Abstract
Sinus tachycardia (ST) is ubiquitous but its presence outside of normal physiological triggers in otherwise healthy individuals remains a commonly encountered phenomenon in medical practice. In many cases, ST can be readily explained by a current medical condition that precipitates an increase in the sinus rate, but ST at rest without physiological triggers may also represent a spectrum of normal. In other cases, ST may not have an easily explainable cause but may represent serious underlying pathology and can be associated with intolerable symptoms. The classification of ST, consideration of possible etiologies, as well as the decisions of when and how to intervene can be difficult. ST can be classified as secondary to a specific, usually treatable, medical condition (e.g. pulmonary embolism, anemia, infection, or hyperthyroidism) or be related to several incompletely-defined conditions (e.g. inappropriate sinus tachycardia, postural tachycardia syndrome, mast cell disorder, or post-COVID syndrome). While cardiologists and cardiac electrophysiologists often evaluate patients with symptoms associated with persistent or paroxysmal ST, an optimal approach remains uncertain. Due to the many possible conditions associated with ST, and an overlap in medical specialists who see these patients, the inclusion of experts in different fields is essential for a more comprehensive understanding. This manuscript is unique in that it was composed by international experts in Neurology, Psychology, Autonomic Medicine, Allergy and Immunology, Exercise Physiology, Pulmonology and Critical Care Medicine, Endocrinology, Cardiology, and Cardiac Electrophysiology in the hope that it will facilitate a more complete understanding and thereby result in the better care of patients with ST.
Keywords: sinus tachycardia, postural orthostatic tachycardia syndrome, inappropriate sinus tachycardia, autonomic dysfunction
Introduction
Despite the frequency of sinus tachycardia and the wide range of clinical presentations, no controlled clinical trials or evidence-based guidelines currently provide direction for initial, appropriate management. Due to the many possible conditions associated with sinus tachycardia, and an overlap in medical specialists who see these patients, the inclusion of experts in different fields is essential for a comprehensive manuscript. The international experts chosen to author this manuscript were selected not only for their expertise in their respective fields, but also to represent the wide range of knowledge from specialties including Neurology, Psychology, Allergy & Immunology, Pulmonology & Critical Care, Endocrinology, Exercise Physiology, Cardiology, and Cardiac Electrophysiology with the goal of creating a “first of its kind” comprehensive multidisciplinary manuscript on sinus tachycardia.
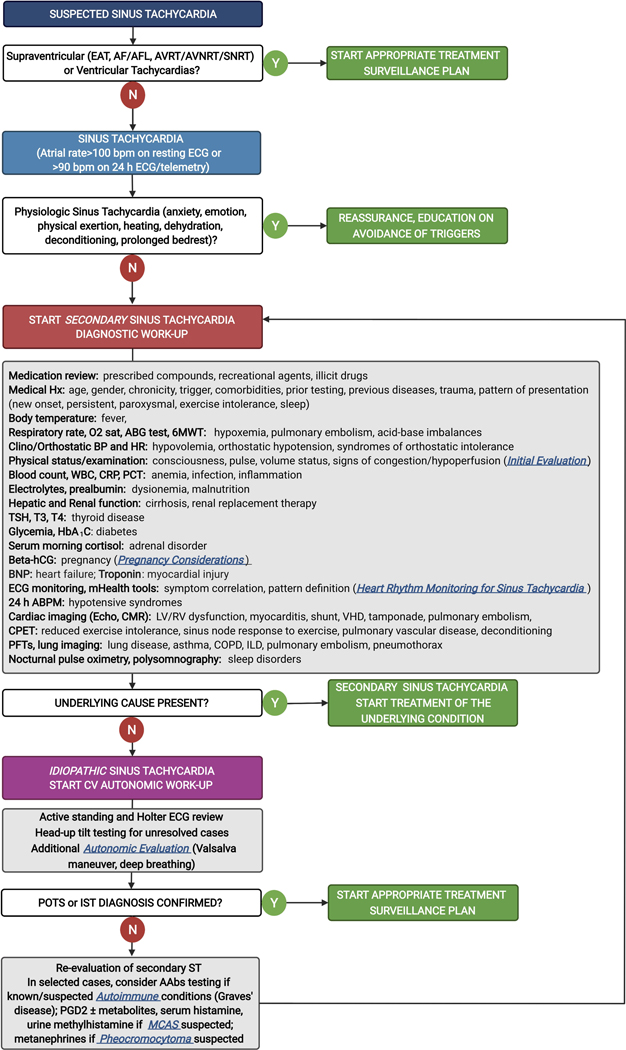
As described here, sinus tachycardia represents a spectrum of problems from a normal variant to a chronic ongoing autonomic condition to an underlying inflammatory or infectious process, among others. Sinus tachycardia may be the cause for adverse outcomes or associated with symptoms; alternatively, it may be a solitary, singular nonspecific “barometer” of one of a multitude of underlying problems. Thus, the initial assessment requires a careful evaluation of the clinical presentation, the length and frequency as well as the intermittent nature of sinus tachycardia and its potential triggers, and careful consideration of one of a number of medical issues described here before attempting to focus specifically on “treatment” of the sinus tachycardia itself (Figure 1).
Figure 1. Evaluation of sinus tachycardia.
AAbs, autoantibodies; ABG, arterial blood gas; ABPM, ambulatory blood pressure monitoring; AF, atrial fibrillation; AFL, atrial flutter; AVNRT, atrioventricular node re-entry tachycardia; AVRT, atrioventricular re-entry tachycardia; BNP, B-type natriuretic peptide; CMR, cardiovascular magnetic resonance; COPD, chronic obstructive pulmonary disease; CPET, cardiopulmonary exercise testing; CRP, C-reactive protein; EAT, ectopic atrial tachycardia; hCG, human chorionic gonadotropin; ILD, interstitial lung disease; IST, inappropriate sinus tachycardia; MCAS, mast cell activation syndrome; LV, left ventricle; PCT, procalcitonin; PFTs, pulmonary function tests; PGD2, prostaglandin D2; POTS, postural orthostatic tachycardia syndrome; RV, right ventricle; SNRT, sinus node re-entry tachycardia; ST, sinus tachycardia; TSH, thyroid-stimulating hormone; VHD, valvular heart disease; WBC, white blood cells; 6MWT, 6-minute walking test.
Sinus tachycardia is the most common arrhythmia. However, it can be a normal, physiologically beneficial, response to physical and psychological stresses driven by an increase in sympathetic activation, circulating catecholamines, and/or a decrease in parasympathetic tone.1 Like fever, sinus tachycardia can be an appropriate response to illness and various disease states and is thus a non-specific clinical sign. While sinus tachycardia is an often expected and normal physiological response, it can exist in the absence of evident intrinsic or extrinsic causes. As such, sinus tachycardia can pose a diagnostic challenge and be a clinical conundrum difficult for electrophysiologists to manage. Differentiating “appropriate” sinus tachycardia from the underpinnings of an ongoing medical problem, an autonomic disturbance, or a channelopathy can be difficult.
For some individuals, sinus tachycardia can be associated with debilitating symptoms affecting functionality, quality of life and well-being, but the relationship of symptoms to tachycardia often remains obscure. This concern is potentiated by population-based studies indicating that baseline heart rate elevations are associated with increased risk of cardiovascular disease, heart failure, cancer, and mortality.2–5 Clinicians are thus faced with the reality that sinus tachycardia, though often physiologic, may be neither benign nor clinically insignificant.
Here, we first define sinus tachycardia and outline its epidemiology, then deconstruct sinus tachycardia by delineating possible mechanisms and propose a practical clinical classification. We consider an initial and subsequent in-depth evaluation of sinus tachycardia to help formulate management strategies with interventions that may improve outcomes based on available evidence.
Definition and Epidemiology
“Tachycardia” carries a textbook definition of a sinus rate >100 bpm.6 However, the recognition of sinus tachycardia is dependent upon what the true “normal” rate is in the general population. The concept that a “normal rate” must be 60–100 bpm is, by most accounts, a construct “for convenience and for uniformity”7 as determined by expert consensus.8 Data influencing this consensus arose from published reports of heart-rate distributions indicating a bell-shaped curve with a rightward skew. One of the earliest published reports of heart rate distributions was a 1940s study of 1000 young aviators showing that heart rates within two standard deviations from the mean ranged from 40–85 bpm for males aged 20–30 and 50–100 bpm for males aged 30–409. Spodick et al., studying 500 asymptomatic healthy adults above age 50, reported the two standard deviations upper limit of heart rates was 93 and 95 bpm for men and women, respectively.10
Contemporary studies of mixed gender confirm this bell-shaped pattern while also showing a clear inflection point of the distribution skew at 85–90 bpm.11 Heart rates above the standard definition of normal are common across large populations with higher heart rates in women compared to men and this can vary by age.12 The percentage of heart rates above 90–100 bpm in population studies ranges from 2.1 to 4.6%.11,13–15 In a large French study of 19,386 healthy subjects (12,123 men) aged 40–69, 3.3% of men and 4.6% of women had rates >100 bpm.13 In a middle-aged Norwegian population of 24,489 subjects (11,773 men), 2.1% of men and 3.2% of women had heart rates >100 bpm.15 The significance of these findings is not fully delineated, as this contains a mixture of patients with and without symptomatology. From (or in spite of) these descriptions, the “normal rate” continues to be attributed to heart rates below 100 bpm.
This description of “normal rate” is overly simplistic and fails to capture attenuation of resting heart rates as people age, gender differences and other influences. “Intrinsic” (denervated) heart rate studies provide insights into these effects. Chemical and surgical denervation unmask the intrinsic rate of the sinus node, resulting in an increase in the resting heart rate to around 90 bpm and above 16–19. In a 1970’s study of chemical denervation (atropine and beta-blockade) in 432 subjects, there was a progressive decrease in the mean intrinsic heart rate with increasing age. The mean rate for those aged 18–30 was >100 bpm and decreased to <100 bpm for those over age 45. Nevertheless, sinus rates still exceeded 100 bpm in >15% of those over age 45.18
These findings raise the question of whether a resting sinus rate >100 bpm is abnormal. The vast majority of patients with sinus tachycardia are asymptomatic. On the other hand, numerous population-based studies indicate that baseline heart rate elevations are associated with increased risk of incident heart disease, cerebrovascular disease, and death.2–5,20–22
In a Norwegian study of 379,843 subjects, there was a positive association between heart rate and all-cause mortality, cardiovascular death, ischemic heart disease, and strokes, but these associations were significantly reduced in an adjusted analysis suggesting that heart rate was not associated independently.23 In the Henry Ford Exercise Test Project, men with a resting heart rate >90 bpm had an increased risk for mortality even after adjusting for fitness (HR 1.22, 95% CI 1.10 – 1.35). There was no association with mortality in women, nor in individuals for cardiovascular events, myocardial infarction after adjustment for fitness.24 In contrast, in the FINRISK study, there was an independent relationship between resting heart rate and cardiovascular disease in both men and women (HR men 1.19 [95% CI 1.11–1.28], HR women 1.21 [95% CI 1.09–1.34]), though adjustment attenuated the cardiovascular risk relationship.25 In the ARIC study, time updated increases in heart rate (5 bpm increase from the preceding visit) were associated with increased risk of death (1.12; 95% CI, 1.10–1.15; P<0.001). There was also a 9% higher risk of myocardial infarction (95% CI, 4%–13%) and a 6% higher risk of stroke (95% CI, 1%–11%).22
Despite these mixed findings, in a meta-analysis of the large population studies, significant increases in relative risk (RR) per 10 bpm increase in resting heart rate were seen with coronary heart disease (RR 1.07, 95% CI: 1.05–1.10), sudden cardiac death (RR 1.09, 95% CI: 1.00–1.18), heart failure (RR 1.18, 95% CI: 1.10–1.27), total stroke (RR 1.06, 95% CI: 1.02–1.10), total cancer (RR 1.14, 95% CI: 1.06–1.23) and all-cause mortality (RR 1.17, 95% CI: 1.14–1.19).2 Whether elevation in resting heart rate predicts future disease or is a manifestation of underlying sub-clinical pathology has not yet been defined fully, though all data point to the fact that patients with a faster heart rates are at higher risk of adverse outcomes.
For patients with underlying heart disease, including active ischemic heart disease and heart failure, sinus tachycardia is associated with an increased risk of morbidity and mortality. Post-myocardial infarction, a statistically significant relationship between heart rate reduction and mortality has been demonstrated consistently. In a meta-analysis of beta-blocker and calcium channel antagonist trials in post-myocardial infarction patients, each 10 bpm reduction in heart rate was associated with 30% reduction in relative risk of cardiac death(P<0.001).26 Similar findings have been seen in heart failure trials. In the INTRINSIC RV trial, each increase in the mean heart rate by 10 bpm was associated with a hazard ratio of 1.34 (p<0.0001, 95% CI 1.19–1.50). Significantly, 20.9% of patients with a mean HR of >90 bpm experienced heart failure hospitalization or death versus 5.9% of patients with heart rates <75 bpm (p <0.0001)27. These data were confirmed in a Portuguese study of 718 patients with heart failure showing that patients with a resting heart rate >70 bpm had a 51% higher risk of death, transplant or ventricular assist device implantation compared to those with a heart rate <70 bpm (HR 1.51, 95% CI 1.95–2.17).28
Insights into whether this increased heart rate is a marker of increased disease or a direct mediator has been offered by the SHIFT trial. In this trial, ivabradine given to lower the heart rate was associated with a reduction in the composite endpoint of cardiovascular death or heart failure hospitalization (HR 0.82, 95% CI 0.75–0.90). The subgroup of patients with a heart rate >87 bpm had the greatest reduction in heart rate with ivabradine and the largest reduction in the composite endpoint (HR 0.75, 95% CI 0.67–0.85). This subgroup analysis showed that ivabradine had little effect in patients with baseline heart rates <75 bpm, suggesting that the benefit of ivabradine in these patients is the result of heart rate reduction and not another secondary effect.29 This same finding of a direct benefit from heart rate attenuation was shown in a meta-analysis of beta-blocker use in heart failure trials.30 These data support the contention that rapid heart rates are directly deleterious in patients with underlying heart disease.
Symptomatic sinus tachycardia syndromes are rare and are represented by Postural Orthostatic Tachycardia Syndrome (POTS) and Inappropriate Sinus Tachycardia (IST). POTS has been shown to have a prevalence of 0.2% in cohort studies31–33 and predominately affects females at a 5:1 ratio.31,34–36 The vast majority are young, with a mean age of presentation between 15–3034,36. The prevalence of IST has been much less well defined. The only estimate of prevalence to date is the OPERA (Oulu Project Elucidating Risk of Atherosclerosis) study that identified an IST prevalence of 1.16%, though this group included symptomatic and asymptomatic patients. In that study, 57% of IST patients were female with a mean age of 47.11 This study failed to capture, by design, the population of IST patients most often seen in clinic (that is, young patients in their 20s), and, as such, gives only limited insight into this disease. At the same time, though IST is commonly attributed to the young, it can affect elderly patients.37 Given the paucity of investigations and data, the true prevalence and natural history of patients with IST remains uncertain.
Classification
Sinus tachycardia can be a normal physiological response to internal and/or external stresses but is excessive when unnecessary physiologically or when exceeding normal bounds. However, there is a wide variation in baseline heart rates by age, sex, mental/emotional state and physical status. Sinus tachycardia rarely sustains for prolonged periods of time but, if it does, it is likely that there is some pathophysiological underpinning.
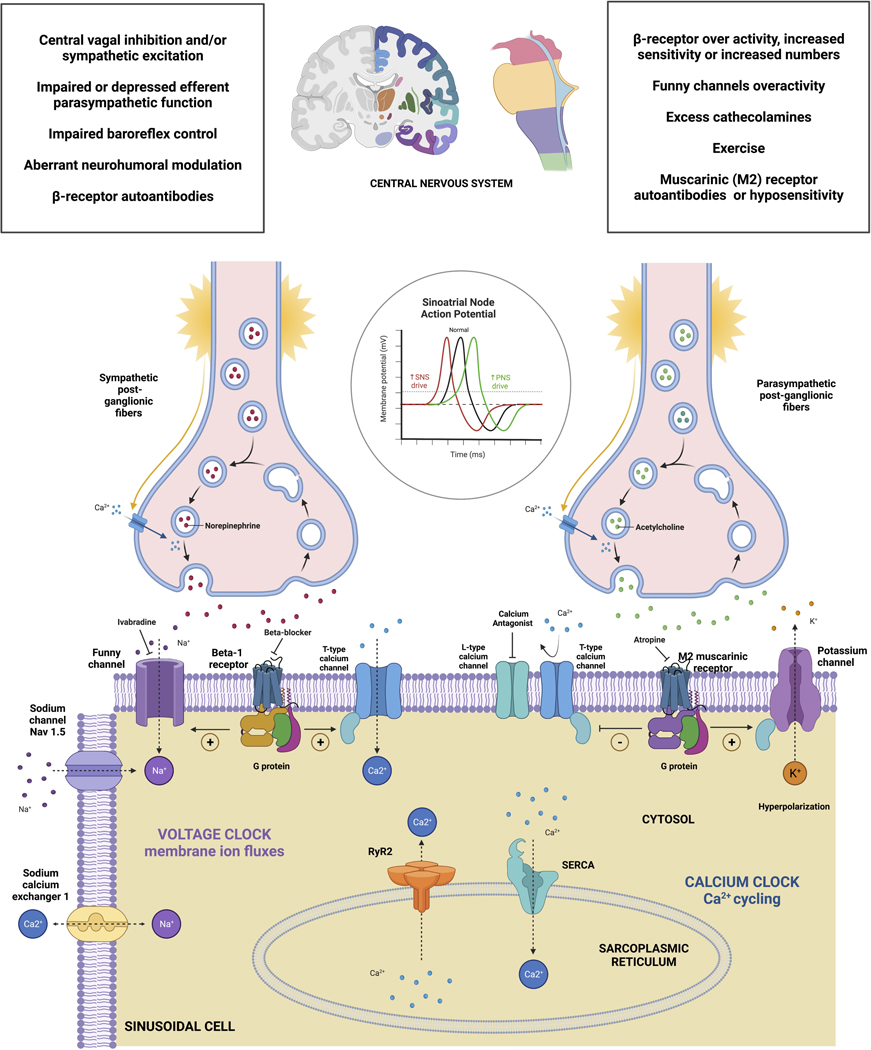
Sinus tachycardia is due to rapid activation of the sinus node produced by a multiplicity of interrelated channels, and receptors (Figure 2). Two main regulating currents are the “calcium clock” and the If (“funny”) current.38 Autonomic influences modulate the intrinsic rate and sympathetic activation or parasympathetic inhibition39 can cause sinus tachycardia. Thus, sinus tachycardia may be due to tonic, or phasic, changes in the autonomic nervous system, circulating neurohormones or due to an underlying pathology of regulating currents of the sinus node.
Figure 2. Autonomic control of the sinoatrial node activity and modulators of autonomic inputs.
Created with Biorender.com
Concerning classification, several questions should be addressed: Is sinus tachycardia a normal physiological response or due to an underlying disorder? What is the pattern of sinus tachycardia presentation? What is the reproducibility and circumstances under which sinus tachycardia occurs? Which symptoms can be attributed to this condition?
Of great importance, is assessment of the relationship of sinus tachycardia to potentially causal circumstances and to its relationship to other commonly observed hemodynamic quantities, such as, blood pressure shifts and respirations. Sinus tachycardia that persists and increases may indicate congestive heart failure, or an evolving underlying infectious or inflammatory condition. Mast cell disorder may present with paroxysms of sinus tachycardia.40 Triggers may be cause for only transient fluctuations in sinus rate including anemia, and pulmonary embolus.
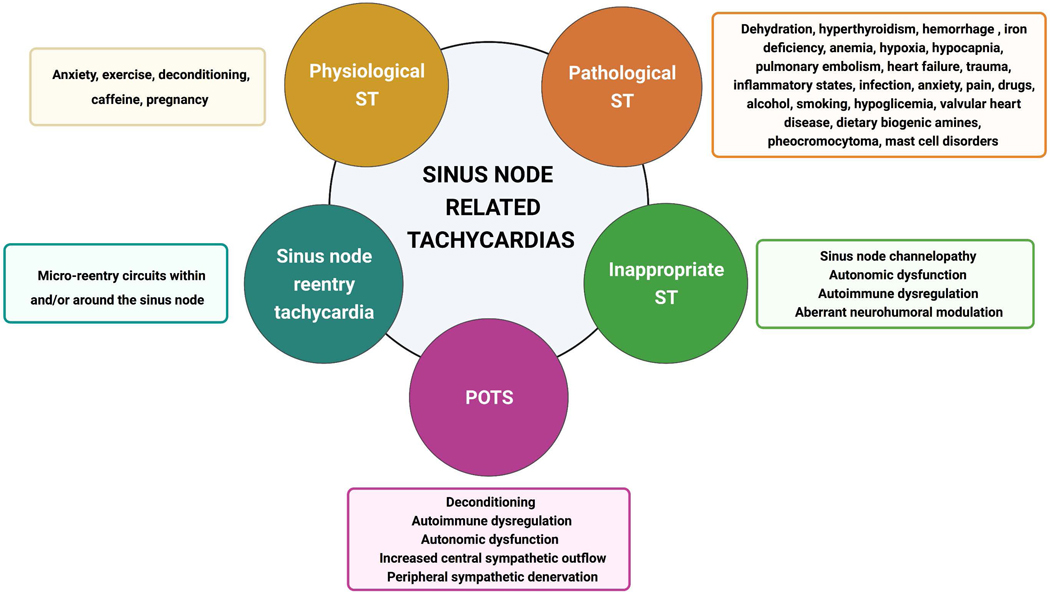
We propose a classification to help determine etiology and, potentially mechanism of sinus tachycardia including physiological sinus tachycardia, pathological sinus tachycardia, sinus node reentry tachycardia (SNRT) (rare), inappropriate sinus tachycardia (IST) and postural orthostatic tachycardia syndrome (POTS) (Figure 3) (Table 1).
Figure 3. Classification of Sinus Tachycardia.
Created with Biorender.com
Table 1.
Categories of Sinus Tachycardia
| Characteristics | Diagnostic Criteria | Therapies | |
|---|---|---|---|
| Physiologic ST | See text | Assess for possible underlying conditions | |
| Pathologic ST | See text | Treatment dependent upon underlying etiology | |
| Sinus Node Reentry | Micro-reentry circuit (rare) | Monitoring for most patients, otherwise medical therapy or catheter ablation | |
| Inappropriate ST | Symptoms along with tachycardia, which can occur even while supine | Resting HR > 100 bpm, Average HR > 90 bpm in 24 hours | Pharmacologic therapy, Invasive management (catheter ablation) |
| Postural Tachycardia Syndrome | Symptoms along with a postural HR increase into tachycardic range | Persistent HR increase ≥ 30 bpm with upright position (≥ 40 bpm in teenage patients), or to ≥120 bpm, in the absence of orthostatic hypotension | Integrated treatment plan, Lifestyle changes, Exercise Rehabilitation, Pharmacologic therapy |
ST = sinus tachycardia. HR = heart rate
Further, we recommend a temporal pattern/episode duration-based classification of sinus tachycardia including new-onset (sudden or gradual), persistent (with/without exacerbation, with/without circadian variation), and paroxysmal (reproducible or sporadic exacerbations) forms (Table 2).
Table 2.
Chronicity of Sinus Tachycardia
| ST pattern | Definition | Clinical features and possible etiologies |
|---|---|---|
| NEW ONSET | New ST episode, of either sudden or gradual onset, usually caused by a precipitating factor or an obvious clinical condition | More frequently expected (proportionate) physiological or pathological ST |
| PERSISTENT | Long-lasting episode of ST |
|
| PAROXYSMAL | Reproducible or sporadic exacerbations of ST starting and ceasing abruptly |
For new-onset sinus tachycardia, a precipitant is likely. Precipitants include well-defined and detectable clinical conditions: dehydration, hyperthyroidism, hemorrhage, iron deficiency anemia, hypoxia, hypocapnia41, pulmonary embolus, heart failure, trauma, inflammatory state42 or infection. For persistent sinus tachycardia, without obvious explanatory trigger, and associated symptoms, such as, deconditioning, consider IST. However, an occult tumor, infection, or underlying cardiovascular disease are possible and should be excluded.
Sinus tachycardia can present with variations in heart rate with rest, sleep and exercise in one of several patterns.43
Reproducible paroxysms of sinus tachycardia with position may be due to a form of orthostatic intolerance (as seen with POTS and OH). Such causal factors have been found in some but not all patients with the often regarded “idiopathic tachycardias”: POTS and IST. Common temporal presentations include recurrent episodes of sinus tachycardia that occur in a pattern consistent with orthostatic positional changes in the case of POTS, and reproducible and often consistent sinus tachycardia at rest, or with minimal disturbance in the case of IST, for which, several clinical presentations have been described. These clinical presentations include persistent elevation in heart rate, spikes in heart rate with minimal activity and heart rate changes with and without exercise.
It should be kept in mind that sinus tachycardia paroxysms may be due to anxiety as in panic disorder44 (epinephrine-mediated), norepinephrine reuptake inhibition45 (a very rare condition; norepinephrine-mediated) or drug intake (e.g. beta-agonists in asthma). Further, sporadic changes in heart rate may be present under conditions of physical or mental stress, and due to sleep disorders or are due to ingestion of stimulant drugs.
Heart Rhythm Monitoring for Sinus Tachycardia
Technological innovations embrace a variety of options to facilitate accurate data collection over select time intervals using a multiplicity of leads potentially to better understand if a tachycardia is indeed sinus tachycardia and to better capture the relationship to activities and symptoms. 46 Conventional diagnostic methods including ambulatory ECG devices (with continuous or intermittent recording abilities) and implantable loop recorders (allowing continuous, long-term ECG monitoring and recording of ECG information either on demand or when an event is sensed by an automated algorithm) are complemented by mobile health technologies, enabling users to monitor, collect and share physiological multiparametric data, including photoplethysmographic and accelerometry-derived parameters.
Selection of an appropriate tool to investigate sinus tachycardia is guided by clinical evaluation. Effective monitoring can provide accurate characterization of the mode and rapidity of onset and offset, duration and temporal patterns in relation to symptoms and activities, and a relationship to position, exercise and other clinical circumstances (e.g., hypoglycemia). The most appropriate method of monitoring must consider the expected frequency of symptoms (daily, weekly, monthly, or more sporadic), diagnostic power, accuracy, reproducibility, discrimination of arrhythmia types, and risk stratification accuracy, with consideration about cost-effectiveness, patient acceptance, degree of automaticity, local availability, and investigator’s experience.47
Documentation that a tachycardia is indeed sinus tachycardia becomes the first step in evaluation but discrimination of non-sinus tachycardias from sinus tachycardia involves more than careful scrutiny of the P-wave morphology. Analysis of mode of onset and termination (abrupt versus gradual), detection of premature atrial complexes at the initiation or termination of tachycardia, temporal patterns of sinus tachycardia (new-onset, paroxysmal or persistent) (Table 1), circadian profile of heart rate and its variability, correlation between patient symptoms and burden of sinus tachycardia, and relationship with exercise and patient position, are indeed important clues captured by ECG monitoring. A 24 hour or 48 hour Holter ECG monitoring can reveal short and frequent episodes of sinus tachycardia, or IST.48
IST is less affected by rest and can be difficult to distinguish from for instance deconditioning which may also show rapid rise in sinus rates with minimal activity. Further, IST may present with various patterns, and at least two distinct populations can be identified based on diurnal heart rate variation which may be useful to classify pathophysiology and ideally provide guidance to specific treatment. An exaggerated increase in heart rate during the morning, might reflect dysfunction extrinsic to the sinus node due to excessive adrenergic surge or hypersensitivity.43
The 2015 Heart Rhythm Society Expert Consensus Statement gives a class IIb recommendation for 24-hour Holter monitor, exercise testing, autonomic and tilt table testing for confirmation of both IST and POTS diagnosis.49 The 2020 Canadian Cardiovascular Society Position Statement on POTS does not recommend the routine use of ECG monitoring although it may be considered to rule out POTS-mimicking conditions (Strong Recommendation, Moderate-Quality Evidence).50
New wearable systems, incorporating specific sensors that acquire biological parameters, besides ECG signals, such as, respiratory frequency, peripheral oxygen saturation, arterial pulse pressure, physical activity, position and others, are transforming conventional ECG monitoring systems into “ambulatory polygraphic monitoring”.51 It is noteworthy that, despite widespread clinical use, there is a surprising lack of research about ECG monitoring data and very little evidence about established reference ranges for common arrhythmias (including sinus node related tachycardias), as well as their prognostic significance and gender differences.52,53 Importantly, although Holter and event monitoring, implantable loop recorders and smart-watches, are widely used, we have no databank of normal monitoring to compare against and to determine how much abrupt change in heart rate or even sinus tachycardia can be considered within the normal range and may be dependent upon features unrelated to any specific underlying clinical circumstance, autonomic disorder or medical condition.
Autonomic Regulation
The cardiac autonomic nervous system exerts exquisite control over the richly innervated sinoatrial node (Figure 2).54,55 Stress to the cardiovascular system may result, via a complex hierarchy of interacting feedback loops in the cardiac neuraxis, to physiologic sinus tachycardia to increase cardiac output. It is the baroreceptors that detect a decreasing BP sending signals afferently to the nucleus tractus solitarious, nucleus ambiguous and caudal ventrolateral medulla. Efferent signals back to the sinoatrial node via the vagus nerve are the pathway to sinus tachycardia. This may be more than a reflexive physiologic tachycardia.
This occurs in volume depletion, infection, pregnancy, heart failure or circulatory or hemorrhagic shock, or in an exaggerated response, as in POTS. In the case of IST, while elevated sinus node automaticity is believed by some to contribute55,56, impaired sympathovagal balance has also been associated with the disorder55.
The contribution of autonomic balance to IST has been evaluated in studies of patients with IST. These studies demonstrated, through pharmacologic autonomic blockade, that sympathetic tone is elevated in IST.57,58 In particular, Morillo et al studied 6 patients with IST using a battery of autonomic testing including heart rate variability analysis, cardiovagal reflex testing using cold face testing, and autonomic blockade with intravenous propranolol and atropine.57 These investigators showed that while patients had higher intrinsic heart rates indicative of increased sinus node automaticity, they had depressed vagal outflow and increased β-adrenergic sensitivity. In contrast, a more recent study of 8 patients with IST compared with 48 patients with POTS and 17 control patients did not show elevated intrinsic heart rates following autonomic blockade to suggest increased sinus node automaticity.58
Sympathovagal imbalance may result from autonomic dysfunction. Perturbations in autonomic regulation that may manifest as IST include diabetic autonomic neuropathy,59 baroreflex impairment,60 β-adrenergic receptor autoantibodies,61 and hyperadrenergic states such as pheochromocytoma62. Data on autoantibodies are not definitive.
Cardiac interventions may either cause or ameliorate IST. An unintended consequence of catheter ablation of arrhythmias may be IST, as several reports describe IST attributed to vagal denervation during ablation for other arrhythmias.63–66 Furthermore, targeted ablation of intrinsic cardiac neurons supplying the sinoatrial node has been evaluated for sinus node dysfunction and vasovagal syncope.67,68 Conversely, while sinus node modification likely also results in denervation, more recent targeted, adjunctive ablation of epicardial fat pads housing cardiac ganglia has been evaluated.69,70 However, as a similar approach has been evaluated for both sinus bradycardia and tachycardia, the mechanism remains unclear and deserving of further study.
Initial Evaluation
The initial evaluation of sinus tachycardia includes a thorough history and symptom assessment, medication review (including illicit drugs), vital sign assessment, physical examination and electrocardiogram (ECG) (Figure 1). The clinical presentation of sinus tachycardia includes temporally associated symptoms, frequency, initiation, and termination of episodes. Common symptoms include, but are not limited to, dizziness, lightheadedness, palpitations (rapid heartbeat), fatigue, anxiety, pain, and weakness71. Particular attention should be made in reviewing medications and supplements that may result in sinus tachycardia either directly (e.g., stimulants) or indirectly such as by causing volume depletion or hypotension (e.g., diuretics). Vital signs and physical exam findings may be within normal limits or may include abnormal findings such as orthostatic vital signs, resting HR >100 bpm, low resting oxygen saturation, pallor, rapid and regular heart rate by palpation of pulse and cardiac auscultation, pleuritic discomfort upon inspiration, stigmata of heart failure (e.g. peripheral edema, decreased breath sounds or pulmonary crackles), infectious signs, and enlarged thyroid on palpation.55
An electrocardiogram (ECG) should be obtained to assess P-wave morphology and to exclude alternative supraventricular tachycardias, such as, atrial tachycardia and sinus node reentry tachycardia.72 Additionally, an ECG may reveal electrocardiographic patterns that may help identify a precipitant for sinus tachycardia. Laboratory assessment to evaluate for explainable causes of sinus tachycardia, such as anemia, hyperthyroidism, hypoglycemia, acute heart failure, dehydration, electrolyte derangements, adrenal dysfunction, drug use (e.g., amphetamines) is recommended.55,72
Autonomic function testing may be valuable in differentiating IST from various forms of autonomic dysfunction, such as, cardiac autonomic neuropathy or autonomic failure.73,74 Cardiac testing, such as, echocardiography, exercise treadmill testing, and electrophysiology studies may provide additional information regarding cardiac structure and function and chronotropic response to exercise72. Prolonged rate and rhythm assessment can be conducted with internal or external ambulatory rhythm monitoring55.
Autonomic Evaluation
Main Points:
The autonomic nervous system exerts significant control over the sinoatrial node, and autonomic dysregulation has been implicated in conditions such as inappropriate sinus tachycardia.
While the evaluation of the autonomic nervous system is still being further explored and has yet to be standardized, current modalities include heart rate variability to paced breathing, Valsalva testing, and quantitative sudomotor axon reflex testing.
The Tilt Table Test has long been a cornerstone of assessing symptoms of orthostatic intolerance and can be particularly useful for sinus tachycardia; it can help differentiate syndromes of sinus tachycardia from other conditions such as vasovagal syncope and orthostatic hypotension with compensatory tachycardia, and it can be combined with ancillary testing such as 12-lead ECG monitoring, measurement of catecholamines, and electroencephalography.
The role of autonomic testing in patients presenting with sinus tachycardia has not been well established. A number of tests have been used to evaluate sympathetic adrenergic, parasympathetic and sympathetic cholinergic function in patients presenting with sinus tachycardia of unknown etiology. The rationale for testing the autonomic nervous system is that several potential mechanisms of autonomic dysregulation have been implicated in IST. These mechanisms include decreased parasympathetic activity, diminished muscarinic receptor function, impaired efferent vagal activation, increased sympathetic activity and baroreceptor dysfunction.55,75 The overlapping features of IST with POTS also suggest autonomic evaluation is helpful in differentiating between these two disorders in some instances where bedside orthostatic vital signs are not able to differentiate. Through assessment of cardiovagal parasympathetic function, cardiovascular sympathetic adrenergic function and sympathetic cholinergic sudomotor function, the role of the autonomic system in the pathophysiology of IST and the contribution to reported symptoms can be evaluated.76,77
In preparation for autonomic testing, and to truly determine the association with IST, patients should stop medications that impact autonomic function for 5 half-lives (if it is safe to do so). Practically, we recommend holding medications for 48 hours before testing, if possible. Autonomic test results will be influenced by a medications across different classes including those with anticholinergic effects (such as tricyclic antidepressants or serotonin-norepinephrine reuptake inhibitors), antihistamines, decongestants, medications with antimuscarinic effects, anti- or pro-hypertensive medications, medications that influence volume status (volume expanders such as fludrocortisone or volume contractors such as furosemide), analgesics (opioids) and anti-inflammatory agents.77,78 Some exceptions are made for medications that could potentiate medical complications, or create withdrawal effects if removed (e.g. levothyroxine, opioids), or if the autonomic testing performed to assess the response on medication or is repeated to assess the effect of a new treatment.77 Parasympathetic autonomic function is typically measured by heart rate variability to paced breathing, a Valsalva maneuver, or the heart rate response to active standing.
Heart rate variability to paced breathing:
As a measure of parasympathetic function, deep breathing with maximum effort is typically performed for 9 cycles, with each cycle paced at 5-seconds for inhalation and 5-seconds for exhalation to assess heart rate variability (HRV). Normal HRV in response to deep breathing is an increase of heart rate during inhalation and a decrease during exhalation, also known as the respiratory sinus arrhythmia. 77,79,80 HRV data are analyzed and compared to normative data that are adjusted for age and gender.77 Heart rate variability declines with age. It is important to measure respiratory effort in order to provide context to the results. Inadequate respiratory effort will result in decreased heart rate variability, which cannot be differentiated from disease. Thus, measurement of respiratory capacity and effort can provide a means to effectively assess respiratory effort. Automated devices that record heart rate variability generally do not analyze respiratory effort and therefore cannot appropriately interpret the results. Detailed spectral analysis of the frequencies of heart rate during rest have been performed exhaustively across many healthy and disease states, but the results have not generally been included in routine clinical testing because they do not often provide additional information relevant to a single patient.81 Heart rate variability might be tested in patients with POTS or IST to determine if there is evidence of parasympathetic dysfunction as the cause of the condition.
Valsalva maneuver:
The Valsalva maneuver is a quantitative assessment of both sympathetic adrenergic and parasympathetic function.77,81,82 The Vasalva maneuver is a deep inspiration, followed by forced exhalation into a tube with a pre-specified resistance of 30–40 mmHg for 15 seconds and is used to assess the cardiovagal heart rate response and the sympathetic adrenergic blood pressure response.77 The heart rate and blood pressure changes during the Valsalva maneuver occur in 4 phases.83 In phase I, the blood pressure increases at the beginning of the expiratory effort due to the sudden increase in intra-thoracic and intra-abdominal pressure leading to compression of the great vessels. This is followed by phase II, which includes both an early and late stage.
During the early stage of phase II the blood pressure decreases steadily secondary to the sustained compression of the vena cava leading to a diminished venous return to the heart, with resulting reduced preload, reduced stroke volume and therefore diminished cardiac output. The fall in blood pressure triggers a compensatory heart rate increase from withdrawal of cardiovagal tone. It also activates baroreceptors in the carotid and aortic arch causing an efferent sympathetic discharge, which leads to increased total peripheral resistance and ultimately a recovery of the blood pressure marking late Phase II.
Phase III is a sudden drop in blood pressure due to a decrease in intra-thoracic pressure once the expiratory effort is terminated. Phase IV is the blood pressure overshoot and depends on cardiac adrenergic tone. Measurement of the changes in blood pressure (through beat-to-beat blood pressure monitoring) provide an assessment of sympathetic adrenergic function.
The Valsalva maneuver can measure parasympathetic function, reported as the Valsalva ratio.77,81 The ratio is measured as the ratio of the maximum heart rate during the maneuver over the minimum heart rate during the 30 seconds after the maneuver. The results are compared to normative data established by age and sex.77,81 As with other measures of autonomic function, there is an age related decline in the Valsalva ratio.
A larger Valsalva ratio indicates the normal functioning of the parasympathetic sympathetic system, while a lower ratio might indicate damage to the parasympathetic nervous system. A few caveats should be noted in the interpretation of the heart rate response to Valsalva. The stimulus for tachycardia during a Valsalva maneuver is the drop in blood pressure. If there is no drop in blood pressure during phase II because of inadequate respiratory effort, there will not be a tachycardia. Automated devices typically do not measure expiratory pressure or beat to beat blood pressures, preventing appropriate interpretation of the heart rate response to a Valsalva maneuver.
Active stand:
An additional test of parasympathetic and sympathetic adrenergic function is the active standing test.83 The active stand is performed after at least 5 minutes baseline recording of blood pressure and heart rate in the relaxed supine resting position. After being supine for 5 minutes, the patient quickly moves to the standing position (within 3 seconds if possible) and stands in an immobile, stable position for 5 minutes. This test causes a shift in blood volume of 300–800 milliliters from the central intravascular compartment to the peripheral vascular system and stimulates both parasympathetic withdrawal and sympathetic activiation.84
With the sudden transfer of blood to the peripheral vascular system there is a rapid increase in the heart rate that peaks at about 3 seconds. This sudden, and transient, tachycardia is mediated by inhibition of vagal tone and is referred to as the exercise reflex. After the initial increase in heart rate, there is a further baroreflex mediated increase in heart rate from the 3 to12 second period due to the gravitationally induced fall in blood pressure. Blood pressure and heart rate achieve a new baseline after approximately 30 seconds. As blood pressure stabilizes, there is a transient period of increased blood pressure with a baroreflex mediated bradycardia that occurs approximately 30 seconds after initiation of standing.84
Measurement of parasympathetic function occurs by taking the ratio of the heart rate at approximately the 15th second (during the period of tachycardia) divided by the heart rate at the 30th second (during the period of bradycardia). Heart rates are measured using the RR interval. The resulting number is known as the 30:15 ratio. The larger the value for the 30:15 ratio, the more ‘intact’ the parasympathetic nervous system is. Abnormalities that can be detected on active standing include evidence of parasympathetic dysfunction, orthostatic hypotension, or postural tachycardia (as seen in POTS).
Tilt table testing:
The tilt table test is used to assess the hemodynamic response to postural change, similar to that seen with active standing. Postural change from supine to upright position leads to venous pooling, a process where 500–1000 ml of blood shift from the thorax into the peripheral vascular compartments (the legs and the abdominal and pelvic regions).77,85,86
Unlike active standing, the tilt table test occurs with the patient supine in a tilted position at 60–80 degrees. This tilted supine position reduces the effects of the muscle pump on blood pressure.85,86 During the tilt table test, both parasympathetic and the sympathetic adrenergic systems are stimulated by gravitational stress. Normally, upon tilt up there is a transient blood pressure drop due to venous pooling, which then triggers the baroreceptors in the carotid and aortic arch to signal a compensatory heart rate increase through withdrawal of parasympathetic tone and an increase in blood pressure due to an increase in sympathetic adrenergic tone.85,87 The following conditions can be identified during tilt table testing: neurally mediated syncope, orthostatic hypotension, postural orthostatic tachycardia syndrome and delayed orthostatic hypotension.88
The tilt table test (TTT) has been a longstanding cornerstone in the assessment of orthostatic intolerance89 and is particularly useful in evaluating sinus tachycardia. One important advantage is the ability to measure concomitant blood pressures, for example, in the case of orthostatic hypotension resulting in a compensatory sinus tachycardia, or sinus tachycardia prior to a vasodepressor response. The TTT can thereby facilitate an accurate diagnosis with precise attribution of symptoms to blood pressure versus heart rate changes, especially for patients with episodes of transient loss of consciousness in addition to sinus tachycardia on cardiac rhythm monitoring.
Many centers use continuous blood pressure monitoring during the TTT instead of, or in supplement to, intermittent arm cuff measurement. This was previously done via invasive radial artery cannulation, but newer methods using beat-to-beat finger plethysmography have largely replaced that. In most TTT protocols, the duration of upright position is at least 10 minutes, and in many centers longer protocols are used, such as 45 minutes90,91.
While the rapidity and pattern of the heart rate increase can be somewhat variable, most centers use an increase of at least 30 bpm within 10 minutes of upright tilt (without drop in blood pressure) to be diagnostic for POTS in patients aged 19 or older, though a longer duration to onset has also been described49,92. Importantly, patients with IST may also display a postural increase in heart rate; mixed forms of IST/POTS are not uncommon74. Ancillary testing can be combined with TTT93. Examples include intermittent 12-lead ECG monitoring to assess for atrial tachycardia mimicking sinus tachycardia94, measurement of plasma catecholamines for suspected hyperadrenergic POTS95, concomitant EEG monitoring to evaluate episodes of unresponsiveness, and provocative maneuvers such as sublingual nitroglycerin or carotid massage in patients who also report episodes of transient loss of consciousness.
Quantitative sudomotor axon reflex test (QSART):
QSART testing evaluates the peripheral sympathetic cholinergic system. Using iontophoresis (the process of pulling a charged medication into the skin via a local electrical current) of acetylcholine, nerves that surround the sweat glands are activated and result in a spreading region of sweat production. The activation of adjacent sweat glands is measured through changes in humidity detected in a multi-compartmental capsule. The measurement of this ‘axon-reflex’ mediated sweating provides an accurate measure of local peripheral sympathetic cholinergic function.96
Standard QSART recording sites are the ventral forearm, lateral proximal leg, medial distal leg and dorsum of the foot. In a normal QSART sweating increases from baseline levels with a delay of 1–2 minutes after the initiation of iontophoresis. The delay in sweat production is due to the axon-reflex dependent spreading of sweat production which is followed by a steadily increasing sweat production 5 minutes after stimulation, followed by a slow decrease in sweating during recovery. Abnormal responses are reported as an increase, a decrease or absent sweat response. As an example, patients with a length-dependent neuropathy (such as in diabetes) would loss of sweating in the distal recording sites.97,98 Patients with POTS may show a distal, or non-length dependent loss of sweat production.
Selected Etiology: Autoimmune
Main Points:
A mechanistic relationship between autoantibodies or autoimmune conditions causing sinus tachycardia is not established.
Circulating autoantibodies affecting cardiovascular G-protein coupled receptors may be found in patients with inappropriate sinus tachycardia and POTS.
Although autoantibodies activating adrenergic receptors or blocking muscarinic receptors may initiate sinus tachycardia, such autoantibodies are also detected in normal healthy controls without sinus tachycardia.
The role of autoimmune mechanisms to cause sinus tachycardia is uncertain.
The hypothetical autoimmune etiology of sinus tachycardia has traditionally focused on sympathetic and parasympathetic innervation of sinus node. However, other mechanisms of action can affect sinus rate. These effects may be exerted directly on sinus node or operate distally and be manifest via the more traditional neurocardiac axis. Most notable, and defined, is Graves’ hyperthyroidism. The presence of thyroid stimulating immunoglobulins cause elevated thyroid hormones, especially T3, altered K+ channel activity and sinus tachycardia. This tachycardia, subtle or obvious, generally responds to reduction of T399. Circulating autoantibodies to beta-1 and 2 adrenergic and the M2 receptors can modulate activity of targeted cardiac receptors and may be related to a reported persistence of sinus tachycardia following normalization of thyroid hormone100.
There is circumstantial evidence that some patients with POTS or IST have an autoimmune basis for their condition. Emerging evidence suggests that some patients with IST have circulating autoantibodies to the beta-adrenergic receptor.101 Autoantibodies directed to the alpha 1 and beta-1 and beta-2 adrenergic receptors were described using bioassay and specific receptor transfected reporter-cell-based assays102–104. Additional autoantibodies have been identified in some subjects to the angiotensin AT1R and to the muscarinic M2R and M3R105,106. These autoantibodies may be non-specific as they can exist in the general population. Induced alpha-1 and beta-1 adrenergic receptor autoantibodies in rabbits resulted in an exaggerated pulse response to 60° tilt in these animals. Blockade using a proteolysis resistant decoy peptide mimicking putative epitopes for these autoantibodies returned the postural pulse response to normal, supporting a pathophysiological role for autoantibodies in this animal model107.
Use of activity assays is limiting since role of autoantibodies involves not on their orthosteric (direct) effect on the target receptor but rather on their variable allosteric (indirect) effect of decreasing the impact of the normal ligand (both norepinephrine and Ang II) binding on their target receptor; while the beta-1 and beta-2 adrenergic autoantibodies affect endogenous nor-epinephrine response on these receptors103. Autoantibodies to M2 and M3 receptors may also exist. The hypothesis that POTS and IST has an autoimmune basis requires further evidence from adequate animal models and subsequent human studies.
The relationship of several well-known autoimmune diseases includes Sjogren’s, lupus108, and other autoimmune conditions109 but more multi-center large-scale studies are needed to confirm these associations.110 Some subjects with idiopathic postural hypotension and tachycardia harbor autoantibodies in variable degrees, but any relationship of these autoantibodies with tachycardia remains speculative.111
Selected Etiology: Mast Cell Disorders
Main Points:
Mast cells may inappropriately release chemical mediators that elicit an array of symptoms. In particular, symptoms include rapid heart beating, allergic reactions, skin rashes, and gastrointestinal complaints, and is termed ‘mast cell activation disorder’ (MCAD).
MCAD is uncommon but may cause a clinical picture resembling postural orthostatic tachycardia syndrome (POTS).
MCAD symptoms tend to occur episodically (‘flares’) and differs from mastocytosis which tends to be more persistent and characterized by elevated tryptase levels.
No single laboratory finding is diagnostic of MCAD, but an elevated circulating prostaglandin (D2 or F2alpha), especially when accompanied by increased histamine marker (plasma histamine, urine methylhistamine), is highly suggestive. Tryptase is only infrequently elevated.
Mast cell cells synthesize various vasoactive mediators and release them into the circulation potentially initiating sinus tachycardia.112,113 These include histamine stored in mast cell granules and released immediately following activation, as well as prostaglandin D2, cysteinyl leukotrienes and platelet activating factor synthesized de novo from membrane lipids within about 5–10 minutes after activation. Mast cells can synthesize cytokines, including TNF-alpha that can cause tachycardia by an undefined by likely inflammatory mechanism.112,114
Histamine, the best known and most studied mast cell mediator can drop systolic and diastolic blood pressure, increase cardiac output and catecholamines, and cause sinus tachycardia115. All 4 known histamine receptors are expressed in the heart; chronotropic effects of histamine have been linked to H2 receptor activation116. Therefore, the mechanism of histamine-induced tachycardia probably involves a compensatory mechanism to vasodilation, associated with increased catecholamine response as well as a direct chronotropic effect.
Tachycardia is a cardinal manifestation of anaphylaxis, associated with systemic mast cell activation.117 Anaphylaxis may be due to identifiable IgE-mediated triggers (e.g., food, drug or venom allergy), IgE independent triggers (e.g. exercise, radiocontrast) or an idiopathic cause. Tachycardia in anaphylaxis, associated with hypotension, flushing, itching, hives, angioedema and respiratory compromise is episodic and transient resolving once treated. Therefore, tachycardia occurring in isolation without other symptoms should not be directly attributed to mast cell activation. Furthermore, drugs used to treat anaphylaxis, such as, epinephrine and glucocorticoids can themselves cause sinus tachycardia.
Mastocytosis, a clonal disorder of the hematopoietic mast cell progenitor associated with a somatic gain of function mutation in KIT gene)118, is characterized by abnormal proliferation and accumulation of mast cells in tissues, such as skin and bone marrow, and is frequently associated with mast cell activation symptoms and sinus tachycardia. It has cutaneous and systemic forms, both of which are associated with mast cell activation symptoms119,120.
DIAGNOSIS:
Systemic mastocytosis should be diagnosed by tissue (often bone marrow) biopsy. Lifetime risk of anaphylaxis in mastocytosis is significantly increased in adults (approximately 49%). 120,121 In addition, patients may have milder episodes not reaching the severity of anaphylaxis, such as, flushing and tachycardia associated with lightheadedness. Therefore, in patients presenting with unexplained episodic mast cell activation symptoms and tachycardia, mastocytosis should be considered in differential diagnosis.
Patients with suspected mastocytosis should have a thorough skin examination to look for typical maculopapular lesions of mastocytosis (urticaria pigmentosa). A tryptase measurement is recommended. Tryptase is a mediator relatively specific to the mast cell lineage. Normal median tryptase level is approximately 5 ng/ml, while a baseline tryptase level >20 ng/ml often indicates increased mast cell burden and is a minor diagnostic criterion for mastocytosis. Tryptase is a good marker of mast cell activation and anaphylaxis. A tryptase level measured within 4 hours of a suspected mast cell activation episode can help differentiate mast cell activation from other causes of tachycardia and hypotension. A formula of acute event related tryptase increase of 20% from baseline level +2 ng/ml has been proposed as a clinically significant confirmation of mast cell activation121,122.
Apart from well-defined scenarios of mastocytosis and anaphylaxis, mast cell activation syndrome has been used to describe patients with episodic mast cell activation who may or may not meet the definition of anaphylaxis122–124. Proposed diagnostic criteria for mast cell activation syndrome include: (i) episodic symptoms consistent with mast cell activation in at least 2 of the following organ systems: cardiovascular (including tachycardia), respiratory, gastrointestinal, skin and nasoocular,; (ii) documented elevation in mast cell mediators during a symptomatic phase (preferably serum tryptase, but may also include less standardized tests of urinary histamine, PGD2 and LTC4 metabolites); and (iii) a positive response of symptoms with drugs targeting mast cell mediators (such as H1 and H2 receptor antagonists, leukotriene blockers, cromolyn).
An alternative broader diagnostic scheme125 can lead to overdiagnosis of mast cell activation syndrome as has been described in case reports of POTS and hypermobile Ehlers-Danlos syndrome. However, objective documentation of mast cell activation by morphology or biochemical markers are missing in these reports.126,127 One study compared POTS patients with mast cell activation symptoms (flushing, shortness of breath, headache, lightheadedness, excessive diuresis, and gastrointestinal symptoms) and elevated urine N-methylhistamine to POTS patients with episodic flushing but normal urine methylhistamine127. Heart rate responses to upright posture were similar in both groups and were increased similarly as compared to healthy controls. No differences were found in catecholamine levels between POTS with or without MCA symptoms. The MCA plus POTS group had higher systolic blood pressures compared with POTS and normal control groups. Many patients with POTS and dysautonomia may have similar symptoms encountered in MCAS, including flushing, lightheadedness, and gastrointestinal motility disturbances, such as, diarrhea113.
Selected Etiology: Pheochromocytoma
Main Points:
Pheochromocytomas are rare catecholamine- secreting tumors.
Clinical presentation is variable. With increased frequency of abdominal imaging many pheochromocytomas are discovered incidentally. When symptoms are present, they are non- specific and include multiple cardiovascular manifestations including – but not limited to- hypertension and sinus tachycardia.
Diagnosis involves biochemical work up followed by imaging for tumor localization.
Surgical resection is the treatment of choice after adequate medical preparation. Multidisciplinary team approach is essential in the care of these patients and cardiology expertise is needed to help manage the associated arrhythmias and potential cardiovascular complications.
Pheochromocytomas are catecholamine-secreting tumors arising from the chromaffin cells of the adrenal medulla. Extra-adrenal catecholamine secreting tumors arising from sympathetic ganglia are termed paragangliomas128. Paragangliomas have different malignancy risk and association with familial syndromes from pheochromocytoma, though clinical presentation and management of both are typically similar.128 Both are neuroendocrine tumors.
Pheochromocytoma is rare (estimated prevalence 0.1–0.6%).129,130 The characteristics and behavior of pheochromocytoma vary based on how the tumor was discovered: whether from investigation of a symptomatic individual (27% of the pheochromocytomas in one series), versus as an incidental finding on abdominal imaging (61%), versus case-based detection due to family history of a genetic mutation in a proband (12%). The latter two presentations are typically associated with smaller sized tumors.131
Pheochromocytoma is typically diagnosed in the third or fourth decade (but can present at a younger age in the setting of familial syndromes), with equal frequency in men and women. Ten percent can be malignant and 40% are familial (Von-Hippel-Lindau disease, Multiple Endocrine Neoplasia type 2A and 2B, Neurofibromatosis type 1, Carney Triad, Succinate Dehydrogenase gene Mutations, plus a few other rare genetic forms).128
With the increasing frequency of diagnostic abdominal imaging, many pheochromocytoma cases are now found incidentally and 50% are asymptomatic. When symptoms are present, they are typically paroxysmal.128 The classic triad of symptoms, include paroxysmal headaches, perspiration and palpitations/tachycardia. The presence of the entire triad is more specific for pheochromocytoma but less sensitive.132
Symptoms of pheochromocytoma are non-specific and include, but are not limited to, palpitations, pallor, lightheadedness, sweating, cold hands and feet. Cardiomyopathy, heart failure, and myocarditis are presentations in which pheochromocytoma can go unrecognized.128
The biochemical phenotype of the tumor can explain symptomatology. Epinephrine secreting tumors, leading to paroxysmal hypertension and brief tachyarrhythmias which could lead to acute cardiovascular decompensation. Norepinephrine secreting pheochromocytomas store and release norepinephrine in a continuous manner leading to persistent HTN and tachyarrhythmia. There can be a mixed picture.133
Pheochromocytomas have various cardiovascular manifestations. In one series 56% of patients had hypertension, of which, 32% had persistent HTN and 24% had paroxysmal HTN. Orthostatic hypotension is possible. Eight percent had labile blood pressure. Other cardiovascular manifestations included palpitations (36%), angina (32%), syncope (20%) and dyspnea (16%). Twenty percent of patients in this series underwent emergency cardiac catheterization due to concern for possible acute coronary syndrome, all had normal coronary arteries. Electrocardiogram changes described included wide QRS tachycardia, junctional or sinus tachycardia.134
In a recent study of 650 patients with pheochromocytoma seen between 2004 and 2019, 10.9% were found to have some form of tachyarrhythmia, of whom, 98.6% developed sinus tachycardia at some point, 11.3% developed atrial fibrillation, 5.6% developed atrial flutter, and 4.2% developed ventricular tachycardia.133
Excessive activation of the adrenoreceptors in the sinoatrial node by excess circulating catecholamines leads to sinus tachycardia.133 Presentations could also include exercise-induced palpitations and chest discomfort,135 exercise induced ventricular tachycardia (VT),136 recurrent VT,137,138QT prolongation and monomorphic VT.139 Isolated junctional tachycardia was the first manifestation in a child.140 Pheochromocytoma-induced atrial tachycardia has been reported.141
DIAGNOSIS:
In addition to history and physical exam, diagnosis involves biochemical testing including 24-hour urine collection for fractionated metanephrines or plasma free metanephrines with the patient supine for at least 30 minutes (beware of some medications and situations that can lead to false positive results).142 Clonidine suppression test can be used to confirm the diagnosis of pheochromocytoma if there is a concern about false positive elevation of plasma fractionated metanephrines.128
Once confirmed biochemically imaging is required for tumor localization. CT of the abdomen is typically the initial imaging procedures of choice. MRI can be used if there is a contraindication to CT or there is concern about metastatic disease, or for detection of skull base and neck paragangliomas.142 An iodine-123- metaiodobenzylguanidine radioisotope scan (MIBG) is indicated if there is concern for metastatic disease and if radiotherapy with an iodine-123- metaiodobenzylguanidine radioisotope is considered for treatment.142
TREATMENT:
Surgical tumor resection after adequate medical preparation, is the treatment of choice. Pre-operative use of alpha blockers for at least 7–14 days (often with addition of beta blockers 3–4 days later) is necessary, including those who are asymptomatic.128
Regarding tachyarrhythmia management in the setting of pheochromocytoma, a recent study suggested treatment of sinus tachycardia be initiated based on the blood pressure status and clinical urgency.133 If a patient with sinus tachycardia is normotensive, therapy with oral ivabradine could be initiated, however cost may limit its availability. Calcium channel blockers such as verapamil and diltiazem are an alternative option if alpha then beta blockade is not effective or if a patient is normotensive or hypotensive. Those are to be avoided in patients with heart failure. If a pheochromocytoma patient with sinus tachycardia is hypotensive, initial treatment is with IV fluids.133
Pheochromocytoma has been described as the “Great Masquerader”.143 It should be on the differential diagnosis of angina, cardiomyopathy, sinus tachycardia and arrhythmias. Clinicians need to have a high index of suspicion for pheochromocytoma and cardiologists need to be involved in the care of these patients.
Selected Etiology: Critical Illness and Pulmonary disease
Main Points:
In critically ill patients, new-onset sinus tachycardia, the most common ECG abnormality in the intensive care unit, is related to a wide range of underlying causes and has been associated with increased risk of cardiac events in this setting.
Sinus tachycardia is a common cardiovascular finding in acute pulmonary illnesses such as pulmonary embolism, pneumonia, and COPD exacerbation.
In ambulatory COPD patients, the chronotropic response to hypoxemia and bronchodilator therapy play important roles in increasing the risk for sinus tachycardia.
Impaired lung function is associated with subtle manifestation of cardiovascular autonomic dysfunction including elevated heart rates.
Sinus tachycardia is the most common ECG abnormality in the intensive care unit (ICU). New-onset sinus tachycardia in critically ill patients is related to a wide range of underlying causes including shock, acute respiratory failure, uncontrolled infections, hemorrhage, and delirium144. Overall, a high heart rate predicts ICU mortality144–146. In critically ill patients at high-risk for cardiac events by the revised Goldman index, elevated heart rate is associated with increased risk for major cardiac events147. In a single center open label randomized phase II trial involving patients in septic shock on high dose norepinephrine, targeted heart rate (between 80 bpm and 94 bpm) was achieved in all patients randomized to receive intravenous esmolol compared with those in the control group without increased adverse events and with favorable hemodynamic parameters (increased systemic vascular resistance, increased left ventricular stroke work and reduced norepinephrine requirements)148.
Ivabradine blocks the hyperpolarization-activated cyclic nucleotide-gated (HCN) channels thereby reducing heart rate without affecting myocardial contractility149. In experimental animal studies with septic shock, heart reduction by Ivabradine did not appear to improve cardiac function or vascular responsiveness to vasopressors150, but may have a beneficial effect on microcirculation151. In a phase II randomized clinical trial, Ivabradine (5 mg by orally twice daily) did not achieve the target reduction in heart rate (10 bpm) and did not improve hemodynamics or vasopressor use compared to a control group. Until further research provides conclusive evidence regarding the utility of heart rate reduction in critically ill patients152, sinus tachycardia remains a key hemodynamic marker that alerts clinicians to worse or deteriorating illness and predicts increased mortality.
Sinus Tachycardia and Pulmonary Disease
Tachycardia is one of seven variables incorporated in the clinical decision rule for the diagnosis of acute pulmonary embolism (Well’s score)153–155. Sinus tachycardia is also a common cardiovascular finding in other acute pulmonary illnesses including pneumonia154, chronic obstructive pulmonary disease (COPD) exacerbation and interstitial lung disease with acute presentation156,157. In ambulatory COPD patients, the prevalence of sinus tachycardia varies from 2% up to 38%158,159. The chronotropic response to hypoxemia and bronchodilator therapy plays important roles in increasing the risk for sinus tachycardia in COPD patients160,161. In a meta-analysis of randomized controlled trials of beta agonist therapy in COPD and asthma, the relative risk for sinus tachycardia with beta agonists was 3.1 (95%CI: 1.7–5.5)162. Overall, sinus tachycardia in lung disease is addressed by the appropriate management of the underlying pulmonary illness.
The autonomic nervous system is directly involved in the homeostasis of the respiratory system and it has been shown that subtle manifestations of cardiovascular autonomic dysfunction, including elevated resting heart rate, are significantly associated with impaired lung function and are independent predictors of chronic obstructive pulmonary disease in the middle-aged population163.
Selected Etiology: Post-COVID-19
Main Points:
Inappropriate Sinus Tachycardia (IST) and Postural Orthostatic Tachycardia Syndrome (POTS) can occur in patients with persistent COVID symptoms (“Long COVID”) and may be a part of less specific and multi-organ Post-Acute COVID-19 Sequelae (PACS).
In PACS, other causes of sinus tachycardia, such as, anemia, fever, hypovolemia, deconditioning, cardiac disease, pulmonary embolism, asthma or hyperthyroidism must be excluded.
Cardiovascular autonomic testing including active standing, tilt testing, deep breathing and other autonomic tests, plus prolonged ECG monitoring are recommended to facilitate a correct diagnosis.
Treatment of post-COVID tachycardia, presenting as IST and/or POTS, is not well established but ivabradine and beta-blockers might be considered as potential therapies.
Multi-organ damage in acute coronavirus disease-19 (COVID-19) is usually associated with transient sinus tachycardia164, which resolves as the patient recovers; however, prolonged tachycardia that persists after COVID-19 can pose a diagnostic conundrum. Case reports have described chronic (“long-haul”) post-COVID-19 symptoms reminiscent of those in POTS or IST165,166. Both these conditions are characterized not only by abnormal pulse accelerations or constantly increased heart rate but also by a variety of non-specific symptoms such as headache, cognitive dysfunction, and fatigue. 55,167 The most affected patients remain severely limited.
Although POTS and IST may create a diagnostic dilemma for an inexperienced clinician74, different causes of sinus tachycardia may overlap. The long-haul post-COVID syndrome may also include non-dysautonomic causes of tachycardia, including general post-viral deconditioning, anemia, hypovolemia, heart failure, myocarditis, pulmonary embolism, asthma, lung fibrosis, autoimmune conditions (e.g., lupus, Sjögren’s disease, vasculitis, hyperthyroidism), or neurodegenerative and neuroinflammatory disorders. Components of the autonomic nervous system such as the sympathetic noradrenergic system, the parasympathetic cholinergic system, and the sympathetic adrenergic system might play important roles in post-COVID tachycardia; however, the states of activation or inhibition of these sub-systems and their associations with neuroendocrine and immune mechanisms have not yet been characterized comprehensively.168
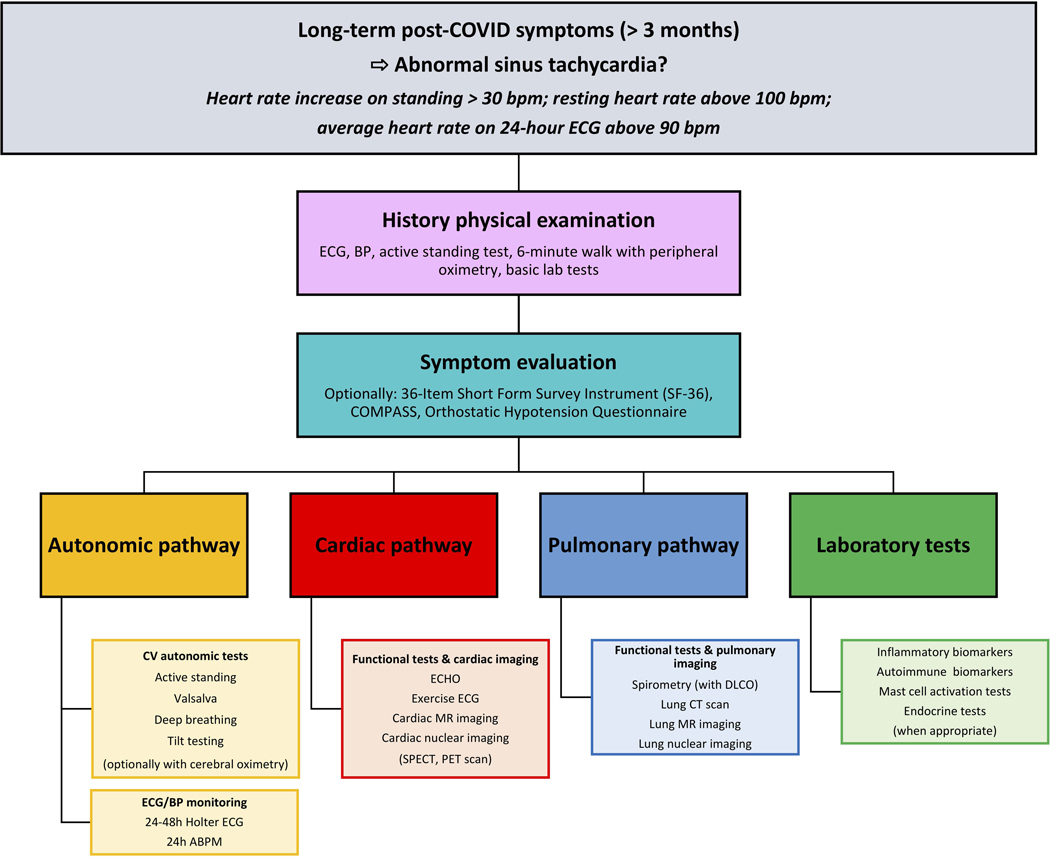
First (physical examination) and second (symptom evaluation) layers are mandatory (Figure 4). Third layer should be adapted to the primary and predominant clinical manifestations. If no pulmonary/respiratory symptoms are present, this part of the study algorithm could be omitted.
Figure 4. Work-up of sinus tachycardia following COVID-19.
Resting ECG, active standing test of at least 5 minutes or up to the intolerance level, 6-minute walk with peripheral saturation monitoring (using pulse oximetry) plus basic laboratory tests to exclude obvious abnormalities such as anemia, infection, electrolyte derangement, renal failure is recommended.
Patients should undergo cardiovascular autonomic testing (tilt testing and additional autonomic tests) plus appropriate cardiac workup (Figure 4). Cardiac imaging should include echocardiography, exercise ECG, cardiovascular MR (CMR) and, optionally, cardiac CT, stress CMR perfusion or cardiac nuclear imaging, if symptoms suggestive of angina pectoris are present.
In the next step, patients should be evaluated according to the “pulmonary pathway”, if symptoms suggestive of respiratory etiology are present. Finally, the patients should be investigated for the presence of traditional autoimmune and inflammatory plasma biomarkers, as multiple reports suggest triggering of autoimmunity in the post-acute phase of COVID-19 infection169.169. Consequently, selected patients with high clinical suspicion of concomitant autoimmune disorder should be screened for the presence of autoantibodies. In patients with signs suggesting mast cell activation disorder, appropriate laboratory tests: plasma tryptase, plasma prostaglandin D2, plasma histamine, and urine methylhistamine125 should be considered. Endocrine tests (e.g., thyroid gland function tests) may complement the laboratory workup, if appropriate.
Management: Lifestyle Changes
Main Points:
Lifestyle management can help alleviate conditions of symptomatic sinus tachycardia, and should be considered in all patients who do not have prohibiting contraindications.
Increasing dietary salt intake to facilitate blood volume expansion can be considered and has been shown in patients with postural tachycardia syndrome to increase plasma volume and decrease the heart rate upon standing, though long term effectiveness is yet to be determined.
Compression garments can be considered to help mitigate the translocation of excessive fluid from upper to lower parts of the body during upright posture, and has been shown to reduce heart rates and improve symptoms during a tilt table test in patients with postural tachycardia syndrome.
IST is a potentially troublesome problem. Apart from a sense of rapid heart beating, affected patients often also experience a wide range of associated complaints including fatigue, lightheadedness, exertional intolerance, muscle weakness and gastrointestinal issues49,75,88,167. In this regard, the pathophysiological relationships between sinus tachycardia and non-cardiac symptoms remains unknown; nevertheless, treatment efforts principally focus on ameliorating the tachycardia with the hope that non-cardiac symptoms will also improve.
Clinically, ‘Idiopathic sinus tachycardia’ comprises two principal diagnostic categories, POTS and IST.49,50,75,167,170 POTS is excessive sinus tachycardia triggered by minimal physical activity, especially movement to upright posture.36,50,125,171 171.
Patient education and management of expectations is crucial to enlisting and maintaining patient compliance.
- Certain teachable points merit attention in POTS and perhaps in IST.
- Understand basic physiology of postural change and how to diminish abrupt blood volume shifts,
- Recognize that circulating blood volume is usually lowest in the mornings, emphasizing the utility of morning volume enhancement by fluid bolus,
- Focus on smaller meals and lower carbohydrate intake to minimize splanchnic vascular dilation
- Avoid prolonged bed rest or immobilization to maintain physical conditioning.
- Encourage physical counter-maneuvers (leg crossing, muscle tensing) to enhance venous return.
Anecdotal evidence in POTS and idiopathic sinus tachycardia, favors avoidance of caffeine, alcohol, and other stimulant agents, including certain prescribed drugs. In MCA patients, low histamine diets and other patient-specific dietary elements should be avoided.
Blood volume expansion with increased dietary salt intake, electrolyte fluids and water bolus therapy are recommended, although with limited evidence of effectiveness (especially in IST). A study of dietary salt intake in patients with POTS reported that high sodium intake increases plasma volume, lowers standing plasma norepinephrine, and decreases the change in heart rate withstanding172 but long-term benefits are not established to indicate benefit over risk.
In the case of dietary salt, a goal of 6–10 grams/day has been proposed but may be difficult to achieve. Salt supplementation may be taken as dietary salt at the table (not in the cooking if the affected individual cooks for others), as tablets or capsules. However, gastrointestinal upset may be a limitation. Some gel-coated capsules are better tolerated but can be more expensive. Hypertension is rarely a concern in this largely young population but may become a problem as they age. Significant fluid intake is essential to optimize the effect of dietary salt. A target of 2.5–3 L/day of water and/or low carbohydrate electrolyte drinks is reasonable.
Exercise is an HRS Class IIA indication for POTS but remains a target of study49,50,125,170,173; its value in IST is unknown.49,50,125 Initially, supine or semi-recumbent exercise is recommended to minimize adverse effects of excessive orthostatic tachycardia early in the treatment program. The preferred modalities are use of a rowing machine, a recumbent cycle, swimming, and lower body isometrics. Exercise sessions should last at least 30 minutes each, 4–5 times per week. The first signs of improvement can take 4–6 weeks to appear.174 Incremental advancement of duration and intensity may be expected to vary from individual to individual, and may be hampered by musculoskeletal co-morbidities (e.g., Ehlers-Danlos Syndrome). Exercise treatment should be presented as a lifestyle change since it may be needed for a prolonged time. About half of patients may no longer meet POTS criteria within 3 months.
Compression garments targeting the large vascular beds of the lower extremities (i.e., thighs, buttocks and splanchnic bed) may diminish translocation of excessive fluid from upper to lower parts of the body during upright posture.49,50,170 A study in patients with POTS reported that abdominal and lower body compression reduced heart rates and improved symptoms during a Tilt Table Test, with some benefit from abdominal compression alone as well.175
Avoid symptom exacerbation triggered by prolonged heat exposure.
In summary, idiopathic sinus tachycardia can be a disabling condition. It tends to occur primarily in the young, particularly young women. Although life-style modifications may prove helpful, treatment is suboptimal, and many affected individuals experience lengthy periods of time during which their quality-of-life is adversely impacted.
Management: Psychological Factors and Intervention
Main Points:
Symptomatic sinus tachycardia can significantly affect a patient’s quality of life, and evaluating and addressing a patient’s mental wellbeing is an important part of patient care.
The biopsychosocial model provides an understanding of symptoms related to sinus tachycardia in the absence of anatomical or disease-related causes.
Integrated treatment programs, based on cognitive behavioral therapy, encompassing multiple providers such as physicians, psychologists, physical therapists, occupational therapists, and nurses can be considered to provide intensive care for patients with postural tachycardia syndrome.
There are two aspects to consider regarding the role of psychological factors and intervention for patients with sinus tachycardia: education about the symptom experience, and treatment with cognitive behavioral therapy. First, patients with mild symptoms may respond to education, reassurance, and lifestyle changes provided in office, such as the recommendation to avoid common triggers. Although avoidance makes sense in theory to prevent the symptoms, it may be unrealistic for patients with frequent or more severe symptoms, or more psychosocial impairment.176 Patients may not be aware of what the triggers are, they may be unavoidable, or may be associated with higher levels of fear/anxiety. This approach also sets up a problematic cycle of disability where quality-of-life is affected by both avoidance of the symptoms and fear of experiencing the symptoms.177 Anticipatory anxiety, a normal response to a distressing stimulus, increases attention to the somatic experience, intensifies sympathetic reactivity, and results in worsening symptoms and greater impairment. Second, patients with sinus tachycardia who do not respond to education and reassurance benefit from a referral for outpatient cognitive behavioral therapy, whether they have a mental health diagnosis or not. Cognitive behavioral therapy is an active, skills-based intervention that teaches modification of maladaptive thoughts, actions, and attention to somatic symptoms that lead to greater impairment and reduced function.
The biopsychosocial model provides an understanding of symptoms related to sinus tachycardia in the absence of anatomical or disease-related causes. In response to biological stressors, such as, dehydration or exertion, psychological stressors, such as anxiety or excitement, or social stressors such as performance expectation or symptom response, the sympathetic nervous system activates and produces symptoms frequently associated with tachycardia including dizziness, altered vision, or nausea.
Patients with maladaptive or avoidant coping styles perceive this as dangerous, leading to increased fear and distress, and avoidance of situations where symptoms may occur. The benefit of cognitive behavioral therapy is that it provides concrete skills patients can use to manage psychological and social stressors by changing their behavioral and cognitive responses to symptoms through engagement in active strategies such as counterpressure, distraction, relaxation, and cognitive reframing. In addition, patients learn skills to manage physical stressors and improve adherence to health habits including hydration and increased salt intake.
Case reports and small-scale studies indicate that cognitive behavioral therapy techniques, including biofeedback assisted relaxation, cognitive reframing, and systematic desensitization, are effective in improving function and reducing episodes in adult patients with syncope178–181, as well as reduction of panic episodes in patients with heart disease. Randomized controlled trials are needed to clarify when patients are most likely to benefit and what techniques are most effective, as well as further investigation in the pediatric population.
Integrated Treatment Programs
The interpretation of autonomic symptoms related to sinus tachycardia, such as, elevated heart rate or lightheadedness as dangerous may lead to significant fear and the curtailment of day-to-day activities. Up to 25% of adult patients with POTS were found to be unable to work182 and studies have shown that adolescents and adults with POTS frequently experience depression, anxiety, anxiety sensitivity, as well as significant disability in response to their sinus tachycardia183,184. Patients with significant distress and impairment related to sinus tachycardia who do not respond to initial treatments, such as pharmacotherapy and/or cognitive behavioral therapy, may need a more intensive approach to the management of symptoms through an integrated treatment program.
Integrated treatment programs, based on cognitive behavioral therapy, and with multiple providers including physicians, psychologists, physical therapists, occupational therapists, and nurses have been used to provide intensive care for patients with POTS, generally in an inpatient 3–6-week program. The goals of intervention are similar to outpatient cognitive behavioral therapy: to decrease distress and improve function, and patients are provided with intensive daily support in a structured setting to meet these goals.
Cognitive behavioral treatment is provided that may include exposure to fearful triggers such as being in an upright position, becoming overheated from exertion, exercise, being physically away from parents or caregivers who might “rescue” patients in the case of increased symptoms, engagement in social interactions, or participation in academic activities. Physical therapy coupled with heart rate monitoring and reassurance is designed to improve conditioning in a safe environment.
A key to rehabilitation is physical therapy, which provides gradual step-wise conditioning and exposure to increased heart rate, while patients learn strategies to cope with changes in somatic symptoms that have been conditioned to provoke significant anxiety/panic.185 Occupational therapy assists patients in returning to academic or work environments that require pacing and planning and has been an integral part of the success of these programs. Programs may offer intensive education for parents and caregivers for adolescent patients, as parents are often extremely worried about these symptoms as well.186,187 The debilitation observed in patients with POTS has been likened to the functional disability observed in patients with chronic pain and the interdisciplinary pain rehabilitation program structure has been utilized to treat patients with POTS successfully.188 The successful outcomes of interdisciplinary pain programs have been extensively researched for adolescents and adults.189–191
Management: Exercise and Rehabilitation
Main Points:
Aerobic-based exercise training that emphasizes submaximal intensity effort and tailored progression can be safe, confidence reinforcing, and an effective non-pharmacological intervention for the long-term management of signs and symptoms associated with sinus tachycardia.
Individualized exercise training for patients with sinus tachycardia should be developed based on current physical abilities, which for most, can be safely evaluated during maximal effort clinical exercise stress testing.
Exercise training in patients with sinus tachycardia should emphasize the importance of avoiding rapid short bursts of high intensity exertion both in gym/recreational settings and during activities of daily living.
Improvements in physical conditioning and symptom severity gained through exercise training are typically slow to develop initially, but become more noticeable and sustained when exercise training is adhered to long-term.
Properly dosed exercise training can be an effective non-pharmacologic therapeutic intervention for reversing severe muscle disuse atrophy and restoring normal exercise cardiac rate and rhythm caused by a continuous period of bedrest in otherwise healthy adults192–194. Similarly, exercise training has also proven effective for physical reconditioning and lessening episodes of sinus tachycardia triggered by hemodynamic, postural, and/or physical stressors in patients with autonomic nervous system conditions and no underlying familial or personal history of heart disease49,185,192,195,196.
Accordingly, in patients where dangerous underlying rhythm disorders and coronary ischemia are not likely to explain suddenly present recurring episodes of sinus tachycardia provoked by physical stress, such as those with POTS, a progressive and individualized exercise training plan should be initiated early on in the course of treatment49,90,185,192,195,196. If possible, supervised training is preferable to maximize improvements in functional capacity and to lend patients confidence that exercise training is safe49,185.
Aerobic Exercise and Training
The main component of the physical reconditioning plan should feature aerobic exercise training initially prescribed as mild-to-moderate intensity approximately corresponding to target HR zones within 50–75% of maximal HR or HR reserve calculated from exercise stress test measurements (Table 3).49,185,196 If patients are takingβ-blocker therapy, this medication should be taken on the day of testing so that HR training zones prescribed reflect effects of this therapy. In instances where the exercise stress test is unavailable, training intensity may be recommended based on approximate equivalents using the Rating of Perceived Exertion (RPE) scale (Table 3).49,185,196 In all other cases, patients should be encouraged to monitor their HR during exercise bouts using a commercially available electronic wearable device at prescribed intensities.
Table 3.
Core components of an exercise and rehabilitation program
| Time | Aerobic | ||||
|---|---|---|---|---|---|
|
| |||||
| Exercise Intensity | RPE | Target HR | Exercise Frequency/Duration | Exercise Mode | |
| Month 1 | mild-to-moderate | 11–13 | 50–75% HR max or reserve | 3–4 times per week (alt. days), 20–30 min per session | (semi)recumbent bicycle |
| Month 2 | moderate | 13–14 | up to 75% HR max or reserve | extend duration by 1–5 min every 3–4 weeks, add a bout of exercise per week every 3–4 weeks | gradually progress to rowing, swimming, or upright cycling |
| Month 3 | moderate-to-high | 14–16 | 75–85% HR max or reserve | ≥3–4 times per week, 45–60 min per session | treadmill walking or jogging |
RPE, rating of perceived exertion (subjective rating of the entire cardio workout on a scale of 6–20: 6 is very, very easy; 11 is fairly easy; 13 is somewhat hard; 15 is hard; 17 is very hard; 19 is very, very hard). HR, heart rate. Additional time should be added to each exercise session to allow for at least a 5 minutes warm up and 5 minutes cool down both performed at low intensity (e.g., RPE=6–9).
The initial frequency of exercise should be prescribed at 3–4 times per week for 20–30 minutes per session (not including time needed for warm up and cool down) using a stationary (semi)recumbent bicycle for at least 3–4 weeks. Other exercises involving horizontal leg position movements, such as rowing or swimming are also options, but may be less tolerable to patients due to whole body metabolic and blood flow demands. Therefore, the use of (semi)recumbent training is critical in initial phases, allowing patients to familiarize themselves with aerobic exercise while avoiding the full effect that gravity has on peripheral-to-central hemodynamic transport experienced in the upright posture.
As patient fitness and comfort with exercise gradually improve, components of the exercise plan should be intermittently modified on a planned schedule to promote continual training progression (Table 3). Program modifications should be conservative in order to not overwhelm patients and to prevent the possibility of excessive delayed onset muscle soreness and symptomology. Changes can include increasing the intensity, extending session durations, adding exercise bouts per week, and/or changing the modality (Table 3). Ultimately, the long-term goal is for patients to be able to perform activities of daily living and should they choose, comfortably exercise 45–60 minutes per session on most days of the week with minimal episodes of sinus tachycardia and symptomology.
Resistance Exercise and Training
After a firm level of comfort has been established with aerobic exercise training, the addition of resistance training to the program focusing on seated lower body and core work can also prove beneficial (Table 3). This is because lower body resistance training can help further strengthen the skeletal muscle pump and venous return to aid in counteracting orthostasis.
For those who are unfamiliar with weight training, a personal trainer who is familiar with precautions needed for working with patients with autonomic nervous system conditions is recommended to maintain safety and help utilize proper form and technique on each machine. If access to seated resistance training equipment is unavailable, patients may consider performing modified versions of exercises while seated/lying on the floor until physical conditioning and knowledge of how this type of training affects HR and symptoms collectively improve.
Special Considerations
Patients with POTS and the Ehlers-Danlos Syndrome (hypermobility type) usually have joint hypermobility, joint instability complications, and widespread musculoskeletal pain. If not carefully considered, these clinical signs and symptoms can limit their ability to regularly participate in an exercise program. Patients should start with non or low-weight bearing exercise, such as low cadence (semi)recumbent cycling, swimming with the aid of a floatation device, or rowing at mild-intensity levels. Elbow and knee braces should also be worn during exercise for joint protection. Similar to above, exercise progression should be slowly and carefully instituted, and each increment depends on the successful completion of the last. Physical therapy should be incorporated along with supervised exercise training in this particular patient population to avoid worsening joint damage, joint instability, and pain.
Management: Pharmacologic Treatment
Main Points:
While suppression of tachycardia is generally considered in conditions of symptomatic sinus tachycardia, in some cases symptoms may not be completely alleviated.
Pharmacologic agents targeting differing mechanisms have been used, such as betablockers, ivabradine, pyridostigmine, fludrocortisone, and midodrine among others.
Pharmacologic therapy is generally used in conjunction with other treatment modalities such as lifestyle changes, exercise training, and cognitive behavioral therapy.
Treatment of sinus tachycardia should first target underlying conditions such as hyperthyroidism, adrenal insufficiency, infections, hypotension, and drug side effects. However, in sinus tachycardia may be unexplained, idiopathic or “dysautonomic”. The two most common forms of sinus tachycardia for which the underlying condition is not well understood are POTS 49,50,170 and IST49,55.
Palpitations in POTS and IST are often caused by sinus tachycardia (or catecholamine effects) but suppressing sinus tachycardia with drugs or ablation may not always eliminate palpitations and other symptoms. Presumably the residual symptoms are due to anxiety, increased cardiac awareness (hypervigilance), or possibly increased contractility due to sympathetic drive.
Patients with POTS or IST frequently present with symptoms not involving the cardiovascular system. In the case of POTS this has led to the concept of POTS Pure (hemodynamic symptoms alone) and POTS Plus (associated with other systemic symptoms). It is not always easy to distinguish between POTS and IST, and their treatments differ in some respects. Physicians should openly discuss these issues with patients: successfully suppressing sinus tachycardia may not suppress palpitations, and successfully suppressing palpitations might not remove all or even most troublesome symptoms. In some or most cases, a slight reduction in heart rate can improve symptoms, but a more aggressive reduction in heart rate can worsen symptoms.
Postural Orthostatic Tachycardia Syndrome49,50,170
Several pharmacologic approaches have been tried, and many are successful in short term trials. However, none has been tested in longer term studies. These drugs may affect the heart rate response but may not improve symptoms.
Midodrine50 is a prodrug metabolized to a peripheral alpha-1 adrenergic receptor agonist that enhances vasoconstriction and venoconstriction, with subsequent increases in venous return, cardiac preload, and stroke volume. It is short acting and should be taken tid (2.5–15 mg/dose), but not within a few hours of lying down or going to sleep. A single dose can be used for acute symptom management. Midodrine, in theory, is likely to benefit patients with a tendency to hypotension and of lesser benefit in patients with hyperadrenergic symptoms, although it may lead to an indirect decrease in adrenergic tone. Scalp tingling and ‘goosebumps’ are common annoying side effects.
Propranolol at low doses (10–20 mg qid) may improve exercise capacity and symptom control. In a randomized trial197, higher doses were less effective at symptoms improvement (although more effective at heart rate control), and long-acting formulations are generally ineffective in controlling symptoms. Side effects may include worsening of exercise tolerance and fatigue.
Pyridostigmine is a peripheral acetylcholinesterase inhibitor that increases synaptic acetylcholine and may be helpful when used in combination with a beta-blocker to reduce orthostatic tachycardia and improve symptoms. It frequently causes diarrhea and may be particularly well tolerated in patients with constipation. It also can contribute to bladder irritability. This can be started at a dose of 30 mg TID and can often safely be increased up to 60–90 mg TID.
Fludrocortisone promotes fluid retention and may increase plasma volume and therefore maintain orthostatic preload. Typical doses range from 0.05–0.3 mg/day, as higher doses may be associated with greater side effects. Side effects include edema, hypokalemia, aggravation of migraines, and possibly osteoporosis in young women. It may exacerbate migraine frequency and symptoms. Plasma electrolyte concentrations, especially potassium, should be monitored.
Ivabradine may be an effective alternative particularly when the patient has prominent symptomatic orthostatic tachycardia and is a non-responder to, or intolerant of, beta blockers. A study of ivabradine in patients with hyperadrenergic POTS reported a reduction in heart rate, improvement in quality-of-life, and a strong trend in reduction of norepinephrine levels upon standing.95 A typical starting dose is 5 mg BID, with titration up or down in the range of 2.5–7.5 mg BID.
Central sympatholytic drugs are occasionally used, particularly in patients with hyperadrenergic POTS, but may not be tolerated in patients with any degree of orthostatic hypotension. They have prominent side effects that include sedation and dry mouth. Drugs include clonidine 0.1 to 0.2 mg up to 3 times per day, and methyldopa 125 mg to 250 mg once or twice daily. The longer half-life of methyldopa or a slow-release clonidine patch is often better tolerated than short-acting oral clonidine.
Desmopressin, octreotide, atomoxetine, and other interventions have been considered with less convincing evidence.
Inappropriate Sinus Tachycardia49,55
Pacemaker function of the sinus node is the result of over a dozen ion channels, of which, If, the funny current, is the most important. Sinus rate is importantly modulated by the sympathetic and parasympathetic nervous system.
Ivabradine is an If inhibitor that reliably reduces heart rate and most symptoms of palpitations. However, the studies to date198 have been either acute randomized trials198 have been either acute randomized trials199 or longer-term open label follow-up studies. This is an important limitation because we do not know how much symptoms may fluctuate or diminish over time. Nonetheless, the drug is generally well-tolerated when given in doses of 2.5–7.5 mg twice daily. Beta blockers can also be used. In a small, randomized crossover trial both metoprolol and ivabradine suppressed sinus tachycardia, but ivabradine was moderately more effective, and the doses of metoprolol that were used frequently caused symptomatic hypotension200.
Generally speaking, the treatment of POTS and IST has improved markedly in the past 15 years. When used with comprehensive lifestyle modification and strong supportive measures most patients respond sufficiently well to return towards a normal life. However, substantial clinician empathy and effort, and patient persistence, are essential requirements in order to achieve treatment benefit.
Management: Invasive management
Main Points:
Invasive approaches to ablate the sinus node to mollify sinus tachycardia are not established; such an approach may cause more harm than good and must be considered only for the most refractory inappropriate sinus tachycardia patients.
For patients with inappropriate sinus tachycardia and POTS, emerging technologies that modify sinus rate without affecting the sinus node directly (sinus node sparing hybrid ablation) may prove to be an effective and safe therapeutic option in patients with symptomatic drug-resistant IST and POTS. but these techniques are not yet established to treat sinus tachycardia.
No controlled trial of interventional techniques to modify the sinus node show symptom improvement; the relationship between sinus rate and symptoms is not robust.
Techniques to identify which patient may benefit from sinus node modification are not available.
For patients with sinus tachycardia that is normal or physiologic in response to a condition, management should be directed to the underlying cause, and invasive management is not attempted. Invasive management can be considered in refractory cases of inappropriate sinus tachycardia who have failed appropriate and optimal medical therapy and lifestyle interventions. Ablation should be considered as the last resort in treating these patients. Since these IST patients have a generally benign prognosis and only a very narrow group of patients likely benefit from invasive management, sinus node modification, surgical ablation, and sympathetic denervation are not recommended (Class III recommendation) as routine intervention for IST.49
Ablation is targeted to limit or stop sympathetic inputs into the sinus node, and surrogate markers of this reduced effect such as heart rate reduction, are used as clinical endpoints. However, heart rate control does not always correlate to symptom improvement. If invasive management of IST is being considered, ablation should be reserved for patients with disabling palpitations as their predominant symptom during episodes of tachycardia, rather than other symptoms such as shortness of breath or chest pain201.
With invasive management, patients should also be informed that repeat ablation attempts are frequently required and carry a significant risk of need for permanent pacemaker along with the prospect of pacemaker generator changes over the course of patients’ lives. In patients with POTS, sinus node modification is simply harmful. These patients frequently have inappropriate vasodilation or insufficient vasoconstriction resulting from autonomic pathology, and ablation in this cohort will prevent the sinus node’s ability to increase the heart rate during position change. By limiting the compensatory mechanism to maintain cardiac output in POTS patients, severe orthostatic hypotension can result.75,202 While tachycardia-mediated cardiomyopathy from IST has been reported in isolated case reports, these cases were responsive to ivabradine 203,204, and so invasive management also is not recommended in this group.
Endocardial ablation
While the diagnosis of IST is usually based on ECG and ambulatory monitoring, even with the pursuit of invasive management, an electrophysiology study should be performed to rule out other supraventricular arrhythmia mechanisms prior to sinus node modification. The sinus node, or sinoatrial nodal complex, extends in a cranial-to-caudal orientation along the superolateral epicardial right atrial wall at the superior vena cava (SVC) - right atrial junction.205 Typically, faster sinus node rates involve the superior aspect of the sinus node, and ablation is typically targeted here.206,207(Appendix 1) Ablation of the entire sinus node is no longer performed, due to the development of junctional rhythm requiring permanent pacemaker206.
Epicardial ablation and autonomic modulation
When the endocardial approach is unsuccessful, usually due to the proximity of the sinus node to the phrenic nerve in about 18% of cases, then alternate approaches should be employed.208,209 An epicardial approach from a subxiphoid pericardial access may allow for effective ablation because the sinus node is predominantly epicardial.210,211 In addition, endocardial target sites that showed phrenic nerve capture may correspond to epicardial sites without phrenic nerve limitations. In those instances where the phrenic nerve requires protection, a pericardial balloon can be deployed to push the phrenic nerve from the targeted ablation site.212–214 Typically, a deflectable sheath is used with an anterior approach for pericardial access to facilitate the positioning of the balloon along the lateral right atrium.212 Minimally invasive thoracoscopic surgery with use of bipolar radiofrequency clamp or cryoablation, 75,205,215–219, isolation of the superior right atrium from the rest of the atrium217,218,220, and inferior vena cava isolation 205,217 are alternative approaches.
Autonomic modulation can be performed during a minimally invasive approach, with concurrent ablation of epicardial fat pads to modify autonomic input into the sinus node region.215,217,220 Case reports of stellate ganglion block and renal sympathetic denervation have also been described75,221–223. While some patients have had successful lowering of heart rate over months of follow-up, one case series showed lack of reduction in the heart rate during exercise, suggesting the hormonal release of catecholamines may be able to overcome sympathetic nerve blockade.223
In a multicenter study of 255 consecutive patients (235 females, 25.9±3.8 years), a sinus node sparing hybrid thoracoscopic ablation procedure for drug-resistant IST or POTS using a minimally invasive bipolar radiofrequency ablation approach was performed targeting superior and inferior vena cavae and the crista terminalis224. Normal sinus rhythm was restored with symptom resolution so that medications were stopped. After 4.1±1.8 years, normal sinus function with chronotropic response to exercise remained present.
Procedural Outcomes
Procedural endpoints can include a decrease of >20–25% or >30 bpm from the maximum heart rate observed during isoproterenol infusion, reduction of maximum sinus rate to < 120 bpm during isoproterenol infusion, lowering of the resting sinus rate to < 90 bpm, or a caudal shift in sinus node activation.201,202,206,207,212,225–228
The acute success rate of sinus node modification varies from 66 – 100% depending on the endpoint definition.201,202,206,207,215,225–228 However, these are small studies, with the largest series having 39 patients201. In a systematic review of 110 patients undergoing catheter ablation for IST, 30% of patients still required rate or rhythm control medication after the procedure and 20% needed re-ablation208. Recurrence rates may be higher in those patients with longer history of IST201. The success rate is likely lower in patients with concurrent features of POTS with probable worsening of symptoms post ablation due to loss of the compensatory heart rate increase needed to maintain cardiac output.75,202
Complications
Complications vary from 0–14% but the true occurrence rate is limited by the small studies.201,202,206,207,215,225–228 Approximately 27% of patients will have a tachyarrhythmia that is not IST. Besides sinus node dysfunction including sinus pauses, common arrhythmias post-ablation includes atrial tachycardias, comprising 71% of all non-IST arrhythmias; these may also require catheter ablation206,210,225,228. Junctional rhythm is also frequent, with 42% of patients having this arrhythmia, usually with recovery of a higher right atrial rhythm within 24 hours.206,208,213 In a meta-analysis from 2017, 10% of patients required a new pacemaker implantation which was for sinus node dysfunction, junctional rhythm, and chronotropic incompetence.208 Right phrenic nerve injury with diaphragmatic paralysis206,228, pericarditis205,213,229, SVC stenosis206,226,230 and cardiac tamponade during pericardial balloon placement requiring open-chest repair212 have all been reported.
While the experience with invasive therapy for IST is limited to small case series, the procedure is able to limit sympathetic input into the sinus node leading to heart rate reduction, but not always symptom improvement. Randomized controlled clinical trials are required to evaluate the efficacy of this treatment option. Invasive management of IST at this point is a treatment of last resort and it should only be considered after failure of lifestyle and medical therapy including ivabradine in patients with disabling symptoms. If invasive management is ultimately pursued, patients need to be informed of potential complications, including other arrhythmias, possible need for permanent pacemaker, and phrenic nerve injury.
Pediatric Considerations
Main Points:
Maximal sinus tachycardia is age dependent decreasing most rapidly early in life but continuing to decrease thereafter.
CO= HR x Stroke volume. Stroke volume (SV) is inversely related to HR. Thus, CO increases with HR provided SV remains sufficient. This dependence becomes most evident with orthostasis when venous return and SV are reduced due to gravitational blood pooling, and increasing sinus tachycardia reduces CO above approximately 120–130 bpm regardless of the cause of sinus tachycardia.
A common brief transient form of orthostatic hypotension, known as “initial orthostatic hypotension”, occurs in 20–25% of healthy young volunteers and may be associated with transient reflex sinus tachycardia into the POTS range within the first minute of standing. Care should be taken when differentiating POTS from mechanisms that result in hypotension such as initial orthostatic hypotension or vasovagal syncope.
“Normal” Maximum Heart Rate Declines with Age
The most rapid AV nodal conducted sinus tachycardia can exceed 240 bpm in a highly stressed neonate. Even unstressed infants have a wide range of normal HR with the 99th percentile in the 180 bpm range shortly after birth, decreasing to 160 bpm at 1 year, 110 bpm by age 12, and 100 bpm by 18231. Resting heart rate is inversely related to the lifespan among homeothermic mammals and within individual species232.
“Normal” Sinus Tachycardia Response to Physiological Stressors in Children???
Daily “normal” physical stressors should drive HR higher. Thus, orthostatic stress, regulated by arterial and cardiopulmonary baroreflexes, normally increases HR by <43 bpm (under age 19)233 with tilt or quiet standing. However, if HR exceeds 120–130 bpm during orthostatic stress, cardiac output (CO) becomes progressively compromised234. Indeed, Guyton235 showed (in dogs) an increase in CO up to a transitional HR (dependent on venous return) above which CO decreases.
The second daily normal physical stressor is exercise. CO increases, in part, due to the skeletal muscle pump236. Parasympathetic withdrawal and sympathetic activation are predominantly driven by the exercise pressor reflex comprising central command, mechanoreflexes and metaboreflexes237, and modulated by baroreflex reset to accommodate normal pressure and HR changes.238
Syndromes of sinus tachycardia in children
A common brief transient form of orthostatic hypotension, known as “initial orthostatic hypotension”, occurs in 20–25% of healthy young volunteers and may be associated with transient reflex sinus tachycardia into the POTS range within the first minute of standing239. Care should be taken when differentiating POTS from mechanisms that result in hypotension such as initial orthostatic hypotension or vasovagal syncope. 240 241. Another group of younger patients with IST caused by abnormalities of the If channel is tachycardic at rest which remains or increases due to sympathetic activation when upright.234. Finally, upright hyperventilation seems rather common and produces a POTS-like tachycardia in some children.242
Pregnancy Considerations
After conception, release of pregnancy related hormones is associated with vasodilation with reduction in total peripheral resistance with subsequent increase in cardiac output.
During pregnancy, heart rate increases via baroreceptor-mediated alteration in autonomic balance escalating until the third trimester. The upper limit of resting heart rate during pregnancy is normally ≤100 bpm in small 243,244 and large245,246 studies when measured recumbent after a resting period; these findings have been corroborated by a meta-analysis.247 A large cohort study using pulse oximetry-derived data, however, indicates heart rates can exceed 100 bpm from 18 weeks gestation (>105 bpm from 28 weeks of gestation) in >10% of observations during healthy pregnancy.248
We recommend that any pregnant women with persistent resting sinus rates >100 bpm detected on routinely should be further evaluated especially if accompanied by symptoms to rule out heart failure and other causes of sinus tachycardia. Our standards practice is to perform a full blood count, CRP, renal function, thyroid function, cardiac troponin, NT pro-BNP, plasma metanepherines and echocardiogram. In the absence of an identifiable cause, IST should be considered.
However, normalization of heart rate in the immediate post-partum period can occur249,250. It has been postulated that pregnancy-related inappropriate sinus tachycardia (PRIST) reflects a distinct phenotype of IST resulting from hormonally mediated alterations in baroreceptor reflex sensitivity and/or sinus node sensitivity.251–254
IST induced by pregnancy is associated with debilitating symptoms, frequent emergency department/hospitalization visits occur with higher rates of induction of labor.250
A single case of possible IST-induced cardiomyopathy during pregnancy has been reported (likely it was cardiomyopathy causing sinus tachycardia)255 a recent cohort study did not demonstrate evidence of an increase in maternal death/major adverse cardiac events (MACE) or peri-natal mortality associated with IST in pregnancy.250
Regarding treatment strategies for IST during pregnancy, no evidence supports a specific treatment. However maintaining hydration, balanced diet, appropriate thermoregulation and regular, symptom-limited, exercise are reasonable. Medications should only be started after a detailed discussion of the risk/benefit balance with the individual.
If pharmacological therapies are to be used β-blockers (bisoprolol, metoprolol, propanolol) at lowest doses as necessary to alleviate symptoms. There are no human safety data on use of ivabradine in pregnancy; some animal data suggest fetotoxicity. The European Cardiac Society considers the use of ivabradine to be contra-indicated in pregnancy.256 β-blockers can be continued during early post-partum period (even if the mother is breast feeding) although symptom resolution generally occurs with delivery.
Sinus tachycardia may occur during the third trimester of pregnancy in women with the supine hypotensive syndrome257–262. In this condition, there is a marked drop in blood pressure in the supine position, in part, due to compression of the inferior vena cava (and, potentially decreased aortic flow due to aortic compressions) with substantial hypotension and reflex sinus tachycardia that is associated with symptoms of dizziness, chest or abdominal discomfort, dyspnea, faintness, nausea, headache, fatigue, pallor, cyanosis, anxiety, need to flex hips, unconsciousness, cardiac arrest.
Symptoms may precede or follow changes in blood pressure. The sinus tachycardia that is present in this circumstance may blunted to some extent by vagal or utero cardiac reflex. There may be blunted baroreflex activity and a blunted response to Valsalva efficiency associated with an incomplete compensatory heart rate response to drop in blood pressure. The syndrome itself occurs in 2.5–20% of women such supine hypotension with sinus tachycardia and occur in asymptomatic women at a greater rate.
Asymptomatic women with supine hypotension tend to have much greater azygous blood flow to compensate for impairment in flow that is present in the inferior vena cava. Women with large pelvic masses may also develop the same type of problem. Removal of the tumor or delivery of the fetus is the treatment, but care must be taken for patients undergoing spinal anesthesia for C-sections in the supine position to prevent hemodynamic collapse. Symptoms and hypotension can be assuaged by lying in a lateral position; the left lateral position is better to prevent liver congestion.
Gaps in Knowledge
Surprisingly, there are extensive gaps in our knowledge about how to identify pathologic sinus tachycardia, link symptoms to sinus tachycardia, determine the relationship between the autonomic nervous system and sinus tachycardia, assess channelopathies that may be responsible for sinus tachycardia and to determine an approach to resolve the underlying problems and sinus tachycardia itself for resolution in adverse outcomes that are associated with its presence.
Conclusion
Sinus tachycardia is the most common arrhythmia that is often nothing more than an appropriate response to physical activity. However, sinus tachycardia can become a substantial problem that is a clinical manifestation of substantial underlying pathology and/or reflective of the presence of autonomic dysfunction that requires careful and complete evaluation leading to specific and substantial interventions. Sinus tachycardia may be a persistent or paroxysmal problem for which electrophysiologists are often consulted with the thought that sinus tachycardia may represent POTS or IST.
In this comprehensive state of the art review, we address critical issues that help distinguish different manifestations of sinus tachycardia and address therapeutic interventions and emerging technologies that may lead to the better care of patients.
Supplementary Material
Non-standard Abbreviations and Acronyms:
- ST
sinus tachycardia
- POTS
postural orthostatic tachycardia syndrome
- IST
inappropriate sinus tachycardia
- QSART
quantitative sudomotor axon reflex test
- TTT
Tilt Table Test
Footnotes
Disclosures
None
References
- 1.DiFrancesco D. Pacemaker mechanisms in cardiac tissue. Annu Rev Physiol. 1993;55:455–472. doi: 10.1146/annurev.ph.55.030193.002323 [DOI] [PubMed] [Google Scholar]
- 2.Aune D, Sen A, o’Hartaigh B, Janszky I, Romundstad PR, Tonstad S, Vatten LJ. Resting heart rate and the risk of cardiovascular disease, total cancer, and all-cause mortality - A systematic review and dose-response meta-analysis of prospective studies. Nutr Metab Cardiovasc Dis. 2017;27:504–517. doi: 10.1016/j.numecd.2017.04.004 [DOI] [PubMed] [Google Scholar]
- 3.Levine HJ. Rest heart rate and life expectancy. J Am Coll Cardiol. 1997;30:1104–1106. doi: 10.1016/s0735-1097(97)00246-5 [DOI] [PubMed] [Google Scholar]
- 4.Singh BN. Morbidity and mortality in cardiovascular disorders: impact of reduced heart rate. J Cardiovasc Pharmacol Ther. 2001;6:313–331. doi: 10.1177/107424840100600401 [DOI] [PubMed] [Google Scholar]
- 5.Fox K, Borer JS, Camm AJ, Danchin N, Ferrari R, Lopez Sendon JL, Steg PG, Tardif JC, Tavazzi L, Tendera M, et al. Resting heart rate in cardiovascular disease. J Am Coll Cardiol. 2007;50:823–830. doi: 10.1016/j.jacc.2007.04.079 [DOI] [PubMed] [Google Scholar]
- 6.Hall JE. Guyton and hall textbook of medical physiology. 14. ed. Philadelphia: Elsevier; 2020. [Google Scholar]
- 7.Kossmann CE. The normal electrocardiogram. Circulation. 1953;8:920–936. doi: 10.1161/01.cir.8.6.920 [DOI] [PubMed] [Google Scholar]
- 8.ASSOCIATION CCOTNYH. Nomenclature and Criteria for Diagnosis of Diseases of the Heart and Blood Vessels. Journal of the American Medical Association. 1953;153:891-891. doi: 10.1001/jama.1953.02940260115033 [DOI] [Google Scholar]
- 9.Packard JM, Graettinger JS, Graybiel A. Analysis of the electrocardiograms obtained from 1000 young healthy aviators; ten year follow-up. Circulation. 1954;10:384–400. doi: 10.1161/01.cir.10.3.384 [DOI] [PubMed] [Google Scholar]
- 10.Spodick DH, Raju P, Bishop RL, Rifkin RD. Operational definition of normal sinus heart rate. Am J Cardiol. 1992;69:1245–1246. doi: 10.1016/0002-9149(92)90947-w [DOI] [PubMed] [Google Scholar]
- 11.Still AM, Raatikainen P, Ylitalo A, Kauma H, Ikaheimo M, Antero Kesaniemi Y, Huikuri HV. Prevalence, characteristics and natural course of inappropriate sinus tachycardia. Europace. 2005;7:104–112. doi: 10.1016/j.eupc.2004.12.007 [DOI] [PubMed] [Google Scholar]
- 12.Brignole M, Rivasi G, Sutton R, Kenny RA, Morillo CA, Sheldon R, Raj SR, Ungar A, Furlan R, van Dijk G, et al. Low-blood pressure phenotype underpins the tendency to reflex syncope. J Hypertens. 2021;39:1319–1325. doi: 10.1097/HJH.0000000000002800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benetos A, Rudnichi A, Thomas F, Safar M, Guize L. Influence of heart rate on mortality in a French population: role of age, gender, and blood pressure. Hypertension. 1999;33:44–52. doi: 10.1161/01.hyp.33.1.44 [DOI] [PubMed] [Google Scholar]
- 14.Pfister R, Michels G, Sharp SJ, Luben R, Wareham NJ, Khaw KT. Resting heart rate and incident heart failure in apparently healthy men and women in the EPIC-Norfolk study. Eur J Heart Fail. 2012;14:1163–1170. doi: 10.1093/eurjhf/hfs104 [DOI] [PubMed] [Google Scholar]
- 15.Sharashova E, Wilsgaard T, Mathiesen EB, Lochen ML, Njolstad I, Brenn T. Resting heart rate predicts incident myocardial infarction, atrial fibrillation, ischaemic stroke and death in the general population: the Tromso Study. J Epidemiol Community Health. 2016;70:902–909. doi: 10.1136/jech-2015-206663 [DOI] [PubMed] [Google Scholar]
- 16.Beck W, Barnard CN, Schrire V. Heart rate after cardiac transplantation. Circulation. 1969;40:437–445. doi: 10.1161/01.cir.40.4.437 [DOI] [PubMed] [Google Scholar]
- 17.Critchley WR, Yonan N, Shaw SM, Fildes JE. Heart rate after cardiac transplantation-lessons from the tortoise and the shrew. Transplantation. 2013;95:259–265. doi: 10.1097/TP.0b013e31826bc42a [DOI] [PubMed] [Google Scholar]
- 18.Jose AD, Collison D. The normal range and determinants of the intrinsic heart rate in man. Cardiovasc Res. 1970;4:160–167. doi: 10.1093/cvr/4.2.160 [DOI] [PubMed] [Google Scholar]
- 19.Endres S, Mayuga KA, de Cristofaro A, Taneja T, Goldberger JJ, Kadish AH. Age and gender difference in ST height at rest and after double autonomic blockade in normal adults. Ann Noninvasive Electrocardiol. 2006;11:253–258. doi: 10.1111/j.1542-474X.2006.00112.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nauman J, Nilsen TI, Wisloff U, Vatten LJ. Combined effect of resting heart rate and physical activity on ischaemic heart disease: mortality follow-up in a population study (the HUNT study, Norway). J Epidemiol Community Health. 2010;64:175–181. doi: 10.1136/jech.2009.093088 [DOI] [PubMed] [Google Scholar]
- 21.Sharashova E, Wilsgaard T, Lochen ML, Mathiesen EB, Njolstad I, Brenn T. Resting heart rate trajectories and myocardial infarction, atrial fibrillation, ischaemic stroke and death in the general population: The Tromso Study. Eur J Prev Cardiol. 2017;24:748–759. doi: 10.1177/2047487316688983 [DOI] [PubMed] [Google Scholar]
- 22.Vazir A, Claggett B, Cheng S, Skali H, Shah A, Agulair D, Ballantyne CM, Vardeny O, Solomon SD. Association of Resting Heart Rate and Temporal Changes in Heart Rate With Outcomes in Participants of the Atherosclerosis Risk in Communities Study. JAMA Cardiol. 2018;3:200–206. doi: 10.1001/jamacardio.2017.4974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tverdal A, Hjellvik V, Selmer R. Heart rate and mortality from cardiovascular causes: a 12 year follow-up study of 379,843 men and women aged 40–45 years. Eur Heart J. 2008;29:2772–2781. doi: 10.1093/eurheartj/ehn435 [DOI] [PubMed] [Google Scholar]
- 24.Aladin AI, Whelton SP, Al-Mallah MH, Blaha MJ, Keteyian SJ, Juraschek SP, Rubin J, Brawner CA, Michos ED. Relation of resting heart rate to risk for all-cause mortality by gender after considering exercise capacity (the Henry Ford exercise testing project). Am J Cardiol. 2014;114:1701–1706. doi: 10.1016/j.amjcard.2014.08.042 [DOI] [PubMed] [Google Scholar]
- 25.Cooney MT, Vartiainen E, Laatikainen T, Juolevi A, Dudina A, Graham IM. Elevated resting heart rate is an independent risk factor for cardiovascular disease in healthy men and women. Am Heart J. 2010;159:612–619 e613. doi: 10.1016/j.ahj.2009.12.029 [DOI] [PubMed] [Google Scholar]
- 26.Cucherat M. Quantitative relationship between resting heart rate reduction and magnitude of clinical benefits in post-myocardial infarction: a meta-regression of randomized clinical trials. Eur Heart J. 2007;28:3012–3019. doi: 10.1093/eurheartj/ehm489 [DOI] [PubMed] [Google Scholar]
- 27.Ahmadi-Kashani M, Kessler DJ, Day J, Bunch TJ, Stolen KQ, Brown S, Sbaity S, Olshansky B, Investigators IRS. Heart rate predicts outcomes in an implantable cardioverter-defibrillator population. Circulation. 2009;120:2040–2045. doi: 10.1161/CIRCULATIONAHA.108.847608 [DOI] [PubMed] [Google Scholar]
- 28.Santos M, West E, Skali H, Forman DE, Nadruz WJ, Shah AM. Resting Heart Rate and Chronotropic Response to Exercise: Prognostic Implications in Heart Failure Across the Left Ventricular Ejection Fraction Spectrum. J Card Fail. 2018;24:753–762. doi: 10.1016/j.cardfail.2018.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Swedberg K, Komajda M, Bohm M, Borer J, Robertson M, Tavazzi L, Ford I, Investigators S. Effects on outcomes of heart rate reduction by ivabradine in patients with congestive heart failure: is there an influence of beta-blocker dose?: findings from the SHIFT (Systolic Heart failure treatment with the I(f) inhibitor ivabradine Trial) study. J Am Coll Cardiol. 2012;59:1938–1945. doi: 10.1016/j.jacc.2012.01.020 [DOI] [PubMed] [Google Scholar]
- 30.Flannery G, Gehrig-Mills R, Billah B, Krum H. Analysis of randomized controlled trials on the effect of magnitude of heart rate reduction on clinical outcomes in patients with systolic chronic heart failure receiving beta-blockers. Am J Cardiol. 2008;101:865–869. doi: 10.1016/j.amjcard.2007.11.023 [DOI] [PubMed] [Google Scholar]
- 31.Low PA, Opfer-Gehrking TL, Textor SC, Benarroch EE, Shen WK, Schondorf R, Suarez GA, Rummans TA. Postural tachycardia syndrome (POTS). Neurology. 1995;45:S19–25. [PubMed] [Google Scholar]
- 32.Schondorf R, Benoit J, Wein T, Phaneuf D. Orthostatic intolerance in the chronic fatigue syndrome. J Auton Nerv Syst. 1999;75:192–201. doi: 10.1016/s0165-1838(98)00177-5 [DOI] [PubMed] [Google Scholar]
- 33.Vernino S, Bourne KM, Stiles LE, Grubb BP, Fedorowski A, Stewart JM, Arnold AC, Pace LA, Axelsson J, Boris JR, et al. Postural orthostatic tachycardia syndrome (POTS): State of the science and clinical care from a 2019 National Institutes of Health Expert Consensus Meeting - Part 1. Auton Neurosci. 2021:102828. doi: 10.1016/j.autneu.2021.102828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thieben MJ, Sandroni P, Sletten DM, Benrud-Larson LM, Fealey RD, Vernino S, Lennon VA, Shen WK, Low PA. Postural orthostatic tachycardia syndrome: the Mayo clinic experience. Mayo Clin Proc. 2007;82:308–313. doi: 10.4065/82.3.308 [DOI] [PubMed] [Google Scholar]
- 35.Schondorf R, Low PA. Idiopathic postural orthostatic tachycardia syndrome: an attenuated form of acute pandysautonomia? Neurology. 1993;43:132–137. doi: 10.1212/wnl.43.1_part_1.132 [DOI] [PubMed] [Google Scholar]
- 36.Low PA, Sandroni P, Joyner M, Shen WK. Postural tachycardia syndrome (POTS). J Cardiovasc Electrophysiol. 2009;20:352–358. doi: 10.1111/j.1540-8167.2008.01407.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lopera G, Castellanos A, Moleiro F, Huikuri HV, Myerburg RJ. Chronic inappropriate sinus tachycardia in elderly females. Ann Noninvasive Electrocardiol. 2003;8:139–143. doi: 10.1046/j.1542-474x.2003.08208.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lakatta EG, DiFrancesco D. What keeps us ticking: a funny current, a calcium clock, or both? J Mol Cell Cardiol. 2009;47:157–170. doi: 10.1016/j.yjmcc.2009.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Palma JA, Benarroch EE. Neural control of the heart: recent concepts and clinical correlations. Neurology. 2014;83:261–271. doi: 10.1212/WNL.0000000000000605 [DOI] [PubMed] [Google Scholar]
- 40.Afrin LB, Self S, Menk J, Lazarchick J. Characterization of Mast Cell Activation Syndrome. Am J Med Sci. 2017;353:207–215. doi: 10.1016/j.amjms.2016.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marshall JM. Peripheral chemoreceptors and cardiovascular regulation. Physiol Rev. 1994;74:543–594. doi: 10.1152/physrev.1994.74.3.543 [DOI] [PubMed] [Google Scholar]
- 42.Amorim MR, de Deus JL, Cazuza RA, Mota CMD, da Silva LEV, Borges GS, Batalhao ME, Carnio EC, Branco LGS. Neuroinflammation in the NTS is associated with changes in cardiovascular reflexes during systemic inflammation. J Neuroinflammation. 2019;16:125. doi: 10.1186/s12974-019-1512-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rubenstein JC, Freher M, Kadish A, Goldberger JJ. Diurnal heart rate patterns in inappropriate sinus tachycardia. Pacing Clin Electrophysiol. 2010;33:911–919. doi: 10.1111/j.1540-8159.2010.02725.x [DOI] [PubMed] [Google Scholar]
- 44.Esler M, Alvarenga M, Lambert G, Kaye D, Hastings J, Jennings G, Morris M, Schwarz R, Richards J. Cardiac sympathetic nerve biology and brain monoamine turnover in panic disorder. Ann N Y Acad Sci. 2004;1018:505–514. doi: 10.1196/annals.1296.062 [DOI] [PubMed] [Google Scholar]
- 45.Shannon JR, Flattem NL, Jordan J, Jacob G, Black BK, Biaggioni I, Blakely RD, Robertson D. Orthostatic intolerance and tachycardia associated with norepinephrine-transporter deficiency. N Engl J Med. 2000;342:541–549. doi: 10.1056/NEJM200002243420803 [DOI] [PubMed] [Google Scholar]
- 46.Varma N, Cygankiewicz I, Turakhia M, Heidbuchel H, Hu Y, Chen LY, Couderc JP, Cronin EM, Estep JD, Grieten L, et al. 2021 ISHNE/ HRS/ EHRA/ APHRS collaborative statement on mHealth in Arrhythmia Management: Digital Medical Tools for Heart Rhythm Professionals: From the International Society for Holter and Noninvasive Electrocardiology/Heart Rhythm Society/European Heart Rhythm Association/Asia Pacific Heart Rhythm Society. Ann Noninvasive Electrocardiol. 2021;26:e12795. doi: 10.1111/anec.12795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Steinberg JS, Varma N, Cygankiewicz I, Aziz P, Balsam P, Baranchuk A, Cantillon DJ, Dilaveris P, Dubner SJ, El-Sherif N, et al. 2017 ISHNE-HRS expert consensus statement on ambulatory ECG and external cardiac monitoring/telemetry. Ann Noninvasive Electrocardiol. 2017;22. doi: 10.1111/anec.12447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yusuf S, Camm AJ. The sinus tachycardias. Nat Clin Pract Cardiovasc Med. 2005;2:44–52. doi: 10.1038/ncpcardio0068 [DOI] [PubMed] [Google Scholar]
- 49.Sheldon RS, Grubb BP 2nd, Olshansky B, Shen WK, Calkins H, Brignole M, Raj SR, Krahn AD, Morillo CA, Stewart JM, et al. 2015 heart rhythm society expert consensus statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope. Heart Rhythm. 2015;12:e41–63. doi: 10.1016/j.hrthm.2015.03.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raj SR, Guzman JC, Harvey P, Richer L, Schondorf R, Seifer C, Thibodeau-Jarry N, Sheldon RS. Canadian Cardiovascular Society Position Statement on Postural Orthostatic Tachycardia Syndrome (POTS) and Related Disorders of Chronic Orthostatic Intolerance. Can J Cardiol. 2020;36:357–372. doi: 10.1016/j.cjca.2019.12.024 [DOI] [PubMed] [Google Scholar]
- 51.Locati ET. New directions for ambulatory monitoring following 2017 HRS-ISHNE expert consensus. J Electrocardiol. 2017;50:828–832. doi: 10.1016/j.jelectrocard.2017.08.009 [DOI] [PubMed] [Google Scholar]
- 52.Williams CB, Andrade JG, Hawkins NM, Cheung C, Krahn A, Laksman ZW, Bennett MT, Heilbron B, Chakrabarti S, Yeung-Lai-Wah JA, et al. Establishing reference ranges for ambulatory electrocardiography parameters: meta-analysis. Heart. 2020;106:1732–1739. doi: 10.1136/heartjnl-2020-316925 [DOI] [PubMed] [Google Scholar]
- 53.Johnson LSB, Conen D. Can you feel the beat? How to define reference ranges for ambulatory heart rhythm monitoring. Heart. 2020;106:1708–1709. doi: 10.1136/heartjnl-2020-317361 [DOI] [PubMed] [Google Scholar]
- 54.Hanna P, Dacey MJ, Brennan J, Moss A, Robbins S, Achanta S, Biscola NP, Swid MA, Rajendran PS, Mori S, et al. Innervation and Neuronal Control of the Mammalian Sinoatrial Node a Comprehensive Atlas. Circ Res. 2021;128:1279–1296. doi: 10.1161/circresaha.120.318458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Olshansky B, Sullivan RM. Inappropriate sinus tachycardia. Europace. 2019;21:194–207. doi: 10.1093/europace/euy128 [DOI] [PubMed] [Google Scholar]
- 56.Baruscotti M, Bianco E, Bucchi A, DiFrancesco D. Current understanding of the pathophysiological mechanisms responsible for inappropriate sinus tachycardia: role of the If “funny” current. J Interv Card Electrophysiol. 2016;46:19–28. doi: 10.1007/s10840-015-0097-y [DOI] [PubMed] [Google Scholar]
- 57.Morillo CA, Klein GJ, Thakur RK, Li H, Zardini M, Yee R. Mechanism of’inappropriate’sinus tachycardia. Role of sympathovagal balance. Circulation. 1994;90:873–877. [DOI] [PubMed] [Google Scholar]
- 58.Nwazue VC, Paranjape SY, Black BK, Biaggioni I, Diedrich A, Dupont WD, Robertson D, Raj SR. Postural tachycardia syndrome and inappropriate sinus tachycardia: role of autonomic modulation and sinus node automaticity. Journal of the American Heart Association. 2014;3:e000700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ewing DJ, Neilson J, Travis P. New method for assessing cardiac parasympathetic activity using 24 hour electrocardiograms. Heart. 1984;52:396–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Leon H, Guzman JC, Kuusela T, Dillenburg R, Kamath M, Morillo CA. Impaired baroreflex gain in patients with inappropriate sinus tachycardia. Journal of cardiovascular electrophysiology. 2005;16:64–68. [DOI] [PubMed] [Google Scholar]
- 61.Chiale PA, Garro HA, Schmidberg J, Sánchez RA, Acunzo RS, Lago M, Levy G, Levin M. Inappropriate sinus tachycardia may be related to an immunologic disorder involving cardiac β andrenergic receptors. Heart Rhythm. 2006;3:1182–1186. [DOI] [PubMed] [Google Scholar]
- 62.Liao W-B, Liu C-F, Chiang C-W, Kung C-T, Lee C-W. Cardiovascular manifestations of pheochromocytoma. The American journal of emergency medicine. 2000;18:622–625. [DOI] [PubMed] [Google Scholar]
- 63.Moreira JM, Curimbaba J, Filho HC, Pimenta J. Persistent inappropriate sinus tachycardia after radiofrequency ablation of left lateral accessory pathway. Journal of cardiovascular electrophysiology. 2006;17:678–681. [DOI] [PubMed] [Google Scholar]
- 64.Skeberis V, Simonis F, Tsakonas K, Celiker AA, Andries E, Brugada P. Inappropriate sinus tachycardia following radiofrequency ablation of AV nodal tachycardia: incidence and clinical significance. Pacing and Clinical Electrophysiology. 1994;17:924–927. [DOI] [PubMed] [Google Scholar]
- 65.Pappone C, Stabile G, Oreto G, De Simone A, Rillo M, Mazzone P, Cappato R, Chierchia S. Inappropriate sinus tachycardia after radiofrequency ablation of para‐Hisian accessory pathways. Journal of cardiovascular electrophysiology. 1997;8:1357–1365. [DOI] [PubMed] [Google Scholar]
- 66.Kocovic DZ, Harada T, Shea JB, Soroff D, Friedman PL. Alterations of heart rate and of heart rate variability after radiofrequency catheter ablation of supraventricular tachycardia. Delineation of parasympathetic pathways in the human heart. Circulation. 1993;88:1671–1681. [DOI] [PubMed] [Google Scholar]
- 67.Debruyne P, Rossenbacker T, Collienne C, Roosen J, Ector B, Janssens L, Charlier F, Vankelecom B, Dewilde W, Wijns W. Unifocal right-sided ablation treatment for neurally mediated syncope and functional sinus node dysfunction under computed tomographic guidance. Circulation: Arrhythmia and Electrophysiology. 2018;11:e006604. [DOI] [PubMed] [Google Scholar]
- 68.Qin M, Zhang Y, Liu X, Jiang WF, Wu SH, Po S. Atrial Ganglionated Plexus Modification: A Novel Approach to Treat Symptomatic Sinus Bradycardia. JACC Clin Electrophysiol. 2017;3:950–959. doi: 10.1016/j.jacep.2017.01.022 [DOI] [PubMed] [Google Scholar]
- 69.Khiabani AJ, Greenberg JW, Hansalia VH, Schuessler RB, Melby SJ, Damiano RJ Jr. Late Outcomes of Surgical Ablation for Inappropriate Sinus Tachycardia. The Annals of thoracic surgery. 2019;108:1162–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Taketani T, Wolf RK, Garrett JV. Partial cardiac denervation and sinus node modification for inappropriate sinus tachycardia. The Annals of thoracic surgery. 2007;84:652–654. [DOI] [PubMed] [Google Scholar]
- 71.Olshansky B, Sullivan RM. Conventional management of inappropriate sinus tachycardia. J Interv Card Electrophysiol. 2016;46:43–45. doi: 10.1007/s10840-015-0034-0 [DOI] [PubMed] [Google Scholar]
- 72.Femenía F, Baranchuk A, Morillo CA. Inappropriate sinus tachycardia: current therapeutic options. Cardiol Rev. 2012;20:8–14. doi: 10.1097/CRD.0b013e31822f0b3e [DOI] [PubMed] [Google Scholar]
- 73.Jones PK, Gibbons CH. The role of autonomic testing in syncope. Auton Neurosci. 2014;184:40–45. doi: 10.1016/j.autneu.2014.05.011 [DOI] [PubMed] [Google Scholar]
- 74.Feigofsky S, Fedorowski A. Defining Cardiac Dysautonomia - Different Types, Overlap Syndromes; Case-based Presentations. J Atr Fibrillation. 2020;13:2403. doi: 10.4022/jafib.2403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Olshansky B, Sullivan RM. Inappropriate sinus tachycardia. J Am Coll Cardiol. 2013;61:793–801. doi: 10.1016/j.jacc.2012.07.074 [DOI] [PubMed] [Google Scholar]
- 76.Low PA, Vernino S, Suarez G. Autonomic dysfunction in peripheral nerve disease. Muscle Nerve. 2003;27:646–661. [DOI] [PubMed] [Google Scholar]
- 77.Low PA. Testing the autonomic nervous system. SeminNeurol. 2003;23:407–421. [DOI] [PubMed] [Google Scholar]
- 78.Low PA. Prevalence of orthostatic hypotension. ClinAutonRes. 2008;18 Suppl 1:8–13. [DOI] [PubMed] [Google Scholar]
- 79.Angelone A, Coulter NA. Respiratory sinus arrhythmia: a frequency dependent phenomenon. JApplPhysiol. 1964;19:479–482. [DOI] [PubMed] [Google Scholar]
- 80.Davies CTM, Neilson JMM. Sinus arrhythmia in man at rest. JApplPhysiol. 1967;22:947–955. [DOI] [PubMed] [Google Scholar]
- 81.Hilz MJ, Dutsch M. Quantitative studies of autonomic function. Muscle Nerve. 2006;33:6–20. [DOI] [PubMed] [Google Scholar]
- 82.Sandroni P, Benarroch EE, Low PA. Pharmacological dissection of components of the Valsalva maneuver in adrenergic failure. JApplPhysiol. 1991;71:1563–1567. [DOI] [PubMed] [Google Scholar]
- 83.Gibbons C, Freeman R. The evaluation of small fiber function-autonomic and quantitative sensory testing. NeurolClin. 2004;22:683–702, vii. [DOI] [PubMed] [Google Scholar]
- 84.Ewing DJ, Hume L, Campbell IW, Murray A, Neilson JM, Clarke BF. Autonomic mechanisms in the initial heart rate response to standing. Journal of Applied Physiology: Respiratory,Environmental & Exercise Physiology. 1980;49:809–814. [DOI] [PubMed] [Google Scholar]
- 85.Wieling W, Borst C, Karemaker JM, Dunning AJ . Testing for autonomic neuropathy: initial heart rate response to active and passive changes of posture. Clinical Physiology. 1985;5 Suppl 5:23–27. [PubMed] [Google Scholar]
- 86.Ajsmit A, Wieling W, Karemaker JM. Clinical approach to cardiovascular reflex testing. ClinicalScience. 1996;91:108–112. [DOI] [PubMed] [Google Scholar]
- 87.Sprangers RL, van Lieshout JJ, Karemaker JM, Wesseling KH, Wieling W. Circulatory responses to stand up: discrimination between the effects of respiration, orthostasis and exercise. ClinPhysiol. 1991;11:221–230. [DOI] [PubMed] [Google Scholar]
- 88.Freeman R, Wieling W, Axelrod FB, Benditt DG, Benarroch E, Biaggioni I, Cheshire WP, Chelimsky T, Cortelli P, Gibbons CH, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Auton Neurosci. 2011;161:46–48. doi: 10.1016/j.autneu.2011.02.004 [DOI] [PubMed] [Google Scholar]
- 89.Sutton R, Fedorowski A, Olshansky B, Gert van Dijk J, Abe H, Brignole M, de Lange F, Kenny RA, Lim PB, Moya A, et al. Tilt testing remains a valuable asset. Eur Heart J. 2021;42:1654–1660. doi: 10.1093/eurheartj/ehab084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vajapey R, Hutt Centeno E, Van Iterson EH, Ahmed HM, Mayuga KA. ST-segment changes during tilt table testing for postural tachycardia syndrome: correlation with exercise stress test results. Clin Auton Res. 2020;30:79–83. doi: 10.1007/s10286-019-00633-9 [DOI] [PubMed] [Google Scholar]
- 91.Abi-Samra F, Maloney JD, Fouad-Tarazi FM, Castle LW. The usefulness of head-up tilt testing and hemodynamic investigations in the workup of syncope of unknown origin. Pacing Clin Electrophysiol. 1988;11:1202–1214. doi: 10.1111/j.1540-8159.1988.tb03973.x [DOI] [PubMed] [Google Scholar]
- 92.Mayuga KA, Butters KB, Fouad-Tarazi F. Early versus late postural tachycardia: a re-evaluation of a syndrome. Clin Auton Res. 2008;18:155–157. doi: 10.1007/s10286-008-0472-1 [DOI] [PubMed] [Google Scholar]
- 93.Brignole M, Moya A, de Lange FJ, Deharo JC, Elliott PM, Fanciulli A, Fedorowski A, Furlan R, Kenny RA, Martin A, et al. Practical Instructions for the 2018 ESC Guidelines for the diagnosis and management of syncope. Eur Heart J. 2018;39:e43–e80. doi: 10.1093/eurheartj/ehy071 [DOI] [PubMed] [Google Scholar]
- 94.Mahalwar G, Courson J, Bhargava M, Mayuga KA. When only the best will do: 12-lead ECG differentiates atrial tachycardia from sinus tachycardia during Tilt Table Testing for presumed Postural Tachycardia Syndrome. Journal of the American College of Cardiology. 2021;77:2983–2983. doi: doi: 10.1016/S0735-1097(21)04338-234112328 [DOI] [Google Scholar]
- 95.Taub PR, Zadourian A, Lo HC, Ormiston CK, Golshan S, Hsu JC. Randomized Trial of Ivabradine in Patients With Hyperadrenergic Postural Orthostatic Tachycardia Syndrome. Journal of the American College of Cardiology. 2021;77:861–871. doi: doi: 10.1016/j.jacc.2020.12.029 [DOI] [PubMed] [Google Scholar]
- 96.Low PA, Caskey PE, Tuck RR, Fealey RD, Dyck PJ. Quantitative sudomotor axon reflex test in normal and neuropathic subjects. AnnNeurol. 1983;14:573–580. [DOI] [PubMed] [Google Scholar]
- 97.Kennedy WR, Sakuta M, Sutherland D, Goetz FC. Quantitation of the sweating deficiency in diabetes mellitus. AnnNeurol. 1984;15:482–488. [DOI] [PubMed] [Google Scholar]
- 98.Gibbons CH, Freeman R, Tecilazich F, Dinh T, Lyons TE, Gnardellis C, Veves A. The evolving natural history of neurophysiologic function in patients with well-controlled diabetes. Journal of the peripheral nervous system : JPNS. 2013;18:153–161. doi: 10.1111/jns5.12021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.De Riva C, Virgili F, Frigato F. Na+, K+ ATPase activity in red cells predicts the recurrence of hyperthyroidism in patients with Graves’ disease. J Endocrinol Invest. 1995;18:683–689. doi: 10.1007/BF03349789 [DOI] [PubMed] [Google Scholar]
- 100.Galloway A, Li H, Vanderlinde-Wood M, Khan M, Benbrook A, Liles C, Zillner C, Rao V, Cunningham MW, Yu X, et al. Activating autoantibodies to the beta1/2-adrenergic and M2 muscarinic receptors associate with atrial tachyarrhythmias in patients with hyperthyroidism. Endocrine. 2015;49:457–463. doi: 10.1007/s12020-014-0495-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chiale PA, Garro HA, Schmidberg J, Sanchez RA, Acunzo RS, Lago M, Levy G, Levin M. Inappropriate sinus tachycardia may be related to an immunologic disorder involving cardiac beta andrenergic receptors. Heart Rhythm. 2006;3:1182–1186. doi: 10.1016/j.hrthm.2006.06.011 [DOI] [PubMed] [Google Scholar]
- 102.Li H, Yu X, Liles C, Khan M, Vanderlinde-Wood M, Galloway A, Zillner C, Benbrook A, Reim S, Collier D, et al. Autoimmune basis for postural tachycardia syndrome. J Am Heart Assoc. 2014;3:e000755. doi: 10.1161/JAHA.113.000755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fedorowski A, Li H, Yu X, Koelsch KA, Harris VM, Liles C, Murphy TA, Quadri SMS, Scofield RH, Sutton R, et al. Antiadrenergic autoimmunity in postural tachycardia syndrome. Europace. 2017;19:1211–1219. doi: 10.1093/europace/euw154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kharraziha I, Axelsson J, Ricci F, Di Martino G, Persson M, Sutton R, Fedorowski A, Hamrefors V. Serum Activity Against G Protein-Coupled Receptors and Severity of Orthostatic Symptoms in Postural Orthostatic Tachycardia Syndrome. J Am Heart Assoc. 2020;9:e015989. doi: 10.1161/JAHA.120.015989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yu X, Li H, Murphy TA, Nuss Z, Liles J, Liles C, Aston CE, Raj SR, Fedorowski A, Kem DC. Angiotensin II Type 1 Receptor Autoantibodies in Postural Tachycardia Syndrome. J Am Heart Assoc. 2018;7. doi: 10.1161/JAHA.117.008351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gunning WT 3rd, Kvale H, Kramer PM, Karabin BL, Grubb BP. Postural Orthostatic Tachycardia Syndrome Is Associated With Elevated G-Protein Coupled Receptor Autoantibodies. J Am Heart Assoc. 2019;8:e013602. doi: 10.1161/JAHA.119.013602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li H, Zhang G, Zhou L, Nuss Z, Beel M, Hines B, Murphy T, Liles J, Zhang L, Kem DC, et al. Adrenergic Autoantibody-Induced Postural Tachycardia Syndrome in Rabbits. J Am Heart Assoc. 2019;8:e013006. doi: 10.1161/JAHA.119.013006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Blitshteyn S. Autoimmune markers and autoimmune disorders in patients with postural tachycardia syndrome (POTS). Lupus. 2015;24:1364–1369. doi: 10.1177/0961203315587566 [DOI] [PubMed] [Google Scholar]
- 109.Schofield JR, Chemali KR. Intravenous Immunoglobulin Therapy in Refractory Autoimmune Dysautonomias: A Retrospective Analysis of 38 Patients. Am J Ther. 2019;26:570–582. doi: 10.1097/MJT.0000000000000778 [DOI] [PubMed] [Google Scholar]
- 110.Lee HC, Huang KT, Wang XL, Shen WK. Autoantibodies and cardiac arrhythmias. Heart Rhythm. 2011;8:1788–1795. doi: 10.1016/j.hrthm.2011.06.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ruzieh M, Batizy L, Dasa O, Oostra C, Grubb B. The role of autoantibodies in the syndromes of orthostatic intolerance: a systematic review. Scand Cardiovasc J. 2017;51:243–247. doi: 10.1080/14017431.2017.1355068 [DOI] [PubMed] [Google Scholar]
- 112.Theoharides TC, Valent P, Akin C. Mast Cells, Mastocytosis, and Related Disorders. N Engl J Med. 2015;373:163–172. doi: 10.1056/NEJMra1409760 [DOI] [PubMed] [Google Scholar]
- 113.Kohno R, Cannom DS, Olshansky B, Xi SC, Krishnappa D, Adkisson WO, Norby FL, Fedorowski A, Benditt DG. Mast Cell Activation Disorder and Postural Orthostatic Tachycardia Syndrome: A Clinical Association. J Am Heart Assoc. 2021:e021002. doi: 10.1161/JAHA.121.021002 [DOI] [PMC free article] [PubMed]
- 114.Gardiner SM, Kemp PA, March JE, Woolley J, Bennett T. The influence of antibodies to TNF-alpha and IL-1beta on haemodynamic responses to the cytokines, and to lipopolysaccharide, in conscious rats. Br J Pharmacol. 1998;125:1543–1550. doi: 10.1038/sj.bjp.0702250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Vigorito C, Russo P, Picotti GB, Chiariello M, Poto S, Marone G. Cardiovascular effects of histamine infusion in man. J Cardiovasc Pharmacol. 1983;5:531–537. doi: 10.1097/00005344-198307000-00004 [DOI] [PubMed] [Google Scholar]
- 116.Gergs U, Bernhardt G, Buchwalow IB, Edler H, Froba J, Keller M, Kirchhefer U, Kohler F, Misslinger N, Wache H, et al. Initial Characterization of Transgenic Mice Overexpressing Human Histamine H2 Receptors. J Pharmacol Exp Ther. 2019;369:129–141. doi: 10.1124/jpet.118.255711 [DOI] [PubMed] [Google Scholar]
- 117.Sampson HA, Munoz-Furlong A, Campbell RL, Adkinson NF Jr, Bock SA, Branum A, Brown SG, Camargo CA Jr, Cydulka R, Galli SJ, et al. Second symposium on the definition and management of anaphylaxis: summary report--Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol. 2006;117:391–397. doi: 10.1016/j.jaci.2005.12.1303 [DOI] [PubMed] [Google Scholar]
- 118.Valent P, Akin C, Metcalfe DD. Mastocytosis: 2016 updated WHO classification and novel emerging treatment concepts. Blood. 2017;129:1420–1427. doi: 10.1182/blood-2016-09-731893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Valent P, Escribano L, Broesby-Olsen S, Hartmann K, Grattan C, Brockow K, Niedoszytko M, Nedoszytko B, Oude Elberink JN, Kristensen T, et al. Proposed diagnostic algorithm for patients with suspected mastocytosis: a proposal of the European Competence Network on Mastocytosis. Allergy. 2014;69:1267–1274. doi: 10.1111/all.12436 [DOI] [PubMed] [Google Scholar]
- 120.Brockow K, Jofer C, Behrendt H, Ring J. Anaphylaxis in patients with mastocytosis: a study on history, clinical features and risk factors in 120 patients. Allergy. 2008;63:226–232. doi: 10.1111/j.1398-9995.2007.01569.x [DOI] [PubMed] [Google Scholar]
- 121.Valent P, Bonadonna P, Hartmann K, Broesby-Olsen S, Brockow K, Butterfield JH, Triggiani M, Lyons JJ, Oude Elberink JNG, Arock M, et al. Why the 20% + 2 Tryptase Formula Is a Diagnostic Gold Standard for Severe Systemic Mast Cell Activation and Mast Cell Activation Syndrome. Int Arch Allergy Immunol. 2019;180:44–51. doi: 10.1159/000501079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Valent P, Akin C, Arock M, Brockow K, Butterfield JH, Carter MC, Castells M, Escribano L, Hartmann K, Lieberman P, et al. Definitions, criteria and global classification of mast cell disorders with special reference to mast cell activation syndromes: a consensus proposal. Int Arch Allergy Immunol. 2012;157:215–225. doi: 10.1159/000328760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Akin C, Valent P, Metcalfe DD. Mast cell activation syndrome: Proposed diagnostic criteria. J Allergy Clin Immunol. 2010;126:1099–1104 e1094. doi: 10.1016/j.jaci.2010.08.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Afrin LB, Ackerley MB, Bluestein LS, Brewer JH, Brook JB, Buchanan AD, Cuni JR, Davey WP, Dempsey TT, Dorff SR, et al. Diagnosis of mast cell activation syndrome: a global “consensus-2”. Diagnosis (Berl). 2021;8:137–152. doi: 10.1515/dx-2020-0005 [DOI] [PubMed] [Google Scholar]
- 125.Afrin LB, Ackerley MB, Bluestein LS, Brewer JH, Brook JB, Buchanan AD, Cuni JR, Davey WP, Dempsey TT, Dorff SR, et al. Diagnosis of mast cell activation syndrome: a global “consensus-2”. Diagnosis (Berl). 2020. doi: 10.1515/dx-2020-0005 [DOI] [PubMed]
- 126.Kohn A, Chang C. The Relationship Between Hypermobile Ehlers-Danlos Syndrome (hEDS), Postural Orthostatic Tachycardia Syndrome (POTS), and Mast Cell Activation Syndrome (MCAS). Clin Rev Allergy Immunol. 2020;58:273–297. doi: 10.1007/s12016-019-08755-8 [DOI] [PubMed] [Google Scholar]
- 127.Shibao C, Arzubiaga C, Roberts LJ, 2nd, Raj S, Black B, Harris P, Biaggioni I. Hyperadrenergic postural tachycardia syndrome in mast cell activation disorders. Hypertension. 2005;45:385–390. doi: 10.1161/01.HYP.0000158259.68614.40 [DOI] [PubMed] [Google Scholar]
- 128.Chapter Young W. 16: Endocrine Hypertension. In. Melmed SPK, Larsen P, Kronenberg H. Williams Textbook Of Endocrinology. 13th ed. Elsevier, 2016: 556–588 [Google Scholar]
- 129.Anderson GH Jr, Blakeman N, Streeten DH. The effect of age on prevalence of secondary forms of hypertension in 4429 consecutively referred patients. J Hypertens. 1994;12:609–615. doi: 10.1097/00004872-199405000-00015 [DOI] [PubMed] [Google Scholar]
- 130.Omura M, Saito J, Yamaguchi K, Kakuta Y, Nishikawa T. Prospective study on the prevalence of secondary hypertension among hypertensive patients visiting a general outpatient clinic in Japan. Hypertens Res. 2004;27:193–202. doi: 10.1291/hypres.27.193 [DOI] [PubMed] [Google Scholar]
- 131.Gruber LM, Hartman RP, Thompson GB, McKenzie TJ, Lyden ML, Dy BM, Young WF, Bancos I. Pheochromocytoma Characteristics and Behavior Differ Depending on Method of Discovery. J Clin Endocrinol Metab. 2019;104:1386–1393. doi: 10.1210/jc.2018-01707 [DOI] [PubMed] [Google Scholar]
- 132.Stein PP, Black HR. A simplified diagnostic approach to pheochromocytoma. A review of the literature and report of one institution’s experience. Medicine (Baltimore). 1991;70:46–66. doi: 10.1097/00005792-199101000-00004 [DOI] [PubMed] [Google Scholar]
- 133.Nazari MA, Rosenblum JS, Haigney MC, Rosing DR, Pacak K. Pathophysiology and Acute Management of Tachyarrhythmias in Pheochromocytoma: JACC Review Topic of the Week. J Am Coll Cardiol. 2020;76:451–464. doi: 10.1016/j.jacc.2020.04.080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Liao WB, Liu CF, Chiang CW, Kung CT, Lee CW. Cardiovascular manifestations of pheochromocytoma. Am J Emerg Med. 2000;18:622–625. doi: 10.1053/ajem.2000.7341 [DOI] [PubMed] [Google Scholar]
- 135.Delekta J, Riahi S, Eschen O. Rare cause of ventricular tachycardia: Pheochromocytoma. J Cardiol Cases. 2015;11:62–65. doi: 10.1016/j.jccase.2014.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Michaels RD, Hays JH, O’Brian JT, Shakir KM. Pheochromocytoma associated ventricular tachycardia blocked with atenolol. J Endocrinol Invest. 1990;13:943–947. doi: 10.1007/BF03349666 [DOI] [PubMed] [Google Scholar]
- 137.Li J, Huang K, Jiang P, Chen Y, Gan H, Su X. Recurrent ventricular tachycardia as initial presentation of pheochromocytoma: A case report and literature review. J Electrocardiol. 2020;59:112–115. doi: 10.1016/j.jelectrocard.2020.02.004 [DOI] [PubMed] [Google Scholar]
- 138.ter Bekke RM, Crijns HJ, Kroon AA, Gorgels AP. Pheochromocytoma-induced ventricular tachycardia and reversible cardiomyopathy. Int J Cardiol. 2011;147:145–146. doi: 10.1016/j.ijcard.2009.11.020 [DOI] [PubMed] [Google Scholar]
- 139.Paulin FL, Klein GJ, Gula LJ, Skanes AC, Yee R, Krahn AD. QT prolongation and monomorphic VT caused by pheochromocytoma. J Cardiovasc Electrophysiol. 2009;20:931–934. doi: 10.1111/j.1540-8167.2008.01405.x [DOI] [PubMed] [Google Scholar]
- 140.Kim JJ, Valdes SO, Kertesz NJ, Cannon BC. Isolated junctional tachycardia in a patient with pheochromocytoma: an unusual presentation of an uncommon disease. Pediatr Cardiol. 2008;29:986–988. doi: 10.1007/s00246-007-9134-7 [DOI] [PubMed] [Google Scholar]
- 141.Shawa H, Bajaj M, Cunningham GR. Pheochromocytoma-induced atrial tachycardia leading to cardiogenic shock and cardiac arrest: resolution with atrioventricular node ablation and pacemaker placement. Tex Heart Inst J. 2014;41:660–663. doi: 10.14503/THIJ-13-3692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lenders JW, Duh QY, Eisenhofer G, Gimenez-Roqueplo AP, Grebe SK, Murad MH, Naruse M, Pacak K, Young WF Jr, Endocrine S. Pheochromocytoma and paraganglioma: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2014;99:1915–1942. doi: 10.1210/jc.2014-1498 [DOI] [PubMed] [Google Scholar]
- 143.Reyes HA, Paquin JJ, Harris DM. Pheochromocytoma, “the Great Masquerader,” Presenting as Severe Acute Decompensated Heart Failure in a Young Patient. Case Rep Cardiol. 2018;2018:8767801. doi: 10.1155/2018/8767801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Park S, Kim DG, Suh GY, Park WJ, Jang SH, Hwang YI, Han SJ, Jeong HH, Lee CH, Jung KS. Significance of new-onset prolonged sinus tachycardia in a medical intensive care unit: a prospective observational study. J Crit Care. 2011;26:534 e531–534 e538. doi: 10.1016/j.jcrc.2011.01.001 [DOI] [PubMed] [Google Scholar]
- 145.Parker MM, Shelhamer JH, Natanson C, Alling DW, Parrillo JE. Serial cardiovascular variables in survivors and nonsurvivors of human septic shock: heart rate as an early predictor of prognosis. Crit Care Med. 1987;15:923–929. doi: 10.1097/00003246-198710000-00006 [DOI] [PubMed] [Google Scholar]
- 146.Hoke RS, Muller-Werdan U, Lautenschlager C, Werdan K, Ebelt H. Heart rate as an independent risk factor in patients with multiple organ dysfunction: a prospective, observational study. Clin Res Cardiol. 2012;101:139–147. doi: 10.1007/s00392-011-0375-3 [DOI] [PubMed] [Google Scholar]
- 147.Sander O, Welters ID, Foex P, Sear JW. Impact of prolonged elevated heart rate on incidence of major cardiac events in critically ill patients with a high risk of cardiac complications. Crit Care Med. 2005;33:81–88; discussion 241–242. doi: 10.1097/01.ccm.0000150028.64264.14 [DOI] [PubMed] [Google Scholar]
- 148.Morelli A, Ertmer C, Westphal M, Rehberg S, Kampmeier T, Ligges S, Orecchioni A, D’Egidio A, D’Ippoliti F, Raffone C, et al. Effect of heart rate control with esmolol on hemodynamic and clinical outcomes in patients with septic shock: a randomized clinical trial. JAMA. 2013;310:1683–1691. doi: 10.1001/jama.2013.278477 [DOI] [PubMed] [Google Scholar]
- 149.Herrmann S, Schnorr S, Ludwig A. HCN channels--modulators of cardiac and neuronal excitability. Int J Mol Sci. 2015;16:1429–1447. doi: 10.3390/ijms16011429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wei C, Al Kattani N, Louis H, Albuisson E, Levy B, Kimmoun A. If Channel Inhibition With Ivabradine Does Not Improve Cardiac and Vascular Function in Experimental Septic Shock. Shock. 2016;46:297–303. doi: 10.1097/SHK.0000000000000593 [DOI] [PubMed] [Google Scholar]
- 151.Miranda ML, Balarini MM, Balthazar DS, Paes LS, Santos MS, Bouskela E. Ivabradine Attenuates the Microcirculatory Derangements Evoked by Experimental Sepsis. Anesthesiology. 2017;126:140–149. doi: 10.1097/ALN.0000000000001431 [DOI] [PubMed] [Google Scholar]
- 152.Nuding S, Schroder J, Presek P, Wienke A, Muller-Werdan U, Ebelt H, Werdan K. Reducing Elevated Heart Rates in Patients with Multiple Organ Dysfunction Syndrome with The If (Funny Channel Current) Inhibitor Ivabradine. Shock. 2018;49:402–411. doi: 10.1097/SHK.0000000000000992 [DOI] [PubMed] [Google Scholar]
- 153.Wells PS, Anderson DR, Rodger M, Ginsberg JS, Kearon C, Gent M, Turpie AG, Bormanis J, Weitz J, Chamberlain M, et al. Derivation of a simple clinical model to categorize patients probability of pulmonary embolism: increasing the models utility with the SimpliRED D-dimer. Thromb Haemost. 2000;83:416–420. [PubMed] [Google Scholar]
- 154.Stein PD, Matta F, Ekkah M, Saleh T, Janjua M, Patel YR, Khadra H. Electrocardiogram in pneumonia. Am J Cardiol. 2012;110:1836–1840. doi: 10.1016/j.amjcard.2012.08.019 [DOI] [PubMed] [Google Scholar]
- 155.Gibson NS, Sohne M, Kruip MJ, Tick LW, Gerdes VE, Bossuyt PM, Wells PS, Buller HR, Christopher study i. Further validation and simplification of the Wells clinical decision rule in pulmonary embolism. Thromb Haemost. 2008;99:229–234. doi: 10.1160/TH07-05-0321 [DOI] [PubMed] [Google Scholar]
- 156.Grinblat J, Mechlis S, Lewitus Z. Organizing pneumonia-like process: an unusual observation in steroid responsive cases with features of chronic interstitial pneumonia. Chest. 1981;80:259–263. doi: 10.1378/chest.80.3.259 [DOI] [PubMed] [Google Scholar]
- 157.Mavridou D, Laws D. Respiratory bronchiolitis associated interstitial lung disease (RB-ILD): a case of an acute presentation. Thorax. 2004;59:910–911. doi: 10.1136/thx.2003.011080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Warnier MJ, Rutten FH, Numans ME, Kors JA, Tan HL, de Boer A, Hoes AW, De Bruin ML. Electrocardiographic characteristics of patients with chronic obstructive pulmonary disease. COPD. 2013;10:62–71. doi: 10.3109/15412555.2012.727918 [DOI] [PubMed] [Google Scholar]
- 159.Shih HT, Webb CR, Conway WA, Peterson E, Tilley B, Goldstein S. Frequency and significance of cardiac arrhythmias in chronic obstructive lung disease. Chest. 1988;94:44–48. doi: 10.1378/chest.94.1.44 [DOI] [PubMed] [Google Scholar]
- 160.Tirlapur VG, Mir MA. Nocturnal hypoxemia and associated electrocardiographic changes in patients with chronic obstructive airways disease. N Engl J Med. 1982;306:125–130. doi: 10.1056/NEJM198201213060301 [DOI] [PubMed] [Google Scholar]
- 161.Simon PM, Taha BH, Dempsey JA, Skatrud JB, Iber C. Role of vagal feedback from the lung in hypoxic-induced tachycardia in humans. J Appl Physiol (1985). 1995;78:1522–1530. doi: 10.1152/jappl.1995.78.4.1522 [DOI] [PubMed] [Google Scholar]
- 162.Salpeter SR, Ormiston TM, Salpeter EE. Cardiovascular effects of beta-agonists in patients with asthma and COPD: a meta-analysis. Chest. 2004;125:2309–2321. doi: 10.1378/chest.125.6.2309 [DOI] [PubMed] [Google Scholar]
- 163.Ricci F, Wollmer P, Engström G, Fedorowski A, Hamrefors V. Markers of cardiovascular autonomic dysfunction predict COPD in middle-aged subjects. European Respiratory Journal. 2018;51:1702481. doi: 10.1183/13993003.02481-2017 [DOI] [PubMed] [Google Scholar]
- 164.Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA. 2020;324:782–793. doi: 10.1001/jama.2020.12839 [DOI] [PubMed] [Google Scholar]
- 165.Miglis MG, Prieto T, Shaik R, Muppidi S, Sinn DI, Jaradeh S. A case report of postural tachycardia syndrome after COVID-19. Clin Auton Res. 2020;30:449–451. doi: 10.1007/s10286-020-00727-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Johansson M, Stahlberg M, Runold M, Nygren-Bonnier M, Nilsson J, Olshansky B, Bruchfeld J, Fedorowski A. Long-Haul Post-COVID-19 Symptoms Presenting as a Variant of Postural Orthostatic Tachycardia Syndrome: The Swedish Experience. JACC Case Rep. 2021;3:573–580. doi: 10.1016/j.jaccas.2021.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Fedorowski A. Postural orthostatic tachycardia syndrome: clinical presentation, aetiology and management. J Intern Med. 2019;285:352–366. doi: 10.1111/joim.12852 [DOI] [PubMed] [Google Scholar]
- 168.Goldstein DA. The possible association between COVID-19 and postural tachycardia syndrome. Heart Rhythm. 2021. doi: 10.1016/j.hrthm.2020.12.007 [DOI] [PMC free article] [PubMed]
- 169.Canas CA. The triggering of post-COVID-19 autoimmunity phenomena could be associated with both transient immunosuppression and an inappropriate form of immune reconstitution in susceptible individuals. Med Hypotheses. 2020;145:110345. doi: 10.1016/j.mehy.2020.110345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Olshansky B, Cannom D, Fedorowski A, Stewart J, Gibbons C, Sutton R, Shen WK, Muldowney J, Chung TH, Feigofsky S, et al. Postural Orthostatic Tachycardia Syndrome (POTS): A critical assessment. Prog Cardiovasc Dis. 2020;63:263–270. doi: 10.1016/j.pcad.2020.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Valent P, Akin C, Bonadonna P, Hartmann K, Brockow K, Niedoszytko M, Nedoszytko B, Siebenhaar F, Sperr WR, Oude Elberink JNG, et al. Proposed Diagnostic Algorithm for Patients with Suspected Mast Cell Activation Syndrome. J Allergy Clin Immunol Pract. 2019;7:1125–1133 e1121. doi: 10.1016/j.jaip.2019.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Garland EM, Gamboa A, Nwazue VC, Celedonio JE, Paranjape SY, Black BK, Okamoto LE, Shibao CA, Biaggioni I, Robertson D, et al. Effect of High Dietary Sodium Intake in Patients With Postural Tachycardia Syndrome. J Am Coll Cardiol. 2021;77:2174–2184. doi: 10.1016/j.jacc.2021.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.George SA, Bivens TB, Howden EJ, Saleem Y, Galbreath MM, Hendrickson D, Fu Q, Levine BD. The international POTS registry: Evaluating the efficacy of an exercise training intervention in a community setting. Heart Rhythm. 2016;13:943–950. doi: 10.1016/j.hrthm.2015.12.012 [DOI] [PubMed] [Google Scholar]
- 174.Raj SR. Row, row, row your way to treating postural tachycardia syndrome. Heart Rhythm. 2016;13:951–952. doi: 10.1016/j.hrthm.2015.12.039 [DOI] [PubMed] [Google Scholar]
- 175.Bourne KM, Sheldon RS, Hall J, Lloyd M, Kogut K, Sheikh N, Jorge J, Ng J, Exner DV, Tyberg JV, et al. Compression Garment Reduces Orthostatic Tachycardia and Symptoms in Patients With Postural Orthostatic Tachycardia Syndrome. J Am Coll Cardiol. 2021;77:285–296. doi: 10.1016/j.jacc.2020.11.040 [DOI] [PubMed] [Google Scholar]
- 176.Flint B, Baker C, Freeston M, Newton JL. Level of psychosocial impairment predicts early response to treatment in vasovagal syncope. Europace. 2009;11:231–236. doi: 10.1093/europace/eun332 [DOI] [PubMed] [Google Scholar]
- 177.Gracie J, Baker C, Freeston MH, Newton JL. The role of psychological factors in the aetiology and treatment of vasovagal syncope. Indian Pacing Electrophysiol J. 2004;4:79–84. [PMC free article] [PubMed] [Google Scholar]
- 178.Newton JL, Kenny RA, Baker CR. Cognitive behavioural therapy as a potential treatment for vasovagal/neurocardiogenic syncope--a pilot study. Europace. 2003;5:299–301. doi: 10.1016/s1099-5129(03)00030-8 [DOI] [PubMed] [Google Scholar]
- 179.McGrady AV, Bush EG, Grubb BP. Outcome of biofeedback-assisted relaxation for neurocardiogenic syncope and headache: a clinical replication series. Appl Psychophysiol Biofeedback. 1997;22:63–72. doi: 10.1023/a:1026241826589 [DOI] [PubMed] [Google Scholar]
- 180.Van Dijk N, Velzeboer SC, Destree-Vonk A, Linzer M, Wieling W. Psychological treatment of malignant vasovagal syncope due to bloodphobia. Pacing Clin Electrophysiol. 2001;24:122–124. doi: 10.1046/j.1460-9592.2001.00122.x [DOI] [PubMed] [Google Scholar]
- 181.Sabin N. The use of applied tension and cognitive therapy to manage syncope (common faint) in an older adult. Aging Ment Health. 2001;5:92–94. doi: 10.1080/13607860020020690 [DOI] [PubMed] [Google Scholar]
- 182.Benrud-Larson LM, Dewar MS, Sandroni P, Rummans TA, Haythornthwaite JA, Low PA. Quality of life in patients with postural tachycardia syndrome. Mayo Clin Proc. 2002;77:531–537. doi: 10.4065/77.6.531 [DOI] [PubMed] [Google Scholar]
- 183.Bruce BK, Weiss KE, Harrison TE, Allman DA, Petersen MA, Luedkte CA, Fischer PR. Interdisciplinary Treatment of Maladaptive Behaviors Associated with Postural Orthostatic Tachycardia Syndrome (POTS): A Case Report. J Clin Psychol Med Settings. 2016;23:147–159. doi: 10.1007/s10880-015-9438-3 [DOI] [PubMed] [Google Scholar]
- 184.McTate EA, Weiss KE. Psychosocial Dimensions and Functioning in Youth With Postural Orthostatic Tachycardia Syndrome. Clin Pediatr (Phila). 2016;55:979–982. doi: 10.1177/0009922815616890 [DOI] [PubMed] [Google Scholar]
- 185.Fu Q, Levine BD. Exercise and non-pharmacological treatment of POTS. Auton Neurosci. 2018;215:20–27. doi: 10.1016/j.autneu.2018.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Keating EM, Antiel RM, Weiss KE, Wallace D, Antiel SJ, Fischer PR, Junghans-Rutelonis AN, Harbeck-Weber C. Parental Perceptions of Pediatric Pain and POTS-Related Disability. Clin Pediatr (Phila). 2017;56:1185–1192. doi: 10.1177/0009922816681137 [DOI] [PubMed] [Google Scholar]
- 187.Weiss KE, Junghans-Rutelonis AN, Aaron RV, Harbeck-Weber C, McTate E, Luedtke C, Bruce BK. Improving Distress and Behaviors for Parents of Adolescents With Chronic Pain Enrolled in an Intensive Interdisciplinary Pain Program. Clin J Pain. 2019;35:772–779. doi: 10.1097/AJP.0000000000000737 [DOI] [PubMed] [Google Scholar]
- 188.Bruce BK, Harrison TE, Bee SM, Luedtke CA, Porter CJ, Fischer PR, Hayes SE, Allman DA, Ale CM, Weiss KE. Improvement in Functioning and Psychological Distress in Adolescents With Postural Orthostatic Tachycardia Syndrome Following Interdisciplinary Treatment. Clin Pediatr (Phila). 2016;55:1300–1304. doi: 10.1177/0009922816638663 [DOI] [PubMed] [Google Scholar]
- 189.Hechler T, Kanstrup M, Holley AL, Simons LE, Wicksell R, Hirschfeld G, Zernikow B. Systematic Review on Intensive Interdisciplinary Pain Treatment of Children With Chronic Pain. Pediatrics. 2015;136:115–127. doi: 10.1542/peds.2014-3319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Bruce BK, Ale CM, Harrison TE, Bee S, Luedtke C, Geske J, Weiss KE. Getting Back to Living: Further Evidence for the Efficacy of an Interdisciplinary Pediatric Pain Treatment Program. Clin J Pain. 2017;33:535–542. doi: 10.1097/AJP.0000000000000433 [DOI] [PubMed] [Google Scholar]
- 191.Gatchel RJ, McGeary DD, McGeary CA, Lippe B. Interdisciplinary chronic pain management: past, present, and future. Am Psychol. 2014;69:119–130. doi: 10.1037/a0035514 [DOI] [PubMed] [Google Scholar]
- 192.Joyner MJ, Masuki S. POTS versus deconditioning: the same or different? Clinical Autonomic Research. 2008;18:300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.CONVERTINO VA BLOOMFIELD SA, GREENLEAF JE. An overview of the issues: physiological effects of bed rest and restricted physical activity. Medicine & Science in Sports & Exercise. 1997;29:187–190. [DOI] [PubMed] [Google Scholar]
- 194.Joyner MJ, Masuki S. POTS versus deconditioning: the same or different? Clin Auton Res. 2008;18:300–307. doi: 10.1007/s10286-008-0487-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Parsaik A, Allison TG, Singer W, Sletten DM, Joyner MJ, Benarroch EE, Low PA, Sandroni P. Deconditioning in patients with orthostatic intolerance. Neurology. 2012;79:1435–1439. doi: 10.1212/WNL.0b013e31826d5f95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Van Iterson EH, Laffin LJ, Mayuga KA, Hutt Centeno E, Ahmed T, Cho L, Ahmed HM. High Submaximal Exercise Heart Rate Impacts Exercise Intolerance in the Postural Orthostatic Tachycardia Syndrome. J Cardiopulm Rehabil Prev. 2020;40:195–201. doi: 10.1097/HCR.0000000000000485 [DOI] [PubMed] [Google Scholar]
- 197.Raj SR, Black BK, Biaggioni I, Paranjape SY, Ramirez M, Dupont WD, Robertson D. Propranolol decreases tachycardia and improves symptoms in the postural tachycardia syndrome: less is more. Circulation. 2009;120:725–734. doi: 10.1161/CIRCULATIONAHA.108.846501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Mathew ST, Po SS, Thadani U. Inappropriate sinus tachycardia-symptom and heart rate reduction with ivabradine: A pooled analysis of prospective studies. Heart Rhythm. 2018;15:240–247. doi: 10.1016/j.hrthm.2017.10.004 [DOI] [PubMed] [Google Scholar]
- 199.Cappato R, Castelvecchio S, Ricci C, Bianco E, Vitali-Serdoz L, Gnecchi-Ruscone T, Pittalis M, De Ambroggi L, Baruscotti M, Gaeta M, et al. Clinical efficacy of ivabradine in patients with inappropriate sinus tachycardia: a prospective, randomized, placebo-controlled, double-blind, crossover evaluation. J Am Coll Cardiol. 2012;60:1323–1329. doi: 10.1016/j.jacc.2012.06.031 [DOI] [PubMed] [Google Scholar]
- 200.Ptaszynski P, Kaczmarek K, Ruta J, Klingenheben T, Wranicz JK. Metoprolol succinate vs. ivabradine in the treatment of inappropriate sinus tachycardia in patients unresponsive to previous pharmacological therapy. Europace. 2013;15:116–121. doi: 10.1093/europace/eus204 [DOI] [PubMed] [Google Scholar]
- 201.Marrouche NF, Beheiry S, Tomassoni G, Cole C, Bash D, Dresing T, Saliba W, Abdul-Karim A, Tchou P, Schweikert R, et al. Three-dimensional nonfluoroscopic mapping and ablation of inappropriate sinus tachycardia. Procedural strategies and long-term outcome. J Am Coll Cardiol. 2002;39:1046–1054. doi: 10.1016/s0735-1097(02)01703-5 [DOI] [PubMed] [Google Scholar]
- 202.Shen WK, Low PA, Jahangir A, Munger TM, Friedman PA, Osborn MJ, Stanton MS, Packer DL, Rea RF, Hammill SC. Is sinus node modification appropriate for inappropriate sinus tachycardia with features of postural orthostatic tachycardia syndrome? Pacing Clin Electrophysiol. 2001;24:217–230. doi: 10.1046/j.1460-9592.2001.00217.x [DOI] [PubMed] [Google Scholar]
- 203.Romeo E, Grimaldi N, Sarubbi B, D’Alto M, Santarpia G, Scognamiglio G, Russo MG, Calabro R. A pediatric case of cardiomyopathy induced by inappropriate sinus tachycardia: efficacy of ivabradine. Pediatr Cardiol. 2011;32:842–845. doi: 10.1007/s00246-011-9964-1 [DOI] [PubMed] [Google Scholar]
- 204.Winum PF, Cayla G, Rubini M, Beck L, Messner-Pellenc P. A case of cardiomyopathy induced by inappropriate sinus tachycardia and cured by ivabradine. Pacing Clin Electrophysiol. 2009;32:942–944. doi: 10.1111/j.1540-8159.2009.02414.x [DOI] [PubMed] [Google Scholar]
- 205.de Asmundis C, Chierchia GB, Sieira J, Stroker E, Umbrain V, Poelaert J, Brugada P, La Meir M. Sinus Node Sparing Novel Hybrid Approach for Treatment of Inappropriate Sinus Tachycardia/Postural Orthostatic Sinus Tachycardia With New Electrophysiological Finding. Am J Cardiol. 2019;124:224–232. doi: 10.1016/j.amjcard.2019.04.019 [DOI] [PubMed] [Google Scholar]
- 206.Lee RJ, Kalman JM, Fitzpatrick AP, Epstein LM, Fisher WG, Olgin JE, Lesh MD, Scheinman MM. Radiofrequency catheter modification of the sinus node for “inappropriate” sinus tachycardia. Circulation. 1995;92:2919–2928. doi: 10.1161/01.cir.92.10.2919 [DOI] [PubMed] [Google Scholar]
- 207.Takemoto M, Mukai Y, Inoue S, Matoba T, Nishizaka M, Ide T, Chishaki A, Sunagawa K. Usefulness of non-contact mapping for radiofrequency catheter ablation of inappropriate sinus tachycardia: new procedural strategy and long-term clinical outcome. Intern Med. 2012;51:357–362. doi: 10.2169/internalmedicine.51.5882 [DOI] [PubMed] [Google Scholar]
- 208.Rodriguez-Manero M, Kreidieh B, Al Rifai M, Ibarra-Cortez S, Schurmann P, Alvarez PA, Fernandez-Lopez XA, Garcia-Seara J, Martinez-Sande L, Gonzalez-Juanatey JR, et al. Ablation of Inappropriate Sinus Tachycardia: A Systematic Review of the Literature. JACC Clin Electrophysiol. 2017;3:253–265. doi: 10.1016/j.jacep.2016.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Sanchez-Quintana D, Cabrera JA, Climent V, Farre J, Weiglein A, Ho SY. How close are the phrenic nerves to cardiac structures? Implications for cardiac interventionalists. J Cardiovasc Electrophysiol. 2005;16:309–313. doi: 10.1046/j.1540-8167.2005.40759.x [DOI] [PubMed] [Google Scholar]
- 210.Gianni C, Di Biase L, Mohanty S, Gokoglan Y, Gunes MF, Horton R, Hranitzky PM, Burkhardt JD, Natale A. Catheter ablation of inappropriate sinus tachycardia. J Interv Card Electrophysiol. 2016;46:63–69. doi: 10.1007/s10840-015-0040-2 [DOI] [PubMed] [Google Scholar]
- 211.Koplan BA, Parkash R, Couper G, Stevenson WG. Combined epicardial-endocardial approach to ablation of inappropriate sinus tachycardia. J Cardiovasc Electrophysiol. 2004;15:237–240. doi: 10.1046/j.1540-8167.2004.03370.x [DOI] [PubMed] [Google Scholar]
- 212.Ibarra-Cortez SH, Rodriguez-Manero M, Kreidieh B, Schurmann P, Dave AS, Valderrabano M. Strategies for phrenic nerve preservation during ablation of inappropriate sinus tachycardia. Heart Rhythm. 2016;13:1238–1245. doi: 10.1016/j.hrthm.2016.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Jacobson JT, Kraus A, Lee R, Goldberger JJ. Epicardial/endocardial sinus node ablation after failed endocardial ablation for the treatment of inappropriate sinus tachycardia. J Cardiovasc Electrophysiol. 2014;25:236–241. doi: 10.1111/jce.12318 [DOI] [PubMed] [Google Scholar]
- 214.Rubenstein JC, Kim MH, Jacobson JT. A novel method for sinus node modification and phrenic nerve protection in resistant cases. J Cardiovasc Electrophysiol. 2009;20:689–691. doi: 10.1111/j.1540-8167.2008.01383.x [DOI] [PubMed] [Google Scholar]
- 215.Aalaei-Andabili SH, Miles WM, Burkart TA, Panna ME, Conti JB, McKillop MS, Beaver TM. Minimally invasive thoracoscopic surgery is an effective approach for treating inappropriate sinus tachycardia. J Cardiovasc Electrophysiol. 2019;30:1297–1303. doi: 10.1111/jce.13970 [DOI] [PubMed] [Google Scholar]
- 216.Beaver TM, Miles WM, Conti JB, Kogan A, Burkart TA, Woo GW, Saxonhouse SJ. Minimally invasive ablation of a migrating focus of inappropriate sinus tachycardia. J Thorac Cardiovasc Surg. 2010;139:506–507. doi: 10.1016/j.jtcvs.2009.01.002 [DOI] [PubMed] [Google Scholar]
- 217.Khiabani AJ, Greenberg JW, Hansalia VH, Schuessler RB, Melby SJ, Damiano RJ Jr., Late Outcomes of Surgical Ablation for Inappropriate Sinus Tachycardia. Ann Thorac Surg. 2019;108:1162–1168. doi: 10.1016/j.athoracsur.2019.03.103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Kreisel D, Bailey M, Lindsay BD, Damiano RJ Jr. A minimally invasive surgical treatment for inappropriate sinus tachycardia. J Thorac Cardiovasc Surg. 2005;130:598–599. doi: 10.1016/j.jtcvs.2004.12.026 [DOI] [PubMed] [Google Scholar]
- 219.Schleifer JW, Jaroszewski DE, Shah N, Scott LR. Long-term follow-up of minimally invasive video-assisted thoracoscopic surgery with epicardial radiofrequency ablation for complex cases of inappropriate sinus tachycardia. HeartRhythm Case Rep. 2015;1:477–480. doi: 10.1016/j.hrcr.2015.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Taketani T, Wolf RK, Garrett JV. Partial cardiac denervation and sinus node modification for inappropriate sinus tachycardia. Ann Thorac Surg. 2007;84:652–654. doi: 10.1016/j.athoracsur.2007.03.027 [DOI] [PubMed] [Google Scholar]
- 221.Huang HD, Tamarisa R, Mathur N, Alam M, Makkar A, Birnbaum Y, Afshar-Kharaghan H. Stellate ganglion block: a therapeutic alternative for patients with medically refractory inappropriate sinus tachycardia? J Electrocardiol. 2013;46:693–696. doi: 10.1016/j.jelectrocard.2012.12.010 [DOI] [PubMed] [Google Scholar]
- 222.Kiuchi MG, Souto HB, Kiuchi T, Chen S. Case Report: Renal Sympathetic Denervation as a Tool for the Treatment of Refractory Inappropriate Sinus Tachycardia. Medicine (Baltimore). 2015;94:e2094. doi: 10.1097/MD.0000000000002094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Cha YM, Li X, Yang M, Han J, Wu G, Kapa SC, McLeod CJ, Noseworthy PA, Mulpuru SK, Asirvatham SJ, et al. Stellate ganglion block and cardiac sympathetic denervation in patients with inappropriate sinus tachycardia. J Cardiovasc Electrophysiol. 2019;30:2920–2928. doi: 10.1111/jce.14233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.de Asmundis C, Chierchia GB, Lakkireddy D, Romeya A, Okum E, Gandhi G, Sieira J, Vloka M, Jones SD, Shah H, et al. Sinus node sparing novel hybrid approach for treatment of inappropriate sinus tachycardia/postural sinus tachycardia: multicenter experience. J Interv Card Electrophysiol. 2021. doi: 10.1007/s10840-021-01044-5 [DOI] [PMC free article] [PubMed]
- 225.Frankel DS, Lin D, Anastasio N, Mountantonakis SE, Dixit S, Gerstenfeld EP, Hutchinson MD, Riley MP, Marchlinski FE, Callans DJ. Frequent additional tachyarrhythmias in patients with inappropriate sinus tachycardia undergoing sinus node modification: an important cause of symptom recurrence. J Cardiovasc Electrophysiol. 2012;23:835–839. doi: 10.1111/j.1540-8167.2012.02297.x [DOI] [PubMed] [Google Scholar]
- 226.Leonelli FM, Pisano E, Requarth JA, Potenza D, Tomassoni G, O’Connor W, Natale A. Frequency of superior vena cava syndrome following radiofrequency modification of the sinus node and its management. Am J Cardiol. 2000;85:771–774, A779. doi: 10.1016/s0002-9149(99)00860-7 [DOI] [PubMed] [Google Scholar]
- 227.Lin D, Garcia F, Jacobson J, Gerstenfeld EP, Dixit S, Verdino R, Callans DJ, Marchlinski FE. Use of noncontact mapping and saline-cooled ablation catheter for sinus node modification in medically refractory inappropriate sinus tachycardia. Pacing Clin Electrophysiol. 2007;30:236–242. doi: 10.1111/j.1540-8159.2007.00655.x [DOI] [PubMed] [Google Scholar]
- 228.Man KC, Knight B, Tse HF, Pelosi F, Michaud GF, Flemming M, Strickberger SA, Morady F. Radiofrequency catheter ablation of inappropriate sinus tachycardia guided by activation mapping. J Am Coll Cardiol. 2000;35:451–457. doi: 10.1016/s0735-1097(99)00546-x [DOI] [PubMed] [Google Scholar]
- 229.Bonhomme CE, Deger FT, Schultz J, Hsu SS. Radiofrequency catheter ablation using non-contact mapping for inappropriate sinus tachycardia. J Interv Card Electrophysiol. 2004;10:159–163. doi: 10.1023/B:JICE.0000019270.34716.56 [DOI] [PubMed] [Google Scholar]
- 230.Callans DJ, Ren JF, Schwartzman D, Gottlieb CD, Chaudhry FA, Marchlinski FE. Narrowing of the superior vena cava-right atrium junction during radiofrequency catheter ablation for inappropriate sinus tachycardia: analysis with intracardiac echocardiography. J Am Coll Cardiol. 1999;33:1667–1670. doi: 10.1016/s0735-1097(99)00047-9 [DOI] [PubMed] [Google Scholar]
- 231.Fleming S, Thompson M, Stevens R, Heneghan C, Pluddemann A, Maconochie I, Tarassenko L, Mant D. Normal ranges of heart rate and respiratory rate in children from birth to 18 years of age: a systematic review of observational studies. Lancet. 2011;377:1011–1018. doi: 10.1016/S0140-6736(10)62226-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Zhang GQ, Zhang W. Heart rate, lifespan, and mortality risk. Ageing Res Rev. 2009;8:52–60. doi: 10.1016/j.arr.2008.10.001 [DOI] [PubMed] [Google Scholar]
- 233.Singer W, Sletten DM, Opfer-Gehrking TL, Brands CK, Fischer PR, Low PA. Postural tachycardia in children and adolescents: what is abnormal? J Pediatr. 2012;160:222–226. doi: 10.1016/j.jpeds.2011.08.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Stewart JM, Medow MS, Visintainer P, Sutton R. When Sinus Tachycardia Becomes Too Much: Negative Effects of Excessive Upright Tachycardia on Cardiac Output in Vasovagal Syncope, Postural Tachycardia Syndrome, and Inappropriate Sinus Tachycardia. Circ Arrhythm Electrophysiol. 2020;13:e007744. doi: 10.1161/CIRCEP.119.007744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Sugimoto T, Sagawa K, Guyton AC. Effect of tachycardia on cardiac output during normal and increased venous return. Am J Physiol. 1966;211:288–292. doi: 10.1152/ajplegacy.1966.211.2.288 [DOI] [PubMed] [Google Scholar]
- 236.Stegall FH. Muscle Pumping in the Dependent Leg. Circulation Research. 1966;19:180–190. doi: 10.1161/01.RES.19.1.180 [DOI] [Google Scholar]
- 237.Fisher JP. Autonomic control of the heart during exercise in humans: role of skeletal muscle afferents. Exp Physiol. 2014;99:300–305. doi: 10.1113/expphysiol.2013.074377 [DOI] [PubMed] [Google Scholar]
- 238.Raven PB, Young BE, Fadel PJ. Arterial Baroreflex Resetting During Exercise in Humans: Underlying Signaling Mechanisms. Exerc Sport Sci Rev 2019;47:129–141. doi: 10.1249/JES.0000000000000190 [DOI] [PubMed] [Google Scholar]
- 239.Stewart JM, Javaid S, Fialkoff T, Tuma-Marcella B, Visintainer P, Terilli C, Medow MS. Initial Orthostatic Hypotension Causes (Transient) Postural Tachycardia. J Am Coll Cardiol. 2019;74:1271–1273. doi: 10.1016/j.jacc.2019.06.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Task Force for the D, Management of S, European Society of C, European Heart Rhythm A, Heart Failure A, Heart Rhythm S, Moya A, Sutton R, Ammirati F, Blanc JJ, et al. Guidelines for the diagnosis and management of syncope (version 2009). Eur Heart J. 2009;30:2631–2671. doi: 10.1093/eurheartj/ehp298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Medow MS, Merchant S, Suggs M, Terilli C, O’Donnell-Smith B, Stewart JM. Postural Heart Rate Changes in Young Patients With Vasovagal Syncope. Pediatrics. 2017;139. doi: 10.1542/peds.2016-3189 [DOI] [PMC free article] [PubMed]
- 242.Stewart JM, Pianosi P, Shaban MA, Terilli C, Svistunova M, Visintainer P, Medow MS. Postural Hyperventilation as a Cause of Postural Tachycardia Syndrome: Increased Systemic Vascular Resistance and Decreased Cardiac Output When Upright in All Postural Tachycardia Syndrome Variants. J Am Heart Assoc. 2018;7. doi: 10.1161/JAHA.118.008854 [DOI] [PMC free article] [PubMed]
- 243.Mahendru AA, Everett TR, Wilkinson IB, Lees CC, McEniery CM. A longitudinal study of maternal cardiovascular function from preconception to the postpartum period. J Hypertens. 2014;32:849–856. doi: 10.1097/HJH.0000000000000090 [DOI] [PubMed] [Google Scholar]
- 244.Savu O, Jurcut R, Giusca S, van Mieghem T, Gussi I, Popescu BA, Ginghina C, Rademakers F, Deprest J, Voigt JU. Morphological and functional adaptation of the maternal heart during pregnancy. Circ Cardiovasc Imaging. 2012;5:289–297. doi: 10.1161/CIRCIMAGING.111.970012 [DOI] [PubMed] [Google Scholar]
- 245.Guy GP, Ling HZ, Machuca M, Poon LC, Nicolaides KH. Effect of change in posture on maternal functional hemodynamics at 35–37 weeks’ gestation. Ultrasound Obstet Gynecol. 2018;51:368–374. doi: 10.1002/uog.17466 [DOI] [PubMed] [Google Scholar]
- 246.Turan OM, De Paco C, Kametas N, Khaw A, Nicolaides KH. Effect of parity on maternal cardiac function during the first trimester of pregnancy. Ultrasound Obstet Gynecol. 2008;32:849–854. doi: 10.1002/uog.5354 [DOI] [PubMed] [Google Scholar]
- 247.Loerup L, Pullon RM, Birks J, Fleming S, Mackillop LH, Gerry S, Watkinson PJ. Trends of blood pressure and heart rate in normal pregnancies: a systematic review and meta-analysis. BMC Med. 2019;17:167. doi: 10.1186/s12916-019-1399-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Green LJ, Mackillop LH, Salvi D, Pullon R, Loerup L, Tarassenko L, Mossop J, Edwards C, Gerry S, Birks J, et al. Gestation-Specific Vital Sign Reference Ranges in Pregnancy. Obstet Gynecol. 2020;135:653–664. doi: 10.1097/AOG.0000000000003721 [DOI] [PubMed] [Google Scholar]
- 249.Belham M, Patient C, Pickett J. Inappropriate sinus tachycardia in pregnancy: a benign phenomena? BMJ Case Rep. 2017;2017. doi: 10.1136/bcr-2016-217026 [DOI] [PMC free article] [PubMed]
- 250.Sharp A PC, Pickett J, Belham M. Pregnancy-related inappropriate sinus tachycardia: A cohort analysis of maternal and fetal outcomes. Obstetric Medicine. 2021. doi: 10.1177/1753495X21990196 [DOI] [PMC free article] [PubMed]
- 251.Jarvis SS, Shibata S, Bivens TB, Okada Y, Casey BM, Levine BD, Fu Q. Sympathetic activation during early pregnancy in humans. J Physiol. 2012;590:3535–3543. doi: 10.1113/jphysiol.2012.228262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Leduc L, Wasserstrum N, Spillman T, Cotton DB. Baroreflex function in normal pregnancy. Am J Obstet Gynecol. 1991;165:886–890. doi: 10.1016/0002-9378(91)90433-r [DOI] [PubMed] [Google Scholar]
- 253.Minson CT, Halliwill JR, Young TM, Joyner MJ. Influence of the menstrual cycle on sympathetic activity, baroreflex sensitivity, and vascular transduction in young women. Circulation. 2000;101:862–868. doi: 10.1161/01.cir.101.8.862 [DOI] [PubMed] [Google Scholar]
- 254.El Khoury N, Mathieu S, Marger L, Ross J, El Gebeily G, Ethier N, Fiset C. Upregulation of the hyperpolarization-activated current increases pacemaker activity of the sinoatrial node and heart rate during pregnancy in mice. Circulation. 2013;127:2009–2020. doi: 10.1161/CIRCULATIONAHA.113.001689 [DOI] [PubMed] [Google Scholar]
- 255.Sag S, Coskun H, Baran I, Gullulu S, Aydinlar A. Inappropriate sinus tachycardia-induced cardiomyopathy during pregnancy and successful treatment with ivabradine. Anatol J Cardiol. 2016;16:212–213. doi: 10.14744/AnatolJCardiol.2016.6813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Regitz-Zagrosek V, Roos-Hesselink JW, Bauersachs J, Blomstrom-Lundqvist C, Cifkova R, De Bonis M, Iung B, Johnson MR, Kintscher U, Kranke P, et al. 2018 ESC Guidelines for the management of cardiovascular diseases during pregnancy. Eur Heart J. 2018;39:3165–3241. doi: 10.1093/eurheartj/ehy340 [DOI] [PubMed] [Google Scholar]
- 257.Howard BK, Goodson JH, Mengert WF. Supine hypotensive syndrome in late pregnancy. Obstet Gynecol. 1953;1:371–377. [PubMed] [Google Scholar]
- 258.Kinsella SM, Lohmann G. Supine hypotensive syndrome. Obstet Gynecol. 1994;83:774–788. [PubMed] [Google Scholar]
- 259.Humphries A, Mirjalili SA, Tarr GP, Thompson JMD, Stone P. Hemodynamic changes in women with symptoms of supine hypotensive syndrome. Acta Obstet Gynecol Scand. 2020;99:631–636. doi: 10.1111/aogs.13789 [DOI] [PubMed] [Google Scholar]
- 260.Hughes EJ, Price AN, McCabe L, Hiscocks S, Waite L, Green E, Hutter J, Pegoretti K, Cordero-Grande L, Edwards AD, et al. The effect of maternal position on venous return for pregnant women during MRI. NMR Biomed. 2021;34:e4475. doi: 10.1002/nbm.4475 [DOI] [PubMed] [Google Scholar]
- 261.Holmes F. The supine hypotensive syndrome. Its importance to the anaesthetist. Anaesthesia. 1960;15:298–306. doi: 10.1111/j.1365-2044.1960.tb13341.x [DOI] [PubMed] [Google Scholar]
- 262.Lanni SM, Tillinghast J, Silver HM. Hemodynamic changes and baroreflex gain in the supine hypotensive syndrome. Am J Obstet Gynecol. 2002;187:1636–1641. doi: 10.1067/mob.2002.127304 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.