Abstract
Background
Rifampin is recommended as adjunctive therapy for patients with a Staphylococcus aureus prosthetic joint infection (PJI) managed with debridement, antibiotics, and implant retention (DAIR), with no solid consensus on the optimal duration of therapy. Our study assessed the effectiveness and optimal duration of rifampin for S aureus PJI using Veterans Health Administration (VHA) data.
Methods
We conducted a retrospective cohort study of patients with S aureus PJI managed with DAIR between 2003 and 2019 in VHA hospitals. Patients who died within 14 days after DAIR were excluded. The primary outcome was a time to microbiological recurrence from 15 days up to 2 years after DAIR. Rifampin use was analyzed as a time-varying exposure, and time-dependent hazard ratios (HRs) for recurrence were calculated according to the duration of rifampin treatment.
Results
Among 4624 patients, 842 (18.2%) received at least 1 dose of rifampin; 1785 (38.6%) experienced recurrence within 2 years. Rifampin treatment was associated with significantly lower HRs for recurrence during the first 90 days of treatment (HR, 0.60 [95% confidence interval {CI}, .45–.79]) and between days 91 and 180 (HR, 0.16 [95% CI, .04–.66]) but no statistically significant protective effect was observed with longer than 180 days (HR, 0.57 [95% CI, .18–1.81]). The benefit of rifampin was observed for subgroups including knee PJI, methicillin-susceptible or -resistant S aureus infection, and early or late PJI.
Conclusions
This study supports current guidelines that recommend adjunctive rifampin use for up to 6 months among patients with S aureus PJI treated with DAIR.
Keywords: antibiotics and implant retention, debridement, prosthetic joint infection, rifampin, Staphylococcus aureus, Veterans Health Administration
In this retrospective cohort study, rifampin treatment was associated with significantly lower hazard ratios for microbiological recurrence during the first 180 days of treatment among patients with staphylococcal prosthetic joint infections treated with debridement, antibiotics, and implant retention.
Prosthetic joint infection (PJI) is a challenging complication after total joint arthroplasty [1]. Biofilm-forming pathogens, such as Staphylococcus aureus, are responsible for a large proportion of PJIs and are difficult to eradicate with standard antibiotic regimens [2, 3].
Rifampin has been used as an adjunctive antibiotic primarily in staphylococcal PJI treatment since the 1990s based on its favorable activity against staphylococcal biofilms [4] and after a randomized controlled trial (RCT) conducted by Zimmerli et al showed the effectiveness of adjunctive rifampin [5]. Supported by that RCT and other observational studies [6, 7], the Infectious Diseases Society of America (IDSA) treatment guidelines recommended rifampin as an adjunctive antibiotic for staphylococcal PJI, particularly among patients managed with debridement, antibiotics, and implant retention (DAIR) [8]. Despite the recommendation, the clinical role of adjunctive rifampin remains controversial [4, 9]. A recent systematic review and meta-analysis suggested that rifampin was associated with a 10% higher success rate for staphylococcal PJI [10]. However, those results need to be interpreted cautiously because most of the included studies were observational studies with <500 patients, and each study used different inclusion and exclusion criteria and outcome definitions. More recently, a multicenter observational study conducted mainly in European countries showed a 70% reduction in treatment failure in patients who received adjunctive rifampin among 669 patients with acute staphylococcal PJI [11]. While those studies have supported the use of rifampin, there are still unanswered questions such as the optimal duration of rifampin treatment, and which patient groups benefit most from adjunctive rifampin treatment. To address those questions, we conducted a retrospective study using Veterans Health Administration (VHA) data to assess the effectiveness and optimal duration of rifampin therapy for patients with S aureus PJI managed with DAIR.
METHODS
Patient Consent Statement
The institutional review board of the University of Iowa and the Iowa City Veterans Affairs Health Care System Research and Development Committee approved this study and granted a waiver for informed consent (IRB ID#201112749).
Study Design and Patient Population
This retrospective cohort study included all patients admitted to acute care VHA hospitals for DAIR to manage S aureus PJI from 2003 to 2019. We defined S aureus PJI as the growth of S aureus from synovial tissue, synovial fluid, surgical wound, or blood culture 14 days before or after DAIR. We only included patients who survived for at least 15 days after DAIR, as it was unlikely that rifampin would have directly affected the outcome of patients who died early after DAIR. Among the 49 patients who died within 14 days from DAIR and therefore were excluded from this study, no patient received rifampin.
Data Source
Data on demographics, microbiology, pharmacy, laboratory, International Classification of Diseases, Ninth Revision (ICD-9) or Tenth Revision (ICD-10) procedure codes, and Current Procedural Terminology (CPT) codes were extracted from the Veterans Affairs (VA) Corporate Data Warehouse databases and accessed through the VA Informatics and Computing Infrastructure. Patients with hip or knee PJI managed with DAIR were identified using ICD procedure codes or CPT codes used in a previous study [12] (Supplementary Table 1). If a patient experienced 2 or more episodes of S aureus PJI managed with DAIR during the study period, only the first episode was included.
Definitions
The primary outcome of this study was microbiological recurrence in the time period between 15 days and 2 years after DAIR, accounting for mortality as a competing risk event. Recurrence was defined as the isolation of S aureus from synovial fluid, joint tissue, or wound at the site of surgery or blood during the follow-up period. As a secondary outcome, the composite outcome of recurrence and all-cause mortality was also examined during the follow-up period. Any inpatient or outpatient rifampin use starting after day 15 from DAIR was collected. Duration of rifampin treatment was counted continuously unless there was a gap longer than 30 days during treatment.
ICD-9/10 diagnostic codes were used to identify comorbidities (Supplementary Table 1). Using methodology from a previous study, the modified Acute Physiology and Chronic Health Evaluation (mAPACHE) III score was calculated from vital signs and laboratory values within 24 hours before or after DAIR [13]. Any missing laboratory values were assumed to be normal to calculate the mAPACHE III score. We assumed that any anti-staphylococcal oral antibiotic prescription within 90 days from DAIR was a continued treatment because we were unable to obtain reliable outpatient parenteral antimicrobial therapy (OPAT) data.
Statistical Analyses
Summary statistics were computed using frequencies and percentages for categorical variables and means with standard deviations for continuous variables. A cumulative incidence function plot was used to describe the probability of recurrence over time for the entire total cohort. Patients who did not experience recurrence 2 years after DAIR were censored. We analyzed rifampin use as a time-dependent exposure using days 15 after DAIR as time = 0. This avoided immortal time bias in associations and allowed us to explore associations between duration of rifampin exposure and recurrence. Using a step function, we separated active rifampin treatment as: R1(t) = 1 if active rifampin treatment for t ≥ 1 day but ≤90 days, R2(t) = 1 if active rifampin treatment for t > 90 days but ≤180 days, and R3(t) = 1 if active rifampin treatment for t > 180 days up to 2 years. Otherwise, Rj(t) = 0 including time prior to rifampin treatment [14]. Days 90 and 180 were selected based on the guideline-recommended treatment durations of rifampin and companion oral antibiotics for 3 months after hip surgery and 6 months after knee surgery [8]. We also used a time-dependent covariate approach to estimate the effects of rifampin exposure over varying follow-up intervals. This allowed us to examine whether rifampin exposure was associated with lower hazards of recurrence at later follow-up time points, after the rifampin was stopped, among patients without recurrence while receiving rifampin. We created 2 time-dependent coefficients for postrifampin (PR) treatment periods: PR1(t) = 1 for a postrifampin period after rifampin treatment for t ≤ 90 days and PR2(t) = 1 for a postrifampin period after rifampin treatment for t > 90 days. We then fitted Fine-Gray subdistribution hazard regression models to estimate the hazard ratios (HRs) for various timings of rifampin treatment (first 90 days, 91–180 days, >180 days, posttreatment after ≤90 days of rifampin, or posttreatment after >90 days of rifampin) on recurrence while accounting for mortality as a competing event [15, 16]. The multivariate model also adjusted for patient demographic characteristics, body mass index, comorbidities, geographic area of VA medical center, methicillin susceptibility, and mAPACHE III score at the time of DAIR. Variables were included in the risk-adjustment model if there were bivariate associations with the outcomes at P < .1. Variables were further selected for the final model with a backward elimination strategy. For the secondary analysis, we assessed the time to the composite outcome of recurrence or all-cause mortality during the follow-up period using multivariate Cox regression models using the same time-dependent variables for rifampin treatment and covariates used for the primary outcome.
We also conducted several subgroup analyses according to the location of PJI (knee or hip), drug susceptibility of S aureus (methicillin-susceptible S aureus [MSSA] or methicillin-resistant S aureus [MRSA]), and limiting to early PJI (ie, PJI within 90 days after initial total joint arthroplasty) or late PJI (ie, PJI after 90 days of DAIR).
All inferential analyses were 2-tailed, and a P value < .05 was considered statistically significant. All analyses were performed using SAS version 9.4 software (SAS Institute, Cary, North Carolina).
RESULTS
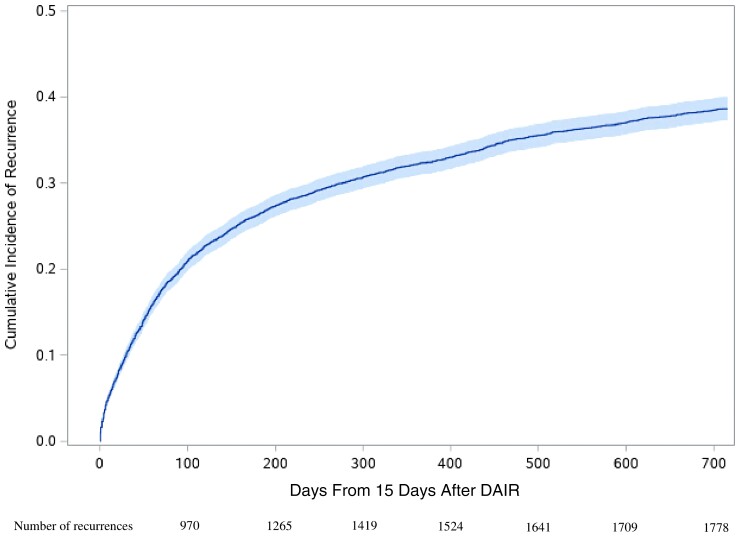
In total, 4624 patients from 101 VHA hospitals were included. Of these, 842 (18.2%) received at least 1 dose of rifampin for S aureus PJI (Table 1) during the study period. In contrast to only 14.5% of patients who received rifampin in 2003 or 2004, 21.6% of patients received rifampin in 2017–2019. The median duration of rifampin treatment was 36 days (interquartile range, 13–105 days). Among patients who received rifampin, 83.0% started within 14 days after DAIR. During the follow-up period, we identified 2084 patients with composite outcomes of recurrence or death (45.1%). Of these, 1785 patients had recurrence (85.7%), and 562 patients died (27.0%). Figure 1 shows the cumulative incidence function plot of recurrence during the follow-up period. More than half of all recurrences happened within the first 100 days (54.3%). The numbers of events and patient-days according to rifampin treatment status are described in Supplementary Table 2.
Table 1.
Basic Characteristics of the Cohort, Stratified by the Composite Outcome of Recurrence or Death
| Characteristic | Total (N = 4624) | Patients Who Had the Composite Outcome (n = 2084) | Patients Who Did Not Have the Composite Outcome (n = 2540) |
|---|---|---|---|
| Age, y, mean (SD) | 64.3 (11.3) | 65.4 (11.3) | 63.3 (11.1) |
| Male sex | 4466 (96.6) | 2016 (96.7) | 2450 (96.5) |
| Race | |||
| White | 3391 (73.3) | 1531 (73.5) | 1860 (73.2) |
| Black | 830 (18.0) | 355 (17.0) | 475 (18.7) |
| Other | 403 (8.7) | 198 (9.5) | 205 (8.1) |
| BMI, kg/m2 | |||
| Underweight (BMI <20) | 44 (1.1) | 25 (1.3) | 19 (0.9) |
| Normal (BMI ≥20 and <25) | 541 (13.2) | 291 (15.6) | 250 (11.2) |
| Overweight (BMI ≥25 and <30) | 1161 (28.4) | 519 (27.8) | 642 (28.8) |
| Obese (BMI = 30) | 2346 (57.3) | 1030 (55.2) | 1316 (59.1) |
| Geographic area | |||
| Northeast | 605 (13.1) | 248 (11.9) | 357 (14.1) |
| South | 1778 (38.5) | 793 (38.1) | 985 (38.8) |
| Central | 1271 (27.5) | 585 (28.1) | 686 (27.0) |
| West | 970 (21.0) | 458 (22.0) | 512 (20.2) |
| Year of PJI | |||
| 2003–2004 | 401 (8.7) | 202 (9.7) | 199 (7.8) |
| 2005–2007 | 820 (17.7) | 387 (18.6) | 433 (17.1) |
| 2008–2010 | 857 (18.5) | 406 (19.5) | 451 (17.8) |
| 2011–2013 | 828 (17.9) | 357 (17.1) | 471 (18.5) |
| 2014–2016 | 820 (17.7) | 354 (17.0) | 466 (18.4) |
| 2017–2019 | 898 (19.4) | 378 (18.1) | 520 (20.5) |
| Joint | |||
| Knee | 3779 (81.7) | 1706 (81.9) | 2073 (81.6) |
| Hip | 813 (17.6) | 362 (17.4) | 451 (17.8) |
| Timing of PJI from previous joint surgery | |||
| Early (within 90 d) | 1150 (24.9) | 445 (21.4) | 705 (27.7) |
| Late (after 90 d) | 1056 (22.8) | 458 (22.0) | 598 (23.5) |
| Unknown | 2418 (52.3) | 1181 (56.7) | 1237 (48.7) |
| Modified APACHE III score | |||
| ≤21 | 3276 (70.9) | 1285 (61.7) | 1991 (78.4) |
| 22–32 | 902 (19.5) | 511 (24.5) | 391 (15.4) |
| 33–45 | 341 (7.4) | 209 (10.0) | 132 (5.2) |
| >45 | 105 (2.3) | 79 (3.8) | 26 (1.0) |
| Staphylococcus aureus susceptibility | |||
| MSSA | 3405 (73.6) | 1456 (69.9) | 1949 (76.7) |
| MRSA | 1219 (26.4) | 628 (30.1) | 591 (23.3) |
| Polymicrobial infection | 1111 (24.3) | 485 (23.9) | 626 (24.7) |
| Comorbidities | |||
| Myocardial infarction | 367 (8.0) | 198 (9.5) | 169 (6.7) |
| Congestive heart failure | 755 (16.4) | 457 (22.0) | 298 (11.8) |
| COPD | 1433 (31.2) | 699 (33.7) | 734 (29.1) |
| Peripheral vascular disease | 925 (20.1) | 528 (25.4) | 397 (15.8) |
| Cerebrovascular disease | 665 (14.5) | 341 (16.4) | 324 (12.9) |
| Dementia | 86 (1.9) | 41 (2.0) | 45 (1.8) |
| Hemiplegia | 160 (3.5) | 98 (4.7) | 62 (2.5) |
| Autoimmune disease | 294 (6.4) | 153 (7.4) | 141 (5.6) |
| Diabetes mellitus | 1792 (39.0) | 929 (44.8) | 863 (34.3) |
| Peptic ulcer | 253 (5.5) | 137 (6.6) | 116 (4.6) |
| Liver disease | 670 (14.6) | 362 (17.4) | 308 (12.2) |
| Chronic kidney disease | 648 (14.1) | 376 (18.1) | 272 (10.8) |
| Solid cancer | 805 (17.5) | 433 (20.9) | 372 (14.8) |
| Hematologic malignancy | 106 (2.3) | 69 (3.3) | 37 (1.5) |
| HIV/AIDS | 51 (1.1) | 27 (1.3) | 24 (1.0) |
| Duration of rifampin treatment from day 15 after DAIR | |||
| 0 d | 3782 (81.8) | 1742 (83.6) | 2040 (80.3) |
| 1–90 d | 610 (13.2) | 290 (13.9) | 320 (12.6) |
| 91–180 d | 143 (3.1) | 33 (1.6) | 110 (4.3) |
| 181–731 d | 89 (1.9) | 19 (0.9) | 70 (2.8) |
| Duration of antibiotic treatment from day 15 after DAIR | |||
| ≤90 | 3602 (77.9) | 1802 (86.5) | 1800 (70.9) |
| 91–180 d | 286 (6.2) | 114 (5.5) | 172 (6.8) |
| 181–270 d | 164 (3.6) | 62 (3.0) | 102 (4.0) |
| ≥271 d | 572 (12.4) | 106 (5.1) | 466 (18.4) |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: APACHE, Acute Physiology and Chronic Health Evaluation; BMI, body mass index; COPD, chronic obstructive pulmonary disease; DAIR, debridement, antibiotics, and implant retention; HIV, human immunodeficiency virus; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-susceptible Staphylococcus aureus; PJI, prosthetic joint infection; SD, standard deviation.
Figure 1.
Crude cumulative incidence function plot for recurrence. Abbreviation: DAIR, debridement, antibiotics, and implant retention.
In the multivariate Fine-Gray subdistribution hazard regression model, rifampin treatment was associated with a significantly lower hazard of recurrence during the first 90 days (HR, 0.60 [95% confidence interval {CI}, .45–.79]) and days 91–180 (HR, 0.16 [95% CI, .04–.66]), but no statistically significant protective effect was observed for continued rifampin use after 180 days (HR, 0.57 [95% CI, .18–1.81]) (Table 2). HRs of postrifampin treatment were not significant (PR1(t) = 1: HR, 1.12 [95% CI, .95–1.31] and PR2(t) = 1: HR, 1.07 [95% CI, .75–1.51]) (Table 2), indicating that rifampin was associated with lower recurrence while it was used, but not with lower rates of additional recurrences after it was stopped. Of note, patients who received at least 1 dose of rifampin received longer antistaphylococcal treatment (Supplementary Table 3). In addition to rifampin treatment, being overweight and diagnosed in a more recent year were associated with lower hazards of recurrence. In contrast, higher mAPACHE III score, MRSA PJI, and having comorbidities were associated with higher HR for recurrence (Table 2).
Table 2.
Bivariate and Multivariate Cox Regression Model to Estimate Recurrence
| Groups | Crude HR (95% CI) | Adjusted HR (95% CI) |
|---|---|---|
| Rifampin treatment | ||
| No rifampin | Reference | Reference |
| Active rifampin treatment (days 1–90) | 0.57 (.43–.75) | 0.60 (.45–.79) |
| Active rifampin treatment (days 91–180) | 0.15 (.04–.59) | 0.16 (.04–.66) |
| Active rifampin treatment (days 181–731) | 0.43 (.14–1.37) | 0.57 (.18–1.81) |
| Postrifampin treatment (total ≤90 d) | 1.09 (.93–1.27) | 1.12 (.95–1.31) |
| Postrifampin treatment (total >90 d) | 1.03 (.74–1.42) | 1.07 (.75–1.51) |
| Age, y | ||
| <45 | Reference | Reference |
| 45–54 | 1.51 (1.05–2.16) | 1.33 (.93–1.90) |
| 55–64 | 1.52 (1.07–2.14) | 1.30 (.93–1.82) |
| 65–74 | 1.49 (1.04–2.12) | 1.20 (.85–1.69) |
| 75–84 | 1.51 (1.05–2.18) | 1.10 (.76–1.57) |
| ≥85 | 1.85 (1.24–2.77) | 1.06 (.70–1.63) |
| Sex | ||
| Male | 0.98 (.75–1.27) | NAa |
| Female | Reference | NAa |
| Race | ||
| White | 1.01 (.85–1.21) | 1.16 (.95–1.42) |
| Black | 0.91 (.74–1.12) | 1.03 (.82–1.29) |
| Other | Reference | Reference |
| BMI, kg/m2 | ||
| Normal (BMI ≥20 and <25) | Reference | Reference |
| Underweight (BMI <20) | 0.96 (.64–1.44) | 1.08 (.71–1.64) |
| Overweight (BMI ≥25 and <30) | 0.79 (.69–.92) | 0.83 (.70–.97) |
| Obese (BMI ≥30) | 0.81 (.70–.92) | 0.88 (.76–1.02) |
| Year of PJI | ||
| 2003–2004 | Reference | Reference |
| 2005–2007 | 0.85 (.70–1.03) | 0.92 (.75–1.13) |
| 2008–2010 | 0.82 (.68–1.00) | 0.90 (.73–1.09) |
| 2011–2013 | 0.73 (.61–.89) | 0.73 (.60–.90) |
| 2014–2016 | 0.70 (.57–.85) | 0.69 (.56–.85) |
| 2017–2019 | 0.68 (.56–.83) | 0.66 (.54–.81) |
| Geographic area | ||
| Northeast | 0.86 (.73–1.03) | NAa |
| South | 0.92 (.81–1.05) | NAa |
| Central | 0.97 (.85–1.10) | NAa |
| West | Reference | NAa |
| Modified APACHE III score | ||
| ≤21 | Reference | Reference |
| 22–32 | 1.43 (1.27–1.62) | 1.30 (1.15–1.48) |
| 33–45 | 1.62 (1.37–1.91) | 1.37 (1.15–1.64) |
| >45 | 2.27 (1.76–2.94) | 1.86 (1.41–2.46) |
| Staphylococcus aureus susceptibility | ||
| MSSA | Reference | Reference |
| MRSA | 1.32 (1.18–1.47) | 1.27 (1.13–1.42) |
| Polymicrobial infection | 0.96 (.86–1.07) | NAa |
| Joint | ||
| Knee | 0.78 (.48–1.28) | NAa |
| Hip | 0.77 (.47–1.28) | NAa |
| Timing of PJI from previous joint surgery | ||
| Early (within 90 d) | 0.85 (.74–.97) | NAa |
| Late (after 90 d) | 0.93 (.83–1.06) | NAa |
| Unknown | Reference | NAa |
| Comorbidities | ||
| Congestive heart failure | 1.29 (1.14–1.47) | 1.18 (1.04–1.35) |
| Peripheral vascular disease | 1.20 (1.07–1.35) | 1.22 (1.08–1.37) |
| Hemiplegia | 1.40 (1.13–1.74) | 1.37 (1.09–1.72) |
| Diabetes mellitus | 1.15 (1.03–1.27) | 1.19 (1.06–1.32) |
| Liver disease | 1.18 (1.04–1.34) | 1.15 (1.01–1.32) |
| Solid cancer | 1.15 (1.02–1.31) | 1.19 (1.05–1.34) |
| Peptic ulcer | 1.15 (.96–1.37) | 1.18 (.98–1.42) |
| Myocardial infarction | 1.04 (.89–1.23) | NAa |
| COPD | 0.97 (.88–1.08) | NAa |
| Cerebrovascular disease | 0.93 (.82–1.06) | NAa |
| Autoimmune disease | 0.95 (.80–1.14) | NAa |
| Hematologic malignancy | 1.09 (.82–1.45) | NAa |
| Chronic kidney disease | 1.06 (.94–1.21) | NAa |
Abbreviations: APACHE, Acute Physiology and Chronic Health Evaluation; BMI, body mass index; CI, confidence interval; COPD, chronic obstructive pulmonary disease; HR, hazard ratio; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-susceptible Staphylococcus aureus; NA, not applicable; PJI, prosthetic joint infection.
Variable was not selected in the final multivariate model.
In the subgroup analysis for knee PJI, there was a significant protective effect of rifampin on recurrence during the first 90 days of treatment (HR, 0.55 [95% CI, .40–.76]) and days 91–180 (HR, 0.19 [95% CI, .05–.75]) but not seen after 180 days of treatment or in the post–rifampin treatment periods (Table 3). In contrast, there was not a statistically significant effect of rifampin among patients with hip PJI (days 1–90: HR, 0.74 [95% CI, .40–1.38]). In the subgroup analysis by the susceptibilities of S aureus, rifampin treatment was associated with a lower hazard of recurrence among patients with MSSA PJI (HR, 0.62 [95% CI, .43–.89]) and among patients with MRSA PJI (HR, 0.58 [95% CI, .37–.90]) during the first 90 days of rifampin treatment but not afterward (Table 3). When we stratified the cohort by the timing of the PJI (early vs late), rifampin treatment was associated with a significantly lower hazard of recurrence compared to no rifampin during the first 90 days of rifampin treatment (HR, 0.47 [95% CI, .30–.74] for early PJI and HR, 0.33 [95% CI, .16–.67] for late PJI) (Table 3).
Table 3.
Hazard Ratios of Various Timing of Rifampin Treatment and Postrifampin Treatment for Recurrence Across Subgroup Analyses
| Groups | Active Rifampin Treatment | Postrifampin Treatment | |||
|---|---|---|---|---|---|
| Active Rifampin Treatment: Days 1–90 (n = 842) | Active Rifampin Treatment: Days 91–180 (n = 231) | Active Rifampin Treatment: Days 181–731 (n = 88) | Post–Rifampin Treatment: Total ≤90 d (n = 546) | Post Rifampin Treatment: Total >90 d (n = 226) | |
| Total cohort (N = 4624) | 0.60 (.45–.79) | 0.16 (.04–.66) | 0.57 (.18–1.81) | 1.12 (.95–1.31) | 1.07 (.75–1.51) |
| Knee (n = 3779) | 0.55 (.40–.76) | 0.19 (.05–.75) | 0.61 (.19–1.92) | 1.12 (.94–1.33) | 1.17 (.82–1.67) |
| Hip (n = 813) | 0.74 (.40–1.38) | (0–∞)a | (0–∞)a | 1.16 (.79–1.72) | 0.42 (.10–1.72) |
| MSSA (n = 3405) | 0.62 (.43–.89) | 0.26 (.07–1.04) | 0.55 (.13–2.22) | 1.18 (.97–1.45) | 1.01 (.67–1.53) |
| MRSA (n = 1219) | 0.58 (.37–.90) | (0–∞)a | 0.65 (.09–4.99) | 1.01 (.78–1.30) | 1.21 (.65–2.26) |
| Early PJI (n = 1150) | 0.47 (.30–.74) | (0–∞)a | 1.14 (.28–4.70) | 1.07 (.81–1.40) | 0.68 (.38–1.21) |
| Late PJI (n = 1056) | 0.33 (.16–.67) | 0.23 (.03–1.66) | (0–∞)a | 0.94 (.69–1.29) | 1.45 (.83–2.53) |
Data are presented as hazard ratio (95% confidence interval).
Abbreviations: MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-susceptible Staphylococcus aureus; PJI, prosthetic joint infection.
Hazard ratio and confidence interval could not be reliably estimated in subgroups having few patients during a given time period and no recurrences.
As a secondary analysis, we analyzed the effect of rifampin on the composite outcome of recurrence or mortality (Supplementary Table 4). Similar to the results of semi-competing risk analysis, there was a statistically significant protective effect of rifampin during the first 90 days of treatment (HR, 0.51 [95% CI, .39–.68]) and day 91–180 of treatment (HR, 0.14 [95% CI, .04–.58]) but not after 180 days and posttreatment.
DISCUSSION
Our study found that receipt of adjunctive rifampin was associated with a significantly lower hazard of recurrence for S aureus PJI compared with no rifampin. The protective effect was statistically significant during the first 180 days of treatment, supporting guideline recommendations for duration of rifampin use. The benefit of rifampin was seen in multiple subgroups including patients with knee PJI, MSSA infection, MRSA infection, and early or late PJI.
Findings from our study are consistent with a previous RCT among patients treated with DAIR for staphylococcal orthopedic implant–related infections [5]. Our findings are also in line with the recent multicenter observational study by Beldman et al [11]. Our study differed from their study in several important definitions. First, we limited our cohort to S aureus PJI, whereas Beldman et al included all Staphylococcus species. Second, we defined recurrence as a microbiological failure with the detection of S aureus in culture, whereas Beldman et al defined recurrence as the need for any further surgical procedure related to infection. We elected to use microbiological failure as our outcome because additional debridement is sometimes required for PJI treatment, even if a patient is on appropriate antibiotic treatment. Additionally, some patients might not have been healthy enough for additional surgical treatment. We acknowledge that our approach might have missed some recurrences without microbiological confirmation or included some patients with S aureus bacteremia from a source not related to PJI. More importantly, Beldman et al defined receipt of chronic antibiotic suppression (CAS) as a treatment failure. CAS is a noncurative strategy to prevent PJI recurrence [17, 18]. It is reported that more than one-third of patients aged >80 years with PJI were treated with CAS, and 60% of them were event-free at 24 months [19]. In practice, treatment with CAS does not necessarily mean the patient failed treatment.
The protective effect of rifampin was statistically significant throughout subgroups except for patients with hip PJI. This may be due to a clinical effect or small sample sizes in these subgroups. In the previous study by Beldman et al, the most prominent effect of rifampin was observed in patients with knee PJI compared to patients with hip PJI, although rifampin was protective for patients with hip PJI [11]. The difference may be from the analytical method in that we used rifampin treatment as a time-dependent variable. Patients with hip PJI might have received a shorter duration of rifampin therapy compared to knee PJI according to IDSA guidelines, and a smaller number of patients with hip PJI were included in this study. Considering those factors, the power to detect the difference might have been smaller in patients with hip PJI.
Our study suggested that rifampin treatment was beneficial up to 180 days of treatment. Although the hazard ratio after 180 days of treatment was 0.57, which appeared protective, it was not statistically significant. A longer duration of rifampin therapy was associated with better clinical outcome in a previous small multicenter observational study [20], but that study was criticized because of the concern for immortal time bias [21]. We addressed immortal time bias by including rifampin use as a time-dependent exposure in our study. A small RCT by Lora-Tamayo et al that compared 8 weeks of levofloxacin plus rifampin versus traditional treatment found that short duration of combination therapy might be noninferior to traditional treatment [22]. That RCT was not able to exclude the benefit of long-term treatment for S aureus knee PJI. We believe our results support the recommendation of rifampin treatment for 6 months for knee PJI, while the effectiveness and optimal duration of rifampin treatment for hip PJI need to be investigated in future studies.
The IDSA guidelines recommending adjunctive rifampin therapy for patients with staphylococcal PJI treated with DAIR were published in 2013, which was midway through our cohort study [8]. Yet, we found that in the years 2017–2019, only 21.6% of our patients received rifampin. Thus, there may be a need for quality improvement projects and implementation science research studies to improve the use of rifampin in this patient population.
Our study has several limitations. First, there is a possibility for residual confounding related to unmeasured risk factors such as adequacy of debridement, choice and duration of additional antibiotics, use of topical antibiotic during DAIR, physician treatment preference, and treatment adherence. We elected not to analyze combination antibiotic treatment duration because we were unable to obtain the duration of OPAT, which would be the majority of initial antibiotic therapy for PJI in the United States, due to our inability to evaluate data on antibiotics used outside the VHA system. Since a recent RCT failed to show noninferiority of 6 weeks of antibiotic treatment over 12 weeks for PJI [23], the duration of antibiotic treatment could have affected the outcome. Second, we were unable to capture positive culture results outside the VHA system; therefore, there would be a possibility of missing recurrences that happened outside the VHA system. Third, we did not evaluate some aspects of rifampin treatment, such as the dose of rifampin treatment, or adverse reactions potentially related to antibiotic therapy. Fourth, we could not obtain the resistance pattern of S aureus to rifampin, which could be important for the effect of rifampin therapy. We were also unable to determine if recurrence was caused by the same strain of S aureus or infection by a different strain. Fifth, although this was a very large multicenter cohort study, we may have been underpowered to find statistically significant differences in our subgroup analyses. Furthermore, as only 88 patients received rifampin for >180 days, we may have very limited power to detect the benefit of longer rifampin treatment. Finally, since our study used a VHA database that includes mainly older male patients, there could be concern about its generalizability to different patient populations. Nevertheless, our study is an important addition to the PJI literature since it included 19 years of data from 101 VHA hospitals and analyzed validated clinical outcomes among multiple subgroups. We used rifampin treatment as a time-dependent variable, which allowed us to assess the duration of rifampin treatment.
In conclusion, adjunctive rifampin use was associated with a significantly lower hazard of microbiological recurrence in the 6 months following DAIR for S aureus PJI, compared with no rifampin. The protective effect was prominent within the first 180 days of rifampin treatment and was present throughout most subgroups. This study supports the current guidelines, which recommend the use of adjunctive rifampin for up to 6 months among patients with S aureus PJI treated with DAIR. Given the low rate of rifampin use in this cohort, wider use of adjunctive rifampin for S aureus PJI after DAIR should be encouraged to improve the outcome.
Supplementary Material
Contributor Information
Hiroyuki Suzuki, Center for Access and Delivery Research and Evaluation, Iowa City Veterans Affairs Health Care System, Iowa City, Iowa, USA; Department of Internal Medicine, University of Iowa Carver College of Medicine, Iowa City, Iowa, USA.
Michihiko Goto, Center for Access and Delivery Research and Evaluation, Iowa City Veterans Affairs Health Care System, Iowa City, Iowa, USA; Department of Internal Medicine, University of Iowa Carver College of Medicine, Iowa City, Iowa, USA.
Rajeshwari Nair, Department of Internal Medicine, University of Iowa Carver College of Medicine, Iowa City, Iowa, USA.
Daniel J Livorsi, Center for Access and Delivery Research and Evaluation, Iowa City Veterans Affairs Health Care System, Iowa City, Iowa, USA; Department of Internal Medicine, University of Iowa Carver College of Medicine, Iowa City, Iowa, USA.
Poorani Sekar, Department of Internal Medicine, University of Iowa Carver College of Medicine, Iowa City, Iowa, USA.
Michael E Ohl, Center for Access and Delivery Research and Evaluation, Iowa City Veterans Affairs Health Care System, Iowa City, Iowa, USA; Department of Internal Medicine, University of Iowa Carver College of Medicine, Iowa City, Iowa, USA.
Daniel J Diekema, Department of Internal Medicine, University of Iowa Carver College of Medicine, Iowa City, Iowa, USA.
Eli N Perencevich, Center for Access and Delivery Research and Evaluation, Iowa City Veterans Affairs Health Care System, Iowa City, Iowa, USA; Department of Internal Medicine, University of Iowa Carver College of Medicine, Iowa City, Iowa, USA.
Bruce Alexander, Center for Access and Delivery Research and Evaluation, Iowa City Veterans Affairs Health Care System, Iowa City, Iowa, USA.
Michael P Jones, Center for Access and Delivery Research and Evaluation, Iowa City Veterans Affairs Health Care System, Iowa City, Iowa, USA; Department of Biostatistics, University of Iowa College of Public Health, Iowa City, Iowa, USA.
Jennifer S McDaniel, Department of Epidemiology, University of Iowa College of Public Health, Iowa City, Iowa, USA.
Marin L Schweizer, Center for Access and Delivery Research and Evaluation, Iowa City Veterans Affairs Health Care System, Iowa City, Iowa, USA; Department of Internal Medicine, University of Iowa Carver College of Medicine, Iowa City, Iowa, USA.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Disclaimer. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
Financial support. This study was funded by a Veterans Affairs Health Services Research and Development Career Development Award (CDA 11-215; principal investigator: M. L. S.).
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Anguita-Alonso P, Hanssen AD, Patel R. Prosthetic joint infection. Expert Rev Anti Infect Ther 2005; 3:797–804. [DOI] [PubMed] [Google Scholar]
- 2. Croes S, Deurenberg RH, Boumans ML, Beisser PS, Neef C, Stobberingh EE. Staphylococcus aureus biofilm formation at the physiologic glucose concentration depends on the S. aureus lineage. BMC Microbiol 2009; 9:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Muszanska AK, Nejadnik MR, Chen Y, et al. . Bacterial adhesion forces with substratum surfaces and the susceptibility of biofilms to antibiotics. Antimicrob Agents Chemother 2012; 56:4961–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zimmerli W, Sendi P. Role of rifampin against staphylococcal biofilm infections in vitro, in animal models, and in orthopedic-device-related infections. Antimicrob Agents Chemother 2019; 63:e01746-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zimmerli W, Widmer AF, Blatter M, Frei R, Ochsner PE. Role of rifampin for treatment of orthopedic implant-related staphylococcal infections: a randomized controlled trial. Foreign-Body Infection (FBI) Study Group. JAMA 1998; 279:1537–41. [DOI] [PubMed] [Google Scholar]
- 6. Trebse R, Pisot V, Trampuz A. Treatment of infected retained implants. J Bone Joint Surg Br 2005; 87:249–56. [DOI] [PubMed] [Google Scholar]
- 7. Widmer AF, Gaechter A, Ochsner PE, Zimmerli W. Antimicrobial treatment of orthopedic implant–related infections with rifampin combinations. Clin Infect Dis 1992; 14:1251–3. [DOI] [PubMed] [Google Scholar]
- 8. Osmon DR, Berbari EF, Berendt AR, et al. . Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2013; 56:e1–25. [DOI] [PubMed] [Google Scholar]
- 9. Karlsen Ø E, Borgen P, Bragnes B, et al. . Rifampin combination therapy in staphylococcal prosthetic joint infections: a randomized controlled trial. J Orthop Surg Res 2020; 15:365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Scheper H, Gerritsen LM, Pijls BG, Van Asten SA, Visser LG, De Boer MGJ. Outcome of debridement, antibiotics, and implant retention for staphylococcal hip and knee prosthetic joint infections, focused on rifampicin use: a systematic review and meta-analysis. Open Forum Infect Dis 2021; 8:ofab298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Beldman M, Löwik C, Soriano A, et al. . If, when, and how to use rifampin in acute staphylococcal periprosthetic joint infections, a multicentre observational study. Clin Infect Dis 2021; 73:1634–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Boyle KK, Kapadia M, Landy DC, Henry MW, Miller AO, Westrich GH. Utilization of debridement, antibiotics, and implant retention for infection after total joint arthroplasty over a decade in the United States. J Arthroplasty 2020; 35:2210–6. [DOI] [PubMed] [Google Scholar]
- 13. Fortis S, O'Shea AMJ, Beck BF, et al. . An automated computerized critical illness severity scoring system derived from APACHE III: modified APACHE. J Crit Care 2018; 48:237–42. [DOI] [PubMed] [Google Scholar]
- 14. Zhang Z, Reinikainen J, Adeleke KA, Pieterse ME, Groothuis-Oudshoorn CGM. Time-varying covariates and coefficients in Cox regression models. Ann Transl Med 2018; 6:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Haneuse S, Lee KH. Semi-competing risks data analysis: accounting for death as a competing risk when the outcome of interest is nonterminal. Circ Cardiovasc Qual Outcomes 2016; 9:322–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation 2016; 133:601–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cobo J, Escudero-Sanchez R. Suppressive antibiotic treatment in prosthetic joint infections: a perspective. Antibiotics (Basel) 2021; 10:743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Escudero-Sanchez R, Senneville E, Digumber M, et al. . Suppressive antibiotic therapy in prosthetic joint infections: a multicentre cohort study. Clin Microbiol Infect 2020; 26:499–505. [DOI] [PubMed] [Google Scholar]
- 19. Prendki V, Zeller V, Passeron D, et al. . Outcome of patients over 80 years of age on prolonged suppressive antibiotic therapy for at least 6 months for prosthetic joint infection. Int J Infect Dis 2014; 29:184–9. [DOI] [PubMed] [Google Scholar]
- 20. Becker A, Kreitmann L, Triffaut-Fillit C, et al. . Duration of rifampin therapy is a key determinant of improved outcomes in early-onset acute prosthetic joint infection due to Staphylococcus treated with a debridement, antibiotics and implant retention (DAIR): a retrospective multicenter study in France. J Bone Jt Infect 2020; 5:28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Scheper H, de Boer MGJ. Comment on “Duration of rifampin therapy is a key determinant of improved outcomes in early-onset acute prosthetic joint infection due to Staphylococcus treated with a debridement, antibiotics and implant retention (DAIR): a retrospective multicenter study in France” by Becker et al (2020). J Bone Jt Infect 2020; 6:17–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lora-Tamayo J, Euba G, Cobo J, et al. . Short- versus long-duration levofloxacin plus rifampicin for acute staphylococcal prosthetic joint infection managed with implant retention: a randomised clinical trial. Int J Antimicrob Agents 2016; 48:310–6. [DOI] [PubMed] [Google Scholar]
- 23. Bernard L, Arvieux C, Brunschweiler B, et al. . Antibiotic therapy for 6 or 12 weeks for prosthetic joint infection. N Engl J Med 2021; 384:1991–2001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.