Abstract
Recent decades have witnessed increased agricultural production to match the global demand for food fueled by population increase. Conventional agricultural practices are heavily reliant on artificial fertilizers that have numerous human and environmental health effects. Cognizant of this, sustainability researchers and environmentalists have increased their focus on other crop fertilization mechanisms. Biofertilizers are microbial formulations constituted of indigenous plant growth-promoting rhizobacteria (PGPR) that directly or indirectly promote plant growth through the solubilization of soil nutrients, and the production of plant growth-stimulating hormones and iron-sequestering metabolites called siderophores. Biofertilizers have continually been studied, recommended, and even successfully adopted for the production of many crops in the world. These microbial products hold massive potential as sustainable crop production tools, especially in the wake of climate change that is partly fueled by artificial fertilizers. Despite the growing interest in the technology, its full potential has not yet been achieved and utilization still seems to be in infancy. There is a need to shed light on the past, current, and future prospects of biofertilizers to increase their understanding and utility. This review evaluates the history of PGPR biofertilizers, assesses their present utilization, and critically advocates their future in sustainable crop production. It, therefore, updates our understanding of the evolution of PGPR biofertilizers in crop production. Such information can facilitate the evaluation of their potential and ultimately pave the way for increased exploitation.
Keywords: biofertilizers, sustainable agriculture, plant growth-promoting rhizobacteria, microbial stimulants, microbial formulations
Introduction
The earth will be home to about 10 billion people by 2050 and a lot of pressure will be mounted on the existing food resources (United Nations, 2015). Although global crop production can be achieved through agricultural intensification, this will escalate reliance on chemical agro-inputs like fertilizers that pose several environmental effects (Vassilev et al., 2015; Abhilash et al., 2016b). For instance, chemical fertilizers are extensively associated with greenhouse gas emissions that fuel global warming and climatic changes (e.g., Kahrl et al., 2010; Mapanda et al., 2011; Carmo et al., 2013). Similarly, the eutrophication of several water bodies and the destabilization of aquatic ecosystems have several times been attributed to fertilizer runoffs from agricultural fields (Melo et al., 2012; Deepa and Venkateswaran, 2018; Zhang et al., 2018). Ironically, long-term artificial fertilization can also include the overall deterioration of soil productivity and quality through acidification (Neog, 2018; Bai et al., 2020; Yan et al., 2020).
Owing to the aforementioned challenges, the exploration of alternative crop fertilization mechanisms is mounting worldwide in an attempt to develop sustainable food production systems. The exploitation of plant microbiomes has particularly gathered surmountable interest in this regard. Among the most interesting plant microbiomes are the plant growth-promoting rhizobacteria (PGPR) that present several advantageous functions in plant rhizospheres, from nutrients solubilization (Ibarra-Galeana et al., 2017; Borgi et al., 2020; Verma et al., 2020), to suppression of plant diseases (e.g., Rizvi et al., 2017; Agisha et al., 2019; Bektas and Kusek, 2021; Jayakumar et al., 2021), nitrogen (N2) fixation (Bahulikar et al., 2014; Hara et al., 2020), and improved phytochemical composition (Rizvi et al., 2022a), among others. Biofertilizers are microbial formulations of PGPR strains that can either be immobilized or trapped on inert carrier materials to enhance plant growth and soil fertility (Aloo et al., 2022a). Over the decades, considerable strides have been made to understand, investigate and formulate various PGPR as alternative crop fertilization tools (e.g., Htwe et al., 2019; Paliya et al., 2019; Bangash et al., 2021; Barin et al., 2022; Aloo et al., 2022b). The yield of various crops can be increased by about 25% and the use of inorganic N and P fertilizers be reduced by about 25–50 and 25% through biofertilizer application (Khan and Chattopadhyay, 2009; Saber et al., 2012).
The utilization of biofertilizers dates back to the 1980s when the first Rhizobium formulations were patented and marketed in Germany (Nobbe and Hiltner, 1986). Several developments have been made through the decades and today, biofertilizer formulations are applied for the production of several crops entirely or with reduced usage of artificial fertilizers as presented in Section 4. Despite these developments, biofertilizer technology is yet to be exploited to its maximum potential. It is important to increase our knowledge of biofertilizers and their massive potential in the sustainability of our food production systems to increase their utilization. Herein, we evaluate the history of PGPR biofertilizers, assess their present utilization status from a global perspective, and critically propound on their future in sustainable crop production. This can update our understanding of the evolution of PGPR biofertilizers in crop production. We believe that such information will provide a good starting point for debate, and intensive global efforts to harness these bio-resources as biotechnological-based solutions for sustainable crop production systems. This work has been modified from a previous preprint (Aloo et al., 2020).
Overview of rhizobacterial biofertilizers and types
The meaning of biofertilizers has evolved for several decades, with many interpretations. The term has therefore received several different definitions over time (Table 1), reflecting the development of our comprehension of them. Most scholars consider PGPR as a biofertilizer because of their positive influences on the plant rhizospheres that can generally stimulate plant growth. However, Riaz et al. (2020) advance that PGPR and biofertilizers should not be used interchangeably since not all PGPR are biofertilizers.
Table 1.
Common definitions of biofertilizers from different literature.
| Literature | Provided definition |
|---|---|
| Mazid and Khan (2015) | A biologically-active product or microbial inoculant/formulation with one or several beneficial microbes, conserving and mobilizing crop nutrients in soil. |
| Vessey (2003) | A preparation with one or several microbial species capable of mobilizing essential plant nutrients from non-usable to usable forms. |
| Malusá et al. (2012) | A formulation with one or several microbes that enhance soil fertility and promote plant growth by availing nutrients and increasing plant access to nutrients. |
| Bisen et al. (2015) | A unique, environmentally-friendly, and cheap alternative to artificial fertilizers that improve soil health and crop productivity sustainably. |
| Sahu and Brahmaprakash (2016) | A formulation/preparation with latent/living microorganisms with long-term storage, ease of handling, and delivery of effective microbes from the laboratory to the field for crop application. |
| Tomer et al. (2016) | A microbial inoculant that colonizes the rhizosphere and improves plant growth by enhancing plant nutrient availability and accessibility. |
| Simarmata et al. (2016) | A product with several beneficial microbes for improving soil productivity through nitrogen (N) fixation, solubilization of P, and plant growth stimulation through the synthesis of plant growth-promoting (PGP) substances. |
| Nair and Brahmaprakash (2017) | A mixture/product containing an active ingredient and inactive/inert substances. |
| Kumar et al. (2014) | A formulation or a biological product that contains microbes that can improve nutrient solubility in soil and fix atmospheric N and/or enhance crop yield. |
| Bhardwaj et al. (2014) | A formulation made of beneficial microbes and/or biological products and can enhance nutrient solubility in soil or fix atmospheric N and/or has the potential of enhancing crop yield. |
| Brahmaprakash and Sahu (2012) | A preparation of beneficial microbes that can boost plant growth or fertilizer that can meet the nutritional requirements of crops microbiologically. |
| Atieno et al. (2020) | Products containing beneficial microorganisms that enhance soil fertility and crop productivity. |
| Riaz et al. (2020) | Formulations of living microbial cells as single or multiple strains that promote plant growth by increasing nutrient availability and acquisition. |
This is probably because the efficient PGPR must be formulated into products that can be applied to plants/soils to stimulate plant growth to qualify as biofertilizers. Nevertheless, the major components of biofertilizers are PGPR whose activities generally contribute to the overall increment, concentration, and accessibility of plant nutrients in plant rhizospheres. Herein, we adopt the definition of biofertilizers as active microbial agents that stimulate plant growth by improving nutrient availability in plant rhizosphere(s). Other synonymous terminologies with biofertilizers are microbial inoculants or bioformulations, bioinoculants, microbial cultures, and bacterial fertilizers or inoculants (Figure 1).
Figure 1.
Terminologies used interchangeably with microbial biofertilizers.
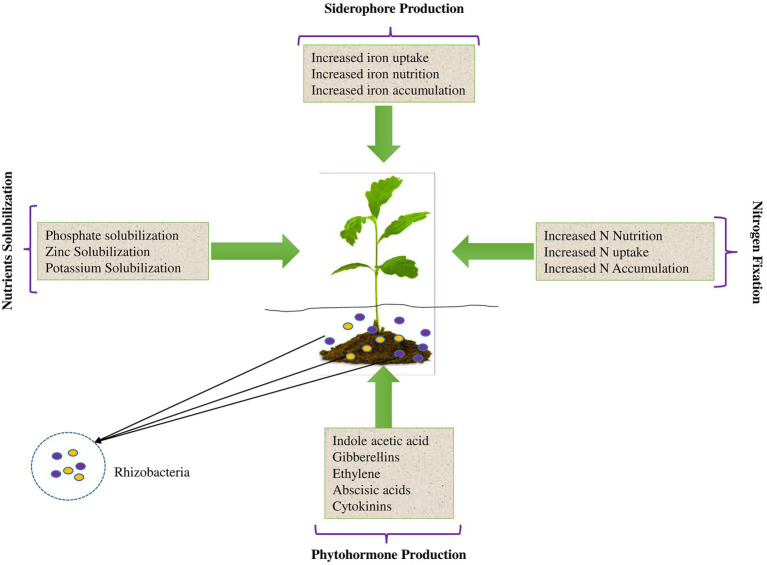
There are several types of biofertilizers depending on their functions in plant rhizospheres. Notably, a single biofertilizer can consist of a single PGPR strain with single or multiple PGP traits, or microbial consortia with multifarious PGP traits. A simulation of the various functions of biofertilizers in PGP is shown in Figure 2 and subsections 2.1 to 2.5 highlight the various types of PGPR biofertilizers. Nevertheless, the different types of biofertilizers normally function synergistically and offer an effective and environmentally-friendly solution for achieving food security while minimizing environmental impacts. Consequently, biofertilizers and PGPR are largely documented as significant factors in integrated soil nutrient management for sustainable crop production as discussed throughout this review.
Figure 2.
A simulation of the various functions of biofertilizers in plant growth promotion.
Nitrogen-fixing biofertilizers
Nitrogen-fixing biofertilizers are currently the most common in the global market and their demand is anticipated to grow by a further 11.9% compounding annual growth rate (CAGR) to reach about USD 4.5 billion by 2026 up from the current USD 2.1 billion (Markets and Markets, 2020). Nitrogen-fixing biofertilizers comprise bacteria that carry out biological N2 fixation (BNF) and boost soil N supply to crops. The legume N2-fixing rhizobia have been researched for decades and shown to increase the quantity of fixed N in inoculated plants relative to un-inoculated ones (Koskey et al., 2017; Sanyal et al., 2020; Gedamu et al., 2021; Ketema and Tefera, 2022). Previous inputs of fixed N for red clover, alfalfa, soybean, pea, and cowpea were estimated to range from 23 to 335 kg ha−1 year−1 (Thies et al., 1995; Wani et al., 1995). The variabilities in terms of quantities of fixed N depend much on the type of legume-rhizobia symbiosis which is dictated by several factors like the legume cultivars and genotypes (Gunnabo et al., 2020; Riah et al., 2021), as well as the geographical distributions (Ramoneda et al., 2020).
According to Herridge et al. (2008), rhizobial inoculants can reduce the annual N fertilization costs by approximately USD 29 ha−1. This scenario demonstrates the importance of N2-fixing rhizobacteria as biofertilizers. Nevertheless, there is a need to perform field trials of new strains for suitability and adaptability before application as inoculants. Besides, N2-fixing biofertilizers are widely investigated for leguminous plants, and more efforts are required to demonstrate their potential in non-leguminous crops using asymbiotic diazotrophs like Azospirillum, Azotobacter, Gluconaceotobacter, and Burkholderia. Earlier studies by Melchiorre et al. (2011) and Hungria et al. (2006) both established that the yield of grains in Brazil, and Argentina, respectively, could reach close to 5 t ha−1 each season through rhizobia-mediated BNF. Similarly, annual N2 fixation rates of approximately 40 kg N ha−1 are documented in Australian soils (Unkovich and Baldock, 2008). Nevertheless, the contribution of asymbiotically fixed N in crop fields largely remains unestablished. More research is necessary, especially for crops like cereals, vegetables, and tubers, considering they contribute to the bulk of human food.
Some efficient N-fixing strains such as Rhizobium and Azotobacter spp. have successfully been formulated into commercial biofertilizers (Adeleke et al., 2019). However, the commercially available N biofertilizers mostly consist of Rhizobium and a few other bacteria such as Azotobacter, and Azospirillum species and are widely applicable to legume crops as presented in Section 4 (Vassilev et al., 2015; Adeleke et al., 2019). Nevertheless, inoculating crops and farms with such biofertilizers can meet the required N levels by plants and substantially reduce the application of artificial fertilizers (Aloo et al., 2022a).
Phosphorus and potassium solubilizing biofertilizers
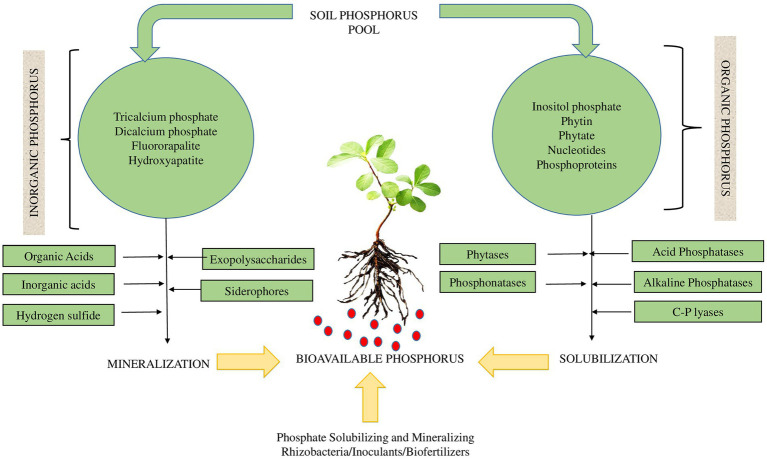
Apart from N-fixation, biofertilizers can also solubilize plant nutrients in soil and facilitate their bioavailability and crop uptake (e.g., Ahmad et al., 2019; Abdelmoteleb and Gonzalez-Mendoza, 2020; El-Deen et al., 2020). Recent biofertilizer forecasts have favored the increased uptake of phosphatic biofertilizers owing to their ability to increase soil P availability and their biocontrol attributes for crop pests (Soumare et al., 2020). The solubilization of P is however dependent on the P forms in soil, whether organic or inorganic (Figure 3).
Figure 3.
Phosphorus solubilization mechanisms depending on the types of available soil P.
Since P deficiency is inherent in numerous agricultural soils, such organisms are largely proposed as potential P biofertilizers. Despite the growing literature, research concerning their application as biofertilizers is still limited and generally inconsistent. Since the economically-mineable P deposits are limited (Cordell et al., 2009; Vassilev et al., 2015), it is doubtless that phosphatic biofertilizers can significantly enhance crop yields, and that the use of P solubilizing biofertilizers (PSB) as bioinoculants can open up a new horizon for sustaining soil P levels and by large, sustainable crop production (Rizvi et al., 2021a).
Similarly, potassium solubilizing biofertilizers (KSB) are equally important in crop production since these are also often limiting in agricultural soils. The K-solubilizing capacity of PGPR from K-bearing rocks through acidification has widely been investigated, thus KSB have a significant role in enhancing crop growth and productivity, for instance, wheat (Laxita and Shruti, 2020), maize (Akintokun et al., 2019; Imran et al., 2020), tomatoes (Reyes-Castillo et al., 2019; Raji and Thangavelu, 2021), and many others. These reports show that these bacteria can significantly improve germination, uptake of nutrients, growth, and crop yields under both controlled and uncontrolled conditions. Although K solubilization may not entirely fulfill plant K requirements like chemical fertilizers, studies show that this novel approach may significantly improve K availability in croplands (Huda et al., 2007; Imran et al., 2020; Laxita and Shruti, 2020). Furthermore, the application of KSB to agricultural soils as biofertilizers can greatly cut the use of artificial fertilizers and are eco-friendly approaches to crop production. Native KSB are especially emerging as a viable technology for mitigating K deficits in agricultural soils. The diversity, solubilizing abilities, and mechanisms of KSB are extensively reviewed by Sattar et al. (2019) and Ahmad et al. (2016). Despite the burgeoning literature, little is still known about the efficacy of KSB and how they can stimulate plant growth in different climates. Meena et al. (2018) advance that KSB are valuable resources for mitigating K-deficiencies in agricultural farms but experimental results on their field efficacy are still grossly inadequate. More research is needed to enhance their usability. This, and related knowledge will undoubtedly help in comprehending their value as bioinoculants for practical field applications.
Zinc solubilizing biofertilizers
Zinc solubilizing biofertilizers (ZSB) are equally important in crop production owing to worldwide Zn deficiency in soils. Such deficiency is prevalent in most arable lands caused by nutrient mining due to crop harvesting (Cakmak et al., 2017). Although chemical Zn fertilizers are often employed to augment these deficits at the recommended rates of approximately 5 kg ha−1 Zn, However, synthetic fertilizers are costly and do not readily get converted into plant-usable forms (Montalvo et al., 2016). Recent literature advances in rhizobacterial Zn solubilization (e.g., Joshi et al., 2013; Hussain et al., 2015; Kamran et al., 2017; Perumal et al., 2019) suggest that the field application of ZSB in the can increase Zn uptake by plants, and subsequently, improve their growth and yields. In an investigation by Naz et al. (2016), Pseudomonas, Azotobacter, Azospirillum, and Rhizobium species were shown to significantly enhance Zn uptake in wheat. Similarly, Sharma et al. (2012) studied 134 bacilli from the soybean (G. max) rhizosphere for Zn solubilization and established that the isolates greatly enhanced the concentration of Zn in the inoculated crops relative to the un-inoculated ones. Similarly, several ZSB like Pantoea dispersa, P. fragi, P. agglomerans, Rhizobium sp., and E. cloacae from the sugarcane and wheat rhizospheres were recently shown to improve the Zn contents and growth of potted wheat (Kamran et al., 2017). A more recent greenhouse trial by Dinesh et al. (2018) that evaluated several rhizospheric ZSB for their effects on soil and plant Zn contents revealed that the concentration in soil and plants was greater in treated plants than in non-treated ones. In India, Goteti et al. (2013) bacterized potted maize seeds with Zn-solubilizing Pseudomonas that significantly enhanced Zn uptake and concentration. Reports also exist for the Zn solubilizing abilities and increased Zn uptake following inoculation of wheat by Pseudomonads (Joshi et al., 2013), maize by Bacillus (Hussain et al., 2015), wheat and soybean by B. aryabhattai (Ramesh et al., 2014), and rice by several ZSB (Perumal et al., 2019).
Iron sequestering biofertilizers
Some biofertilizers can sequester iron (Fe) through special mechanisms using metabolites called siderophores with a high affinity for Fe in low-Fe environments (Wang et al., 2022). After the formation of the Fe3 + −microbial siderophores complexes formed in the microbial membrane, the former is reduced to Fe2+ which is subsequently freed into the cell through an input mechanism. In this process, plants access and directly assimilate the Fe2+ from bacterial siderophores from the Fe-siderophore complexes or through ligand exchange reactions (Yehuda et al., 1996). Siderophore production is a typical example of Fe nutrition enhancement by rhizobacterial inoculants in biofertilizers and owing to its indisputable significance, should be given more attention (Aloo et al., 2019).
Phytostimulators
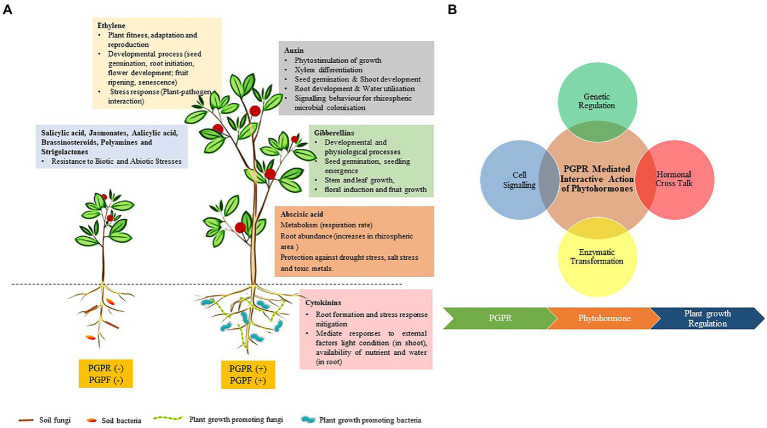
Still, other biofertilizers can promote plant growth through phytohormone production and plant growth stimulation in many ways (Figure 4). Such biofertilizers are largely known as phytostimulators owing to the various roles they play in stimulating the growth of crops through the production of phytohormones. The most common phytohormones are auxins, gibberellins, and cytokinin. Although very small amounts of phytohormones are produced by PGPR, they are still very crucial for plant metabolic processes, including those that modulate plant growth (e.g., Kalimuthu et al., 2019; Haerani et al., 2021) and plant tolerance to various abiotic stresses (Mahmoody and Noori, 2014; Santhi et al., 2021). Among the most potential PGPR that can function as biofertilizers due to phytohormone production are Azospirillum (e.g., Coniglio et al., 2019) and Bacillus spp. (Kang et al., 2019; Bandopadhyay, 2020), and many others. Owing to the importance of phytostimulation, such PGPR are viable candidates for PGP as biofertilizers, especially if they can also solubilize plant nutrients and/or fix N to improve plant nutrition.
Figure 4.
(A) Plant growth promotion through the production of different phytohormones and (B) PGPR-mediated plant growth promotion is governed through a complex network of cell signaling, genetic regulation, hormonal cross-talk, and enzymatic transformation. The PGPR generates multiple stimuli through the synthesis of phytohormones. These phytohormones interact through phosphorylation cascade or activating a secondary messenger which leads to the regulation of genes affecting hormone biosynthesis and developmental process in plants (Khan et al., 2020).
History of rhizobacterial biofertilizers
Whereas the application of microbial formulations is generally considered a modern and novel biotechnological agricultural approach in, crop inoculation with efficient PGPR for yield improvement is a century-old practice. The 1st attempts at rhizobacterial formulation date back to the late 18th century when a French scientist called Jean-Baptiste Boussingault (1801–1887) recognized that plant growth was proportional to N quantities. This observation was later linked to the reduction of N2 to ammonium and the 1st commercial biofertilizer Nitragin® made from Rhizobium was produced (Nobbe and Hiltner, 1986). These were the first commercial formulations of PGPR that were patented and marketed over a century ago (Nobbe and Hiltner, 1986).
Due to the inconsistent performance of bioformulations relative to artificial fertilizers, the use of biofertilizers slowed down but picked up after subsequent decades of research that produced encouraging greenhouse results using Pseudomonas spp. (Kloepper et al., 1980). Large-scale field trials were performed using Azotobacter and Bacillus spp. on more than 35 million ha of land in the former Soviet Union in 1958 (Cooper, 1959), but the impact of bacterization was relatively unsatisfactory. Nevertheless, the commercialization of Rhizobium formulations continued in the 19th century (Fages, 1992), and extended globally thereafter (Deaker et al., 2004). A lot of biofertilizers have since been formulated and marketed worldwide attempts have also been made to formulate bacterial soil-fertilizing preparations for non-legume crops. The 1st preparation “Alinit” based on B. ellenbachensis was introduced in Germany to promote cereal growth (Caron, 1897).
Rhizobacterial inoculations in parts of Southern Africa can be traced back to 1963 after successful soybean nodulation efficiency by native Bradyrhizobium and Rhizobium inoculants (Shurtleff and Aoyagi, 2018). Thereafter, a natural soybean nodulating variety called Nitrozam was formulated for use in Zambia and other African countries (Raimi et al., 2021). These concerted efforts massively increased soybean cultivation by 48% from 6,550 ha in 1984 to 22,780 ha in 1992. Additionally, about US$100,000 worth of Nitrozam was sold during this period. In South Africa, the biofertilizer market rapidly expanded in 1952, and to date, the country has one of the most established biofertilizer markets and regulations in the whole of Africa. The marketing and application of N2-fixing rhizobial biofertilizers in legume production have since been practiced for years. Globally, the total area of legumes under treatment with biofertilizers yearly was over 40 million ha by the year 2000 (Phillips, 2004), half of which was used for soybean production (Catroux et al., 2001). There are more success stories of legume inoculants in different parts of the world (El-Wakeil and El-Sebai, 2009; Ngakou et al., 2009; Gomare et al., 2013). The production and marketing of rhizobial inoculants for legume production have thus been practiced for decades, somewhat decreasing the need for chemical fertilizers in several countries around the world. The development of new biofertilizer bioformulations continues to expand, and the future of the technology seems bright.
The current state of rhizobacterial biofertilizers
There is a burgeoning literature on the current application of microbial products as biofertilizers and agricultural inputs. Nearly 170 establishments in 24 countries commercialize biofertilizers and possess factories that produce, and market microbe-based fertilizers at both small and large scales (Bharti et al., 2017). The marketing of rhizobial inoculants has particularly been practiced for several decades now to partially eliminate the application of artificial fertilizers (Paudyal and Gupta, 2018). However, the full potential of many potential biofertilizers is largely untapped. Likewise, biofertilizer commercialization remains low globally, albeit steadily increasing.
In developed countries where artificial agricultural inputs are fairly cheap, the use of PGPR is less prioritized but is albeit growing. In 2013, the highest demand for biofertilizers was highest in North America and projections were that the entire Asia-Pacific biofertilizer market would show the maximum growth from 2014 to 2019 and lead in biofertilizer consumption worldwide (Markets and Markets, 2014). The consumption of biofertilizers is reportedly growing in countries such as Canada, Argentina, China, India, Europe, and the United States of America (USA) due to tax exemptions, and input subsidies, among other incentives (Markets and Markets, 2019). Such approaches have generally served to expand the global biofertilizer market, but more efforts are still required.
The advancement of research around the globe on the diversity, functions, and potentials of native rhizobacteria has stimulated the selection and isolation of efficient PGPR, and several biofertilizer formulations are already produced and commercialized for use in different countries across the globe (Table 2). The most progressive and dominant biofertilizer market in the world is Europe, where biofertilizer demand has grown at a CAGR of 12.3% from approximately US$2566 million in 2012 to US$4582 million in 2017 (Chandrasekhar, 2014). The global biofertilizer market was worth US$1.06 million in 2016 and was estimated to hit US$2 billion in 2019 and over US$3.8 billion in 2026, at a CAGR of 11.2% (Markets and Markets, 2020). The global increase in demand for biofertilizers has greatly been influenced by the growing demand for organic food products.
Table 2.
Examples of commercial biofertilizer products in some countries around the world.
| Country | Product | Organisms | Manufacturer | Crop | References |
|---|---|---|---|---|---|
| Argentina | Liquid PSA | P. aurantiaca | Laboratorios BioAgro S.A. | Wheat | Celador-Lera et al. (2018) |
| Zadspirillum | Azospirillum brasilense | Semillera Guasch SRL | Maize | Celador-Lera et al. (2018) | |
| Rhizo Liq | Bradyrhizobium sp., Mesorhizobium ciceri, Rhizobium spp. | Rhizobacter | Chickpea, Soybean, Common bean, green gram, Groundnut | Adeleke et al. (2019) | |
| Australia | Bio-N | Azotobacter spp. | Nutri-Tech solution | Not stated | Adeleke et al. (2019) |
| Myco-Tea | Azotobacter chroococcum, B. polymyxa | Nutri-Tech solution | Tea | Adeleke et al. (2019) | |
| Twin N | Azorhizobium sp., Azoarcus sp., Azospirillum sp. | Mapleton Int. Ltd | Not stated | Adeleke et al. (2019) | |
| Brazil | Bioativo | PGPR consortia | Embrafros Ltda | Beans, maize, sugarcane, rice, cereals | Odoh et al. (2019) |
| Canada | Rhizocell GC Nodulator | B. amyloliquefaciens IT 45, B. japonicum | Lallen and plant care BASF Inc. | Beans, maize, carrot, rice, cotton | Odoh et al. (2019) |
| Vault HP | Bradyrhizobium sp. | BASF | Not stated | Adeleke et al. (2019) | |
| China | CBF | Bacillus mucilaginosus, B. subtilis | China Bio-Fertilizer AG | Various cereals | Celador-Lera et al. (2018) |
| Colombia | Fe Sol B | Not mentioned | Agri Life Bio Solutions | Not stated | Mishra and Arora (2016) |
| Germany | FZB 24 fl, BactofilA 10 | B. amyloliquefaciens, B. megaterium, P. fluorescens | AbiTEP GmbH | Vegetables, cereals | Odoh et al. (2019) |
| Hungary | BactoFil A10 | A. brasilense, Azotobacter vinelandii, B. megaterium | AGRObio | Maize | Mustafa et al. (2019) |
| India | Ajay Azospirillum | Azospirillum | Ajay Biotech | Cereals | Celador-Lera et al. (2018) |
| Greenmax AgroTech Life Biomix, Biodinc, G max PGPR | Azotobacter, P. fluorescens | Biomax | Various crops | Odoh et al. (2019) | |
| Fe Sol B | Not mentioned | Agri Life Bio Solutions | Not mentioned | Mishra and Arora (2016) | |
| Symbion van plus | B.megaterium | T. Stanes and Co. Ltd | Not mentioned | Celador-Lera et al. (2018) | |
| Kenya | Biofix | Rhizobia | MEA Fertilizer Ltd | Not mentioned | Adeleke et al. (2019) |
| Kefrifix | Not mentioned | KFRI | Not mentioned | Raimi et al. (2021) | |
| Nigeria | Nodumax | Bradyrhizobia | IITA | Not mentioned | Tairo and Ndakidemi (2014), Adeleke et al. (2019) |
| Russia | Azobacterium | Azobacterium brasilense | JSC Industrial Innovations | Wheat, barley, maize, | Celador-Lera et al. (2018) |
| South Africa | Organico | Rhizobium, Enterobacter spp., Bacillus spp., Stenotrophomonas, Pseudomonas | Amka Products (Pty) Ltd | Not mentioned | Adeleke et al. (2019) |
| Azo-N, Azo-N-Plus | A.brasiliense, A. lipoferum | Biocontrol Products Ltd | Not mentiomne | Raimi (2018) | |
| Lifeforce, Firstbase, Biostart, Landbac, Composter, Waterbac | Bacillus spp., | Microbial solution (Psty) Ltd | Not stated | Mohammadi and Sohrabi (2012) | |
| Histick | B. japonicum | BASF | Not stated | Tairo and Ndakidemi (2014) | |
| N-Soy | B.japoniucm | Biocontrol Products Ltd | Not stated | Tairo and Ndakidemi (2014) | |
| Soilfix | Brevibacillus laterosporus, Paenibacillus chitinolyticus | Biocontrol Products Ltd | Not stated | Grady et al. (2016) | |
| Organico | Bacillus sp. | Amka Products | Not stated | Raimi (2018) | |
| Bac-up | B. subtilis | Biocontrol Products Ltd | Not stated | Adeleke et al. (2019) | |
| Spain | InomixR | B. polymyxa, B. subtilis | Lab (Labiotech) | Cereals | Odoh et al. (2019) |
| Vita Soil | PGPR consortia | Symborg | Not stated | Sekar et al. (2016) | |
| Thailand | BioPlant | Streptomyces, Nitrobacter, Clostridium, Bacillus, Aerobacter, Achromobacter, Nitrosomonas | Artemis & Angelio Co. Ltd. | Not stated | Adeleke et al. (2019) |
| United Kingdom | Ammnite A 100 | Azotobacter, Bacillus, Rhizobium, Pseudomonas | Cleveland biotech | Cucumber, tomato, pepper | Odoh et al. (2019) |
| Legume Fix | Rhizobium sp., B. japonicum. | Legume Technology | Common bean, Soybean | Adeleke et al. (2019) | |
| Twin N | Azorhizobium sp., Azoarcus sp., Azospirillum sp. | Mapleton Int. Ltd | Not mentioned | Adeleke et al. (2019) | |
| Uruguay | Nitrasec | Rhizobium sp. | Lage y Cia | Not mentioned | Adeleke et al. (2019) |
| United States | Inogro | 30 bacterial species | Flozyme Corporation | Rice | Celador-Lera et al. (2018) |
| Vault NP | B. japonicum | Becker Underwood | Not mentioned | Adeleke et al. (2019) | |
| Chickpea Nodulator | Mesorhizobium cicero | Becker Underwood | Chickpea | Adeleke et al. (2019) | |
| Cowpea Inoculant | Rhizobia | Becker Underwood | Cowpea | Adeleke et al. (2019) | |
| PHC Biopak | B. subtilis, B. azotofixans, B. megaterium, B. licheniformis, B. thuringiensis, B. polymyxa, | Plant Health Care Inc. | Not mentioned | Adeleke et al. (2019) | |
| Complete Plus | Bacillus strains | Plant Health Care | Various crops | Mustafa et al. (2019) | |
| Quickroots | B. amyloliquefaciens | Monsanto | Wheat, common bean | Celador-Lera et al. (2018) |
Although there exist many reports on the formulation and/or commercialization and application of rhizobacterial biofertilizers in several parts of the world, only a few reports indicate their application and commercialization in Africa. The PGPR inoculant technology has little or no impact on crop productivity in developing countries since it is either not practiced or the poor quality of available inoculants (Bashan, 1998). According to Aloo et al. (2021), the potential benefits of biofertilizers have largely been untapped in Africa due to inadequate regulatory frameworks and several other challenges. Most biofertilizers are commercialized for use in Asia, Europe, and the USA but in Africa, most commercialization and application occur only in South Africa. In East Africa, the production and use of biofertilizers are pronounced in Kenya which is the manufacturer of Biofix which can effectively inoculate 15 kg of common bean seeds per ha at approximately US$1.25 in comparison to 90 kg of artificial N fertilizer required for the same number of seeds per ha at US$12.50 (Raimi et al., 2021). However, Biofix and other biofertilizers are still largely underutilized in Kenya, probably due to a lack of awareness and other technology adoption challenges. Current and future initiatives are anticipated to improve the uptake of biofertilizers in Africa (Raimi et al., 2021). However, more efforts are needed to boost the consumption of these microbial products and promote the sustainability of global food production systems (Aloo et al., 2021).
The worldwide market for biofertilizers is presently largely dominated by legume and N2-fixing inoculants (Vassilev et al., 2015). While rhizobial inoculants currently dominate the global biofertilizer market, PSB, and other bioinoculants occupy less than 30% altogether (Transparency Market Research, 2022). Nevertheless, P-, K-, and Zn-based biofertilizers are now developing into significant bioinoculants to address soil nutrient deficiencies, and KSM are already commonly used as inoculants in some countries with K-deficient croplands (Teotia et al., 2016). India is reportedly the 4th largest consumer of K bioinoculants globally while countries like Brazil, the USA, and China come first in the overall consumption of these microbial products (Matich, 2016).
Unlike rhizobial biofertilizers, PSB like Pseudomonas, Bacillus, and diazotrophs like Azospirillum have neither been used as much nor at a large scale (Lesueur et al., 2016). Most of the commercially-available non-rhizobial PGPR inoculants consist of Azospirillum as free-living N2 fixers or Bacilli as PSB (Herrmann and Lesueur, 2013). The application of non-rhizobial biofertilizers has a less significant impact on global food production probably because of several bottlenecks that exist in biofertilizer uptake and use relative to well-documented PGP functions. Yet, PGPR like PSB are essential candidates for improving legume P nutrition for efficient nodulation (Zaidi et al., 2017). The global biofertilizers market for crop production is projected to grow from US$2.02 billion in 2022 to US$4.47 billion by 2029, at a CAGR of 12.04% from 2022 to 2029 (Fortune Business Insights, 2022). Still, there is a need for more efforts for adequate market infiltration and application.
The future of biofertilizers
The incorporation of biofertilizers as fundamental components of agricultural practices is quickly gathering momentum globally. These microbial products are already in use in some countries and are expected to become more popular in the future. The global and future expansion of the biofertilizer market will largely be driven by the need to increase food production sustainably. With an ever-increasing demand for organic food products, growing awareness of sustainable agricultural practices, and promotion of cleaner production methods for reducing soil contamination, land degradation, and water pollution the market growth for biofertilizers will continue to grow over the coming years (Abhilash et al., 2016a; Anand et al., 2022; Vaishnav et al., 2022).
Forecasts are that the present global market for biofertilizers which was approximated at US$396 million in 2018 will grow at a CAGR of 10.9% to escalate to US$4448.97 million by 2028 (Vintage Market Research, 2022). Further indications are that the global biofertilizer market which was valued at close to US$ 3.0 billion in 2020 will grow at a CAGR of 12.2% and reach about US$5 billion by 2031 (Transparency Market Research, 2022). The number of investigations targeting the isolation, identification, and evaluation of the capacity of PGPR with the potential of being transformed into inoculants for a variety of crops is equally expanding (Vatsyayan and Ghosh, 2013; Datta et al., 2015; Tan et al., 2015; Koskey et al., 2017; Anand et al., 2022; Aloo et al., 2022b). It is, therefore, realistic to expect that widespread biofertilizer usage will soon offer countless approaches to the progression of sustainable crop production systems.
For the extensive utilization of biofertilizers, proper regulatory and legal frameworks will be required in place of the existing ones that are currently stringent and hinder their proper utilization. Fortunately, regulatory authorities are increasingly encouraging the implementation of alternative crop fertilization mechanisms to promote the development of sustainable agricultural technologies. Recognizing the need for a specific legislative framework for biofertilizers in Europe, the European Commission has proposed to amend existing regulations (European Parliament and Council of the European Union, 2016). Such initiatives will eventually relax the stringent regulatory frameworks and enable the widespread adoption of these microbial resources.
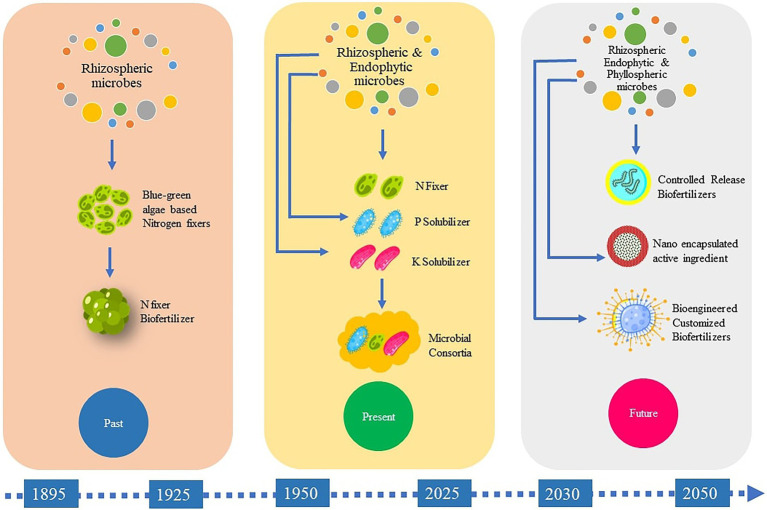
While a number of the existing biofertilizers are mainly composed of natural rhizobacterial strains chosen for their PGP qualities, the development of genetically-modified inoculants that are likely to be more efficient at plant growth stimulation is required. Still, the biggest hurdle will be for scientists to convince society and regulatory authorities worldwide that such genetically-engineered organisms are harmless. Our current ability to manipulate and exploit the plant microbiome in situ similarly remains limited, and more investigations are required to facilitate their large-scale application and commercialization. The inoculant industry is faced with various challenges in making formulations with prolonged shelf lives. Progress into developing formulations with improved shelf lives, broad spectra of action, and reliable field performance will therefore hasten the commercialization of this technology (Nakkreen et al., 2005). In this regard, new approaches should be evaluated to develop formulations with longer shelf lives. Micro-encapsulation is one viable approach but most experiments in this regard have been restricted to laboratories and the standardization of this technology for industrial and field applications should be pursued. The future of biofertilizer technology depends a lot on developing efficient PGP strains. This is quite challenging but continued research in this area will eventually pave the way for this (Figure 5).
Figure 5.
Schematic representation of the past present and future of biofertilizer development.
Investigation on N-fixing and PSB is developing fairly well, unlike for K solubilizers despite K being among the important macronutrients for plant development. More research in this regard will promote their application and utilization as bioinoculants in the future. Future research should additionally focus on the development of inoculants that can tolerate unfavorable environmental conditions for applicability in stressful environments, especially for inoculants that have been shown to relieve plants of metal stress (Rizvi et al., 2019, 2020, 2022b), salinity stress (Shahid et al., 2021), and cold stress (Rizvi et al., 2021b). More research is needed on the practical aspects of large-scale formulation and production to develop stable, effective, and state-of-the-art bioformulations. Microbial consortia offer multiple PGP traits for producing novel biofertilizer formulations as substitutes for artificial inputs (Singh et al., 2020; Cakmaksi et al., 2021; Vaishnav and Singh, 2021).
The interactions among plants and biofertilizer inoculants will require further studies and new approaches. Future research should additionally include careful selection of rhizosphere microbiota, and their in-situ testing for use as plant inoculants. It is expected that the identification of effective microbiomes in different soil types and climates will be extremely helpful in this regard. To improve this strategy, establishing a global database of effective plant microbiomes will be an important milestone toward successful translational research. A lot of obstacles remain to be overcome before this can fully be realized. For instance, several formulations based on such microbes have been developed for applications to different crops worldwide. However, inoculation results are often inconsistent and dependent on the prevailing local soil and plant-related properties, altogether necessitating the optimization of each system.
The application of biotechnology and the improvement of biofertilizer regulations will facilitate in designing of more effective and reliable rhizobial bioformulations as biofertilizers. To design suitable rhizobial formulations, we must use modern technologies to increase our understanding of plant-microbe interactions (Jain et al., 2021). For example, multi-omics approaches can greatly help us to comprehend complex plant-microbial symbioses to design suitable bioformulations for particular soils and crops (Kaul et al., 2016; López-Mondéjar et al., 2017; Ding et al., 2021; Yamazaki et al., 2021). These novel approaches will with time enhance the complete characterization of PGPR and their influence on plant nutrient acquisition and other PGP traits to facilitate their application. Thus, these should be prioritized for research.
Finally, it will be important to identify the challenges in the production and application of biofertilizers and strategies to address such problems. For example, the field efficiency of biofertilizers is dependent on crop species, soil complexity, and climatic conditions. Research on suitable biofertilizers should in the future be handled by agronomists that understand the nexus between crops, climatic conditions, and nutrients in various parts of the world. Besides, genomic engineering can be necessary for manipulating indigenous PGPR with suitable genes for enhanced expression of biofertilization functions for field applications. Additionally, particular additives could improve product stability, shelf life, and field efficiency.
Conclusion
The greatest global challenge in the 21st century is to invent and implement sustainable agricultural practices. This can only be achieved if we accommodate changing and advanced technologies such as the use of efficient rhizobacterial biofertilizers. The present discussion is useful for the development of sustainable agricultural systems. The use of these bio-resources though has been practiced in several parts of the globe is still low but the results are encouraging and there is room for development to boost their efficiency. With time, the practice will certainly grow and projections are that biofertilizers will have massive market potential soon. Researchers, agricultural institutions, and universities can fast-track biofertilizer development and promote their usage and adaptation for sustainable agricultural practices. If issues linked to regulation, policy development, and social acceptability of biofertilizer products can simultaneously be addressed, these bio-based tools can potentially and significantly contribute to sustainable agricultural productivity.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
This research was originally published as a preprint: doi: 10.20944/preprints202009.0650.v1.
References
- Abdelmoteleb A., Gonzalez-Mendoza D. (2020). Isolation and identification of phosphate solubilizing bacillus spp. from Tamarix ramosissima rhizosphere and their effect on growth of Phaseolus vulgaris under salinity stress. Geomicrobiol J. 37, 901–908. doi: 10.1080/01490451.2020.1795321 [DOI] [Google Scholar]
- Abhilash P., Dubey R. K., Tripathi V., Gupta V. K., Singh H. B. (2016a). Plant growth-promoting microorganisms for environmental sustainability. Trends Biotechnol. 34, 847–850. doi: 10.1016/j.tibtech.2016.05.005 [DOI] [PubMed] [Google Scholar]
- Abhilash P., Tripathi V., Edrisi S. A., Dubey R. K., Bakshi M., Dubey P. K., et al. (2016b). Sustainability of crop production from polluted lands. Energy Ecol. Environ. 1, 54–65. doi: 10.1007/s40974-016-0007-x [DOI] [Google Scholar]
- Adeleke R. A., Raimi A. R., Roopnarain A., Mokubedi S. M. (2019). “Status and prospects of bacterial inoculants for sustainable Management of Agroecosystems” in Biofertilizers for Sustainable Agriculture and Environment. eds. Giri B., Prasad R., Wu Q. S., Varma A. (Cham: Springer International Publishing; ), 137–172. [Google Scholar]
- Agisha V. N., Kumar A., Eapen S. J., Sheoran N., Suseelabhai R. (2019). Broad-spectrum antimicrobial activity of volatile organic compounds from endophytic pseudomonas putida BP25 against diverse plant pathogens. Biocontrol Sci. Technol. 29, 1069–1089. doi: 10.1080/09583157.2019.1657067 [DOI] [Google Scholar]
- Ahmad M., Adil Z., Hussain A., Mumtaz M. Z., Nafees M., Ahmad I., et al. (2019). Potential of phosphate solubilizing bacillus strains for improving growth and nutrient uptake in mungbean and maize crops. Pak. J. Agric. Sci. 56, 283–289. doi: 10.21162/PAKJAS/19.7455 [DOI] [Google Scholar]
- Ahmad M., Nadeem S. M., Naveed M., Zahir Z. A. (2016). “Potassium-solubilizing microorganisms and their application in agriculture” in Potassium Solubilizing Microorganisms for Sustainable Agriculture. eds. Meena V., Maurya B., Verma J., Meena R. (New Delhi: Springer; ), 293–313. [Google Scholar]
- Akintokun A. K., Ezaka E., Akintokun P. O., Shittu O. B., Taiwo L. B. (2019). Isolation, screening and response of maize to plant growth promoting. Sci. Agric. Bohem. 50, 181–190. doi: 10.2478/sab-2019-0025 [DOI] [Google Scholar]
- Aloo B. N., Makumba B. A., Mbega E. R. (2019). The potential of bacilli rhizobacteria for sustainable crop production and environmental sustainability. Microbiol. Res. 219, 26–39. doi: 10.1016/j.micres.2018.10.011, PMID: [DOI] [PubMed] [Google Scholar]
- Aloo B. N., Makumba B. A., Mbega E. R. (2020). Plant growth promoting rhizobacterial biofertilizers for sustainable crop production: The past, present, and future. Preprints 2020090650. [DOI] [PMC free article] [PubMed]
- Aloo B. N., Mbega E. R., Makumba B. A., Tumuhairwe J. B. (2022b). Effects of carrier materials and storage temperatures on the viability and stability of three biofertilizer inoculants obtained from potato (Solanum tuberosum L.) Rhizosphere. Agriculture 12:140. doi: 10.3390/agriculture12020140 [DOI] [Google Scholar]
- Aloo B. N., Mbega E. R., Tumuhairwe J. B., Makumba B. A. (2021). Advancement and practical applications of rhizobacterial biofertilizers for sustainable crop production in sub-Saharan Africa. Agric. Food Secur. 10, 1–12. doi: 10.1186/s40066-021-00333-6 [DOI] [Google Scholar]
- Aloo B. N., Mbega E., Tumuhairwe J., Makumba B. (2022a). “Microbial biostimulants for crop production: industry advances, bottlenecks, and future prospects” in Microbial biostimulants for Sustainable Agriculture and Environmental Bioremediation. eds. Inamuddin C. O. A., Ahamed M. I., Altalhi T. (Boca Raton: CRC Press; ), 177–198. [Google Scholar]
- Anand U., Vaishnav A., Sharma S. K., Sahu J., Ahmad S., Sunita K., et al. (2022). Current advances and research prospects for agricultural and industrial uses of microbial strains available in world collections. Sci. Total Environ. 842:156641. doi: 10.1016/j.scitotenv.2022.156641, PMID: [DOI] [PubMed] [Google Scholar]
- Atieno M., Herrmann L., Nguyen H. T., Phan H. T., Nguyen N. K., Srean P., et al. (2020). Assessment of biofertilizer use for sustainable agriculture in the great Mekong region. J. Environ. Manage. 275:111300. doi: 10.1016/j.jenvman.2020.111300, PMID: [DOI] [PubMed] [Google Scholar]
- Bahulikar R. A., Torres-Jerez I., Worley E., Craven K., Udvardi M. (2014). Diversity of nitrogen-fixing bacteria associated with switchgrass in the native tallgrass prairie of northern Oklahoma. Appl. Environ. Microbiol. 80, 5636–5643. doi: 10.1128/AEM.02091-14, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Y. C., Chang Y. Y., Hussain M., Lu B., Zhang J. P., Song X. B., et al. (2020). Soil chemical and microbiological properties are changed by long-term chemical fertilizers that limit ecosystem functioning. Microorganisms 8:694. doi: 10.3390/microorganisms8050694, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandopadhyay S. (2020). Application of plant growth promoting bacillus thuringiensis as biofertilizer on Abelmoschus esculentus plants under field condition. J. Pure Appl. Microbiol. 14, 1287–1294. doi: 10.22207/JPAM.14.2.24 [DOI] [Google Scholar]
- Bangash N., Mahmood S., Akhtar S., Hayat M. T., Gulzar S., Khalid A. (2021). Formulation of biofertilizer for improving growth and yield of wheat in rain dependent farming system. Environ. Technol. Innov. 24:101806. doi: 10.1016/j.eti.2021.101806 [DOI] [Google Scholar]
- Barin M., Asadzadeh F., Hosseini M., Hammer E. C., Vetukuri R. R., Vahedi R. (2022). Optimization of biofertilizer formulation for phosphorus solubilizing by Pseudomonas fluorescens Ur21 via response surface methodology. PRO 10:650. doi: 10.3390/pr10040650 [DOI] [Google Scholar]
- Bashan Y., Puente M. E., Myrold D. D., Toledo G. (1998). In vitro transfer of fixed nitrogen from diazotrophic filamentous cyanobacteria to black mangrove seedlings. FEMS Microbiol. Ecol. 26, 165–170. doi: 10.1111/j.1574-6941.1998.tb00502.x [DOI] [Google Scholar]
- Bektas I., Kusek M. (2021). Biological control of onion basal rot disease using phosphate solubilising rhizobacteria. Biocontrol Sci. Technol. 31, 190–205. doi: 10.1080/09583157.2020.1839381 [DOI] [Google Scholar]
- Bhardwaj D., Ansari M. W., Sahoo R. K., Tuteja N. (2014). Biofertilizers function as key player in sustainable agriculture by improving soil fertility, plant tolerance and crop productivity. Microb. Cell Fact. 13, 1–10. doi: 10.1186/1475-2859-13-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharti N., Sharma S. K., Saini S., Verma A., Nimonkar V., Prakash O. (2017). “Microbial plant probiotics: problems in application and formulation” in Probiotics and Plant Health. eds. Kumar V., Kumar M., Sharma S., Prasad R. (Singapore: Springer; ), 317–335. [Google Scholar]
- Bisen K., Keswani C., Mishra S., Saxena A., Rakshit A., Singh H. (2015). “Unrealized potential of seed biopriming for versatile agriculture” in Nutrient Use Efficiency: From Basics to Advances. eds. Rakshit A., Singh H. B., Sen A. (New Delhi: Springer; ), 193–206. [Google Scholar]
- Borgi M. A., Saidi I., Moula A., Rhimi S., Rhimi M. (2020). The attractive Serratia plymuthica BMA1 strain with high rock phosphate-solubilizing activity and its effect on the growth and phosphorus uptake by Vicia faba L. Plants Geomicrobiol. J. 37, 437–445. doi: 10.1080/01490451.2020.1716892 [DOI] [Google Scholar]
- Brahmaprakash G., Sahu P. K. (2012). Biofertilizers for sustainability. J. Indian Inst. Sci. 92, 37–62. [Google Scholar]
- Cakmak I., McLaughlin M. J., White P. (2017). Zinc for better crop production and human health. Plant Soil 411, 1–4. doi: 10.1007/s11104-016-3166-9 [DOI] [Google Scholar]
- Cakmaksi R., Akcura S., Mustafa E. (2021). Effect of co-inoculation of multi-traits bacteria based bio-formulations on the growth, yield and enzyme activities of tea. J. Agric. Nat. Resour. 8, 594–604. doi: 10.30910/turkjans.807411 [DOI] [Google Scholar]
- Carmo J. B. D., Filoso S., Zotelli L. C., De Sousa Neto E. R., Pitombo L. M., Duarte-Neto P. J., et al. (2013). Infield greenhouse gas emissions from sugarcane soils in Brazil: effects from synthetic and organic fertilizer application and crop trash accumulation. Glob. Change Biol. Bioenergy 5, 267–280. doi: 10.1111/j.1757-1707.2012.01199.x [DOI] [Google Scholar]
- Caron A. (1897). Culture of Bacteria. U.S. patent no. 679600. Washington, DC: U.S. Patent and Trademark Office [Google Scholar]
- Catroux G., Hartmann A., Revellin C. (2001). Trends in rhizobial inoculant production and use. Plant Soil 230, 21–30. doi: 10.1023/A:1004777115628 [DOI] [Google Scholar]
- Celador-Lera L., Jiménez-Gómez A., Menéndez E., Rivas R. (2018). “Biofertilizers based on bacterial Endophytes isolated from cereals: potential solution to enhance these crops” in Stress Management and Agricultural Sustainability. ed. Meena V. S. (Singapore: Springer; ), 175–203. [Google Scholar]
- Chandrasekhar K. (2014). Europe bio fertilizer market is expected to reach $4,582.2 million in 2017 new report. MicroMarket monitor. Available at: http://www.micromarketmonitor.com/market/europe-bio-fertilizer-4637178345.html (Accessed July 2, 2022).
- Coniglio A., Mora V., Puente M., Cassán F. (2019). “Azospirillum as biofertilizer for sustainable agriculture: Azospirillum brasilense AZ39 as a model of PGPR and field traceability,” in Microbial Probiotics for Agricultural Systems. Sustainability in Plant and Crop Protection, eds. Zúñiga-Dávila D., González-Andrés F., Ormeño-Orrillo E., (Cham, Springer; ) pp. 45–70. [Google Scholar]
- Cooper R. (1959). Bacterial fertilizers in the Soviet Union. Soil Fert. 22, 327–333. [Google Scholar]
- Cordell D., Drangert J. O., White S. (2009). The story of phosphorus: global food security and food for thought. Glob. Environ. Change 19, 292–305. doi: 10.1016/j.gloenvcha.2008.10.009 [DOI] [Google Scholar]
- Datta A., Singh R. K., Tabassum S. (2015). Isolation, characterization and growth of rhizobium strains under optimum conditions for effective biofertilizer production. Int. J. Pharm. Sci. Rev. Res. 32, 199–208. [Google Scholar]
- Deaker R., Roughley R. J., Kennedy I. R. (2004). Legume seed inoculation technology—a review. Soil Biol. Biochem. 36, 1275–1288. doi: 10.1016/j.soilbio.2004.04.009 [DOI] [Google Scholar]
- Deepa S., Venkateswaran S. (2018). Appraisal of groundwater quality in upper Manimuktha sub basin, Vellar river, Tamil Nadu, India by using water quality index (WQI) and multivariate statistical techniques. Model. Earth Syst. Environ. 4, 1165–1180. doi: 10.1007/s40808-018-0468-3 [DOI] [Google Scholar]
- Dinesh R., Srinivasan V., Hamza S., Sarathambal C., Anke Gowda S. J., Ganeshamurthy A. N., et al. (2018). Isolation and characterization of potential Zn solubilizing bacteria from soil and its effects on soil Zn release rates, soil available Zn and plant Zn content. Geoderma 321, 173–186. doi: 10.1016/j.geoderma.2018.02.013 [DOI] [Google Scholar]
- Ding Y., Yi Z., Fang Y., He S., Li Y., He K., et al. (2021). Multi-Omics reveal the efficient phosphate-solubilizing mechanism of bacteria on rocky soil. Front. Microbiol. 12:761972. doi: 10.3389/fmicb.2021.761972, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Deen S. R. O., El-Azeem A., Samy A., Abd Elwahab A. F., Mabrouk S. S. (2020). Effects of phosphate solubilizing microorganisms on wheat yield and phosphatase activity. Egypt. J. Med. Microbiol. 55, 71–86. doi: 10.21608/ejm.2020.20675.1137 [DOI] [Google Scholar]
- El-Wakeil N. E., El-Sebai T. N. (2009). Role of biofertilizer on faba bean growth, yield, and its effect on bean aphid and the associated predators. Arch. Phytopathol. Plant Prot. 42, 1144–1153. doi: 10.1080/03235400701650882 [DOI] [Google Scholar]
- European Parliament and Council of the European Union (2016). Proposal for a regulation of the European Parliament and of the council laying down rules on the making available on the market of CE marked Fertilising products and amending regulations (ec) no 1069/2009 and (ec) no 1107/2009 (regulation no. COM(2016)157). Available at: https://www.eumonitor.eu/9353000/1/j9vvik7m1c3gyxp/vk2hefenu2z2 (Accessed July 3, 2022).
- Fages J. (1992). An industrial view of Azospirillum inoculants: formulation and application technology. Symbiosis 13, 15–26. [Google Scholar]
- Fortune Business Insights (2022). Biofertilizer market size, share & COVID-19 impact analysis, by type (nitrogen fixing, phosphate Solubilizers, others), by microorganism (rhizobium, Azotobacter, Azospirillum, pseudomonas, bacillus, VAM, others), by application (seed treatment, soil treatment, others), by crop type (cereals, Pulses & Oilseeds, Fruits & Vegetables, others), and regional forecast, 2022-2029. Available at: https://www.fortunebusinessinsights.com/industry-reports/biofertilizers-market-100413 (Accessed July 3, 2022).
- Gedamu S. A., Tsegaye E. A., Beyene T. F. (2021). Effect of rhizobial inoculants on yield and yield components of faba bean (Vicia fabae L.) on vertisol of Wereillu District, South Wollo. Ethiop. CABI Agric. Biosci. 2, 1–10. doi: 10.1186/s43170-021-00025-y [DOI] [Google Scholar]
- Gomare K., Mese M., Shetkar Y. (2013). Isolation of rhizobium and cost effective production of biofertilizer. Indian J. Life Sci. 2:49. [Google Scholar]
- Goteti P. K., Emmanuel L. D. A., Desai S., Shaik M. H. A. (2013). Prospective zinc Solubilising bacteria for enhanced nutrient uptake and growth promotion in maize (Zea mays L.). Int. J. Microbiol. 2013:869697. doi: 10.1155/2013/869697, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady E. N., MacDonald J., Liu L., Richman A., Yuan Z. C. (2016). Current knowledge and perspectives of Paenibacillus: a review. Microb. Cell Fact. 15, 1–18. doi: 10.1186/s12934-016-0603-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnabo A., van Heerwaarden J., Geurts R., Wolde-Meskel E., Degefu T., Giller K. (2020). Symbiotic interactions between chickpea (Cicer arietinum L.) genotypes and Mesorhizobium strains. Symbiosis 82, 235–248. doi: 10.1007/s13199-020-00724-6 [DOI] [Google Scholar]
- Haerani N., Syam’Un E., Rasyid B., Haring F. (2021). Isolation and characterization of N-fixing and IAA producing rhizobacteria from two rice field agro-ecosystems in South Sulawesi. Indonesia Biodivers J. Biol. Divers. 22, 2497–2503. doi: 10.13057/biodiv/d220506 [DOI] [Google Scholar]
- Hara S., Takashi M., Sawa W., Yasuhiro K., Taichi K., Kiyoshi Y., et al. (2020). Identification of nitrogen-fixing Bradyrhizobium associated with roots of field-grown sorghum by Metagenome and proteome. Front. Microbiol. 10:47. doi: 10.3389/fmicb.2019.00407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herridge D. F., Peoples M. B., Boddey R. M. (2008). Global inputs of biological nitrogen fixation in agricultural systems. Plant Soil 311, 1–18. doi: 10.1007/s11104-008-9668-3 [DOI] [Google Scholar]
- Herrmann L., Lesueur D. (2013). Challenges of formulation and quality of biofertilizers for successful inoculation. Appl. Microbiol. Biotechnol. 97, 8859–8873. doi: 10.1007/s00253-013-5228-8, PMID: [DOI] [PubMed] [Google Scholar]
- Htwe A. Z., Moh S. M., Soe K. M., Moe K., Yamakawa T. (2019). Effects of biofertilizer produced from Bradyrhizobium and Streptomyces griseoflavus on plant growth, nodulation, nitrogen fixation, nutrient uptake, and seed yield of Mung bean, cowpea, and soybean. Agronomy 9:77. doi: 10.3390/agronomy9020077 [DOI] [Google Scholar]
- Huda S., Sujauddin M., Shafinat S., Uddin M. (2007). Effects of phosphorus and potassium addition on growth and nodulation of Dalbergia sissoo in the nursery. J. Forest Res. 18, 279–282. doi: 10.1007/s11676-007-0056-2 [DOI] [Google Scholar]
- Hungria M., Franchini J. C., Campo R. J., Crispino C. C., Moraes J. Z., Sibaldelli R. N., et al. (2006). Nitrogen nutrition of soybean in Brazil: contributions of biological N2 fixation and N fertilizer to grain yield. Can. J. Plant Sci. 86, 927–939. doi: 10.4141/P05-098 [DOI] [Google Scholar]
- Hussain A., Arshad M., Zahir Z. A., Asghar M. (2015). Prospects of zinc solubilizing bacteria for enhancing growth of maize. Pak. J. Agric. Sci. 52, 915–922. [Google Scholar]
- Ibarra-Galeana J. A., Castro-Martínez C., Fierro-Coronado R. A., Armenta-Bojórquez A. D., Maldonado-Mendoza I. E. (2017). Characterization of phosphate-solubilizing bacteria exhibiting the potential for growth promotion and phosphorus nutrition improvement in maize (Zea mays L.) in calcareous soils of Sinaloa. Mexico. Ann. Microbiol. 67, 801–811. doi: 10.1007/s13213-017-1308-9 [DOI] [Google Scholar]
- Imran M., Shahzad S. M., Arif M. S., Yasmeen T., Ali B., Tanveer A. (2020). Inoculation of potassium solubilizing bacteria with different potassium fertilization sources mediates maize growth and productivity. Pak. J. Agric. Sci. 57, 1045–1055. [Google Scholar]
- Jain A., Singh H. B., Das S. (2021). Deciphering plant-microbe crosstalk through proteomics studies. Microbiol. Res. 242:126590. doi: 10.1016/j.micres.2020.126590, PMID: [DOI] [PubMed] [Google Scholar]
- Jayakumar V., Sundar A. R., Viswanathan R. (2021). Biocontrol of Colletotrichum falcatum with volatile metabolites produced by endophytic bacteria and profiling VOCs by headspace SPME coupled with GC–MS. Sugar Tech 23, 94–107. doi: 10.1007/s12355-020-00891-2 [DOI] [Google Scholar]
- Joshi D., Negi G., Vaid S., Sharma A. (2013). Enhancement of wheat growth and Zn content in grains by zinc solubilizing bacteria. Int. J. Environ. Agric. Biotechnol. 6, 363–370. doi: 10.5958/j.2230-732X.6.3.004 [DOI] [Google Scholar]
- Kahrl F., Li Y., Su Y., Tennigkeit T., Wilkes A., Xu J. (2010). Greenhouse gas emissions from nitrogen fertilizer use in China. Environ. Sci. Pol. 13, 688–694. doi: 10.1016/j.envsci.2010.07.006 [DOI] [Google Scholar]
- Kalimuthu R., Suresh P., Varatharaju G., Balasubramanian N., Rajasekaran K. M., Shanmugaiah V. (2019). Isolation and characterization of Indole acetic acid [IAA] producing tomato Rhizobacterium pseudomonas sp VSMKU4050 and its potential for plant growth promotion. Int. J. Curr. Microbiol. Appl. Sci. 8, 443–455. doi: 10.20546/ijcmas.2019.806.050 [DOI] [Google Scholar]
- Kamran S., Shahid I., Baig D. N., Rizwan M., Malik K. A., Mehnaz S. (2017). Contribution of zinc solubilizing bacteria in growth promotion and zinc content of wheat. Front. Microbiol. 8:2593. doi: 10.3389/fmicb.2017.02593, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S.-M., Khan A. L., Waqas M., Asaf S., Lee K.-E., Park Y.-G., et al. (2019). Integrated phytohormone production by the plant growth-promoting rhizobacterium bacillus tequilensis SSB07 induced thermotolerance in soybean. J. Plant Interact. 14, 416–423. doi: 10.1080/17429145.2019.1640294 [DOI] [Google Scholar]
- Kaul S., Sharma T., Dhar K. (2016). “Omics” tools for better understanding the plant–endophyte interactions. Front. Plant Sci. 7:955. doi: 10.3389/fpls.2016.00955, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketema P., Tefera T. (2022). Effectiveness of rhizobium strains on faba bean (Vicia fabae L.) at Gumer district, highland area of Southern Ethiopia. Ukraine J. Ecol. 12, 13–18. [Google Scholar]
- Khan N., Bano A., Ali S., Babar M. (2020). Crosstalk amongst phytohormones from planta and PGPR under biotic and abiotic stresses. Plant Growth Regul. 90, 189–203. doi: 10.1007/s10725-020-00571-x [DOI] [Google Scholar]
- Khan S., Chattopadhyay N. (2009). Effect of inorganic and biofertilizers on chilli. J. Crop Weed 5, 191–196. [Google Scholar]
- Kloepper J. W., Leong J., Teintze M., Schroth M. N. (1980). Enhanced plant growth by siderophores produced by plant growth-promoting rhizobacteria. Nature 286, 885–886. doi: 10.1038/286885a0 [DOI] [Google Scholar]
- Koskey G., Mburu S. W., Njeru E. M., Kimiti J. M., Ombori O., Maingi J. M. (2017). Potential of native rhizobia in enhancing nitrogen fixation and yields of climbing beans (Phaseolus vulgaris L.) in contrasting environments of eastern Kenya. Front. Plant Sci. 8:443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Bauddh K., Barman S., Singh R. P. (2014). Amendments of microbial biofertilizers and organic substances reduces requirement of urea and DAP with enhanced nutrient availability and productivity of wheat (Triticum aestivum L.). Ecol. Eng. 71, 432–437. doi: 10.1016/j.ecoleng.2014.07.007 [DOI] [Google Scholar]
- Laxita L., Shruti S. (2020). Isolation and characterization of potassium solubilizing microorganisms from South Gujarat region and their effects on wheat plant. Mukta Shabad 9, 7483–7496. [Google Scholar]
- Lesueur D., Deaker R., Herrmann L., Bräu L., Jansa J. (2016). “The production and potential of biofertilizers to improve crop yields” in Bioformulations: For Sustainable Agriculture. eds. Arora N. K., Mehnaz S., Balestrini R. (New Delhi: Springer; ), 71–92. doi: 10.1007/978-81-322-2779-3_4 [DOI] [Google Scholar]
- López-Mondéjar R., Kostovčík M., Lladó S., Carro L., García-Fraile P. (2017). “Exploring the plant microbiome through multi-omics approaches” in Probiotics in Agroecosystem. eds. Kumar V., Kumar M., Sharma S., Prasad R. (Singapore: Springer; ), 233–268. [Google Scholar]
- Mahmoody M., Noori M. (2014). Effect of gibberellic acid on growth and development plants and its relationship with abiotic stress. Int. J. Farming Allied Sci. 3, 717–721. [Google Scholar]
- Malusá E., Sas-Paszt L., Ciesielska J. (2012). Technologies for beneficial microorganisms inocula used as biofertilizers. Sci. World J. 2012:491206, 1–12. doi: 10.1100/2012/491206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mapanda F., Wuta M., Nyamangara J., Rees R. M. (2011). Effects of organic and mineral fertilizer nitrogen on greenhouse gas emissions and plant-captured carbon under maize cropping in Zimbabwe. Plant Soil 343, 67–81. doi: 10.1007/s11104-011-0753-7 [DOI] [Google Scholar]
- Markets and Markets (2019). Biofertilizer market by form (liquid, carrier-based), mode of application (soil treatment, seed treatment), crop type, type (nitrogen-fixing, phosphates solubilizing and mobilizing, potash solubilizing and mobilizing), region-global forecast to 2025, 2019. Available at: https://www.marketsandmarkets.com/Market-Reports/compound-biofertilizers-customized-fertilizers-market-856.html (Accessed September 1, 2020).
- Markets and Markets (2020). Markets and markets, biofertilizer market by form (liquid, carrier-based), mode of application (soil treatment, seed treatment), crop type, type (nitrogen-fixing, phosphates solubilizing and mobilizing, potash solubilizing and mobilizing), region-global forecast to 2026. Available at: https://www.researchandmarkets.com/reports/5334050/global-biofertilizers-market-by-form-liquid (Accessed May17, 2022).
- Markets and Markets (2014). Global biofertilizer markets by types, application and geography-trends and forecast. Available at: MarketResearch.com (Accessed July 3, 2022).
- Matich T. (2016). Potash investing. What is potash. Investing news network. Available at: https://investingnews.com/daily/resource-investing/agriculture-investing/potash-investing/what-is-potash/ (Accessed July11, 2022).
- Mazid M., Khan T. A. (2015). Future of bio-fertilizers in Indian agriculture: an overview. Int. J. Agric. Res. 3, 10–23. [Google Scholar]
- Meena V. S., Maurya B. R., Meena S. K., Mishra P. K., Bisht J. K., Pattanayak A. (2018). Potassium solubilization: Strategies to mitigate potassium deficiency in agricultural soils. Glob. J. Biol. Agric. Health Sci. 7, 1–3. doi: 10.24105/2319-5584.100003 [DOI] [Google Scholar]
- Melchiorre M., De Luca M. J., Gonzalez Anta G., Suarez P., Lopez C., Lascano R., et al. (2011). Evaluation of Bradyrhizobia strains isolated from field-grown soybean plants in Argentina as improved inoculants. Biol. Fertil. Soils 47, 81–89. doi: 10.1007/s00374-010-0503-7 [DOI] [Google Scholar]
- Melo A., Pinto E., Aguiar A., Mansilha C., Pinho O., Ferreira I. M. P. L. V. O. (2012). Impact of intensive horticulture practices on groundwater content of nitrates, sodium, potassium, and pesticides. Environ. Monit. Assess. 184, 4539–4551. doi: 10.1007/s10661-011-2283-4, PMID: [DOI] [PubMed] [Google Scholar]
- Mishra J., Arora N. K. (2016). “Bioformulations for plant growth promotion and combating phytopathogens: a sustainable approach” in Bioformulations: For sustainable agriculture. eds. Arora N., Mehnaz S., Balestrini R. (New Delhi: Springer; ), 3–33. [Google Scholar]
- Mohammadi K., Sohrabi Y. (2012). Bacterial biofertilizers for sustainable crop production: a review. ARPN J. Agric. Biol. Sci. 7, 307–316. [Google Scholar]
- Montalvo D., Degryse F., da Silva R. C., Baird R., McLaughlin M. J. (2016). “Agronomic effectiveness of zinc sources as micronutrient fertilizer” in Advances in Agronomy. ed. Sparks D. L. (Cambridge, MA: Academic Press; ) [Google Scholar]
- Mustafa S., Kabir S., Shabbir U., Batool R. (2019). Plant growth promoting rhizobacteria in sustainable agriculture: from theoretical to pragmatic approach. Symbiosis 78, 115–123. doi: 10.1007/s13199-019-00602-w [DOI] [Google Scholar]
- Nair S. S., Brahmaprakash G. (2017). Development and standardization of effervescent biofertilizer consortial tablets for french bean (Phaseolus vulgaris L.). Mysore J. Agric. Sci. 51, 373–384. [Google Scholar]
- Nakkreen S., Fernando D. W. G., Siddiqui Z. A. (2005). “Plant growth promoting rhizobacteria formulations and its scope in commercialization for the management of pests and diseases” in Biocontrol and biofertilization. ed. Siddiqui Z. A. (Dordrecht: Springer; ), 257–296. [Google Scholar]
- Naz I., Ahmad H., Khokhar S. N., Khan K., Shah A. H. (2016). Impact of zinc solubilizing bacteria on zinc contents of wheat. Am. Euras. J. Agric. Environ. Sci. 16, 449–454. [Google Scholar]
- Neog R. (2018). Assessing the impact of chemical fertilizers on soil acidification: a study on Jorhat district of Assam. India. Agric. Sci. Digest 38, 270–274. doi: 10.18805/ag.D-4220 [DOI] [Google Scholar]
- Ngakou A., Megueni C., Ousseni H., Massai A. (2009). Study on the isolation and characterization of rhizobia strains as biofertilizer tools for growth improvement of four grain legumes in Ngaoundéré-Cameroon. Int. J. Biol. Chem. 3, 1078–1089. doi: 10.4314/ijbcs.v3i5.51086 [DOI] [Google Scholar]
- Nobbe F., Hiltner L. (1986). Inoculation of the soil for cultivating leguminous plants. U.S. patent no. 570:813. Washington, DC: U.S. Patent and Trademark Office. [Google Scholar]
- Odoh C. K., Eze C. N., Akpi U. K., Unah V. U. (2019). Plant growth promoting rhizobacteria (PGPR): a novel agent for sustainable food production. Am. J. Agric. Biol. Sci. 14, 35–54. doi: 10.3844/ajabssp.2019.35.54 [DOI] [Google Scholar]
- Paliya S., Mandpe A., Kumar S., Kumar M. S. (2019). Enhanced nodulation and higher germination using sludge ash as a carrier for biofertilizer production. J. Environ. Manag. 250:109523. doi: 10.1016/j.jenvman.2019.109523, PMID: [DOI] [PubMed] [Google Scholar]
- Paudyal S. P., Gupta V. (2018). Substitution of chemical fertilizer nitrogen through rhizobium inoculation technology. Our Nat. 16, 43–47. doi: 10.3126/on.v16i1.22121 [DOI] [Google Scholar]
- Perumal M. D., Selvi D., Chitdeshwari T., Balachandar D. (2019). Enhanced zinc nutrient and enzyme activity of rice crop by zinc solubilizing bacteria with Zn sources in Zn deficient Rice soil. Madras Agric. J. 106, 171–177. doi: 10.29321/MAJ.2019.000242 [DOI] [Google Scholar]
- Phillips P. W. (2004). An economic assessment of the global inoculant industry. Crop Manage. 3, 1–10. doi: 10.1094/CM-2004-0301-08-RV [DOI] [Google Scholar]
- Raimi A. (2018). Quality assessment of commercial Biofertilisers and the awareness of smallholder farmers in Gauteng province, South Africa. Dissertation/master's thesis. Gauteng (South Africa): University of South Africa.
- Raimi A., Roopnarain A., Adeleke R. (2021). Biofertilizer production in Africa: current status, factors impeding adoption and strategies for success. Sci. Afr. 11:e00694. doi: 10.1016/j.sciaf.2021.e00694 [DOI] [Google Scholar]
- Raji M., Thangavelu M. (2021). Isolation and screening of potassium solubilizing bacteria from saxicolous habitat and their impact on tomato growth in different soil types. Arch. Microbiol. 203, 3147–3161. doi: 10.1007/s00203-021-02284-9, PMID: [DOI] [PubMed] [Google Scholar]
- Ramesh A., Sharma S. K., Sharma M. P., Yadav N., Joshi O. P. (2014). Inoculation of zinc solubilizing bacillus aryabhattai strains for improved growth, mobilization and biofortification of zinc in soybean and wheat cultivated in Vertisols of Central India. Appl. Soil Ecol. 73, 87–96. doi: 10.1016/j.apsoil.2013.08.009 [DOI] [Google Scholar]
- Ramoneda J., Roux J. J. L., Frossard E., Frey B., Gamper H. A. (2020). Geographical patterns of root nodule bacterial diversity in cultivated and wild populations of a woody legume crop. FEMS Microbiol. Ecol. 96:fiaa145. doi: 10.1093/femsec/fiaa145, PMID: [DOI] [PubMed] [Google Scholar]
- Reyes-Castillo A., Gerding M., Oyarzúa P., Zagal E., Gerding J., Fischer S. (2019). Plant growth-promoting rhizobacteria able to improve NPK availability: selection, identification and effects on tomato growth. Chil. J. Agric. Res. 79, 473–485. doi: 10.4067/S0718-58392019000300473 [DOI] [Google Scholar]
- Riah N., de Lajudie P., Béna G., Heulin K., Djekoun A. (2021). Variability in symbiotic efficiency with respect to the growth of pea and lentil inoculated with various rhizobial genotypes originating from sub-humid and semi-arid regions of eastern Algeria. Symbiosis 85, 371–384. doi: 10.1007/s13199-021-00821-0 [DOI] [Google Scholar]
- Riaz U., Mehdi S. M., Iqbal S., Khalid H. I., Qadir A. A., Anum W., et al. (2020). “Bio-fertilizers: eco-friendly approach for plant and soil environment” in Bioremediation and Biotechnology. eds. Hakeem K., Bhat R., Qadri H. (Cham: Springer; ), 189–213. [Google Scholar]
- Rizvi A., Ahmed B., Khan M. S., El-Beltagi H. S., Umar S., Lee J. (2022a). Bioprospecting plant growth promoting rhizobacteria for enhancing the biological properties and phytochemical composition of medicinally important crops. Molecules 27:1407. doi: 10.3390/molecules27041407, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizvi A., Ahmed B., Khan B., Rajput V. D., Umar S., Minkina T., et al. (2022b). Maize associated bacterial microbiome linked mitigation of heavy metal stress: a multidimensional detoxification approach. Environ. Exp. Bot. 200:104911. doi: 10.1016/j.envexpbot.2022.104911 [DOI] [Google Scholar]
- Rizvi A., Ahmed B., Khan M. S., Umar S., Lee J. (2021a). Sorghum-phosphate solubilizers interactions: crop nutrition, biotic stress alleviation, and yield optimization. Front. Plant Sci. 12:746780. doi: 10.3389/fpls.2021.746780, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizvi A., Ahmed B., Khan M. S., Umar S., Lee J. (2021b). Psychrophilic bacterial phosphate-biofertilizers: a novel extremophile for sustainable crop production under cold environment. Microorganisms 9:2451. doi: 10.3390/microorganisms9122451, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizvi A., Ahmed B., Zaidi A., Khan M. (2019). Bioreduction of toxicity influenced by bioactive molecules secreted under metal stress by Azotobacter chroococcum. Ecotoxicology 28, 302–322. doi: 10.1007/s10646-019-02023-3, PMID: [DOI] [PubMed] [Google Scholar]
- Rizvi A., Zaidi A., Ameen F., Ahmed B., AlKahtani M. D., Khan M. S. (2020). Heavy metal induced stress on wheat: phytotoxicity and microbiological management. RSC Adv. 10, 38379–38403. doi: 10.1039/D0RA05610C, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizvi A., Zaidi A., Khan M., Saif S., Ahmed B., Shahid M. (2017). Growth Improvement and Management of Vegetable Diseases by Plant Growth-promoting Rhizobacteria in Microbial Strategies for Vegetable Production, (Cham, Springer; ) [Google Scholar]
- Saber Z., Pirdashti H., Esmaeili M., Abbasian A., Heidarzadeh A. (2012). Response of wheat growth parameters to co-inoculation of plant growth promoting rhizobacteria (PGPR) and different levels of inorganic nitrogen and phosphorus. World Appl. Sci. J. 16, 213–219. [Google Scholar]
- Sahu P. K., Brahmaprakash G. P. (2016). “Formulations of biofertilizers – approaches and advances” in Microbial Inoculants in Sustainable Agricultural Productivity: Functional Applications. eds. Singh D. P., Singh H. B., Prabha R. (New Delhi: Springer; ), 179–198. [Google Scholar]
- Santhi C., Rajesh M., Ramesh S., Muralikrishna K., Gangaraj K., Payal G. (2021). Genome-wide exploration of auxin response factors (ARFs) and their expression dynamics in response to abiotic stresses and growth regulators in coconut (Cocos nucifera L.). plant. Gene 28:100344. doi: 10.1016/j.plgene.2021.100344 [DOI] [Google Scholar]
- Sanyal D., Osorno J. M., Chatterjee A. (2020). Influence of rhizobium inoculation on dry bean yield and symbiotic nitrogen fixation potential. J. Plant Nutr. 43, 798–810. doi: 10.1080/01904167.2020.1711946 [DOI] [Google Scholar]
- Sattar A., Naveed M., Ali M., Zahir Z. A., Nadeem S. M., Yaseen M., et al. (2019). Perspectives of potassium solubilizing microbes in sustainable food production system: a review. Appl. Soil Ecol. 133, 146–159. doi: 10.1016/j.apsoil.2018.09.012 [DOI] [Google Scholar]
- Sekar J., Raj R., Prabavathy V. (2016). “Microbial consortial products for sustainable agriculture: commercialization and regulatory issues in India” in Agriculturally Important Microorganisms. eds. Singh H., Sarma B., Keswani C. (Singapore: Springer; ), 107–132. [Google Scholar]
- Shahid M., Ameen F., Maheshwari H. S., Ahmed B., AlNadhari S., Khan M. S. (2021). Colonization of Vigna radiata by a halotolerant bacterium Kosakonia sacchari improves the ionic balance, stressor metabolites, antioxidant status and yield under NaCl stress. Appl. Soil Ecol. 158:103809. doi: 10.1016/j.apsoil.2020.103809 [DOI] [Google Scholar]
- Sharma S. K. M. P., Ramesh A., Joshi O. P. (2012). Characterization of zinc-solubilizing bacillus isolates and their potential to influence zinc assimilation in soybean seeds. J. Microbiol. Biotechnol. 22, 352–359. doi: 10.4014/jmb.1106.05063, PMID: [DOI] [PubMed] [Google Scholar]
- Shurtleff W., Aoyagi A. (2018). History of Research on Nitrogen Fixation in Soybeans (1887–2018). Lafayette, CA: Soyinfo Center. [Google Scholar]
- Simarmata T., Turmuktini T., Fitriatin B. N., Setiawati M. R. (2016). Application of bioameliorant and biofertilizers to increase the soil health and rice productivity. HAYATI J. Biosci. 23, 181–184. doi: 10.1016/j.hjb.2017.01.001 [DOI] [Google Scholar]
- Singh J., Singh A. V., Upadhayay V. K., Khan A. (2020). Comparative evaluation of developed carrier based bio-formulations bearing multifarious PGP properties and their effect on shelf life under different storage conditions. Environ. Ecol. 38, 96–103. [Google Scholar]
- Soumare A., Boubekri K., Lyamlouli K., Hafidi M., Ouhdouch Y., Kouisni L. (2020). From isolation of phosphate solubilizing microbes to their formulation and use as biofertilizers: status and needs. Front. Bioeng. Biotechnol. 7:425. doi: 10.3389/fbioe.2019.00425, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tairo E. V., Ndakidemi P. A. (2014). Macronutrients uptake in soybean as affected by Bradyrhizobium japonicum inoculation and phosphorus (P) supplements. Am. J. Plant Sci. 5, 488–496. doi: 10.4236/ajps.2014.54063 [DOI] [Google Scholar]
- Tan K., Radziah O., Halimi M., Khairuddin A., Shamsuddin Z. (2015). Assessment of plant growth-promoting rhizobacteria (PGPR) and rhizobia as multi-strain biofertilizer on growth and N2 fixation of rice plant. Aust. J. Crop. Sci. 9, 1257–1264. [Google Scholar]
- Teotia P., Kumar V., Kumar M., Shrivastava N., Varma A. (2016). “Rhizosphere microbes: potassium solubilization and crop productivity–present and future aspects” in Potassium Solubilizing Microorganisms for Sustainable Agriculture. eds. Meena V., Maurya B., Verma J., Meena R. (New Delhi: Springer; ), 315–325. doi: 10.1007/978-81-322-2776-2_22 [DOI] [Google Scholar]
- Thies J., Woomer P., Singleton P. (1995). Enrichment of Bradyrhizobium spp populations in soil due to cropping of the homologous host legume. Soil Biol. Biochem. 27, 633–636. doi: 10.1016/0038-0717(95)98643-3 [DOI] [Google Scholar]
- Tomer S., Suyal D. C., Goel R. (2016). “biofertilizers: a timely approach for sustainable agriculture” in Plant-microbe Interaction: an Approach to Sustainable Agriculture. ed. Choudhary D. K. (Singapore: Springer; ) [Google Scholar]
- Transparency Market Research (2022). Biofertilizers market (product: nitrogen fixing, phosphate mobilizing, and potassium mobilizing; and application: Cereals & Grains, Fruits & Vegetables, oil Seeds & Pulses, and others [nursery and turf]) – global industry analysis, size, share, growth, trends, and forecast, 2021–2031. Available at: https://www.transparencymarketresearch.com/pressrelease/globalbiofertilizersmarket.htm (Accessed July 11, 2022).
- United Nations (2015). Department of Economic and Social Affairs, Population Division. World population prospects: the 2015 revision, key findings and advance tables (ESA/P/WP.241; p. 66). United Nations. Available at: https://population.un.org/wpp/publications/files/key_findings_wpp_2015.pdf (Accessed July 13, 2022).
- Unkovich M., Baldock J. (2008). Measurement of asymbiotic N2 fixation in Australian agriculture. Soil Biol. Biochem. 40, 2915–2921. doi: 10.1016/j.soilbio.2008.08.021 [DOI] [Google Scholar]
- Vaishnav A., Kumar R., Singh H. B., Sarma B. K. (2022). Extending the benefits of PGPR to bioremediation of nitrile pollution in crop lands for enhancing crop productivity. Sci. Total Environ. 826:154170. doi: 10.1016/j.scitotenv.2022.154170, PMID: [DOI] [PubMed] [Google Scholar]
- Vaishnav A., Singh H. B. (2021). New and Future Developments in Microbial Biotechnology and Bioengineering. Amsterdam: Elsevier. [Google Scholar]
- Vassilev N., Vassilev M., Lopez A., Martos V., Reyes A., Maksimovic I., et al. (2015). Unexploited potential of some biotechnological techniques for biofertilizer production and formulation. Appl. Microbiol. Biotechnol. 99, 4983–4996. doi: 10.1007/s00253-015-6656-4, PMID: [DOI] [PubMed] [Google Scholar]
- Vatsyayan N., Ghosh A. K. (2013). Isolation and characterization of microbes with biofertilizer potential. J. Environ. Sci. 7, 5–9. [Google Scholar]
- Verma M., Singh A., Dwivedi D. H., Arora N. K. (2020). Zinc and phosphate solubilizing rhizobium radiobacter (LB2) for enhancing quality and yield of loose leaf lettuce in saline soil. Environ. Sustain. 3, 209–218. doi: 10.1007/s42398-020-00110-4 [DOI] [Google Scholar]
- Vessey J. K. (2003). Plant growth promoting rhizobacteria as biofertilizers. Plant Soil 255, 571–586. doi: 10.1023/A:1026037216893 [DOI] [Google Scholar]
- Vintage Market Research (2022). Global biofertilizers market to witness a CAGR growth of 10.9% market is projected to worth of USD 4448.97 million by 2028 key players change the view of the global face of industry. Available at: https://www.globenewswire.com/news-release/2022/04/01/2414570/0/en/Global-Biofertilizers-Market-to-witness-a-CAGR-Growth-of-10-9-Market-is-Projected-to-Worth-of-USD-4448-97-Million-by-2028-Key-Players-Change-the-View-of-the-Global-Face-of-Industry.html (Accessed July 3, 2022).
- Wang Y., Zhang G., Huang Y., Guo M., Song J., Zhang T., et al. (2022). A potential biofertilizer-Siderophilic bacteria isolated from the Rhizosphere of Paris polyphylla var. yunnanensis. Front. Microbiol. 13:870413. doi: 10.3389/fmicb.2022.870413, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wani S., Rupela O., Lee K. (1995). “Sustainable agriculture in the semi-arid tropics through biological nitrogen fixation in grain legumes” in Management of Biological Nitrogen Fixation for the Development of more Productive and Sustainable Agricultural Systems. eds. Ladha J. K., Peoples M. B. (Singapore: Dordrecht; ) [Google Scholar]
- Yamazaki S., Mardani-Korrani H., Kaida R., Ochiai K., Kobayashi M., Nagano A. J., et al. (2021). Field multi-omics analysis reveals a close association between bacterial communities and mineral properties in the soybean rhizosphere. Sci. Rep. 11, 1–16. doi: 10.1038/s41598-021-87384-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan P., Wu L., Wang D., Fu J., Shen C., Li X., et al. (2020). Soil acidification in Chinese tea plantations. Sci. Total Environ. 715:136963. doi: 10.1016/j.scitotenv.2020.136963, PMID: [DOI] [PubMed] [Google Scholar]
- Yehuda Z., Shenker M., Romheld V., Marschner H., Hadar Y., Chen Y. (1996). The role of ligand exchange in the uptake of iron from microbial siderophores by gramineous plants. Plant Physiol. 112, 1273–1280. doi: 10.1104/pp.112.3.1273, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidi A., Khan M. S., Rizvi A., Saif S., Ahmad B., Shahid M. (2017). “Role of phosphate-solubilizing bacteria in legume improvement” in Microbes for Legume Improvement. eds. Zaidi A., Khan M. S. (Musarrat: Springer International Publishing; ), 175–197. [Google Scholar]
- Zhang Q., Wang H., Wang L. (2018). Tracing nitrate pollution sources and transformations in the over-exploited groundwater region of North China using stable isotopes. J. Contam. Hydrol. 218, 1–9. doi: 10.1016/j.jconhyd.2018.06.001, PMID: [DOI] [PubMed] [Google Scholar]