Abstract
Purpose
There have been autoimmune mechanisms for the pathogenesis of severe asthma (SA) involving epithelial autoantigen-specific antibodies. This study aimed to find the function of these antibodies in the formation of eosinophil extracellular traps (EETs), contributing to the development of SA.
Methods
Patients with SA (n = 11), those with patients with nonsevere asthma (NSA, n = 41), and healthy controls (HCs, n = 26) were recruited to evaluate levels of epithelial antigens and autoantigen-specific antibodies. Moreover, the significance of epithelial autoantigen-specific antibodies in association with EET production was investigated ex vivo and in vivo.
Results
Significantly higher levels of serum cytokeratin (CK) 18 and CK18-specific IgG were observed in patients with SA than in those with NSA (P = 0.001 and P = 0.031, respectively), while no differences were found in serum CK19 or CK19-specific immunoglobulin G (IgG). Moreover, levels of serum CK18 were positively correlated with total eosinophil counts (r = 0.276, P = 0.048) in asthmatics, while a negative correlation was noted between levels of serum CK18 and forced expiratory volume in 1 second (FEV1) %. In the presence of CK18-specific IgG, peripheral eosinophils from asthmatics released EETs, which further increased CK18 production from airway epithelial cells. In severe asthmatic mice, CK18 expression and CK18-specific IgG production were enhanced in the lungs, where EET treatment enhanced CK18 expression and CK18-specific IgG production, either of which was not suppressed by dexamethasone.
Conclusions
These suggest that EETs could enhance epithelial autoantigen (CK18)-induced autoimmune responses, further stimulating EET production and type 2 airway responses, which is a new therapeutic target for SA.
Keywords: Asthma, keratin, autoantigens, epithelium, immunoglobulin G, eosinophils, extracellular traps, autoimmunity
INTRODUCTION
Asthma is a chronic inflammatory disease with various phenotypes which is characterized by hyperresponsiveness and inflammation in the airways.1 To date, most asthmatics have been controlled by regular use of inhaled corticosteroids (ICS) with/without long-acting beta2-agonists (LABA); however, patients with severe asthma (SA; 5%–10% among asthmatics) are suffering from frequent asthma exacerbations even on regular anti-inflammatory medications, indicating that they have distinct clinical presentations and poor responses to current medications.2 Patients with SA were found to show persistent blood/sputum eosinophilia, high fractional exhaled nitric oxide levels and frequent asthma exacerbation rates.3,4 Although extensive studies have been conducted to understand its pathogenic mechanism, it remains unsolved, and some other pathways are suggested in its pathogenesis.
The airway epithelium is the first barrier regulating immune responses to environmental factors. Indeed, emerging evidence suggests that epithelial dysfunction is involved in asthma pathogenesis.5 The epithelial layer was more activated and thicker in patients with SA than in those with nonsevere asthma (NSA) in association with abnormal cellular processes.6,7 Many factors have been shown to contribute to a thickened epithelium, which provides a microenvironment for persistent airway inflammation.8,9 Moreover, the bronchial epithelium is more susceptible to apoptosis, and activated caspases could contribute to the cleavage of structural proteins.10,11,12 Regarding autoimmune mechanisms, the existence of epithelial cell-derived autoantigens, including cytokeratin (CK), and the pathogenic role of circulating autoantibodies to epithelial antigens have been identified in patients with nonallergic asthma, contributing to poor clinical outcomes.13,14
Eosinophils are major effector cells driving immune responses against bacterial or fungal infections by releasing extracellular traps (composed of a complex meshwork of chromatin filaments and cytotoxic granule proteins).15,16 However, overproduction of eosinophil extracellular traps (EETs) has been reported in relation to the pathology of upper and lower airway diseases.17,18 In particular, these traps have been demonstrated to be involved in the development of SA by stimulating the airway epithelium to enhance type 2/eosinophilic inflammation via releasing interleukin (IL)-33 and thymic stromal lymphopoietin.19,20 Moreover, EETs could induce asthma progression via mediating autoimmune responses in SA; sputum autoantibodies collected from severe asthmatics could trigger eosinophil cytolysis and EET formation in SA.21 Although multiple factors induce EET formation, the role of epithelial antigen-specific autoantibodies remains unclear.
Here, we hypothesized that epithelial antigen-specific autoantibodies may contribute to airway inflammation. The present study enumerated: 1) the levels of epithelial antigens and epithelial antigen-specific antibodies in patients with SA compared to NSA; 2) the effect of epithelial antigen-specific antibodies on EET formation ex vivo; 3) the effect of EETs on epithelial antigen and epithelial antigen-specific antibody production in vivo.
MATERIALS AND METHODS
Patient cohorts and clinical parameters
This study was approved by the Institutional Review Board of Ajou University Hospital (AJIRB-GEN-SMP-13-108). All study subjects provided written informed consent at the time of recruitment. To evaluate the significance of epithelial antigens and epithelial antigen-specific antibodies in the pathogenesis of asthma, patients with SA (n = 11), those with NSA (n = 41), and healthy controls (HCs; n = 26) were enrolled. We recruited healthy subjects who had no history of any allergic disease, including asthma and rhinitis. Asthmatics were diagnosed by recurrent episodes of wheezing, dyspnea, and cough as well as evidence of airway hyperresponsiveness (AHR) to methacholine or airway reversibility. In addition, SA was defined according to the definition of the European Respiratory Society/American Thoracic Society guidelines.2 The patients with SA had maintained a medium-to-high dose of ICS-LABA, but had experienced > 2 times of asthma exacerbations requiring systemic steroids (higher than 15 mg/day of prednisolone-equivalent for more than 3 days); the patients with NSA had maintained a medium dose of ICS-LABA. No one had occupational asthma. No one was a current smoker; the prevalence of ex-smoker was not different between severe and nonsevere asthmatics (Table). However, anyone had used systemic steroids, when we collected eosinophils from their peripheral blood. The degree of airway obstruction was evaluated using spirometry. The degree of AHR was examined by methacholine bronchial challenge tests. Atopic status was defined as at least 1 positive result on skin prick tests for common inhalant allergens (Bencard, Bradford, UK). Total immunoglobulin (Ig) E was measured using the ImmunoCAP system (ThermoFisher Scientific, Waltham, MA, USA). The total eosinophil count was evaluated using a hematology analyzer (Beckman Coulter, Fullerton, CA, USA).
Table. Demographic data of the study subjects.
| Variables | Healthy controls (n = 26) | Asthmatic patients (n = 52) | P value | NSA (n = 41) | SA (n = 11) | P value |
|---|---|---|---|---|---|---|
| Age (yr) | 40.1 ± 9.8 | 46.1 ± 14.5 | 0.061 | 44.4 ± 14.9 | 52.4 ± 11.5 | 0.108 |
| Female sex (%) | 26.9 | 61.5 | 0.086 | 61.0 | 63.6 | 0.872 |
| Atopy (%) | 35.0 | 48.1 | 0.073 | 51.2 | 36.4 | 0.381 |
| Ex-smoker (%) | 25.5 | 38.5 | 0.240 | 18.2 | 27.5 | 0.530 |
| Baseline FEV1 (%) | ND | 80.6 ± 23.2 | ND | 84.3 ± 20.0 | 66.4 ± 29.6 | 0.021 |
| PC20 (mg/mL) | ND | 1.6 ± 2.0 | ND | 1.8 ± 2.1 | 1.0 ± 0.7 | 0.398 |
| Total IgE (kU/L) | 115.3 ± 184.6 | 275.6 ± 308.4 | 0.027 | 181.7 ± 147.0 | 300.7 ± 335.9 | 0.260 |
| TEC (cells/μL) | ND | 323.4 ± 273.0 | ND | 304.9 ± 254.1 | 391.7 ± 339.5 | 0.443 |
| Sputum Eos (%) | ND | 26.1 ± 31.4 | ND | 24.3 ± 31.9 | 31.7 ± 30.6 | 0.525 |
P values were obtained by Pearson’s χ2 test for categorical variables and by Student’s t-test for continuous variables.
NSA, nonsevere asthma; SA, severe asthma; FEV1, forced expiratory volume in 1 second; methacholine PC20, the provocative concentration of methacholine required to cause a 20% fall in FEV1; IgE, immunoglobulin E; TEC, total eosinophil count; Eos, eosinophil; ND, no data.
Measurement of total IgG, epithelial antigens, and epithelial antigen-specific antibodies
Blood samples from asthmatics and HCs were collected in BD Vacutainer serum tubes with spray-coated silica (BD Biosciences, Franklin Lakes, NJ, USA). To isolate the serum, blood was centrifuged at 2,000 rpm and 20°C for 10 minutes. Total IgG was measured by immunoturbidimetric assays using a COBAS INTEGRA 800 (Roche, Marlborough, MA, USA). Levels of human CK18 and CK19 (2 major structural autoantigens within airway epithelial cells [AEC]) were measured using enzyme-linked immunosorbent assay (ELISA) kits (LSBio, Seattle, WA, USA) according to the manufacturer’s recommendations. To detect levels of serum CK18/CK19-specific autoantibodies, recombinant human CK18 and CK19 (10 μg/mL; Abcam, Waltham, MA, USA) were coated on 96-well microplates (R&D Systems, Minneapolis, MN, USA) for 24 hours at 4°C. After washes, the plate was blocked by using 1% bovine serum albumin (BSA; Sigma-Aldrich, St. Louis, MO, USA) in phosphate-buffered saline (PBS) for 1 hour at room temperature. After washing to remove the blocking solution, human sera (50 μL) were incubated for 2 hours. Then, biotinylated anti-human IgG (2 μg/mL; Sigma-Aldrich) was added for 2 hours. The horseradish peroxidase (HRP)-conjugated streptavidin (Sigma-Aldrich) was incubated for 30 minutes, and 3,3′, 5,5′ tetramethylbenzidine (TMB) substrate solution (BD Biosciences) was added for 30 minutes. Optical density was detected by using a microplate reader (BioTek, Winooski, VT, USA) at 450 nm.
Isolation of peripheral eosinophils and extracellular traps
Blood samples from patients with SA were collected in BD Vacutainer tubes containing acid citrate dextrose solution (BD Biosciences). Blood was layered on a Lymphoprep™ Solution (Axis-Shield, Oslo, Norway), followed by centrifugation at 879 g and 20°C for 25 minutes without brakes. Then, eosinophils were isolated from the fraction containing red blood cells and granulocytes using the Eosinophil Isolation kit and MACS Column (Miltenyi Biotec Inc., Auburn, CA, USA) according to the manufacturer’s instructions. To induce EET formation, eosinophils were stimulated with 100 nM phorbol myristate acetate (PMA; Sigma-Aldrich) for 4 hours. Each well was washed twice with a serum-free medium to eliminate PMA and EET-dissociated molecules. Micrococcal nuclease (ThermoFisher Scientific) was added to degrade the complex of EETs at 37°C for 20 minutes. Isolated EETs were quantified by measuring the DNA concentration using the Quant-iT™ PicoGreen dsDNA kit (Invitrogen, Paisley, UK). Protein concentrations were evaluated using the QuantiPro BCA Assay kit (Sigma-Aldrich).
Measurement of dsDNA and eosinophil granule protein
To measure eosinophil-derived DNA and eosinophil-derived neurotoxin (EDN), eosinophils were seeded on 48-well plates at a concentration of 5 × 105 cells/well. Cells were treated with 10 μg/mL epithelial antigen-specific antibodies for 18 hours. Eosinophils cultured in the medium without treatment served as background control. We harvested the supernatant and attached eosinophils, which were incubated with micrococcal nuclease at 37°C for 20 minutes. DNA levels were evaluated with Quant-iT™ PicoGreen dsDNA kit. Levels of human EDN were measured using ELISA kits (SKIMS-BIO Co., Seoul, Korea) according to the manufacturer’s recommendations.
Airway epithelial cell culture and stimulation
A549 cells (American Type Culture Collection, Manassas, VA, USA) were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS), 100 IU/mL penicillin, and 50 μg/mL streptomycin. Cells were grown at 37°C in humidified air with 5% CO2. To investigate epithelial antigen expression, A549 cells were treated with a constant dose of 10 μg/mL EETs (based on protein concentration) for 48 hours at 37°C in humidified air with 5% CO2.
In vivo experiments
All experimental protocols were approved by the Institutional Animal Care and Use Committee of Ajou University (IACUC-2019-0024). Female 6-week-old BALB/c wild-type mice (Orient BIO, Seongnam, Korea) were maintained under specific pathogen-free conditions. To induce SA, mice were sensitized twice for 2 weeks using saline containing 75 μg of ovalbumin (OVA; Sigma-Aldrich) with 2 mg of alum (Sigma-Aldrich) by intraperitoneal injection and challenged 5 times using 6% OVA by super mesh nebulizers (KTMEB Inc., Seoul, Korea). Mouse dexamethasone (2 μg; Sigma-Aldrich) was administered 1 hour prior to each challenge. To generate an EET-mediated mouse model, mice were treated twice every 1 week for 1 month with intranasal administration of 0.1 or 1 μg of EETs, and 2 μg of dexamethasone by intraperitoneal injection. To isolate EETs, mouse blood was obtained from OVA-induced asthmatic mice, and peripheral eosinophils were collected by using Anti-Siglec-F MicroBeads (Miltenyi Biotec Inc.) for magnetic-activated cell sorting. Other procedures were conducted in the same manner as in human samples. To measure AHR, the flexiVent System (SCIREQ, Montreal, Canada) was used. Each mouse was injected with aerosol methacholine (Sigma-Aldrich) at increasing concentrations (0, 1.56, 3.12, 6.25, and 12.5 mg/mL), and the peak airway responses to the inhaled methacholine were recorded. For differential cell counting, bronchoalveolar lavage fluid (BALF) was collected and then centrifuged at 3,000 rpm for 5 minutes. The cells were stained using hematoxylin (Dako Products, Carpinteria, CA, USA) and Eosin Y (Sigma-Aldrich). Supernatants were collected and stored at -80°C for further analysis. Concentrations of IL-13 were measured using ELISA kits (R&D Systems) according to the manufacturer’s recommendations. For histological analysis, lung tissues were perfused with PBS and fixed with 4% paraformaldehyde. Then, we prepared a paraffin block, cut it into 4-µm-thick sections, and stained it using Periodic Acid-Schiff Kit (Sigma-Aldrich), according to the manufacturer’s instructions. Lung tissues were investigated using ImageJ (National Institutes of Health, Bethesda, MD, USA).
Measurement of total IgG and CK18-specific IgG antibodies in mice
Anti-mouse IgG (Sigma-Aldrich) antibodies were coated on 96 well microplates to evaluate total serum IgG level, while recombinant mouse CK18 (Abcam) was attached on the microplates prior to putting mouse serum samples to investigate CK18-specific IgG for 24 hours at 4°C. The plate was blocked by using 1% BSA in PBS for 1 hour at room temperature. Then, HRP-conjugated rabbit anti-mouse IgG (Santa Cruz, Dallas, TX, USA) was used for immunoglobulin detection overnight at 4°C. To detect the optical density, a microplate reader (BioTek) was set at 450 nm.
Western blot analysis
CK18, CK19, p38, and phospho-p38 expressions in cell lysates were evaluated by immunoblot analysis. Relative expression of each protein to actin or glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was evaluated. Anti-CK18 antibody (Santa Cruz), anti-CK19 antibody (Santa Cruz), anti-p38 antibody (Cell Signaling, Minneapolis, MN, USA), anti-phospho-p38 antibody (Cell Signaling), anti-GAPDH antibody (Santa Cruz) and anti-Actin antibody (Santa Cruz) were used.
Immunofluorescence analysis
Samples on glass coverslips were fixed with 4% paraformaldehyde (Sigma-Aldrich) for 15 minutes at room temperature. Then, the samples were washed twice with PBS and blocked using 1% BSA in PBS for 30 minutes. After incubation, the samples were treated with anti-CK18 (Cell Signaling) and anti-CK19 (Cell Signaling) IgG overnight at 4°C, decanted into the primary antibody mixture, and washed 3 times for 5 minutes each wash. Secondary antibodies Alexa Fluor 488 anti-rabbit IgG (ThermoFisher Scientific) or Alexa Fluor 594 anti-mouse IgG (ThermoFisher Scientific) were incubated with samples for 2 hours at room temperature in the dark. For DNA staining, 1 μg/mL 4′,6-diamidino-2-phenylindole (DAPI; ThermoFisher Scientific) was used.
Statistical analysis
All statistical analysis were performed using IBM SPSS software, version 26.0 (IBM Corp., Armonk, NY, USA). Differences between 2 groups were analyzed by Student’s t-test. In addition, comparisons between data from multiple groups were made by using one-way ANOVA with Bonferroni’s post hoc test. P values of < 0.05 were considered statistically significant. GraphPad Prism 8.0 software (GraphPad Inc., San Diego, CA, USA) was used to create graphs.
RESULTS
Increased levels of serum CK18 (not CK19) in patients with SA
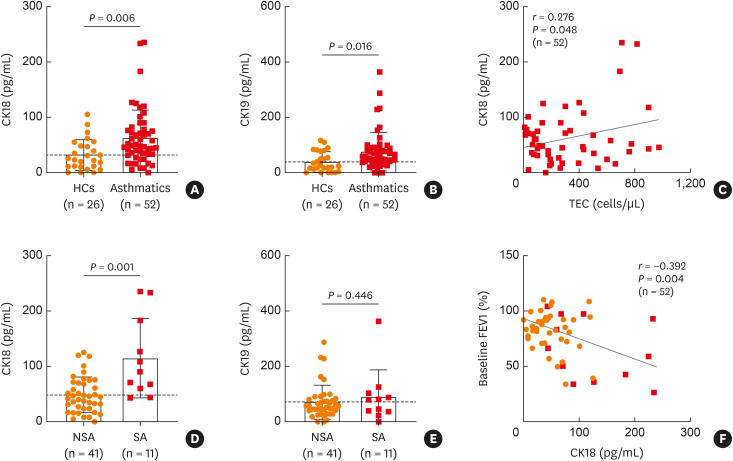
Demographic data of the study subjects are depicted in Table. In asthmatic patients, significantly elevated levels of CK18 (P = 0.006) and CK19 (P = 0.016) in sera were noted compared to HCs (Fig. 1A and B). Moreover, total eosinophil count was positively correlated with the serum level of CK18 (r = 0.276, P = 0.048; Fig. 1C), but not with CK19 (data not shown). When asthmatics were classified into patients with SA and those with NSA, significantly higher levels of serum CK18 (P = 0.001; Fig. 1D), but not serum CK19 (P = 0.446; Fig. 1E), were found in patients with SA than in those with NSA. Furthermore, the serum CK18 level was negatively correlated with baseline forced expiratory volume in 1 second (FEV1) % (r = −0.392, P = 0.004; Fig. 1F).
Fig. 1. Comparisons of circulating CK18/CK19 levels in association with blood eosinophil counts and FEV1% in the study subjects. Levels of (A) CK18 and (B) CK19 in sera from asthmatic patients as well as from HCs. Data are presented as mean ± SD. P values were obtained by Student’s t-tests. (C) A positive correlation between levels of CK18 and TEC. Data are represented as Pearson correlation coefficient r (P value). Comparisons of serum levels of (D) CK18 and (E) CK19 between patients with SA and NSA. Data are presented as mean ± SD. P values were obtained by Student’s t-tests. (F) A negative correlation between levels of CK18 and FEV1% values. Data are presented as Pearson correlation coefficient r (P value).
CK, cytokeratin; FEV1, forced expiratory volume in 1 second; HCs, healthy control subjects; SD, standard deviation; SA, severe asthma; NSA, nonsevere asthma; TEC, total eosinophil count.
Elevated levels of CK18-specific IgG in patients with SA
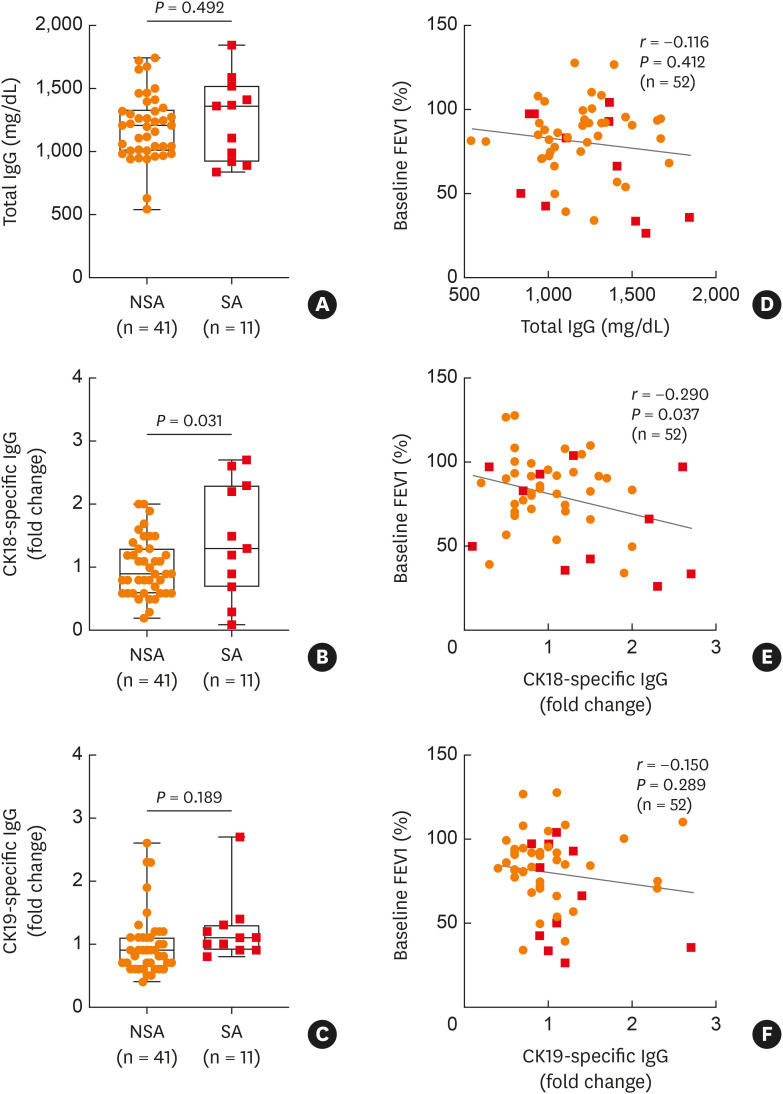
In addition to measurement of epithelial antigens, the present study evaluated total circulating IgG and epithelial CK18-specific IgG antibodies in sera of asthmatics. As a result, levels of total IgG were not significantly different between patients with SA and those with NSA (P = 0.492; Fig. 2A). However, levels of CK18-specific IgG were markedly higher in patients with SA than in those with NSA (P = 0.031; Fig. 2B), while no significant differences were noted in CK19-specific IgG levels between the 2 groups (P = 0.189; Fig. 2C). Here, we further investigated associations between lung function and total IgG/CK18-specific IgG/CK19-specific IgG, and only found a negative correlation between levels of serum CK18-specific IgG and FEV1% (Fig. 2D-F).
Fig. 2. Comparisons of serum CK18/CK19-specific IgG levels with asthma severity. (A) Levels of total IgG. Levels of (B) CK18- and (C) CK19-specific IgG in sera of patients with NSA and those with SA. Data are presented as box plots. P values were obtained by Student’s t-tests. Correlations of baseline FEV1% with levels of (D) total IgG, (E) CK18-specific IgG, and (F) CK19-specific IgG. Data are presented as Pearson correlation coefficient r (P value).
CK, cytokeratin; IgG, immunoglobulin G; NSA, nonsevere asthma; SA, severe asthma; FEV1, forced expiratory volume in 1 second.
Eosinophil degranulation induced by CK18-specific IgG antibody
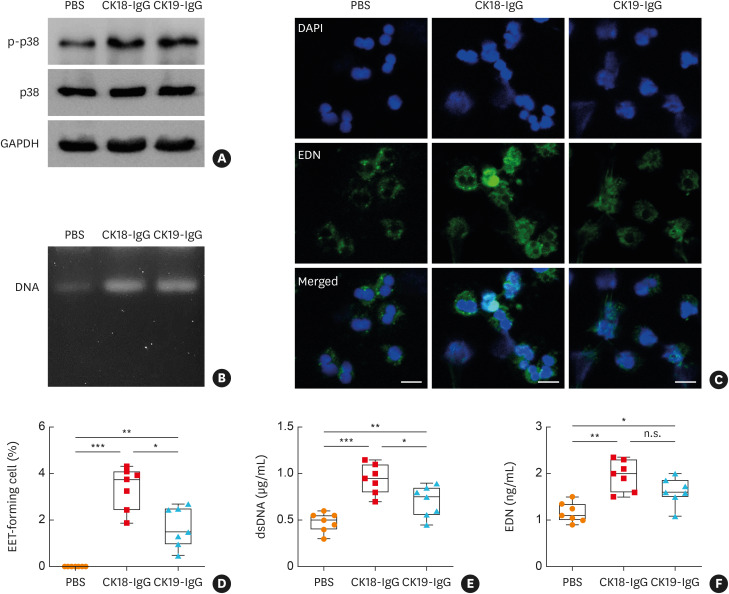
To clarify the function of CK18-specific IgG in airway inflammation, it was evaluated whether these molecules could induce EET formation. When peripheral eosinophils from patients with SA were treated with antibodies, both CK18- and CK19-specific antibodies enhanced phosphorylation of p38 in the cells (Fig. 3A). Moreover, dsDNA was detected in cell culture supernatants (Fig. 3B). By using confocal microscopy, significantly increased EET-forming cells were observed by antibody treatment (Fig. 3C and D). CK18-specific IgG antibody more significantly produced extracellular DNA and EDN from eosinophils compared to CK19-specific IgG (Fig. 3E and F). Furthermore, these EETs could enhance the expression of 2 epithelial antigens including CK18 and CK19 in AECs visualized by immunocytochemistry (Supplementary Fig. S1).
Fig. 3. Effects of epithelial antigen (CK18)-specific IgG antibody on EET formation in eosinophils ex vivo. (A) Phosphorylation of p38 in human peripheral eosinophils. (B) Extracellular DNA from the cells observed by agarose gel electrophoresis. (C) Confocal microscopic images of eosinophil degranulation stained with DAPI (blue) and EDN (green). Scale bar, 20 µm. (D) Quantification of EET-forming cells. Concentrations of (E) dsDNA and (F) EDN released from the cells. Data are presented as box plots (n = 5).
CK, cytokeratin; IgG, immunoglobulin G; EETs, eosinophil extracellular traps; DAPI, 4′,6-diamidino-2-phenylindole; EDN, eosinophil-derived neurotoxin; ANOVA, analysis of variance; n.s., not significant.
*P < 0.05, **P < 0.01, and ***P < 0.001 were obtained by one-way ANOVA with Bonferroni’s post hoc test.
The effect of EETs on CK18/CK18-specific IgG production in a mouse model of SA
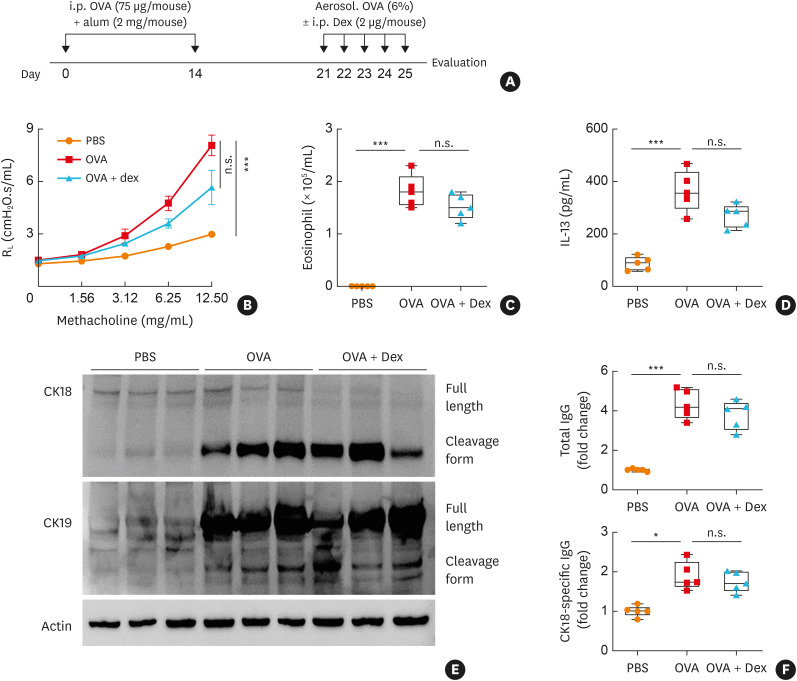
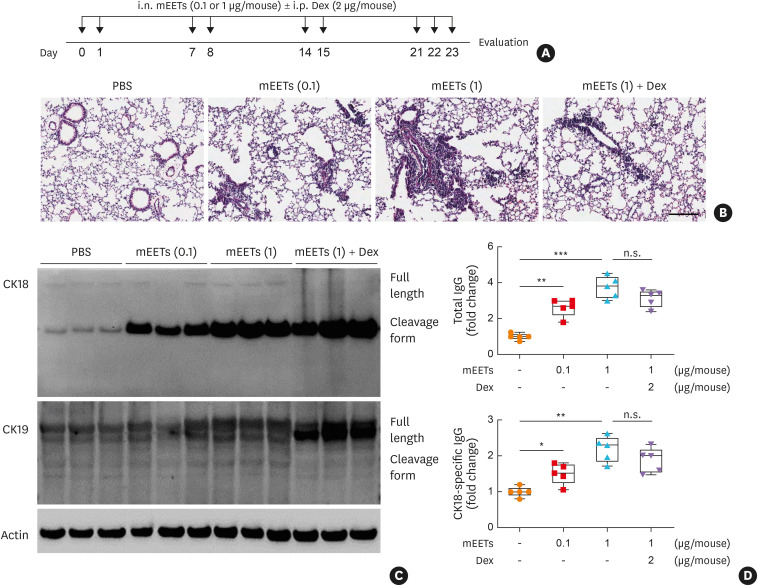
To validate changes in CK18 and CK18-specific IgG production in vivo, a mouse model of SA was established as depicted in Fig. 4A. In this model, significantly enhanced airway responsiveness with steroid insensitivity was shown (Fig. 4B). In addition, eosinophil numbers as well as IL-13 concentrations were markedly elevated in BALF; however, dexamethasone could not completely attenuate eosinophilia and cytokine production (Fig. 4C and D). In the lungs of severe asthmatic mice, significantly increased expression of CK18 and CK19 was noted (Fig. 4E, Supplementary Fig. S2). Furthermore, higher levels of total IgG and CK18-specific IgG were observed in the sera (Fig. 4F). To demonstrate the role of EETs in SA, airway inflammation was induced as shown in Fig. 5A. As a result, EETs markedly induced airway inflammation in the lung tissues (Fig. 5B). In addition, after EET treatment, expression of CK18 was up-regulated, and the levels of total IgG and CK18-specific IgG increased in the lung tissues in a dose-dependent manner, which were not suppressed by dexamethasone treatment (Fig. 5C and D, Supplementary Fig. S3).
Fig. 4. In vivo validation of CK18/CK18-specific IgG production in a mouse model of severe asthma. (A) Schematic for establishing SA in mice. (B) Changes in AHR. (C) Total eosinophil numbers in BALF. (D) Concentrations of IL-13 in BALF. (E) Expression of CK18 and CK19 in the lung tissues. (F) Levels of total IgG and CK18-specific IgG in sera. Data are presented as box plots (n = 5).
CK, cytokeratin; IgG, immunoglobulin G; SA, severe asthma; AHR, airway hyperresponsiveness; BALF, bronchoalveolar lavage fluid; IL, interleukin; n.s., not significant; Dex, dexamethasone; OVA, ovalbumin; PBS, phosphate-buffered saline; ANOVA, analysis of variance.
*P < 0.05 and ***P < 0.001 were obtained by one-way ANOVA with Bonferroni’s post hoc test.
Fig. 5. EETs-induced CK18/CK18-specific IgG production in a mouse model of severe asthma in vivo. (A) Schematic for mEET treatment. (B) Histological analysis of lung tissues stained with hematoxylin and eosin. Scale bar, 200 µm. (C) Expression of CK18 and CK19 in the lung tissues. (D) Levels of total IgG and CK18-specific IgG in sera. Data are presented as box plots (n = 5).
EETs, eosinophil extracellular traps; CK, cytokeratin; IgG, immunoglobulin G; mEETs, mouse-derived eosinophil extracellular traps; i.n., intranasal; i.p. intraperitoneal; Dex, dexamethasone; PBS, phosphate-buffered saline; n.s., not significant.
*P < 0.05, **P < 0.01, and ***P < 0.001 were obtained by one-way ANOVA with Bonferroni’s post hoc test.
DISCUSSION
This is the first study to demonstrate the autoimmune inflammatory axis (EET-induced CK18 and CK18-specific IgG production)-mediated type 2 responses in SA. Here, we observed significantly higher levels of serum CK18 and CK18-specific IgG in patients with SA. Moreover, the CK18-specific antibody was able to induce eosinophil degranulation, leading to EET formation. These EETs further stimulated AECs to release CK18, enhancing eosinophilic inflammation (forming a vicious circle) in SA. These findings may provide new insight into an autoimmune airway mechanism in patients with treatment-refractory SA, which may be mediated by close interplays between AECs and EETs.
CK, including CK18 and CK19, is a cytoskeletal structure protein mainly expressed in AECs, including both bronchial and lung alveolar epithelial cells.22 Previously, our group identified CK18 as a bronchial epithelial antigen in patients with nonallergic asthma (presenting severe clinical phenotype).13 Increased levels of serum CK18-specific IgG were reported in patients with aspirin-exacerbated respiratory disease (AERD); patients with higher levels of CK18-specific IgG had poor lung function.23 In addition, increased expression of CK19 from AECs and increased serum levels of CK19-specific IgG have been demonstrated in patients with isocyanate-induced occupational asthma.24,25 The present study demonstrated that serum levels of circulating CK18 and CK18-specific IgG were significantly higher in patients with SA than in those with NSA (no differences were noted in serum levels of circulating CK19 and CK19-specific IgG). Considering the pathogenic mechanisms of isocyanate-induced asthma, ROS/neutrophil activation is one of the major mechanisms involved in airway inflammation. Increased CK19 expression (sensitive to ROS injury) along with the subsequent generation of CK19-specific IgG may be a major epithelial autoantigen-induced autoimmune mechanism, while SA is characterized by type 2/eosinophilic (including EET-mediated) inflammation; therefore, CK18 may be a major epithelial autoantigen to induce autoimmune mechanisms, further enhancing EET-mediated type 2 airway inflammation. It is known that CK18 undergoes reorganization processes during epithelial cell apoptosis.11,26 Moreover, apoptosis leads to degradation of structure proteins,12 and caspase3-dependent apoptosis causes detachment of epithelial cells in the airway mucosa of asthmatics.10,27 The present study showed a significantly higher expression of the cleavage form of CK18 in the lungs of severe asthmatic mice. Taken together, these findings may explain that CK18 could be a major epithelial autoantigen in SA, even though we could not measure levels of caspase-cleaved CK18.
In addition to structure proteins, specific antibodies against these epithelial antigens have also been found in asthma. Previous studies have shown significantly higher prevalence rates of CK18-specific IgG in patients with AERD.23 The present study demonstrated elevated levels of CK18-specific IgG in patients with SA than in those with NSA; significantly negative correlations were noted between levels of CK18-specific IgG levels and FEV1%, suggesting that CK18-specific IgG may contribute to asthma severity. These findings are comparable to those of our previous studies in patients with AERD, which are characterized by moderate-to-severe asthmatic symptoms and persistent type 2/eosinophilic inflammation in upper and lower airway mucosa.28,29,30 The mechanism of how epithelial antigens induce autoantibody production needs to be further clarified. The present study did not confirm the presence of B-cell clusters as the source of autoantibody generation, although some studies have demonstrated this finding in chronic inflammatory diseases such as rheumatoid arthritis and allergic granulomatosis.31,32,33 However, antigen-binding characteristics of circulating IgG autoantibodies to CK18 were reported in asthmatic patients.34 Furthermore, immune cells including T cell/B cell/antigen presenting cells were localized in the airway mucosa of asthmatics presenting the autoimmune phenotype (presence of autoantibodies in airway secretion), which was associated with eosinophilic infiltration.21,35
Eosinophils are predominantly found in patients with SA, contributing to 2 major events, such as airway inflammation and remodeling, followed by airway obstruction.36,37 In particular, EETs have recently been suggested as a biomarker of SA, activating AECs and type 2 innate immune cells (ILC2) and further enhancing type 2/eosinophilic airway inflammation.38 Sputum autoantibodies have been revealed to stimulate eosinophils to release EETs with steroid resistance, although several factors are shown to induce EET formation.21 Similar to previous studies, ours has demonstrated that CK18-specific IgG antibody could enhance EET formation ex vivo, indicating that the presence of CK18-specific IgG may contribute to EET-mediated type 2 airway inflammation, presenting persistent eosinophilia and steroid resistance in patients with SA. Therefore, we suggest that a novel auto-inflammatory pathway (the epithelial CK18-specific IgG autoantibody-EETs axis) may play a role in enhancing type 2/eosinophilic inflammation in treatment-refractory severe asthmatics having persistent eosinophilia. Eosinophil granule proteins have cytotoxic effects by triggering tissue injury and cell damage.18,39,40 These granule proteins are commonly released, but specific granules are secreted when eosinophils respond to various stimuli.41,42 Especially, EDN stimulates AECs to release mediators involved in the breakdown of extracellular matrix,43 while ECP reduces cell viability and enhances apoptosis by caspase activation.44 Taken together, eosinophil granule proteins in EETs may play an important role in epithelial CK18 production in SA.
The present study has some limitations. We could not demonstrate a direct relationship between CK18-specific IgG levels and local EET formation in patients with SA. However, EETs were localized in endobronchial biopsy specimens of allergic asthmatics15 as well as in endoscopic sinus surgery specimens in patients with severe chronic rhinosinusitis.45 These findings support the active role of EET formation in SA; however, EET-mediated type 2 airway inflammation is not effectively controlled by corticosteroid or anti-IL-5 treatment.37,46 Further investigations are needed to identify new therapeutic agents to overcome this autoimmune-type 2 inflammatory axis (the epithelial CK18-specific IgG autoantibody-EETs axis). It is suggested that inhibition of eosinophil granule proteins may have a potential benefit for asthma treatment.47 Therefore, among eosinophil granule proteins contained in the EETs, anti-ECP or EDN therapy may be a new target to overcome this pathway.
In conclusion, the present study suggests an autoimmune mechanism linked to the type 2 inflammation pathway involved in epithelial autoantigen (CK18)-specific IgG-induced EET formation in the pathogenesis of type 2/eosinophilic airway inflammation of SA. Regulation of this axis could be a new therapeutic option for treatment-refractory severe asthmatics with persistent eosinophilia.
ACKNOWLEDGMENTS
This study was supported by a grant from the Korean Health Technology R & D Project, Ministry of Health & Welfare, Republic of Korea (HR16C0001). The confocal laser scanning microscope (LSM710) used in this study was supported by a Korea Basic Science Institute (National Research Facilities and Equipment Center) grant funded by the Ministry of Education (grant no. 2019R1A6C1010003).
Footnotes
Disclosure: There are no financial or other issues that might lead to conflict of interest.
SUPPLEMENTARY MATERIALS
Confocal images of epithelial antigen expression in airway epithelial cells with or without EET treatment. DAPI (blue), CK18 (red), and CK19 (yellow). Scale bar, 200 µm.
Expression of CK18 (red) and CK19 (green) in severe asthmatic mice with or without steroid treatment. Scale bar, 50 µm.
Expression of CK18 (red) and CK19 (green) in mice stimulated with mEETs. Scale bar, 50 µm.
References
- 1.Cevhertas L, Ogulur I, Maurer DJ, Burla D, Ding M, Jansen K, et al. Advances and recent developments in asthma in 2020. Allergy. 2020;75:3124–3146. doi: 10.1111/all.14607. [DOI] [PubMed] [Google Scholar]
- 2.Holguin F, Cardet JC, Chung KF, Diver S, Ferreira DS, Fitzpatrick A, et al. Management of severe asthma: a European Respiratory Society/American Thoracic Society guideline. Eur Respir J. 2020;55:1900588. doi: 10.1183/13993003.00588-2019. [DOI] [PubMed] [Google Scholar]
- 3.Soma T, Iemura H, Naito E, Miyauchi S, Uchida Y, Nakagome K, et al. Implication of fraction of exhaled nitric oxide and blood eosinophil count in severe asthma. Allergol Int. 2018;67S:S3–11. doi: 10.1016/j.alit.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 4.Price DB, Bosnic-Anticevich S, Pavord ID, Roche N, Halpin DM, Bjermer L, et al. Association of elevated fractional exhaled nitric oxide concentration and blood eosinophil count with severe asthma exacerbations. Clin Transl Allergy. 2019;9:41. doi: 10.1186/s13601-019-0282-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heijink IH, Kuchibhotla VN, Roffel MP, Maes T, Knight DA, Sayers I, et al. Epithelial cell dysfunction, a major driver of asthma development. Allergy. 2020;75:1902–1917. doi: 10.1111/all.14421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen L, e X, Tarsi J, Ramkumar T, Horiuchi TK, Cochran R, et al. Epithelial cell proliferation contributes to airway remodeling in severe asthma. Am J Respir Crit Care Med. 2007;176:138–145. doi: 10.1164/rccm.200607-1062OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bourdin A, Neveu D, Vachier I, Paganin F, Godard P, Chanez P. Specificity of basement membrane thickening in severe asthma. J Allergy Clin Immunol. 2007;119:1367–1374. doi: 10.1016/j.jaci.2007.01.055. [DOI] [PubMed] [Google Scholar]
- 8.Holgate ST, Davies DE, Puddicombe S, Richter A, Lackie P, Lordan J, et al. Mechanisms of airway epithelial damage: epithelial-mesenchymal interactions in the pathogenesis of asthma. Eur Respir J Suppl. 2003;44:24s–29s. doi: 10.1183/09031936.03.00000803. [DOI] [PubMed] [Google Scholar]
- 9.Yang Y, Jia M, Ou Y, Adcock IM, Yao X. Mechanisms and biomarkers of airway epithelial cell damage in asthma: a review. Clin Respir J. 2021;15:1027–1045. doi: 10.1111/crj.13407. [DOI] [PubMed] [Google Scholar]
- 10.Bucchieri F, Puddicombe SM, Lordan JL, Richter A, Buchanan D, Wilson SJ, et al. Asthmatic bronchial epithelium is more susceptible to oxidant-induced apoptosis. Am J Respir Cell Mol Biol. 2002;27:179–185. doi: 10.1165/ajrcmb.27.2.4699. [DOI] [PubMed] [Google Scholar]
- 11.Caulín C, Salvesen GS, Oshima RG. Caspase cleavage of keratin 18 and reorganization of intermediate filaments during epithelial cell apoptosis. J Cell Biol. 1997;138:1379–1394. doi: 10.1083/jcb.138.6.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.White SR, Williams P, Wojcik KR, Sun S, Hiemstra PS, Rabe KF, et al. Initiation of apoptosis by actin cytoskeletal derangement in human airway epithelial cells. Am J Respir Cell Mol Biol. 2001;24:282–294. doi: 10.1165/ajrcmb.24.3.3995. [DOI] [PubMed] [Google Scholar]
- 13.Nahm DH, Lee YE, Yim EJ, Park HS, Yim H, Kang Y, et al. Identification of cytokeratin 18 as a bronchial epithelial autoantigen associated with nonallergic asthma. Am J Respir Crit Care Med. 2002;165:1536–1539. doi: 10.1164/rccm.200201-009OC. [DOI] [PubMed] [Google Scholar]
- 14.Kwon B, Lee HA, Choi GS, Ye YM, Nahm DH, Park HS. Increased IgG antibody-induced cytotoxicity against airway epithelial cells in patients with nonallergic asthma. J Clin Immunol. 2009;29:517–523. doi: 10.1007/s10875-009-9276-x. [DOI] [PubMed] [Google Scholar]
- 15.Dworski R, Simon HU, Hoskins A, Yousefi S. Eosinophil and neutrophil extracellular DNA traps in human allergic asthmatic airways. J Allergy Clin Immunol. 2011;127:1260–1266. doi: 10.1016/j.jaci.2010.12.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ueki S, Melo RC, Ghiran I, Spencer LA, Dvorak AM, Weller PF. Eosinophil extracellular DNA trap cell death mediates lytic release of free secretion-competent eosinophil granules in humans. Blood. 2013;121:2074–2083. doi: 10.1182/blood-2012-05-432088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mukherjee M, Lacy P, Ueki S. Eosinophil extracellular traps and inflammatory pathologies-untangling the web! Front Immunol. 2018;9:2763. doi: 10.3389/fimmu.2018.02763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi Y, Sim S, Park HS. Distinct functions of eosinophils in severe asthma with type 2 phenotype: clinical implications. Korean J Intern Med. 2020;35:823–833. doi: 10.3904/kjim.2020.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi Y, Le Pham D, Lee DH, Lee SH, Kim SH, Park HS. Biological function of eosinophil extracellular traps in patients with severe eosinophilic asthma. Exp Mol Med. 2018;50:1–8. doi: 10.1038/s12276-018-0136-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi Y, Kim YM, Lee HR, Mun J, Sim S, Lee DH, et al. Eosinophil extracellular traps activate type 2 innate lymphoid cells through stimulating airway epithelium in severe asthma. Allergy. 2020;75:95–103. doi: 10.1111/all.13997. [DOI] [PubMed] [Google Scholar]
- 21.Mukherjee M, Bulir DC, Radford K, Kjarsgaard M, Huang CM, Jacobsen EA, et al. Sputum autoantibodies in patients with severe eosinophilic asthma. J Allergy Clin Immunol. 2018;141:1269–1279. doi: 10.1016/j.jaci.2017.06.033. [DOI] [PubMed] [Google Scholar]
- 22.Chu PG, Weiss LM. Keratin expression in human tissues and neoplasms. Histopathology. 2002;40:403–439. doi: 10.1046/j.1365-2559.2002.01387.x. [DOI] [PubMed] [Google Scholar]
- 23.Ye YM, Nahm DH, Kim SH, Kim SH, Choi JH, Suh CH, et al. Circulating autoantibodies in patients with aspirin-intolerant asthma: an epiphenomenon related to airway inflammation. J Korean Med Sci. 2006;21:412–417. doi: 10.3346/jkms.2006.21.3.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi JH, Nahm DH, Kim SH, Kim YS, Suh CH, Park HS, et al. Increased levels of IgG to cytokeratin 19 in sera of patients with toluene diisocyanate-induced asthma. Ann Allergy Asthma Immunol. 2004;93:293–298. doi: 10.1016/S1081-1206(10)61504-9. [DOI] [PubMed] [Google Scholar]
- 25.Ye YM, Nahm DH, Kim CW, Kim HR, Hong CS, Park CS, et al. Cytokeratin autoantibodies: useful serologic markers for toluene diisocyanate-induced asthma. Yonsei Med J. 2006;47:773–781. doi: 10.3349/ymj.2006.47.6.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leers MP, Kölgen W, Björklund V, Bergman T, Tribbick G, Persson B, et al. Immunocytochemical detection and mapping of a cytokeratin 18 neo-epitope exposed during early apoptosis. J Pathol. 1999;187:567–572. doi: 10.1002/(SICI)1096-9896(199904)187:5<567::AID-PATH288>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 27.Benayoun L, Letuve S, Druilhe A, Boczkowski J, Dombret MC, Mechighel P, et al. Regulation of peroxisome proliferator-activated receptor gamma expression in human asthmatic airways: relationship with proliferation, apoptosis, and airway remodeling. Am J Respir Crit Care Med. 2001;164:1487–1494. doi: 10.1164/ajrccm.164.8.2101070. [DOI] [PubMed] [Google Scholar]
- 28.Choi GS, Kim JH, Shin YS, Ye YM, Kim SH, Park HS. Eosinophil activation and novel mediators in the aspirin-induced nasal response in AERD. Clin Exp Allergy. 2013;43:730–740. doi: 10.1111/cea.12096. [DOI] [PubMed] [Google Scholar]
- 29.Trinh HK, Pham DL, Choi Y, Kim HM, Kim SH, Park HS. Epithelial folliculin enhances airway inflammation in aspirin-exacerbated respiratory disease. Clin Exp Allergy. 2018;48:1464–1473. doi: 10.1111/cea.13253. [DOI] [PubMed] [Google Scholar]
- 30.Ban GY, Kim SH, Park HS. Persistent eosinophilic inflammation in adult asthmatics with high serum and urine levels of leukotriene E4 . J Asthma Allergy. 2021;14:1219–1230. doi: 10.2147/JAA.S325499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salomonsson S, Larsson P, Tengnér P, Mellquist E, Hjelmström P, Wahren-Herlenius M. Expression of the B cell-attracting chemokine CXCL13 in the target organ and autoantibody production in ectopic lymphoid tissue in the chronic inflammatory disease Sjögren’s syndrome. Scand J Immunol. 2002;55:336–342. doi: 10.1046/j.1365-3083.2002.01058.x. [DOI] [PubMed] [Google Scholar]
- 32.Rangel-Moreno J, Hartson L, Navarro C, Gaxiola M, Selman M, Randall TD. Inducible bronchus-associated lymphoid tissue (iBALT) in patients with pulmonary complications of rheumatoid arthritis. J Clin Invest. 2006;116:3183–3194. doi: 10.1172/JCI28756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wenzel SE, Vitari CA, Shende M, Strollo DC, Larkin A, Yousem SA. Asthmatic granulomatosis: a novel disease with asthmatic and granulomatous features. Am J Respir Crit Care Med. 2012;186:501–507. doi: 10.1164/rccm.201203-0476OC. [DOI] [PubMed] [Google Scholar]
- 34.Yim H, Kim JE, Shin JY, Ye YM, Park HS, Nahm DH. Antigen-binding characteristics of circulating IgG autoantibodies to cytokeratin 18 protein in patients with nonallergic asthma. J Korean Med Sci. 2006;21:652–655. doi: 10.3346/jkms.2006.21.4.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mukherjee M, Nair P. Autoimmune responses in severe asthma. Allergy Asthma Immunol Res. 2018;10:428–447. doi: 10.4168/aair.2018.10.5.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bakakos A, Loukides S, Bakakos P. Severe eosinophilic asthma. J Clin Med. 2019;8:1375. doi: 10.3390/jcm8091375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee Y, Quoc QL, Park HS. Biomarkers for severe asthma: lessons from longitudinal cohort studies. Allergy Asthma Immunol Res. 2021;13:375–389. doi: 10.4168/aair.2021.13.3.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Granger V, Taillé C, Roach D, Letuvé S, Dupin C, Hamidi F, et al. Circulating neutrophil and eosinophil extracellular traps are markers of severe asthma. Allergy. 2020;75:699–702. doi: 10.1111/all.14059. [DOI] [PubMed] [Google Scholar]
- 39.Acharya KR, Ackerman SJ. Eosinophil granule proteins: form and function. J Biol Chem. 2014;289:17406–17415. doi: 10.1074/jbc.R113.546218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gigon L, Yousefi S, Karaulov A, Simon HU. Mechanisms of toxicity mediated by neutrophil and eosinophil granule proteins. Allergol Int. 2021;70:30–38. doi: 10.1016/j.alit.2020.11.003. [DOI] [PubMed] [Google Scholar]
- 41.Dvorak AM, Estrella P, Ishizaka T. Vesicular transport of peroxidase in human eosinophilic myelocytes. Clin Exp Allergy. 1994;24:10–18. doi: 10.1111/j.1365-2222.1994.tb00910.x. [DOI] [PubMed] [Google Scholar]
- 42.Melo RC, Spencer LA, Perez SA, Ghiran I, Dvorak AM, Weller PF. Human eosinophils secrete preformed, granule-stored interleukin-4 through distinct vesicular compartments. Traffic. 2005;6:1047–1057. doi: 10.1111/j.1600-0854.2005.00344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsuda T, Maeda Y, Nishide M, Koyama S, Hayama Y, Nojima S, et al. Eosinophil-derived neurotoxin enhances airway remodeling in eosinophilic chronic rhinosinusitis and correlates with disease severity. Int Immunol. 2019;31:33–40. doi: 10.1093/intimm/dxy061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chang KC, Lo CW, Fan TC, Chang MD, Shu CW, Chang CH, et al. TNF-alpha mediates eosinophil cationic protein-induced apoptosis in BEAS-2B cells. BMC Cell Biol. 2010;11:6. doi: 10.1186/1471-2121-11-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hwang CS, Park SC, Cho HJ, Park DJ, Yoon JH, Kim CH. Eosinophil extracellular trap formation is closely associated with disease severity in chronic rhinosinusitis regardless of nasal polyp status. Sci Rep. 2019;9:8061. doi: 10.1038/s41598-019-44627-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ban GY, Kim SC, Lee HY, Ye YM, Shin YS, Park HS. Risk factors predicting severe asthma exacerbations in adult asthmatics: a real-world clinical evidence. Allergy Asthma Immunol Res. 2021;13:420–434. doi: 10.4168/aair.2021.13.3.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koh GC, Shek LP, Goh DY, Van Bever H, Koh DS. Eosinophil cationic protein: is it useful in asthma? A systematic review. Respir Med. 2007;101:696–705. doi: 10.1016/j.rmed.2006.08.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Confocal images of epithelial antigen expression in airway epithelial cells with or without EET treatment. DAPI (blue), CK18 (red), and CK19 (yellow). Scale bar, 200 µm.
Expression of CK18 (red) and CK19 (green) in severe asthmatic mice with or without steroid treatment. Scale bar, 50 µm.
Expression of CK18 (red) and CK19 (green) in mice stimulated with mEETs. Scale bar, 50 µm.