Abstract
Invasive ants are usually harmful taxa and are considered a potential problem to biodiversity due to their negative ecological impacts, as they can outcompete native ant species. Ten such species are reported in Brazil. In this study, we report for the first time the Asian tramp ant Technomyrmex vitiensis Mann, 1921 at the municipality of Oiapoque, in the Brazilian Amazon. The colony studied contained workers, intercastes, males and larvae, which provided sperm structure and cytogenetic data. Considering the unprecedented report of the genus Technomyrmex as well as the recent finding of the primarily Australian genus Leptomyrmex in Brazil, we present a revised key for the workers of Brazilian Dolichoderinae genera. Technomyrmex vitiensis presented 2n = 16 chromosomes; all metacentrics and comparative cytogenetics on the genus is provided. A single rDNA 18S site located in intrachromosomal region was observed in this species, which is a common trait in ants. The spermatozoa of T. vitiensis had a filiform shape, with 78.13 (± 1.96) μm of total length and 11.43 (± 0.51) μm of nucleus length. Total and nucleus sperm size length fit with the known variation observed in other ant species. The occurrence of T. vitiensis in Brazil is probably a result of traffic between French Guiana and the Amapá state. Cytogenetics and sperm structures of T. vitiensis enhance the biological knowledge of this tramp species. We highlight the scarce knowledge of ant diversity in the state of Amapá and the consequences that the presence of this species may have in this region.
Keywords: Amazon, Biogeography, Invasive ant, Karyotype, Spermatozoa, Guiana shield
BACKGROUND
Ants from the genus Technomyrmex Mayr are usually arboreal or sub-arboreal and are primarily distributed in the Old World (92 spp., Bolton 2007 2022), with only a couple of extant species occurring natively in the Neotropics, namely Technomyrmex fulvus (Wheeler) and Technomyrmex gorgona Fernández and Guerrero, making it one of the various ant genera with a puzzling biogeographic pattern. Two Dominican amber fossils are currently attributed to Technomyrmex (Brandão et al. 1999); however, there is still debat as to whether or not they truly belong to this genus (Bolton 2007; Fernández and Guerrero 2008). Along with the two native extant Neotropical species, three other tramp have been recorded in the New World (Fernández and Guerrero 2008; Janicki et al. 2016; Guénard et al. 2017). Basic information concerning the biology and taxonomy of these species is scarce (Bolton 2007; Delabie et al. 2011). Moreover, these tramp Technomyrmex are morphologically similar and belong to the albipes group of species –two of them from the pallipes complex, T. pallipes (Smith) and T. difficilis Forel, and the other two from the albipes complex, T. albipes (Smith) and T. vitiensis (Mann) (Bolton 2007). Their similarities have led to much taxonomic confusion, mostly among T. albipes, T. vitiensis, and T. difficilis, usually with all of them being identified as T. albipes (Bolton 2007; Wetterer 2013).
Integrative studies using different tools for complementary biological information are useful for understanding species (Schlick-Steiner et al. 2010). In insects, classical cytogenetics have proven useful in several species (Gokhman and Kuznetsova 2006), such as in bees (Tavares et al. 2017), dragonflies and damselflies (Kuznetsova and Golub 2020), grasshoppers (Bidau and Martí 2010), and ants (Lorite and Palomeque 2010; Mariano et al. 2019). Banding techniques and fluorescence in situ hybridization (FISH) have also proven to be useful for comparative purposes, including general insights into Formicidae and specific groups such as leaf-cutting ants (reviewed by Teixeira et al. 2021).
Structural sperm analysis also provides important data for species characterization. Sperm, for example, varies immensely in size and shape across the animal kingdom and is the most diverse cell type known (Pitnick et al. 2009). In insects, sperm varies in length from 7 μm (Uzbekov et al. 2017) to nearly 6 cm (Pitnick et al. 1995). Structural sperm characteristics may be useful for differentiating sympatric, cryptic species, such as the ants Neoponera inversa (Smith) and Neoponera villosa (Fabricius) (Barcellos et al. 2015), which are often confused by many researchers (Fernandes et al. 2014). The difference in their sperm nuclear size confirms that these are different species (Barcellos et al. 2015).
In this study we report T. vitiensis in Brazil, the first report of the genus for the country, and provide an updated identification key to the Brazilian Dolichoderinae genera based on workers. We also present classical and molecular cytogenetic data and sperm structure, which significantly improves the biological characterization of this tramp ant.
MATERIALS AND METHODS
One fragment of a Technomyrmex colony was collected by active search during a field campaign in March 2020 in urban areas of Oiapoque, state of Amapá, extreme north of Brazil (3.843167, -51.833328). Sampling permission was given by the Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio) to Luísa Antônia Campos Barros (SISBIO accession number 62598). The nest containing workers, brood, and males was transported and maintained in an artificial nest in the laboratory. Honey and water were provided to the workers. Some adult specimens were stored in alcohol and maintained as vouchers in the Laboratório de Morfofisiologia e Imunoparasitologia, at the Universidade Federal do Amapá, Oiapoque, Amapá, Brazil. Six dry and mounted specimens were deposited in the Coleção Entomológica do Laboratório de Coleoptera (CELC), at Universidade Federal de Viçosa, Minas Gerais, Brazil (an intercaste, UFV-LABECOL-010782; a male, UFV-LABECOL-010784; and four workers, UFV-LABECOL-010997, UFV-LABECOL-010996, UFV-LABECOL-010995, UFV-LABECOL-010783). One dry and pinned worker was deposited in the Museu de Zoologia da Universidade Federal de São Paulo (MZSP), São Paulo, Brazil (ANTWEB1047072) and one dry and pinned worker in the Museu Paraense Emílio Goeldi (MPEG), Belém, Pará, Brazil (ANTWEB1047073). The remaining brood and males of the colony were used subsequent cytogenetic and sperm analyses. To identify the species as T. vitiensis, we used a combination of identification keys and Dolichoderinae genus and Technomyrmex species (Shattuck 1992; Palacio and Fernández 2003; Bolton 2007; Fernández and Guerrero 2008; Baccaro et al. 2015), then consulted Dr. Jacques H. C. Delabie.
Mitotic chromosomes were obtained from the cerebral ganglia of five larvae of workers after meconium elimination using the protocol described by Imai et al. (1988) and additional improvements (see Imai 2017). This protocol also allowed us to detect heterochromatin using Giemsa staining without C-banding treatment. The chromosome number and morphology of metaphases were analyzed using conventional 4% Giemsa staining. Chromosomes were arranged based on the ratio of the chromosomes’ arm lengths (r = long arm/short arm), according to the classification proposed by Levan et al. (1964). Chromosomes were organized using Adobe Photoshop 2021® and 10 metaphases were measured using Image Pro Plus® in order to determine the chromosomal morphology.
Physical mapping of 18S ribosomal genes was performed by FISH following the protocol of Pinkel et al. (1986). The 18S rDNA probes were amplified via PCR (polymerase chain reaction) using Melipona quinquefasciata Lepeletier primers rDNA 18SF1 (5'-GTC ATA GCT TTG TCT CAA AGA-3') and 18SR1.1 (5'-CGC AAA TGA AAC TTT TTT AAT CT-3') (Pereira 2006) and genomic DNA from the ant Camponotus rufipes (Fabricius). Gene amplification followed Pereira (2006). 18S rDNA probes were labeled by an indirect method using digoxigenin-11-dUTP using DIG-Nick Translation Mix (Roche Applied Science, Mannheim, Germany), and the FISH signals were detected with anti-digoxigenin-rhodamine (Roche Applied Science), following the manufacturer’s protocol.
The sperm structures of three males of T. vitiensis were also analyzed. For this, males were individually dissected using glass slides in 0.9% saline solution. Sperm extracted from the seminal vesicles of each individual were spread on clean, air-dried histological slides at room temperature. The samples were initially stained with the fluorochrome 4',6-diamidino-2-phenylindole (DAPI) (0.6 μg/mL) for 30 minutes, washed in Sörensen pH 7.0 buffer, and mounted with coverslips and 50% sucrose to measure the nuclei. After removing the fluorochrome, the slides were stained with Giemsa 4% for 30 minutes, then washed in running water and photographed under a light microscope to measure the total length of the sperm. The average total and nucleus length of spermatozoa were randomly measured using 20 cells per individual. Measurements were conducted using the software Image Pro Plus®.
Fluorescence images were analyzed and photographed using an epifluorescent microscope Olympus BX60 attached to an image system QColor Olympus® with WU (330–385 nm), and WG (510–550 nm) filters for the fluorochromes DAPI and rhodamine, respectively. Giemsa images were photographed under a light microscope.
Images of the adult specimens (Figs. 1 and 2) were made in an Olympus SZ60 stereomicroscope with an 2x auxiliary lens and an attached Canon 1100D DSLR camera. A slightly modified version of the dome illumination system presented in Kawada and Buffington (2016) was used for image acquisition. Light microscope images of disarticulated body parts were made in a Leica Galen III with the same DSLR attached. Images were edited in GIMP (Kimball and Mattis 1996) and scales were added in ImageJ (Schneider et al. 2012) calibrated by measurements made in the specimens during image acquisition. Individual high-definition images from each specimen were uploaded to AntWeb Version 8.56.1 (2021, www.antweb.org), accompanied by their unique identifiers, which are presented in the respective figure captions. Various images composing the plates that illustrate the keys have been gathered from antweb.org and are credited to each photographer accordingly under the figure captions; those not credited to a photographer were taken by us.
Fig. 1.
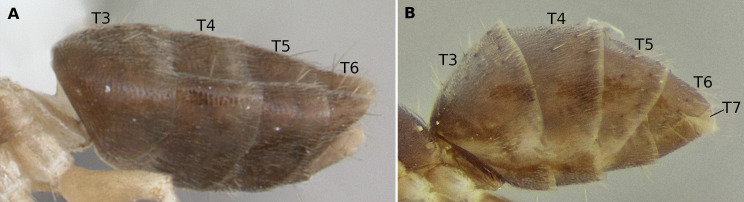
Worker (UFV-LABECOL-010783) and intercaste (UFV-LABECOL-010782) of Technomyrmex vitiensis. (A) full-face view of worker, (B) profile view of worker, (C) dorsal view of worker, (D) vertex of intercaste, white triangles point to ocelli, (E and F) mesosoma of worker and intercaste, respectively, arrowhead indicating barely differentiated mesoscutellum. Abbreviations: T3–T7, tergites three to seven. Scale bars: A, D, E and F = 0.2 mm; B and C = 0.5 mm.
Fig. 2.
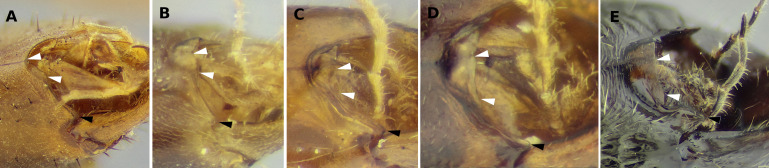
Technomyrmex vitiensis male (UFV-LABECOL-010784). (A) full-face view, (B) profile view, (C) fore wing, (D) sternite 9, (E) tergite 9, (F, G, and H) genital capsule, dorsal, ventral and lateral views, respectively, (I) volsella, (J, and K) entire penisvalva and detail of dented ventral margin, respectively. Scale bars:A= 0.2 mm, B and C = 0.5 mm, D, E, H and J = 0.1 mm. Scale bar in H serves for F and G as well.
Genitalia was dissected, disarticulated and mounted in PVLG (polyvinyl alcohol-lactic acid-glycerol) medium (Omar et al. 1979) between a pair of 8 mm round cover glasses. Excessive muscle fibers were removed physically during dissection, sclerites were not treated with KOH. A paper card was glued at the margin of the cover glasses so that the genitalia could be pinned and maintained together with the male.
RESULTS
The focal colony for this study was collected during the collection of Crematogaster spp. on tree branches of an exotic Lauraceae denominated as “caneleira” in the region, possibly corresponding to Cinnamomum verum Presl. In the most recent identification guide to the Brazilian genera of ants (Baccaro et al. 2015), the sampled specimens smoothly keyed into Tapinoma Foerster. Among Dolichoderinae genera, Tapinoma and Technomyrmex are the only to have a petiole overlapped and concealed by the anteriorly projected first gaster tergite and with a reduced or absent node (Bolton 2007). According to Shattuck (1992), Technomyrmex differs from most other Dolichoderinae genera, including Tapinoma, by the morphology of the gaster. In Technomyrmex the gaster has a visible fifth tergite (or abdominal tergite 7, T7 in Fig. 1), dorsally or at most posteriorly facing, as opposed to a reflexed fifth gaster tergite which faces ventrally and is concealed by the fourth tergite, leaving the gaster with only four apparent tergites (Shattuck 1992; Bolton 2003). This difference is used to discriminate between the two genera in the Neotropical identification key of Palacio and Fernández (2003), but not in the Brazilian guide (Baccaro et al. 2015), as Technomyrmex had not been recorded for the country before the present study. Gastral morphology confirms our sampled specimens belong to Technomyrmex. Other characters such as a single pair of pronotal setae, mesonotum lacking setae, an angled mesonotum in profile view (as opposed to an evenly curved one), and the propodeum shape, with the dorsal and the descending margins meeting in a sharp angle, narrow down the identification in the species level to T. vitiensis (Figs. 1, 2) (Bolton 2007; Fernández and Guerrero 2008). The current number of 111 genera recorded in Brazil so far (Fernández et al. 2021) is therefore increased to 112. Information concerning winged males, ergatomorph wingless males, and winged females are available for T. vitiensis (Pech and Bezděk 2016; Väänänen et al. 2018), with similarities between winged males (Väänänen et al. 2018).
Since the publication of Baccaro et al. (2015), two genera of dolichoderines have been added to the country’s fauna, Leptomyrmex Mayr (Boudinot et al. 2016) and Technomyrmex (this study), rendering out of date the Dolichoderinae key of that guide. Technomyrmex identification can be made as explained above. Looking for several specimens is preferable, as dry pinned dolichoderines often have the gaster deformed, thus making difficult to separate between Technomyrmex and Tapinoma. Also, although not ideal, considering that to date only T. vitiensis has been recorded for Brazil, identification of the genus in the country can rely on that species idiosyncrasies as well. Leptomyrmex relictus Boudinot, Probst, Brandão, Feitosa and Ward is unique among Neotropical ants and is, in fact, more likely to be confused with the genus Camponotus Mayr than with other dolichoderines in the region due to its large size, uncommon in the subfamily. Similarities are very superficial though, and it can be easily distinguished from any Camponotus by having a metapleural gland orifice, and by lacking an acidopore on the fifth gaster sternite. From the remaining dolichoderines, Leptomyrmex relictus can be distinguished by the following features or their combination: absence of spines, lamellae or angles in the mesosoma and petiole; pilosity conspicuous, composed of semierect, short, thick and dark setae, contrasting to body’s light coloration (on most parts); hypostoma medially notched; total body length large (more than 7 mm) with hind legs longer than body (Boudinot et al. 2016).
Below we present an updated key to the Brazilian Dolichoderinae genera. The key is largely based on those of Baccaro et al. (2015) and Palacio and Fernández (2003), also making use of the diagnostic characters given in Shattuck (1992) and Bolton (2007). It is presented in English below and in Portuguese in the supplementary material.
Key to Brazilian Dolichoderinae genera based on workers
1. Large or very large ants, easily larger than 5 mm and usually reaching 10 mm or more ................. 2
-Small or medium-sized ants, rarely reaching 5 mm and never as large as 10 mm ................. 3
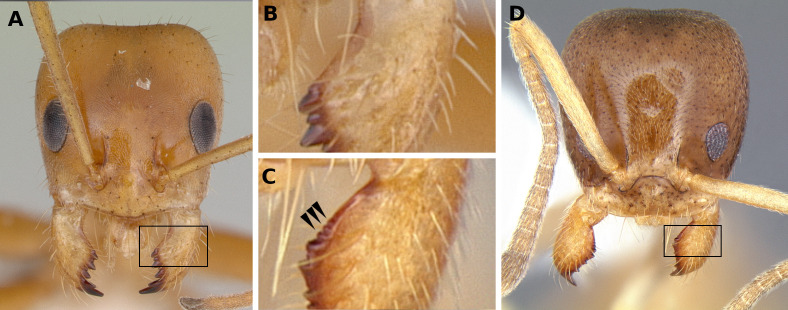
2(1). Mesosoma without spines, lamellae or well-defined angles. Hypostoma with a deep medial incision (Fig. 6A). Integument delicate. Pilosity composed of thick, short and erect dark setae (Fig. 3A) ................. Leptomyrmex Mayr
-Mesosoma, usually on the propodeum, having spines, lamellae or well-defined angles. Hypostoma without a deep medial incision, having at most a minute one (Fig. 6C–E). Usually with thick and sculptured integument. Pilosity varied (Fig. 3B and 3C) ................. Dolichoderus Lund (in part)
3(1). Petiolar node vestigial or completely absent (Fig. 4A) ................. 4
-Petiolar node clearly present, sometimes very small (Fig. 4B and 4C) ................. 5
4(3). Gaster with four visible tergites (Fig. 5A) ................. Tapinoma Foerster
-Gaster with five visible tergites (Fig. 5B) ................. Technomyrmex Mayr
5(3). Mesosoma, usually on the propodeum, having spines, lamellae or well-defined angles (Fig. 3B and 3C). Usually having thick and sculptured integument. Hypostoma with a pair of dentiform projections anterolaterally (Fig. 6D and 6E), sometimes reduced (Fig. 6C) ................. Dolichoderus Lund (in part) (Note 1)
-Pronotum without spines, lamellae or well-defined angles; propodeum sometimes with a single medial coniform projection (Fig. 4C), but never a pair of spines or lamellae between the dorsal and the descending faces. Integument never thickened, usually fragile and unsculptured. Hypostoma without dentiform projections anterolaterally (Fig. 6A and 6B) ................. 6
6(5). Propodeum having a single, dorso-medial coniform to spiniform projection (Fig. 4C). Third maxillary palpomere elongate, having approximately the size of palpomeres 4 to 6 combined. Psamophore sometimes present (Fig. 7A) (Note 2) ................. Dorymyrmex Mayr
-Propodeum without spiniform or coniform projections, or sharp angles (Fig. 4A and 4B), at most having defined dorsal and descending surfaces (Fig. 1E). Third maxillary palpomere not conspicuously elongate in relation to the others. Psamophore absent (Fig. 7B) ................. 7
7(6). Eyes absent. Pale colored species. Gaster tube-like, abdominal segments 4 to 6 (second to fourth of gaster) being relatively elongate ................. Anillidris Santschi (Fig. 8) (Note 3)
-Eyes present. Rarely pale coloured species. Gastral tergites not particularly elongate, not tube-like, abdominal segments 5 and 6 (third and fourth of gaster) much smaller than 3 and 4 (first and second of gaster) ................. 8
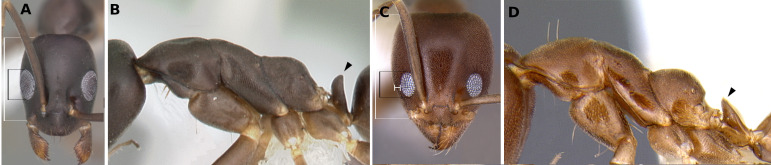
8(7). Propodeal spiracle usually conspicuously large (Fig. 4B). First gastral tergite overhanging petiole on dorsal view (often only partially). Basal margin of mandible edentate, without teeth or minute serrations (Fig. 9A and 9B). Two to 12 long clypeal setae anteriorly projected over and beyond the anterior clypeal margin ................. Forelius Emery
-Propodeal spiracle small and inconspicuous (Fig. 4A and 4C). First gastral tergite does not overhang the petiole on dorsal view. Basal margin of mandible either edentate or having teeth or minute serrations (Fig. 9B and 9C). Two to six long clypeal setae anteriorly projected over and beyond the anterior clypeal margin ................. 9
9(8). Eye large, about a third of lateral head margin. Scape very long, surpassing the posterior margin of head by about one third of its length. Outer margins of eyes reach the lateral margins of the head in full-face view (Fig. 10A). In profile view, anterior margin of petiolar node convex and posterior margin either flat or mildly concave, so that apex of node tends to be slightly inclined posteriorly. Dorsum of mesosoma and metasoma devoid of erect setae (Fig. 10B) ................. Gracilidris Wild and Cuezzo
-Without the above combination of characters. Eyes always smaller than a third of lateral head margin. Scape not reaching the vertexal margin (less common) or surpassing it by less than a third of its length (more common). Outer margins of eyes not reaching the lateral margins of the head in full-face view (Fig. 10C). In profile view, tip of petiolar node usually anteriorly inclined. Dorsum of mesosoma and metasoma usually having erect setae, sometimes abundant (Fig. 11A), sometimes only a few (Fig. 10D) ................. 10
10(9). Mesonotum usually higher than pronotum in profile. Erect setae on the mesosoma, when present, not forming evident symmetrical pairs on the sclerites (Fig. 11A and 11B). Medial section of anterior clypeal margin usually convex or flat (Fig. 11E and 11F). Petiole node usually not compressed anteroposteriorly, node (only tergite) as long as tall or longer than tall in profile (Fig. 11A and 11B) ................. Azteca Forel
-Mesonotum usually below the maximum level of the pronotum in profile. Erect setae on the mesosoma, when present, usually a few, paired, and easily countable (Fig. 11C and 11D) (Note 4). Medial portion of anterior clypeal margin usually mildly concave (Fig. 11G and 11H). Petiole node anteroposteriorly compressed, taller than long in profile view (Fig. 11C and 11D) (Note 5) ................. Linepithema Mayr
Fig. 3.
Profile view of A, Leptomyrmex relictus (ANTWEB1032698); B, Dolichoderus attelaboides (CASENT0249660, www.antweb.org, image by Will Ericson); C, Dolichoderus bispinosus (CASENT0173833, www.antweb.org, image by April Nobile). Scale bars = 2 mm.
Fig. 4.
Zoomed profile of propodeum and petiole of A, Tapinoma ramulorum; B, Forelius alw02 (CASENT0173741, www.antweb.org, image by April Nobile); and C, Dorymyrmex az03 (CASENT0172961, www.antweb.org, image by April Nobile). Dashes pointing to petiole nodes (even if vestigial as in A) and triangles to propodeal spiracles.
Fig. 5.
Zoomed profile of petiole and gaster of A, Tapinoma atriceps (CASENT0173743, www.antweb.org, image by April Nobile); and B, Technomyrmex vitiensis (UFV-LABECOL-010783), with abdominal tergites 3–7 indicated, T7 hidden in Tapinoma.
Fig. 6.
Oblique lateroventral view of head of A, Leptomyrmex relictus (ANTWEB1032698); B, Gracilidris pombero (UFV-LABECOL-007570); C, Dolichoderus ghilianii (ANTWEB1038704); D, Dolichoderus debilis (UFV-LABECOL-010644); E, Dolichoderus attelaboides (UFV-LABECOL-005856). Black triangles indicate lateral edges of hypostomae (with absence or presence of teeth) and white triangles point to their median portions (with or without incision).
Fig. 7.
Zoomed profile of head of A, Dorymyrmex brunneus (CASENT0192698, www.antweb.org, image by Erin Prado); and B, Linepithema oblongum (CASENT0106974, www.antweb.org, image by Alexander Wild). Maxillary palpomere 3 is indicated (mxp3) and hypertrophied in Dorymyrmex (maxillary palpomeres 1 and 2 are not seen in image A). Arrowhead indicates one of the setae that forms the psamophore.
Fig. 8.
Full-face view (A) and profile view (B) of Anillidris bruchi (UFV-LABECOL-004260).
Fig. 9.
Full-face views and details of mandible basal margins. A and B, Forelius nigriventris (CASENT0173738, www.antweb.org, image by April Nobile); C and D, Linepithema keiteli (CASENT0106975, www.antweb.org, image by Alexander Wild).
Fig. 10.
Full-face view and profile view of A and B, Gracilidris pombero (CASENT0010797, www.antweb.org, image by April Nobile); and C and D, Linepithema neotropicum (CASENT0106968, www.antweb.org, image by Alexander Wild).
Fig. 11.
Profile (A–D) and full-face (E–H) views of Azteca and Linepithema. From A–D, black bars are the upper limit of pronota, small white bars the upper limit of mesonota and thin long white bars show proper alignment of the specimens, with lower limits of meso-and metapleurae sitting on the line. White triangles in E–H point to medial portion of anterior clypeal margins. A and E, Azteca alfari (INBIOCRI001280538, www.antweb.org, images by Will Ericson); B and F, Azteca lanuginosa (UFV-LABECOL-000274, www.antweb.org, images by Ricardo Solar); C and G, Linepithema oblongum (CASENT0106974, www.antweb.org, images by Alex Wild); D and H, Linepithema neotropicum (CASENT0106968, www.antweb.org, images by Alex Wild).
Note 1. We observed in the examined material that the hypostoma lateral teeth of Dolichoderus vary from almost absent to very large, so that we recommend caution with the use of this character.
Note 2. The psamophore consists of elongate setae of similar size and arranged in a defined pattern ventrally on the head (Cuezzo and Guerrero 2012, Fig. 7A, black triangle). It is extremely developed in some species, however is discrete in size and number of setae in the most commonly sampled species in Brazil.
Note 3. Anillidris Santschi is a peculiar genus unlikely to be confused with any Dolichoderinae. However, it is superficially similar to Acropyga Roger, a genus of subterranean formicines. Acropyga can be separate from Anillidris by the presence of the acidopore, a synapomorphy of the Formicinae, however, if the gaster is poorly preserved, other traits can help separating the two genera. Acropyga in the Neotropics often have reduced number of antennomeres, while Anillidris has 12; their heads tend to be subquadrate, while it is evidently longer than wide in Anillidris; they have small eyes, sometimes single-faceted, while Anillidris is entirely blind; their gasters, although sometimes large, is never tubuliform as in Anillidris. Lastly, Acropyga species are much more commonly found than Anillidris bruchi Santschi, the single species in the genus and one of the rarest in the Neotropics.
Note 4. A recently described species, Linepithema hirsutum (Escárraga and Guerrero 2016), has numerous erect setae across the body, a unique feature in the genus. There are, however, no records of L. hirsutum outside Colombia to this date.
Note 5. Linepithema aztecoides Wild has a low petiolar node, more similar to that attributed to Azteca in the couplet, however it matches Linepithema in the other characters of the couplet.
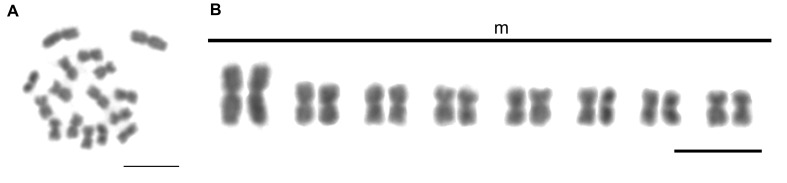
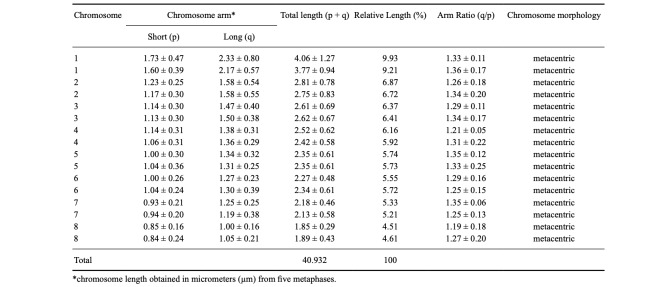
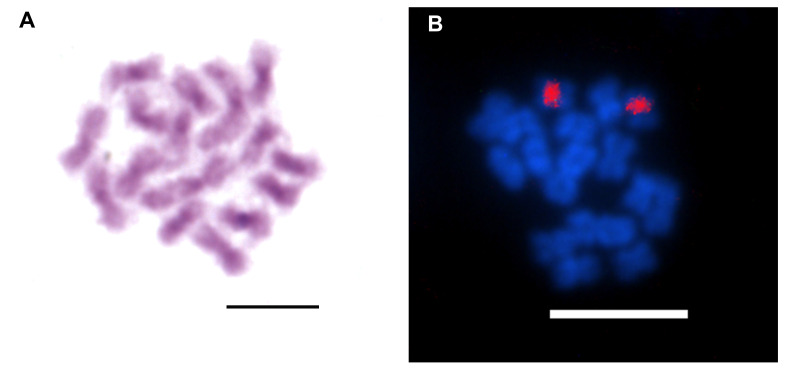
Concerning cytogenetic analysis, T. vitiensis presented 2n = 16 chromosomes, all metacentrics (Fig. 12, Tables 1, 2). The biggest chromosome pair is notably larger than the rest, being, for example, 40% larger than the second bigger pair. Heterochromatic blocks were observed on centromeric and pericentromeric regions of the chromosomes (Fig. 13A). 18S rDNA clusters were detected on centromeric/pericentromeric region of a single chromosome pair (Fig. 13B).
Fig. 12.
Diploid metaphase (A), and karyotype (B) of Technomyrmex vitiensis (2n = 16; 2n = 16m) from Oiapoque, state of Amapá, Brazil. Scale bars = 5 μm.
Table 1.
Karyomorphometric analysis of the tramp ant Technomyrmex vitiensis Mann, 1921 (2n = 16) from Oiapoque, state of Amapá. Brazil
Table 2.
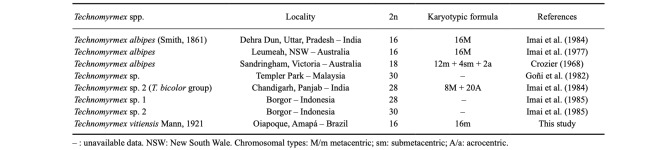
Cytogenetic data of the ant genus Technomyrmex Mayr, 1872. Localities of collection sites, diploid chromosome numbers (2n), diploid karyotypic formulae, and references. Chromosome morphology was used according to literature published data. Chromosome morphology was used according to literature published data
Fig. 13.
Diploid metaphases of Technomyrmex vitiensis submitted to (A) Giemsa staining showing heterochromatic pattern in centromeric/pericentromeric regions on chromosomes, and (B) Fluorescence in situ hybridization with 18S rDNA probe (red blocks). Scale bars = 5 μm.
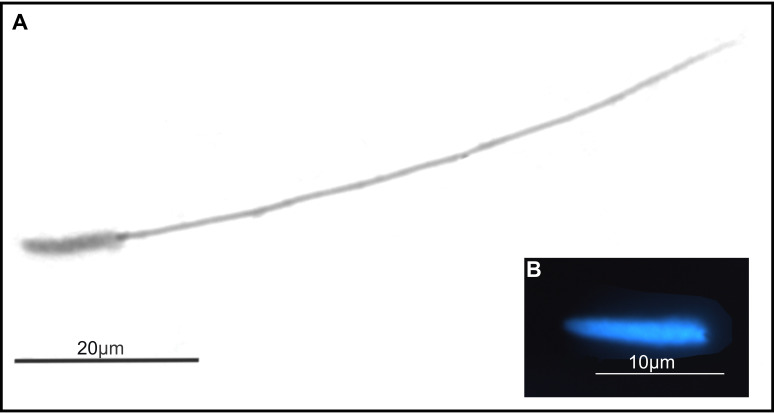
The spermatozoa of T. vitiensis appeared filiform, having a total length of 78.13 (± 1.96) μm and 11.43 (± 0.51) μm of nucleus (Fig. 14).
Fig. 14.
Sperm structure of Technomyrmex vitiensis. (A) sperm stained with Giemsa and (B) nucleus stained with DAPI fluorochrome.
DISCUSSION
Biological aspects of Technomyrmex vitiensis: cytogenetics and sperm analyzes
Cytogenetic data in Technomyrmex are available for five taxa with chromosome numbers ranging from 2n = 16 to 30 (Table 2). Technomyrmex albipes, included in the same group as T. vitiensis, presented 2n = 16, all metacentrics, in populations from Australia (Imai et al. 1977) and India (Imai et al. 1984). In contrast, another population of T. albipes from Australia showed 2n = 18 (2n = 12m + 4sm + 2a) (Crozier 1968). In addition, in the Australian karyotype of T. albipes analyzed by Crozier (1968), most of the chromosomes were metacentrics and the larger chromosome pair was considered submetacentric. According to Imai et al. (1984), the karyotype 2n = 18 seems to be a derivation of 2n = 16, as a consequence of a centric fission and a pericentric inversion. Nevertheless, these authors highlighted the importance of additional investigations to understand if the karyotype 2n = 18 is polymorphic or belongs to a sibling species.
Technomyrmex vitiensis in Brazil showed the same karyotypic formula (2n = 16m) as T. albipes from Australia and India (Imai et al. 1977 1984). Nevertheless, contrasting with T. vitiensis of this study, the three larger pairs of chromosomes in both populations of T. albipes are similar in size. In addition, the first pair of chromosomes in the population of T. albipes with 2n = 18 is not notably different in size from the other chromosomes and differs in chromosomal morphology compared to T. vitiensis. The karyotypic dissimilarity among the T. vitiensis and T. albipes populations seems to corroborate the distinct species status between them.
The chromosome number associated with the heterochromatin distribution is in accordance with the species with n ≤ 12 (Imai et al. 1988). Heterochromatin usually has repetitive elements, such as satellite repeats and transposable elements (Allshire and Madhani 2018) and its amplification in centromeric/pericentromeric regions has been suggested to occur in bees possibly due to mobile elements (Rocha and Pompolo 1998; Cunha et al. 2020). Heterochromatin remodeling leading to chromosome size alteration is an adequate possibility to explain size variations found in chromosomes of T. vitiensis (from Oiapoque) and T. albipes (from India and Australia). Future investigations on the composition of this heterochromatin might bring a clearer idea of the karyotypic evolution paths that led to the size variation observed between these two species.
The intrachromosomal location of ribosomal genes, as observed in T. vitiensis, is the most common pattern in Formicidae and influences its restriction to a single chromosome pair, since the rearrangements that lead to dispersion of rDNA genes in karyotype are less likely to occur in intrachromosomal regions as reviewed by Teixeira et al. (2021).
Sperm length and shape were similar to those observed in other ants such as in the genera Neoponera Emery (Barcellos et al. 2015), Pachycondyla Smith (Cuquetto-Leite et al. 2017), Solenopsis Westwood (Lino-Neto and Dolder 2002), and many Attina (fungus-farming ants) (Baer et al. 2009). Sperm length in Formicidae ranges from 53 μm in Pseudomyrmex termitarius (Smith) (Moya et al. 2007) to 230.49 μm in Apterostigma sp. 2 (Baer et al. 2009). Detailed ultrastructure data are available for other Dolichoderinae, which includes Tapinoma sessile (Say), Dorymyrmex insanus (Buckley) (as Conomyrma insana), and Dorymyrmex wheeleri (Kusnezov) (as Conomyrma wheeleri) (Wheeler et al. 1990); however, total length for those species were not presented.
Dispersal pattern of Technomyrmex in South America
Eight introduced invasive species are reported in French Guiana, in the Guiana shield (Talaga et al. 2015), including T. vitiensis, which is native from southeast Asia (continental and insular). Due to transport and introduction, T. vitiensis also occurs on islands in the Indian Ocean, Madagascar, Kenya; on islands in the Pacific Ocean; and in some European countries, including France. By the end of the last century, T. vitiensis had already been found across the world, mainly in greenhouses (Pospischil 2011). Among exotic ant species, T. vitiensis is considered the most common in tropical greenhouses using tropical environmental conditions in continental Europe (Väänänen et al. 2018). The first record in the Americas is from California, USA (Bolton 2007). Technomyrmex vitiensis was first reported in South America in 2009 in French Guiana and it was supposedly imported from France due to the intense “French connection” between the two localities (Delabie et al. 2011). Another possibility is the introduction from Southeast Asia, as farmers from the region are immigrants in the French Guiana population. To the best of our knowledge, there are no previous records of the species in Brazil.
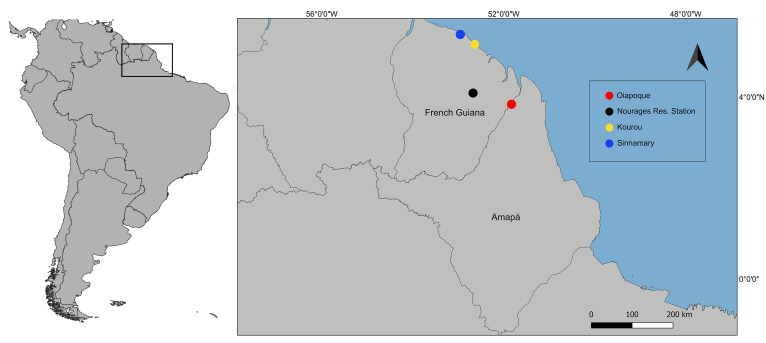
The occurrence of T. vitiensis in French Guiana is reported for three localities: Nouragues Research Station in the forest about 100 km from the Atlantic coast (Delabie et al. 2011), Sinnamary, and Kourou on bromeliads Aechmea aquilega (Salisb.) in coastal urban areas (Talaga et al. 2015). Interestingly, the collected colony in this study was not found in bromeliads, suggesting that the species can nest in different places. The presence of T. vitiensis in Oiapoque, Brazil (Fig. 15) is probably another consequence of the interchange of people between French Guiana and Oiapoque as reported by Almeida and Rauber (2017), which can now be linked to interchange effects on the fauna.
Fig. 15.
Map of the sampling locality of the tramp ant Technomyrmex vitiensis in Oiapoque, Brazil (red circle) and previous records from French Guiana in the forest surrounding the Nouragues Research Station (black circle), in the coastal urban areas in Kourou (yellow circle), and Sinnamary (blue circle).
Technomyrmex vitiensis is widespread in Central Europe and across the world in greenhouses (Pospischil 2011). The rapid dispersion of T. vitiensis to Oiapoque, hypothesized by Delabie et al. (2011) to have been due to intense travel, is supported in this study. However, considering the intense flow between Oiapoque and the capital of the state, Macapá, the presence of this tramp ant should be considered very likely in that locality, which is connected to all major Brazilian cities through the airport and naval port. Although negative ecological impacts of T. albipes have been shown (Dejean et al. 2010), it is still unknown whether T. vitiensis can cause such impacts in the Amazon region, which includes, besides rainforests, other environments such as mangroves and savannahs. The detection of exotic fauna in Amazon environments is important for wildlife monitoring because interaction with native species can generate unpredictable effects on biodiversity.
CONCLUSIONS
In this study, we reported for the first time the genus Technomyrmex, species T. vitiensis, in Brazil and provided an updated key to the Brazilian Dolichoderinae genera. We presented morphological, karyological and sperm structure information of T. vitiensis from northern Brazil, in the Amazon region, providing basic biological data and expanding the distribution of an important tramp species. We emphasize the significance of cytogenetic data for understanding complex taxonomic groups and chromosomal evolution in ants. Our data highlight the importance of further studies on ant biodiversity and conservation in the state of Amapá especially considering the paucity of ant information in the region when compared to nearby localities, such as French Guiana (Franco et al. 2019) and the state of Pará, Brazil (Albuquerque et al. 2021).
Supplementary materials
The key to the workers of the Brazilian Dolichoderine genera, presented in English in the main text, is translated to Portuguese below aiming to maximize its use among researchers and amateurs.
Acknowledgments
We are grateful to Jacques H. C. Delabie for confirming species identification. Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) funded JCMC and GAT. CNPq has funded RBL. Financial support for this study was provided by the Programa de Auxílio ao Pesquisador –PAPESQ/UNIFAP and by “Investissement d’Avenir” grants managed by the French Agence Nationale de la Recherche (DRIIHM ref. ANR-11-LABX-0010 and CEBA, ref. ANR-10-LABX-25-01), by the PO-FEDER 2014-2020, Région Guyane (BiNG, ref. GY0007194).
Footnotes
Authors’ contributions: L.A.C.B and J.C.M.C. conceived the study; L.A.C.B, R.B.L., and H.J.A.C.A. collected the samples; L.A.C.B., H.J.A.C.A, J.O., J.C.M.C. contributed with reagents, materials and analysis tools; L.A.C.B., G.A.T., and H.J.A.C.A conducted cytogenetic experiments; L.A.C.B. conducted sperm analyses; JCMC conducted taxonomic analyses; all the authors analyzed the data and contributed to drafting and revising the manuscript.
Competing interests: All authors declare that they have no conflict of interest.
Availability of data and materials: The material analyzed is available at the Coleção Entomológica do Laboratório de Coleoptera (CELC), Universidade Federal de Viçosa.
Consent for publication: Not applicable.
Ethics approval consent to participate: The sampling permission was conceded by the Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio) to Luísa Antônia Campos Barros (SISBIO accession number 62598).
References
- Albuquerque EZ, Prado LP, Andrade-Silva J, Siqueira ELS, Sampaio KLDS, Alves D, ... Silva RR. 2021. Ants of the State of Para, Brazil: a historical and comprehensive dataset of a key biodiversity hotspot in the Amazon Basin. Zootaxa 5001:1–83. doi:10.11646/zootaxa.5001.1.1. . [DOI] [PubMed]
- Allshire RC, Madhani HD. 2018. Ten principles of heterochromatin formation and function. Nat Rev Mol Cell Biol 19(4):229–244. doi:10.1038/nrm.2017.119. . [DOI] [PMC free article] [PubMed]
- Almeida CS, Rauber AL. 2017. Oiapoque, aqui começa o Brasil: a fronteira em construção e os desafios do Desenvolvimento Regional. Redes 22(1):474–493. doi:10.17058/redes.v22i1.8532.
- AntWeb Version 8.56.1. 2021. California Academy of Science. Available at: https://www.antweb.org. Accessed 4 June 2021.
- Baccaro FB, Feitosa RM, Fernández F, Fernandes IO, Izzo TJ, Souza JD, Solar R (eds). 2015. Guia para os gêneros de formigas do Brasil. Editora INPA, Manaus, Brazil.
- Baer B, Dijkstra MB, Mueller UG, Nash DR, Boomsma JJ. 2009. Sperm length evolution in the fungus-growing ants. Behav Ecol 20:38–45. doi:10.1093/beheco/arn112.
- Barcellos MS, Martins LC, Cossolin JF, Serrão JE, Delabie JH, Lino-Neto J. 2015. Testes and spermatozoa as characters for distinguishing two ant species of the genus Neoponera (Hymenoptera: Formicidae). Fla Entomol 98(4):1254–1256. doi:10.1653/024.098.0441.
- Bidau CJ, Martí DA. 2010. 110 years of orthopteran cytogenetics, the chromosomal evolutionary viewpoint, and Michael White’s signal contributions to the field. J Orthoptera Res 19(2):165–182. doi:10.1665/034.019.0202.
- Bolton B. 2003. Synopsis and classification of Formicidae. Mem Am Entomol Inst 71:1–370.
- Bolton B. 2007. Taxonomy of the dolichoderine ant genus Technomyrmex Mayr (Hymenoptera: Formicidae) based on the worker caste. Contrib Am Entomol Inst 35(1):1–150. doi:10.15468/mhkmav.
- Bolton B. 2022. An online catalog of the ants of the world. Available at: http://antcat.org. Accessed 15 Jan. 2022.
- Boudinot BE, Probst RS, Brandão CRF, Feitosa RM, Ward PS. 2016. Out of the Neotropics: newly discovered relictual species sheds light on the biogeographical history of spider ants (Leptomyrmex, Dolichoderinae, Formicidae). Syst Entomol 41(3):658–671. doi:10.1111/syen.12181.
- Brandão CRF, Baroni-Urbani C, Wagensberg J, Yamamoto CI. 1999. New Technomyrmex in Dominican amber (Hymenoptera: Formicidae), with a reappraisal of Dolichoderinae phylogeny. Entomol Scandinavica 29:411–428. doi:10.1163/187631298X00041.
- Crozier RH. 1968. Cytotaxonomic Studies on some Australian Dolichoderine Ants (Hymenoptera: Formicidae). Caryologia 21(3):241–259. doi:10.1080/00087114.1968.10796302.
- Cuezzo F, Guerrero RJ. 2012. The ant genus Dorymyrmex Mayr (Hymenoptera: Formicidae: Dolichoderinae) in Colombia. Psyche 2012 Article ID 516058. doi:10.1155/2012/516058.
- Cunha MS, Campos LAO, Lopes DM. 2020. Insights into the heterochromatin evolution in the genus Melipona (Apidae: Meliponini). Insectes Soc 67(3):391–398. doi:10.1590/S1415-47571998000100008.
- Cuquetto-Leite L, Dolder H, Oliveira PS, Santo NE, Lino-Neto J, Mancini KC. 2017. Structural and ultrastructural characterization of the spermatozoa in Pachycondyla striata and P. marginata. Insectes Soc 64(2):209–217. doi:10.1007/s00040-016-0533-8.
- Dejean A, Fisher BL, Corbara B, Rarevohitra R, Randrianaivo R, Rajemison B, Leponce M. 2010. Spatial distribution of dominant arboreal ants in a Malagasy coastal rainforest: gaps and presence of an invasive species. PLoS ONE 5(2):1–7. doi:10.1371/journal. pone.0009319. . [DOI] [PMC free article] [PubMed]
- Delabie JH, Groc S, Dejean A. 2011. The tramp ant Technomyrmex vitiensis (Hymenoptera: Formicidae: Dolichoderinae) on South America. Fla Entomol 94(3):688–689. doi:10.1653/024.094.0335.
- Escárraga M, Guerrero RJ. 2016. The ant genus Linepithema (Formicidae: Dolichoderinae) in Colombia. Zootaxa 4208:446–458. doi:10.11646/zootaxa.4208.5.3. . [DOI] [PubMed]
- Fernandes IO, Oliveira ML, Delabie JHC. 2014. Description of two new species in the Neotropical Pachycondyla foetida complex (Hymenoptera: Formicidae: Ponerinae) and taxonomic notes on the genus. Myrmecol News 19:133–163.
- Fernández F, Guerrero RJ. 2008. Technomyrmex (Formicidae: Dolichoderinae) in the New World: synopsis and description of a new species. Rev Colomb Entomol 34(1):110–115. doi:10.25100/socolen.v34i1.9261.
- Fernández F, Guerrero RJ, Sánchez-Restrepo AF. 2021. Systematics and diversity of Neotropical ants. Rev Colomb Entomol 47(1):1–20. doi:10.25100/socolen.v47i1.11082.
- Franco W, Ladino N, Delabie JH, Dejean A, Orivel J, Fichaux M, Groc S, Leponce M, Feitosa RM. 2019. First checklist of the ants (Hymenoptera: Formicidae) of French Guiana. Zootaxa 4674(5):509–543. doi:10.11646/zootaxa.4674.5.2. . [DOI] [PubMed]
- Gokhman VE, Kuznetsova VG. 2006. Comparative insect karyology: current state and applications. Entomol Rev 86(3):352–368. doi:10.1134/S0013873806030110.
- Goñi B, Imai HT, Kubota M, Kondo M, Yong HS, Tso YP. 1982. Chromosome observations of tropical ants in Western Malaysia and Singapore. Annual Report of the National Institute of Genetics 32:71–73.
- Guénard B, Weiser M, Gomez K, Narula N, Economo EP. 2017. The Global Ant Biodiversity Informatics (GABI) database: Synthesizing data on the geographic distribution of ant species (Hymenoptera: Formicidae). Myrmecol News 24:83–89.
- Imai HT. 2017. A manual for ant chromosome preparations (an improved air-drying method) and Giemsa staining. Chromosome Sci 19(1-4):57–66. doi:10.11352/scr.19.57.
- Imai HT, Crozier RH, Taylor RW. 1977. Karyotype evolution in Australian ants. Chromosoma 59:341–393. doi:10.1007/BF0032 7974.
- Imai HT, Urbani CB, Kubota M, Sharma GP, Narasimhanna MN, Das BC, ... Rajasekarasetty MR. 1984. Karyological survey of Indian ants. Jpn J Genet 59(1):1–32. doi:10.1266/jjg.59.1.
- Imai HT, Kubota M, Brown WL Jr, Ihara M, Tohari M, Pronata RI. 1985. Chromosome observations on tropical ants from Indonesia. Annu Rep Natl Inst Genet (Japan) 35:46–48.
- Imai H, Taylor RW, Crosland MW, Crozier RH. 1988. Modes of spontaneous chromossomal mutation and karyotype evolution in ants with reference to the minimum interaction hypothesis. Jpn J Genet 63:159–185. doi:10.1266/jjg.63.159. . [DOI] [PubMed]
- Janicki J, Narula N, Ziegler M, Guénard B, Economo EP. 2016. Visualizing and interacting with large-volume biodiversity data using client-server web-mapping applications: The design and implementation of antmaps.org. Ecol Inform 32:185–193. doi:10.1016/j.ecoinf.2016.02.006.
- Kawada R, Buffington ML. 2016. A Scalable and Modular Dome Illumination System for Scientific Microphotography on a Budget. PLoS ONE 11(5):1–20. doi:10.1371/journal.pone. 0153426. . [DOI] [PMC free article] [PubMed]
- Kimball S, Mattis P. 1996. GIMP –The GNU Image Manipulation Program. Available at: https://www.gimp.org. Accessed 1 Sept. 2021.
- Kuznetsova VG, Golub NV. 2020. A checklist of chromosome numbers and a review of karyotype variation in Odonata of the world. Comp Cytogenet 14(4):501–540. doi:10.3897/CompCytogen. v14.i4.57062. . [DOI] [PMC free article] [PubMed]
- Levan A, Fredga K, Sandberg A. 1964. Nomenclature for centromeric position on chromosomes. Hereditas 52:201–220. doi:10.1111/j.1601-5223.1964.tb01953.x.
- Lino-Neto J, Dolder H. 2002. Sperm structure and ultrastructure of the fire ant Solenopsis invicta (Buren) (Hymenoptera, Formicidae). Tissue Cell 34(2):124–128. doi:10.1016/S0040-8166(02)00026-5. . [DOI] [PubMed]
- Lorite P, Palomeque T. 2010. Karyotype evolution in ants (Hymenoptera: Formicidae), with a review of the known ant chromosome numbers. Myrmecol News 13(1):89–102.
- Mariano CSF, Barros LAC, Velasco YM, Guimarães IN, Pompolo SG, Delabie JHC. 2019. Citogenética de hormigas de la región neotropical. In: Fernández F, Guerrero R, Delsinne T (Eds) Hormigas de Colombia. Universidad Nacional de Colombia, Bogotá, pp. 131–157.
- Moya J, Mancini K, Lino‐Neto J, Delabie J, Dolder H. 2007. Sperm ultrastructure of five species of the Neotropical ant genus Pseudomyrmex (Hymenoptera: Formicidae). Acta Zool 88(3):181–187. doi:10.1111/j.1463-6395.2007.00264.x.
- Omar MB, Bolland L, Heather WA. 1979. A permanent mounting medium for fungi. Bull Br Mycol Soc 13(1):13–32. . [PubMed]
- Palacio EE, Fernández F. 2003. Claves para las subfamilias y géneros. pp. 233–260. In: Fernández, F. (ed.) 2003. Introducción a las hormigas de la región Neotropical. Bogotá: Instituto de Investigación de Recursos Biológicos Alexander von Humboldt, xxvi + 424 pp. doi:10.13140/2.1.2183.8401.
- Pereira JOP. 2006. Diversidade genética da abelha sem ferrão Melipona quinquefasciata baseada no sequenciamento das regiões ITS1 parcial e 18S do DNA ribossômico nuclear. Master thesis, Universidade Federal do Ceará, Fortaleza, Brazil.
- Pinkel D, Straume T, Gray JW. 1986. Cytogenetic analysis using quantitative, high-sensitivity, fluorescence hybridization. Proc Natl Acad Sci USA 83:2934–2938. doi:10.1073/pnas.83.9.2934. . [DOI] [PMC free article] [PubMed]
- Pitnick S, Spicer GS, Markow TA. 1995. How long is a giant sperm? Nature 375(6527):109–109. doi:10.1038/375109a0. . [DOI] [PubMed]
- Pitnick S, Hosken DJ, Birkhead TR. 2009. Sperm morphological diversity. Sperm biology, pp. 69–149. doi:10.1016/B978-0-12-372568-4.00003-3.
- Pech P, Bezděk A. 2016. Ergatomorph wingless males in Technomyrmex vitiensis Mann, 1921 (Hymenoptera: Formicidae). J Hymenopt Res 53:25–34. doi:10.3897/jhr.53.8904.
- Pospischil R. 2011. Role of tropical greenhouses for introduction and establishment of foreign ant species (Hymenoptera: Formicidae) in Central Europe. In: 7th International Conference on Urban Pests (ICUP), Ouro Preto, BR, 7-10 Aug. 2011.
- Rocha MP, Pompolo SG. 1998. Karyotypes and heterochromatin variation (C-bands) in Melipona species (Hymenoptera, Apidae, Meliponinae). Genet Mol Biol 21:41–45. doi:10.1590/S1415-47571998000100008.
- Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675. doi:10.1038/nmeth.2089. . [DOI] [PMC free article] [PubMed]
- Schlick-Steiner BC, Steiner FM, Seifert B, Stauffer C, Christian E, Crozier RH. 2010. Integrative taxonomy: a multisource approach to exploring biodiversity. Annu Rev Entomol 55:421–438. doi:10.1146/annurev-ento-112408-085432. . [DOI] [PubMed]
- Shattuck SO. 1992. Generic revision of the ant subfamily Dolichoderinae (Hymenoptera: Formicidae). Sociobiology 21:1–181.
- Talaga S, Delabie JHC, Dézerald O, Salas-Lopez A, Petitclerc F, Leroy C, Dejean A, Hérault B, Céréghino R. 2015. A bromeliad species reveals invasive ant presence in urban areas of French Guiana. Ecol Indic 58:1–7. doi:10.1016/j.ecolind.2015.05.027.
- Tavares MG, Lopes DM, Campos LAO. 2017. An overview of cytogenetics of the tribe Meliponini (Hymenoptera: Apidae). Genetica 145:241–258. doi:10.1007/s10709-017-9961-2. . [DOI] [PubMed]
- Teixeira GA, Aguiar HJAC, Petitclerc F, Orivel J, Lopes DM, Barros LAC. 2021. Evolutionary insights into the genomic organization of major ribosomal DNA in ant chromosomes. Insect Mol Biol 30(3):340–354. doi:10.1111/imb.12699. . [DOI] [PubMed]
- Uzbekov R, Burlaud-Gaillard J, Garanina AS, Bressac C. 2017. The length of a short sperm: elongation and shortening during spermiogenesis in Cotesia congregata (Hymenoptera, Braconidae). Arthropod Struct Dev 46(2):265–273. doi:10.1016/j.asd.2016.11.011. . [DOI] [PubMed]
- Väänänen S, Vepsäläinen K, Vepsäläinen V. 2018. Technomyrmex vitiensis Mann, 1921 (Hymenoptera, Formicidae, Dolichoderinae), a new exotic tramp ant in Finland. Sahlbergia 24(1):14–19.
- Wetterer JK. 2013. Worldwide spread of the difficult white-footed ant, Technomyrmex difficilis (Hymenoptera: Formicidae). Myrmecol News 18:93–97.
- Wheeler DE, Crichton EG, Krutzsch PH. 1990. Comparative ultrastructure of ant spermatozoa (Formicidae: Hymenoptera). J Morphol 206(3):343–350. doi:10.1002/jmor.1052060311. . [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The key to the workers of the Brazilian Dolichoderine genera, presented in English in the main text, is translated to Portuguese below aiming to maximize its use among researchers and amateurs.