Abstract
People with tuberculosis (TB) are at risk of major adverse cardiovascular events. We estimated the prevalence of cardiovascular risk (CVR) factors among people with active TB in Africa. This was a systematic review and meta-analysis of studies from Africa. We searched EMBASE, MEDLINE through PubMed, Web of Science, the Cochrane Central Register of Controlled Trials, mRCTs, Clinical trials.gov, and International Clinical Trials Registry Platform from inception to 31st December 2021. Among 110 eligible studies, 79 (238,316 participants) were included in the meta-analysis for smoking, 67 (52,793 participants) for current alcohol use, 30 (31,450 participants) for hazardous alcohol use, 51 (37,879 participants) for diabetes mellitus (DM), 19 (18,211 participants) for hypertension and 18 (13,910 participants) for obesity. The pooled prevalence was 26.0% (95% confidence interval 22.0–29.0) for smoking, 30.0% (25.0–35.0) for any current alcohol use, 21.0% (17.0–26.0) for hazardous alcohol use, 14.0% (9.0–18.0) for hypertension, 7.0% (6.0–9.0) for DM, and 4.0% (2.0–5.0) for obesity. Cost-effective strategies are needed to screen for CVR factors among people with active TB in Africa.
Subject terms: Cardiology, Risk factors
Introduction
The global burden of cardiovascular disease (CVD) has nearly doubled in the last two decades from 271 to 523 million cases between 1990 and 20191. In Africa, the burden of CVD has increased due to the rise in traditional cardiovascular risk (CVR) factors2. CVD accounts for 13% of all deaths and 37% of non-communicable disease-related deaths in sub-Saharan Africa3. At the same time, the region is still grappling with a high incidence of infectious diseases such as HIV and tuberculosis (TB). Accordingly, a convergence of cardiovascular and infectious diseases is observed in African countries4.
Africa contributed 25% of the global TB cases in 20195. The interaction between TB and CVD is complex. Latent TB infection increases the risk of hypertension independent of body mass index, HIV infection and serum cholesterol6. Mycobacterium tuberculosis can also directly cause myocarditis, aortitis and pericarditis7. As such, people with active TB have a 51% higher risk for major adverse cardiovascular events than controls8. Several observational studies show that people with active TB are at a higher risk of ischemic stroke9, myocardial infarction10, peripheral artery disease11, deep venous thrombosis, pulmonary embolism and venous thromboembolism12. There is need to characterise the burden of CVD risk factors among people with active TB because CVD risk factors can synergistically increase the risk for CVD-related mortality among these people. Moreover, CVD accounts for 20% of deaths among survivors of TB after TB treatment completion13. On the one hand, CVR factors such as smoking14,15, alcohol use16,17 and diabetes mellitus (DM)18,19 are risk factors for TB infection and poor TB outcomes. On the other hand, obesity is protective against TB infection and adverse outcomes20,21.
While the prevalence of DM in TB in Africa is estimated at 7.7–9.0%22,23, the burden of other CVR factors in active TB in Africa is not well characterised. Determining the burden of CVR factors in active TB in Africa informs the need, if any, of integrating CVR scoring and risk modification interventions in routine TB care. We, therefore, determined the prevalence of CVR factors among people with active TB in Africa.
Methods
Search strategy and selection criteria
We performed a systematic review and meta-analysis of studies reporting the prevalence of CVR factors among people with active TB in Africa. The CVR factors of focus were hypertension, DM, dyslipidaemia (lipid abnormalities), obesity, physical inactivity, alcohol use and smoking. The study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines24. The study protocol was developed following the PRISMA-P guidelines25 and registered on PROSPERO (registration number: CRD42021245395).
We searched for all studies published from inception to 31st December 2020 and later updated the search to 31st December 2021 (Supplementary Material Table 10). The following databases were comprehensively searched: EMBASE, MEDLINE through PubMed, Web of Science, the Cochrane Central Register of Controlled Trials (CENTRAL), mRCTs, Clinical trials.gov, and International Clinical Trials Registry Platform (ICTRP). The following medical subject headings (MeSH) terms were used: “prevalence” OR “incidence” OR “burden” AND “hypertension”, “diabetes”, “pre-diabetes”, “cardiovascular risk factors”, “metabolic syndrome” “hyperlipidaemia”, “dyslipidemia”, “cholesterol”, “hypercholesterolemia”, “low density lipoprotein”, “triglycerides”, “alcohol”, “smoking”, “cigarette”, “obesity”, “overweight”, “physical inactivity”, AND ”tuberculosis”, “TB”, “PTB”, AND “Africa” OR the individual names of the African countries. The search was limited to studies published in English and French; the predominant languages used in Africa.
We included prospective, cross-sectional, retrospective, and interventional studies reporting the prevalence (or for which the proportion could be calculated) of any of hypertension, DM, lipid abnormalities, obesity, physical inactivity, alcohol use and smoking among people with active TB in Africa. We excluded case reports, case series with subjects less than 10, opinion papers, qualitative research, letters to the editor, comments, conference proceedings, policy papers, reviews and meta-analyses, study protocols without baseline data, and animal studies.
After the database search, duplicates were removed using the Healthcare Databases Advanced Search program (National Institute for Health and Care Excellence, UK). Thereafter, articles were reviewed by title and abstract by two reviewers (JBB and RO) to remove articles that are unrelated to the study question. The full text of the articles that passed this initial screen were then retrieved and assessed by two investigators independently (JBB and FB) (Supplementary Table 7). Any disagreements were resolved by consensus. Data were extracted by three independent reviewers (JBB, FB and RO) with Microsoft Excel® using a data abstraction form. The form captured study design, year of publication, number of participants, country where the study was conducted, type of TB by drug resistance profile, criteria used for classifying individuals as having a given CVR factor and reported prevalence (or proportion) of the CVR factors. Any variation in the extracted data by the reviewers was discussed and resolved by consensus.
The primary study outcome was the prevalence of any current alcohol use, smoking, hypertension, DM, lipid abnormalities, obesity and physical inactivity among people with active TB in Africa. Hypertension was defined as a systolic blood pressure (SBP) ≥ 140 mmHg and/or diastolic blood pressure (DBP) ≥ 90 mmHg or use of anti-hypertension medication or “known patient with hypertension”. Obesity was defined as a body mass index (BMI) of ≥ 30 kg per m2. DM was defined as glycated haemoglobin (HbA1c) level ≥ 6.5% or fasting blood sugar (FBS) ≥ 126 mg/dl or 2 h plasma glucose of ≥ 200 mg/dl after oral glucose tolerance test or random blood sugar (RBS) ≥ 11.1 mmol/l with symptoms of DM or use of DM medication. Hazardous alcohol use was operationally defined a posteriori as any of: daily use of alcohol, consumption of alcohol on ≥ 3 days of the week, studies describing users as "chronic drinker”, “misusing alcohol”, or reporting a prevalence of “alcoholism", alcohol dependence measured by the Mini International Neuropsychiatric Interview, Alcohol Use Disorders Identification Test (AUDIT) score of ≥ 826, and the cut down, anger, guilt and eye-opener (CAGE) questionnaire score of ≥ 227. Any history of smoking was considered in estimating the prevalence of smoking.
Using a tool by Hoy et al.28 for assessing risk of bias in prevalence studies, two independent reviewers (RO and FB) evaluated the quality of the studies for risk of bias and risk was graded as low (> 8), moderate (5–8) and high (< 5) (Supplementary Table 6).
Statistical analysis
Data were analysed using STATA 16.0 (StataCorp LLC, Texas, USA). Heterogeneity of the data was assessed using the Q statistic and I2 index and the corresponding p-value. Heterogeneity was considered as low (I2 = 0–25%), moderate (I2 = 26–50%), or high (I2 > 50%). Depending on the heterogeneity of the data, random-effect (for I2 ≥ 50%) or fixed-effect (for I2 < 50%) models were used to determine the pooled prevalence of a given CVR factor presented as a proportion and the corresponding 95% confidence interval. Forest plots were used to present the results of the meta-analysis. Publication bias was assessed visually using funnel plots and statistically using Egger’s regression test. We further determined whether the observed asymmetry was due to publication bias via enhanced-contour funnel plots after the trim-and-fill method. A sensitivity analysis was performed for the prevalence of a given CVR by region, among studies within the funnel plot (Supplementary Material: Figs. 1–6), among people with drug resistant TB (DRTB), and risk of bias of the study. We further performed meta-regression analyses to assess sources of heterogeneity (Supplementary Material: Table 8). A two-tailed p < 0.05 was considered statistically significant.
Results
Study characteristics
We identified 110 eligible studies (Supplementary Fig. 7). The mean (standard deviation) risk of bias score was 7.9 (1.4). Majority of studies (63.6%, 70/110) had a score of ≥ 8. The study summary statistics are shown in Table 1. Among these studies, 79 (238,316 participants) were included in the meta-analysis for smoking, 67 (52,793 participants) for current alcohol use, 30 (31,450 participants) for hazardous alcohol use, 51 (37,879 participants) for DM, 19 (18,211 participants) for hypertension and 18 (13,910 participants) for obesity. Two studies (4320 participants)29,30 reported on lipid abnormalities and another two studies31,32 reported data on physical inactivity (2247 participants). Therefore, meta-analyses for lipid abnormalities and physical inactivity were not performed. Supplementary Tables 1–5 show characteristics of studies included in the meta-analyses for each CVR factor.
Table 1.
Summary Statistics for the prevalence of cardiovascular risk factors among people with active TB in Africa.
| Cardiovascular risk factor | No. studies | No. participants | Pooled prevalence (%) | 95% confidence interval (CI) (%) | Heterogeneity (I2) (%) | pheterogeneity | pEgger’s |
|---|---|---|---|---|---|---|---|
| Smoking | |||||||
| Overall | 79 | 238,316 | 26 | 22–29 | 99.6 | < 0.001 | 0.005 |
| Studies in funnel plot | 15 | 199,641 | 22 | 21.8–22.2 | 4.01 | < 0.001 | 0.235 |
| DRTB only | 10 | 3751 | 27 | 16–37 | 98.4 | < 0.001 | |
| Southern Africa | 35 | 219,397 | 30 | 25–36 | 99.7 | < 0.001 | |
| West Africa | 16 | 10,294 | 18 | 10–25 | 99.3 | < 0.001 | |
| East Africa | 23 | 7823 | 21 | 16–26 | 98.3 | < 0.001 | |
| Northern Africa | 4 | 507 | 45 | 29–61 | 92.7 | < 0.001 | |
| Current alcohol use | |||||||
| Overall | 67 | 52,793 | 30 | 25–35 | 99.5 | < 0.001 | 0.161 |
| Studies in funnel plot | 15 | 4382 | 28 | 26–29 | 0.0 | < 0.001 | 0.421 |
| DRTB only | 10 | 3356 | 27 | 16–38 | 98.6 | < 0.001 | |
| Southern Africa | 28 | 37,327 | 29 | 21–36 | 99.7 | < 0.001 | |
| West Africa | 12 | 7502 | 29 | 15–43 | 99.6 | < 0.001 | |
| East Africa | 24 | 7596 | 32 | 24–40 | 98.8 | < 0.001 | |
| Hazardous alcohol use | |||||||
| Overall | 30 | 31,450 | 21 | 17–26 | 99.1 | < 0.001 | 0.001 |
| Studies in funnel plot | 5 | 3978 | 21 | 20–22 | 35.1 | < 0.001 | 0.535 |
| DRTB only | 3 | 733 | 15 | 2–28 | 97.1 | < 0.001 | |
| Southern Africa | 17 | 26,586 | 21 | 16–27 | 99.3 | < 0.001 | |
| West Africa | 3 | 1703 | 13 | 2–25 | 96.8 | < 0.001 | |
| East Africa | 8 | 2793 | 22 | 11–33 | 98.6 | < 0.001 | |
| Diabetes | |||||||
| Overall | 51 | 37,879 | 7 | 6–9 | 98.5 | < 0.001 | < 0.001 |
| Studies in funnel plot | 18 | 10,922 | 4 | 4–4 | 0.0 | < 0.001 | 0.787 |
| DRTB only | 9 | 3587 | 7 | 2–12 | 97.6 | < 0.001 | |
| Southern Africa | 19 | 19,029 | 6 | 4–7 | 94.2 | < 0.001 | |
| West Africa | 11 | 6914 | 6 | 3–10 | 96.5 | < 0.001 | |
| East Africa | 19 | 11,702 | 9 | 5–13 | 99.1 | < 0.001 | |
| Hypertension | |||||||
| Overall | 19 | 18,211 | 14 | 9–18 | 99.1 | < 0.001 | 0.022 |
| Studies in funnel plot | 7 | 6001 | 8 | 7.8–9.2 | 0.0 | 0.470 | 0.203 |
| DRTB only | 6 | 2008 | 13 | 4–22 | 97.3 | < 0.001 | |
| Southern Africa | 11 | 14,601 | 14 | 8–19 | 99.3 | < 0.001 | |
| West Africa | 4 | 2044 | 6 | 1–11 | 94.3 | < 0.001 | |
| East Africa | 4 | 1566 | 22 | 12–33 | 96.5 | < 0.001 | |
| Obesity | |||||||
| Overall | 18 | 13,910 | 4 | 2–5 | 97.0 | < 0.001 | 0.002 |
| Studies in funnel plot | 8 | 1026 | 1.5 | 0.9–2.1 | 34.8 | 0.150 | 0.010 |
| Southern Africa | 10 | 9661 | 5 | 3–7 | 90.4 | < 0.001 | |
| West Africa | 4 | 1962 | 5 | 1–10 | 98.0 | < 0.001 | |
| East Africa | 4 | 2287 | 2 | 0–3 | 81.9 | < 0.001 | |
Prevalence of smoking
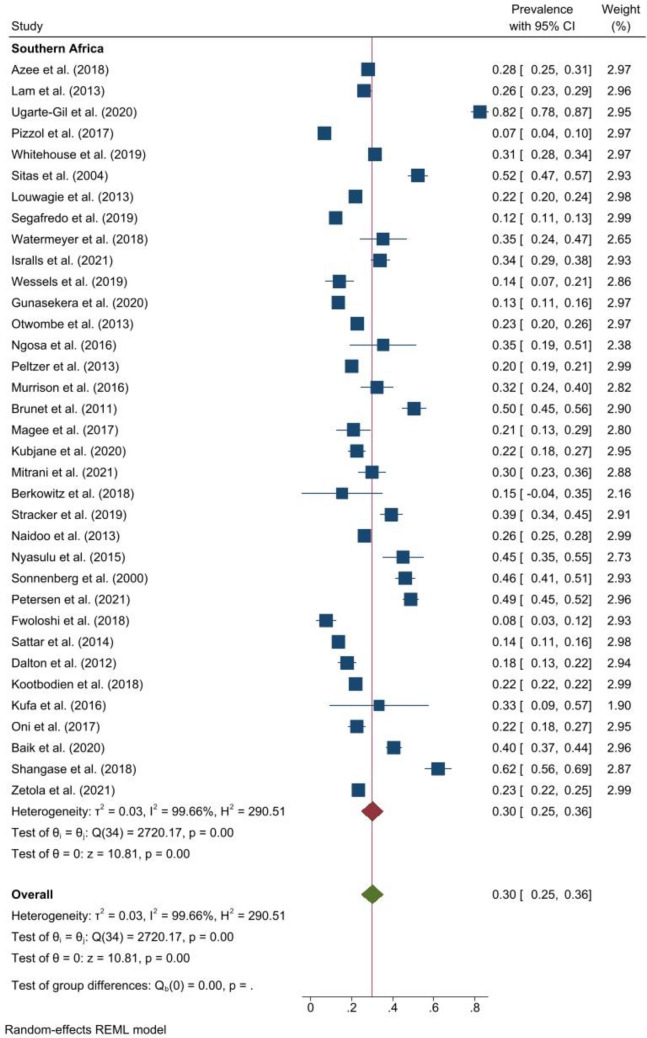
The pooled prevalence of any history of smoking was 26.0% (95% CI 22.0–29.0, I2 = 99.6%, p < 0.001) and ranged from 1.4% in Uganda33 to 82.5% in South Africa34. Among people with DRTB, the pooled prevalence of smoking was 27.0% (95% CI 16.0–37.0, I2 = 98.4%, p < 0.001)35–44. The pooled prevalence of any history of smoking was 45.0% (95% CI 29.0–61.0, I2 = 92.7%, p < 0.001) in Northern Africa, 30.0% (95% CI 25.0–36.0, I2 = 99.7%, p < 0.001) in Southern Africa (Fig. 1), 21.0% (95% CI 16.0–26.0, I2 = 98.3%, p < 0.001) in East Africa, and 18.0% (95% CI 10.0–25.0, I2 = 99.3%, p < 0.001) in West Africa. One study in Central Africa reported the prevalence at 30.0%45. Figure 2 shows the forest plot for the prevalence of smoking in Western, Eastern, and Northern Africa. Among studies within the funnel plot, the pooled prevalence of smoking was 22.0% (95% CI 22.0–22.0, I2 = 4.0%, p < 0.001). Similarly, among studies with low risk of bias, the pooled prevalence was 22.0% (95% CI 14.0–31.0, I2 = 99.8%, p < 0.001) and 27.0% (95% CI 23.0–31.0, I2 = 99.1%, p < 0.001) among studies with moderate risk of bias.
Figure 1.
Forest plot showing the pooled prevalence of smoking among people with active TB in Southern Africa.
Figure 2.
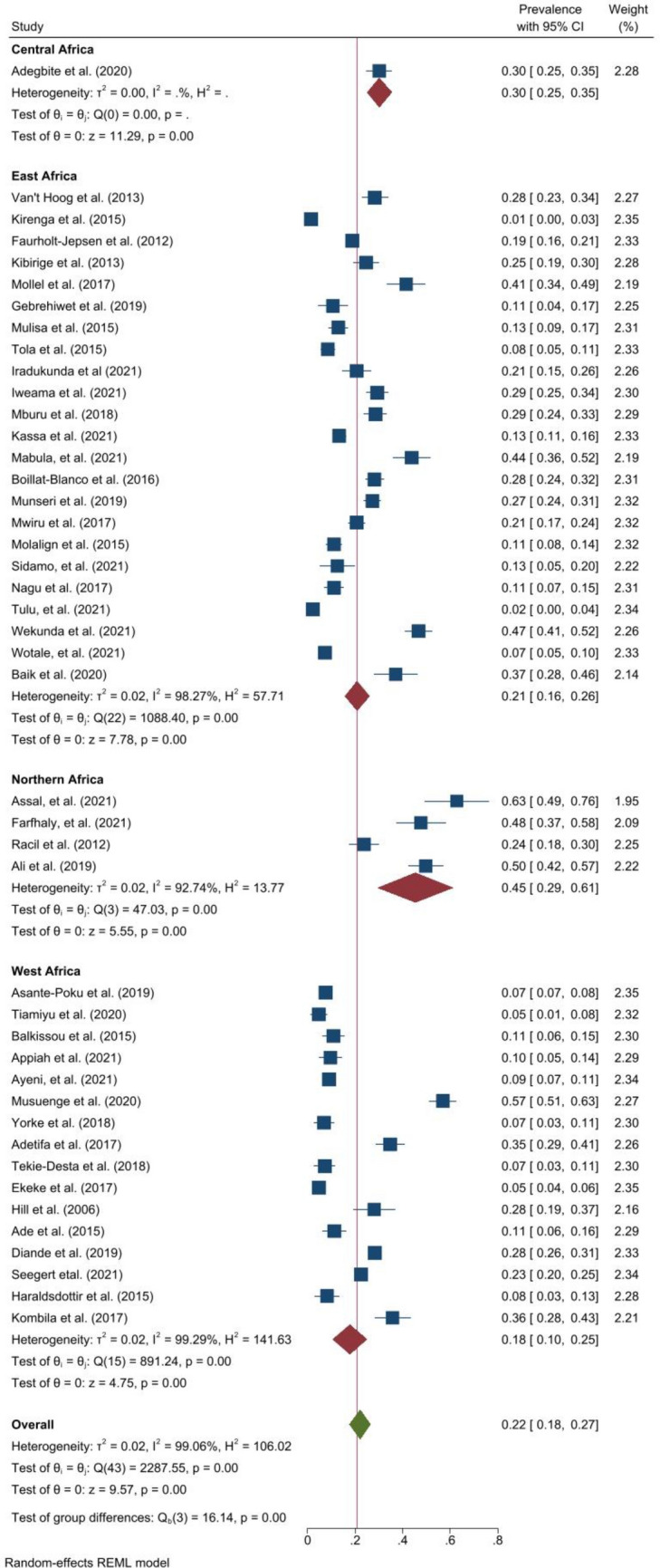
Forest plot showing the pooled prevalence of smoking among people with active TB in East, West, Northern and Central Africa.
Prevalence of alcohol use
The pooled prevalence of any current alcohol use (Fig. 3) was 30.0% (25.0–35.0, I2 = 99.5%, p < 0.001). The prevalence ranged from 3.3% in South Africa46 to 97.8% in another study from South Africa47. In DRTB, the pooled prevalence of any current use was 27.0% (95% CI 16.0–38.0, I2 = 98.6%, p < 0.001)35,36,38,40,42,43,46,48–50. Across the regions, the pooled prevalence of any current alcohol use was highest in East Africa but similar in other regions; that is, 32.0% (95% CI 24.0–40.0, I2 = 98.8%, p < 0.001) in East Africa, 29.0% (95% CI 21.0–36.0, I2 = 99.7%, p < 0.001) in Southern Africa, and 29.0% (95% CI 15.0–43.0, I2 = 99.6%, p < 0.001) in West Africa. In Northern Africa, two studies reported a prevalence of 25.0–37.0%51,52. For studies within the funnel plot, the pooled prevalence of any current was 28.0% (95% CI 26.0–29.0, I2 = 0.0%, p < 0.001). Among studies with low risk of bias, the pooled prevalence was 29.0% (95% CI 20.0–38.0, I2 = 99.8%, p < 0.001) and 30.0% (95% CI 24.0–36.0, I2 = 99.2%, p < 0.001) among studies with moderate risk of bias.
Figure 3.
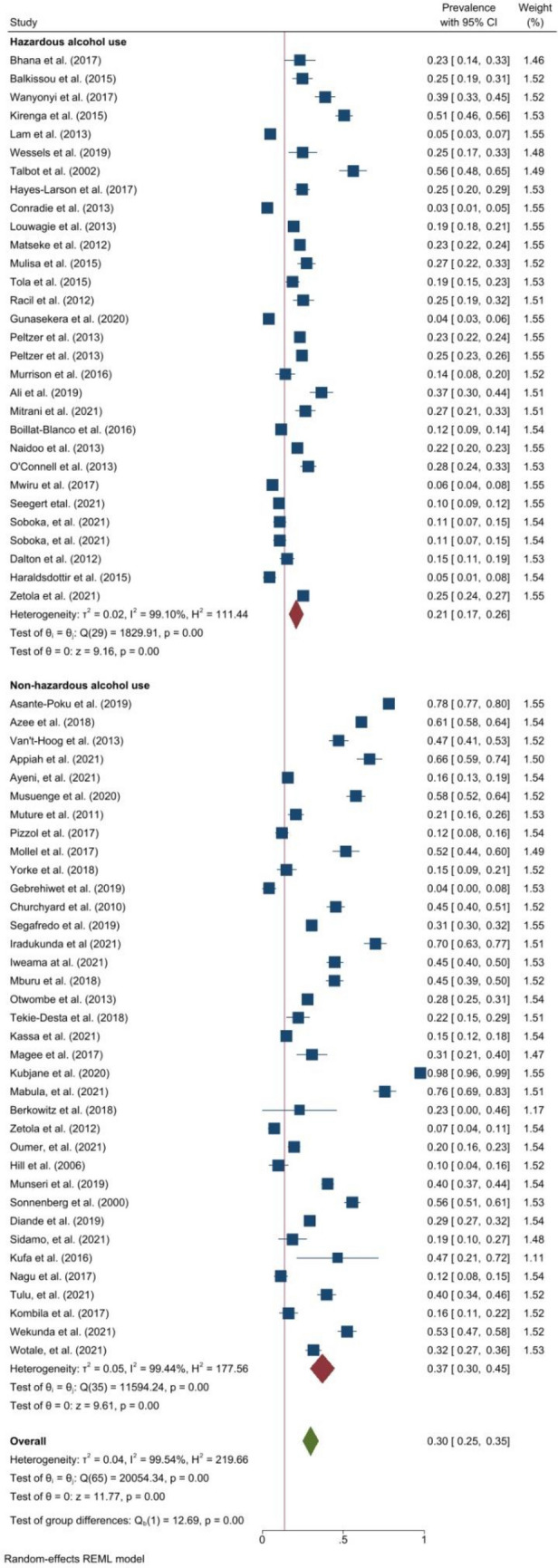
Forest plot showing the prevalence of alcohol use among people with active TB in Africa.
Prevalence of hazardous alcohol use
The pooled prevalence of hazardous alcohol use was 21.0% (95% CI 17.0–26.0, I2 = 99.1%, p < 0.001). The estimates were similar among studies within the funnel plot, low and moderate risk of bias. That is, 21.0% (95% CI 20.0–22.0, I2 = 35.1%, p < 0.001) among studies within the funnel plot, 21.0% (95% CI 16.0–25.0, I2 = 98.3%, p < 0.001) in studies with low risk of bias, and 21.0% (95% CI 14.0–29.0, I2 = 99.2%, p < 0.001) in studies with moderate risk of bias. Only three studies36,46,48 reported the prevalence of hazardous alcohol use in DRTB with a pooled prevalence of 15.0% (95% CI 2.0–28.0, I2 = 97.1%, p = 0.03).
Prevalence of DM
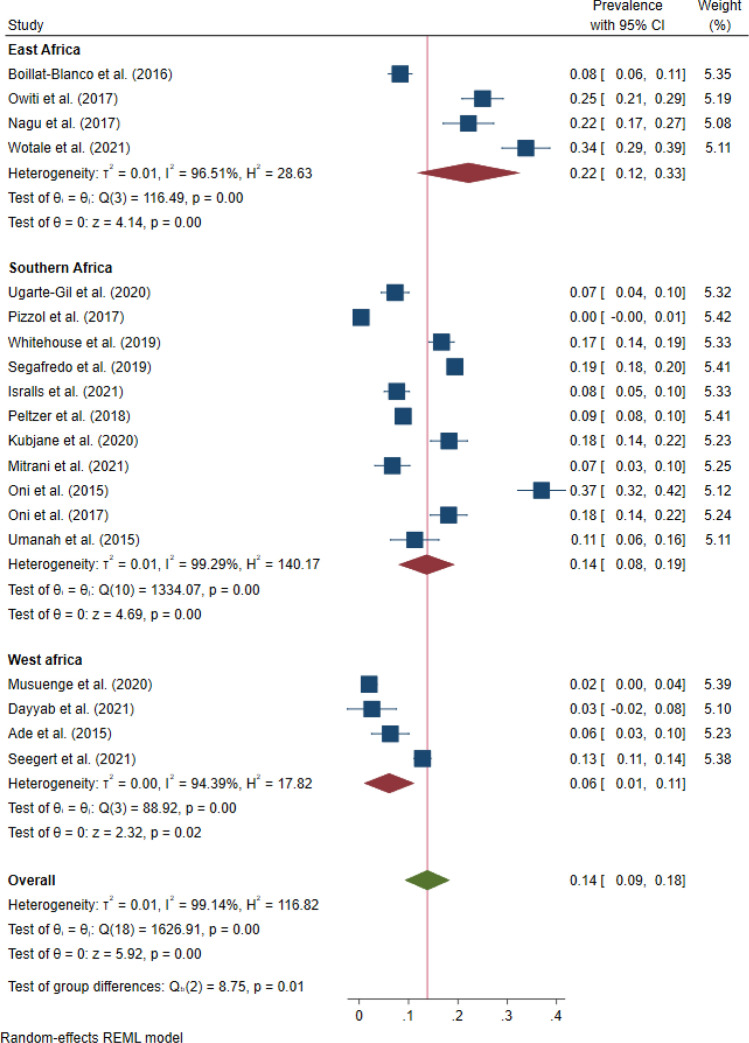
The pooled prevalence of DM (Fig. 4) was 7.0% (95% CI 6.0–9.0, I2 = 98.5%, p < 0.001). The prevalence of DM ranged from 1.0% in studies from Uganda53, Mozambique54 and Cameroon55 to 37.2% in Kenya56. However, Mburu et al.56 used HbA1c of > 6.0% as the cut-off for defining DM in Kenya. Among patients with DRTB, the pooled prevalence was also 7.0% (95% CI 2.0–12.0, I2 = 97.6%, p < 0.001)35–37,39,42,44,48,57. The pooled prevalence of DM was highest in East Africa at 9.0% (95% CI 5.0–13.0, I2 = 99.1%, p < 0.001) and was similar in West Africa (6.0% (95% CI 3.0–10.0, I2 = 96.5%, p < 0.001)) and Southern Africa at 6.0% (4.0–7.0, I2 = 94.2%, p < 0.001). In Northern Africa, the prevalence of DM was reported by two studies at 16.5%51 and 15.7%58. The pooled prevalence of DM was 4.0% (95% CI 4.0–4.0, I2 = 0.0%, p < 0.001) among studies within the funnel plot. Among studies with low risk of bias, the pooled prevalence was 8.0% (95% CI 6.0–10.0, I2 = 96.8%, p < 0.001) and 7.0% (95% CI 5.0–10.0, I2 = 98.5%, p < 0.001) among studies with moderate risk of bias.
Figure 4.
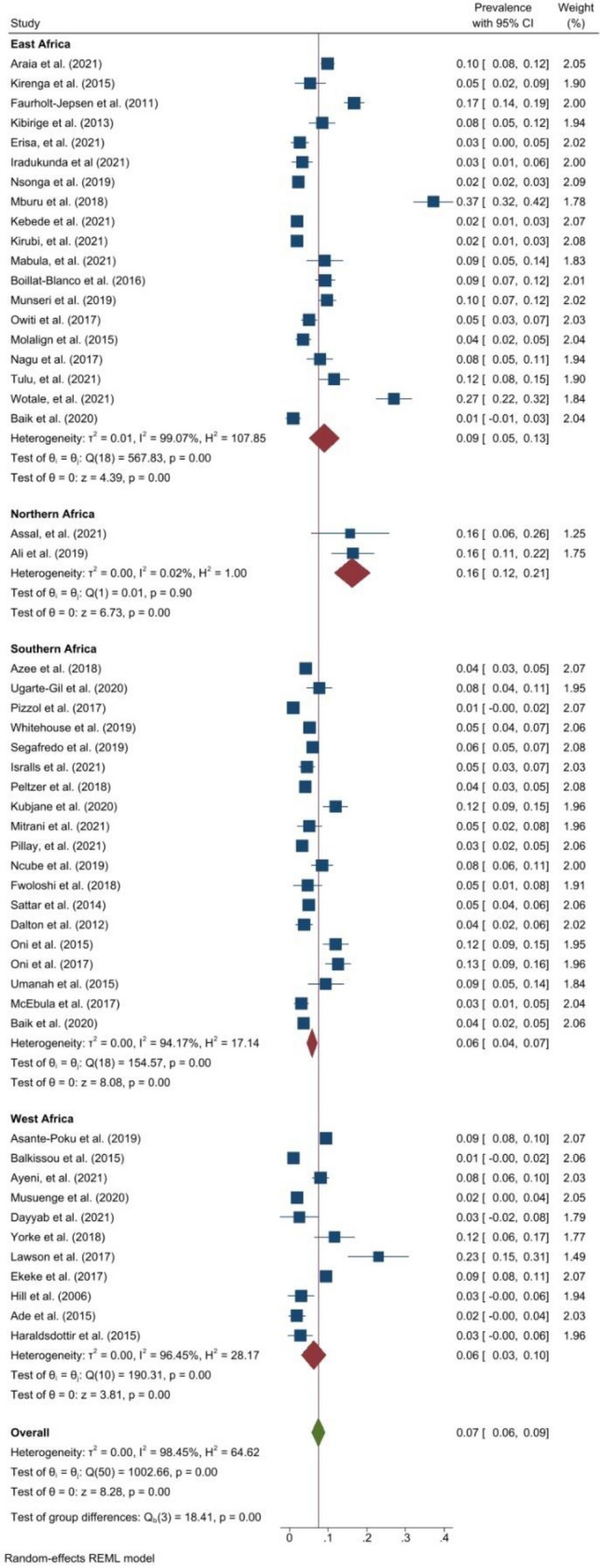
Forest plot showing the prevalence of DM among people with active TB in Africa.
Prevalence of hypertension
The pooled prevalence of hypertension (Fig. 5) was 14.0% (95% CI 9.0–18.0, I2 = 99.1%, p < 0.001). The prevalence of hypertension ranged from 0.3% in Mozambique54 to 37.0% in South Africa59. The prevalence in DRTB was 13.0% (95% CI 4.0–22.0, I2 = 97.3%, p < 0.001) in six studies37,42,44,48,57,60. East Africa had the highest pooled prevalence of hypertension at 22.0% (95% CI 12.0–33.0, I2 = 96.5%, p < 0.001) followed by Southern Africa at 14.0% (95% CI 8.0–19.0, I2 = 99.3%, p < 0.001) and West Africa at 6.0% (95% CI 1.0–11.0, I2 = 94.4%, p < 0.001). Among studies within the funnel plot, the pooled prevalence of hypertension was 9.0% (95% CI 8.0–9.0, I2 = 0.0%, p < 0.001). Among studies with low risk of bias, the pooled prevalence was 14.0% (95% CI 10.0–18.0, I2 = 98.9%, p < 0.001) and 13.0% (95% CI 4.0–23.0, I2 = 98.5%, p < 0.001) among studies with moderate risk of bias.
Figure 5.
Forest plot showing prevalence of hypertension among people with active TB in Africa.
Prevalence of obesity
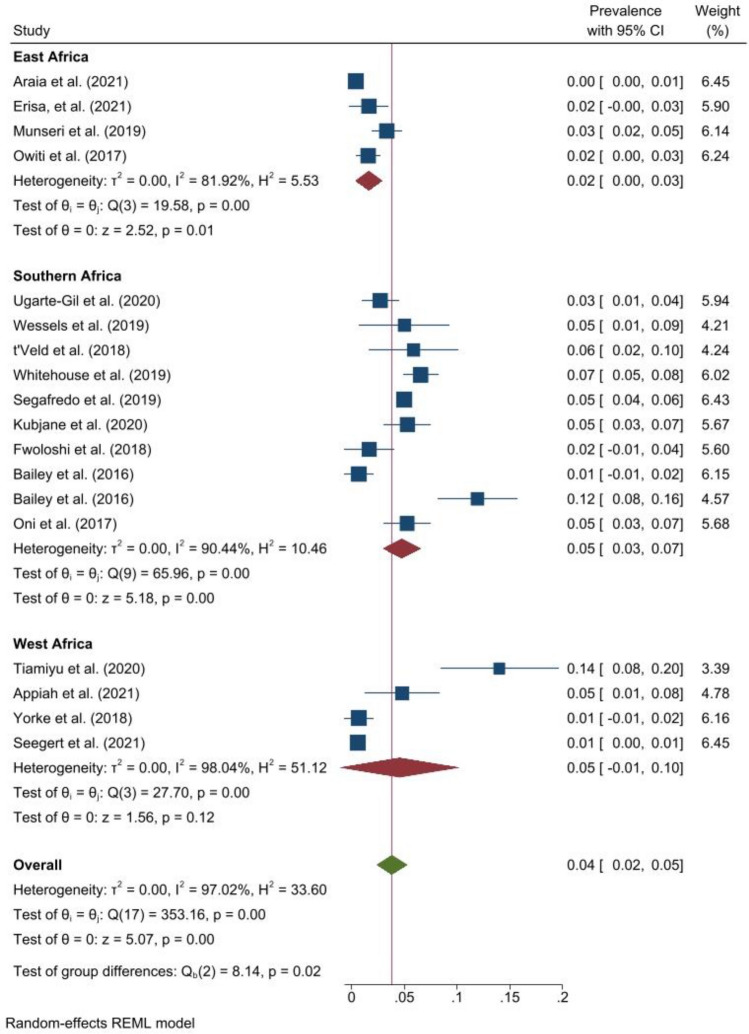
The pooled prevalence of obesity was 4.0% (95% CI 2.0–5.0, I2 = 97.0%, p < 0.001) (Fig. 6). The prevalence ranged from 0.4% in Eritrea61 to 14.0% in Nigeria62. One study reported a prevalence of 7.0% (95% CI 5.0–8.0) among people with DRTB37. The pooled prevalence of obesity in Southern Africa was 5.0% (95% CI 3.0–7.0, I2 = 90.4%, p < 0.001), 5.0% (95% CI 1.0–10.0, I2 = 98.0, p < 0.001 ) in West Africa and 2.0% (95% CI 0.0–3.0, I2 = 81.9%, p < 0.001) in East Africa. The pooled prevalence of obesity was 2.0% (95% CI 1.0–2.0, I2 = 34.8%, p = 0.150) among studies within the funnel plot. Among studies with low risk of bias, the pooled prevalence was 5.0% (95% CI 3.0–7.0, I2 = 97.6%, p < 0.001) and 2.0% (95% CI 1.0–3.0, I2 = 77.3%, p < 0.001) among studies with moderate risk of bias.
Figure 6.
Forest plot showing prevalence of obesity among people with active TB in Africa.
Prevalence of lipid abnormalities and physical inactivity
With regards to lipid abnormalities, 67% of people with TB had low high density lipoproteins in Nigeria29 while only 1.7% self-reported any lipid abnormalities in South Africa30. Physical inactivity, defined as a sedentary occupation and “no physical exercise”, was reported in 49.8% of people with TB in Nigeria and 69.9% in Tanzania, respectively31,32. In the study from Nigeria, 32.9% had moderate activity and 17.3% had occupations associated with vigorous activity.
Heterogeneity and publication bias
There was high heterogeneity (I2 > 50%) in the estimation of the pooled prevalence of all CVR factors (Table 1) except for studies within the funnel plots. In the meta-regression analysis (Supplementary Material Table 8), there were significant differences in the prevalence of hypertension and smoking across the regions of Africa. Current alcohol use also varied between studies that reported hazardous and non-hazardous use. Visually, the funnel plots (Supplementary Fig. 8) were asymmetrical, suggesting overall publication bias for the studies included in the meta-analyses. This was confirmed by Egger’s test for smoking (p = 0.005), hazardous alcohol use (p = 0.001), DM (p < 0.001), hypertension (p = 0.022) and obesity (p = 0.002) but not current alcohol use (p = 0.161). The enhanced-contour funnel plots after the trim-and-fill method further demonstrated publication bias in the estimates for the prevalence of obesity and current alcohol use (Supplementary Material Fig. 9 and Table 9).
Discussion
In this systematic review and meta-analysis, we have provided the first comprehensive estimates for the burden of traditional CVR factors among people with active TB in Africa. The prevalence was 26.0% for smoking, 30.0% for any current alcohol use, 21.0% for hazardous alcohol use, 14.0% for hypertension, 7.0% for DM, and 4.0% for obesity. There was substantial heterogeneity in these estimates, and this could be explained by variations in the prevalence by regions of Africa and publication bias. Accordingly, East Africa had the highest prevalence of hypertension and DM while Northern Africa had the highest prevalence of smoking. People with active DRTB had comparable estimates in the prevalence of all CVR factors.
The prevalence of smoking and DM in this study is higher than what is reported in the general African population for smoking (8.3%)63 and undiagnosed DM (3.9–5.4%)64. This is possibly because smoking65 and DM66 are well established risk factors for active TB. Moreover, smoking interacts with DM to synergistically increase the risk for TB67. The high prevalence of smoking and DM in active TB is concerning because both increase the risk of adverse TB treatment outcomes14,68. It was interesting to observe similar prevalence of DM in DRTB even when the criteria for the diagnosis of DM in DRTB was unknown in four of the five studies included in the sub-analysis for DM35,36,39,57. Therefore, although the true burden of DM in DRTB in Africa remains largely unknown, it is likely to be similar to that among people with susceptible TB. Our findings suggest a need for cost effective strategies for screening for DM and smoking among people with active TB in Africa. The prevalence of DM in active TB in our study is comparable to that reported by Alebel (9.0%)22, Noubiap (8.0%)23 et al. in Africa. For smoking, our estimate is slightly higher than the prevalence among people with TB in Bangladesh and Pakistan (23%) which are TB high-burdened countries69. This is likely because their study focused on daily and current smoking only.
Our findings for the prevalence of hypertension and obesity are in agreement with the global estimates for the prevalence of hypertension (0.7–38.3%)70 and obesity (5.9%)20 in active TB. However, these estimates are lower than what is reported in the general population for hypertension (30.0–42.0%) and obesity (21.0%) in Africa71,72. A low prevalence of obesity in active TB should be expected since undernourishment accounts for the most global TB cases5 while obesity is protective against active TB20. With regards to hypertension, it is likely that HIV, age and obesity status influence the blood pressure dynamics in TB. HIV has been associated with 25% lower risk for hypertension73. In our meta-analysis for hypertension, HIV co-infection was reported among > 30% of participants in eight of the twelve studies for which HIV status data were reported, although estimates of hypertension by HIV status were not reported in most of the studies. Additionally, TB is predominantly a disease of young individuals while hypertension is more prevalent in older individuals in Africa5,74. Despite the relatively low prevalence of hypertension in active TB in Africa, screening is warranted due to the low levels of hypertension awareness, treatment initiation and blood pressure (Bp) control in Africa75. The low rate of hypertension treatment rates could explain why hypertension was most prevalent in East Africa—where the rate of hypertension treatment is lowest in Africa75.
Estimating the prevalence of alcohol use in Africa is difficult. This is because the types of alcoholic drinks are varied (often home brewed) and few studies use high-quality quantification of alcohol use76. Further, over 60% of “current drinkers” in Africa are heavy episodic drinkers (defined as consumption of ≥ 60 g of pure alcohol on at least one occasion at least once per month)77. Therefore, our estimate of the prevalence of any current alcohol use may not be very reliable. However, the estimate for hazardous alcohol use is likely to be more reliable. All studies (except five) included in the analysis for hazardous alcohol use used validated tools or quantified alcohol use by amount and/or frequency. Our estimate of hazardous alcohol use is similar to the prevalence of alcohol use disorder among people with TB in Africa (24.0%) reported by Necho et al.78. Alcohol use increases the risk for TB infection16 and poor TB outcomes76. Therefore, the high prevalence of hazardous alcohol use suggests a need for integrating validated tools in screening for alcohol use disorders among people at risk of TB and people with TB.
Our estimates should be interpreted in the context of some limitations. Firstly, there was significant heterogeneity and publication bias across most estimates. Therefore, the overall pooled estimates were over-estimated particularly due to publication bias. Our sensitivity analyses showed lower prevalence among studies within the funnel plots. Secondly, the prevalence for smoking may have been overestimated since any history of smoking (other than current smoking only) was considered in the pooled prevalence. However, the CVR posed by smoking is higher in former smokers than “never smokers”79,80. Therefore, assessing for any history of smoking as a CVR factor is justified. Moreover, half of the studies included in our analysis reported current smoking. Thirdly, transient hyperglycaemia can confound the estimates for DM. In our analysis, two studies demonstrated a decline in the prevalence of DM when measurements are taken at baseline and months into TB treatment47,81. Other two studies that assessed the prevalence at least a month into TB treatment report a prevalence of 2–3%82,83. Therefore, transient hyperglycaemia could spuriously increase the prevalence of DM. Nonetheless, hyperglycaemia does not resolve at follow-up in 50% of people with TB and hyperglycaemia at baseline84. More studies are needed to determine whether transient hyperglycaemia in TB heralds new onset DM or predicts future risk for DM. Regarding hypertension, none of the studies purposely set out to determine the prevalence of hypertension. Further, some studies based the diagnosis on single blood pressure measurements59,85, and the criteria were unknown in some other studies54,57,62. There is therefore a risk of selection and misclassification bias in the estimate for the prevalence of hypertension. Nonetheless the range of the prevalence of hypertension in our analysis is similar to that reported by Seegert et al.70 in a global systematic review. Importantly, CVR factors in Africa may be different to those in high-income countries. As such, the synergistic effect of aging, stress, illiteracy, poor health systems and poverty on CVD in Africa needs to be further evaluated among people with active TB86.
Notwithstanding the limitations, this analysis provides the first comprehensive prevalence of most traditional CVR factors among people with active TB in Africa. We further explored differences by TB drug resistance status and regions of Africa in the sub-analyses. Moreover, our study provides an updated estimate for the prevalence of DM in TB in Africa.
In conclusion, the prevalence of smoking, hazardous alcohol use and DM was high among people with TB in Africa. The findings suggest a need for screening for these CVR factors in this population. Although the prevalence of hypertension was low relative to regional estimates, screening is warranted because of the low awareness levels previously reported in the region. Prospective studies are needed to determine the role of CVR scoring among people with TB to prevent future CVD. More studies evaluating the burden of lipid abnormalities and physical inactivity among people with TB in Africa are needed as well.
Supplementary Information
Author contributions
J.B.B.—Conceptualisation, developing study protocol, methodology, data accrual, formal analysis, interpretation of results, drafting manuscript, manuscript revision, final approval. R.O.—Methodology, data accrual, formal analysis, interpretation of results, manuscript revision, final approvals. P.B.—Interpretation of results, drafting manuscript, manuscript revision, final approval. E.O.—Interpretation of results, manuscript revision, final approval. F.B.—Developing study protocol, data accrual, interpretation of results, manuscript revision, final approval.
Data availability
All data generated or analysed during this study are included in this article and its supplementary information files.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-20833-0.
References
- 1.Roth GA, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: Update from the GBD 2019 Study. J. Am. Coll. Cardiol. 2020;76:2982–3021. doi: 10.1016/j.jacc.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hamid S, Groot W, Pavlova M. Trends in cardiovascular diseases and associated risks in sub-Saharan Africa: A review of the evidence for Ghana, Nigeria, South Africa, Sudan and Tanzania. Aging Male. 2019;22:169–176. doi: 10.1080/13685538.2019.1582621. [DOI] [PubMed] [Google Scholar]
- 3.Yuyun, M. F., Sliwa, K., Kengne, A. P., Mocumbi, A. O. & Bukhman, G. Cardiovascular diseases in sub-Saharan Africa compared to high-income countries: An epidemiological perspective. Glob. Heart15, (2020). [DOI] [PMC free article] [PubMed]
- 4.Wong EB, et al. Convergence of infectious and non-communicable disease epidemics in rural South Africa: A cross-sectional, population-based multimorbidity study. Lancet Glob. Health. 2021;9:e967–e976. doi: 10.1016/S2214-109X(21)00176-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organisation. Global Tuberculosis Report 2020.
- 6.Mandieka E, Saleh D, Chokshi AK, Rivera AS, Feinstein MJ. Latent tuberculosis infection and elevated incidence of hypertension. J. Am. Heart Assoc. 2020;9:e019144. doi: 10.1161/JAHA.120.019144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.López-López JP, et al. Tuberculosis and the heart. J. Am. Heart Assoc. 2021;10:e019435. doi: 10.1161/JAHA.120.019435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Basham CA, Smith SJ, Romanowski K, Johnston JC. Cardiovascular morbidity and mortality among persons diagnosed with tuberculosis: A systematic review and meta-analysis. PLoS ONE. 2020;15:e0235821. doi: 10.1371/journal.pone.0235821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sheu J-J, Chiou H-Y, Kang J-H, Chen Y-H, Lin H-C. Tuberculosis and the risk of ischemic stroke. Stroke. 2010;41:244–249. doi: 10.1161/STROKEAHA.109.567735. [DOI] [PubMed] [Google Scholar]
- 10.Huaman MA, et al. Tuberculosis and risk of acute myocardial infarction: A propensity score-matched analysis. Epidemiol. Infect. 2017;145:1363–1367. doi: 10.1017/S0950268817000279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang S-H, et al. Tuberculosis increases the risk of peripheral arterial disease: A nationwide population-based study. Respirology. 2017;22:1670–1676. doi: 10.1111/resp.13117. [DOI] [PubMed] [Google Scholar]
- 12.Danwang C, Bigna JJ, Awana AP, Nzalie RN-T, Robert A. Global epidemiology of venous thromboembolism in people with active tuberculosis: A systematic review and meta-analysis. J. Thromb. Thrombolysis. 2021;51:502–512. doi: 10.1007/s11239-020-02211-7. [DOI] [PubMed] [Google Scholar]
- 13.Romanowski K, et al. Long-term all-cause mortality in people treated for tuberculosis: A systematic review and meta-analysis. Lancet Infect. Dis. 2019;19:1129–1137. doi: 10.1016/S1473-3099(19)30309-3. [DOI] [PubMed] [Google Scholar]
- 14.Burusie A, Enquesilassie F, Addissie A, Dessalegn B, Lamaro T. Effect of smoking on tuberculosis treatment outcomes: A systematic review and meta-analysis. PLoS ONE. 2020;15:e0239333. doi: 10.1371/journal.pone.0239333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bates MN, et al. Risk of tuberculosis from exposure to tobacco smoke: A systematic review and meta-analysis. Arch Intern. Med. 2007;167:335–342. doi: 10.1001/archinte.167.4.335. [DOI] [PubMed] [Google Scholar]
- 16.Imtiaz, S. et al. Alcohol consumption as a risk factor for tuberculosis: Meta-analyses and burden of disease. Eur. Respir. J. 50, (2017). [DOI] [PMC free article] [PubMed]
- 17.Simou E, Britton J, Leonardi-Bee J. Alcohol consumption and risk of tuberculosis: A systematic review and meta-analysis. Int. J. Tuberc. Lung Dis. 2018;22:1277–1285. doi: 10.5588/ijtld.18.0092. [DOI] [PubMed] [Google Scholar]
- 18.Al-Rifai RH, Pearson F, Critchley JA, Abu-Raddad LJ. Association between diabetes mellitus and active tuberculosis: A systematic review and meta-analysis. PLoS ONE. 2017;12:e0187967. doi: 10.1371/journal.pone.0187967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huangfu P, Ugarte-Gil C, Golub J, Pearson F, Critchley J. The effects of diabetes on tuberculosis treatment outcomes: An updated systematic review and meta-analysis. Int. J. Tuberc. Lung Dis. 2019;23:783–796. doi: 10.5588/ijtld.18.0433. [DOI] [PubMed] [Google Scholar]
- 20.Badawi A, Gregg B, Vasileva D. Systematic analysis for the relationship between obesity and tuberculosis. Public Health. 2020;186:246–256. doi: 10.1016/j.puhe.2020.06.054. [DOI] [PubMed] [Google Scholar]
- 21.Yen Y-F, et al. Association of body mass index with tuberculosis mortality: A population-based follow-up study. Medicine. 2016;95:e2300. doi: 10.1097/MD.0000000000002300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alebel A, et al. Prevalence of diabetes mellitus among tuberculosis patients in Sub-Saharan Africa: A systematic review and meta-analysis of observational studies. BMC Infect. Dis. 2019;19:254. doi: 10.1186/s12879-019-3892-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noubiap JJ, et al. Global prevalence of diabetes in active tuberculosis: A systematic review and meta-analysis of data from 2·3 million patients with tuberculosis. Lancet Glob. Health. 2019;7:e448–e460. doi: 10.1016/S2214-109X(18)30487-X. [DOI] [PubMed] [Google Scholar]
- 24.Moher D, Liberati A, Tetzlaff J, Altman DG, Prisma Group Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moher D, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Conigrave KM, Hall WD, Saunders JB. The AUDIT questionnaire: Choosing a cut-off score. Addiction. 1995;90:1349–1356. doi: 10.1046/j.1360-0443.1995.901013496.x. [DOI] [PubMed] [Google Scholar]
- 27.Ewing JA. Detecting alcoholism: The CAGE questionnaire. JAMA. 1984;252:1905–1907. doi: 10.1001/jama.252.14.1905. [DOI] [PubMed] [Google Scholar]
- 28.Hoy D, et al. Assessing risk of bias in prevalence studies: Modification of an existing tool and evidence of interrater agreement. J. Clin. Epidemiol. 2012;65:934–939. doi: 10.1016/j.jclinepi.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 29.Lawson L, et al. Tuberculosis and diabetes in Nigerian patients with and without HIV. Int. J. Infect. Dis. 2017;61:121–125. doi: 10.1016/j.ijid.2017.06.014. [DOI] [PubMed] [Google Scholar]
- 30.Peltzer, K. Tuberculosis non-communicable disease comorbidity and multimorbidity in public primary care patients in South Africa. Afr. J. Prim. Health Care Fam. Med. 10, (2018). [DOI] [PMC free article] [PubMed]
- 31.Ekeke, N. et al. Screening for diabetes mellitus among tuberculosis patients in Southern Nigeria: A multi-centre implementation study under programme settings. Sci. Rep. 7, (2017). [DOI] [PMC free article] [PubMed]
- 32.Mabula PL, et al. Prevalence and risk factors for diabetes mellitus among tuberculosis patients in Moshi Municipal Council, Kilimanjaro Tanzania. East Afr. Health Res. J. 2021;5:69–74. doi: 10.24248/eahrj.v5i1.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kirenga, B. J. et al. Tuberculosis risk factors among tuberculosis patients in Kampala, Uganda: Implications for tuberculosis control. BMC Public Health15, (2015). [DOI] [PMC free article] [PubMed]
- 34.Ugarte-Gil C, et al. Diabetes mellitus among pulmonary tuberculosis patients from 4 tuberculosis-endemic countries: The TANDEM study. Clin. Infect. Dis. 2020;70:780–788. doi: 10.1093/cid/ciz284. [DOI] [PubMed] [Google Scholar]
- 35.Azeez A, Ndege J, Mutambayi R. Associated factors with unsuccessful tuberculosis treatment outcomes among tuberculosis/HIV coinfected patients with drug-resistant tuberculosis. Int. J. Mycobacteriol. 2018;7:347. doi: 10.4103/ijmy.ijmy_140_18. [DOI] [PubMed] [Google Scholar]
- 36.Dalton T, et al. Prevalence of and risk factors for resistance to second-line drugs in people with multidrug-resistant tuberculosis in eight countries: A prospective cohort study. Lancet. 2012;380:1406–1417. doi: 10.1016/S0140-6736(12)60734-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Whitehouse ER, Perrin N, Levitt N, Hill M, Farley JE. Cardiovascular risk prevalence in South Africans with drug-resistant tuberculosis: A cross-sectional study. Int. J. Tuberc. Lung Dis. 2019;23:587–593. doi: 10.5588/ijtld.18.0374. [DOI] [PubMed] [Google Scholar]
- 38.Magee, M. J. et al. Diabetes mellitus, smoking status, and rate of sputum culture conversion in patients with multidrug-resistant tuberculosis: A cohort study from the Country of Georgia. PLoS One9, (2014). [DOI] [PMC free article] [PubMed]
- 39.Molalign, S. & Wencheko, E. Risk factors of mortality in patients with multi-drug resistant TB. EJHD29, (2015).
- 40.Mollel EW, Chilongola JO. Predictors for mortality among multidrug-resistant tuberculosis patients in Tanzania. J. Trop. Med. 2017;2017:e9241238. doi: 10.1155/2017/9241238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shangase, Z., Tsoka-Gwegweni, J. M. & Okem, A. Smoking prevalence among inpatients with drug resistant tuberculosis in KwaZulu-Natal, South Africa. Tobacco Induced Diseases. 16, (2018).
- 42.Wotale TW, Terefe AN, Fufa JA. Modeling time to death of patients with multidrug-resistant tuberculosis at Saint Peter’s Specialized Hospital. J. Res. Health Sci. 2021;21:e00513. doi: 10.34172/jrhs.2021.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sidamo T, Shibeshi W, Yimer G, Aklillu E, Engidawork E. Explorative analysis of treatment outcomes of levofloxacin-and moxifloxacin-based regimens and outcome predictors in Ethiopian MDR-TB patients: A prospective observational cohort study. Infect. Drug Resistance. 2021;14:5473. doi: 10.2147/IDR.S342964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Isralls S, Baisley K, Ngam E, Grant AD, Millard J. QT interval prolongation in people treated with Bedaquiline for drug-resistant tuberculosis under programmatic conditions: A retrospective cohort study. Open Forum Infect. Dis. 2021;8:ofab413. doi: 10.1093/ofid/ofab413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Adegbite BR, et al. Epidemiological, mycobacteriological, and clinical characteristics of smoking pulmonary tuberculosis patients, in Lambaréné, Gabon: A cross-sectional study. Am. J. Trop. Med. Hyg. 2020;103:2501–2505. doi: 10.4269/ajtmh.20-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Conradie F, et al. Prevalence and incidence of symmetrical symptomatic peripheral neuropathy in patients with multidrugresistant TB. S. Afr. Med. J. 2014;104:24–26. doi: 10.7196/samj.6455. [DOI] [PubMed] [Google Scholar]
- 47.Kubjane M, et al. Tuberculosis, human immunodeficiency virus, and the association with transient hyperglycemia in Periurban South Africa. Clin. Infect. Dis. 2020;71:1080–1088. doi: 10.1093/cid/ciz928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mitrani L, et al. Diverse clinical and social circumstances: Developing patient-centred care for DR-TB patients in South Africa. Public Health Action. 2021;11:120–125. doi: 10.5588/pha.20.0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oumer N, Atnafu DD, Worku GT, Tsehay AK. Determinants of multi-drug resistant tuberculosis in four treatment centers of Eastern Amhara, Ethiopia: A case-control study. J. Infect. Developing Countries. 2021;15:687–695. doi: 10.3855/jidc.13265. [DOI] [PubMed] [Google Scholar]
- 50.Kassa GM, Merid MW, Muluneh AG. Khat chewing and clinical conditions determine the epidemiology of primary drug resistance tuberculosis in Amhara Region of Ethiopia: A multicenter study. Infect Drug Resist. 2021;14:2449–2460. doi: 10.2147/IDR.S316268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ali, M. H., Alrasheedy, A. A., Hassali, M. A., Kibuule, D. & Godman, B. Predictors of multidrug-resistant tuberculosis (MDR-TB) in Sudan. Antibiotics (Basel). 8, (2019). [DOI] [PMC free article] [PubMed]
- 52.Racil H, Mami M, Chabbou A. Predictive factors for recurrence of pulmonary tuberculosis in Tunisia: A retrospective study. Rev. Mal. Respir. 2012;29:412–418. doi: 10.1016/j.rmr.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 53.Baik Y, et al. A clinical score for identifying active tuberculosis while awaiting microbiological results: Development and validation of a multivariable prediction model in sub-Saharan Africa. PLoS Med. 2020;17:e1003420. doi: 10.1371/journal.pmed.1003420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pizzol D, et al. Prevalence of diabetes mellitus in newly diagnosed pulmonary tuberculosis in Beira, Mozambique. Afr. Health Sci. 2017;17:773–779. doi: 10.4314/ahs.v17i3.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Balkissou A, et al. Residual pleural opacity in patients treated for pleural tuberculosis in Yaounde. Rev. Pneumol. Clin. 2015;72:115–121. doi: 10.1016/j.pneumo.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 56.Mburu JW, Kingwara L, Ester M, Andrew N. Prognostic factors among TB and TB/DM comorbidity among patients on short course regimen within Nairobi and Kiambu counties in Kenya. J. Clin. Tuberculosis Other Mycobacterial Diseases. 2018;12:9–13. doi: 10.1016/j.jctube.2018.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Umanah T, Ncayiyana J, Padanilam X, Nyasulu PS. Treatment outcomes in multidrug resistant tuberculosis-human immunodeficiency virus Co-infected patients on anti-retroviral therapy at Sizwe Tropical Disease Hospital Johannesburg, South Africa. BMC Infect. Dis. 2015;15:478. doi: 10.1186/s12879-015-1214-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Assal GME, AbdelFattah EB, Nabil MM. Characteristics and outcome determinants in patients with pulmonary tuberculosis in ICU. Egypt. J. Chest Diseases Tuberculosis. 2021;70:89. [Google Scholar]
- 59.Oni T, et al. Patterns of HIV, TB, and non-communicable disease multi-morbidity in peri-urban South Africa—A cross sectional study. BMC Infect. Dis. 2015;15:20. doi: 10.1186/s12879-015-0750-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dayyab FM, Iliyasu G, Ahmad BG, Habib AG. Early safety and efficacy of linezolid-based combination therapy among patients with drug-resistant tuberculosis in North-western Nigeria. Int. J. Mycobacteriol. 2021;10:129–135. doi: 10.4103/ijmy.ijmy_57_21. [DOI] [PubMed] [Google Scholar]
- 61.Araia ZZ, et al. Diabetes mellitus and its associated factors in tuberculosis patients in maekel region, eritrea: Analytical cross-sectional study. Diabetes Metabolic Syndrome Obesity Targets Ther. 2021;14:515. doi: 10.2147/DMSO.S293557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Musuenge BB, Poda GG, Chen P-C. Nutritional status of patients with tuberculosis and associated factors in the Health Centre Region of Burkina Faso. Nutrients. 2020;12:2540. doi: 10.3390/nu12092540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Murphy JD, Liu B, Parascandola M. Smoking and HIV in Sub-Saharan Africa: A 25-country analysis of the demographic health surveys. Nicotine Tob. Res. 2019;21:1093–1102. doi: 10.1093/ntr/nty176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dessie G, et al. A systematic analysis on prevalence and sub-regional distribution of undiagnosed diabetes mellitus among adults in African countries. J. Diabetes Metab. Disord. 2020;19:1931–1941. doi: 10.1007/s40200-020-00635-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Amere GA, Nayak P, Salindri AD, Narayan KMV, Magee MJ. Contribution of smoking to tuberculosis incidence and mortality in high-tuberculosis-burden countries. Am. J. Epidemiol. 2018;187:1846–1855. doi: 10.1093/aje/kwy081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kyu HH, et al. The global burden of tuberculosis: Results from the Global Burden of Disease Study 2015. Lancet. Infect. Dis. 2018;18:261–284. doi: 10.1016/S1473-3099(17)30703-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wagnew F, et al. Meta-analysis of the prevalence of tuberculosis in diabetic patients and its association with cigarette smoking in African and Asian countries. BMC. Res. Notes. 2018;11:298. doi: 10.1186/s13104-018-3390-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Baker MA, et al. The impact of diabetes on tuberculosis treatment outcomes: A systematic review. BMC Med. 2011;9:81. doi: 10.1186/1741-7015-9-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Marshall, A.-M. et al. Smoking prevalence among tuberculosis patients: A crosssectional study in Bangladesh and Pakistan. Tob. Induc. Dis. 18, (2020). [DOI] [PMC free article] [PubMed]
- 70.Seegert AB, Rudolf F, Wejse C, Neupane D. Tuberculosis and hypertension—A systematic review of the literature. Int. J. Infect. Dis. 2017;56:54–61. doi: 10.1016/j.ijid.2016.12.016. [DOI] [PubMed] [Google Scholar]
- 71.Ataklte F, et al. Burden of undiagnosed hypertension in sub-Saharan Africa. Hypertension. 2015;65:291–298. doi: 10.1161/HYPERTENSIONAHA.114.04394. [DOI] [PubMed] [Google Scholar]
- 72.Akpa OM, et al. Regional patterns and association between obesity and hypertension in Africa: Evidence from the H3Africa Chair study. Hypertension. 2020;75:1167–1178. doi: 10.1161/HYPERTENSIONAHA.119.14147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Davis K, et al. Association between HIV infection and hypertension: A global systematic review and meta-analysis of cross-sectional studies. BMC Med. 2021;19:105. doi: 10.1186/s12916-021-01978-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bosu WK, Reilly ST, Aheto JMK, Zucchelli E. Hypertension in older adults in Africa: A systematic review and meta-analysis. PLoS ONE. 2019;14:e0214934. doi: 10.1371/journal.pone.0214934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kayima J, Wanyenze RK, Katamba A, Leontsini E, Nuwaha F. Hypertension awareness, treatment and control in Africa: A systematic review. BMC Cardiovasc. Disord. 2013;13:1–11. doi: 10.1186/1471-2261-13-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ragan EJ, et al. The impact of alcohol use on tuberculosis treatment outcomes: A systematic review and meta-analysis. Int. J. Tuberc. Lung Dis. 2020;24:73–82. doi: 10.5588/ijtld.19.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.World Health Organization . Global Status Report on Alcohol and Health 2018. World Health Organization; 2019. [Google Scholar]
- 78.Necho M, et al. Prevalence and associated factors for alcohol use disorder among tuberculosis patients: A systematic review and meta-analysis study. Substance Abuse Treatment Prevent. Policy. 2021;16:2. doi: 10.1186/s13011-020-00335-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mons U, et al. Impact of smoking and smoking cessation on cardiovascular events and mortality among older adults: Meta-analysis of individual participant data from prospective cohort studies of the CHANCES consortium. BMJ. 2015;350:h1551. doi: 10.1136/bmj.h1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Leopold JA, Antman EM. Ideal cardiovascular health in former smokers. J. Clin. Med. 2021;10:2450. doi: 10.3390/jcm10112450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Boillat-Blanco N, et al. Transient hyperglycemia in patients with tuberculosis in tanzania: implications for diabetes screening algorithms. J. Infect. Dis. 2016;213:1163–1172. doi: 10.1093/infdis/jiv568. [DOI] [PubMed] [Google Scholar]
- 82.Ade S, et al. Low prevalence of diabetes mellitus in patients with tuberculosis in Cotonou, Benin. Public Health Action. 2015;5:147–149. doi: 10.5588/pha.14.0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mcebula V, Crowther NJ, Nagel SE, George JA. Diabetes and abnormal glucose tolerance in subjects with tuberculosis in a South African urban center. Int. J. Tuberc. Lung Dis. 2017;21:208–213. doi: 10.5588/ijtld.15.0831. [DOI] [PubMed] [Google Scholar]
- 84.Menon S, et al. The epidemiology of tuberculosis-associated hyperglycemia in individuals newly screened for type 2 diabetes mellitus: Systematic review and meta-analysis. BMC Infect. Dis. 2020;20:937. doi: 10.1186/s12879-020-05512-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Owiti P, et al. Diabetes and pre-diabetes in tuberculosis patients in western Kenya using point-of-care glycated haemoglobin. Public Health Action. 2017;7:147–154. doi: 10.5588/pha.16.0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nzali FN, Temgoua MN, Tochie JN, Choukem SP. Lifestyle and Epidemiology: Poverty and Cardiovascular Diseases a Double Burden in African Populations. IntechOpen; 2021. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this article and its supplementary information files.