Abstract
Purpose
The beneficial effects of a combination therapy using Bifidobacterium longum and galactooligosaccharide (GOS) for the treatment of atopic dermatitis (AD) have not been elucidated.
Methods
Gene expressions of interleukin (IL)-4 and IL-13 from peripheral blood mononuclear cells and fecal abundance of B. longum from 12-month-old infants were evaluated. Human primary epidermal keratinocytes (HEKs) and hairless mice were treated with B. longum, GOS, B. longum-derived extracellular vesicles (BLEVs), dinitrochlorobenzene (DNCB), or a synbiotic mixture of B. longum and GOS. Expression of epidermal barrier proteins and cytokines as well as serum immunoglobulin E (IgE) levels were analyzed in HEKs and mice. Dermatitis scores, transepidermal water loss (TEWL), epidermal thickness, and fecal B. longum abundance were evaluated in mice.
Results
Fecal abundance of B. longum was negatively correlated with blood IL-13 expression in infants. B. longum or BLEVs increased expression of filaggrin (FLG) and loricrin (LOR) in HEKs. B. longum increased the efficacy of GOS to upregulate FLG and LOR expressions in HEKs. Oral administration of GOS increased fecal abundance of B. longum in mice. Oral administration of B. longum attenuated DNCB-induced skin inflammation, abnormal TEWL, AD-like skin, and deficiency of epidermal barrier proteins. Moreover, the combination of B. longum and GOS showed greater effects to improve DNCB-induced skin inflammation, abnormal TEWL, AD-like skin, serum IgE levels, IL-4 over-expression, and the deficiency of epidermal barrier proteins than the administration of B. longum alone.
Conclusions
B. longum and GOS improve DNCB-induced skin barrier dysfunction and AD-like skin.
Keywords: Atopic dermatitis, Bifidobacterium longum, prebiotics, probiotics, synbiotics
INTRODUCTION
Recent insights into the pathophysiology of atopic dermatitis (AD) have focused on the pivotal roles of gut microbial dysbiosis and skin barrier dysfunction.1,2,3 It has been reported that children with eczema have low numbers of Lactobacillus, Bifidobacterium (B), and Bacteroides in the gut.4 Additionally, previous studies have suggested that oral administration of probiotics and prebiotics, such as galactooligosaccharide (GOS), has beneficial effects on the treatment of AD.5,6,7 Skin barrier dysfunction is a characteristic finding of AD and considered an initial step in the development of allergic diseases.3,8,9 Thus, maintaining normal skin barrier function is important for preventing and controlling AD.1,10,11
B. longum is one of the dominant microbial strains in the gut of vaginally-delivered and breast-fed infants.12 It has been suggested that B. longum inhibits the expression of T helper type 2 (Th2) cytokines and reduces immunoglobulin E (IgE) levels.13 GOS provides energy sources for intestinal microbes and produces short-chain fatty acids (SCFAs) that stimulate the growth of beneficial microorganisms.7,14,15 It has been demonstrated that oral administration of GOS attenuates dinitrochlorobenzene (DNCB)-induced dermatitis symptoms via changes in the intestinal microbial community and inhibition of Th2 cytokines.16 Therefore, it is supposed that oral administration of probiotics or prebiotics has beneficial effects on AD. However, specific mechanisms underlying the beneficial effects of probiotics and prebiotics have not been fully understood. Recently, it has also been proposed that a combination therapy with both probiotics and prebiotics is more effective than a single therapy of probiotics or prebiotics alone for the treatment of AD.17 However, it has not been clarified whether the combination therapy is more effective than a single therapy of probiotics or prebiotics alone for the treatment of AD.
Therefore, the present study examined whether B. longum modulates epidermal barrier proteins. Also, the beneficial effects of a synbiotic mixture (B. longum and GOS) on skin barrier dysfunction and AD-like skin were investigated.
MATERIALS AND METHODS
Human subjects and analysis of blood and fecal samples
Blood and fecal samples were collected from Korean 12-month-old infants who had never been administered oral antibiotics. Peripheral blood mononuclear cells (PBMCs) were purified from peripheral blood using standard Ficoll-PaqueTM PLUS (GE Healthcare, Chicago, IL, USA) gradient centrifugation according to the manufacturer’s instructions. Gene expressions of interleukin (IL)-4 and IL-13 from PBMC were evaluated using real-time reverse transcriptase-polymerase chain reaction (RT-PCR). This study was approved by the Institutional Review Board (IRB) of Samsung Medical Center in Seoul (IRB No. 2016-12-111), and written informed consent was obtained from each parent prior to participation in this study.
Preparation of B. longum and GOS
The B. longum KACC 91563 (GenBank accession number CBG0412011-1) was provided by the National Institute of Animal Science, Rural Development Administration, Jeonju, Korea, and was freeze-dried in a skim-milk-based protectant. The GOS was purchased from Biosynth Carbosynth (Campton, Berkshire, UK). They were isolated from feces of healthy Korean infants and anaerobically cultured at 37ºC.
Preparation and isolation of B. longum-derived extracellular vesicles (BLEVs)
B. longum was grown in tryptic soy broth, and 5 × 106 colony-forming units (CFU) /flask (150 cm2) of B. longum were incubated with differentiated human primary epidermal keratinocytes (HEKs) in serum-free Epilife™ cell culture medium (Life Technologies, Grand Island, NY, USA) for 48 hours. Then, culture medium was centrifuged at 8,000 rpm for 20 minutes. The supernatant was filtered using a 0.22-μm bottle-top filter to remove cellular debris and stored at −80°C until use. Non-conditioned control medium was generated in the same manner as above, except that no cells were cultured on the plates. Protein concentrations of BLEVs were measured using a Bicinchoninic Protein Assay Kit (ThermoFisher Scientific, Rockford, IL, USA).
Human primary keratinocytes culture
The HEKs were derived from neonatal foreskin and grown in serum-free EpiLife™ cell culture medium (Life Technologies) containing 0.06 mmol/L calcium chloride, 1% human keratinocyte growth supplement S7 (Life Technologies), and 1% gentamicin/amphotericin as described previously.9 To investigate the effects of the B. longum, GOS, and a synbiotic mixture (B. longum and GOS) on the mRNA expression of filaggrin (FLG) and loricrin (LOR), HEKs were differentiated in the presence of 1.3 mmol/L CaCl2 for 3 days. Cells were then incubated with B. longum (0.01, 0.05, or 0.1 CFU/cell), GOS (0.01%), a synbiotic mixture, a combination of IL-4 (5 ng/mL, R&D Systems, Minneapolis, MN, USA) and IL-13 (5 ng/mL, R&D Systems), or interferon (IFN)-γ (10 ng/mL, R&D Systems) for additional 24 hours. To investigate the effects of BLEVs on the expression of FLG and LOR, HEKs were differentiated in the presence of 1.3 mmol/L CaCl2 for 3 days. HEKs were then incubated with BLEVs (0, 25, 50, or 100 ng/mL) for additional 24 hours. All reagents including B. longum, GOS, cytokines, and BLEVs were diluted using the serum-free EpiLife™ cell culture medium (Life Technologies) for in vitro experiments.
Animal study
Hairless mice (Crl: SKH1-Hrhr, female, 12-week-old, Orient Bio Incorporation, Seongnam, Korea) were applied with 100 μL of 0.75% DNCB (Sigma-Aldrich Inc, St. Louis, MO, USA) in propylene glycol:EtOH (7:3 v/v) to the dorsal skin once daily for 4 weeks, while mice in the non-treatment group were treated with propylene glycol:EtOH (7:3 v/v) alone. The DNCB-treated mice were divided into 3 groups: the DNCB alone group, DNCB skin treatment plus oral administration of B. longum group, and DNCB plus synbiotics (oral administration of a combination of B. longum and GOS) group. The B. longum (107 CFU per mouse) and GOS (8 mg per mouse) were administered orally once a day from days 7 to 28. The dose was chosen in terms of previous animal study results, where B. longum was administered in doses ranging between 1 × 107 and 5 × 109 CFU/day.18,19 Mice were housed in a temperature-controlled animal room (24°C ± 2°C) under a 12 h/12 h light–dark cycle. They were fed standard laboratory food and water during the experiments. All experiments were approved by the Institutional Animal Care and Use Committee of Samsung Medical Center, Seoul, Korea (Certification No. SMC-20201113001).
Transepidermal water loss (TEWL) and dermatitis score were evaluated on days 0, 7, 14, 21, and 28. On each measurement day, a device from GPower Inc. (Seoul, Korea) was used to obtain 3 measurements of TEWL in the dorsal skin of each mouse, and the mean values were calculated. The severity of erythema/hemorrhage, scarring/dryness, excoriation/erosion, and edema was scored as 0 (none), 1 (mild), 2 (moderate), or 3 (severe). The dermatitis score was defined as the sum of these individual scores. All mice were euthanized using carbon dioxide on the final day of the experimental schedule, and 2 skin biopsy specimens (4 mm) were obtained from each animal. The skin biopsy specimens were submerged immediately into either TRI Reagent® (Sigma-Aldrich Inc) or 4% buffered formalin for real-time RT-PCR and immunostaining. Sections from formalin-fixed, paraffin-embedded skin were stained with hematoxylin and eosin (H&E), and epidermal thickness was measured under a microscope. Blood samples were obtained from mice, and total serum IgE concentration was measured using enzyme-linked immunosorbent assay (ELISA) kits (eBioscience Inc., San Diego, CA, USA).
RNA preparation and real time RT-PCR
The skin biopsy specimens of each mouse in TRI Reagent® (Sigma-Aldrich Inc) were homogenized, and chloroform was added. After centrifugation, the aqueous phase was transferred to a new tube and isopropanol was added. After centrifugation, the supernatant was removed and 70% ethanol was added to the RNA pellet. RNeasy Mini Kits (Qiagen, Valencia, CA, USA) were used according to the manufacturer’s protocol to isolate RNA from keratinocytes or the skin biopsies of mice. The RNA was reverse transcribed into cDNA using SuperScript® VILOTM MasterMix according to the manufacturer’s protocol (Life Technologies). The cDNA was analyzed using real-time quantitative-PCR and an ABI prism 7900 real-time PCR instrument (Applied Biosystems, ThermoFisher Scientific) as described previously.20 Primers and probes for GAPDH, IL-4, IL-5, IL-10, IL-12, IL-13, IL-33, transforming growth factor (TGF)-β, FLG, LOR, transglutaminase (TGM)-3, keratin (KRT)-1, KRT-10, and desmoglein (DSG)-1 were purchased from Applied Biosystems and normalized to the GAPDH mRNA levels.
Western blot
For Western blot analysis, 20 μg of total proteins extracted from HEKs were separated on 4%–20% Tris-Glycine gel (Invitrogen, Carlsbad, CA, USA) and transferred to the nitrocellulose membrane (Invitrogen). Membranes were blocked for 1 hour at room temperature in 5% non-fat milk. Primary antibodies were diluted with their respective blocking buffers and incubated overnight at 4°C. Antibodies against FLG (1:200 dilution, Cat# sc-66192, Santa Cruz Biotechnology Inc., Dallas, TX, USA), and β-actin (1:5,000 dilution, Cat# A-5441, Sigma-Aldrich) were used as primary antibodies. Washing was performed with TBS 0.1% Tween 20 (TBS-T) before addition of goat anti-mouse IgG HRP-conjugated secondary antibody (1:5,000 diluted, Cat# ab205719, Abcam) for 1 hour at room temperature. After incubating secondary antibody and washing with TBS-T, chemiluminescence detection was performed using a chemiluminescence kit (Amersham ECL Western Blotting Kit, GE Healthcare) and developed on Automatic X-RAY Film Processor JP-33 (JPI Healthcare, Korea). The densitometry of the detected protein bands was calculated using ImageJ software, version 1.52 (National Institutes of Health, Bethesda, MD, USA).
Immunofluorescence staining
Mouse skin sections were fixed and blocked with 5% bovine serum albumin and Super Block (ScyTek Laboratories, Logan, UT, USA). Slides were stained with an antibody against FLG (1:500 dilution, Cat# 905804, BioLegend, San Diego, CA, USA). Nuclei were visualized with DAPI, and wheat germ agglutinin-conjugated FITC was used to stain the cytoskeleton. The slides were visualized using fluorescent microscopy (Leica, Wetzler, Germany). Images were collected at 400x magnification, and the mean fluorescence intensity was measured using Slidebook 6.0 (Intelligent Imaging Innovations, Denver, CO, USA).
Analysis of the gut microbiome
Total DNA from fecal samples obtained from infants and mice was extracted using a FastDNA® SPIN Kit for Soil (MP Biomedicals, Santa Ana, CA, USA). Then, PCR amplification of the DNA was performed using fusion primers targeting the V3 to V4 regions of the 16S rRNA gene with the extracted DNA. The PCR amplification, DNA extraction, and sequencing were carried out at Chunlab, Inc. (Seoul, Korea) using the Illumina MiSeq Sequencing system (Illumina, San Diego, CA, USA) as described previously.21 After the QC pass, paired-end sequence data were merged using the fastq mergepairs command of VSEARCH version 2.13.4 with default parameters.22 Chimeric reads were filtered on reads with <97% similarity by reference-based chimeric detection using the UCHIME algorithm and the non-chimeric 16S rRNA database from EzBioCloud.23
Statistical analysis
All statistical analyses were conducted using GraphPad Prism, version 9 (San Diego, CA, USA) and SPSS 27.0 software (SPSS Inc., Armonk, NY, USA). In cases where 2 groups were compared, data were analyzed using a paired or unpaired Student’s t test. Correlations between 2 variables were calculated using Spearman’s correlation. Statistical differences between 3 or more groups were determined using a one-way ANOVA, and significant differences were determined by a Tukey-Kramer post hoc test. All error bars represent the SEM.
RESULTS
The fecal proportion of B. longum was negatively correlated with IL-13 from PBMCs in 12-month-old infants.
A total of 17 infants were enrolled, and the demographic findings are presented in Table. We found that the proportion of fecal B. longum among total fecal bacteria was negatively correlated with gene expression of IL-13 from PBMC (r = −0.501, P < 0.05) (Supplementary Fig. S1A). The relationship between the proportion of fecal B. longum and gene expression of IL-4 from PBMC failed to reach statistical significance (r = −0.448, P =0.08) (Supplementary Fig. S1B).
Table. Clinical characteristics of the subjects (n = 17).
| Characteristics | Values | |
|---|---|---|
| Sex (boys, %) | 14 (82.4) | |
| Birth weight (kg) | 3.2 ± 0.3 | |
| Intrauterine period (wk)* | 39.0 ± 1.9 | |
| Presence of atopic dermatitis | 4 (23.5) | |
| Birth type | ||
| Vaginal delivery | 7 (41.2) | |
| Cesarean section | 10 (58.8) | |
| Paternal history of allergic diseases | ||
| Atopic dermatitis | 2 (11.8) | |
| Allergic rhinitis | 3 (17.6) | |
| Asthma | 0 (0.0) | |
| Maternal history of allergic diseases | ||
| Atopic dermatitis | 4 (23.5) | |
| Allergic rhinitis | 11 (64.7) | |
| Asthma | 2 (11.8) | |
Data are presented as number (%) or mean ± standard deviation.
B. longum increased the efficacy of GOS to upregulate expression of epidermal barrier proteins in cultured human primary keratinocytes.
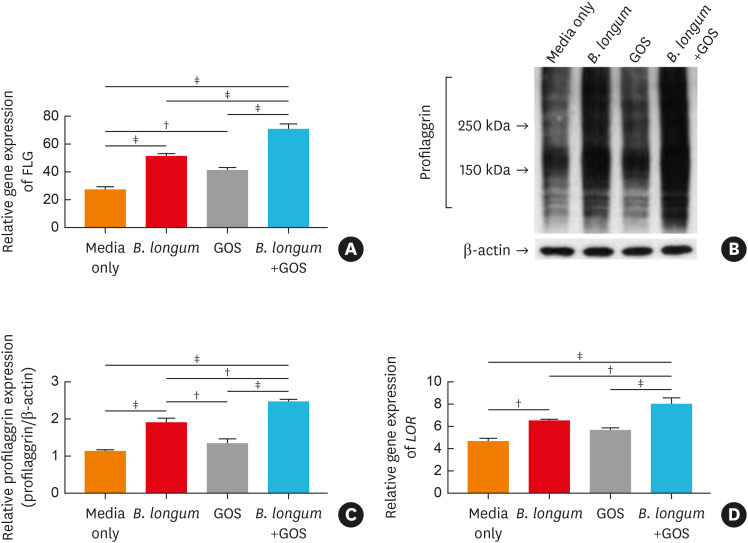
We examined whether B. longum and GOS modulate the expression of epidermal barrier proteins in HEKs. As depicted in Supplementary Fig. S2, gene expression of FLG was significantly increased in HEKs incubated with 0.05 CFU/cell (P < 0.001) or 0.1 CFU/cell (P < 0.05) of B. longum compared to cells culutred in media alone (Supplementary Fig. S2A). Additionally, gene expression of FLG was significantly increased in HEKs incubated with 0.01% GOS compared to cells cultured in media alone (P < 0.01) (Fig. 1A). Furthermore, FLG expression was significantly increased in HEKs treated with a synbiotic mixture (0.05 CFU/cell of B. longum and 0.01% GOS) compared to cells treated with 0.05 CFU/cell of B. longum or 0.01% GOS alone (all P < 0.001) (Fig. 1A). These findings were confirmed at the protein level using Western blotting (Fig. 1B and C). Similarly, various concentrations of B. longum (0.01, 0.05, or 0.1 CFU/cell) significantly increased gene expression of LOR in HEKs (P < 0.001, < 0.001, and < 0.01, respectively) (Supplementary Fig. S2B). Gene expression of LOR was also significantly upregulated in HEKs treated with a synbiotic mixture (0.05 CFU/cell of B. longum and 0.01% GOS) compared to cells treated with 0.05 CFU/cell of B. longum or 0.01% GOS alone (P < 0.01 and < 0.001) (Fig. 1D). Gene expression of FLG and LOR was significantly reduced by Th2 cytokines (all P < 0.01) and increased by IFN-γ (all P < 0.001) (Supplementary Fig. S2A and B).
Fig. 1. The effects of B. longum, GOS, or synbiotic mixtures of B. longum and GOS on expression of epidermal barrier proteins in human primary keratinocytes. Gene (A) and protein (B and C) expression of FLG and gene expression of LOR (D) in cultured HEKs were evaluated by RT-PCR and Western blotting. Data are representative of 3 independent experimental replicates. The data are shown as the mean ± SEM.
FLG, filaggrin; B. longum, Bifidobacterium longum; GOS, galactooligosaccharide; LOR, loricrin.
†P < 0.01, ‡P < 0.001 by a one-way ANOVA with Tukey-Kramer’s post hoc test.
BLEVs upregulated gene expression of epidermal barrier proteins in cultured human primary keratinocyte.
As shown in Supplementary Fig. S3A, gene expressions of FLG was significantly increased in HEKs treated with 50 ng/mL BLEVs compared to cells cultured in media alone (P < 0.01). Similarly, gene expression of LOR was significantly increased in HEKs treated with 50 ng/mL of BLEVs compared to cells cultured in media alone (P < 0.001) (Supplementary Fig. S3B). Furthermore, gene expressions of FLG and LOR were significantly decreased in HEKs treated with Th2 cytokines (P < 0.001 and < 0.05, respectively) (Supplementary Fig. S3A and B), but increased significantly in HEKs treated with IFN-γ (all P < 0.001) (Supplementary Fig. S3A and B) compared to cells cultured in media alone.
A combination of B. longum and GOS had greater effects on improving DNCB-induced AD-like skin and skin barrier dysfunction than B. longum in a murine model.
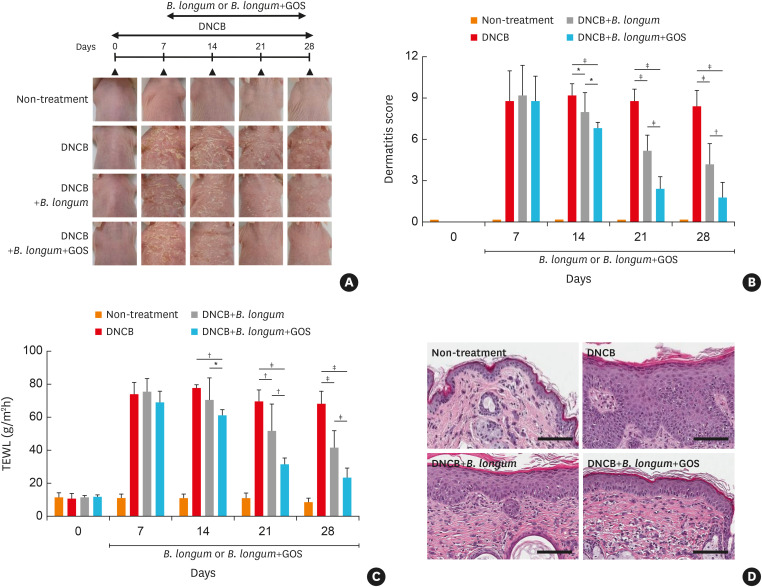
On days 14, 21, and 28, dermatitis scores were significantly decreased in mice treated with DNCB plus B. longum compared to those treated with DNCB alone (P < 0.05, < 0.001, and < 0.001, respectively) (Fig. 2A and B). Moreover, dermatitis scores on days 14, 21, and 28 were further reduced in DNCB-treated mice fed with the combination of B. longum and GOS compared to those treated with DNCB alone (all P < 0.001) or DNCB plus B. longum (P < 0.05, < 0.001, and < 0.01, respectively) (Fig. 2A and B).
Fig. 2. The effects of B. longum or synbiotic mixtures of B. longum and GOS on DNCB-induced atopic dermatitis-like skin and skin barrier dysfunction in a murine model. Hairless mice were treated with DNCB for the initial 7 days and continually treated with DNCB alone, DNCB with B. longum or DNCB with synbiotics (B. longum with GOS) for additional 21 days. Experimental design and features of mouse skin are shown (A). Dermatitis scores (B), TEWL (C), and H&E staining (original magnification, ×800) (D) were evaluated. The data are shown as the mean ± SEM of 2 independent experiments (n = 5 mice per group; scale bar = 100 μm).
B. longum, Bifidobacterium longum; GOS, galactooligosaccharide; DNCB, dinitrochlorobenzene; TEWL, transepidermal water loss.
*P < 0.05, †P < 0.01, ‡P < 0.001 by one-way ANOVA with the Tukey-Kramer test.
In addition, on days 21 and 28, TEWL was significantly decreased in mouse skin treated with DNCB plus B. longum compared to that treated with DNCB alone on days 21 and 28 (P < 0.01 and < 0.001) (Fig. 2C). TEWL was significantly decreased on days 14, 21, and 28 in DNCB-treated mice fed with the combination of B. longum and GOS compared to those treated with DNCB alone (P < 0.01, < 0.001, and < 0.001, respectively) or DNCB plus B. longum (P < 0.05, < 0.01, and < 0.001, respectively) (Fig. 2C). Epidermal thickness was significantly increased in mice treated with DNCB compared to the control mice (non-treatment) (P < 0.001) (Fig. 2D, Supplementary Fig. S4A). However, epidermal thickness was significantly decreased in mice treated with DNCB plus B. longum compared to those treated with DNCB alone (P < 0.001) (Supplementary Fig. S4A). Moreover, epidermal thickness was significantly decreased in DNCB-treated mice fed with the combination of B. longum and GOS compared to those treated with DNCB plus B. longum (P < 0.001) (Supplementary Fig. S4A) or DNCB only (P < 0.001) (Supplementary Fig. S4A).
B. longum was not detected in fecal samples from mice not administered B. longum. The proportion of fecal B. longum among total fecal bacteria was significantly increased in mice treated with the combination of B. longum and GOS compared to those treated with B. longum alone (P < 0.001) (Supplementary Fig. S4B).
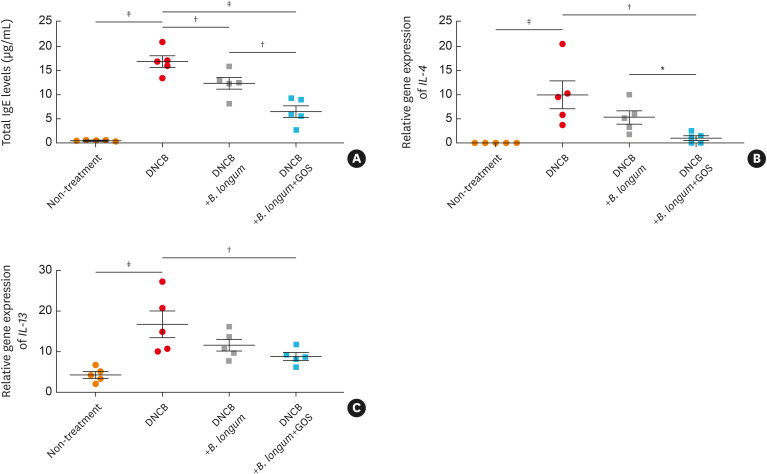
B. longum had beneficial effects with GOS to attenuate DNCB-induced expression of IgE and Th2 cytokines in a murine model.
In the present study, serum total IgE concentration was significantly increased in mice treated with DNCB compared to the control mice (non-treatment) (P < 0.001), but significantly decreased in those treated with B. longum (P < 0.01) (Fig. 3A). Moreover, serum IgE concentrations were significantly reduced in mice treated with the combination of B. longum and GOS compared to those treated with B. longum (P < 0.01) or DNCB alone (P < 0.001) (Fig. 3A). These findings indicate that B. longum attenuates DNCB-induced skin barrier dysfunction and a synbiotic mixture of B. longum and GOS further improves skin barrier dysfunction.
Fig. 3. The effects of B. longum and GOS on expression of IgE and T helper type 2 cytokines in a murine model. Serum IgE levels were measured by ELISA (A). Total RNAs were isolated from mouse skin, and gene expressions of IL-4 (B), and IL-13 (C) were examined by reverse transcriptase-polymerase chain reaction. The data are shown as the mean ± SEM of 2 independent experiments. Each point indicates individual mice, n = 5 mice per group.
IgE, immunoglobulin E; DNCB, dinitrochlorobenzene; B. longum, Bifidobacterium longum; GOS, galactooligosaccharide; IL, interleukin.
*P < 0.05, †P < 0.01, ‡P < 0.001 by one-way ANOVA with the Tukey-Kramer test.
Gene expressions of IL-4 (Fig. 3B, IL-13 (Fig. 3C) and IL-5 (Supplementary Fig. S5A) were significantly increased in mouse skin treated with DNCB (P < 0.001, < 0.001, and < 0.01, respectively), but significantly decreased in that treated with the combination of B. longum and GOS compared to that treated with DNCB alone (all P < 0.01). Also, gene expression of IL-4 was significantly decreased in mouse skin treated with the combination of B. longum and GOS compared to skin treated with B. longum alone (P < 0.05) (Fig. 3B). Moreover, gene expression of IL-5 was significantly decreased in mouse skin treated with B. longum compared to that treated with DNCB only (P < 0.05) (Supplementary Fig. S5A). No difference was observed in gene expression of IL-33 among the experimental groups (Supplementary Fig. S5B). Gene expressions of IL-10 was not significantly decreased in mouse skin treated with DNCB (Supplementary Fig. S5C), but TGF-β gene expression was significantly decreased in that treated with DNCB (P < 0.05) (Supplementary Fig. S5D). Gene expressions of IL-10 was significantly increased in mouse skin treated with the combination of B. longum and GOS compared to that treated with B. longum (P < 0.05) or DNCB alone (P < 0.05) (Supplementary Fig. S5C). Similarly, gene expression of TGF-β was significantly increased in mouse skin treated with the combination of B. longum and GOS compared to that treated with DNCB alone (P < 0.05) (Supplementary Fig. S5D). However, IL-12 gene expression was not significantly affected by DNCB, B. longum, or the combination of B. longum and GOS (Supplementary Fig. S5E).
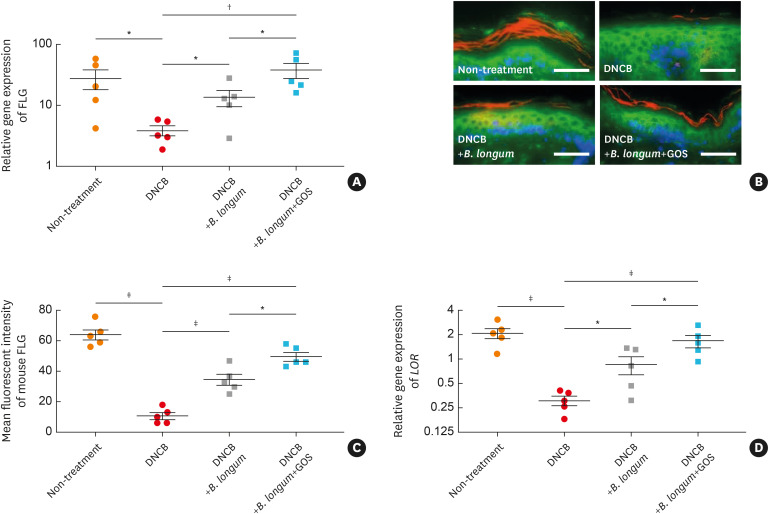
B. longum and GOS recover DNCB-induced inhibition of epidermal barrier proteins in murine skin.
Gene expression of FLG was significantly decreased in mouse skin treated with DNCB alone compared to untreated mouse skin (P < 0.05) (Fig. 4A). However, gene expression of FLG was significantly increased in mouse skin treated with B. longum compared to that treated with DNCB alone (P < 0.05) (Fig. 4A). Moreover, FLG gene expression was further increased in mouse skin treated with the combination of B. longum and GOS compared to that treated with DNCB (P < 0.01) or B. longum (P < 0.05) (Fig. 4A). These findings were confirmed at protein levels using immunofluorescent staining (Fig. 4B and C). Similarly, gene expressions of LOR (P < 0.001, Fig. 4D), KRT-10 (P < 0.001, Supplementary Fig. S6A), and DSG-1(P < 0.05, Supplementary Fig. S6B) were significantly decreased in mouse skin treated with DNCB compared to that untreated. However, gene expressions of LOR (P < 0.05, Fig. 4D), KRT-10 (P < 0.05, Supplementary Fig. S6A), and DSG-1 (P < 0.05, Supplementary Fig. S6B) were significantly increased in mouse skin treated with B. longum compared to that treated with DNCB alone. Gene expressions of DSG-1 (P < 0.05, Supplementary Fig. S6B) and KRT-1 (P < 0.01, Supplementary Fig. S6C) were increased in mouse skin treated with the combination of B. longum and GOS compared to that treated with DNCB alone. Moreover, gene expressions of LOR (P < 0.05 and < 0.001, Fig. 4D) and KRT-10 (P < 0.01 and < 0.001, Supplementary Fig. S6A) were further increased in mouse skin treated with the combination of B. longum and GOS compared to that treated with B. longum or DNCB alone. In contrast, gene expression of TGM-3 was significantly increased in mouse skin treated with DNCB compared to untreated mouse skin (P < 0.001), but significantly decreased in that treated with B. longum and synbiotics compared to that treated with DNCB alone (P < 0.05 and < 0.01) (Supplementary Fig. S6D).
Fig. 4. The effects of B. longum or synbiotic mixtures of B. longum and GOS on epidermal barrier proteins in DNCB-induced atopic dermatitis-like skin. Gene (A) and protein (B and C) expressions of FLG and gene expression of LOR (D) were evaluated by reverse transcriptase-polymerase chain reaction and immunostaining. FLG was visualized with Cy3 (red). Wheat germ agglutinin-conjugated FITC (green) was used to stain the cytoskeleton. Nuclei were visualized with DAPI (blue). The data are shown as the mean ± SEM of 2 independent experiments. Each point indicates individual mice (n = 5 mice per group; scale bar = 100 μm).
FLG, filaggrin; DNCB, dinitrochlorobenzene; B. longum, Bifidobacterium longum; GOS, galactooligosaccharide; LOR, loricrin.
*P < 0.05, †P < 0.01, ‡P < 0.001 by one-way ANOVA with Tukey-Kramer test.
DISCUSSION
In the present study, we initially presented a negative correlation between abundance of fecal B. longum and levels of Th2 cytokines, such as IL-4 and IL-13, from PBMC in infants. This finding suggests that increased abundance of B. longum is associated with decreased expression of Th2 cytokines, which cause skin barrier dysfunction and AD.2,20 Therefore, this observation led us to perform the current study because it is supposed that B. longum improves AD skin by the inhibition of Th2 cytokines.
Our in vitro data showed that both B. longum and BLEVs upregulated epidermal barrier proteins and that B. longum produced beneficial effects for GOS to upregulate epidermal barrier proteins. Moreover, B. longum and GOS recovered DNCB-induced skin barrier dysfunction and AD-like skin in vivo. These effects could be explained by the role of GOS on the growth of gut microbiota. It has been known that prebiotic fermentation products, including SCFAs and peptidoglycans, are utilized as substrates for microorganisms.7 It has also been reported that oral administration of prebiotics increases the proportions of Bacteroidetes, beneficial gut microflora.16 In the present study, the proportion of fecal B. longum was 2.5-fold greater in mice administered the combination of B. longum and GOS than in those administered B. longum alone. This finding supports that oral administration of GOS enhances the abundance of B. longum and plays important roles in the beneficial clinical effects of the synbiotic mixture.
In the present study, dermal application of DNCB increased serum IgE concentration and induced gene expression of IL-4, IL-5, and IL-13 in mouse skin. Dermal application of DNCB also downregulated expression of FLG, LOR, KRT-10, and DSG-1 in mouse skin, and increased TGM-3 expression, the latter of which has been reported to promote skin inflammation in AD as an autoallergen.24 These findings are compatible with those of previous reports that show DNCB induces overexpression of Th2 cytokines and downregulation of epidermal barrier proteins.25,26 Importantly, our in vivo data indicate that oral administration of B. longum mitigates DNCB-induced expression of Th2 cytokines and deficiency of epidermal barrier proteins. In a recent animal study, the expressions of procollagen type 1 and collagen type 1 were significantly increased in the mice fed with the combination of B. longum and GOS.27 These findings suggest that oral administration of B. longum could be an effective strategy for the treatment of AD because Th2 cytokines and lack of epidermal barrier proteins cause skin barrier dysfunction and induce AD.1,2,27,28 Indeed, our data showed that oral administration of B. longum attenuated skin barrier dysfunction and AD-like skin. Furthermore, the combination of B. longum and GOS had greater effects in improving DNCB-induced skin barrier dysfunction and AD-like skin compared to the treatment of B. longum alone.
Of note, it is inferred that the direct and indirect effects of probiotics and synbiotics had similar effects on skin barrier proteins in the present study, because in vitro and animal models represented the administration of those probiotic materials through skin and gut, respectively. It is noteworthy that in vitro models using HEKs and mice treated with probiotics or synbiotics had a similar outcome on gene expression of skin barrier proteins in the current study. In accordance with previous studies using HEKs, our in vitro study may indicate that molecular mechanisms mediated by probiotics or synbiotics to attenuate skin inflammation and enhance skin barrier function are similar in the skin and the gut.29,30 It is postulated that synbiotics can enhance epidermal barrier through a direct pathway as well as a pathway through Th2 cytokines because B. longum and GOS increased epidermal barrier proteins in in vitro experiments. However, little is known about how probiotics directly act on epidermal barrier in the inflamed skin, and thus further research is needed before reaching conclusions on these mechanisms.
It has been suggested that oral administration of synbiotics not only increases the expression of immunoregulatory cytokines, such as IL-10 and TGF-β,31,32,33 but also inhibits IFN-γ and IL-12 expression.34,35,36 Our present data showed that B. longum and GOS increased the expression of IL-10 and TGF-β, while they did not modulate IL-12 expression. Although the pathway of T-cell trafficking within tissues is not well known, animal experiments showed that CD4+ T cells preferentially migrate within the inflamed dermis in the direction of local collagen fibers.37 Previous reports showed that oral administration of probiotics increased Treg cells, which induces expression of IL-10 and TGF-β.5,38 Therefore, oral synbiotics therapy may improve DNCB-induced skin barrier dysfunction and AD-like skin by facilitating migration of Treg cells and inhibit expression of not only Th2 but also Th1 cytokines, which have shown beneficial effects on epidermal barrier proteins.39 These findings support a previous report that the ingestion of synbiotics is effective in the treatment of pediatric AD.17 However, anti-inflammatory effects of these probiotics and synbiotics on AD symptoms need to be examined in mice at different ages.
Our in vivo data suggest the existence of gut-to-skin crosstalk because oral administration of B. longum and synbiotics improved skin barrier dysfunction and AD-like skin. A recent study reported that oral administration of probiotics-derived EVs reduced skin inflammation and expression of IL-4 in a mouse model,40 potentially indicating a bacterial EVs-mediated interaction between microbiota and skin.40,41 Bacterial EVs have been shown to be involved in intercellular communication throughout the entire body as they are able to pass through tight junctions.42 Another explanation for our findings is that microbial fermentation metabolites as well as microbiome-derived Toll-like receptors (TLRs) can enter the bloodstream through enterocytes and affect distant organs.14,43,44 In the current study, we demonstrated that BLEVs upregulated gene expression levels of FLG and LOR in cultured HEKs. Moreover, it has been reported that EVs activate dendritic cells (DCs) and CD4+ T cells through the modulation of TLRs.45 It is evident that activated DCs promote differentiation of Th0 cells into Treg cells, which produce IL-10 and TGF-β.46 Therefore, we suggest that bacterial EVs from intestinal bacteria modulate skin inflammation by activating DCs and T cells as well as by direct invasion into skin through the blood stream. However, little is known about the kinetics and mechanisms of EVs in vivo.
In conclusion, our present study demonstrates that oral administration of B. longum can attenuate DNCB-induced skin barrier dysfunction and AD-like skin. Additionally, the combination of B. longum and GOS has greater effects in improving DNCB-induced skin barrier dysfunction and AD-like skin than the administration of B. longum alone. Oral supplementation of synbiotics may be more effective than a single therapy with probiotics or GOS alone for the treatment of AD.
ACKNOWLEDGMENTS
This work was supported by Lotte Foods Co., Ltd, Seoul, Korea, and by the Korea Environment Industry and Technology Institute (KEITI) through the Environmental Health Action Program funded by the Korean Ministry of Environment (MOE; 2017001360006).
Footnotes
Disclosure: Youngbae Noh, Sangjong Kim, and Phyrim Lee are employed by Lotte R&D Center. The other authors declare that there are no potential conflicts of interests with respect to the authorship and/or publication of this article.
SUPPLEMENTARY MATERIALS
Correlation between the proportion of fecal B. longum among total bacteria and gene expression of T helper type 2 cytokines from peripheral blood mononuclear cell in infants. The proportion of fecal B. longum was negatively correlated with gene expression of IL-13 (A), and the relationship between fecal B. longum and gene expression of IL-4 approached statistical significance (B). Correlation was calculated by means of Spearman correlation.
Effects of B. longum on expression of epidermal barrier proteins in human primary keratinocytes. Gene expression of FLG (A) and LOR (B) in cultured human primary epidermal keratinocytes were evaluated by reverse transcriptase-polymerase chain reaction. Data are representative of 3 independent experimental replicates. The data are shown as the mean ± SEM.
Effects of BLEVs of B. longum and galactooligosaccharide on expression of epidermal barrier proteins in human primary keratinocytes. Gene expression of FLG (A) and LOR (B) in cultured HEKs were evaluated by RT-PCR. Data are representative of 3 independent experimental replicates. The data are shown as the mean ± SEM.
Effects of B. longum and GOS in a murine model. Epidermal thickness (A) and fecal abundance of B. longum (B) were evaluated. The data are shown as the mean ± SEM of two independent experiments, n = 5 mice per group.
Effects of B. longum and GOS on cytokine expression in a murine model. Gene expressions of IL-5 (A), IL-33 (B), IL-10 (C), TGF-β (D), and IL-12 (E) were examined by reverse transcriptase-polymerase chain reaction. The data are shown as the mean ± SEM of two independent experiments. Each point indicates individual mice, n = 5 mice per group.
Effects of B. longum and GOS on expression of epidermal barrier proteins in a murine model. Gene expression of KRT-10 (A) DSG-1 (B), KRT-1 (C), and TGM-3 (D) were evaluated by reverse transcriptase-polymerase chain reaction. The data are shown as the mean ± SEM of 2 independent experiments. Each point indicates individual mice, n = 5 mice per group.
References
- 1.Ahn K, Kim BE, Kim J, Leung DY. Recent advances in atopic dermatitis. Curr Opin Immunol. 2020;66:14–21. doi: 10.1016/j.coi.2020.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim J, Kim BE, Leung DY. Pathophysiology of atopic dermatitis: Clinical implications. Allergy Asthma Proc. 2019;40:84–92. doi: 10.2500/aap.2019.40.4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sugita K, Akdis CA. Recent developments and advances in atopic dermatitis and food allergy. Allergol Int. 2020;69:204–214. doi: 10.1016/j.alit.2019.08.013. [DOI] [PubMed] [Google Scholar]
- 4.Chan CW, Wong RS, Law PT, Wong CL, Tsui SK, Tang WP, et al. Environmental factors associated with altered gut microbiota in children with eczema: a systematic review. Int J Mol Sci. 2016;17:1147. doi: 10.3390/ijms17071147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim J, Lee BS, Kim B, Na I, Lee J, Lee JY, et al. Identification of atopic dermatitis phenotypes with good responses to probiotics (Lactobacillus plantarum CJLP133) in children. Benef Microbes. 2017;8:755–761. doi: 10.3920/BM2017.0034. [DOI] [PubMed] [Google Scholar]
- 6.Han Y, Kim B, Ban J, Lee J, Kim BJ, Choi BS, et al. A randomized trial of Lactobacillus plantarum CJLP133 for the treatment of atopic dermatitis. Pediatr Allergy Immunol. 2012;23:667–673. doi: 10.1111/pai.12010. [DOI] [PubMed] [Google Scholar]
- 7.Davani-Davari D, Negahdaripour M, Karimzadeh I, Seifan M, Mohkam M, Masoumi SJ, et al. Prebiotics: definition, types, sources, mechanisms, and clinical applications. Foods. 2019;8:92. doi: 10.3390/foods8030092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goleva E, Berdyshev E, Leung DY. Epithelial barrier repair and prevention of allergy. J Clin Invest. 2019;129:1463–1474. doi: 10.1172/JCI124608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim BE, Kim J, Goleva E, Berdyshev E, Lee J, Vang KA, et al. Particulate matter causes skin barrier dysfunction. JCI Insight. 2018;6:e145185. doi: 10.1172/jci.insight.145185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Egawa G, Kabashima K. Barrier dysfunction in the skin allergy. Allergol Int. 2018;67:3–11. doi: 10.1016/j.alit.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 11.Leung DY. New insights into atopic dermatitis: role of skin barrier and immune dysregulation. Allergol Int. 2013;62:151–161. doi: 10.2332/allergolint.13-RAI-0564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang B, Chen Y, Stanton C, Ross RP, Lee YK, Zhao J, et al. Bifidobacterium and Lactobacillus composition at species level and gut microbiota diversity in infants before 6 weeks. Int J Mol Sci. 2019;20:3306. doi: 10.3390/ijms20133306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi M, Lee Y, Lee NK, Bae CH, Park DC, Paik HD, et al. Immunomodulatory effects by Bifidobacterium longum KACC 91563 in mouse splenocytes and macrophages. J Microbiol Biotechnol. 2019;29:1739–1744. doi: 10.4014/jmb.1812.12002. [DOI] [PubMed] [Google Scholar]
- 14.Kim JE, Kim HS. Microbiome of the skin and gut in atopic dermatitis (AD): understanding the pathophysiology and finding novel management strategies. J Clin Med. 2019;8:444. doi: 10.3390/jcm8040444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirata SI, Kunisawa J. Gut microbiome, metabolome, and allergic diseases. Allergol Int. 2017;66:523–528. doi: 10.1016/j.alit.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 16.Kim JA, Kim SH, Kim IS, Yu DY, Kim GI, Moon YS, et al. Galectin-9 induced by dietary prebiotics regulates immunomodulation to reduce atopic dermatitis symptoms in 1-chloro-2,4-dinitrobenzene (DNCB)-treated NC/Nga mice. J Microbiol Biotechnol. 2020;30:1343–1354. doi: 10.4014/jmb.2005.05017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang YS, Trivedi MK, Jha A, Lin YF, Dimaano L, García-Romero MT. Synbiotics for prevention and treatment of atopic dermatitis: a meta-analysis of randomized clinical trials. JAMA Pediatr. 2016;170:236–242. doi: 10.1001/jamapediatrics.2015.3943. [DOI] [PubMed] [Google Scholar]
- 18.Zhou L, Liu D, Xie Y, Yao X, Li Y. Bifidobacterium infantis induces protective colonic PD-L1 and Foxp3 regulatory T cells in an acute murine experimental model of inflammatory bowel disease. Gut Liver. 2019;13:430–439. doi: 10.5009/gnl18316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim JH, Jeun EJ, Hong CP, Kim SH, Jang MS, Lee EJ, et al. Extracellular vesicle-derived protein from Bifidobacterium longum alleviates food allergy through mast cell suppression. J Allergy Clin Immunol. 2016;137:507–516.e8. doi: 10.1016/j.jaci.2015.08.016. [DOI] [PubMed] [Google Scholar]
- 20.Nomura I, Goleva E, Howell MD, Hamid QA, Ong PY, Hall CF, et al. Cytokine milieu of atopic dermatitis, as compared to psoriasis, skin prevents induction of innate immune response genes. J Immunol. 2003;171:3262–3269. doi: 10.4049/jimmunol.171.6.3262. [DOI] [PubMed] [Google Scholar]
- 21.Choi JG, Huh E, Kim N, Kim DH, Oh MS. High-throughput 16S rRNA gene sequencing reveals that 6-hydroxydopamine affects gut microbial environment. PLoS One. 2019;14:e0217194. doi: 10.1371/journal.pone.0217194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rognes T, Flouri T, Nichols B, Quince C, Mahé F. VSEARCH: a versatile open source tool for metagenomics. PeerJ. 2016;4:e2584. doi: 10.7717/peerj.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics. 2011;27:2194–2200. doi: 10.1093/bioinformatics/btr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Su H, Luo Y, Sun J, Liu X, Ling S, Xu B, et al. Transglutaminase 3 promotes skin inflammation in atopic dermatitis by activating monocyte-derived dendritic cells via DC-SIGN. J Invest Dermatol. 2020;140:370–379.e8. doi: 10.1016/j.jid.2019.07.703. [DOI] [PubMed] [Google Scholar]
- 25.Kim SH, Seong GS, Choung SY. Fermented morinda citrifolia (Noni) alleviates DNCB-Induced atopic dermatitis in NC/Nga mice through modulating immune balance and skin barrier function. Nutrients. 2020;12:249. doi: 10.3390/nu12010249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bai XY, Liu P, Chai YW, Wang Y, Ren SH, Li YY, et al. Artesunate attenuates 2, 4-dinitrochlorobenzene-induced atopic dermatitis by down-regulating Th17 cell responses in BALB/c mice. Eur J Pharmacol. 2020;874:173020. doi: 10.1016/j.ejphar.2020.173020. [DOI] [PubMed] [Google Scholar]
- 27.Kim D, Lee KR, Kim NR, Park SJ, Lee M, Kim OK. Combination of Bifidobacterium longum and galacto-oligosaccharide protects the skin from photoaging. J Med Food. 2021;24:606–616. doi: 10.1089/jmf.2021.K.0032. [DOI] [PubMed] [Google Scholar]
- 28.Kim BE, Leung DY. Significance of skin barrier dysfunction in atopic dermatitis. Allergy Asthma Immunol Res. 2018;10:207–215. doi: 10.4168/aair.2018.10.3.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Szöllősi AG, Gueniche A, Jammayrac O, Szabó-Papp J, Blanchard C, Vasas N, et al. Bifidobacterium longum extract exerts pro-differentiating effects on human epidermal keratinocytes, in vitro . Exp Dermatol. 2017;26:92–94. doi: 10.1111/exd.13130. [DOI] [PubMed] [Google Scholar]
- 30.Sultana R, McBain AJ, O’Neill CA. Strain-dependent augmentation of tight-junction barrier function in human primary epidermal keratinocytes by Lactobacillus and Bifidobacterium lysates. Appl Environ Microbiol. 2013;79:4887–4894. doi: 10.1128/AEM.00982-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Komai T, Inoue M, Okamura T, Morita K, Iwasaki Y, Sumitomo S, et al. Transforming growth factor-β and interleukin-10 synergistically regulate humoral immunity via modulating metabolic signals. Front Immunol. 2018;9:1364. doi: 10.3389/fimmu.2018.01364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hsu P, Santner-Nanan B, Hu M, Skarratt K, Lee CH, Stormon M, et al. IL-10 potentiates differentiation of human induced regulatory T cells via STAT3 and Foxo1. J Immunol. 2015;195:3665–3674. doi: 10.4049/jimmunol.1402898. [DOI] [PubMed] [Google Scholar]
- 33.Kazemi A, Soltani S, Ghorabi S, Keshtkar A, Daneshzad E, Nasri F, et al. Effect of probiotic and synbiotic supplementation on inflammatory markers in health and disease status: a systematic review and meta-analysis of clinical trials. Clin Nutr. 2020;39:789–819. doi: 10.1016/j.clnu.2019.04.004. [DOI] [PubMed] [Google Scholar]
- 34.Hofeld BC, Puppala VK, Tyagi S, Ahn KW, Anger A, Jia S, et al. Lactobacillus plantarum 299v probiotic supplementation in men with stable coronary artery disease suppresses systemic inflammation. Sci Rep. 2021;11:3972. doi: 10.1038/s41598-021-83252-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lorea Baroja M, Kirjavainen PV, Hekmat S, Reid G. Anti-inflammatory effects of probiotic yogurt in inflammatory bowel disease patients. Clin Exp Immunol. 2007;149:470–479. doi: 10.1111/j.1365-2249.2007.03434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prakoeswa CR, Herwanto N, Prameswari R, Astari L, Sawitri S, Hidayati AN, et al. Lactobacillus plantarum IS-10506 supplementation reduced SCORAD in children with atopic dermatitis. Benef Microbes. 2017;8:833–840. doi: 10.3920/BM2017.0011. [DOI] [PubMed] [Google Scholar]
- 37.Ali N, Rosenblum MD. Regulatory T cells in skin. Immunology. 2017;152:372–381. doi: 10.1111/imm.12791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bieber K, Sun S, Witte M, Kasprick A, Beltsiou F, Behnen M, et al. Regulatory T cells suppress inflammation and blistering in pemphigoid diseases. Front Immunol. 2017;8:1628. doi: 10.3389/fimmu.2017.01628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marras L, Caputo M, Bisicchia S, Soato M, Bertolino G, Vaccaro S, et al. The role of Bifidobacteria in predictive and preventive medicine: a focus on eczema and hypercholesterolemia. Microorganisms. 2021;9:836. doi: 10.3390/microorganisms9040836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim MH, Choi SJ, Choi HI, Choi JP, Park HK, Kim EK, et al. Lactobacillus plantarum-derived extracellular vesicles protect atopic dermatitis induced by staphylococcus aureus-derived extracellular vesicles. Allergy Asthma Immunol Res. 2018;10:516–532. doi: 10.4168/aair.2018.10.5.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jang SC, Kim SR, Yoon YJ, Park KS, Kim JH, Lee J, et al. In vivo kinetic biodistribution of nano-sized outer membrane vesicles derived from bacteria. Small. 2015;11:456–461. doi: 10.1002/smll.201401803. [DOI] [PubMed] [Google Scholar]
- 42.Haas-Neill S, Forsythe P. A budding relationship: bacterial extracellular vesicles in the microbiota-gut-brain axis. Int J Mol Sci. 2020;21:8899. doi: 10.3390/ijms21238899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zheng D, Liwinski T, Elinav E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020;30:492–506. doi: 10.1038/s41422-020-0332-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Price AE, Shamardani K, Lugo KA, Deguine J, Roberts AW, Lee BL, et al. A map of Toll-like receptor expression in the intestinal epithelium reveals distinct spatial, cell type-specific, and temporal patterns. Immunity. 2018;49:560–575.e6. doi: 10.1016/j.immuni.2018.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ahmadi Badi S, Khatami SH, Irani SH, Siadat SD. Induction effects of Bacteroides fragilis derived outer membrane vesicles on Toll Like Receptor 2, Toll Like Receptor 4 genes expression and cytokines concentration in human intestinal epithelial cells. Cell J. 2019;21:57–61. doi: 10.22074/cellj.2019.5750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Molina-Tijeras JA, Gálvez J, Rodríguez-Cabezas ME. The immunomodulatory properties of extracellular vesicles derived from probiotics: a novel approach for the management of gastrointestinal diseases. Nutrients. 2019;11:1038. doi: 10.3390/nu11051038. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Correlation between the proportion of fecal B. longum among total bacteria and gene expression of T helper type 2 cytokines from peripheral blood mononuclear cell in infants. The proportion of fecal B. longum was negatively correlated with gene expression of IL-13 (A), and the relationship between fecal B. longum and gene expression of IL-4 approached statistical significance (B). Correlation was calculated by means of Spearman correlation.
Effects of B. longum on expression of epidermal barrier proteins in human primary keratinocytes. Gene expression of FLG (A) and LOR (B) in cultured human primary epidermal keratinocytes were evaluated by reverse transcriptase-polymerase chain reaction. Data are representative of 3 independent experimental replicates. The data are shown as the mean ± SEM.
Effects of BLEVs of B. longum and galactooligosaccharide on expression of epidermal barrier proteins in human primary keratinocytes. Gene expression of FLG (A) and LOR (B) in cultured HEKs were evaluated by RT-PCR. Data are representative of 3 independent experimental replicates. The data are shown as the mean ± SEM.
Effects of B. longum and GOS in a murine model. Epidermal thickness (A) and fecal abundance of B. longum (B) were evaluated. The data are shown as the mean ± SEM of two independent experiments, n = 5 mice per group.
Effects of B. longum and GOS on cytokine expression in a murine model. Gene expressions of IL-5 (A), IL-33 (B), IL-10 (C), TGF-β (D), and IL-12 (E) were examined by reverse transcriptase-polymerase chain reaction. The data are shown as the mean ± SEM of two independent experiments. Each point indicates individual mice, n = 5 mice per group.
Effects of B. longum and GOS on expression of epidermal barrier proteins in a murine model. Gene expression of KRT-10 (A) DSG-1 (B), KRT-1 (C), and TGM-3 (D) were evaluated by reverse transcriptase-polymerase chain reaction. The data are shown as the mean ± SEM of 2 independent experiments. Each point indicates individual mice, n = 5 mice per group.