Abstract
Objective
Evidence supports the efficacy of coronary computed tomography angiography (CCTA)-based risk scores in cardiovascular risk stratification of patients with suspected coronary artery disease (CAD). We aimed to compare two CCTA-based risk score algorithms, Leiden and Confirm scores, in patients with diabetes mellitus (DM) and suspected CAD.
Materials and Methods
This single-center prospective cohort study consecutively included 1241 DM patients (54.1% male, 60.2 ± 10.4 years) referred for CCTA for suspected CAD in 2015–2017. Leiden and Confirm scores were calculated and stratified as < 5 (reference), 5–20, and > 20 for Leiden and < 14.3 (reference), 14.3–19.5, and > 19.5 for Confirm. Major adverse cardiovascular events (MACE) were defined as the composite outcomes of cardiovascular death, nonfatal myocardial infarction (MI), stroke, and unstable angina requiring hospitalization. The Cox model and Kaplan–Meier method were used to evaluate the effect size of the risk scores on MACE. The area under the curve (AUC) at the median follow-up time was also compared between score algorithms.
Results
During a median follow-up of 31 months (interquartile range, 27.6–37.3 months), 131 of MACE were recorded, including 17 cardiovascular deaths, 28 nonfatal MIs, 64 unstable anginas requiring hospitalization, and 22 strokes. An incremental incidence of MACE was observed in both Leiden and Confirm scores, with an increase in the scores (log-rank p < 0.001). In the multivariable analysis, compared with Leiden score < 5, the hazard ratios for Leiden scores of 5–20 and > 20 were 2.37 (95% confidence interval [CI]: 1.53–3.69; p < 0.001) and 4.39 (95% CI: 2.40–8.01; p < 0.001), respectively, while the Confirm score did not demonstrate a statistically significant association with the risk of MACE. The Leiden score showed a greater AUC of 0.840 compared to 0.777 for the Confirm score (p < 0.001).
Conclusion
CCTA-based risk score algorithms could be used as reliable cardiovascular risk predictors in patients with DM and suspected CAD, among which the Leiden score outperformed the Confirm score in predicting MACE.
Keywords: Coronary computed tomography angiography, Diabetes mellitus, Coronary artery disease, Risk stratification
INTRODUCTION
Diabetes mellitus (DM) has developed into a global health problem and social concern that cannot be ignored due to its increasing prevalence and mortality [1,2]. The increased risk of adverse cardiovascular events among DM patients emphasizes the need to assess asymptomatic DM patients for cardiovascular disease risk because early identification and stratification may lead to appropriate management and better prognosis [3,4,5]. The 2019 European Society of Cardiology/European Association for the Study of Diabetes (ESC/EASD) and the 2020 American College of Cardiology/American Heart Association (ACC/AHA) guidelines highlighted the importance of cardiovascular risk assessment for patients with DM, and coronary computed tomography angiography (CCTA) was recommended as a first-line assessment [6].
Meanwhile, the CCTA-based risk score was introduced to quantify risk among patients with suspected coronary artery disease (CAD), which has been proven to be associated with prognosis in the long and short term [7,8,9]. However, it remains unknown which CT-based risk score is the most appropriate for patients with diabetes. To this end, we compared the predictive value of two classic score algorithms, the Leiden risk score [8] and the Confirm comprehensive score [10], in patients with DM and suspected CAD.
MATERIALS AND METHODS
Patients
Informed consent was obtained from all patients. The study protocol followed the ethical guidelines of the 1975 Declaration of Helsinki, and the local ethics committee approved this prospective observational study (IRB No. S2020-255-01).
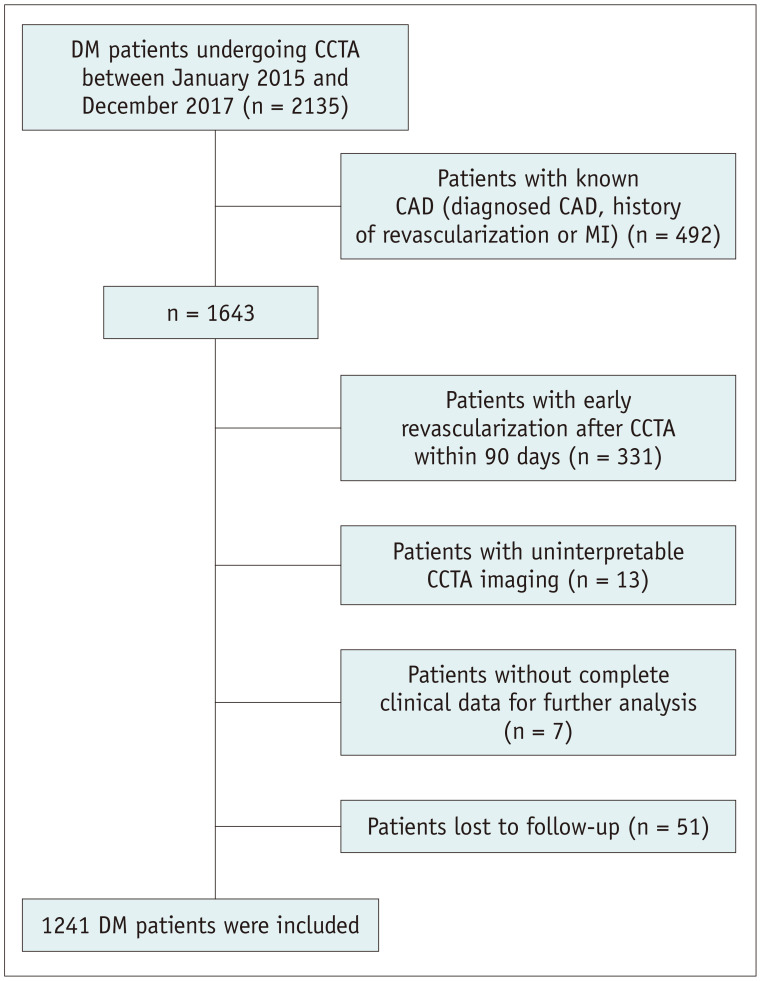
This was a single-center, prospective, and observational cohort study. Patients with DM undergoing CCTA examination due to suspected CAD at our institution between January 2015 and December 2017 were consecutively enrolled. Of the 1643 patients with DM and suspected CAD, 331 patients with early revascularization within 90 days after CCTA, 13 patients with uninterpretable CCTA imaging, 7 patients without complete clinical data for further analysis, and 51 patients lost to follow-up were excluded, leaving 1241 patients in the current analysis (Fig. 1).
Fig. 1. Flow chart of the study population.
CAD = coronary artery disease, CCTA = coronary computed tomography angiography, DM = diabetes mellitus, MI = myocardial infarction
Clinical Data
Demographic and clinical characteristics were collected from the electronic medical record system. According to the 2019 American Diabetes Association guidelines [11], DM was defined as fasting glucose ≥ 7.0 mmol/L, 2-hours plasma glucose ≥ 11.1 mmol/L during the oral glucose tolerance test, A1C ≥ 6.5% (48 mmol/mol), or use of oral hypoglycemic agents/insulin. A structured interview was conducted to collect information on the manifestation of cardiac risk factors before CCTA, as follows: 1) hypertension (systolic blood pressure ≥ 140 mm Hg or diastolic blood pressure ≥ 90 mm Hg or receiving antihypertensive therapy), 2) hyperlipidemia (total serum cholesterol ≥ 230 mg/dL or serum triglycerides ≥ 200 mg/dL or treatment with lipid-lowering medication), 3) family history of CAD (presence of CAD in the first-degree relative at < 55 years in males and < 65 years in females), and 4) smoking (current or previous smoking within the last 3 months of CCTA). The treatment and management of all patients were conducted at the physician’s discretion.
CCTA Acquisition and Interpretation
CCTA scans were performed using a dual-source scanner (Somatom Definition Flash CT; Siemens Medical Solutions), and a Siemens workstation (Syngo.via VB10B, Siemens Healthcare) was used for postprocessing. Two experienced experts blinded to the clinical outcomes interpreted the CCTA images and analyzed all coronary segments based on the 17-segment modified AHA classification [12]. A third expert was introduced in cases of disagreement, and the final consensus dataset was used for subsequent analyses.
Stenosis severity was assessed and defined as the percentage of stenosis and proximal adjacent normal vessel lumen: normal (0%), minimal (1%–24%), mild (25%–49%), moderate (50%–69%), severe (70%–99%), and occlusion (100%) by visual assessment. The CAD-Reporting and Data System (RADS) system [13] was introduced as a quantitative index of stenosis severity. Plaque composition was classified as calcified (plaques with a high density of > 130 Hounsfield unit [HU]), noncalcified plaque (having lower density compared with the contrast-enhanced lumen) and mixed plaque (both calcified and noncalcified elements existed). Adverse plaque characteristics were recognized as spotty calcification [14] (a < 3 mm calcified plaque surrounded by a noncalcified component), low CT attenuation plaques [15] (average attenuation < 30 HU), positive remodeling [16] (the maximal diameter of the outer vessel at the plaque was 10% greater than the mean of the proximal and distal normal vessel reference), and the napkin ring sign [16] (a ring-like comparative higher attenuation plaque tissue with a low attenuation central area). A high-risk plaque (HRP) was diagnosed if at least two adverse plaque characteristics were present [17,18].
CCTA-Based Risk Scores
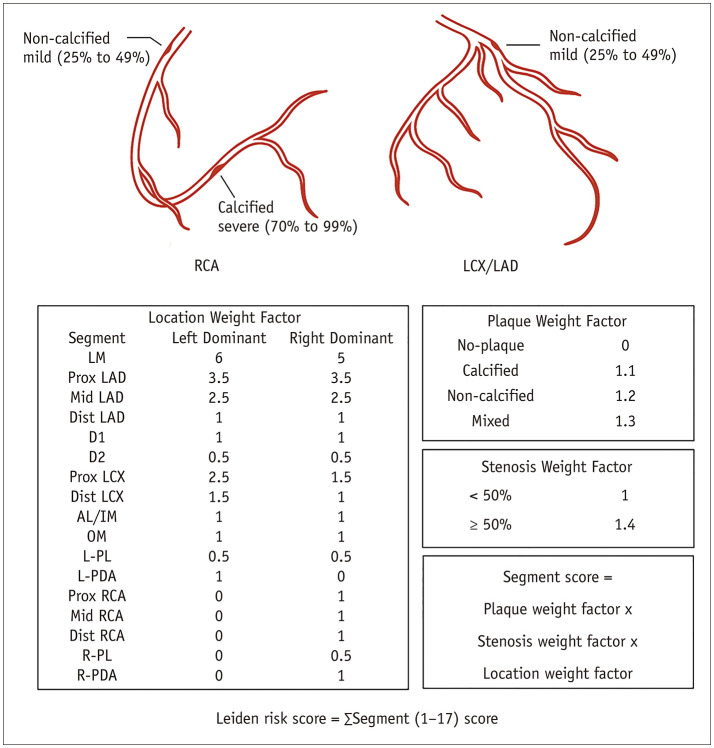
The Leiden score, a comprehensive evaluation of all coronary segments observed on CCTA, incorporating plaque location (i.e., location weight factor, range 0–6), severity (i.e., stenosis weight factor, range 1–1.4), and composition (i.e., plaque weight factor, range 0–1.3), was calculated for each patient as described (Fig. 2) [8]. Based on the final calculated scores, the included patients were stratified into low (< 5), moderate (5–20) and high-risk (> 20) groups, as previously described [8,19].
Fig. 2. Leiden score calculation.
Leiden score was calculated by summation of segment score quantified as plaque weight factor × stenosis weight factor × location weight factor, i.e., a right dominant system with a non-calcified plaque with < 50% stenosis in the Prox RCA (1 × 1.2 × 1) + a calcified plaque with > 50% stenosis in the distal right coronary artery (1 × 1.1 × 1.4) + a non-calcified plaque with < 50% stenosis in the Prox left anterior descending artery (3.5 × 1.2 × 1), so the Leiden score is 6.94. AL = anterolateral, Dist = distal, D1 = diagonal 1, D2 = diagonal 2, IM = intermediate, LAD = left anterior descending coronary artery, LCX = left circumflex coronary artery, LM = left main, L-PDA = left posterior descending artery, L-PL = left posterolateral, OM = obtuse marginal, Prox = proximal, RCA = right coronary artery, R-PDA = right posterior descending artery, R-PL = right posterolateral
The Confirm comprehensive score, CT-based score and clinical risk score were also incorporated. For the CT-based score component, proximal segments (left main coronary artery, proximal and mid-left anterior descending artery, proximal left circumflex artery, first obtuse marginal branch, and proximal and mid-right coronary artery) of calcified or mixed plaques and proximal segments of > 50% stenosis were graded [10]. The National Cholesterol Education Program (NCEP) ATP III score [20] was added as the clinical risk component to obtain the final Confirm score as follows: ln (NCEP ATP III score)/0.235 + 2.83 number of proximal segments with calcified or mixed plaques (2 at most) + 2.76 number of proximal segments with stenosis > 50% (2 at most). Patients were stratified into low (< 14.3), moderate (14.3–19.5), and high-risk (> 19.5) groups based on the recommended cutoff of the Confirm score, as previously described [10].
Outcomes
Follow-up data were collected from electronic medical records or patient interviews for at least 90 days after the CCTA examination. Adverse events and relevant treatments were recorded and evaluated independently by two experienced cardiologists. The primary outcome of interest was major adverse cardiovascular events (MACE), defined as composite outcomes of cardiovascular death, nonfatal myocardial infarction (MI), stroke, and unstable angina requiring hospitalization.
Statistical Analysis
Statistical analysis was performed using SPSS version 26.0 (IBM Corp.) and R version 3.6.3 (The R Foundation for Statistical Computing). Continuous variables with a normal distribution are represented as the mean ± standard deviation, and non-normally distributed continuous variables are expressed as medians (interquartile range). Categorical variables are presented as frequencies and percentages. Inter-observer and intra-observer agreements in obtaining CCTA risk scores were determined using intraclass correlation coefficients (ICC), and the following criteria were used to evaluate the magnitudes of the ICCs: poor agreement (0.01–0.20), fair agreement (0.21–0.40), moderate agreement (0.41–0.60), good agreement (0.61–0.80), and excellent agreement (0.81–1.00). Kaplan–Meier analysis and log-rank test were used to estimate and compare the cumulative probability of MACE between the score groups. Multivariable analysis was performed using Cox proportional hazards method [21]. The area under the curve (AUC) from the time-dependent receiver operating characteristic curve (tdROC) at the median follow-up time was compared between the score algorithms to determine their discrimination performance. Statistical significance was set at a p value < 0.05, considered statistically significant.
RESULTS
Study Population
The final study population comprised 1241 patients (54.1% male) with a mean age of 60.2 ± 10.4 years. The major cardiovascular risk factors were hypertension (66.4%) and hyperlipidemia (53.6%). Information on cardiovascular agents at baseline is presented in Table 1. Diabetes treatment was categorized into diet only (20.0%), oral hypoglycemic agents (72.6%), and insulin (24.2%). According to the severity of CAD noted on CCTA, most patients had no significant stenosis (CAD-RADS 1–3, 67.6%), whereas 15.3% had no CAD. HRPs were also detected in 66 patients. Compared to those without MACE, patients in the event group had significantly higher Leiden and Confirm scores (p < 0.001).
Table 1. Baseline Characteristics.
| Characteristic | Total (n = 1241) | Event | P | |||
|---|---|---|---|---|---|---|
| Yes (n = 131) | No (n = 1110) | |||||
| Age, years | 60.2 ± 10.4 | 63.3 ± 11.4 | 59.9 ± 10.2 | 0.001 | ||
| Male | 671 (54.1) | 79 (60.3) | 592 (53.3) | 0.130 | ||
| Body mass index, kg/m2 | 26.2 ± 3.6 | 26.2 ± 3.8 | 26.2 ± 3.6 | 0.779 | ||
| Cardiac risk factors | ||||||
| Hypertension | 824 (66.4) | 92 (70.2) | 732 (65.9) | 0.326 | ||
| Hyperlipidemia | 665 (53.6) | 87 (66.4) | 578 (52.1) | 0.002 | ||
| Smoking | 340 (27.4) | 45 (34.4) | 295 (26.6) | 0.059 | ||
| Family history of CAD | 295 (23.8) | 28 (21.4) | 267 (24.1) | 0.496 | ||
| CCTA findings | ||||||
| CAD-RADS | ||||||
| 0 | 190 (15.3) | 5 (3.8) | 185(16.7) | < 0.001 | ||
| 1 | 121 (9.8) | 4 (3.1) | 117 (10.5) | 0.006 | ||
| 2 | 502 (40.5) | 41 (31.3) | 461 (41.5) | 0.024 | ||
| 3 | 215 (17.3) | 27 (20.6) | 188 (16.9) | 0.293 | ||
| 4 | 189 (15.2) | 49 (37.4) | 140 (12.6) | < 0.001 | ||
| 5 | 24 (1.9) | 5 (3.8) | 19 (1.7) | 0.098 | ||
| Leiden risk score | 5.1 (1.2–11.1) | 10.6 (5.1–14.6) | 4.6 (1.2–10.6) | < 0.001 | ||
| Confirm score | 13.4 (8.4–18.0) | 16.5 (12.4–22.4) | 12.9 (7.5–17.3) | < 0.001 | ||
| High-risk plaque | 66 (5.3) | 23 (17.6) | 43 (3.9) | < 0.001 | ||
| Medication | ||||||
| Anti-platelet | 480 (38.7) | 45 (34.4) | 435 (39.2) | 0.280 | ||
| Beta blocker | 408 (32.9) | 40 (30.5) | 368 (33.2) | 0.550 | ||
| ACEI/ARB | 287 (23.1) | 35 (26.7) | 252 (22.7) | 0.300 | ||
| Statin | 482 (38.8) | 64 (48.9) | 418 (37.7) | 0.013 | ||
| Calcium channel blocker | 262 (21.1) | 32 (24.4) | 230 (20.7) | 0.330 | ||
| Diabetic treatment | ||||||
| Diet only | 248 (20.0) | 22 (16.8) | 226 (20.4) | 0.330 | ||
| Oral hypoglycemic agent | 901 (72.6) | 95 (72.5) | 806 (72.6) | 0.980 | ||
| Insulin | 300 (24.2) | 34 (26.0) | 266 (24.0) | 0.610 | ||
Values are mean ± standard deviation, number of patients (%), or median (interquartile range). ACEI = angiotensin-converting enzyme inhibitors, ARB = angiotension II receptor blockers, CAD = coronary artery disease, CCTA = coronary computed tomography angiography, RADS = Reporting and Data System
Inter- and Intra-Observer Agreement for CCTA Risk Scores
The ICC for inter-observer agreement for the Leiden score was 0.942, while that for the intra-observer agreement was 0.957, as also mentioned in a previous study [22]. For the Confirm score, inter- and intra-observer agreement were excellent, with ICC values of 0.921 and 0.913, respectively.
Cumulative Incidence of MACE
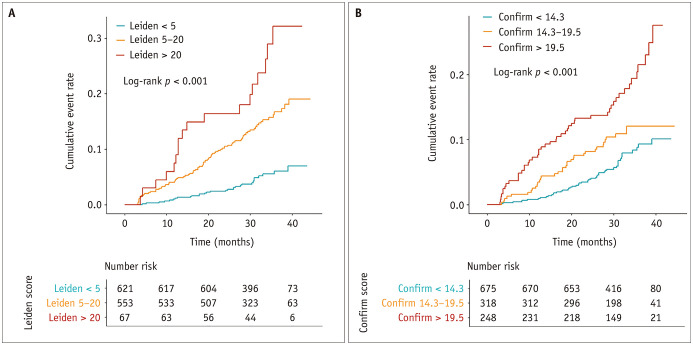
In total, 131 MACE (17 cardiovascular deaths, 28 nonfatal MI, 22 strokes, and 64 unstable angina requiring hospitalization) occurred during a median follow-up duration of 31 months (interquartile range, 27.6–37.3 months). The relationship between the risk scores and cumulative rates of MACE is shown in Figure 3.
Fig. 3. Cumulative incidence of MACE according to Leiden score (A) and Confirm comprehensive score (B).
A. Cumulative incidence of MACE according to Leiden score. B. Cumulative incidence of MACE according to Confirm comprehensive score. MACE = major adverse cardiovascular events
For the Leiden score algorithm, the MACE rates were 1.8, 5.8, and 10.7 per 100 person-years in the low-, mid-, and high-score groups, respectively (log-rank p < 0.001). A similar trend was observed in MACE rates stratified by the Confirm score algorithm, which showed 2.7, 4.2, and 7.7 per 100 person-years in the low-, mid-, and high-score groups, respectively (log-rank p < 0.001).
Univariable and Multivariable Analysis of MACE Risk
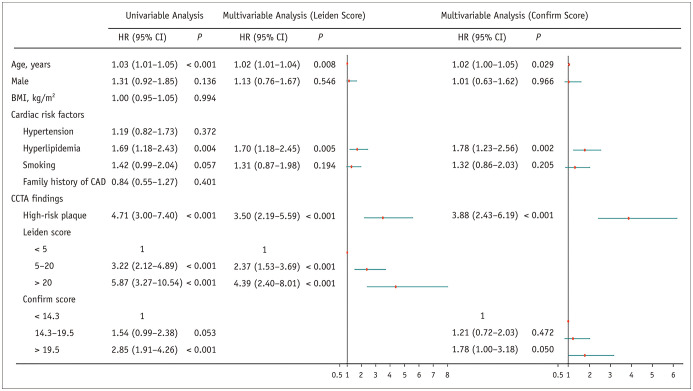
In univariable analyses, age {hazard ratio (HR), 1.03 (95% confidence interval [CI]: 1.01–1.05); p < 0.001}, hyperlipidemia (HR, 1.69 [95% CI: 1.18–2.43]; p = 0.004), and HRP (HR, 4.71 [95% CI: 3.00–7.40]; p < 0.001) were associated with MACE (Fig. 4). Patients with higher Leiden or Confirm scores had a higher risk of MACE (Fig. 4).
Fig. 4. Univariable and multivariable analysis of MACE risk.
BMI = body mass index, CAD = coronary artery disease, CCTA = coronary computed tomography angiography, CI = confidence interval, HR = hazard ratio, MACE = major adverse cardiovascular events
In the multivariable analysis, the Leiden score was still significantly associated with MACE after adjustment for age, sex, hyperlipidemia, smoking, and HRP. Compared with Leiden score < 5, the HR for the Leiden score was 2.37 (95% CI: 1.53–3.69; p < 0.001) for the 5–20 group and 4.39 (95% CI: 2.40–8.01; p < 0.001) for the > 20 group. A weaker increase in risk with an increasing Confirm score was noted, although the results were not statistically significant (Fig. 4).
Discrimination Performance of Risk Scores
A time-dependent receiver operating characteristic curve at the median follow-up time was used to evaluate the discrimination performance of the risk-scoring models, as shown in Figure 5. The AUC for the discrimination of those who had and did not have MACE was 0.840 for the Leiden score, which was significantly higher than 0.777 for the Confirm score (p < 0.001). The NCEP ATP III score did not show incremental benefits over the Leiden score regarding discrimination performance (AUCNCEP ATP III + Leiden vs. AUCLeiden: 0.826 vs. 0.840; p = 0.151).
Fig. 5. Discrimination performance of different CT-based score algorithms.
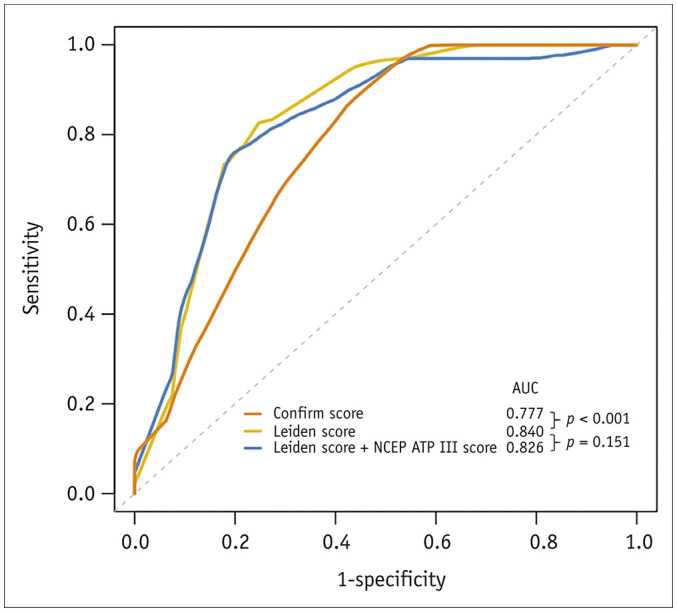
AUC = area under the curve, NCEP = National Cholesterol Education Program
DISCUSSION
The present study demonstrated that CT-based risk score algorithms could be used as reliable cardiovascular risk predictors in DM patients with suspected CAD. Moreover, the CCTA-based Leiden score outperformed the Confirm score in predicting MACE. These findings provide supporting evidence for using the Leiden score in the risk stratification of patients with DM.
A large amount of evidence has conferred the potential value of CCTA in cardiovascular risk stratification in the DM population [5,23,24] from anatomy to functionality. Previous studies have addressed the relationship between plaque or stenosis characteristics identified by CCTA and DM prognosis, such as obstructive CAD [25], vulnerable plaque [26], and atherosclerosis extent [27,28]. Additionally, a longitudinal assessment based on serial CCTA found that the presence of DM had an incremental impact on coronary plaque progression [29], which might be associated with subsequent cardiac events [30]. However, the FACTOR-64 Randomized Clinical Trial [31] denied that CCTA improved prognosis in DM patients, as the outcome event rates did not differ between the CCTA and control groups (6.2% [28 events] vs. 7.6% [34 events]; HR: 0.80, 95% CI: 0.49–1.32, p = 0.380) among asymptomatic patients with DM. A probable cause is that the management decision and stratification made by CCTA might be insufficient for patients with DM because only patients with the mild proximal disease to severe proximal or distal CAD by CCTA or a > 10 coronary artery calcium score were recommended for aggressive care in the FACTOR-64 trial. There may be more testing power to support the superiority of CCTA [32], as functional testing, such as CT-derived fractional flow reserve (CT-FFR) [33], or advanced imaging markers, such as the perivascular fat attenuation index, develop. Overall, our results concur with most previous findings that support the feasibility of CCTA-derived information in DM population stratification. It was noted that, in our cohort, approximately half of the included subjects had less than 50% stenosis, presenting a comparatively low-to-intermediate risk population. However, a relatively higher MACE incidence rate of 10.6% was observed in the present study, which may result from a broadened definition of MACE, such as stroke. This was based on the premise that patients with diabetes had a similar risk of MI to the risk observed in the general population, despite their increased risk of ischemic stroke and death, as reported in a recent study [24]. Moreover, an extended observation duration of median follow-up was conducted, which may also contribute to the high incidence of MACE.
There are many kinds of CCTA-based scores in clinical practice. The calculation method and focus scope are diversified: segment involvement score (SIS) and 3-vessel score focus on the segments or vessels involved, respectively; CAD-RADS and segment stenosis score (SSS) solely evaluate the degree of stenosis. Other scoring models, such as the CT-Leaman score and Confirm and Leiden scores, integrate multiple atherosclerosis characteristics (e.g., presence, composition, stenosis, location, dominance, and extent), permitting comprehensive and systematic assessment. A previous study conducted by Hadamitzky et al. [4] observed that a higher SIS in the DM group was significantly associated with hard cardiac events. Further analysis [28] found significant prognostic value for the SIS and SSS compared to the Framingham score and illustrated incremental benefit compared to clinical risk factors [23]. Moreover, according to a combined cohort from the Leiden University Medical Center and the CONFIRM registry with 5-year follow-up data [19], including 732 DM patients who were 1:1 propensity-matched with 732 non-DM patients by age, sex, and cardiovascular risk factors, semiquantitative CCTA risk scores were independently associated with the primary endpoint. Further analysis demonstrated that the Leiden score had a better discrimination performance than any stenosis ≥ 50% and ≥ 70% in predicting MACE. However, the Leiden score was comparable to other qualitative risk scores such as the CAD-RADS, SIS, and SSS. Of DM, diffuse CAD and microcirculation disturbances are prone to occur owing to their unique vasculopathies. Thus, a global assessment of all coronary artery segments, such as the Leiden score, may be preferred in patients with DM. On the other hand, the Confirm score was obtained from the proximal segments of the coronary artery while incorporating clinical risk factors. Therefore, the introduction of clinical risk factors may also be incrementally beneficial to the Leiden score, as demonstrated by the Confirm scoring system. In our study, however, negative results were obtained: the NCEP ATP III score could not improve the discrimination performance beyond the Leiden score. This may be because the NCEP ATP III score is not an optimal clinical assessment for diabetes, and the risk posed by diabetes may be far greater than that shown by the NCEP ATP III score. Further research is required to confirm an appropriate clinical assessment approach for patients with DM.
Although specific anatomical features were incorporated into the Leiden score system, this comprehensive algorithm has potential links to functional conditions. For the global assessment of all coronary segments, the Leiden score corresponds to the blood flow profile of the whole coronary tree, which is associated with decreased microcirculation and diffuseness of epicardial atherosclerosis in diabetic patients, indirectly reflecting the overall myocardial blood flow (MBF). A recent study [34] demonstrated a lower coronary flow reserve and microvascular resistance reserve in DM patients in the absence of significant disease than in non-diabetic patients, reflecting impaired vasoreactivity of the microcirculation in these patients. Additionally, changes in microvascular function seem to precede microangiopathy, whereas structural microvascular damage eventually induces symptoms and future cardiovascular events [35]. Further studies have also elaborated on lower MBF in patients with DM [36]. However, to date, only a few studies have focused on this topic. Future applications of advanced technologies such as CT-FFR may provide new evidence for functional assessment of DM.
The present study has certain limitations. Since some of the clinical information was collected retrospectively, it is subject to inherent limitations regarding information insufficiency and data analysis, which may lead to potential bias and impact the effect size of the target variables. Second, although consecutive patients were included in the present study, a few patients with DM did not undergo CCTA examination because of patient preference, cost, comorbidities, or other considerations that may cause selection bias. Third, the DM duration was not recorded, although a longer duration of DM was associated with an increased CAD burden and a higher rate of MACE. However, nearly 75% of patients managed DM with diet or oral hypoglycemic agents to lower glucose levels, indicating a generally moderate condition of DM and less heterogeneity in the present study population.
In conclusion, the CCTA-based risk score algorithms could be used as reliable cardiovascular risk predictors in patients with DM and suspected CAD, among which the Leiden score outperformed the Confirm score in predicting MACE.
Footnotes
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Junjie Yang.
- Data curation: Zinuan Liu, Jing Jing.
- Formal analysis: Zinuan Liu, Yipu Ding.
- Funding acquisition: Yundai Chen, Junjie Yang.
- Methodology: Guanhua Dou, Xi Wang, Dongkai Shan, Bai He.
- Project administration: Junjie Yang.
- Supervision: Yundai Chen, Junjie Yang.
- Writing—original draft: Zinuan Liu, Yipu Ding.
- Writing—review & editing: Yundai Chen, Junjie Yang.
Funding Statement: This work was supported by grants from the National Key R&D Program of China (2016YFC1300304 and 2021YFC2500505) and Medical Big Data Program of PLAGH (2019MBD-035).
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
References
- 1.Emerging Risk Factors Collaboration. Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215–2222. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tancredi M, Rosengren A, Svensson AM, Kosiborod M, Pivodic A, Gudbjörnsdottir S, et al. Excess mortality among persons with type 2 diabetes. N Engl J Med. 2015;373:1720–1732. doi: 10.1056/NEJMoa1504347. [DOI] [PubMed] [Google Scholar]
- 3.Kang SH, Park GM, Lee SW, Yun SC, Kim YH, Cho YR, et al. Long-term prognostic value of coronary CT angiography in asymptomatic type 2 diabetes mellitus. JACC Cardiovasc Imaging. 2016;9:1292–1300. doi: 10.1016/j.jcmg.2016.01.040. [DOI] [PubMed] [Google Scholar]
- 4.Hadamitzky M, Hein F, Meyer T, Bischoff B, Martinoff S, Schömig A, et al. Prognostic value of coronary computed tomographic angiography in diabetic patients without known coronary artery disease. Diabetes Care. 2010;33:1358–1363. doi: 10.2337/dc09-2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blanke P, Naoum C, Ahmadi A, Cheruvu C, Soon J, Arepalli C, et al. Long-term prognostic utility of coronary CT angiography in stable patients with diabetes mellitus. JACC Cardiovasc Imaging. 2016;9:1280–1288. doi: 10.1016/j.jcmg.2015.12.027. [DOI] [PubMed] [Google Scholar]
- 6.Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, et al. 2019 ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41:255–323. doi: 10.1093/eurheartj/ehz486. [DOI] [PubMed] [Google Scholar]
- 7.Min JK, Shaw LJ, Devereux RB, Okin PM, Weinsaft JW, Russo DJ, et al. Prognostic value of multidetector coronary computed tomographic angiography for prediction of all-cause mortality. J Am Coll Cardiol. 2007;50:1161–1170. doi: 10.1016/j.jacc.2007.03.067. [DOI] [PubMed] [Google Scholar]
- 8.van Rosendael AR, Shaw LJ, Xie JX, Dimitriu-Leen AC, Smit JM, Scholte AJ, et al. Superior risk stratification with coronary computed tomography angiography using a comprehensive atherosclerotic risk score. JACC Cardiovasc Imaging. 2019;12:1987–1997. doi: 10.1016/j.jcmg.2018.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deseive S, Shaw LJ, Min JK, Achenbach S, Andreini D, Al-Mallah MH, et al. Improved 5-year prediction of all-cause mortality by coronary CT angiography applying the CONFIRM score. Eur Heart J Cardiovasc Imaging. 2017;18:286–293. doi: 10.1093/ehjci/jew195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hadamitzky M, Achenbach S, Al-Mallah M, Berman D, Budoff M, Cademartiri F, et al. Optimized prognostic score for coronary computed tomographic angiography: results from the CONFIRM registry (COronary CT Angiography EvaluatioN For Clinical Outcomes: an InteRnational Multicenter Registry) J Am Coll Cardiol. 2013;62:468–476. doi: 10.1016/j.jacc.2013.04.064. [DOI] [PubMed] [Google Scholar]
- 11.American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2019. Diabetes Care. 2019;42(Suppl 1):S13–S28. doi: 10.2337/dc19-S002. [DOI] [PubMed] [Google Scholar]
- 12.Austen WG, Edwards JE, Frye RL, Gensini GG, Gott VL, Griffith LS, et al. A reporting system on patients evaluated for coronary artery disease. Report of the Ad Hoc Committee for Grading of Coronary Artery Disease, Council on Cardiovascular Surgery, American Heart Association. Circulation. 1975;51(4 Suppl):5–40. doi: 10.1161/01.cir.51.4.5. [DOI] [PubMed] [Google Scholar]
- 13.Cury RC, Abbara S, Achenbach S, Agatston A, Berman DS, Budoff MJ, et al. Coronary artery disease - Reporting and data system (CAD-RADS): an expert consensus document of SCCT, ACR and NASCI: endorsed by the ACC. JACC Cardiovasc Imaging. 2016;9:1099–1113. doi: 10.1016/j.jcmg.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 14.Ehara S, Kobayashi Y, Yoshiyama M, Shimada K, Shimada Y, Fukuda D, et al. Spotty calcification typifies the culprit plaque in patients with acute myocardial infarction: an intravascular ultrasound study. Circulation. 2004;110:3424–3429. doi: 10.1161/01.CIR.0000148131.41425.E9. [DOI] [PubMed] [Google Scholar]
- 15.Motoyama S, Kondo T, Sarai M, Sugiura A, Harigaya H, Sato T, et al. Multislice computed tomographic characteristics of coronary lesions in acute coronary syndromes. J Am Coll Cardiol. 2007;50:319–326. doi: 10.1016/j.jacc.2007.03.044. [DOI] [PubMed] [Google Scholar]
- 16.Kashiwagi M, Tanaka A, Kitabata H, Tsujioka H, Kataiwa H, Komukai K, et al. Feasibility of noninvasive assessment of thin-cap fibroatheroma by multidetector computed tomography. JACC Cardiovasc Imaging. 2009;2:1412–1419. doi: 10.1016/j.jcmg.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 17.Williams MC, Moss AJ, Dweck M, Adamson PD, Alam S, Hunter A, et al. Coronary artery plaque characteristics associated with adverse outcomes in the SCOT-HEART study. J Am Coll Cardiol. 2019;73:291–301. doi: 10.1016/j.jacc.2018.10.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferencik M, Mayrhofer T, Bittner DO, Emami H, Puchner SB, Lu MT, et al. Use of high-risk coronary atherosclerotic plaque detection for risk stratification of patients with stable chest pain: a secondary analysis of the PROMISE randomized clinical trial. JAMA Cardiol. 2018;3:144–152. doi: 10.1001/jamacardio.2017.4973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van den Hoogen IJ, van Rosendael AR, Lin FY, Lu Y, Dimitriu-Leen AC, Smit JM, et al. Coronary atherosclerosis scoring with semiquantitative CCTA risk scores for prediction of major adverse cardiac events: propensity score-based analysis of diabetic and non-diabetic patients. J Cardiovasc Comput Tomogr. 2020;14:251–257. doi: 10.1016/j.jcct.2019.11.015. [DOI] [PubMed] [Google Scholar]
- 20.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 21.Park SH, Han K, Park SY. Mistakes to avoid for accurate and transparent reporting of survival analysis in imaging research. Korean J Radiol. 2021;22:1587–1593. doi: 10.3348/kjr.2021.0579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Z, Ding Y, Dou G, Yang X, Wang X, Shan D, et al. Impact of atherosclerotic extent on clinical outcome for diabetic patients with non-obstructive coronary artery disease. Atherosclerosis Plus. 2021;44:10–17. doi: 10.1016/j.athplu.2021.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee KY, Hwang BH, Kim TH, Kim CJ, Kim JJ, Choo EH, et al. Computed tomography angiography images of coronary artery stenosis provide a better prediction of risk than traditional risk factors in asymptomatic individuals with type 2 diabetes: a long-term study of clinical outcomes. Diabetes Care. 2017;40:1241–1248. doi: 10.2337/dc16-1844. [DOI] [PubMed] [Google Scholar]
- 24.Olesen KKW, Madsen M, Gyldenkerne C, Thrane PG, Thim T, Jensen LO, et al. Ten-year cardiovascular risk in diabetes patients without obstructive coronary artery disease: a retrospective Western Denmark cohort study. Cardiovasc Diabetol. 2021;20:23. doi: 10.1186/s12933-021-01212-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim JJ, Hwang BH, Choi IJ, Choo EH, Lim S, Kim JK, et al. Impact of diabetes duration on the extent and severity of coronary atheroma burden and long-term clinical outcome in asymptomatic type 2 diabetic patients: evaluation by Coronary CT angiography. Eur Heart J Cardiovasc Imaging. 2015;16:1065–1073. doi: 10.1093/ehjci/jev106. [DOI] [PubMed] [Google Scholar]
- 26.Heinsen LJ, Pararajasingam G, Andersen TR, Auscher S, Sheta HM, Precht H, et al. High-risk coronary artery plaque in asymptomatic patients with type 2 diabetes: clinical risk factors and coronary artery calcium score. Cardiovasc Diabetol. 2021;20:164. doi: 10.1186/s12933-021-01350-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Finck T, Will A, Hendrich E, Martinoff S, Hadamitzky M. Coronary computed tomography angiography as a tool for long-term cardiovascular risk stratification in diabetic patients. Heart Vessels. 2019;34:1086–1095. doi: 10.1007/s00380-018-01339-0. [DOI] [PubMed] [Google Scholar]
- 28.Nadjiri J, Hausleiter J, Deseive S, Will A, Hendrich E, Martinoff S, et al. Prognostic value of coronary CT angiography in diabetic patients: a 5-year follow up study. Int J Cardiovasc Imaging. 2016;32:483–491. doi: 10.1007/s10554-015-0785-9. [DOI] [PubMed] [Google Scholar]
- 29.Won KB, Lee SE, Lee BK, Park HB, Heo R, Rizvi A, et al. Longitudinal assessment of coronary plaque volume change related to glycemic status using serial coronary computed tomography angiography: a PARADIGM (Progression of AtheRosclerotic PlAque DetermIned by Computed TomoGraphic Angiography Imaging) substudy. J Cardiovasc Comput Tomogr. 2019;13:142–147. doi: 10.1016/j.jcct.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 30.Yang J, Dou G, Tesche C, De Cecco CN, Jacobs BE, Schoepf UJ, et al. Progression of coronary atherosclerotic plaque burden and relationship with adverse cardiovascular event in asymptomatic diabetic patients. BMC Cardiovasc Disord. 2019;19:39. doi: 10.1186/s12872-019-1016-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muhlestein JB, Lappé DL, Lima JA, Rosen BD, May HT, Knight S, et al. Effect of screening for coronary artery disease using CT angiography on mortality and cardiac events in high-risk patients with diabetes: the FACTOR-64 randomized clinical trial. JAMA. 2014;312:2234–2243. doi: 10.1001/jama.2014.15825. [DOI] [PubMed] [Google Scholar]
- 32.Shan D, Yang J, Chen Y. Noninvasive cardiac imaging technologies in detecting coronary artery disease: from research to clinical practice. Cardiol Plus. 2020;5:13–20. [Google Scholar]
- 33.Xue Y, Zheng MW, Hou Y, Zhou F, Li JH, Wang YN, et al. Influence of diabetes mellitus on the diagnostic performance of machine learning-based coronary CT angiography-derived fractional flow reserve: a multicenter study. Eur Radiol. 2022;32:3778–3789. doi: 10.1007/s00330-021-08468-7. [DOI] [PubMed] [Google Scholar]
- 34.Gallinoro E, Paolisso P, Candreva A, Bermpeis K, Fabbricatore D, Esposito G, et al. Microvascular dysfunction in patients with type II diabetes mellitus: invasive assessment of absolute coronary blood flow and microvascular resistance reserve. Front Cardiovasc Med. 2021;8:765071. doi: 10.3389/fcvm.2021.765071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sezer M, Kocaaga M, Aslanger E, Atici A, Demirkiran A, Bugra Z, et al. Bimodal pattern of coronary microvascular involvement in diabetes mellitus. J Am Heart Assoc. 2016;5:e003995. doi: 10.1161/JAHA.116.003995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rasmussen IKB, Hasbak P, von Scholten BJ, Laursen JC, Zobel EH, Jorge Diaz L, et al. Non-invasive assessment of temporal changes in myocardial microvascular function in persons with type 2 diabetes and healthy controls. Diabet Med. 2021;38:e14517. doi: 10.1111/dme.14517. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.