 and A3 ResD binding sites. (A) Lanes 1 to 4, 122-bp template DNA fragment from an EcoRI digestion of pXH38 containing only ResD binding site A3 (Fig. 3B, section 5; lanes 5 to 8, 163-bp template DNA fragment from an EcoRI digestion of pXH37 containing only ResD binding site A2 (Fig. 3B, section 3); lanes 9 and 10, 224-bp template DNA from an EcoRI digestion of pXH43 containing ResD binding sites A2 and A3 (Fig. 3B, section 1). (B) Lanes 1 to 3, 409-bp DNA fragment from a PvuII digestion of pXH37 containing only ResD binding site A2 (Fig. 3B, section 4). WT, wild type; −, absent; +, present; M, 200-nt RNA marker. ResD was added at 100 pmol. ResE was added at 100 pmol.
DISCUSSION
In vivo expression of ctaA is dependent on resD (23). DNase I footprinting experiments indicated that ResD or ResD∼P protected three regions in the ctaAB intercistronic region and that the affinity of ResD binding varied at each site, as did the effect of phosphorylation of ResD on DNA binding. The bases protected by ResD at each site were independent of ResD phosphorylation. Promoter deletion analysis showed that ResD binding at site A1 is independent of the other sites. DNA containing the two binding sites closest to the ctaA coding regions A2 and A3 were required for full ctaA promoter expression, and site A2 was essential for expression. An enhanced affinity of ResD∼P for site A2 in the presence of ResD binding site A3 on the same fragment was noted and considered important for full in vivo promoter activity using the promoter fusions containing sites A2 and A3 compared to results with lacZ promoter fusions with only site A2 (29).
The ctaA promoter region required for full promoter expression (bp −152 to +72) contains two promoters; one is a ςA promoter, and the second promoter requires a developmental sigma factor.
The number of in vitro transcripts obtained (one or two) varied, as did the relative concentration of each transcript, depending on the growth stage of the culture from which the RNAP was isolated. RNAP from vegetative cells (T−2) produced a transcript solely from the upstream Pv promoter (Fig. 7, lane 9). The concentration of the Pv transcript relative to that of the Ps promoter was highest using RNAP from cells at T0, and that ratio decreased with RNAP from later-stationary-phase cultures (Fig. 4). Conversely, RNAP from cultures 4 or 5 h later (T4 or T5) was capable of Ps transcription only. We showed that core RNAP plus ςA and ResD∼P was sufficient for in vitro transcription from the Pv promoter but not for that from Ps. The decrease in in vitro expression of Pv relative to that of Ps using RNAP from later-stage cultures is consistent with data which showed that, although ςA is present in the cells during stationary growth and is associated with the core polymerase at T0, it is released from the core RNAP between T2 and T3 (2). The in vitro transcription data also corroborate the in vivo expression data from a lacZ fusion containing only A2, which was induced during late exponential and early stationary growth but failed to increase further, unlike expression from the full promoter fusion (A2 and A3) which continued to increase for 4 h or more into stationary growth. The form of RNAP required for Ps activation was not present in either the spo0A or sigE mutant strain. As Spo0A is required for sigE transcription, these data suggest that SigE, or a sigma factor dependent on SigE transcription, is required for transcription of the ctaA Ps promoter.
Based on sequence analysis of a compilation of 35 SigE-requiring promoters, the following consensus sequence for SigE binding was determined: ATa(18 to 16 bp)cATAca-T, where capital letters represent highly conserved positions and lowercase letters indicate less well conserved positions (J. Helmann, personal communication). The ctaA Ps promoter sequence, tTc(18 bp)tATAaa-T, has 100% conservation of the highly conserved positions in the −10 region consensus (or five out of seven of the positions of the complete SigE −10 region consensus), which suggests that it is likely a SigE promoter.
One of the mysteries of ResD regulation is how and why ResD can recognize and selectively regulate one set of promoters during aerobic respiration and a second set of promoters during anaerobic growth. The in vitro transcription data from the ctaA promoter alone indicate that ResD is capable of activation of promoters controlled by at least two different sigma factors. As the mechanism of ResD activation of additional ResD-requiring promoters is examined, the importance of the ability to facilitate expression of promoters requiring different RNAP holoenzymes to the diverse roles of ResD may be determined. It should be noted here that another B. subtilis response regulator, Spo0A, activates transcription from promoters controlled by different sigma factors, namely, spoIIA, which requires EςH and spoIIG, or spoIIE, which require EςA.
The Role of ResD∼P in ctaA Pv and Ps promoter expression.
Unphosphorylated ResD binds promoters that have been shown to require the resD gene for in vivo activation. This raised the question of the role of ResD phosphorylation in transcription activation. Our results showed that adding ResD to the in vitro reaction mixtures stimulated expression from both Pv and Ps when T0 RNAP was used (Fig. 4A, lanes 1 and 2), although the transcriptional stimulation was greater with ResD∼P (Fig. 4A, lane 3). It is also of interest that very low levels of Pv and Ps transcripts could be detected with T0 RNAP without ResD or ResD∼P. Together, these data suggest that each promoter functions at a low level in the presence of the correct RNAP holoenzyme and that ResD and to a greater extent ResD∼P increase that expression.
Certain other response regulators can bind template DNA without phosphorylation. ResD and ResE are paralogues of PhoP and PhoR. Like ResD, PhoP binds promoter DNA in the unphosphorylated state. Unlike ResD, PhoP is unable to initiate or stimulate transcription of Pho regulon promoters in vitro without being phosphorylated. UhpA, the response regulator for the E. coli uhpT promoter, also binds promoter DNA in vitro in the unphosphorylated state. In this case, phosphorylation of UhpA for transcriptional activation of uhpT is not required when UhpA is overexpressed in vivo (19).
In summary, ctaA is transcribed from two promoters. Maximal induction from each promoter requires ResD∼P in vitro or in vivo. The activation of each promoter requires specific ResD binding sequences and apparently different forms of RNAP holoenzyme. The significance of (i) low-level transcription of each promoter by its specific holoenzyme (independent of ResD) and (ii) the apparent low level of in vitro induction with unphosphorylated ResD is unclear. Perhaps these low levels of ctaA transcription may contribute to the appearance of aa3 terminal oxidase during exponential growth before the impressive ctaA induction, which requires ResD∼P.
ACKNOWLEDGMENTS
This work was supported by a grant (GM33471) from the National Institutes of Health to F.M.H.
We thank Charles Moran for strains, John Helmann for purified sigma, and both for information concerning SigE consensus data. We thank Shaozhgen Xie for purification of certain RNAP preparations and Ying Qi for construction of MH5654.
REFERENCES
- 1.Chestnut R S, Bookstein C, Hulett F M. Separate promoters direct expression of phoAIII, a member of the Bacillus subtilis alkaline phosphatase multigene family, during phosphate starvation and sporulation. Mol Microbiol. 1991;5:2181–2190. doi: 10.1111/j.1365-2958.1991.tb02148.x. [DOI] [PubMed] [Google Scholar]
- 1a.Cosby W M, Zuber P. Regulation of Bacillus subtilis sigmaH (spoOH) and AbrB in response to changes in external pH. J Bacteriol. 1997;179:6778–6787. doi: 10.1128/jb.179.21.6778-6787.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ju J, Mitchell T, Peters III H, Haldenwang W G. Sigma factor displacement from RNA polymerase during Bacillus subtilis sporulation. J Bacteriol. 1999;181:4969–4977. doi: 10.1128/jb.181.16.4969-4977.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kroos L, Yu Y. Regulation of sigma factor activity during Bacillus subtilis development. Curr Opin Microbiol. 2000;3:553–560. doi: 10.1016/s1369-5274(00)00140-5. [DOI] [PubMed] [Google Scholar]
- 4.Kunst F, Ogasawara N, Moszer I, Albertini A M, Alloni G, Azevedo V, Bertero M G, Bessieres P, Bolotin A, Borchert S, Borriss R, Boursier L, Brans A, Braun M, Brignell S C, Bron S, Brouillet S, Bruschi C V, Caldwell B, Capuano V, Carter N M, Choi S K, Codani J J, Connerton I F, Danchin A, et al. The complete genome sequence of the gram-positive bacterium Bacillus subtilis. Nature. 1997;390:249–256. doi: 10.1038/36786. [DOI] [PubMed] [Google Scholar]
- 5.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 6.Le Brun N E, Bengtsson J, Hederstedt L. Genes required for cytochrome c synthesis in Bacillus subtilis. Mol Microbiol. 2000;36:638–650. doi: 10.1046/j.1365-2958.2000.01883.x. [DOI] [PubMed] [Google Scholar]
- 7.Leighton T J, Doi R H. The stability of messenger ribonucleic acid during sporulation in Bacillus subtilis. J Biol Chem. 1971;246:3189–3195. [PubMed] [Google Scholar]
- 8.Linn T G, Greenleaf A L, Shorenstein R G, Losick R. Loss of the sigma activity of RNA polymerase of Bacillus subtilis during sporulation. Proc Natl Acad Sci USA. 1973;70:1865–1869. doi: 10.1073/pnas.70.6.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu J, Cosby W M, Zuber P. Role of lon and ClpX in the post-translational regulation of a sigma subunit of RNA polymerase required for cellular differentiation in Bacillus subtilis. Mol Microbiol. 1999;33:415–428. doi: 10.1046/j.1365-2958.1999.01489.x. [DOI] [PubMed] [Google Scholar]
- 10.Liu J, Zuber P. The ClpX protein of Bacillus subtilis indirectly influences RNA polymerase holoenzyme composition and directly stimulates sigma H dependent transcription. Mol Microbiol. 2000;37:885–897. doi: 10.1046/j.1365-2958.2000.02053.x. [DOI] [PubMed] [Google Scholar]
- 11.Liu W, Hulett F M. Bacillus subtilis PhoP binds to the phoB tandem promoter exclusively within the phosphate starvation-inducible promoter. J Bacteriol. 1997;179:6302–6310. doi: 10.1128/jb.179.20.6302-6310.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu X, Taber H. Catabolite regulation of the Bacillus subtilis ctaBCDEF gene cluster. J Bacteriol. 1998;180:6154–6163. doi: 10.1128/jb.180.23.6154-6163.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lonetto M, Gribskov M, Gross C A. The sigma 70 family: sequence conservation and evolutionary relationships. J Bacteriol. 1992;174:3843–3849. doi: 10.1128/jb.174.12.3843-3849.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mooney R A, Landick R. RNA polymerase unveiled. Cell. 1999;98:687–690. doi: 10.1016/s0092-8674(00)81483-x. [DOI] [PubMed] [Google Scholar]
- 15.Mueller J P, Taber H W. Structure and expression of the cytochrome aa3 regulatory gene ctaA of Bacillus subtilis. J Bacteriol. 1989;171:4979–4986. doi: 10.1128/jb.171.9.4979-4986.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakano M, Zhu Y, LaCelle M, Zhang X, Hulett F M. Interaction of ResD with regulatory regions of anaerobically induced genes in Bacillus subtilis. Mol Microbiol. 2000;37:1198–1207. doi: 10.1046/j.1365-2958.2000.02075.x. [DOI] [PubMed] [Google Scholar]
- 17.Nakano M, Zu Y, Liu J, Reyes D, Yoshikawa H, Zuber P. Mutations conferring amino acid residue substitutions in the carboxy-terminal domain of RNA polymerase alpha can suppress clpX and clpP with respect to developmentally regulated transcription in Bacillus subtilis. Mol Microbiol. 2000;37:869–884. doi: 10.1046/j.1365-2958.2000.02052.x. [DOI] [PubMed] [Google Scholar]
- 18.Nakano M M, Hulett F M. Adaptation of Bacillus subtilis to oxygen limitation. FEMS Microbiol Lett. 1997;157:1–7. doi: 10.1111/j.1574-6968.1997.tb12744.x. [DOI] [PubMed] [Google Scholar]
- 19.Olekhnovich I N, Dahl J L, Kadner R J. Separate contributions of UhpA and CAP to activation of transcription of the uhpT promoter of Escherichia coli. J Mol Biol. 1999;292:973–986. doi: 10.1006/jmbi.1999.3127. [DOI] [PubMed] [Google Scholar]
- 20.Qi Y, Hulett F M. PhoP-P and RNA polymerase sigmaA holoenzyme are sufficient for transcription of Pho regulon promoters in Bacillus subtilis: PhoP-P activator sites within the coding region stimulate transcription in vitro. Mol Microbiol. 1998;28:1187–1197. doi: 10.1046/j.1365-2958.1998.00882.x. [DOI] [PubMed] [Google Scholar]
- 21.Qi Y, Hulett F M. Role of Pho-P in transcriptional regulation of genes involved in cell wall anionic polymer biosynthesis in Bacillus subtilis. J Bacteriol. 1998;180:4007–4010. doi: 10.1128/jb.180.15.4007-4010.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saraste M, Metso T, Nakari T, Jalli T, Lauraeus M, van der Oost J. The Bacillus subtilis cytochrome-c oxidase. Variations on a conserved protein theme. Eur J Biochem. 1991;195:517–525. doi: 10.1111/j.1432-1033.1991.tb15732.x. [DOI] [PubMed] [Google Scholar]
- 23.Sun G, Sharkova E, Chesnut R, Birkey S, Duggan M F, Sorokin A, Pujic P, Ehrlich S D, Hulett F M. Regulators of aerobic and anaerobic respiration in Bacillus subtilis. J Bacteriol. 1996;178:1374–1385. doi: 10.1128/jb.178.5.1374-1385.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Svensson B, Hederstedt L. Bacillus subtilis CtaA is a heme-containing membrane protein involved in heme A biosynthesis. J Bacteriol. 1994;176:6663–6671. doi: 10.1128/jb.176.21.6663-6671.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Svensson B, Lubben M, Hederstedt L. Bacillus subtilis CtaA and CtaB function in haem A biosynthesis. Mol Microbiol. 1993;10:193–201. doi: 10.1111/j.1365-2958.1993.tb00915.x. [DOI] [PubMed] [Google Scholar]
- 26.Tjian R, Losick R. An immunological assay for the sigma subunit of RNA polymerase in extracts of vegetative and sporulating Bacillus subtilis. Proc Natl Acad Sci USA. 1974;71:2872–2876. doi: 10.1073/pnas.71.7.2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uptain S, Kane C, Chamberlin M. Basic mechanisms of transcript elongation and its regulation. Annu Rev Biochem. 1997;66:117–172. doi: 10.1146/annurev.biochem.66.1.117. [DOI] [PubMed] [Google Scholar]
- 28.Winstedt L, von Waschenfildt C. Terminal oxidases of Bacillus subtilis strain 168: one quinol oxidase, cytochrome aa3 or cytochrome bd, is required for aerobic growth. J Bacteriol. 2000;182:6557–6564. doi: 10.1128/jb.182.23.6557-6564.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang X, Hulett F M. ResD signal transduction regulator of aerobic respiration in Bacillus subtilis; ctaA promoter regulation. Mol Microbiol. 2000;37:1208–1219. doi: 10.1046/j.1365-2958.2000.02076.x. [DOI] [PubMed] [Google Scholar]