Abstract
Non-human primates (NHPs) represent one of the most important models for pre-clinical studies of novel biomedical interventions. In contrast with small animal models, however, widespread utilization of NHPs is restricted by cost, logistics, and availability. Therefore, we sought to develop a translational primatized mouse model, akin to a humanized mouse, to allow for high-throughput in vivo experimentation leveraged to inform large animal immunology-based studies. We found that adult rhesus macaque mobilized blood (AMb) CD34+ enriched hematopoietic stem and progenitor cells (HSPCs) engrafted at low but persistent levels in immune-deficient mice harboring transgenes for human (NHP cross-reactive) GM-CSF and IL3, but did not in mice with wild-type murine cytokines lacking NHP cross-reactivity. To enhance engraftment, fetal liver-derived HSPCs were selected as the infusion product based on an increased CD34hi fraction compared to AMb and bone marrow. Coupled with co-transplantation of rhesus fetal thymic fragments beneath the mouse kidney capsule, fetal liver-derived HSPC infusion in cytokine-transgenic mice yielded robust multilineage lymphohematopoietic engraftment. The emergent immune system recapitulated that of the fetal monkey, with similar relative frequencies of lymphocyte, granulocyte, and monocyte subsets within the thymic, secondary lymphoid, and peripheral compartments. Importantly, while exhibiting a predominantly naïve phenotype, in vitro functional assays demonstrated robust cellular activation in response to non-specific and allogenic stimuli. This primatized mouse represents a viable and translatable model for the study of hematopoietic stem cell physiology, immune development, and functional immunology in NHPs.
Keywords: Rhesus macaque, immune system, hematopoietic stem cells, primatized mouse, chimerism
Graphical Abstract
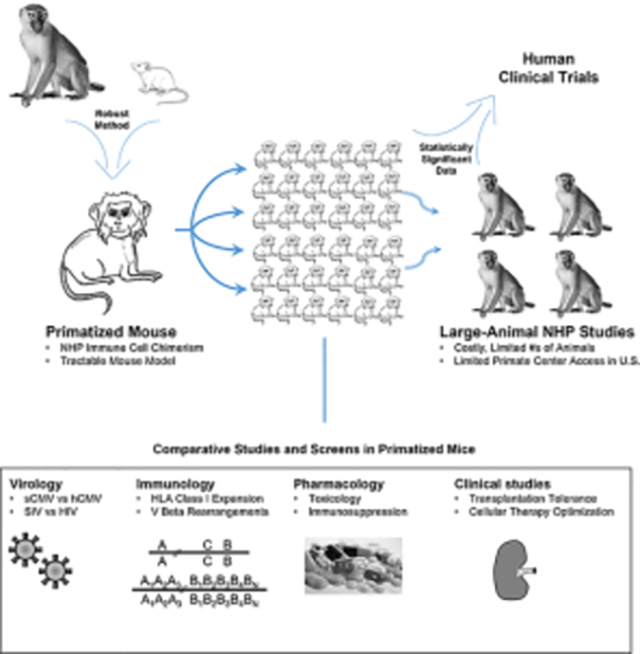
Summary Sentence
Engraftment of rhesus macaque hematopoietic tissues in immune-deficient mice yields a robust BLT/NeoThy-type primatized mouse model for studying non-human primate hematopoiesis and immune function in vivo.
1. Introduction
Humanized mouse models have enabled important discoveries involving human hematopoietic engraftment[1] and leukocyte development.[2] In recent years, humanized mice have become a useful tool for pre-clinical experimental study of human immune function.[3] Similarly, non-human primates (NHPs), such as the rhesus macaque (Macaca mulatta), serve as important large-animal models for pre-clinical translational studies of novel therapeutics given their biological similarities to humans.[4] However, the high cost and prolonged timelines associated with NHP experimentation are critical barriers to their effective and efficient use in large-scale biomedical research.
We sought to develop a mouse model with an NHP immune system akin to BLT/NeoThy-type humanized mice,[5–7] to investigate, in a high-throughput manner, the relationship between NHP hematopoietic stem/progenitor (HSPC) ontogeny stage and engraftment, as well as leukocyte function. Such a primatized model would allow for investigation of factors that influence NHP hematopoietic engraftment, and enable more efficient large-animal studies in transplant immunology and virology.
There are a limited number of prior published descriptions of NHP-derived hematopoiesis and leukocyte development in immune-deficient mice, each with significant limitations precluding widespread use of existing models.[8–11] However, to our knowledge, there have been no reports of BLT/NeoThy-type primatiziation utilizing IV injection of HSPCs with surgical transplantation of NHP thymic tissue, the current gold-standard for humanization studies. In a recent study, Radtke et al. utilized a powerful next-generation host, the MISTRG mouse, harboring multiple human hematopoietic transgenes, including SIRPα, M-CSF, Thrombopoietin, GM-CSF, and IL-3 on a RAG2−/− IL2Rg−/− background.[12] Though this is important work in the field, unfortunately, this host strain is not widely-available commercially, precluding their widespread use. Additionally, in this particular case, the authors’ most robust induction of hematopoietic engraftment relied on use of a rare FACS-sorted HSPC population, resulting in a low-throughput model and thus complicating its feasibility for large-scale studies. Furthermore, although the primatized mice developed T lymphocytes via the native murine thymus, these cells harbored limited functional capacity due to a lack of NHP MHC selection cues. This deficiency resulted in a suboptimal model for study of NHP MHC-restricted immune responses.
This limitation, and those of other cited attempts, illustrates the importance of tissue implantation coupled with cellular transfusion for succesfull primatization. Crucially, mouse hosts must have a sufficient transgenic background to allow for robust HSPC engraftment and primatization. In our study, we aimed to develop a tractable BLT/NeoThy-type primatized mouse model to use in pre-clinical studies of hematopoietic engraftment, immunologic development, and leukocyte function.
2. Materials and Methods
2.1. Tissue Processing and Cell Purification
Experiments were approved by the Animal Care and Use Committees of the University of Wisconsin-Madison School of Medicine and Public Health, the College of Letters and Sciences and the Vice Chancellor’s Office for Research and Graduate Education. NHP fetal tissues were obtained from fetuses 96–100 days gestation that went to necropsy during the course of other research studies. NHP fetal liver was processed by macerating the tissue over a 100μm cell strainer, and leukocytes were collected with Lymphocyte Separation Medium (Corning, Manassas, VA). The amount of tissue was similar in quantity to human fetal tissue at the timepoint typically used for humanized mice, and therefore an approximately similar number of mice per donor can be derived from NHP and human fetuses. HSPCs were enriched using NHP-CD34-APC antibody (clone 563, BD Biosciences, San Jose, CA) and MACS anti-APC beads (Miltenyi, Bergisch Gladbach, Germany). NHP thymus was processed from fetal rhesus macaques by placing necropsied thymus in cold media, removing extraneous tissue, then dissecting into 1mm × 1mm fragments. Cells and tissue were cryopreserved in CryoStor medium (Stem Cell Technologies, Vancouver, BC Canada).
2.2. Primatization Surgeries and Secondary Transplantation
Primatized mice were generated similarly to previously published humanization reports.[7, 13] Briefly, 6–10-week-old male and female NBSGW, NOG-EXL, or NSG-SGM3 mice were IV injected with 1×105–1×106 CD34+ rhesus cells. Cryopreserved rhesus fetal thymus fragments were surgically implanted under the mouse kidney capsule. Mice also received IV injection of αCD2 antibody (clone LoCD2a,100μg) at days 0 and 7 post-surgery, as previously described.[7] All mice were treated with Buprenorphine SR at day 0, and with Baytril antibiotic for 10 days post-surgery.
2.3. Mouse Blood Collection and Immunoprofiling by Flow Cytometry
Peripheral mouse blood was sampled via retro-orbital bleed using heparin-coated capillary tubes (Thermo Fisher Scientific, Waltham, MA) into microtubes containing 150μl of 2% dextran/dPBS−/− and 150μl of 0.5%-Heparin solution (Sigma Aldrich, Saint Louis, MO). After 20 minutes of settling, the leukocyte-containing upper layer was spun down at 400g × 5 minutes and resuspended in ACK red blood cell lysis buffer (Thermo Fisher Scientific, Waltham, MA), then washed for downstream analysis. Cells were stained for NHP-CD45 (clone D058–1283) and immune subset markers (additional marker and clone information available in Supplemental Materials). Upon necropsy of recipient mice, fresh splenocytes, lymph node tissue, and bone marrow (BM) were isolated, stained with the aforementioned antibodies, and fixed in 4% PFA. Flow cytometric data were acquired using an LSR II or FACSymphony cytometer (BD Biosciences, San Jose, CA). Data analysis was performed using FlowJo software (Treestar, San Carlos, CA). Serum was collected by placing blood in an empty tube, allowing for clotting over 20 minutes, then centrifuging at 1000g × 10 minutes.
2.4. Mixed Lymphocyte Reactions and Stimulation Assays
Freshly isolated splenocytes (responder cells) from primatized mice were collected, labeled with Cell Trace Violet (CTV) (Thermo Fisher Scientific, Waltham, MA), then plated for 5 days under various stimulatory conditions. Recombinant human IL-2 (PeproTech, Cranbury, NJ) was added to a subset of MLR wells at a concentration of 100ng/well.
3. Results and Discussion
3.1. Low-level engraftment of adult HSPCs in transgenic immune-deficient mice
We first tested engraftment of a commonly available NHP HSPC source, adult mobilized blood (AMb), in an immune-deficient NSG-variant mouse, the NBSGW.[7, 14] Using magnetic beads, we sorted CD34+ cells from AMb and IV injected into naïve NBSGW mice (Fig. 1A). Peripheral blood engraftment was monitored by presence of NHP-CD45+ cells. After 18 weeks, we were unable to detect any engraftment of primate cells after infusion of AMb product within this host (data not shown).
Figure 1. Low-Level Engraftment of Adult Mobilized Blood HSPCs in Immune-Deficient Mice.
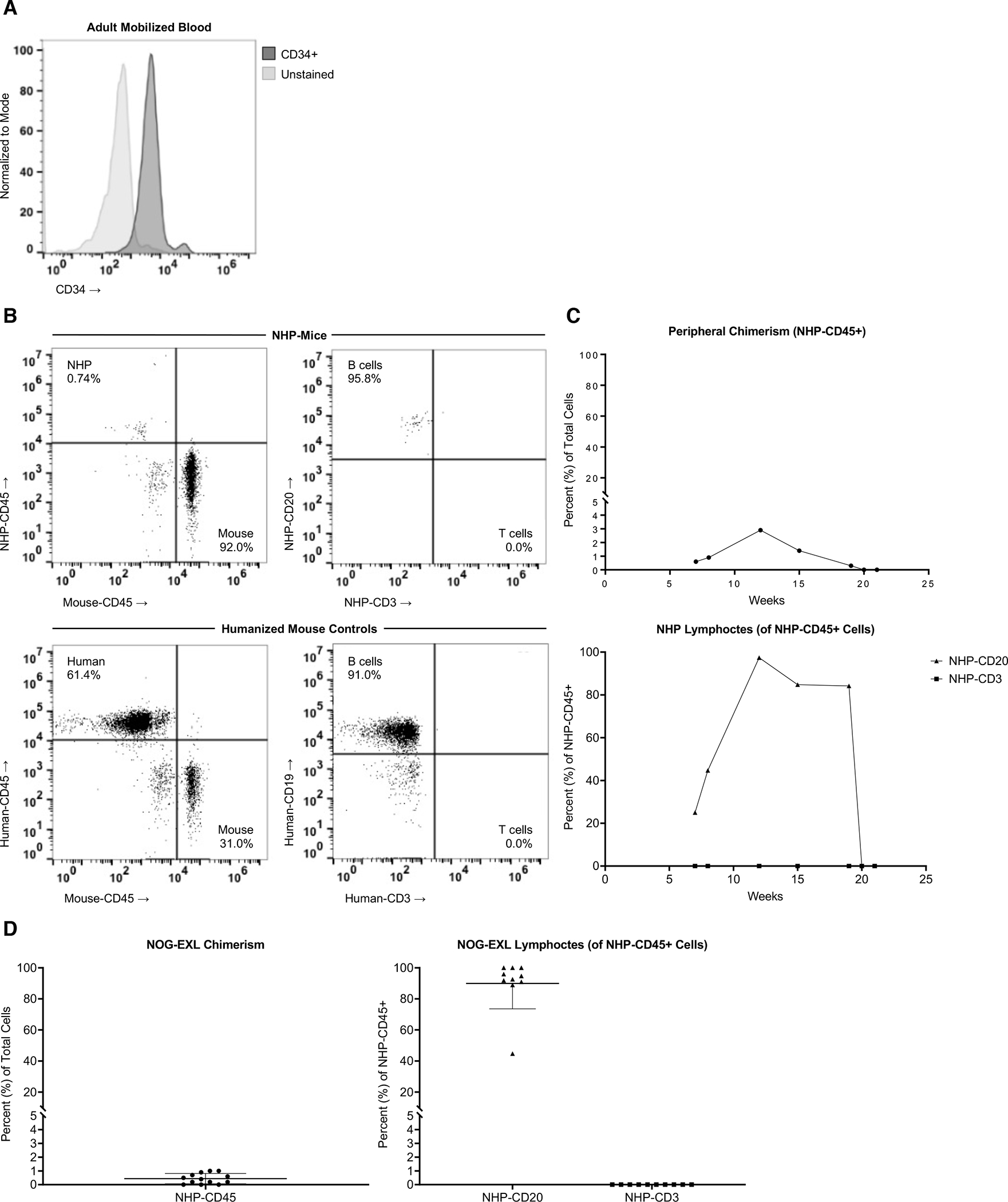
(A) Frozen mobilized blood from a male adult rhesus was processed via MACS beads to isolate CD34+ cells for injection. Post-enrichment purity in this example was 91.9%. 1×105–1×106 cells were IV injected per mouse for all experiments. (B) Representative flow plots of NHP mice (top) showing overall NHP-CD45 vs. Mouse-CD45 engraftment and CD20+ B cell and CD3+ T cell frequency of the NHP-CD45+ cells, compared to human cord blood CD34+ injected controls (bottom). 1×105 CD34+ cells were injected, and 55cGY-irradiated NOG-EXL strain was used for both sets of mice. NOG-EXL mice harboring human IL3 and GM-CSF transgenes were injected with 1×106 NHP-CD34+ cells per animal and monitored for chimerism (representative animal shown in C) over time in multiple animals (D). Results in D are shown at 8–11 weeks post-injection, compiled from n=2 separate experiments. Value of ≤0.1 and/or <4 positive events were considered background and listed as 0% engraftment.
Next, the NOG-EXL recipient strain was evaluated. This strain incorporates two transgenic human cytokines (GM-CSF and IL3) that have a high degree of homology and subsequent cross-reactivity with NHPs. We hypothesized that the human-NHP cross-reactive cytokines would be sufficient to enhance the engraftment potential of rhesus AMb HSPCs. This is supported by observations in the NOG-EXL and similar strains (e.g., NSG-SGM3, MISTRG) of enhanced human engraftment and chimerism, which was similarly demonstrated in one prior primatization study.[9]
IV injection of 1×105–1×106 CD34+ AMb cells resulted in moderately durable engraftment in irradiated NOG-EXL mice vs. humanized mice made with umbilical cord-derived CD34+ HSPCs (Fig. 1B,C). Engrafted NHP leukocytes demonstrated CD20+ B cell predominance with an absence of T cells. The results were reproducible (Fig. 1D), indicating the possibility of stable engraftment of adult NHP HSPC populations. However, low-level engraftment and lack of T cell development may impose limits on the use of this model for hematopoietic studies or leukocyte functional assays.
The paucity of T cells was attributed to the absence of thymic implantation. This is consistent with several reported studies,[15] as well as our prior experience.[7] Despite this limited durability, we established a framework for subsequent experiments. Specifically, we successfully identified a transgenic host and confirmed the importance of thymic implantation for developing a functional and MHC-restricted/donor-matched peripheral immune compartment. Our results with adults cells do not preclude successful engraftment of these cells in future primatized mouse model iterations.
3.2. Robust primatization of transgenic immune deficient mice
To enhance the applicability of our model, we developed new methods to establish a more robust primatized immune system within the NOG-EXL host. Several sources of HSPCs were evaluated, including adult BM and a variety of fetal cells (100 days gestation) in addition to the AMb described above (Sup. Fig. 1A,B). CD34+ cells were magnetically enriched to >90% purity prior to cytometric phenotyping. The fetal-derived samples had significant numbers of CD34hi cells, which are associated with augmented engraftment, compared to AMb (Fig. 2A).[16, 17] Importantly, these CD34hi cells are known to contain the HSPCs subsets with the greatest hematopoietic potential, described previously to be the lineagenegCD34hiCD45midCD117+CD45RA−CD90+ fraction.[18] Based on these findings, we focused on determining if fetal-derived HSPCs would be well-suited for the induction of NHP chimerism in our transgenic host. Another important practical consideration is that at the fetal developmental age there should be a sufficient quantity of CD34+ cells and thymus tissue to allow for BLT-(fetal tissue) type primatization of multiple hosts from a single fetal rhesus donor.
Figure 2. Robust Engraftment of Fetal Liver HSPCs and Thymic Tissue in NOG-EXL Mice.
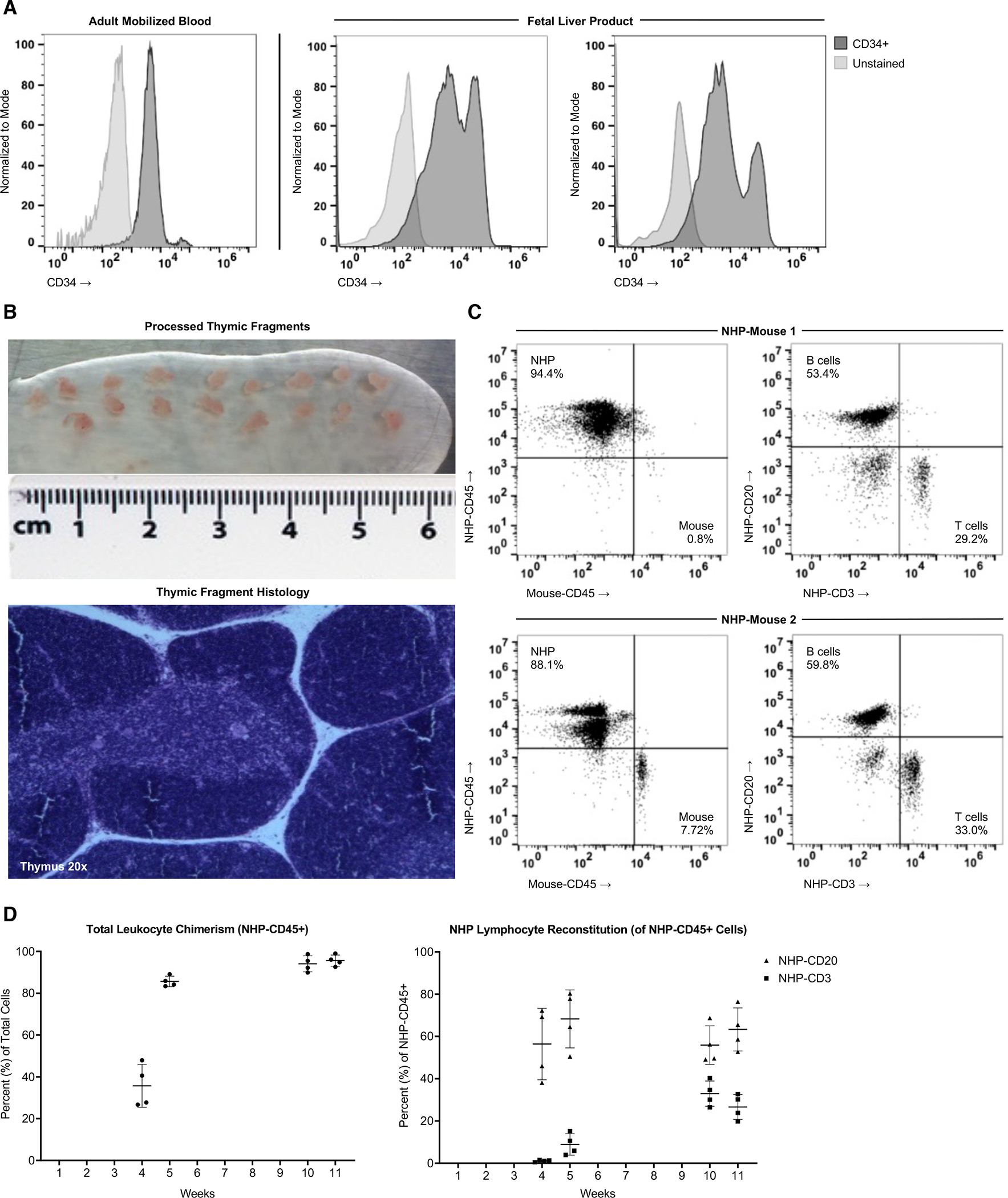
(A) NHP-CD34 hematopoietic stem and progenitor cell (HSPC) purity was measured following MACS-purification of rhesus mobilized blood (left, negative control = unstained cells) and fetal liver (right, negative control = negative MACS fraction), including CD34lo and CD34hi populations. Mobilized blood was from the same donor but a separate purification than shown in Figure 1. (B) Thymus tissue was dissected into 1mm × 1mm fragments suitable for primatization experiments, and histological analysis of H&E stained thymus sections shows anatomical structures required for T cell development (medulla, cortex, Hassell’s corpuscles). (C) Two NOG-EXL mice were transplanted with a common source of fetal rhesus thymus tissue and IV injected with 1×106 CD34+ cells isolated from fetal liver of the same donor. Overall engraftment (NHP-CD45 vs mouse-CD45) at week 11 post-surgery is shown on the left, and the proportion of the NHP-CD45 cells expressing the B cell marker CD20 and T cell marker CD3 is shown on the right. (D) Engraftment kinetics are shown from week 4 post-primatization surgery to week 11 in a cohort of 6 mice made from a second tissue source, started at the same time. Two mice were analyzed at all timepoints, two were removed after 5 weeks due to premature death, and two were included in the analysis at 10 and 11 weeks (logistical issues preclude their analyses at early timepoints).
To determine the promise of this HSPC source, we IV injected 1×106 cryopreserved fetal liver-derived CD34+ cells into the irradiated NOG-EXL host. Additionally, fragments of cryopreserved fetal thymus were surgically implanted under the kidney capsule after cell infusion, as multiple prior studies have demonstrated the utility of thymic organoid co-transplantation as an essential component of T cell development and maturation in BLT/NeoThy-type humanized mice (Fig. 2B).[7, 13] To facilitate removal of “passenger thymocytes,” known to contribute to graft-vs-host-disease (GVHD), recipient mice were injected with anti-CD2 antibody, which binds and depletes fetal NHP thymoctyes (Sup. Fig. 1C). Implementation of this protocol yielded robust multilineage chimerism of NHP-CD45+ cells in all animals (Fig. 2C,D), for long durations (20 weeks at study completion) without apparent GVHD. We anticipate that future studies will determine the incidence of GHVD in longer-term studies in this model, as this is an important complication associated with humanization of mice and, indeed, could be leveraged in this model to better understand the mechanisms of GVHD pathogenesis.
Engraftment was demonstrated in both CD20+ and CD3+ lymphocytes, with a significant increase in T cell percentages over time (Fig. 2D). This indicates de novo T cell development in the thymic organoid, which is explored further below via examination of developing thymocytes (Fig. 4). Typical ratios of CD4+ and CD8+ T cells were present in the peripheral blood, with a majority of cells demonstrating a naïve phenotype (CD28+CD95−), similar to the fetal NHP immune system (Fig. 3A,B; Sup. Fig. 2A). In contrast, the antigen-experienced NHP adult has higher relative percentages of central (CD28+CD95+) and effector memory (CD28−CD95+) T cells. Interestingly, CCR7 expression on CD4+ T cells in primatized mice and primary NHP fetuses demonstrated marked enhancement compared to adult NHPs (Sup. Fig. 2B). CCR7 is known to be involved in thymic homing during murine immune development, raising the possibility that upregulation is related to tissue-specific chemotaxis and T cell maturation. However, further exploration is needed. [19]
Figure 4. Fetal Thymic Organoid, Secondary Lymphoid and Tissue-Resident Immune Cells.
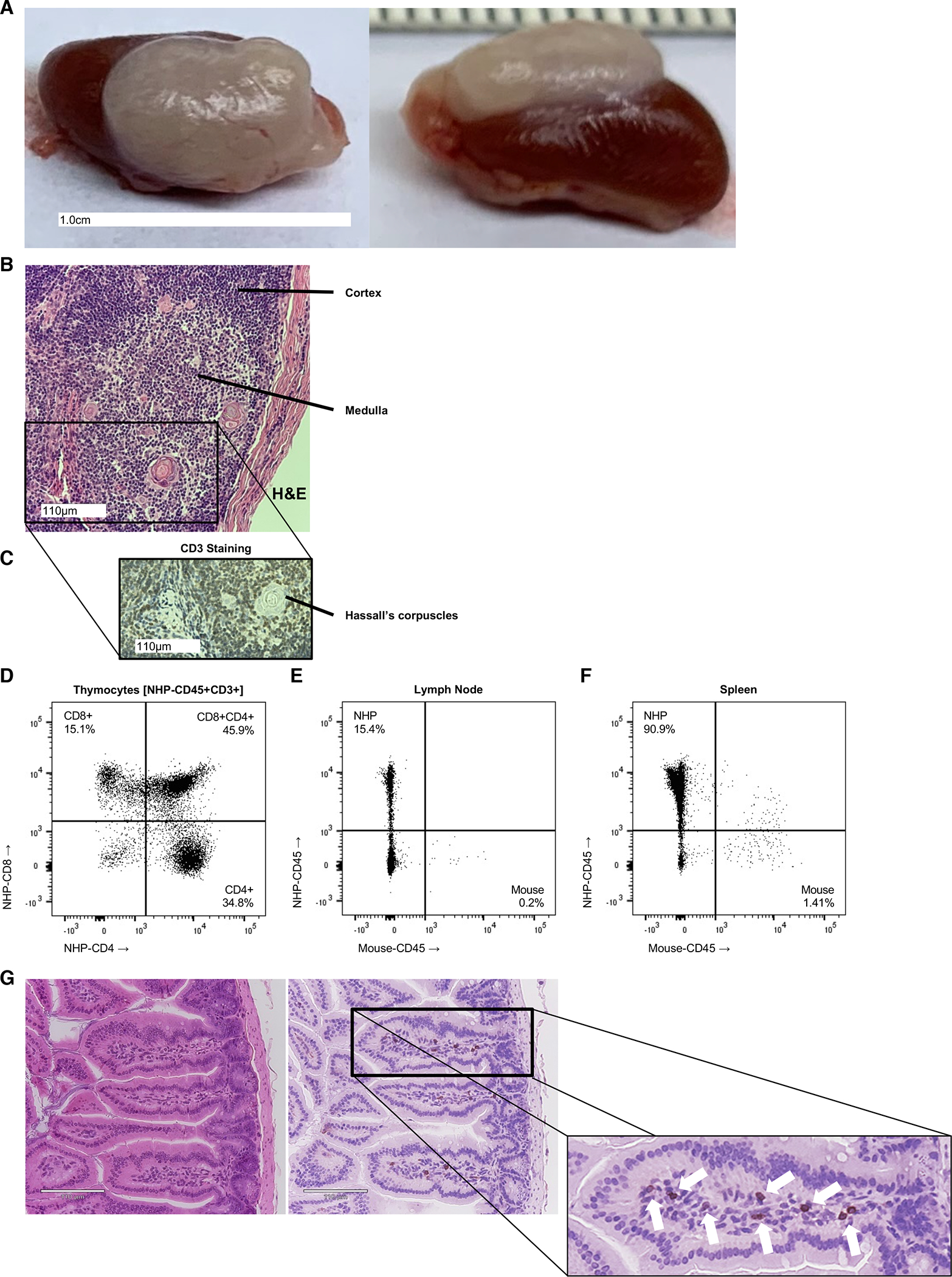
Thymic organoids formed in engrafted NHP mice (A) shown grossly, with (B) H&E staining including well-organized medullary and cortical regions, as well as Hassall’s corpuscles, consistent with native thymic histology. (C) Anti-CD3 (rhesus) staining identifies the presence of diffuse T cell distribution within the cortex and medulla, which is supported by (D) flow cytometric analysis of CD4+ and CD8+, including developing double positive CD4+CD8+ subsets within the thymic organoid of a fully engrafted primatized mouse. Secondary lymphoid tissue, including lymph node (E) and spleen (F) are populated with NHP-derived leukocytes (NHP-CD45+) as demonstrated by flow cytometric analysis of NHP-CD45+ staining. (G) Cross-sectional H&E and anti-CD3 stained histology reveals tissue-resident T and non-T lymphocytes (denoted by arrows) within the intestinal villi of the mature NHP mouse.
Figure 3. Primatization Yielded Multilineage Reconstitution of the Peripheral Immune System.
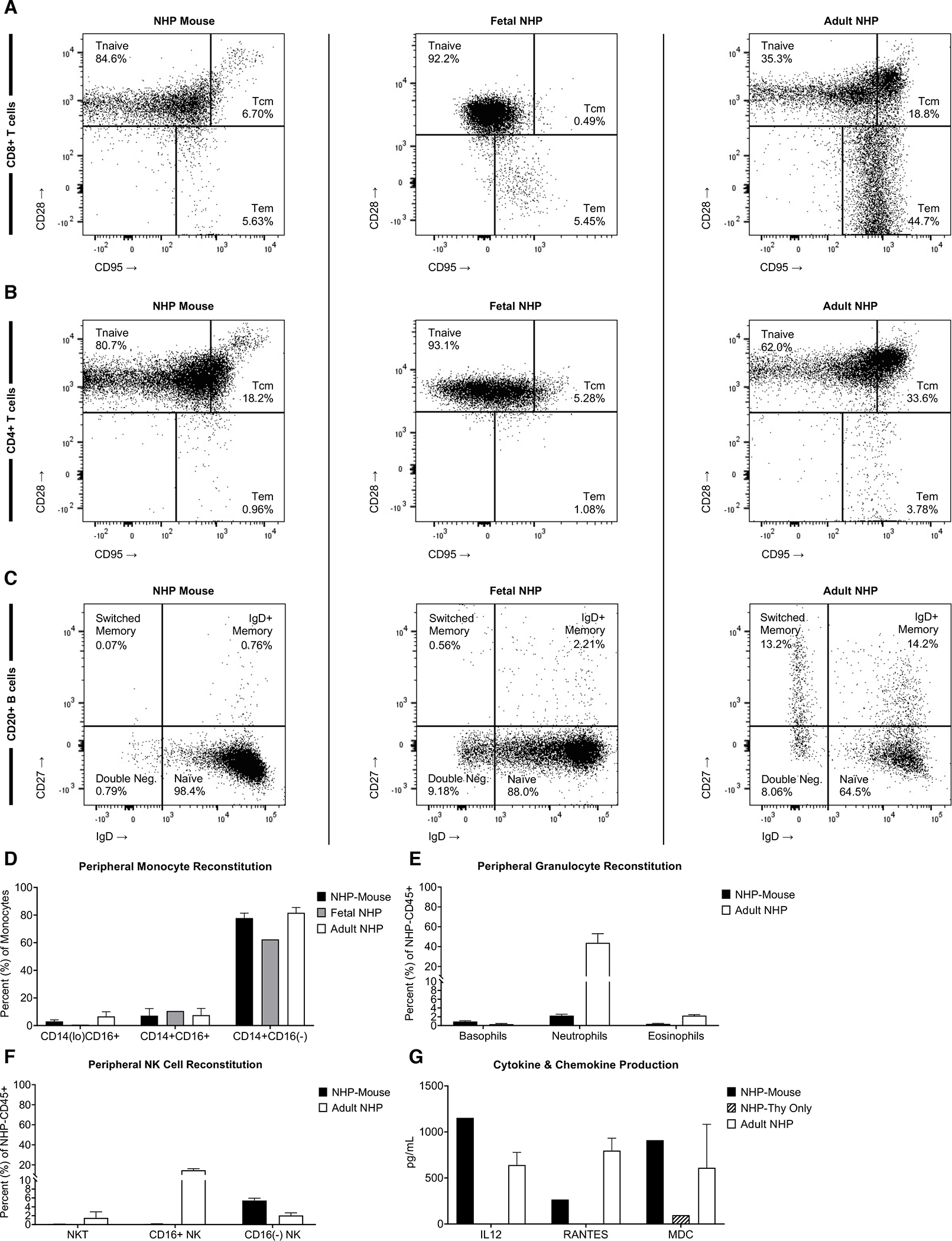
Flow cytometric analyses of peripheral CD8+ T cell (A), CD4+ T cell (B), and CD20+ B cell (C) subsets were performed on gated NHP-CD45+ cells from engrafted NHP-mice at 20 weeks post-infusion, which was compared to fetal (96 days gestation) and adult (15-years-old) adult NHP peripheral blood controls. Representative flow plots are shown here, summaritive bar graphs from multiple analyses are presented in Supplemental Figure 2. (D) Peripheral recovery of non-classical (NHP-CD45+CD3−CD20−HLA-DR+CD14loCD16+), intermediate (NHP-CD45+CD3−CD20−HLA-DR+CD14+CD16+), and classical (NHP-CD45+CD3−CD20−HLA-DR+CD14+CD16−) monocyte subsets in NHP-mice were compared to fetal and adult NHP controls. (E) NHP-mouse granulocyte recovery, including basophils (NHP-CD45+CD20−CD3−NKG2A−HLA-DR−CD14−CD123hiCD66−CD33loCD39mid), neutrophils (NHP-CD45+CD20−CD3−NKG2A−HLA-DR−CD14+CD123−CD66+CD33hiCD39lo), and eosinophils (NHP-CD45+CD20−CD3−NKG2A−HLA-DR−CD14−CD123loCD66hiCD33loCD39+), were compared to adult NHP controls. (F) Peripheral NHP-CD45+Lineage-NKG2A+ NK cell subsets in the NHP mouse were compared to adult NHP controls. (G) Serum samples from NHP mice 12–16 weeks post-primatization were analyzed by 29-plex NHP Luminex panel for presense of cytokines and chemokines vs control that received a thymus implantation surgery but no CD34+ injection.
Also consistent with the fetal immunoprofile, the B cell compartment within our NHP mice was largely naïve (CD27−IgD+) (Fig. 3C; Sup. Fig. 2C). NHP-derived monocytes (Fig. 3D), granulocytes (Fig. 3E), and CD16neg NK cells (Fig. 3F) were also present. NK cell frequency was suboptimal, comparable to conventional humanized BLT mice, but potentially could be improved in future iterations of the model via administration of exogenous IL-15,[20] for example, or other cytokines supportive of NK cell development, homeostasis, and function. Further, animals developed comparable levels of the inflammatory cytokine, IL-12, as well as chemokines, RANTES and MDC, vs. adult NHP controls (Fig. 3G). Taken together, these findings demonstrate robust reconstitution of a diverse, multilineage immune system following hematopoietic engraftment. In contrast to prior primatization reports, the multilineage nature of this engraftment has wide-reaching implications for studies of immunologic maturation and function.
3.3. Fetal thymic organoid, secondary lymphoid repopulation, and tissue- resident immune cells
The transplanted fetal NHP thymus fragment produces a large thymic organoid in the recipient mice (Fig. 4A), similar to previous humanized mice reports from our group and others[5, 7]. The histologic structure was similar to that seen in primary fetal thymus (Fig. 2D), with well-developed Hassall’s corpuscles (Fig. 4B) as well as CD3+ thymocytes within the cortical and medullary regions (Fig. 4C). Flow cytometric analysis revealed developing double-positive CD4+CD8+ thymocytes, as well as single-positive CD4+ and CD8+ T cells (Fig. 4D), similar to the primary fetal thymus (Sup. Fig. 3A). Importantly, secondary lymphoid tissues (lymph nodes and spleen) were also repopulated with NHP-derived lymphocytes (Fig. 4E,F), consisting of both CD20+ B cell and CD3+ T cell populations (Sup. Fig. 3B), as well as CD8+ and CD4+ T cell subsets (Sup. Fig 3C). Furthermore, tissue-resident NHP lymphocytes were found, albeit at low levels, within end organs involved in immune surveillance and defense (e.g., intestine), as demonstrated by lymphocyte aggregation and positive CD3 staining (Fig. 4G). The tissue-resident chimerism could be increased in future iterations of the model, similar to how addition of transgenic cytokines improves such chimerism in humanized mice.[21] Reconstitution of primary and secondary lymphoid organs are an essential component of immunologic maturation and function, which underscores the utility of this model for future virology studies, including investigation of acquired immunodeficiencies. This notion is supported by preliminary data demonstrating SIVmac239 replication within primatized mouse splenocytes (Sup. Fig. 4D).
3.4. Bone marrow engraftment and secondary transplantation
To assess the efficiency of true hematopoietic stem cell (HSC) engraftment within the mouse BM, we isolated BM from the femur, and performed flow cytometric analysis of specific markers associated with HSC identity. The BM contained a rich population of lineage-negative CD34+CD38loCD45RAnegCD90+ cells (Fig. 5A). To test the viability and functionality of these HSPCs, total BM was secondarily transplanted into two strains of transgenic immune-deficient mice (Fig. 5B). The NSG-SGM3 variant includes transgenic human SCF in addition to IL-3 and GM-CSF present in the NOG-EXL strain. Importantly, secondary engraftment was evident in both strains, demonstrating the strong engraftment potential of the transplanted HSPCs. Also of interest, the NSG-SGM3 model yielded a marked increase in secondary NHP-CD45+ chimerism, likely due to the addition of human SCF, warranting future studies. The durable primary and secondary engraftment within these models suggests true HSC engraftment, and allows for future studies of hematopoietic potential of HSPC subsets. It also creates the potential for testing novel non-myeloablative conditioning regimens prior to large animal implementation.
Figure 5. Hematopoietic Engraftment in NHP Mouse Bone Marrow and Secondary Transplantation.
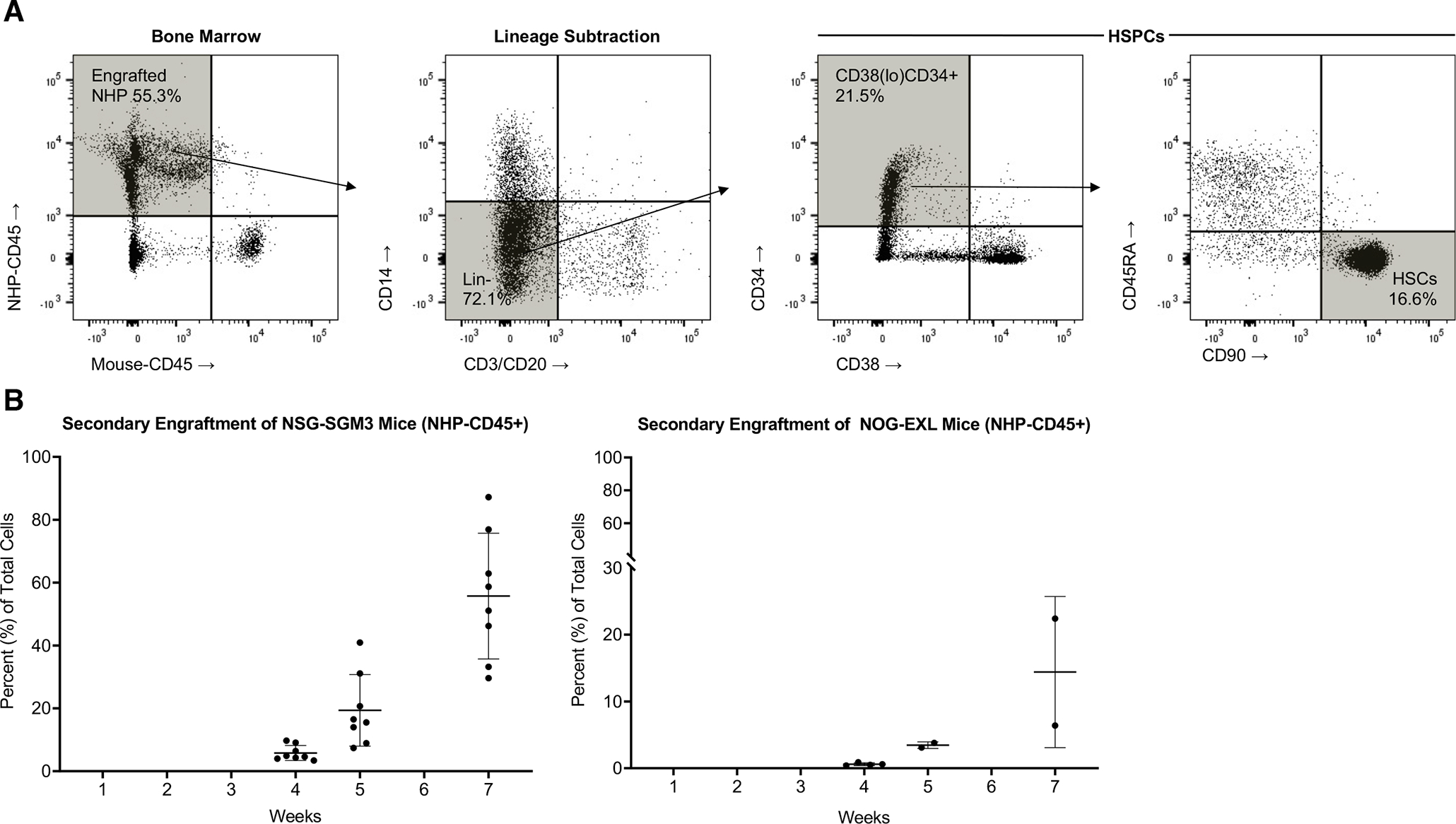
(A) Bone marrow (BM) was harvested from NHP mouse femurs and analyzed for hematopoietic stem and progenitor markers (lineage negative CD38(lo)CD34+ and CD38(lo)CD34+CD45RA-CD90+ HSPC subsets, values given as a percentage of NHP-CD45+ cells). (B) Total BM cells were harvested from NHP mouse femurs and transplanted via IV injection into secondary, naïve NSG-SGM3 mice, which have human IL3 and GM-CSF transgenes, similar to the NOG-EXL, but also harbor a human SCF transgene. 2×106 total cells injected per animal, n=8, 5 males and 3 females. NOG-EXL mice were also injected (2×106 total cells per animal) and monitored for engraftment over the course of 7 weeks. N=4 male mice, two mice died between weeks 4 and 5.
3.5. Development of functional immune cells
Having established durable engraftment and immune reconstitution, we sought to validate the translational potential of the model in functional NHP immunologic studies. We conducted a series of stimulation assays to characterize the functional fitness of the peripheral T cell compartment. Splenocytes from primatized mice were exposed in vitro to mitogens (PMA/Ionomycin and PHA) to evaluate T cell proliferative capacity. After five days in culture, stimulated T cells were found to have undergone robust, multigenerational proliferation, indicating responsiveness to non-specific stimuli (Fig. 6A). Based on these findings, mixed lymphocyte reactions (MLRs) were set up with isolated splenocytes in an attempt to induce an MHC-restricted allo-specific immune response (Fig. 6B). As expected, robust T cell activation and proliferation were induced in the presence of MHC-disparate irradiated adult NHP cells. This allogeneic response was increased upon introduction of the inflammatory cytokine IL-2 (Fig. 6B, right panel), mimicking the hostile post-allogenic transplant environment. Importantly, activated T cells produced IFNᵧ and TNFα in response to allogeneic stimulation (Fig. 6C), further demonstrating the development of functional T cells in the NHP mice. These findings have notable implications in the field of transplant immunology, which is highly dependent on NHP studies given the disparate nature of small animal and human allo-immune responses. Moreover, the morbidity and mortality of human trials is a barrier to the development of robust immunomodulating therapies in transplantation, further emphasizing the importance of effective large animal models. The uncovering of conserved allo-immune responses in these primatized mice opens the door for extensive experimentation in a proven translationally-relevant species, which will allow for more efficient and precise large animal studies and subsequent human clinical implementation.
Figure 6. NHP Mice Immune Cell Function.
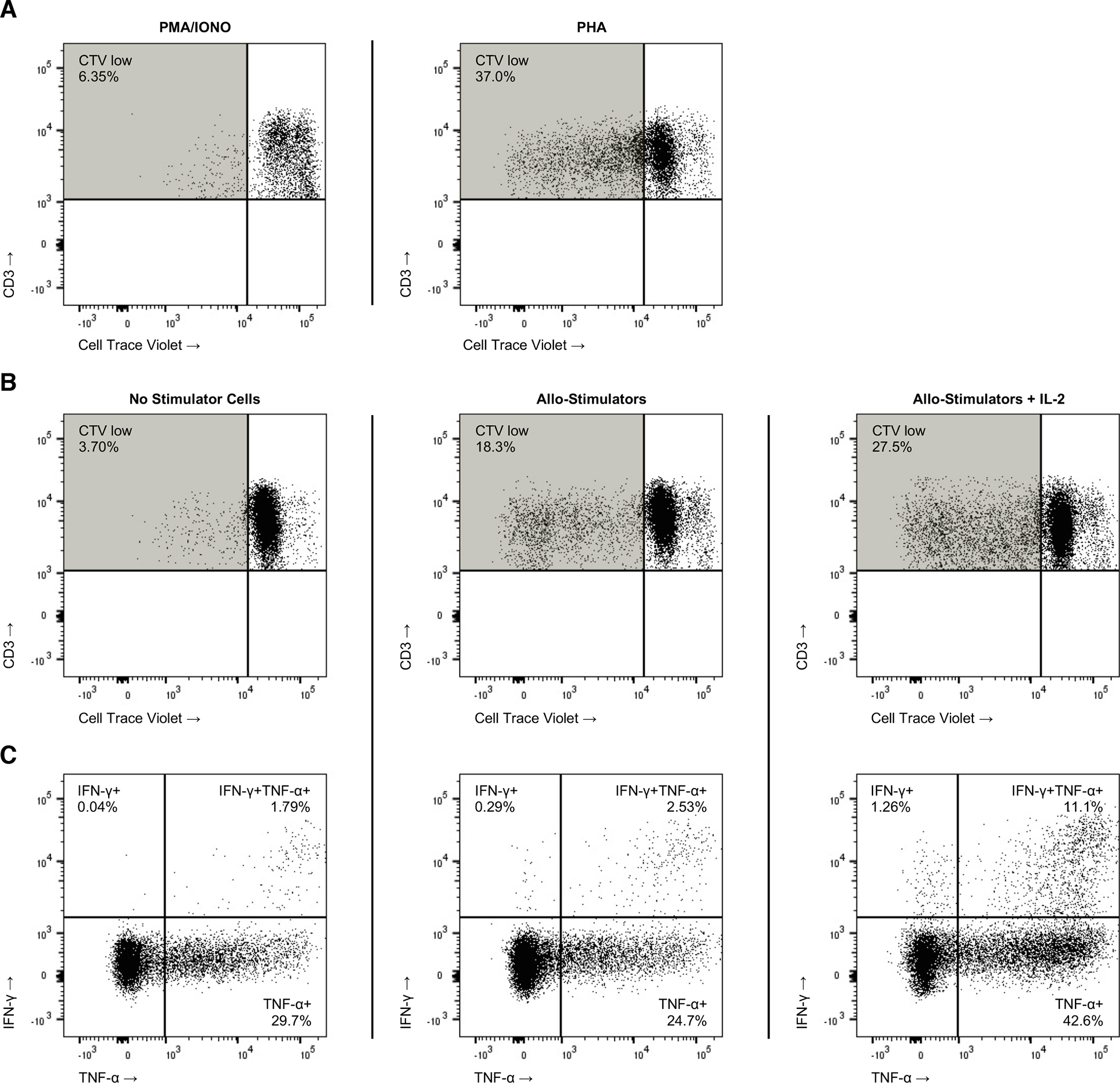
Splenocytes from primary NHP mice were harvested, labeled with Cell Trace Violet (CTV), and then stimulated with 10x PMA/Ionomycin or PHA (10ug/ml), which (A) resulted in robust T cell proliferation [CTV(lo)] in culture. A mixed lymphocyte reaction (MLR) was performed with isolated NHP mouse splenocytes mixed in a 2:1 dilution with counter-labeled, irradiated adult NHP PBMC (B) yielding multiple cycles of in vitro T cell proliferation in response to allogenic stimulation; IL-2 (100ng) was added for one condition as an additional inflammatory stimulus present in the post-transplantation microenvironment. At the termination of the MLR, cells were additionally assayed (C) by intracellular flow cytometry for interferon gamma (IFNγ) and tumor necrosis factor alpha (TNFα) production.
To conclude, we successfully established a durable primatized mouse model by transplanting fetal liver-derived CD34+ HSPCs and thymic fragments into a novel and commercially available transgenic murine host. Although there have been previous reports of mouse primatization, this represents the first model with robust multilineage chimerism and immune reconstitution within multiple physiologic compartments, mirroring the donor primate. This model is well-poised for multiple applications within the fields of stem cell biology, developmental immunology, virology, and transplant immunology, all of which currently rely on NHP models. The primatized mouse can be leveraged as an efficient, cost-effective mechanism to inform implementation of novel therapeutic or diagnostic NHP studies, thus minimizing the risk and logistical burden of large animal models. Further, creation of primatized mice with other NHP species (e.g., cynomolgus macaques), and/or utilization of neonatal tissues for primatization of NeoThy (non-fetal, neonatal tissue) animals will enable novel studies of species-specific immune responses and ontogeny-associated immune cell development and function. By accelerating the pace of NHP studies through the utilization of this primatized model, we hope that impactful and potentially life-saving approaches can be more rapidly translated to the bedside, thus more-efficiently advancing the field of clinical medicine.
Supplementary Material
Acknowledgments
We thank Mr. Logan Vosler and Kim Weisgrau for their technical assistance, and Heidi F. Little for scientific illustration. NSG-SGM3 mice were a generous gift from Leonard Shultz at the Jackson Laboratory. NOG-EXL mice were provided by Taconic Biosciences. This study utilized animals and tissues from the Wisconsin National Primate Research Center (WNPRC) and was supported in part by NCRR grant P51OD011106 and P30CA014520. This work was supported by pilot funding from the WNPRC and the University of Wisconsin-Madison (UW) Institute for Clinical and Translational Research (NCATS CTSA grant Ul1TR002373), the Wisconsin Alumni Research Foundation, NIH NIAID 75N93021C00004 and NIH NHLBI U01HL134764 to M.E.B., NIH NIAID T32AI125231 award to C.J.L., NIH R21 HD099576 and R01 HD103443 to T.G.G., NIH NHLBI U01HL134655 to J.A.T., the UW Carbone Cancer Center Support Grant P30 CA014520. The authors thank Dana Maya for manuscript editing assistance, and the UW Department of Surgery Histology Core Lab, and certified Histotechnician Sierra Raglin HTL (ASCP) for histological processing staining, in addition to Dr. Susan Thibeault PhD, CCC-SLP, PI of the DOS Histology Core, and Lab Supervisor, Sara Dutton Sackett, PhD.
Abbreviations
- AMb
Adult mobilized blood
- BM
Bone marrow
- BLT
Bone marrow, liver, thymus
- CCR7
C-C chemokine receptor type 7
- CTFR
Cell Trace Far Red
- CTV
Cell Trace Violet
- FACS
Flow cytometry staining buffer
- GM-CSF
Granulocyte-macrophage colony-stimulating factor
- HSCT
Hematopoietic stem cell transplant
- HSPC
Hematopoietic stem and progenitor cells
- IFN
Interferon
- IL
Interleukin
- IV
Intravenous
- MDC
Macrophage-derived chemokine
- MFI
Mean fluorescence intensity
- MHC
Major histocompatibility complex
- MISTRG
Mouse strain [C;129S4-Rag2tm1.1FlvCsf1tm1(CSF1)FlvCsf2/Il3tm1.1(CSF2,IL3)Flv Thpotm1.1(TPO)FlvIl2rgtm1.1FlvTg(SIRPA)1Flv/J]
- MLR
Mixed lymphocyte reaction
- NBSGW
Mouse strain [NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ (NSG) crossed with C57BL/6J-KitW−41J/J (C57BL/6.KitW41)]
- NeoThy
Neonatal thymus
- NHP
Non-human primate
- NK
Natural killer cells
- NOG-EXL
Mouse strain [NOD.Cg-Prkdcscid Il2rgtm1Sug Tg(SV40/HTLV-IL3,CSF2)10–7Jic/JicTac]
- NSG
Mouse strain [NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ]
- NSG-SGM3
Mouse strain [NOD.Cg-PrkdcscidIl2rgtm1WjlTg(CMV-IL3,CSF2,KITLG)1Eav/ MloySzJ]
- PBMC
Peripheral blood mononuclear cells
- PHA
Phytohemagglutinin-L
- PMA
Phorbol 12-myristate-13-acetate
- RANTES
Regulated upon activation, normal T cell expressed and presumably secreted
- SCID
Severe combined immunodeficient mice
- TNFα
Tumor necrosis factor alpha
Footnotes
Conflict of Interest Disclosure
M.E.B is a consultant for Taconic Biosciences; the other authors declare no conflict of interest.
References
- 1.Kamel-Reid S and Dick J (1988) Engraftment of immune-deficient mice with human hematopoietic stem cells. Science 242, 1706–1709. [DOI] [PubMed] [Google Scholar]
- 2.Mold JE, Venkatasubrahmanyam S, Burt TD, Michaëlsson J, Rivera JM, Galkina SA, Weinberg K, Stoddart CA, McCune JM (2010) Fetal and Adult Hematopoietic Stem Cells Give Rise to Distinct T Cell Lineages in Humans. Science 330, 1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baenziger S, Tussiwand R, Schlaepfer E, Mazzucchelli L, Heikenwalder M, Kurrer MO, Behnke S, Frey J, Oxenius A, Joller H, Aguzzi A, Manz MG, Speck RF (2006) Disseminated and sustained HIV infection in CD34+ cord blood cell-transplanted Rag2−/−gamma c−/− mice. Proc Natl Acad Sci U S A 103, 15951–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maufort JP, Israel JS, Brown ME, Kempton SJ, Albano NJ, Zeng W, Kelnhofer LE, Reynolds MR, Perrin ES, Sanchez RJ, Sluvkin II, Thomson JA, Poore SO (2019) Major Histocompatibility Complex-Matched Arteries Have Similar Patency to Autologous Arteries in a Mauritian Cynomolgus Macaque Major Histocompatibility Complex-Defined Transplant Model. J Am Heart Assoc 8, e012135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Melkus MW, Estes JD, Padgett-Thomas A, Gatlin J, Denton PW, Othieno FA, Wege AK, Haase AT, Garcia JV (2006) Humanized mice mount specific adaptive and innate immune responses to EBV and TSST-1. Nat Med 12, 1316–22. [DOI] [PubMed] [Google Scholar]
- 6.Lan P, Tonomura N, Shimizu A, Wang S, Yang YG (2006) Reconstitution of a functional human immune system in immunodeficient mice through combined human fetal thymus/liver and CD34+ cell transplantation. Blood 108, 487–92. [DOI] [PubMed] [Google Scholar]
- 7.Brown ME, Zhou Y, McIntosh BE, Norman IG, Lou HE, Biermann M, Sullivan JA, Kamp TJ, Thomson JA, Anagnostopoulos PV, Burlingham WJ (2018) A Humanized Mouse Model Generated Using Surplus Neonatal Tissue. Stem Cell Reports 10, 1175–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Binhazim AA, Rizvi TA, Coghlan LG, Lew K, Schmidt R, Wong PK (1996) Rhesus thymic/liver xenografts in severe combined immunodeficient mice: immunologic reconstitution and intrathymic infection with simian immunodeficiency virus. Lab Invest 75, 339–48. [PubMed] [Google Scholar]
- 9.Radtke S, Chan Y-Y, Sippel TR, Kiem H-P, Rongvaux A (2019) MISTRG mice support engraftment and assessment of nonhuman primate hematopoietic stem and progenitor cells. Experimental Hematology 70, 31–41.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horn PA, Thomasson BM, Wood BL, Andrews RG, Morris JC, Kiem HP (2003) Distinct hematopoietic stem/progenitor cell populations are responsible for repopulating NOD/SCID mice compared with nonhuman primates. Blood 102, 4329–35. [DOI] [PubMed] [Google Scholar]
- 11.Soumeya A, Alisa T, Porntip C, In Hyun P, Alice P, Aïssa B, Lucie T, Edouard De D, Anais P, Marine G-L, Francis R, Stéphane P, Gerard T, Suthat F, George QD, Emmanuel P, Stany C, Philippe L, Leïla M-C (2015) Transplantation of Macaca cynomolgus iPS-derived hematopoietic cells in NSG immunodeficient mice. Haematologica 100, e428–e431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rongvaux A, Willinger T, Martinek J, Strowig T, Gearty SV, Teichmann LL, Saito Y, Marches F, Halene S, Palucka AK, Manz MG, Flavell RA (2014) Development and function of human innate immune cells in a humanized mouse model. Nat Biotechnol 32, 364–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalscheuer H, Danzl N, Onoe T, Faust T, Winchester R, Goland R, Greenberg E, Spitzer TR, Savage DG, Tahara H, Choi G, Yang Y-G, Sykes M (2012) A Model for Personalized in Vivo Analysis of Human Immune Responsiveness. Science Translational Medicine 4, 125ra30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McIntosh BE, Brown ME, Duffin BM, Maufort JP, Vereide DT, Slukvin II, Thomson JA (2015) Nonirradiated NOD,B6.SCID Il2rγ−/− Kit(W41/W41) (NBSGW) mice support multilineage engraftment of human hematopoietic cells. Stem Cell Reports 4, 171–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pearson T, Greiner DL, Shultz LD (2008) Creation of “humanized” mice to study human immunity. Curr Protoc Immunol Chapter 15, Unit-15.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu AG, Michejda M, Mazumder A, Meehan KR, Menendez FA, Tchabo JG, Slack R, Johnson MP, Bellanti JA (1999) Analysis and characterization of hematopoietic progenitor cells from fetal bone marrow, adult bone marrow, peripheral blood, and cord blood. Pediatr Res 46, 163–9. [DOI] [PubMed] [Google Scholar]
- 17.DiGiusto DL, Lee R, Moon J, Moss K, O’Toole T, Voytovich A, Webster D, Mule JJ (1996) Hematopoietic potential of cryopreserved and ex vivo manipulated umbilical cord blood progenitor cells evaluated in vitro and in vivo. Blood 87, 1261–71. [PubMed] [Google Scholar]
- 18.Radtke S, Adair JE, Giese MA, Chan Y-Y, Norgaard ZK, Enstrom M, Haworth KG, Schefter LE, Kiem H-P (2017) A distinct hematopoietic stem cell population for rapid multilineage engraftment in nonhuman primates. Science Translational Medicine 9, eaan1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calderón L and Boehm T (2011) Three chemokine receptors cooperatively regulate homing of hematopoietic progenitors to the embryonic mouse thymus. Proc Natl Acad Sci U S A 108, 7517–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brehm MA, Aryee K-E, Bruzenksi L, Greiner DL, Shultz LD, Keck J (2018) Transgenic expression of human IL15 in NOD-<em>scid IL2rg</em><em><sup>null</sup></em> (NSG) mice enhances the development and survival of functional human NK cells. The Journal of Immunology 200, 103.20. [Google Scholar]
- 21.Vaidya S, St. Louis P, Burzenski L, Greiner DL, Brehm MA, Shultz LD (2020) Enhanced development of functional human innate immune cells in a novel mouse FLT3<sup>null</sup> NSG mouse strain expressing human FLT3L. The Journal of Immunology 204, 223.24. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


