Abstract
A recombinant cosmid containing genes involved in Klebsiella pneumoniae C3 core lipopolysaccharide biosynthesis was identified by its ability to confer bacteriocin 28b resistance to Escherichia coli K-12. The recombinant cosmid contains 12 genes, the whole waa gene cluster, flanked by kbl and coaD genes, as was found in E. coli K-12. PCR amplification analysis showed that this cluster is conserved in representative K. pneumoniae strains. Partial nucleotide sequence determination showed that the same genes and gene order are found in K. pneumoniae subsp. ozaenae, for which the core chemical structure is known. Complementation analysis of known waa mutants from E. coli K-12 and/or Salmonella enterica led to the identification of genes involved in biosynthesis of the inner core backbone that are shared by these three members of the Enterobacteriaceae. K. pneumoniae orf10 mutants showed a two-log-fold reduction in a mice virulence assay and a strong decrease in capsule amount. Analysis of a constructed K. pneumoniae waaE deletion mutant suggests that the WaaE protein is involved in the transfer of the branch β-d-Glc to the O-4 position of l-glycero-d-manno-heptose I, a feature shared by K. pneumoniae, Proteus mirabilis, and Yersinia enterocolitica.
In gram-negative bacteria the lipopolysaccharide (LPS) is one of the major structural and immunodominant molecules of the outer membrane. It consists of three domains: lipid A, core oligosaccharide, and O-specific antigen or O side chain. The genetics of O antigen and core biosynthesis have been intensively studied in the Enterobacteriaceae and other gram-negative bacteria (1, 12, 20, 42). Studies on characterization of the genes involved in LPS core biosynthesis in Escherichia coli and Salmonella enterica have shown that these genes are usually found clustered in a region of the chromosome, the waa (rfa) gene cluster (20). (The nomenclature proposed by Reeves et al. [37] for proteins and genes involved in core LPS biosynthesis is used in this work, with the original reported name in parentheses.) Studies concerning waa genes have been carried out in other gram-negative bacteria, such as Bordetella, where it has been shown that genes involved in the biosynthesis of the O antigen and LPS core are found in the same cluster (1).
Klebsiella spp., particularly Klebsiella pneumoniae, are important causes of nosocomial infections (10). K. pneumoniae infections occur in almost all body sites but occur with higher incidence in the urinary and respiratory tracts. The main populations at risk are neonates and predisposed and/or immunocompromised hosts (10, 22). K. pneumoniae typically has both LPS (O antigen) and capsule (K antigen) on its cell surface, and both contribute to the pathogenicity of this species (47, 56).
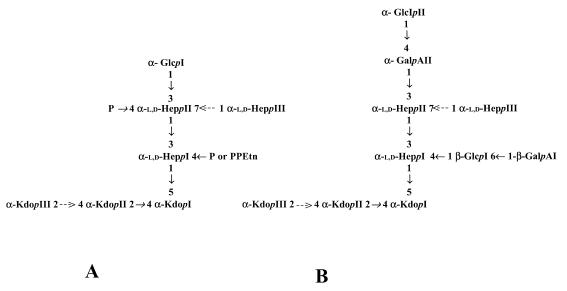
Comparison of the core LPS structure in K. pneumoniae (43, 45) and E. coli and S. enterica (20) reveals differences in both the inner and outer core structures (Fig. 1). In all these species, the inner core structure contains two residues of 3-deoxy-d-manno-octulopyranosonic acid (Kdop) and three residues of l-glycero-d-manno-heptopyranose (HeppI, HeppII, and HeppIII). The most striking differences are the absence of phosphoryl groups, the substitution of HeppI at the O-4 position by a β-d-galactopyranosonic acid-(1→6)-β-d-glucopyranose [β-d-GalpAI-(1→6)-β-d-GlcpI] disaccharide, and substitution of HeppII at the O-3 position by an α-d-GalpAII residue (43, 45).
FIG. 1.
Comparative structure of the chemotype Rc core LPS of E. coli and S. enterica (A) (20) versus K. pneumoniae strain RFK-11 (B) (43, 45). In K. pneumoniae RFK-11 (O8−:K−), only two Kdop residues were detected (42). In K. pneumoniae R20 (O1−:K20−), a GlcpN residue substituted with a tetraglycan of α-1,2-linked d-glycero-d-manno-heptopyranose was found instead of Glcpll (44). Dashed arrows denote modifications that are either nonstoichiometric or are confined to a particular core LPS type. P, phosphate; PPEtn, ethanolamine pyrophosphate.
In order to characterize the K. pneumoniae waa locus, we used the Serratia marcescens bacteriocin 28b (51) as a screening tool. This bacteriocin binds to the LPS core and outer membrane proteins OmpA and OmpF of sensitive E. coli cells (11). It was previously shown that bacteriocin 28b is a useful tool for identifying recombinant plasmids or cosmids harboring structural genes for small Ail-like outer membrane proteins (18). We have also shown that expression in E. coli K-12 of genes coding for enzymes involved in S. marcescens O antigen (39) and LPS core biosynthesis (17) confer a bacteriocin-resistant phenotype.
In this work we report the isolation and partial genetic characterization of the complete waa gene cluster involved in K. pneumoniae LPS core biosynthesis.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
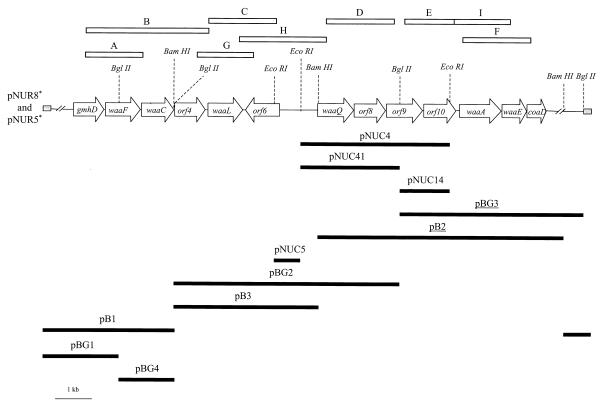
Bacterial strains (Table 1) were grown in Luria-Bertani (LB) Miller broth and LB Miller agar (31). LB media were supplemented with tetracycline (25 μg/ml), ampicillin (100 μg/ml), chloramphenicol (20 μg/ml), and kanamycin (50 μg/ml) when needed. The physical maps of the plasmids used in this study are shown in Fig. 2.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s)a | Source or reference |
|---|---|---|
| K. pneumoniae | ||
| C3 | O1:K66 | 56 |
| NC17 | C3-derived yibD mutant | This work |
| NC20 | C3-derived waaL mutant | This work |
| 52145 | O1:K2 | 32 |
| NC16 | 52145-derived waaE mutant | This work |
| NC18 | 52145-derived yibD mutant | This work |
| NC19 | 52145-derived waaQ mutant | This work |
| RFK-11 | O1−:K2− | 43 |
| E. coli | ||
| NM554 | recA13 araD139 Δ(ara-leu)7696 Δ(lac)X74 galE15 galK16 hsdR2 rpsL mcrA mcrB | 36 |
| DH5α | F−endA hsdR17(rK− mK+) supE44 thi-1 recA1 gyrA96 φ80lacZM15 Δ(argF lacZYA)U169 | 19 |
| XI1Blue | recA1 endA1 gyrA96 thi-1 hsdR17 supE44 relA1 lac (F′ proAB lacIqZΔM15 Tn10) | Stratagene |
| CJB26 | waaA::kan recA harboring plasmid pJSC2 | 6 |
| S. enterica serovar Typhimurium | ||
| SA1377 | waaC630 | 7 |
| SL3789 | waaF511 | 38 |
| SL3790 | waaF511 gal1-851 | 38 |
| SL3768 | waaG471 | 38 |
| SL3854 | waaG489 gal1-851 | 30 |
| SL3749 | waaL | 30 |
| SA2381 | galE409 | 38 |
| Cosmids and, plasmids | ||
| pWSK | Ampr vector | 54 |
| pLA2917 | Tetr Kmr cosmid vector | 2 |
| pNUR8 | Recombinant pLA2917 harboring the K. pneumoniae waa gene cluster | This work |
| pNUR5 | Recombinant pLA2917 harboring the K. pneumoniae waa gene cluster | This work |
| pNUC4 | EcoRI 4.5-kb insert from pNUR8 containing waaQ and orf8-orf9 | This work |
| pNUC41 | EcoRI-BglII 2.9-kb insert from pNUC4 containing waaQ and orf8 | This work |
| pNUC14 | BglII-EcoRI 1.5-kb insert from pNUC4 | This work |
| pBG3 | BglII 5.9-kb insert from pNUR8 containing orf10 waaAE and coaD | This work |
| pB2 | BamHl-BglII 7.3-kb insert from pNUR8 containing waaQ orf8-orf9-orf10 waaAE and coaD | This work |
| pNUC5 | EcoRI 1-kb insert from pNUR8 | This work |
| pBG2 | BglII 6.9-kb insert from pNUR8 containing waaL orf6 waaQ and orf8 | This work |
| pB3 | BamHI 4.3-kb insert from pNUR8 containing waaL and orf6 | This work |
| pB1 | pNUR8 BamHI deletion derivative containing gmhD and waaFC | This work |
| pBG1 | BglII 3.0-kb insert from pB1 containing gmhD | This work |
| pBG4 | BglII 1.6-kb insert from pB1 containing waaC | This work |
| pKO3 | CmrsacB temperature-sensitive replication | 29 |
| pKO3Δorf10 | pKO3 containing the engineered orf10 deletion | This work |
| pKO3ΔwaaE | pKO3 containing the engineered waaE deletion | This work |
| pKO3ΔwaaQ | pKO3 containing the engineered waaQ deletion | This work |
| pSF100 | pir-dependent replicon | 39 |
| pSF100ΔwaaL | pSF100 containing an internal fragment of the waaL gene | This work |
| pGEMT | Ampr plasmid vector | Promega |
| pGEMT-WaaC | waaC gene in pGEMT | This work |
| pGEMT-WaaF | waaF gene in pGEMT | This work |
| pGEMT-WaaA | waaA gene in pGEMT | This work |
| pGEMT-WaaE | waaE gene in pGEMT | This work |
| pGEMT-orf8 | orf8 in pGEMT | This work |
| pGEMT-orf10 | orf10 in pGEMT | This work |
| pGEMT-WaaQ | waaQ gene in pGEMT | This work |
| pGEMT-WaaL | waaL gene in pGEMT | |
| pGLU | lgtF from H. ducreyi | 12 |
| pJSC2 | Cmr temperature sensitive for replication plasmid containing E. coli waaA | 6 |
Ampr, ampicillin resistance; Cmr, chloramphenicol resistance; Kmr, kanamycin resistance; Tetr, tetracycline resistance.
FIG. 2.
Physical map of plasmids used in this study. Plasmids conferring high- and low-level bacteriocin 28b resistance on E. coli NM554 are denoted by asterisks and underlined letters, respectively. The right-side BglII site corresponds to the junction between the insert and vector cosmid in recombinant cosmid pNUR8.
General DNA methods.
DNA restriction endonucleases, T4 DNA ligase, E. coli DNA polymerase (Klenow fragment), and alkaline phosphatase were used as recommended by the suppliers. K. pneumoniae C3 genomic DNA was isolated and partially digested with Sau3A as described by Sambrook et al. (40). Cosmid pLA2917 (2) was digested with BglII, dephosphorylated, and ligated to Sau3A genomic fragments. Gigapack GoldIII (Stratagene) was used for DNA packaging, and packaged DNA was transduced into E. coli NM554. K. pneumoniae C3 genomic DNA recombinant clones were selected on LB agar plates containing tetracycline. The pNUC plasmid series were constructed by ligation of pNUR8-derived DNA fragments into pWSK29 (54) (Fig. 2). Plasmids of the pBG and pB series were obtained by ligation of pNUR8-derived BglII and BamHI fragments, respectively, into pLA2917 (Fig. 2). Plasmids subcloned in vector pGEMT were constructed by ligation to the vector of PCR-amplified products as follows: pGEMT-WaaF (5′-GAAAGCCCGAAACTGTTTGA-3′ and 5′-TCACCCGTTCGACGCTTTTA-3′), pGEMT-WaaC (5′-GTTTAAATCGGCATTAGTCC-3′ and 5′-CATTACTGAAGGCGTAATAG-3′), pGEMT-orf6 (5′-GGGTGATTAATTTTTCCCCA-3′ and 5′-GCGGTCTATAACAAACGCAA-3′), pGEMT-WaaA (5′-CGCCTGTACTTTCCGTTTAC-3′ and 5′-CATAAAGCGTCCGAGAAAAT-3′), pGEMT-WaaE (5′-TGCTTTATACCACCCTACT-3′ and 5′-GATAAACGACCACTCTTTG-3′), pGEMT-WaaL (5′-GGGTGATTAATTTTTCCCCA-3′ and 5′-GCGGTCTATAACAAACGCAA-3′), and pGEMT-WaaQ (5′-CACCTGATACCCGTATTCCAC-3′ and 5′-CGCTGGTTATCAATGGCGTTG-3′).
Bacteriocin 28b production and sensitivity assay.
Bacteriocin 28b was prepared as previously described (51). The overlay test was used for qualitative bacteriocin sensitivity assays, and quantitative bacteriocin sensitivity assays were performed as previously described (11).
Southern blot hybridization.
The DNA fragment containing the waaA and waaE genes from S. marcescens was labeled with digoxigenin as described by the manufacturer (Boehringer Mannheim). BamHI-digested pNUR8 DNA was electrophoresed, denatured, and transferred to a Hybond B membrane. After being baked, the membrane was prehybridized and hybridized in 5× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)–0.5% blocking reagent (Boehringer Mannheim)–0.1% Sarkosyl–0.02% sodium dodecyl sulfate (SDS). Washing, antibody incubation, and signal detection with p-nitroblue tetrazolium chloride and 5-bromo-4-chloro-3-indolylphosphate were done as recommended by the manufacturer (Boehringer Mannheim).
LPS isolation and analysis.
Cultures for analysis of LPS were grown in tryptic soy agar at 37°C. LPS was purified by the method of Westphal and Jann (55). For screening purposes LPS was obtained after proteinase K digestion of whole cells (23). LPS samples were separated by SDS-polyacrylamide gel electrophoresis (PAGE) or SDS-Tricine-PAGE and visualized by silver staining as previously described (34, 50). For chemical analyses, purified LPS was hydrolyzed with 1 N trifluoroacetic acid for 4 h at 100°C. Monosaccharides were analyzed as their alditol acetate derivatives by gas-liquid chromatography on an Rtx-2330 column (Restek Corp.). Alditol acetate monosaccharides were obtained by a previously described derivatization procedure (53). Colorimetric analysis of 2-keto-3-deoxyoctulosonic acid (Kdo) was performed by the method of Karkhanis et al. (26).
DNA sequencing and computer analysis of sequence data.
Double-stranded DNA sequencing was performed by using the Sanger dideoxy-chain termination method (41) with the Abi Prism dye terminator cycle sequencing kit (Perkin- Elmer). The pNUC plasmids were sequenced from both ends using the M13 and reverse M13 primers. The nucleotide sequence of cosmid pLA2917 is not available, so we determined the nucleotide sequence around the BglII cloning site (2) to be able to design primers CSPLA (5′-GACTGGGCGGTTTTATGG-3′) and RCSPLA (5′-CCATCTTGTTCAATCATGCG-3′); these primers were used to determine the nucleotide sequence of pBG and pB plasmids. Other sequence-derived oligonucleotides were used to extend the nucleotide sequence and to close the junctions between subclones. Primers used for DNA sequencing were purchased from Pharmacia LKB Biotechnology. The DNA sequence was translated in all six frames, and all open reading frames (ORFs) of greater than 100 bp were inspected. Deduced amino acid sequences were compared to those of DNA translated in all six frames from nonredundant GenBank and EMBL database entries by using the BLAST (3, 4) and FASTA (33) network service at the National Center for Biotechnology Information and the European Biotechnology Information, respectively. The Genetics Computer Group package (Madison, Wis.) Terminator program was used for prediction of possible terminator sequences. Clustal W (46) was used for multiple sequence alignments. Hydropathy profiles were calculated according to the guidelines of Kyte and Doolitle (27). The TopPredII program (8) was used to identify predicted protein transmembrane domains.
K. pneumoniae orf10, waaE, waaQ, and waaL mutant construction.
To obtain mutant strains K. pneumoniae NC16, NC17, NC18, and NC19, the method of Link et al. (29) was used to create chromosomal in-frame waa deletions. Briefly, pBG3 and primer pairs A (5′-CGCGGATCCCCCGGACGGTGACTACCTGAT-3′) and B (5′-CCCATCCACTAAACTTAAACATGTAGACGGCAGCCACGATGC-3′) and C (5′-TGTTTAAGTTTAGTGGATGGGTTCCGTTTACGCTGGCGCCTG-3′) and D (5′-CGCGGATCCTGGCGATCACCAGCGGGATCT-3′) were used in two sets of asymmetric PCRs to amplify DNA fragments of 582 (AB) and 608 (CD) bp, respectively. DNA fragment AB contains nucleotide 10327, inside orf9, to nucleotide 10908, corresponding to the 14th codon of orf10. DNA fragment CD contains nucleotide 11791, corresponding to the first base of codon 270 of orf10, to nucleotide 12398, inside the waaA gene. DNA fragments AB and CD were annealed at their overlapping region (underlined letters in primers B and C) and amplified by PCR as a single fragment using primers A and D. The fusion product was purified, BamHI digested (the BamHI site is double-underlined in primers A and D), ligated into BamHI-digested and phosphatase-treated pKO3 vector (29), electroporated into E. coli DH5α, and plated on chloramphenicol plates at 30°C to obtain plasmid pKO3Δorf10. Plasmid pBG3 and primer pairs A1 (5′-CGCGGATCCCACCGCAAGCTGCTGGAAAA-3′) and B1 (5′-CCCATCCACTAAACTTAAACAGCTTTTGCGGCTGCTCATTC-3′) and C1 (5′-TGTTTAAGTTTAGTGGATGGGGTGGTCAACGCGCAATATAC-3′) and D1 (5′-CGCGGATCCTCCTTCACCAGTGATGAGGA-3′) were used to obtain plasmid pKO3ΔwaaE containing an internally deleted waaE gene (the first 6 codons, a 7-codon tag, and the last 24 codons) flanked by 541 bp upstream and 409 bp downstream. Plasmid pNUC41 and primer pairs A2 (5′-CGCAGATCTCACCTGATACCCGTATTCCAC-3′) and B2 (5′-CCCATCCACTAAACTTAAACACAGCTTAATGACCAGGATCCG-3′) and C2 (5′-TGTTTAAGTTTAGTGGATGGGGCTATCAACACCAACACCGAC-3′) and D2 (5′-CGCAGATCTCGCTGGTTATCAATGGCGTTG-3′) (the BglII site is double-underlined in primers A2 and D2) were used to obtain plasmid pK03ΔwaaQ containing an internally deleted waaQ gene (the first 21 codons, a 7-codon tag, and the last 28 codons) flanked by 570 bp upstream and 547 bp downstream. Mutants NC17 and NC18 were constructed by gene replacement using plasmid pK03Δorf10 essentially as described (29). Plasmid pK03ΔwaaE and pK03ΔwaaQ were used to construct mutants NC16 and NC19, respectively. The waaL mutant was constructed by a single recombination procedure. Briefly, primers NUK5K2 (5′-GATATCGCGTGTATCTGACCGGGT-3′) and NUC514 (5′-GATATCCCACGATGCCGCCTTTCA-3′) (the EcoRV tag is double-underlined) were used to amplify a 657-bp waaL internal fragment from plasmid pB3. The fragment was cloned into the pir-dependent replication vector pSF100 (39) to obtain pSF100ΔwaaL.
Nucleotide sequence accession number.
The nucleotide sequence of the K. pneumoniae C3 cluster containing gmhD, waaF, waaC, orf4, waaL, orf6, waaQ, orf8, orf9, yibD, waaA, waaE, and coaD genes has been deposited in GenBank under accession no. AF146532 (see Fig. 2).
RESULTS AND DISCUSSION
Cloning of the K. pneumoniae waa gene cluster.
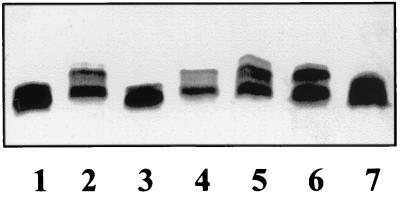
Comparison of the core LPS structure in K. pneumoniae (43, 45) and E. coli (20) reveals differences in both the inner and outer core structures. In bacteriocin 28b-sensitive E. coli cells, the core LPS is involved in bacteriocin binding (11). We anticipated that expression in the E. coli background of K. pneumoniae genes involved in core LPS biosynthesis could confer a bacteriocin-resistant phenotype. A K. pneumoniae C3 (56) cosmid gene bank was constructed, introduced into E. coli galE strain NM554, and screened for bacteriocin 28b resistance. LPS isolated from bacteriocin-resistant clones was analyzed by SDS-Tricine-PAGE to identify clones containing putative waa genes. Core LPS from two clones, pNUR8 and pNUR5, migrated faster than core LPS from E. coli NM554 harboring vector pLA2917 (Fig. 3, lanes 1, 2, and 3). The LPS phenotype suggests that genes from pNUR8 and pNUR5 produce a truncated NM554 core LPS or increase the negative charge of the molecule. EcoRI-digested recombinant cosmid pNUR8 and pNUR5 DNAs hybridized with a DNA probe containing the S. marcescens waaA (kdtA) and waaE (kdtX) genes (17), suggesting that waaA or waaE homologues were present in these recombinant cosmids.
FIG. 3.
E. coli NM554 harboring plasmids pNUR8 (lane 1), pNUR5 (lane 3), vector pLA2917 (lane 2), pBG3 (lane 4), pBG1 (lane 5), pNUC4 (lane 6), and pBG2 (lane 7). Similar results were obtained using the E. coli DH5α background.
Isolation of the minimum region required for bacteriocin 28b resistance.
Restriction analysis showed that the insert in recombinant cosmids pNUR8 and pNUR5 shared the region depicted in Fig. 2. Several subclones were constructed (Fig. 2) from pNUR8, and the level of bacteriocin 28b resistance was tested in E. coli NM554 harboring these plasmids. Only recombinant cosmids pNUR8 and pNUR5 conferred high levels of bacteriocin resistance, with cells surviving at 4 bacteriocin units. E. coli NM554 harboring either pBG3 or pB2 showed an intermediate level of bacteriocin resistance, with 50% of cells surviving at 1.7 bacteriocin units (Fig. 2). Plasmid pBG3 also modified the pattern of LPS mobility in SDS-Tricine-PAGE (Fig. 3, lane 4). Plasmids not conferring any bacteriocin resistance did not show strong changes in the pattern of LPS in SDS-Tricine-PAGE (Fig. 3, lanes 5, 6, and 7). These results suggest that genes present in pBG3 alter the core LPS, leading to partial bacteriocin 28b resistance.
Sequencing of the K. pneumoniae waa genes.
The subclones shown in Fig. 2 were used to identify the putative waa genes present in pNUR8. Analysis of the 16,222-bp nucleotide sequence revealed 12 ORFs putatively related to core LPS biosynthesis. Sequences defining putative ribosome binding sites were found upstream of each of the ORF start codons. Data summarizing the location of the ORFs and the characteristics of the putative encoded proteins are shown in Table 2. The distances between the stop and start codons between the successive ORF pairs orf1-orf2, orf2-orf3, and orf3-orf4 were 9, 3, and 0 bp, respectively. No Rho-independent transcription termination sequences were found between orf4 and orf5. This organization suggests that the first five ORFs constitute a transcriptional unit. orf6 apparently corresponds to a monocistronic gene transcribed in the opposite direction to that of the orf1-orf5 operon. In agreement with this genetic organization, the sequences GGGCCGTCAGCGGCCCTTTTT (between nucleotides 5027 and 5047) and GGGCCGCTGACGGCCCTTTTT (between nucleotides 5022 and 5042, complementary strand) were found. These two sequences suggest the presence of Rho-independent transcription termination sites between orf6 and the orf1-orf5 operon.
TABLE 2.
Characteristics of the K. pneumoniae C3 waa gene cluster and downstream coaD (kdtB) (16) gene
| Locus | Base positions | % G plus C | Size of protein encoded (kDa) | pIa | GRAVYb |
|---|---|---|---|---|---|
| orf1 (gmhD)c | 356–231 | 55.60 | 32.202 | 4.84 | −0.421 |
| orf2 (waaF) | 1241–2299 | 61.11 | 39.226 | 8.14 | −0.188 |
| orf3 (waaC) | 2303–3274 | 64.12 | 35.969 | 9.54 | −0.215 |
| orf4 | 3274–4182 | 52.70 | 34.596 | 9.27 | −0.120 |
| orf5 (waaL) | 4247–5329 | 60.96 | 40.146 | 9.59 | 0.613 |
| orf6 | 5373–6413 comp | 52.93 | 39.124 | 8.94 | −0.048 |
| orf7 (waaQ) | 7540–8616 | 60.93 | 40.306 | 9.21 | −0.168 |
| orf8 | 8613–9740 | 62.96 | 42.559 | 8.68 | −0.267 |
| orf9 | 9743–10837 | 61.39 | 40.985 | 9.16 | −0.276 |
| orf10 | 10867–11856 | 55.26 | 38.783 | 7.77 | −0.482 |
| orf11 (waaA) | 11948–13222 | 59.10 | 47.677 | 9.60 | −0.079 |
| orf12 (waaE) | 13222–13998 | 59.74 | 29.268 | 8.77 | −0.282 |
| orf13 (coaD) | 13995–14474 | 56.27 | 17.676 | 7.06 | 0.149 |
Isoelectric point of the protein was calculated using ProtParam at the ExPassy server.
Grand average hydropathicity of the protein calculated using the Kyte and Doolitle method (27).
Truncated ORF.
Analysis of the intergenic regions between the other ORFs showed that (i) the first base of the stop codon corresponds to the second base of the start codon for the ORF pairs orf7-orf8 and orf12-orf13; (ii) the orf11 termination codon and orf12 start codon are adjacent; and (iii) the distance between the stop and start codons of the orf pairs orf8-orf9, orf9-orf10, and orf10-orf11 were of 2, 28, and 91 bp, respectively. No sequences resembling Rho-independent transcription termination signals were detected in the intergenic regions between orf9-orf10 and orf10-orf11. These data suggest that orf7 to orf13 are transcriptionally coupled.
No E. coli ς70 promoter consensus-like sequences were found, suggesting that ORFs are expressed from a vector promoter or from promoters recognized by alternative ς factors.
Organization of the waa gene cluster in different K. pneumoniae strains.
To date, the core LPS chemical structures for two strains of K. pneumoniae have been reported. The outer core structure is different in the two strains; the α-d-GalpAll residue is substituted at the O-4 position by a α-d-Glcp residue in strain RFK-11 (43) and by a α-d,d-Hepp(1→2)-α-d,d-Hepp-(1→2)-α-d,d-Hepp-(1→2)-α-d,d-Hepp-(1→6)-d-GlcpN pentasaccharide in strain R20 (45). In order to determine the variability of the waa region in K. pneumoniae we designed five sets of sequence-derived oligonucleotides. Using genomic DNA from the C3 strain as template, these oligonucleotides generated PCR amplification DNA fragments (fragments A through F) of 1.58 (A), 2.89 (B), 2.07 (C), 2.14 (D), 1.56 (E), and 2.41 (F) kbp (Fig. 2). Amplification products A, B, C, D, E, and F were also obtained when using as template genomic DNA from K. pneumoniae subsp. pneumoniae strains KT751 (O1:K1), KT760 (O3:K11), KT768 (O4:K42), KT769 (O5:K57), KT771 (O7:K67), KT776 (O12:K80), 52145d (O1:K2), and RFK-11 (O8−:K−). The same amplification products were obtained from Klebsiella rhinoscleromatis KT755 (O2:K3) and K. pneumoniae subsp. ozaenae RFK-11 (O8−:K−).
Since the core structure of K. pneumoniae RFK-11 is known, we designed additional oligonucleotides that allowed amplification of DNA fragments (fragments G through I) of 1.45 (G), 2.7 (H), and 1.79 (I) kbp (Fig. 2) when using genomic DNA from either strain C3 or strain RFK-11 as template. PCR-amplified fragments A to I obtained using genomic DNA from strain RFK-11 as template were sequenced from both ends to obtain the nucleotide sequence of the junction between each ORF. Analysis of the sequence data showed that the same ORFs are found in strains C3 and RFK-11, with more than 99% identity at the nucleotide level. This result strongly suggests that the reported waa sequence is responsible for the biosynthesis of the RFK-11 core LPS. Nevertheless, we do not rule out the possibility that genes located outside the reported waa gene cluster could also be involved in K. pneumoniae core biosynthesis. Our results suggest that the waa genes and their organization are well conserved among representative strains of K. pneumoniae (these strains include serotypes O1, O2, O3, O4, O5, O7, O8, and O12). These results agree with a previous study showing that 89.4% of clinical isolates of Klebsiella reacted with the genus-specific monoclonal antibody V/9-5, which recognizes an epitope of the outer core region of Klebsiella LPS (49). Important differences in other Klebsiella waa gene clusters should be expected, since only 50% of isolates of the O1 serogroup were found to react with monoclonal antibody V/9-5 (49).
K. pneumoniae genes involved in inner core biosynthesis.
The inner core backbone has been found to be the same in all the Enterobacteriaceae species studied so far. Thus, we expected to find four genes coding for proteins highly similar to the known bifunctional CMP-Kdo:lipid A Kdo transferase (WaaA or KdtA), ADP-l-glycero-d-manno-heptose epimerase (GmhD or RfaD), ADP-heptose-LPS heptosyltransferase I (WaaC or RfaC), and ADP-heptose-LPS heptosyltransferase II (WaaF or RfaF). The 5′-truncated orf1 and the orf2, orf3, and orf11 ORFs had high levels of amino acid identity to the known enterobacterial GmhD (95 to 96%), WaaF (82 to 88%), WaaC (82 to 88%), and WaaA (83%) proteins, respectively (9, 17, 20, 21).
Complementation analyses of known inner core backbone mutants were performed to test the functions of these genes. E. coli strain CJB26 harbors a kanamycin determinant inserted in the waaA gene and a wild-type waaA gene in a temperature-sensitive plasmid (pJSC2), leading to a growth-temperature-sensitive phenotype. Plasmid pGEMT-WaaA was transformed into E. coli CJB26 at 30°C. Transformant colonies able to grow at 44°C in LB media containing kanamycin and ampicillin were tested for chloramphenicol sensitivity. Plasmid DNA isolation and analysis from one of the chloramphenicol-sensitive colonies showed the presence of plasmid pGEMT-WaaA and the absence of plasmid pJSC2. This experiment strongly suggests that orf11 corresponds to the K. pneumoniae waaA gene.
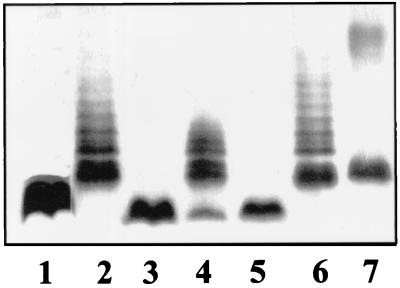
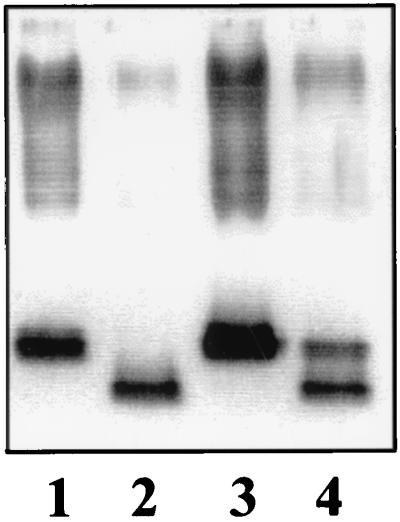
LPS from S. enterica serovar Typhimurium strains SA1377 (waaC630) and SL3789 (waaF511) transformed with plasmid pB1 migrated more slowly in SDS-Tricine-PAGE and even showed several higher molecular weight bands (Fig. 4, lanes 2 and 6). Furthermore, plasmids pGEMT-WaaC and pGEMT-WaaF complemented strains SA1377 and SL3789, respectively (Fig. 4, lanes 4 and 7). The differences in the smooth LPS banding pattern in strain SA1377 complemented with pBI and pGEMT-WaaC, and strain SL3789 complemented with pBI and pGEMT-WaaF could be due to plasmid copy number and/or to differences in vector promoter read-through. As expected, plasmid pBG1 did not complement strain SA1377 (Fig. 4, lane 3) or strain SL3789 (data not shown). This result strongly suggests that K. pneumoniae waaC and waaF code for ADP-heptose-LPS heptosyltransferase I and ADP-heptose-LPS heptosyltransferase II, respectively.
FIG. 4.
SDS-Tricine-PAGE analysis of LPS from S. enterica serovar Typhimurium SA1377 (waaC630) (lane 1), SA1377 (pB1) (lane 2), SA1377 (pBG1) (lane 3), SA1377 (pGEMT-WaaC) (lane 4), SL3789 (waaF511) (lane 5), SL3789 (pB1) (lane 6), and SL3789 (pGEMT-WaaF) (lane 7).
The protein encoded by orf7 showed significant levels of identity (42 to 43%) and similarity (60 to 61%) to the known enterobacterial ADP-heptose-LPS heptosyltransferase III (WaaQ) (20). Plasmid pK03ΔwaaQ was used to construct an in-frame tagged deletion mutant (NC19) as previously described (29). LPS obtained from mutant NC19 contained O antigen and migrated slightly faster than that of the parent 52145 strain in SDS-Tricine-PAGE (data not shown), as previously described for a Haemphilus ducreyi waaQ mutant (12). This LPS also showed a reduction in l-glycero-d-manno-heptose (l,d-Hep) (Table 3). On the other hand, no d,d-Hep was detected in the wild-type 52145 strain (Table 3), suggesting that the α-d,d-Hepp-(1→2)-α-d,d-Hepp-(1→2)-α-d,d-Hepp(1→2)-α-d,d-Hepp heptan found in strain R20 is absent from strain 52145. Taken together, these results suggest that waaQ codes for ADP-heptose-LPS heptosyltransferase III.
TABLE 3.
Chemical composition of LPS from K. pneumoniae wild-type strain 52145 and from waaE and waaQ mutantsa
| LPS from K. pneumoniae | Gal | GalAb | Glcb | l,d-Hepb | Kdoc |
|---|---|---|---|---|---|
| 52145 (wild type) | 919 | 83 | 387 | 228 | 218 |
| NC16 (waaE) | 650 | 35 | 161 | 245 | 230 |
| NC16 (pGEMT-WaaE) | 1,030 | 90 | 470 | 240 | 260 |
| NC19 (waaQ) | 959 | 84 | 344 | 100 | 220 |
| NC19 (pGEMT-WaaQ) | 1,010 | 77 | 379 | 235 | 215 |
Monosaccharide amounts are expressed in nanomoles of LPS per milligram.
Assayed by gas-liquid chromatography.
Assayed by the colorimetric method.
Other K. pneumoniae genes involved in core biosynthesis.
The remaining ORFs, except orf10 and orf11, encoded proteins with low levels of similarity to proteins known to be involved in the biosynthesis of the core LPS of E. coli or S. enterica. The deduced 302-amino-acid protein encoded by orf4 showed significant levels of amino acid similarity and identity to WaaZ proteins from S. enterica (43 and 23%) and E. coli core types K-12 and R2 (42 and 16%). It has been suggested that the WaaZ protein could be involved in the addition of KdopIII (20), although its function remains unknown. Interestingly, a KdopIII residue has been found in the core structure of K. pneumoniae subsp. pneumoniae strain R20 (45). The presence of a KdopIII residue has not been reported in strain RFK-11, but its presence in nonstoichiometric amounts cannot be ruled out.
Position-iterated BLAST (4) searches identified enterobacterial ADP-heptose-LPS heptosyltransferases related to the orf6-encoded protein. On the other hand, the orf6-encoded protein was found to share similarities (25% identity and 45% similarity) with putative ADP-heptose-LPS heptosyltransferase WaaU from E. coli K-12 (44). It has been suggested that the E. coli K-12 WaaU transfers l,d-HepIV to GlcIII in the outer core LPS (20). Four d-glycero-d-manno heptoses constitute the distal end of core LPS in K. pneumoniae R20 (45), but these residues were not found in strain RFK-11 or strain C3 (Table 3). On this basis, we speculate that orf6 could encode a putative heptosyltransferase involved in the addition of a fourth l,d-Hepp residue.
The deduced 375-amino-acid protein encoded by orf8 was found to share limited similarity with WaaG proteins from Pseudomonas aeruginosa (accession number O33426), S. enterica (20), and E. coli K-12 (44), R2, R-3, and R-4 (20, 21). The orf8-encoded protein and the WaaG proteins belong to the retaining glycosyltransferase family 4 (http://afmb.cnrs-mrs.fr/∼pedro/CAZY/db.html). WaaG protein is reported to be a glucosyltransferase involved in the α-1→3 linkage of d-Glcpl to HeppII in E. coli and S. enterica (20). In K. pneumoniae strains RFK-11 and R20 the HeppII at the O-3 position is substituted by an α-d-GalpAII residue (43, 45). Thus, it was expected that the role of the K. pneumoniae C3 orf8-encoded product would be different from that of the WaaG proteins. To test this hypothesis S. enterica SL3768 (waaG471), a chemotype Rd1 O antigen-deficient strain, was transformed with plasmid pGEMT-Orf8. The transformed strain was not complemented by orf8, as judged by SDS-Tricine-PAGE analysis of LPS (data not shown).
The deduced 364-amino-acid protein encoded by orf9 showed limited similarity to the WlaE protein from Campylobacter jejuni (14) and lower levels of similarity to several other putative glycosyltransferase proteins from different gram-negative bacteria. The levels of similarity among these proteins are too low to deduce a putative function for orf9.
Lipid A core:O-antigen polymer ligase.
The deduced 360-amino-acid protein encoded by orf5 did not show high levels of amino acid similarity to other proteins in the databases. However, TopPred2 analysis of this protein predicted 10 membrane-spanning domains, suggesting an integral membrane location. The distribution of these putative transmembrane domains along the protein sequence and the protein hydropathy profile were found to be very similar to those of WaaL proteins. WaaL proteins are responsible for the ligation of O-antigen polymer to the lipid A core (20, 21). The pir-dependent plasmid pSF100ΔwaaL was introduced into K. pneumoniae C3 to obtain strain NC20 by homologous recombination. Southern blot experiments with appropriate probes confirmed that strain NC20 harbors two incomplete copies of the waaL gene. Analysis by SDS-PAGE overloaded with LPS samples showed that no O antigen was present in LPS obtained from mutant NC20, while no differences were observed in the core region (Fig. 5, lane 2). On the other hand, mutant NC20 was complemented by plasmid pGEMT-WaaL (Fig. 5, lane 3). These results suggest that orf5 corresponds to the waaL gene coding for the K. pneumoniae C3 lipid A core:O-antigen polymer ligase. Plasmid pGEMT-WaaL was introduced into S. enterica SL3749 (waaL), and analysis of its LPS by SDS-Tricine-PAGE showed no complementation of the waaL mutation (data not shown). It has been previously shown that the waaL gene from E. coli K-12 does not complement an S. enterica waaL mutant and that the waaL gene from E. coli R2 complements waaL mutants from E. coli K-12 and S. enterica with different efficiencies (20, 21). The core attachment site for O antigen is unknown for K. pneumoniae. Our results suggest that differences in overall core LPS structure and O-antigen attachment sites preclude functional complementation of the S. enterica waaL mutant by its K. pneumoniae homologue.
FIG. 5.
SDS-PAGE analysis of LPS from K. pneumoniae 52145 (wild type) (lane 1), NC20 (waaL) (lane 2), and NC20 (pGEMT-WaaL).
Characterization of K. pneumoniae orf10.
The deduced 329-amino-acid protein encoded by orf10 was found to be similar (38.2 and 29.4% similarity and identity, respectively) to the hypothetical YibD protein from E. coli (44). The YibD-like K. pneumoniae protein also showed significant levels of amino acid similarity to other proteins, mainly glycosyltransferases, involved in biosynthesis of polysaccharides and teichoic acid. The putative E. coli K-12 yibD gene is found outside and upstream of the waa gene cluster. To determine if K. pneumoniae orf10 is involved in core oligosaccharide biosynthesis, an in-frame tagged deletion mutant was constructed. Plasmid pKO3Δorf10 containing the engineered deletion was used in gene replacement experiments as previously described (29) in K. pneumoniae C3 (O1:K66) and 52145 (O1:K2). Mutant strains NC17 and NC18 (orf10 deletion mutants from C3 and 52145, respectively) were isolated. LPS from K. pneumoniae NC17, NC18, and their wild-type strains were isolated. No differences were seen between wild-type and mutant LPS with SDS-Tricine-PAGE. Furthermore, no differences in sugar composition were found between these LPS samples. These results suggest that orf10 is not involved in K. pneumoniae core LPS biosynthesis, at least under the growth conditions used in this study. Nevertheless, a role for the yibD gene in the addition of a labile core LPS component cannot be ruled out.
Strains NC17 and NC18 became partially resistant (5-log decrease in their efficiencies of plating) to K-antigen-specific bacteriophages FC3-9 (for K66) and Φ2 (for K2) but were fully sensitive to O1-antigen-specific bacteriophages like FC3-1 (48). The partial resistance to specific K-antigen phages (FC3-9 for K66 and Φ2 for K2) suggested that orf10 could be involved in capsule production. When we analyzed the amount of K66 or K2 by enzyme-linked immunosorbent assay, using specific antibodies and whole cells (5) in the mutant strains NC17 and NC18, we found that there was at least a 90% reduction in their amounts compared to those of their respective wild-type strains. Enzyme-linked immunosorbent assay of culture supernatants of the mutant strains showed a similar reduction in the amount of unbound capsule material. On the other hand, strain 52145 (O1:K2) showed a 50% lethal dose (LD50) in mice of 102, while the NC18 mutant showed a large increase in its LD50 in mice (8 × 104) that correlates with a capsule reduction in the mutant. A fully unencapsulated mutant from 52145 showed an LD50 in mice of 5 × 106. Mutant strains NC17 and NC18 complemented with plasmid DNA containing a complete orf10 gene showed the same characteristics as their respective wild-type strains (full sensitivity to FC3-9 and Φ2 bacteriophages and an LD50 in mice of 102). Capsule was extracted from wild-type strain 52145 and mutant strain NC18. Neutral sugar and uronic acid analyses revealed essentially the same composition and molar ratios for Glc, mannose (Man), and glucuronic acid (GlcA) for both capsules. Thus, it appears that the orf10-encoded protein is responsible for proper capsule amount production by an unknown mechanism.
Characterization of the K. pneumoniae waaE gene.
The deduced 258-amino-acid protein encoded by orf12 showed substantial levels of amino acid identity and similarity to protein WaaE (KdtX) (70 and 80%) from S. marcescens (17), LgtF protein from H. ducreyi (44 and 66%) (12), LgtF protein from Neisseria meningitidis (34 and 51%) (25), and HIO653 from Haemophilus influenzae (51 and 66%) (13). The LgtF protein has been shown to be a transferase that adds a d-Glcp residue to Heppl of the lipooligosaccharide (LOS) inner core (12, 25). It has also been shown that a d-Glcp residue is attached via a β-1,4 linkage to Heppl in the LOS inner core of H. ducreyi and the LPS inner cores of K. pneumoniae strains RFK-11 and R20 (43, 45). Hydrophobic cluster analysis (15) between WaaE and LgtF proteins suggests that both proteins share extensive secondary structure similarity. Furthermore, both proteins belong to the inverting glycosyltransferase family 2 (http://cnrs-mrs.fr/∼pedro/CAZY/db.html).
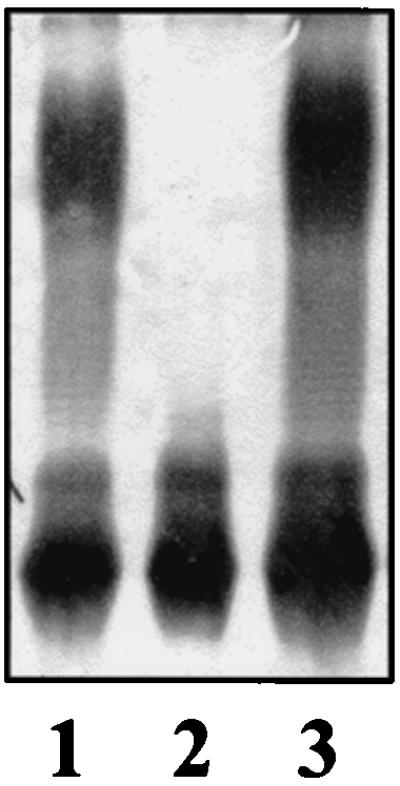
To determine the function of the K. pneumoniae waaE gene, an in-frame tagged deletion was constructed. Plasmid pKO3ΔwaaE, containing the engineered deletion, was used to introduce the waaE deletion into K. pneumoniae 52145 by double recombination, as described previously (29). Candidate mutants were screened by PCR, and one of them (strain NC16) was further proved to contain the desired mutation by nucleotide sequence determination of the waaA-coaD region. LPS from strains 52145 (wild type) and NC16 (waaE) were extracted and analyzed by SDS-Tricine-PAGE. The result obtained (Fig. 6, lanes 1 and 2) shows that the core LPS from strain NC16 migrates faster than that of the wild-type strain, and it appears that the mutant LPS still contains O antigen, although in smaller amounts than wild-type LPS. The monosaccharide composition was determined for both wild-type and waaE mutant LPS, and the results obtained show a marked reduction in Glc and galacturonic acid (GalA) content and similar amounts of Kdo, Hep, and galactose (Gal) (Table 3). The LPS from mutant strain NC16 transformed with pGEMT-WaaE showed a wild-type migration pattern (Fig. 6, lane 3) and a wild-type sugar composition (Table 3). According to the known core structure of K. pneumoniae RFK-11, a glucosyltransferase defect affecting the branch β-d-Glcpl-(1→4)-l-d-Heppl would lead to at least a 50% reduction in GalA. On the other hand, a glucosyltransferase defect affecting α-d- GlcpII-(1→4)-α-d-GalpAII would not prevent the addition of the two GalA residues (Fig. 1). The reduction in GalA in the LPS from the NC16 mutant suggests that the waaE mutant LPS has a defect affecting the branch β-d-GalpA-(1→6)-β-d-Glcp disaccharide linked to α-l,d-Heppl. To test the WaaE putative function, the mutant NC16 was transformed with plasmid pGLU containing the lgtF gene from H. ducreyi. LPS from NC16 (pGLU) showed two core LPS bands migrating to the mutant and wild-type core LPS positions, respectively (Fig. 6, lane 4). This result shows that the lgtF gene partially complements the waaE mutation. The lack of full complementation could be due to low efficiency of recognition of the lgtF promoter in the K. pneumoniae genetic background. On the other hand, an α-1,2 linkage between Hepplll and Heppll residues is found in Helicobacter pylori (12), while an α-1,7 linkage is found in K. pneumoniae (43, 45). Thus, structural differences around the Heppl recognized by LgtF and WaaE proteins could also be responsible for the partial complementation observed. These results suggest that waaE codes for the glucosyltransferase that attaches the d-Glcpl residue via a β-1,4 linkage to l,d-Heppl. On the other hand, the O1 antigen of the wild-type LPS is a d-galactan and the presence of Gal in both mutant and wild-type LPS (Fig. 6 and Table 3) clearly shows that a waaE defect does not prevent O1-antigen ligation.
FIG. 6.
SDS-Tricine-PAGE analysis of LPS from K. pneumoniae 52145 (wild type) (lane 1), NC16 (waaE) (lane 2), NC16 (pGEMT-WaaE) (lane 3), and NC16 (pGLU) (lane 4).
Comparison of the known waa gene clusters in Enterobacteriaceae.
The results presented in this work suggest that the 12 genes of the K. pneumoniae waa gene cluster are well conserved in representative strains of Klebsiella sp. These genes are organized in two main operons transcribed in the same direction; only orf6 is apparently monocistronic, being located between the two operons and transcribed in the opposite direction. Furthermore, analysis of nucleotide sequences surrounding the described K. pneumoniae waa gene cluster allowed detection of ORFs similar to kbl (upstream) and coaD and fpg (downstream). This analysis indicates that the waa gene cluster is similarly located in both E. coli K-12 and K. pneumoniae. No sequences similar to the antitermination JUMPStart (Just Upstream of Many Polysaccharide-associated Starts) (24, 28) sequence were found in the 1,126-bp intergenic region between the divergently transcribed waaQ operon and monocistronic orf6. The JUMPStart sequence has been found upstream of the waaQ operon in E. coli and S. enterica (20, 24). This difference suggests that in the K. pneumoniae waaQ operon there are fewer functional terminator elements or that another unknown antitermination mechanism is used.
The reported core LPS structures of K. pneumoniae, E. coli, and S. enterica (20, 43, 45) have a common inner core backbone structure (Fig. 1). As expected, we found four genes involved in epimerization (gmhD) and transfer of Hepl (waaC), Hepll (waaF), and Heplll (waaQ) and a fifth gene coding for the transfer of the Kdo residues. However, a prominent feature is the absence of phosphoryl substituents in the K. pneumoniae inner core LPS and substitution of Hepl at the O-4 position by a β-d-GalpA-(1→6)-β-d-Glcp disaccharide (43, 45). As expected, no genes similar to those involved in phosphoryl modification of Hepl and Hepll (waaP and waaY) were found in the K. pneumoniae waa gene cluster. The waaE gene, which codes for the transferase involved in the addition of the branched d-GlcpI residue via a β-1,4 linkage to Heppl, is located just downstream from the waaA gene. A waaE gene is also found downstream of the waaA gene in S. marcescens (17), strongly suggesting the presence of a branched d-Glc residue linked to Heppl by a β-1,4 linkage in this species. On the other hand, a branched d-Glc residue linked via a β-1,4 linkage to Heppl has also been found in Proteus mirabilis (52) and Yersinia enterocolitica (35), strongly suggesting that similar waaE genes exist in these two species.
ACKNOWLEDGMENTS
This work was supported by DGICYT and Plan Nacional de I + D grants (Ministerio de Educación y Cultura [Spain]) and from Generalitat de Catalunya. N.C., N.A., N.C., L.I., and M.A. have predoctoral fellowships from Ministerio de Educación y Cultura (Spain), Generalitat de Catalunya, and Universitat de Barcelona.
We thank K. E. Sanderson (Salmonella Genetic Stock Center) for providing Salmonella strains. We also thank Maite Polo for her technical assistance.
REFERENCES
- 1.Allen A G, Maskell D J. The identification, cloning and mutagenesis of a genetic locus required for lipopolysaccharide biosynthesis in Bordetella pertussis. Mol Microbiol. 1996;19:37–52. doi: 10.1046/j.1365-2958.1996.354877.x. [DOI] [PubMed] [Google Scholar]
- 2.Allen L N, Hanson R S. Construction of broad-host-range cosmid cloning vectors: identification of genes necessary for growth of Methylobacterium organophilum on methanol. J Bacteriol. 1985;161:955–962. doi: 10.1128/jb.161.3.955-962.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altschul S F, Gish W, Miller W, Myers E W, Lipman D. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 4.Altschul S F, Madden T L, Schäffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alvarez D, Merino S, Tomás J M, Benedi V J, Alberti S. Capsular polysaccharide is a major complement resistance factor in lipopolysaccharide O side chain-deficient Klebsiella pneumoniae clinical isolates. Infect Immun. 2000;68:953–955. doi: 10.1128/iai.68.2.953-955.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belunis C J, Clementz T, Carty S M, Raetz C H R. Inhibition of lipopolysaccharide biosynthesis and cell growth following inactivation of the kdtA gene in Escherichia coli. J Biol Chem. 1995;270:27646–27652. doi: 10.1074/jbc.270.46.27646. [DOI] [PubMed] [Google Scholar]
- 7.Chatterjee A K, Ross H, Sanderson K E. Leakage of periplasmic enzymes from lipopolysaccharide-defective mutants of Salmonella typhimurium. Can J Microbiol. 1976;22:1549–1560. doi: 10.1139/m76-227. [DOI] [PubMed] [Google Scholar]
- 8.Claros M G, von Heijne G. Prediction of transmembrane segments in integral membrane proteins, and putative topologies, using several algorithms. Comput Appl Biosci. 1994;10:685–686. doi: 10.1093/bioinformatics/10.6.685. [DOI] [PubMed] [Google Scholar]
- 9.Clementz T, Raetz C H R. A gene coding for 3-deoxy-d-manno-octulosonic-acid transferase in Escherichia coli: identification, mapping, cloning, and sequencing. J Biol Chem. 1991;266:9687–9696. [PubMed] [Google Scholar]
- 10.Emori T G, Gaynes R P. An overview of nosocomial infections, including the role of the microbiology laboratory. Clin Microbiol Rev. 1993;6:428–442. doi: 10.1128/cmr.6.4.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Enfedaque J, Ferrer S, Guasch J F, Tomás J, Regué M. Bacteriocin 28b from Serratia marcescens N28b: identification of Escherichia coli surface components involved in bacteriocin binding and translocation. Can J Microbiol. 1996;42:19–26. doi: 10.1139/m96-004. [DOI] [PubMed] [Google Scholar]
- 12.Filiatrault M J, Gibson B G, Schilling B, Sun S, Munson R S, Campagnari A A. Construction and characterization of Haemophilus ducreyi lipooligosaccharide (LOS) mutants defective in expression of heptosyltransferase III and beta 1,4-glucosyltransferase: identification of LOS glycoforms containing lactosamine repeats. Infect Immun. 2000;68:3352–3361. doi: 10.1128/iai.68.6.3352-3361.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fleischmann R D, Adams M D, White O, Clayton R A, Kirkness E E, Kerlavage A R, Bult C J, Tomb J F, Dougherty B A, Merrick J M, McKenney K, Sutton G, FitzHugh W, Fields C, Gocayne J D, Scott J, Shirley R, Liu L L, Glodek A, Kelley J M, Weidman J F, Phillips C A, Spriggs T, Hedblom E, Cotton M D, Utterback T R, Hanna M C, Nguyen D T, Saudek D M, Brandon R C, Fine L D, Fritchman J L, Fuhrmann J L, Geoghagen N S M, Gnehm C L, McDonald L A, Small K V, Fraser C M, Smith H O, Venter J C. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science. 1995;269:496–512. doi: 10.1126/science.7542800. [DOI] [PubMed] [Google Scholar]
- 14.Fry B N, Korolok V, ten Brinke J A, Pennings M T T, Zalm R, Teunis B J J, Coloe P J, van der Zeijs A M. The lipopolysaccharide biosynthesis locus of Campylobacter jejuni 81116. Microbiology. 1988;144:2049–2061. doi: 10.1099/00221287-144-8-2049. [DOI] [PubMed] [Google Scholar]
- 15.Gaboriaud C, Bissery V, Benchetrit T, Mornon J P. Hydrophobic cluster analysis: an efficient new way to compare and analyse amino acid sequences. FEBS Lett. 1987;224:149–155. doi: 10.1016/0014-5793(87)80439-8. [DOI] [PubMed] [Google Scholar]
- 16.Geerlof A, Lewendon A, Shaw W V. Purification and characterization of phosphopantetheine adenylyltransferase from Escherichia coli. J Biol Chem. 1999;274:27105–27111. doi: 10.1074/jbc.274.38.27105. [DOI] [PubMed] [Google Scholar]
- 17.Guasch J F, Piqué N, Climent N, Ferrer S, Merino S, Rubirés X, Tomás J M, Regué M. Cloning and characterization of two Serratia marcescens genes involved in core lipopolysaccharide biosynthesis. J Bacteriol. 1996;178:5741–5747. doi: 10.1128/jb.178.19.5741-5747.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guasch J F, Ferrer S, Enfedaque J, Viejo M B, Regué M. A 17 kDa outer-membrane protein (Omp4) from Serratia marcescens confers partial resistance to bacteriocin 28b when expressed in Escherichia coli. Microbiology. 1995;141:2535–2542. doi: 10.1099/13500872-141-10-2535. [DOI] [PubMed] [Google Scholar]
- 19.Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983;166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 20.Heinrichs D E, Yethon J A, Whitfield C. Molecular basis for structural diversity in the core regions of the lipopolysaccharides of Escherichia coli and Salmonella enterica. Mol Microbiol. 1998;30:221–232. doi: 10.1046/j.1365-2958.1998.01063.x. [DOI] [PubMed] [Google Scholar]
- 21.Heinrichs D E, Monteiro M A, Perry M B, Whitfield C. The assembly system for the lipopolysaccharide R2 core-type of Escherichia coli is a hybrid of those found in Escherichia coli K-12 and Salmonella enterica. J Biol Chem. 1998;273:8849–8859. doi: 10.1074/jbc.273.15.8849. [DOI] [PubMed] [Google Scholar]
- 22.Hervás J A, Alomar A, Salvá F, Reina J, Benedí V J. Neonatal sepsis and meningitis in Mallorca (Spain), 1977–1991. Clin Infect Dis. 1993;16:719–724. doi: 10.1093/clind/16.5.719. [DOI] [PubMed] [Google Scholar]
- 23.Hitchcock P J, Brown T M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983;154:269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hobbs M, Reeves P R. The JUMPstart sequence: a 39 bp element common to several polysaccharide gene clusters. Mol Microbiol. 1994;12:855–856. doi: 10.1111/j.1365-2958.1994.tb01071.x. [DOI] [PubMed] [Google Scholar]
- 25.Kahler C M, Carlson R W, Rahman M M, Martin L E, Stephens D S. Two glycosyltransferase genes, lgtF and rfaK, constitute the lipooligosaccharide (ice inner core extension) biosynthesis operon of Neisseria meningitidis. J Bacteriol. 1996;178:6677–6684. doi: 10.1128/jb.178.23.6677-6684.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karkhanis Y D, Zeltner J Y, Jackson J J, Carlo D J. A new improved microassay to determine 2-keto-3-deoxyoctonate in lipopolysaccharide of gram-negative bacteria. Anal Biochem. 1978;85:595–601. doi: 10.1016/0003-2697(78)90260-9. [DOI] [PubMed] [Google Scholar]
- 27.Kyte J, Doolittle R F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- 28.Leeds J A, Welch R A. Enhancing transcription through the Escherichia coli hemolysin operon, hlyCBAD: RfaH and upstream JUMPstart DNA sequences function together via postinitiation mechanism. J Bacteriol. 1997;179:3519–3527. doi: 10.1128/jb.179.11.3519-3527.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Link A J, Phillips D, Church G M. Methods for generating precise deletions and insertions in the genome of wild-type Escherichia coli: application to open reading frame characterization. J Bacteriol. 1997;179:6228–6237. doi: 10.1128/jb.179.20.6228-6237.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.MacLachlan P R, Sanderson K E. Transformation of Salmonella typhimurium with plasmid DNA: differences between rough and smooth strains. J Bacteriol. 1985;161:442–445. doi: 10.1128/jb.161.1.442-445.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 32.Nassif X, Fournier J M, Arondel J, Sansonetti P J. Mucoid phenotype of Klebsiella pneumoniae is a plasmid-encoded virulence factor. Infect Immun. 1989;57:546–552. doi: 10.1128/iai.57.2.546-552.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pearson W R, Lipman D J. Improved tools for biological sequence comparison. Proc Natl Acad Sci USA. 1988;85:2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pradel E, Schnaitman C A. Effect of the rfaH (sfrB) and temperature on the expression of the rfa genes of Escherichia coli K-12. J Bacteriol. 1991;173:6428–6431. doi: 10.1128/jb.173.20.6428-6431.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Radziejewska-Lebrecht J, Shashkov A S, Stroobant V, Wartenberg K, Wart C, Mayer H. The inner core region of Yersinia enterocolitica. Eur J Biochem. 1994;221:343–351. doi: 10.1111/j.1432-1033.1994.tb18746.x. [DOI] [PubMed] [Google Scholar]
- 36.Raleigh E A, Murray N E, Revel H, Blumenthal R M, Westaway D, Reith A D, Rigby P W J, Elhai J, Hanahan D. McrA and McrB restriction phenotypes of some E. coli strains and implications for gene cloning. Nucleic Acids Res. 1988;16:1563–1575. doi: 10.1093/nar/16.4.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reeves P R, Hobbs M, Valvano M A, Skurnik M, Whitfield C, Coplin D, Kido N, Klena J, Maskell D, Raetz C R H, Rick P D. Bacterial polysaccharide synthesis and gene nomenclature. Trends Microbiol. 1996;4:495–503. doi: 10.1016/s0966-842x(97)82912-5. [DOI] [PubMed] [Google Scholar]
- 38.Roantree R J, Kuo T T, MacPhee D G. The effect of defined lipopolysaccharide core defects upon antibiotic resistances of Salmonella typhimurium. J Gen Microbiol. 1977;103:223–234. doi: 10.1099/00221287-103-2-223. [DOI] [PubMed] [Google Scholar]
- 39.Rubirés X, Saigi F, Piqué N, Climent N, Merino S, Albertí S, Tomás J M, Regué M. A gene (wbbL) from Serratia marcescens N28b (O4) complements the rfb-50 mutation of Escherichia coli K-12 derivatives. J Bacteriol. 1997;179:7581–7586. doi: 10.1128/jb.179.23.7581-7586.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 41.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schnaitman C L, Klena J D. Genetics of lipopolysaccharide biosynthesis in enteric bacteria. Microbiol Rev. 1993;57:655–682. doi: 10.1128/mr.57.3.655-682.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Severn W B, Kelly R F, Richards J C, Whitfield C. Structure of the core oligosaccharide in serotype O8 lipopolysaccharide from Klebsiella pneumoniae. J Bacteriol. 1996;178:1731–1741. doi: 10.1128/jb.178.6.1731-1741.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sofia H J, Burland V, Daniels D L, Plunkert III G, Blattner F R. Analysis of the Escherichia coli genome. V. DNA sequence of the region from 76.0 to 81.5 minutes. Nucleic Acids Res. 1994;22:2576–2586. doi: 10.1093/nar/22.13.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Süsskind M, Brade L, Brade H, Holst O. Identification of a novel heptoglycan of α1→2-linked d-glycero-d-manno-heptopyranose: chemical and antigenic structure of lipopolysaccharides from Klebsiella pneumoniae ssp. pneumoniae rough strain R20 (O1−:K20−) J Biol Chem. 1998;273:7006–7017. doi: 10.1074/jbc.273.12.7006. [DOI] [PubMed] [Google Scholar]
- 46.Thompson J D, Higgins D G, Gibson T J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tomas J M, Camprubi S, Merino S, Davey M R, Williams P. Surface exposure of O1 serotype lipopolysaccharide in Klebsiella pneumoniae strains expressing different K antigens. Infect Immun. 1991;59:2006–2011. doi: 10.1128/iai.59.6.2006-2011.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tomas J M, Jofre J. Lipopolysaccharide-specific bacteriophage for Klebsiella pneumoniae C. J Bacteriol. 1985;162:1276–1279. doi: 10.1128/jb.162.3.1276-1279.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Trautmann M, Ruhnke M, Rukavina T, Held T K, Cross A S, Marre R, Whitfield C. O-antigen seroepidemiology of Klebsiella clinical isolates and implications for immunoprophylaxis of Klebsiella infections. Clin Diagn Lab Immunol. 1997;4:550–555. doi: 10.1128/cdli.4.5.550-555.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tsai C M, Frasch C E. A sensitive silver stain for detecting lipopolysaccharide in polyacrylamide gels. Anal Biochem. 1982;119:115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- 51.Viejo M B, Ferrer S, Enfedaque J, Regué M. Cloning and DNA sequence analysis of a bacteriocin gene from Serratia marcescens. J Gen Microbiol. 1992;138:1737–1743. doi: 10.1099/00221287-138-8-1737. [DOI] [PubMed] [Google Scholar]
- 52.Vinogradov E, Radziejewska-Lebrecht J, Kaca W. The structure of the carbohydrate backbone of core-lipid A region of the lipopolysaccharides from Proteus mirabilis wild-type strain S1959 (serotype O3) and its Ra mutant R110/1959. Eur J Biochem. 2000;267:262–268. doi: 10.1046/j.1432-1327.2000.01001.x. [DOI] [PubMed] [Google Scholar]
- 53.Vinogradov E V, Holst O, Thomas-Oates J E, Broady K W, Brade H. The structure of the O-antigenic polysaccharide from lipopolysaccharide of Vibrio cholerae strain H11 (non-O1) Eur J Biochem. 1992;210:491–498. doi: 10.1111/j.1432-1033.1992.tb17447.x. [DOI] [PubMed] [Google Scholar]
- 54.Wang R F, Kushner S R. Construction of versatile low-copy-number vectors for cloning, sequencing and gene expression in Escherichia coli. Gene. 1991;109:195–199. [PubMed] [Google Scholar]
- 55.Westphal O, Jann K. Bacterial lipopolysaccharides: extraction with phenol-water and further applications of the procedure. Methods Carbohydr Chem. 1965;5:83–91. [Google Scholar]
- 56.Williams P, Tomás J M. The pathogenicity of Klebsiella pneumoniae. Rev Med Microbiol. 1990;1:196–204. [Google Scholar]