Abstract
The attack by the bph-encoded biphenyl dioxygenase of Burkholderia sp. strain LB400 on a number of symmetrical ortho-substituted biphenyls or quasi ortho-substituted biphenyl analogues has been investigated. 2,2′-Difluoro-, 2,2′-dibromo-, 2,2′-dinitro-, and 2,2′-dihydroxybiphenyl were accepted as substrates. Dioxygenation of all of these compounds showed a strong preference for the semisubstituted pair of vicinal ortho and meta carbons, leading to the formation of 2′-substituted 2,3-dihydroxybiphenyls by subsequent elimination of HX (X = F, Br, NO2, or OH). All of these products were further metabolized by 2,3-dihydroxybiphenyl 1,2-dioxygenases of Burkholderia sp. strain LB400 or of Rhodococcus globerulus P6. Dibenzofuran and dibenzodioxin, which may be regarded as analogues of doubly ortho-substituted biphenyls or diphenylethers, respectively, were attacked at the “quasi ortho” carbon (the angular position 4a) and its neighbor. This shows that an aromatic ring-hydroxylating dioxygenase of class IIB is able to attack angular carbons. The catechols formed, 2,3,2′-trihydroxybiphenyl and 2,3,2′-trihydroxydiphenylether, were further metabolized by 2,3-dihydroxybiphenyl 1,2-dioxygenase. While angular attack by the biphenyl dioxygenase was the main route of dibenzodioxin oxidation, lateral dioxygenation leading to dihydrodiols was the major reaction with dibenzofuran. These results indicate that this enzyme is capable of hydroxylating ortho or angular carbons carrying a variety of substituents which exert electron-withdrawing inductive effects. They also support the view that the conversions of phenols into catechols by ring-hydroxylating dioxygenases, such as the transformation of 2,2′-dihydroxybiphenyl into 2,3,2′-trihydroxybiphenyl, are the results of di- rather than of monooxygenations. Lateral dioxygenation of dibenzofuran and subsequent dehydrogenation and extradiol dioxygenation by a number of biphenyl-degrading strains yielded intensely colored dead-end products. Thus, dibenzofuran can be a useful chromogenic indicator for the activity of the first three enzymes of biphenyl catabolic pathways.
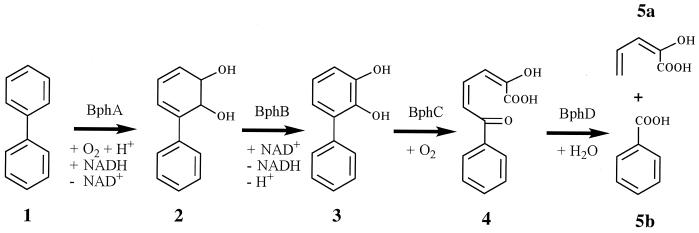
Ring-hydroxylating dioxygenases are key enzymes of the aerobic bacterial catabolism of aromatic compounds. Generally, this family of enzymes has been shown to be quite versatile with respect to the substrates accepted as well as to the type of reactions catalyzed (11, 28). The prototype reaction supported is the addition of two hydroxy groups to vicinal carbons of aromatic rings which, usually via a subsequent dehydrogenation, leads to the formation of catechols (Fig. 1). The hydroxy groups enable and direct fission of the aromatic ring by extra- or intradiol dioxygenases which cleave the carbon-carbon bonds either between or adjacent to these substituents (Fig. 1). We previously characterized the dioxygenations of chlorinated biphenyls (Cl-Bs) as catalyzed by two bacterial enzymes, the bph-encoded biphenyl dioxygenases (BphAs) of Burkholderia sp. strain LB400 and of Rhodococcus globerulus P6 (26, 33, 35). In the course of those studies, we found that the enzyme of strain LB400 is able to attack chlorinated ortho carbons of some biphenyls, an observation also made by other investigators (21, 33, 35). This specific type of attack, leading to elimination of the ortho chlorine, is a theoretically interesting and practically useful property of the enzyme which facilitates further microbial degradation of chlorinated substrates by reducing the toxicity, increasing the aqueous solubility, and enhancing the enzymatic turnover of metabolites. This led us to investigate whether the enzyme is able to catalyze the same type of dioxygenation with other ortho-substituted biphenyls and with other aromatic compounds which can be regarded as quasi ortho-substituted biphenyl analogues.
FIG. 1.
Attack on an aromatic compound by a ring-hydroxylating dioxygenase and subsequent catabolic reactions, exemplified by biphenyl (e.g., see references 1, 6, 14, 20, and 25). Enzymes; BphA, biphenyl 2,3-dioxygenase; BphB, biphenyl-2,3-dihydrodiol 2,3-dehydrogenase; BphC, 2,3-dihydroxybiphenyl 1,2-dioxygenase; BphD, 2-hydroxy-6-oxo-6-phenylhexa-2,4-dienoate hydrolase. Compounds: 1, biphenyl; 2, biphenyl-2,3-dihydrodiol; 3, 2,3-dihydroxybiphenyl; 4, 2-hydroxy-6-oxo-6-phenylhexa-2,4-dienoic acid; 5a, 2-hydroxypenta-2,4-dienoic acid; 5b, benzoic acid.
MATERIALS AND METHODS
Chemicals.
Halogenated biphenyls (>98% purity) were obtained from Lancaster Synthesis (White Lund, Morecambe, England), Promochem (Wesel, Germany) or Restek (Sulzbach, Germany). 2,2′-Dinitro-biphenyl [2,2′-di(NO2)-B] (>99% purity), 2,2′-dihydroxybiphenyl [2,2′-di(OH)-B] (>99% purity), and dibenzofuran (DBF) (>99% purity) were obtained from Merck, Fluka, and Aldrich, respectively. Dibenzo-p-dioxin (DBD) (Promochem) and 2,3,2′-trihydroxybiphenyl [2,3,2′-tri(OH)-B] were of >99 and >95% purity and were kindly provided by D. Pieper.
Bacterial strains, plasmids, and culture conditions.
The Escherichia coli strains used in this study were BL21(DE3)/pLysS (40) containing either pAIA111, pAIA15, or pAIA50 and MV1190 (41) harboring pJA94. pAIA111 contains bphA1A2A3A4, pAIA15 harbors bphC, and pAIA50 carries bphA1A2A3A4BC of Burkholderia sp. strain LB400. pJA94 contains bphC2 of R. globerulus P6. The constructions of pAIA111 (26), pAIA15 (35), pAIA50 (34), and pJA94 (3) have been described previously. Bacteria were grown in Luria-Bertani medium (30) at 37°C unless otherwise indicated. If appropriate, chloramphenicol and/or ampicillin at a concentration of 20 or 50 μg/ml, respectively, was used for selection.
Preparation of resting cells.
Preparation of resting cells was carried out as previously described (34).
Degradation of aromatic compounds by BphA and analysis of products.
Resting cell suspensions (1 ml; optical density at 600 nm [OD600] = 15) of E. coli BL21(DE3)/pLysS/pAIA111 were incubated on a rotary shaker with nominal substrate concentrations of either 1 mM [2,2′-di(NO2)-B, 2,2′-di(OH)-B, DBF, DBD] or 2 mM (2,2′-difluorobiphenyl, 2,2′-dichlorobiphenyl, 2,2′-dibromobiphenyl [2,2′-diF-B, 2,2′-diCl-B, 2,2′-diBr-B, respectively]) for 6 h at 30°C. The reaction mixtures were extracted with an equal volume of ethyl acetate. The organic layer was reextracted with 1 volume of 50 mM sodium phosphate buffer, pH 7.5, and dried over magnesium sulfate. To obtain butylboronate derivatives, the solvent was removed from 200 μl of extract, and the residue was redissolved in 80 μl of acetone. Twenty milliliters of a 2-mg/ml solution of n-butylboronic acid in acetone was added, and the solution was incubated at 50°C for 10 min (22). To obtain trimethylsilyl (TMS) derivatives, the solvent was removed from 100 μl of extract, the residue was redissolved in 50 μl of N,O-bis-trimethylsilyl-trifluoroacetamide and trimethylchlorosilane (99:1 [vol/vol]), and the solution was incubated at 70°C for 30 min. After derivatization, mixtures were evaporated to dryness under a stream of nitrogen and dissolved in 10 μl of n-octane or cyclohexane. Samples (1 μl) were injected in the splitless mode into a gas chromatography-mass spectrometry (GC-MS) system: either a Hewlett-Packard 5890 series II gas chromatograph with an Rtx1 column (Restek, Bellefonte, Pa.) coupled to a Hewlett-Packard 5989 mass spectrometer, a Shimadzu GC-17A gas chromatograph with an XTI-5 column (Restek) coupled to a Shimadzu QP-5000 mass spectrometer, or a Fisons 8060 series 8000 gas chromatograph with a BPX5 column (SGE, Austin, Tex.) coupled to a Fisons Trio 1000 mass spectrometer. The carrier gas was helium. Mass spectrometers were operated in the electron ionization mode.
HPLC-UV analysis of metabolites.
High-performance liquid chromatography (HPLC)-UV analysis was carried out as previously described (34).
Degradation of aromatic compounds by BphA, BphB, and BphC or by BphA and BphC and analysis of products.
Resting cell suspensions (1 ml; OD600 = 15) of E. coli BL21(DE3)/pLysS/pAIA50 were incubated for 6 h as described above with a 125 μM nominal concentration of substrate. In the experiments without BphB, resting cell suspensions (1 ml; OD600 = 20) of E. coli BL21(DE3)/pLysS/pAIA111 were first incubated for 6 h with a nominal substrate concentration of 150 μM [2,2′-di(NO2)-B, 2,2′-di(OH)-B, DBF, DBD] or 300 μM (2,2′-diF-B, 2,2′-diCl-B, 2,2′-diBr-B). Subsequently, they were further incubated with 2 μl of a crude extract of E. coli MV1190 harboring pJA94 (kindly provided by M. Prucha) or with 1 volume of a resting cell suspension (1 ml; OD600 = 20) of E. coli BL21(DE3)/pLysS/pAIA15. The formation of meta cleavage products (MCPs) was monitored at intervals between 1 min and 6 h by UV-visible spectral scanning of the assay mixtures with a Beckman model DU-70 spectrophotometer.
RESULTS AND DISCUSSION
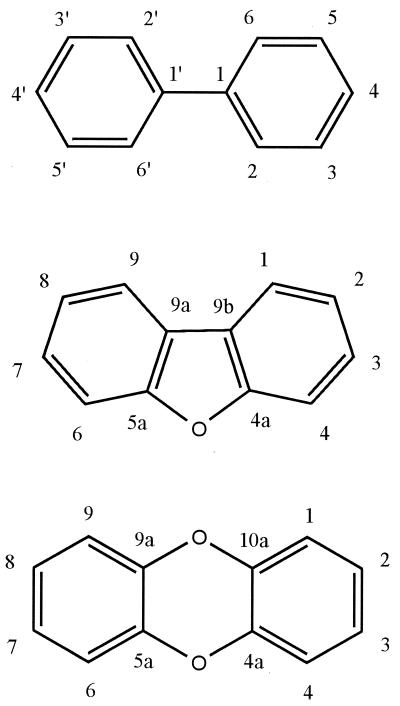
The structural formulas of the cores of the investigated compounds are shown in Fig. 2. Incubations of substrates were carried out with resting recombinant E. coli cells synthesizing BphA of Burkholderia sp. strain LB400. The supernatants of these incubations were extracted with ethyl acetate, and the products were analyzed by GC-MS. 2,2′-Substituted biphenyls were used as substrates to make sure that attack by BphA, if any, is directed towards the ortho-substituted ring.
FIG. 2.
Structural formulas and carbon atom numbering of biphenyl (top), DBF (middle), and DBD (bottom).
Halogenated ortho carbons.
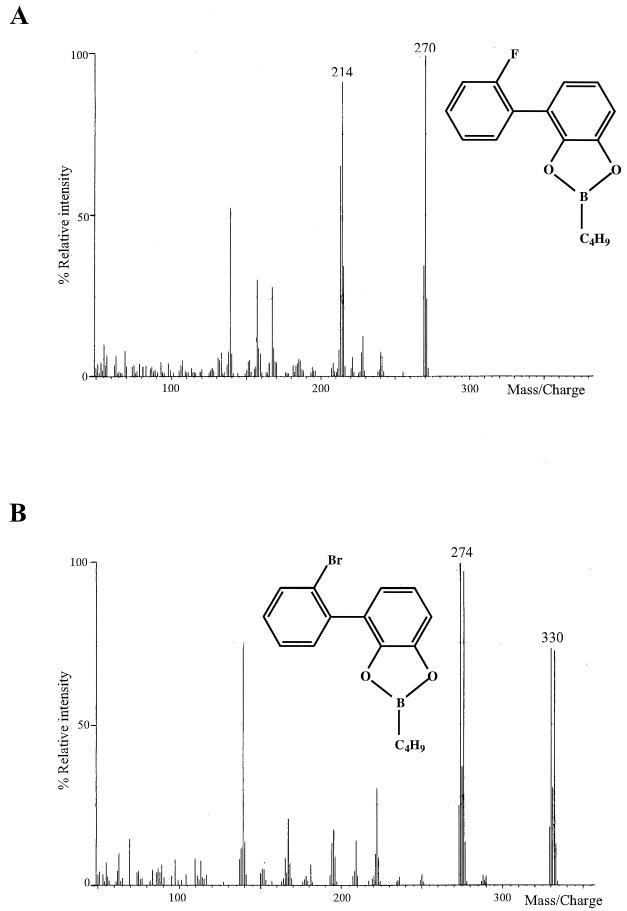
As previously shown for 2,2′-diCl-B (21, 33), 2,2′-diF- and 2,2′-diBr-B were also attacked by BphA. The iodinated analogue was not available. Dioxygenation products were analyzed after derivatization with the bifunctional reagent n-butylboronic acid (Table 1). In each case one main metabolite was detected. The mass spectra of the n-butylboronates of these products are shown in Fig. 3. For steric reasons, these cyclic esters can only be formed with vicinal hydroxy groups. The molecular ions and the fragment ions (M-56)+ (loss of C4H8) were the only prominent positively charged species, indicating the formation of catechols (22). In accordance with this, the loss of one of the substrate halogens was clearly visible. Independent evidence for catechol formation was obtained by dehydrogenase-independent conversion of the products of the BphA-catalyzed reaction into ring fission compounds by an extradiol-cleaving dihydroxybiphenyl dioxygenase (BphC). The λmax values of the MCPs observed (Table 2) are typical for ortho-substituted 2-hydroxy-6-oxo-6-phenylhexa-2,4-dienoates (34). The specificity of BphCs for ortho, meta-dihydroxybiphenyls, together with the loss of one halogen, indicates initial hydroxylation at ortho and meta carbons at the halogenated side of the ring, yielding 2,3-dihydroxy-2′-halobiphenyls. Formally, the direct product of such a hydroxylation would be a compound that contains a dearomatized ring with a halohydroxy-substituted carbon. Due to these characteristics, such a compound is expected to be chemically highly unstable and to undergo one or both of two possible eliminations. These reactions could take place either simultaneously with dioxygenation or briefly thereafter, in the latter case giving rise to a short-lived reaction intermediate. Without participation of the enzyme, an elimination of hydrogen halogenide is expected, as (near neutral pH) a halide ion represents a better leaving group than a hydroxy ion. However, in the case of involvement of the enzyme, an elimination of water could be the preferred reaction. Both reaction pathways regenerate the aromatic system, the first yielding a 2,3-dihydroxylated ring, the second leading to a 2-halo-3-hydroxy-substituted ring. In GC-MS analyses employing derivatization with a bifunctional reagent, the latter type of compound will probably not be detected. Studies using monofunctional derivatization indicated a strong preference for the first type of elimination, as only catechols were detected as reaction products (19, 29, 33).
TABLE 1.
Catabolites formed by BphA from halogenated biphenyls and analysis of their n-butylboronates by GC-MS
| Substratea | Products
|
|||
|---|---|---|---|---|
| tr (min)b | Relative yieldc (%) | Prominent ionsd (m/z) | Type of compound | |
| 2,2′-DiF-B | 19.5 | 10–15 | 290 | 2,2′-DiF-B-5,6-DHDe |
| 290-68 | ||||
| 20.7 | 85–90 | 270 | 2,3-Di(OH)-2′-F-B | |
| 270-56 | ||||
| 2,2′-DiCl-B | 22.6 | 100 | 286 | 2,3-Di(OH)-2′-Cl-B |
| 286-56 | ||||
| 2,2′-DiBr-B | 23.7 | 100 | 330 | 2,3-Di(OH)-2′-Br-B |
| 330-56 | ||||
B, biphenyl.
tr values refer to the XTI-5 column (see Materials and Methods).
Deduced from total ion chromatogram peak areas.
Ions known to be typical for the type of compound given are underlined.
Tentative assignment of hydroxylated carbons.
FIG. 3.
Mass spectra of n-butylboronate derivatives of dioxygenation products. Major metabolites of 2,2′-difluoro- (A) and 2,2′-dibromo-biphenyl (B) are shown. The assigned structures are shown as inserts.
TABLE 2.
Electron spectroscopic characteristics of meta ring cleavage products formed from different ortho-substituted biphenyls by the combined actions of BphA and BphC
| Substrate | Characteristics of products
|
|
|---|---|---|
| λmax (nm) | Amaxa | |
| 2,2′-DiF-Bb | 396d | 1.5d |
| 2,2′-DiCl-Bb | 392d | 1.3d |
| 2,2′-DiBr-Bb | 392d | 0.9d |
| 2,2′-Di(NO2)-Bc | 388e | 1.3e |
Amax, absorption at λmax.
Nominal substrate concentration of 300 μM; B, biphenyl.
Nominal substrate concentration of 150 μM.
Products formed by BphA were incubated for 5 min with extracts of E. coli cells containing BphC2 of R. globerulus P6.
Products formed by BphA were incubated for 5 min with E. coli cells containing BphC of Burkholderia sp. strain LB400.
With 2,2′-diF-B as substrate, a compound yielding a mass spectrum consistent with that of a difluorinated dihydrodiol (DHD) was detected as a minor product (Table 1). Presumably, this metabolite was formed by dioxygenation at ortho and meta carbons 5 and 6. In any case, the results indicate a general strong preference for the dioxygenation of ortho and meta carbons at the substituted side of the ortho-halogenated ring.
Nitrogenated ortho carbons.
Potential products of 2,2′-di(NO2)-B were analyzed after derivatization with the monofunctional reagent trimethylchlorosilane. Essentially, only a single product was observed (Table 3). Its mass spectrum indicated formation of a di(OH)-mono(NO2)-B. Direct formation of an MCP upon incubation with BphC (Table 2) provided independent evidence for catechol formation. Following the arguments outlined above, the catechol was identified as 2,3-(diOH)-2′-(NO2)-B.
TABLE 3.
Catabolites formed by BphA from different substituted biphenyls and structurally related compounds and analysis of their TMS derivatives by GC-MS
| Substrate | Products
|
||||
|---|---|---|---|---|---|
| No. | tr (min)b | Relative yieldc (%) | Prominent ionsd (m/z) (relative abundance [% base peak]) | Type of compound | |
| 2,2′-Di(NO2)-Ba | 1 | 25.4 | 100 | 375 (M+, 40), 360 (3), 270 (51), 242 (5), 146 (8), 73 (100) | 2,3-Di(OH)-2′-(NO2)-B |
| 2,2′-Di(OH)-B | 1 | 23.2 | 10–20 | 418 (M+, 16), 315 (24), 299 (2), 73 (100) | Tri(OH)-B |
| 2 | 23.3 | 80–90 | 418 (M+, 13), 403 (3), 315 (51), 299 (4), 73 (100) | 2,3,2′-Tri(OH)-B | |
| DBF | 1 | 22.2 | 2–5 | 256 (M+, 100), 241 (100), 225 (84), 73 (18) | Mono(OH)-DBF |
| 2 | 22.7 | 5–10 | 256 (M+, 69), 241 (100), 225 (6), 73 (19) | Mono(OH)-DBF | |
| 3 | 23.3 | 10–15 | 418 (M+, 11), 403 (1), 315 (34), 299 (2), 73 (100) | 2,3,2′-Tri(OH)-B | |
| 4 | 23.5 | 55–65 | 346 (M+, 34), 256 (10), 241 (12), 184 (12), 168 (15), 156 (25), 147 (34), 139 (15), 73 (100) | DBF-1,2-DHDe | |
| 5 | 23.6 | 10–20 | 346 (M+, 19), 315 (3), 256 (3), 241 (14), 184 (15), 168 (12), 156 (11), 147 (21), 139 (12), 73 (100) | DBF-3,4-DHDe | |
| DBD | 1 | 24.5 | 85–95 | 434 (M+, 12), 419 (2), 331 (35), 315 (3), 73 (100) | 2,3,2′-Tri(OH)-DPE |
| 2 | 24.9 | 5–15 | 362 (M+, 10), 272 (23), 257 (33), 166 (40), 73 (100) | DBD-1,2-DHDe | |
B, biphenyl.
tr values refer to the BPX5 column (see Materials and Methods).
Deduced from total ion chromatogram peak areas. Minor peaks are neglected.
Ions known to be typical for the type of compound given are underlined.
Tentative assignment of hydroxylated carbons.
Oxygenated ortho carbons.
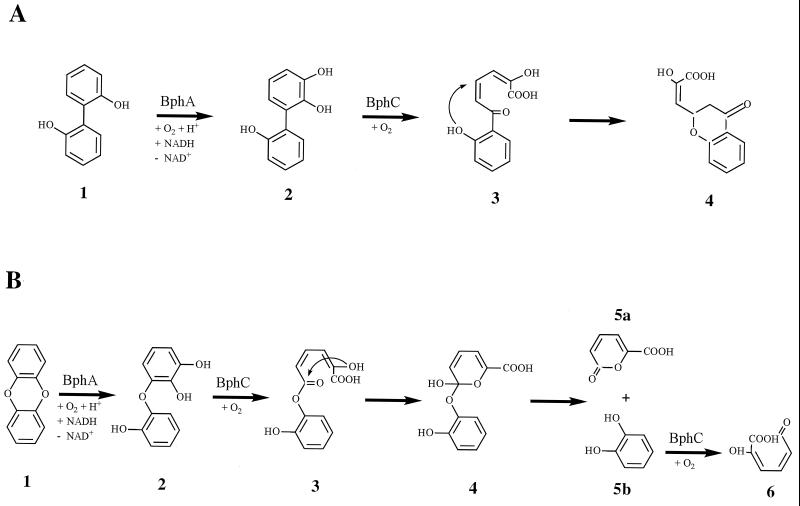
Two dioxygenation products were found with 2,2′-di(OH)-B (Table 3). A tri(OH)-B, as identified by GC-MS, was formed as a major metabolite. The retention time (tr) and mass spectrum of this metabolite (a TMS derivative) were identical to those of an authentic reference of 2,3,2′-tri(OH)-B (not shown). This compound has been shown to be cleaved by the BphC of Pseudomonas sp. strain HBP1 (23). Furthermore, it represents an analogue of 2,3-di(OH)-2′-Cl-B, which is accepted by the BphC of strain LB400 (33, 34). When the dioxygenation products of 2,2′-di(OH)-B were incubated with an E. coli strain synthesizing this enzyme, the appearance of a yellow coloration, suggesting the formation of an MCP, was transiently observed. It has previously been described (23, 39) that the MCP derived from 2,3,2′-tri(OH)-B is unstable and is further converted into 3-(chroman-4-on-2-yl)-pyruvate by an intramolecular cyclization (Fig. 4A). The formation of this follow-up product was verified by its tr (HPLC) and UV spectrum in comparison with those of the end product formed by ring fission of authentic 2,3,2′-triOH-B (not shown).
FIG. 4.
Formation and further conversion of unstable extradiol cleavage products. Enzymes: BphA, biphenyl 2,3-dioxygenase; BphC, 2,3-dihydroxybiphenyl 1,2-dioxygenase. (A) Formation and further conversion of 2,3,2′-trihydroxybiphenyl from 2,2′-dihydroxybiphenyl. Compounds: 1, 2,2′-dihydroxybiphenyl; 2, 2,3,2′-trihydroxybiphenyl; 3, 2-hydroxy-6-(2-hydroxyphenyl)-6-oxo-hexa-2,4-dienoic acid; 4, 2-hydroxy-3-(4-oxo-chroman-2-yl)-acrylic acid. (B) Formation and further conversion of 2,3,2′-trihydroxydiphenylether from DBD. Compounds: 1, DBD; 2, 2,3,2′-trihydroxydiphenylether; 3, 2-hydroxy-hexa-2,4-diene-dioic acid 6-(2-hydroxyphenyl) ester; 4, 6-hydroxy-6-(2-hydroxyphenoxy)-6H-pyran-2-carboxylic acid; 5a, 2-pyrone-6-carboxylic acid; 5b, catechol; 6, 2-hydroxymuconic semialdehyde.
A second tri(OH)-B was observed as a minor product of 2,2′-di(OH)-B (Table 3). It may have been generated by dioxygenation at unsubstituted vicinal carbons (presumably positions 5 and 6), yielding a DHD, followed by the elimination of water or TMS-OH, respectively. DHDs are more stable than compounds with dihydroxylated carbons (hydrated carbonyl compounds) but also tend to undergo elimination reactions that reform the energetically favorable aromatic system (8). Such eliminations may occur during metabolite extraction, particularly under acidic conditions (8), during derivatization, or during GC. However, we note that traces of the respective precursor DHDs were not detected.
DBF may be regarded as a biphenyl derivative in which carbons 2 and 2′ are oxygenated in a specific way, namely by formation of an ether bridge. This has two important steric consequences. Firstly, it keeps the two benzene rings in a planar conformation. Neither ortho-substituted nor unsubstituted biphenyls possess a planar conformation in solution (16, 32). Secondly, it somewhat distorts the positions of the two rings with respect to the carbon-carbon bond linking them.
The total ion chromatogram obtained after incubation of DBF with BphA showed several products (Table 3). Two of them (no. 4 and 5) yielded mass spectra consistent with DHDs. This was confirmed by HPLC analysis of the underivatized dioxygenation products. The UV spectrum of the major peak (λmax = 228 ± 3 and 308 ± 3 nm, respectively) was identical to literature data on DBF-cis-1,2-DHD (15). The UV spectrum of another peak was similar and thus consistent with a second DHD.
A minor DBF product (no. 3) yielded the mass spectrum of a tri(OH)-B. Only the attack of DBF at carbons 4 and 4a can yield a tri(OH)-B. Stabilization of the initial product by cleavage of the ether bridge would yield such a metabolite, namely 2,3,2′-tri(OH)-B. Indeed, the tr (GC) and mass spectrum of the tri(OH)-B were identical to those of the tri(OH)-B formed from 2,2′-di(OH)-B and authentic 2,3,2′-tri(OH)-B.
Two mono(OH)-DBFs were additionally found as by-products of DBF oxidation. The possibility cannot be excluded that these compounds originate from monooxygenations, but to our knowledge convincing evidence for monooxygenation at an aromatic carbon by this family of enzymes has not yet been provided (see below). Thus, it appears more likely that the mono(OH)-DBFs are dehydration products of the observed DHDs or of dihydroxylation at carbons 4 and 4a. The elimination of water is not expected to be preferred as a spontaneous secondary reaction of the latter dihydroxylation, but a participation of the enzyme could favor this pathway.
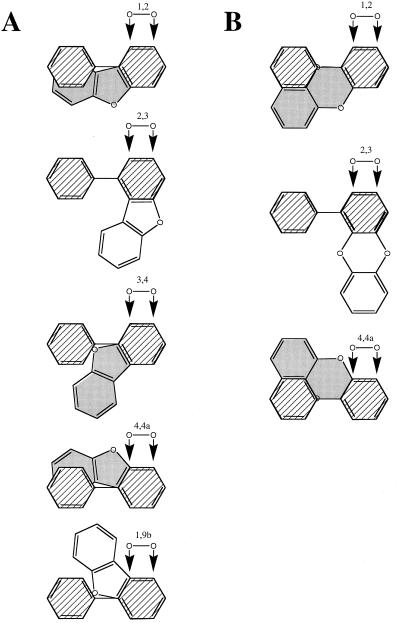
Our results show that the DBF molecule is not preferentially dioxygenated at positions 4 and 4a but that the main product originates from attack at carbons 1 and 2. Electronic factors modulate the reactivities of the different carbons (12). However, the observed high regiospecificities of dioxygenations of molecules such as biphenyl, DBF, and DBD cannot primarily be explained by the relatively small differences in electron densities among different pairs of vicinal carbons. This indicates a predominant role of the steric fit of the compounds into the enzyme's substrate binding pocket. The different discrete positions a DBF molecule must occupy at the BphA active site relative to the activated dioxygen in order to give rise to all theoretically possible dioxygenation products are schematically depicted in Fig. 5A (dioxygenation at the “internal” bonds connecting carbons 4a and 9b or 5a and 9a, respectively, is neglected as, to our knowledge, it has never been described). It is obvious that the two orientations that are most similar to that of a biphenyl molecule (attack at carbons 2 and 3) lead to dioxygenation either at carbons 1 and 2 or at carbons 4 and 4a. As the oxygen appears to enhance the reactivity of carbon 4a (12), the observed preference for the lateral dioxygenation suggests that the respective positioning of the molecule is sterically more favorable. The formation of the third dioxygenation product requires an orientation of the DBF molecule which more strongly deviates from that of the 2,3-dioxygenated biphenyl (Fig. 5A). We note that 2,5,2′,5′-tetraCl-B is 3,4-dioxygenated by this enzyme (21) and that the substrate orientation required for this reaction is similar to those that would yield 2,3- or 3,4-dioxygenation of the DBF molecule.
FIG. 5.
Schematic representation of the various positions a DBF (A) or a DBD (B) molecule must occupy in the BphA active site relative to the activated dioxygen species for dioxygenation of the different vicinal carbons. The numbers of the atoms that would be attacked are indicated. The orientation of a biphenyl molecule (hatched), when dioxygenated at its major site, is shown for comparison. Note that for biphenyl the plane of the nonoxidized ring may rotate relative to the plane of the oxidized ring. DBF and DBD molecules in positions that are probably tolerated and lead to dioxygenations (see text) are shown shaded.
Incubation of DBF with BphA, BphB, and BphC of strain LB400, but not with BphA and BphC alone, yielded an apparently large quantity of one or more stable deep yellow MCPs (λmax = 464 ± 3 nm; Amax = 4.2). BphB and BphC appear to be specific or to have a strong preference for biphenyl metabolites dihydroxylated at ortho and meta carbons (4, 17, 21). As carbons 1 and 2 or 4 and 4a of the DBF molecule are the equivalents of ortho and meta carbons of the biphenyl molecule and as a stable MCP was not formed in the absence of BphB, the deep yellow compound is probably a follow-up product of DBF-1,2-DHD. We note that Selifonov et al. (37) reported an absorption maximum of 470 nm for the MCP of 1,2-di(OH)-DBF, which is close to our value. The deep yellow product was not further converted in the presence of the subsequent pathway enzyme, 2-hydroxy-6-oxo-6-phenylhexa-2,4-dienoate hydrolase or BphD (see Fig. 1). Burkholderia sp. strain LB400 itself and 80% of a number of other natural biphenyl catabolic isolates (5) also converted DBF into this compound which was not further transformed. Thus, DBF may often be useful as a convenient chromogenic indicator for the activity of the first three enzymes of wild-type or recombinant biphenyl degraders in the presence of BphD or the complete catabolic pathway.
In DBD, like in DBF, the planes of the two benzene rings are locked in a planar conformation, but as they are connected via two ether bridges, their positioning relative to one another deviates from the biphenyl molecule more strongly than in DBF (see Fig. 2). DBD was converted into a single major product (Table 3). Its mass spectrum indicated that this compound is a trihydroxylated diphenylether [tri(OH)-DPE]. Thus, the dioxygenolytic attack yielding this metabolite was directed against carbons 4 and 4a, and the product is 2,3,2′-tri(OH)-DPE. A DHD was found as a minor metabolite (Table 3). Sterically, DHD formation by hydroxylation at positions 1 and 2 appears more likely than at positions 2 and 3 (Fig. 5B).
When the DBD reaction mixture produced by BphA was incubated with the BphC of strain LB400, formation of a stable yellow product (λmax = 375 ± 2 nm) indicated meta cleavage. This corroborates catechol formation by BphA and furthermore shows that 2,3,2′-tri(OH)-DPE is a substrate for this BphC. Ring fission is expected to occur between carbons 1 and 2. The resulting metabolite, by analogy with the MCP of 2,3-di(OH)-DPE (27), is expected to be unstable. An intramolecular cyclization followed by an elimination, as described by Pfeifer et al. (27), would yield 2-pyrone-6-carboxylic acid and catechol (Fig. 4B). This carboxylic acid has indeed been identified as a follow-up product of meta fission of 2,3,2′-tri(OH)-DPE (D. Pieper, personal communication). Catechol is known to be cleaved by the BphC of strain LB400 to yield 2-hydroxymuconic semialdehyde (Fig. 4B) (17). The λmax value observed in our experiments is identical to the value for this compound.
Unlike DBF, DBD was preferentially dioxygenated at the angular position. However, product quantities, as deduced from total ion chromatogram peak areas, suggest that the absolute yields of angular dioxygenation were similar with both compounds and that the lateral attack was strongly disfavored with DBD.
Implications.
Bacterial aromatic ring-hydroxylating dioxygenases have been shown to oxidize, in addition to several core compounds, a large number of differently substituted derivatives. This variety of substrates, together with the frequent formation of more than a single product from a given compound, results in over 300 dioxygenase-produced metabolites that are currently known (11). Although unsubstituted vicinal aromatic carbons are the typical target sites for dioxygenation, the reported number of exceptions is continuously increasing. All three possible types of dioxygenative attack of vicinally substituted aromatic rings have been reported, namely dihydroxylation at un-, semi-, and fully substituted carbon pairs. Substituents at carbons that have been shown or postulated to be target sites for dioxygenation include condensed aromatic rings (18); linear (9, 19, 36) and circular aliphatic residues (10); carboxy (19, 29, 42), sulfo (24), hydroxy (19, 38), alkoxy (19), and phenoxy groups (13); halogens (7, 19, 21, 29, 33); and nitro (2) and amino groups (19, 31). If the substituents (X) are good leaving groups, dioxygenation leads to the elimination of HX. This is generally assumed to be a spontaneous reaction, but acid-base catalysis by the enzyme may well facilitate such eliminations. Indeed, arguments outlined below support such a participation for the elimination of water.
Recently, Bressler and Fedorak (12) pointed out that angular dioxygenations seem to be favored by a high electronegativity of one of the neighbors of the angular carbon, suggesting that formation of a negatively charged reaction intermediate is favored by electron-withdrawing groups at this position. This suggests that the same effect could be responsible for the preferential 2,3-dioxygenation of 2-substituted biphenyls investigated in the present study. However, a predominant influence in some cases of steric factors apparently manifests itself in the findings that the strain LB400 dioxygenase preferentially oxidizes the unsubstituted ring of 2-monoCl-B (34) and that biphenyl dioxygenases that share a high degree of sequence similarity with the LB400 enzyme, and therefore are likely to employ the same catalytic mechanism, do not hydroxylate chlorinated carbons of a wide range of Cl-Bs tested (26; our unpublished results).
Possibly not all dioxygenations involving hydroxylated carbons have been recognized as such and have been assumed to be monooxygenations. The net result of such an attack is the conversion of a phenol into a catechol. Compelling evidence has been provided for monooxygenations of a variety of target atoms by aromatic ring-hydroxylating dioxygenases (11, 28). However, to our knowledge, monooxygenation at aromatic carbons by this family of enzymes has never been unequivocally demonstrated. Spain and coworkers (38) showed that a toluene dioxygenase is responsible for the seeming monooxygenation of phenol and derivatives by Pseudomonas putida F1. They investigated this reaction by 18O labeling and found that the original oxygen of the substrate was retained in the resulting catechol. This result, however, still does not rule out the possibility of a dioxygenation if the subsequent elimination of water takes places in a highly stereospecific manner as a result of a protonation mediated by the enzyme. Indeed, these authors favored this interpretation. Our present results strongly support this view. They clearly show that the conversions of differently substituted biphenyls and biphenyl analogues into catechols by the investigated biphenyl dioxygenase take place via dioxygenation. These conversions include hydroxylations of halo-, nitro-, or phenoxy-substituted carbons. By analogy, it appears very likely that hydroxy-substituted carbons are also hydroxylated by the enzyme.
ACKNOWLEDGMENTS
We thank Silke Backhaus, Christian Hesse, Sabine Witt, and María Elena Ortiz for assistance in HPLC-UV and GC-MS analyses, Matthias Prucha for BphC2 of R. globerulus P6, and Dietmar Pieper for gifts of substrates and helpful discussions.
This work was supported by the following grants: DGIP of the Universidad Técnica Federico Santa María, CONICYT and FONDECYT 1990808 to M.S., FONDECYT 7990001 to M.S. and B.H., and BMBF WTZ CHL 001/98 BIO to B.H.
REFERENCES
- 1.Ahmed M, Focht D D. Degradation of polychlorinated biphenyls by two species of Achromobacter. Can J Microbiol. 1973;19:47–52. doi: 10.1139/m73-007. [DOI] [PubMed] [Google Scholar]
- 2.An D, Gibson D T, Spain J C. Oxidative release of nitrite from 2-nitrotoluene by a three-component enzyme system from Pseudomonas sp. strain JS42. J Bacteriol. 1994;176:7462–7467. doi: 10.1128/jb.176.24.7462-7467.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asturias J A, Eltis L D, Prucha M, Timmis K N. Analysis of three 2,3-dihydroxybiphenyl 1,2-dioxygenases found in Rhodococcus globerulus P6. Identification of a new family of extradiol dioxygenases. J Biol Chem. 1994;269:7807–7815. [PubMed] [Google Scholar]
- 4.Barriault D, Vedadi M, Powlowski J, Sylvestre M. cis-2,3-dihydro-2,3-dihydroxybiphenyl dehydrogenase and cis-1,2-dihydro-1,2-dihydroxynaphthalene dehydrogenase catalyze dehydrogenation of the same range of substrates. Biochem Biophys Res Commun. 1999;260:181–187. doi: 10.1006/bbrc.1999.0706. [DOI] [PubMed] [Google Scholar]
- 5.Bartels F, Backhaus S, Moore E R B, Timmis K N, Hofer B. Occurrence and expression of glutathione S-transferase-encoding bphK genes in Burkholderia sp. strain LB400 and other biphenyl-utilizing bacteria. Microbiology. 1999;145:2821–2834. doi: 10.1099/00221287-145-10-2821. [DOI] [PubMed] [Google Scholar]
- 6.Bedard D L, Haberl M L. Influence of chlorine substitution pattern on the degradation of polychlorinated biphenyls by eight bacterial strains. Microb Ecol. 1990;20:87–102. doi: 10.1007/BF02543870. [DOI] [PubMed] [Google Scholar]
- 7.Beil S, Happe B, Timmis K N, Pieper D H. Genetic and biochemical characterization of the broad spectrum chlorobenzene dioxygenase from Burkholderia sp. strain PS12: dechlorination of 1,2,4,5-tetrachlorobenzene. Eur J Biochem. 1997;247:190–199. doi: 10.1111/j.1432-1033.1997.00190.x. [DOI] [PubMed] [Google Scholar]
- 8.Boyd D R, Blacker J, Byrne B, Dalton H, Hand M V, Kelly S C, More O'Ferrall R A, Nagaraja Rao S, Sharma N D, Sheldrake G N. Acid-catalysed aromatisation of benzene cis-1,2-dihydrodiols: a carbocation transition state poorly stabilised by resonance. J Chem Soc Chem Commun. 1994;1994:313–314. [Google Scholar]
- 9.Boyd D R, Sharma N D, Brannigan I N, Haughey S A, Malone J F, Clarke D A, Dalton H. Dioxygenase-catalysed formation of cis/trans-dihydrodiol metabolites of mono- and bi-cyclic heteroarenes. Chem Commun. 1996;1996:2361–2363. [Google Scholar]
- 10.Boyd D R, Sharma N D, Evans T A, Groocock M, Malone J F, Stevenson P J, Dalton H. Toluene dioxygenase-catalysed oxidation route to angular cis-monohydrodiols and other bioproducts from bacterial metabolism of 1,2-dihydrobenzocyclobutene and derivatives. J Chem Soc Perkin Trans I. 1997;1997:1879–1886. [Google Scholar]
- 11.Boyd D R, Sheldrake G N. The dioxygenase-catalyzed formation of vicinal cis-diols. Nat Prod Rep. 1998;15:309–325. [Google Scholar]
- 12.Bressler D C, Fedorak P M. Bacterial metabolism of fluorene, dibenzofuran, dibenzothiophene, and carbazole. Can J Microbiol. 2000;46:397–409. [PubMed] [Google Scholar]
- 13.Bünz P V, Cook A M. Dibenzofuran 4,4a-dioxygenase from Sphingomonas sp. strain RW1: angular dioxygenation by a three-component enzyme system. J Bacteriol. 1993;175:6467–6475. doi: 10.1128/jb.175.20.6467-6475.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Catelani D, Colombi A, Sorlini C, Treccani V. 2-Hydroxy-6-oxo-6-phenylhexa-2,4-dienoate: the meta-cleavage product from 2,3-dihydroxybiphenyl by Pseudomonas putida. Biochem J. 1973;134:1063–1066. doi: 10.1042/bj1341063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cerniglia C E, Morgan J C, Gibson D T. Bacterial and fungal oxidation of dibenzofuran. Biochem J. 1979;180:175–185. doi: 10.1042/bj1800175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eaton V J, Steele D. Dihedral angle of biphenyl in solution and the molecular force field. J Chem Soc Faraday Trans II. 1973;1973:1601–1608. [Google Scholar]
- 17.Eltis L D, Hofmann B, Hecht H-J, Lünsdorf H, Timmis K N. Purification and crystallization of 2,3-dihydroxybiphenyl 1,2-dioxygenase. J Biol Chem. 1993;268:2727–2732. [PubMed] [Google Scholar]
- 18.Engesser K H, Strubel V, Christoglou K, Fischer P, Rast H G. Dioxygenolytic cleavage of aryl ether bonds: 1,10-dihydro-1,10-dihydroxyfluoren-9-one, a novel arene dihydrodiol as evidence for angular dioxygenation of dibenzofuran. FEMS Microbiol Lett. 1989;53:205–209. doi: 10.1016/0378-1097(89)90392-3. [DOI] [PubMed] [Google Scholar]
- 19.Fetzner S, Müller R, Lingens F. Purification and some properties of 2-halobenzoate 1,2-dioxygenase, a two-component enzyme system from Pseudomonas cepacia 2CBS. J Bacteriol. 1992;174:279–290. doi: 10.1128/jb.174.1.279-290.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Furukawa K, Matsumura F, Tonomura K. Alcaligenes and Acinetobacter capable of degrading polychlorinated biphenyls. Agric Biol Chem. 1978;42:543–548. [Google Scholar]
- 21.Haddock J D, Horton J R, Gibson D T. Dihydroxylation and dechlorination of chlorinated biphenyls by purified biphenyl 2,3-dioxygenase from Pseudomonas sp. strain LB400. J Bacteriol. 1995;177:20–26. doi: 10.1128/jb.177.1.20-26.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kirsch N H, Stan H-J. Gas chromatographic-mass spectrometric determination of chlorinated cis-1,2-dihydroxycyclohexadienes and chlorocatechols as their boronates. J Chromatogr A. 1994;684:277–287. [Google Scholar]
- 23.Kohler H-P E, Schmid A, van der Maarel M. Metabolism of 2,2′-dihydroxybiphenyl by Pseudomonas sp. strain HBP1: production and consumption of 2,2′,3-trihydroxybiphenyl. J Bacteriol. 1993;175:1621–1628. doi: 10.1128/jb.175.6.1621-1628.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Locher H H, Leisinger T, Cook A M. 4-Sulphobenzoate 3,4-dioxygenase. Purification and properties of a desulphonative two-component enzyme system from Comamonas testosteroni T-2. Biochem J. 1991;274:833–842. doi: 10.1042/bj2740833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Massé R, Messier F, Péloquin L, Ayotte C, Sylvestre M. Microbial biodegradation of 4-chlorobiphenyl, a model compound of chlorinated biphenyls. Appl Environ Microbiol. 1984;47:947–951. doi: 10.1128/aem.47.5.947-951.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McKay D B, Seeger M, Zielinski M, Hofer B, Timmis K N. Heterologous expression of biphenyl dioxygenase-encoding genes from a gram-positive broad-spectrum PCB degrader and characterization of chlorobiphenyl oxidation by the gene products. J Bacteriol. 1997;179:1924–1930. doi: 10.1128/jb.179.6.1924-1930.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pfeifer F, Trüper H G, Klein J, Schacht S. Degradation of diphenylether by Pseudomonas cepacia Et4: enzymatic release of phenol from 2,3-dihydroxydiphenylether. Arch Microbiol. 1993;159:323–329. doi: 10.1007/BF00290914. [DOI] [PubMed] [Google Scholar]
- 28.Resnick S M, Lee K, Gibson D T. Diverse reactions catalyzed by naphthalene dioxygenase from Pseudomonas sp. strain NCIB 9816. J Ind Microbiol. 1996;17:438–457. [Google Scholar]
- 29.Romanov V, Hausinger R. Pseudomonas aeruginosa 142 uses a three-component ortho-halobenzoate 1,2-dioxygenase for metabolism of 2,4-dichloro- and 2-chlorobenzoate. J Bacteriol. 1994;176:3368–3374. doi: 10.1128/jb.176.11.3368-3374.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 31.Sato S I, Nam J W, Kasuga K, Nojiri H, Yamane H, Omori T. Identification and characterization of genes encoding carbazole 1,9a-dioxygenase in Pseudomonas sp. strain CA10. J Bacteriol. 1997;179:4850–4858. doi: 10.1128/jb.179.15.4850-4858.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmid E D, Brosa B. Determination of conjugation and angle of twist in biphenyls by Raman intensity. J Chem Phys. 1972;56:6267–6268. [Google Scholar]
- 33.Seeger M, Timmis K N, Hofer B. Degradation of chlorobiphenyls catalyzed by the bph-encoded biphenyl-2,3-dioxygenase and biphenyl-2,3-dihydrodiol-2,3-dehydrogenase of Pseudomonas sp. LB400. FEMS Microbiol Lett. 1995;133:259–264. doi: 10.1111/j.1574-6968.1995.tb07894.x. [DOI] [PubMed] [Google Scholar]
- 34.Seeger M, Timmis K N, Hofer B. Conversion of chlorobiphenyls into phenylhexadienoates and benzoates by the enzymes of the upper pathway for polychlorobiphenyl degradation encoded by the bph locus of Pseudomonas sp. strain LB400. Appl Environ Microbiol. 1995;61:761–768. doi: 10.1128/aem.61.7.2654-2658.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seeger M, Zielinski M, Timmis K N, Hofer B. Regiospecificity of dioxygenation of di- to pentachlorobiphenyls and their degradation to chlorobenzoates by the bph-encoded catabolic pathway of Burkholderia sp. strain LB400. Appl Environ Microbiol. 1999;65:3614–3621. doi: 10.1128/aem.65.8.3614-3621.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Selifonov S A, Gurst J E, Wackett L P. Regioselective dioxygenation of ortho-trifluoromethylbenzoate by Pseudomonas aeruginosa 142: evidence for 1,2-dioxygenation as a mechanism in ortho-halobenzoate dehalogenation. Biochem Biophys Res Commun. 1995;213:759–767. doi: 10.1006/bbrc.1995.2195. [DOI] [PubMed] [Google Scholar]
- 37.Selifonov S A, Slepen'kin A V, Adanin V M, Nefedova M Y, Starovoitov I I. Oxidation of dibenzofuran by pseudomonads harboring plasmids for naphthalene degradation. Mikrobiologiya. 1991;60:67–71. [PubMed] [Google Scholar]
- 38.Spain J C, Zylstra G J, Blake C K, Gibson D T. Monohydroxylation of phenol and 2,5-dichlorophenol by toluene dioxygenase in Pseudomonas putida F1. Appl Environ Microbiol. 1989;55:2648–2652. doi: 10.1128/aem.55.10.2648-2652.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Strubel V, Engesser K-H, Fischer P, Knackmuss H-J. 3-(2-Hydroxyphenyl)catechol as substrate for proximal meta ring cleavage in dibenzofuran degradation by Brevibacterium sp. strain DPO 1361. J Bacteriol. 1991;173:1932–1937. doi: 10.1128/jb.173.6.1932-1937.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Studier F W. Use of bacteriophage T7 lysozyme to improve an inducible T7 expression system. J Mol Biol. 1991;219:37–44. doi: 10.1016/0022-2836(91)90855-z. [DOI] [PubMed] [Google Scholar]
- 41.Vieira J, Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- 42.Yamaguchi M, Fujisawa H J. Purification and characterization of an oxygenase component in benzoate 1,2-dioxygenase system from Pseudomonas arvilla C-1. J Biol Chem. 1980;255:5058–5063. [PubMed] [Google Scholar]