Abstract
Introduction
As the COVID-19 pandemic evolves, new effective treatment options are essential for reducing morbidity and mortality as well as the strain placed on the healthcare system. Since publication of our initial review on hyperbaric oxygen treatment (HBOT) for hypoxaemic COVID-19 patients, interest in HBOT for COVID-19 has grown and additional studies have been published.
Methods
For this living systematic review update the previously published search strategy (excluding Google Scholar) was adopted with an extension from 1 February 2021 to 1 April 2022. Study inclusion criteria, data extraction, risk of bias estimation and dispute resolution methods were repeated.
Results
Two new studies enrolling 127 patients were included in this update, taking the total to eight studies with 224 patients. Both new studies were randomised controlled trials, one at moderate and one at high risk of bias. Across these eight studies, 114 patients were treated with HBOT. All reported improved clinical outcomes without observation of any serious adverse events. Meta-analysis remained unjustified given the high heterogeneity between studies and incomplete reporting.
Conclusions
This updated living systematic review provides further evidence on the safety and effectiveness of HBOT to treat acute hypoxaemic COVID-19 patients.
Keywords: Hyperbaric medicine, Hypoxia, Infection, SARS-CoV-2
Introduction
More than two years following the first reported case of COVID-19, the SARS-CoV-2 virus has infected over 482 million individuals worldwide, causing over 6.1 million deaths as of 28 March 2022.[ 1] While global vaccination efforts are underway, there are varying rates of both access to and compliance with COVID-19 vaccines across the globe,[ 2 , 3] and the efficacy of the vaccines for new variants of concern remains unclear.[ 4 , 5] Even if COVID-19 eventually becomes endemic, morbidity levels, death rates, and the susceptible proportion of the population are unpredictable.6 Endemic infections can still cause disruptive waves as variants emerge.[ 6] The clinical experience to date suggests that 15 to 20% of COVID-19 patients require oxygen supplementation, and the mortality rate is 20 to 25% of patients requiring intubation and ventilation.[ 7 - 11] Finding treatments to help patients avoid extended hospital stays and intensive care unit (ICU) admission can also help the healthcare system to maintain capacity and recover from surgical and procedural backlogs incurred from the progression of the pandemic. To improve our global efforts to combat COVID-19, there is significant value in assessing novel treatment modalities that show promise in improving clinical outcomes and that could benefit patients in the future.[ 6 , 12]
In 2021, we published a systematic review of the efficacy and safety of hyperbaric oxygen treatment (HBOT) for COVID-19 patients.[ 13] Based on the limited available literature at the time, it was concluded that emerging data may suggest "HBOT is safe and may be a promising intervention to optimise treatment and outcomes in hypoxaemic COVID-19 patients".[ 13] Interest in HBOT for COVID-19 has continued to grow and further clinical evidence is emerging. Given the importance of providing up-to-date evidence to clinicians, policymakers, and patients, particularly in the context of a global pandemic, the original systematic review has been transitioned to a living review. A living systematic review is "a systematic review which is continually updated, incorporating relevant new evidence as it becomes available."[ 14] Active monitoring of the evidence through monthly searches, followed by incorporation and dissemination of any new information that is identified, facilitates timely and up-to-date guidance to clinicians and decision-makers.[ 14] This report is the first update of the original review.
This living systematic review aims to provide an up-to-date synthesis of the available evidence on the efficacy and safety of HBOT for COVID-19 patients to inform clinical decision-making.
Methods
PROTOCOL
The protocols for the original systematic review (CRD42020209933) and for the current living systematic review (CRD42022309553) were registered with the International Prospective Register of Systematic Reviews (PROSPERO). This update is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 Checklist.[ 15]
LIVING SYSTEMATIC REVIEW
We followed the same methods used in the original systematic review.[ 13] These are briefly summarised in Box 1. The search strategies are provided in *Appendix 1 (30.7KB, pdf) . This update repeated the search strategy as previously published (excluding Google Scholar), but updated to 1 April 2022.
Box 1.
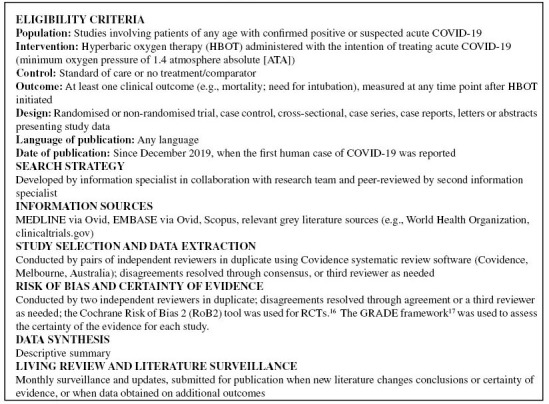
Summary of systematic review methods
For included randomised controlled trials, the Cochrane Risk of Bias 2 (RoB2) tool was used.[ 16] RoB2 assesses whether an individual study has a lower or higher risk of bias according to five domains: bias arising from the randomisation process, bias due to deviations from intended interventions, bias due to missing outcome data, bias in measurement of the outcome, and bias in selection of the reported results.[ 16] The tool also provides an overall risk of bias judgement of low/high/some concerns.[ 16]
The certainty of the evidence for included comparative studies was assessed using the GRADE framework.17 GRADE considers five domains (risk of bias, indirectness, inconsistency, imprecision, and publication bias), and rates the certainty of the evidence as high, moderate, low or very low.[ 17]
Results
STUDY SELECTION
The updated literature search identified 80 potential studies for inclusion, of which 13 were duplicates, and two met inclusion criteria after abstract and full-text screening (Figure 1). A total of eight studies were included in this review (six from the previous review and two from this update).
Figure 1.
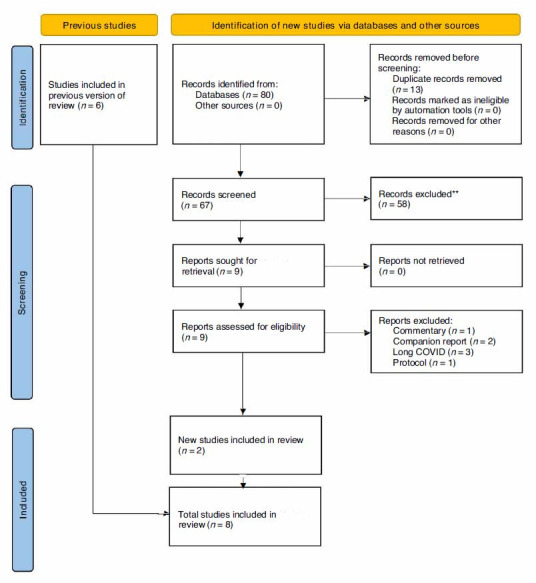
PRISMA Flow Diagram
STUDY AND PATIENT CHARACTERISTICS
An overview of studies included and patient characteristics from both the initial and current updated review is presented in Table 1 and *Appendices 2 (72.2KB, pdf) and 3 (81.8KB, pdf) . Further details on the included studies in this update are available in Table 2. Both the studies identified in this update were conducted outside of North America: one in in Argentina and the other in Russia.
Table 1. Comparison of study and patient characteristics, initial review to current update; atm abs − atmospheres absolute; HBOT – hyperbaric oxygen treatment .
| Parameter | Initial review | Current update | Total |
| Studies (n) | 6 | 2 | 8 |
| Study design | |||
| Case report (n) | 2 | 0 | 2 |
| Case series (n) | 3 | 0 | 3 |
| Cohort study (n) | 1 | 0 | 1 |
| Randomised controlled trial (n) | 0 | 2 | 2 |
| Patient characteristics | |||
| Total patients (n) | 97 | 127 | 224 |
| Patients treated with HBOT (n) | 37 | 77 | 114 |
| Female (%) | 12 (12.4) | 58 (45.7) | 70 (31.3) |
| Age range (years) | 24–87 | NR | 24–87 |
| Intervention details | |||
| Length of sessions (minutes) | 60–100 | 40–90 | 40−100 |
| Mean number of sessions | 1–7 | 4−6 | 1−7 |
| Pressure range kPa / atm abs | 152–203 / 1.5–2.0 | 141–162 / 1.4–1.6 | 141–203 / 1.4–2.0 |
Table 2. Characteristics of new studies included in this update; HBOT – hyperbaric oxygen treatment; ICU – intensive care unit; PCR – polymerase chain reaction; RCT – randomised controlled trial .
| Reference | Study design | Inclusion criteria | Exclusion criteria | Intervention | Control |
| Cannellotto[18] | RCT, n = 40 (20 per group), three centres | “Patients in emergency department or ICU, > 18 years of age, with confirmed diagnosis of COVID-19 by PCR or nasal swab, with pneumonia with oxygen dependence and no previous hospitalisation within the last 6 months.” | “Patients unable to give consent, were pregnant or breast feeding, required mechanical ventilation, were unable to maintain prolonged sitting position (≥ 2 h) or had contraindications for HBOT.” | Monoplace 147 kPa 90 minutes ≥ 5 sessions Once daily | Standard of care |
| Petrikov[19] | RCT, n = 87 (57 HBOT, 30 control), single centre Two HBOT subgroups based on start of HBOT after admission: Group 1 (≤ 7 days): n = 28 Group 2 ( > 7 days): n = 24 | Patient admitted to hospital and clinical diagnosis of COVID-19 | Not reported | Monoplace 142−162 kPa 40 minutes Number of sessions and frequency not reported | Standard of care |
This update identified two randomised controlled trials, one which is single centre[ 19] and the other which is multicentre.[ 18] Across all eight included studies, there were 224 patients (initial review: n = 97; update: n = 127). Of these, 114 were treated with HBOT (initial review: n = 37; update: n = 77). HBOT sessions ranged from 40 to 100 minutes, and the number of sessions ranged from one to seven. The pressure used ranged from 141-203 kPa (1.4-2.0 atmospheres absolute [atm abs]).
RISK OF BIAS
In the multicentre randomised controlled trial,[ 18] risk of bias was found to be low across all domains but one ("risk of bias due to deviations from the intended interventions") rendering an overall risk of bias assessment of "some concerns" with high certainty of evidence. In the single centre randomised controlled trial,[ 19] risk of bias was either found to be of some or high concern across each domain except for the domain "risk of bias due to missing outcome data", which was deemed low risk. Overall, this study[ 19] was rated as high risk of bias with moderate certainty of evidence. The risk of bias assessment for each study is provided in *Appendix 4 (24.1KB, pdf) .
EFFECTIVENESS OF HBOT FOR COVID-19
The two studies in this update assessed clinical outcomes (Table 3). Petrikov's study also assessed certain biological outcomes.[ 19] Improvements in all outcomes assessed for patients who were treated with HBOT compared to the control group were observed by both studies (Table 3). Across all eight studies, improvements were observed for a number of clinical outcomes for HBOT at pressures anywhere between 141 and 203 kPa (1.4 and 2.0 atm abs), including: in-hospital survival, median days to recovery, oxygen saturation, respiratory rate, shortness of breath, need for respiratory support, and walking distance (*Appendix 5 (107.2KB, pdf) ). Five studies[ 18 - 22] reported patients treated with HBOT were able to avoid mechanical ventilation and one study reported improvement in the ordinal clinical outcomes scale.[ 19] Figure 2 summarises the number of patients who improved versus did not improve for each outcome, where data were available.
Table 3. Characteristics of new studies included in this update; HBOT – hyperbaric oxygen treatment; ICU – intensive care unit; PCR – polymerase chain reaction; RCT – randomised controlled trial .
| Reference | Patients (n) | Timing of outcome measurement | HBOT sessions Mean (SD) | Biological outcomes | Imaging outcomes | Safety outcomes |
| Cannellotto[18] | 40 (20 per group) | Within 30 days after admission | 6.2 (1.2) | Nil | Nil | Ear discomfort (n=1) |
| Clinical outcomes | Primary outcome - proportion of patients that recovered from hypoxaemia (SpO2 ≥ 93%) Control group: Day 3, 1 (8%); Day 5, 8 (40%); Day 10, 13 (65%); Day 15, 16 (80%) HBOT group: Day 3, 11 (55%); Day 5, 19 (95%); Day 10, 20 (100%); Day 15, 20 (100%) Odds ratio (OR) of recovery from hypoxaemia (SpO2 ≥ 93%) for HBOT vs. control group Day 3, 23.2 (95% CI 1.6 to 329.6, P = 0.001); Day 5, 28.5 (95% CI 1.8 to 447.4, P < 0.001) | |||||
| Co-primary outcome - median time to recovery HBOT group: median (IQR) 3 (1.0–4.5) days; Control group: 9 (5.5–12.5) days (P < 0.010) | ||||||
| Secondary outcomes Acute respiratory distress Control group: 3 (15%); HBOT group: 3 (15%) (P = 0.61) Mechanical ventilationControl group: 3 (15%); HBOT group: 1 (5%) (P = 0.61) Death Control group: 1 (5%), HBOT group: 1 (5%) (P = 1.00) | ||||||
| Petrikov[19] | 87 total 57 randomised to HBOT 30 randomised to control | Assessed over course of HBOT | Subgroup 1: 5.1 (2.5) | Blood malone dialdehyde decreased (HBOT group) from mean (SD) 4.34 (0.52) μmol·l-1 prior to HBOT to 3.98 (0.48) μmol·l-1 at day 7 Total antioxidant activity decreased (HBOT group) from 1.26 (0.28) mmol·l-1 to 1.21 (0.05) mmol·l-1 | Nil | Claustrophobia (n=1) |
| HBOT group divided into two subgroups based on start of HBOT after admission: Group 1 (≤ 7 days), n = 28 Group 2 (> 7 days), n = 24 | Subgroup 2: 4.2 (2.0) | Open circuit potential of platinum electrode decreased (HBOT group) from -22.78 (24.58) mV to -30.45 (15.32) mV Apoptotic lymphocytes showed no significant change | Pain in the ears (n=4) | |||
| Clinical outcomes | Oxygen saturation (SpO2), Mean (SD) Control group: Day 1, 92.6 (4.2); Day 3, 90.4 (4.9); Day 7, 91.3 (4.8); Day 14, 93.4 (4.7) HBOT group 1: Day 1, 91.9 (5.3); Day 3, 91.4 (3.8); Day 7, 92.1 (3.9); Day 14*, 96.1 +/- 2.8 (*P < 0.05 vs. baseline and vs. control) | |||||
| National Early Warning Score (NEWS2), Mean (SD) Control group: Day 4, 4.7 (2.3) points; Day 10, 4.0 (2.3) points HBOT group 1: Before HBOT, 4.4 (2.2) points; After HBOT, 1.2 (1.7) points (P < 0.05 for difference from the baseline in the HBOT group and from the control group value at day 10) | ||||||
| Ordinal scale for clinical improvement score, Mean (SD) Control group: Day 4, 4.1 (0.7) points; Day 10, 3.9 (0.8) points HBOT group 1: Before HBOT, 4.2 (0.7) points; After HBOT, 3.0 (0.6) points (P < 0.05 for difference from the baseline in the HBOT group and from the control group value at day 10) | ||||||
| Respiratory support (defined as oxygen supplementation through nasal cannulae or face mask with a flow of 3–6 l·min-1, in severe cases using high-flow oxygen therapy or non-invasive lung ventilation) Non-invasive ventilation/high-flow oxygen therapy (P not reported) Control group: Day 4, 10 (33.3%); Day 10, 8 (26.7%) HBOT group 1: Before HBOT, 9 (32.1%); After HBOT, 0 (0%) Oxygen supplementation with flow rate 3–6 l·min-1 (P not reported) Control group: Day 4, 14 (46.7%); Day 10, 13 (43.3%) HBOT group 1: Before HBOT, 15 (53.6%); After HBOT, 5 (17.9%) No respiratory support required (P not reported) Control group: Day 4, 6 (20%); Day 10, 9 (30%) HBOT group 1: Before HBOT, 4 (14.3%); After HBOT, 23 (82.1%) | ||||||
Figure 2.
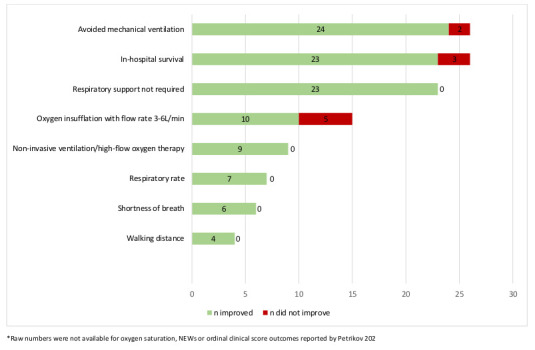
Summary of clinical outcomes for COVID-19 patients treated with HBOT, when reported, across all eight reviewed studies
Discussion
This living systematic review update identified two new studies published since the completion of the original review. As of this update, there are now eight studies which have assessed the efficacy and safety of HBOT for treating patients with COVID-19. Of note, the two new studies included in this update are the first randomised controlled trials published. Although continued investigation through rigorously conducted multicentre randomised controlled trials is still needed to draw definitive conclusions, the available evidence suggests HBOT may be an effective adjunctive treatment for COVID-19. It is difficult to comment definitively on safety on the basis of treating 114 patients but no serious adverse events have been reported in the reviewed studies.
In addition to the risk of bias present in the available studies, another challenge when assessing the effectiveness of HBOT for acute hypoxaemic COVID-19 patients is the lack of consensus in outcomes selection in the existing literature. We found a wide range of observed clinical outcomes, such as improved oxygen saturation, respiratory rate, walking distance, and in-hospital survival as well as avoiding the need for mechanical ventilation. The hyperbaric medicine community should develop a minimum set of core outcomes to be used in every study on HBOT for acute COVID-19. This could incorporate the World Health Organization ordinal COVID-19 scale that captures the main patient-centred outcomes into a single tool.[ 23 , 24] Consistent reporting of individual patient data across studies would also be beneficial for supporting future meta-analyses.
Based on published evidence, HBOT is a promising therapeutic option that could contribute to reduce the strain new variants continue to place on the healthcare system based on the ability to improve oxygenation without the need for intubation or mechanical ventilation. The published evidence reports a positive clinical effect of HBOT for acute hypoxaemic COVID-19 patients regardless of their specific HBOT regimen. Importantly, most studies found this positive clinical effect after just a few days, typically less than seven days. Interestingly, a number of reports suggest the mechanisms of action of HBOT in COVID-19 patients may include immunoregulatory effects in addition to correcting the oxygen debt.[ 25 - 29] These suggestions may have implications for other septic conditions for future research.
Even if the incidence of Omicron, the current dominant variant reduces, it is likely that new variants will continue emerge and their potential impact is unpredictable.[ 30] There are varying rates of both access to and compliance with COVID-19 vaccines across the globe,[ 2 , 3] and vaccine efficacy has been shown to wane over time.[ 31] Although current vaccines offer a certain immunity against new variants of concern, the protection level varies.[ 4 , 5] Despite intense research and some therapeutic progress, more low-cost, safe, effective and scalable treatment options are needed.[ 32] HBOT is an already approved drug/intervention for non-COVID-19 indications that is minimally invasive. It can be employed across a wide range of case-severity, unlike other interventions, which may be limited to narrow patient subgroups or time frames.[ 33 - 39] HBOT would not be subject to supply chain disruptions and product shortages, which have been observed throughout the pandemic for pharmaceutical interventions.[ 40] Of course, HBOT has some limitations such as chamber availability and requirement for transfer to a chamber from the hospital ward which should be acknowledged.
This living systematic review will be updated as required following monthly repetition of our search strategy. Our narrative review will be modified and meta-analysis will be performed as appropriate. The updated review will be submitted for publication when new literature changes the conclusions and/or certainty of evidence or when data are obtained on additional outcomes.
LIMITATIONS
This review is subject to several limitations. First, there are discrepancies in the quality of reporting between studies. Designing reporting guidelines specific to hyperbaric medicine is paramount to improvement in the quality of publication. Secondly, findings may be subject to various degrees of bias found in this review. In addition, a few studies that are likely to be relevant to our review are completed but not yet published according to registration databases.
Conclusions
This updated living systematic review provides further evidence on the promising effectiveness of HBOT to treat hypoxaemic acute COVID-19 patients.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
Acknowledgements
George Djaiani, MD, for his contributions to the original systematic review.
Conflict of interest and funding
Dr Tricco was funded by a Tier 2 Canada Research Chair in Knowledge Synthesis. Dr Boet was supported by The Ottawa Hospital Anesthesia Alternate Funds Association and the Faculty of Medicine, University of Ottawa with a Tier 2 Clinical Research Chair. No conflicts of interest were declared.
Contributor Information
Sylvain Boet, Department of Anesthesiology and Pain Medicine, The Ottawa Hospital, Ottawa, Canada; Clinical Epidemiology Program, The Ottawa Hospital Research Institute, Ottawa, Canada; Francophone Affairs, Faculty of Medicine, University of Ottawa, Ottawa, Canada.
Cole Etherington, Clinical Epidemiology Program, The Ottawa Hospital Research Institute, Ottawa, Canada.
Nibras Ghanmi, Department of Public Health Sciences, Faculty of Health Sciences, Queens University, Ontario, Canada.
Paul Ioudovski, Department of Public Health Sciences, Faculty of Health Sciences, Queens University, Ontario, Canada.
Andrea C Tricco, Li Ka Shing Knowledge Institute, St Michael’s Hospital, Unity Health Toronto, Toronto, Ontario, Canada; Epidemiology Division and Institute of Health Policy, Management, and Evaluation, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada; Queen’s Collaboration for Health Care Quality: A JBI Centre of Excellence, Ontario, Canada.
Lindsey Sikora, Health Sciences Library, University of Ottawa, Ottawa, Canada.
Rita Katznelson, Department of Anesthesia and Pain Management, University Health Network, Toronto, Ontario, Canada.
References
- World Health Organization WHO coronavirus disease (COVID-19) dashboard; c2022. [cited 2022 Mar 28]. Available from: https://covid19.who.int/.
- Our World in Data . Coronavirus (COVID-19) vaccinations; c2022. [cited 2022 Mar 28]. Available from: https://ourworldindata.org/covid-vaccinations.
- Dolgin E. Omicron is supercharging the COVID vaccine booster debate. Nature. 2021;Dec 2:Online ahead of print. doi: 10.1038/d41586-021-03592-2. [DOI] [PubMed] [Google Scholar]
- Karim SSA, Karim QA. Omicron SARS-CoV-2 variant: a new chapter in the COVID-19 pandemic. Lancet. 2021;398(10317):2126–28. doi: 10.1016/S0140-6736(21)02758-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torjesen I. Covid-19: Omicron may be more transmissible than other variants and partly resistant to existing vaccines, scientists fear. BMJ. 2021;375:n2943. doi: 10.1136/bmj.n2943. [DOI] [PubMed] [Google Scholar]
- Katzourakis A. COVID-19: endemic doesn’t mean harmless. Nature. 2022;601:485. doi: 10.1038/d41586-022-00155-x. [DOI] [PubMed] [Google Scholar]
- Guan W-J, Ni Z-Y, Hu Y, Liang W-H, Ou C-Q, He J-X, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–20. doi: 10.1056/NEJMoz2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling L, So C, Shum HP, Chan PKS, Lai CKC, Kandamby DH, et al. Critically ill patients with COVID-19 in Hong Kong: a multicentre retrospective observational cohort study. Crit Care Resusc. 2020;22:119–25. doi: 10.51893/2020.2.oa1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatraju PK, Ghassemieh BJ, Nichols M, Kim R, Jerome KR, Nalla AK, et al. COVID-19 in critically ill patients in the Seattle region – case series. N Engl J Med. 2020;382:2012–22. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–62. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Sun J, Dai Z, Deng H, Li X, Huang Q, et al. Prevalence and severity of coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. J Clin Virol. 2020;127:104371. doi: 10.1016/j.jcv.2020.104371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby J. Hyperbaric oxygen therapy for patients with COVID-19. Emerg Med J. 2022;39:86–7. doi: 10.1136/emermed-2021-212015. [DOI] [PubMed] [Google Scholar]
- Boet S, Etherington C, Djaiani G, Tricco AC, Sikora L, Katznelson R. Efficacy and safety of hyperbaric oxygen treatment in SARS-COV-2 (COVID-19) pneumonia: a systematic review. Diving Hyperb Med. 2021;51:271–81. doi: 10.28920/dhm51.3.271-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooker J, Synnot A, McDonald S, Elliott J, Turner T, Hodder R, et al. Guidance for the production and publication of Cochrane living systematic reviews: Cochrane reviews in living mode; c2019. [cited 2022 Apr 19]. Available from: https://community.cochrane.org/sites/default/files/uploads/inline-files/Transform/201912_LSR_Revised_Guidance.pdf
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:I4898. doi: 10.1136/bmj.I4898. [DOI] [PubMed] [Google Scholar]
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction – GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64:383–94. doi: 10.1016/j.clinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- Cannellotto M, Duarte M, Keller G, Larrea R, Cunto E, Chediack V, et al. Hyperbaric oxygen as an adjuvant treatment for patients with COVID-19 severe hypoxaemia: a randomised controlled trial. Emerg Med J. 2022;39:88–93. doi: 10.1136/emermed-2021-211253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrikov SS, Evseev AK, Levina OA, Shabanov AK, Kulabukhov VV, Kutrovskaya NY, et al. Hyperbaric oxygen therapy in patients with COVID-19. Gen Reanimatol. 2021; 16: 4- 18. doi: 10.15360/1813-9779-2020-6-4-18. [cited 2022 May 20]. Available from: https://www.reanimatology.com/rmt/article/view/1983/1460 [DOI] [Google Scholar]
- Gorenstein SA, Castellano ML, Slone ES, Gillette B, Liu H, Alsamarraie C, et al. Hyperbaric oxygen therapy for COVID-19 patients with respiratory distress: treated cases versus propensity-matched controls. Undersea Hyperb Med. 2020;47:405–13. doi: 10.22462/01.03.2020.1. [DOI] [PubMed] [Google Scholar]
- Thibodeaux K, Speyrer M, Raza A, Yaakov R, Serena TE, et al. Hyperbaric oxygen therapy in preventing mechanical ventilation in COVID-19 patients: a retrospective case series. J Wound Care. 2020;29(Sup5a):S4–8. doi: 10.12968/jowc.2020.29.Sup5a.S4. [DOI] [PubMed] [Google Scholar]
- Guo D, Pan S, Wang MM, Guo Y. Hyperbaric oxygen therapy may be effective to improve hypoxemia in patients with severe COVID-2019 pneumonia: two case reports. Undersea Hyperb Med. 2020;47:181–7. doi: 10.22462/04.06.2020.2. [DOI] [PubMed] [Google Scholar]
- World Health Organization . WHO R&D blueprint novel coronavirus COVID-19 therapeutic trial synopsis; c2022. [cited 2022 Apr 19]. Available from: http://www.moh.gov.sa/en/CoronaNew/PressReleases/Pages/default.aspx.
- WHO working group on the clinical characterisation and management of COVID-19 infection. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis. 2020; 20: e192- 7. doi: 10.1016/S1473-3099(20)30483-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinomiya N, Asai Y. Hyperbaric oxygenation therapy: molecular mechanisms and clinical applications. Singapore: Springer Nature: 2019. [Google Scholar]
- Boet S, Martin L, Cheng-Boivin O, Etherington C, Louge P, Pignel R, et al. Can preventive hyperbaric oxygen therapy optimise surgical outcome?: a systematic review of randomised controlled trials. Eur J Anaesthesiol. 2020;37:636–48. doi: 10.1097/EJA.0000000000001219. [DOI] [PubMed] [Google Scholar]
- Thom SR. Oxidative stress is fundamental to hyperbaric oxygen therapy. J Appl Physiol (1985). 2009;106:988–95. doi: 10.1152/japplphysiol.91004.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simsek K, Sadir S, Oter S. The relation of hyperbaric oxygen with oxidative stress-reactive molecules in action. Oxid Antioxid Med Sci. 2015;4:17–22. doi: 10.5455/oams.010415.rv.016. [DOI] [Google Scholar]
- Feldmeier JJ, Kirby JP, Buckey JC, Denham D, Evangelista J, Gelly H, et al. Rationale, study design considerations, and protocol recommendations for treating COVID-19 patients with hyperbaric oxygen; c2022. [cited 2022 Apr 19]. Available from: https://www.uhms.org/images/MiscDocs/Rational_and_study_design_for_treating_COVID_patients_with_HBO2.pdf
- Dhama K, Kumar SK, Iqbal MI, Tiwari R, Sharun K. SARS-CoV-2 existence in sewage and wastewater: a global public health concern? J Environ Manage. 2021; 280: 111825. 10.1016/j.jenvman.2020.111825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naaber P, Tserel L, Kangro K, Sepp E, Jürjenson V, Adamson A, et al. Dynamics of antibody response to BNT162b2 vaccine after six months: a longitudinal prospective study. Lancet Reg Health Eur. 2021;10:100208. doi: 10.1016/j.lanepe.2021.100208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehtabchi S. COVID-19 evidence bites: the NNT; c2020. [cited 2020 Sep 30]. Available from: https://www.thennt.com/review-covid-analysis-2020/.
- Wang M, Wu T, Zuo Z, You Y, Yang X, Pan L, et al. Evaluation of current medical approaches for COVID-19: a systematic review and meta-analysis. BMJ Support Palliat Care. 2021;11:45–52. doi: 10.1136/bmjspcare-2020-002554. [DOI] [PubMed] [Google Scholar]
- Jorda A, Siller-Matula JM, Zeitlinger M, Jilma B, Gelbenegger G. Anticoagulant treatment regimens in patients with COVID-19: a meta-analysis. Clin Pharmacol Ther. 2022;111:614–23. doi: 10.1002/cpt.2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega-Paz L, Galli M, Capodanno D, Franchi F, Rollini F, Bikdeli B, et al. Safety and efficacy of different prophylactic anticoagulation dosing regimens in critically and non-critically ill patients with COVID-19: a systematic review and meta-analysis of randomized controlled trials. Eur Hear J Cardiovasc Pharmacother. 2021;8:677–86. doi: 10.1093/ehjcvp/pvab070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sholzberg M, Tang GH, Rahhal H, AlHamzah M, Kreuziger LB, Áinle FN, et al. Effectiveness of therapeutic heparin versus prophylactic heparin on death, mechanical ventilation, or intensive care unit admission in moderately ill patients with COVID-19 admitted to hospital: RAPID randomised clinical trial. BMJ. 2021;375:n2400. doi: 10.1136/bmj.n2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ATTACC Investigators . ACTIV-4a Investigators . REMAP-CAP Investigators . Lawler PR, Goligher EC, Berger JS, et al. Therapeutic anticoagulation with heparin in noncritically ill patients with COVID-19. N Engl J Med. 2021;385:790–802. doi: 10.1056/NEJMoa2105911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh AK, Majumdar S, Singh R, Misra A. Role of corticosteroid in the management of COVID-19: a systemic review and a clinician’s perspective. Diabetes Metab Syndr. 2020;14:971–8. doi: 10.1016/j.dsx.2020.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RECOVERY Collaborative Group . Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, et al. Dexamethasone in hospitalized patients with COVID-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuman AG, Fox ER, Unguru Y. COVID-19 and drug shortages: a call to action. J Manag Care Spec Pharm. 2020;26:945–7. doi: 10.18553/jmcp.2020.26.8.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


