Abstract
Objective
To quantify unused opioids among adult and pediatric patients discharged from the emergency department (ED) or ambulatory care settings with a prescription for acute pain.
Methods
We searched MEDLINE, Embase, CINHAL, PsycINFO, the Cochrane Library, and the gray literature from inception to April 29, 2021. We included observational studies in which any patient with an acutely painful condition received a prescription for an opioid on discharge from an outpatient care setting, and unused opioids were quantified. Two reviewers screened records for eligibility, extracted data, and conducted the quality assessment. Where possible, we pooled data and otherwise described the results of studies narratively. Total unused prescriptions were synthesized using a weighted average. Random effects models were used, and heterogeneity was measured by the I2 statistic. Our primary outcome was the quantity of unused opioid medication available after receiving a prescription for acute pain. Secondary outcomes were the proportion of patients with unused opioids following a prescription, the proportion of patients using no opioids, morphine equivalents of unused opioids, and factors associated with leftover opioids.
Results
In this systematic review and meta‐analysis of 9 studies in emergency and ambulatory care settings, 59.6% of prescribed opioids remained unused; pediatric patients had 69.3% of their prescriptions remaining, compared to 54.6% among adult patients. The highest proportion of unused opioids was found following dental extractions (82.6%).
Conclusions and Relevance
More than 50% of opioids remain unused following prescriptions for acute pain. Responsible prescribing must be accompanied by education on safer use, storage, and disposal.
Keywords: acute pain, opioid usage, outpatient, pain medications
1. INTRODUCTION
1.1. Background
The opioid crisis has highlighted the intertwined complexities of pain management, prescribing practices, and risk while continuing to exact a severe toll, currently exacerbated by the COVID‐19 pandemic. 1 In 2020 alone, 68,630 Americans died from opioid overdose and nearly one‐quarter of those deaths involved prescription opioids. 2 Within this context, efforts to prevent opioid‐related harms must be a priority.
Clinicians in the emergency department (ED) and ambulatory care settings often treat patients with acutely painful conditions and are therefore important players in the responsible prescribing of opioids. As the risks associated with opioids have become more apparent, rates of prescribing have decreased over time, including in the ED. 3 , 4 , 5 However, an additional critical factor in responsible prescribing is knowing how much to prescribe, which, to date, remains unclear. 6
1.2. Importance
Following treatment in the ED, primary studies have reported that between 49% and 93% of patients have some leftover opioids, with up to 68% of the total prescribed pills remaining unused. 7 , 8 , 9 Evidence from systematic reviews evaluating post‐operative populations suggests that among patients prescribed an opioid, 31%–71% of their prescription remains unused, 67% to 92% of patients reported unused opioids, and fewer than 9% of patients appropriately dispose of unused opioids. 10 , 11 , 12 , 13 Given the high proportion of unused pills, consideration must be given to the potential for diversion of opioids for non‐medical use and associated downstream effects, such as the development of opioid use disorder. 14 , 15 , 16 Importantly, evidence suggests that the most common source of opioids among those who report using prescription opioids not prescribed to them is a friend or relative's leftover prescription, and this applies to adolescents, young adults, and adults. 17 , 18
1.3. Goals of this investigation
With a shared scope of prescribing opioids for acutely painful, self‐limiting conditions, we examined evidence from both the ED and ambulatory settings 19 to address 4 key objectives. Among patients discharged from the ED or ambulatory care settings, we aimed to determine: (1) the proportion of unused opioids available following a prescription for acute pain, (2) the number of opioids used by the patient for acute pain, (3) the factors associated with a high or low proportion of unused opioids, and (4) why the full dose is not used and what patients do with unused opioids.
2. METHODS
We adhered to the standard systematic review methodology recommended by Cochrane 20 and followed a predefined, registered protocol (PROSPERO #CRD42021257448). 21
2.1. Search strategy
A research librarian (E.W.) developed a peer‐reviewed search strategy 22 and searched five databases from inception to April 29, 2021: Ovid MEDLINE, Ovid Embase, Cochrane Library, Ovid PsycINFO, and CINHAL via EBSCOhost. We searched key gray literature sources from the Canadian Agency for Drug and Technologies in Health's recommended Grey Matters list, 23 reference lists of relevant and included studies, and Google Scholar to conduct a forward citation search based on included studies. The search strategy for MEDLINE is available in the Supporting Information Methods.
2.2. Study selection
Two reviewers (M.P.D. and S.Z.G.) independently screened records in DistillerSR (Evidence Partners, Ottawa, Canada) in a 2‐stage process, guided by predefined eligibility criteria outlined below. Titles and abstracts were screened using the liberal‐accelerated approach, in which any record identified as relevant by 1 reviewer advanced to full‐text screening, and a second reviewer confirmed or refuted all exclusions. 24 , 25 All full‐text records were screened by 2 reviewers for eligibility. Forms were pilot tested on a sample of studies (n = 100) before screening (available from authors on request). Pilot testing indicated fair agreement between reviewers (Cohen's κ = 0.34).
We included observational studies in which any patient (adult or pediatric) with an acutely painful condition received a prescription for an opioid medication on discharge from an outpatient care setting (including the ED), and the amount of unused opioids was quantified. Acute pain was defined to include new acute conditions or acute pain resulting from day surgery, provided the underlying condition was not treated with opioids. We considered ambulatory and primary care settings, including EDs, urgent care centers, community clinics, and day surgeries conducted in clinics. Our primary outcome was the quantity of unused opioid medication available after receiving a prescription for acute pain, measured as a percentage. Secondary outcomes were the proportion of patients with unused opioids following a prescription (patients not filling opioid prescriptions plus patients filling their prescriptions and reporting unused opioids, divided by the number of patients prescribed an opioid upon discharge), the proportion of patients using no opioids, morphine equivalents of unused opioids, 26 and clinical and/or demographic characteristics associated with leftover opioids, including reasons for not using or stopping the use of opioids and storage and disposal characteristics. Relevant studies published in English or French were included.
2.3. Data extraction and quality assessment
Data were extracted using standardized forms, entered into Excel (Microsoft) by 1 reviewer (S.S.) and verified for accuracy and completeness by another (M.P.D.). 27 Pilot testing was conducted by both reviewers to ensure consistent and adequate use of the form. Extracted data included study and population characteristics, details of the opioid exposure, and outcomes reported.
Two reviewers (M.P.D. and S.S.) independently assessed the methodological quality of included studies and resolved discrepancies through discussion. We used design‐specific JBI (formerly Joanna Briggs Institute) Critical Appraisal Tools for uncontrolled cohort studies, modified from the case series checklist, 28 and analytical cross‐sectional studies. 29
2.4. Data analysis and synthesis
Where possible, we pooled data and otherwise described the results of studies narratively and in evidence tables. Total unused prescriptions were synthesized using a weighted average (weighted on sample size for each study). Because total number of prescriptions in each study was unknown, it was not possible to get precision estimates around the proportions, thus weighted averages are displayed without confidence intervals. Averages were computed overall as well as broken down by setting and age category. Proportions of patients with unused opioids were statistically pooled using the Freeman‐Tukey double arc sine method of pooling prevalences. 30 Random effects were used with heterogeneity being measured by the I2 statistic. Pooling was done overall for all studies as well as sub‐grouped by setting and age category. The analysis was completed using Stata 17.0 (StataCorp). Because of insufficient data, morphine equivalents of unused opioids were not calculated.
3. RESULTS
3.1. Description of included studies
We identified and screened 6057 records in our search, of which 273 full‐text articles were assessed for eligibility, and 9 studies 13 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 (n = 2742 participants) were included (Figure S1). 39 Study characteristics are presented in Table 1. Five studies (55.6%) 13 , 31 , 32 , 33 , 34 were conducted in the ED and 4 studies (44.4%) 35 , 36 , 37 , 38 were conducted in ambulatory clinics. Three studies (33.3%) 13 , 31 , 32 included mixed populations from the ED, 3 studies (33.3%) included patients with fractures (1 from the ED; 33 2 from orthopedics clinics), 34 , 35 2 studies (22.2%) 37 , 38 included dental extractions, and 1 study (11.1%) 36 included patients with burn injuries. Five studies (55.6%) 34 , 35 , 36 , 37 , 38 were conducted in pediatric‐only (<18 years) or pediatric and young adult populations (conducted in both pediatric and adult populations with an upper age limit of 30 years) and the other 4 studies (44.4%) 13 , 31 , 32 , 33 were in adult‐only populations. The median sample size was 89 (interquartile ratio [IQR] = 81, 142). Key characteristics and results of the included studies are described in Table 2.
TABLE 1.
Summary of included studies (n = 9)
| Characteristic | No. (%) |
|---|---|
| Study design | |
| Uncontrolled cohort | 7 (77.8%) |
| Cross‐sectional | 2 (22.2%) |
| Sample size | |
| Total | 2,742 |
| Median (IQR) | 89 (81, 142) |
| Range | 48–1,513 |
| Participant demographics a | |
| Mean age (years) | 25.8 |
| Pediatric or pediatric + young adult populations b | 5 (55.6%) |
| Adult‐only population | 4 (44.4%) |
| Sex (% female) | 53.4% |
| Race | |
| White | 83.7% |
| Non‐White c | 16.3% |
| Country | |
| United States | 7 (77.8%) |
| Canada | 1 (11.1%) |
| China | 1 (11.1%) |
| Setting | |
| ED | 5 (55.6%) |
| Ambulatory clinic | 4 (44.4%) |
| Dental clinic | 2 (22.2%) |
| Burn center | 1 (11.1%) |
| Orthopedic clinic | 1 (11.1%) |
| Opioid use indication | |
| Multiple indications (ED setting) | 3 (33.3%) |
| Fracture | 3 (33.3%) |
| Dental extraction | 2 (22.2%) |
| Burn injury | 1 (11.1%) |
| Duration of follow‐up | |
| Median (IQR), days | 21 (14, 21) |
| Outcome ascertainment | |
| Self‐report | 7 (77.8%) |
| Parent/caregiver report | 2 (22.2%) |
Abbreviations: ED, emergency department; IQR, interquartile ratio
Age reported in 8 studies (range encompasses categories from 0–2 years to 80–100 years); race reported in 3 studies.
Pediatric populations refer to studies conducted exclusively with participants <18 years; pediatric and young adult populations refer to studies conducted in both pediatric and adult populations with an upper age limit of 30 years.
Reported categories for race included Asian, Black or African American, Native Hawaiian or Other, Pacific Islander, more than one race, unknown, and other.
TABLE 2.
Key characteristics and results of included studies
| Author, publication year, study years, country | Study design, follow‐up duration no. of participants analyzed (% female), age, y | Indication or pain diagnosis | Opioid(s) prescribed, dose, duration | Opioids used/prescribed | Proportion of prescription unused | Patients with any unused opioids (%) | Quality assessment score (JBI tool) |
|---|---|---|---|---|---|---|---|
| Mixed ED populations | |||||||
|
Shi, 2020 31 2018 United States |
Uncontrolled cohort 21 days 98 (53) Mean (SD): 51 (18) |
Extremity: 32.7% Chest/back: 21.4% Renal colic: 20.4% Abdominal: 18.4% Facial/head: 7.1% |
Hydrocodone‐acetaminophen: 56.1% Oxycodone‐acetaminophen: 29.6% Oxycodone: 8.2% Tramadol: 5.1% Morphine immediate release: 1.0% NR NR |
NR/60 Median MME |
33.4% |
58.2% 9.2% used no opioids |
6/10 |
|
Daoust, 2018 13 2016–2017 Canada |
Uncontrolled cohort 14 days 627 (47.8) Mean (SD): 51.0 (15.9) |
Musculoskeletal: 44% Fracture: 19.1% Renal colic: 17.0% Abdominal: 6.0% Other: 13.9% |
Morphine: 43.6% Oxycodone: 40.5% Hydromorphone: 15.9% NR (converted to 5 mg morphine equivalent tablets) NR |
7/30 Median MME |
68.6% |
NR 5% used no opioids |
6/10 |
|
Yang, 2020 32 2018 United States |
Cross‐sectional 30 days 89 (51.7) Mean (SD): 51.9 (NR) |
NR |
NR NR NR |
NR | 41.6% |
64.0% 14.6% used no opioids |
4/8 |
| Fractures | |||||||
|
Zhu, 2018 33 2017 China |
Uncontrolled cohort 30 days 1513 (54.4) Range: 18–100 |
Dislocation or displaced fracture: 50.5% Avulsion fracture: 25.9% Non‐displaced fracture: 23.6% (ED presentations) |
Codeine‐ibuprofen (13 mg): 49.0% Oxycodone‐acetaminophen (5 mg): 40.3% Sustained‐release tramadol (100 mg): 10.7% NR |
7.2/14.7 Mean no. pills |
51.0% |
71.1% 10% used no opioids |
7/10 |
|
Nelson, 2019 34 NR United States |
Uncontrolled cohort 21 days 81 (38.3) Mean (SD): 6.1 (2.1) |
Supracondylar humeral fracture: 100% (ED presentations) |
Oxycodone: 100% Weight‐based dosing NR |
4.8/19.8 Mean no. doses |
75.9% |
NR 22% used no opioids |
8/10 |
|
Stillwagon, 2020 35 2017–2018 United States |
Uncontrolled cohort 21 days 63 (56) Mean (SD): 4.8 (1.9) |
Supracondylar humeral fracture: 100% (orthopedics clinic) |
Oxycodone: 100% Weight‐based dosing NR |
4/17.2 Mean no. doses |
77% | NR | 5/10 |
| Ambulatory clinics | |||||||
|
Shahi, 2020 36 2019 United States |
Cross‐sectional 14 days 142 (56.5 a ) Mean (SD): 2.7 (NR) |
Burn injuries: 100% |
Oxycodone: 70.0% Hydrocodone‐acetaminophen: 30.0% NR NR |
4/8 Median no. doses |
50.0% | 86.9% | 2/8 |
|
Resnick, 2019 37 2018 United States |
Uncontrolled cohort 7 days 81 (56) Mean (SD): 19.4 (7.7) |
Third molar extraction |
Oxycodone (5 mg): 100% 1 tablet every 6 h as needed (6 tablets total) |
0.04/6 Mean no. tablets (total study population) 3.3/6 Mean no. tablets per patient who took any opioids |
96.0% |
NR 93.0% used no opioids |
4/10 |
|
Weiland, 2015 38 2012–2013 United States |
Uncontrolled cohort 7 days 48 (67) Mean (SD): 19.6 (4.2) |
Third molar extraction |
Hydrocodone‐acetaminophen (Vicodin, Lorcet, Norco): NR Oxycodone‐acetaminophen (Percocet): NR NR NR |
8/20 Median no. tablets |
60.0% |
NR 10.4% used no opioids |
4/10 |
Abbreviations: ED, emergency department; MME, morphine milligram equivalents; NR, not reported; SD, standard deviation; y, years.
aBased on patients who received opioids, not total study sample.
3.2. Methodological quality of included studies
Critical appraisal of uncontrolled cohort studies (n = 7) 13 , 31 , 33 , 34 , 35 , 37 , 38 ranged from 4 to 8 out of 10 checklist items met. Common limitations in this group of studies were the reliance on self‐report to ascertain outcomes, low or unclear participation rates among eligible patients, and inadequate levels of participant follow‐up. Both cross‐sectional studies 32 , 36 were of low methodological quality, with 2 and 4 of 8 checklist items met. Failure to account for confounding factors, and not using valid, reliable measures to assess exposure and outcomes were driving factors.
3.3. Proportion of prescription unused
Across all 9 studies, the weighted average of prescribed opioids that remained unused was 56.9% (Table 3). When investigating differences by population, the percentage of unused opioids ranged from 50.0% for patients with burn injuries to 82.6% for patients undergoing dental extraction. Pediatric patients had a weighted average of 69.3% of their opioid prescription remaining, whereas adult patients had 54.6%.
TABLE 3.
Percent of prescription unused (weighted averages, weighted by sample size)
| Study, year | Patients (n) | Unused prescriptions (%) a | |
|---|---|---|---|
| All studies | Total (weighted average) | 2742 | 56.9 |
| By population | |||
| Mixed emergency | Yang, 2020 32 | 89 | 41.6 |
| Shi, 2020 31 | 98 | 33.4 | |
| Daoust, 2018 13 | 627 | 68.6 | |
| Total (weighted average) | 814 | 61.4 | |
| Fracture | Stillwagon, 2020 35 | 63 | 77.0 |
| Nelson, 2019 34 | 81 | 75.9 | |
| Zhu, 2018 33 | 1513 | 51.0 | |
| Total (weighted average) | 1657 | 53.2 | |
| Ambulatory | Shahi, 2020 36 | 142 | 50.0 |
| Total (weighted average) | 142 | 50.0 | |
| Dental | Weiland, 2015 38 | 48 | 60.0 |
| Resnick, 2019 37 | 81 | 96.0 | |
| Total (weighted average) | 129 | 82.6 | |
| By age category | |||
| Pediatric | Weiland, 2015 38 | 48 | 60.0 |
| Resnick, 2019 37 | 81 | 96.0 | |
| Shahi, 2020 36 | 142 | 50.0 | |
| Stillwagon, 2020 35 | 63 | 77.0 | |
| Nelson, 2019 34 | 81 | 75.9 | |
| Total (weighted average) | 415 | 69.3 | |
| Adult | Shi, 2020 31 | 98 | 33.4 |
| Daoust, 2018 13 | 627 | 68.6 | |
| Yang, 2020 32 | 89 | 41.6 | |
| Zhu, 2018 33 | 1513 | 51.0 | |
| Total (weighted average) | 2327 | 54.6 |
Precision estimates were not possible due to nature of outcome.
3.4. Proportion of patients with unused opioids
Four 31 , 32 , 33 , 36 studies reported the proportion of patients with unused opioids, with an overall weighted average of 68% (95% confidence interval [CI], 60%–77%) (Figure 1). In 3 studies in the ED, the proportion was 66% (95% CI, 57%–74%), compared to 87% (68%–95%) in 1 study of a non‐ED setting (burn center). The 3 investigations in the ED were all conducted in adult populations, and the non‐ED study was conducted in pediatrics (Figures S2 and S3).
FIGURE 1.
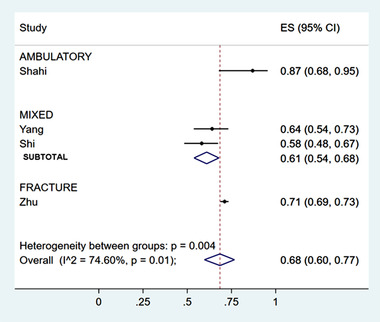
Proportion of patients with unused opioids, by clinical population
Across 7 studies reporting data, 13 , 31 , 32 , 33 , 34 , 37 , 38 21% (95% CI, 8%–37%) of patients used none of their prescribed opioids (Figure 2). This proportion was highest among studies of dental extractions (65% [95% CI, 56%–73%]); however, this is subject to influence by 1 outlier, 37 reporting 93% (95% CI, 85%–97%) of patients using no opioids. Comparing age categories, 42% (95% CI, 1%–93%) of pediatric patients used no opioids, compared to 9% (95% CI, 5%–13%) of adult populations. Again, the pediatric data are influenced by the same outlier study. 37 Of patients presenting to the ED, 11% (95% CI, 7%–16%) reported no opioid use at home (Figures S4 and S5). One study reported on patients not filling their prescriptions: of the 58.2% of patients with unused opioids, 49.0% had some of their prescription remaining, and 9.2% did not fill their prescriptions. 31
FIGURE 2.
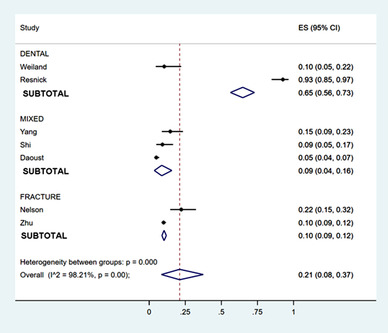
Proportion of patients who used no opioids, by clinical population
3.5. Characteristics associated with unused opioids
A description of factors associated with opioid use, including reasons for not using or stopping use of opioids, is presented in Table S1. Among mixed populations presenting to the ED, both Shi et al 31 and Daoust et al 13 report a large excess of pills prescribed to patients with renal colic and abdominal pain. In patients with fractures, Zhu et al 33 found significantly higher opioid consumption with dislocation or displaced fractures when compared with non‐displaced or avulsion; fractures of the wrist or forearm; ankle, tibia, or fibula; and elbow or humerus were associated with higher opioid consumption than fractures at other locations (P < 0.001). Nelson et al 34 and Stillwagon et al 35 found no significant differences in opioid consumption by fracture type, age, or sex.
Three studies 32 , 36 , 38 reported on storage of opioids (Table S2). The most common location was a bathroom or medicine cabinet (31.0%–56.3%), and the majority of the time, the storage location was unlocked (61.5%–97.6%). Two studies 31 , 32 reported on disposal of unused opioids, with planned disposal methods including throwing them in the garbage (21.4%), disposing of them in the sink or toilet (12.5%–42.9%), or returning them to a pharmacy or police station (14.3%–17.5%). Notably, Shi reported that 70.0% of respondents planned to keep their unused opioids for future use. 31
4. DISCUSSION
In our analysis of 9 studies in which opioid consumption was tracked after discharge from emergency or ambulatory care, over half of prescribed opioid pills went unused, almost 60% of patients had unused pills, and 1 of 5 patients used none of their opioids. The quantity of unused opioids was higher in dental settings and among pediatric and young adult populations. These findings demonstrate a high level of unused opioids available in the community, following emergency and ambulatory care for acutely painful conditions, reinforcing concerns about the potential for diversion of leftover pills for non‐medical use.
Judicious prescribing is a cornerstone of responsible patient care. Our finding that over half of pills prescribed are unused should be viewed as a call to action. Current recommendations for acute pain management suggest that patients receive the lowest effective dose: a 3‐day supply (approximately 10 pills) is typically sufficient, and more than 7 days is rarely required for most acutely painful conditions treated in the ED and other ambulatory settings. 40 , 41 , 42 Although opioid prescribing has declined since its peak in 2012, 43 , 44 most studies in our review (median publication date, 2019) were still reporting prescriptions in excess of these guidelines, even though actual patient usage fell within the recommended range of 3–7 days. A shift to prescribing lower quantities is still needed, but equally importantly, this will need to be accompanied by safety planning—education on safe storage and disposal of medications—and close follow‐up, including education on an appropriate course of action if the quantity of opioids provided is not enough.
The quantity of unused opioids varied by indication. Dental pain was associated with the highest proportion of unused opioids, consistent with long‐standing calls to reduce prescribing in this population. As in other specialties, prescribing in dentistry has been declining over time 45 ; however, rates remain high. 46 , 47 Miller et al 46 found that 74.9% of analgesic prescriptions written between 2013 and 2018 were for opioids; in an American national survey of dentists, 47 50% of those reporting prescribing opioids were doing so in amounts exceeding what was required, and 69% reported having had patients who had diverted or used their opioids non‐medically. These numbers are alarming and can have significant consequences for the safety of patients and the community. The American Dental Association, as well as the Choosing Wisely Canada campaign with the Canadian Association of Hospital Dentists, has recommended the use of a non‐opioid analgesic for post‐operative pain, with an opioid considered only if the first‐line treatment is not sufficient. Furthermore, they recommend a limited number of tablets dispensed, appropriate patient education with respect to use and disposal, and daily dispensing and/or delayed prescriptions. 48 , 49 , 50 These guidelines provide a practical and safe approach that could also be applied in other settings, such as in the ED.
In the ED, renal colic and abdominal pain were associated with the highest quantities of unused opioids. A recent meta‐analysis of 63 studies of the effectiveness of interventions to reduce opioid prescribing in the ED found that although education, policy, and guideline interventions were successful at reducing the rate of prescriptions (6‐month step change, –33.31%; 95% CI, –39.67% to –26.94%), interventions to reduce prescribed opioid quantities were not effective. 5 This is mirrored by findings that laws limiting opioid prescribing have not had a substantial impact on reducing excessive prescribing. 51 , 52 An identified challenge in achieving success in this area is being able to determine the appropriate quantity according to indication and patient need, 6 and our review results can contribute to that evidence base.
Pediatric patients had an alarming 70% of their prescriptions remaining, compared with 55% in adults. Although the possibility for diversion is a risk regardless of who receives the prescription, children and youth rarely live in isolation, potentially increasing the risk to caregivers, siblings, and friends. Equally concerning, 42% of pediatric patients used no opioids, whereas this proportion was 9% among adults. This raises the questions of whether children and youth require a different approach to opioid prescribing than adults; perhaps the original indications for opioid prescribing in the pediatric studies were inaccurate or, importantly, families are afraid to use opioids and are undertreating their child's pain. In children with fractures, ibuprofen has been found to have similar analgesic effectiveness to oxycodone with a more favorable adverse effect profile, suggesting that non‐opioid alternatives may be used for at‐home pain management. 53 Although this review cannot answer these important questions, this will be an important future line of inquiry.
Responsible prescribing practices must also include provision of patient education on safe storage and disposal. Our results demonstrate that there are both large quantities of unused opioids and that these unused opioids are not being safely stored or disposed of. The 2 studies in our sample that investigated education on the use of opioids reported that only approximately 25% of patients or caregivers received counselling on safe storage and disposal of opioids. 31 , 36 There is a clear need for improvement in providing patients and families with resources, such as the tools available through knowledge mobilization agencies such as Solutions for Kids in Pain 54 and Translating Emergency Knowledge for Kids. 55
Despite trends showing an overall decline in the prescribing of opioids, opioid‐related harms and deaths have continued to increase. 56 As a result, it is incumbent on all health care professionals that prescribe opioids to think deliberately on what a “safer prescribing dose” means in relation to each patient encounter. The safer prescribing dose will need to be individualized based on patient‐specific factors (eg, allergies, presence of comorbidities, current opioid tolerance) as well as injury‐specific factors (eg, type of injury, severity, expected time required to resolve). Beyond these contextual factors, all health care prescribers should also implement key initiatives in their management plans that have been shown to reduce the harms associated with prescription opioids. For example, optimizing non‐opioid pharmacotherapy and non‐pharmacological therapy as first‐line options and using opioids only if first‐line therapies are insufficient or the acute traumatic injury is of a severe nature. If opioids are required, prescribers should be very careful in limiting the number of tablets dispensed, ensuring appropriate patient education with respect to use and disposal, and in particular scenarios, use daily dispensing and/or delayed prescriptions.
Diversion of unused opioids for non‐medical use remains a serious concern because of the potential of opioid toxicity and/or the development of an opioid use disorder. 5 Although ensuring the safety of the patient receiving an opioid is vital, it is important to consider that unused opioids pose a risk not only to them, but also to all others who have access to them. Knowing that both significant quantities of opioids remain unused, and that the most common source of opioids among individuals reporting non‐medical prescription opioid use is from a family member or friend, the importance of responsible prescribing and patient education regarding safe storage and disposal cannot be overlooked.
5. LIMITATIONS
The studies included in our review tended to be of lower methodological quality, limited by reliance on self‐reported, unvalidated data. Sample sizes were generally small, and there was heterogeneity in outcomes measured, limiting the amount and quality of data that could be pooled. However, we conducted our review to high methodological standards, and our findings mirror what has been found in other specialties, providing support for the validity of our findings. Notably, one study with 81 participants was an outlier, reporting 96% of opioids remaining unused. 37 This may be attributable to standardized prescriptions for and education about non‐opioid analgesics in addition to the opioids received.
In summary, almost 60% of opioids prescribed for acutely painful conditions remain unused after receiving a prescription from the ED or ambulatory care setting. One in 5 patients did not use a single opioid pill from their prescription. Specifically, dental procedure‐related prescriptions and those for children seem to have the largest amount of leftover opioids. Tailoring prescriptions according to patient indication, considering safety planning, and providing patient education on safer use, storage, and disposal are essential pieces in balancing the risks and benefits associated with opioid use.
AUTHOR CONTRIBUTIONS
Michele P. Dyson, Kathryn Dong, William Sevcik, Lisa Hartling, and Samina Ali conceived the study, designed the review, and obtained research funding. Michele P. Dyson supervised the conduct of the review, including data collection, extraction, synthesis, and quality assessment. Samir Z. Graham and Sabrina Saba contributed to data collection, data extraction, and quality assessment. Michele P. Dyson drafted the manuscript and all authors critically reviewed the draft and contributed important intellectual content. Michele P. Dyson takes responsibility for the study as a whole.
CONFLICTS OF INTEREST
Kathryn Dong receives a medical leadership salary from Alberta Health Services and has received committee membership‐related honoraria from the College of Physicians and Surgeons of Canada and the Edmonton Zone Medical Staff Association. Lisa Hartling is supported by a Tier 1 Canada Research Chair in Knowledge Synthesis and Translation and is a distinguished researcher with the Stollery Science Laboratory. The other authors made no disclosures.
Supporting information
ACKNOWLEDGMENTS
We thank Erica Wright for developing and running the search strategy and Ben Vandermeer for planning and conducting the statistical analyses. Research reported in this publication was supported by the Alberta Health Services Emergency Strategic Clinical Network (ESCN). The content is solely the responsibility of the authors and does not necessarily represent the views of the ESCN Scientific Office or Alberta Health Services. The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Dyson MP, Dong K, Sevcik W, et al. Quantifying unused opioids following emergency and ambulatory care: A systematic review and meta‐analysis. JACEP Open. 2022;3:e12822. 10.1002/emp2.12822
REFERENCES
- 1. Special Advisory Committee on the Epidemic of Opioid Overdoses . Opioid‐ and stimulant‐related Harms in Canada. Ottawa: Public Health Agency of Canada; December 2021. Accessed January 27, 2022. Available at: https://health‐infobase.canada.ca/substance‐related‐harms/opioids‐stimulants
- 2. National Institute on Drug Abuse . Overdose Death Rates. January 20, 2022. Accessed January 27, 2022. Available at: https://nida.nih.gov/drug‐topics/trends‐statistics/overdose‐death‐rates [Google Scholar]
- 3. Canadian Institute for Health Information . Opioid Prescribing in Canada: How Are Practices Changing? CIHI; 2019. Accessed January 27, 2022. Available at: https://www.cihi.ca/sites/default/files/document/opioid‐prescribing‐canada‐trends‐en‐web.pdf [Google Scholar]
- 4. Hatten BW, Cantrill SV, Dubin JS, et al. Clinical policy: critical issues related to opioids in adult patients presenting to the emergency department. Ann Emerg Med. 2020;76:313‐e39. [DOI] [PubMed] [Google Scholar]
- 5. Daoust R, Paquet J, Marquis M, et al. Evaluation of interventions to reduce opioid prescribing for patients discharged from the emergency department: a systematic review and meta‐analysis. JAMA Network Open. 2022;5(1):e2143425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kilaru AS, Lowenstein M, Agarwal AK. Optimizing opioid prescriptions for patients in the emergency department – how much is almost never? JAMA Network Open. 2022;5(1):e2143433. [DOI] [PubMed] [Google Scholar]
- 7. McCarthy DM, Kim HS, Hur SI, et al. Patient‐reported opioid pill consumption after an ED visit: how many pills are people using? Pain Med. 2021;22:292‐302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shi R, Quinones A, Bair J, et al. Patient utilization of prescription opioids after discharge from the emergency department. Am J Emerg Med. 2020;38:1568‐1571. [DOI] [PubMed] [Google Scholar]
- 9. Daoust R, Paquet J, Cournoyer A, et al. Quantity of opioids consumed following an emergency department visit for acute pain: a Canadian prospective cohort study. BMJ Open. 2018;8:e022649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schirle L, Stone AL, Morris MC, et al. Leftover opioids following adult surgical procedures: a systematic review and meta‐analysis. Syst Rev. 2020;9:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Arwi GA, Schug SA. Potential for harm associated with discharge opioids after hospital stay: a systematic review. Drugs. 2020;80:573‐585. [DOI] [PubMed] [Google Scholar]
- 12. Sheth U, Mehta M, Huyke F, Terry MA, Tjong VK. Opioid use after common sports medicine procedures: a systematic review. Sports Health. 2020;12:225‐233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bicket MC, Long JJ, Pronovost PJ, Alexander GC, Wu CL. Prescription opioid analgesics commonly unused after surgery: a systematic review. JAMA Surgery. 2017;152:1066‐1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ahrari M, Ali S, Hartling L, et al. Nonmedical opioid use after short‐term therapeutic exposure in children: a systematic review. Pediatrics. 2021;148(6):e2021051927. [DOI] [PubMed] [Google Scholar]
- 15. Moreno MA, Furtner F, Rivara FP. Adolescent opioid abuse. Arch Pediatr Adolesc Med. 2012;166:880. [DOI] [PubMed] [Google Scholar]
- 16. Garbutt JM, Kulka K, Dodd S, Sterkel R, Plax K. Opioids in adolescents’ homes: prevalence, caregiver attitudes, and risk reduction opportunities. Acad Pediatr. 2019;19:103‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hudgins JD, Porter JJ, Monuteaux MC, Bourgeois FT. Prescription opioid use and misuse among adolescents and young adults in the United States: a national survey study. PLoS Med. 2019;16(11):e1002922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lipari RN, Hughes AH. How people obtain the prescription pain relievers they misuse. The CBHSQ Report: January 12, 2017. Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration, Rockville, MD. [PubMed]
- 19. Borgundvaag B, McLeod S, Khuu W, Varner C, Tadrous M, Gomes T. Opioid prescribing and adverse events in opioid‐naïve patients treated by emergency physicians versus family physicians: a population‐based cohort study. CMAJ Open. 2018;6:E110‐E117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Higgins JPT, Thomas J, Chandler J, et al. (Eds.). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Accessed January 27, 2022. Available from www.training.cochrane.org/handbook [Google Scholar]
- 21. National Institute for Health Research . PROSPERO International prospective register of systematic reviews. Accessed January 27, 2022. Available at: https://www.crd.york.ac.uk/prospero/
- 22. McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol. 2016;75:40‐46. [DOI] [PubMed] [Google Scholar]
- 23. The Canadian Agency for Drugs and Technologies in Health . Grey Matters: a practical tool for searching health‐related grey literature. Updated April 2019. Accessed January 27, 2022. Available at: https://www.cadth.ca/grey‐matters‐practical‐tool‐searching‐health‐related‐grey‐literature‐0
- 24. Khangura S, Konnyu K, Cushman R, Grimshaw J, Moher D. Evidence summaries: the evolution of a rapid review approach. Syst Rev. 2012;1(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. O'Blenis P, One simple way to speed up your screening process. Accessed January 27, 2022. Available at: https://blog.evidencepartners.com/one‐simple‐way‐to‐speed‐up‐your‐screening‐process
- 26. Berdine HJ, Nesbit SA. Equianalgesic dosing of opioids. J Pain Palliat Care Pharmacother. 2006;20:79‐84. [PubMed] [Google Scholar]
- 27. Buscemi N, Hartling L, Vandermeer B, Tjosvold L, Klassen T. Single data extraction generated more errors than double data extraction in systematic reviews. J Clin Epidemiol. 2006;59:697‐703. [DOI] [PubMed] [Google Scholar]
- 28. Munn Z, Barker T, Moola S, et al. Methodological quality of case series studies: an introduction to the JBI critical appraisal tool. JBI Evid Synth. 2020;18(10):2127‐2133. 10.11124/JBISRIR-D-19-00099 [DOI] [PubMed] [Google Scholar]
- 29. Moola S, Munn Z, Tufanaru C, et al. Chapter 7: systematic reviews of etiology and risk. In: Aromataris E, Munn Z (Eds.). JBI Manual for Evidence Synthesis. JBI, 2020. Accessed January 27, 2022. Available from https://synthesismanual.jbi.global [Google Scholar]
- 30. Barendregt JJ, Doi SA, Lee YY, Norman RE, Vos T. Meta‐analysis of prevalence. J Epidemiol Community Health. 2013;67(11):974‐978. [DOI] [PubMed] [Google Scholar]
- 31. Shi R, Quinones A, Bair J, et al. Patient utilization of prescription opioids after discharge from the emergency department. Am J Emerg Med. 2020;38:1568‐1571. [DOI] [PubMed] [Google Scholar]
- 32. Yang C, Stilley JAW, Bedy SMC, Goddard KB, Sampson CS. Leftover narcotic analgesics among emergency department patients and methods of disposal. JACEP Open. 2020;1:1486‐1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhu H, Gao Y, Zhang C, Zheng X. A prospective evaluation of patient‐reported opioid utilization after nonoperative treatment of fractures and dislocations. J Bone Joint Surg Am. 2018;100(1):177‐183. [DOI] [PubMed] [Google Scholar]
- 34. Nelson SE, Adams AJ, Buczek MJ, Anthony CA, Shah AS. Postoperative pain and opioid use in children with supracondylar humeral fractures. J Bone Joint Surg Am. 2019;101(1):19‐26. [DOI] [PubMed] [Google Scholar]
- 35. Stillwagon MR, Feinstein S, Nichols B, Andrews PN, Vergun AD. Pain control and medication use in children following closed reduction and percutaneous pinning of supracondylar humerus fractures: are we still overprescribing opioids? J Pediatr Orthop. 2020;40(10):543‐548. [DOI] [PubMed] [Google Scholar]
- 36. Shahi N, Meier M, Phillips R, et al. Pain management for pediatric burns in the outpatient setting: a changing paradigm? J Burn Care Res. 2020;41(4):814‐819. [DOI] [PubMed] [Google Scholar]
- 37. Resnick CM, Calabrese CE, Afshar S, Padwa BL. Do oral and maxillofacial surgeons over‐prescribe opioids after extraction of asymptomatic third molars? J Oral Maxillofac Surg. 2019;77:1332‐1336. [DOI] [PubMed] [Google Scholar]
- 38. Weiland BM, Wach AG, Kanar BP, et al. Use of opioid pain relievers following extraction of third molars. Compend Contin Educ Dent. 2015;36(2):107‐111. [PubMed] [Google Scholar]
- 39. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Health Quality Ontario. Quality Standards: Opioid Prescribing for Acute Pain. Care for People 15 Years of Age and Older. Accessed March 5, 2022. Available at: https://www.hqontario.ca/portals/0/documents/evidence/quality‐standards/qs‐opioid‐acute‐pain‐clinician‐guide‐en.pdf
- 41. Choosing Wisely Canada. Opioid Wisely. Accessed March 5, 2022. Available at: https://choosingwiselycanada.org/campaign/opioid‐wisely/
- 42. Ali S, Drendel AL. Bottom Line Recommendations: Pain Treatment. Translating Emergency Knowledge for Kids. Accessed March 5, 2022. Available at: https://trekk.ca/system/assets/assets/attachments/535/original/2021‐03‐16_Pain_Treatment_v2.0.pdf?1620411251
- 43. Smith BC, Vigotsky AD, Apkarian AV, et al. Temporal factors associated with opioid prescriptions for patients with pain conditions in an urban emergency department. JAMA Netw Open. 2020;3(3):e200802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dowell D, Haegerich T, Chou R. No shortcuts to safer opioid prescribing. N Engl J Med. 2019;380(24):2285‐2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Okunev I, Frantsve‐Hawley J, Tranby E. Trends in national opioid prescribing for dental procedures among patients enrolled in Medicaid. J Am Dent Assoc. 2021;152(8):622‐630.e3. [DOI] [PubMed] [Google Scholar]
- 46. Miller CS, Ke C, Witty JT, et al. Prescribing patterns of opioid analgesics in a dental setting: 2013–2018. Oral Surg Oral Med Oral Pathol Oral Radiol. 2020;130(4):402‐410. [DOI] [PubMed] [Google Scholar]
- 47. Heron MJ, Nwokorie NA, O'Connor B, et al. Survey of opioid prescribing among dentists indicates need for more effective education regarding pain management. J Am Dent Assoc. 2022;153(2):110‐119. [DOI] [PubMed] [Google Scholar]
- 48. American Dental Association . Policy on Opioid Prescribing. Accessed March 22, 2022. Available at: https://www.ada.org/about/governance/current‐policies#substanceusedisorders
- 49. American Dental Association . Statement on the Use of Opioids in the Treatment of Dental Pain. Accessed March 22, 2022.d Available at: https://www.ada.org/about/governance/current‐policies#subtanceusedisorders
- 50. Canadian Association of Hospital Dentists . Eight Things Dentists and Patients Should Question. Choosing Wisely Canada. Accessed March 6, 2022. Available at: https://choosingwiselycanada.org/hospital‐dentistry/
- 51. Chua KP, Kimmel L, Brummett CM. Disappointing early results from opioid prescribing limits for acute pain. JAMA Surgery. 2020;155(5):375‐376. [DOI] [PubMed] [Google Scholar]
- 52. Davis CS, Lieberman AJ. Laws limiting prescribing and dispensing of opioids in the United States, 1989–2019. Addiction. 2021;116(7):1817‐1827. [DOI] [PubMed] [Google Scholar]
- 53. Ali S, Manaloor R, Johnson DW, et al. An observational cohort study comparing ibuprofen and oxycodone in children with fractures. PLoS One. 2021;16(9):e0257021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Reiter E, Ali S. So you have been prescribed an opioid? Solutions for Kids in Pain. Accessed March 6, 2022. Available at: https://kidsinpain.ca/wp‐content/uploads/2021/04/ED_opioids_19Apr2021_Final.pdf
- 55. Dyson M, Wright K, Saidhersi Z. Opioids – Information for Parents, Youth, and Clinicians. Translating Emergency Knowledge for Kids. Accessed March 6, 2022. Available at: https://trekk.ca/resources?utf8=%E2%9C%93&tag_id=D000701&external_resource_type=All
- 56. Canadian Institute for Health Information. Opioid prescribing in Canada: How are practices changing? CIHI; 2019. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


