Abstract
Purpose
Interleukin (IL)-17A plays a critical role in the pathogenesis of allergic airway inflammation. Yet, the exact roles of IL-17A in asthma are still controversial. Thus, the aim of this study was to dissect the roles of IL-17A in toluene diisocyanate (TDI)-induced mixed granulocytic asthma and to assess the effects of neutralizing antibody in different effector phases on TDI-induced asthma.
Methods
IL-17A functions in allergic airway inflammation were evaluated using mice deficient in IL-17A (Il17a−/−) or IL-17A monoclonal antibody (IL-17A mab, intraperitoneally, 50 μg per mouse, 100 μg per mouse). Moreover, the effects of exogenous recombinant IL (rIL)-17A in vivo (murine rIL-17A, intranasally, 1 μg per mouse) and in vitro (human rIL-17A, 100 ng/mL) were investigated.
Results
TDI-induced mixed granulocytic airway inflammation was IL-17A-dependent because airway hyperreactivity, neutrophil and eosinophil infiltration, airway smooth muscle thickness, epithelium injury, dysfunctional T helper (Th) 2 and Th17 responses, granulocytic chemokine production and mucus overproduction were more markedly reduced in the Il17a−/− mice or by IL-17A neutralization during the sensitization phase of wild-type (WT) mice. By contrast, IL-17A neutralization during the antigen-challenge phase aggravated TDI-induced eosinophils recruitment, with markedly elevated Th2 response. In line with this, instillation of rIL-17 during antigen sensitization exacerbated airway inflammation by promoting neutrophils aggregation, while rIL-17A during the antigen-challenge phase protected the mice from TDI-induced airway eosinophilia. Moreover, rIL-17A exerted distinct effects on eosinophil- or neutrophil-related signatures in vitro.
Conclusions
Our data demonstrated that IL-17A was required for the initiation of TDI-induced asthma, but functioned as a negative regulator of established allergic inflammation, suggesting that early abrogation of IL-17A signaling, but not late IL-17A neutralization, may prevent the progression of TDI-induced asthma and could be used as a therapeutic strategy for severe asthmatics in clinical settings.
Keywords: Asthma, toluene diisocyanate, early intervention, IL-17A, dual effects
INTRODUCTION
Asthma has increased dramatically in prevalence and severity over the last 3 decades, currently affecting about 334 million individuals at all ages around the world.1 Work-relatedness accounts for 15%–33% in adult-onset asthma cases, but delays in diagnosis remain common and lead to declined lung function and poor prognosis.2 Severe asthma represents 10% of the asthmatic population, but has greater morbidity and mortality, posing a greater financial burden on the healthcare system. As one of the most commonly reported causes of occupational asthma, toluene diisocyanate (TDI) exhibited the capacity to induce mixed granulocytic inflammation characterized by a larger number of neutrophils and a smaller number of eosinophils infiltrating into the airways, which responses poorly to both systemic and inhaled steroid treatment.3,4 Evidence from clinical and in vivo investigations revealed that distinct subgroups of CD4+ T lymphocytes (especially T helper [Th] 2 and Th17 cells), with their secreted mediators, play preeminent roles in the steroid irresponsiveness of mixed granulocytic asthma.3,5,6 Thus, a better understanding of mechanisms underpinning TDI-induced mixed granulocytic airway inflammation is of great importance to the precise therapy of refractory asthma.
Interleukin-17A (IL-17A, namely IL-17) is a cytokine mainly produced by Th17 cells, a Th-cell lineage distinct from Th1 and Th2 cells, which is negatively regulated by interferon-γ (IFN-γ) and IL-4.7 Although the role of IL-17A in allergic airway inflammation has been extensively investigated in animal models and patients, the exact roles of IL-17A in asthma are still in dispute. Increased expression of IL-17A was detected in the airways of allergic asthmatic subjects8 and positively linked to asthma severity and steroid resistance.9 In addition, blockade of IL-17A signaling using gene-deficient mice or neutralizing antibody abrogates house dust mite (HDM)- or ovalbumin (OVA)-induced airway hyperresponsiveness (AHR), airway eosinophil and neutrophil recruitment and remodeling.10,11,12 Yet, other researchers reported that IL-17A acts as a negative regulator of established allergic asthma and exogenous IL-17A could markedly blunt OVA-induced AHR, Th2 response and lung eosinophil aggregation.13,14 Even the latest study found that IL-17A blocking antibody significantly inhibited airway neutrophil infiltration, but promoted eosinophil accumulation in a HDM/complete Freunds adjunvant (CFA)-induced mixed granulocytic asthma model.15 In addition, IL-17A directly enhances IL-13-driven airway inflammation.16,17 Indeed, researchers have already detected elevated IL-17A concentrations in TDI-induced asthma model and discovered that IL-17A neutralization could alleviate TDI-induced airway inflammation and remodeling.18,19 Conversely, we previously demonstrated that anti-IL-17A-neutralizing antibody evidently aggravated TDI-induced allergic airway inflammation through amplifying Th2 responses and eosinophil infiltration,3 which seems opposite to the results by using Il17a−/− mice.20,21 These conflicted results addressed the crucial but controversial roles of IL-17A in asthma, yet how IL-17A contributes to the pathogenesis of TDI-induced asthma is still incompletely understood.
Over the past decades, great advances have been made in the generation of neutralizing antibodies that target IL-17A signaling directly or indirectly, which sparked a series of clinical trials to examine whether this approach is effective in several refractory diseases including rheumatoid arthritis, severe asthma, psoriasis, psoriatic arthritis, uveitis and Crohn disease.22,23 In 2010, Hueber and coworkers demonstrated that a human IL-17A monoclonal antibody, AIN457, improved the severity of psoriasis, rheumatoid arthritis and uveitis.22 However, Busse et Al.23 reported that inhibition of IL-17RA by using brodalumab, a monoclonal antibody, did not exert a therapeutic effect on the symptoms of asthmatics. Actually, our research group found that elevated IL-17A was detected in the serum of early-onset severe asthmatics, but not in the serum of late-onset severe asthmatics, and positively correlated to sputum neutrophils.24 In addition, our preliminary results demonstrated that in vivo deficiency of IL-17A attenuates TDI-induced experimental asthma. All these drove us to propose our hypothesis that IL-17A conveys distinct effects on TDI-induced asthma during different effector phases and that intervention of IL-17A signaling during different phases would produce different effects on TDI asthma. Thus, the purpose of this study is: 1) to elucidate the functional roles of IL-17A in TDI-induced asthma and 2) to assess the effects of IL-17A neutralizing antibody on TDI-induced asthma during different effector phases.
MATERIALS AND METHODS
Animals
For this study, 6- to 8-week-old female C57BL/6 mice were supplied by Guangdong Medical Laboratory Animal Center and female Il17a−/− mice on C57BL/6 background (generated by Prof. Yoichiro Iwakura and provided as a gift by Prof. Eyal Raz, University of California, San Diego, CA, USA) were bred in-house. All mice were housed in a specific pathogen-free facility with 12 hours dark/light cycles (temperature 23°C ± 2°C, humidity range 40%–70%, 12 hours light/dark cycle [lighting, 7:00–19:00]). Water and food were provided ad libitum. All animal experiments described here complied with the guidelines of the Committee on the Use and Care of Animals of Shenzhen People’s Hospital (Shenzhen, China), and were approved by the Animal Subjects Committee of Shenzhen People’s Hospital. The genotyping of Il17a−/− mice was carried out using the following polymerase chain reaction (PCR) primers: primer 1, 5′-ACTCTTCATCCACCTCACACGA-3′; primer 2, 5′-GCCATGATATAGACGTTGTGGC-3′; primer 3, 5′-CAGCATCAGAGACTAGAAGGGA-3′. Primers 1 and 2 were used to detect wild-type alleles (1.3 kb), and primers 1 and 3 were used to detect mutant alleles (0.5 kb).
Reagents
Toluene diisocyanate (toluene-2, 4-diisocyanate, ≥ 98.0%), methacholine and acetone were obtained from Sigma-Aldrich (Shanghai, China). Anti-IL-17A monoclonal antibody (IL-17A mab, #BP0173) and mouse IgG1 isotype control antibody (#BP0083) were purchased from Bio X Cell (Lebanon, PA, USA). Recombinant mouse IL-17A (#210-17) and human IL-17A (#200-17) were purchased from PeproTech (Rocky Hill, NJ, USA). Multiplex immunoassays for IL-4, IL-5, IL-13, IL-17A, IL-6, IL-18, CCL11, CXCL1 and CSF-3 and enzyme-linked immunosorbent assay (ELISA) kits for IL-17F and total immunoglobulin (Ig) E was purchased from eBioscience (San Diego, CA, USA). TRIzol reagents for extracting total RNA and gene expression analysis were purchased from Takara (Guangzhou, China).
TDI-induced asthma model
TDI-induced asthma model was prepared according to our previous work.3 C57BL/6 and Il17a−/− mice were dermally sensitized with 0.3% TDI on days 1 and 8. After that, on days 15, 18 and 21, the mice were placed in a horizontal rectangle chamber and challenged through compressed air nebulization (NE-C28; Omron, Kyoto, Japan). TDI was diluted in a mixture of 3 volumes of olive oil and 2 volumes of acetone for the sensitization and 4 volumes of olive oil and 1 volume of acetone for the challenge. Control mice were sensitized and challenged with the same amount of vehicle.
Treatment
IL-17A mab (Bio X Cell: 50 μg/mouse and 100 μg/mouse) or mouse IgG isotype control antibody (50 μg/mouse and 100 μg/mouse) was administered separately via the intraperitoneal route.13,19 Recombinant mouse IL-17A (rmIL-17A; PeproTech) was dissolved in sterile phosphate buffered saline (PBS) and instilled at a dose of 1 μg/mouse per time.13 During allergen sensitization, IL-17A mab and rmIL-17A were given every 3 days beginning from the first to the second immunization for 3 times after each sensitization in total (marked as “TDI + IL-17A mab/S” and “TDI + recombinant IL [rIL]-17A/S”), while during the TDI challenge phase, IL-17A mab and rmIL-17A were given immediately after each inhalation (marked as “TDI + IL-17A mab/C” and “TDI + rIL-17A/C”).
Cell culture
The BEAS-2B cells cultured in Dulbecco’s Modified Eagle Medium (DMEM) medium supplemented with 10% calf serum were grown to 70%–80% confluent and then treated with TDI (2 mM) + PBS or TDI (2 mM) + IL-17A (100 ng/mL) for 24 hours; thereafter, collected for detecting gene expression by quantitative PCR.
Airway responsiveness measurement
Twenty-four hours after the last challenge, measurements of lung resistance (RL) were taken on anaesthetized and mechanically ventilated (Buxco Electronics, Troy, NY, USA) mice in response to increasing doses of methacholine by ultrasonic nebulization (6.25, 12.5, 25 and 50 mg/mL).
Sample collection
Mice were euthanized with overdoses of pentobarbital (100 mg/kg, intraperitoneally [i.p.]) 24 hours after the last airway challenge. Blood samples were taken from the retroorbital plexus/sinus and placed at room temperature for 1 hour; they were centrifuged (3,000 g, 20 minutes) and their supernatants were harvested for detection of total IgE using an ELISA kit according to the manufacturer’s instructions. After blood was taken, the lungs were lavaged twice in situ with 0.8 mL prewarmed sterile saline (0.9% NaCl) and the recovered fluid was pooled. Cell counts were determined for each bronchoalveolar lavage fluid (BALF) sample, and differential cell counts were performed on cytospin preparations stained with haematoxylin and eosin (H&E). The remaining fluid was centrifuged (1,000 g, 10 minutes) and supernatants were stored at −80ºC for further analysis.
Histopathological analysis
The left lung was harvested, fixed overnight in 4% neutral formalin and embedded in paraffin. Sections (4 μm) were stained with H&E and periodic acid-Schiff (PAS). Airway inflammation, PAS stainning and cellular infiltration were assigned a random code to blind the examiner to the identity of each specimen. PAS-positive epithelial cells of the total epithelial cells were counted to obtain a percentage and compare groups. The 10–16 image fields of 8 sections from 5–8 mice per group were analyzed. Thickness of airway smooth muscle was measured as previously described.3 Briefly, the thickness of the peribronchial smooth muscle layer (the transverse diameter) in large airways was measured from the innermost aspect to the outermost aspect of the circumferential smooth muscle layer. The 10–24 image fields of eight sections from 5–8 mice per group were analyzed.
Cytokine and chemokine analysis
Levels of cytokines and chemokines in BALF, including Th2-related IL-4, IL-5, IL-13, Th17-related IL-17A, IL-17F, Th17 cell maturation associated IL-6, neutrophil chemokine IL-18 and eosinophil attractants CCL11, were detected using multiplex immunoassay or ELISA kits according to the manufacturer’s specifications.
Gene expression analysis
Total RNA was extracted with an RNAiso Plus kit and reverse-transcribed to complementary DNA in the presence of PrimeScript™ RT reagent kit. Real-time PCR was carried out in a 20 µL reaction system using SYBR Green Premix Ex Taq (Takara) by LightCycler 480 Fast Real-Time PCR System. The primers used were listed in Table.
Table. Primer sequences for quantitative PCR.
| Gene | Species | Forward sequence (5′→3′) | Reverse sequence (5′→3′) |
|---|---|---|---|
| β-actin | Murine | GGCTGTATTCCCCTCCATCG | CCAGTTGGTAACAATGCCATGT |
| Muc5ac | Murine | CAGGACTCTCTGAAATCGTACCA | AAGGCTCGTACCACAGGGA |
| Il4 | Murine | ACGAGGTCACAGGAGAAGGGA | AGCCCTACAGACGAGCTCACTC |
| Il5 | Murine | CTGGCCTCA AACTGGTAATGTAG | ATGAGGGGGAGGGAGTATAACTC |
| Il13 | Murine | CCTCTGACCCTTAAGGAGCTTAT | CGTTGCACAGGGGAGTCT |
| Il17a | Murine | GAGAGCTTCATCTGTGTCTCTG | GCGCCAAGGGAGTTAAAGAC |
| Il17f | Murine | CGTGAAACAGCCATGGTCAAG | GGGACAGAAATGCCCTGGTT |
| Ccl11 | Murine | TGCTCACGGTCACTTCCTTC | CTTGAAGACTATGGCTTTCAGGGTG |
| Clca3 | Murine | AGGAAAACCCCAAGCAGTG | GCACCGACGAACTTGATTTT |
| Cxc11 | Murine | AACCGAAGTCATAGCCACACT | CCGTTACTTGGGGACACCTT |
| Cxcl3 | Murine | CACCCAGACAGAAGTCATAGCC | CCGTTGGGATGGATCGCTTT |
| Csf3 | Murine | GTGCTGCTGGAGCAGTTGT | TCGGGATCCCCAGAGAGT |
| Tbet | Murine | GGACGATCATCTGGGTCACATTGT | GCCAGGGAACCGCTTATATG |
| Gata3 | Murine | CTACCGGGTTCGGATGTAAGTCG | GTTCACACACTCCCTGCCTTCT |
| Rorc | Murine | ACAACAGCAGCAAGTGATGG | CCTGGATTTATCCCTGCTGA |
| β-actin | Human | GAGACCGCGTCCGCC | ATCATCATCCATGGTGAGCTGG |
| Il4 | Human | CACAGAGCAGAAGAACACAACTG | GCGAGTGTCCTTCTCATGGT |
| Il5 | Human | TGACTTTTGGAAGGGGAGACC | AACATGGATGAACCCCGCTT |
| Il13 | Human | ATGGCGCTTTTGTTGACCAC | ATTGCAGAGCGGAGCCTTC |
| Il17a | Human | TCTCATAGCAGGCACAAACTCA | AGCAGTAGCAGTGACACCAAT |
| Il17f | Human | TTGGACCGCTGAACTTGTGG | AAGTACTTGACCATGGCTGGG |
| IL1β | Human | GCTCGCCAGTGAAATGATGG | CTGGAAGGAGCACTTCATCTGT |
| Ccl11 | Human | ACCACCTGCTGCTTTAACCT | CTTGAAGATCACAGCTTTCTGGG |
| Ccl24 | Human | CTGTTACCTCCGGGTCCTTT | GATGATGTGGTGGGCACAGA |
| Cxcl1 | Human | AACCGAAGTCATAGCCACACTC | AGGAACAGCCACCAGTGAG |
| Cxcl3 | Human | AAGATACTGAACAAGGGGAGCAC | TTTTCAGCTCTGGTAAGGGCA |
| Csf3 | Human | AGAGCTTCCTGCTCAAGTGC | TGGCACACTCACTCACCAG |
| Tbet | Human | CTGGATGCGCCAGGAAGTT | TGGAGCACAATCATCTGGGTC |
| Gata3 | Human | CAGCACAGGCAGGGAGTG | AGCCTTCGCTTGGGCTTAAT |
| Rorc | Human | AAGAAGACCCACACCTCACAAA | GCACCCCTCACAGGTGATAA |
PCR, polymerase chain reaction.
Statistical analysis
Data are expressed as mean ± standard error of the mean. Results were interpreted using one-way analysis of variance and LSD-t with SPSS version 22.0 (SPSS, Chicago, IL, USA). A value of P < 0.05 was considered statistically significant.
RESULTS
IL-17A is required for the initiation of TDI-induced experimental asthma
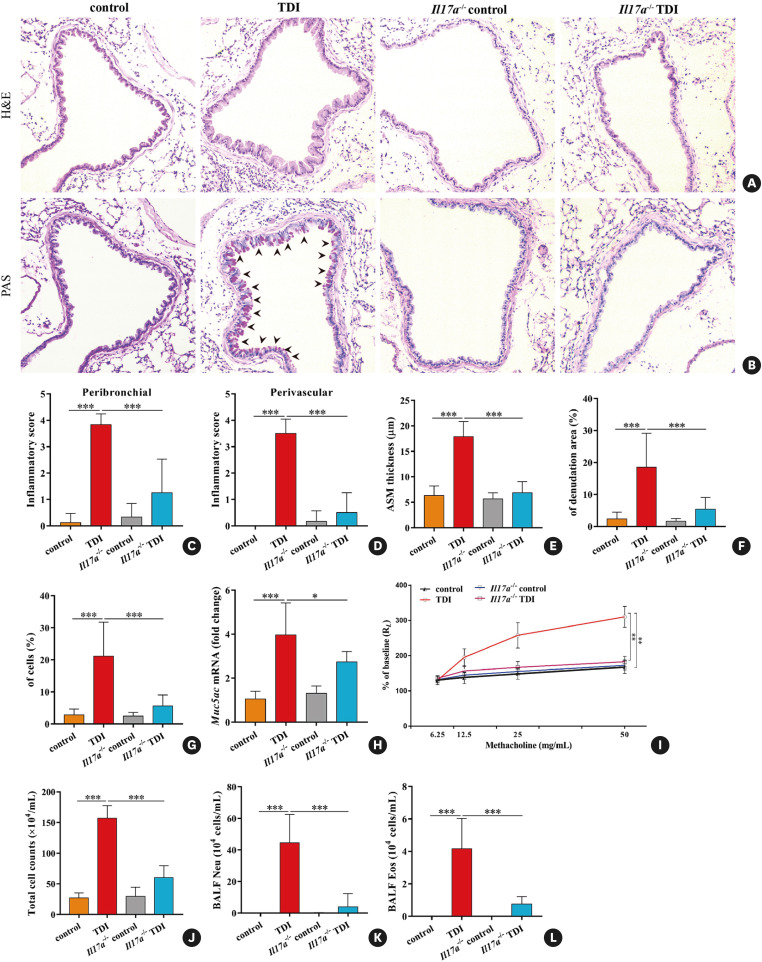
To directly test the role of IL-17A in TDI-induced asthma, we sensitized Il17a−/− and wild-type (WT) mice on days 1 and 8 with 0.3% TDI (dermally immunized on the dorsum of each ear), followed by 3 challenges with 3% TDI on day 15, 18 and 21 (Supplementary Fig. S1A). TDI-inhaled WT mice exhibited a robust neutrophil and eosinophil infiltration into airway compared to the controls. However, TDI-induced airway inflammation, epithelial hyperplasia, AHR, smooth muscle thickening, and epithelium denudation were markedly blunted by IL-17A deficiency (Fig. 1A-F and I). Likewise, we found a reduction in neutrophil and eosinophil recruitment in BALF of TDI-sensitized and challenged Il17a−/− mice compared to WT mice (Fig. 1J, K and L).
Fig. 1. IL-17A deficiency alleviates TDI-induced mixed granulocytic asthma. (A) Representative H&E-stained lung sections of different groups. (B) Representative PAS-stained lung sections of different groups. Original magnification was 200×. (C, D) Semi-quantification of airway inflammation was performed (n = 5–8). (E, F) Analysis of ASM thickness and epithelial denudation was performed (n = 5–8). (G) Semi-quantification of PAS-positive staining was determined by counting the number of PAS-positive epithelial cells (n = 5–8). (H) Expression of Muc5ac gene (quantitative PCR) in the lungs (n = 3). (I) Airway hyperresponsiveness was measured by lung resistance (RL). Results are shown as percentage of baseline value (n = 4). (J, K) Numbers of total inflammatory cells, neutrophils and eosinophils in BALF (n = 5–8).
IL, interleukin; TDI, toluene diisocyanate; H&E, haematoxylin and eosin; PAS, periodic acid-Schiff; ASM, airway smooth muscle; PCR, polymerase chain reaction; BALF, bronchoalveolar lavage fluid.
*P < 0.05; **P < 0.01; ***P < 0.001.
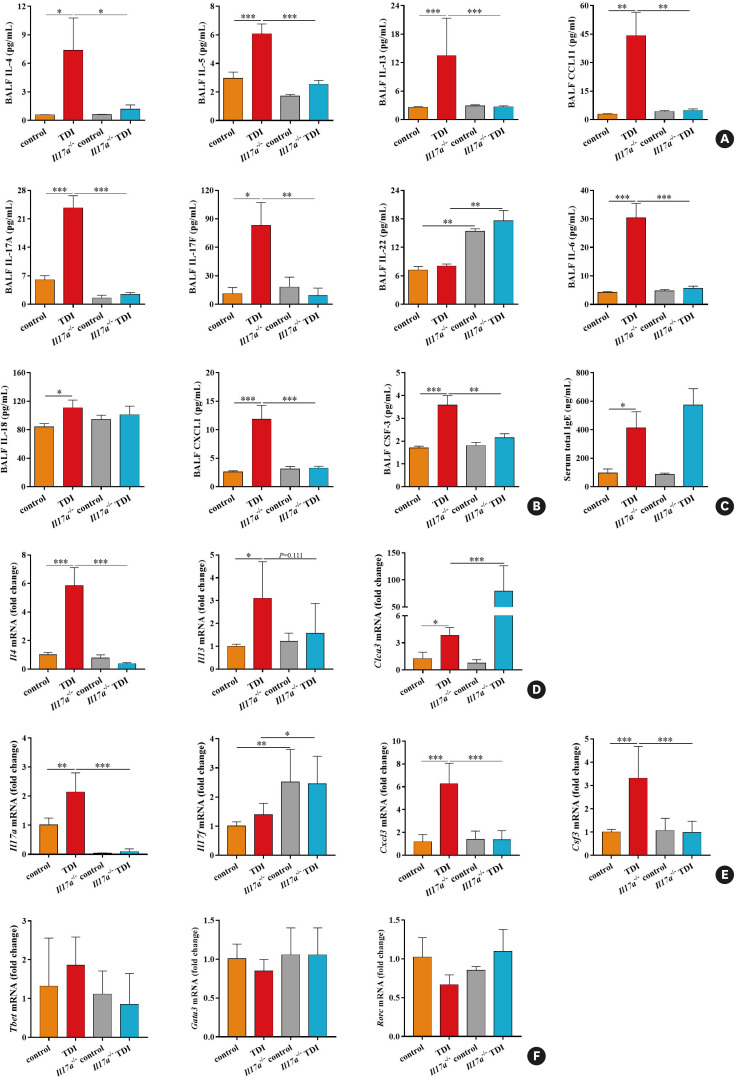
To further explore how IL-17A orchestrates TDI-induced allergic responses, we analyzed the BALF and lung tissues from TDI-exposed WT mice and Il17a−/− mice by multiple assays and quantitative real-time PCR. BALF levels of Th2-related IL-4, IL-5, IL-13 and Th17-related IL-17A, IL-17F, IL-6 were significantly reduced in Il17a−/− mice compared to WT mice after TDI challenge (Fig. 2A and B). Other cytokines, including eosinophil chemoattractants CCL11, neutrophil chemokines CXCL1 and CSF-3, were markedly blunted in the absence of IL-17A. Meanwhile, IL-17A deficiency promoted IL-22 production in BALF, but had no effects on the secretion of BALF IL-18 and serum total IgE (Fig. 2A-C). Consequently, the mRNA levels of Th2 markers Il4, Il5, Clca3 and Th17 markers Il17a, Cxcl3, Csf3 were extensively up-regulated by TDI, of which Il4, Il17a, Cxcl3, and Csf3 mRNA levels were suppressed by the lack of IL-17A. Yet, IL-17A knockout had no effect on the expression of Il5 mRNA, but led to increases in Clca3 and Il17f mRNA (Fig. 2D and E). To further investigate the effect of IL-17A deficiency on the differentiation of Th1, Th2 and Th17 cells, we assessed their key transcription factors T-bet, Gata3 and Rorc mRNA levels and found that both TDI exposure and removal of IL-17A did not alter their gene expression patterns (Fig. 2F). Hence, IL-17A is required to develop a robust allergic response because TDI-induced AHR, airway inflammatory cells infiltration, mucus overproduction, Th2/Th17-related cytokines and chemokines production were impaired in the mice lacking IL-17A.
Fig. 2. Reduced granulocytic chemokines and Th2/Th17 markers in mice deficient in IL-17A. (A) BALF levels of IL-4, IL-5, IL-13, and eosinophils attractant CCL11 were quantified by multiplex immunoassays (n = 5–8). (B) BALF levels of IL-17A, IL-17F, IL-6, IL-18, as well as neutrophils attractants CXCL1 and CSF-3 were quantified by multiplex immunoassays or ELISA (n = 5–8). (C) Serum total IgE concentrations were determined by ELISA (n = 5–8). (D) Whole lung tissue expression of Th2 markers Il4, Il5, and Clca3 was assessed by quantitative PCR (n = 3). (E) Whole lung tissue expression of the Th17 markers Cxcl3 and Csf3, as well as Il17a and Il17f was assessed by quantitative PCR (n = 3). (F) Whole lung tissue expression of transcription factors Tbet, Gata3, and Rorc was assessed by quantitative PCR (n = 3).
Th, T helper; IL, interleukin; BALF, bronchoalveolar lavage fluid; ELISA, enzyme-linked immunosorbent assay; Ig, immunoglobulin; PCR, polymerase chain reaction; TDI, toluene diisocyanate.
*P < 0.05; **P < 0.01; ***P < 0.001.
Neutralizing IL-17A during antigen sensitization or challenge plays different roles in TDI-induced allergic asthma
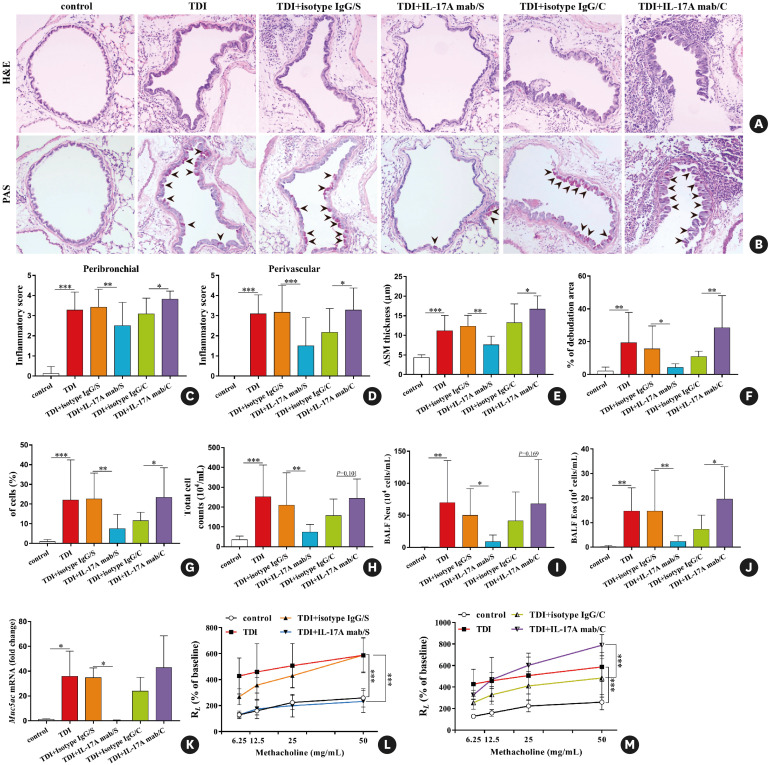
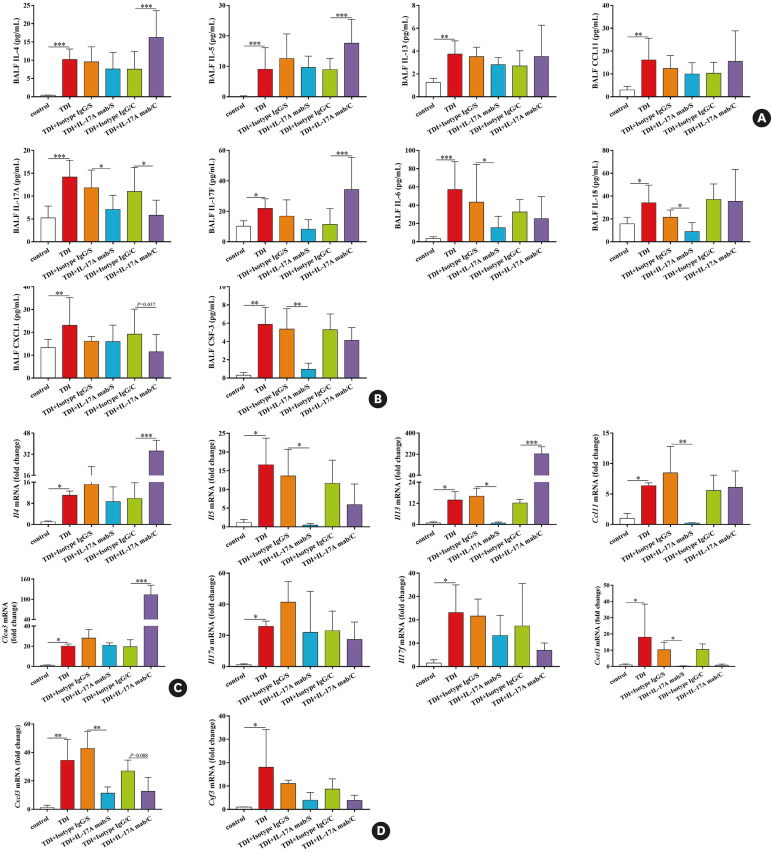
To test our hypothesis, IL-17A monoclonal antibody at a dose of 100 μg/mouse was given to the mice during antigen sensitization or after each airway challenge (Supplementary Fig. S1C). As expected, we observed different outcomes after neutralizing IL-17A during different effector phases in TDI-inhaled mice. In line with our previous study, administration of IL-17A mab during antigen challenge exacerbated TDI-induced AHR and inflammation, resulted in more severe epithelial cell hyperplasia and denudation, and drove greater numbers of eosinophils into the airways (Fig. 3). In the meantime, we detected larger amounts of Th2-related IL-4, IL-5 and Th17-related IL-17F but lower level of CXCL1 in BALF after the mice were treated with IL-17A mab during antigen challenge, whereas levels of IL-13, IL-6, IL-18 and CSF-3 in TDI asthmatic mice were not affected (Fig. 4A and B). In line with this, TDI-induced increased mRNA expression of Th2 markers Il4, Il13 and Clca3 was enhanced by anti-IL-17A during antigen challenge, while Cxcl1, Cxcl3 and Csf3 expression patterns remained unchanged (Fig. 4C and D). The effects of isotype IgG, IL-17A mab and rIL-17A on naive mice were shown in Supplementary Fig. S2. Actually, IL-17 mab at a lower dose of 50 μg/mouse was also used in TDI-induced asthma model and the results were shown in Supplementary Fig. S3 and S4.
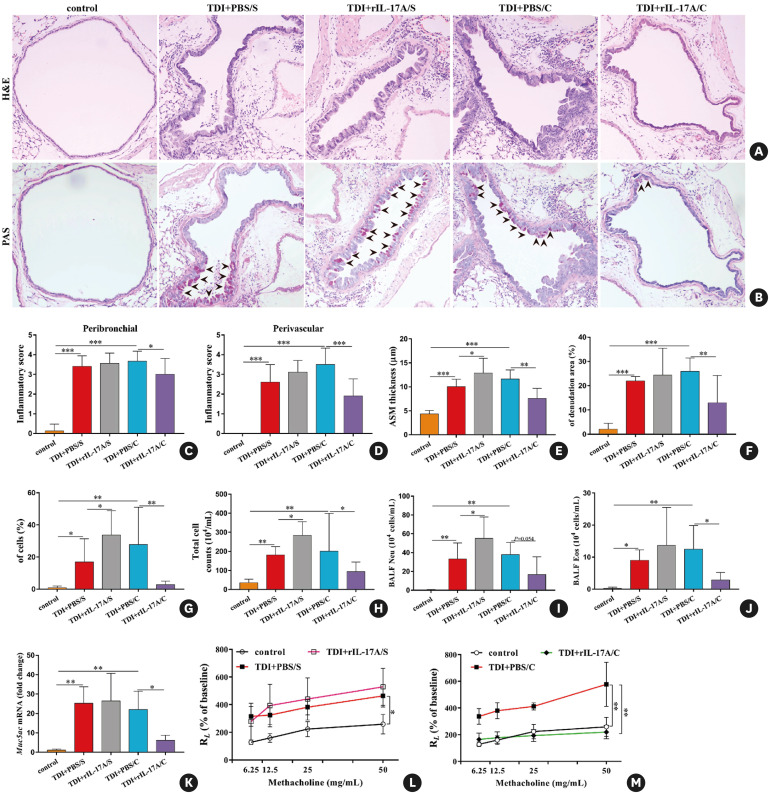
Fig. 3. Neutralization of IL-17A during antigen immunization and antigen challenge exerts distinct effects on TDI-elicited airway hyperreactivity and inflammation. (A, B) Representative H&E- and PAS-stained lung sections of different groups. Original magnification was 200×. (C, D) Semi-quantification of airway inflammation was performed (n = 8–10). (E, F) Analysis of ASM thickness and epithelial denudation was performed (n = 8–10). (G) Semi-quantification of PAS staining was performed (n = 8–10). (H-J) Numbers of total inflammatory cells, neutrophils and eosinophils in BALF (n = 8–10). (K) Expression of Muc5ac gene (quantitative PCR) in the whole lung (n = 3). (L, M) Airway hyperresponsiveness was measured by lung resistance (RL). Results are shown as percentage of baseline value (n = 5).
IL, interleukin; TDI, toluene diisocyanate; H&E, haematoxylin and eosin; PAS, periodic acid-Schiff; ASM, airway smooth muscle; BALF, bronchoalveolar lavage fluid; PCR, polymerase chain reaction; Ig, immunoglobulin.
*P < 0.05; **P < 0.01; ***P < 0.001.
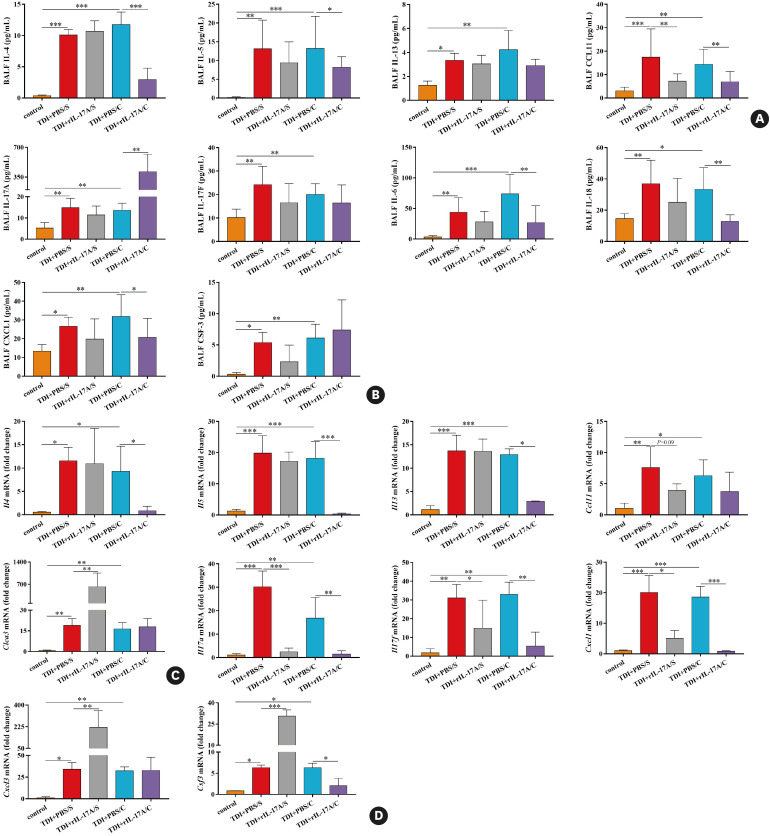
Fig. 4. Neutralizing IL-17A during different effector phases displays different capacity to orchestrate Th2 and Th17 responses. (A) BALF levels of IL-4, IL-5, IL-13, and eosinophils attractant CCL11 were quantified by multiplex immunoassays (n = 8–10). (B) BALF levels of IL-17A, IL-17F, IL-6, IL-18, as well as neutrophils attractants CXCL1 and CSF-3 were quantified by multiplex immunoassays or ELISA (n = 8–10). (C) Whole lung tissue expression of the Th2 markers Il4, Il5, Il13, Ccl11 and Clca3 was assessed by quantitative PCR (n = 3). (D) Whole lung tissue expression of the Th17 markers Cxcl1, Cxcl3 and Csf3 as well as Il17a and Il17f, was assessed by quantitative PCR (n = 3).
IL, interleukin; Th, T helper; BALF, bronchoalveolar lavage fluid; ELISA, enzyme-linked immunosorbent assay; PCR, polymerase chain reaction; TDI, toluene diisocyanate.
*P < 0.05; **P < 0.01; ***P < 0.001.
On the other hand, blocking IL-17A during TDI sensitization exerted a list of protective functions. Intraperitoneal injection of IL-17A mab at a dose of 100 μg/per mouse per time during immunization for a total 3 times (Supplementary Fig. S1B) resulted in decreased airway inflammation and AHR, extensively compromised epithelial hyperplasia, goblet cell metaplasia and mucus production, as well as a smaller number of neutrophils in BALF, while airway eosinophil recruitment was not inhibited (Fig. 3). Moreover, IL-17A mab treatment during sensitization inhibited the release of IL-6, IL-18 and CSF-3 in BALF, while IL-4, IL-5, IL-13, CCL11, IL-17F and CXCL1 in BALF did not show obvious differences between mice treated with isotype IgG and mice treated with IL-17A mab during the antigen-immunized phase (Fig. 4A and B). Accordingly, TDI-induced increased gene expression of Th17 markers Cxcl1 and Cxcl3 was down-regulated by neutralizing IL-17A during immunization. Interestingly, IL-17A mab treatment during the antigen-sensitized phase also inhibited mRNA expression patterns of Th2 markers Il5, Il13 and Ccl11 in the lungs of each TDI-inhaled mouse (Fig. 4C and D).
Instillation of exogenous IL-17A during different effector phases exhibits distinct capacity for orchestrating TDI-induced airway inflammation
To further confirm aforementioned results, subsequently, murine recombinant IL-17A (rmIL-17A) at a dos of 1 μg/mouse were respectively given to the mice during antigen sensitization or antigen challenge (Supplementary Fig. S1D and E). Likewise, we observed distinct results from TDI-exposed mice treated by instillation of exogenous IL-17A during antigen sensitization or antigen challenge. Without exerting any effects on airway epithelia denudation, airway eosinophilia and lung Muc5ac mRNA expression (Fig. 5F, J and K), rmIL-17A during antigen sensitization aggravated TDI-induced AHR and inflammation, led to more severe goblet cell metaplasia and airway smooth muscle thickening, and attracted greater numbers of neutrophils into the airways (Fig. 5A-E, G-I and L-M). Administration of rmIL-17A during antigen immunization did not affect the secretion of IL-4, IL-5, IL-13, IL-17F, IL-6 or IL-18 in BALF, but decreased levels of BALF CCL11 and lung Ccl11, Il17f as well as Cxcl1 mRNA (Fig. 6A, C and D). At the same time, we detected markedly enhanced mRNA expression of neutrophil-active chemokine Cxcl3 and Csf3, eosinophils attractant Clca3 in the lung homogenates (Fig. 6D), though BALF levels of CXCL1 and CSF-3 in TDI asthmatic mice was not affected by rmIL-17A (Fig. 6B).
Fig. 5. Inhalation of exogenous IL-17A during the antigen-immunized or antigen-challenge phase has opposite effects on TDI-induced airway hyperreactivity and inflammation. (A, B) Representative H&E- and PAS-stained lung sections of different groups. Original magnification 200×. (C, D) Semi-quantification of airway inflammation was performed (n = 6–10). (E, F) Analysis of ASM thickness and epithelial denudation was performed (n = 6–10). (G) Semi-quantification of PAS staining was performed (n = 6–10). (H-J) Numbers of total inflammatory cells, neutrophils and eosinophils in BALF (n = 6–10). (K) Expression of Muc5ac gene (quantitative PCR) in the whole lung (n = 3). (L, M) Airway hyperresponsiveness was measured by lung resistance (RL). Results are shown as percentage of baseline value (n = 5).
IL, interleukin; TDI, toluene diisocyanate; H&E, haematoxylin and eosin; PAS, periodic acid-Schiff; ASM, airway smooth muscle; BALF, bronchoalveolar lavage fluid; PCR, polymerase chain reaction; PBS, phosphate buffered saline.
*P < 0.05; **P < 0.01; ***P < 0.001.
Fig. 6. Exogenous IL-17A shows distinct roles for TDI-induced Th2 and Th17 responses during different effector phases. (A) BALF levels of IL-4, IL-5, IL-13, and eosinophils attractant CCL11 were quantified by multiplex immunoassays (n = 6–8). (B) BALF levels of IL-17A, IL-17F, IL-6, IL-18, as well as the neutrophils attractants CXCL1 and CSF-3 were quantified by multiplex immunoassays or ELISA (n = 6–8). (C) Whole lung tissue expression of Th2 markers Il4, Il5, Il13, Ccl11 and Clca3 was assessed by quantitative PCR (n = 3). (D) Whole lung tissue expression of the Th17 markers Cxcl1, Cxcl3 and Csf3 as well as Il17a and Il17f was assessed by quantitative PCR (n = 3).
IL, interleukin; TDI, toluene diisocyanate; Th, T helper; BALF, bronchoalveolar lavage fluid; ELISA, enzyme-linked immunosorbent assay; PCR, polymerase chain reaction; PBS, phosphate buffered saline.
*P < 0.05; **P < 0.01; ***P < 0.001.
By contrast, inhalation of exogenous rmIL-17A during antigen challenge period displayed the capacity to inhibit Th2 responses and airway eosinophilia. Plus, exogenous rmIL-17A in TDI challenge alleviated TDI-elicited airway hyperreactivity, airway epithelium denudation airway smooth muscle thickening as well as goblet cell metaplasia and mucus production, coupled with less granulocyte aggregation (especially eosinophils) around the airway (Fig. 5). Furthermore, the concentrations of IL-4, IL-5, CCL11, IL-6, IL-18 and CXCL1 in BALF were markedly reduced by rmIL-17 administration, yet BALF levels of IL-13, IL-17F and CSF-3 was not inhibited (Fig. 6A and B). Moreover, TDI-induced increased gene expression of Th2 markers Il4, Il5 and Il13, together with Th17 markers Il17a, Il17f, Cxcl1 and Csf3, was down-regulated by exogenous IL-17A inhalation during the antigen-challenged phase, while mRNA expression of other genes including Ccl11, Clca3 or Cxcl3 was not affected (Fig. 6C and D).
Exogenous human recombinant IL-17A (rhIL-17A) exerted distinct biological effects on TDI-induced airway inflammation in vitro
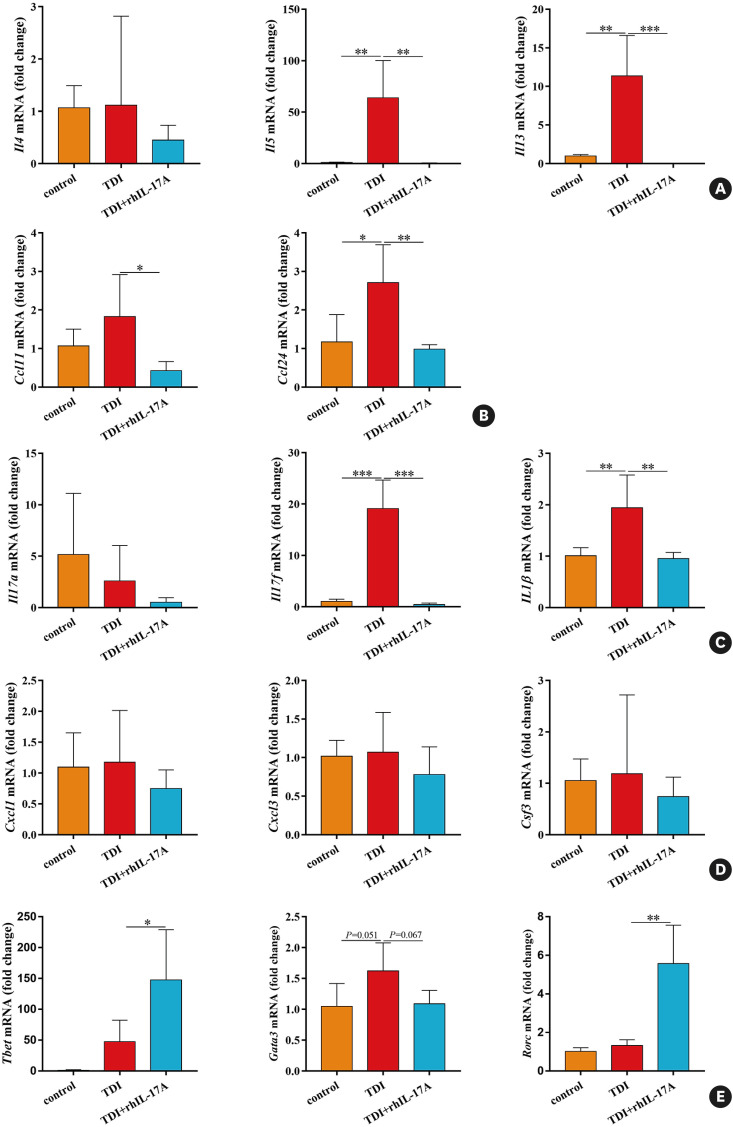
Th2 and Th17 signatures would result in distinct gene expression patterns in lung epithelia. Finally, we evaluated the effects of exogenous rhIL-17A on TDI-treated human bronchial epithelial cell line (BEAS-2B). As expected, TDI stimulation (2 mM) markedly increased the gene expression of the Th2 markers Il5, Il13, Ccl11 and Ccl24 as well as of the Th17 markers Il17f and Il1β in BEAS-2B, but did not affect the mRNA expression of Il4, Il17a, Cxcl1, Cxcl3, Csf3, Tbet, Gata3 or Rorc. In addition, rhIL-17A at a dosage of 100 ng/mL suppressed TDI-induced increased gene expression of the Th2 markers Il5, Il13, Ccl11 and Ccl24 as well as of the Th17 markers Il17f and Il1β in BEAS-2B, and caused a declined trend for TDI-raised Gata3 mRNA, though not significant (Fig. 7). Yet, rhIL-17A had no effect on the mRNA expression of Il4, Il17a, Cxcl1, Cxcl3 or Csf3 in TDI-treated BEAS-2B, and it seemed that rhIL-17A gave rise to an augmented effect of Tbet and Rorc gene expression (Fig. 7).
Fig. 7. Recombinant IL-17A exerts distinct effects on TDI-induced Th2 and Th17 signatures in vitro. The BEAS-2B cells cultured in DMEM medium supplemented with 10% calf serum were grown to 70%–80% confluent and then treated with TDI (2 mM) + PBS or TDI (2 mM) + IL-17A (100 ng/mL) for 24 hours, and then collected for detecting genes expression by quantitative PCR. (A, B) Expression of the Th2 markers Il4, Il5, Il13, Ccl11 and Ccl24 in human bronchial epithelial cell line was assessed by quantitative PCR (n = 4). (C, D) Expression of Th17 markers Cxcl1, Cxcl3, Csf3 as well as Il17a and Il17f in human bronchial epithelium was assessed by quantitative PCR (n = 4). (E) Expression of the transcription factors Tbet, Gata3 and Rorc in human bronchial epithelial cell line was assessed by quantitative PCR (n = 4).
IL, interleukin; TDI, toluene diisocyanate; Th, T helper; DMEM, Dulbecco’s Modified Eagle Medium; PBS, phosphate buffered saline; PCR, polymerase chain reaction.
*P < 0.05; **P < 0.01; ***P < 0.001.
DISCUSSION
In the present study, we discovered that IL-17A deficiency relieved TDI-induced allergic asthma. Then, we interfered with IL-17A signaling using neutralizing antibody or exogenous IL-17A during the immunization or in the antigen challenge, demonstrating that IL-17A exhibits distinct capacities to orchestrate airway inflammation during different effector phases.
Although eosinophil played a preeminent role in the pathogenesis of allergic asthma, increasing evidence demonstrates that mixed granulocytic airway inflammation is also recognized as a cardinal feature of TDI-induced severe asthma.25 IL-17A is a potent neutrophil-mobilizing cytokine, which attracts granulocytes into the mucosal surface and promotes neutrophil recruitment by prompting the release of neutrophil-modulating mediators such as IL-1β, IL-6 and granulocyte-colony stimulating factor from structural cells.26 Increased expression of IL-17A has been proposed to be associated with airway mixed granulocytic inflammation and declined lung function in severe asthmatics,27,28,29 supporting that IL-17A is critically implicated in the physiopathology of severe asthma. Kim et al.18 demonstrated the important role for IL-17A in the pathogenesis of TDI-induced asthma and discovered that IL-17A neutralization dramatically alleviated TDI-induced airway inflammation.18,19 In the current study using Il17a−/− mice, we generated an asthma model to explore the functional role of IL-17A in the development of mixed granulocytic asthma in response to TDI. Intriguingly, the TDI-exposed Il17a−/− mice exhibited declined AHR, airway neutrophil and eosinophil aggregation, together with diminished Th2- and Th17-related cytokines in BALF when compared to WT mice, indicating that IL-17A plays a proinflammatory role in the initiation of TDI-induced experimental allergic asthma. In addition, TDI-induced mucus overproduction and airway smooth muscle (ASM) thickening were suppressed by the lack of IL-17A. This is in agreement with the results of several published studies showing that deficiency in IL-17RA or IL-17A leads to an impaired neutrophilic response to allergens, lack of AHR and reduced airway remodeling,20,30 but seems opposite to our previous findings that blocking IL-17A with neutralizing antibody during antigen challenge aggregated TDI-induced responses.3 Indeed, our group examined whether IL-17A is modulated in severe asthmatics and found that higher serum IL-17A levels were detected in severe early-onset asthmatics compared to severe late-onset asthmatics,24 suggesting that IL-17A might contribute to the initiation of allergic responses in severe asthma. Given that different mechanisms are involved during antigen sensitization and challenge phases of allergic asthma,13,31 we set to test the hypothesis that IL-17A exerts distinct effects on TDI-induced asthma during different effector phases.
Subsequently, we investigated the functional role of IL-17A during the sensitization and challenge of TDI-induced asthma. On one hand, we showed that IL-17A neutralization in WT mice during sensitization diminishes allergic inflammation, which indicates that IL-17A is required to develop allergic airway inflammation. On the other hand, IL-17A neutralization during the challenge phase exacerbates the disease, indicating a tissue protective role for IL-17A. Consistent with this, we found out that exogenous IL-17A given during TDI immunization acts to promote airway neutrophilia, while rIL-17A given with the TDI challenge protects from lung eosinophil recruitment. These results demonstrated that IL-17A prompts the initiation of TDI-induced asthma, but functions as a negative regulator in established allergic inflammation. Our data are consistent with a study demonstrating that blocking IL-17 signaling by using IL-17RA-deficient mice significantly attenuated OVA-induced airway inflammatory responses, while neutralizing IL-17A during the allergen-challenge phase markedly aggravated airway eosinophil infiltration that could be ameliorated by rIL-17A.13 The authors ascribed the negative regulatory function of IL-17A to its dependence on IL-4 signaling and a direct inhibitory effect of this cytokine on dendritic cells, IL-5 and IL-13 production.13 Furthermore, in 2017, the same group reported this similar phenomenon in a HDM-induced asthma murine model.11 Coincidentally, Hellings et al.31 also found that neutralizing IL-17A in the challenge period worsened OVA-induced airway eosinophilia through the up-regulation of BALF IL-4, IL-5 and IL-13. Additionally, these literatures also revealed that suppression of IL-17A signaling during allergen immunization by using gene-knockout mice impaired OVA/HDM-induced neutrophilic influx into the airways.11,13,31 Several researchers have also discovered that IL-17A neutralization during allergen sensitization alleviated TDI-induced airway hyperreactivity and inflammation.19 They attributed these phenomenon to the capacity of IL-17A to directly induce maturity, activation and recruitment of neutrophils11,13,31 as well as initiate allergic responses.21 This interpretation is in agreement with the work of Wilson et al.20 proposing that airway allergic sensitization primed Th17-dependent neutrophilia in a lipopolysaccharide (LPS)/OVA -induced asthma model. Actually, the effect of IL-17A in neutrophils is also indirect by means of activating structural cells, including airway epithelial cells, fibroblasts and ASM cells, to produce the related cytokines and chemokines that in turn interact with neutrophils.32 Moreover, other researchers found that antigen-specific T cell sensitization is impaired in Il17a−/− mice, causing the suppression of allergic cellular and humoral responses.21 All these findings support a notion that IL-17A functions differently during different effector phases of asthma. As aforementioned, studies have reported higher levels of IL-17A in bronchial biopsies, sputum, and serum of severe asthmatics as compared to mild asthmatics.8,27,28,29 In fact, an IL-17A level of 20 pg/mL in serum was identified as an independent risk factor for severe asthma.29 Thus, in our opinion, higher IL-17A in sputum or serum could be used as a biomarker for distinguishing severe asthma from mild or moderate asthma. Plus, we speculated that IL-17A mab could be administered when the asthmatics initially need systemic steroid treatment, in line with the findings of a work conducted in a HDM/CFA-induced mixed granulocytic asthma model showing that combined administration of anti-IL-17A and systemic corticosteroid significantly attenuated both overall and neutrophilic airway inflammation.15 Actually, the challenging task of targeting severe asthma is further complicated by the complex pathobiology of asthma., but it still hits us that interfere with IL-17A signaling during an earlier phase would be more beneficial to severe asthmatics in clinical practice. However, the exact time-point of intervention is needed to be further explored in the future.
The levels of Th2 or Th17 cytokines could reflect immune homeostasis and indicate Th2 or Th17 predominance during inflammatory process. TDI-induced asthma is characterized by a larger number of neutrophils and a handful of eosinophil influx into the airways, with combined Th2 and Th17 responses.3 In the present study, dysregulation of Th2 and Th17 responses in TDI-exposed mice were decreased by IL-17A deficiency, indicating that IL-17A is a crucial regulator of Th2/Th17 responses in TDI asthma. Also, IL-17A blockade during immunization not only inhibits TDI-induced release of IL-6, IL-18 and CSF-3 in BALF, but also suppresses the mRNA expression of Cxcl1 and Cxcl3, while blocking IL-17A during challenge amplifies levels of IL-4 and IL-5 in BALF as well as lung gene expression of Il4, Il13 and Clca3, suggesting that IL-17A prompts TDI-induced airway neutrophilia and Th17 response during sensitization, but restricts airway eosinophilia and Th2 response during TDI challenge. In addition, in vitro exogenous IL-17 seems to inhibit Th2 response, but augment Th17 response. In agreement with our data, studies have revealed that IL-17A blocking antibody inhibited Th17 response and neutrophil aggregation, but promoted Th2 response and eosinophil accumulation in a HDM/CFA- or OVA-induced mixed granulocytic asthma model.15,31 Actually, researchers have demonstrated that Th2 and Th17 inflammatory pathways are reciprocally regulated in asthma, which means that therapies targeting Th2 or Th17 cytokines can lead to amplification of the opposing pathway.33 Moreover, IL-17A could drive an airway inflammatory phenotype shift from airway eosinophilia to neutrophilia in LPS/OVA-induced asthma model.34 Thus it is evident that IL-17A exerts distinct effects on TDI-induced airway neutrophilia and eosinophilia. Yet, TDI exposure has no effects on BALF levels of IL-22 or IL-33 in vivo, while IL-17A deficiency increases the secretion of IL-22 in BALF, which might be attributed to its crosstalk with IL-17A.35 This suggests that compensatory mechanisms are possibly involved in the mutual regulation of IL-17A and IL-22 in the pathogenesis of asthma, which may account for the failure of neutralizing antibody targeting IL-17RA to improve the symptoms of severe asthmatics.23 Specifically targeting pathogenic Th17 cells would be more appropriate and attractive in the future treatment of refractory asthma.
In conclusion, IL-17A is required for the onset of TDI-induced allergic asthma, but functions as a negative regulator of established allergic inflammation. Importantly, these data suggested that earlier abrogation of IL-17A signaling, but not late IL-17A neutralization, prevents the progression of TDI-induced asthma and could be used as a therapeutic strategy for severe asthmatics in clinical practice.
ACKNOWLEDGMENTS
This study was supported by National Natural Science Foundation of China (82100023, 82170042, 818 71266 and 82103941), China Postdoctoral Science Foundation (2021M691243, 2022M712186), and Shenzhen Science Technology Program (JCYJ20210324114400001 and JCYJ20210324114400002).
Footnotes
Disclosure: There are no financial or other issues that might lead to conflict of interest.
SUPPLEMENTARY MATERIALS
Experimental treatment schedules. (A) Mice were dermally sensitized with 0.3% TDI on days 1 and 8. On days 15, 18 and 21, the mice were challenged with 3% TDI through compressed air nebulization. (B) Mice were dermally sensitized and challenged with TDI. Anti-IL-17A monoclonal antibody or the isotype control antibody was administered separately via the intraperitoneal route at the doses of 50 μg/mouse per time or 100 μg/mouse per time every three days beginning from the first sensitization to second sensitization. (C) Anti-IL-17A monoclonal antibody or the isotype control antibody was administered separately via the intraperitoneal route at the doses of 50 μg/mouse per time or 100 μg/mouse per time immediately after each airway challenge. (D) Mice were dermally sensitized and challenged with TDI. Recombinant IL-17A was instilled at the dose of 1 μg/mouse per time every three days beginning from the first sensitization to second sensitization. (E) Recombinant IL-17A was instilled at the dose of 1 μg/mouse per time immediately after each airway challenge.
None of the antibodies affected airway inflammation in naive mice. C57BL/6 mice were treated with mouse isotype IgG1 control (100 μg/per mouse, i.p.), IL-17A mab (100 μg/per mouse, i.p.), rIL-17A (1 μg/per mouse, i.n.) or vehicle control once daily every other 2 days for a total of 3 times. The vehicle used to dissolve the blocking antibodies was PBS. Analysis was performed one day after the last treatment. (A) Representative H&E-stained lung sections of different treatment groups. No inflammation was seen around the airways of all enrolled mice. Original magnification was 200×. (B-D) Total and differential inflammatory cell counts in BALF (n = 3). There were no significant differences among all groups. (E) Airway hyperresponsiveness was measured by lung resistance (RL). Results are shown as percentage of baseline value (n = 3).
IL-17A blockade (50 μg/per mouse) during TDI immunization and challenge exert distinct effects on airway hyperreactivity and inflammation. (A, B) Representative H&E- and PAS-stained lung sections of different groups. Original magnification was 200×. (C, D) Semi-quantification of airway inflammation was performed (n = 8–10). (E, F) Analysis of ASM thickness and epithelial denudation was performed (n = 8–10). (G) Semi-quantification of PAS staining was performed (n = 8–10). (H) Expression of Muc5ac gene (quantitative PCR) in the whole lung (n = 3). (I-K) Numbers of total inflammatory cells, neutrophils and eosinophils in BALF (n = 8–10).
Neutralization of IL-17A (50 μg/per mouse) during different effector phases display distinct capacity for modulating Th2 and Th17 signatures. (A, B) Whole lung tissue expression of Th2 markers Il4, Il5, Il13, as well as Ccl11 and Clca3 was assessed by quantitative PCR (n = 3). (C, D) Whole lung tissue expression of Th17 markers Il17a and Il17f as well as Cxcl1, Cxcl3 and Csf3 was assessed by quantitative PCR (n = 3).
References
- 1.Papi A, Brightling C, Pedersen SE, Reddel HK. Asthma. Lancet. 2018;391:783–800. doi: 10.1016/S0140-6736(17)33311-1. [DOI] [PubMed] [Google Scholar]
- 2.Lau A, Tarlo SM. Update on the management of occupational asthma and work-exacerbated asthma. Allergy Asthma Immunol Res. 2019;11:188–200. doi: 10.4168/aair.2019.11.2.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen R, Zhang Q, Chen S, Tang H, Huang P, Wei S, et al. IL-17F, rather than IL-17A, underlies airway inflammation in a steroid-insensitive toluene diisocyanate-induced asthma model. Eur Respir J. 2019;53:1801510. doi: 10.1183/13993003.01510-2018. [DOI] [PubMed] [Google Scholar]
- 4.Chen S, Chen Z, Deng Y, Zha S, Yu L, Li D, et al. Prevention of IL-6 signaling ameliorates toluene diisocyanate-induced steroid-resistant asthma. Allergol Int. 2022;71:73–82. doi: 10.1016/j.alit.2021.07.004. [DOI] [PubMed] [Google Scholar]
- 5.Lummus ZL, Wisnewski AV, Bernstein DI. Pathogenesis and disease mechanisms of occupational asthma. Immunol Allergy Clin North Am. 2011;31:699–716. doi: 10.1016/j.iac.2011.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Irvin C, Zafar I, Good J, Rollins D, Christianson C, Gorska MM, et al. Increased frequency of dual-positive TH2/TH17 cells in bronchoalveolar lavage fluid characterizes a population of patients with severe asthma. J Allergy Clin Immunol. 2014;134:1175–1186.e7. doi: 10.1016/j.jaci.2014.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iwakura Y, Ishigame H, Saijo S, Nakae S. Functional specialization of interleukin-17 family members. Immunity. 2011;34:149–162. doi: 10.1016/j.immuni.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 8.Molet S, Hamid Q, Davoine F, Nutku E, Taha R, Pagé N, et al. IL-17 is increased in asthmatic airways and induces human bronchial fibroblasts to produce cytokines. J Allergy Clin Immunol. 2001;108:430–438. doi: 10.1067/mai.2001.117929. [DOI] [PubMed] [Google Scholar]
- 9.Chesné J, Braza F, Mahay G, Brouard S, Aronica M, Magnan A. IL-17 in severe asthma. Where do we stand? Am J Respir Crit Care Med. 2014;190:1094–1101. doi: 10.1164/rccm.201405-0859PP. [DOI] [PubMed] [Google Scholar]
- 10.Lu S, Li H, Gao R, Gao X, Xu F, Wang Q, et al. IL-17A, but not IL-17F, is indispensable for airway vascular remodeling induced by exaggerated Th17 cell responses in prolonged ovalbumin-challenged mice. J Immunol. 2015;194:3557–3566. doi: 10.4049/jimmunol.1400829. [DOI] [PubMed] [Google Scholar]
- 11.Chenuet P, Fauconnier L, Madouri F, Marchiol T, Rouxel N, Ledru A, et al. Neutralization of either IL-17A or IL-17F is sufficient to inhibit house dust mite induced allergic asthma in mice. Clin Sci (Lond) 2017;131:2533–2548. doi: 10.1042/CS20171034. [DOI] [PubMed] [Google Scholar]
- 12.Yang XO, Chang SH, Park H, Nurieva R, Shah B, Acero L, et al. Regulation of inflammatory responses by IL-17F. J Exp Med. 2008;205:1063–1075. doi: 10.1084/jem.20071978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schnyder-Candrian S, Togbe D, Couillin I, Mercier I, Brombacher F, Quesniaux V, et al. Interleukin-17 is a negative regulator of established allergic asthma. J Exp Med. 2006;203:2715–2725. doi: 10.1084/jem.20061401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tian BP, Hua W, Xia LX, Jin Y, Lan F, Lee JJ, et al. Exogenous interleukin-17A inhibits eosinophil differentiation and alleviates allergic airway inflammation. Am J Respir Cell Mol Biol. 2015;52:459–470. doi: 10.1165/rcmb.2014-0097OC. [DOI] [PubMed] [Google Scholar]
- 15.Menson KE, Mank MM, Reed LF, Walton CJ, Van Der Vliet KE, Ather JL, et al. Therapeutic efficacy of IL-17A neutralization with corticosteroid treatment in a model of antigen-driven mixed-granulocytic asthma. Am J Physiol Lung Cell Mol Physiol. 2020;319:L693–L709. doi: 10.1152/ajplung.00204.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kinyanjui MW, Shan J, Nakada EM, Qureshi ST, Fixman ED. Dose-dependent effects of IL-17 on IL-13-induced airway inflammatory responses and airway hyperresponsiveness. J Immunol. 2013;190:3859–3868. doi: 10.4049/jimmunol.1200506. [DOI] [PubMed] [Google Scholar]
- 17.Hall SL, Baker T, Lajoie S, Richgels PK, Yang Y, McAlees JW, et al. IL-17A enhances IL-13 activity by enhancing IL-13-induced signal transducer and activator of transcription 6 activation. J Allergy Clin Immunol. 2017;139:462–471.e14. doi: 10.1016/j.jaci.2016.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim SR, Lee KS, Park SJ, Min KH, Lee KY, Choe YH, et al. PTEN down-regulates IL-17 expression in a murine model of toluene diisocyanate-induced airway disease. J Immunol. 2007;179:6820–6829. doi: 10.4049/jimmunol.179.10.6820. [DOI] [PubMed] [Google Scholar]
- 19.Liu SY, Wang WZ, Yen CL, Tsai MY, Yang PW, Wang JY, et al. Leukocyte nicotinamide adenine dinucleotide phosphate-reduced oxidase is required for isocyanate-induced lung inflammation. J Allergy Clin Immunol. 2011;127:1014–1023. doi: 10.1016/j.jaci.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 20.Wilson RH, Whitehead GS, Nakano H, Free ME, Kolls JK, Cook DN. Allergic sensitization through the airway primes Th17-dependent neutrophilia and airway hyperresponsiveness. Am J Respir Crit Care Med. 2009;180:720–730. doi: 10.1164/rccm.200904-0573OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakae S, Komiyama Y, Nambu A, Sudo K, Iwase M, Homma I, et al. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity. 2002;17:375–387. doi: 10.1016/s1074-7613(02)00391-6. [DOI] [PubMed] [Google Scholar]
- 22.Hueber W, Patel DD, Dryja T, Wright AM, Koroleva I, Bruin G, et al. Effects of AIN457, a fully human antibody to interleukin-17A, on psoriasis, rheumatoid arthritis, and uveitis. Sci Transl Med. 2010;2:52ra72. doi: 10.1126/scitranslmed.3001107. [DOI] [PubMed] [Google Scholar]
- 23.Busse WW, Holgate S, Kerwin E, Chon Y, Feng J, Lin J, et al. Randomized, double-blind, placebo-controlled study of brodalumab, a human anti-IL-17 receptor monoclonal antibody, in moderate to severe asthma. Am J Respir Crit Care Med. 2013;188:1294–1302. doi: 10.1164/rccm.201212-2318OC. [DOI] [PubMed] [Google Scholar]
- 24.Chen D, Zhang Y, Yao C, Li B, Li S, Liu W, et al. Increased levels of serum IL-17 and induced sputum neutrophil percentage are associated with severe early-onset asthma in adults. Allergy Asthma Clin Immunol. 2021;17:64. doi: 10.1186/s13223-021-00568-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Vooght V, Smulders S, Haenen S, Belmans J, Opdenakker G, Verbeken E, et al. Neutrophil and eosinophil granulocytes as key players in a mouse model of chemical-induced asthma. Toxicol Sci. 2013;131:406–418. doi: 10.1093/toxsci/kfs308. [DOI] [PubMed] [Google Scholar]
- 26.Nembrini C, Marsland BJ, Kopf M. IL-17-producing T cells in lung immunity and inflammation. J Allergy Clin Immunol. 2009;123:986–994. doi: 10.1016/j.jaci.2009.03.033. [DOI] [PubMed] [Google Scholar]
- 27.Al-Ramli W, Préfontaine D, Chouiali F, Martin JG, Olivenstein R, Lemière C, et al. T(H)17-associated cytokines (IL-17A and IL-17F) in severe asthma. J Allergy Clin Immunol. 2009;123:1185–1187. doi: 10.1016/j.jaci.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 28.Bullens DM, Truyen E, Coteur L, Dilissen E, Hellings PW, Dupont LJ, et al. IL-17 mRNA in sputum of asthmatic patients: linking T cell driven inflammation and granulocytic influx? Respir Res. 2006;7:135. doi: 10.1186/1465-9921-7-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Agache I, Ciobanu C, Agache C, Anghel M. Increased serum IL-17 is an independent risk factor for severe asthma. Respir Med. 2010;104:1131–1137. doi: 10.1016/j.rmed.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 30.Zhao J, Lloyd CM, Noble A. Th17 responses in chronic allergic airway inflammation abrogate regulatory T-cell-mediated tolerance and contribute to airway remodeling. Mucosal Immunol. 2013;6:335–346. doi: 10.1038/mi.2012.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hellings PW, Kasran A, Liu Z, Vandekerckhove P, Wuyts A, Overbergh L, et al. Interleukin-17 orchestrates the granulocyte influx into airways after allergen inhalation in a mouse model of allergic asthma. Am J Respir Cell Mol Biol. 2003;28:42–50. doi: 10.1165/rcmb.4832. [DOI] [PubMed] [Google Scholar]
- 32.Rahmawati SF, Te Velde M, Kerstjens HA, Dömling AS, Groves MR, Gosens R. Pharmacological rationale for targeting IL-17 in asthma. Front Allergy. 2021;2:694514. doi: 10.3389/falgy.2021.694514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choy DF, Hart KM, Borthwick LA, Shikotra A, Nagarkar DR, Siddiqui S, et al. TH2 and TH17 inflammatory pathways are reciprocally regulated in asthma. Sci Transl Med. 2015;7:301ra129. doi: 10.1126/scitranslmed.aab3142. [DOI] [PubMed] [Google Scholar]
- 34.Zhao S, Jiang Y, Yang X, Guo D, Wang Y, Wang J, et al. Lipopolysaccharides promote a shift from Th2-derived airway eosinophilic inflammation to Th17-derived neutrophilic inflammation in an ovalbumin-sensitized murine asthma model. J Asthma. 2017;54:447–455. doi: 10.1080/02770903.2016.1223687. [DOI] [PubMed] [Google Scholar]
- 35.Besnard AG, Sabat R, Dumoutier L, Renauld JC, Willart M, Lambrecht B, et al. Dual role of IL-22 in allergic airway inflammation and its cross-talk with IL-17A. Am J Respir Crit Care Med. 2011;183:1153–1163. doi: 10.1164/rccm.201008-1383OC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Experimental treatment schedules. (A) Mice were dermally sensitized with 0.3% TDI on days 1 and 8. On days 15, 18 and 21, the mice were challenged with 3% TDI through compressed air nebulization. (B) Mice were dermally sensitized and challenged with TDI. Anti-IL-17A monoclonal antibody or the isotype control antibody was administered separately via the intraperitoneal route at the doses of 50 μg/mouse per time or 100 μg/mouse per time every three days beginning from the first sensitization to second sensitization. (C) Anti-IL-17A monoclonal antibody or the isotype control antibody was administered separately via the intraperitoneal route at the doses of 50 μg/mouse per time or 100 μg/mouse per time immediately after each airway challenge. (D) Mice were dermally sensitized and challenged with TDI. Recombinant IL-17A was instilled at the dose of 1 μg/mouse per time every three days beginning from the first sensitization to second sensitization. (E) Recombinant IL-17A was instilled at the dose of 1 μg/mouse per time immediately after each airway challenge.
None of the antibodies affected airway inflammation in naive mice. C57BL/6 mice were treated with mouse isotype IgG1 control (100 μg/per mouse, i.p.), IL-17A mab (100 μg/per mouse, i.p.), rIL-17A (1 μg/per mouse, i.n.) or vehicle control once daily every other 2 days for a total of 3 times. The vehicle used to dissolve the blocking antibodies was PBS. Analysis was performed one day after the last treatment. (A) Representative H&E-stained lung sections of different treatment groups. No inflammation was seen around the airways of all enrolled mice. Original magnification was 200×. (B-D) Total and differential inflammatory cell counts in BALF (n = 3). There were no significant differences among all groups. (E) Airway hyperresponsiveness was measured by lung resistance (RL). Results are shown as percentage of baseline value (n = 3).
IL-17A blockade (50 μg/per mouse) during TDI immunization and challenge exert distinct effects on airway hyperreactivity and inflammation. (A, B) Representative H&E- and PAS-stained lung sections of different groups. Original magnification was 200×. (C, D) Semi-quantification of airway inflammation was performed (n = 8–10). (E, F) Analysis of ASM thickness and epithelial denudation was performed (n = 8–10). (G) Semi-quantification of PAS staining was performed (n = 8–10). (H) Expression of Muc5ac gene (quantitative PCR) in the whole lung (n = 3). (I-K) Numbers of total inflammatory cells, neutrophils and eosinophils in BALF (n = 8–10).
Neutralization of IL-17A (50 μg/per mouse) during different effector phases display distinct capacity for modulating Th2 and Th17 signatures. (A, B) Whole lung tissue expression of Th2 markers Il4, Il5, Il13, as well as Ccl11 and Clca3 was assessed by quantitative PCR (n = 3). (C, D) Whole lung tissue expression of Th17 markers Il17a and Il17f as well as Cxcl1, Cxcl3 and Csf3 was assessed by quantitative PCR (n = 3).