Abstract
Objective
Anterior cervical corpectomy and fusion (ACCF) has been widely used in the treatment of cervical spondylotic myelopathy (CSM) but is accompanied by unavoidable motion loss and destruction of vertebra. We aim to evaluate the range of motion (ROM) of caprine cervical spine constructs implanted with cervical artificial disc and vertebra system (ADVS). The purpose of this study was to investigate the biomechanical properties of the ADVS from an in vivo caprine cervical spine non-fusion model.
Methods
Twelve goats were randomly divided into ADVS or control group, with 6 animals in each group. The animals in the ADVS group were implanted with ADVS at the C4 level. The cervical spine constructs were harvested 6 months after the operation. The ROM of cervical spine specimens in the ADVS group was recorded. Biomechanical testing of the specimens in the control group were conducted to evaluate the ROM of the cervical spine specimens under intact and fixed condition (C3-C5) by an anterior plate, respectively.
Results
The biomechanical outcomes showed that the ROM of the levels (C3-C5) implanted with ADVS was maintained. The ROM in the adjacent level (C2-3) did not increase significantly comparing with intact group.
Conclusions
In general, ADVS could preserve the ROM of operative levels and could reconstruct the height of the vertebra. ADVS did not increase the ROM of upper adjacent level. This device provides a non-fusion method for the treatment of patients suffering from CSM. However, improvements on the design of ADVS are still needed.
Translational potential statement
This study introduced a novel cervical spinal implant, which was designed to have the ability of motion preservation and vertebra construction. Our study provided a non-fusion procedure in the treatment of CSM after ACCF.
Keywords: Anterior cervical corpectomy and fusion, Cervical artificial disc and vertebra system, Biomechanics, Range of motion, Non-fusion
1. Introduction
Cervical spondylotic myelopathy (CSM), which can cause spinal cord injury, is a common disease with several therapeutic options [[1], [2], [3]]. Anterior cervical corpectomy and fusion (ACCF) is an effective treatment for CSM [[4], [5], [6], [7]]. This procedure usually fuses three or more cervical vertebrae by using an anterior plate and bone graft or titanium mesh cage after decompression by corpectomy [8]. ACCF allows direct removal of the compression to the spinal cord and provides the cervical spine with a stable biomechanical environment via anterior arthrodesis at the operative levels. Although ACCF has been reported to exhibit good neurologic recovery [9], and restoration of cervical alignment in patients with CSM, the long-segment solid fusion inevitably sacrifices partial motor function of the cervical spine. The loss of motion at the operative levels can lead to an increased range of motion (ROM) of the adjacent intervertebral space [10,11], which can possibly lead to adjacent segment disease and resulting in CSM in those patients [[12], [13], [14]].
Non-fusion device such as artificial cervical disc has been demonstrated maintaining the ROM at the operative levels for the prevention of adjacent segment disease [15]. Previous studies have reported superior long-term neurological or radiographic outcomes of patients undergoing artificial cervical disc replacement compared with anterior cervical fusion [16]. However, due to its design specifically for cervical disc, artificial cervical disc is infeasible for patients with CSM resulting from vertebral lesions [17] or ossification of the posterior longitudinal ligament [18]. In such cases, ACCF is the frequent surgical option. Considering that preservation of motor function of the intervertebral disc is necessary while reconstructing the load-bearing function of the vertebral body, we designed a novel cervical artificial disc and vertebra system (ADVS) to restore the intervertebral motion and maintain the height of the vertebral body after cervical corpectomy.
Previous studies have reported that the cervical spine of goat is an ideal model for spinal implants [19,20]. We thus established an in vivo cervical spine non-fusion model by implantation of an ADVS specially designed for goat. We hypothesis that ADVS can maintain mobility of operational level and reduce the ROM of adjacent segment. The aim of this study is to evaluate the ROM of non-fusion caprine cervical spine constructs implanted with ADVS 6 months after the operation in comparison with intact and anterior plate fixed cervical spine models.
2. Materials and methods
2.1. Characteristics of ADVS
ADVS for caprine cervical spine was designed containing three parts, an upper and lower artificial disc and a vertebra (China patent number: 201410072192.7) (Fig. 1). These parts are three-dimensional (3D) printed titanium-aluminum alloy (Ti6Al4V) (Bright Laser Rapid Prototyping Technology Co. Ltd., Xi’an, China). The prosthesis is assembled before implantation. Each disc is first fixed on to the artificial vertebra and then rotated 90° until the plate is anterior. The endplates of the vertebral bodies correspond to the discs. The chamfered structure of the upper and lower intervertebral disc surfaces aids bone formation. The anti-dislocation structure of the intervertebral disc component is “L" shaped, with a shallow spherical core with a height of 2 mm in the middle, surrounded by a shallow spherical fossa joint in the vertebral body. The spherical core and fossa form a spherical fossa joint, which embodies the prosthesis' mobility: 6 degrees of flexion, extension, left and right lateral bending, and 360 degrees of axial rotation between the intervertebral disc and the vertebral body. The “L" shaped anti-dislocation structure of the disc is unique to ADVS, as it prevents disc and vertebral body dislocation while still allowing for some mobility between the two. The articulation of this implant is metal on metal.
Figure 1.
Photographs of ADVS. The artificial disc has an anterior plate (a, b). The angle between anterior plate and disc is 84° in upper artificial disc (A), 100° in lower artificial disc (b). A core (10 mm in diameter, 2 mm in height) is in the below-center of the disc surrounding with L-structure anteriorly & posteriorly for preventing the dislocation of disc from the vertebra (a, b). Two screw holes (3.5 mm in diameter) are designed in the middle of the anterior plate (c). Several zigzag crests are on the above surface of the disc (c). Four unicortical self-tapping screws (3.4 mm in diameter, 14–16 mm in length) are used for the fixation (d). The artificial vertebra is a quadrangular column (width: 14 mm; length: 35 mm; depth: 14 mm). Articular fovea matching to the core of the disc is designed cranially & caudally of the artificial vertebra (e, f, g). This hemisphere socket joint allows 18° ROM in flexion-extension and lateral bending, 360° ROM in rotation. Several tubes for bone graft are designed laterally (h).
2.2. Surgical procedure and postoperative care
All animal surgeries and experimental procedures were conducted following the protocols approved by the ethics committee of Xi’an Jiaotong University (XJTULAC2014-405). A total of 12 male goats (18.5 ± 2.5 month, 35.34 ± 3.2 kg) were included in this study, 6 in the ADVS group with implantation of the ADVS and 6 in the non-surgical group, which were treated as controls.
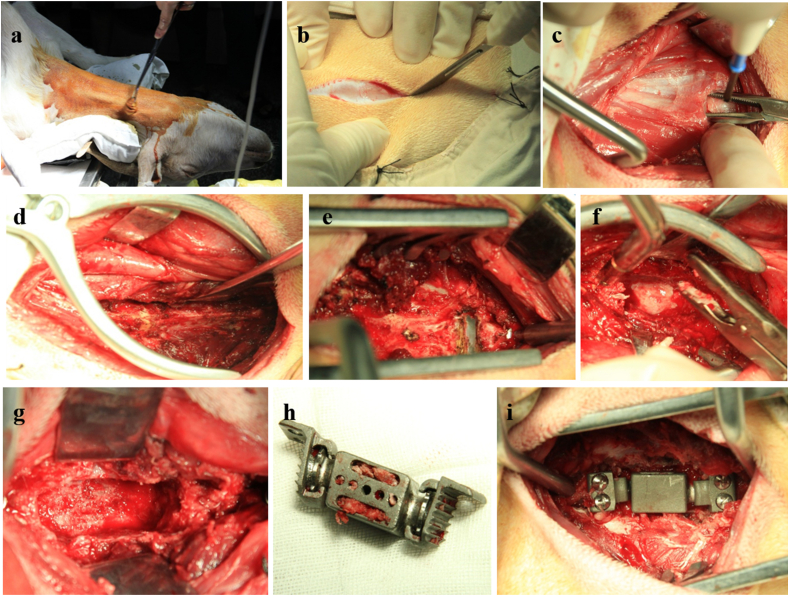
Operation in the ADVS group was performed under aseptic conditions. The goat in the ADVS group underwent a three-day fast for gastrointestinal tract emptying and a subcutaneous injection of atropine (0.02 mg/kg) before intravenous anesthesia to reduce the tracheal secretions. Thiopental sodium solution (2.5%) was used as anesthetic with an initial dose of 10 ml and gradually added during the operation. Anteroposterior and lateral radiographs for the exclusion of skeletal abnormalities were taken when the goat displayed a satisfying anesthetic effect. The goat was placed in a supine position. Penicillin sodium (100 mg/kg) was injected via the venous channel, which was established via the auricular vein. A negative pressure aspirator and sputum suction apparatus were used to remove the blood and for respiratory caring. The ADVS was implanted at the C4 level after the resection of C4 vertebral body, and the surgical process was recorded (Fig. 2). Before closing the wound, 500 ml metronidazole solution (5 mg/ml) was used to douche the operative region. The implantation was performed at C4 with reasons. The adjacent levels would be C1-2 and C4-5 if C3 was implanted. However, C1-2 is not appropriate for testing ROM because it has a very large ROM under a very small torque, rending the ROM of the other levels inaccurate. C5 was not chosen because the markers below could not be recorded. Finally, we decided to implant the prosthesis at C4 and observed the ROM of the upper adjacent level (C2-3).
Figure 2.
Process of implantation of ADVS. After sterilization, a right-sided anterolateral retropharyngeal approach to the cervical spine was used (a, b). C4 vertebral body was exposed following the incision and separation of soft tissue using high-frequency electrosurgical equipment (c, d). The median crests of C3 and C5 were localized by positioning needle, respectively (e). The C4 vertebra corpectomy was performed accompanied with the discectomy of C3-4 and C4-5 by using bone rongeur or nucleus pulposus forceps (f). A decompression groove (approximately width: 15 mm; length: 35 mm; depth: 15 mm) was made in the middle of C4 vertebra body (g). The assembled ADVS was filled with bone grafts and implanted into the decompression groove (h). Four unicortical self-tapping screws were fixed with a 20° trajectory in cranial and caudal direction, respectively (i).
Penicillin sodium (100 mg/kg) was administered for an additional three days after the operation. The dressing was changed once a day until the wound healed. All 12 goats were kept in a special animal care center (De Sheng Husbandry, Sanyuan County, Xi’an) and were euthanized six months after the operation.
2.3. Image examination
All images were taken while the goats were under general anesthesia. Immediate postoperative anteroposterior and lateral radiographs (QDR-2000; Hologic, Waltham, MA, USA.) were taken for inspecting the position of the ADVS. Computed tomography (CT) (GE Medical Systems, Milwaukee, Wisconsin) images with the slice thickness of 0.625 mm were obtained to observe the implant in detail by using 3D reconstruction model. Magnetic resonance imaging (MRI) of 1.5 T (MAGNETOM Amira, Siemens, Germany) was taken to make sure there was no compression to the spinal cord.
2.4. Biomechanical testing
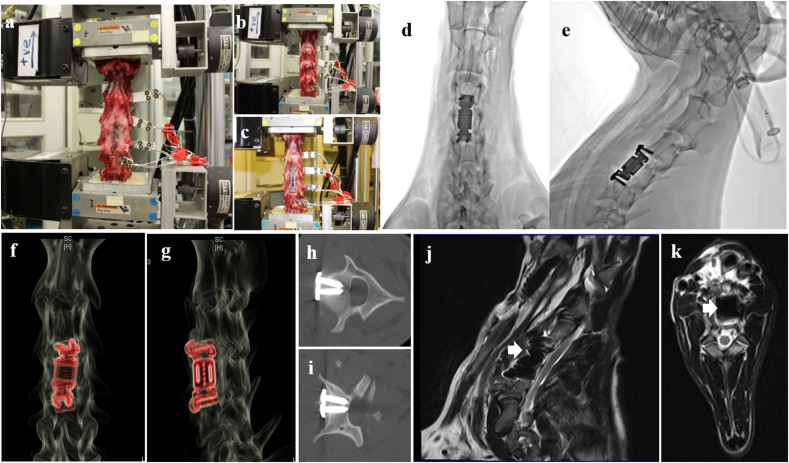
All 12 goats’ cervical specimens (C1–C7) were harvested six months after the operation. The surrounding muscle and soft tissue were removed with caution, keeping the facet joints and interspinous ligaments intact. The specimens were preserved in a −20 °C lab refrigerator. Before testing, the fresh frozen goat cervical spine specimens were thawed at room temperature and kept hydrated using 0.9% saline-soaked gauzes. C1 and C2 as well as C6 and C7 were fixed together with several nails to increase the contacting area between the specimen and fixing material for the enhancement of embedding. The top and bottom vertebrae were embedded in a square cylinder containing mixture of N (3-dimethylaminopropyl)-1, 3-propylenediamine and bisphenol A-(epichlorohydrin) (1:1) to maintain the specimens in a neutral position. Four light-emitting diodes (LEDs) were firmly fixed with screws to the C2, C3, C4 and C5 vertebra. There were 16 LEDs in total (Fig. 3a, b, 3c).
Figure 3.
Photograph of biomechanics testing and radiological results. a: biomechanics testing of ADVS group; b: intact group; c: fixation group. Postoperative anteroposterior (d) and lateral (e) radiographs showed that ADVS was implanted at C4, no dislocation was observed. Postoperative CT of hardware enhanced model of 3D reconstruction anteroposterior (f) and laterally (g) images showed ADVS reconstructed the height of vertebra after corpectomy of C4. Screws were implanted into C3 vertebral body (h) and C5 vertebral body (i) respectively, no screw broke into the spinal canal. Sagittal (j) and axial (k) view of postoperative MRI T2 images showed that no compression to the spinal cord was observed.
Flexibility test was performed on a servohydraulic materials testing machine (MTS 858 Bionix machine, MTS System Inc., Minneapolis, MN, USA). Without any axial preload, a 2.5 N m torque was applied to the C1 and C2 vertebrae, producing a ROM of the specimen in flexion-extension, lateral bending and axial rotation. During the test, movements of the LEDs on C2, C3, C4 and C5 were recorded using an optoelectronic three-dimensional motion capture system with three cameras (OPTOTRAK CERTUS, Northern Digital Inc., Waterloo, Canada). This device has a three-dimensional precision of 0.1 mm, a resolution of 0.01 mm and a sampling frequency of 100 Hz. The positions of the LEDs recorded by OPTOTRAK CERTUS were converted into ROM using a program written in Matlab (MathWorks, Natick, MA, USA).
Specimens in the control group were first tested as an intact group and were then tested as a fixation group in which each cervical spine was fixed by using anterior plate (Fule Science & Technology Development Co., Ltd, Beijing, China) from C3 to C5 (Fig. 3a, 3b, 3c). In total, three groups (intact group, fixation group and ADVS group) were included in this study. The primary data, the instantaneous coordinates of the marks, were converted into the load–displacement curve and ROM curve of C2-3, C3-4, C4-5 and C2-5 in flexion, extension, lateral bending (left and right) and axial rotation (left and right). ROM was obtained for all the specimens. Five loading cycles were completed. The third circle was used for data analysis.
2.5. Statistical analysis
The data were analyzed using SPSS software (version 19.0; SPSS Inc., Chicago, IL, USA). The results are presented as the means ± SD. GraphPad Prism 5.01 (GraphPad Software, Inc.) was used to create the histogram. The data for ROM were analyzed using one-way analysis of variance (ANOVA) and the least significant difference (LSD) post-hoc test. A P-Value less than 0.05 was considered statistically significant.
3. Results
3.1. Radiological results
Anteroposterior and lateral plain films were taken immediately after the operation. The results showed that the ADVS was implanted in C4 and that the screws were fixed in C3 and C5. No dislocation of the prosthesis or loosened/fractured screws were observed. Postoperative CT showed that the ADVS reconstructed the vertebra. The upper and lower artificial discs were in the C3-4 or C4-5 intervertebral spaces and firmly attached to lower endplate of C3 and upper endplate of C5 (Fig. 3d and e). 3D-CT detected that all prosthesis and screws were in good positions (Fig. 3f, g, 3h, 3i). The postoperative MRI confirmed that the diameter of spinal canal at the operative level did not change suggesting that there was no compression to spinal cord (Fig. 3j and k).
3.2. Biomechanical results
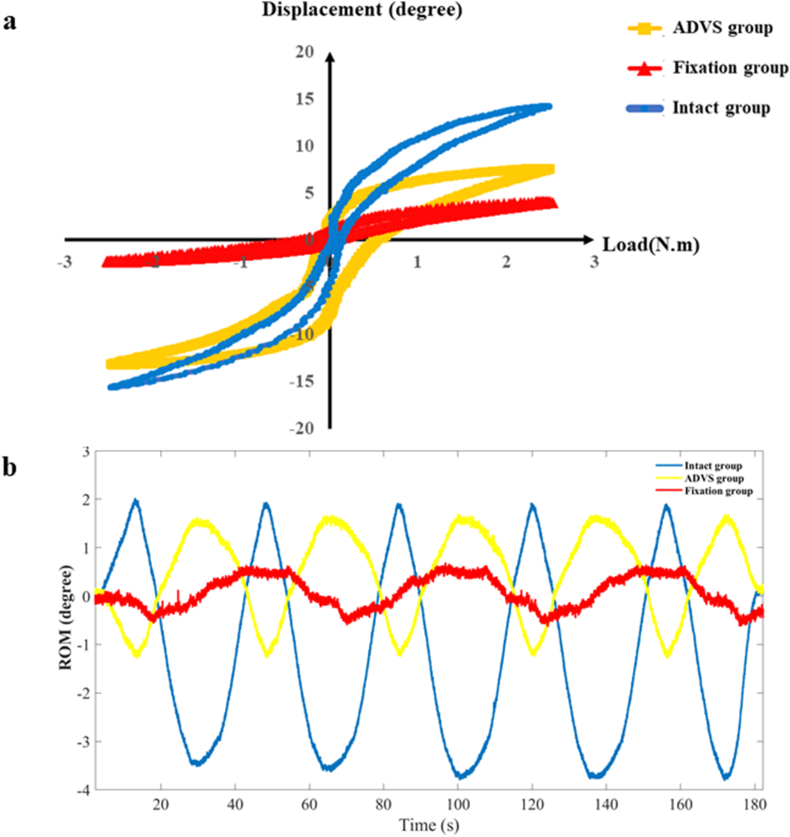
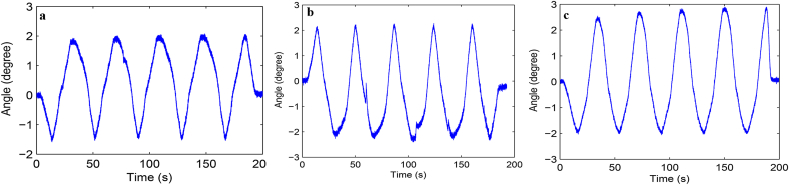
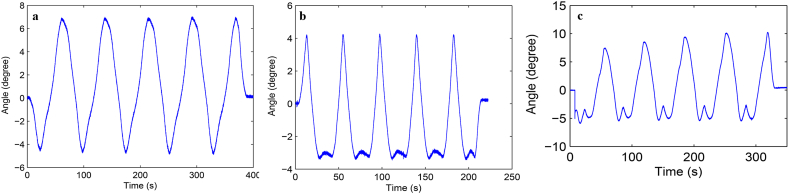
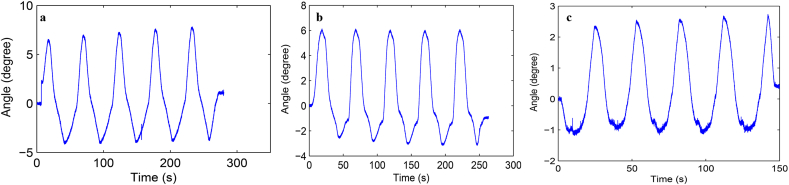
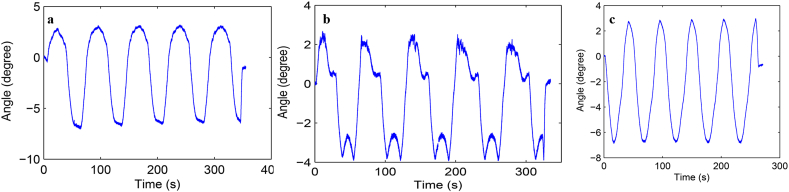
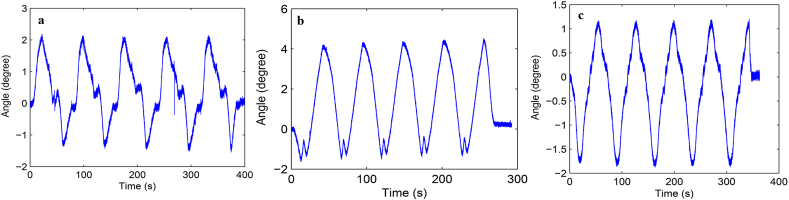
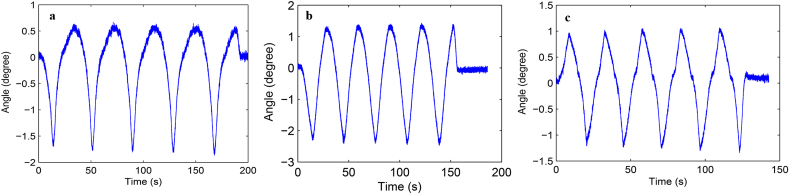
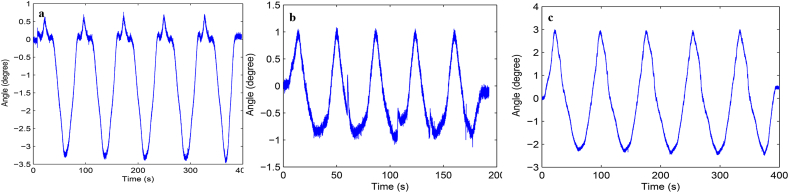
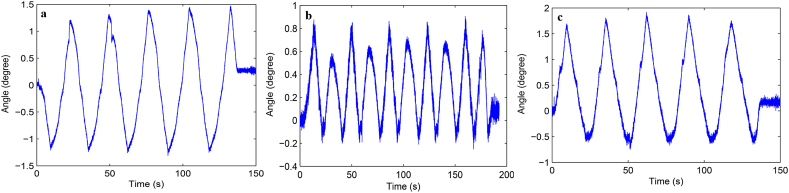
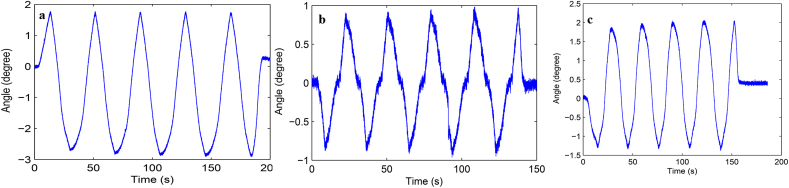
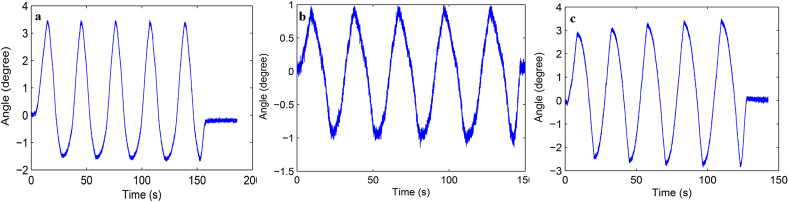
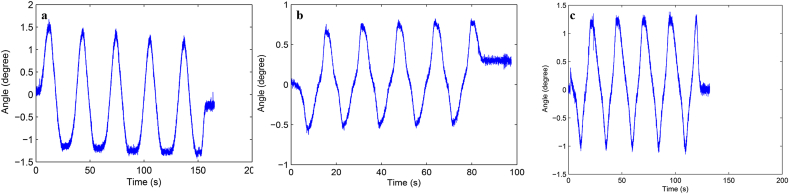
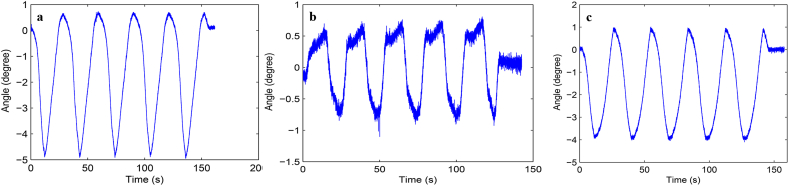
Load-displacement curve and ROM curve of C2-3, C3-4, C4-5 and C2-5 segments in flexion-extension, lateral bending axial rotation were showed in Fig. 4. Total ROM of three degrees-of-freedom for each segment were recorded (Supplementary Figs. 1–12).
Figure 4.
A typical load–displacement curve (a) and ROM curve (b) of C2-5 axial rotation.
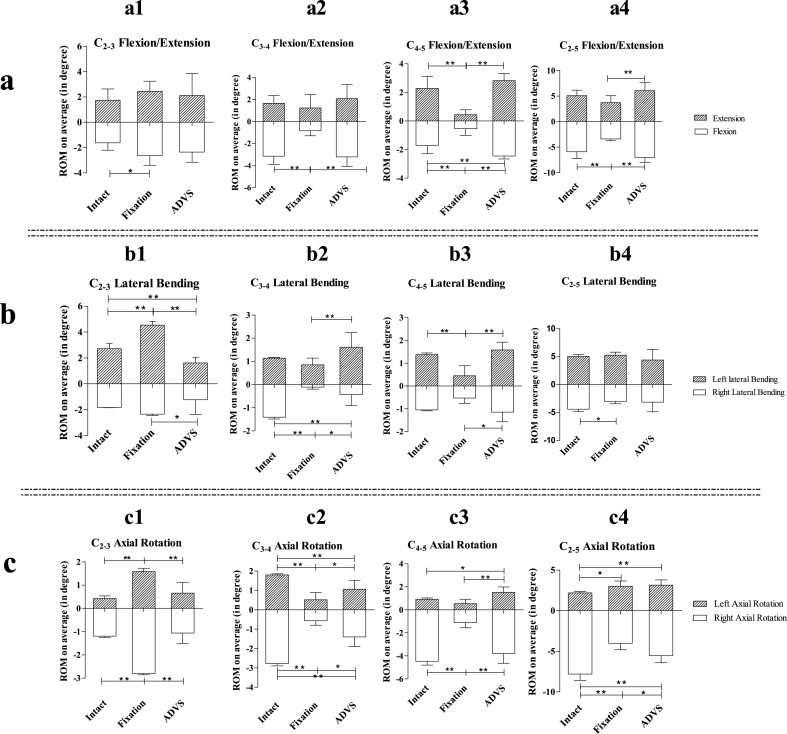
3.3. C2-3 ROM
Significantly increased C2-3 ROM in flexion was observed in the fixation group compared with intact group (p = 0.027). C2-3 ROM in extension in the intact, fixation and ADVS groups did not differ in pairwise comparisons (p > 0.05) (Fig. 5a1). C2-3 ROM in left lateral bending in the intact, fixation and ADVS groups were significantly different (intact VS fixation, p < 0.01; intact VS ADVS, p = 0.01; fixation VS ADVS, p < 0.01). The C2-3 ROM in right lateral bending in the fixation group was significantly larger than ADVS group (Fig. 5b1). Significantly increased C2-3 ROM in right and left axial rotation in the fixation group were observed in comparison with the intact and ADVS groups (intact VS fusion, p < 0.01; fusion VS ADVS, p < 0.01) (Fig. 5c1).
Figure 5.
Average ROM of C2-3, C3-4, C4-5, C2-5 intervertebral space. a: flexion/extension; a1 C2-3 flexion/extension; a2: C3-4 flexion/extension; a3: C4-5 flexion/extension; a4: C2-5 flexion/extension. b: lateral bending; b1 C2-3 lateral bending; b2: C3-4 lateral bending; b3: C4-5 lateral bending; b4: C2-5 lateral bending. c: axial rotation; c1 C2-3 axial rotation; c2: C3-4 axial rotation; c3: C4-5 axial rotation; c4: C2-5 axial rotation. One asterisk means that there is statistically significant difference and p < 0.05. Two asterisks mean that there is statistically significant difference and p < 0.01.
3.4. C3-4 ROM
Significantly decreased C3-4 ROM in the flexion in fixation group was observed comparing with the intact and ADVS groups (fixation VS intact, p < 0.01; fixation VS ADVS, p < 0.01) (Fig. 5a2); however, no difference was detected in C3-4 ROM in extension in the pairwise comparison among the intact, fixation and ADVS groups (p > 0.05) (Fig. 5a2). Significant increased C3-4 ROM in left lateral bending was only observed between fixation group and ADVS group (p < 0.01) (Fig. 5b2). Significant differences in C3-4 ROM right lateral bending were observed in pairwise comparisons among the intact group, fixation and ADVS groups (intact VS fixation, p < 0.01; intact VS ADVS, p < 0.01; fixation VS ADVS, p = 0.35) (Fig. 5b2). Significantly decreased C3-4 ROM in left and right axial rotation in the fixation and ADVS group were observed compared with the intact group (left axial rotation: fixation VS intact, p < 0.01; ADVS VS intact, p < 0.01; right axial rotation: fixation VS intact, p < 0.01; ADVS VS intact, p < 0.01) (Fig. 5c2). Significant differences in left and right axial rotations also existed in a comparison between the ADVS group and the anterior fixation group (left axial rotation: p = 0.014; right axial rotation: p = 0.016) (Fig. 5c2).
3.5. C4-5 ROM
C4-5 ROM decreased significantly in flexion & extension in the fixation group compared with the intact group and ADVS group (fusion VS intact, p < 0.01; fusion VS ADVS, p < 0.01), but increased significantly in flexion in the ADVS group compared with the intact group (p < 0.01) (Fig. 5a3). Significant decrease of C4-5 ROM in left lateral bending in the fixation group compared with intact and ADVS groups were detected (fixation VS intact, p < 0.01; fixation VS ADVS, p < 0.01) (Fig. 5b3). Similarly, the fixation group showed a significantly decreased C4-5 ROM in right lateral bending compared with the ADVS group (p = 0.024) (Fig. 5b3). C4-5 ROM in left axial rotation in the ADVS group increased significantly compared with the intact and fixation groups (ADVS VS intact, p = 0.011; ADVS VS fixation, p < 0.01) (Fig. 5c3). C4-5 ROM in right rotation in the fixation group decreased significantly compared with the intact and ADVS groups (fixation VS intact, p < 0.01; fixation VS ADVS, p < 0.01) (Fig. 5c3).
3.6. C2-5 ROM
Significantly decreased C2-5 ROM in the flexion in fixation group was detected compared with the intact group and ADVS groups (fixation VS intact, p < 0.01; fixation VS ADVS, p < 0.01) (Fig. 5a4). C2-5 ROM in extension in the fixation group decreased significantly compared with the ADVS group (p < 0.01) (Fig. 5a4). No difference in left lateral bending of C2-5 ROM was observed in pairwise comparisons among the intact, fixation and ADVS groups (p > 0.05) (Fig. 5b4). A significant difference in right lateral bending was only detected in a comparison between the intact and fixation groups (p = 0.036) (Fig. 5b4). C2-5 ROM in left axial rotation in the fixation and ADVS groups were significantly increased compared with the intact group (fixation VS intact, p = 0.013; ADVS VS intact, p < 0.01) (Fig. 5c4). Significant differences were observed in right axial rotation in pairwise comparisons among the intact, fixation and ADVS groups (intact VS fixation, p < 0.01; intact VS ADVS, p < 0.01; fixation VS ADVS, p = 0.014) (Fig. 5c4).
4. Discussion
ACCF has been widely used for the treatment of CSM [5]. Solid fusion and decompression to the spinal cord have been considered successful outcomes of this procedure [6,21]; however ACCF is, in fact, the second-best choice when corpectomy must be performed for decompression and anterior fusion must be achieved to maintain a stable biomechanical environment. In theory, the loss of motion of the operative levels resulting from rigid fusion redistributes into adjacent segments [11]. Studies have reported increased ROM of adjacent levels and high pressures in adjacent discs and facet joints [11,22,23]. For maximum preservation of the normal function of the spine, non-fusion implant is advocated and has been used in the clinic for decades [24]. Motion preservation prostheses, such as artificial cervical disc, have demonstrated superiority compared to interbody fusion devices [25,26]. Unlike solid fusion, ACD replacement could preserve the dynamic function of the intervertebral space. Consequently, the ROM of adjacent levels and the pressure in the adjacent discs and facet joints will not increase accordingly [26]; however, artificial cervical disc could only be appropriate for the reconstruction of intervertebral disc instead of a functional spinal unit. To preserve the ROM after corpectomy, ADVS is designed to restore the motion of the intervertebral segment.
This study investigated the three degrees-of-freedom ROM of the caprine cervical spine under three conditions. Theoretically, the ROM in left lateral bending should be equal to the right lateral bending and the ROM in left axial rotation should be equal to the right axial rotation [10]. However, in our study, the left ROM was not equivalent to the right ROM. Possible reasons for this discrepancy is that the movement behavior of a specimen does not always have to be symmetrical. The ROM in flexion of the three groups was not equal to the extension because goats have larger ROM in flexion than extension [27].
The fixation procedure contributed significantly to the change of ROM of the operative levels (C3–C5). In our study, the ROM in the fixation group was not completely lost because the fixed model did not have rigid fusion. Despite this finding, the ROM of C3-4 and C4-5 decreased in the fixation group and experienced little change in the ADVS group compared with the intact group, suggesting that ADVS could preserve the dynamic function of the intervertebral space. Although ADVS was designed to have ROM levels of 360° in axial rotation and 12° in one-side lateral bending, the ROM levels of the ADVS group were dramatically decreased in right lateral bending and right axial rotation in C3-4 and were increased in flexion and left axial rotation in C4-5 compared with the intact group. Because the goat had a flexible neck after the operation, the motion of the cervical spine six months of implantation might contribute to the slight positional abnormality of the ADVS resulting in different ROM values in different directions.
The ROM of the adjacent level (C2-3) in the fixation group was significantly increased indicating the compensation of the loss of motion of the fixed level, that is, the loss of motion of the operative segments redistributed to the adjacent level; however, we could not conclude that the ROM in C5-6 had presented a similar situation because no markers were fixed to C6. The ROM of C2-3 in all directions except left lateral bending maintained by ADVS did not differ from those of the intact cervical spine.
Generally, the total ROM would have compensated when the motion of the fixed level was lost; however, the total ROM (C2-5) of the fixation group was decreased dramatically in all directions except left lateral bending. The reason for this phenomenon may due to that 6 months of implantation is not too long enough for the caprine spine to compensate the ROM lost in the fixed levels. Interestingly, the ROM of the ADVS group also decreased in some directions suggesting that improvement of the ADVS is still needed.
The limitations of our study must be acknowledged. First, this study was conducted in vivo in animals. Consequently, disparities compared to the human anatomical structure were unavoidable. Second, the observation time of this non-fusion model was just 6 months and long-term in vivo observation is needed. Third, metal-on-metal has historically been a poor bearing surface, and a friction test is necessary for the pre-clinical evaluation for this new implant. The metal ions in the blood of the animals should be examined in the next stage. Forth, the C5-6 ROM was not reported due to technical limitations.
In conclusion, ADVS was designed for the preservation of motion at the operative levels and for the reconstruction of the vertebral body. The biomechanical properties of ADVS from an in vivo caprine cervical spine model showed that ROM values at the levels affected by implantation of this prosthesis were maintained. The radiographic outcomes suggested that the height of the vertebra was restored without spinal cord compression. Overall, this non-fusion device provides a new, promising model for the treatment of patients suffering from CSM.
Authors contributions
Xijing He and Jun Dong contributed to the study conception and design. Material preparation were performed by Yuan Sun, Xi Li, Pei Han, Lei Jin, Sen Yu and Zhentao Yu. Data collection and analysis were performed by Baobao Liang, Ruoxi Liu, Sihua Huang, Chen Wang, Yabing Song, Hao Wu, Huanjin Song and Liying Fan. The first draft of the manuscript was written by Jun Dong. Xijing He, Chun Zhang and Jun Dong contributed to the animal operation. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Declaration of competing interest
The authors declare that they have no conflict of interests in this manuscript. No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript.
Acknowledgments
We thank Professor William Lu and Mr. Steven Chan from Hongkong University for providing the MTS of biomechanical testing and Ms. Xiyuan Wu from surgical laboratory of the Second Affiliated Hospital of Xian Jiaotong University for animal preparing.
Footnotes
National Natural Science Foundation of China (32071327), Key Project of Research and development Program of Shaanxi Province (2020SF-190) and Special Fund of the Second Affiliated Hospital of Xi'an Jiaotong University (2020YJ(ZYTS)188) were received in support of this work.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jot.2022.07.005.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Fig. S1 ROM curve in flexion and extension of C2-3 intervertebral space. a: intact group; b: fixation group; c: ADVS group.
Fig. S10 ROM curve in flexion and extension of C2-5 intervertebral space. a: intact group; b: fixation group; c: ADVS group.
Fig. S11 ROM curve in lateral bending of C2-5 intervertebral space. a: intact group; b: fixation group; c: ADVS group.
Fig. S12 ROM curve in axial rotation of C2-5 intervertebral space. a: intact group; b: fixation group; c: ADVS group.
Fig. S2 ROM curve in lateral bending of C2-3 intervertebral space. a: intact group; b: fixation group; c: ADVS group.
Fig. S3 ROM curve in axial rotation of C2-3 intervertebral space. a: intact group; b: fixation group; c: ADVS group.
Fig. S4 ROM curve in flexion and extension of C3-4 intervertebral space. a: intact group; b: fixation group; c: ADVS group.
Fig. S5 ROM curve in lateral bending of C3-4 intervertebral space. a: intact group; b: fixation group; c: ADVS group.
Fig. S6 ROM curve in axial rotation of C3-4 intervertebral space. a: intact group; b: fixation group; c: ADVS group.
Fig. S7 ROM curve in flexion and extension of C4-5 intervertebral space. a: intact group; b: fixation group; c: ADVS group.
Fig. S8 ROM curve in lateral bending of C4-5 intervertebral space. a: intact group; b: fixation group; c: ADVS group.
Fig. S9 ROM curve in axial rotation of C4-5 intervertebral space. a: intact group; b: fixation group; c: ADVS group.
References
- 1.Hu J.H., Chen F., Qiu G.X., Sun T.S., Yang H.L., Shen H.Y., et al. Jingshu Keli for treating cervical spondylotic radiculopathy: the first multicenter, randomized, controlled clinical trial. J. Orthop. Translat. 2021;27:44–56. doi: 10.1016/j.jot.2020.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ji W., Zhang Y.J., Zhou F., Mao H.Q., Yang H.L., Liu T. Comparing clinical outcomes of using 3 versus 5 titanium miniplates in laminoplasty for multilevel cervical myelopathy: a prospective cohort study. J. Orthop. Translat. 2020;20:67–72. doi: 10.1016/j.jot.2019.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu X.H., Xu X.L., Liu Q., Ding J.Y., Liu J.H., Huang Z.P., et al. Unilateral cervical spinal cord injury induces bone loss and metabolic changes in non-human primates (Macaca fascicularis) J. Orthop. Translat. 2021;29:113–122. doi: 10.1016/j.jot.2021.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nassr A., Aleem I.S., Eck J.C., Woods B., Ponnappan R.K., Donaldson W.F., 3rd, et al. Does resection of the posterior longitudinal ligament impact the incidence of C5 palsy after cervical corpectomy procedures?: a review of 459 consecutive cases. Spine. 2017;42(7) doi: 10.1097/BRS.0000000000001806. E392-e97. [DOI] [PubMed] [Google Scholar]
- 5.Galivanche A.R., Gala R., Bagi P.S., Boylan A.J., Dussik C.M., Coutinho P.D., et al. Perioperative outcomes in 17,947 patients undergoing 2-level anterior cervical discectomy and fusion versus 1-level anterior cervical corpectomy for treatment of cervical degenerative conditions: a propensity score matched national surgical quality improvement program analysis. Neurospine. 2020;17(4):871–878. doi: 10.14245/ns.2040134.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cui S.B., Nasser A.A.E., Ma L., Su P.Q., Su D.Y., Liao Z.H. Analysis of the morphometric change in the uncinate process of the cervical spondylosis patients: a study of radiological anatomy. J. Orthop. Translat. 2020;24:32–38. doi: 10.1016/j.jot.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joo P.Y., Zhu J.R., Kammien A.J., Gouzoulis M.J., Arnold P.M., Grauer J.N. Clinical outcomes following one-, two-, three-, and four-level anterior cervical discectomy and fusion: a national database study. Spine J : off. j. N. Am. Spine Soc. 2022;22(4):542–548. doi: 10.1016/j.spinee.2021.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Zdeblick T.A., Bohlman H.H. Cervical kyphosis and myelopathy. Treatment by anterior corpectomy and strut-grafting. J Bone Joint Surg Am. 1989;71(2):170–182. [PubMed] [Google Scholar]
- 9.Farber S.H., Mauler D.J., Sagar S., Pacult M.A., Walker C.T., Bohl M.A., et al. Perioperative and swallowing outcomes in patients undergoing 4-and 5-level anterior cervical discectomy and fusion. J Neurosurg Spine. 2021;34(6):849–856. doi: 10.3171/2020.10.SPINE201307. [DOI] [PubMed] [Google Scholar]
- 10.Yu Y., Li J.S., Guo T., Lang Z., Kang J.D., Cheng L.M., et al. Normal intervertebral segment rotation of the subaxial cervical spine: an in vivo study of dynamic neck motions. J. Orthop. Translat. 2019;18:32–39. doi: 10.1016/j.jot.2018.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen C., Yuchi C.X., Gao Z.W., Ma X.L., Zhao D., Li J.W., et al. Comparative analysis of the biomechanics of the adjacent segments after minimally invasive cervical surgeries versus anterior cervical discectomy and fusion: a finite element study. J. Orthop. Translat. 2020;23:107–112. doi: 10.1016/j.jot.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang X., Bartels R., Donk R., Arts M.P., Goedmakers C.M.W., Vleggeert-Lankamp C.L.A. The association of cervical sagittal alignment with adjacent segment degeneration. Eur Spine J. 2020;29(11):2655–2664. doi: 10.1007/s00586-019-06157-0. [DOI] [PubMed] [Google Scholar]
- 13.Hilibrand A.S., Carlson G.D., Palumbo M.A., Jones P.K., Bohlman H.H. Radiculopathy and myelopathy at segments adjacent to the site of a previous anterior cervical arthrodesis. J. Bone Jt. Surg. Am. 1999;81(4):519–528. doi: 10.2106/00004623-199904000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Li X., Li G., Luk K.D.-K., Hu Y. Neurorestoratology evidence in an animal model with cervical spondylotic myelopathy. J Neurorestoratol. 2017;5:21–29. [Google Scholar]
- 15.Cao S., Zhao Y., Sun Y., Li W., Zhou F., Zhang F., et al. Single-level cervical arthroplasty with prodisc-C vivo artificial disc: five-year follow-up results from one center. Spine. 2022;47(2):122–127. doi: 10.1097/BRS.0000000000004119. [DOI] [PubMed] [Google Scholar]
- 16.Burkus J.K., Traynelis V.C., Haid R.W., Jr., Mummaneni P.V. Clinical and radiographic analysis of an artificial cervical disc: 7-year follow-up from the Prestige prospective randomized controlled clinical trial: clinical article. J Neurosurg Spine. 2014;21(4):516–528. doi: 10.3171/2014.6.SPINE13996. [DOI] [PubMed] [Google Scholar]
- 17.Yan N., Yu S., Hou T., Gu G., Zhang H., Zhao S., et al. Cervical spondylotic myelopathy caused by single-level vertebral spontaneous fusion. PLoS One. 2014;9(11) doi: 10.1371/journal.pone.0112423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kommu R., Sahu B.P., Purohit A.K. Surgical outcome in patients with cervical ossified posterior longitudinal ligament: a single institutional experience. Asian j. Neurosurg. 2014;9(4):196–202. doi: 10.4103/1793-5482.146602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peterson J.M., Chlebek C., Clough A.M., Wells A.K., Batzinger K.E., Houston J.M., et al. Stiffness matters: Part II-the effects of plate stiffness on load-sharing and the progression of fusion following anterior cervical discectomy and fusion in vivo. Spine. 2018;43(18) doi: 10.1097/BRS.0000000000002644. E1069-e76. [DOI] [PubMed] [Google Scholar]
- 20.Chu L., Li R., Liao Z., Yang Y., Dai J., Zhang K., et al. Highly effective bone fusion induced by the interbody cage made of calcium silicate/polyetheretherketone in a goat model. ACS Biomater Sci Eng. 2019;5(5):2409–2416. doi: 10.1021/acsbiomaterials.8b01193. [DOI] [PubMed] [Google Scholar]
- 21.Wang S., Xiang Y., Wang X., Li H., Hou Y., Zhao H., et al. Anterior corpectomy comparing to posterior decompression surgery for the treatment of multi-level ossification of posterior longitudinal ligament: a meta-analysis. Int J Surg. 2017;40:91–96. doi: 10.1016/j.ijsu.2017.02.058. [DOI] [PubMed] [Google Scholar]
- 22.Daentzer D., Welke B., Hurschler C., Husmann N., Jansen C., Flamme C.H., et al. In vitro-analysis of kinematics and intradiscal pressures in cervical arthroplasty versus fusion - a biomechanical study in a sheep model with two semi-constrained prosthesis. Biomed Eng Online. 2015;14(1):27. doi: 10.1186/s12938-015-0018-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lubelski D., Healy A.T., Mageswaran P., Colbrunn R., Schlenk R.P. Analysis of adjacent-segment cervical kinematics: the role of construct length and the dorsal ligamentous complex. J Neurosurg Spine. 2020;32(1):15–22. doi: 10.3171/2019.7.SPINE19279. [DOI] [PubMed] [Google Scholar]
- 24.Wigfield C.C., Gill S.S., Nelson R.J., Metcalf N.H., Robertson J.T. The new Frenchay artificial cervical joint: results from a two-year pilot study. Spine. 2002;27(22):2446–2452. doi: 10.1097/00007632-200211150-00006. [DOI] [PubMed] [Google Scholar]
- 25.Nunley P.D., Coric D., Frank K.A., Stone M.B. Cervical disc arthroplasty: current evidence and real-world application. Neurosurgery. 2018;83(6):1087–1106. doi: 10.1093/neuros/nyx579. [DOI] [PubMed] [Google Scholar]
- 26.Sasso R.C., Best N.M. Cervical kinematics after fusion and bryan disc arthroplasty. J Spinal Disord Tech. 2008;21(1):19–22. doi: 10.1097/BSD.0b013e3180500778. [DOI] [PubMed] [Google Scholar]
- 27.DeVries Watson N.A., Gandhi A.A., Fredericks D.C., Smucker J.D., Grosland N.M. Sheep cervical spine biomechanics: a finite element study. Iowa Orthop J. 2014;34:137–143. [PMC free article] [PubMed] [Google Scholar]