Abstract
Intervertebral disc extrusion associated with extensive epidural hemorrhage (DEEH) is a well-documented pathological condition in veterinary medicine. This retrospective study aimed to evaluate the prevalence and clinical features of DEEH in a population of French Bulldogs affected by intervertebral disc extrusion (n=75), compare the findings with those from a group of Dachshunds (n=98) and identify possible predictive factors of DEEH and outcomes in surgically treated patients. The study showed that the prevalence of DEEH observed in Dachshunds (11.2% [95% confidence interval [CI]: 5.7–19.2%]) was significantly lower than that observed in French Bulldogs (41.3% [95% CI: 30.1–53.3%]). The multiple logistic regression model highlighted that the patients presenting with an acute onset of clinical signs (>24 hr) (odds ratio [OR]: 13.08; 95% CI: 4.63–37.03, P=0.00), presence of clinical signs progression (OR: 5.04; P=0.01), and French Bulldogs (OR: 5.15; 95% CI: 1.71–15.54, P=0.00) were at increased risk of developing DEEH. Secondary analysis showed that patients with DEEH were at an increased risk of being non-ambulatory at discharge (OR: 3.43; P=0.017). Overall, the surgically treated patients had favorable outcomes.
Keywords: Dachshund, extensive epidural hemorrhage, French Bulldog, intervertebral disc extrusion
Intervertebral disc extrusion associated with extensive epidural hemorrhage (DEEH) is a pathological condition well documented in veterinary medicine [9, 11, 20, 25,26,27,28]. Hemorrhage at the time of intervertebral disc extrusion is probably caused by a rupture of the ventral internal vertebral venous plexus, which lies over the intervertebral disc space on the floor of the vertebral canal and is covered by epidural fat [7]. It is hypothesized that rupture of the venous plexuses associated with disc extrusion can lead to dispersion of disc material and blood along with several vertebral segments and, consequently, cause massive and extensive spinal cord compression. Hemorrhage and disc material dispersion worsens spinal cord compression and enhances spinal cord injury [27]. The clinical features, imaging findings, and outcomes have been described in dogs. In particular, dogs with DEEH showed rapid progression towards severe neurological dysfunction, and the prognosis after surgery did not appear to be different from that of dogs without epidural hemorrhage associated with intervertebral disc extrusion. However, a large number of cases required extensive hemilaminectomy involving all the compressed spinal segments [17, 26]. Recently, it has been demonstrated that paraplegic medium-to large-breed dogs with DEEH have a less favorable outcome after surgical decompression than paraplegic dogs with intervertebral disc extrusion [28].
To the best of our knowledge, the prevalence, clinical features, and outcome of DEEH have not been evaluated in chondrodystrophic breeds in veterinary medicine: French Bulldogs and Dachshunds are predisposed to developing intervertebral disc extrusion and have a high allele frequency for 12-FGF4 retrogene, implicated in intervertebral disc degeneration [4, 22]. French Bulldogs are also demonstrated to be likely to developing intervertebral disc extrusion associated with epidural hemorrhage (prevalence of 66%) [9]. We hypothesized that the prevalence of DEEH is higher in French Bulldogs compared to Dachshunds. We tried to evaluate the clinical aspects of DEEH compared to intervertebral disc extrusions without extensive epidural hemorrhage; we also hypothesized that the overall outcome in surgically treated patients could be favourable, despite the demanding surgical technique and despite the severity of neurological signs.
MATERIALS AND METHODS
The study included a population of French Bulldogs admitted to the Valdinievole Veterinary Clinic (2009–2018) and a group of Dachshunds admitted to the same institution (2016–2018). The medical and surgical records of both groups were retrospectively reviewed. Dogs diagnosed with intervertebral disc extrusion involving the thoracolumbar and lumbosacral regions using magnetic resonance imaging (MRI) and who underwent surgical decompression were considered for inclusion. The inclusion criteria were as follows: signalment, history, neurological examination, complete blood analysis, MRI features, surgical procedures and findings, ambulatory status at discharge, and follow-up of at least 3 months.
History and neurological examination
The rate of onset of clinical signs was considered hyperacute (within 24 hr) and acute (>24 hr) [17]. The progression of clinical signs (deterioration of at least one neurological grade since the initial presentation) was also considered. The neurological grade was defined as grade I if the only clinical sign was hyperesthesia on spinal palpation without neurological deficits. In addition, ambulatory paraparesis with delayed limb postural reactions (patients can rise and take steps without assistance) was classified as grade II, non-ambulatory paraparesis (patients are not able to rise and take steps unassisted) as grade III, paraplegia with intact deep pain perception as grade IV, and paraplegia with loss of deep pain perception as grade V [24]. Neurological assessment was performed by a board-certified neurologist (M. B.) or neurology resident (F. P.).
The time from initial presentation to the MRI examination and subsequent surgery was also considered.
All dogs required an MRI of the thoracolumbar spine (Esaote VetMR Grande 0.25 T, Genoa, Italy). T2 weighted (slice thickness of 3–5 mm, repetition time (TR) 2,680–5,300 msec, echo time (TE) 90–120 msec), and T1 weighted (T1W) (slice thickness 4–5 mm, TR 450–630 msec, TE 14–30 msec) sequences were acquired in the sagittal and transverse planes before intravenous administration of 0.2 mg/kg gadoteric acid (Dotarem, Guerbet Laboratories, Villepinte, FR). Sagittal and transverse T1W sequences were acquired after contrast administration. Patients were included if MRI and surgery confirmed the presence of intervertebral disc extrusion associated with or without hemorrhage. The MRI criteria to define hemorrhage were predominantly T1W hyperintense signals of the epidural material (subacute stage of the hemorrhage) mixed with the extruded disc material. GET2* sequences were not considered relevant because of the low-field MRI. The complete obliteration of the subarachnoid space and spinal cord distortion on T2 sagittal and transverse images, with >50% of the vertebral canal occupied by the epidural material, was considered significant for spinal cord compression on MRI and confirmed in surgical records. Sagittal and transverse spinal cord compressions were evaluated for every slice in MRI studies. Degree of spinal cord damage (expressed as ratio of the T2W hyperintense endomedullary signal length to the L2 vertebral body length) (T2SCH) was also evaluated. All MRI studies were evaluated by a board-certified neurologist (M. B.) and a neurology resident (F. P.) (Fig. 1).
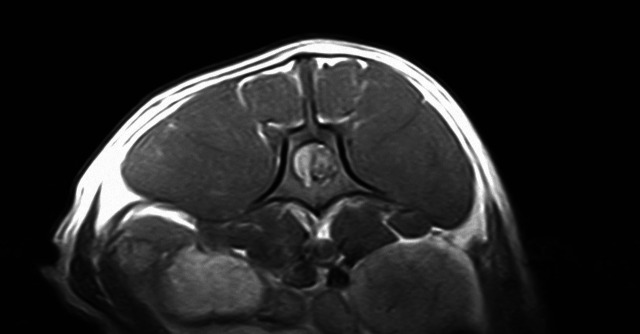
Fig. 1.
Transverse T1W magnetic resonance image of a L1–L2 intervertebral disc extrusion associated with epidural hemorrhage in a French Bulldog. The image is at the level of L4 vertebral body showing spinal cord compression on the right side due to disc material mixed with hemorrhage.
Surgical treatment and follow up
Immediately after the MRI procedure, the dogs underwent surgical decompression via hemi- or mini-hemilaminectomy [14] (and one dorsal laminectomy case at the L7–S1 level) based on the imaging findings. The majority of cases were treated using the least invasive technique, mini-hemilaminectomy. In a few selected cases, a hemilaminectomy was performed based on the surgeon’s choice (the main criterion for choosing hemilaminectomy instead of minihemilaminectomy was the dorsal positon of the extruded disc material and/or hemorrhage, which was considered better addressed removing the vertebral articular processes). All surgical procedures were performed by a board-certified neurologist and experienced neurosurgeon (M. B.). The presence of epidural hemorrhage was confirmed macroscopically during surgery. Hemorrhage was considered secondary to intervertebral disc extrusion only if the presence of epidural material represented by extruded nucleus pulposus mixed with clots and blood was ascertained. Decompression involved all vertebral segments with evidence of spinal cord compression on MRI. Any rupture of the venous sinuses that occurred during surgery was recorded and was not considered part of the bleeding caused by the extrusion itself.
Standard postoperative care consisted of analgesics (opioids), anti-inflammatory drugs (steroids or NSAIDs), and antibiotics (amoxicillin-clavulanate or cephalosporins); the neurological assessment was performed every 12 hr after surgery, at discharge (criteria for discharge varied individually), and 1 month and 3 months after surgery. In line with the results of Penning et al. [23], the outcome was considered good if the dog regained normal voluntary ambulation, regained ambulation with mild deficits (neurological grade II), or showed resolution of the thoracolumbar pain in cases classified as grade I at presentation and poor if the dog did not regain voluntary ambulation or was not able to walk unassisted. The time the dogs took to show a good or poor outcome after the surgery was classified as short (<15 days), medium (>15 and ≤30 days), and long (>30 days). Follow-up was obtained through neurological assessment.
Statistical analysis
All the variables collected were described by mean and standard deviation when normally distributed. The median and interquartile ranges were used in case of skewed data. Categorical variables were expressed as counts and percentages. The distributions of variables were investigated by the Shapiro–Wilk test. Bivariate analyses were conducted to identify statistically significant differences between the two groups. The chi-squared and Wilcoxon–Mann–Whitney tests were used for categorical and continuous variables, respectively. The prevalence rates of DEEH in the two groups were estimated by calculating the 95% confidence interval using the Clopper-Pearson method.
A multiple logistic regression model was developed to identify predictors of DEEH. Variables under the hypothesis and known potential confounders were introduced into the model. Model fit was verified using standardized residual analysis and the Hosmer–Lemeshow goodness of fit test. The final model selection was made using the Akaike Information Criterion.
Predictors of the ambulatory status at discharge were investigated using multiple logistic regression similar to the analysis of the main endpoint, while the recovery time predictors were studied using a multiple ordinal regression model.
As a sensitivity analysis, stepwise backward regression models with a 0.2 elimination threshold criterion were developed for each of the three endpoints.
RESULTS
Primary analysis
Signalment, history, and clinical signs: A total of 75 French Bulldogs, including 53 males and 22 females with a median age of 4 years (range 2–8), and 98 Dachshunds, including 57 males and 41 females with a median age of 6 years (range 2–14), were included in the study. Blood tests of all dogs revealed no significant abnormalities. The onset of clinical signs was hyperacute in 35/75 French Bulldogs and 45/98 Dachshunds. The clinical signs since presentation progressed to a worse clinical status by at least one grade in 16/75 French Bulldogs and 9/89 Dachshunds. The neurological grade at presentation was I in 9/75 French Bulldogs and 19/98 Dachshunds, II in 27/75 French Bulldogs and 29/98 Dachsunds, III in 10/75 French Bulldogs and 14/98 Dachshunds, IV in 28/75 French Bulldogs and 31/98 Duchshunds, V in 1/75 French Bulldogs and 5/98 Dachshunds. The median neurological grade at presentation was IV and II for both French Bulldogs and Dachshunds with and without DEEH respectively.
The mean time from initial presentation of clinical signs and the MRI examination was between 12 and 36 hr for French Bulldogs and between 12 and 48 hr for Dachshunds.
Diagnostic imaging and surgical treatment: The prevalence of DEEH in French Bulldogs was 41.3% (95% CI: 30.1–53.3%), while in Dachshunds, the prevalence was 11.2% (95% CI: 5.7–19.2%).
We found that the majority of dogs with DEEH (80.64% French Bulldogs and 90% of Dachshunds) had an associated endomedullary damage, seen as T2W hyperintensity on sagittal T2W images, compared to the non-DEEH group (25% French Bulldogs and 32% Dachshunds). The overall population had an endomedullary damage not greater than three times the lenght of the L2 vertebral body. We found that the presence of T2SCH was not statistically associated with the ambulatory status at discharge and partially with the recovery time (only T2SCH grade 1 was statistically associated with a longer recovery time: OR: 2.12, P=0.038). Nevertheless the T2SCH was statistically associated with a severe neurological grade at presentation (grade 4 and 5) and the presence of DEEH (OR:9.45, P=0.00 and OR: 2.45 and P=0.03, respectively).
The extension of DEEH required decompression of two to four vertebral sites in all dogs (when the degree of spinal cord compression was judged >50% according to the MRI findings). In all dogs without epidural hemorrhage (both French Bulldogs and Dachshunds), hemi- or mini-hemilaminectomy limited to two vertebral segments adjacent to the involved disc was considered sufficient to achieve complete spinal cord decompression [11]. The most common vertebral site of intervertebral disc extrusion in French Bulldogs was the lumbar region (50/75), while the most common site of intervertebral disc extrusion was the thoracolumbar region in Dachshunds (53/98). Major complications were not recorded in patients with DEEH.
Ambulatory status at discharge and recovery: There were 13/75 French Bulldogs and 22/98 Dachshunds who were non-ambulatory at discharge.
The time to recover normal neurological status or the median time to recover with residual mild neurological deficits (classified as neurological grade II) was ≤15 days for 60/75 French Bulldogs and 78/98 Dachshunds, over 30 days for 13/75 French Bulldogs and 19/98 Dachshunds, two French Bulldogs, and one Dachshund did not recover voluntary ambulation. A summary of the main clinical signs and imaging findings is shown in Table 1.
Table 1. Summary of the main clinical and imaging findings in the population studied.
| Onset |
PCS |
SDE |
Gender |
AAD |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hyperacute | Acute | Total | Yes | No | Total | T | L | T-L | Total | M | F | Total | Yes | No | Total | |
| Bulldogs | 35 | 40 | 75 | 16 | 59 | 75 | 10 | 50 | 15 | 75 | 53 | 22 | 75 | 62 | 13 | 75 |
| Daschunds | 45 | 53 | 98 | 9 | 89 | 98 | 53 | 31 | 14 | 98 | 57 | 41 | 98 | 76 | 22 | 98 |
| Total | 80 | 93 | 173 | 25 | 148 | 173 | 63 | 81 | 29 | 173 | 110 | 63 | 173 | 138 | 35 | 173 |
| Pr | 0.922 | 0.024 | 0.000 | 0.090 | 0.407 | |||||||||||
PCS, progression of clinical signs; SDE, site of intervertebral disc extrusion; AAD, ambulation at discharge; T, thoracic region; L, lumbar region; T-L, thoraco-lumbar region; M, male; F, female; Pr, probability.
Secondary analysis
The multiple logistic regression model highlighted that patients presenting with an acute onset of clinical signs (>24 hr) (OR: 13.08; 95% CI: 4.63–37.03), presence of clinical signs progression (OR: 5.04; 95% CI: 1.50–16.89), and belonging to the French Bulldogs group (OR: 5.15; 95% CI: 1.71–15.54) were at increased risk of developing DEEH.
The Hosmer–Lemeshow test indicated goodness of fit (P=0.16). Similarly, the low regression of Pearson residuals versus the linear model prediction showed good model stability.
Population-based marginal extimations of prevalence were 31.0% (95% CI: 15.9–46.1%) and 8.0% (95% CI: 2.1–14.0%) for French Bulldogs and Dachshunds, respectively.
Secondary multivariate analysis demonstrated how the odds of being ambulatory at discharge decreased by 85% (P=0.03) in cases of disc extrusion site in the lumbar region compared to that in the thoracic region, independent of the presence of DEEH.
Similarly, the ordinal model showed that ambulatory status at discharge did correlate with the presence of DEEH: secondary analysis showed that patients with DEEH were at an increased risk of being non-ambulatory at discharge (OR: 3.43; P=0.017). Moreover, in our population, patients without progression of clinical signs at presentation might have a shorter recovery time and less chance of being not ambulatory at discharge, with a decrease in risk of 69% (OR: 0.31; P=0.01).
Exploratory analysis
Further exploratory regression models were used to evaluate the possible interactions between the presence of DEEH and other factors. In dogs with DEEH, those without progression of clinical signs at presentation might have a higher probability of not being ambulatory at discharge (OR: 84.131; 95% CI: 2.74–2,583.021, P=0.01). Moreover, in dogs with DEEH, those with lumbar intervertebral disc extrusion had a shorter recovery time than those with thoracic intervertebral disc extrusion (OR: 0.14; 95% CI: 0.02–0.77, P=0.02), while those without progression of clinical signs had a longer recovery time (OR: 12.10; 95% CI: 1.97–74.31) than those with clinical signs progression.
In dogs with DEEH, Dachshunds had a longer recovery time than French Bulldogs (OR: 5.34; 95% CI: 1.16–24.58).
DISCUSSION
Intervertebral disc extrusion and DEEH are well-described pathological conditions in veterinary medicine [5, 6, 9, 10, 16, 26, 28]. However, to the best of our knowledge, the prevalence of DEEH and its clinical features in specific breeds has not been evaluated. Despite the high prevalence of DEEH in our canine population, this pathology is not common in humans [12]. It has been hypothesized that the pathophysiology of epidural hemorrhage in human medicine, apart from trauma, could include a violent cough or sneeze with spine flexion, epidural venous distension, increase in intradiscal pressure, disc extrusion, and venous rupture; other anatomical factors may play a role in the pathophysiology of the canine population, and further studies are needed to elucidate this.
In this study, two different canine chondrodystrophic breeds (French Bulldog and Dachshund) prone to developing intervertebral disc extrusions were included [4, 22]. We sought to identify and compare the prevalence and clinical features of DEEH between the two populations. A very recent study evaluated the prevalence and clinical features of thoraco-lumbar intervertebral disc extrusion associated epidural hemorrhage in dogs. Among the breeds evaluated, it was found the highest prevalence in French Bulldogs (66%); Dachshunds were less likely to present epidural haemorrhage associated with intervertebral disc extrusion (10%) [9].
The authors found a high prevalence (41.3%) of DEEH in French Bulldogs, which is in line with previous preliminary findings in the literature. French Bulldogs became a popular breed in Europe and the United States. Mayousse et al. reported a high prevalence of extradural hemorrhage or hematoma (27.8% of all cases diagnosed with intervertebral disc extrusion) in a large study on the prevalence of neurological diseases in French Bulldogs [18].
DEEH affects different canine breeds. A previous study [17] reported 31/46 chondrodystrophic dogs in the study population, including 5/46 French Bulldogs and 6/46 Dachshunds. Tartarelli et al. [26] reported 23 cases, including only 1/23 French Bulldogs and 2/23 Dachshunds. The study population included some large-breed dogs in addition to the usual chondrodystrophic small breeds, and this study tried to clarify the prevalence of the disease. Macias and Applewhite [2, 16] reported this pathology in Dobermanns, who are highly predisposed to an inherited bleeding disorder (von Willebrand’s disease) [19]; in the present population coagulation profile was normal, although specific tests (i.e. Von Willebrand Factor determination) were not perfomed.
It is clinically noteworthy that, in this study, patients presenting with an acute onset of clinical signs, having clinical signs progression since the presentation, and belonging to the French Bulldog group were at an increased risk of having DEEH. This should alert the clinician of the possible more demanding treatment of the condition compared to the usual intervertebral disc extrusion. The acute onset of clinical signs is caused by rupture of the venous sinuses and consequent bleeding that contributes to and worsens spinal cord compression. The extruded disc material also has a direct effect on spinal cord compression. The subsequent progression of clinical signs could be explained by impairment of spinal cord perfusion and ischemic damage, and red blood cell breakdown products could further exacerbate the inflammatory cascade and enhance spinal cord injury [3, 28]. Nevertheless, it is not possible to exclude the possibility that the progression of clinical signs could be due to further extrusion of disc material and secondary rupture of the venous plexus.
The authors could not find a significant association between the presence of DEEH and the site of intervertebral disc extrusion in either breed; however, in dogs with DEEH, those with lumbar intervertebral disc extrusion had a shorter recovery time than those with thoracic intervertebral disc extrusion. Lumbar vertebral sites are reported to be more susceptible to disc extrusion associated with hemorrhage [17]. In line with our findings, Aikawa [1] reported that French Bulldogs were more likely to develop intervertebral disc extrusions at the lumbar sites. This could provide important information for clinicians regarding the presentation and post-surgical outcome of lumbar DEEH.
The authors found that the presence of endomedullary spinal cord T2W hyperintensity was associated with a severe neurological grade at presentation (grade 4 and 5). Endomedullary MRI signal changes associated with intervertebral disc extrusion have been investigated in several studies and associated with necrosis, inflammation, hemorrhage, edema [13, 15, 21]. The presence of T2W spinal cord hyperintensity has been correlated with the severity of neurological signs at presentation [15, 21], and this is in line with our findings. The value of spinal cord T2W hyperintensity as a prognostic factor is not clear in veterinary literature [21]. Although some studies demonstrated that T2W hyperintensity correlated well with outcome [13, 15], other studies did not supported this association [8]. This discrepancy has been attributed to the use of different magnetic resonance field strength: high field might detect even mild spinal cord changes thus lowering the significance of the damage. Using a low filed MRI, only T2SCH grade 1 in our study was statistically associated with a longer recovery time; grade 2 and 3 were not, but a statistical observation suggests that a greater sample would have confirmed the significance; nevertheless, we couldn’t find an association between the T2SCH with the ambulatory status at discharge. Thus our study might partially confirm the utility of T2W spinal cord changes to predict the outcome.
The presence of DEEH was associated with the presence of T2W spinal cord hyperintensity in this study, suggesting that extensive haemorrhage leads not only to mechanical spinal cord compression but also to cytotoxic effect due to red blood cell breakdown products starting the inflammatory cascade. The overall result could enhance spinal cord damage, as proposed in other studies [9].
More than two vertebral sites required decompression in all patients with DEEH, suggesting that the presence of hemorrhage could be more demanding in terms of time and surgical effort. Major complications were not recorded in our patients with DEEH, suggesting that surgically addressing multiple intervertebral sites did not lead to vertebral instability, as demonstrated by Tartarelli [26], or predisposition to infection at the three-month follow-up. This contrasts with a recent study of a population of middle-to large-sized dogs with DEEH who experienced complications linked to the challenge of nursing care, such as aspiration pneumonia [28]. This is unlikely to have occurred in the present population of small-sized dogs.
Patients with DEEH are at an increased risk of being non-ambulatory at discharge, and spinal cord injury together with a massive spinal cord compression can lead to a slower recovery of ambulation; nevertheless, the outcome of the patients with and without DEEH does not differ substantially and is in line with that described in the literature.
This study has some limitations. First, it is retrospective in nature. The sample consisted of dogs bred in Italy, which may not represent breeds on an international level. Further studies with a larger number of patients could enhance or add evidence to the results of the present study.
Conclusion
In the present study, DEEH was more common in French Bulldogs than in Dachshunds.
Clinicians should be aware that in the absence of relevant complications related to the surgical procedures, the presence of DEEH does not seem to affect the outcome compared to dogs with intervertebral disc extrusion without the presence of extensive epidural hemorrhage. Important clinical information is that the progression of clinical signs from the initial presentation and the rate of onset of clinical signs are predictors of DEEH. Patients with DEEH are at an increased risk of being non-ambulatory at discharge. Clinicians should be aware that DEEH is a relatively frequent condition in French Bulldogs. The surgical approach to such a condition could be more technically demanding (approach to multiple spinal segments) and, thus, eventually more time consuming than addressing intervertebral disc extrusion without DEEH.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.Aikawa T, Shibata M, Asano M, Hara Y, Tagawa M, Orima H. 2014. A comparison of thoracolumbar intervertebral disc extrusion in French Bulldogs and Dachshunds and association with congenital vertebral anomalies. Vet Surg 43: 301–307. doi: 10.1111/j.1532-950X.2014.12102.x [DOI] [PubMed] [Google Scholar]
- 2.Applewhite AA, Wilkens BE, McDonald DE, Radasch RM, Barstad RD. 1999. Potential central nervous system complications of von Willebrand’s disease. J Am Anim Hosp Assoc 35: 423–429. doi: 10.5326/15473317-35-5-423 [DOI] [PubMed] [Google Scholar]
- 3.Bakker NA, Veeger NJGM, Vergeer RA, Groen RJ. 2015. Prognosis after spinal cord and cauda compression in spontaneous spinal epidural hematomas. Neurology 84: 1894–1903. doi: 10.1212/WNL.0000000000001545 [DOI] [PubMed] [Google Scholar]
- 4.Batcher K, Dickinson P, Giuffrida M, Sturges B, Vernau K, Knipe M, Rasouliha SH, Drögemüller C, Leeb T, Maciejczyk K, Jenkins CA, Mellersh C, Bannasch D. 2019. Phenotipic effects of FGF4 retrogenes on intervertebral disc disease in dogs. Genes (Basel) 10: E435. doi: 10.3390/genes10060435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergknut N, Egenvall A, Hagman R, Gustås P, Hazewinkel HAW, Meij BP, Lagerstedt AS. 2012. Incidence of intervertebral disk degeneration-related diseases and associated mortality rates in dogs. J Am Vet Med Assoc 240: 1300–1309. doi: 10.2460/javma.240.11.1300 [DOI] [PubMed] [Google Scholar]
- 6.Besalti O, Ozak A, Pekcan Z, Tong S, Eminaga S, Tacal T. 2005. The role of extruded disk material in thoracolumbar intervertebral disk disease: a retrospective study in 40 dogs. Can Vet J 46: 814–820. [PMC free article] [PubMed] [Google Scholar]
- 7.Bezuidenhout AJ. 2013. Veins. p. 531. In: Miller’s Anatomy of the Dog, 4th ed. (Evans HE, de Lahunta A, eds.), Elsevier, St. Louis. [Google Scholar]
- 8.Boekhoff TM, Flieshardt C, Ensinger EM, Fork M, Kramer S, Tipold A. 2012. Quantitative magnetic resonance imaging characteristics: evaluation of prognostic value in the dog as a translational model for spinal cord injury. J Spinal Disord Tech 25: E81–E87. doi: 10.1097/BSD.0b013e31823f2f55 [DOI] [PubMed] [Google Scholar]
- 9.Bridges J, Windsor R, Stewart SD, Fuerher-Senecal L, Khanna C. 2022. Prevalence and clinical features of thoracolumbar intervertebral disc-associated epidural hemorrhage in dogs. J Vet Intern Med (in press). doi: 10.1111/jvim.16442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brisson BA. 2010. Intervertebral disc disease in dogs. Vet Clin North Am Small Anim Pract 40: 829–858. doi: 10.1016/j.cvsm.2010.06.001 [DOI] [PubMed] [Google Scholar]
- 11.Cerda-Gonzalez S, Olby NJ. 2006. Fecal incontinence associated with epidural spinal hematoma and intervertebral disk extrusion in a dog. J Am Vet Med Assoc 228: 230–235. doi: 10.2460/javma.228.2.230 [DOI] [PubMed] [Google Scholar]
- 12.Giri PJ, Sharma MS, Jaiswal AK, Behari S, Jain VK. 2006. Extruded lumbar disc associated with epidural hematoma. Case report. J Neurosurg 104 Suppl: 282–284. [DOI] [PubMed] [Google Scholar]
- 13.Ito D, Matsunaga S, Jeffery ND, Sasaki N, Nishimura R, Mochizuki M, Kasahara M, Fujiwara R, Ogawa H. 2005. Prognostic value of magnetic resonance imaging in dogs with paraplegia caused by thoracolumbar intervertebral disk extrusion: 77 cases (2000–2003). J Am Vet Med Assoc 227: 1454–1460. doi: 10.2460/javma.2005.227.1454 [DOI] [PubMed] [Google Scholar]
- 14.Jeffery ND. 1988. Treatment of acute and chronic thoracolumbar disc disease by ‘mini hemilaminectomy’. J Small Anim Pract 29: 611–616. doi: 10.1111/j.1748-5827.1988.tb02181.x [DOI] [Google Scholar]
- 15.Levine JM, Fosgate GT, Chen AV, Rushing R, Nghiem PP, Platt SR, Bagley RS, Kent M, Hicks DG, Young BD, Schatzberg SJ. 2009. Magnetic resonance imaging in dogs with neurologic impairment due to acute thoracic and lumbar intervertebral disk herniation. J Vet Intern Med 23: 1220–1226. doi: 10.1111/j.1939-1676.2009.0393.x [DOI] [PubMed] [Google Scholar]
- 16.Macias C, Mckee WM, May C, Innes JF. 2002. Thoracolumbar disc disease in large dogs: a study of 99 cases. J Small Anim Pract 43: 439–446. doi: 10.1111/j.1748-5827.2002.tb00010.x [DOI] [PubMed] [Google Scholar]
- 17.Mateo I, Lorenzo V, Foradada L, Muñoz A. 2011. Clinical, pathologic, and magnetic resonance imaging characteristics of canine disc extrusion accompanied by epidural hemorrhage or inflammation. Vet Radiol Ultrasound 52: 17–24. [PubMed] [Google Scholar]
- 18.Mayousse V, Desquilbet L, Jeandel A, Blot S. 2017. Prevalence of neurological disorders in French bulldog: a retrospective study of 343 cases (2002-2016). BMC Vet Res 13: 212. doi: 10.1186/s12917-017-1132-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moser J, Meyers KM, Russon RH. 1996. Inheritance of von Willebrand factor deficiency in Doberman pinschers. J Am Vet Med Assoc 209: 1103–1106. [PubMed] [Google Scholar]
- 20.Olby NJ, Muñana KR, Sharp NJH, Thrall DE. 2000. The computed tomographic appearance of acute thoracolumbar intervertebral disc herniations in dogs. Vet Radiol Ultrasound 41: 396–402. doi: 10.1111/j.1740-8261.2000.tb01860.x [DOI] [PubMed] [Google Scholar]
- 21.Olby NJ, da Costa RC, Levine JM, Stein VM. Canine Spinal Cord Injury Consortium (CANSORT SCI). 2020. Prognostic Factors in Canine Acute Intervertebral Disc Disease. Front Vet Sci 7: 596059. doi: 10.3389/fvets.2020.596059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parker HG, VonHoldt BM, Quignon P, Margulies EH, Shao S, Mosher DS, Spady TC, Elkahloun A, Cargill M, Jones PG, Maslen CL, Acland GM, Sutter NB, Kuroki K, Bustamante CD, Wayne RK, Ostrander EA. 2009. An expressed fgf4 retrogene is associated with breed-defining chondrodysplasia in domestic dogs. Science 325: 995–998. doi: 10.1126/science.1173275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Penning V, Platt SR, Dennis R, Cappello R, Adams V. 2006. Association of spinal cord compression seen on magnetic resonance imaging with clinical outcome in 67 dogs with thoracolumbar intervertebral disc extrusion. J Small Anim Pract 47: 644–650. doi: 10.1111/j.1748-5827.2006.00252.x [DOI] [PubMed] [Google Scholar]
- 24.Scott HW, McKee WM. 1999. Laminectomy for 34 dogs with thoracolumbar intervertebral disc disease and loss of deep pain perception. J Small Anim Pract 40: 417–422. doi: 10.1111/j.1748-5827.1999.tb03114.x [DOI] [PubMed] [Google Scholar]
- 25.Suran JN, Durham A, Mai W, Seiler GS. 2011. Contrast enhancement of extradural compressive material on magnetic resonance imaging. Vet Radiol Ultrasound 52: 10–16. [PubMed] [Google Scholar]
- 26.Tartarelli CL, Baroni M, Borghi M. 2005. Thoracolumbar disc extrusion associated with extensive epidural haemorrhage: a retrospective study of 23 dogs. J Small Anim Pract 46: 485–490. doi: 10.1111/j.1748-5827.2005.tb00277.x [DOI] [PubMed] [Google Scholar]
- 27.Tidwell AS, Specht A, Blaeser L, Kent M. 2002. Magnetic resonance imaging features of extradural hematomas associated with intervertebral disc herniation in a dog. Vet Radiol Ultrasound 43: 319–324. doi: 10.1111/j.1740-8261.2002.tb01011.x [DOI] [PubMed] [Google Scholar]
- 28.Woelfel CW, Robertson JB, Mariani CL, Muñana KR, Early PJ, Olby NJ. 2021. Outcomes and prognostic indicators in 59 paraplegic medium to large breed dogs with extensive epidural hemorrhage secondary to thoracolumbar disc extrusion. Vet Surg 50: 527–536. doi: 10.1111/vsu.13592 [DOI] [PubMed] [Google Scholar]