Abstract
Rationale & Objective
Chronic kidney disease (CKD) is associated with impaired physical performance. However, the association between albuminuria, a marker of vascular endothelial dysfunction, and physical performance has not been fully characterized. We hypothesized that estimated glomerular filtration rate (eGFR) and albuminuria would be independently associated with physical performance.
Study Design
Cross-sectional analysis.
Setting & Participants
A total of 571 adults with and without CKD.
Predictors
Creatinine-based eGFR (eGFRCr) and cystatin C-based eGFR (eGFRCysC) and urine albumin to creatinine ratio (UACR).
Outcome
Short Physical Performance Battery (SPPB).
Analytical Approach
Univariate and multivariable logistic regression models were used to examine associations of eGFR and UACR with impaired physical performance.
Results
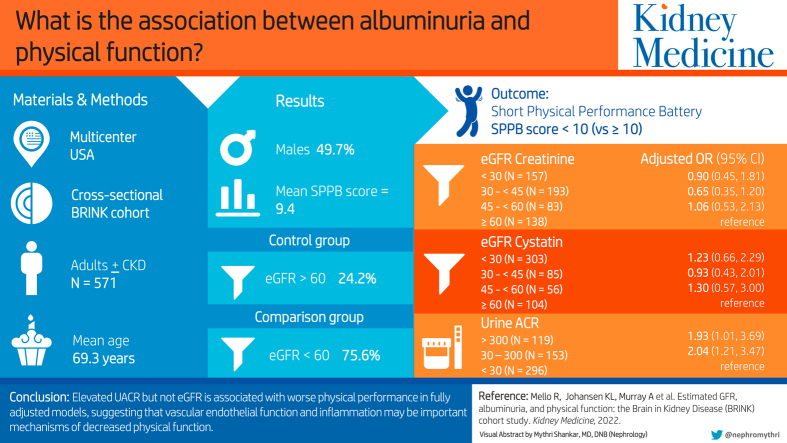
Of the 571 participants (mean age, 69.3 years), 157 (27.5%) had eGFRCr (mL/min/1.73m2) <30, 276 (48.3%) had eGFRCr 30-<60, and 138 (24.2%) had eGFRCr ≥60; 303 (55.3%) participants had eGFRcysC <30, 141 (25.7%) had eGFRcysC 30-<60, and 104 (19.0%) had eGFRcysC ≥60. Impaired physical performance was observed in 222 (38.9%) participants. Separate univariate analyses showed that lower eGFRCr, lower eGFRCysC, and higher UACR were associated with higher odds of impaired physical performance. In the adjusted model with eGFRCr or eGFRCysC, UACR, and covariates, UACR retained a statistically significant association with impaired physical performance (adjusted odds ratio [OR], 2.04; 95% confidence interval [CI], 1.21-3.47 for UACR from 30-300 mg/g vs <30 mg/g and adjusted OR, 1.93; 95% CI, 1.01-3.69 for UACR >300 mg/g vs <30 mg/g), but eGFRCr and eGFRCysC did not.
Limitations
Cross-sectional analysis, estimated rather than measured GFR.
Conclusions
Only UACR was associated with worse physical performance in the fully adjusted model, suggesting that vascular endothelial function and inflammation may be important mechanisms of decreased physical function. Similar results were found using eGFRCr or eGFRCysC, suggesting that confounding based on muscle mass does not explain the lack of an association between eGFRCr and physical performance.
Index Words: Albuminuria, eGFR, physical performance, frailty, CKD
Visual Abstract
Plain-Language Summary.
The kidney research community has recognized the need to focus on patient-centered measures in patients with chronic kidney disease (CKD), with the goal of improving the quality of life. Better physical performance is associated with greater independence and improved quality of life. The association between CKD and physical performance is complex given the state of increased inflammation and comorbid conditions. In this study, we found that albuminuria was associated with decreased physcial performance after adjusting for potential confounders and that kidney function estimated using creatinine-based estimated glomerular filtration rate (eGFR) or cystatin C-based eGFR were not. This finding suggests that albuminuria and endothelial function may be important therapeutic targets to reduce the risk of impaired physical function in patients with CKD.
Half of the US population is expected to develop chronic kidney disease (CKD) during their lifetime,1 and nearly 500 million adults are living with CKD globally.2 The association between CKD and a higher risk of mortality has been well described;3, 4, 5 however, the kidney research community has recognized a need to focus on patient-centered measures, such as maintaining higher levels of physical function. Better physical performance is associated with greater independence and better patient-reported quality of life,6 and worse physical performance is associated with poor clinical outcomes and mortality in patients with CKD.7,8
The association between CKD and outcomes such as physical performance may be complicated given the multitude of potential contributors, such as decreased glomerular filtration rate (GFR), a state of heightened inflammation, and comorbid conditions. Therefore, the identification of factors associated with decreased physical performance may help in the clinical care and decision-making for patients with CKD. Furthermore, CKD has been linked to a higher incidence and prevalence of frailty, which includes physical activity and walking speed.9,10 However, whether these associations are related to the degree of reduction in GFR is unclear. Other studies have reported an association between lower estimated GFR (eGFR) and decreased physical performance.11, 12, 13 In the Health, Aging, and Body Composition (Health ABC) study, individuals with higher cystatin C (CysC) levels had a higher risk of functional limitation independent of comorbid conditions, but the association was attenuated by markers of inflammation.13 Importantly, this study included few patients with advanced CKD. In participants of Invecchiare in Chianti (InCHIANTI), another cohort study of community-dwelling elders, lower creatinine clearance was associated with slower walking speed and reduced quadriceps strength in a graded manner.14 Conversely, Walker et al15 found no association between creatinine-based eGFR (eGFRCr) and physical function in participants with advanced CKD in the Canadian Frailty Observation and Interventions Trial (CANFit), whereas diabetes was strongly associated with lower physical performance. Among participants in the Cardiovascular Health Study (CHS), lower eGFR estimated using cystatin C (eGFRCysC) was associated with a higher incidence and prevalence of frailty, but eGFRCr was not,10 suggesting that the lack of association between eGFR and outcomes that include measures of physical performance might be related to confounding based on the association between serum creatinine concentration (and thus eGFRCr) and muscle mass.
In addition to the uncertainty regarding a graded association between eGFR and physical performance, the association between albuminuria and physical performance has not been well explored. Albuminuria is associated with inflammation in patients with CKD16 and has been shown to be strongly associated with progression of kidney disease, progression of cardiovascular disease, cognitive impairment, and mortality.17, 18, 19, 20 However, few previous studies evaluating the relationship between CKD and physical performance have included measures of albuminuria. Interestingly, one of the few studies investigating the associations between CKD and functional status that included albuminuria and eGFR in their multivariable analyses found that albuminuria, but not eGFR, was associated with slow gait speed.21
To better understand the relatioship among kidney function, albuminuria, and physical performance, we examined the association between eGFRCr and reduced physical performance with and without albuminuria in the models, measured as urine albumin to creatinine ratio (UACR). We also examined whether these associations differed when GFR was estimated using cystatin C rather than using creatinine.
Methods
Study Participants
The Brain in Kidney Disease (BRINK) study is a multisite observational cohort study that was designed to examine the association between CKD and cognitive impairment, as well as other geriatric outcomes. Participants included 574 community-dwelling adults in Minnesota of ≥45 years of age.22 To ensure that participants with a wide range of eGFR were included, participants were enrolled in groups based on eGFR: <45 mL/min/1.73m2, 45-<60 mL/min/1.73m2, and ≥60 mL/min/1.73m2 (controls). No participant had received a transplant or was undergoing any form of kidney replacement therapy at the time of enrollment. The Institutional Review Boards of each institution approved the study, and all participants provided written informed consent (Veterans Administration IRB #4364-B, Hennepin Healthcare Research Institute IRB #11-3393, University of Minnesota IRB #1203M11122, Health Partners IRB #A12-282).
Laboratory and Clinical Evaluation
Laboratory values obtained at the baseline assessment included serum creatinine (calibrated to IDMS), cystatin C, hemoglobin, albumin, hemoglobin A1c, and urine albumin and creatinine concentrations. Nonfasting blood samples were obtained and stored at -80° within 2 hours of collection. All analyses were performed centrally by a CLIA-certified laboratory. Cystatin C was measured as a 2-plex by Luminex using magnetic beads (R&D Systems). The CKD-EPI equation was used to calculate eGFRCr without inclusion of the coefficient for Black race. The CKD-EPI cystatin C equation was used to calculate eGFRcysC.23, 24, 25
Comorbid conditions, including a history of stroke or transient ischemic attack (TIA), were obtained from the participants’ electronic medical records and medical history questionnaires. Diabetes mellitus (DM) was defined as a nonfasting glucose level of ≥200 mg/dL, A1c of ≥6.5%, self-reported DM, or the prescription of DM medications. For this analysis, “controlled” diabetes was defined as a hemoglobin A1c of <7.5%. Body mass index (BMI) was calculated from measured height and weight. Blood pressure was measured 3 times using an automated cuff while the participant sat at rest. Years of education, tobacco use history, cardiovascular disease (CVD), and congestive heart failure (CHF) were obtained from the medical history questionnaire.22,26
Physical Performance
Physical performance was assessed using the Short Physical Performance Battery (SPPB),27 which includes tests of balance, chair standing, and gait speed. Balance is assessed while participants stand with their feet in a side-by-side position, a semi-tandem position, and a tandem position. Participants are asked to stand from a chair 5 times as quickly as they are able without using their arms, and the time taken to stand is recorded in seconds. Gait speed (meters/seconds) is measured over 4 meters at the participants’ usual speed. Each test is scored on a scale of 0-4 (with 4 indicating best performance) and summed for a maximum total score of 12. We considered an SPPB score of ≥10 to represent normal physical performance. The same cutoff was used in a previous study investigating physical performance in patients with CKD.15 Further, an SPPB score of <10 has been shown to predict admission to a long-term care facility and all-cause mortality among older community-dwelling adults.27,28
Statistical Analysis
This cross-sectional analysis was performed using baseline data from the BRINK study cohort. The primary predictors were eGFRCr, eGFRCysC, and UACR. The primary outcome was an SPPB score of <10 (vs SPPB score of ≥10); the secondary outcomes were total SPPB score and gait speed. Covariates examined included sex, Black race (vs other races), hemoglobin ≤12 g/dL vs > 12 g/dL, serum bicarbonate <20 mEq/L vs ≥20 mEq/L, tobacco use (never, former, or current), serum albumin <4 g/dL vs ≥4 g/dL, BMI <25, 25-<30, or ≥30 kg/m2, pulse pressure (systolic blood pressure minus diastolic blood pressure) as a continuous variable, DM (none, controlled, or not controlled), and history (yes or no) of CHF, CVD, and stroke or TIA. Age and age2 were included as continuous variables given a nonlinear association between age and SPPB.
Univariate logistic regression models were used to assess whether eGFRCr, eGFRCysC, UACR, or other covariates were associated with low SPPB scores. Multivariable logistic regression was then used to examine the association between the primary predictors and low SPPB score adjusted for potential confounders. Four multivariable models were developed: 1) eGFRCr without UACR, 2) eGFRCr with UACR, 3) eGFRCysC without UACR, and 4) eGFRCysC with UACR.
To test for differential associations between covariates and low SPPB scores according to eGFRCr, eGFRCysC, or UACR levels in the overall BRINK cohort, separate logistic regression models for the binary SPPB outcome were fit for each primary predictor and covariate with the following interaction terms: 4-level eGFRCr, 4-level eGFRCysC, and 3-level UACR. Interactions between 3-level UACR and both 4-level eGFRCr and 4-level eGFRCysC were also modeled. Multiple linear regression models were used to evaluate the associations between primary predicators and secondary outcomes of total SPPB score and gait speed.
In the sensitivity analyses, ordinal multivariable logistic regression models were used to evaluate the associations of primary predictors and covariates with a 4-level ordinal SBBP score (0-6, 7-9, 10-11, and 12) rather than a dichotomous variable. Sensitivity analysis models for primary and secondary outcomes included the aforementioned logistic and linear regression models limited to participants with eGFR <60 mL/min/1.73m2. Logistic and linear regression models with UACR modeled as continuous on the natural log (Ln) scale—instead of as a 3-level categorical variable—were also run as a sensitivity analysis. An alpha significance level of 0.05 identified statistically significant results. No adjustments for multiple comparisons were applied.29 All statistical analyses were conducted using SAS software (v9.4, copyright © 2016 by SAS Institute, Inc.).
Results
Participant Characteristics
Three participants were missing baseline SPPB scores and were excluded; thus, 571 participants were included in the analytical cohort. Table 1 summarizes the participants’ characteristics and SPPB scores overall and by eGFRCr category. The mean age of the participants was 69.3 ± 9.8 years, 49.7% were male, and 15.8% were Black. Of the 571 participants, 433 (75.8%) had eGFRCr <60 mL/min/1.73m2 and 138 (24.2%) had eGFRCr >60 mL/min/1.73m2 (controls). Within the CKD group (eGFR <60 mL/min/1.73m2), 157 (27.5%) had eGFRCr <30 mL/min/1.73m2, 193 (33.8%) had eGFRCr 30-≤ 45 mL/min/1.73m2, and 83 (14.5%) had eGFRCr 45-< 60 mL/min/1.73m2. The mean SPPB score for the cohort was 9.4. Overall, 222 participants (38.9%) had impaired physical performance (SPPB score <10). Stratified by CKD or no CKD, 43.4% (188/433) of participants in the CKD group had reduced physical performance compared with 24.6% (34/138) of participants without CKD.
Table 1.
Characteristics of the Cohort Overall (N = 571) and by eGFRCr Category
| Outcome Covariates and Predictors |
Overall N = 571 |
eGFRCr <30 N = 157 |
eGFRCr 30 – <45 N = 193 |
eGFRCr 45 –<60 N = 83 |
eGFRCr ≥ 60 N = 138 |
|---|---|---|---|---|---|
| SPPB group: binary N (%) | |||||
| 0-9 | 222 (38.9) | 82 (52.2) | 73 (37.8) | 33 (39.8) | 34 (24.6) |
| 10-12 | 349 (61.1) | 75 (47.8) | 120 (62.2) | 50 (60.2) | 104 (75.4) |
| SPPB group: 4-level N (%) | |||||
| 0-6 | 87 (15.2) | 32 (20.4) | 29 (15.0) | 14 (16.9) | 12 (8.7) |
| 7-9 | 135 (23.6) | 50 (31.9) | 44 (22.8) | 19 (22.9) | 22 (15.9) |
| 10-11 | 189 (33.1) | 50 (31.9) | 67 (34.7) | 26 (31.3) | 46 (33.3) |
| 12 | 160 (28.0) | 25 (15.9) | 53 (27.5) | 24 (28.9) | 58 (42.0) |
| SPPB total score: mean (SD) | 9.4 (2.7) | 8.6 (3.0) | 9.5 (2.5) | 9.3 (2.8) | 10.2 (2.3) |
| Gait speed: m/sec, mean (SD) | 0.96 (0.27) | 0.92 (0.27) | 0.93 (0.26) | 0.96 (0.30) | 1.03 (0.26) |
| eGFRCrgroup: mL/min/1.73m2, 4-level N (%) | |||||
| <30 | 157 (27.5) | 157 (100) | |||
| 30-<45 | 193 (33.8) | 193 (100) | |||
| 45-<60 | 83 (14.5) | 83 (100) | |||
| ≥60 | 138 (24.2) | 138 (100) | |||
| eGFRCr: mL/min/1.73m2, mean (SD) | 45.0 (23.2) | 21.2 (6.1) | 36.8 (4.1) | 50.9 (3.9) | 80.1 (12.3) |
| eGFRCysC: mL/min/1.73m2, 4-level N (%) | |||||
| < 30 | 303 (55.3) | 126 (84.0) | 94 (50.0) | 23 (28.8) | 60 (46.2) |
| 30-<45 | 85 (15.5) | 19 (12.7) | 35 (18.6) | 28 (35.0) | 3 (2.3) |
| 45-<60 | 56 (10.2) | 2 (1.3) | 34 (18.1) | 11 (13.8) | 9 (6.9) |
| ≥60 | 104 (19.0) | 3 (2.0) | 25 (13.3) | 18 (22.5) | 58 (44.6) |
| eGFRCysC: mL/min/1.73m2, mean (SD) | 36.1 (31.9) | 19.5 (15.4) | 33.7 (24.6) | 42.9 (29.0) | 54.5 (44.1) |
| UACR group: mg/g, 3-level N (%) | |||||
| <30 | 296 (52.1) | 33 (21.2) | 91 (47.2) | 56 (68.3) | 116 (84.7) |
| 30-300 | 153 (26.9) | 52 (33.3) | 63 (32.6) | 20 (24.4) | 18 (13.1) |
| >300 | 119 (21.0) | 71 (45.5) | 39 (20.2) | 6 (7.3) | 3 (2.2) |
| UACR: mg/g, median [IQR] | 26.3 [0, 193] | 229 [35, 1250] | 38.5 [0.0, 189] | 0.0 [0.0, 43] | 0.0 [0.0, 11] |
| Hgb: g/dL, N (%) | |||||
| ≤12 | 172 (30.2) | 91 (58.0) | 56 (29.2) | 13 (15.8) | 12 (8.7) |
| >12 | 397 (69.8) | 66 (42.0) | 136 (70.8) | 69 (84.2) | 126 (91.3) |
| Hgb: g/dL, mean (SD) | 12.95 (1.71) | 11.8 (1.8) | 13.0 (1.6) | 13.4 (1.4) | 13.8 (1.2) |
| Bicarbonate (CO2): mEq/L, N (%) | |||||
| <20 | 24 (4.2) | 18 (11.5) | 5 (2.6) | 0 (0.0) | 1 (0.7) |
| ≥20 | 546 (95.8) | 139 (88.5) | 188 (97.4) | 82 (100.0) | 137 (99.3) |
| Bicarbonate (CO2): mEq/L mean (SD) | 25.8 (3.4) | 24.4 (3.8) | 25.4 (3.0) | 26.2 (2.7) | 27.6 (2.9) |
| SBP group: mmHg, 3-level N (%) | |||||
| <120 | 154 (27.1) | 41 (26.3) | 45 (23.3) | 26 (31.3) | 42 (30.7) |
| 120-<140 | 218 (38.3) | 44 (28.2) | 80 (41.5) | 36 (43.4) | 58 (42.3) |
| ≥140 | 197 (34.6) | 71 (45.5) | 68 (35.2) | 21 (25.3) | 37 (27.0) |
| SBP: mmHg, mean (SD) | 132.3 (18.6) | 136.7 (21.8) | 133.1 (17.2) | 129.2 (16.5) | 128.1 (16.9) |
| Pulse pressure: mmHg | 63.5 (16.7) | 67.9 (19.2) | 64.3 (16.3) | 60.6 (15.3) | 59.0 (13.4) |
| Serum albumin group: mg/dL, N (%) | |||||
| <4 | 121 (21.3) | 58 (37.2) | 37 (19.2) | 16 (19.5) | 10 (7.3) |
| ≥4 | 448 (78.7) | 98 (62.8) | 156 (80.8) | 66 (80.5) | 128 (92.7) |
| Serum albumin: mg/dL mean (SD) | 4.2 (0.39) | 4.1 (0.45) | 4.3 (0.38) | 4.3 (0.36) | 4.3 (0.29) |
| BMI group: kg/m2, 3-level N (%) | |||||
| Not overweight: BMI <25 | 107 (18.8) | 29 (18.6) | 29 (15.0) | 11 (13.4) | 38 (27.7) |
| Overweight: BMI 25-<30 | 160 (28.2) | 45 (28.8) | 49 (25.4) | 27 (32.9) | 39 (28.5) |
| Obese: BMI ≥30 | 301 (53.0) | 82 (52.6) | 115 (59.6) | 44 (53.7) | 60 (43.8) |
| BMI: kg/m2, mean (SD) | 31.4 (7.3) | 31.3 (6.8) | 32.3 (7.5) | 32.5 (8.1) | 29.6 (6.6) |
| Tobacco use group: N (%) | |||||
| Never | 247 (43.3) | 53 (33.8) | 80 (41.5) | 42 (50.6) | 72 (52.2) |
| Previous | 265 (46.4) | 78 (49.7) | 96 (49.7) | 38 (45.8) | 53 (38.4) |
| Current | 59 (10.3) | 26 (16.6) | 17 (8.8) | 3 (3.6) | 13 (9.4) |
| Diabetes group, N (%) | |||||
| No DM | 296 (51.8) | 62 (39.5) | 107 (55.4) | 46 (55.4) | 81 (58.7) |
| Controlled DM, A1c <7.5% | 163 (28.6) | 61 (38.8) | 45 (23.3) | 23 (27.7) | 34 (24.6) |
| Uncontrolled DM, A1c ≥7.5% | 112 (19.6) | 34 (21.7) | 41 (21.2) | 14 (16.9) | 23 (16.7) |
| History of CVD | |||||
| No | 330 (57.8) | 70 (44.6) | 98 (50.8) | 53 (63.9) | 109 (79.0) |
| Yes | 241 (42.2) | 87 (55.4) | 95 (49.2) | 30 (36.1) | 29 (21.0) |
| History of CHF | |||||
| No | 489 (85.6) | 128 (81.5) | 156 (80.8) | 72 (86.8) | 133 (96.4) |
| Yes | 82 (14.4) | 29 (18.5) | 37 (19.2) | 11 (13.2) | 5 (3.6) |
| History of stroke/TIA | |||||
| No | 478 (83.7) | 126 (80.3) | 157 (81.3) | 70 (84.3) | 125 (90.6) |
| Yes | 93 (16.3) | 31 (19.7) | 36 (18.7) | 13 (15.7) | 13 (9.4) |
| Age: mean (SD) | 69.3 (9.8) | 68.7 (10.3) | 70.5 (9.5) | 70.6 (9.7) | 67.3 (9.3) |
| Years of education: mean (SD) | 14.3 (2.8) | 13.4 (2.7) | 14.1 (2.8) | 14.7 (2.9) | 15.2 (2.5) |
| Black race: N (%) | 90 (15.8) | 43 (27.4) | 20 (10.4) | 7 (8.4) | 20 (14.5) |
| Sex: N (%) | |||||
| Male | 284 (49.7) | 92 (58.6) | 89 (46.1) | 46 (55.4) | 57 (41.3) |
| Female | 287 (50.3) | 65 (41.4) | 104 (53.9) | 37 (44.6) | 81 (58.7) |
Abbreviations: BMI, body mass index; CHF, congestive heart failure; CVD, cardiovascular disease; GFRCr, creatinine-based estimated glomerular filtration rate; Hgb, hemoglobin; IQR; interquartile range; SBP, systolic blood pressure; SD, standard deviation; SPPB, Short Physical Performance Battery; TIA, transient ischemic attack; UACR, urine albumin to creatinine ratio.
Twenty-three participants were missing cystatin C values at baseline. Thus, 548 participants were included in analyses using eGFRCysC. Of note, among the participants with both creatinine and cystatin C values (n = 548), more participants were classified as having CKD as defined by an eGFR of <60 mL/min/1.73m2 and estimated with eGFRCysC (444, 81.0%) than when estimated with eGFRCr (433, 75.8%). Table S1 summarizes the participants’ characteristics and SPPB scores by UACR category for the 568 participants with nonmissing UACR data.
Univariate Analyses of the Association Between Kidney Function and Physical Performance
In separate univariate analyses, lower eGFRCr and eGFRcysC and higher UACR were associated with an increased odds of lower physical performance (Table 2). For example, eGFRCr 45-<60, 30–<45, and <30 mL/min/1.73m2 were associated with a 2.02- (95% CI, 1.12-3.63), 1.86- (95% CI, 1.15-3.02), and 3.34-fold (95% CI, 2.03-5.50) higher odds, respectively, of a low SPPB score (SPPB score <10) compared with participants with eGFRCr >60 mL/min/1.73m2. Results were similar for eGFRCysC. UACR 30-300 and >300 mg/g were associated with higher odds of low SPPB score (OR, 2.54; 95% CI, 1.70-3.80 and OR, 2.17; 95% CI, 1.40-3.37) compared with participants with UACR <30 mg/g. Results were similar when SPPB was treated as a 4-level ordinal variable (Table S2). For example, eGFRCr 45-<60, 30-<45, and <30 mL/min/1.73m2 were associated with 1.96 (95% CI, 1.19-3.23), 1.90 (95% CI, 1.27-2.84), and 3.32 (95% CI, 2.17-5.07) higher proportional odds of being grouped in the lower SPPB category vs the higher category, respectively. Similarly, UACR 30-300 and >300 mg/g vs lower UACR categories were associated with higher proportional odds of lower SPPB scores (proportional OR, 2.43; 95% CI, 1.70-3.47 and OR, 2.21; 95% CI, 1.50-3.25).
Table 2.
Comparison of the Univariate and Multivariable Associations Between Different Measures of Kidney Function and Low Physical Function (SPPB Score < 10)
| Measure of Kidney Function | Unadjusted OR (95% CI) | Adjusteda OR (95% CI) in Models Without UACR | Adjusteda OR (95% CI) in Models with UACR |
|---|---|---|---|
| eGFRCrmL/min/1.73m2reference = ≥60 | |||
| <30 | 3.34 (2.03, 5.50) | 1.23 (0.64, 2.36) | 0.90 (0.45, 1.81) |
| 30-<45 | 1.86 (1.15, 3.02) | 0.81 (0.45, 1.47) | 0.65 (0.35, 1.20) |
| 45-<60 | 2.02 (1.12, 3.63) | 1.16 (0.58, 2.31) | 1.06 (0.53, 2.13) |
| UACR mg/g reference = <30 | |||
| >300 | 2.17 (1.40, 3.37) | 1.93 (1.01, 3.69)b | |
| 30-300 | 2.54 (1.70, 3.80) | 2.04 (1.21, 3.47)b | |
| eGFRCysCmL/min/1.73m2reference = ≥60 | |||
| <30 | 2.61 (1.57, 4.34) | 1.35 (0.73, 2.48) | 1.23 (0.66, 2.29) |
| 30-<45 | 1.91 (1.01, 3.61) | 1.01 (0.48, 2.15) | 0.93 (0.43, 2.01) |
| 45-<60 | 2.16 (1.07, 4.36) | 1.33 (0.58, 3.04) | 1.30 (0.57, 3.00) |
| UACR mg/g reference = <30 | |||
| >300 | 1.62 (0.86, 3.05)c | ||
| 30-300 | 1.84 (1.09, 3.11)c |
Abbreviations: CI, confidence interval; GFRCr, creatinine-based estimated glomerular filtration rate; eGFRCysC, cystatin C-based estimated glomerular filtration rate; OR, odds ratio; UACR, urine albumin to creatinine ratio.
Model adjusted for hemoglobin, serum bicarbonate, serum albumin, body mass index, diabetes (none, controlled, not controlled), pulse pressure, cardiovascular disease, congestive heart failure, stroke or transient ischemic attack, smoking status (current, former, never), race (black vs other), sex, age, age2,years of education.
Adjusted OR (95% CI) for UACR in model with eGFRCr and covariates listed above.
Adjusted OR (95% CI) for UACR in model with eGFRCysC and covariates listed above.
Multivariable Analyses of the Association Between Kidney Function and Physical Performance
In multivariable analysis, with and without UACR in the model, eGFRCr was no longer significantly associated with low SPPB scores (Table 2). Conversely, a higher UACR maintained a significant association with lower SPPB scores when adjusted for eGFRCr and covariates. A UACR of 30-300 mg/g vs <30 mg/g was associated with twice the odds of a low SPPB score (adjusted OR, 2.04; 95% CI, 1.21-3.47), and a UACR of >300 mg/g vs UACR <30 mg/g was associated with low SPPB scores (adjusted OR, 1.93; 95% CI, 1.01-3.69) (Table 2). Significant risk factors for lower physical performance included female sex, obesity (BMI ≥30 kg/m2), low bicarbonate (<20 mEq/L), uncontrolled and controlled DM, history of CVD, and history of CHF (Table 3). Similar results were found in models exploring the association between eGFRCysC and SPPB. In the fully adjusted model with both eGFRCysC and UACR, eGFRCysC was not associated with low SPPB scores, whereas a UACR of 30-300 mg/g was associated with higher odds of a low SPPB score compared with UACR of <30 mg/g (adjusted OR, 1.84; 95% CI, 1.09-3.11).
Table 3.
Association Between Covariates and Physical Function in Models Using eGFRCr and eGFRcysC
| Predictor or Covariate | Adjusted OR for Model Using eGFRCr | 95% Confidence Limits |
P | Adjusted OR for Model Using eGFRCysC | 95% Confidence Limits |
P | ||
|---|---|---|---|---|---|---|---|---|
| UACR: mg/g, 30-00 vs <30 | 2.04 | 1.21 | 3.47 | 0.008 | 1.84 | 1.09 | 3.11 | 0.02 |
| UACR: mg,/g, > 300 vs <30 | 1.93 | 1.01 | 3.69 | 0.05 | 1.62 | 0.86 | 3.05 | 0.14 |
| Hgb: g/dL, ≤ 12 vs >12 | 1.37 | 0.85 | 2.23 | 0.20 | 1.36 | 0.84 | 2.20 | 0.21 |
| Bicarbonate (CO2): mEq/L, < 20 vs ≥ 20 | 3.50 | 1.27 | 9.64 | 0.02 | 3.26 | 1.21 | 8.80 | 0.02 |
| Serum albumin: mg/dL, < 4 vs ≥4 | 1.57 | 0.92 | 2.67 | 0.09 | 1.56 | 0.91 | 2.69 | 0.11 |
| BMI: kg/m2, OW: 25 – <30 vs not OW: <25 | 1.83 | 0.95 | 3.52 | 0.07 | 1.88 | 0.97 | 3.65 | 0.06 |
| BMI: kg/m2, obese: ≥ 30 vs not OW: <25 | 2.61 | 1.37 | 4.98 | 0.004 | 2.54 | 1.31 | 4.91 | 0.006 |
| DM (A1c < 7.5%) controlled vs no DM | 2.08 | 1.24 | 3.48 | 0.006 | 2.23 | 1.32 | 3.78 | 0.003 |
| DM not controlled (A1c ≥ 7.5%) vs no DM | 1.96 | 1.11 | 3.48 | 0.02 | 2.07 | 1.15 | 3.73 | 0.02 |
| Pulse Pressure: 1 mmHg increase | 0.99 | 0.97 | 1.00 | 0.04 | 0.98 | 0.97 | 1.00 | 0.03 |
| CVD: yes vs no | 1.59 | 1.02 | 2.48 | 0.04 | 1.46 | 0.93 | 2.27 | 0.10 |
| CHF: yes vs no | 2.13 | 1.19 | 3.81 | 0.01 | 2.29 | 1.26 | 4.16 | 0.006 |
| Stroke/TIA: yes vs no | 1.64 | 0.93 | 2.89 | 0.09 | 1.49 | 0.83 | 2.68 | 0.18 |
| Current smoker vs never smoked | 1.24 | 0.58 | 2.64 | 0.58 | 1.40 | 0.63 | 3.11 | 0.41 |
| Previous smoker vs never smoked | 0.88 | 0.56 | 1.39 | 0.58 | 0.86 | 0.54 | 1.37 | 0.52 |
| Black race: yes vs no | 1.38 | 0.73 | 2.59 | 0.32 | 1.52 | 0.74 | 3.12 | 0.25 |
| Sex: female vs male | 1.92 | 1.23 | 2.98 | 0.004 | 1.81 | 1.16 | 2.83 | 0.009 |
| Age, each additional year | 0.91 | 0.70 | 1.19 | 0.50 | 0.90 | 0.69 | 1.18 | 0.45 |
| Age2 | 1.001 | 0.999 | 1.003 | 0.18 | 1.001 | 0.999 | 1.003 | 0.16 |
| Years of education, each additional year | 0.88 | 0.81 | 0.95 | 0.002 | 0.89 | 0.81 | 0.96 | 0.004 |
Abbreviations: BMI, body mass index; CHF, congestive heart failure; CVD, cardiovascular disease; DM, diabetes mellitus; GFRCr, creatinine-based estimated glomerular filtration rate; eGFRCysC, cystatin C-based estimated glomerular filtration rate; OR, odds ratio; TIA, transient ischemic attack; UACR, urine albumin to creatinine ratio.
The results were similar when the analysis was restricted to participants with eGFR <60 mL/min/1.73m2. In either adjusted model (eGFRCr and eGFRCysC), eGFR was not significantly associated with low SPPB score. In contrast, a UACR of 30-300 mg/g vs UACR <30 mg/g was associated with higher odds of low SPPB score in the model adjusted for eGFRCr and covariates, but not in the model adjusted for eGFRCysC and covariates. In models with Ln UACR instead of the 3-level categorical UACR, eGFR was not significant in either adjusted model; Ln UACR was significantly associated with lower SPPB scores in the model adjusted for eGFRCr and covariates (adjusted OR, 1.11; 95% CI, 1.01, 1.22) and was marginally associated with lower SPPB scores in the model adjusted for eGFRCysC and covariates (adjusted OR, 1.09; 95% CI, 0.99, 1.19). In ordinal logistic regression models, a UACR of 30-300 was associated with higher proportional odds of lower SPPB scores (proportional adjusted OR, 1.64; 95% CI, 1.08 -2.48) vs a lower UACR category in the model with eGFRCr; this association was marginally significant in the ordinal logistic regression model with eGFRCysC (proportional adjusted OR, 1.49; 95% CI, 0.99, 2.26). No significant interactions were found between covariates and eGFR (both creatinine and cystatin C based) and UACR, nor between UACR and either eGFR variables.
LS Means SPPB scores for the 3 measures of kidney function, adjusted for all covariates in the logistic regression models, are shown in Table S3. Adjusted mean SPPB score was similar across the eGFRCr and eGFRCysC categories. The adjusted mean SPPB score was slightly higher for participants with <30 mg/g albuminuria than for those with 30-300 or >300 mg/g (LS Mean [SE]: 8.23 [0.35], 7.62 [0.34], and 7.67 [0.37], respectively). In a secondary analysis evaluating the association between measures of kidney function and gait speed, rather than SPPB, only eGFRCysC <30 mL/min/1.73m2 was associated with slower gait speeds compared with eGFRCysC >60 mL/min/1.73m2 after adjusting for the covariates in the logistic regression models (P = 0.03). Higher categories of eGFRCysC were not significantly associated with slower gait speeds. Lower eGFRCr and higher UACR categories were not associated with slower gait speeds (Table S4).
Discussion
In this analysis of BRINK study participants with a broad range of kidney function, lower eGFR—as measured using creatinine and cystatin C—and higher UACR were associated with decreased physical performance in univariate analyses. However, only UACR was associated with decreased SPPB score after adjusting for other risk factors.
This result differs from some, but not all, previous analyses exploring the association between CKD and physical performance. In an analysis of participants enrolled in the Systolic Blood Pressure Intervention Trial, Wolfgram et al21 investigated associations of albuminuria and eGFR with measures of functional status. In fully adjusted models, albuminuria was associated with gait speed but not reported physical function, whereas eGFR level was not associated with any functional measure. Similarly, Walker et al15 did not find a graded association between eGFRCr and physical function in the CANFit study of patients with advanced CKD, but this study did not include an assessment of albuminuria. Conversely, a prospective analysis performed by Fried et al13 as part of the Health ABC study found an association between eGFRCysC and reported functional impairment, although the association was no longer significant after adjusting for inflammatory markers. Dalrymple et al’s10 finding of an association between eGFRCysC but not eGFRCr in the CHS cohort supports the hypothesis that the confounding of eGFRCr by muscle mass complicates studies of eGFR and physical performance, although that study evaluated frailty rather than physical function.
Although we found a graded association between eGFR (estimated by both creatinine and cystatin C) and physical performance on univariate analysis, the significance of the association did not persist after adjusting for key comorbid conditions that were more prevalent among those with CKD, such as DM and heart failure, and for some possible sequelae of CKD, such as hypoalbuminemia and acidosis. Our cohort included more individuals with advanced CKD than the CHS cohort. Therefore, one possible explanation for the differing eGFRcysC results may be that lower physical performance (or frailty) is more common among patients with CKD in general and that decreased performance may not be strongly associated with eGFR level per se. Another potential explanation for this finding is that albuminuria, which may indicate inflammation, and acidosis may be mechanisms by which CKD leads to poor performance. Adjusting for these in our models may have negated the eGFR association found in the univariate models.
The impact of proteinuria may explain at least some of the different results on the association between CKD and physical function. Most studies have not included albuminuria in their models, despite its likelihood of being more common in patients with advanced CKD. For example, Zhou et al12 examined the relationship between body composition and measured GFR with multiple measures of physical performance in patients with an eGFR of <30 mL/min/1.73m2 and found an association between eGFR and physical performance. However, whether this relationship would have changed with the addition of albuminuria measures remains unknown because it was not included in these models. In a cross-sectional analysis of 120 patients in Japan that included eGFRCr and urine protein,11 Hiraki et al found that eGFRCr was associated with multiple measures of physical performance, whereas the urine protein to creatinine ratio was only associated with single leg balance testing. However, in our study, only albuminuria remained associated with low physical performance after adjusting for potential confounders. This difference may be due to our use of SPPB: a summary performance measure that may be more sensitive than individual measures of physical performance, such as single leg balance. Indeed, when gait speed alone was evaluated in our study as a secondary outcome, the association between UACR and this physical performance measure was no longer apparent.
The present study provides an opportunity to examine the relationship between different measures of eGFR together with albuminuria and thus help elucidate the relationship between CKD and physcial performance using a high-quality dataset with detailed data collection and very less missing data across a range of eGFR values. The hightened inflammatory state in CKD has been implicated in the association between CKD and many poor outcomes,30 and similar associations with several poor outcomes have been found in association with proteinuria.17, 18, 19, 20 Physical function in patients with CKD may be similarly impacted by proteinuria, with measured albuminuria acting as a surrogate for endothelial inflammation and dysfunction. In support of this hypothesis, Fried et al13 found that an association between eGFR and reported functional status was no longer significant when markers of inflammation were added to the model. The fact that the relationship between albuminuria and physical performance persisted despite extensive adjustment for multiple risk factors suggests that at least some of the the decline in physical performance noted in previous studies that did not include an assessment of proteinuria may have been related to endothelial dysfunction. These findings underscore the importance of exploring albuminuria as a biomarker of reduced physical function in clinical settings, as well as its use in predicting declines in physical performance.
Although our study provides additional insights into the association between kidney disease and physical function, several limitations deserve mention. First, this cross-sectional analysis can only provide information on possible assocations, and our results cannot be used to infer causality. Second, this study uses estimated rather than measured GFR. Although we were able to compare 2 different approaches to estimating GFR using creatinine and cystatin C, potential confounding by muscle mass and inflammation may have persisted. Third, a majority of the participants were of White race, and approximately 16% of the participants were of Black race. Given that kidney disease disproportionately affects non-White patients, addional studies inclusive of more non-White participants are needed. Fourth, we assessed physical function using SPPB alone. While this test is validated and includes multiple dimensions of physical function, it is susceptible to ceiling effects in higher-functioning individuals and does not capture all facets of physical function. Finally, although we were able to include many variables and potential confounders in our analysis, the effects of additional unmeasured confounders may have persisted.
In conclusion, we found that albuminuria was associated with decreased physical function performance after adjusting for potential confounders, but eGFRCr or eGFRCysC were not. Additional studies on the longitudinal association between albuminuria and change in physcial function over time are needed to determine whether baseline proteinuria predicts functional decline or whether the association varies with time, particularly with the initiation of kidney replacement therapy.
Article Information
Authors’ Full Names and Academic Degrees
Ryan Mello, MD, PhD, Kirsten L Johansen, MD, Anne Murray, MD, MSc, Cynthia Davey, MS, and Allyson Hart, MD, MSc.
Authors’ Contributions
Study design: RM, KJ, AM, AH; BRINK cohort design and data acquisition: AM, CD; Statistical analysis: CD; Data interpretation: RM, KJ, AM, AH. Mentorship and supervision: AH, CD, KJ. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Support
This research was supported by the National Institute of Aging R01AG037551 and RF1AG058729, the National Institutes of Health’s National Center for Advancing Translational Science grant UL1TR002494, and by the National Institute of Digestive and Diabetes and Kidney Disease R01DK107269-01A1. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH Health’s National Center for Advancing Translational Sciences.
Financial Disclosure
Dr Hart has conducted research funded by CSL Behring and Atara that is not related to the content of this analysis. The other authors have no conflicts of interest to report.
Prior Presentation
“Associations of eGFR and Albuminuria with Physical Performance” Poster presentation at the American Society of Nephrology Kidney Week Virtual Meeting, October 29-November 4, 2021.
Peer Review
Received March 30, 2022. Evaluated by 2 external peer reviewers, with direct editorial input from the Statistical Editor and the Editor-in-Chief. Accepted in revised form July 7, 2022.
Footnotes
Complete author and article information provided before references.
Table S1: Descriptive Statistics of Outcome, Predictors, and Covariates: Overall (N = 568 with UACR Data) and by UACR Group
Table S2: Comparison of the Univariate and Multivariable Association Between Different Measures of Kidney Function and Low Physical Function Modeled as a 4-Level Ordinal Outcome
Table S3: LS Means for SPPB Score According to Kidney Function Category
Table S4: Association Between Covariates and Gait Speed in Models Using eGFRCr and eGFRcysC
Supplementary Material
Tables S1-S4
References
- 1.Grams M.E., Chow E.K., Segev D.L., Coresh J. Lifetime incidence of CKD stages 3-5 in the United States. Am J Kidney Dis. 2013;62(2):245–252. doi: 10.1053/j.ajkd.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mills K.T., Xu Y., Zhang W., et al. A systematic analysis of worldwide population-based data on the global burden of chronic kidney disease in 2010. Kidney Int. 2015;88(5):950–957. doi: 10.1038/ki.2015.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raymond N.T., Zehnder D., Smith S.C., Stinson J.A., Lehnert H., Higgins R.M. Elevated relative mortality risk with mild-to-moderate chronic kidney disease decreases with age. Nephrol Dial Transplant. 2007;22(11):3214–3220. doi: 10.1093/ndt/gfm396. [DOI] [PubMed] [Google Scholar]
- 4.Marks A., Macleod C., McAteer A., et al. Chronic kidney disease, a useful trigger for proactive primary care? Mortality results from a large U.K. cohort. Fam Pract. 2013;30(3):282–289. doi: 10.1093/fampra/cms079. [DOI] [PubMed] [Google Scholar]
- 5.Go A.S., Chertow G.M., Fan D., McCulloch C.E., Hsu C. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004 23;351(13):1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 6.Fusco O., Ferrini A., Santoro M., Lo Monaco M.R., Gambassi G., Cesari M. Physical function and perceived quality of life in older persons. Aging Clin Exp Res. 2012;24(1):68–73. doi: 10.1007/BF03325356. [DOI] [PubMed] [Google Scholar]
- 7.Painter P., Roshanravan B. The association of physical activity and physical function with clinical outcomes in adults with chronic kidney disease. Curr Opin Nephrol Hypertens. 2013;22(6):615–623. doi: 10.1097/MNH.0b013e328365b43a. [DOI] [PubMed] [Google Scholar]
- 8.MacKinnon H.J., Wilkinson T.J., Clarke A.L., et al. The association of physical function and physical activity with all-cause mortality and adverse clinical outcomes in nondialysis chronic kidney disease: a systematic review. Ther Adv Chronic Dis. 2018;9(11):209–226. doi: 10.1177/2040622318785575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roshanravan B., Khatri M., Robinson-Cohen C., et al. A prospective study of frailty in nephrology-referred patients with CKD. Am J Kidney Dis. 2012;60(6):912–921. doi: 10.1053/j.ajkd.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dalrymple L.S., Katz R., Rifkin D.E., et al. Kidney function and prevalent and incident frailty. Clin J Am Soc Nephrol. 2013;8(12):2091–2099. doi: 10.2215/CJN.02870313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hiraki K., Yasuda T., Hotta C., et al. Decreased physical function in pre-dialysis patients with chronic kidney disease. Clin Exp Nephrol. 2013;17(2):225–231. doi: 10.1007/s10157-012-0681-8. [DOI] [PubMed] [Google Scholar]
- 12.Zhou Y., Hellberg M., Svensson P., Höglund P., Clyne N. Sarcopenia and relationships between muscle mass, measured glomerular filtration rate and physical function in patients with chronic kidney disease stages 3-5. Nephrol Dial Transplant. 2018;33(2):342–348. doi: 10.1093/ndt/gfw466. [DOI] [PubMed] [Google Scholar]
- 13.Fried L.F., Lee J.S., Shlipak M., et al. Chronic kidney disease and functional limitation in older people: health, aging and body composition study. J Am Geriatr Soc. 2006;54(5):750–756. doi: 10.1111/j.1532-5415.2006.00727.x. [DOI] [PubMed] [Google Scholar]
- 14.Roshanravan B., Patel K.V., Robinson-Cohen C., et al. Creatinine clearance, walking speed, and muscle atrophy: a cohort study. Am J Kidney Dis. 2015;65(5):737–747. doi: 10.1053/j.ajkd.2014.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walker S.R., Brar R., Eng F., et al. Frailty and physical function in chronic kidney disease: the CanFIT study. Can J Kidney Health Dis. 2015 Sep 5;2:32. doi: 10.1186/s40697-015-0067-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gupta J., Mitra N., Kanetsky P.A., et al. Association between albuminuria, kidney function, and inflammatory biomarker profile in CKD in CRIC. Clin J Am Soc Nephrol. 2012;7(12):1938–1946. doi: 10.2215/CJN.03500412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruggenenti P., Perna A., Mosconi L., Pisoni R., Remuzzi G. Urinary protein excretion rate is the best independent predictor of ESRF in non-diabetic proteinuric chronic nephropathies. "Gruppo Italiano di Studi Epidemiologici in Nefrologia" (GISEN) Kidney Int. 1998;53(5):1209–1216. doi: 10.1046/j.1523-1755.1998.00874.x. [DOI] [PubMed] [Google Scholar]
- 18.Perkovic V., Verdon C., Ninomiya T., et al. The relationship between proteinuria and coronary risk: a systematic review and meta-analysis. PLoS Med. 2008;5(10) doi: 10.1371/journal.pmed.0050207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Llewellyn D.J., Langa K.M., Friedland R.P., Lang I.A. Serum albumin concentration and cognitive impairment. Curr Alzheimer Res. 2010;7(1):91–96. doi: 10.2174/156720510790274392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grimm R.H., Jr., Svendsen K.H., Kasiske B., Keane W.F., Wahi M.M. Proteinuria is a risk factor for mortality over 10 years of follow-up. MRFIT Research Group. Multiple Risk Factor Intervention Trial. Kidney Int Suppl. 1997;63:S10–S14. [PubMed] [Google Scholar]
- 21.Wolfgram D.F., Garcia K., Evans G., et al. Association of albuminuria and estimated glomerular filtration rate with functional performance measures in older adults with chronic kidney disease. Am J Nephrol. 2017;45(2):172–179. doi: 10.1159/000455388. [DOI] [PubMed] [Google Scholar]
- 22.Murray A.M., Bell E.J., Tupper D.E., et al. The Brain in Kidney Disease (BRINK) cohort study: design and baseline cognitive function. Am J Kidney Dis. 2016;67(4):593–600. doi: 10.1053/j.ajkd.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levey A.S., Stevens L.A., Schmid C.H., et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inker L.A., Schmid C.H., Tighiouart H., et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367(1):20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bragg-Gresham J., Zhang X., Le D., et al. Prevalence of chronic kidney disease among Black individuals in the US after removal of the Black race coefficient from a glomerular filtration rate estimating equation. JAMA Netw Open. 2021;4(1) doi: 10.1001/jamanetworkopen.2020.35636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vemuri P., Knopman D.S., Jack C.R., Jr., et al. Association of kidney function biomarkers with brain MRI findings: the BRINK study. J Alzheimers Dis. 2017;55(3):1069–1082. doi: 10.3233/JAD-160834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guralnik J.M., Simonsick E.M., Ferrucci L., et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 28.Pavasini R., Guralnik J., Brown J.C., et al. Short physical performance battery and all-cause mortality: systematic review and meta-analysis. BMC Med. 2016;14(1):215. doi: 10.1186/s12916-016-0763-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rothman K.J. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1(1):43–46. [PubMed] [Google Scholar]
- 30.Kaysen G.A. The microinflammatory state in uremia: causes and potential consequences. J Am Soc Nephrol. 2001;12(7):1549–1557. doi: 10.1681/ASN.V1271549. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1-S4