Abstract
MicroRNAs (miRNAs) modulate physiological responses by repressing the expression of gene networks. We found that global deletion of microRNA-7 (miR-7), the most enriched miRNA in the hypothalamus, causes obesity in mice. Targeted deletion of miR-7 in Single-minded homolog 1 (Sim1) neurons, a critical component of the hypothalamic melanocortin pathway, causes hyperphagia, obesity and increased linear growth, mirroring Sim1 and Melanocortin-4 receptor (MC4R) haplo-insufficiency in mice and humans. We identified Snca (α-Synuclein) and Igsf8 (Immunoglobulin Superfamily Member 8) as miR-7 target genes that act in Sim1 neurons to regulate body weight and endocrine axes. In humans, MIR-7-1 is located in the last intron of HNRNPK, whose promoter drives the expression of both genes. Genetic variants at the HNRNPK locus that reduce its expression are associated with increased height and truncal fat mass. These findings demonstrate that miR-7 suppresses gene networks involved in the hypothalamic melanocortin pathway to regulate mammalian energy homeostasis.
Subject terms: Obesity, Hypothalamus, miRNAs
The hypothalamus controls systemic energy homeostasis. Here, the authors show that loss of microRNA-7 in hypothalamic neurons causes obesity, increased growth and endocrine dysfunction in mice, and report genetic evidence that links microRNA-7 to height and adiposity in humans.
Introduction
Energy homeostasis is maintained by neurons in the hypothalamus that modulate eating behaviour, pituitary hormone secretion, and autonomic nervous system activation in response to changes in nutrient availability1,2. Hypothalamic circuits involving leptin-responsive neurons in the arcuate nucleus (ARC) that express Pro-opiomelanocortin (POMC) and Agouti-related protein (AgRP) play a pivotal role in energy homeostasis and the defence against starvation in mice and humans3. These ARC neurons synapse onto Melanocortin-4 receptor (MC4R)-expressing neurons in the paraventricular nucleus of the hypothalamus (PVN). POMC-derived peptides are endogenous agonists of MC4R, whose activation leads to reduced food intake and increased energy expenditure in the fed state. In the fasted state, AgRP (an endogenous MC4R antagonist) drives an increase in food intake4.
Single-minded homology 1 (SIM1) is a basic helix-loop-helix transcription factor required for the migration and differentiation of PVN neurons, which express MC4R and the neuropeptides oxytocin (OXT), arginine vasopressin (AVP), corticotropin-releasing hormone (CRH), thyrotropin-releasing hormone (TRH), and somatostatin5. These neurons project to the posterior pituitary and the hypophyseal portal system to regulate hormone secretion, and to the hindbrain and spinal cord to regulate feeding and autonomic tone6,7. Mice and humans with MC4R and SIM1 haplo-insufficiency develop hyperphagia, obesity and increased linear growth8–14.
In humans, candidate gene and exome sequencing studies have highlighted the prominent role of the leptin-melanocortin pathway in the regulation of energy homeostasis15,16. Moreover, weight loss drugs targeting the melanocortin pathway have recently been licensed for the management of severe obesity caused by genetic disorders affecting this pathway17–19. However, a substantial proportion of the heritability of obesity remains unexplained20. It is well-recognized that a large portion of the noncoding genome is functional and harbours genetic variants that may contribute to Mendelian disorders or influence complex traits21. Nonetheless, the interpretation of noncoding genetic variants in humans presents challenges, including the identification of disease-relevant cell types and the dissection of underlying mechanisms in the appropriate in vivo models.
MicroRNAs (miRNAs) are small noncoding RNAs that repress gene expression at the post-transcriptional level. Mammals express hundreds of distinct miRNAs, each with hundreds of target transcripts22. By regulating the expression of networks of genes, miRNAs modulate the function of multiple cellular processes simultaneously. MiR-7 is one of the most evolutionarily conserved miRNAs, with a mature miRNA sequence that is conserved from invertebrates to humans23. MiR-7 is highly expressed in the brain, pituitary, and pancreatic β-cells24–26. We have previously shown that miR-7 regulates gonadotropin and prolactin production in the pituitary27,28 and insulin secretion in pancreatic β-cells29. In the brain, miR-7 promotes neuronal differentiation and survival30,31. It is the most highly enriched miRNA in the hypothalamus32 and displays a distinct expression pattern in the ARC and PVN33, suggesting that it may be involved in the control of body weight by melanocortin circuits.
In this study, we carried out an in vivo genetic screen to systematically examine whether hypothalamic miR-7 regulates energy homeostasis. Mice with a global knockout of all three members of the miR-7 family (miR-7a1, miR-7a2, and miR-7b) exhibited obesity on a standard chow diet, which was exacerbated on a high-fat diet (HFD), demonstrating that miR-7 is essential for the control of energy balance. To identify the specific cell types mediating these effects, we deleted the miR-7 family in neurons expressing the Leptin receptor (Lepr), Pomc, Agrp and Sim1. We found that Sim1-cre;mir-7fl/fl mice exhibited severe obesity due to increased food intake and decreased energy expenditure, associated with increased linear growth and increased insulin secretion. These phenotypes recapitulate SIM1 haploinsufficiency in mice and humans, and are likely explained by reduced expression of MC4R. In addition, Sim1-cre;mir-7fl/fl mice exhibited reduced corticosterone levels and increased fluid intake due to impaired CRH and AVP function. We further identified Snca (α-Synuclein) and Igsf8 (Immunoglobulin Superfamily Member 8) as miR-7 target genes whose overexpression in Sim1 neurons induces weight gain and endocrine abnormalities. In humans, MIR7-1 is located in the last intron of HNRNPK, whose promoter drives the expression of both genes. By interrogating genome-wide association studies, we found that variants at the HNRNPK locus that reduce its expression are associated with increased height and truncal fat mass. Together, these studies demonstrate that miR-7 suppresses gene networks involved in the hypothalamic melanocortin pathway to regulate mammalian energy homeostasis.
Results
Genetic disruption of mir-7 leads to severe obesity in mice
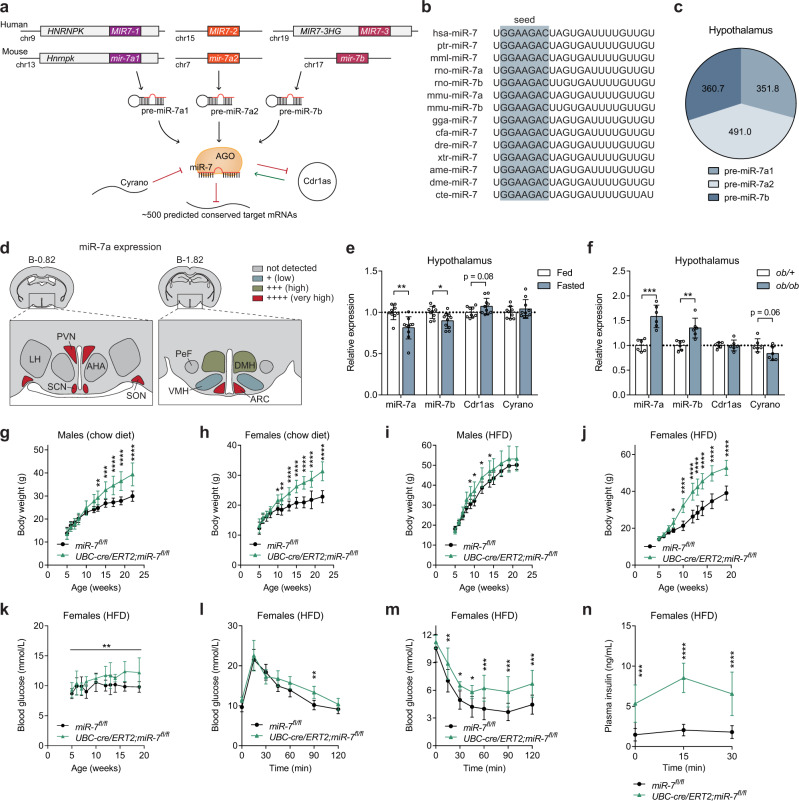
In mice and humans, the miR-7 family comprises three precursors (miR-7a1/7-1, miR-7a2/7-2, and miR-7b/7-3), which are encoded by different genomic loci but possess identical seed sequences with which they regulate the same repertoire of targets34 (Fig. 1a, b). We found that the hypothalamus expresses miR-7a1, miR-7a2, and miR-7b at approximately equal levels (Fig. 1c). Specifically, miR-7 is highly enriched in the ARC and PVN of the hypothalamus (Fig. 1d). Hypothalamic miR-7 was regulated by the metabolic state, with reduced expression during fasting (Fig. 1e) and increased expression in obese ob/ob mice (Fig. 1f). In the brain, miR-7 is implicated in a unique regulatory noncoding RNA network, in which the long noncoding RNA, Cyrano, degrades miR-7 and prevents miR-7-mediated destabilisation of the circular RNA, Cdr1as35. Cdr1as acts reciprocally by sponging miR-7 through its >70 binding sites, a process, which protects miR-7 from degradation and is thought to regulate its transport within neurons36. We found that Cdr1as and Cyrano showed trends to be inversely correlated with miR-7 expression in the hypothalamus (Fig. 1e, f), consistent with the previously described relationships in this regulatory network.
Fig. 1. Severe obesity in mice lacking miR-7.
a miR-7 genomic loci and organisation of the neuronal regulatory network comprising Cyrano, Cdr1as, and miR-7. Primary miRNA transcripts are processed into precursor miRNA (pre-miR), and then into mature miRNA (miR), which is loaded onto an AGO protein to interact with miRNA binding sites. b miR-7 sequences in different species. The grey box indicates the seed sequence. hsa, Homo sapiens; ptr, Pan troglodytes; mml, Macaca mulatta; rno, Rattus norvegicus; mmu, Mus musculus; gga, Gallus gallus; cfa, Canis familiaris; dre, Danio rerio; xtr, Xenopus tropicalis; ame, Apis mellifera; dme, Drosophila melanogaster; cte, Capitella teleta. c Contribution of each miR-7 precursor to the absolute expression of miR-7 in the hypothalamus. Values indicate copies/pg RNA. d miR-7 expression in hypothalamic nuclei, based on published data33. AHA anterior hypothalamic area, ARC arcuate nucleus, DMH dorsomedial hypothalamus, LH lateral hypothalamus, PeF perifornical nucleus, PVN paraventricular nucleus, SCN suprachiasmatic nucleus, SON supraoptic nucleus, VMH ventromedial hypothalamus. Brain coordinates indicate distance from Bregma (“B”) in mm. e–f Hypothalamic gene expression in fed and fasted (relative to fed) (n = 9 and 10 animals) (e) or ob/+ and ob/ob mice (relative to ob/+) (n = 6 animals) (f). (g–j) Body weight of chow-fed male (n = 8 and 10 animals) (g), chow-fed female (n = 12 and 8 animals) (h), HFD-fed male (n = 12 and 8 animals) (i), and HFD-fed female (n = 10 animals) (j) mir-7fl/fl and UBC-cre/ERT2;mir-7fl/fl mice. k–n Random fed blood glucose (n = 10 animals) (k), blood glucose during an ipGTT (n = 8 animals) (l), blood glucose during an ipITT (n = 10 animals) (m), and plasma insulin during an ipGTT (n = 7 animals) (n) in HFD-fed female mir-7fl/fl and UBC-cre/ERT2; mir-7fl/fl mice. Data are presented as mean ± SD, where error bars are present. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; no asterisk indicates P > 0.05; two-tailed t test (e, f) or 2-way repeated measures ANOVA with Sidak’s multiple comparisons test (g, h, i, j, l, m, n) or 2-way repeated measures ANOVA (k). Source data are provided as a Source Data file.
To investigate the physiological role of miR-7, we generated mice with loxP sites flanking the mir-7a1, mir-7a2, and mir-7b alleles (hereafter referred to as mir-7fl/fl) and crossed these with mice harbouring a UBC-cre/ERT2 allele to introduce a global, tamoxifen-inducible knockout of all three members of the miR-7 family (Supplementary Fig. 1a). Both male and female UBC-cre/ERT2; mir-7fl/fl mice exhibited obesity on chow diet (Fig. 1g, h) and high-fat diet (HFD) (Fig. 1i, j), demonstrating that the miR-7 family plays an important role in the regulation of body weight. We also measured random fed blood glucose concentrations over time and performed intraperitoneal glucose tolerance tests (ipGTT) and intraperitoneal insulin tolerance tests (ipITT). Blood glucose levels were unchanged or slightly elevated in chow-fed UBC-cre/ERT2;mir-7fl/fl mice, whereas these mice exhibited improved glucose tolerance in association with increased insulin secretion and normal insulin sensitivity (Supplementary Fig. 1b–h). In HFD-fed UBC-cre/ERT2;mir-7fl/fl mice, blood glucose levels were unchanged in males (Supplementary Fig. 1i) and increased in females (Fig. 1k). Glucose tolerance was unchanged or only slightly impaired (Fig. 1l, Supplementary Fig. 1j) in spite of severe insulin resistance (Fig. 1m, Supplementary Fig. 1k), due to increased insulin secretion (Fig. 1n).
Deletion of mir-7 in Sim1 neurons drives hyperphagia, weight gain and increased insulin secretion
To identify the specific hypothalamic cell populations involved in the miR-7-mediated control of energy homeostasis, we generated conditional knockout mouse models by crossing mir-7fl/fl mice with mice harbouring a Lepr-cre, Pomc-cre, Agrp-cre, or Sim1-cre allele (Fig. 2a) and performed a comprehensive metabolic phenotyping screen in males and females fed a chow diet or HFD. The most prominent obesity phenotype occurred upon the Sim1 neuron-specific loss of miR-7 (Fig. 2b–f). When fed a regular chow diet, body weight was increased in Sim1-cre;mir-7fl/fl females, but not males (Fig. 2b, c). When challenged with chronic HFD feeding, obesity was significantly exacerbated in Sim1-cre;mir-7fl/fl mice of both sexes (Fig. 2d, e). We verified that the Sim1-cre transgene itself did not affect the body weight of HFD-fed mice (Supplementary Fig. 2a, b). Comparison of the body weight between male and female animals revealed that miR-7 in Sim1 neurons is required to maintain the sexual dimorphism of body weight in mice. In both chow- and HFD-fed mice, mir-7fl/fl females had a lower body weight than mir-7fl/fl males, whereas Sim1-cre;mir-7fl/fl females displayed an increase in body weight that matched the body weight of male mice (Fig. 2g, h). HFD-fed Sim1-cre;mir-7fl/fl females exhibited an increase in fat mass and lean mass (Fig. 2i), as well as increased mass of adipose tissue depots (Fig. 2j). In chow-fed Sim1-cre;mir-7fl/fl females studied in metabolic cages, food intake was unchanged (Supplementary Fig. 2c, d) but energy expenditure was reduced (Supplementary Fig. 2e, f), without any changes in respiratory exchange ratio (RER) or locomotor activity (Supplementary Fig. 2g–j). In metabolic cage experiments performed one week after the onset of HFD feeding, Sim1-cre;mir-7fl/fl females exhibited increased food intake (Fig. 2k, l) and RER (Supplementary Fig. 2k, l) compared to mir-7fl/fl controls, while energy expenditure and locomotor activity were unaffected (Fig. 2m–p). No changes in metabolic measurements were detected in males (Supplementary Fig. 2m–t). The expression of Pgc1a (Peroxisome proliferator-activated receptor gamma co-activator-1) in interscapular brown adipose tissue (iBAT) was significantly reduced and expression of Ucp1 (Uncoupling protein 1) nominally reduced (p = 0.06) in Sim1-cre;mir-7fl/fl mice, compared to mir-7fl/fl control mice (Supplementary Fig. 2u). We also detected upregulation of Adrb3 (encoding the β-3 adrenergic receptor), which has previously been observed as a compensatory response to a blockade of sympathetic input37,38. These results suggest that reduced sympathetic activation of iBAT contributes to the lower energy expenditure in Sim1-cre;mir-7fl/fl mice.
Fig. 2. Obesity, hyperphagia, and increased insulin secretion upon deletion of mir-7 in Sim1 neurons.
a Experimental strategy to assess the functions of miR-7 in hypothalamic neuron populations. b–e Body weight of chow-fed male (n = 15 and 13 animals) (b), chow-fed female (n = 8 and 11 animals) (c), HFD-fed male (n = 10 and 19 animals) (d), and HFD-fed female (n = 18 and 13 animals) (e) mir-7fl/fl and Sim1-cre;mir-7fl/fl mice. f Representative photograph of HFD-fed female mir-7fl/fl and Sim1-cre;mir-7fl/fl mice. g–h Male and female body weight in 20 week-old chow-fed (g) and HFD-fed (h) mir-7fl/fl and Sim1-cre;mir-7fl/fl mice, using data shown in Fig. 2b–e. Dashed line indicates the body weight of mir-7fl/fl males. i–j Lean and fat mass (n = 8 animals) (i) and adipose depot mass (n = 4 animals) (j) of HFD-fed female mir-7fl/fl and Sim1-cre;mir-7fl/fl mice. k–p 72-hour metabolic cage measurements of HFD-fed female mir-7fl/fl and Sim1-cre;mir-7fl/fl mice (n = 7 animals). (k) Cumulative food intake; (l) average food intake per 12-h phase; (m) hourly energy expenditure; (n) average energy expenditure per 12-hour phase; (o) cumulative locomotor activity; (p) average locomotor activity per 12-hour phase. Dark phases are shaded in grey. q–t Random fed blood glucose (n = 8 and 11 animals) (q), blood glucose during an ipGTT (n = 8 and 11 animals) (r), blood glucose during an ipITT (n = 6 and 9 animals) (s), and plasma insulin during an ipGTT (n = 6 animals) (t) in chow-fed female mir-7fl/fl and Sim1-cre;mir-7fl/fl mice. u–x Random fed blood glucose (n = 18 and 13 animals) (u), blood glucose during an ipGTT (n = 16 and 12 animals) (v), blood glucose during an ipITT (n = 16 and 12 animals) (w), and plasma insulin during an ipGTT (n = 7 animals) (x) in HFD-fed female mir-7fl/fl and Sim1-cre;mir-7fl/fl mice. Data are presented as mean ± SD. *P < 0.05; **P < 0.01; ***P < 0.001, ****P < 0.0001; no asterisk indicates P > 0.05; 2-way repeated measures ANOVA with Sidak’s multiple comparisons test (b, c, d, e, g, h, l, n, p, r, s, t, v, w, x), two-tailed t test i, j, or 2-way repeated measures ANOVA (k, m, o, q, u). Source data are provided as a Source Data file.
On chow diet, Sim1-cre;mir-7fl/fl females displayed no change in random fed blood glucose levels compared to mir-7fl/fl controls (Fig. 2q). However, glucose tolerance was improved at 8–9 weeks of age (Fig. 2r), prior to any changes in body weight (Fig. 2c). This improvement in glucose clearance was not due to changes in insulin sensitivity (Fig. 2s) but correlated with increased glucose-stimulated insulin secretion (Fig. 2t). In HFD-fed Sim1-cre; mir-7fl/fl females, random fed blood glucose levels were consistently elevated compared to mir-7fl/fl controls (Fig. 2u). However, at 8–9 weeks of age, glucose tolerance was unchanged (Fig. 2v). This maintenance of normal glucose tolerance, in spite of obesity (Fig. 2e) and insulin resistance (Fig. 2w), was correlated with marked hyperinsulinemia in Sim1-cre;mir-7fl/fl mice (Fig. 2x). Measurements of glucose homeostasis in male Sim1-cre;mir-7fl/fl mice revealed only non-significant trends (Supplementary Fig. 2v–aa). To verify that the hyperinsulinemic phenotype was not caused by aberrant Cre recombinase activity and mir-7 deletion in β-cells29, we performed immunofluorescence analysis of the LSL-tdTomato reporter allele in the pancreas of Sim1-cre;LSL-tdTomato mice and observed no recombination (Supplementary Fig. 2ab). Furthermore, miR-7 expression was unchanged in the pancreatic islets of Sim1-cre;mir-7fl/fl mice versus mir-7fl/fl controls (Supplementary Fig. 2ac). Thus, miR-7 acts cooperatively in pancreatic β-cells29 and Sim1 neurons to regulate insulin secretion. Sim1- and MC4R-expressing neurons control insulin secretion by regulating autonomic tone39,40, and deficiency of Sim1 or MC4R results in hyperinsulinemia in mice and humans41–43.
Deletion of mir-7 in Sim1 neurons causes neuroendocrine abnormalities
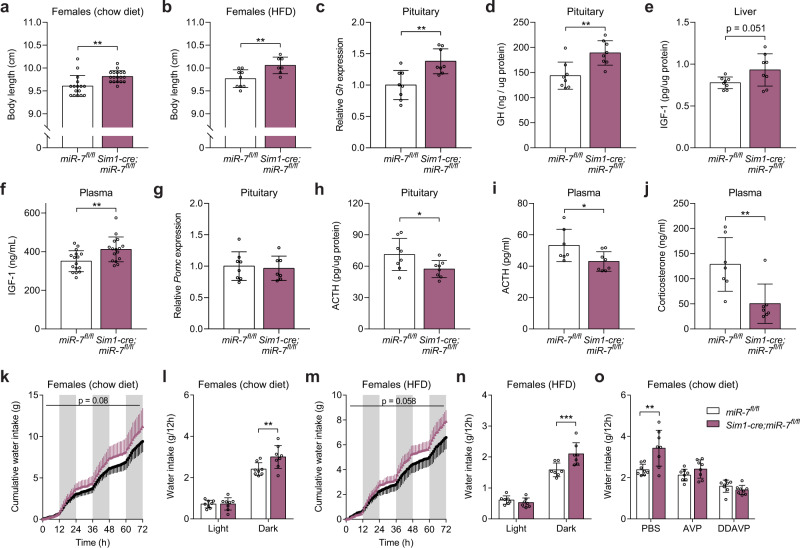
Subsets of Sim1 neurons release neuropeptides from axon terminals in the posterior pituitary and hypophyseal portal system to control endocrine axes involved in growth, stress response, and fluid homeostasis. We found that the loss of miR-7 increased linear growth in chow-fed females but not males (Fig. 3a, Supplementary Fig. 3a), and in both HFD-fed males and females (Fig. 3b, Supplementary Fig. 3b). Despite their obesity, we found increased levels of growth hormone (GH) mRNA and protein in the pituitary, as well as increased insulin-like growth factor 1 (IGF-1) protein levels in the liver and plasma of Sim1-cre;mir-7fl/fl mice compared to mir-7fl/fl controls (Fig. 3c–f). These findings align with results in human MC4R deficiency, where the pulsatile secretion of GH is retained compared to weight-matched controls42. Our findings in Sim1-cre;mir-7fl/fl mice indicate that increased linear growth may be a consequence of both reduced somatostatin (increasing GH secretion) and increased insulin secretion, as also seen in mice and humans with MC4R deficiency39,42.
Fig. 3. Neuroendocrine abnormalities in mice lacking miR-7 in Sim1 neurons.
a–b Body length of chow-fed female (n = 16 and 18 animals) (a) and HFD-fed female (n = 9 and 7 animals) (b) mir-7fl/fl and Sim1-cre;mir-7fl/fl mice. c–j Pituitary Gh expression (relative to mir-7fl/fl) (n = 8 animals) (c), pituitary GH content (n = 8 animals) (d), liver IGF-1 content (n = 8 animals) (e), plasma IGF-1 concentration (n = 16 animals) (f), pituitary Pomc expression (relative to mir-7fl/fl) (n = 8 and 7 animals) (g), pituitary ACTH content (n = 8 animals) (h), plasma ACTH concentration (n = 8 and 7 animals) (i), and plasma corticosterone concentration (n = 7 animals) (j), in chow-fed mir-7fl/fl and Sim1-cre; mir-7fl/fl mice. k–n 72-hour metabolic cage measurements of chow-fed (n = 8 animals) (k–l) and HFD-fed (n = 7 animals) (m-n) female mir-7fl/fl and Sim1-cre;mir-7fl/fl mice. k, m cumulative water intake; (l, n) average water intake per 12-hour phase. Dark phases are shaded in grey. o Water intake of mir-7fl/fl and Sim1-cre;mir-7fl/fl mice during a 12-hour dark phase following intraperitoneal injection of PBS (phosphate-buffered saline), AVP ((Arg8)-Vasopressin), or DDAVP (Desmopressin) (n = 8 animals). Data are presented as mean ± SD. *P < 0.05; **P < 0.01; ***P < 0.001; no asterisk indicates P > 0.05; two-tailed t test (a–j), 2-way repeated measures ANOVA (k, m), or 2-way repeated measures ANOVA with Sidak’s multiple comparisons test (l, n, o). Source data are provided as a Source Data file.
In Sim1-cre;mir-7fl/fl mice, pituitary Pomc expression was unchanged (Fig. 3g), but pituitary adrenocorticotropic hormone (ACTH) content, plasma ACTH levels, and plasma corticosterone levels were reduced (Fig. 3h–j), indicating a suppression of the hypothalamic-pituitary-adrenal (HPA) stress-response axis downstream of CRH-expressing Sim1 neurons.
In metabolic cages, Sim1-cre;mir-7fl/fl mice exhibited increased water intake compared to mir-7fl/fl controls (Fig. 3k–n, Supplementary Fig. 3c, d). We found that this phenotype could be reversed by administration of AVP or Desmopressin (DDAVP), a synthetic vasopressin analog that acts on the V2 receptor to mediate the antidiuretic effect of AVP in renal tubular cells (Fig. 3o). These results suggest that inadequate levels of AVP released from Sim1 neurons caused impaired water retention and excess water intake in Sim1-cre;mir-7fl/fl mice.
Deletion of mir-7 in POMC- and LepR-expressing neurons causes weight gain
We also performed the metabolic phenotyping screen (Fig. 2a) in the other conditional knockout mouse models. In Pomc-cre;mir-7fl/fl mice, we observed no change in body weight on chow diet (Supplementary Fig. 4a, b), but detected a slight exacerbation of HFD-induced obesity in females, with a similar trend toward weight gain in males (Supplementary Fig. 4c, d). In metabolic cage experiments, we measured no differences in food intake, water intake, energy expenditure, RER, or locomotor activity of chow-fed males or females (Supplementary Fig. 4e–x). Finally, we found slightly elevated blood glucose levels and impaired glucose tolerance in Pomc-cre;mir-7fl/fl females, but not males (Supplementary Fig. 4y–ah). Loss of miR-7 in LepR-expressing neurons yielded a similar phenotype as the POMC neuron-specific ablation of mir-7 (Supplementary Fig. 5a–ah). In Agrp-cre;mir-7fl/fl mice, we observed no changes in body weight or glucose homeostasis in chow or HFD-fed mice of either sex (Supplementary Fig. 6a–ah). These data indicate that loss of miR-7 in POMC and LepR neurons leads to a mild exacerbation of diet-induced obesity and impaired glucose tolerance, whereas miR-7 does not appear to modulate any metabolic functions of AgRP neurons.
Reduced neuron abundance and neuropeptide expression in Sim1 neurons lacking miR-7
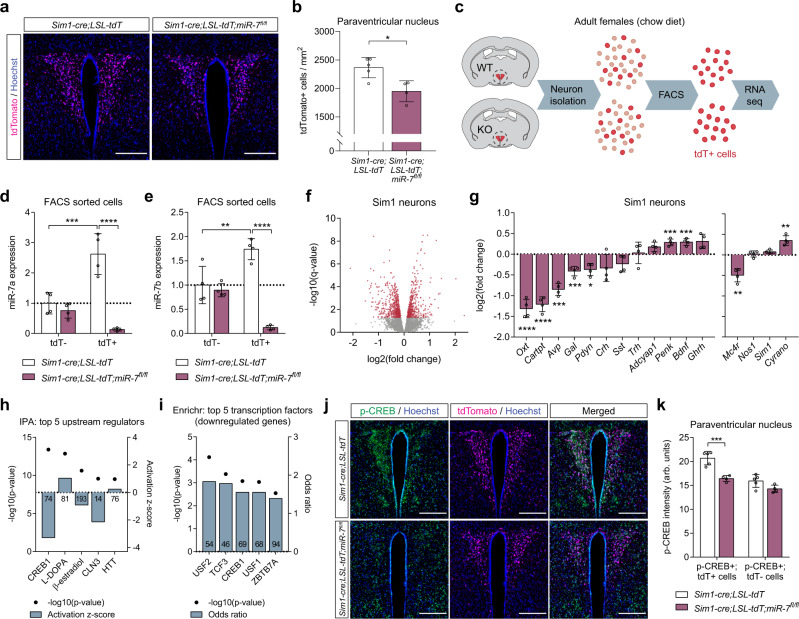
Sim1 haploinsufficiency in mice is associated with a 24% reduction in the number of PVN neurons8. We examined the PVN in mice expressing tdTomato in Sim1 neurons and observed an 18% loss of Sim1 neurons in Sim1-cre; LSL-tdTomato; mir-7fl/fl mice compared to Sim1-cre;LSL-tdTomato control mice (Fig. 4a, b). Whilst neuronal loss may contribute to the metabolic phenotype, it is notable that postnatal deletion of Sim1 induces a similar phenotype without affecting cell number10,44. We next performed RNA sequencing of FACS-sorted Sim1 neurons from Sim1-cre;LSL-tdTomato and Sim1-cre;LSL-tdTomato;mir-7fl/fl mice (Fig. 4c). In control mice, expression of miR-7a and miR-7b was enriched in tdTomato+ neurons compared to tdTomato- neurons of the hypothalamus (Fig. 4d, e). Furthermore, miR-7 expression was only reduced in tdTomato+, but not tdTomato- neurons of Sim1-cre;LSL-tdTomato;mir-7fl/fl mice compared to controls (Fig. 4d, e).
Fig. 4. Cellular dysfunction of Sim1 neurons upon mir-7 ablation.
a–b Representative image (scale bar: 250 μm) (a) and quantification (n = 5 and 4 animals) (b) of tdTomato-positive cells within the PVN of Sim1-cre;LSL-tdTomato and Sim1-cre;LSL-tdTomato;mir-7fl/fl mice. Magenta, tdTomato; blue, Hoechst. c Schematic of sample preparation for RNA sequencing of Sim1 neurons from Sim1-cre;LSL-tdTomato (“WT”) and Sim1-cre;LSL-tdTomato;mir-7fl/fl (“KO”) mice. d–e miR-7a (d) and miR-7b (e) expression in tdTomato-negative and tdTomato-positive FACS-sorted cells from hypothalamus of Sim1-cre;LSL-tdTomato and Sim1-cre;LSL-tdTomato;mir-7fl/fl mice (n = 4 samples, with 4 mice pooled per sample). Expression is relative to tdT-negative cells from Sim1-cre;LSL-tdTomato mice. f –log10(q-value) and log2(fold change) of all expressed genes in Sim1-cre;LSL-tdTomato;mir-7fl/fl versus Sim1-cre;LSL-tdTomato RNA sequencing data. Significantly regulated genes (q < 0.05) are indicated in red. g log2(fold change) of key neuropeptide-encoding genes (left panel), and Mc4r, Nos1, Sim1, and Cyrano (right panel) in Sim1-cre;LSL-tdTomato;mir-7fl/fl versus Sim1-cre;LSL-tdTomato RNA sequencing data (n = 4 samples, with 4 mice pooled per sample). h –log10(p-value) and activation z-scores of top upstream regulators identified by QIAGEN Ingenuity Pathway Analysis of Sim1-cre;LSL-tdTomato;mir-7fl/fl versus Sim1-cre;LSL-tdTomato RNA sequencing data. Numbers in the bars indicate the number of genes contributing to the prediction. i –log10(p-value) and odds ratio of top transcription factors identified by Enrichr “ENCODE and ChEA Consensus Transcription Factors from ChIP-X” of downregulated genes in Sim1-cre;LSL-tdTomato;mir-7fl/fl versus Sim1-cre;LSL-tdTomato RNA sequencing data. Numbers in the bars indicate the number of genes contributing to the prediction. j–k Representative immunofluorescence (scale bar: 250 μm) (j) and quantification (n = 5 and 4 animals) (k) of p-CREB intensity in p-CREB+ /tdTomato+ cells and p-CREB+ /tdTomato– cells in the PVN of Sim1-cre;LSL-tdTomato and Sim1-cre;LSL-tdTomato;mir-7fl/fl mice. Green, p-CREB; magenta, tdTomato; blue, Hoechst. Data are presented as mean ± SD, where error bars are present. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; no asterisk indicates P > 0.05; two-tailed t test (b), 2-way ANOVA with Sidak’s multiple comparisons test (d, e, k), or Fisher’s exact test (h, i). *q < 0.05; **q < 0.01; ***q < 0.001; ****q < 0.0001; quasi-likelihood (QL) differential expression test (f, g). Source data are provided as a Source Data file.
RNA sequencing revealed 522 upregulated transcripts and 651 downregulated transcripts in Sim1 neurons lacking miR-7 (Fig. 4f). In particular, we observed reduced expression of Mc4r and several key neuropeptides, including Oxt, Avp, and Prodynorphin (Pdyn) (Fig. 4g). An unbiased QIAGEN Ingenuity Pathway Analysis (IPA) of RNA sequencing data identified CREB1 as the top predicted regulator of gene expression changes in Sim1-cre;LSL-tdTomato;mir-7fl/fl versus Sim1-cre;LSL-tdTomato mice, with a negative z-score indicating inhibited function (Fig. 4h). In addition, CREB1 was among the top 5 Enrichr results from “ENCODE and ChEA Consensus Transcription Factors from ChIP-X” for significantly downregulated genes (Fig. 4i). Immunofluorescence analysis of phosphorylated CREB (p-CREB) in Sim1 neurons revealed reduced intensity of p-CREB signal in double-positive p-CREB+/tdTomato+ cells, but not in p-CREB+/tdTomato- cells in Sim1-cre;LSL-tdTomato;mir-7fl/fl mice compared to control mice (Fig. 4j, k). CREB mediates the effects of canonical MC4R signalling through the Gαs–cAMP–PKA cascade45–47, and mice with genetic deletion of Creb1 in Sim1 neurons develop obesity, impaired thermogenesis, and reduced AVP expression48. Therefore, reduced CREB activation likely contributes to the metabolic defects in mice lacking miR-7 in Sim1 neurons.
No change was found in the expression of Nos1, a marker of the Sim1 neuron subpopulation that contains the OXT-expressing neurons49. This suggests that the downregulation of specific neuropeptides such as Oxt represents a gene expression defect, rather than a subtype-specific loss of cell populations (Fig. 4g). Immunofluorescence analysis of OXT in Sim1 neurons confirmed that subtype-specific cell loss was not responsible for the downregulation of Oxt expression, as the number of OXT+ neurons was reduced to a similar extent as that of Sim1 neurons (Supplementary Fig. 7a, b). PDYN and MC4R are produced in distinct subsets of Sim1 neurons that act independently to reduce feeding6; additionally, both AVP- and OXT-expressing neurons have been implicated in Sim1 neuron-induced satiety9,50. Thus, dysfunction of several Sim1 neuron subpopulations likely contributes to the hyperphagic obesity of Sim1-cre;mir-7fl/fl mice.
In the brain, miR-7 forms a unique regulatory network with Cdr1as and Cyrano. While Cyrano has been previously shown to induce degradation of miR-7 in the hypothalamus, a reciprocal effect of miR-7 on hypothalamic Cyrano expression was not observed35. Interestingly, Cyrano was 1.3-fold upregulated in Sim1 neurons lacking miR-7 (Fig. 4g). As we have found that miR-7 is enriched in Sim1 neurons compared to the rest of the hypothalamus (Fig. 4d, e), it is plausible that its particularly high expression in Sim1 neurons enables miR-7 to regulate Cyrano in a cell type-specific manner that is not detectable at the whole-tissue level.
The miR-7 target genes Snca and Igsf8 act in Sim1 neurons to regulate body weight
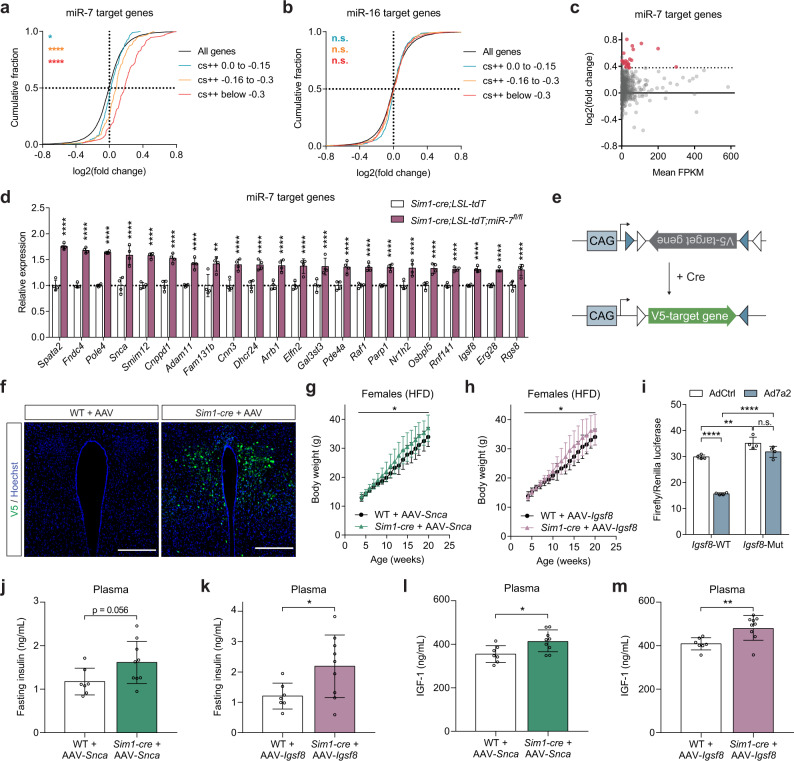
MiRNAs directly repress target transcripts possessing a specific miRNA binding site in their 3’UTR. We found that mRNAs with a conserved 8mer, 7mer-m8, or 7mer-A1 binding site for miR-7 in their 3’UTR (classified by TargetScanMouse release 7.251) were preferentially upregulated in Sim1 neurons of Sim1-cre;LSL-tdTomato;mir-7fl/fl mice compared to Sim1-cre;LSL-tdTomato controls (Fig. 5a). In comparison, predicted targets of miR-16, which is ubiquitously expressed and is abundant in the mouse hypothalamus52, were not enriched (Fig. 5b). After applying a threshold of 1.3-fold upregulation, RNA sequencing revealed 22 predicted target genes of miR-7 (Fig. 5c, d). In mammals, miRNAs predominantly regulate their target protein levels through destabilization and degradation of their target mRNA; as such, regulation of target mRNA expression and protein translation is tightly correlated53. By manipulating miR-7 expression in neuronal cells, we confirmed that miR-7 regulates its target mRNA and protein levels in a consistent manner (Supplementary Fig. 8a–d).
Fig. 5. Identification of miR-7 target genes involved in Sim1 neuron function.
a–b Cumulative frequency distribution of mRNA changes for predicted targets of miR-7 (a) or miR-16 (b) in Sim1-cre;LSL-tdTomato;mir-7fl/fl versus Sim1-cre;LSL-tdTomato RNA sequencing data. Targets are binned by cumulative weighted context ++ score (cs + +) and compared to all expressed genes. Number of genes per bin for miR-7: black, 16830; blue, 225; orange, 149; red, 112. Number of genes per bin for miR-16: black, 16830; blue, 332; orange, 408; red, 425. c log2(fold change) and mean FPKM (fragments per kilobase of transcript per million fragments mapped) of all predicted conserved miR-7 target genes in Sim1-cre;LSL-tdTomato;mir-7fl/fl versus Sim1-cre;LSL-tdTomato RNA sequencing data. Targets with >1.3-fold upregulation are shown in red. d Expression (relative to Sim1-cre;LSL-tdTomato) of upregulated targets in Sim1-cre;LSL-tdTomato;mir-7fl/fl versus Sim1-cre;LSL-tdTomato RNA sequencing data (n = 4 samples, with 4 mice pooled per sample). e Strategy of Cre-dependent target overexpression using a pAAV-FLEx vector. f V5 immunofluorescence in the PVN of WT (representative of 3 mice) and Sim1-cre mice (representative of 3 mice), 4 months after injection with AAV-Snca (scale bar: 250 μm). Green, V5; Blue, Hoechst. g–h Body weight of HFD-fed female WT and Sim1-cre mice injected with AAV-Snca (n = 7 and 9 animals) (g) or AAV-Igsf8 (n = 7 and 9 animals) (h). i Luciferase assay of cells expressing the WT or mutated 3’UTR of Igsf8, with or without miR-7 overexpression (n = 4; representative of two independent experiments). j–m Plasma insulin (j–k) and IGF-1 (l–m) in WT and Sim1-cre mice injected with AAV-Snca (n = 7 and 9 animals) (j, l) or AAV-Igsf8 (n = 7 and 9 animals) (k, m). Data are presented as mean ± SD, where error bars are present. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; no asterisk or n.s. indicates P > 0.05; Kruskal-Wallis test with Dunn’s multiple comparisons test (a, b), 2-way repeated measures ANOVA (g, h; genotype-by-time interaction), 2-way ANOVA with Sidak’s multiple comparisons test (i), or two-tailed t test (j–m). **q < 0.01; ****q < 0.0001; quasi-likelihood (QL) differential expression test (c, d). Source data are provided as a Source Data file.
To investigate the role of the identified miR-7 target genes in Sim1 neuron function, we performed AAV-mediated, Cre-dependent overexpression of selected targets in Sim1 neurons using a pAAV-FLEx vector in an AAV-PHP.eB capsid that efficiently transduces the CNS upon intravenous administration54,55 (Fig. 5e). For this analysis, we selected seven of the 22 target genes that were upregulated upon mir-7 ablation: the five most upregulated genes (Spata2, Fndc4, Pole4, Snca, and Smim12), as well as Arrb1 (the gene encoding Beta-arrestin-1), due to its known role in MC4R signalling56–58, and Igsf8, which had the highest baseline expression among the upregulated target genes and is known to modulate neuronal function59,60. We verified that these constructs induced expression that persisted in the PVN for at least 4 months after injection (Fig. 5f, Supplementary Fig. 8e, f). Comparing the body weight of AAV-injected Sim1-cre mice versus AAV-injected wild-type littermates, we found that overexpression of Snca (Fig. 5g) and Igsf8 (Fig. 5h) caused an exacerbation of HFD-induced obesity, whereas overexpression of Spata2, Pole4, Fndc4, Smim12, or Arrb1 had no impact on body weight (Supplementary Fig. 8g–k). Both Snca (encoding α-Synuclein) and Igsf8 (encoding Immunoglobulin Superfamily Member 8) contain a conserved 8-mer binding site for miR-7, but only Snca has been previously confirmed as a direct miR-7 target gene29,31. We therefore cloned the 3’UTR of Igsf8, containing either the wild-type or mutated miR-7 binding sites, into a luciferase reporter construct and confirmed by luciferase assay that Igsf8 is a direct target of miR-7 (Fig. 5i). In addition to their effects on body weight, overexpression of both Snca and Igsf8 in Sim1 neurons led to an increase in plasma insulin (Fig. 5j, k) and plasma IGF-1 (Fig. 5l, m), whereas no differences were observed upon overexpression of Spata2, Pole4, Fndc4, Smim12, or Arrb1 (Supplementary Fig. 8l–p). The fact that, among all investigated targets, only Snca and Igsf8 have a detectable function in Sim1 neurons may be related to their enriched expression in these cells; Snca and Igsf8 display the highest baseline expression out of the 22 upregulated targets in Sim1 neurons (Supplementary Fig. 8q). Corticosterone levels and water intake were unchanged upon overexpression of Snca or Igsf8 (Supplementary Fig. 8r–u), suggesting that the function of CRH- and AVP-expressing Sim1 neurons was not impaired by these targets. Food intake was slightly increased in mice with Igsf8 overexpression (Supplementary Fig. 8v, w), while energy expenditure was slightly reduced in mice with Snca overexpression (Supplementary Fig. 8x, y). Taken together, overexpression of Snca and Igsf8 in Sim1 neurons each partly recapitulates the phenotype of Sim1-cre;mir-7fl/fl mice. These data identify Snca and Igsf8 as direct targets of miR-7 that play a functional role in Sim1 neurons to regulate body weight and endocrine axes.
Human variants in the locus encompassing MIR7-1 are associated with height and adiposity
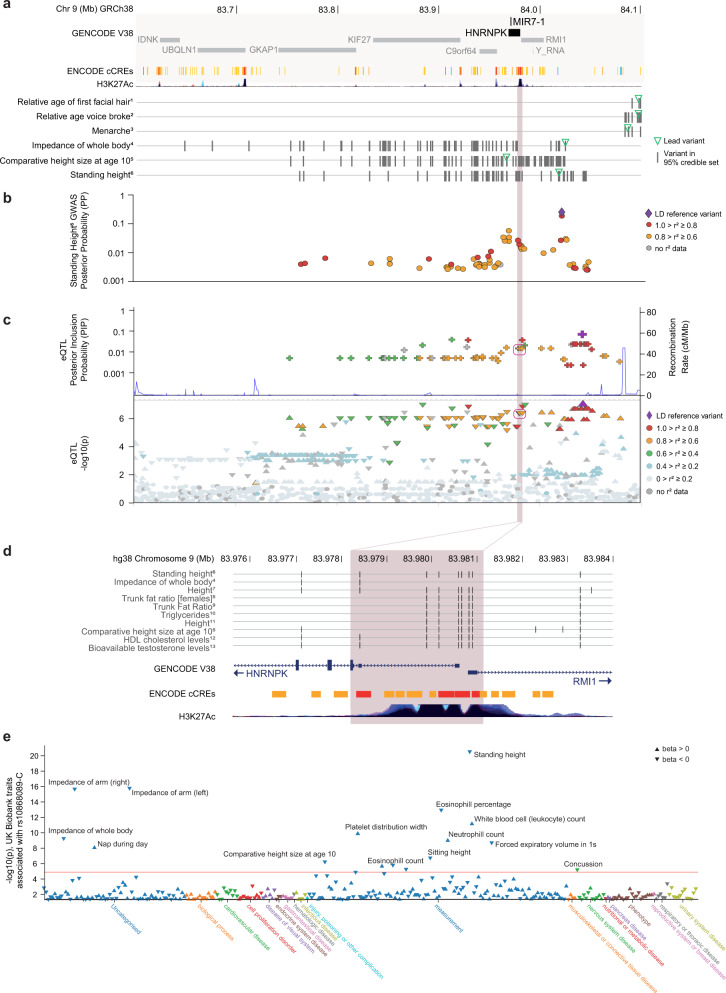
MIR7-1/mir-7a1 is located in the last intron of a ubiquitously expressed gene, HNRNPK (Heterogeneous Nuclear Riboucleoprotein K), an RNA-binding protein that complexes with pre-mRNAs in the nucleus to influence their processing and transport. MIR7-1 is co-transcribed with its host gene, and its tissue specificity is achieved by inhibition of miRNA maturation in non-neuronal cells61–63. To investigate the potential contribution of variation in miR-7 expression to human adiposity, height and metabolic traits, we interrogated Genome-Wide Association Studies (GWAS) and expression quantitative trait loci (eQTL) using publicly available resources which collate numerous large datasets (OpenTargets64, FIVEx65, PhenomeXcan66).
Loci linked to HNRNPK by the Open Targets locus-to-gene pipeline67 were associated with several traits including height, trunk fat ratio and triglyceride levels (GWAS threshold P < 5 × 10−8) (Fig. 6a, Supplementary Data 1). Credible sets contained 7 variants overlapping putative HNRNPK promoter regions immediately 5’ of the HNRNPK coding region (Fig. 6b). In neuronal cells, increased transcription of Hnrnpk induces an equivalent increase in pre-miR-7a1 and mature miR-7a levels61; therefore, variants affecting transcription of HNRNPK would similarly affect miR-7 expression. To investigate whether human noncoding variants were associated with altered expression of HNRNPK, we interrogated data from a series of human eQTL datasets. Among single-variant eQTL summary statistics reporting nominal association (P < 0.05) with HNRNPK expression65, 22 of the 24 studies showed that variants were associated with decreased expression, including four datasets derived from brain samples. Six variants overlapping the HNRNPK 5’ promotor region were part of the credible set (monocyte, BLUEPRINT study; posterior inclusion probability, PIP = 0.016 for each of these 6 variants, pairwise r2 > 0.9; credible set size = 72) (Fig. 6c, d); that is, evidence for a putative causal variant rather than in linkage disequilibrium (LD) with a causal variant. Open Targets reported colocalisation of this monocyte eQTL with height and comparative height at age 10. The directions of effect at these loci are consistent with decreased HNRNPK expression associated with increased height and trunk fat percentage (Fig. 6e). We conclude that common variants in the region upstream of HNRNPK are associated with decreased HNRNPK expression and increased height and trunk fat in humans.
Fig. 6. Association of human variants in the locus encompassing MIR7-1 with height, adiposity and related traits.
a MIR7-1 is located in the last intron of HNRNPK (dark grey, GENCODE V38; chr9:83.6-84.1 Mb). Tracks display ENCODE candidate cis-Regulatory Elements (ENCODE cCREs112; red, promotor-like; orange, proximal enhancer-like; yellow: distal enhancer-like; blue: CTCF-only) and histone acetylation (H3K27Ac) tracks (UCSC browser hg38, (http://genome.ucsc.edu113). Tracks labelled with phenotypic traits (UK Biobank Neale v2, 2018) show the position of fine-mapped variants with Open Targets variant-to-gene (V2G) annotations for HNRNPK within this chromosomal region. Trait superscripts 1-6 refer to study-locus summary statistics in Supplementary Data 1b. Summary statistics for study-locus associations which had an Open Targets locus-to-gene (L2G) annotation for HNRNPK are provided in Supplementary Data 1. b Fine-mapping of GWAS locus for standing height, shown as posterior probability (PP) for each variant in the 95% credible set (UK Biobank-derived trait NEALE2_50_raw, lead variant chr9:84,020,284 G > C; data from Open Targets; Supplementary Data 1). The shaded area indicates a region where credible variants overlap with predicted regulatory regions immediately 5’ of HNRNPK. Six of these seven variants are in high pairwise LD (r2 > 0.95). c LocusZoom of the reported cis-eQTL for HNRNPK expression in monocytes (BLUEPRINT); data from FIVEx, https://fivex.sph.umich.edu. Open Targets reported evidence of colocalisation with standing height (UKB Neale v2, 2018; coloc114, H4 = 0.81, QTL beta = 0.04). eQTL minimum p-value was at rs10868089-C (chr9:84,042,868 T > C, LD reference variant). d Zoomed-in view showing predicted ENCODE cis-Regulatory Elements immediately 5’ of HNRNPK (chr9:83.976-83.984 Mb). Displays variants which are contained in a credible set of a selected trait (for UK Biobank-derived traits) or are in linkage disequilibrium (r2 > 0.8) with the lead variant (for non-UK Biobank studies). Trait superscripts 4-13 refer to study-locus summary statistics in Supplementary Data 1b. e Single-variant summary statistics (p-value and direction of effect) for the HNRNPK eQTL lead variant shown in (c) (rs10868089-C, chr9:84042868 T > C) obtained from Open Targets. Points represent UK Biobank-derived traits (UK Biobank Neale v2, 2018) with single-variant P < 0.05.
Additionally, we used PhenomeXcan66 to interrogate gene-tissue-trait associations for the host gene HNRNPK and target genes IGSF8 and SNCA. For each gene, we identified consensus associations across GTEx tissues and investigated the direction of effect for human traits (Supplementary Data 2). For top-ranked relevant traits, we inspected the direction of effect per tissue (Supplementary Data 2). We found that increased expression of HNRNPK is associated with reduced height and adiposity traits (Supplementary Data 2a–e), while increased expression of IGSF8 tends to be associated with increased height and adiposity traits (Supplementary Data 2f). SNCA expression was not consistently associated with increases or decreases in height or adiposity traits (Supplementary Data 2i–j). However, the per-tissue associations of SNCA expression with the two top-ranked height-related traits (each showing different direction of effect in consensus analysis across tissues) suggest that higher expression of SNCA in some neural and endocrine tissues (including pituitary, pancreas, adipose tissues, nucleus accumbens, and basal ganglia) is associated with increased height (Supplementary Data 2k). Therefore, we found that the direction of effect for associations between variants that alter the expression of HNRNPK and IGSF8, and SNCA in some cell types, are consistent with the mouse phenotypes observed.
Discussion
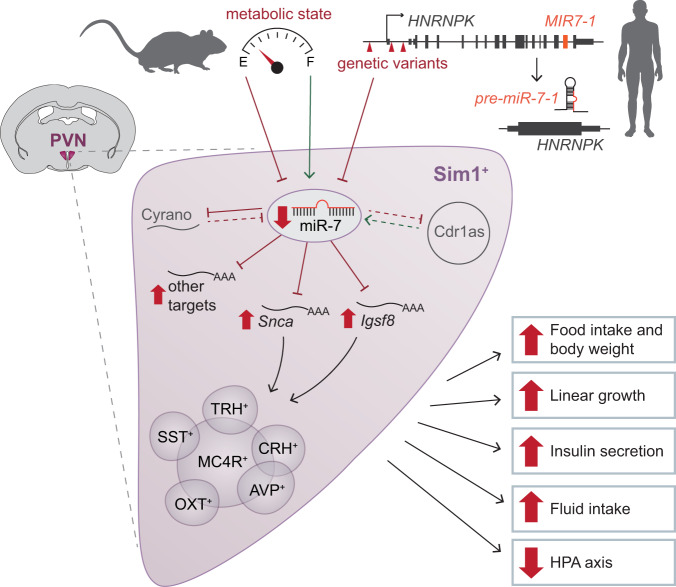
The evolutionary conservation of miR-7, the redundancy of three independently regulated miR-7-encoding genes, and its tissue-specific expression pattern suggest that this miRNA family carries out an important functional role in the hypothalamus. Here, by deploying a systematic approach to investigate the physiological consequences of mir-7 deletion from several hypothalamic cell populations critical to the regulation of mammalian energy homeostasis, we discovered that miR-7 plays an essential role in Sim1 neurons to maintain energy homeostasis (Fig. 7). The slight weight gain observed in Pomc-cre;mir-7fl/fl and Lepr-cre;mir-7fl/fl mice, combined with the absence of weight gain in Agrp-cre;mir-7fl/fl mice, is consistent with miR-7 coordinately regulating gene expression responses to defend against starvation, in keeping with the regulation of this microRNA by states of negative and positive energy balance.
Fig. 7. The role of miR-7 in Sim1 neurons to regulate mammalian energy homeostasis and neuroendocrine function.
In mice, hypothalamic expression of miR-7 is regulated by metabolic state. In humans, variants in the locus encompassing miR-7 are associated with reduced expression of HNRNPK/MIR-7-1. miR-7 regulates the expression of the noncoding RNAs Cyrano and Cdr1as (dashed lines indicate previously published data35). Loss of miR-7 expression in Sim1 neurons leads to upregulation of miR-7 target genes, including Snca and Igsf8, to disrupt the function of multiple Sim1 neuron subpopulations. As a result, the absence of miR-7 causes hyperphagic obesity, increased linear growth, increased insulin secretion, increased fluid intake, and suppressed HPA axis function. Green arrows indicate a positive effect on expression; red blunt-headed lines indicate a negative effect on expression.
Although several miRNAs have been implicated in peripheral metabolism, only a few studies have linked miRNAs to the hypothalamic control of energy homeostasis68–71. A previous study overexpressing a miR-7 sponge in POMC neurons found only a minor effect on body weight70, in line with our results upon genetic deletion of mir-7 in these neurons. Here, by examining the effects of mir-7 deletion in Lepr, Pomc, Agrp and Sim1-expressing neurons, we demonstrate that miR-7 in Sim1 neurons plays a critical role in energy balance control. The indispensability of miR-7 in the melanocortin circuit controlling body weight is remarkable, as deletion of individual miRNAs rarely causes marked phenotypes72,73.
The loss of miR-7 from Sim1 neurons induces severe obesity, increased energy intake, decreased energy expenditure, increased linear growth, and hyperinsulinemia due to increased insulin secretion. These phenotypes precisely mirror those seen in mice and humans with SIM1 haploinsufficiency. Amongst the different neuronal populations in the PVN, the effects of miR-7 on food intake, energy expenditure, linear growth and insulin secretion are likely to be mediated by the disrupted function of MC4R-expressing neurons (Fig. 7). These phenotypes overlap with those seen in humans (and mice) with loss-of-function mutations in MC4R, which represent the commonest monogenic cause of obesity. Similarly, disruption of genes which affect the development and/or function of hypothalamic melanocortin circuits cause obesity and increased growth in multiple species74–76. To that end, it is notable that the miR-7 sequence is highly conserved across zebrafish, mice, and humans and may similarly regulate the melanocortin pathway across these species. In ob/ob mice with leptin deficiency and an associated reduction in MC4R signalling, the observed upregulation of hypothalamic miR-7 expression may represent a compensatory mechanism to dampen the obesogenic effect of a suppressed melanocortin system.
This study demonstrates that miR-7 is required for the sexual dimorphism of body weight control, as female mice lacking miR-7 in Sim1 neurons exhibit enhanced obesity and ultimately attain the same body weight as male mice. Estrogens contribute to the sexual dimorphism of obesity susceptibility, such that males and ovariectomized females are more obesity-prone than intact female mice77,78. Furthermore, estrogen has recently been shown to regulate melanocortin circuits79. Notably, we have previously reported a reciprocal relationship between miR-7 and estradiol, in which estradiol regulates miR-7 expression28 and mir-7a2–/– mice develop hypogonadotropic hypogonadism and suppression of circulating estradiol27. Further studies are warranted to determine whether miR-7 plays a role in the protective effects of estrogens on diet-induced obesity. The sex-specific effects of miR-7 depletion may also arise from the fact that Sim1 neurons themselves contribute to energy balance control in a sexually dimorphic manner. For example, it has been shown that both Sim1 deficiency and Sim1 neuron ablation elicit greater obesity in females compared to males10,44,80.
UBC-cre/ERT2; mir-7fl/fl mice displayed a more pronounced obesity phenotype than Sim1-cre;mir-7fl/fl mice, suggesting that miR-7 in other unidentified cell population(s) or an additive effect of miR-7 in multiple hypothalamic cell types may be implicated in weight regulation. In addition to the ARC and PVN, miR-7 is also highly expressed in the suprachiasmatic nucleus (SCN) and the supraoptic nucleus (SON). The SCN is the circadian pacemaker in mammals; it controls behavioural, neuroendocrine, and autonomic functions to coordinate the daily rhythm of neural and metabolic processes81. As SCN disruption leads to metabolic dysfunction and obesity82–84, loss of miR-7 in this hypothalamic region may account for the stronger obesity phenotype of UBC-cre/ERT2;miR-7fl/fl mice compared to Sim1-cre;miR-7fl/fl mice. The SON is comprised of magnocellular OXT- and AVP-expressing neurons that release these neuropeptides into circulation through axon terminals in the posterior pituitary. Sim1 is expressed in neurons of both the PVN and SON, as these two populations arise from a common developmental lineage5,43. Thus, deletion of mir-7 in the SON of Sim1-cre;miR-7fl/fl mice may contribute to phenotypes related to OXT and AVP release, such as water intake.
Using an in vivo, Cre-dependent viral overexpression approach, we identified Igsf8 and Snca as direct miR-7 targets that are individually sufficient to regulate energy homeostasis and neuroendocrine axes through Sim1 neurons. IgSF8 localizes to presynaptic axon terminals60 and has been recently described to regulate synaptic function in hippocampal circuits59. In line with our findings of increased plasma insulin upon Igsf8 overexpression, Igsf8 knockout mice have been reported to exhibit impaired glucose tolerance by the International Mouse Phenotyping Consortium (www.mousephenotype.org)85. Alpha-synuclein is known for its pathological aggregation in neurodegenerative disorders and is associated with dopaminergic neuron loss in Parkinson’s disease86. While its physiological function is only partly understood, accumulation of α-synuclein has been linked to ER stress, altered mitochondrial function, impaired vesicle trafficking, and synaptic dysfunction87. Of note, the overexpression of these two target genes did not fully recapitulate the obesity phenotype induced by mir-7 deletion. These intermediate phenotypes likely reflect the fact that miRNAs typically act via the cumulative contributions of several target genes to simultaneously regulate numerous cellular processes in a sensitized background such as metabolic stress conditions88. In genetic mouse models harbouring mutated miRNA binding sites within target genes, only rare targets containing multiple binding sites tend to elicit strong phenotypes89, whereas most targets typically have negligible or partial contribution to the miRNA phenotypes90. The fact that Snca and Igsf8 overexpression did not affect corticosterone levels or water intake may suggest that other targets mediate the effects of miR-7 in specific Sim1 neuron subpopulations. Alternatively, as miR-7 is known to regulate neuronal development30, the post-developmental timing of AAV-mediated target overexpression may have precluded the detection of important developmental roles for these targets in specific Sim1 neuron subpopulations.
By interrogating genome-wide association studies based on multiple cohorts involving >500,000 people, as well as large-scale eQTL resources, we found that variants at the HNRNPK/MIR7-1 locus were associated with increased height and truncal fat and with other related measures such as bioelectrical impedance. We also observed associations with phenotypes that are directly modulated by the degree of adiposity, in particular the timing of puberty. Several studies have shown that variants associated with body mass index (BMI) or obesity are also associated with the timing of pubertal onset (recorded by age of menarche in females or timing of voice break in males91,92). These associations with the timing of puberty could also be linked to the necessity of miR-7 for the maintenance of pituitary hormone production and fertility27,28. Recognised limitations of current genetic resources include uncertainty in predicting causal genes at GWAS and eQTL loci67, a lack of validation data for the predicted direction of tissue-specific gene expression93, and distinguishing causal genes in the presence of regulatory pleiotropy66,94. Further work is therefore warranted to delineate the roles of genetic regulation by individual alleles and other mechanisms on the expression of HNRNPK and intron-embedded MIR7-1, as well as RMI1 (located head-to-head on the opposite strand) and other proximal genes, and to assess their role in the regulation of metabolic traits. Additional studies are also needed to test whether variants in the loci surrounding IGSF8 and SNCA are associated with anthropometric and/or metabolic traits and whether rare variants in these genes are associated with obesity in clinical cohorts.
In summary, we demonstrate that miR-7 regulates the hypothalamic melanocortin pathway. It is clear that quantitative variation in MC4R signalling is a major regulator of human body weight, as loss-of-function MC4R mutations cause obesity13 and gain-of-function mutations in MC4R protect against obesity58. Moreover, MC4R agonists lead to weight loss in people with obesity due to genetic disruption of the melanocortin pathway17–19. In view of our findings, dissection of the gene network regulated by miR-7 may reveal new insights into the regulation of mammalian energy homeostasis and identify potential targets for weight loss therapy.
Methods
Mouse strains and animal husbandry
All animal experiments were approved by the Kantonale Veterinäramt Zürich (Veterinary Office of the Canton of Zürich). Pomc-cre (B6.FVB-Tg(Pomc-cre)1Lowl/J)95, Lepr-cre (B6.129-Leprtm2(cre)Rck/J)96, Agrp-cre (STOCK Agrptm1(cre)Lowl/J)97, Sim1-cre (B6.FVB(129 × 1)-Tg(Sim1-cre)1Lowl/J)43, UBC-cre/ERT2 (B6.Cg-Ndor1Tg(UBC-cre/ERT2)1Ejb/1J)98, and Rosa26-LSL-tdTomato (B6.Cg-Gt(ROSA)26Sortm14(CAG-tdTomato)Hze/J)99 mice were purchased from The Jackson Laboratory. Agrp-cre mice were backcrossed five times into a C57BL/6 background before being used for experiments. Generation of mir-7a1fl/fl, mir-7a2fl/fl, mir-7bfl/fl mice has been previously described27,29, and mice harbouring all three floxed alleles are referred to as mir-7fl/fl mice. All mice were maintained on a C57BL/6 background. Mice were housed in a pathogen-free animal facility at the Institute of Molecular Health Sciences at ETH Zürich, in a temperature-controlled room (22 °C) with 55% humidity and a 12-hour light/12-hour dark cycle (lights on from 06:00 to 18:00). Mice were fed a standard laboratory chow diet (Kliba Nafag 3437; containing 3 kcal/g metabolizable energy with 4.5% fat, 18.5% protein, and 38% carbohydrate [w/w]) or high-fat diet (HFD) (SAFE diets 260HF; containing 5.5 kcal/g metabolizable energy with 36% fat, 20% protein, 37% carbohydrate, and 18% sucrose [w/w]). Feeding with HFD began at 4–5 weeks of age. Weekly body weight and blood glucose measurements were performed between 4–25 weeks of age. All other measurements were performed between 8–25 weeks of age. Mice were monitored for signs of distress at least three times per week, or twice daily during metabolic cage experiments. Euthanasia was performed by placing mice in a plastic container that was slowly flooded with carbon dioxide. After breathing stopped, the head of the mouse was removed to ensure death.
Tamoxifen administration
Four-week-old mir-7fl/fl and UBC-cre/ERT2; mir-7fl/fl littermate mice were administered daily intraperitoneal injections of 2 mg tamoxifen (T5648, Sigma), dissolved at a concentration of 20 mg/ml in 10% ethanol/90% corn oil, for 5 days. In HFD-fed cohorts, feeding with HFD began one week after the last injection.
Body composition and length
Fat and lean mass composition was measured using an EchoMRI instrument. Fat pad weights were determined by post-mortem dissection. Body length was measured as the distance from nose to base of tail.
Metabolic cage measurements
Food intake, water intake, energy expenditure, locomotor activity, and RER were measured in up to 16 cages simultaneously using the PhenoMaster home cage phenotyping system (TSE Systems) in the ETH Phenomics Center Phenotyping Unit. After two days of habituation, measurements were recorded for 72 hours. Data are presented as both the hourly data over the 72 hour period as well as the average values across all three recorded light (06:00–18:00) and dark phases (18:00–06:00). For water intake measurements upon PBS, AVP, and DDAVP administration, injections were performed over a period of 20 minutes between 17:20 and 17:40. Mice were injected intraperitoneally with PBS, 0.16 µg/g body weight AVP (H-1775, Bachem), or 0.25 µg/g body weight DDAVP (H-7675, Bachem). Water intake was subsequently recorded for 12 h during the dark phase.
Blood glucose measurements, ipGTT, and ipITT
Blood glucose was measured from a tail nick using a Contour XT glucometer (Bayer). For ipGTT, mice were fasted for 6 hours prior to intraperitoneal injection with D-glucose in PBS (2.5 g/kg body weight for chow-fed mice or 1.25 g/kg body weight for HFD-fed mice). For ipITT, mice were fasted for 6 hours prior to intraperitoneal injection with insulin (I9278, Sigma) in PBS (0.5 U/kg body weight for chow-fed mice or 0.7 U/kg body weight for HFD-fed mice).
Hormone measurements in plasma and tissues
To collect tissues for hormone measurements, mice were euthanized by CO2 inhalation and tissues were dissected, flash frozen on dry ice, and stored at −80 °C. Tissues were homogenized in PBS supplemented with protease inhibitor (11697498001, Roche) and lysed by sonication. Protein concentration was measured by BCA assay. Blood was collected from a tail nick into a tube containing EDTA (0.5 mM final concentration) and plasma was separated by centrifugation at 8000 rcf for 8 minutes. For plasma insulin measurements, mice were fasted for 6 hours prior to blood collection.
The following assays were used for hormone measurements: ACTH Ultrasensitive lumELISA (AC562T-100, Calbiotech), Corticosterone ELISA (ADI-900-097, Enzo), Growth Hormone ELISA (EZRMGH-45K, Millipore), IGF-I/IGF-1 Quantikine ELISA (MG100, R&D Systems), and Ultrasensitive Insulin ELISA (80-INSRTU, Alpco).
Cryosections and immunofluorescence
Mice were euthanized by CO2 inhalation and perfused intracardially with 10 mL of PBS, followed by 10 mL of 4% paraformaldehyde. Tissues were dissected and fixed in 4% paraformaldehyde for 24 h at 4 °C, followed by 30% sucrose for at least 24 hours at 4 °C. Samples were then frozen in O.C.T. compound (361603E, VWR), cryosectioned, mounted onto glass slides, and stored at −80 °C.
Cryosections (30 μm) were permeabilized in 95% ethanol at −20 °C for 5 minutes. For immunostainings with anti-Oxytocin or anti-V5 antibodies, antigen retrieval was performed in 10 mM sodium citrate buffer (pH 6.0) for 10 minutes at 95 °C. Sections were blocked at room temperature in PBS containing 0.1% Triton X-100, 1% bovine serum albumin, and 5% goat serum for 1 hour. Primary antibodies were applied at 4 °C overnight and secondary antibodies were applied at room temperature for 1 hour. Hoechst dye was applied to stain nuclei, and then slides were mounted using CC/Mount (C9368, Sigma).
Imaging was performed with a 20x objective on a Pannoramic 250 Slide Scanner (3D Histech). Image quantification was performed using QuPath software version 0.1.2100 by an experimenter who was blinded to the genotypes. Quantification of tdTomato+ and p-CREB+ cells within the PVN was performed in three sections per mouse, at 120 μm intervals between −0.7 mm to −0.96 mm to bregma101, and is reported as the average per mouse. P-CREB staining intensity was determined as the mean grey value within each p-CREB+ cell, from which the background value (mean grey value of p-CREB-negative regions) was subtracted.
Quantification of microRNA and mRNA by qPCR
Mice were euthanized by CO2 inhalation and tissues were dissected, frozen on dry ice, and stored at −80 °C. Pancreatic islet isolation was performed as previously described102. RNA isolation was performed with TRI reagent (T9424, Sigma) according to the manufacturer’s protocol. For mRNA measurements, reverse transcription was performed with High Capacity cDNA Reverse Transcription kit (4368814, Applied Biosystems) and qPCR was performed with SYBR Fast Universal Mastermix (KK4600, Kapa) and gene-specific primers (listed in Supplementary Table 1) in a LC480 II Lightcycler (Roche). MicroRNA measurements were performed with TaqMan microRNA assay kits (4440887, Applied Biosystems). Measurements of mRNA were normalised to 36b4, and miRNA measurements were normalised to sno-202. Absolute quantification of miR-7a and miR-7b was determined using a spike-in of synthetic RNA comprised of the mature miR-7a or miR-7b sequences. To differentiate between absolute levels of miR-7a1 and miR-7a2, mature miR-7a was quantified in the hypothalamus of mir-7a2+/+ and mir-7a2–/– mice.
Neuron isolation, FACS, and RNA extraction
Hypothalami from four 8–10 week old female mice were pooled for each sample. The hypothalamus was dissected in a manner that included the entire PVN region and excluded the SON region (which also contains Sim1-expressing neurons). Tissues were dissected into cold Hibernate-A (A1247501, Gibco) and cut into small pieces with a scalpel blade. Tissues were then incubated in dissociation media (Hibernate-A minus calcium (HA-CA, Brainbits) with 2 mg/ml papain (LS003120, Worthington), 100 U/ml DNase I (LK003172, Worthington), 0.5 mM GlutaMAX (35050-038, Gibco), 5% w/v trehalose (T0167, Sigma), 0.5 mM EDTA, 5.5 mM L-cysteine, and 0.067 mM β-mercaptoethanol) for 60 minutes at 37 °C with agitation. After the papain digest, the dissociation media was replaced with pre-warmed trituration media (Hibernate A with 1x B27 supplement (17504044, Gibco), 0.5 mM GlutaMAX, and 5% w/v trehalose). Samples were triturated using a 200 μL pipette, and then pelleted by centrifugation at 100 rcf for 10 minutes. Media was replaced with fresh trituration media and the trituration and centrifugation steps were repeated. The cell pellet was washed once with FACS media (Leibowitz’s L15 medium (21083027, Gibco) with 10 mM Hepes, 1 mg/mL bovine serum albumin (126579, Calbiochem), 5% trehalose, and 50 U/mL DNase I) to remove traces of phenol red, and then was resuspended in FACS media. Prior to sorting, cells were passed through a 35 μm mesh filter (352235, Falcon). Dead cells were stained with Sytox blue (S34857, Invitrogen). Sorting was performed by the ETH Zürich Flow Cytometry Core Facility with a BD FACSAriaIII Cell Sorter. 10% of the sorted cells were used for microRNA quantification by qPCR. For RNA sequencing, the remaining 90% of tdTomato+ cells were used for RNA isolation with PicoPure RNA Isolation Kit (KIT0204, Applied Biosystems) according to manufacturers’ instructions with DNase treatment (79254, Qiagen).
RNA sequencing and analysis
Library preparation and sequencing were performed by the Functional Genomics Center Zürich. Sequencing libraries were prepared by SmartSeq2 and sequencing was performed with the Illumina NovaSeq 6000. Raw reads were cleaned by removing adapter sequences, trimming low quality ends, and filtering reads with low quality (phred quality <20) using Trimmomatic (Version 0.36)103. Sequence pseudo alignment of the resulting high-quality reads to the mouse reference genome (GRCm38.p6 assembly) and quantification of gene level expression (gene models from GENCODE release M32) were carried out using Kallisto (Version 0.44)104. Differentially expressed (DE) genes were identified using the R package edgeR105 from Bioconductor Version 20, using a generalised linear model (glm) regression, a quasi-likelihood (QL) differential expression test and the trimmed means of M-values (TMM) normalisation. Predicted conserved miR-7 and miR-16 targets were retrieved from TargetScanMouse release 7.2. The criteria for target site conservation in TargetScanMouse7.2 is defined by phylogenetic branch length, with each site type having a different threshold for conservation (8mer ≥ 0.6; 7mer-m8 ≥ 1.8; 7mer-1A ≥ 2.5)51.
Cell culture
SH-SY5Y cells (94030304, Sigma) were cultured in a 1:1 mixture of MEM (10370021, Gibco) and Ham’s F-12 Nutrient Mix (11765054, Gibco), supplemented with 10% fetal bovine serum (F9665, Sigma), 0.5% GlutaMAX (35050061), 0.5% sodium pyruvate (11360070, Gibco), and 1% pen strep (11548876, Gibco). GH3 cells (CCL-82.1, ATCC) were cultured in Ham’s F-12K media (21127030, Gibco) supplemented with 15% horse serum (26050088, Gibco), 2.5% fetal bovine serum (F9665, Sigma), and 1% pen strep (11548876, Gibco). Cells were plated at a density of 50,000 cells/cm2 for experiments.
Luciferase assay
To assess the interaction of miR-7 with the 3’UTR of Igsf8, we utilized the pmirGLO vector (E1330, Promega), which expresses firefly luciferase (as an experimental reporter that is subject to the effect of miRNA regulation) and Renilla luciferase (as an internal control). 160 bp fragments of the Igsf8 3’UTR containing either the WT (GTCTTCCA) or mutated (GCCCTTCA) binding site for miR-7 were do novo synthesized (Genscript; sequences listed in Supplementary Table 2) and cloned into SacI/XbaI sites of pmirGLO. GH3 cells on poly-D-lysine coated plates (P6407, Sigma) were transduced with miR-7a2 and control recombinant adenoviruses29, and then were transfected 24 hours later with pmirGLO-Igsf8-WT or pmirGLO-Igsf8-Mut using Lipofectamine 2000 (11668019, Invitrogen). Cells were harvested and assayed 24 hours post-transfection using the Dual-Luciferase Reporter Assay System (Promega). Firefly luciferase activity was normalized to the activity of the Renilla luciferase control.
Target gene AAV production and administration
pAAV-FLEx-GFP was a gift from Edward Boyden (Addgene plasmid #28304). Annealed oligo cloning was performed to replace the GFP sequence with new multiple cloning sites containing SpeI/NsiI sites and an N-terminal or C-terminal V5 tag (oligo sequences listed in Supplementary Table 3). Target genes were PCR amplified from cDNA of mouse brain (primers listed in Supplementary Table 3) and inserted into the SpeI/NsiI sites of the pAAV-FLEx-N-V5 or pAAV-FLEx-C-V5 vector. The resulting plasmids were transfected with or without a Cre-expressing plasmid (gift from Tyler Jacks; Addgene plasmid #17408) into GH3 cells using Lipofectamine 2000 (11668019, Invitrogen) and were harvested 48 hours post-transfection for verification of V5 expression by Western blot. Production of AAV-PHP.eB viral vectors was performed by the EPFL Bertarelli Foundation Gene Therapy Platform. For each construct, AAV-PHP.eB vectors were generated by transient transfection of the HEKExpress XLG1.0 cell line (ExcellGene SA) maintained in suspension in Freestyle F17 medium supplemented with 4 mM GlutaMAX (ThermoFisher), as previously described106. The pUCmini-iCAP-PHP.eB was a gift from Viviana Gradinaru (Addgene plasmid #103005). Transfection conditions were defined as previously described107. For vector production, cells were maintained for 7 days after transfection in medium supplemented with 4 mM valproic acid (Sigma). Cell supernatant was collected on days 4 and 7 after transfection and the cell pellet was collected on day 7. DNA present in the cell pellet was eliminated by SAN nuclease digestion (ArcticZymes). For downstream purification, AAV particles present in both the cell pellet and culture medium were isolated by affinity chromatography using an AKTA Pure chromatography apparatus (GE Healthcare) connected to a 5 mL pre-packed POROS CaptureSelect AAV9 column (ThermoFisher). Buffer exchange (PBS supplemented with 0.001% Pluronic F-68 (Gibco)) and vector concentration were performed using a 100 kDa cut-off Amicon Ultra-15 device (Millipore). The final concentration of genome-containing AAV particles in the vector suspension was determined by digital PCR (QIAcuity Digital PCR System, QIAGEN) using TaqMan primers specific for the WPRE sequence. AAV vectors were administered intravenously to 3–4 week-old mice at a dose of 2.5 × 1011 vg/mouse.
Western blot
Cells were washed once with PBS, then lysed in RIPA buffer (150 nM NaCl, 1% Triton-X, 50 mM Tris, 0.5% sodium deoxycholate, 0.1% SDS) supplemented with cOmplete, EDTA-free Protease inhibitors (11873580001, Roche). Protein concentrations were measured by bicinchoninic acid assay. Proteins were separated by SDS-PAGE, transferred to nitrocellulose membranes, and blocked in 5% milk in Tris-buffered saline with Tween-20 (TBS-T) for 1 h. Membranes were incubated with primary antibody at 4 °C overnight followed by secondary antibody at room temperature for 1 h, and were developed using ECL Western Blotting Substrate. For protein quantification, densitometry was performed using ImageJ version 1.53c and values were normalised to GAPDH. Uncropped images of blots are provided in the Source Data file.
Antibodies
The following antibodies were used for immunofluorescence: guinea pig anti-insulin (A056401, Dako, 1:1000), rabbit anti-phospho-CREB (Ser133) (9198, Cell Signaling, 1:250), rabbit anti-Oxytocin (T-4084, Peninsula Laboratories, 1:1000), mouse anti-V5 tag (R960-25, Invitrogen, 1:250), goat anti-guinea pig conjugated to Alexa Fluor 488 (A11073, Invitrogen, 1:500), goat anti-rabbit conjugated to Cy5 (A10523, Invitrogen, 1:500), and goat anti-mouse conjugated to Alexa647 (115-605-003, Jackson ImmunoResearch, 1:500). The following antibodies were used for Western blot: mouse anti-V5 tag (R960-25, Invitrogen, 1:5000), goat anti-IgSF8 (AF3117, R&D Systems, 1:500), mouse anti-Alpha-synuclein (610786, BD Biosciences, 1:500), rabbit anti-Beta-arrestin (ab32099, Abcam, 1:1000), rabbit anti-c-Raf (53745, Cell Signalling, 1:500), rabbit anti-GAPDH (2118, Cell Signalling, 1:1000), goat anti-rabbit conjugated to HRP (401393, Sigma, 1:10,000), rabbit anti-goat conjugated to HRP (401515, Sigma, 1:10,000), and goat anti-mouse conjugated to HRP (401253, Sigma, 1:10,000).
Statistics
Statistical analysis was performed using GraphPad Prism 7. Data are presented as mean ± standard deviation (where error bars are present). Statistical tests are indicated in the figure legends. Sample size (n) represents number of animals or number of wells in a multi-well culture plate, unless otherwise stated. A p value of less than 0.05 was considered statistically significant.
Open Targets Genetics GWAS loci and gene prioritization
We defined a list of relevant human traits (anthropometry, adiposity, puberty and other related traits) prior to selection and analysis of GWAS loci. We used the pre-computed Open Targets Genetics (Version 5)64 locus-to-gene (L2G) resource67 to obtain all GWAS loci (locus-trait associations, p < 10−8) that had an L2G annotation for HNRNPK. Gene prioritization scores and Partial gene prioritization scores were inspected at each study-locus for distance, eQTL, colocalisation and other evidence used by the L2G pipeline to prioritize genes at a GWAS locus (https://genetics.opentargets.org/study-locus/[Study-ID]/[lead-variant-ID]). Fine-mapping results (credible sets, available for UK Biobank Neale v2 GWAS108) and linkage disequilibrium (LD) with respect to lead variants were downloaded from Open Targets LocusPlot variant-to-gene (V2G) tables.
FIVEx eQTL repository
We obtained eQTL summary statistics and credible sets from the FIVEx (Functional Interpretation and Visualization of Expression) instance at https://fivex.sph.umich.edu65 (accessed 25 October 2021), which is based on data from the EBI eETL Catalogue109. In this FIVEx instance, credible sets (posterior inclusion probability, PIP) are calculated using SuSiE110, which aims to highlight significantly associated variants in the presence of high LD. We used the FIVEx gene-search facility for HNRNPK cis-eQTLs and found one reported significant eQTL (BLUEPRINT: monocyte). We used the LocusZoom utility in FIVEx to obtain PIP and -log(P) plots for the credible set. Linkage disequilibrium is displayed using the European (EUR) population. Single- variant cis-eQTL nominal associations (P < 0.05) with HNRNPK expression were obtained from the FIVEx single-variant view for variant rs796004 C > T (chr9:83,979,883 C > T).
PhenomeXcan analysis of genes-tissues-traits in human biobanks
We interrogated the PhenomeXcan repository66 (http://phenomexcan.org) which aims to prioritize likely causal gene-trait associations by synthesizing GWAS on 4,091 traits with transcriptome regulation data from 49 tissues in GTEx v8. The GWAS traits are predominantly from the Neale v2 GWAS resource108 using UK Biobank data111. For each gene (host gene HNRNPK, and target genes IGSF8 and SNCA) we performed a PhenomeXscan search (P value<0.05) using two different thresholds of regional colocalization probability, RCP > 0.1 and RCP > 0.01 (less conservative)66. Among top-ranked traits (by p-value) with a relevant phenotype, we performed a PhenomeXscan_SingleTissue search among all tissues (p < 0.05) and inspected the direction of effect in each tissue.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
We thank Regina Kubsch for excellent technical and animal husbandry assistance, and Dr. Pamuditha Silva for isolation of pancreatic islets. We thank the Functional Genomics Center Zürich (FGCZ), the ETH Phenomics Center (EPIC), the ETH Zürich Flow Cytometry Core Facility (E-FCCF), the ETH Zürich Scientific Center for Optical and Electron Microscopy (ScopeM), and the EPFL Bertarelli Foundation Gene Therapy Platform for support. This work was supported as a part of NCCR RNA & Disease, a National Centre of Competence in Research, funded by the Swiss National Science Foundation (grant numbers 141735 and 182880, to M.S.) M.P.L. was supported by a Canadian Institutes of Health Research (CIHR) Doctoral Foreign Study Award (DFS-152262). I.S.F. was supported by Wellcome (207462/Z/17/Z), the NIHR (National Institute for Health Research) Cambridge Biomedical Research Centre, Fondation Botnar, the Bernard Wolfe Health Neuroscience Endowment and a NIHR Senior Investigator Award.
Source data
Author contributions
M.P.L. and M.S. conceived the experimental studies and interpreted the results. M.P.L. and S.G. performed experiments. K.L. performed the human genetic analyses. I.S.F. contributed to interpretation of the results in mice and humans. M.P.L., I.S.F. and M.S. wrote the manuscript. All authors read and provided input on the manuscript.
Peer review
Peer review information
Nature Communications thanks Yingchang Lu, Sean Hartig and the other, anonymous, reviewer for their contribution to the peer review of this work. Peer reviewer reports are available.
Data availability
RNA sequencing data have been deposited to the European Nucleotide Archive (accession ID PRJEB48660). The following third-party data sources were used in this study: mouse reference genome GRCm38.p6 assembly (https://www.ncbi.nlm.nih.gov/assembly/GCF_000001635.26/), Open Targets Genetics (https://genetics.opentargets.org), FIVEx (https://fivex.sph.umich.edu), PhenomeXcan (http://phenomexcan.org), PhenomeXcan fastENLOC results (https://github.com/hakyimlab/phenomexcan), PrediXcan GTEx v8 expression predictors (https://www.predictdb.org), GTEx v8 eQTLs (accessible via https://www.gtexportal.org/home/datasets). All other data generated or analysed during this study are included in this published article (and its supplementary information files). Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-022-33367-w.
References
- 1.Gautron L, Elmquist JK, Williams KW. Neural control of energy balance: Translating circuits to therapies. Cell. 2015;161:133–145. doi: 10.1016/j.cell.2015.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clemmensen C, et al. Gut-Brain Cross-Talk in Metabolic Control. Cell. 2017;168:758–774. doi: 10.1016/j.cell.2017.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friedman JM. Leptin and the endocrine control of energy balance. Nat. Metab. 2019;1:754–764. doi: 10.1038/s42255-019-0095-y. [DOI] [PubMed] [Google Scholar]
- 4.Yeo, G. S. H. et al. The melanocortin pathway and energy homeostasis: From discovery to obesity therapy. Mol. Metab. 101206 (2021). [DOI] [PMC free article] [PubMed]
- 5.Michaud JL, Rosenquist T, May NR, Fan CM. Development of neuroendocrine lineages requires the bHLH-PAS transcription factor SIM1. Genes Dev. 1998;12:3264–3275. doi: 10.1101/gad.12.20.3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li MM, et al. The Paraventricular Hypothalamus Regulates Satiety and Prevents Obesity via Two Genetically Distinct Circuits. Neuron. 2019;102:653–667. doi: 10.1016/j.neuron.2019.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gold RM, Jones AP, Sawchenko PE, Kapatos G. Paraventricular area: Critical focus of a longitudinal neurocircuitry mediating food intake. Physiol. Behav. 1977;18:1111–1119. doi: 10.1016/0031-9384(77)90019-1. [DOI] [PubMed] [Google Scholar]
- 8.Michaud JL, et al. Sim1 haploinsufficiency causes hyperphagia, obesity and reduction of the paraventricular nucleus of the hypothalamus. Hum. Mol. Genet. 2001;10:1465–1473. doi: 10.1093/hmg/10.14.1465. [DOI] [PubMed] [Google Scholar]
- 9.Kublaoui BM, Gemelli T, Tolson KP, Wang Y, Zinn AR. Oxytocin Deficiency Mediates Hyperphagic Obesity of Sim1 Haploinsufficient Mice. Mol. Endocrinol. 2008;22:1723–1734. doi: 10.1210/me.2008-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tolson KP, et al. Postnatal Sim1 deficiency causes hyperphagic obesity and reduced Mc4r and oxytocin expression. J. Neurosci. 2010;30:3803–3812. doi: 10.1523/JNEUROSCI.5444-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holder JL, et al. Sim1 gene dosage modulates the homeostatic feeding response to increased dietary fat in mice. Am. J. Physiol. - Endocrinol. Metab. 2004;287:105–113. doi: 10.1152/ajpendo.00446.2003. [DOI] [PubMed] [Google Scholar]
- 12.Ramachandrappa S, et al. Rare variants in single-minded 1 (SIM1) are associated with severe obesity. J. Clin. Invest. 2013;123:3042–3050. doi: 10.1172/JCI68016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farooqi IS, et al. Clinical Spectrum of Obesity and Mutations in the Melanocortin 4 Receptor Gene. N. Engl. J. Med. 2003;348:1085–1095. doi: 10.1056/NEJMoa022050. [DOI] [PubMed] [Google Scholar]
- 14.Huszar D, et al. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88:131–141. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- 15.Van Der Klaauw AA, Farooqi IS. The hunger genes: Pathways to obesity. Cell. 2015;161:119–132. doi: 10.1016/j.cell.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 16.Loos, R. J. F. & Yeo, G. S. H. The genetics of obesity: from discovery to biology. Nat. Rev. Genet. (2021). 10.1038/s41576-021-00414-z [DOI] [PMC free article] [PubMed]
- 17.Kühnen P, et al. Proopiomelanocortin Deficiency Treated with a Melanocortin-4 Receptor Agonist. N. Engl. J. Med. 2016;375:240–246. doi: 10.1056/NEJMoa1512693. [DOI] [PubMed] [Google Scholar]
- 18.Clément K, et al. MC4R agonism promotes durable weight loss in patients with leptin receptor deficiency. Nat. Med. 2018;24:551–555. doi: 10.1038/s41591-018-0015-9. [DOI] [PubMed] [Google Scholar]
- 19.Clément K, et al. Efficacy and safety of setmelanotide, an MC4R agonist, in individuals with severe obesity due to LEPR or POMC deficiency: single-arm, open-label, multicentre, phase 3 trials. Lancet Diabetes Endocrinol. 2020;8:960–970. doi: 10.1016/S2213-8587(20)30364-8. [DOI] [PubMed] [Google Scholar]
- 20.Barroso I, McCarthy MI. The Genetic Basis of Metabolic Disease. Cell. 2019;177:146–161. doi: 10.1016/j.cell.2019.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.French JD, Edwards SL. The Role of Noncoding Variants in Heritable Disease. Trends Genet. 2020;36:880–891. doi: 10.1016/j.tig.2020.07.004. [DOI] [PubMed] [Google Scholar]
- 22.Friedman RC, Farh KKH, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prochnik SE, Rokhsar DS, Aboobaker AA. Evidence for a microRNA expansion in the bilaterian ancestor. Dev. Genes Evol. 2007;217:73–77. doi: 10.1007/s00427-006-0116-1. [DOI] [PubMed] [Google Scholar]
- 24.Farh KK-H, et al. The widespread impact of mammalian MicroRNAs on mRNA repression and evolution. Science. 2005;310:1817–1821. doi: 10.1126/science.1121158. [DOI] [PubMed] [Google Scholar]
- 25.Landgraf P, et al. A Mammalian microRNA Expression Atlas Based on Small RNA Library Sequencing. Cell. 2007;129:1401–1414. doi: 10.1016/j.cell.2007.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Correa-Medina M, et al. MicroRNA miR-7 is preferentially expressed in endocrine cells of the developing and adult human pancreas. Gene Expr. Patterns. 2009;9:193–199. doi: 10.1016/j.gep.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 27.Ahmed K, et al. Loss of microRNA-7a2 induces hypogonadotropic hypogonadism and infertility. J. Clin. Invest. 2017;127:1061–1074. doi: 10.1172/JCI90031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.LaPierre MP, et al. MicroRNA-7a2 Regulates Prolactin in Developing Lactotrophs and Prolactinoma Cells. Endocrinology. 2021;162:bqaa220. doi: 10.1210/endocr/bqaa220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Latreille M, et al. MicroRNA-7a regulates pancreatic β cell function. J. Clin. Invest. 2014;124:2722–2735. doi: 10.1172/JCI73066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pollock A, Bian S, Zhang C, Chen Z, Sun T. Growth of the developing cerebral cortex is controlled by MicroRNA-7 through the p53 pathway. Cell Rep. 2014;7:1184–1196. doi: 10.1016/j.celrep.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McMillan KJ, et al. Loss of MicroRNA-7 Regulation Leads to α-Synuclein Accumulation and Dopaminergic Neuronal Loss In Vivo. Mol. Ther. 2017;25:2404–2414. doi: 10.1016/j.ymthe.2017.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bak M, et al. MicroRNA expression in the adult mouse central nervous system. RNA. 2008;14:432–444. doi: 10.1261/rna.783108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herzer S, Silahtaroglu A, Meister B. Locked Nucleic Acid-Based In Situ Hybridisation Reveals miR-7a as a Hypothalamus-Enriched MicroRNA with a Distinct Expression Pattern. J. Neuroendocrinol. 2012;24:1492–1504. doi: 10.1111/j.1365-2826.2012.02358.x. [DOI] [PubMed] [Google Scholar]
- 34.Bartel DP. Metazoan MicroRNAs. Cell. 2018;173:20–51. doi: 10.1016/j.cell.2018.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kleaveland B, Shi CY, Stefano J, Bartel DP. A Network of Noncoding Regulatory RNAs Acts in the Mammalian Brain. Cell. 2018;174:350–362. doi: 10.1016/j.cell.2018.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Piwecka M, et al. Loss of a mammalian circular RNA locus causes miRNA deregulation and affects brain function. Science. 2017;357:eaam8526. doi: 10.1126/science.aam8526. [DOI] [PubMed] [Google Scholar]
- 37.Sinden DS, et al. Knockout of the X-linked Fgf13 in the hypothalamic paraventricular nucleus impairs sympathetic output to brown fat and causes obesity. FASEB J. 2019;33:11579–11594. doi: 10.1096/fj.201901178R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Granneman JG, Lahners KN. Differential adrenergic regulation of beta 1- and beta 3-adrenoreceptor messenger ribonucleic acids in adipose tissues. Endocrinology. 1992;130:109–114. doi: 10.1210/endo.130.1.1309320. [DOI] [PubMed] [Google Scholar]
- 39.Fan W, et al. The Central Melanocortin System Can Directly Regulate Serum Insulin Levels. Endocrinology. 2000;141:3072–3079. doi: 10.1210/endo.141.9.7665. [DOI] [PubMed] [Google Scholar]
- 40.Kublaoui BM, Holder JL, Gemelli T, Zinn AR. Sim1 Haploinsufficiency Impairs Melanocortin-Mediated Anorexia and Activation of Paraventricular Nucleus Neurons. Mol. Endocrinol. 2006;20:2483–2492. doi: 10.1210/me.2005-0483. [DOI] [PubMed] [Google Scholar]
- 41.Greenfield JR, et al. Modulation of Blood Pressure by Central Melanocortinergic Pathways. N. Engl. J. Med. 2009;360:44–52. doi: 10.1056/NEJMoa0803085. [DOI] [PubMed] [Google Scholar]
- 42.Martinelli CE, et al. Obesity due to melanocortin 4 receptor (MC4R) deficiency is associated with increased linear growth and final height, fasting hyperinsulinemia, and incompletely suppressed growth hormone secretion. J. Clin. Endocrinol. Metab. 2011;96:181–188. doi: 10.1210/jc.2010-1369. [DOI] [PubMed] [Google Scholar]
- 43.Balthasar N, et al. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123:493–505. doi: 10.1016/j.cell.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 44.Tolson KP, et al. Inducible neuronal inactivation of Sim1 in adult mice causes hyperphagic obesity. Endocrinology. 2014;155:2436–2444. doi: 10.1210/en.2013-2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harris M, et al. Transcriptional regulation of the thyrotropin-releasing hormone gene by leptin and melanocortin signaling. J. Clin. Invest. 2001;107:111–120. doi: 10.1172/JCI10741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sarkar S, Légrádi G, Lechan RM. Intracerebroventricular administration of α-melanocyte stimulating hormone increases phosphorylation of CREB in TRH- and CRH-producing neurons of the hypothalamic paraventricular nucleus. Brain Res. 2002;945:50–59. doi: 10.1016/s0006-8993(02)02619-7. [DOI] [PubMed] [Google Scholar]
- 47.Caruso C, et al. Melanocortin 4 receptor activation induces brain-derived neurotrophic factor expression in rat astrocytes through cyclic AMP - Protein kinase A pathway. Mol. Cell. Endocrinol. 2012;348:47–54. doi: 10.1016/j.mce.2011.07.036. [DOI] [PubMed] [Google Scholar]
- 48.Chiappini F, Cunha LL, Harris JC, Hollenberg AN. Lack of cAMP-response Element-binding Protein 1 in the Hypothalamus Causes Obesity. J. Biol. Chem. 2011;286:8094–8105. doi: 10.1074/jbc.M110.178186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sutton AK, et al. Control of food intake and energy expenditure by Nos1 neurons of the paraventricular hypothalamus. J. Neurosci. 2014;34:15306–15318. doi: 10.1523/JNEUROSCI.0226-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pei H, Sutton AK, Burnett KH, Fuller PM, Olson DP. AVP neurons in the paraventricular nucleus of the hypothalamus regulate feeding. Mol. Metab. 2014;3:209–215. doi: 10.1016/j.molmet.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Agarwal V, Bell GW, Nam J-W, Bartel DP. Predicting effective microRNA target sites in mammalian mRNAs. Elife. 2015;4:e05005. doi: 10.7554/eLife.05005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Choi J-W, et al. MicroRNA profiling in the mouse hypothalamus reveals oxytocin-regulating microRNA. J. Neurochem. 2013;126:331–337. doi: 10.1111/jnc.12308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guo H, Ingolia NT, Weissman JS, Bartel DP. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature. 2010;466:835–840. doi: 10.1038/nature09267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chan KY, et al. Engineered AAVs for efficient noninvasive gene delivery to the central and peripheral nervous systems. Nat. Neurosci. 2017;20:1172–1179. doi: 10.1038/nn.4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Luoni M, et al. Whole brain delivery of an instability-prone Mecp2 transgene improves behavioral and molecular pathological defects in mouse models of Rett syndrome. Elife. 2020;9:1–30. doi: 10.7554/eLife.52629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Breit A, et al. The natural inverse agonist agouti-related protein induces arrestin-mediated endocytosis of melanocortin−3 and −4 receptors. J. Biol. Chem. 2006;281:37447–37456. doi: 10.1074/jbc.M605982200. [DOI] [PubMed] [Google Scholar]
- 57.Shinyama H, Masuzaki H, Fang H, Flier JS. Regulation of melanocortin-4 receptor signaling: Agonist-mediated desensitization and internalization. Endocrinology. 2003;144:1301–1314. doi: 10.1210/en.2002-220931. [DOI] [PubMed] [Google Scholar]
- 58.Lotta LA, et al. Human Gain-of-Function MC4R Variants Show Signaling Bias and Protect against Obesity. Cell. 2019;177:597–607.e9. doi: 10.1016/j.cell.2019.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Apóstolo N, et al. Synapse type-specific proteomic dissection identifies IgSF8 as a hippocampal CA3 microcircuit organizer. Nat. Commun. 2020;11:5171. doi: 10.1038/s41467-020-18956-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ray A, Treloar HB. IgSF8: A developmentally and functionally regulated cell adhesion molecule in olfactory sensory neuron axons and synapses. Mol. Cell. Neurosci. 2012;50:238–249. doi: 10.1016/j.mcn.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fernández-de Frutos M, et al. MicroRNA 7 Impairs Insulin Signaling and Regulates Aβ Levels through Posttranscriptional Regulation of the Insulin Receptor Substrate 2, Insulin Receptor, Insulin-Degrading Enzyme, and Liver X Receptor Pathway. Mol. Cell. Biol. 2019;39:1–19. doi: 10.1128/MCB.00170-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Choudhury NR, et al. Tissue-specific control of brain-enriched miR-7 biogenesis. Genes Dev. 2013;27:24–38. doi: 10.1101/gad.199190.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kumar, S., Velasco, A. D. R. & Michlewski, G. Oleic Acid induces miR-7 processing through remodelling of pri-miR-7/protein complex. J. Mol. Biol. (2017).10.1016/j.jmb.2017.05.001 [DOI] [PMC free article] [PubMed]
- 64.Ghoussaini M, et al. Open Targets Genetics: Systematic identification of trait-associated genes using large-scale genetics and functional genomics. Nucleic Acids Res. 2021;49:D1311–D1320. doi: 10.1093/nar/gkaa840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kwong, A. et al. FIVEx: an interactive eQTL browser across public datasets. Bioinformatics btab614 (2021). 10.1093/bioinformatics/btab614 [DOI] [PMC free article] [PubMed]
- 66.Pividori M, et al. PhenomeXcan: Mapping the genome to the phenome through the transcriptome. Sci. Adv. 2020;6:1–12. doi: 10.1126/sciadv.aba2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mountjoy, E. et al. An open approach to systematically prioritize causal variants and genes at all published human GWAS trait-associated loci. Nat. Genet. (2021). 10.1038/s41588-021-00945-5 [DOI] [PMC free article] [PubMed]
- 68.Vinnikov IA, et al. Hypothalamic miR-103 Protects from Hyperphagic Obesity in Mice. J. Neurosci. 2014;34:10659–10674. doi: 10.1523/JNEUROSCI.4251-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Crépin D, et al. The over-expression of miR-200a in the hypothalamus of ob/ob mice is linked to leptin and insulin signaling impairment. Mol. Cell. Endocrinol. 2014;384:1–11. doi: 10.1016/j.mce.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 70.Gao Y, et al. MicroRNA miR-7 and miR-17-92 in the Arcuate Nucleus of Mouse Hypothalamus Regulate Sex-Specific Diet-Induced Obesity. Mol. Neurobiol. 2019;56:7508–7521. doi: 10.1007/s12035-019-1618-y. [DOI] [PubMed] [Google Scholar]
- 71.Ma Y, et al. Neuronal miR-29a protects from obesity in adult mice. Mol. Metab. 2022;61:101507. doi: 10.1016/j.molmet.2022.101507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ebert MS, Sharp PA. Roles for MicroRNAs in Conferring Robustness to Biological Processes. Cell. 2012;149:515–524. doi: 10.1016/j.cell.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Miska EA, et al. Most Caenorhabditis elegans microRNAs are individually not essential for development or viability. PLoS Genet. 2007;3:2395–2403. doi: 10.1371/journal.pgen.0030215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.van der Klaauw AA, et al. Human Semaphorin 3 Variants Link Melanocortin Circuit Development and Energy Balance. Cell. 2019;176:729–742.e18. doi: 10.1016/j.cell.2018.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sebag JA, Zhang C, Hinkle PM, Bradshaw AM, Cone RD. Developmental Control of the Melanocortin-4 Receptor by MRAP2 Proteins in Zebrafish. Science. 2013;341:278–281. doi: 10.1126/science.1232995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Asai M, et al. Loss of function of the melanocortin 2 receptor accessory protein 2 is associated with mammalian obesity. Science. 2013;341:275–278. doi: 10.1126/science.1233000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rogers NH, Li JWP, Strissel KJ, Obin MS, Greenberg AS. Reduced energy expenditure and increased inflammation are early events in the development of ovariectomy-induced obesity. Endocrinology. 2009;150:2161–2168. doi: 10.1210/en.2008-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shi H, Seeley RJ, Clegg DJ. Sexual differences in the control of energy homeostasis. Front. Neuroendocrinol. 2009;30:396–404. doi: 10.1016/j.yfrne.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Krause WC, et al. Oestrogen engages brain MC4R signalling to drive physical activity in female mice. Nature. 2021;599:131–135. doi: 10.1038/s41586-021-04010-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xi, D., Gandhi, N., Lai, M. & Kublaoui, B. M. Ablation of Sim1 neurons causes obesity through hyperphagia and reduced energy expenditure. PLoS One7, e36453 (2012). [DOI] [PMC free article] [PubMed]
- 81.Hastings MH, Maywood ES, Brancaccio M. Generation of circadian rhythms in the suprachiasmatic nucleus. Nat. Rev. Neurosci. 2018;19:453–469. doi: 10.1038/s41583-018-0026-z. [DOI] [PubMed] [Google Scholar]
- 82.Adlanmerini M, et al. REV-ERB nuclear receptors in the suprachiasmatic nucleus control circadian period and restrict diet-induced obesity. Sci. Adv. 2021;7:1–13. doi: 10.1126/sciadv.abh2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Grippo RM, et al. Dopamine Signaling in the Suprachiasmatic Nucleus Enables Weight Gain Associated with Hedonic Feeding. Curr. Biol. 2020;30:196–208.e8. doi: 10.1016/j.cub.2019.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Coomans CP, et al. The suprachiasmatic nucleus controls circadian energy metabolism and hepatic insulin sensitivity. Diabetes. 2013;62:1102–1108. doi: 10.2337/db12-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dickinson ME, et al. High-throughput discovery of novel developmental phenotypes. Nature. 2016;537:508–514. doi: 10.1038/nature19356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bridi JC, Hirth F. Mechanisms of α-Synuclein Induced Synaptopathy in Parkinson’s Disease. Front. Neurosci. 2018;12:1–18. doi: 10.3389/fnins.2018.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bernal-Conde LD, et al. Alpha-Synuclein Physiology and Pathology: A Perspective on Cellular Structures and Organelles. Front. Neurosci. 2020;13:1399. doi: 10.3389/fnins.2019.01399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Peláez N, Carthew RW. Biological robustness and the role of microRNAs: a network perspective. Curr. Top. Dev. Biol. 2012;99:237–255. doi: 10.1016/B978-0-12-387038-4.00009-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Title, A. C. et al. Genetic dissection of the miR-200–Zeb1 axis reveals its importance in tumor differentiation and invasion. Nat. Commun. 9, 4671 (2018). [DOI] [PMC free article] [PubMed]
- 90.Goga A, et al. miR-802 regulates Paneth cell function and enterocyte differentiation in the mouse small intestine. Nat. Commun. 2021;12:1–18. doi: 10.1038/s41467-021-23298-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Perry JRB, et al. Parent-of-origin-specific allelic associations among 106 genomic loci for age at menarche. Nature. 2014;514:92–97. doi: 10.1038/nature13545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Elks CE, et al. Thirty new loci for age at menarche identified by a meta-analysis of genome-wide association studies. Nat. Genet. 2010;42:1077–1085. doi: 10.1038/ng.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li B, et al. Evaluation of PrediXcan for prioritizing GWAS associations and predicting gene expression. Pac. Symp. Biocomput. 2018;23:448–459. [PMC free article] [PubMed] [Google Scholar]
- 94.GTEx Consortium. The GTEx Consortium atlas of genetic regulatory effects across human tissues. Science. 2020;369:1318–1330. doi: 10.1126/science.aaz1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.McHugh TJ, et al. Dentate Gyrus NMDA Receptors Mediate Rapid Pattern Separation in the Hippocampal Network. Science. 2007;317:94–99. doi: 10.1126/science.1140263. [DOI] [PubMed] [Google Scholar]
- 96.DeFalco J, et al. Virus-assisted mapping of neural inputs to a feeding center in the hypothalamus. Science. 2001;291:2608–2613. doi: 10.1126/science.1056602. [DOI] [PubMed] [Google Scholar]
- 97.Tong Q, Ye C-P, Jones JE, Elmquist JK, Lowell BB. Synaptic release of GABA by AgRP neurons is required for normal regulation of energy balance. Nat. Neurosci. 2008;11:998–1000. doi: 10.1038/nn.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ruzankina Y, et al. Deletion of the Developmentally Essential Gene ATR in Adult Mice Leads to Age-Related Phenotypes and Stem Cell Loss. Cell Stem Cell. 2007;1:113–126. doi: 10.1016/j.stem.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Madisen L, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bankhead P, et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017;7:16878. doi: 10.1038/s41598-017-17204-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Paxinos, G. & Franklin, K. The Mouse Brain in Stereotaxic Coordinates. (Academic, 2001).
- 102.Zmuda, E. J., Powell, C. A. & Hai, T. A Method for Murine Islet Isolation and Subcapsular Kidney Transplantation. J. Vis. Exp. e2096 (2011). 10.3791/2096 [DOI] [PMC free article] [PubMed]
- 103.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bray NL, Pimentel H, Melsted P, Pachter L. Near-optimal probabilistic RNA-seq quantification. Nat. Biotechnol. 2016;34:525–527. doi: 10.1038/nbt.3519. [DOI] [PubMed] [Google Scholar]
- 105.Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Blessing D, et al. Scalable Production of AAV Vectors in Orbitally Shaken HEK293 Cells. Mol. Ther. - Methods Clin. Dev. 2019;13:14–26. doi: 10.1016/j.omtm.2018.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Challis RC, et al. Systemic AAV vectors for widespread and targeted gene delivery in rodents. Nat. Protoc. 2019;14:379–414. doi: 10.1038/s41596-018-0097-3. [DOI] [PubMed] [Google Scholar]
- 108.Neale, B. M. UK Biobank GWAS Results. http://www.nealelab.is/uk-biobank/. http://www.nealelab.is/uk-biobank/http://www.nealelab.is/uk-biobank/ (2018).
- 109.Kerimov N, et al. A compendium of uniformly processed human gene expression and splicing quantitative trait loci. Nat. Genet. 2021;53:1290–1299. doi: 10.1038/s41588-021-00924-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang G, Sarkar A, Carbonetto P, Stephens M. A simple new approach to variable selection in regression, with application to genetic fine mapping. J. R. Stat. Soc. Ser. B Stat. Methodol. 2020;82:1273–1300. doi: 10.1111/rssb.12388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bycroft C, et al. The UK Biobank resource with deep phenotyping and genomic data. Nature. 2018;562:203–209. doi: 10.1038/s41586-018-0579-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.ENCODE Project Consortium. et al. Expanded encyclopaedias of DNA elements in the human and mouse genomes. Nature. 2020;583:699–710. doi: 10.1038/s41586-020-2493-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kent WJ, et al. The Human Genome Browser at UCSC. Genome Res. 2002;12:996–1006. doi: 10.1101/gr.229102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Giambartolomei C, et al. Bayesian Test for Colocalisation between Pairs of Genetic Association Studies Using Summary Statistics. PLoS Genet. 2014;10:e1004383. doi: 10.1371/journal.pgen.1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
RNA sequencing data have been deposited to the European Nucleotide Archive (accession ID PRJEB48660). The following third-party data sources were used in this study: mouse reference genome GRCm38.p6 assembly (https://www.ncbi.nlm.nih.gov/assembly/GCF_000001635.26/), Open Targets Genetics (https://genetics.opentargets.org), FIVEx (https://fivex.sph.umich.edu), PhenomeXcan (http://phenomexcan.org), PhenomeXcan fastENLOC results (https://github.com/hakyimlab/phenomexcan), PrediXcan GTEx v8 expression predictors (https://www.predictdb.org), GTEx v8 eQTLs (accessible via https://www.gtexportal.org/home/datasets). All other data generated or analysed during this study are included in this published article (and its supplementary information files). Source data are provided with this paper.