Introduction
Necrobiosis lipoidica (NL) is a rare, idiopathic granulomatous disorder that classically presents as well-circumscribed, red to yellow plaques on the distal legs.1 The etiology of NL remains unclear, but suggested mechanisms involve vascular disturbance with microangiopathy or immune complex deposition. Abnormalities in collagen and impaired neutrophil migration have also been described.2
Treatment for NL is challenging, and data evaluating treatment efficacy are lacking. High-potency topical steroids are often prescribed as first-line treatment. Therapeutic options for refractory disease include intralesional steroids, topical immunomodulators, topical psoralen-ultraviolet A photochemotherapy, hydroxychloroquine, or tumor necrosis factor-alpha inhibitors. Pentoxifylline, a hemorheologic agent with anti-inflammatory properties, has also been associated with resolution of NL.3
More recently, Janus kinase (JAK) inhibitors have emerged as a promising therapy for granulomatous disorders4 and NL.5, 6, 7 Herein, we describe a case of NL with remarkable improvement after the use of topical 1.5% ruxolitinib cream (Opzelura, Incyte Corp) but not compounded topical tofacitinib.
Case report
A 19-year-old woman with a 5-year history of NL on her bilateral shins presented for ongoing disease management. Her NL was previously refractory to treatment with high-potency topical steroids, pimecrolimus cream, hydroxychloroquine, methotrexate, and pentoxifylline. Physical examination showed well-circumscribed, red to orange shiny sclerosing plaques with areas of central atrophy on her right medial and anterior shin and smaller plaques on her left medial and anterior shin (Fig 1, A). The patient was started on pentoxifylline 400 mg twice daily, hydroxychloroquine 200 mg twice daily, and after 1 month of minimal improvement, compounded topical tofacitinib 2.0% cream twice daily was added.
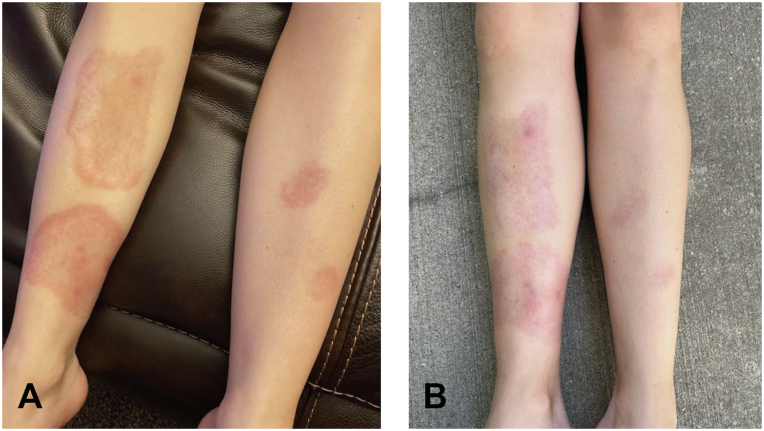
Fig 1.
A, Clinical image of the patient’s necrobiosis lipoidica at initial presentation, previously treated with multiple therapeutic modalities. B, Clinical response of the patient’s necrobiosis lipoidica after 3 months of treatment with topical ruxolitinib 1.5% cream and pentoxifylline.
Follow-up at 3 months demonstrated partial improvement of her lower extremity plaques. Hydroxychloroquine was held due to a syncopal episode, and the patient continued treatment with compounded tofacitinib cream and pentoxifylline with partial improvement.
After 13 months of treatment, the patient was switched to topical ruxolitinib 1.5% cream twice daily and continued on pentoxifylline. Follow-up at 3 months later showed dramatic improvement in the color and size of her lower extremity plaques (Fig 1, B).
Discussion
The clinical response of this patient’s NL to ruxolitinib expands on emerging data that JAK inhibitors are a promising treatment for granulomatous disorders. In granulomatous disorders, cytokines recruit and activate macrophages via JAK-signal transducer and activator of transcription (STAT) signaling.8 JAK-STAT signaling may be constitutively activated at low levels in NL,7 and JAK inhibition can therefore suppress immune cell activation and T-cell-mediated inflammation.8 Case reports have similarly described improvement in NL after treatment with JAK inhibitors.5, 6, 7,9
Selective enzyme inhibition may contribute to the differences in therapeutic response seen between tofacitinib and ruxolitinib. Tofacitinib preferentially inhibits JAK1 and JAK3, while ruxolitinib inhibits JAK1 and JAK2.8 Tofacitinib’s compounded formulation may have also limited its efficacy. Poor drug formulation uniformity and subtherapeutic doses have been reported with compounded medications.10
JAK inhibitors are a promising treatment for granulomatous diseases. Further research is needed to understand the role of JAK-STAT signaling in NL and to elucidate its underlying pathophysiology and molecular pathways. These data will provide insight about new targeted treatments for patients with granulomatous disorders.
Conflicts of interest
None disclosed.
Footnotes
Funding sources: None.
IRB approval status: Not applicable.
Consent for publication of all patient photographs and medical information was provided by the authors at the time of article submission to the journal stating that all patients gave consent for their photographs and medical information to be published in print and online with the understanding that this information may be publicly available.
References
- 1.Hashemi D.A., Brown-Joel Z.O., Tkachenko E., et al. Clinical features and comorbidities of patients with necrobiosis lipoidica with or without diabetes. JAMA Dermatol. 2019;155(4):455–459. doi: 10.1001/jamadermatol.2018.5635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sibbald C., Reid S., Alavi A. Necrobiosis lipoidica. Dermatol Clin. 2015;33(3):343–360. doi: 10.1016/j.det.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Wee E., Kelly R. Pentoxifylline: an effective therapy for necrobiosis lipoidica. Australas J Dermatol. 2017;58(1):65–68. doi: 10.1111/ajd.12420. [DOI] [PubMed] [Google Scholar]
- 4.Chapman S., Gold L.S., Lim H.W. Janus kinase inhibitors in dermatology: part II. A comprehensive review. J Am Acad Dermatol. 2022;86(2):414–422. doi: 10.1016/j.jaad.2021.06.873. [DOI] [PubMed] [Google Scholar]
- 5.Lee J.J., English J.C., III Improvement in ulcerative necrobiosis lipoidica after Janus kinase-inhibitor therapy for polycythemia vera. JAMA Dermatol. 2018;154(6):733–734. doi: 10.1001/jamadermatol.2018.0756. [DOI] [PubMed] [Google Scholar]
- 6.Barbet-Massin M.A., Rigalleau V., Blanco P., et al. Remission of necrobiosis lipoidica diabeticorum with a JAK1/2 inhibitor: a case report. Diabetes Metab. 2021;47(4) doi: 10.1016/j.diabet.2020.01.001. [DOI] [PubMed] [Google Scholar]
- 7.Damsky W., Singh K., Galan A., King B. Treatment of necrobiosis lipoidica with combination Janus kinase inhibition and intralesional corticosteroid. JAAD Case Rep. 2020;6(2):133–135. doi: 10.1016/j.jdcr.2019.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwartz D.M., Kanno Y., Villarino A., Ward M., Gadina M., O'Shea J.J. JAK inhibition as a therapeutic strategy for immune and inflammatory diseases. Nat Rev Drug Discov. 2017;17(1):78. doi: 10.1038/nrd.2017.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Janssen S., Jansen T.M. Ulcerated necrobiosis lipoidica successfully treated with tofacitinib. Int J Dermatol. 2022;61(6):739–741. doi: 10.1111/ijd.15960. [DOI] [PubMed] [Google Scholar]
- 10.Gudeman J., Jozwiakowski M., Chollet J., Randell M. Potential risks of pharmacy compounding. Drugs R D. 2013;13(1):1–8. doi: 10.1007/s40268-013-0005-9. [DOI] [PMC free article] [PubMed] [Google Scholar]