Abstract
Ultrasound monitoring, both in the form of Doppler and 2D echocardiography, has been used post-dive to detect decompression bubbles circulating in the bloodstream. With large variability in both bubble time course and loads, it has been hypothesised that shorter periods between imaging, or even continuous imaging, could provide more accurate post-dive assessments. However, while considering applications of ultrasound imaging post-decompression, it may also be prudent to consider the possibility of ultrasound-induced bioeffects. Clinical ultrasound studies using microbubble contrast agents have shown bioeffect generation with acoustic powers much lower than those used in post-dive monitoring. However, to date no studies have specifically investigated potential bioeffect generation from continuous post-dive echocardiography. This review discusses what can be drawn from the current ultrasound and diving literature on the safety of bubble sonication and highlights areas where more studies are needed. An overview of the ultrasound-bubble mechanisms that lead to bioeffects and analyses of ultrasound contrast agent studies on bioeffect generation in the pulmonary and cardiovascular systems are provided to illustrate how bubbles under ultrasound can cause damage within the body. Along with clinical ultrasound studies, studies investigating the effects of decompression bubbles under ultrasound are analysed and open questions regarding continuous post-dive monitoring safety are discussed.
Keywords: Bubbles, Cavitation, Decompression, Decompression research, Venous gas emboli
Introduction
Decompression sickness (DCS) is a condition caused by the formation and growth of bubbles from dissolved inert gases in the tissues when the body experiences decompression. The effects of DCS vary from symptoms such as skin itching, joint pain, numbness, and dizziness,[ 1 , 2] to rare but severe outcomes, such as coma or even death.[ 3] In the case of scuba diving, divers breathe gas at ambient pressure throughout the dive. As pressure increases with depth, so do the partial pressures of the inert gases breathed. This results in a pressure gradient from the inspired gas in the lungs to the rest of the body's tissues, which are saturated at sea level. During ascent, the pressure gradient reverses, and supersaturation can drive gas out of solution, resulting in bubbles in the tissues and bloodstream during and after decompression. Bubbles continue to appear in the venous blood for two to three hours post-dive and may cause problems by blocking blood vessels, mechanically distorting tissues, and inducing inflammatory cascades.[ 1]
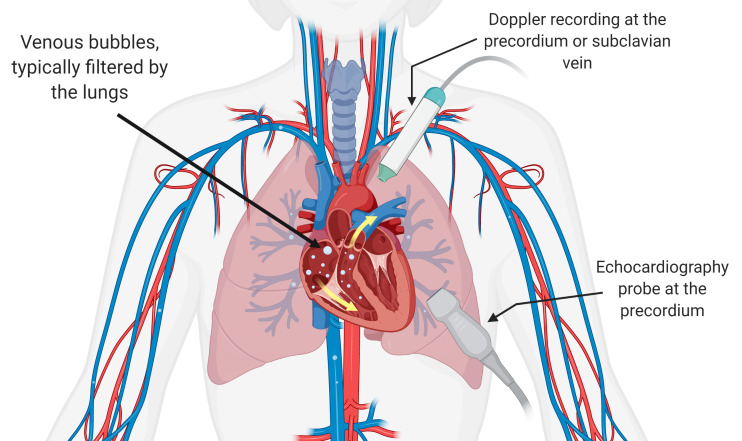
Ultrasound monitoring, both in the form of Doppler and 2D echocardiography, has been used post-dive to detect decompression bubbles in the bloodstream, termed 'venous gas emboli' or VGE. Doppler ultrasound was first used in 1968 to detect intravascular decompression bubbles and became the predominant method for detecting VGE in divers.[ 4] In this case, VGE are detected aurally by employing continuous-wave Doppler detection with a single-element transducer with a separate transducer used as a receiver. This high-frequency sound is reflected by moving intravascular decompression bubbles and results in received chirp-like signals in the auditory range, which can be detected by a trained listener and used to provide a bubble grade.[ 5] More recently, 2D echocardiography using a transducer array has been employed to visualise VGE in the heart. As with Doppler, the evaluation of these cardiac images allows raters to score circulating VGE and provide either a bubble grade or, more recently, employ frame-based bubble counting to evaluate VGE load.[ 6] Although data acquisition is more difficult, training for 2D echocardiography image evaluation is relatively quick,[ 6 , 7] unlike training for Doppler VGE detection, and this ease of training has shown 2D echocardiography to be a more economical form of evaluation compared to Doppler.[ 7] As a result, 2D echocardiography has quickly grown in popularity for post-dive decompression bubble analysis. These two methods of VGE detection are illustrated in Figure 1.
Figure 1.
Venous gas emboli circulating post-dive can be detected using ultrasound via Doppler precordial or subclavian recording (audio) and precordial apical 4-four chamber view echocardiography (video). Note the differing probe placement for the two detection methods
Post-dive VGE analysis is used as a tool for evaluating a diver's likelihood for developing decompression sickness. While VGE analysis cannot be used on its own to determine whether a diver will develop DCS, a lack of VGE is a good indication a diver will not develop DCS.[ 8] Also, despite the low specificity of VGE analysis, there is a definite positive association between VGE load and DCS incidence, with higher VGE grades corresponding to an increase in DCS risk.[ 9] Thus, ultrasound imaging provides a method for screening divers for DCS risk and can be used both for diving physiology research and in the development of decompression schedules for specific diving profiles. From the early use of Doppler in the 1970s to more recent echocardiography studies, it is well-established that there exists large variability in VGE loads not only for different dive profiles but also between subjects and for the same subject undergoing the same controlled dive profile.[ 10 - 12] Additionally, the time course of VGE varies significantly post-dive, so that regular monitoring intervals are paramount for correct quantification.[ 12 - 14] As such, continuous ultrasound monitoring could provide a more accurate post-dive assessment. The development of smaller, more portable echocardiography devices has increased the feasibility of this continuous monitoring. Continuous in-suit Doppler has been employed by NASA for bubble detection,[ 15] but this method has not yet been used for 2D echocardiography.
The increasing popularity of 2D echocardiography for post-dive monitoring and a push towards shorter intervals between image acquisitions or even continuous monitoring demands an evaluation of the safety of these methods. Ultrasound is considered the safest imaging modality to date; however, precautions still need to be taken when seeking to increase sonication time under abnormal imaging conditions, such as in the presence of bubbles in the tissues and bloodstream. In the realm of clinical ultrasound, established guidelines have resulted in the thermal index (TI) to avoid tissue heating and the mechanical index (MI) to avoid mechanical effects of ultrasonic waves on tissues. An MI safety limit of 1.9 is imposed during normal ultrasonic conditions, but more recent studies have shown that, in the presence of microbubble ultrasound vascular contrast agents, bioeffects, such as microvascular leakage, petechiae, cardiomyocyte death, and premature ventricular contraction, occur at much lower MIs.[ 16]
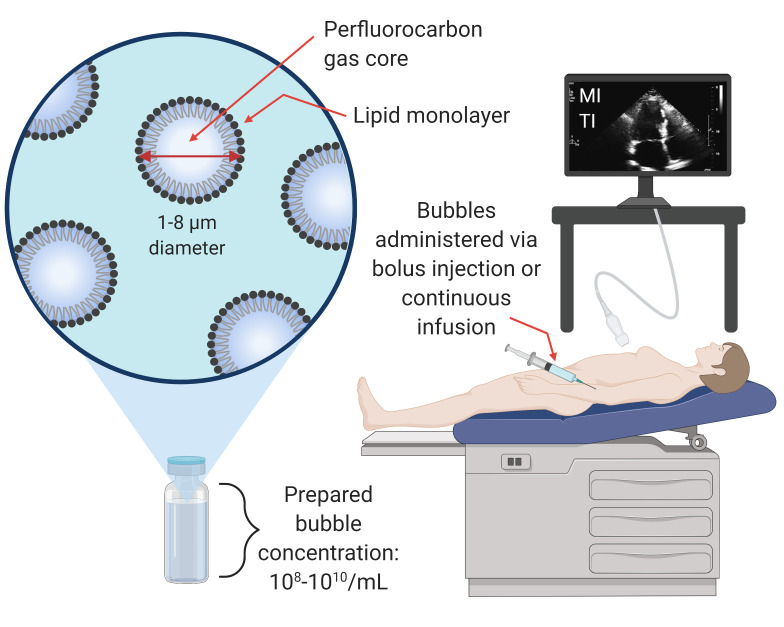
Microbubble ultrasound vascular contrast agents are small bubbles with an outer lipid shell and an inner gas core. Clinically, they are injected intravenously and most often used as an echogenic source to provide high contrast ultrasonic images of organ structure or blood volume and perfusion to an organ of interest. Studies have also investigated their use for gas transport, such as oxygen delivery to tissues,[ 17 - 19] and gas scavenging.[ 20] Free, unencapsulated bubbles have also been used clinically as contrast agents. For example, agitated saline is used in echocardiography to detect patent foramen ovale (PFO).[ 21] The properties and clinical use of encapsulated microbubbles can be seen in Figure 2.
Figure 2.
Clinical use and properties of ultrasound microbubble contrast agents
Contrast agent manufacturer guidelines recommend setting the default MI to below 0.4 (Sonovue™)[ 22] or below 0.8 (Definity™, Optison™)[ 23 , 24] for the safe use of microbubble contrast agents. Nevertheless, physicians still occasionally utilise a short sonication pulse at a higher MI (> 1.0) to momentarily break microbubbles in the field of view, before returning to low MI imaging (destruction-reperfusion technique for perfusion quantification).[ 25] To date, significant bioeffects from contrast imaging in humans have not been observed; however, due to bioeffects observed in some preclinical studies, the World Federation for Ultrasound in Medicine and Biology (WFUMB) has proposed that contrast imaging should be performed at an MI of less than 0.4 when possible to reduce the likelihood of bioeffects.[ 26]
2D echocardiography post-dive typically uses continuous imaging at > 1.2 MI to achieve higher quality images, which is significantly higher than the proposed 0.4 MI suggestion for imaging ultrasound contrast microbubbles. The properties of decompression bubbles, such as bloodstream concentrations and diameter distributions, are largely unknown and still debated, making direct comparisons between contrast agent microbubbles and VGE difficult; however, previous research showing the activation of gas bodies with ultrasound provides a reason to approach the sonication of gas-containing tissues with caution.[ 27] No studies to date have investigated potential mechanically induced bioeffects at the MIs used for post-dive evaluation.
This review aims to discuss what can be drawn from the current ultrasound and diving literature on this topic, and identify areas where more studies are needed. First, we provide an overview of ultrasound safety, introduce microbubble vascular contrast agents and summarise their dynamics under ultrasound that can lead to bioeffects. Next, we review the ultrasound bioeffects literature, focusing on the pulmonary and cardiovascular systems of special interest to diving physiology. Finally, we consider previous studies combining diving and low-frequency ultrasound and discuss open questions regarding the safety of post-dive echocardiography.
Ultrasound bioeffect mechanisms
HOW BUBBLES BEHAVE UNDER ULTRASOUND
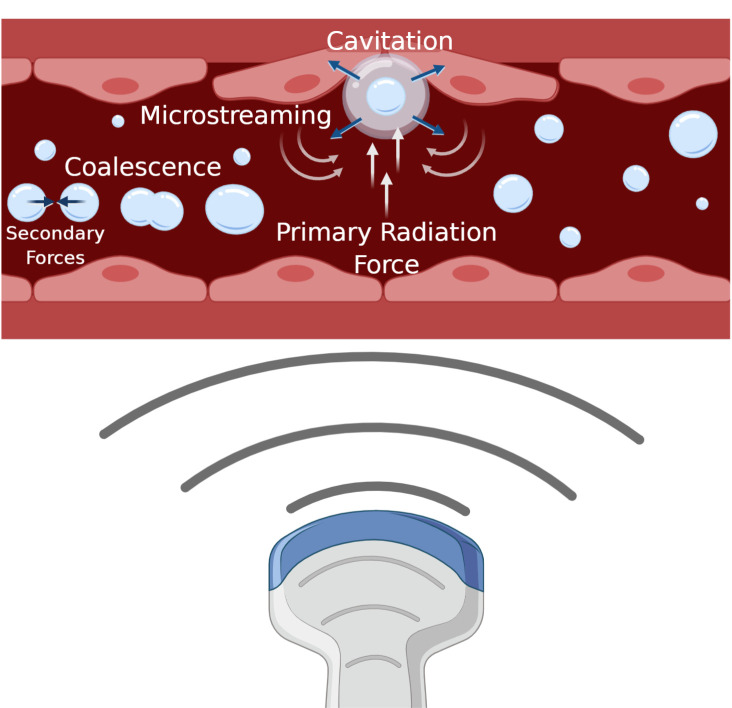
Bubbles under ultrasound experience different mechanical effects depending on the surrounding environment and the ultrasound parameters used. The main mechanical effects of bubbles under ultrasound are described below, along with the type of bioeffects each may generate. These are also graphically depicted in an idealised blood vessel schematic in Figure 3.
Figure 3.
Microbubble behavior in a blood vessel under ultrasound sonication. Four mechanical effects of microbubbles are illustrated: microbubbles experience a push in the direction of ultrasound propagation (primary radiation force); can undergo cavitation depending on the sonication parameters (frequency match to their resonance diameter, transmit amplitude) which shrinks and expands the bubble; this oscillation creates local flow disturbances (microstreaming); and bubbles can coalesce (secondary radiation force)
Cavitation
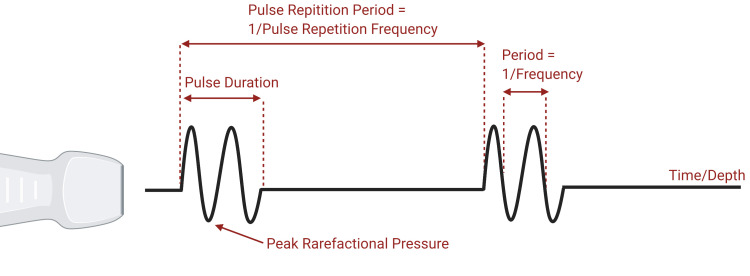
Ultrasound imaging employs sound, in the form of pressure waves, to produce images. Pressure waves emitted from a transducer propagate and, when reflected off interfaces with different acoustic impedance, are received by the same transducer to form an image. The body is composed of tissues and water, which are incompressible. Gas, however, is compressible, and bubbles excited with a pressure wave will shrink during periods of increased pressure and expand during periods of rarefaction. Since VGE are bubbles in blood, surrounded by incompressible liquid, small VGE can expand and shrink under ultrasound. The properties of sound waves including the definition of various acoustic parameters can be seen in Figure 4.
Figure 4.
Properties of an ultrasonic wave. Note that horizontal distance represents time. Peak rarefactional pressure, pulse duration, pulse repetition period, and period are illustrated
Acoustic cavitation is the expansion and contraction of a gas bubble within a sound field. When a bubble in liquid is exposed to an acoustic field, that bubble will oscillate around an equilibrium radius. Two types of oscillation can occur depending on the acoustic field insonifying the bubble: stable (non-inertial) cavitation and inertial cavitation. Under stable cavitation, a bubble undergoes repetitive oscillation over multiple acoustic cycles. When the acoustic amplitude is increased, oscillating bubbles reach a point where there is greater bubble expansion than there is contraction. This leads to the rapid growth and then violent collapse of the bubble (with the bubble fragmenting and gas dissolving into the surrounding fluid) in a process known as inertial cavitation. Both forms of cavitation can result in bioeffects; in some cases these effects can have harmful unintended consequences, but they may also be purposefully elicited in therapeutic settings. During stable cavitation, oscillating bubbles produce heat and cause localised shear stress or microstreaming of fluid near the bubble.[ 28] While sometimes undesirable, the physical effects from stable cavitation are utilised in therapeutic settings to produce pores in membranes for transporting of genetic material in a process called sonoporation[ 29] or to lyse blood clots.[ 30] Sustained stable cavitation, in the absence of unstable cavitation, is also used to temporarily open the blood-brain barrier[ 31] and the amount of stable cavitation has also been shown to correlate with the concentration of therapeutic agents delivered via focused ultrasound blood-brain barrier opening.[ 32] The collapse associated with inertial cavitation produces violent effects such as localised but extreme temperature rises and high-velocity liquid jets that cause mechanical damage.[ 33] Inertial cavitation can produce harmful effects such as micro-vessel rupture[ 34 , 35] and blood cell rupture.[ 36] As with stable cavitation, however, the effects of inertial cavitation are used therapeutically. Inertial cavitation can be used to fractionate tissue,[ 37 , 38] with applications in tumor ablation, open the blood-brain barrier with some bubble diameters,[ 39] release drugs from micelles,[ 40] and can be precisely controlled for sustained sonoporation.[ 41]
Although the above studies deal with encapsulated microbubbles, it should be noted that the lipid layer of the bubble is not what enables cavitation or other bubble mechanics. While sonication of free bubbles, such as saline, does not cause bioeffects,[ 42] it is not the bubbles themselves but rather their size away from resonance and timescale that prevent bioeffect generation. Free bubbles are capable of cavitation at even lower pressures than stiff-shelled encapsulated bubbles.[ 43] When not under supersaturated conditions, however, these unencapsulated bubbles have half-lives of only a few seconds.[ 44]
Microstreaming
As bubbles rapidly expand and contract during stable cavitation, fluid flow can be generated near the bubble in a process known as microstreaming. This flow around the oscillating bubble can impose shear stress on surrounding surfaces and result in cell death.[ 45 , 46] The stress exerted via microstreaming can also be used therapeutically; for example, to open membrane pores for therapeutic agent delivery via sonoporation.[ 47]
Radiation forces and coalescence
As ultrasound waves propagate through a medium, they have an associated momentum that can be imparted onto objects in their path. If an object in the beam's path is free to move, the imparted momentum will result in the translation of the object in the direction of the beam.[ 48] This imparted force is known as the primary radiation force. Bubbles pushed hard enough with this force may attain high speeds, and collisions with these high-speed bubbles have been proposed to be the cause of cell lysis[ 49] and clot lysis.[ 30] The pushing of microbubbles can also be used to localise and concentrate contrast agents near vessel walls to assist in the delivery of targeted agents.[ 50]
As microbubbles oscillate, they act as a secondary source of sound.[ 48] This source of sound is associated with another radiation force referred to as the secondary radiation force, which can cause attraction between nearby microbubbles or even other nearby particles. When two bubbles are close enough to one another via the primary and secondary radiation forces, they may fuse together as a single bubble in a process known as coalescence. The coalescence of microbubbles occurs because of the thinning of the bubble film. As encapsulated bubbles expand under an ultrasonic field, the flow between the bubbles creates a pressure reduction, and the two bubbles will move closer towards each other.[ 33] Once the bubbles are adjacent, their expansion will cause the pressure in the film between them to increase, which results in the thinning and flattening of the bubble surfaces.[ 51] The continued bubble expansion leads to the drainage of the film until it reaches a critical thickness.[ 51] At this point, the film ruptures and the bubbles coalesce into a single bubble. Whereas free bubbles coalesce more readily during collisions without the use of ultrasound, the resulting radiation forces from ultrasound make the coalescence of encapsulated bubbles much more likely.[ 52] The use of the secondary radiation force may allow for the combination of therapeutic agents encapsulated in microbubbles or may be used to aid in concentrating agents to a targeted area.[ 50]
RELEVANCE OF THE MECHANICAL INDEX
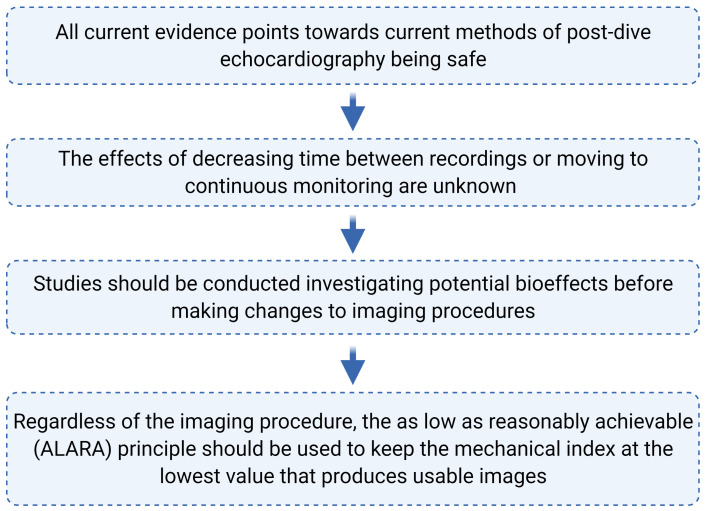
The mechanical index is used to infer the risk of nonthermal mechanical effects during diagnostic ultrasound. Apfel and Holland developed this metric using theoretical and experimental observations to determine the acoustic pressure amplitude required to cause an optimally sized bubble to undergo inertial cavitation.[ 53] In their work, a threshold level of 0.7 MI was reported for initiating inertial cavitation. Interestingly, because the FDA guidelines are based upon the acoustic output in use commercially prior to the 1976 FDA Medical Device Amendments (by law), although the MI computation is derived from Apfel and Holland, the FDA MI guideline is 1.9. It is important to note that the MI calculation is based only on the threshold for generating inertial cavitation for free bubbles and not on the severity of effects resulting from inertial cavitation.[ 54]
The FDA defines the MI as the ratio of the peak rarefactional negative pressure (in MPa) adjusted for tissue attenuation (derated by 0.3 dB.MHz-cm-1 and the square root of the center frequency of the wave (in megahertz (MHz)), thus = Equation 1.
Equation 1.
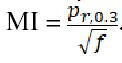
The FDA defines the MI as the ratio of the peak rarefactional negative pressure (in MPa) adjusted for tissue attenuation (derated by 0.3 dB.MHz-cm-1 and the square root of the center frequency of the wave (in megahertz (MHz)), thus =
From this equation, at a set peak rarefactional negative pressure, lower frequencies lead to higher MI values, indicating a higher possibility of inertial cavitation. This is because cavitation is more likely under long wavelength stimulation (low frequencies) when bubbles have more time to expand and is less likely under short wavelength stimulation (high frequencies) when sufficient time is not provided for bubble growth.[ 33]
It is important to note the conditions under which the MI was developed. First, there is an assumption of pre-existing microscopic gas nuclei in the body.[ 53] While this is an accurate assumption for gas containing bodies such as the lungs and intestine, the use of microbubble contrast agents, and potentially even the case of circulating decompression bubbles, it proves to be less applicable for tissues not known to contain gas,[ 55] such as most soft tissues including muscle, fat, and cardiac tissue. Second, the MI assumes the existence of optimally sized bubbles in vivo.[ 53] In some situations, this may be a reasonable assumption, such as in the case of contrast agents where the bubble size distribution is at least known initially. Most tissues, however, do not contain these pre-existing, optimally sized bubbles, meaning that the MI is not necessarily a good predictor of in vivo cavitation.[ 56] In the case of decompression sickness, bubbles are present, but their size is debated. VGE with diameters above 20-30 µm have been detected using 2D echocardiography, and theoretical calculations and new imaging techniques, such as a dual-frequency system for detecting and sizing bubbles,[ 57] also predict the presence of smaller bubbles < 10 µm.[ 58] One study, for example, detected microbubbles in the 1-10 µm diameter range in swine following hyperbaric chamber dives.[ 59] Third, the MI was developed assuming only a single acoustic period of sonication typical of traditional imaging schemes. This is not the case for some forms of ultrasound imaging, such as Doppler and acoustic radiation force impulse imaging, that employ several hundred acoustic periods.
There is debate about the validity of using the MI as a predictor of cavitation. This metric only accounts for the onset of inertial cavitation and does not include other cavitation events such as subharmonic emissions from stable cavitation, and it is a poor predictor of ultrasound contrast agent rupture.[ 60] As a result, other cavitation metrics have been proposed, the most notable being the cavitation index Equation 2.
Equation 2.
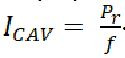
As a result, other cavitation metrics have been proposed, the most notable being the cavitation index.
This seeks to describe the cavitation process as a whole.60 Under this metric, the likelihood of ultrasound contrast agent rupture increases for ICAV > 0.02.[ 60] Aside from the issue of the MI not accounting for other cavitation events and tissues without pre-existing, optimally sized bubbles, this measurement system also only considers peak rarefactional pressure and frequency. It is important to note that other factors, such as sonication time, pulse duration, pulse repetition frequency (PRF), and even the waveform shape, also contribute to the likelihood of cavitation and the occurrence and severity of bioeffects.[ 54] Computational studies have been conducted investigating the effect of increased pulse durations on the inertial cavitation threshold. Church found that under the sonication of liquids, such as urine, water, or blood, increased pulse durations reduced the cavitation threshold as much as 6-24%, although the effect on tissue was minor.[ 56] Compared to some experimental data, inertial cavitation thresholds generated under the MI method do not always agree with the frequency response.[ 61] From this disagreement, some alternative methods have been proposed such as modifying the frequency exponent in the MI equation[ 56] or adopting a two-criterion model that considers both the inertial cavitation and also a fixed value for the maximum radius a bubble may attain during expansion.[ 61]
Despite its inaccuracies and over-simplifications, the MI remains a useful metric for evaluating the threshold for inertial cavitation and bioeffect production in certain scenarios. For example, when diagnostic B-mode imaging, which employs only a few acoustic periods, is used on gas containing bodies, the MI may provide a useful way to indicate frequency and acoustic pressure combinations that are more likely to lead to cavitation-induced bioeffects. Guidelines have been released advising caution using MIs above 0.4 for diagnostic imaging of tissues with gas-containing bodies,[ 26] which is significantly below the FDA's 1.9 MI guideline and the commonly used 1.2 MI for post-dive echocardiography. In the following discussion of experimental studies evaluating organ bioeffects under diagnostic imaging, the MI will be strongly considered, although other sonication factors that play a role in bioeffect production, such as sonication duration, will also be discussed.
Pulmonary and cardiovascular bioeffects
Although the papers discussed in this section do not focus on ultrasound as it relates to scuba diving, the pulmonary and cardiovascular systems are directly sonicated during post-dive echocardiography, making it useful to understand how they may be affected by ultrasound sonication. It is important to note, however, that the pulmonary and cardiovascular systems are not the only systems that suffer from ultrasound-induced bioeffects. Although they will not be discussed in detail here, the intestines, kidneys, bones, and even nervous system experience unique effects under ultrasound.[ 28] Since this review is focused on post-dive echocardiography, however, the discussion below will be kept to the two most relevant systems. It should be noted that while this section discusses negative effects the pulmonary and cardiovascular systems may experience under ultrasound, overall ultrasound is considered the safest imaging modality. The effects described below serve as a cautionary tale for the use of continuous ultrasound without prior safety investigations, as they demonstrate harm from unusual sonication circumstances (i.e., in the presence of gas bodies, at high pressures, etc).
PULMONARY BIOEFFECTS
Although the lungs are not the focus of post-dive 2D echocardiography, they can receive exposure as the beam passes through the chest wall to the heart. Whereas the cardiovascular system contains circulating VGE post-dive that provide a potential source of gas cavitation under ultrasound, the lungs are comprised of pre-existing gas bodies. This makes it important to consult the literature on the potential for bioeffect generation in the lungs, especially when considering extending the duration of post-dive echocardiography.
Many murine studies have found that lung haemorrhage is possible under diagnostically relevant levels of pulsed ultrasound sonication, with typical thresholds between 0.4 and 1.4 MPa peak rarefactional pressure, frequencies from 1.1 MHz to 12.0 MHz, and an MI range of 0.37-1.0.[ 62 - 66] This pulmonary capillary haemorrhaging resulting from sonication has been shown to correlate with the length of comet-tail artifacts,[ 64 , 65] suggesting that these artifacts may be used to indicate developing damage during imaging. These results illustrate the potential for lung haemorrhage to occur in rats and mice at MIs much lower than the 1.9 MI FDA guideline. The sonication frequency, however, does not appear to be a strong factor in determining the haemorrhage threshold, making the MI a poor predictor for damage.[ 65 , 67] Despite haemorrhage occurring at low sonication pressures in murine models, some researchers have speculated that the mouse is a poor model for damage that could occur during human diagnostic imaging.[ 68 , 69] This is substantiated by cross-species studies that have found less damage occurring in larger animals, such as rabbits and pigs, compared to rats and mice at the same sonication parameters.[ 68 , 70] Zachary and O'Brien concluded that a species' sensitivity to ultrasound is likely determined by anatomical and physical properties such as alveolar diameter, thickness of alveolar septa, lung compliance, and pleural thickness,[ 70] which all differ significantly between humans and rodents. It is also important to note that these studies investigating lung haemorrhage thresholds focus ultrasound directly on the lungs, whereas lung ultrasound exposure during echocardiography is more incidental (and currently of short duration).
To determine whether the results of small animal studies are applicable to humans, researchers have investigated the effects of diagnostic imaging on both human and monkey lungs. Damage has been shown to be possible with clinical diagnostic settings in monkeys, but only minimal damage was found using the maximum diagnostic ultrasound settings.[ 71] A study on 50 human subjects undergoing clinical echocardiography at 1.3 MI found no lung damage, leading the authors to conclude that human lungs are not as sensitive as those of animals.[ 72]
The mechanism by which ultrasound causes lung haemorrhage is not well understood. The interaction of ultrasound and alveolar gas is likely the primary cause of lung damage, as determined by the low sensitivity of fetal swine lungs to ultrasound compared to adult lungs since fetal lungs contain no gas.[ 73] Although the interaction with gas is the likely cause, inertial cavitation is not believed to be the mechanism by which gas causes damage. Evidence for this includes the lack of frequency dependence on the haemorrhage threshold,[ 65] the lack of difference in lung damage due to positive or negative peak pressures (the use of negative peak pressures should lead to more damage if inertial cavitation was the mechanism),[ 74] and the lack of effect of hydrostatic pressure on damage.[ 75] Although the exact form of gas body activation leading to haemorrhage remains unknown,[ 76] hypothesised mechanisms include the acoustic radiation surface pressure at the tissue-air interface.[ 77]
Although it appears that lung damage due to human echocardiography under typical clinical conditions is unlikely, the effect of increasing sonication time should be considered. Murine studies have found that increased exposure duration increases the surface area of lung lesions resulting from ultrasound.[ 78 , 79] Even with the same total sonication on-time, longer exposure durations can lead to greater haemorrhage and a lower sonication threshold.[ 79] The effect of exposure duration is so significant in determining the occurrence and extent of lung damage that its inclusion into the MI equation for lung sonication has been suggested.[ 67] Still, it should be noted that the previously mentioned human clinical diagnostic study performed echocardiography for as long as 50 minutes and still found no lung haemorrhage.[ 72] Overall, diagnostic echocardiography in humans seems unlikely to cause lung damage using clinical settings, but it may be wise to exercise caution when implementing long exposure durations.
CARDIOVASCULAR BIOEFFECTS
Although there is a lack of research regarding the interaction of decompression bubbles and echocardiography, there is extensive research on an interesting parallel: the use of microbubble contrast agents during echocardiography. In contrast echocardiography, microbubbles are introduced into the bloodstream, where they are confined to the vasculature, as an echogenic source to provide higher quality images. When sonicated, these contrast agents have the potential to cavitate and induce bioeffects through the mechanisms previously described. To better understand the effects of cavitating bubbles in the cardiovascular system, this section will provide a literature review of the bioeffects elicited under diagnostic ultrasound conditions in both the heart and bloodstream along with a discussion of the safety of contrast echocardiography.
Cardiac bioeffects
Human and animal studies have revealed the production of many cardiac bioeffects when exposing contrast agents to diagnostic imaging conditions. Examples of generated bioeffects include capillary rupture,[ 35 , 80 , 81] premature ventricular contraction,[ 80 , 82 - 85] ventricular damage,[ 34] cardiac bio-marker release,[ 86 , 87] and mortality.[ 84] There is great variation in the settings that elicit these bioeffects, however. Mortality, for example, occurred only in extreme conditions far removed from traditional echocardiography: continuous ultrasound focused on the heart at a low frequency, maximum MI, a continuous bolus injection of contrast agents, and a sonication duration of over 9 minutes.[ 84] Unlike the production of pulmonary capillary haemorrhage, many cardiac studies have found a strong MI dependence on cardiac bioeffect production. One study, for example, found a strong damage dependence on the MI in rats where damage occurred slightly below 0.4 MI and increased with increasing MI.[ 35] Similar low MI thresholds have been found in contrast echocardiography rat studies: microvascular leakage occurred with exposure above 0.3 MI,[ 80] higher rates of mortality occurred with pressures above 0.6 MPa at 1.3 MHz (above 0.53 MI),[ 84] and premature ventricular contraction occurred with thresholds between 0.3 and 0.77 MI.[ 80 , 83 , 84] Larger animal and human studies, however, have found higher thresholds required for generating bioeffects. In an open-heart canine model, capillary rupture occurred with both 1.0 and 1.8 MI, although significantly more damage was produced with 1.8 MI.[ 81] Ex vivo rabbit heart sonication with microbubbles showed damage occurring with an MI greater than 0.8 and more damage occurred when using a lower frequency,[ 34] an outcome the MI model predicts. Human models show even greater thresholds. In one human clinical contrast echocardiography study, an MI of 1.5 elicited premature ventricular contraction whereas a 1.1 MI did not.[ 85] Another study found increased release of the cardiac bio-markers troponin I, creatine kinase myocardial band (CK-MB), and myoglobin in the coronary sinus, suggesting microscale damage to cardiomyocytes, when imaging at 1.5 MI in triggered second harmonic mode but not with a mode that implemented an alternating low-high combination where 0.2 MI was interrupted with 10 images at 1.7 MI every minute.[ 87]
The MI is not the only relevant setting in relation to damage, however. Some studies have shown that sonication time impacts the amount of damage produced. At high pressures, mortality has been shown to gradually increase as the sonication time increases from nine to 30 minutes[ 84] and bio-marker release increases with time up to 15 minutes.[ 87] Bioeffect generation during contrast echocardiography also depends on the concentration and infusion rate of microbubbles. Increased infusion rates are associated with greater premature ventricular contraction[ 82 , 85] and greater microbubble dosages are known to produce more capillary leakage.[ 80]
Despite the above studies that found bioeffect production with contrast echocardiography, human[ 88] and animal[ 86] studies have found no negative impacts from intermittent ECG-triggered contrast echocardiography at MIs around 1.0, and multiple reviews have concluded that contrast echocardiography has been shown to be safe in regards to the fairly insignificant findings of many studies.[ 25 , 89] Several major retrospective studies have found no increased risk of negative effects from the use of contrast agents during echocardiography.[ 90 - 93] The above studies also have several limitations that hinder their applicability to clinical settings. First, several of the studies employ contrast dosages much higher than those used clinically.[ 35 , 80 , 84 , 86] Many studies are also conducted on small animals or ex vivo organs,[ 34 , 35 , 80 , 81 , 86] meaning the studied hearts likely received greater ultrasound organ coverage or less tissue attenuation than would be present in clinical human use. Lastly, the studies on human subjects concede that the study population is more likely to experience arrythmias than healthy individuals,[ 82] potentially skewing results. Even so, contrast agent product inserts warn of potential arrythmia generation with MIs above 0.8[ 22 - 24] and caution has been recommended when using moderate and high MIs in contrast echocardiography.[ 25]
Vascular bioeffects
The use of contrast agents in vasculature provides an interesting parallel to sonication of circulating decompression bubbles. Since microbubble contrast agents travel through the bloodstream after injection, it is important to consider the potential interaction of these bubbles with blood cells under ultrasound sonication. Haemolysis, the destruction of blood cells, has been found with the sonication of microbubble-containing vasculature. Animal studies with contrast agents have found inertial cavitation to be the primary mechanism for haemolysis, as indicated by the strong correlation between the amount of haemolysis and the amount of inertial cavitation recorded using a cavitation detection system.[ 36 , 94 , 95] Increasing the dissolved oxygen (in normobaric conditions) in the blood, introducing more cavitation nuclei, also leads to greater inertial cavitation and greater haemolysis,[ 96] supporting inertial cavitation as the mechanism causing haemolysis. The amount of haemolysis occurring also shows a strong frequency effect where lower frequencies produce greater haemolysis, and the amount of haemolysis increases with increasing MI.[ 95 , 97] Despite this, even when sonicating in vitro blood at MIs > 1.9, much greater than what would be used clinically, the levels of haemolysis produced are less than 5%[ 97 , 98] or almost indistinguishable from sham treatments.[ 36] Other studies have simply found no evidence of haemolysis even at maximum diagnostic settings.[ 99] The high thresholds necessary to invoke even minimal red blood cell destruction with contrast agents suggests that harmful levels of haemolysis are unlikely during diagnostic conditions.[ 28 , 36 , 76 , 97 , 98]
Relevance to diving
PREVIOUS STUDIES
Sonar and diagnostic ultrasound use vastly different parameters and are not comparable exposures (but we include this section for completeness). Of particular note, sonar typically uses frequencies in the kilohertz (kHz) range, which is much lower than the MHz frequencies used in diagnostic ultrasound. Sonar often transmits long or continuous signals, whereas diagnostic ultrasound most often uses pulsed sequences. The exposure in the studies in this section also occur during the dive bottom time, instead of post-dive. Despite the differences from post-dive echocardiography, the discussion of sonar exposure to divers still offers interesting insights into the potential interactions of decompression bubbles and ultrasonic waves. Several experimental studies have investigated the potential for decompression bubbles to grow under sonar. A computational study investigated the potential for bubbles of 1-10 µm initial radius in dissolved gas concentrations of 100-223% to grow under low-frequency ultrasound.[ 100] They found that under these conditions, sound pressures greater than 210 dB re 1 µPa (31.6 kPa) resulted in rapid bubble growth to sizes large enough to block capillaries and other small blood vessels, but that pressures below 190 dB re 1 µPa (3.16 kPa) were unlikely to result in bubble growth.[ 100] Supporting the conclusions of this study, several animal or ex vivo tissue studies under simulated dives found the potential for bubble growth under sufficiently high sound pressures. Prawns in 203 kPa hyperbaric conditions exposed to sound at 37 kHz and 1.4-2.8 MPa during a 10-minute bottom time presented bubbles for a longer period of time and with higher mean volumes than those not exposed to sound.[ 101] Bubble growth was also found in supersaturated ex vivo blood and tissues when exposed to 37 kHz sound at pressures above 50 kPa.[ 102] Even sound pressures below 3.16 kPa have been found to elicit bubble growth in supersaturated conditions. Rats experiencing a simulated diving profile in a hyperbaric chamber that were exposed to 1.7 kPa sound at 37 kHz for the 60-minute bottom time produced larger bubbles and higher bubble densities than those with no sound exposure.[ 103]
Not only has sonar been shown to increase the amount or size of decompression bubbles, it has also led to increased damage or mortality in some studies. Immersed explanted pig lungs exposed to 22 and 36 kHz at 1 kPa and 0.8 kPa, respectively, incurred pulmonary microhaemorrhages.[ 104] A recent study found that rats exposed to diving profiles and 8 kHz sound experienced 20% mortality (vs. no deaths in the diving control group) and rats exposed to 8 kHz and to 15 kHz sound experienced higher rates of neurological decompression sickness.[ 105]
Few human studies exist to compare to the findings of animal and ex vivo tissue studies. Two case studies of divers exposed to continuous underwater sound reveal potentially recurrent harmful non-auditory effects such as lightheadedness, agitation, and the inability to concentrate, but these effects are difficult to validate.[ 106] Other human studies show that harmful effects from sonar exposure during dives are unlikely, but also conclude that further studies should be conducted for adequate conclusions.[ 107 , 108]
OPEN QUESTIONS
Although comparisons can be made between post-dive echocardiography and the use of both clinical diagnostic imaging with contrast agents and with sonar exposure during dives, there are too many parameter differences for either to provide a true parallel. Current post-dive protocols stipulate that measurements should be conducted for 120 minutes from completion of the decompression period, that an initial measurement should be made within 15 minutes following decompression followed by measurement intervals of no more than 20 minutes, and that sonication intensities and scan durations kept as low as reasonably achievable.[ 14] It should be noted that protocols such as these have been used for decades and adverse reactions in divers have not been reported. These conclusions are outlined in Figure 5.
Figure 5.
Current conclusions regarding post-dive monitoring safety
The above studies on pulmonary exposure during diagnostic echocardiography on humans or large mammals appear to indicate that damage is unlikely during typical clinical conditions,[ 68 , 70 , 72] even potentially during extended imaging durations. Post-dive, however, the pulmonary system plays an important role in filtering out circulating VGE. The lungs post-dive could be more sensitive to the effects of ultrasound due to the presence of VGE, which could in turn hinder this filtering capacity.
The results from clinical contrast echocardiography studies are difficult to interpret. Many studies indicate cardiovascular damage is possible at clinical settings with the introduction of microbubble contrast agents,[ 80 - 83 , 85 , 87] but most of these studies were conducted on small animals with contrast agent concentrations larger than typically used in therapeutic procedures.[ 80 , 81 , 83 , 86] Even with high VGE concentrations, individual bubbles can normally be detected on echocardiograms, indicating potential VGE concentrations much lower than the bubble concentrations used in ultrasound contrast agent procedures; however, there are varying radii of VGE and small circulating bubbles, or stationary tissue bubbles if present, that may not be picked up by echocardiography, making concentrations unknowable. Additionally, dissolved gas in the plasma from tissue supersaturation is not detectable with echocardiography, further complicating the question of gas concentration within the bloodstream. Ultrasound contrast agents are also confined to the vasculature, whereas VGE probably arise in the microcirculation of supersaturated tissues where extravascular bubble formation is also likely to be occurring. Most studies indicate that higher pressures and lower frequencies (higher MIs) result in more damage,[ 34 , 35 , 81 , 84 , 85] which does lead to the question of whether the typical 1-2 MHz and 1.2 MI post-dive echocardiography could result in damage from cavitating decompression bubbles. Human studies resulting in cardiovascular damage or premature ventricular contraction from contrast echocardiography were also conducted on populations more likely to experience cardiovascular difficulties.[ 82] Finally, contrast echocardiography studies have indicated higher occurrences and greater damage with extended sonication times,[ 78 , 79 , 84 , 87] indicating that extended post-dive sonication times could potentially result in a greater risk of bioeffects.
Studies on diving humans and animals exposed to sonar may leave the most unanswered questions, although this sonar sonication is very different to diagnostic ultrasound. These studies indicate that decompression bubbles in supersaturated conditions can grow when exposed to ultrasound[ 100 - 103] and potentially result in more severe decompression sickness.[ 104 , 105] These studies, however, use a much lower sonication frequency, and therefore higher MI, than that used in diagnostic imaging. Whereas the sonar studies focus on ultrasound in the low kHz range, echocardiography uses frequencies on the order of 1 MHz. Although it was previously thought that bubbles would most strongly oscillate when exposed to their resonant frequency, meaning that bubbles with low µm diameters would respond most strongly to MHz ultrasound, new studies have shown that lower frequency ultrasound, such as 250 kHz, causes bubbles to expand to more than 30 times their equilibrium size.[ 109] This raises the question as to whether sonar might cause bubbles to oscillate more strongly than diagnostic frequencies, meaning that the expansion seen in sonar conditions could potentially be less likely for diagnostic conditions. There are also questions as to whether supersaturated tissues exposed to ultrasound during dive bottom times would be more likely to grow or produce more bubbles than tissues that have decompressed post-dive, or whether the presence of circulating bubbles that result from the decompression could lead to stronger effects from ultrasound. The location of the sonication probe also differs from sonar studies and diagnostic studies; in diagnostic imaging, the probe is placed directly on the skin of the patient, giving them direct ultrasound exposure, whereas when humans and animals are exposed to sonar, the transducer is typically much further away. Lastly, the pulse repetition frequencies differ greatly between sonar exposure and diagnostic imaging. Sonar uses much lower pulse repetition frequencies than diagnostic imaging, meaning that patients under diagnostic imaging are subject to more frequent ultrasound exposure. These numerous considerations make it difficult to assess the potential hazards of continuous post-dive echocardiography.
Conclusion
Ultrasound has the potential to generate bioeffects in divers through sonar and in the pulmonary and cardiovascular systems through diagnostic ultrasound imaging, especially under conditions of high acoustic pressure, low frequency, and long duration sonication. Despite this, no research has been conducted on the safety of echocardiography for the evaluation of VGE load post-dive. Although the above research offers interesting insights into the role of ultrasound in bioeffect production and areas of possible concern, no conclusive statements can be made regarding the safety of continuous post-dive echocardiography. Since little information is known, sonication pressures should ideally be kept as low as reasonably achievable (the ALARA principle) to avoid any potential bioeffects. To avoid cavitation-related effects, sonication frequency should also be kept as high as possible. Further studies should also be conducted investigating the potential for post-dive echocardiography to produce bioeffects in divers.
Footnotes
Acknowledgements
Figures 1−5 were created using BioRender.com.
Conflicts of interest and funding
V.P. gratefully acknowledges funding from the Divers Alert Network as DAN Scholar (grant #DAN-UNC-1), as well as the Department of the Navy, Office of Naval Research under ONR award number N00014-20-1-2590. No conflicts of interest were declared.
Contributor Information
Erica P McCune, Joint Department of Biomedical Engineering, The University of North Carolina at Chapel Hill and North Carolina State University, Chapel Hill, NC, USA.
David Q Le, Joint Department of Biomedical Engineering, The University of North Carolina at Chapel Hill and North Carolina State University, Chapel Hill, NC, USA.
Peter Lindholm, Department of Emergency Medicine, School of Medicine, University of California, San Diego, La Jolla, CA, USA.
Kathryn R Nightingale, Department of Biomedical Engineering, Duke University, Durham, NC, USA.
Paul A Dayton, Joint Department of Biomedical Engineering, The University of North Carolina at Chapel Hill and North Carolina State University, Chapel Hill, NC, USA.
Virginie Papadopoulou, Joint Department of Biomedical Engineering, The University of North Carolina at Chapel Hill and North Carolina State University, Chapel Hill, NC, USA.
References
- Levett DZH, Millar IL. Bubble trouble: a review of diving physiology and disease. Postgrad Med J. 2008;84:571–8. doi: 10.1136/pgmj.2008.068320. [DOI] [PubMed] [Google Scholar]
- Mitchell SJ, Bennett MH, Moon RE. Decompression sickness and arterial gas embolism. N Eng J Med. 2022;386:1254–64. doi: 10.1056/NEJMra2116554. [DOI] [PubMed] [Google Scholar]
- Papadopoulou V, Tang M-X, Balestra C, Eckersley RJ, Karapantsios TD. Circulatory bubble dynamics: from physical to biological aspects. Adv Colloid Interface Sci. 2014;206:239–49. doi: 10.1016/j.cis.2014.01.017. [DOI] [PubMed] [Google Scholar]
- Spencer MP. Detection of embolism with doppler ultrasound. Echocardiography. 1996;13:519–28. doi: 10.1111/j.1540-8175.1996.tb00930.x. [DOI] [PubMed] [Google Scholar]
- Spencer MP, Johanson DC. Investigation of new principles for human decompression schedules using Doppler ultrasound blood bubble detection. Technical Report to ONR on Contract N00014-73-C-0094 Seattle: Institute for Environmental Medicine and Physiology; 1974. [Google Scholar]
- Germonpré P, Papadopoulou V, Hemelryck W, Obeid G, Lafère P, Eckersley RJ, et al. The use of portable 2D echocardiography and ‘frame-based’ bubble counting as a tool to evaluate diving decompression stress. Diving Hyperb Med. 2014;44:5–13. [PubMed] [Google Scholar]
- Eftedal O, Brubakk AO. Agreement between trained and untrained observers in grading intravascular bubble signals in ultrasonic images. Undersea Hyperb Med. 1997;24:293–9. [PubMed] [Google Scholar]
- Conkin J, Powell MR, Foster PP, Waligora JM. Information about venous gas emboli improves prediction of hypobaric decompression sickness. Aviat Space Environ Med. 1998;69:8–16. [PubMed] [Google Scholar]
- Eftedal OS, Lydersen S, Brubakk AO. The relationship between venous gas bubbles and adverse effects of decompression after air dives. Undersea Hyperb Med. 2007;34:99–105. [PubMed] [Google Scholar]
- Carturan D, Boussuges A, Vanuxem P, Bar-Hen A, Burnet H, Gardette B. Ascent rate, age, maximal oxygen uptake, adiposity, and circulating venous bubbles after diving. J Appl Physiol. 2002;93:1349–56. doi: 10.1152/japplphysiol.00723.1999. [DOI] [PubMed] [Google Scholar]
- Gennser M, Jurd KM, Blogg SL. Pre-dive exercise and post-dive evolution of venous gas emboli. Aviat Space Environ Med. 2012;83:30–4. doi: 10.3357/asem.2893.2012. [DOI] [PubMed] [Google Scholar]
- Papadopoulou V, Germonpré P, Cosgrove D, Eckersley RJ, Dayton PA, Obeid G, et al. Variability in circulating gas emboli after a same scuba diving exposure. Eur J Appl Physiol. 2018;118:1255–64. doi: 10.1007/s00421-018-3854-7. [DOI] [PubMed] [Google Scholar]
- Blogg SL, Gennser M. The need for optimisation of post-dive ultrasound monitoring to properly evaluate the evolution of venous gas emboli. Diving Hyperb Med. 2011;41:139. [PubMed] [Google Scholar]
- Møllerløkken A, Blogg SL, Doolette DJ, Nishi RY, Pollock NW. Consensus guidelines for the use of ultrasound for diving research. Diving Hyperb Med. 2016; 46: 26- 32. [cited 2022 March 25]. Available from: https://www.dhmjournal.com/images/IndividArticles/46March/Mollerlokken_dhm.46.1.26-32.pdf. [PubMed] [Google Scholar]
- Gernhardt ML, Acock KE, Conkin J, Hamilton D. Operational evaluation of in-suit doppler (NEEMO-DOPPLER). National Aeronautics and Space Administration; 2003. [cited 2022 Mar 25]. Study information available from: https://lsda.jsc.nasa.gov/Experiment/exper/1069.
- Miller DL, Averkiou MA, Brayman AA, Everbach EC, Holland CK, Wible JH, et al. Bioeffects considerations for diagnostic ultrasound contrast agents. J Ultrasound Med. 2008;27:611–36. doi: 10.7863/jum.2008.27.4.611. [DOI] [PubMed] [Google Scholar]
- Lundgren CEG, Bergoe GW, Tyssebotn IM. Intravascular fluorocarbon-stabilized microbubbles protect against fatal anemia in rats. Artif Cells Blood Substit Immobil Biotechnol. 2006;34:473–86. doi: 10.1080/10731190600769271. [DOI] [PubMed] [Google Scholar]
- Tyssebotn IM, Lundgren CEG, Olszowka AJ, Bergoe GW. Hypoxia due to shunts in pig lung treated with O2 and fluorocarbon-derived intravascular microbubbles. Artif Cells Blood Substit Immobil Biotechnol. 2010;38:79–89. doi: 10.3109/10731191003634679. [DOI] [PubMed] [Google Scholar]
- Kheir JN, Scharp LA, Borden MA, Swanson EJ, Loxley A, Reese JH, et al. Oxygen gas-filled microparticles provide intravenous oxygen delivery. Sci Transl Med. 2012;4:140ra88. doi: 10.1126/scitranslmed.3003679. [DOI] [PubMed] [Google Scholar]
- Radhakrishnan K, Holland CK, Haworth KJ. Scavenging dissolved oxygen via acoustic droplet vaporization. Ultrason Sonochem. 2016;31:394–403. doi: 10.1016/j.ultsonch.2016.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto FJ. When and how to diagnose patent foramen ovale. Heart. 2005;91:438–40. doi: 10.1136/hrt.2004.052233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumason (sulfur hexafluoride lipid-type a microspheres) for injectable suspension, for intravenous use or intravesical use. Monroe Township (NJ): Bracco Diagnostics Inc; 2014. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/203684s002lbl.pdf. [Google Scholar]
- Optison: perflutren protein-type A microspheres injectable suspension. St. Louis (MO): Mallinckrodt Inc; 2012. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/020899s015lbl.pdf. [Google Scholar]
- Definity: (perflutren lipid microsphere) injectable suspension. N.Billerica (MA): Lantheus Medical Imaging; 2011. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/021064s011lbl.pdf. [Google Scholar]
- Chong WK, Papadopoulou V, Dayton PA. Imaging with ultrasound contrast agents: current status and future. Abdom Radiol (NY). 2018;43:762–72. doi: 10.1007/s00261-018-1516-1. [DOI] [PubMed] [Google Scholar]
- Barnett SB, Duck F, Ziskin M. Recommendations on the safe use of ultrasound contrast agents. Ultrasound Med Biol. 2007;33:173–4. doi: 10.1016/j.ultrasmedbio.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Miller DL. Implications of gas body activation for medical ultrasonics [Abstract]. J Acoust Soc Am. 1989;86 Suppl 1:S28. doi: 10.1121/1.2027442. [DOI] [Google Scholar]
- Dalecki D. Mechanical bioeffects of ultrasound. Annu Rev Biomed Eng. 2004;6:229–48. doi: 10.1146/annurev.bioeng.6.040803.140126. [DOI] [PubMed] [Google Scholar]
- van Wamel A, Kooiman K, Harteveld M, Emmer M, ten Cate FJ, Versluis M, et al. Vibrating microbubbles poking individual cells: drug transfer into cells via sonoporation. J Control Release. 2006;112:149–55. doi: 10.1016/j.jconrel.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Bader KB, Gruber MJ, Holland CK. Shaken and stirred: mechanisms of ultrasound-enhanced thrombolysis. Ultrasound Med Biol. 2015;41:187–96. doi: 10.1016/j.ultrasmedbio.2014.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T, Samiotaki G, Wang S, Acosta C, Chen CC, Konofagou EE. Acoustic cavitation-based monitoring of the reversibility and permeability of ultrasound-induced blood-brain barrier opening. Phys Med Biol. 2015;60:9079–94. doi: 10.1088/0031-9155/60/23/9079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Zhang X, Ye D, Laforest R, Williamson J, Liu Y, et al. Cavitation dose painting for focused ultrasound-induced blood-brain barrier disruption. Sci Rep. 2019;9:2840. doi: 10.1038/s41598-019-39090-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar H, Pagel PS. Potential adverse ultrasound-related biological effects: a critical review. Anesthesiology. 2011;115:1109–24. doi: 10.1097/ALN.0b013e31822fd1f1. [DOI] [PubMed] [Google Scholar]
- Ay T, Havaux X, Van Camp G, Campanelli B, Gisellu G, Pasquet A, et al. Destruction of contrast microbubbles by ultrasound: effects on myocardial function, coronary perfusion pressure, and microvascular integrity. Circulation. 2001;104:461–6. doi: 10.1161/hc3001.092038. [DOI] [PubMed] [Google Scholar]
- Skyba DM, Price RJ, Linka AZ, Skalak TC, Kaul S. Direct in vivo visualization of intravascular destruction of microbubbles by ultrasound and its local effects on tissue. Circulation. 1998;98:290–3. doi: 10.1161/01.cir.98.4.290. [DOI] [PubMed] [Google Scholar]
- Everbach EC, Makin IR, Azadniv M, Meltzer RS. Correlation of ultrasound-induced hemolysis with cavitation detector output in vitro. Ultrasound Med Biol. 1997;23:619–24. doi: 10.1016/s0301-5629(97)00039-2. [DOI] [PubMed] [Google Scholar]
- Dubinsky TJ, Khokhlova TD, Khokhlova V, Schade GR. Histotripsy: the next generation of high-intensity focused ultrasound for focal prostate cancer therapy. J Ultrasound Med. 2020;39:1057–67. doi: 10.1002/jum.15191. [DOI] [PubMed] [Google Scholar]
- Bader KB, Vlaisavljevich E, Maxwell AD. For whom the bubble grows: Physical principles of bubble nucleation and dynamics in histotripsy ultrasound therapy. Ultrasound Med Biol. 2019;45:1056–80. doi: 10.1016/j.ultrasmedbio.2018.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung Y, Vlachos F, Feshitan JA, Borden MA, Konofagou EE. The mechanism of interaction between focused ultrasound and microbubbles in blood-brain barrier opening in mice. J Acoust Soc Am. 2011;130:3059–67. doi: 10.1121/1.3646905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husseini GA, Diaz de la Rosa MA, Richardson ES, Christensen DA, Pitt WG. The role of cavitation in acoustically activated drug delivery. J Control Release. 2005;107:253–61. doi: 10.1016/j.jconrel.2005.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo C, Desjouy C, Chen S, Lee J, Inserra C, Béra J, et al. Stabilizing in vitro ultrasound-mediated gene transfection by regulating cavitation. Ultrason Sonochem. 2014;21:833–9. doi: 10.1016/j.ultsonch.2013.10.017. [DOI] [PubMed] [Google Scholar]
- Wible JH Jr, Galen KP, Wojdyla JK, Hughes MS, Klibanov AL, Brandenburger GH. Microbubbles induce renal hemorrhage when exposed to diagnostic ultrasound in anesthetized rats. Ultrasound Med Biol. 2002;28:1535–46. doi: 10.1016/s0301-5629(02)00651-8. [DOI] [PubMed] [Google Scholar]
- Church CC, Brayman AA. Can the presence of contrast media promote ultrasound bioeffects. In: Thomsen HS, Muller RN, Mattrey RF, editors Trends in contrast media. Medical radiology. Berlin, Heidelberg: Springer; 1999. p. 413- 22. [Google Scholar]
- Nanda NC. History of echocardiographic contrast agents. Clin Cardiol. 1997;20:I7–11. doi: 10.1002/clc.4960201304. [DOI] [PubMed] [Google Scholar]
- Clarke PR, Hill CR. Physical and chemical aspects of ultrasonic disruption of cells. J Acoust Soc Am. 1970;47:649–53. doi: 10.1121/1.1911940. [DOI] [PubMed] [Google Scholar]
- Rooney JA. Hemolysis near an ultrasonically pulsating gas bubble. Science. 1970;169:869–71. doi: 10.1126/science.169.3948.869. [DOI] [PubMed] [Google Scholar]
- Marmottant P, Hilgenfeldt S. Controlled vesicle deformation and lysis by single oscillating bubbles. Nature. 2003;423:153–6. doi: 10.1038/nature01613. [DOI] [PubMed] [Google Scholar]
- Stride E. Physical principles of microbubbles for ultrasound imaging and therapy. Cerebrovasc Dis. 2009;27 Suppl 2:1–13. doi: 10.1159/000203122. [DOI] [PubMed] [Google Scholar]
- Miller DL, Thomas RM, Williams AR. Mechanisms for hemolysis by ultrasonic cavitation in the rotating exposure system. Ultrasound Med Biol. 1991;17:171–8. doi: 10.1016/0301-5629(91)90124-f. [DOI] [PubMed] [Google Scholar]
- Dayton P, Klibanov A, Brandenburger G, Ferrara K. Acoustic radiation force in vivo: a mechanism to assist targeting of microbubbles. Ultrasound Med Biol. 1999;25:1195–201. doi: 10.1016/s0301-5629(99)00062-9. [DOI] [PubMed] [Google Scholar]
- Postema M, van Wamel A, Lancée CT, de Jong N. Ultrasound-induced encapsulated microbubble phenomena. Ultrasound Med Biol. 2004;30:827–40. doi: 10.1016/j.ultrasmedbio.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Postema M, Marmottant P, Lancee CT, Versluis M, Hilgenfeldt S, de Jong N. Ultrasound-induced coalescence of free gas microbubbles. IEEE Ultrasonics Symposium. Piscataway (NJ): IEEE; 2004. doi: 10.1109/ULTSYM.2004.1417653 [DOI] [Google Scholar]
- Apfel RE, Holland CK. Gauging the likelihood of cavitation from short-pulse, low-duty cycle diagnostic ultrasound . Ultrasound Med Biol. 1991;17:179–85. doi: 10.1016/0301-5629(91)90125-g. [DOI] [PubMed] [Google Scholar]
- American Institute of Ultrasound in Medicine Section 7 – discussion of the mechanical index and other exposure parameters. J Ultrasound Med. 2000; 19: 143-8, 154-68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nightingale KR, Church CC, Harris G, Wear KA, Bailey MR, Carson PL, et al. Conditionally increased acoustic pressures in nonfetal diagnostic ultrasound examinations without contrast agents: a preliminary assessment. J Ultrasound Med. 2015;34:1–41. doi: 10.7863/ultra.34.7.15.13.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church CC, Labuda C, Nightingale K. A theoretical study of inertial cavitation from acoustic radiation force impulse (ARFI) imaging and implications for the mechanical index. Ultrasound Med Biol. 2015;41:472–85. doi: 10.1016/j.ultrasmedbio.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckey JC, Knaus DA, Alvarenga DL, Kenton MA, Magari PJ. Dual-frequency ultrasound for detecting and sizing bubbles. Acta Astronaut. 2005;56:1041–7. doi: 10.1016/j.actaastro.2005.01.032. [DOI] [PubMed] [Google Scholar]
- Papadopoulou V, Tang M-X, Balestra C, Eckersley RJ, Karapantsios TD. Circulatory bubble dynamics: from physical to biological aspects. Adv Colloid Interface Sci. 2014;206:239–49. doi: 10.1016/j.cis.2014.01.017. [DOI] [PubMed] [Google Scholar]
- Swan JG, Bollinger BD, Donoghue TG, Wilbur JC, Phillips SD, Alvarenga DL, et al. Microbubble detection following hyperbaric chamber dives using dual-frequency ultrasound. J Appl Physiol (1985). 2011;111:1323–8. doi: 10.1152/japplphysiol.01203.2010. [DOI] [PubMed] [Google Scholar]
- Bader KB, Holland CK. Gauging the likelihood of stable cavitation from ultrasound contrast agents. Phys Med Biol. 2013;58:127–44. doi: 10.1088/0031-9155/58/1/127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church CC, Miller DL. A two-criterion model for microvascular bio-effects induced in vivo by contrast microbubbles exposed to medical ultrasound. Ultrasound Med Biol. 2016;42:1385–98. doi: 10.1016/j.ultrasmedbio.2016.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Child SZ, Hartman CL, Schery LA, Carstensen EL. Lung damage from exposure to pulsed ultrasound. Ultrasound Med Biol. 1990;16:817–25. doi: 10.1016/0301-5629(90)90046-f. [DOI] [PubMed] [Google Scholar]
- Baggs R, Penney DP, Cox C, Child SZ, Raeman CH, Dalecki D, et al. Thresholds for ultrasonically induced lung hemorrhage in neonatal swine. Ultrasound Med Biol. 1996;22:119–28. doi: 10.1016/0301-5629(95)02035-7. [DOI] [PubMed] [Google Scholar]
- Miller DL. Induction of pulmonary hemorrhage in rats during diagnostic ultrasound. Ultrasound Med Biol. 2012;38:1476–82. doi: 10.1016/j.ultrasmedbio.2012.04.004. [DOI] [PubMed] [Google Scholar]
- Miller DL, Dou C, Raghavendran K. Dependence of thresholds for pulmonary capillary hemorrhage on diagnostic ultrasound frequency. Ultrasound Med Biol. 2015;41:1640–50. doi: 10.1016/j.ultrasmedbio.2015.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raeman CH, Child SZ, Dalecki D, Cox C, Dalecki EL. Exposure-time dependence of the threshold for ultrasonically induced murine lung hemorrhage. Ultrasound Med Biol. 1996;22:139–41. doi: 10.1016/0301-5629(95)02036-5. [DOI] [PubMed] [Google Scholar]
- Church CC, O’Brien WD. Evaluation of the threshold for lung hemorrhage by diagnostic ultrasound and a proposed new safety index. Ultrasound Med Biol. 2007;33:810–8. doi: 10.1016/j.ultrasmedbio.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien WD Jr, Zachary JF. Comparison of mouse and rabbit lung damage exposure to 30 kHz ultrasound. Ultrasound Med Biol. 1994;20:299–307. doi: 10.1016/0301-5629(94)90070-1. [DOI] [PubMed] [Google Scholar]
- O’Brien WD Jr, Zachary JF. Mouse lung damage from exposure to 30 kHz ultrasound. Ultrasound Med Biol. 1994;20:287–97. doi: 10.1016/0301-5629(94)90069-8. [DOI] [PubMed] [Google Scholar]
- Zachary JF, O’Brien WD Jr. Lung lesions induced by continuous- and pulsed-wave (diagnostic) ultrasound in mice, rabbits, and pigs. Vet Pathol. 1995;32:43–54. doi: 10.1177/030098589503200106. [DOI] [PubMed] [Google Scholar]
- Tarantal AF, Canfield DR. Ultrasound-induced lung hemorrhage in the monkey. Ultrasound Med Biol. 1994;20:65–72. doi: 10.1016/0301-5629(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Meltzer RS, Adsumelli R, Risher WH, Hicks GL Jr, Stern DH, Shah PM, et al. Lack of lung hemorrhage in humans after intraoperative transesophageal echocardiography with ultrasound exposure conditions similar to those causing lung hemorrhage in laboratory animals. J Am Soc Echocardiogr. 1998;11:57–60. doi: 10.1016/s0894-7317(98)70120-8. [DOI] [PubMed] [Google Scholar]
- Hartman C, Child SZ, Mayer R, Schenk E, Carstensen EL. Lung damage from exposure to the fields of an electrohydraulic lithotripter. Ultrasound Med Biol. 1990;16:675–9. doi: 10.1016/0301-5629(90)90100-q. [DOI] [PubMed] [Google Scholar]
- Bailey MR, Dalecki D, Child SZ, Raeman CH, Penney DP, Blackstock DT, et al. Bioeffects of positive and negative acoustic pressures in vivo. J Acoust Soc Am. 1996;100:3941–6. doi: 10.1121/1.417340. [DOI] [PubMed] [Google Scholar]
- O’Brien WD Jr, Frizzell LA, Weigel RM, Zachary JF. Ultrasound-induced lung hemorrhage is not caused by inertial cavitation. J Acoust Soc Am. 2000;108:1290–7. doi: 10.1121/1.1287706. [DOI] [PubMed] [Google Scholar]
- Miller DL. Overview of experimental studies of biological effects of medical ultrasound caused by gas body activation and inertial cavitation. Prog Biophys Mol Biol. 2007;93:314–30. doi: 10.1016/j.pbiomolbio.2006.07.027. [DOI] [PubMed] [Google Scholar]
- Miller DL. Mechanisms for induction of pulmonary capillary hemorrhage by diagnostic ultrasound: review and consideration of acoustical radiation surface pressure. Ultrasound Med Biol. 2016;42:2743–57. doi: 10.1016/j.ultrasmedbio.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien WD Jr, Frizzell LA, Schaeffer DJ, Zachary JF. Superthreshold behavior of ultrasound-induced lung hemorrhage in adult mice and rats: role of pulse repetition frequency and exposure duration. Ultrasound Med Biol. 2001;27:267–77. doi: 10.1016/s0301-5629(00)00342-2. [DOI] [PubMed] [Google Scholar]
- Raeman CH, Child SZ, Carstensen EL. Timing of exposures in ultrasonic hemorrhage of murine lung. Ultrasound Med Biol. 1993;19:507–12. doi: 10.7863/jum.1998.17.6.409. [DOI] [PubMed] [Google Scholar]
- Li P, Armstrong WF, Miller DL. Impact of myocardial contrast echocardiography on vascular permeability: comparison of three different contrast agents. Ultrasound Med Biol. 2004;30:83–91. doi: 10.1016/j.ultrasmedbio.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Miller DL, Driscoll EM, Dou C, Armstrong WF, Lucchesi BR. Microvascular permeabilization and cardiomyocyte injury provoked by myocardial contrast echocardiography in a canine model. J Am Coll Cardiol. 2006;47:1464–8. doi: 10.1016/j.jacc.2005.09.078. [DOI] [PubMed] [Google Scholar]
- Chapman S, Windle J, Xie F, McGrain A, Porter TR. Incidence of cardiac arrhythmias with therapeutic versus diagnostic ultrasound and intravenous microbubbles. J Ultrasound Med. 2005;24:1099–107. doi: 10.7863/jum.2005.24.8.1099. [DOI] [PubMed] [Google Scholar]
- Tran TA, Le Guennec JY, Babuty D, Bougnoux P, Tranquart F, Bouakaz A. On the mechanisms of ultrasound contrast agents-induced arrhythmias. Ultrasound Med Biol. 2009;35:1050–6. doi: 10.1016/j.ultrasmedbio.2008.11.015. [DOI] [PubMed] [Google Scholar]
- Vancraeynest D, Havaux X, Pasquet A, Gerber B, Beauloye C, Rafter P, et al. Myocardial injury induced by ultrasound-targeted microbubble destruction: evidence for the contribution of myocardial ischemia. Ultrasound Med Biol. 2009;35:672–9. doi: 10.1016/j.ultrasmedbio.2008.10.005. [DOI] [PubMed] [Google Scholar]
- van Der Wouw PA, Brauns AC, Bailey SE, Powers JE, Wilde AA. Premature ventricular contractions during triggered imaging with ultrasound contrast. J Am Soc Echocardiogr. 2000;13:288–94. doi: 10.1067/mje.2000.103865. [DOI] [PubMed] [Google Scholar]
- Chen S, Kroll MH, Shohet RV, Frenkel P, Mayer SA, Grayburn PA. Bioeffects of myocardial contrast microbubble destruction by echocardiography. Echocardiography. 2002;19:495–500. doi: 10.1046/j.1540-8175.2002.00495.x. [DOI] [PubMed] [Google Scholar]
- Vancraeynest D, Kefer J, Hanet C, Fillee C, Beauloye C, Pasquet A, et al. Release of cardiac bio-markers during high mechanical index contrast-enhanced echocardiography in humans. Eur Heart J. 2007;28:1236–41. doi: 10.1093/eurheartj/ehm051. [DOI] [PubMed] [Google Scholar]
- Raisinghani A, Wei KS, Crouse L, Villanueva F, Feigenbaum H, Schiller NB. Myocardial contrast echocardiography (MCE) with triggered ultrasound does not cause premature ventricular complexes: evidence from PB127 MCE studies. J Am Soc Echocardiogr. 2003;16:1037–42. doi: 10.1016/S0894-7317(03)00549-2. [DOI] [PubMed] [Google Scholar]
- Hayat SA, Senior R. Safety: the heart of the matter. Eur J Echocardiogr. 2005;6:237–7. doi: 10.1016/j.euje.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Abdelmoneim SS, Bernier M, Scott CG, Dhoble A, Ness SAC, Hagen ME, et al. Safety of contrast agent use during stress echocardiography: a 4-year experience from a single-center cohort study of 26,774 patients. JACC Cardiovasc Imaging. 2009;2:1048–56. doi: 10.1016/j.jcmg.2009.03.020. [DOI] [PubMed] [Google Scholar]
- Wei K, Mulvagh SL, Carson L, Davidoff R, Gabriel R, Grimm RA, et al. The safety of deFinity and Optison for ultrasound image enhancement: a retrospective analysis of 78,383 administered contrast doses. J Am Soc Echocardiogr. 2008;21:1202–6. doi: 10.1016/j.echo.2008.07.019. [DOI] [PubMed] [Google Scholar]
- Abdelmoneim SS, Bernier M, Scott CG, Dhoble A, Ness SAC, Hagen ME, et al. Safety of contrast agent use during stress echocardiography in patients with elevated right ventricular systolic pressure: a cohort study. Circ Cardiovasc Imaging. 2010;3:240–8. doi: 10.1161/CIRCIMAGING.109.895029. [DOI] [PubMed] [Google Scholar]
- Dolan MS, Gala SS, Dodla S, Abdelmoneim SS, Xie F, Cloutier D, et al. Safety and efficacy of commercially available ultrasound contrast agents for rest and stress echocardiography a multicenter experience. J Am Coll Cardiol. 2009;53:32–8. doi: 10.1016/j.jacc.2008.08.066. [DOI] [PubMed] [Google Scholar]
- Chen W, Brayman AA, Matula TJ, Crum LA. Inertial cavitation dose and hemolysis produced in vitro with or without Optison. Ultrasound Med Biol. 2003;29:725–37. doi: 10.1016/s0301-5629(03)00013-9. [DOI] [PubMed] [Google Scholar]
- Miller TW, Everbach EC, Cox C, Knapp RR, Brayman AA, Sherman TA. A comparison of the hemolytic potential of Optison and Albunex in whole human blood in vitro: acoustic pressure, ultrasound frequency, donor and passive cavitation detection considerations. Ultrasound Med Biol. 2001;27:709–21. doi: 10.1016/s0301-5629(01)00356-8. [DOI] [PubMed] [Google Scholar]
- Miller TW, Everbach EC, Miller WM, Battaglia LF. Biological and environmental factors affecting ultrasound-induced hemolysis in vitro: 2. Medium dissolved gas (pO2) content. Ultrasound Med Biol. 2003;29:93–102. doi: 10.1016/s0301-5629(02)00562-8. [DOI] [PubMed] [Google Scholar]
- Brayman AA, Strickler PL, Luan H, Barned SL, Raeman CH, Cox C, et al. Hemolysis of 40% hematocrit, Albunex-supplemented human erythrocytes by pulsed ultrasound: frequency, acoustic pressure and pulse length dependence. Ultrasound Med Biol. 1997;23:1237–50. doi: 10.1016/s0301-5629(97)00126-9. [DOI] [PubMed] [Google Scholar]
- Dalecki D, Raeman CH, Child SZ, Cox C, Francis CW, Meltzer RS, et al. Hemolysis in vivo from exposure to pulsed ultrasound. Ultrasound Med Biol. 1997;23:307–13. doi: 10.1016/s0301-5629(96)00203-7. [DOI] [PubMed] [Google Scholar]
- Killam AL, Greener Y, McFerran BA, Maniquis J, Bloom A, Widdler KJ, et al. Lack of bioeffects of ultrasound energy after intravenous administration of FS069 (Optison) in the anesthetized rabbit. J Ultrasound Med. 1998;17:349–56. doi: 10.7863/jum.1998.17.6.349. [DOI] [PubMed] [Google Scholar]
- Crum LA, Mao Y. Acoustically enhanced bubble growth at low frequencies and its implications for human diver and marine mammal safety. J Acoust Soc Am. 1996;99:2898–907. doi: 10.1121/1.414859. [DOI] [PubMed] [Google Scholar]
- Arieli Y, Arieli R, Shupak A. Can high-frequency sound affect gas-bubble dynamics? A study in the intact prawn Palaemon elegans. Ultrasound Med Biol. 2000;26:1511–5. doi: 10.1016/s0301-5629(00)00305-7. [DOI] [PubMed] [Google Scholar]
- Crum LA, Bailey MR, Guan J, Hilmo PR, Kargl SG, Matula TJ, et al. Monitoring bubble growth in supersaturated blood and tissue ex vivo and the relevance to marine mammal bioeffects. Acoust Res Lett Online. 2005;6:214–20. doi: 10.1121/1.1930987. [DOI] [Google Scholar]
- Shupak A, Pratt H, Arieli Y, Tal D. High-frequency sound transmissions under water and risk of decompression sickness. Ultrasound Med Biol. 2003;29:119–25. doi: 10.1016/s0301-5629(02)00683-x. [DOI] [PubMed] [Google Scholar]
- Shupak A, Arieli R, Rosenhause G, Resnick MB, Arieli Y, Adir Y. The effect of low-frequency ultrasound on immersed pig lungs. Ultrasound Med Biol. 1999;25:1439–43. doi: 10.1016/s0301-5629(99)00086-1. [DOI] [PubMed] [Google Scholar]
- Tal D, Shachar-Bener H, Hershkovitz D, Arieli Y, Shupak A. Evidence for the initiation of decompression sickness by exposure to intense underwater sound. J Neurophysiol. 2015;114:1521–9. doi: 10.1152/jn.00466.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steevens CC, Russell KL, Knafelc ME, Smith PF, Hopkins EW, Clark JB. Noise-induced neurologic disturbances in divers exposed to intense water-borne sound: two case reports. Undersea Hyperb Med. 1999;26:261–5. [PubMed] [Google Scholar]
- Smith PF, Hunter J, William L. On the effects of exposure to intense underwater sound on navy divers: a report of a conference on the bio-effects of sound. Interim report. NSMRL Report 80-1. Groton (CT): Naval Submarine Medical Research Laboratory; 1980. [Google Scholar]
- Smith PF. Effects of exposure to intense tones in water while wearing wet-suit hoods. Interim report. NSMRL Report 1120.. Groton (CT): Naval Submarine Medical Research Laboratory; 1988. [Google Scholar]
- Ilovitsh T, Ilovitsh A, Foiret J, Caskey CF, Kusunose J, Fite BZ, et al. Enhanced microbubble contrast agent oscillation following 250 kHz insonation. Sci Rep. 2018;8:16347. doi: 10.1038/s41598-018-34494-5. [DOI] [PMC free article] [PubMed] [Google Scholar]