Abstract
BACKGROUND
Cardiovascular complications have been increasingly recognized in the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) associated coronavirus disease 2019 (COVID-19). Cardiac biomarkers are released because of this ongoing cardiovascular injury and can act as surrogate markers to assess the disease severity.
AIM
To review the variation and utility of these biomarkers in COVID-19 to ascertain their role in diagnosis, prognosis and clinical outcomes of the disease.
METHODS
We performed a literature search in PubMed, Medline and the Reference Citation Analysis (RCA), using the search terms “COVID-19” and “cardiac bioenzymes” or “cardiac biomarkers”. Additionally, we also used the latest reference citation analysis tool to identify more articles.
RESULTS
Cardiac troponin has been consistently elevated in patients with COVID-19 associated myocarditis, and strongly correlated with adverse prognosis. Natri-uretic peptides including brain natriuretic peptide (BNP) and pro-BNP is elevated in patients with COVID-19 associated cardiac injury, irrespective of their prior heart failure status, and independently correlated with worst outcomes. Alongside these traditional biomarkers, novel cardiac bioenzymes including presepsin, soluble ST2 and copeptin, are also increasingly recognized as markers of cardiovascular injury in COVID-19 and can be associated with poor outcomes.
CONCLUSION
Assessment of cardiac bioenzymes at admission and their serial monitoring can help assess the severity of disease and predict mortality in patients with SARS-CoV-2 infection. Future studies are needed to elude the critical importance of novel biomarkers.
Keywords: SARS-CoV-2, Troponin, Brain natriuretic peptide, Prognosis, Outcomes, Heart failure
Core Tip: Cardiac bioenzymes act as surrogate markers for various cardiovascular complications associated with coronavirus disease 2019 (COVID-19). Cardiac bioenzymes at admission and their serial monitoring can help assess the disease severity and predict mortality in patients with COVID-19. This review summarizes the role of these bioenzymes in diagnosis, prognosis and clinical implications on outcomes of various cardiovascular complications associated with COVID-19.
INTRODUCTION
The coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has since infected nearly 500 million people across 200 different countries and killed more than six million people worldwide. Lung injury is the most common presentation seen; however, cardiac injury is another dreaded consequence of this viral disease. Multiple mechanisms of injury have been hypothesized that culminate in widespread inflammation and cytokine storm causing significant cardiovascular dysfunction. A few authors have hypothesized that the inciting events for this injury include microvascular damage in the heart, causing perfusion defects, vessel hyperpermeability, and vasospasm[1-5]. Cardiac biomarkers are released because of this ongoing cardiovascular injury and can act as surrogate markers to assess the disease severity. These biomarkers can be elevated in many cardiac conditions, including acute Myocardial infarction (AMI), heart failure (HF), arrhythmias and cardiomyopathies. Among the available biomarkers, cardiac troponin (cTn) and natriuretic peptides including brain natriuretic peptide (BNP) and N terminal pro-BNP (NT-proBNP), have been extensively studied. Numerous reports from China have noted elevated cTn in COVID-19 patients[6,7]. A major review on cardiac biomarkers in HF emphasized the importance of negative NPs in ruling out HF[8]. In addition, novel biomarkers including soluble ST2 (sST2), Galectin-3 (Gal-3), and copeptin have also been studied. In this review, we aimed to study in detail the various cardiac biomarkers that have been reported in the literature in patients with COVID-19. We also aimed to identify the role of these cardiac biomarkers in diagnosing the impact of cardiac injury and their role in prognostication of morbidity and mortality among patients with COVID-19.
MATERIALS AND METHODS
We conducted an extensive review of the literature of all the studies on patients with COVID-19 associated cardiac injury and cardiac bioenzymes. We screened for articles on cardiac biomarkers in patients with COVID-19 in the MEDLINE/PubMed database. Published articles between November 2019 and March 2022 were reviewed. Keywords for the search criteria included “Coronavirus disease 2019”, OR “COVID-19”, OR “Severe Acute Respiratory Syndrome Coronavirus-2”, OR “cardiac bioenzymes”, OR ‘biomarkers”, OR “prognosis”, OR “heart failure”, OR “myocarditis” OR “outcome”, OR “morbidity”, and “mortality”. We also used the related article search feature and manual search of references to identify further articles. Additionally, we used the latest reference citation analysis tool to screen for more articles. Two independent trained physician reviewers were involved in screening and reviewing relevant articles. As of March 2022, a total of 560 papers were identified. Among them, only 61 papers were eligible to be included (Figure 1). All articles with details on COVID-19 patients with cardiac injury and measured cardiac biomarkers were eligible to be included in this review. We included all articles published in English from all over the world. Independent reviews, editorials, letters, abstracts, preprints, and opinions were excluded. Most studies reporting cardiac biomarkers in patients with COVID-19 were from China, North America, and Europe. The reporting of study design, methodology, data collection, biomarker levels, and measured outcomes were not consistent across all the studies. To simplify the role of each cardiac biomarker with regard to COVID-19 disease diagnosis, prognosis, and mortality, we subdivided this review into three principal sections. The three sections were (1) Studies on the role of cardiac troponin in diagnosing and prognosticating COVID-19 associated myocardial injury and mortality; (2) Studies on the role of natriuretic peptides in diagnosing and prognosticating COVID-19 associated myocardial injury and mortality; and (3) Studies on the role of other biomarkers and novel cardiac biomarkers in diagnosing and prognosticating COVID-19 associated myocardial injury and mortality.
Figure 1.
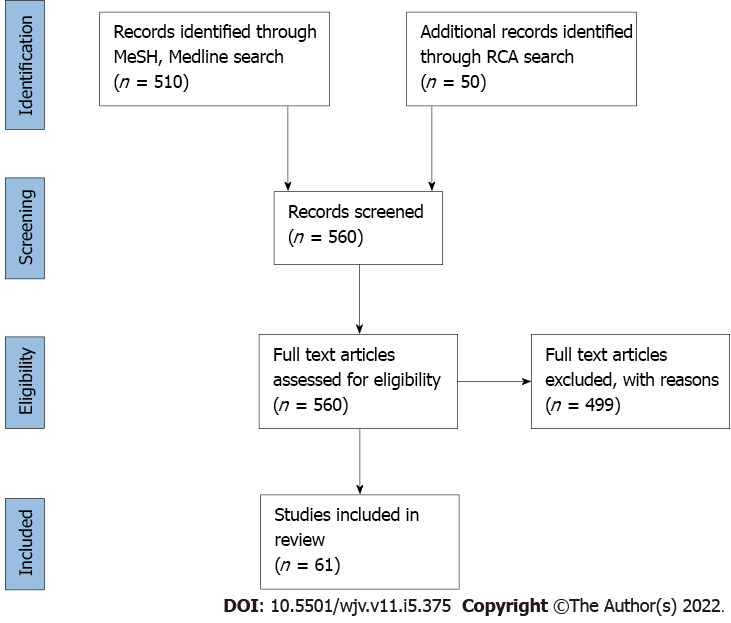
PRISMA diagram of literature search and selection.
Cardiac troponin
Pathophysiology: cTn include troponin T (cTnT) and troponin I (cTnI), which are universally accepted markers of cardiac injury[9]. Cardiac troponin, a regulatory protein complex with three units, is located at the sarcomere thin filament. The inhibitory unit cTnI and a tropomyosin binding unit cTnT are responsible for maintaining a relaxed state when intracellular Ca2+ concentrations are low in diastole. In systole, the rise in intracellular Ca2+ leads to Ca2+ binding to cardiac troponin C (cTnC), releasing inhibition and promoting contraction and ejection[10].
Troponin as a diagnostic marker of cardiovascular injury in COVID-19: In the early phases of the pandemic caused by SARS-CoV-2, the emphasis was on lung damage and treatment of the same. Guidelines from AHA had recommended against the determination of cTnT and cTnI. However, this notion changed in 2020, when Chapman et al[11] published a statement strongly supporting the determination of serum cTnI and cTnT, emphasizing their role as biomarkers for cardiac injury in COVID-19 infected patients. Initially, the exact mechanism leading to serum elevations of these biomarkers was unclear, with several theories being proposed. Recent evidence showed direct infection of cardiac myocytes by SARS-CoV-2[12], leading to a decrease in Angiotensin-converting enzyme 2 (ACE2) and an increase in Angiotensin II (AngII). The dysfunctional signaling leads to necrosis or membrane instability, causing the leak of the bioenzymes[13]. Multiple additional studies[14-16] have reiterated the importance of cardiac troponin as a diagnostic tool and have been summarized in Table 1. Cardiac troponins have been reported to be elevated irrespective of the pattern of cardiac injury and clinical presentation. Levels have been reported to be higher among patients with an ischemic pattern of injury than in non-ischemic injury. The release of cTn has been seen in COVID-19 patients with acute coronary syndrome, tachyarrhythmias, cardiomyopathy, and myocarditis. In COVID-19, patients’ cTn has been used as a marker of inflammation and myocardial injury. A large observational study from New York on patients hospitalized with SARS-CoV-2 showed a positive correlation between elevated cTnT and inflammatory markers[14].
Table 1.
Summary of studies characterizing the role of cardiac troponin
|
Study
|
Type of study
|
Location
|
Number of participants
|
Recommendation
|
| Diagnostic and prognostic utility of troponin | ||||
| Lala et al[14], 2020 | Single center, Observational | New York | 2736 | cTn elevated in patients with primary cardiac etiology including MI. Other etiologies included arrythmias, HF, myocarditis and Takotsubu cardiomyopathy |
| Khaloo et al[15], 2022 | Multicenter, Retrospective | Massachusetts | 2450 | |
| Sandoval et al[17], 2020 | Review | Serial cTn measurement aids in risk stratification | ||
| Almeida et al[18], 2020 | Single center, Retrospective | Brazil | 183 | Elevated cTn measured within first 24 h is associated with worst prognosis. Increased need for MV |
| Maino et al[19], 2021 | Single center, Retrospective | Italy | 189 | |
| Arcari et al[20], 2020 | Multicenter, Observational | Italy | 111 | cTn elevation correlated with poor prognosis and need for MV |
| Role in outcome and mortality | ||||
| Scarl et al[21], 2021 | Single center, Retrospective | Ohio | 81 | In patients with pre-existing comorbidities, CTnI elevation is associated with mortality |
| Lala et al[14], 2020 | Single center, Observational | New York | 2,736 | Threefold increase in mortality in patients with cTnI three times the upper limit of normal |
| Mueller et al[22], 2021 | Review | cTn elevation is associated with significant in hospital adverse events | ||
| Henein et al[23], 2021 | Multicenter, Retrospective | International, mainly European | 748 | |
| Kermali et al[24], 2020 | Systematic review | China | 607 | |
| Arcari et al[25], 2021 | Multicenter, Retrospective | Italy | 252 | cTn significantly elevated in patients with pre-existing comorbidities, and is associated with increased mortality |
| Lombardi et al[26], 2020 | Multicenter,Cross sectional | Italy | 614 | Elevation in cTn associated with mortality. 45.3% patients had elevated cTn and correlated with 71% increase in mortality, and a 2-fold increase in additional complications inlcuding sepsis, PE, AKI |
| Salvatici et al[27], 2020 | Single center, Retrospective | Italy | 523 | cTnT and cTnI remain independent predictors of mortality even after adjusting for potential confounders |
| Al Abbasi et al[28], 2020 | Single center, Retrospective | Florida | 257 | Elevated cTnI in the first 24 h of admission had a significantly higher in hospital mortality, with 89.7% negative predictive value |
cTn: Cardiac troponin; cTnT: Troponin T; cTnI: Troponin I; HF: Heart failure; MV: Mechanical ventilation; PE: Pulmonary embolism; AKI: Acute kidney injury.
Role of troponin as a prognostic indicator of cardiovascular outcomes in COVID-19: Sandoval et al[17] found higher levels of cTn in severe SARS-CoV-2 infection and opined that their serial measurement can aid in the risk stratification of COVID-19 patients. Based on the progression of the disease, they grouped COVID-19 patients in three phases; first - during admission where cTn elevation reflected the comorbidities; second - further rise in cTn with critical acute respiratory distress syndrome (ARDS) and; third - peak cTn with COVID-19 associated complications, including myocarditis and pulmonary embolism. Studies have shown that a high level of cTn and serial up-trending of cTn have been predictive of worse prognosis[18-20]. Troponin levels between 0.03 and 0.09 ng/mL were considered to be predictive of cardiac damage, with levels above 0.09 ng/mL conferring an even higher risk. A few studies utilising high sensitivity troponin have shown that troponin levels above 4 ng/L, 13 ng/L and 37 ng/L to be predictive of mild, severe and critical illness respectively[19]. Similarly, lower levels of cTn at presentation and a downward trend have been consistently reported among the survivors. Importantly COVID-19 patients with prior cardiovascular comorbidities have been at risk of further cardiovascular injury. Among these patients with cardiovascular comorbidities, cTn has been associated with further adverse prognosis.
Role of troponin on outcomes and mortality in COVID-19: Among patients with COVID-19, cTn was higher in deceased patients compared to survivors. Multiple studies have shown a significant correlation between cTn and in-hospital adverse events and mortality even in patients without comorbidities[14,21-28] (Table 1). Multiple studies show that cTnT and cTnI are independent predictors of mortality even after adjusting for confounding factors[29,30]. Scarl et al[21] reported that, in hospitalized patients with pre-existing comorbidities and SARS-CoV-2, there was a significant correlation between serum cTnI level and mortality. Salvatici et al[27] in their study utilising high sensitivity troponin, showed that in hospital survival rates was about 90% when cTnI was normal. The survival rate decreased to 87% when cTnI was above normal but less than 40 ng/L, and further reduced to 59% with cTnI above 40 ng/L. These studies have shown that cTn drawn at admission had a high positive predictive value for serious illness and a high negative predictive value for death. An up-trending cTn among COVID-19 patients is shown to correlate with a twofold increase in complications including sepsis, pulmonary embolism, and acute kidney injury and a threefold increase in mortality. The level of cTn has been shown to correlate with the outcome within 24 h of hospital admission. A study from Florida showed that COVID-19 patients with elevated cTnI levels in the first 24 h of admission had a significantly higher in-hospital mortality as compared to those with a normal cTnI level[28]. Patients with a normal cTnI level at admission had a low risk of worse outcome demonstrating an 89.7% negative predictive value. Similar results were reported by two other studies showing an increased need for invasive mechanical ventilation and risk of death among patients with elevated cTn levels within the first 24 h of admission[18,19]. Therefore, measurement of cTnI after hospitalization for COVID-19, followed by longitudinal monitoring, can help clinicians intercept dynamic changes in the levels of cTnI as a surrogate marker of myocardial injury.
Troponin as a surrogate marker of cardiovascular dysfunction post-discharge in COVID-19: Elevated cTn has been associated with impaired left ventricular relaxation and decline in right ventricular function resulting in long-term sequelae. As a component of Long COVID-19, the persistence of cardiac injury has been reported in young patients following an acute COVID-19 episode until six months. A cross-sectional study of 144 patients who were followed up for 85 d after their recovery from SARS-CoV-2 showed that patients with baseline elevations in cTn had a higher incidence of dyspnea after discharge. These patients also had impaired diastolic dysfunction and elevated pulmonary artery (PA) pressures, as noted by echocardiography. They also had persistence of cTn until mid-follow-up[31]. A rise in the incidence of HF has also been seen in COVID-19 patients with elevated cTn in two large multicenter studies[15,26]. These studies signify that cTn can be used as diagnostic and prognostic tools for long-term cardiac outcomes related to SARS-CoV-2 infection in select subgroup of patients. Despite these observations, clinical judgment should be used to avoid any unnecessary diagnostic and therapeutic interventions triggered by the isolated cTn elevation.
Natriuretic peptides
Pathophysiology: Natriuretic peptides (NPs), including BNP and NT-proBNP, are quantitative biomarkers of hemodynamic myocardial stress and heart failure[32]. Brain natriuretic peptide is a preprohormone which is split into a single peptide and a propeptide (pro-BNP). Natriuretic peptides mediate their biological effects through guanylyl cyclase receptors [natriuretic peptide receptor (NPR)] A, B and C. Stress of the ventricular wall due to volume or pressure overload is the primary inducer of BNP synthesis, which acts on the kidney to induce natriuresis and diuresis[33] Natriuretic peptides are considered one of the initial diagnostic tools in acute HF patients. Historically, studies like TOPCAT[34] have supported its value, and over the past years, NT-proBNP has had a growing role in the standardization of the definition of HF.
Natriuretic peptides as a diagnostic marker of cardiovascular injury in COVID-19: Prior to the advent of SARS-CoV-2, multiple viral infections have been reported to induce HF due to direct viral invasion and pro-inflammatory cytokines leading to sympathetic activation. In SARS-CoV-2, elevation in NPs is a result of inflammatory overdrive, specifically interleukin (IL)-1β, IL-6, and monocyte chemoattractant protein-1 (MCP-1), which can lead to fulminant myocarditis. The rise in NPs is believed to be secondary to hypoxia and cardiac injury. In addition, widespread inflammation and decreased nitric oxide levels result in endothelial dysfunction, which causes heart failure symptoms. This can be a combination of pre-existing cardiac disease and the acute hemodynamic and hypoxemic stress related to COVID-19[32,35]. The use of vasopressor therapy, hypoxia-induced pulmonary vasoconstriction, inflammatory involvement of the myocardium, oxidative stress, and fibrin microthrombi in the vasculature contributes to the release of NPs[36-38]. Across multiple studies, NPs level were high among COVID-19 patients with and without HF. Higher levels of NPs have not been consistently shown to correlate with severe COVID-19 disease. Still, they have been shown to correlate with developing or worsening of heart failure in these patients (Table 2).
Table 2.
Summary of studies characterizing the role of natriuretic peptides
|
Study
|
Type of study
|
Location
|
Number of participants
|
Recommendation
|
| Diagnostic and prognostic utility of natriuretic peptides | ||||
| Arcari et al[20], 2020 | Multicenter, Observational | Italy | 111 | Positive correlation between the rise in NPs and COVID-19 disease severity. Half of these patients had their NP level above the upper limit of normal |
| Caro-Codón et al[40], 2021 | Population | Spain | 396 | In patients with history of HF, elevation in NT-proBNP above the cut-off for normal suggested development of acute HF |
| Gao et al[41], 2020 | Multicenter, Prospective | China | 402 | This study proposed a triple cut point strategy of NT-proBNP (HF unlikely if NT-proBNP < 300pg/L, grey zone 300-900 pg/L and HF likely if > 900 pg/L) for its role in developing acute HF and in determining prognosis. Thirty day mortality in HF group was 40.8%. |
| Sorrentino et al[42], 2020 | Meta-analysis of 13 observational studies | 2248 | Natriuretic peptides have significant prognostic importance in predicting severity of COVID-19 | |
| Yoo et al[43], 2021 | Single center, Retrospective cohort | New York | 679 | In patients without a history of HF, elevated admission NT-proBNP correlated with fewer hospital free, ICU free and ventilator free days compared to those with low NT-proBNP levels |
| Alvarez-Garcia et al[44], 2020 | Single center, Retrospective | New York | 6439 | No difference identified in the level of NP and COVID-19 disease severity |
| Dawson et al[45], 2020 | Meta-analysis | China | 12 studies included | |
| Abdeen et al[46], 2021 | Single center, Retrospective | New Jersey | 230 | |
| Role in outcome and mortality | ||||
| Gao et al[48], 2020 | Single center, Retrospective | China | 102 | Natriuretic peptides independently associated with in-hospital mortality in severe COVID-19 patients. The cut off value predicting in hospital death was 88.64 pg/mL with a 100% sensitivity and 66.7% specificity. |
| Caro-Codón et al[40], 2021 | Population | Spain | 396 | Elevations in NP correlated with in-hospital mortality, even after adjusting for relevant confounders |
| Calvo-Fernández A et al[49], 2021 | Single center, Retrospective | Spain | 872 | Natriuretic peptide elevation is independently related to death or mechanical ventilation in COVID-19 patients |
| Selcuk et al[50], 2021 | Single center, Retrospective | Istanbul | 137 | Among patients who did not have a baseline diagnosis of HF, NPs were independent predictors of mortality. This study used a cut off threshold of 260pg/ml predicting an in-hospital mortality with 82% sensitivity and 93% specificity |
| Iorio et al[51], 2022 | Multicenter, Retrospective observational | Italy | 341 | The level of NP elevation correlated with mortality. Cut off threshold used in this study is 2598 pg/L predicting a 30-d mortality with 91.7% sensitivity and an 80% specificity |
| Belarte-Tornero et al[52], 2021 | Single center, Retrospective | Spain | 129 | |
| Dalia et al[53], 2021 | Systematic review | India | 5967 | Patients with fulminant COVID-19 and elevated NPs had an eight-fold increased risk of acute cardiac injury and death when compared to their counterparts |
| Pranata et al[54], 2020 | Meta analysis | 967 | In patients with HF, natriuretic peptide elevation is associated with disease progression and mortality. This effect was seen even after adjustment for troponin and CKMB | |
| Iorio et al[51], 2022 | Multicenter, Retrospective observational | Italy | 341 | The combined effect of cTn and NT-proBNP was studied in COVID-19 patients. Irrespective of prior HF history, increased mortality was seen in patients with both biomarker elevation. In patients with only one biomarker elevation, case fatality higher in patients with NP elevation |
| Stefanini et al[55], 2020 | Single center, Retrospective | Italy | 397 | |
NP: Natriuretic peptide; NT-proBNP: N terminal pro-brain natriuretic peptide; HF: Heart failure; cTn: Cardiac troponin; COVID-19: Corona virus disease 2019.
Role of natriuretic peptides as a prognostic indicator of cardiovascular complications in HF and COVID-19: In patients with COVID-19 and myocardial injury, the elevation of NPs has been reported consistently[7,36]. Mehra et al[39] suggested that in patients with COVID-19 and cardiac comorbidities, the earliest manifestation of cardiac decompensation is due to diastolic dysfunction. This is secondary to hemodynamic instability and pulmonary complications in the early course of the disease. Subsequently, because of cytokine storm, systolic dysfunction ensues. A large multicenter study from Italy showed a positive correlation between the rise in NPs and associated SARS-CoV-2 severity[20]. Half of the patients in this study had their NP level above the upper limit of normal. Similar results were reported from a large meta-analysis of 13 observational studies, including 2248 patients. The average NT-proBNP among COVID-19 patients with severe disease was 791 pg/mL, vs 160 pg/mL in non severe patients[42]. In patients with pre-existing HF and COVID-19, an elevation of NT-proBNP above the cut-off for normal[32] suggested an acute decompensation of HF, leading to a prolonged hospital stay[40]. Interestingly, in a different study from New York that included 679 patients without a history of HF, elevations in NT-proBNP correlated with longer ICU stay, hospital stay, and the increased need for mechanical ventilation[43]. Negative results were also seen in a few studies which did not identify any difference in the NP levels and COVID-19 severity[44-46]. These were however small studies, and given the lack of a diverse population, the results cannot be generalized.
Role of natriuretic peptide on outcomes and mortality in COVID-19: Heart failure per se is a significant risk factor for developing severe COVID-19[1,2,12]. In a series of 113 patients who died from SARS-CoV-2, HF was the most frequent cause of death after ARDS and sepsis[47]. In a large population study from Spain that enrolled patients with HF, elevation in NT-proBNP above the cut-off for normal was independently associated with mortality, even after adjusting for confounders[40]. Gao et al[48] reported an increased mortality in COVID-19 patients who had an elevated BNP above 88.64 pg/mL, with a 100% sensitivity and a 66.7% specificity. The significance of NPs in predicting mortality among SARS-CoV-2 patients is independent of their HF status. A single-center study with 137 patients without a prior diagnosis of HF showed that elevation of NPs is an independent predictor of mortality[50]. This study used a cut-off value of 260 pg/mL, predicting in-hospital mortality with 82% sensitivity and 93% specificity. It must be noted that the threshold used for NT-pro BNP in this study is lower than the cut-off used in the clinic and clinical trials for the diagnosis of HF. This implies that elevated NT-pro BNP levels even within the upper limit of the normal reference range could indicate an occult cardiac injury in COVID-19 patients. In patients with chronic HF, an elevated pro-BNP above suggested cut off of 2598 pg/mL was associated with an increased odds of 30 d mortality[52]. An extensive systematic review from India, including 5967 patients, found that non-survivors and patients with fulminant SARS-CoV-2 with elevated NPs, had an 8-fold increased risk of acute cardiac injury and death compared to their counterparts[53]. The average NT-proBNP across patients with severe COVID-19 was 1142 pg/mL. Two large center studies from Italy studied the combined role of troponins and NPs in COVID-19 associated disease progression and mortality. They found that patients with dual biomarker elevation had increased mortality, irrespective of their prior HF status[51,55].
Natriuretic peptides as a surrogate marker for new-onset heart failure post-discharge: Elevated NT-proBNP in COVID-19 patients without cardiac comorbidities indicates SARS-CoV-2 mediated cardiac complications. New-onset HF was seen in 23% of hospitalized patients with COVID-19 and was the most frequent cause of death after sepsis and ARDS[47]. A large prospective study in COVID-19 patients[56] used HFA-PEFF score (Heart Failure Association Pre-test assessment, Echocardiography & natriuretic peptide, Functional testing, Final etiology) with a specificity of 93% and a positive predictive value of 98% to rule in HFpEF. These patients had higher NT-proBNP levels when compared to their counterparts. Cardiac biomarkers are known to decline after the resolution of acute infection, as seen in ECHOVID-19 study[57]. On the contrary, persistent biomarker elevation despite infection resolution has been noted in two different studies[58,59]. They also had echocardiographic parameters of ventricular dysfunction. The exact cause of reduced ventricular myocardial function is unknown; however, it is presumed to be secondary to systemic inflammation and ventricular remodeling[60-62]. If the recovery is good, the prognosis is better, else, it predisposes them to the development of HF[63].
Additional biomarkers
Other biomarkers have also been implicated in determining prognosis and predicting mortality in patients with SARS-CoV-2. Significant elevations in creatine kinase MB (CK-MB) and NT-proBNP above the upper limit of normal are seen in critically ill patients with COVID-19, helping in risk stratification[64,65]. An increase in the level of myoglobin (MYO), NT-proBNP, and cTnI correlated with disease severity in patients with SARS-CoV-2[66]. Similar results were seen in two other studies identifying the prognostic significance of myoglobin, procalcitonin, and d-dimer in COVID-19[67,68]. Alongside troponin and natriuretic peptides, elevations in CK-MB and LDH (lactate dehydrogenase) have been shown to predict in-hospital mortality in patients with COVID-19[69]. Similarly, a rise in IL-6 and INR predicted an increased odds of 7-d mortality in patients admitted with SARS-CoV-2[64]. In addition, there is now data on novel emerging biomarkers and their role in predicting the disease severity in COVID-19. Among them, presepsin, growth differentiation factor 15 (GDF-15), soluble ST2, galectin 3, and copeptin have been studied.
Presepsin
Presepsin is a CD14 biomarker released into circulation by pro-inflammatory signals during infection. Through its interaction with T and B cells, it acts as an immunomodulator and has diagnostic and prognostic significance in sepsis[70]. Its role has been implicated alongside natriuretic peptides in the diagnosis of HF. A single-center study with 506 patients showed that presepsin was elevated in patients with acute HF decompensation and correlated with their 6-month mortality[71]. Similar results were seen in another study, with higher presepsin levels correlating with longer ICU stay and increased mortality[72].
Role of presepsin in prognosis and outcomes in COVID-19: Presepsin elevation has been noted in patients with COVID-19, thus serving as a reliable biomarker[73]. Studies have shown a four-to-five-fold increase in serum presepsin, which correlates with disease severity when compared to their counterparts[74-76]. Fukada et al[77] in a small series of patients with COVID-19-related respiratory failure, found that presepsin is more expressed in severe cases than in mild cases. Similar results have been seen in other studies identifying the prognostic importance of presepsin with COVID-19 related disease severity[73,78]. Patients with presepsin values higher than 250 ng/L had a longer ICU stay when compared to the patients with lower values[78]. Park et al[74] suggested that an elevated presepsin level at 717 pg/mL is a significant predictor of 30-d mortality. A threefold rise in presepsin has been identified as a very specific indicator of 30-d mortality[75,79]. Thus, routine assessment of presepsin in COVID-19 may provide valuable clinical information for predicting adverse outcomes, as well as for guiding the clinical and therapeutic decision-making.
Soluble ST2
Soluble ST2 (sST2) is among the most important novel biomarkers for prognosis in HF. Upregulated in states of mechanical strain, it plays an essential role in myocardial hypertrophy and fibrosis. Studies have shown an increase in sST2 gene expression in the presence of cardiac injury. High circulating levels of sST2 are involved in the aberrant inflammatory process of ARDS and have also been linked to acute and chronic HF, myocardial infarction, sepsis, and fibrosis[80]. Among patients with ARDS, sST2 elevations up to ten times the normal expected for HF has been seen, and this correlated with an increase in their mortality[81,82]. As evident in PRIDE study, among the 593 patients admitted with acute dyspnea, sST2 concentrations were higher among those with acute HF[79]. NT-proBNP however outperformed sST2 for acute HF diagnosis (AUC = 0.94 vs 0.80; P < 0.001). In patients with HFpEF, Manzano-Fernández et al[83] showed sST2 to be superior to NT-proBNP for prognosis. In addition, it also strongly correlated to the 30-d, one-year, and four-year mortality. Rehman et al[84] found that values of sST2 correlated with the severity of HF, making it a powerful predictor of mortality. Lassus et al[85] showed that in patients with pre-existing HF, sST2, relative to other biomarkers, is a powerful variable for one-year mortality. Similarly, Breidthardt et al[86] reported that a dynamic change in sST2 value from admission to discharge was a stronger predictor of mortality than baseline values alone.
Role of sST2 in prognosis and outcomes in COVID-19: Soluble ST2 is linked to SARS-CoV-2 viremia and indicators of inflammation, cardiovascular disease, and thrombosis. Omland et al[87] found an association between sST2 and disease severity among patients hospitalized for COVID-19 and was independent of established risk factors. Elevated ST2 concentrations above 37.9 ng/mL correlated with severe disease, with non-survivors having concentration as high as 107 ng/mL. Similar results were seen by Huang et al[88] and Ragusa et al[89], who concluded that sST2 is an important COVID-19 prognostic marker and correlated with disease severity. This association was deemed secondary to pulmonary fibrosis, seen as a complication in COVID-19. Elevations in sST2 Levels strongly correlated with mortality in ICU patients with sepsis secondary to COVID-19[87,90]. Omland et al[87] also noted that elevations in sST2 correlated with poor outcomes on days 3 and 9 of hospitalization among patients with COVID-19.
Galectin-3
Galectin-3 (GAL-3) is a mineralocorticoid receptor-regulated pro-inflammatory molecule. It exhibits a pleiotropic role in mediating infection and inflammation. Gal-3 is a biomarker of fibrosis and inflammation and has been implicated in the development and progression of HF[91]. ARDS is chiefly mediated by releasing IL-1, IL-6, and TNF-α from macrophages, monocytes, and dendritic cells[92]. Gal-3 inhibition has been shown to reduce the release of these cytokines from immune cells[93]. The PRIDE study showed that higher galectin-3 concentration was a strong independent predictor of 60-d mortality and recurrent HF admissions[94]. Shah et al[95] showed that galectin-3 above a median value of 15.0 had a strong prognostic significance in HF and was a significant predictor of 4-year mortality.
Role of galectin-3 in prognosis and outcomes in COVID-19: Multiple studies have shown Gal-3 to be upregulated in patients suffering from severe COVID-19. Among patients with COVID-19, Gal-3 was shown to be considerably higher in bronchoalveolar immune cells in patients with severe disease when compared to those with mild disease[96]. Higher galectin-3 levels were found to be a major predictor of 60-d mortality and recurrent HF hospitalizations. In a study of SARS-CoV-2 associated ARDS patients, high Gal-3 above 35.3 ng/mL was linked to worse outcomes and shorter survival[97].
Copeptin
Copeptin is a surrogate marker for vasopressin release. Copeptin is an arginine-vasopressin (AVP) glycopeptide composed of 39 amino acids. It is released from the neurohypophysis by osmotic or hemodynamic stimulation with AVP, and its plasma levels correlate well. AVP is an antidiuretic and vasoconstrictive hormone. It shows the endogenous stress response and is released by stimuli including hypotension, hypoxia, and infections. However, its circadian rhythm, short half-life, and unstable molecule make it impossible to use it as a biomarker[98]. Copeptin is a more stable peptide, and its level in the blood can be easily detected. The role of copeptin has been implicated in chronic HF. Elevated copeptin levels, especially in HF patients with hyponatremia, has been linked to poor outcomes. Maisel et al[99] noted that patients with elevated copeptin levels had a greater risk of 90-d mortality and HF readmission.
Role of copeptin in prognosis and outcomes in COVID-19: The importance of copeptin as a biomarker in COVID-19 patients has not been very well studied. Gregoriano et al[100] found that the rise in copeptin levels correlated with the disease severity in COVID-19 patients. Copeptin level of 20 pmol/L had an 88.2% sensitivity and a 64.9% specificity for identifying severe disease. Similar results were seen by Hammad et al[101] by using a cut off level of 18.5 pmol/L, yielding a sensitivity of 93.3% and a specificity of 100% for severe COVID-19 disease. In these studies, patients with severe COVID-19 disease were also noted to have increased mortality.
Growth differentiation factor 15
Growth differentiation factor 15 (GDF-15), also known as macrophage inhibitory cytokine (MIC-1), is a member of the transforming growth factor-beta (TGF-β) superfamily that helps tissues survive inflammatory stress. GDF-15 expression outside the reproductive organs is low to absent; it is upregulated in pathological conditions that involve inflammation or oxidative stress, including cancer, cardiovascular, pulmonary, and renal disease[102].
Role of GDF-15 in prognosis and outcomes in COVID-19: In a study of 84 patients with COVID-19, Apfel et al[103] determined that higher circulating levels of GDF-15 correlated with the disease severity. Patients with COVID-19 had an average GDF-15 level of 2051 pg/mL when compared to 582 pg/mL in non COVID patients. GDF-15 levels were higher in patients requiring mechanical ventilation and correlated with increasing oxygen requirements. In a different study by Verhamme et al[102], higher GDF-15 Levels were associated with increased mortality risk. Figure 2 illustrates the variation of different cardiac bioenzymes across different etiologies for cardiovascular dysfunction.
Figure 2.

Studies showing elevation of cardiac bioenzymes across different etiologies of cardiovascular dysfunction.
RESULTS
A total of 560 papers were identified after extensive literature review, as depicted in the PRISMA diagram (Figure 1). Among them, 61 papers were eligible to be included in the review. Cardiac troponin and natriuretic peptides were the most extensively studied of the bioenzymes. The evidence of cardiac troponin as a diagnostic marker for cardiovascular injury in COVID-19 is robust and has been shown on thousands (n = 11290) of patients worldwide, in both prospective and retrospective studies (Table 1). A consistently elevated level of cTn has been reported in COVID-19 patients with mild myocarditis to severe cardiogenic shock. Multiple studies have shown that troponin levels above the 99th percentile of upper limit of normal, to be associated with worse prognosis. Elevated cTn has been shown to correlate with severe disease, higher oxygen requirement, ARDS, the need for respiratory support including noninvasive and invasive mechanical ventilation, the requirement of intensive care unit admission, acute kidney injury, multiorgan failure, sepsis, pulmonary embolism, major bleeding and in-hospital mortality (Table 1). Troponin levels elevated five times the upper limit of normal have shown a 2.5% increase in in-hospital mortality. NPs are the second most studied cardiac biomarker in studies reporting cardiac injury in patients with COVID-19. Multiple studies have echoed a significant positive correlation between the rise in natriuretic peptides and disease severity in SARS-CoV-2[20,40-43] (summary in Table 2). Many of these studies have utilised the cutoff points for NT-proBNP based off the triple cut point strategy from European society guidelines[32] . In patients with pre-existing HF, natriuretic peptides have been independently associated with increased odds of the need for mechanical ventilation and death across studies[40,48-55] (Table 2). Novel biomarkers including presepsin, copeptin, soluble ST2 and galectin have also been implicated as prognostic markers in COVID-19, as detailed in Table 3.
Table 3.
Summary of studies characterizing the role of novel biomarkers in prognosis and outcomes in COVID-19
|
Biomarker in COVID
|
Study
|
Recommendation
|
| Presepsin | Favaloro et al[73] | Presepsin elevation is a reliable biomarker in COVID-19 |
| Park et al[74] | A four-to-five-fold increase in presepsin correlates with disease severity in COVID-19 | |
| Lippi et al[75] | ||
| Koyjit et al[73] | ||
| Fukada et al[77] | Presepsin is elevated in severe cases of SARS-CoV-2 associated respiratory complications | |
| Park et al[74] | Presepsin level at 717pg/ml is a significant predictor of 30-day mortality | |
| Dell’Aquila et al[79] | A threefold rise in presepsin has been identified as a very specific indicator of 30-day mortality | |
| Lippi et al[75] | ||
| Soluble ST2 (sST2) | Omland et al[87] | Robust association between baseline sST2 level and disease severity along with poor outcome |
| Huang et al[88] | Baseline sST2 is associated with a worse prognosis | |
| Ragusa et al[89] | Circulating level of sST2 can be used as a discharge prognosticator | |
| Galectin-3 | Caniglia et al[96] | Gal-3 is considerably higher in bronchoalveolar immune cells in patients with severe COVID-19 disease |
| Portacci et al[97] | Higher galectin-3 is associated with worse outcomes and shorter survival | |
| Copeptin | Gregoriano et al[100] | Serum copeptin level above 20 Pmol/L had sensitivity of > 88% to predict severe COVID-19 |
| Hammad et al[101] | Serum copeptin level above 18.5 Pmol/L had sensitivity of > 93% and specificity of 100% to predict severe COVID-19 | |
| GDF 15 | Apfel et al[103] | Higher levels of GDF-15 correlated with severity of COVID-19 |
Gal-3: Galectin-3; GDF-15: Growth differentiation factor 15; sST2: Soluble ST2.
DISCUSSION
SARS-CoV-2 associated COVID-19 infection is a global disease with multiple clinical manifestations. Cardiovascular complications are a dreaded outcome, and assessment of cardiac bio enzymes is crucial in gauging the disease severity. Our review aims at highlighting the variation in these bio enzymes through the disease process and their role in predicting outcomes.
Troponin has been seen as a robust indicator of cardiovascular injury across aetiologies including myocarditis, coronary syndromes, and cardiomyopathy. Serum cTn above 0.09 ng/mL have been shown to confer a higher risk of cardiovascular injury. Generally, studies have shown that troponin levels above the 99th percentile of upper limit of normal are associated with a worse prognosis. Elevated levels on admission, and serial up-trending carry a high positive predictive value for worse prognosis. Furthermore, long term sequelae with impaired ventricular function and subsequent development of heart failure is seen in a select subset of patients with cTn elevation[103]. Alongside cTn, natriuretic peptides help in prognostication mainly in patients with HF. Elevations in levels above cut off for normal[32] have been associated with worse outcomes. Multiple different studies citing the utility of NPs have been included in this review, and each of them had a unique cut off for HF. Irrespective of the cut-off used, elevated NT-proBNP was independently associated with poor outcomes regardless of the HF status[104]. This finding was common across studies.
The role of a few of these cardiac biomarkers has been studied before. However, our review is unique in its discussion about the role of novel biomarkers including presepsin, soluble ST2, galectin-3 which have not been studied extensively yet. Prior studies have highlighted their importance in HF, but not so much in COVID-19. Our review has consolidated these studies, to mention that these biomarkers, similar to troponin and NPs are elevated in patients with severe COVID-19 and can aid in prognosis[105,106].
Our study has a few limitations too. Majority of the studies that have been included are from China and European countries. This is partly because many studies were originally from Wuhan China, where the pandemic began, opening a possibility that many patients would have been repeated across studies. Another limitation is the nature of these studies, majority were retrospective or observational in nature. The trend of these bio enzymes could not be followed in patients who recovered from the illness. Small sample size of a few of these studies also precludes the generalisability. Hence future large prospective studies with follow up will be beneficial, especially for novel biomarkers.
CONCLUSION
SARS-CoV-2 associated COVID-19 infection undeniably has respiratory complications, however, extensive cardiovascular implications are also seen. Multiple cardiac biomarkers can help predict the severity of the disease and serve as prognostic indicators for outcomes and mortality. Assessment of cardiac bioenzymes at admission and their serial monitoring can help assess the severity of disease and predict mortality in patients with SARS-CoV-2 infection. A more liberal determination of cardiac biomarkers may improve early diagnosis and management of AHF, and other cardiovascular complications. COVID-19 associated myocarditis and HF have sequential effects even after the resolution of primary illness, and hence long-term correlation needs to be studied. In addition, there is emerging data on novel biomarkers, including growth GDF-15, soluble ST2, galectin 3, presepsin, and copeptin, which can aid in evaluation alongside natriuretic peptides and troponins. Further studies are needed to elude the critical importance of these novel markers.
ARTICLE HIGHLIGHTS
Research background
The coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is prevalent worldwide. Though lung injury is the most common presentation, cardiovascular dysfunction is seen on account of the widespread inflammation.
Research motivation
Cardiac biomarkers are released secondary to cardiovascular injury, and can be used as surrogate markers to gauge the disease severity.
Research objectives
To identify the role of individual biomarkers in diagnosing cardiac injury, and implications in determining prognosis and mortality.
Research methods
An extensive literature search was conducted for all studies on patients with COVID-19 associated cardiovascular injury and cardiac bioenzymes. Articles were screened using PubMed/Medline database, additionally reference citation analysis tool was also used. Eligible articles were then included in the study.
Research results
Cardiac troponin was seen as a robust diagnostic marker of cardiovascular injury across studies. Elevated troponin levels correlated with the level of disease severity. Similar results were seen alongside elevations in natriuretic peptides, irrespective of their prior diagnosis of heart failure.
Research conclusions
Multiple cardiac biomarkers can help predict the severity of disease and serve for prognostication purposes. Assessment of bioenzymes at admission and their serial monitoring can help predict mortality in patients with COVID-19.
Research perspectives
New data is emerging on novel biomarkers including soluble ST2, galectin-3, presepsin and copeptin which can further aid in diagnostic evaluation alongside troponins and natriuretic peptides.
Footnotes
Conflict-of-interest statement: All authors report no relevant conflict of interest for this article.
PRISMA 2009 Checklist statement: The authors have read the PRISMA 2009 Checklist, and the manuscript was prepared and revised according to the PRISMA 2009 Checklist.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: April 25, 2022
First decision: May 31, 2022
Article in press: August 10, 2022
Specialty type: Virology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mukhopadhyay A, India; Munteanu C, Romania S-Editor: Wu YXJ L-Editor: A P-Editor: Wu YXJ
Contributor Information
Anjani Muthyala, Department of Internal Medicine, Saint Vincent Hospital, Worcester, MA 01608, United States.
Sandeep Sasidharan, Department of Internal Medicine, Saint Vincent Hospital, Worcester, MA 01608, United States.
Kevin John John, Department of Critical Care, Belivers Church Medical College Hospital, Thiruvalla 689103, Kerela, India.
Amos Lal, Department of Medicine, Division of Pulmonary and Critical Care Medicine, Mayo Clinic, Rochester, MN 55905, United States.
Ajay K Mishra, Department of Cardiology, Saint Vincent Hospital, Worcester, MA 01608, United States. ajay.mishra@stvincenthospital.com.
References
- 1.Inciardi RM, Lupi L, Zaccone G, Italia L, Raffo M, Tomasoni D, et al. Cardiac Involvement in a Patient with Coronavirus Disease 2019 (COVID-19) JAMA Cardio . 2020;5:819–824. doi: 10.1001/jamacardio.2020.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zeng JH, Liu YX, Yuan J, Wang FX, Wu WB, Li JX, et al. First case of COVID-19 complicated with fulminant myocarditis: a case report and insights. Infection. 2020;48:773–777. doi: 10.1007/s15010-020-01424-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu H, Ma F, Wei X, Fang Y. Coronavirus fulminant myocarditis treated with glucocorticoid and human immunoglobulin. Eur Heart J. 2021;42:206. doi: 10.1093/eurheartj/ehaa190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med . 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng YY, Ma YT, Zhang JY, Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17:259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, Wang H, Wan J, Wang X, Lu Z. Cardiovascular Implications of Fatal Outcomes of Patients With Coronavirus Disease 2019 (COVID-19) JAMA Cardiol. 2020;5:811–818. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aleksova A, Sinagra G, Beltrami AP, Pierri A, Ferro F, Janjusevic M, et al. Biomarkers in the management of acute heart failure: state of the art and role in COVID-19 era. ESC Heart Fail . 2021;8:4465–4483. doi: 10.1002/ehf2.13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Solaro CR, Solaro RJ. Implications of the complex biology and micro-environment of cardiac sarcomeres in the use of high affinity troponin antibodies as serum biomarkers for cardiac disorders. J Mol Cell Cardiol. 2020;143:145–158. doi: 10.1016/j.yjmcc.2020.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kobayashi T, Solaro RJ. Calcium, thin filaments, and the integrative biology of cardiac contractility. Annu Rev Physiol. 2005;67:39–67. doi: 10.1146/annurev.physiol.67.040403.114025. [DOI] [PubMed] [Google Scholar]
- 11.Chapman AR, Bularga A, Mills NL. High-Sensitivity Cardiac Troponin Can Be an Ally in the Fight Against COVID-19. Circulation. 2020;141:1733–1735. doi: 10.1161/CIRCULATIONAHA.120.047008. [DOI] [PubMed] [Google Scholar]
- 12.Tavazzi G, Pellegrini C, Maurelli M, Belliato M, Sciutti F, Bottazzi A. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur J Heart Fail . 2020;22:911–915. doi: 10.1002/ejhf.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Solaro RJ, Rosas PC, Langa P, Warren CM, Wolska BM, Goldspink PH. Mechanisms of troponin release into serum in cardiac injury associated with COVID-19 patients. Int J Cardiol Cardiovasc Dis. 2021;1:41–47. doi: 10.46439/cardiology.1.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lala A, Johnson KW, Januzzi JL, Russak AJ, Paranjpe I, Richter F, et al. Prevalence and Impact of Myocardial Injury in Patients Hospitalized With COVID-19 Infection. J Am Coll Cardiol . 2020;76:533–546. doi: 10.1016/j.jacc.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khaloo P, Shaqdan A, Ledesma PA, Uzomah UA, Galvin J, Ptaszek LM, et al. Distinct etiologies of high-sensitivity troponin T elevation predict different mortality risks for patients hospitalized with COVID-19. Int J Cardiol . 2022;351:118–125. doi: 10.1016/j.ijcard.2021.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rezabakhsh A, Sadat-Ebrahimi SR, Ala A, Nabavi SM, Banach M, Ghaffari S. A close-up view of dynamic biomarkers in the setting of COVID-19: Striking focus on cardiovascular system. J Cell Mol Med. 2022;26:274–286. doi: 10.1111/jcmm.17122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sandoval Y, Januzzi JL Jr, Jaffe AS. Cardiac Troponin for Assessment of Myocardial Injury in COVID-19: JACC Review Topic of the Week. J Am Coll Cardiol. 2020;76:1244–1258. doi: 10.1016/j.jacc.2020.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Almeida Junior GLG, Braga F, Jorge JK, Nobre GF, Kalichsztein M, Faria PMP, Bussade B, Penna GL, Alves VO, Pereira MA, Gorgulho PC, Faria MRDSE, Drumond LE, Carpinete FBS, Neno ACLB, Neno ACA. Prognostic Value of Troponin-T and B-Type Natriuretic Peptide in Patients Hospitalized for COVID-19. Arq Bras Cardiol. 2020;115:660–666. doi: 10.36660/abc.20200385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maino A, Di Stasio E, Grimaldi MC, Cappannoli L, Rocco E, Vergallo R, et al. Prevalence and characteristics of myocardial injury during COVID-19 pandemic: A new role for high-sensitive troponin. Int J Cardiol. 2021;338:278–285. doi: 10.1016/j.ijcard.2021.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arcari L, Luciani M, Cacciotti L, Musumeci MB, Spuntarelli V, Pistella E, et al. Incidence and determinants of high-sensitivity troponin and natriuretic peptides elevation at admission in hospitalized COVID-19 pneumonia patients. Intern Emerg Med. 2020;15:1467–1476. doi: 10.1007/s11739-020-02498-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scarl RT, Balada-LIasat JM, Nowacki N, Solaro RJ, Williams J, Li J. Myocardial Injury, Inflam-mation and Prothrombotic Response Are Associated with Outcomes of COVID-19 Patients. Ann Cardiol Vasc Med . 2021;4:1041. [Google Scholar]
- 22.Mueller C, Giannitsis E, Jaffe AS, Huber K, Mair J, Cullen L. Cardiovascular biomarkers in patients with COVID-19. Eur Heart J Acute Cardiovasc Care . 2021;10:310–319. doi: 10.1093/ehjacc/zuab009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henein MY, Mandoli GE, Pastore MC, Ghionzoli N, Hasson F, Nisar MK. Biomarkers Predict In-Hospital Major Adverse Cardiac Events in COVID-19 Patients: A Multicenter International Study. J Clin Med. 2021;10:5863. doi: 10.3390/jcm10245863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kermali M, Khalsa RK, Pillai K, Ismail Z, Harky A. The role of biomarkers in diagnosis of COVID-19 - A systematic review. Life Sci. 2020;254:117788. doi: 10.1016/j.lfs.2020.117788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arcari L, Luciani M, Cacciotti L, Pucci M, Musumeci MB, Pietropaolo L. Coronavirus disease 2019 in patients with cardiovascular disease: clinical features and implications on cardiac biomarkers assessment. J Cardio Med . 2021;22 doi: 10.2459/JCM.0000000000001252. Available from: https://journals.lww.com/jcardiovascularmedicine/Fulltext/2021/11000/Coronavirus_disease_2019_in_patients_with.6.aspx. [DOI] [PubMed] [Google Scholar]
- 26.Lombardi CM, Carubelli V, Iorio A, Inciardi RM, Bellasi A, Canale C, Camporotondo R, Catagnano F, Dalla Vecchia LA, Giovinazzo S, Maccagni G, Mapelli M, Margonato D, Monzo L, Nuzzi V, Oriecuia C, Peveri G, Pozzi A, Provenzale G, Sarullo F, Tomasoni D, Ameri P, Gnecchi M, Leonardi S, Merlo M, Agostoni P, Carugo S, Danzi GB, Guazzi M, La Rovere MT, Mortara A, Piepoli M, Porto I, Sinagra G, Volterrani M, Specchia C, Metra M, Senni M. Association of Troponin Levels With Mortality in Italian Patients Hospitalized With Coronavirus Disease 2019: Results of a Multicenter Study. JAMA Cardiol. 2020;5:1274–1280. doi: 10.1001/jamacardio.2020.3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salvatici M, Barbieri B, Cioffi SMG, Morenghi E, Leone FP, Maura F. Association between cardiac troponin I and mortality in patients with COVID-19. Biomarkers . 2020;25:634–640. doi: 10.1080/1354750X.2020.1831609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Al Abbasi B, Torres P, Ramos-Tuarez F, Dewaswala N, Abdallah A, Chen K. Cardiac Troponin-I and COVID-19: A Prognostic Tool for In-Hospital Mortality. Cardiol Res . 2020;11:398–404. doi: 10.14740/cr1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghossein MA, Driessen RGH, van Rosmalen F, Sels JWEM, Delnoij T, Geyik Z. Serial Assessment of Myocardial Injury Markers in Mechanically Ventilated Patients With SARS-CoV-2 (from the Prospective MaastrICCht Cohort) Am J Cardiol . 2022;170:118–127. doi: 10.1016/j.amjcard.2022.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laouan Brem F, Chaymae M, Rasras H, Merbouh M, Bouazzaoui MA, Bkiyar H, Abda N, Zakaria B, Ismaili N, Housni B, El Ouafi N. Acute Myocardial Injury Assessed by High-Sensitive Cardiac Troponin Predicting Severe Outcomes and Death in Hospitalized Patients with COVID-19 Infection. Clin Appl Thromb Hemost. 2022;28:10760296221090227. doi: 10.1177/10760296221090227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Italia L, Ingallina G, Napolano A, Boccellino A, Belli M, Cannata F. Subclinical myocardial dysfunction in patients recovered from COVID-19. Echocardiography . 2021;38:1778–1786. doi: 10.1111/echo.15215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mueller C, McDonald K, de Boer RA, Maisel A, Cleland JGF, Kozhuharov N. Heart Failure Association of the European Society of Cardiology practical guidance on the use of natriuretic peptide concentrations. Eur J Heart Fail. 2019;21:715–731. doi: 10.1002/ejhf.1494. [DOI] [PubMed] [Google Scholar]
- 33.Schlueter N, de Sterke A, Willmes DM, Spranger J, Jordan J, Birkenfeld AL. Metabolic actions of natriuretic peptides and therapeutic potential in the metabolic syndrome. Pharmacol Ther. 2014;144:12–27. doi: 10.1016/j.pharmthera.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 34.Myhre PL, Vaduganathan M, Claggett BL, Anand IS, Sweitzer NK, Fang JC, O'Meara E, Shah SJ, Desai AS, Lewis EF, Rouleau J, Pitt B, Pfeffer MA, Solomon SD. Association of Natriuretic Peptides With Cardiovascular Prognosis in Heart Failure With Preserved Ejection Fraction: Secondary Analysis of the TOPCAT Randomized Clinical Trial. JAMA Cardiol. 2018;3:1000–1005. doi: 10.1001/jamacardio.2018.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mueller C, Laule-Kilian K, Frana B, Rodriguez D, Scholer A, Schindler C, Perruchoud AP. Use of B-type natriuretic peptide in the management of acute dyspnea in patients with pulmonary disease. Am Heart J. 2006;151:471–477. doi: 10.1016/j.ahj.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 36.Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, Gong W, Liu X, Liang J, Zhao Q, Huang H, Yang B, Huang C. Association of Cardiac Injury With Mortality in Hospitalized Patients With COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thomas-Rüddel D, Winning J, Dickmann P, Ouart D, Kortgen A, Janssens U, Bauer M. Coronavirus disease 2019 (COVID-19): update for anesthesiologists and intensivists March 2020. Anaesthesist. 2021;70:1–10. doi: 10.1007/s00101-020-00760-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bois MC, Boire NA, Layman AJ, Aubry MC, Alexander MP, Roden AC. COVID-19-Associated Nonocclusive Fibrin Microthrombi in the Heart. Circulation . 2021;143:230–243. doi: 10.1161/CIRCULATIONAHA.120.050754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mehra Mandeep R, Ruschitzka Frank. COVID-19 Illness and Heart Failure. JACC: Heart Failure. 2020;8:512–514. doi: 10.1016/j.jchf.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Caro-Codón J, Rey JR, Buño A, Iniesta AM, Rosillo SO, Castrejon-Castrejon S. Characterization of NT-proBNP in a large cohort of COVID-19 patients. Eur J Heart Fail . 2021;23:456–464. doi: 10.1002/ejhf.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gao W, Fan J, Sun D, Yang M, Guo W, Tao L, Zheng J, Zhu J, Wang T, Ren J. Heart Failure Probability and Early Outcomes of Critically Ill Patients With COVID-19: A Prospective, Multicenter Study. Front Cardiovasc Med. 2021;8:738814. doi: 10.3389/fcvm.2021.738814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sorrentino S, Cacia M, Leo I, Polimeni A, Sabatino J, Spaccarotella CAM. B-Type Natriuretic Peptide as Biomarker of COVID-19 Disease Severity-A Meta-Analysis. J Clin Med . 2020;9:2957. doi: 10.3390/jcm9092957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yoo J, Grewal PK, Hotelling J, Papamanoli A, Cao K, Dhaliwal S. Admission Nt_proBNP and outcomes in patients without history of heart failure hospitalized with COVId_19. ESC Heart Fail. 2021;8:4278–4287. doi: 10.1002/ehf2.13548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alvarez-Garcia J, Lee S, Gupta A, Cagliostro M, Joshi AA, Rivas-Lasarte M. Prognostic Impact of Prior Heart Failure in Patients Hospitalized With COVID-19. J Am Coll Cardiol . 2020;76:2334–2348. doi: 10.1016/j.jacc.2020.09.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dawson D, Dominic P, Sheth A, Modi M. Prognostic value of Cardiac Biomarkers in COVID-19 Infection: A Meta-analysis. Res Sq. 2020 doi: 10.1038/s41598-021-84643-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abdeen Y, Kaako A, Alnabulsi M, Okeh A, Meng W, Miller R. The prognostic effect of brain natriuretic peptide levels on outcomes of hospitalized patients with COVID-19. Avicenna J Med. 2021;11:20–26. doi: 10.4103/ajm.ajm_169_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, Ma K, Xu D, Yu H, Wang H, Wang T, Guo W, Chen J, Ding C, Zhang X, Huang J, Han M, Li S, Luo X, Zhao J, Ning Q. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gao L, Jiang D, Wen XS, Cheng XC, Sun M, He B. Prognostic value of NT-proBNP in patients with severe COVID-19. Respir Res. 2020;21:83–83. doi: 10.1186/s12931-020-01352-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Calvo-Fernández A, Izquierdo A, Subirana I, Farré N, Vila J, Durán X, García-Guimaraes M, Valdivielso S, Cabero P, Soler C, García-Ribas C, Rodríguez C, Llagostera M, Mojón D, Vicente M, Solé-González E, Sánchez-Carpintero A, Tevar C, Marrugat J, Vaquerizo B. Markers of myocardial injury in the prediction of short-term COVID-19 prognosis. Rev Esp Cardiol (Engl Ed) 2021;74:576–583. doi: 10.1016/j.rec.2020.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Selcuk M, Keskin M, Cinar T, Gunay N, Dogan S, Cicek V, et al. Prognostic significance of N-Terminal Pro-BNP in patients with COVID-19 pneumonia without previous history of heart failure. Eur Heart J . 2021;42:ehab724.0866. doi: 10.34172/jcvtr.2021.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Iorio A, Lombardi CM, Specchia C, Merlo M, Nuzzi V, Ferraro I, Peveri G, Oriecuia C, Pozzi A, Inciardi RM, Carubelli V, Bellasi A, Canale C, Camporotondo R, Catagnano F, Dalla Vecchia L, Giovinazzo S, Maccagni G, Mapelli M, Margonato D, Monzo L, Provenzale G, Sarullo F, Tomasoni D, Ameri P, Gnecchi M, Leonardi S, Agostoni P, Carugo S, Danzi GB, Guazzi M, La Rovere MT, Mortara A, Piepoli M, Porto I, Volterrani M, Sinagra G, Senni M, Metra M. Combined Role of Troponin and Natriuretic Peptides Measurements in Patients With Covid-19 (from the Cardio-COVID-Italy Multicenter Study) Am J Cardiol. 2022;167:125–132. doi: 10.1016/j.amjcard.2021.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Belarte-Tornero LC, Valdivielso-Moré S, Vicente Elcano M, Solé-González E, Ruíz-Bustillo S, Calvo-Fernández A. Prognostic Implications of Chronic Heart Failure and Utility of NT-proBNP Levels in Heart Failure Patients with SARS-CoV-2 Infection. J Clin Med. 2021;10:323. doi: 10.3390/jcm10020323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dalia T, Lahan S, Ranka S, Acharya P, Gautam A, Goyal A, et al. Impact of congestive heart failure and role of cardiac biomarkers in COVID-19 patients: A systematic review and meta-analysis. Indian Heart J. 2021;73:91–98. doi: 10.1016/j.ihj.2020.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pranata R, Huang I, Lukito AA, Raharjo SB. Elevated N-terminal pro-brain natriuretic peptide is associated with increased mortality in patients with COVID-19: systematic review and meta-analysis. Postgrad Med J. 2020;96:387–391. doi: 10.1136/postgradmedj-2020-137884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stefanini GG, Chiarito M, Ferrante G, Cannata F, Azzolini E, Viggiani G, De Marco A, Briani M, Bocciolone M, Bragato R, Corrada E, Gasparini GL, Marconi M, Monti L, Pagnotta PA, Panico C, Pini D, Regazzoli D, My I, Kallikourdis M, Ciccarelli M, Badalamenti S, Aghemo A, Reimers B, Condorelli G Humanitas COVID-19 Task Force. Early detection of elevated cardiac biomarkers to optimise risk stratification in patients with COVID-19. Heart. 2020;106:1512–1518. doi: 10.1136/heartjnl-2020-317322. [DOI] [PubMed] [Google Scholar]
- 56.Pieske B, Tschöpe C, de Boer RA, Fraser AG, Anker SD, Donal E. How to diagnose heart failure with preserved ejection fraction: the HFA-PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC) Eur Heart J . 2019;40:3297. doi: 10.1093/eurheartj/ehz641. [DOI] [PubMed] [Google Scholar]
- 57.Lassen MCH, Skaarup KG, Lind JN, Alhakak AS, Sengeløv M, Nielsen AB. Recovery of cardiac function following COVID-19 - ECHOVID-19: a prospective longitudinal cohort study. Eur J Heart Fail. 2021;23:1903–1912. doi: 10.1002/ejhf.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Skaarup KG, Lassen MCH, Lind JN, Alhakak AS, Sengeløv M, Nielsen AB, Espersen C, Hauser R, Schöps LB, Holt E, Johansen ND, Modin D, Sharma S, Graff C, Bundgaard H, Hassager C, Jabbari R, Lebech AM, Kirk O, Bødtger U, Lindholm MG, Joseph G, Wiese L, Schiødt FV, Kristiansen OP, Walsted ES, Nielsen OW, Madsen BL, Tønder N, Benfield TL, Jeschke KN, Ulrik CS, Knop FK, Pallisgaard J, Lamberts M, Sivapalan P, Gislason G, Solomon SD, Iversen K, Jensen JUS, Schou M, Biering-Sørensen T. Myocardial Impairment and Acute Respiratory Distress Syndrome in Hospitalized Patients With COVID-19: The ECHOVID-19 Study. JACC Cardiovasc Imaging. 2020;13:2474–2476. doi: 10.1016/j.jcmg.2020.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lassen MCH, Skaarup KG, Lind JN, Alhakak AS, Sengeløv M, Nielsen AB, et al. Echocardiographic abnormalities and predictors of mortality in hospitalized COVID-19 patients: the ECHOVID-19 study. ESC Heart Fail. 2020;7:4189–4197. doi: 10.1002/ehf2.13044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Puntmann VO, Carerj ML, Wieters I, Fahim M, Arendt C, Hoffmann J, Shchendrygina A, Escher F, Vasa-Nicotera M, Zeiher AM, Vehreschild M, Nagel E. Outcomes of Cardiovascular Magnetic Resonance Imaging in Patients Recently Recovered From Coronavirus Disease 2019 (COVID-19) JAMA Cardiol. 2020;5:1265–1273. doi: 10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huang L, Zhao P, Tang D, Zhu T, Han R, Zhan C, Liu W, Zeng H, Tao Q, Xia L. Cardiac Involvement in Patients Recovered From COVID-2019 Identified Using Magnetic Resonance Imaging. JACC Cardiovasc Imaging. 2020;13:2330–2339. doi: 10.1016/j.jcmg.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moody WE, Liu B, Mahmoud-Elsayed HM, Senior J, Lalla SS, Khan-Kheil AM, Brown S, Saif A, Moss A, Bradlow WM, Khoo J, Ahamed M, McAloon C, Hothi SS, Steeds RP. Persisting Adverse Ventricular Remodeling in COVID-19 Survivors: A Longitudinal Echocardiographic Study. J Am Soc Echocardiogr. 2021;34:562–566. doi: 10.1016/j.echo.2021.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Escher F, Westermann D, Gaub R, Pronk J, Bock T, Al-Saadi N. Development of diastolic heart failure in a 6-year follow-up study in patients after acute myocarditis. Heart . 2011;97:709. doi: 10.1136/hrt.2010.199489. [DOI] [PubMed] [Google Scholar]
- 64.Li C, Jiang J, Wang F, Zhou N, Veronese G, Moslehi JJ, Ammirati E, Wang DW. Longitudinal correlation of biomarkers of cardiac injury, inflammation, and coagulation to outcome in hospitalized COVID-19 patients. J Mol Cell Cardiol. 2020;147:74–87. doi: 10.1016/j.yjmcc.2020.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Saleh A, Matsumori A, Abdelrazek S, Eltaweel S, Salous A, Neumann FJ. Myocardial involvement in coronavirus disease 19. Herz. 2020;45:719–725. doi: 10.1007/s00059-020-05001-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Han H, Xie L, Liu R, Yang J, Liu F, Wu K. Analysis of heart injury laboratory parameters in 273 COVID-19 patients in one hospital in Wuhan, China. J Med Virol . 2020;92:819–823. doi: 10.1002/jmv.25809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Qin JJ, Cheng X, Zhou F, Lei F, Akolkar G, Cai J, Zhang XJ, Blet A, Xie J, Zhang P, Liu YM, Huang Z, Zhao LP, Lin L, Xia M, Chen MM, Song X, Bai L, Chen Z, Zhang X, Xiang D, Chen J, Xu Q, Ma X, Touyz RM, Gao C, Wang H, Liu L, Mao W, Luo P, Yan Y, Ye P, Chen M, Chen G, Zhu L, She ZG, Huang X, Yuan Y, Zhang BH, Wang Y, Liu PP, Li H. Redefining Cardiac Biomarkers in Predicting Mortality of Inpatients With COVID-19. Hypertension. 2020;76:1104–1112. doi: 10.1161/HYPERTENSIONAHA.120.15528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wungu CDK, Khaerunnisa S, Putri EAC, Hidayati HB, Qurnianingsih E, Lukitasari L. Meta-analysis of cardiac markers for predictive factors on severity and mortality of COVID-19. Int J Infect Dis . 2021;105:551–559. doi: 10.1016/j.ijid.2021.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shoar S, Hosseini F, Naderan M, Mehta JL. Meta-analysis of Cardiovascular Events and Related Biomarkers Comparing Survivors Versus Non-survivors in Patients With COVID-19. Am J Cardiol. 2020;135:50–61. doi: 10.1016/j.amjcard.2020.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang HS, Hur M, Yi A, Kim H, Lee S, Kim SN. Prognostic value of presepsin in adult patients with sepsis: Systematic review and meta-analysis. PLoS One. 2018;13:e0191486. doi: 10.1371/journal.pone.0191486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nishimura H, Ishii J, Muramatsu T, Harada M, Motoyama S, Matsui S, et al. Presepsin, Soluble CD14 Subtype, Is a Novel Marker of Short-term Mortality in Patients Hospitalized for Worsening Heart Failure. J Cardiac Failure. 2017;23:S61. [Google Scholar]
- 72.Zhou W, Rao H, Ding Q, Lou X, Shen J, Ye B. Soluble CD14 Subtype in Peripheral Blood is a Biomarker for Early Diagnosis of Sepsis. Lab Med. 2020;51:614–619. doi: 10.1093/labmed/lmaa015. [DOI] [PubMed] [Google Scholar]
- 73.Favaloro EJ, Lippi G. Recommendations for Minimal Laboratory Testing Panels in Patients with COVID-19: Potential for Prognostic Monitoring. Semin Thromb Hemost. 2020;46:379–382. doi: 10.1055/s-0040-1709498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Park M, Hur M, Kim H, Lee CH, Lee JH, Kim HW. Prognostic Utility of Procalcitonin, Presepsin, and the VACO Index for Predicting 30-day Mortality in Hospitalized COVID-19 Patients. Ann Lab Med . 2022;42:406–414. doi: 10.3343/alm.2022.42.4.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lippi G, Sanchis-Gomar F, Henry BM. Presepsin value predicts the risk of developing severe/critical COVID-19 illness: results of a pooled analysis. Clin Chem Lab Med. 2022;60:e1–e3. doi: 10.1515/cclm-2021-0848. [DOI] [PubMed] [Google Scholar]
- 76.Koyjit A, Sogut O, Durmus E, Kao1mdan E, Guler EM, Kaplan O. Circulating furin, IL-6, and presepsin levels and disease severity in SARS-CoV-2_infected patients. Science Progress . 2021:104. doi: 10.1177/00368504211026119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fukada A, Kitagawa Y, Matsuoka M, Sakai J, Imai K, Tarumoto N. Presepsin as a predictive biomarker of severity in COVID-19: A case series. J Med Virol . 2021;93:99–101. doi: 10.1002/jmv.26164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zaninotto M, Mion MM, Cosma C, Rinaldi D, Plebani M. Presepsin in risk stratification of SARS-CoV-2 patients. Clin Chim Acta. 2020;507:161–163. doi: 10.1016/j.cca.2020.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dell'Aquila P, Raimondo P, Racanelli V, De Luca P, De Matteis S, Pistone A, Melodia R, Crudele L, Lomazzo D, Solimando AG, Moschetta A, Vacca A, Grasso S, Procacci V, Orso D, Vetrugno L. Integrated lung ultrasound score for early clinical decision-making in patients with COVID-19: results and implications. Ultrasound J. 2022;14:21. doi: 10.1186/s13089-022-00264-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Homsak E, Gruson D. Soluble ST2: A complex and diverse role in several diseases. Clin Chim Acta. 2020;507:75–87. doi: 10.1016/j.cca.2020.04.011. [DOI] [PubMed] [Google Scholar]
- 81.Bajwa EK, Volk JA, Christiani DC, Harris RS, Matthay MA, Thompson BT, Januzzi JL National Heart, Lung and Blood Institute Acute Respiratory Distress Syndrome Network. Prognostic and diagnostic value of plasma soluble suppression of tumorigenicity-2 concentrations in acute respiratory distress syndrome. Crit Care Med. 2013;41:2521–2531. doi: 10.1097/CCM.0b013e3182978f91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bajwa EK, Mebazaa A, Januzzi JL. ST2 in Pulmonary Disease. Am J Cardiol. 2015;115:44B–47B. doi: 10.1016/j.amjcard.2015.01.040. [DOI] [PubMed] [Google Scholar]
- 83.Manzano-Fernández S, Mueller T, Pascual-Figal D, Truong QA, Januzzi JL. Usefulness of soluble concentrations of interleukin family member ST2 as predictor of mortality in patients with acutely decompensated heart failure relative to left ventricular ejection fraction. Am J Cardiol. 2011;107:259–267. doi: 10.1016/j.amjcard.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rehman SU, Mueller T, Januzzi JL Jr. Characteristics of the novel interleukin family biomarker ST2 in patients with acute heart failure. J Am Coll Cardiol. 2008;52:1458–1465. doi: 10.1016/j.jacc.2008.07.042. [DOI] [PubMed] [Google Scholar]
- 85.Lassus J, Gayat E, Mueller C, Peacock WF, Spinar J, Harjola VP. Incremental value of biomarkers to clinical variables for mortality prediction in acutely decompensated heart failure: The Multinational Observational Cohort on Acute Heart Failure (MOCA) study. Int J Cardiol. 2013;168:2186–2194. doi: 10.1016/j.ijcard.2013.01.228. [DOI] [PubMed] [Google Scholar]
- 86.Breidthardt T, Balmelli C, Twerenbold R, Mosimann T, Espinola J, Haaf P, Thalmann G, Moehring B, Mueller M, Meller B, Reichlin T, Murray K, Ziller R, Benkert P, Osswald S, Mueller C. Heart failure therapy-induced early ST2 changes may offer long-term therapy guidance. J Card Fail. 2013;19:821–828. doi: 10.1016/j.cardfail.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 87.Omland T, Prebensen C, Jonassen C, Svensson M, Berdal JE, Seljeflot I, Myhre PL. Soluble ST2 concentrations associate with in-hospital mortality and need for mechanical ventilation in unselected patients with COVID-19. Open Heart. 2021;8 doi: 10.1136/openhrt-2021-001884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Huang J. Comparing biomarkers for COVID-19 disease with commonly associated preexisting conditions and complications. medRxiv. 2020; 2020.10.02.20205609. [Google Scholar]
- 89.Ragusa R, Basta G, Del Turco S, Caselli C. A possible role for ST2 as prognostic biomarker for COVID-19. Vascul Pharmacol. 2021;138:106857. doi: 10.1016/j.vph.2021.106857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hoogerwerf JJ, Tanck MW, van Zoelen MA, Wittebole X, Laterre PF, van der Poll T. Soluble ST2 plasma concentrations predict mortality in severe sepsis. Intensive Care Med. 2010;36:630–637. doi: 10.1007/s00134-010-1773-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Martínez-Martínez E, Calvier L, Fernández-Celis A, Rousseau E, Jurado-López R, Rossoni LV, Jaisser F, Zannad F, Rossignol P, Cachofeiro V, López-Andrés N. Galectin-3 blockade inhibits cardiac inflammation and fibrosis in experimental hyperaldosteronism and hypertension. Hypertension. 2015;66:767–775. doi: 10.1161/HYPERTENSIONAHA.115.05876. [DOI] [PubMed] [Google Scholar]
- 92.Zhang C, Wu Z, Li JW, Zhao H, Wang GQ. Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int J Antimicrob Agents. 2020;55:105954. doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chen SS, Sun LW, Brickner H, Sun PQ. Downregulating galectin-3 inhibits proinflammatory cytokine production by human monocyte-derived dendritic cells via RNA interference. Cell Immunol. 2015;294:44–53. doi: 10.1016/j.cellimm.2015.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Januzzi JL Jr, Peacock WF, Maisel AS, Chae CU, Jesse RL, Baggish AL, O'Donoghue M, Sakhuja R, Chen AA, van Kimmenade RR, Lewandrowski KB, Lloyd-Jones DM, Wu AH. Measurement of the interleukin family member ST2 in patients with acute dyspnea: results from the PRIDE (Pro-Brain Natriuretic Peptide Investigation of Dyspnea in the Emergency Department) study. J Am Coll Cardiol. 2007;50:607–613. doi: 10.1016/j.jacc.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 95.Shah RV, Chen-Tournoux AA, Picard MH, van Kimmenade RR, Januzzi JL. Galectin-3, cardiac structure and function, and long-term mortality in patients with acutely decompensated heart failure. Eur J Heart Fail. 2010;12:826–832. doi: 10.1093/eurjhf/hfq091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Caniglia JL, Asuthkar S, Tsung AJ, Guda MR, Velpula KK. Immunopathology of galectin-3: an increasingly promising target in COVID-19. F1000Res. 2020;9:1078. doi: 10.12688/f1000research.25979.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Portacci A, Diaferia F, Santomasi C, Dragonieri S, Boniello E, Di Serio F. Galectin-3 as prognostic biomarker in patients with COVID-19 acute respiratory failure. Respir Med . 2021;187:106556–106556. doi: 10.1016/j.rmed.2021.106556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Christ-Crain M, Fenske W. Copeptin in the diagnosis of vasopressin-dependent disorders of fluid homeostasis. Nat Rev Endocrinol. 2016;12:168–176. doi: 10.1038/nrendo.2015.224. [DOI] [PubMed] [Google Scholar]
- 99.Maisel A, Xue Y, Shah K, Mueller C, Nowak R, Peacock WF. Increased 90-Day Mortality in Patients With Acute Heart Failure With Elevated Copeptin. Circulation: Heart Failure . 2011;4:613–620. doi: 10.1161/CIRCHEARTFAILURE.110.960096. [DOI] [PubMed] [Google Scholar]
- 100.Gregoriano C, Molitor A, Haag E, Kutz A, Koch D, Haubitz S, Conen A, Bernasconi L, Hammerer-Lercher A, Fux CA, Mueller B, Schuetz P. Activation of Vasopressin System During COVID-19 is Associated With Adverse Clinical Outcomes: An Observational Study. J Endocr Soc. 2021;5:bvab045. doi: 10.1210/jendso/bvab045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hammad R, Elshafei A, Khidr EG, El-Husseiny AA, Gomaa MH, Kotb HG, Eltrawy HH, Farhoud H. Copeptin: a neuroendocrine biomarker of COVID-19 severity. Biomark Med. 2022;16:589–597. doi: 10.2217/bmm-2021-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Verhamme FM, Freeman CM, Brusselle GG, Bracke KR, Curtis JL. GDF-15 in Pulmonary and Critical Care Medicine. Am J Respir Cell Mol Biol. 2019;60:621–628. doi: 10.1165/rcmb.2018-0379TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Apfel T, Riecher-Rössler A. [Do psychiatric patients receive disability pension before adequate diagnostics and treatment? Psychiatr Prax. 2005;32:172–176. doi: 10.1055/s-2004-828496. [DOI] [PubMed] [Google Scholar]
- 104.John KJ, Mishra AK, Ramasamy C, George AA, Selvaraj V, Lal A. Heart failure in COVID-19 patients: Critical care experience. World J Virol. 2022;11:1–19. doi: 10.5501/wjv.v11.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mishra AK, Lal A, Sahu KK, Kranis M, Sargent J. Quantifying and reporting cardiac findings in imaging of COVID-19 patients. Monaldi Arch Chest Dis. 2020;90 doi: 10.4081/monaldi.2020.1394. [DOI] [PubMed] [Google Scholar]
- 106.Philip AM, George LJ, John KJ, George AA, Nayar J, Sahu KK, Selvaraj V, Lal A, Mishra AK. A review of the presentation and outcome of left ventricular thrombus in coronavirus disease 2019 infection. J Clin Transl Res. 2021;7:797–808. [PMC free article] [PubMed] [Google Scholar]


