Key Points
Question
What is the optimal assessment schedule for patients who have been definitively treated for locally advanced head and neck cancer?
Findings
This retrospective cohort study of 673 patients found optimal assessment schedules according to the follow-up time points at which 5% of events occurred. Different schedules of follow-up visits were recommended for nasopharyngeal, human papillomavirus (HPV)-positive oropharyngeal, HPV-negative oropharyngeal, hypopharyngeal, and laryngeal cancer, with a 3-fold difference in the number of required follow-up visits among the groups.
Meaning
The findings of this retrospective study suggest that assessment schedules should be individualized and evidence based to be optimal for assessing cancer recurrence after definitive treatment of different types of head and neck cancer; development of new consensus guidelines is proposed.
Abstract
Importance
In clinical practice, assessment schedules are often arbitrarily determined after definitive treatment of head and neck cancer (HNC), producing heterogeneous and inconsistent surveillance plans.
Objective
To establish an optimal assessment schedule for patients with definitively treated locally advanced HNC, stratified by the primary subsite and HPV status, using a parametric model of standardized event-free survival curves.
Design, Setting, and Participants
This was a retrospective study including 2 tertiary referral hospitals and a total of 673 patients with definitive locoregional treatment of locally advanced HNC (227 patients with nasopharyngeal cancer [NPC]; 237 patients with human papillomavirus-positive oropharyngeal cancer [HPV+ OPC]; 47 patients with HPV-negative [HPV−] OPC; 65 patients with hypopharyngeal cancer [HPC]; and 97 patients with laryngeal cancer [LC]). Patients had received primary treatment in 2008 through 2019. The median (range) follow-up duration was 57.8 (6.4-158.1) months. Data analyses were performed from April to October 2021.
Main Outcomes and Measures
Tumor recurrence and secondary malignant neoplasms. Event-free survival was defined as the period from the end of treatment to occurrence of any event. Event-free survival curves were estimated using a piecewise exponential model and divided into 3 phases of regular follow-up. A 5% event rate criterion determined optimal follow-up time point and interval.
Results
The median (range) age of the 673 patients at HNC diagnosis was 58 (15-83) years; 555 (82.5%) were men; race and ethnicity were not considered. The event rates of NPC, HPV+ OPC, HPV− OPC, HPC, and LC were 18.9% (43 of 227), 14.8% (35 of 237), 36.2% (17 of 47), 44.6% (29 of 65), and 30.9% (30 of 97), respectively. Parametric modeling demonstrated optimal follow-up intervals for HPC, LC, and NPC, respectively, every 2.1, 3.2, and 6.1 months; 3.7, 5.6, and 10.8 months; and 9.1, 13.8, and 26.5 months until 16.5, 16.5 to 25.0, and 25.0 to 99.0 months posttreatment (open follow-up thereafter). For HPV− OPC, assessment was recommended every 2.7, 4.8, and 11.8 months until 16.5, 16.5 to 25.0, and 25 to 99 months posttreatment, respectively. In contrast, HPV+ OPC optimal intervals were every 7.7, 13.7, and 33.7 months until 16.5, 16.5 to 25.0, and 25 to 99 months posttreatment, respectively. Five, 4, 12, 15, and 10 follow-up visits were recommended for NPC, HPV+ OPC, HPV− OPC, HPC, and LC, respectively.
Conclusions and Relevance
This retrospective cohort study using parametric modeling suggests that the HNC assessment schedules should be patient tailored and evidence based to consider primary subsites and HPV status. Given limited health care resources and rising detection rates and costs of HNC, the guidelines offered by these findings could benefit patients and health systems and aid in developing future consensus guidelines.
This retrospective cohort study of more than 600 patients with definitively treated head and neck cancer uses parametric modeling to examine current surveillance guidelines and to propose new assessment schedules.
Introduction
Approximately half of patients with locally advanced head and neck cancer (HNC) experience locoregional recurrence after definitive treatment.1 Additionally, the incidence of a second primary cancer is approximately 2% to 4% per year, with a lifetime risk of 10% to 20%.2 However, posttreatment surveillance strategies remain an ongoing debate because currently no evidence exists to support any follow-up strategy being more effective in detecting recurrence or improving patients’ quality of life.3,4,5,6 Although several medical societies have made recommendations,7,8,9,10 these mainly depended on expert opinions rather than solid evidence. Furthermore, they did not consider the risk of HNC recurrence, which is greatly dependent on the primary subsite, tumor stage, and molecular markers, such as human papillomavirus (HPV) status.11 Therefore, assessment schedules are often determined arbitrarily by physicians in daily clinical practice, which produces heterogeneous and inconsistent plans.9 This approach has been associated with over- and undersurveillance of tumor recurrence, thus imposing unnecessary economic and resource burdens on health care systems.12 In this context, a strong demand for evidence-based risk-tailored guidelines for HNC surveillance exists.
A piecewise exponential model widely used in survival analysis has proven useful in various cancer research studies.13,14,15 Piecewise exponential distribution assumes different hazards in predefined time intervals, reflecting changes in incidence over time.16 This model is a simple and flexible tool for developing an applicable assessment schedule for clinical practice.
In this study, we sought to establish an evidence-based assessment schedule model, stratified by the primary subsite and HPV status, in patients definitively treated for locally advanced HNC. Using piecewise exponential modeling, we determined the optimal assessment time point using a 5% event rate criterion.
Methods
This retrospective study was reviewed and approved by the institutional review boards of the Seoul National University Hospital and the Seoul National University Bundang Hospital. Informed consent was waived per the institutional policies for minimal-risk studies. The methods followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines.
Study Cohort
We retrospectively identified patients who had received definitive locoregional treatment for locally advanced HNC at the Seoul National University Hospital and at the Seoul National University Bundang Hospital in 2008 through 2019. The inclusion criteria were patients with (1) newly diagnosed, histologically proven, and T3 to T4 or node-positive head and neck squamous cell carcinoma of the nasopharynx (NPC), HPV-positive oropharynx cancer (HPV+ OPC), HPV-negative (HPV−)OPC, hypopharynx cancer (HPC), and larynx cancer (LC); (2) who had completed definitive locoregional treatment, including radiotherapy (RT) or surgery, and were followed up for more than 6 months after treatment completion; and (3) who achieved a complete response within 6 months after treatment. Patients who had residual tumor or progressive disease at 6 months after treatment were excluded because the current study aimed to develop a surveillance program for detecting de novo events after gross tumor eradication with primary treatment. Patients initially diagnosed with distant metastases or other malignant neoplasms were also excluded. All data were reported according to the American Joint Committee on Cancer Staging System, 8th edition. Only OPC was divided into 2 distinct groups based on HPV status because HPV+ cancers in other primary subsites are rare (<6%) and their prognostic value has not been proven. A total of 673 patients were included: with NPC, 227 patients; HPV+ OPC, 237 patients; HPV− OPC, 47 patients; HPC, 65 patients; and LC, 97 patients.
Treatment and Follow-up Evaluation
A multidisciplinary head and neck tumor board determined treatment modalities. After primary treatment, all patients underwent close follow-up for 6 months; treatment response was evaluated using positron emission tomography (PET). For patients who achieved a complete response, institutional follow-up protocols were as follows: every 2 to 3 months for the first year after treatment; every 3 to 5 months for the second and third years; every 6 months for the fourth and fifth years; and open follow-up thereafter (eTable 1 in the Supplement). The patients were instructed to promptly visit the hospital if they experienced any unusual symptoms such as pain, palpable lumps, or bleeding. Follow-up evaluation comprised history taking, physical and endoscopic examination, laboratory tests, and radiological assessment, if indicated. Chest imaging was performed annually in heavy smokers.
Event Definitions
Events included tumor recurrence and secondary malignant neoplasms. Local events were defined as those occurring within the head and neck area. Among local events, patients were regarded to have primary tumor recurrence when the disease had the same histologic features and occurred within 2 cm of the primary cancer. Otherwise, patients were considered to have a secondary malignant neoplasm. Distant events were defined as events occurring outside the head and neck areas. Only secondary malignant neoplasms arising from the aerodigestive tract were included in the cohort. Event-free survival (EFS) was defined as the time from the end of the treatment to the occurrence of any event.
Statistical Analysis
A piecewise exponential model was used to construct an evidence-based follow-up protocol for estimating EFS curves. Specifying constant hazards in the predefined time intervals can provide a flexible and applicable assessment schedule based on an EFS curve. We adopted the criterion of planning a subsequent assessment schedule at the time point when 5% of the EFS rate was dropped. The piecewise exponential survival function was defined as follows:
For given knots of survival, time 0 = τ0<τ1<…<τJ = Τ
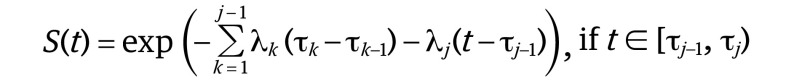 |
where λj,j = 1,…, J are constants that represent hazard at each interval [τj −1, τj).
Because it had a constant hazard value (λj) in each predetermined interval, the resulting follow-up visits were scheduled regularly in each interval. For a given p percentage, the test is scheduled for every
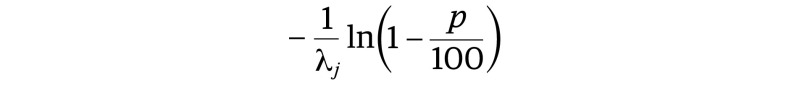 |
time unit in the interval [τj −1, τj). These consistent follow-up schedules are desirable for practical application.
Furthermore, to create unified schedules for the 5 HNC groups, we used the Cox proportional hazard model, with the covariate indicating each group. It adjusts for covariates by prescribing the log hazard ratio to be linear in the covariates, and specifies constant baseline hazards in predefined time intervals, allowing a complete specification of the hazard ratio estimation. The proportional hazard function is defined as follows: λi(t|z) = λ0(t) exp(ztβ), where λ0(t) is a baseline hazard function, and z is a covariate vector. In this study, z is a covariate vector indicating each group.
We selected the knots using the forward selection procedure with the Bayes information criterion (BIC), as used in a previous study on gliomas.13 However, we slightly modified the procedure to prevent selection of redundant knots. We only selected knots such that the difference in the survival probabilities at 2 consecutive knots was larger than 1%, that is, the selected knots {τj, j ∈ {1, …, J}} satisfied the following calculation:
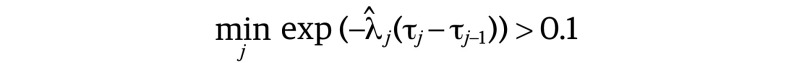 |
All statistical analyses were performed using R, version 4.0.2 (The R Foundation for Statistical Computing); EFS was estimated using the Kaplan-Meier method. We estimated the piecewise constant hazards λj, j = 1, …, J and the regression coefficient, β, using the maximum likelihood estimator and performed a scaled Schoenfeld residuals test to check validity of the proportional hazard assumption before fitting Cox proportional hazard models. Statistical tests were 2-tailed and P values < .05 were considered statistically significant. Data analyses were performed from April to October 2021.
Results
Patient Characteristics
Overall, the patient median (range) age at diagnosis was 58 (15-83) years and most (82.5%; 555 patients) were men, accounting for 70% to 90% of all groups. For the 5 separate groups, median age ranged from 52 to 66 years. Race and ethnicity data were not collected. Definitive concurrent chemoradiotherapy was the most common treatment modality (336 patients; 49.9%), followed by induction chemotherapy plus definitive concurrent chemoradiotherapy (198 patients; 29.4%). Patient characteristics are summarized in Table 1.
Table 1. Characteristics of Patients (N = 673), by Type of Definitively Treated HNC.
| Characteristic | Nasopharynx (n = 227) | HPV-positive oropharynx (n = 237) | HPV-negative oropharynx (n = 47) | Hypopharynx (n = 65) | Larynx (n = 97) |
|---|---|---|---|---|---|
| Age, median (range), y | 52 (15-81) | 59 (37-81) | 63 (49-77) | 66 (47-83) | 64 (40-82) |
| Men | 168 (74.0) | 203 (85.7) | 43 (91.5) | 54 (83.1) | 87 (89.7) |
| Women | 59 (26.0) | 34 (14.3) | 4 (8.5) | 11 (16.9) | 10 (10.3) |
| Performance status | |||||
| ECOG 0-1 | 222 (97.8) | 237 (100) | 45 (95.7) | 56 (86.2) | 85 (87.6) |
| ECOG ≥2 | 5 (2.2) | 0 | 2 (4.3) | 9 (13.8) | 12 (12.4) |
| Tumor stage | |||||
| T1 | 72 (31.7) | 58 (24.5) | 8 (17.0) | 7 (10.8) | 7 (7.2) |
| T2 | 47 (20.7) | 124 (52.3) | 22 (46.8) | 27 (41.5) | 15 (15.5) |
| T3 | 43 (18.9) | 29 (12.2) | 8 (17.0) | 13 (20.0) | 51 (52.6) |
| T4 | 65 (28.6) | 26 (11.0) | 9 (19.2) | 18 (27.7) | 24 (24.7) |
| Nodule stage | |||||
| N0 | 28 (12.3) | 8 (3.4) | 2 (4.3) | 15 (23.1) | 45 (46.4) |
| N1 | 88 (38.8) | 88 (37.1) | 19 (40.4) | 9 (13.9) | 14 (14.4) |
| N2 | 94 (41.4) | 136 (57.4) | 25 (53.2) | 40 (61.5) | 38 (39.2) |
| N3 | 17 (7.5) | 5 (2.1) | 1 (2.1) | 1 (1.5) | 0 |
| Treatment | |||||
| Definitive CCRT | 146 (64.3) | 81 (34.2) | 21 (44.7) | 32 (49.2) | 56 (57.7) |
| Induction chemotherapy and CCRT | 81 (35.7) | 66 (27.9) | 14 (29.8) | 18 (27.7) | 19 (19.6) |
| Surgery and adjuvant RT | 0 | 42 (17.7) | 6 (12.8) | 7 (10.8) | 17 (17.5) |
| Surgery and adjuvant CCRT | 0 | 48 (20.3) | 6 (12.8) | 8 (12.3) | 5 (5.2) |
| RT dose, median (range), Gy | 67.5 (60.0-72.0) | 67.5 (57.0-72.0) | 67.5 (60.0-70.0) | 67.5 (60.0-70.0) | 67.5 (59.4-70.0) |
| Follow-up period, median (range), mo | 59.0 (6.4-157.2) | 58.8 (6.8-158.1) | 51.0 (10.1-135.6) | 52.9 (8.0-148.3) | 54.2 (13.5-156.5) |
Abbreviations: CCRT, concurrent chemoradiotherapy; ECOG, Eastern Cooperative Oncology Group; HNC, head and neck cancer; HPV, human papillomavirus; RT, radiotherapy.
Survival Outcomes
With a median (range) follow-up of 57.8 (6.4-158.1) months, the total event rates of NPC, HPV+ OPC, HPV− OPC, HPC, and LC were 18.9% (43 of 227 patients), 14.8% (35 of 237), 36.2% (17 of 47), 44.6% (29 of 65), and 30.9% (30 of 97), respectively (eTable 2 in the Supplement). The distant event rates of NPC, HPV+ OPC, HPV− OPC, HPC, and LC were 12.8% (29 of 227 patients), 8.4% (20 of 237), 21.3% (10 of 47), 24.6% (16 of 65), and 18.6% (18 of 97), respectively. The most common site of distant events was the lungs, accounting for 43.8% of all distant events. The overall rate of secondary malignant neoplasms in the aerodigestive tract was 3.6%. The EFS curves according to 5 HNC groups are shown in Figure 1. The 2-year EFS rates for NPC, HPV+ OPC, HPV− OPC, HPC, and LC were 87.5%, 91.9%, 73.8%, 69.1%, and 78.1%, respectively. At 5 years, the corresponding rates were 80.6%, 85.6%, 65.6%, 58.1%, and 64.9%, respectively.
Figure 1. Kaplan-Meier Estimation of Event-Free Survival for the 5 Groups of Patients With Definitive Treatment of Locally Advanced Head and Neck Cancer.
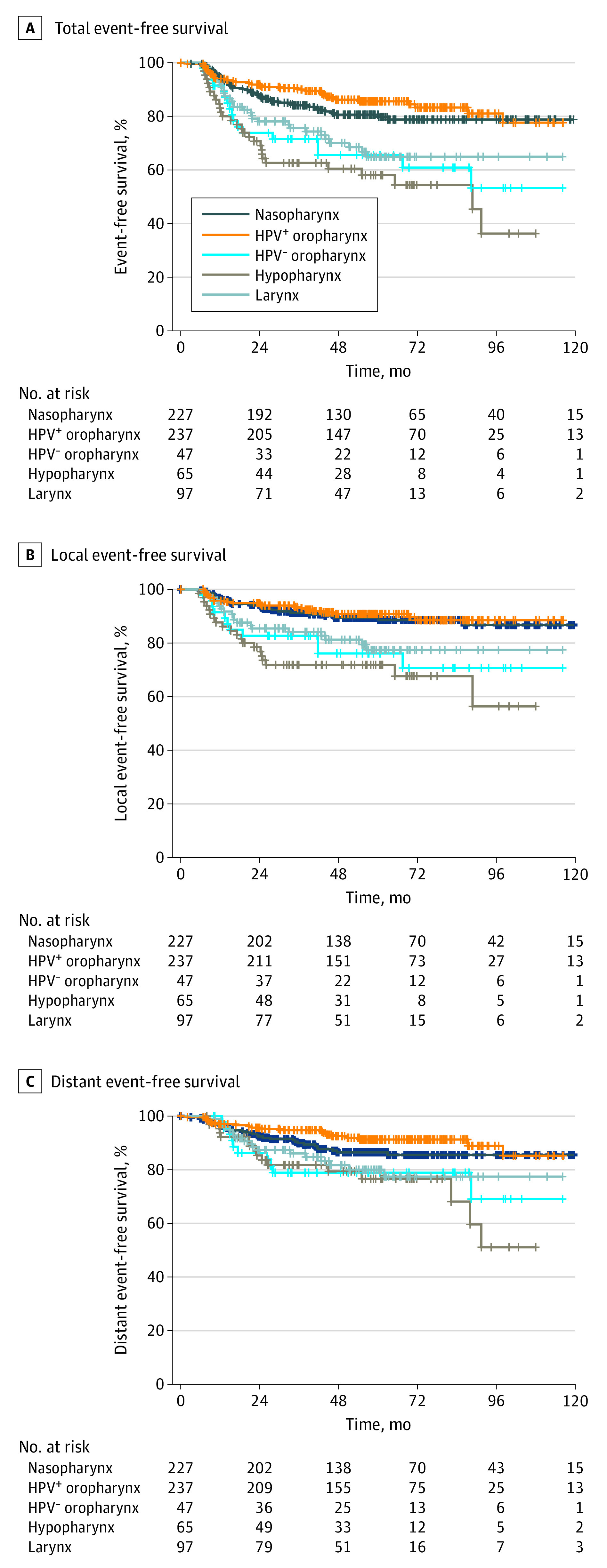
HPV refers to human papillomavirus.
Parametric Models for the Optimal Assessment Schedule
The parametric modeling of the 5 HNC groups is shown in eFigure 1 in the Supplement. The first 6 months from the end of treatment were defined as the period for response evaluation and close follow-up. Then, using the BIC criterion, EFS curves were divided into 3 phases for regular follow-up: first phase, 6.0 to 16.5 months; second phase, 16.5 to 25.0 months; third phase, 25.0 to 99.0 months; and open follow-up thereafter. Each phase had a constant follow-up interval based on the piecewise exponential modeling.
The optimal follow-up interval for NPC was every 6.1, 10.8, and 26.5 months for the first, second, and third phases, respectively (eFigure 1A in the Supplement). For HPV+ OPC, the optimal interval was every 7.7, 13.7, and 33.7 months for the first, second, and third phases, respectively (eFigure 1B in the Supplement). In contrast, HPV− OPC assessment was every 2.7, 4.8, and 11.8 months for first, second, and third phases, respectively (eFigure 1C in the Supplement). The shortest optimal follow-up intervals were for HPC: every 2.1, 3.7, and 9.1 months for the first, second, and third phases, respectively (eFigure 1D in the Supplement). Lastly, the optimal assessment intervals for LC were 3.2, 5.6, and 13.8 months for the first, second, and third phases, respectively (eFigure 1E in the Supplement). After 99.0 months of treatment, open follow-up was recommended for all groups.
Parametric Models for Separated Local and Distant Surveillance
While separating local and distant events, follow-up intervals were extended (eFigures 2 and 3 in the Supplement). Regarding surveillance for local events, the local EFS curves were divided into 3 slightly different phases for regular follow-up, based on the BIC criterion: first phase, 6.0 to 16.5 months; second phase, 16.5 to 25.5 months; and third phase, 25.5 to 99.0 months. The optimal local follow-up interval for NPC was every 9.9, 22.7, and 51.8 months for the first, second, and third phase, respectively. For HPV+ OPC and HPV− OPC, follow-up was recommended every 10.8 and 3.9, 24.7 and 8.8, and 56.4 and 20.2 months for the first, second, and third phases, respectively. The optimal local follow-up interval for HPC was the shortest among all 5 groups: every 2.9, 6.7, and 15.3 months for the first, second, and third phases, respectively. The optimal assessment intervals for LC were 4.7, 10.8, and 24.7 months for the first, second, and third phases, respectively (eFigure 2 in the Supplement).
Regarding surveillance for distant events, distant EFS curves were divided into 2 phases: first phase, 6.0 to 27.5 months; and second phase, 27.5 to 99.0 months. The optimal distant follow-up interval for NPC was every 13.8 and 36.7 months for the first and second phases, respectively. For HPV+ OPC and HPV− OPC, follow-up was recommended every 20.4 and 6.8, and 54.2 and 18.2 months for the first and second phases, respectively. The optimal distant assessment interval for HPC was every 5.8 and 15.4 months for the first and second phases, respectively. Finally, the optimal distant assessment interval for LC was every 8.0 and 21.2 months for the first and second phases, respectively (eFigure 3 in the Supplement).
The summarized assessment schedule guideline for the 5 HNC groups is shown in Table 2 and Figure 2. According to this guideline, the recommended number of follow-up visits after the close follow-up period is 5, 4, 12, 15, and 10 visits for the NPC, HPV+ OPC, HPV− OPC, HPC, and LC groups, respectively.
Table 2. Summary of the Optimal Assessment Schedules After Definitive Treatment of Locally Advanced HNC.
| HNC type | Phase (time) since treatment end | ||||
|---|---|---|---|---|---|
| Close (≤6.0 mo) | First (6.0-16.5 mo) | Second (16.5-25.0 mo) | Third (25.0-99.0 mo) | Open (>99.0 mo) | |
| Nasopharynx | Response evaluation and close follow-up | 6.1 | 10.8 | 26.5 | Open follow-up |
| HPV-positive oropharynx | 7.7 | 13.7 | 33.7 | ||
| HPV-negative oropharynx | 2.7 | 4.8 | 11.8 | ||
| Hypopharynx | 2.1 | 3.7 | 9.1 | ||
| Larynx | 3.2 | 5.6 | 13.8 | ||
Abbreviations: HNC, head and neck cancer; HPV, human papillomavirus.
Figure 2. Recommended Assessment Schedule Guidelines for Patients With Definitive Treatment of Locally Advanced Head and Neck Cancer.
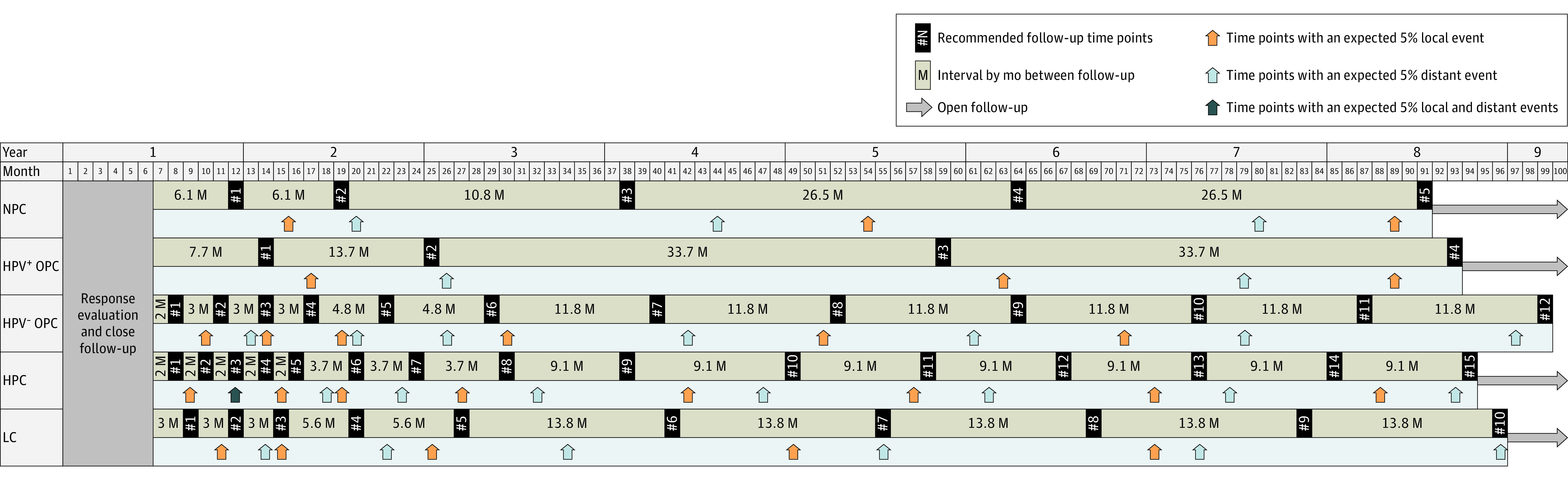
The recommended follow-up time points are indicated by black rectangles; each time point with expected 5% local and distant events is indicated by orange and blue arrows, respectively. For all 5 groups, when more events occurred, more follow-up visits were recommended. HPC refers to hypopharynx cancer; HPV, human papillomavirus; HPV− OPC, human papillomavirus-negative oropharynx cancer; HPV+ OPC, human papillomavirus-positive oropharynx cancer; LC, larynx cancer; and NPC, squamous cell carcinoma of the nasopharynx.
Discussion
Using parametric modeling, this study proposed an optimal assessment schedule guideline for locally advanced HNC by selecting follow-up time points at which 5% of event rates occurred. The 5 HNC groups had substantially different prognoses and therefore require diverse assessment schedules. The HPV+ OPC and NPC groups showed a low risk of events during follow-up, and a total of 4 and 5 follow-up visits were required after treatment, respectively. In contrast, the HPC group had a high risk of events (44.6%) during follow-up, requiring 15 follow-up visits after treatment. Therefore, there was a 3-fold difference in the number of required follow-up visits among the HNC groups. Given that most medical societies provide uniform surveillance guidelines for patients with HNC, these findings are noteworthy. In particular, HPV+ and HPV− OPC are 2 distinct entities that require 4 and 12 follow-up visits, respectively. However, HPV status is rarely considered when determining surveillance plans. The current findings reveal an urgent need for individualized surveillance strategies in patients with HNC.
Generally, published guidelines recommend follow-up every 1 to 3 months for the first year, every 2 to 6 months for the second year, every 4 to 8 months for the third through fifth years, and annually thereafter (eTable 1 in the Supplement).7,8,9,10,17 In other words, the recommended number of follow-up visits for 5 years after treatment ranges from 11 to 27 visits, which is too broad to be followed. These crude recommendations create wide variation in the clinical practice of posttreatment surveillance in patients with HNC.18 Furthermore, it is not possible currently to draw firm conclusions because of a lack of evidence in support of any surveillance strategy.3 The assessment schedule proposed by the present study was generated based on standardized EFS curves, with a criterion of 5% event rates. This evidence-based and sophisticated assessment schedule will be helpful in determining surveillance plans in clinical practice and for further investigating and developing consensus guidelines.
A main conflicting topic in HNC surveillance is whether routine imaging should be performed or not. There is a consensus for the first posttreatment imaging to be performed within 6 months (optimally around 12 weeks) to assess treatment response and establish a baseline.7,17 However, continued imaging for asymptomatic survivors is generally not recommended because there is no proven survival benefit. A study by Ho and colleagues19 reported no difference in 3-year disease-free survival between patients undergoing imaging surveillance and those who received only clinical examination (41% vs 46%; P = .91). A study by Ng and colleagues20 reported no difference in salvage or survival rates between patients who had asymptomatic recurrence detected via routine imaging and those with clinically detected recurrence. Therefore, routine imaging surveillance may cause unnecessary financial burden, radiation exposure, and consumption of limited hospital resources without proven benefits. Nevertheless, many physicians still continue to conduct imaging studies for asymptomatic patients because of the risk of missing recurrent events.21 Recent biomarker tests, such as plasma circulating tumor HPV DNA, can be a convenient and cost-effective alternative to imaging surveillance. A study by Chera and colleagues reported that monitoring of plasma circulating tumor HPV DNA had excellent negative and positive predictive values (100% and 94%, respectively) for posttreatment surveillance.22 The present guidelines were established based on complete clinical examination composed of medical history taking, physical examination, and endoscopic evaluation; biomarker testing is also recommended. On the other hand, imaging studies would be indicated when the patient has symptoms or abnormal findings on physical examination or when clinical examination is inaccessible owing to anatomical structure.7
It is well known that HNC has a substantial economic burden. An estimated $4.2 billion is spent annually in the US,23 and it is expected to increase annually.24 Diagnostic workup and primary treatment are major factors that influence costs; however, they are difficult to reduce. Surveillance is an area of potential reduction in cost burden. A study by Nocon and colleagues evaluated direct costs associated with surveillance in patients with HNC and reported a mean cost of $36 800 per patient, which translated to $11 800 per year for follow-up surveillance.12 This unnecessary financial burden can be reduced using a reasonable assessment schedule. In the current cohort, patients had an average of 13 outpatient visits during the surveillance period (excluding the close follow-up period), according to institutional policy. The present study’s proposed assessment schedule would lower the recommended number of outpatient visits to 4, 5, 10, and 12 times for the HPV+ OPC, NPC, LC, and HPV− OPC groups, respectively, and increase the number of recommended outpatient visits to 15 for the HPC group. Overall, costs associated with HNC surveillance would be lower when following this new guideline, and patients expected to have a poor prognosis would be more frequently evaluated. This guideline may balance clinical benefits and economic burden without compromising oncologic outcomes.
Another essential role of posttreatment surveillance is the management of late complications, rehabilitation, and restoration of psychosocial status.25 The most common late effects of HNC treatment include pain, xerostomia, dental issues, and speech and hearing difficulties.26,27 Untreated late effects can substantially affect patients’ quality of life, and timely intervention should be provided. Also, it should be noted that depression is relatively common for survivors of HNC; its prevalence is approximately 15%.28 Less than half of survivors return to work and regain their social roles. Given the increasing incidence of HPV-associated HNC among young people,29 psychosocial support and regular evaluation are becoming increasingly important. Therefore, health care resources should be reallocated in a way that limits unnecessary follow-up visits while increasing multidisciplinary care, including care from speech, swallowing, and hearing specialists, dentists, and psychologists.
To the best of our knowledge, this is the first report to propose an assessment schedule guideline based on scientific statistical analysis of the actual event rates in a cohort of patients with definitive treatment of HNC. As the number of HNC survivors increases with advancements in screening and treatment modalities, posttreatment surveillance will more greatly affect patients’ lives and health care resources.
Limitations
This study had several limitations that should be considered. First, the number of patients may be insufficient to yield stable results in some groups, which may be associated with over- or underestimation of event rates. We plan to validate this guideline among a larger cohort of patients to assess its clinical applicability. Second, the current approach may delay detection of recurrence because it was designed for follow-up at the time point when 5% of events occurred. A delay can be minimized by counseling patients to promptly visit a hospital in case of any abnormal symptoms; most recurrences are symptomatic and reported with patients’ symptoms.20 High-quality education should be provided in multiple formats, including oral, written, and web-based resources. Third, several other clinical factors should be considered, including patient age, performance status, and smoking history. We recommend determining a patient tailored assessment plan that integrates available prognostic factors based on these guidelines. In particular, the follow-up intervals for patients treated for HPV+ OPC and NPC and/or with high-risk traits may be modified from the recommended follow-up visits. For example, patients with HPC+ OPC who have a smoking history of more than 10 pack-years should be regarded as being of intermediate risk and should be followed up more frequently.
Conclusion
The findings of the retrospective study established an assessment schedule guideline for surveillance of patients definitively treated for locally advanced HNC using standardized EFS curves and parametric modeling. The guidelines, stratified into 5 groups according to the primary subsite and HPV status, showed that each group required substantially different follow-up visits after treatment. Given the limited health care resources and the rising number of patients with HNC, patient tailored and evidence based assessment schedules will benefit both patients and health systems. Further investigation for consensus guidelines is needed, and we hope that the findings of this study will aid in their establishment in the near future.
eTable 1. Recommendations for assessment schedule in head and neck cancer.
eTable 2. The overall incidence of event, progression, and primary malignancies in five subgroups.
eFigure 1. Parametric modeling of total events in (A) nasopharynx cancer, (B) HPV-positive oropharynx cancer, (C) HPV-negative oropharynx cancer, (D) hypopharynx cancer, and (E) larynx cancer
eFigure 2. Parametric modeling of local events in (A) nasopharynx cancer, (B) HPV-positive oropharynx cancer, (C) HPV-negative oropharynx cancer, (D) hypopharynx cancer, and (E) larynx cancer.
eFigure 3. Parametric modeling of distant events in (A) nasopharynx cancer, (B) HPV-positive oropharynx cancer, (C) HPV-negative oropharynx cancer, (D) hypopharynx cancer, and (E) larynx cancer.
References
- 1.Brockstein B, Haraf DJ, Rademaker AW, et al. Patterns of failure, prognostic factors and survival in locoregionally advanced head and neck cancer treated with concomitant chemoradiotherapy: a 9-year, 337-patient, multi-institutional experience. Ann Oncol. 2004;15(8):1179-1186. doi: 10.1093/annonc/mdh308 [DOI] [PubMed] [Google Scholar]
- 2.Morris LGT, Sikora AG, Patel SG, Hayes RB, Ganly I. Second primary cancers after an index head and neck cancer: subsite-specific trends in the era of human papillomavirus-associated oropharyngeal cancer. J Clin Oncol. 2011;29(6):739-746. doi: 10.1200/JCO.2010.31.8311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Szturz P, Van Laer C, Simon C, Van Gestel D, Bourhis J, Vermorken JB. Follow-up of head and neck cancer survivors: tipping the balance of intensity. Front Oncol. 2020;10:688. doi: 10.3389/fonc.2020.00688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kytö E, Haapio E, Minn H, Irjala H. Critical review of the follow-up protocol for head and neck cancer patients. J Laryngol Otol. 2019;133(5):424-429. doi: 10.1017/S0022215119000811 [DOI] [PubMed] [Google Scholar]
- 5.Denaro N, Merlano MC, Russi EG. Follow-up in head and neck cancer: Do more does it mean do better? a systematic review and our proposal based on our experience. Clin Exp Otorhinolaryngol. 2016;9(4):287-297. doi: 10.21053/ceo.2015.00976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Felice F, Musio D, Tombolini V. Follow-up in head and neck cancer: a management dilemma. Adv Otolaryngol. 2015;1-4. doi: 10.1155/2015/703450 [DOI] [Google Scholar]
- 7.National Comprehensive Cancer Network . NCCN Guidelines: Head and Neck Cancer. Accessed January 4, 2022. https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1437
- 8.Machiels JP, René Leemans C, Golusinski W, Grau C, Licitra L, Gregoire V; EHNS Executive Board; ESMO Guidelines Committee . Squamous cell carcinoma of the oral cavity, larynx, oropharynx and hypopharynx: EHNS-ESMO-ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31(11):1462-1475. doi: 10.1016/j.annonc.2020.07.011 [DOI] [PubMed] [Google Scholar]
- 9.Paniello RC, Virgo KS, Johnson MH, Clemente MF, Johnson FE. Practice patterns and clinical guidelines for posttreatment follow-up of head and neck cancers: a comparison of 2 professional societies. Arch Otolaryngol Head Neck Surg. 1999;125(3):309-313. doi: 10.1001/archotol.125.3.309 [DOI] [PubMed] [Google Scholar]
- 10.Simo R, Homer J, Clarke P, et al. Follow-up after treatment for head and neck cancer: United Kingdom National Multidisciplinary Guidelines. J Laryngol Otol. 2016;130(S2):S208-S211. doi: 10.1017/S0022215116000645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morgan RL, Eguchi MM, McDermott J, et al. Comparative effectiveness of posttreatment imaging modalities for Medicare patients with advanced head and neck cancer. Cancer. 2021;127(4):535-543. doi: 10.1002/cncr.33244 [DOI] [PubMed] [Google Scholar]
- 12.Nocon CC, Kennedy A, Jaffe J, Pruitt J, Kuchta K, Bhayani MK. Costs associated with imaging surveillance after treatment for head and neck cancer. JAMA Otolaryngol Head Neck Surg. 2021;147(7):632-637. doi: 10.1001/jamaoto.2021.0835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ji SY, Lee J, Lee JH, et al. Radiological assessment schedule for high-grade glioma patients during the surveillance period using parametric modeling. Neuro Oncol. 2021;23(5):837-847. doi: 10.1093/neuonc/noaa250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dieffenbach BV, Li N, Madenci AL, et al. Incidence of and risk factors for late cholecystectomy in survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Eur J Cancer. 2020;133:4-13. doi: 10.1016/j.ejca.2020.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qin N, Wang Z, Liu Q, et al. Pathogenic germline mutations in DNA repair genes in combination with cancer treatment exposures and risk of subsequent neoplasms among long-term survivors of childhood cancer. J Clin Oncol. 2020;38(24):2728-2740. doi: 10.1200/JCO.19.02760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han G, Schell MJ, Kim J. Improved survival modeling in cancer research using a reduced piecewise exponential approach. Stat Med. 2014;33(1):59-73. doi: 10.1002/sim.5915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roman BR, Goldenberg D, Givi B; Education Committee of American Head and Neck Society (AHNS) . Do you know your guidelines? guideline recommended follow-up and surveillance of head and neck cancer survivors. Head Neck. 2016;38(2):168-174. doi: 10.1002/hed.24100 [DOI] [PubMed] [Google Scholar]
- 18.Johnson FE, Johnson MH, Clemente MF, Paniello RC, Virgo KS. Geographical variation in surveillance strategies after curative-intent surgery for upper aerodigestive tract cancer. Ann Surg Oncol. 2006;13(8):1063-1071. doi: 10.1245/ASO.2006.04.014 [DOI] [PubMed] [Google Scholar]
- 19.Ho AS, Tsao GJ, Chen FW, et al. Impact of positron emission tomography/computed tomography surveillance at 12 and 24 months for detecting head and neck cancer recurrence. Cancer. 2013;119(7):1349-1356. doi: 10.1002/cncr.27892 [DOI] [PubMed] [Google Scholar]
- 20.Ng SP, Pollard C III, Berends J, et al. Usefulness of surveillance imaging in patients with head and neck cancer who are treated with definitive radiotherapy. Cancer. 2019;125(11):1823-1829. doi: 10.1002/cncr.31983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roman BR, Patel SG, Wang MB, et al. Guideline familiarity predicts variation in self-reported use of routine surveillance PET/CT by physicians who treat head and neck cancer. J Natl Compr Canc Netw. 2015;13(1):69-77. doi: 10.6004/jnccn.2015.0010 [DOI] [PubMed] [Google Scholar]
- 22.Chera BS, Kumar S, Shen C, et al. Plasma circulating tumor HPV DNA for the surveillance of cancer recurrence in HPV-associated oropharyngeal cancer. J Clin Oncol. 2020;38(10):1050-1058. doi: 10.1200/JCO.19.02444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dwojak SM, Bhattacharyya N. Incremental and comparative health care expenditures for head and neck cancer in the United States. Laryngoscope. 2014;124(10):2305-2308. doi: 10.1002/lary.24795 [DOI] [PubMed] [Google Scholar]
- 24.Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010-2020. J Natl Cancer Inst. 2011;103(2):117-128. doi: 10.1093/jnci/djq495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pagh A, Vedtofte T, Lynggaard CD, et al. The value of routine follow-up after treatment for head and neck cancer. a national survey from DAHANCA. Acta Oncol. 2013;52(2):277-284. doi: 10.3109/0284186X.2012.741324 [DOI] [PubMed] [Google Scholar]
- 26.Langendijk JA, Doornaert P, Verdonck-de Leeuw IM, Leemans CR, Aaronson NK, Slotman BJ. Impact of late treatment-related toxicity on quality of life among patients with head and neck cancer treated with radiotherapy. J Clin Oncol. 2008;26(22):3770-3776. doi: 10.1200/JCO.2007.14.6647 [DOI] [PubMed] [Google Scholar]
- 27.Muzumder S, Srikantia N, Udayashankar AH, Kainthaje PB, Sebastian MGJ, Raj JM. Late toxicities in locally advanced head and neck squamous cell carcinoma treated with intensity modulated radiation therapy. Radiat Oncol J. 2021;39(3):184-192. doi: 10.3857/roj.2020.00913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen AM, Daly ME, Vazquez E, et al. Depression among long-term survivors of head and neck cancer treated with radiation therapy. JAMA Otolaryngol Head Neck Surg. 2013;139(9):885-889. doi: 10.1001/jamaoto.2013.4072 [DOI] [PubMed] [Google Scholar]
- 29.Gillison ML, Chaturvedi AK, Anderson WF, Fakhry C. Epidemiology of human papillomavirus-positive head and neck squamous cell carcinoma. J Clin Oncol. 2015;33(29):3235-3242. doi: 10.1200/JCO.2015.61.6995 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Recommendations for assessment schedule in head and neck cancer.
eTable 2. The overall incidence of event, progression, and primary malignancies in five subgroups.
eFigure 1. Parametric modeling of total events in (A) nasopharynx cancer, (B) HPV-positive oropharynx cancer, (C) HPV-negative oropharynx cancer, (D) hypopharynx cancer, and (E) larynx cancer
eFigure 2. Parametric modeling of local events in (A) nasopharynx cancer, (B) HPV-positive oropharynx cancer, (C) HPV-negative oropharynx cancer, (D) hypopharynx cancer, and (E) larynx cancer.
eFigure 3. Parametric modeling of distant events in (A) nasopharynx cancer, (B) HPV-positive oropharynx cancer, (C) HPV-negative oropharynx cancer, (D) hypopharynx cancer, and (E) larynx cancer.


