Abstract
Background and Purpose —
Clopidogrel is an antiplatelet drug that is metabolized to its active form by the CYP2C19 enzyme. The CHANCE trial found a significant interaction between loss-of-function allele status for the CYP2C19 gene and the effect of dual antiplatelet therapy (DAPT) with aspirin and clopidogrel on the rate of early recurrent stroke following acute TIA/minor stroke. The POINT trial, similar in design to CHANCE but performed largely in North America and Europe, demonstrated a reduction in early recurrent stroke with DAPT compared to aspirin alone. This substudy was done to evaluate a potential interaction between loss-of-function CYP2C19 alleles and outcome by treatment group in POINT.
Methods —
Of the 269 sites in 10 countries that enrolled patients in POINT, 134 sites participated in this substudy. DNA samples were genotyped for CYP2C19 *2, *3 and *17 alleles and classified as being carriers or non-carriers of loss-of-function alleles. Major ischemia consisted of ischemic stroke, myocardial infarction, or ischemic vascular death.
Results —
932 patients provided analyzable DNA. The rates of major ischemia were 6.7% for the aspirin group vs. 2.3% for the DAPT group (HR 0.33; 95%CI, 0.09–1.21; P=0.09) among carriers of loss-of function allele. The rates of major ischemia were 5.6% for the aspirin group vs. 3.7% for the DAPT group (HR, 0.65; 95%CI, 0.32–1.34; P=0.25) among non-carriers. There was no significant interaction by genotype for major ischemia (P=0.36) or stroke (P=0.33).
Conclusions —
This substudy of POINT found no significant interaction with CYP2C19 loss-of-function carrier status and outcome by treatment group. Failure to confirm the findings from the CHANCE trial may be because the loss-of-function alleles tested are not clinically important in this context or because the two trials had differences in racial/ethnic composition. Additionally, differences between the two trials might be due to chance as our statistical power was limited to 50%.
Keywords: Loss-of-function, alleles, polymorphism, CYP2C19, clopidogrel, aspirin
Introduction
Clopidogrel exerts its antiplatelet effect via binding to the P2Y12 platelet membrane receptor. However, clopidogrel itself is a prodrug that must be converted to an active metabolite to achieve this antiplatelet effect. The cytochrome P450 2C19 enzyme (CYP2C19) is a critical enzyme in the conversion of clopidogrel to this active metabolite. A number of polymorphisms in CYP2C19 have been shown to decrease clopidogrel metabolism. Most studied is the CYP2C19 681G>A (referred to as *2) polymorphism, which is present in about 25% of Caucasians, with a higher prevalence in Black/African American and Asian populations.1 Additional loss-of-function alleles (*3, *4, *5, *8) have also been shown to impair clopidogrel metabolism but are very uncommon (< 5% of subjects).2,3 In 162 healthy subjects treated with clopidogrel, the presence of at least one loss-of-function allele was associated with a 30% reduction in plasma concentrations of the active clopidogrel metabolite and significantly less inhibition of platelet aggregation.3
These genetic polymorphisms appear to have a significant clinical impact. In an analysis of 1477 patients with acute coronary syndromes treated with clopidogrel in the TRITON-TIMI 38 study, patients with loss-of-function alleles (> 95% of which were *2 polymorphisms) had a 53% relative increase in the primary endpoint of stroke, MI, or vascular death (12.1% vs. 8.0%, p=0.01) and a tripling of risk of stent thrombosis (2.6% vs. 0.8%, p=0.02).2 Other studies analyzing only the *2 polymorphism have demonstrated similar findings.1,3 However, a nationwide French registry of patients with acute MI found increased vascular risk only in patients with two loss-of-function alleles and not in those with a single loss-of-function allele.4
The Clopidogrel in High-Risk Patients with Acute Nondisabling Cerebrovascular Events (CHANCE) trial found that, relative to aspirin alone, dual antiplatelet therapy with clopidogrel and aspirin reduced the risk of stroke among Chinese patients with TIA or minor ischemic stroke treated within 24 hours after onset of symptoms.5 In CHANCE, 58.8% of participants were carriers of loss-of-function alleles (*2, *3), and there was a significant interaction between loss-of-function allele status and the effects of dual antiplatelet therapy on the rate of new stroke in the first 90 days of follow-up, with the treatment effect of clopidogrel limited to those without a loss-of-function allele.6
The Platelet-Oriented Inhibition in New TIA and Minor Ischemic Stroke (POINT) trial, similar in design to CHANCE but performed largely in North America and Europe, found a similar reduction in major ischemic events with clopidogrel-aspirin combined, but also found an elevated risk of major hemorrhagic events compared to aspirin alone.7 We sought to test for interaction between variations in CYP2C19 and treatment effects in the multinational, multiethnic POINT trial.
Methods
The data that support the findings of the POINT Trial are available on the NINDS Archived Clinical Research Datasets website, https://www.ninds.nih.gov/Current-Research/Research-Funded-NINDS/Clinical-Research/Archived-Clinical-Research-Datasets. POINT was a multicenter, international, prospective, randomized, double-blind trial enrolling patients with minor ischemic stroke (NIH Stroke Scale [NIHSS] score ≤3)8 or high-risk TIA (ABCD2 score ≥4).9 The trial was approved by institutional review boards and ethics committees according to local and national regulatory requirements; all patients provided written informed consent. Patients were randomized to either clopidogrel at a loading dose of 600 mg on day 1, followed by 75 mg per day, plus aspirin at a dose of 50–325 mg per day, or the same dose range of aspirin alone.
The primary efficacy outcome was the composite of new ischemic vascular events: ischemic stroke, myocardial infarction or ischemic vascular death up to 90 days (major ischemia). Major hemorrhage was defined as symptomatic intracranial hemorrhage, intraocular hemorrhage causing vision loss, transfusion of two or more units of red blood cells or an equivalent of whole blood, hospitalization or prolongation of an existing hospitalization, or death due to hemorrhage.7
This pharmacogenetic substudy of POINT was initiated after trial enrollment was underway. The first blood sample for this substudy was obtained after 1,184 patients had been enrolled in the trial. Of the 269 total sites in 10 countries that enrolled participants in the trial, 134 opted to participate in the substudy; 106 of these sites (80%) consented at least one participant for the substudy. Participating sites shipped individual blood samples to LabCorp Central Laboratory Services for processing, and then samples were shipped periodically from LabCorp to Mayo Clinic Florida in batches for molecular genetic analysis.
Genotyping for CYP2C19 *2, *3 and *17 alleles (rs4244285, rs986893, and rs12248560) was performed using a Drug Metabolism Enzyme TaqMan Allelic Discrimination Assay (Assay ID: C 25986767 70,C 27861809 10, and C 469857 10, respectively) on a QuantStudioTM 7 Flex Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s instructions. Genotype calls were made using QuantStudioTM Real-Time PCR Software (v1.1). Metabolizer phenotypes and loss-of-function carrier status were defined as in the CHANCE trial to allow for direct comparison of results.6 Patients with at least two *2 or *3 alleles (*2/*2, *2/*3, or *3/*3) were classified as poor metabolizers, those with one *2 or *3 (*1/*2 or *1/*3) were classified as intermediate metabolizers, and those without a *2, *3, or *17 allele (*1/*1) were classified as extensive metabolizers. Individuals carrying at least 1 *17 allele (*1/*17 or *17/*17) were classified as ultra-metabolizers.
Statistical Analysis
Based on a pre-specified power analysis, our goal was to have a sample size of about 2,534 pharmacogenetic substudy participants, of whom about 1,267 would be randomized to the clopidogrel-aspirin treatment group. We assumed that the event rate would be about 12% in the clopidogrel-aspirin group, yielding 152 ischemic outcome events, and that about 30% of patients in the clopidogrel-aspirin group would carry the CYP2C19 poor metabolizer variants. Were these assumptions to hold, the two-sided log-rank test for equality of survival curves would have at least 80% power to detect a difference between the risks for carriers receiving clopidogrel-aspirin relative to non-carriers receiving clopidogrel-aspirin at the 0.05 alpha level when the true hazard ratio is a relative risk of 1.64.
Baseline characteristics amongst those who did and did not provide a DNA sample were compared. Cox proportional hazard regression was used to model time to event adjusted for allele type (carrier vs. non-carrier), treatment group (clopidogrel-aspirin vs. aspirin), and the interaction of carrier and treatment group. The hazard ratio and 95% confidence intervals of the treatment group by carrier status were estimated from this model. Similar Cox models were fit adjusting for metabolic phenotype, treatment group, and the interaction of phenotype and treatment group. The following outcome events were modelled: Major Ischemic Events, Ischemic Stroke, Stroke, Major Hemorrhage, and Minor Hemorrhage. Data for participants who did not have a 90-day assessment were censored on the 7-day assessment date, on the date of an event, or on the date of death, whichever came latest. For some models, the number of events or stratum size was too small to estimate a hazard ratio for a particular subgroup stratum, and in these cases, the stratum was retained in the model, but the results are noted as NE, not estimable.
The Cox model of major ischemic events adjusting for allele type, treatment group, and the interaction of carrier and treatment was repeated while adjusting for the following suspected significant confounders: age, time from symptom onset to randomization, Black/African American race, those previously taking aspirin, and those with index TIA versus minor ischemic stroke. Tobacco use was added post hoc as a cofounder of interest based on literature suggesting interaction with clopidogrel. 10,11 A model building approach was used, first considering the association of each potential confounder and the outcome and retaining significant covariates in the final adjusted model.
Results
A total of 22.9% (1,119/4,881) of POINT trial participants also consented to participate in this pharmacogenetic substudy; 932 DNA specimens were available for genotyping. The median age of the population was 63 years (IQR, 53–72). A total of 56.9% (530/932) were men. 70.8% (660/932) were of White race; 23.2% (216/932) Black/African American race; and 6% (56/932) other race. The majority of participants had at least one of the following comorbidities: hypertension (69.3%); diabetes (26.2%); current tobacco use (20.4%); heart disease (9.2%). For 56.8% of participants, the qualifying event was minor stroke. A majority of participants were taking aspirin at the time of enrollment (58.9%); few were taking clopidogrel at the time of enrollment (1.8%). Demographic and clinical characteristics of participants in the substudy were comparable to those of non-participants, and among participants in the substudy, demographic and clinical characteristics of those randomized to aspirin alone were comparable to those randomized to dual antiplatelet therapy (Supplemental Table I).
A total of 28.4% (265/932) of participants were carriers of a loss-of-function CYP2C19 allele. Demographic and clinical characteristics of carriers were comparable to those of non-carriers. (Table 1). In particular, the distribution of self-identified race/ethnicity was comparable between the two groups. The carrier rates for loss-of-function alleles based on race were as follows: 26.5% for White; 30.1% for Black/African American; and 44.6% for other race.
Table 1.
Baseline characteristics among carriers and non-carriers of CYP2C19 loss-of-function alleles by treatment group (N=932)
| Characteristics | Loss-of-Function Carriers | Loss-of-Function Non-Carriers | |||||
|---|---|---|---|---|---|---|---|
| Aspirin (n=341) | Clopidogrel-aspirin (n=326) | Total (n=667) | Aspirin (n=134) | Clopidogrel-aspirin (n=131) | Total (n=265) | ||
| Age in years, median (IQR) | 64(54–73) | 61(51–71) | 63(53–72) | 63(54–73) | 64(54–72) | 63(54–72) | |
| Symptoms to randomization in hours, median (IQR) | 7 (5–10) | 7 (5–10) | 7 (5–10) | 7 (5–10) | 7 (5–10) | 7 (5–10) | |
| Male, no. (%) | 74 (55.2) | 73 (55.7) | 147 (55.5) | 197 (57.8) | 186 (57.1) | 383 (57.4) | |
| White, no (%) | 87 (64.9) | 88 (67.2) | 175 (66.0) | 250 (73.3) | 235 (72.1) | 485 (72.7) | |
| Black/AA, no. (%) | 35 (26.1) | 30 (22.9) | 65 (24.5) | 75 (22.0) | 76 (23.3) | 151 (22.6) | |
| Other, no. (%) | 12 (9.0) | 13 (9.9) | 25 (9.4) | 16 (4.7) | 15 (4.6) | 31 (4.6) | |
| Medical History, no. (%) | |||||||
| Heart disease | 13 (9.7) | 9 (6.9) | 22 (8.3) | 27 (7.9) | 37 (11.4) | 64 (9.6) | |
| Congestive heart failure | 2 (1.5) | 5 (3.8) | 7 (2.7) | 5 (1.5) | 9 (2.8) | 14 (2.1) | |
| Atrial fibrillation | 1 (0.7) | 1 (0.8) | 2 (0.8) | 2 (0.6) | 3 (0.9) | 5 (0.8) | |
| Valvular heartdisease | 1 (0.7) | 1 (0.8) | 2 (0.8) | 6 (1.8) | 5 (1.5) | 11 (1.7) | |
| Hypertension | 90 (67.7) | 91 (69.5) | 181 (68.6) | 235 (69.1) | 230 (70.6) | 465 (69.8) | |
| Diabetes mellitus | 44 (32.8) | 32 (24.6) | 76 (28.8) | 88 (25.8) | 81 (25.0) | 169 (25.4) | |
| Smoking Status, no. (%)* | |||||||
| Non-Smoker | 77 (57.4) | 73 (55.7) | 150 (56.6) | 183 (53.6) | 164 (50.3) | 347 (52.0) | |
| Former Smoker | 35 (26.1) | 30 (22.9) | 65 (24.5) | 86 (25.5) | 93 (28.5) | 179 (26.8) | |
| Current Smoker | 21 (15.6) | 28 (21.3) | 49 (18.4) | 72 (21.1) | 69 (21.1) | 141 (21.1) | |
| Index event, no. (%) | |||||||
| TIA | 47 (35.1) | 63 (48.1) | 110 (41.5) | 153 (44.9) | 139 (42.6) | 292 (43.8) | |
| Minor stroke | 87 (64.9) | 68 (51.9) | 155 (58.5) | 188 (55.1) | 187 (57.4) | 375 (56.2) | |
| Prior medication use, no. (%) | |||||||
| Aspirin | 83 (61.9) | 62 (47.3) | 145 (54.7) | 198 (58.1) | 206 (63.2) | 404 (60.6) | |
| Clopidogrel | 1 (0.8) | 0 (0) | 1 (0.4) | 9 (2.6) | 7 (2.1) | 16 (2.4) | |
| Antihypertensives | 47 (51.6) | 48 (50.5) | 95 (51.1) | 124 (53.9) | 113 (53.6) | 237 (53.7) | |
| Statins | 40 (30.1) | 43 (32.8) | 83 (31.4) | 149 (43.7) | 133 (40.8) | 282 (42.3) | |
| Other lipid lowering Rx | 9 (6.8) | 5 (3.8) | 14 (5.3) | 9 (2.6) | 7 (2.1) | 16 (2.4) | |
One patient is missing smoking status
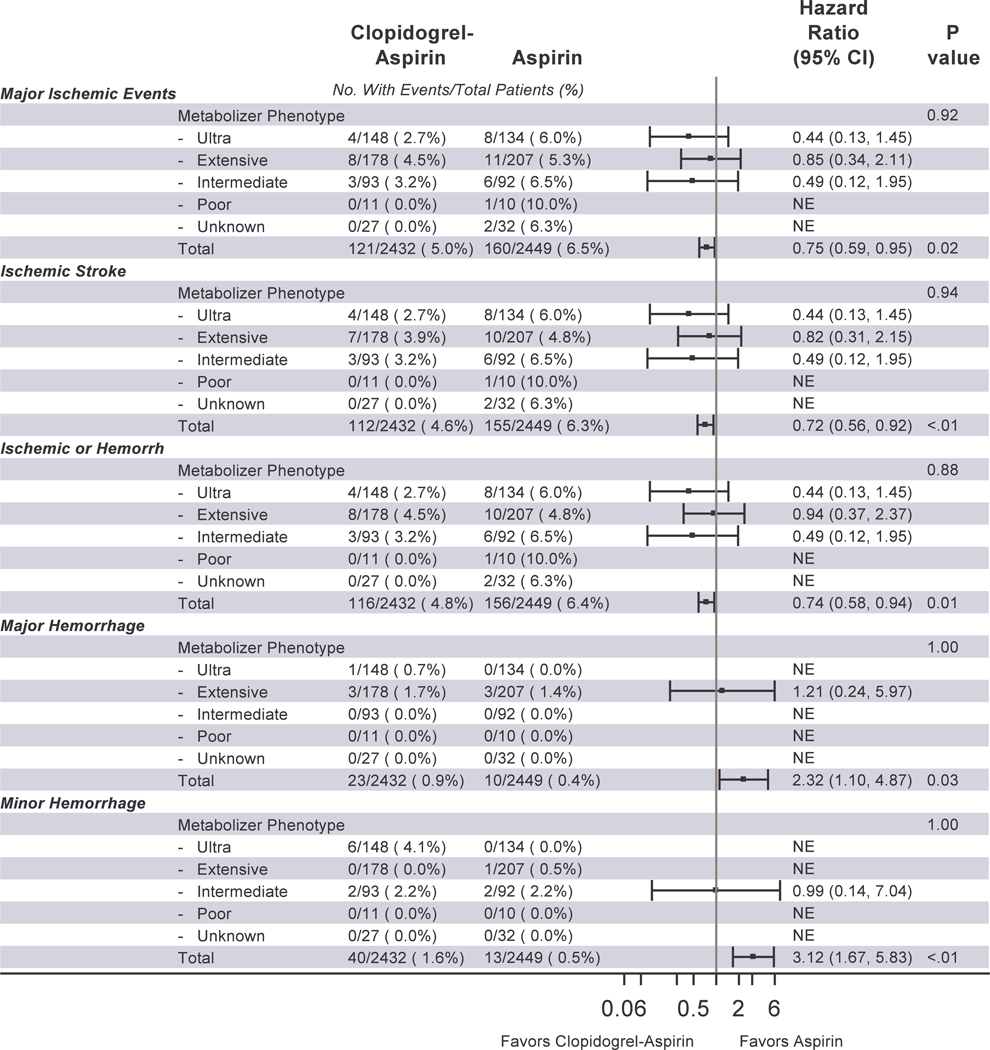
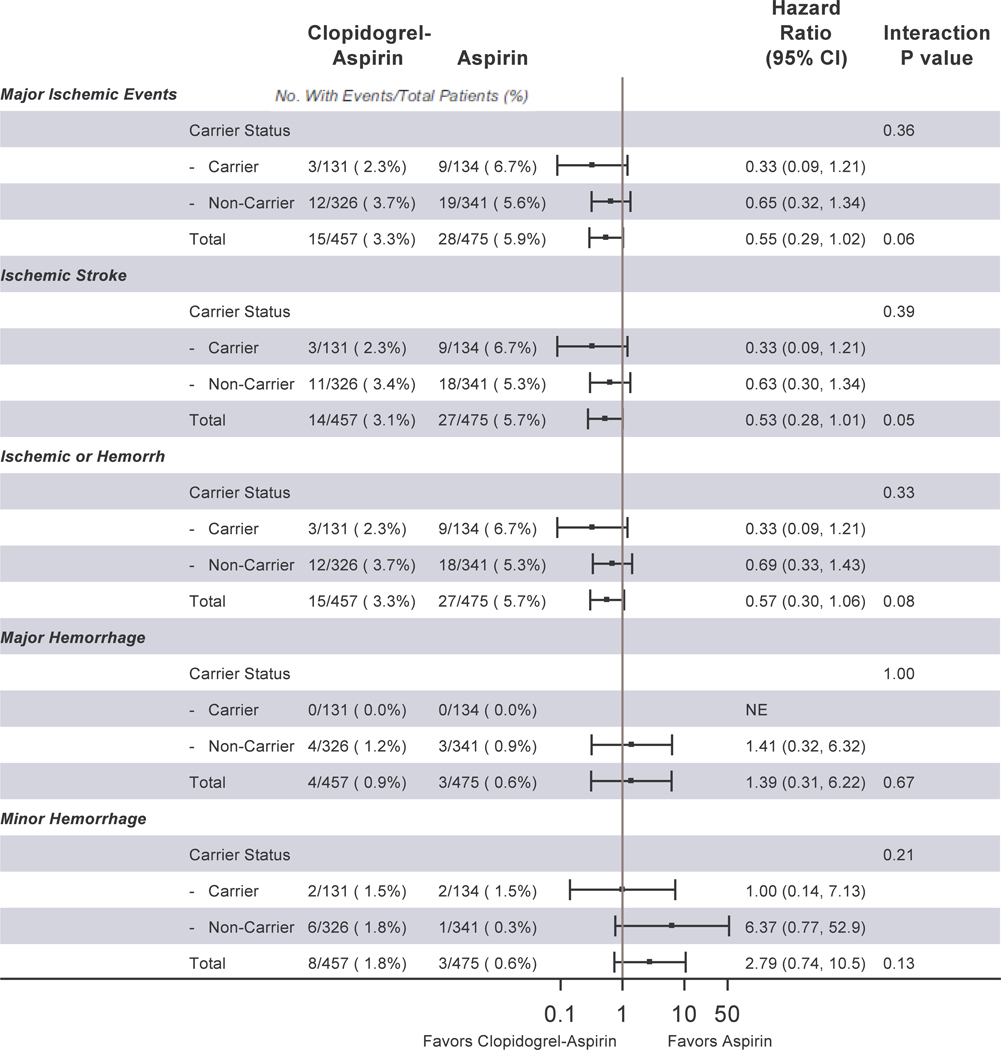
There were 42 major ischemic events overall. Table 2 shows the distribution of major ischemic events by genotype for each of the three CYP2C19 single nucleotide polymorphisms (SNPs). Figure 1 shows the events by metabolizer phenotype. We detected no significant interaction between metabolizer phenotypes and event rates by treatment group for major ischemic events; ischemic stroke; ischemic or hemorrhagic stroke; major hemorrhage; or minor hemorrhage. Figure 2 shows the distribution of clinical events by loss-of-function carrier status. As with metabolizer status, we detected no significant interaction between metabolizer phenotypes and event rates by treatment group for major ischemic events; ischemic stroke; ischemic or hemorrhagic stroke; major hemorrhage; or minor hemorrhage.
Table 2.
Distribution and event rates of major ischemic events by genotype for each of the three CYP2C19 single nucleotide polymorphisms.
| CYP2C19 SNP | Total (N=932) | Aspirin (n=475) | Clopidogrel-Aspirin (n=457) | |||
|---|---|---|---|---|---|---|
| No. with Genotype | Event RateN (%) | No. with Genotype | Event Rate N (%) | No. with Genotype | Event Rate N (%) | |
| CYP2C19*2 (681G>A) | ||||||
| GG | 668 | 31 (4.6%) | 342 | 19 (5.6%) | 326 | 12 (3.7%) |
| GA | 24 | 10 (4.2%) | 123 | 7 (5.7%) | 117 | 3 (2.6%) |
| AA | 24 | 1 (4.2%) | 10 | 1 (10.0%) | 14 | -- |
| CYP2C19*3 (636G>A) | ||||||
| GG | 931 | 42 (4.6%) | 474 | 27 (6.0%) | 457 | 15 (3.2%) |
| GA | 1 | -- | 1 | -- | -- | -- |
| CYP2C19*17 (−806C>T) | ||||||
| CC | 591 | 29 (4.9%) | 309 | 18 (5.8%) | 282 | 11 (3.9%) |
| CT | 306 | 12 (3.9%) | 147 | 8 (5.4%) | 159 | 4 (2.5%) |
| TT | 35 | 1 (2.9%) | 19 | 1 (5.3%) | 16 | -- |
Figure 1.
Major Ischemic Events, Ischemic Stroke, Stroke, Major Hemorrhage, and Minor Hemorrhage in POINT by treatment group and by CYP2C19 metabolizer phenotype (as defined by the CHANCE trial)
Abbreviations: POINT, Platelet-oriented Inhibition in New TIA and minor ischemic stroke trial; CHANCE, Clopidogrel in High-risk patients with Acute Non-disabling Cerebrovascular Events trial; CI, confidence interval; NE, not estimable
Figure 2.
Major Ischemic Events, Ischemic Stroke, Stroke, Major Hemorrhage, and Minor Hemorrhage by treatment group and by CYP2C19 loss-of-function allele carrier status.
Abbreviations: CI, confidence interval; NE, not estimable
A series of subgroup analyses stratified by carrier status is shown in Table 3. There were no significant differences between treatment groups for major ischemic events among either carriers or non-carriers. The treatment group differences remained non-significant after adjusting for race/ethnicity and time from symptom onset (the only covariates related to outcome as determined by the model building procedure). There were no treatment group differences either among carriers or non-carriers for ischemic stroke; ischemic or hemorrhagic stroke; major hemorrhage; or minor hemorrhage. There were no treatment group differences between either carriers or non-carriers for those patients whose qualifying event was TIA, nor was there a difference in those whose qualifying event was minor stroke.
Table 3.
Effect of clopidogrel-aspirin compared with aspirin alone on clinical outcomes stratified by CYP2C19 loss-of-function carrier status.
| Outcomes | Loss-of-Function Carriers | Loss-of-Function Non-Carriers | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Event Rates | Hazard Ratio (95% CI) | P | Event Rates | Hazard Ratio (95% CI) | P | P* | |||||
| Total (n=265) | Aspirin (n=134) | Clopidogrel-Aspirin (n=131) | Total (n=667) | Aspirin (n=341) | Clopidogrel-Aspirin (n=326) | ||||||
| Major Ischemia: ischemic stroke, MI, or ischemic vascular death | 12/256 (4.7%) | 9/134(6.7%) | 3/131 (2.3%) | 0.33 (0.09, 1.21) | 0.09 | 31/667 (4.6%) | 19/341(5.6%) | 12/326(3.7%) | 0.65 (0.32, 1.34) | 0.25 | 0.36 |
| Major Ischemia (adjusted for Black/AA race and time from symptom onset) | 12/256 (4.7%) | 9/134(6.7%) | 3/131 (2.3%) | 0.33 (0.09, 1.22) | 0.10 | 31/667 (4.6%) | 19/341(5.6%) | 12/326(3.7%) | 0.64 (0.31, 1.33) | 0.23 | 0.38 |
| Major Ischemia amongst TIA Cohort (n=402) | 3/110 (2.7%) | 2/47 (4.3%) | 1/63(1.6%) | 0.35 (0.03, 3.88) | 0.39 | 10/292 (3.4%) | 6/153(3.9%) | 4/139(2.9%) | 0.73(0.21, 2.60) | 0.63 | 0.60 |
| Major Ischemia amongst Minor Stroke Cohort (n=530) | 9/155 (5.8%) | 7/87 (8.1%) | 2/68(2.9%) | 0.36(0.07, 1.71) | 0.20 | 21/375 (5.6%) | 13/188(6.9%) | 8/187(4.3%) | 0.60(0.25, 1.46) | 0.26 | 0.56 |
| Major Ischemia amongst current smokers (n=190) | 1/49 (2.0%) | 1/21 (4.8%) | 0/28 (0.0%) | NE | 0.99 | 4/141 (2.8%) | 2/72 (2.8%) | 2/69 (2.9%) | 1.05 (0.15, 7.44) | 0.96 | 0.99 |
| Major Ischemia amongst Non-smokers/Former smokers (n=741) | 11/215 (5.1%) | 8/112 (7.1%) | 3/103 (2.9%) | 0.40 (0.11, 1.50) | 0.17 | 27/526 (5.1%) | 17/269 (6.3%) | 10/257 (3.9%) | 0.61 (0.28, 1.32) | 0.21 | 0.59 |
| Ischemic Stroke | 12/265 (4.5%) | 9/134 (6.7%) | 3/131 (2.3%) | 0.33 (0.09, 1.21) | 0.09 | 29/667 (4.3%) | 18/341 (5.3%) | 11/326 (3.4%) | 0.63 (0.30, 1.34) | 0.23 | 0.39 |
| Ischemic or Hemorrhagic Stroke | 12/265 (4.5%) | 9/134 (6.7%) | 3/131 (2.3%) | 0.33 (0.09, 1.21) | 0.09 | 30/667 (4.5%) | 18/341 (5.3%) | 12/326 (3.7%) | 0.69 (0.33, 1.43) | 0.32 | 0.33 |
| Major Hemorrhage | 0/265 (0%) | 0/134 (0.0%) | 0/131 (0.0%) | NE | 1 | 7/667 (1.0%) | 3/341 (0.9%) | 4/326 (1.2%) | 1.41 (0.32, 6.32) | 0.65 | 1 |
| Minor Hemorrhage | 4/265 (1.5%) | 2/134 (1.5%) | 2/131 (1.5%) | 1.00 (0.14, 7.13) | 1 | 7/667 (1.0%) | 1/341 (0.3%) | 6/236 (1.8%) | 6.37 (0.77, 52.9) | 0.09 | 0.21 |
NE = Not estimable
P-value of the interaction between carrier and treatment group
Discussion
In this substudy of the POINT trial, we did not find a significant interaction between clopidogrel metabolizer status, defined by three CYP2C19 alleles, and differences in rates of major ischemic and hemorrhagic events between aspirin-only and clopidogrel-aspirin treatment groups. The expected loss-of-function effects on rates of major ischemic and hemorrhagic events between aspirin-only and clopidogrel-aspirin combination treatment groups was not observed. This contrasts with CHANCE, where a substantial treatment effect of clopidogrel was seen in non-carriers of loss-of-function alleles, but no effect was seen in carriers.6
There are a number of possible explanations for the conflicting results between this study and the results seen in CHANCE. First, lack of statistical power is one possible explanation for our failure to detect differences in response to treatment based on carrier status. Our study included only 932 patients, compared to the 2,933 that were successfully genotyped in CHANCE. With less than half of the planned sample size and also a lower than expected event rate, the potential for a Type II error is quite high. Recognizing the limitations of post hoc power calculations, we estimate that the power of our study to have been limited to 50%. It is of interest to note that the point estimates from the current investigation were in the direction of demonstrating that patients with the loss-of-function allele had greater relative efficacy of dual antiplatelet therapy, counter to what one would expect pharmacologically. However, the direction of bleeding events was concordant with the expected greater bleeding with dual antiplatelet treatment. That said, given the small number of outcome events in the four groups (those with and without loss-of-function allele receiving single and dual-antiplatelet therapy) one can only confidently say the current study is consistent with the null finding that there was no evidence of an interaction between the loss-of-function allele and dual-antiplatelet therapy.
In addition, the prevalence of loss-of-function allele carriers in CHANCE was 58.8% compared to 28.2% in POINT, which would further reduce the ability to detect a genotype-phenotype interaction. This lower prevalence of loss-of-function carrier status observed in POINT is likely explained by the race/ethnicity differences in study populations, as loss-of-function alleles are more common in Asian populations.12 Thus a limitation of this study is the lack of statistical power to fully exclude the possibility of a clinically significant effect of CYP2C19 genotype on response to acute treatment with dual antiplatelet therapy following TIA or minor ischemic stroke. This lower rate would reduce the ability to detect a genotype-phenotype interaction.
The differences in loading doses of clopidogrel between CHANCE and POINT may be important. In CHANCE, a loading dose of 300 mg was used, compared to a 600 mg loading dose in POINT. A study of platelet aggregation before and after patients received either 600 mg or 300 mg of clopidogrel in addition to aspirin (81 to 325 mg) found that platelet aggregation non-responsiveness was significantly lower after 600 mg compared to the 300 mg dose.13 As most events in both POINT and CHANCE occurred very early in the trials (within the first 48 hours), differential efficacy of the loading dose may have substantially impacted the overall trial results. The higher loading dose in POINT may have negated any significant effect of loss-of-function alleles on early ischemic outcomes. The risk of hemorrhagic complications appeared to relatively constant over the 90-day follow-up period in the POINT trial, suggesting that at a maintenance dose of clopidogrel, CYP2C19 variant status was not clinically important.
Another potential explanation is differences in rates of tobacco use in the two study populations. In POINT, about 20% of participants reported tobacco use, with just over twice that rate reported in CHANCE. Cigarette smoking is known to increase the metabolic activation of clopidogrel by inducing a different cytochrome p450 complex isoenzyme and there is evidence suggesting a reduced or complete lack of benefit of clopidogrel among nonsmokers.10,11 Therefore, the lower sample size of the POINT substudy, the lower prevalence of loss-of-function allele carriers in the POINT population, the higher loading dose used in POINT, and the lower prevalence of tobacco use in POINT may have reduced the ability to identify an interaction between loss-of-function carrier status and outcomes.
In conclusion, we did not find that previously identified gene variants that influence clopidogrel metabolism impacted the efficacy or safety of clopidogrel combined with aspirin for minor stroke or TIA. Although this study was specifically designed to test the findings of the CHANCE trial with regard to response to dual antiplatelet therapy and CYP2C19, our results may be explained by differences in the prevalence of loss-of-function allele carriers and smokers in the study population and the higher loading dose of clopidogrel used in POINT. Other factors including CYP3A4 and CYP1A2 may also be important in metabolizing the prodrug to its active form.14 Thus a more comprehensive genotyping approach may be required to fully assess a possible pharmacogenetic effect in POINT.
Supplementary Material
Acknowledgements
We wish to express our sincere gratitude to the patients and their families who participated in the substudy.
Sources of Funding
The POINT study was funded by the U.S. National Institute of Neurological Disorders and Stroke, National Institutes of Health.
Disclosures
Drs. Meschia and Ross reported funding from the University of California, San Francisco. Drs. Elm, Lindblad, Barsan, Meurer and Johnston, and Ms. Farrant and Ms. Zurita, reported grants from the National Institutes of Health/National Institute of Neurological Disorders and Stroke. Dr. Easton received support from the NIH/NINDS as co-Principal Investigator on the POINT Trial (NCT00991029). POINT received non-financial support for free study drug and placebo from Sanofi. Dr. Easton received research grant support as a consultant from Boehringer Ingelheim for planning and conducting the RESPECT ESUS trial (NCT02239120). Dr. Johnston received institutional support only from AstraZeneca and Sanofi.
Footnotes
Clinical Trial Registration — http://www.clinicaltrials.gov. Unique identifier: NCT00991029.
References
- 1.Shuldiner AR, O’Connell JR, Bliden KP, Gandhi A, Ryan K, Horenstein RB, et al. Association of cytochrome p450 2c19 genotype with the antiplatelet effect and clinical efficacy of clopidogrel therapy. JAMA. 2009; 302:849–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mega JL, Close SL, Wiviott SD, Shen L, Hockett RD, Brandt JT, et al. Cytochrome p-450 polymorphisms and response to clopidogrel. N Engl J Med. 2009; 360:354–362 [DOI] [PubMed] [Google Scholar]
- 3.Collet JP, Hulot JS, Pena A, Villard E, Esteve JB, Silvain J, et al. . Cytochrome p450 2c19 polymorphism in young patients treated with clopidogrel after myocardial infarction: A cohort study. Lancet. 2009; 373:309–317 [DOI] [PubMed] [Google Scholar]
- 4.Simon T, Verstuyft C, Mary-Krause M, Quteineh L, Drouet E, Meneveau N, et al. Genetic determinants of response to clopidogrel and cardiovascular events. N Engl J Med. 2009; 360:363–375 [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, Wang Y, Zhao X, Liu L, Wang D, Wang C, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med. 2013; 369: 11–9. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y, Zhao X, Lin L, Li H, Johnston SC, Lin Y, et al. Association between CYP2C19 loss-of-function allele status and efficacy of clopidogrel for risk reduction among patients with minor stroke or transient ischemic attack. JAMA. 2016; 316:70–8. [DOI] [PubMed] [Google Scholar]
- 7.Johnston SC, Easton JD, Farrant M, Barsan W, Conwit RA, Elm JJ, et al. Clopidogrel and aspirin in acute ischemic stroke and high-risk TIA. N Engl J Med. 2018; 379: 215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lyden P, Brott T, Tilley B, Welch KM, Mascha EJ, Levine S, et al. Improved reliability of the NIH Stroke Scale using video training. Stroke. 1994; 25: 2220–6 [DOI] [PubMed] [Google Scholar]
- 9.Johnston SC, Rothwell PM, Nguyen-Huynh MN, Giles MF, Elkins JS, Bernstein AL, et al. Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet. 2007; 369: 283–92. [DOI] [PubMed] [Google Scholar]
- 10.Gurbel PA, Nolin TD, Tantry US. Clopidogrel efficacy and cigarette smoking status. JAMA. 2012; 307:2495–6. [DOI] [PubMed] [Google Scholar]
- 11.Gurbel PA, Bliden KP, Logan DK, Kereiakes DJ, Lasseter KC, White A, et al. The influence of smoking status on the pharmacokinetics and pharmacodynamics of clopidogrel and prasugrel: the PARADOX study. J Am Coll Cardiol. 2013;62:505–12. [DOI] [PubMed] [Google Scholar]
- 12.Jang JS, Cho KI, Jin HY, Seo JS, Yang TH, Kim DS, et al. Meta-analysis of cytochrome P4502C19 polymorphism and risk of adverse clinical outcomes among coronary artery disease patients of different ethnic groups treated with clopidogrel. Am J Cardiol. 2012; 110: 502–8. [DOI] [PubMed] [Google Scholar]
- 13.Gurbel PA, Bliden KP, Hayes KM, Yoho JA, Herzog WR, Tantry US. The relation of dosing to clopidogrel responsiveness and the incidence of high post-treatment platelet aggregation in patients undergoing coronary stenting. J Am Coll Cardiol. 2005; 45: 1392–6. [DOI] [PubMed] [Google Scholar]
- 14.Ford NF. The metabolism of clopidogrel: CYP2C19 is a minor pathway. J Clin Pharmacol. 2016; 56: 1417–83. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.